David A. Peura 1,5*, Anne Le Moigne 2, Heather Wassel 3 and Charles Pollack 4
|
|
|
- Laureen Robinson
- 5 years ago
- Views:
Transcription
1 Peura et al. BMC Gastroenterology (2018) 18:69 RESEARCH ARTICLE Open Access Sustained efficacy following resolution of frequent heartburn with an over-thecounter regimen of esomeprazole 20 mg or placebo for 14 days: two randomized trials David A. Peura 1,5*, Anne Le Moigne 2, Heather Wassel 3 and Charles Pollack 4 Abstract Background: A two-week course of therapy with an over-the-counter proton-pump inhibitor (PPI) is recommended for frequent heartburn. Limited research has been conducted on the sustained efficacy of short-term PPI therapy after treatment cessation. Esomeprazole 20 mg was evaluated in the seven-day follow-up period after the two-week treatment period using pooled data from two identical randomized, double-blind, placebo-controlled studies. Methods: Adults without confirmed diagnoses of gastroesophageal reflux disease experiencing heartburn at least two days/week in the past four weeks were eligible. Subjects received treatment with esomeprazole 20 mg or placebo once daily for 14 days. Heartburn episodes were documented using daily diaries. Missing data during the two-week treatment period were assumed to be days with heartburn. The proportion of subjects with heartburn resolution while on treatment and during the seven days of follow-up was assessed. Predictors of resolution during this post-treatment period were evaluated using a stepwise logistic regression model. Results: All subjects in the pooled analysis set who reported diary data for at least three follow-up days were analyzed (N = 584). This cut-off was used to ensure that sufficient data were collected for these analyses. Greater run-in heartburn frequency was a significant negative predictor of heartburn resolution during follow-up (P < 0.001). Among the on-treatment efficacy variables, the best predictor of resolution during follow-up was resolution during the last seven days of treatment (odds ratio: 3.81 [95% confidence interval: 2.40, 6.05; P < ]). Conclusions: Lower baseline heartburn frequency and heartburn resolution during the last seven days of treatment were associated with a greater likelihood of heartburn resolution during the seven-day follow-up. Trial registration: Registered at ClinicalTrials.gov June 11, 2011: NCT ; NCT Keywords: Esomeprazole, Proton pump inhibitor, Heartburn, Gastric acid/secretion Background Frequent heartburn and regurgitation occur commonly in the global population, with incidence rates ranging from approximately 10% to 30% in Western countries [1]. Proton-pump inhibitors (PPIs) are highly effective for treating these symptoms [2] and have become * Correspondence: DAP8V@hscmail.mcc.virginia.edu 1 University of Virginia, School of Medicine, Charlottesville, VA 22908, USA 5 Department of Medicine, Division of Gastroenterology and Hepatology, University of Virginia, School of Medicine, 1215 Lee Street, Charlottesville, VA , USA Full list of author information is available at the end of the article available worldwide without a prescription for selftreating frequent heartburn [3, 4]. Esomeprazole is a PPI that has recently become available over-the-counter (OTC) for treating frequent heartburn at a dose of 20 mg once daily for 14 days [5]. The efficacy of esomeprazole in the OTC setting has been demonstrated in two randomized, double-blind, placebo-controlled, phase 3 clinical trials in adults with frequent heartburn [6]. The World Gastroenterology Organization s guidelines for treating frequent heartburn (two or more days/week) recommend a two-week course of treatment with an OTC PPI along with lifestyle and dietary modifications [3]. In The Author(s) Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( applies to the data made available in this article, unless otherwise stated.
2 Peura et al. BMC Gastroenterology (2018) 18:69 Page 2 of 8 the US, OTC esomeprazole 20 mg is approved for 14 days of treatment for frequent heartburn, a treatment course that can be repeated once every four months; however, if symptoms persist or recur within this time frame the individual should consult a physician [3, 5]. The effects of discontinuing PPI therapy have been studied primarily in the context of rebound acid hypersecretion after long-term use and the development of effective discontinuation strategies to reduce unnecessary use of PPIs [7, 8]. However, analyses assessing the degree to which the symptomatic response is sustained after discontinuation of a short-term course of OTC PPIs are limited. Two studies conducted with an OTC PPI failed to show continued benefit during the week after the completion of active treatment [9]. Determining whether the level of heartburn resolution while on treatment has an impact on the duration of response following treatment cessation is therefore important to clinical practice. The current post hoc analyses were conducted with data collected during two phase 3 clinical trials that assessed a 14-day treatment regimen with esomeprazole 20 mg or placebo once daily in subjects experiencing frequent heartburn who are likely to self-treat these symptoms [6]. The primary objective of these analyses was to evaluate the impact of reaching resolution of heartburn at different time points throughout the treatment period on the durability of response during a one-week follow-up period at the end of active treatment. Additionally, a secondary objective was to analyze subject-level data to determine clinical factors that may be related to experiencing a sustained treatment response. Methods NEXT-1 and NEXT-2 were two identical phase 3, randomized, double-blind, placebo-controlled studies designed to determine the efficacy of a 14-day regimen of esomeprazole 20 mg for treating frequent heartburn in subjects who are likely to self-treat with OTC medications without consulting a healthcare provider. Data from these studies, which were registered at ClinicalTrials.gov under identifiers NCT and NCT , were pooled for the current analyses. The methodology for conducting these studies has been published in detail previously [6] and is summarized briefly here. Study design Both studies enrolled otherwise healthy male and female subjects aged 18 years who had experienced frequent heartburn on two or more days per week over the prior four weeks. Exclusion criteria included confirmed diagnosis of gastroesophageal reflux disease (GERD) or any history of erosive esophagitis, current use of prescription medications for GERD, or a need for continuous treatment with prescription histamine H 2 receptor antagonists (H 2 RAs), PPIs, gastric prokinetic drugs, or antacids throughout the study for any indication. Subjects who required more than one 14-day course of PPI therapy over the past four months and three or more 14-day courses of PPI therapy during thepastyearwereexcluded. Informed consent was obtained from all individual participants included in these studies, which were conducted in accordance with the Declaration of Helsinki. The informed consent form and study protocol for each study were approved by Alpha Independent Review Board (San Clemente, California, USA) [6]. Following heartburn medication washout (one or more days for antacids and seven or more days for H 2 RAs and/or PPIs), eligible subjects were enrolled in a seven-day placebo run-in period. After completing the run-in period, subjects who continued to meet the inclusion criteria and were compliant with reporting heartburn symptoms were randomly assigned to 14 days of double-blind treatment with either esomeprazole 20 mg or placebo administered once daily. At the end of the 14-day active treatment period, all subjects subsequently entered a one-week single-blind placebo follow-up period. Because the investigators were aware of this aspect of the study design, only subjects remained blinded to the treatment they were receiving during the follow-up period. Although unblinding the investigators generally carries the risk of introducing bias, the subjects were responsible for recording their symptoms, so sustaining the blind for the subjects would notbeexpectedtohaveanimpactonthereportingoftheir symptoms. Treatments Eligible subjects were randomly assigned to receive 14 days of double-blind treatment with esomeprazole 20 mg capsules (administered as esomeprazole magnesium trihydrate 22.3 mg) or matching placebo. To reflect the instructions for using OTC PPIs, subjects were instructed to take study drugs once daily before eating their morning meal. Subjects were permitted to use antacid tablets (Gelusil tablets; WellSpring Pharmaceutical Corporation, Sarasota, Florida, USA) as a rescue medication for managing breakthrough symptoms; however, the use of other prescription or OTC heartburn treatments was prohibited during the study period. Outcome measures of current analyses The primary efficacy endpoint for both studies was the percentage of heartburn-free 24-h days during the 14 days of treatment as measured by daily self-assessment diaries that utilized an interactive voice response system. Subjects were asked to rate their overall heartburn severity over the past 24 h using the following scale: 0 = none; 1 = mild; 2 = moderate; 3 = severe. The primary results, which demonstrate significant improvements for esomeprazole 20 mg
3 Peura et al. BMC Gastroenterology (2018) 18:69 Page 3 of 8 over placebo on all primary and secondary endpoints, have been previously published [6]. The primary efficacy outcome of interest for the current analyses was resolution of heartburn during the follow-up period, which was defined as one or no days of heartburn. Secondary endpoints for these analyses included heartburn resolution and the number of heartburn-free days during weeks 1 and 2 individually, over the entire two-week treatment period, and during the last seven days of treatment, which included the last seven consecutive days when the subjects received randomized study medication. Resolution was defined as one or no days (week 1 and 2, and last seven days of treatment) or two or fewer (two-week treatment period) days of heartburn during the period of interest. These outcomes were all planned secondary endpoints inthetwophase3trials. Statistical analyses The analyses presented in this report are based on pooled data from the full analysis set from NEXT-1 and NEXT-2. The full analysis set consisted of all subjects who received at least one dose of randomized treatment and had a baseline and at least one post-baseline efficacy measurement recorded. Additionally, subjects in the pooled full analysis set who had reported diary data for three or more days during the seven-day follow-up period were included in these analyses. Because these analyses focused on symptom response during the follow-up period,thiscut-offwasusedtoensurethatadequate data were collected during this period to evaluate heartburn resolution without excluding too many subjects. Stepwise logistic regression models were developed to identify variables during the run-in and on-treatment periods that were the best predictors of resolution in the follow-up period, regardless of treatment group assignment. Variables included in the first model were the number of days with heartburn during the run-in period and heartburn resolution during week 1, week 2, the entire two-week treatment period, and the last seven days of treatment. Variables included in the second model were the number of days with heartburn during the run-in period and number of days without heartburn during week 1, week 2, the entire two-week treatment period, and the last seven days of treatment. A mixed-effect model was also used to determine which variables were good predictors of the number of days with heartburn during the follow-up period. Variables included in this model were the number of days without heartburn during week 1, week 2, the entire two-week treatment period, and the last seven days of treatment. All of the mixed models included the number of days with heartburn during the run-in period as a covariate. The proportion of subjects with heartburn resolution and the number of heartburn-free days during each treatment period investigated are reported with descriptive statistics. Results A total of 584 subjects had complete diary data for at least three days during the placebo follow-up period, representing 89.7% of the total full analysis set population enrolled in the two studies (n = 651). Baseline demographics for this population are presented in Table 1 and are similar to the complete study population for the two trials [6]. Subjects in this subgroup were primarily female (56 57%) and years of age and experienced a mean of 5.7 days of heartburn during the run-in period. In the total study population, 55 58% of subjects were female, and their mean age (43 44 years) and number of run-in heartburn days ( ) were generally consistent with the subgroup used for these analyses. Resolution and number of heartburn-free days During each on-treatment time interval that was analyzed, subjects treated with esomeprazole 20 mg had higher rates of resolution (defined as one or no or two day(s) with heartburn) versus those treated with placebo (Fig. 1). During the last seven days of treatment, 27.2% of subjects treated with esomeprazole 20 mg had resolution of heartburn, compared with 11.0% in the placebo group. Similarly, as shown in Fig. 2, the mean number of heartburn-free days was higher for subjects in the esomeprazole 20 mg group versus the placebo group during each period. During the last seven days of treatment the mean number of heartburn-free days was 3.4 and 2.2 in the esomeprazole 20 mg and placebo groups, respectively. The mean number of heartburn-free days during the entire two-week treatment period for esomeprazole 20 mg and placebo was 6.1 days and 3.9 days, respectively. Table 1 Baseline demographics Characteristic Age, years Esomeprazole 20 mg (n = 294) Placebo (n = 290) Mean (SD) 43.1 (13.0) 44.9 (12.6) Female, n (%) 166 (56.5) 162 (55.9) Race, n (%) White 186 (63.3) 200 (69.0) Black/African American 100 (34.0) 87 (30.0) Asian American Indian/Alaskan native 0 (0) 3 (1.0) 1 (0.3) 0 (0) Native Hawaiian/Pacific Islander 1 (0.3) 0 (0) Other 4 (1.4) 2 (0.7) Run-in Heartburn Frequency, days Mean (SD) 5.7 (1.8) 5.7 (1.8) SD standard deviation
4 Peura et al. BMC Gastroenterology (2018) 18:69 Page 4 of 8 50 Esomeprazole 20 mg (n = 294) Placebo (n = 290) Subjects with heartburn resolution, % Week 1 a Week 2 a Weeks 1 2 b Last 7 days a Fig. 1 Percentage of subjects with heartburn resolution at selected time points during treatment. Days with missing data were assumed to be days with heartburn. a One or no days with heartburn. b Two or fewer days with heartburn Predictors of heartburn resolution during follow-up period Of the subjects who achieved heartburn resolution during the last seven days of treatment, 54.5% maintained resolution in the follow-up period, compared with 19.1% of those who had not achieved resolution during the last seven days of treatment (Fig. 3). These same subjects had a mean of 5.7 days with heartburn during treatment, compared with 10.1 days for subjects not achieving heartburn resolution during the follow-up period (Fig. 4). The stepwise logistic regression model identified several key predictors of heartburn resolution during the placebo follow-up period, independent of treatment randomization to esomeprazole 20 mg or placebo. This model demonstrated that a greater frequency of run-in heartburn was a significant negative predictor of heartburn resolution during the follow-up period, with an adjusted odds ratio (OR) of 0.68 (95% confidence interval [CI]: ) for each additional 1-day increase in heartburn frequency during the run-in phase (P < in both logistic regression models; Table 2). Among the on-treatment efficacy variables included in the stepwise logistic regression model focusing on resolution parameters, the best predictor of heartburn resolution during the follow-up period was 10 Esomeprazole 20 mg (n = 294) Placebo (n = 290) Number of heartburn-free days, mean Week 1 Week 2 Weeks 1 2 Last 7 days Fig. 2 Mean number of heartburn-free days at selected time points during treatment. Days with missing data were assumed to be days with heartburn
5 Peura et al. BMC Gastroenterology (2018) 18:69 Page 5 of Resolution During Follow-up Period No Yes 90 Subjects with resolution during follow-up period, % n = 59 n = 525 n = 112 n = 472 Yes No Yes No 2-Week treatment period a Last 7 days of treatment b Resolution During Treatment Period Fig. 3 Heartburn resolution status at follow-up by status during the on-treatment period. a Two or fewer days with heartburn. b One or no days with heartburn heartburn resolution (one or no days of heartburn) during the last seven days of treatment, with an OR of 3.81 (95% CI: ; P < ; Table 2). For the stepwise logistic regression model that was conducted to identify predictors of heartburn resolution during the follow-up period with independent variables, focusing on the number of heartburn-free days in each treatment period (i.e., week 1, week 2, two weeks of treatment, and the last seven days of treatment), the best predictor of heartburn resolution was the number of days without heartburn during the full two-week treatment period. The OR for this endpoint was 1.24 (95% CI: ; P< ), indicating that while holding the number of days with heartburn during run-in at a fixed value, every 1-day increase in heartburn-free days 15 2-Week Treatment Period Last 7 Days of Treatment Number of days with heartburn, mean Yes No Yes No Resolution a During Follow-up Period Fig. 4 Number of days with heartburn during the on-treatment period by resolution status at follow-up. a One or no days with heartburn
6 Peura et al. BMC Gastroenterology (2018) 18:69 Page 6 of 8 Table 2 Predictors of heartburn resolution during the follow-up period Independent Variables Selected for Final Models Resolution Adjusted Odds Ratio (95% Wald CI) No. of days with HB during run-in period For every 1-day increase in run-in HB frequency 0.68 ( ) Resolution during the last seven days Yes vs. no 3.81 ( ) No. of HB-Free Days Adjusted Odds Ratio (95% Wald CI) No. of days with HB during run-in period For every 1-day increase in run-in HB frequency 0.79 ( ) No. of HB-free days during two weeks of treatment For every 1-day increase in HB-free days 1.24 ( ) CI confidence interval, HB heartburn during the two-week treatment period increased the odds of heartburn resolution during the follow-up period by 24% (Table 2). In a separate mixed model analysis that was conducted to identify predictors of the number of days with heartburn during the follow-up period, the best model included the number of days without heartburn during the entire two-week treatment period. For every additional day without heartburn during the entire two-week treatment period there was a reduction in the number of days with heartburn during the seven-day follow-up period of 0.22 days (95% CI: ; P < ). These results indicate that, for example, holding the number of days with heartburn during the run-in as a constant value, if an individual experienced 10 heartburn-free days while on treatment it is estimated that they would have 2.2 fewer days of heartburn during follow-up than if they had experienced zero heartburn-free days while on treatment. The number of days with heartburn during the seven-day run-in period was also predictive of the number of days with heartburn during the follow-up period. For each additional day of heartburn during the run-in period the number of days with heartburn during the followup period increased by 0.27 days (95% CI: ; P < ). Discussion The current analyses demonstrate that a greater frequency of heartburn prior to initiating treatment with esomeprazole 20 mg was associated with a decreased likelihood of sustained symptom resolution during the seven days after the cessation of treatment. In the publication describing the secondary endpoints of these studies, which also included a post hoc, pooled analysis of heartburn-free days during the follow-up period, the between-group differences on this outcome favored esomeprazole 20 mg significantly over placebo (difference: 5.7 [95% CI: ; P = 0.018) [10]. Additionally, the number of heartburn-free days remained significantly higher (P < 0.001) during the post-treatment period than the run-in phase, indicating that there was no symptomatic rebound at the end of the active treatment phase. To our knowledge, no studies that examined the degree to which treatment response is sustained following the discontinuation of short-term PPI therapy have demonstrated a significant effect after treatment has ended. During two similarly designed studies that were conducted with lansoprazole 15 mg for 14 days for treating frequent heartburn, no significant differences between active treatment and placebo were observed in the percentage of heartburn-free days (44 to 47%) during the 7-day placebo follow-up periods [9]. A prior analysis of two different esomeprazole trials that were conducted to assess the effect of treatment on reflux-related sleep disturbances found that a higher frequency of sleep disturbances during the run-in period was associated with a lower rate of sleep disturbance resolution during the 14-day treatment period [11]. Additionally, an absence of daytime or nighttime heartburn during the active treatment period was associated with a greater likelihood of resolution of reflux-related sleep disturbances. Bardhan et al. demonstrated that subjects receiving an initial two weeks of treatment with ranitidine or omeprazole followed by an additional two- to four-week course of on-demand treatment were able to remain off treatment for a median of 142 days, with only 22% of those receiving omeprazole being transferred to maintenance therapy [12]. Additionally, following four weeks of treatment for non-erosive GERD with rabeprazole 20 mg, continued on-demand treatment during the six months of follow-up was low, with an average intake of rabeprazole of tablets/day [13]. These results suggest a durability of treatment effects following the resolution or significant reduction of reflux symptoms (one or no days/week) with short-term PPI treatment. PPIs treat reflux-related symptoms by irreversibly binding to gastric proton pumps, which suppresses acid secretion, an effect that can last for as long as two days following the cessation of treatment [14]. Pharmacokinetic analyses of esomeprazole 20 mg report a half-life of approximately one hour in plasma [15], which does not provide an accurate representation of the continued pharmacodynamic effects of esomeprazole after treatment has ended. Because
7 Peura et al. BMC Gastroenterology (2018) 18:69 Page 7 of 8 esomeprazole irreversibly binds to gastric proton pumps, the recurrence of symptoms at the end of active treatment is primarily dependent upon new proton pumps being synthesized [14, 16 18]. This process of restoring acid secretion following single dose administration of PPIs has been estimated to have half-times ranging from 14 to 46 h [14, 16, 18, 19]. Rebound acid hypersecretion is felt to underlie the rapid return of dyspeptic/reflux symptoms following the cessation of PPI therapy. This phenomenon has been observed primarily in healthy volunteers [20, 21], and studies conducted in individuals with heartburn have found little evidence of rebound acid hypersecretion or symptom rebound following short-term or on-demand PPI therapy [22, 23]. The potential for rebound acid hypersecretion and symptom rebound in individuals who self-diagnose and self-treat with OTC PPIs could therefore be of potential concern, but it remains to be elucidated what the level of risk is in this population. Importantly, the data presented here suggest there is no evidence of symptom rebound although rebound acid hypersecretion was not studied. In these analyses, the most important predictors of sustained symptom control during the follow-up period were a lower incidence of heartburn frequency at baseline, heartburn resolution during the last seven days of treatment, and a greater number of days without heartburn during the two-week treatment period. In the previously described study of intermittent omeprazole treatment, which is similar to the current study, the most important predictor of sustained symptom control was response to the initial two weeks of treatment [12]. The analyses of predictors of response conducted here included subjects who received both esomeprazole and placebo, so resolution of symptoms following the treatment period in these analyses relates to symptoms during treatment, rather than to a specific treatment (esomeprazole 20 mg vs placebo). However, only a small minority of placebo-treated patients experienced heartburn resolution during the two-week treatment period, and treatment with esomeprazole was the best predictor of symptom resolution during that period [6]. Overall, we think these results can inform decision-making about the most appropriate treatment options for those with heartburn by indicating that individuals who do not achieve resolution of their heartburn during the last week of a two-week course of PPI therapy may require a change in therapy due to the increased risk for recurrences. Furthermore, because of the potential safety concerns associated with high-dose, long-term PPI therapy [3], initiating treatment for heartburn with an OTC PPI in an attempt to limit unnecessary exposure should be considered. Although the data presented here are based on results from two well-controlled phase 3 studies, these analyses have some weaknesses that are worth noting. The data included in these analyses were collected prospectively, but the current analyses were post hoc in nature. Furthermore, whilewehavespeculatedonthemechanismsbehindthe continued resolution of heartburn symptoms after treatment cessation, future prospective research that explores this topic may benefit from monitoring gastric acid secretion during the post-treatment period to determine the degree to which antisecretory effects are sustained in a quantitative as well as a qualitative manner. Finally, the post-treatment follow-up period reported here extended for only seven days, so future studies should aim to evaluate symptom control during a period of time that more accurately reflects real-world use. Conclusions The results of these post hoc analyses indicate that the degree of symptom resolution observed during esomeprazole therapy that is consistent with OTC use is associated with the degree of continued symptom relief following the cessation of treatment. Less-frequent pre-treatment heartburn, achieving heartburn resolution during the last seven days of treatment, and fewer days with heartburn throughout the treatment period were predictors of sustained heartburn resolution during the seven-day follow-up period that subjects entered after discontinuing 14 days of treatment with esomeprazole 20 mg. Among individuals with frequent heartburn who are likely to self-treat their symptoms without consulting a healthcare provider, esomeprazole 20 mg for 14 days in the OTC setting is associated with sustained therapeutic benefits, including after the treatment period has ended. Abbreviations CI: Confidence interval; GERD: Gastroesophageal reflux disease; H 2 RA: Histamine H 2 receptor antagonist; HB: Heartburn; OR: Odds ratio; OTC: Over-the-counter; PPI: Proton-pump inhibitor; SD: Standard deviation Funding The studies were funded by AstraZeneca, which entered into an agreement with Pfizer for the over-the-counter (OTC) rights for NEXIUM (esomeprazole magnesium). Medical writing support was provided by Dennis Stancavish, MA, of Peloton Advantage, LLC, and was funded by Pfizer. This manuscript includes data owned by AstraZeneca. Availability of data and materials The dataset supporting the results of this article is included within the article. The data supporting the conclusions of this article are not available in a public repository but can be obtained upon request from the corresponding author, David A. Peura, MD, in accordance with AstraZeneca and Pfizer Consumer Healthcare policies regarding the sharing of AstraZeneca datasets. Authors contributions Study design: DAP, AL, HW, CP. Data analysis/interpretation: DAP, AL, HW, CP. Critical revision and review of the manuscript: DAP, AL, HW, CP. Project/data management: CP, AL. Statistical analyses: AL, HW. Approval of final draft for submission: DAP, AL, HW, CP. AL, HW, and CP were variously involved in the study design, data analysis/interpretation, project/data management, statistical analyses, critical revision and review of the manuscript, and approval of the final draft for submission.
8 Peura et al. BMC Gastroenterology (2018) 18:69 Page 8 of 8 Ethics approval and consent to participate Informed consent was obtained from all individual participants included in these studies, which were conducted in accordance with the Declaration of Helsinki. The informed consent form and study protocol for each study were approved by Alpha Independent Review Board (San Clemente, California, USA). Competing interests DAP has served as a consultant for AstraZeneca, Pfizer, Takeda, and Horizon. AL is an employee of Pfizer Consumer Healthcare. HW is an employee of Pfizer Consumer Healthcare. CP is a former employee of Pfizer Consumer Healthcare. Publisher s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Author details 1 University of Virginia, School of Medicine, Charlottesville, VA 22908, USA. 2 Clinical Excellence and Biometrics, Pfizer Consumer Healthcare, Madison, NJ 07940, USA. 3 Pfizer Consumer Healthcare, Madison, NJ, USA. 4 Former Senior Director, Global R&D, Pfizer Consumer Healthcare, Madison, NJ 07940, USA. 5 Department of Medicine, Division of Gastroenterology and Hepatology, University of Virginia, School of Medicine, 1215 Lee Street, Charlottesville, VA , USA. Received: 16 November 2017 Accepted: 30 April 2018 References 1. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6): Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;5: CD Hunt R, Quigley E, Abbas Z, Eliakim A, Emmanuel A, Goh KL, et al. Coping with common gastrointestinal symptoms in the community: a global perspective on heartburn, constipation, bloating, and abdominal pain/ discomfort May J Clin Gastroenterol. 2014;48(7): Boardman HF, Heeley G. The role of the pharmacist in the selection and use of over-the-counter proton-pump inhibitors. Int J Clin Pharm. 2015; 37(5): Nexium 24HR [product labeling]. Madison, NJ: Pfizer Consumer Healthcare; Peura DA, Traxler B, Kocun C, Lind T. Esomeprazole treatment of frequent heartburn: two randomized, double-blind, placebo-controlled trials. Postgrad Med. 2014;126(4): Hunfeld NG, Geus WP, Kuipers EJ. Systematic review: rebound acid hypersecretion after therapy with proton pump inhibitors. Aliment Pharmacol Ther. 2007;25(1): Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbol DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6): Kushner PR, Snoddy AM, Gilderman L, Peura DA. Lansoprazole 15 mg once daily for 14 days is effective for treatment of frequent heartburn: results of 2 randomized, placebo-controlled, double-blind studies. Postgrad Med. 2009; 121(4): Peura D, Le Moigne A, Pollack C, Nagy P, Lind T. A 14-day regimen of esomeprazole 20 mg/day for frequent heartburn: durability of effects, symptomatic rebound, and treatment satisfaction. Postgrad Med. 2016; 128(6): Johnson DA, Le Moigne A, Li J, Pollack C, Nagy P. Analysis of clinical predictors of resolution of sleep disturbance related to frequent nighttime heartburn and acid regurgitation symptoms in individuals taking esomeprazole 20 mg or placebo. Clin Drug Investig. 2016;36(7): Bardhan KD, Muller-Lissner S, Bigard MA, Bianchi PG, Ponce J, Hosie J, et al. Symptomatic gastro-oesophageal reflux disease: double blind controlled study of intermittent treatment with omeprazole or ranitidine. The European Study Group. BMJ. 1999;318(7182): Ponce J, Arguello L, Bastida G, Ponce M, Ortiz V, Garrigues V. On-demand therapy with rabeprazole in nonerosive and erosive gastroesophageal reflux disease in clinical practice: effectiveness, health-related quality of life, and patient satisfaction. Dig Dis Sci. 2004;49(6): Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6): Hassan-Alin M, Andersson T, Bredberg E, Rohss K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol. 2000;56(9 10): Gedda K, Scott D, Besancon M, Lorentzon P, Sachs G. Turnover of the gastric H+,K(+)-adenosine triphosphatase alpha subunit and its effect on inhibition of rat gastric acid secretion. Gastroenterology. 1995;109(4): Ferron GM, McKeand W, Mayer PR. Pharmacodynamic modeling of pantoprazole's irreversible effect on gastric acid secretion in humans and rats. J Clin Pharmacol. 2001;41(2): Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+,K+-ATPase activity. J Biol Chem. 1985;260(25): Im WB, Blakeman DP, Davis JP. Irreversible inactivation of rat gastric (H+-K+)-ATPase in vivo by omeprazole. Biochem Biophys Res Commun. 1985;126(1): Reimer C, Sondergaard B, Hilsted L, Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 2009;137(1): Niklasson A, Lindstrom L, Simren M, Lindberg G, Bjornsson E. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double-blind placebo-controlled trial. Am J Gastroenterol. 2010;105(7): Juul-Hansen P, Rydning A. Clinical and pathophysiological consequences of on-demand treatment with PPI in endoscopy-negative reflux disease. Is rebound hypersecretion of acid a problem? Scand J Gastroenterol. 2011; 46(4): Lodrup AB, Reimer C, Bytzer P. Systematic review: symptoms of rebound acid hypersecretion following proton pump inhibitor treatment. Scand J Gastroenterol. 2013;48(5):
1 Introduction. David A. Johnson 1 Anne Le Moigne. Peter Nagy 3
 Clin Drug Investig (2016) 36:531 538 DOI 10.1007/s40261-016-0398-7 ORIGINAL RESEARCH ARTICLE Analysis of Clinical Predictors of Resolution of Sleep Disturbance Related to Frequent Nighttime Heartburn and
Clin Drug Investig (2016) 36:531 538 DOI 10.1007/s40261-016-0398-7 ORIGINAL RESEARCH ARTICLE Analysis of Clinical Predictors of Resolution of Sleep Disturbance Related to Frequent Nighttime Heartburn and
Unmet Needs in the Management of Gastroesophageal Reflux Disease
 Unmet Needs in the Management of Gastroesophageal Reflux Disease Ronnie Fass MD Professor of Medicine Case Western Reserve University Chairman, Division of Gastroenterology and Hepatology Director, Esophageal
Unmet Needs in the Management of Gastroesophageal Reflux Disease Ronnie Fass MD Professor of Medicine Case Western Reserve University Chairman, Division of Gastroenterology and Hepatology Director, Esophageal
Heartburn is a common symptom among adults in
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:553 563 Early Heartburn Relief With Proton Pump Inhibitors: A Systematic Review and Meta-analysis of Clinical Trials KENNETH R. MCQUAID*, and LOREN LAINE
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:553 563 Early Heartburn Relief With Proton Pump Inhibitors: A Systematic Review and Meta-analysis of Clinical Trials KENNETH R. MCQUAID*, and LOREN LAINE
Nexium 24HR. Tools and information for you and your pharmacy team NOW OTC FOR FREQUENT HEARTBURN. Consumer Healthcare Pfizer Inc.
 NOW OTC FOR FREQUENT HEARTBURN w e N Nexium 24HR P H A R M A S S I S T K I T Tools and information for you and your pharmacy team 2014 Pfizer Inc. NXM041468 05/14 Q: What is the indication for Nexium 24HR
NOW OTC FOR FREQUENT HEARTBURN w e N Nexium 24HR P H A R M A S S I S T K I T Tools and information for you and your pharmacy team 2014 Pfizer Inc. NXM041468 05/14 Q: What is the indication for Nexium 24HR
Drug Class Review on Proton Pump Inhibitors
 Drug Class Review on Proton Pump Inhibitors Final Report Update 4 July 2006 Original Report Date: November 2002 Update 1 Report Date: April 2003 Update 2 Report Date: April 2004 Update 3 Report Date: May
Drug Class Review on Proton Pump Inhibitors Final Report Update 4 July 2006 Original Report Date: November 2002 Update 1 Report Date: April 2003 Update 2 Report Date: April 2004 Update 3 Report Date: May
Review article: gastric acidity ) comparison of esomeprazole with other proton pump inhibitors
 Aliment Pharmacol Ther 2003; 17 (Suppl. 1): 10 15. Review article: gastric acidity ) comparison of esomeprazole with other proton pump inhibitors J. G. HATLEBAKK Department of Medicine, Haukeland Sykehus,
Aliment Pharmacol Ther 2003; 17 (Suppl. 1): 10 15. Review article: gastric acidity ) comparison of esomeprazole with other proton pump inhibitors J. G. HATLEBAKK Department of Medicine, Haukeland Sykehus,
GERD: 2014 Dilemmas and Solutions. Ronnie Fass MD, FACP Professor of Medicine Case Western Reserve University
 GERD: 2014 Dilemmas and Solutions Ronnie Fass MD, FACP Professor of Medicine Case Western Reserve University How to Maximize Your PPI Treatment? Improve compliance and adherance Fass R. Am J Gastroenterol.
GERD: 2014 Dilemmas and Solutions Ronnie Fass MD, FACP Professor of Medicine Case Western Reserve University How to Maximize Your PPI Treatment? Improve compliance and adherance Fass R. Am J Gastroenterol.
The long-term safety and established efficacy of proton ORIGINAL ARTICLES
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2004;2:17 21 ORIGINAL ARTICLES Self-Selection and Use Patterns of Over-the-Counter Omeprazole for Frequent Heartburn A. MARK FENDRICK,*, MICHAEL SHAW, BERNARD SCHACHTEL,
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2004;2:17 21 ORIGINAL ARTICLES Self-Selection and Use Patterns of Over-the-Counter Omeprazole for Frequent Heartburn A. MARK FENDRICK,*, MICHAEL SHAW, BERNARD SCHACHTEL,
Intragastric acidity during treatment with esomeprazole 40 mg twice daily or pantoprazole 40 mg twice daily a randomized, two-way crossover study
 Aliment Pharmacol Ther 2005; 21: 963 967. doi: 10.1111/j.1365-2036.2005.02432.x Intragastric acidity during treatment with esomeprazole 40 mg twice daily or pantoprazole 40 mg twice daily a randomized,
Aliment Pharmacol Ther 2005; 21: 963 967. doi: 10.1111/j.1365-2036.2005.02432.x Intragastric acidity during treatment with esomeprazole 40 mg twice daily or pantoprazole 40 mg twice daily a randomized,
Nexium 24HR Pharmacy Training
 Nexium 24HR Pharmacy Training Your pharmacist's advice is required. Always read the label. Use only as directed. If symptoms persist, consult your doctor/ healthcare professional. Pfizer Consumer Healthcare
Nexium 24HR Pharmacy Training Your pharmacist's advice is required. Always read the label. Use only as directed. If symptoms persist, consult your doctor/ healthcare professional. Pfizer Consumer Healthcare
Review article: pharmacology of esomeprazole and comparisons with omeprazole
 Aliment Pharmacol Ther 2003; 17 (Suppl. 1): 5 9. Review article: pharmacology of esomeprazole and comparisons with omeprazole J. DENT Department of Gastroenterology, Hepatology and General Medicine, Royal
Aliment Pharmacol Ther 2003; 17 (Suppl. 1): 5 9. Review article: pharmacology of esomeprazole and comparisons with omeprazole J. DENT Department of Gastroenterology, Hepatology and General Medicine, Royal
Effective Health Care
 Effective Health Care Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease Executive Summary Background Gastroesophageal reflux disease (GERD), defined as weekly heartburn
Effective Health Care Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease Executive Summary Background Gastroesophageal reflux disease (GERD), defined as weekly heartburn
Alginates Extended Abstract
 Alginates Extended Abstract III) Clinical practice guidelines: DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux
Alginates Extended Abstract III) Clinical practice guidelines: DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux
Drug Class Review Proton Pump Inhibitors
 Drug Class Review Proton Pump Inhibitors Evidence Tables April 2009 Update 4: May 2006 Update 3: May 2005 Update 2: April 2004 Update 1: April 2003 Original Report: November 2002 The literature on this
Drug Class Review Proton Pump Inhibitors Evidence Tables April 2009 Update 4: May 2006 Update 3: May 2005 Update 2: April 2004 Update 1: April 2003 Original Report: November 2002 The literature on this
FREQUENTLY ASKED QUESTIONS
 When it comes to your health, it s normal to have a lot of questions. Whether you ve got questions about when and where Nexium 24HR is available to purchase, or how Nexium 24HR works and should be taken,
When it comes to your health, it s normal to have a lot of questions. Whether you ve got questions about when and where Nexium 24HR is available to purchase, or how Nexium 24HR works and should be taken,
Rpts. GENERAL General Schedule (Code GE) Program Prescriber type: Dental Medical Practitioners Nurse practitioners Optometrists Midwives
 Esomeprazole 20mg Name, Restriction, Manner of esomeprazole 20 mg enteric tablet, 30 (8886Q) (029W) Gastric ulcer Peptic ulcer Treatment Phase: Initial treatment The therapy must be for initial treatment
Esomeprazole 20mg Name, Restriction, Manner of esomeprazole 20 mg enteric tablet, 30 (8886Q) (029W) Gastric ulcer Peptic ulcer Treatment Phase: Initial treatment The therapy must be for initial treatment
Many patients with gastroesophageal reflux
 ... HEALTH ECONOMICS... Efficacy and Cost Effectiveness of Lansoprazole Versus Omeprazole in Maintenance Treatment of Symptomatic Gastroesophageal Reflux Disease Eva Vivian, PharmD; Anthony Morreale, PharmD,
... HEALTH ECONOMICS... Efficacy and Cost Effectiveness of Lansoprazole Versus Omeprazole in Maintenance Treatment of Symptomatic Gastroesophageal Reflux Disease Eva Vivian, PharmD; Anthony Morreale, PharmD,
Drug Class Review on Proton Pump Inhibitors
 Drug Class Review on Proton Pump Inhibitors Evidence Tables July 2006 Original Report Date: November 2002 Update 1 Report Date: April 2003 Update 2 Report Date: April 2004 Update 3 Report Date: May 2005
Drug Class Review on Proton Pump Inhibitors Evidence Tables July 2006 Original Report Date: November 2002 Update 1 Report Date: April 2003 Update 2 Report Date: April 2004 Update 3 Report Date: May 2005
Famotidine Extended Abstracts
 Famotidine Extended Abstracts I) Primary literature Summary Ciccone, Decktor, et. al. Efficacy and tolerability of famotidine in preventing heartburn and related symptoms of upper gastrointestinal discomfort.
Famotidine Extended Abstracts I) Primary literature Summary Ciccone, Decktor, et. al. Efficacy and tolerability of famotidine in preventing heartburn and related symptoms of upper gastrointestinal discomfort.
High use of maintenance therapy after triple therapy regimes in Ireland
 High use of maintenance therapy after triple therapy regimes in Ireland K Bennett, H O Connor, M Barry, C O Morain, J Feely Department of Pharmacology & Therapeutics Department of Gastroenterology Trinity
High use of maintenance therapy after triple therapy regimes in Ireland K Bennett, H O Connor, M Barry, C O Morain, J Feely Department of Pharmacology & Therapeutics Department of Gastroenterology Trinity
Drug Class Monograph
 Drug Class Monograph Class: Proton Pump Inhibitors Drugs: Aciphex Sprinkle (rabeprazole), Dexilant (dexlansoprazole), Lansoprazole, Nexium (esomeprazole capsule, esomeprazole granules), Omeprazole, Pantoprazole,
Drug Class Monograph Class: Proton Pump Inhibitors Drugs: Aciphex Sprinkle (rabeprazole), Dexilant (dexlansoprazole), Lansoprazole, Nexium (esomeprazole capsule, esomeprazole granules), Omeprazole, Pantoprazole,
A model of healing of Los Angeles grades C and D reflux oesophagitis: is there an optimal time of acid suppression for maximal healing?
 Alimentary Pharmacology and Therapeutics A model of healing of Los Angeles grades C and D reflux oesophagitis: is there an optimal time of acid suppression for maximal healing? P. O. Katz*, D. A. Johnson
Alimentary Pharmacology and Therapeutics A model of healing of Los Angeles grades C and D reflux oesophagitis: is there an optimal time of acid suppression for maximal healing? P. O. Katz*, D. A. Johnson
Validation of a Four-Graded Scale for Severity of Heartburn in Patients with Symptoms of Gastroesophageal Reflux Disease
 Volume 11 Number 4 2008 VALUE IN HEALTH Validation of a Four-Graded Scale for Severity of Heartburn in Patients with Symptoms of Gastroesophageal Reflux Disease Ola Junghard, PhD, 1 Ingela Wiklund, PhD
Volume 11 Number 4 2008 VALUE IN HEALTH Validation of a Four-Graded Scale for Severity of Heartburn in Patients with Symptoms of Gastroesophageal Reflux Disease Ola Junghard, PhD, 1 Ingela Wiklund, PhD
Committee Approval Date: October 14, 2014 Next Review Date: October 2015
 Medication Policy Manual Topic: esomeprazole-containing medications: - Nexium - Vimovo - esomeprazole strontium Policy No: dru039 Date of Origin: May 2001 Committee Approval Date: October 14, 2014 Next
Medication Policy Manual Topic: esomeprazole-containing medications: - Nexium - Vimovo - esomeprazole strontium Policy No: dru039 Date of Origin: May 2001 Committee Approval Date: October 14, 2014 Next
Omeprazole 10mg. Name, Restriction, Manner of administration and form OMEPRAZOLE omeprazole 10 mg enteric tablet, 30 (8332M) Max. Qty.
 Omeprazole 10mg Name, Restriction, Manner of administration and form omeprazole 10 mg enteric tablet, 30 (8332M) Gastro-oesophageal reflux disease Name, Restriction, Manner of administration and form omeprazole
Omeprazole 10mg Name, Restriction, Manner of administration and form omeprazole 10 mg enteric tablet, 30 (8332M) Gastro-oesophageal reflux disease Name, Restriction, Manner of administration and form omeprazole
PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW
 PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW DATE OF AUTHORISATION: AUTHORISING GP: PRESCRIBING SUPPORT TECHNICIAN: SUMMARY Dyspepsia refers to a broad range of symptoms related
PRESCRIBING SUPPORT TEAM AUDIT: PROTON PUMP INHIBITOR PRESCRIBING REVIEW DATE OF AUTHORISATION: AUTHORISING GP: PRESCRIBING SUPPORT TECHNICIAN: SUMMARY Dyspepsia refers to a broad range of symptoms related
Appropriate Use of Proton Pump Inhibitors (PPIs) Anderson Mabour, Pharm.D., BCPS Clinical Pharmacy Specialist
 Appropriate Use of Proton Pump Inhibitors (PPIs) Anderson Mabour, Pharm.D., BCPS Clinical Pharmacy Specialist Disclosures I have no actual or potential conflicts of interest to report in relation to this
Appropriate Use of Proton Pump Inhibitors (PPIs) Anderson Mabour, Pharm.D., BCPS Clinical Pharmacy Specialist Disclosures I have no actual or potential conflicts of interest to report in relation to this
SELF CARE OF HEARTBURN
 O P I N I O N SelfCare 2010;1(2):77-82 In each issue, UK General Practitioner Dr. James Kennedy considers a common medical problem and summarises the pragmatic evidence-based advice that can be offered
O P I N I O N SelfCare 2010;1(2):77-82 In each issue, UK General Practitioner Dr. James Kennedy considers a common medical problem and summarises the pragmatic evidence-based advice that can be offered
Proton Pump Inhibitors. Description. Section: Prescription Drugs Effective Date: July 1, 2014
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.09.01 Subject: Proton Pump Inhibitors Page: 1 of 7 Last Review Date: June 12, 2014 Proton Pump Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.09.01 Subject: Proton Pump Inhibitors Page: 1 of 7 Last Review Date: June 12, 2014 Proton Pump Inhibitors
Review article: immediate-release proton-pump inhibitor therapy potential advantages
 Aliment Pharmacol Ther 25; 22 (Suppl. 3): 25 3. Review article: immediate-release proton-pump inhibitor therapy potential advantages C. W. HOWDEN Division of Gastroenterology, Northwestern University Feinberg
Aliment Pharmacol Ther 25; 22 (Suppl. 3): 25 3. Review article: immediate-release proton-pump inhibitor therapy potential advantages C. W. HOWDEN Division of Gastroenterology, Northwestern University Feinberg
Understanding gastro-oesophageal reflux disease: a patient-cluster analysis
 ORIGINAL PAPER Understanding gastro-oesophageal reflux disease: a patient-cluster analysis A. King, 1 C. MacDonald, 1 C. Örn 2 doi: 10.1111/j.1742-1241.2008.01929.x OnlineOpen: This article is available
ORIGINAL PAPER Understanding gastro-oesophageal reflux disease: a patient-cluster analysis A. King, 1 C. MacDonald, 1 C. Örn 2 doi: 10.1111/j.1742-1241.2008.01929.x OnlineOpen: This article is available
Clinical Study Placebo-Controlled Discontinuation of Long-Term Acid-Suppressant Therapy: A Randomised Trial in General Practice
 International Family Medicine Volume 2015, Article ID 175436, 7 pages http://dx.doi.org/10.1155/2015/175436 Clinical Study Placebo-Controlled Discontinuation of Long-Term Acid-Suppressant Therapy: A Randomised
International Family Medicine Volume 2015, Article ID 175436, 7 pages http://dx.doi.org/10.1155/2015/175436 Clinical Study Placebo-Controlled Discontinuation of Long-Term Acid-Suppressant Therapy: A Randomised
Page 1. Objectives. The Role of the Pharmacist as Gatekeeper to the Appropriate Use of OTC PPI Therapy in Frequent Heartburn
 Page 1 The Role of the Pharmacist as Gatekeeper to the Appropriate Use of OTC PPI Therapy in The Role of the Pharmacist as Gatekeeper to the Appropriate Use of OTC PPI Therapy in Colin W. Howden, MD, FRCP
Page 1 The Role of the Pharmacist as Gatekeeper to the Appropriate Use of OTC PPI Therapy in The Role of the Pharmacist as Gatekeeper to the Appropriate Use of OTC PPI Therapy in Colin W. Howden, MD, FRCP
Four-Day Bravo ph Capsule Monitoring With and Without Proton Pump Inhibitor Therapy
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:1083 1088 Four-Day Bravo ph Capsule Monitoring With and Without Proton Pump Inhibitor Therapy IKUO HIRANO, QING ZHANG, JOHN E. PANDOLFINO, and PETER J. KAHRILAS
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2005;3:1083 1088 Four-Day Bravo ph Capsule Monitoring With and Without Proton Pump Inhibitor Therapy IKUO HIRANO, QING ZHANG, JOHN E. PANDOLFINO, and PETER J. KAHRILAS
Identifying patients who may benefit from stepping down PPI treatment
 CLINICAL AUDIT Identifying patients who may benefit from stepping down PPI treatment Valid to January 2024 bpac nz better medicin e This audit identifies patients who are prescribed the proton pump inhibitor
CLINICAL AUDIT Identifying patients who may benefit from stepping down PPI treatment Valid to January 2024 bpac nz better medicin e This audit identifies patients who are prescribed the proton pump inhibitor
Proton Pump Inhibitor De-prescribing Guidance
 Amendment History Proton Pump Inhibitor De-prescribing Guidance VERSION DATE AMENDMENT HISTORY 1.0 2013 Previous version 2.0 September 2015 Comments Amendment to Flow chart and addition of Rationale page
Amendment History Proton Pump Inhibitor De-prescribing Guidance VERSION DATE AMENDMENT HISTORY 1.0 2013 Previous version 2.0 September 2015 Comments Amendment to Flow chart and addition of Rationale page
Disclosures. Proton Pump Inhibitors Deprescribing? Deprescribing PPI Objectives. Deprescribing. Proton Pump Inhibitors (PPI) 5/28/2018.
 Proton Pump Inhibitors Deprescribing? None Disclosures Chad Burski, MD Assistant Professor of Medicine UAB Gastroenterology Deprescribing PPI Objectives AR Why? Who? How? The mechanism of action of Proton
Proton Pump Inhibitors Deprescribing? None Disclosures Chad Burski, MD Assistant Professor of Medicine UAB Gastroenterology Deprescribing PPI Objectives AR Why? Who? How? The mechanism of action of Proton
GASTROINTESTINAL AND ANTIEMETIC DRUGS. Submitted by: Shaema M. Ali
 GASTROINTESTINAL AND ANTIEMETIC DRUGS Submitted by: Shaema M. Ali GASTROINTESTINAL AND ANTIEMETIC DRUGS by: Shaema M. Ali There are four common medical conditions involving the GI system 1) peptic ulcers
GASTROINTESTINAL AND ANTIEMETIC DRUGS Submitted by: Shaema M. Ali GASTROINTESTINAL AND ANTIEMETIC DRUGS by: Shaema M. Ali There are four common medical conditions involving the GI system 1) peptic ulcers
Fast Facts In OTC PPI s
 Page 1 Fast Facts in OTC PPI s Fast Facts in OTC PPIs James M. Scheiman, MD Professor of Internal Medicine Division of Gastroenterology University of Michigan Medical School Ann Arbor, Michigan This program
Page 1 Fast Facts in OTC PPI s Fast Facts in OTC PPIs James M. Scheiman, MD Professor of Internal Medicine Division of Gastroenterology University of Michigan Medical School Ann Arbor, Michigan This program
Fast Facts In OTC PPI s
 Page 1 Fast Facts in OTC PPI s Fast Facts in OTC PPIs James M. Scheiman, MD Professor of Internal Medicine Division of Gastroenterology University of Michigan Medical School Ann Arbor, Michigan This program
Page 1 Fast Facts in OTC PPI s Fast Facts in OTC PPIs James M. Scheiman, MD Professor of Internal Medicine Division of Gastroenterology University of Michigan Medical School Ann Arbor, Michigan This program
11/19/2012. Comparison between PPIs G CELL. Risk ratio (95% CI) Patient subgroup. gastrin. S-form of omeprazole. Acid sensitive. coated.
 REGULATION OF GASTRIC ACID SECRETION Comparison between PPIs Omeprazole Lansoprazole Rabeprazole Pantoprazole Esomeprazole gastrin G CELL + Acid sensitive Yes T1/2 30-60 minutes Main elimination Enteric
REGULATION OF GASTRIC ACID SECRETION Comparison between PPIs Omeprazole Lansoprazole Rabeprazole Pantoprazole Esomeprazole gastrin G CELL + Acid sensitive Yes T1/2 30-60 minutes Main elimination Enteric
Oral esomeprazole vs. intravenous pantoprazole: a comparison of the effect on intragastric ph in healthy subjects
 Aliment Pharmacol Ther 2003; 18: 705 711. doi: 10.1046/j.1365-2036.2003.01743.x Oral esomeprazole vs. intravenous pantoprazole: a comparison of the effect on intragastric in healthy subjects D. ARMSTRONG*,
Aliment Pharmacol Ther 2003; 18: 705 711. doi: 10.1046/j.1365-2036.2003.01743.x Oral esomeprazole vs. intravenous pantoprazole: a comparison of the effect on intragastric in healthy subjects D. ARMSTRONG*,
Proton Pump Inhibitor Clinical Trials: Focus On Lansoprazole In The Treatment Of Gastroesophageal Reflux Disease And Frequent Heartburn
 ISPUB.COM The Internet Journal of Advanced Nursing Practice Volume 11 Number 1 Proton Pump Inhibitor Clinical Trials: Focus On Lansoprazole In The Treatment Of Gastroesophageal J Pallentino Citation J
ISPUB.COM The Internet Journal of Advanced Nursing Practice Volume 11 Number 1 Proton Pump Inhibitor Clinical Trials: Focus On Lansoprazole In The Treatment Of Gastroesophageal J Pallentino Citation J
Optimal Management of GERD with Dexlansoprazole - Extended plasma concentration and dosing flexibility with a dual delayed release PPI
 Optimal Management of GERD with Dexlansoprazole - Extended plasma concentration and dosing flexibility with a dual delayed release PPI Jun Heng Lee, M.D. Samsung Medical Center, Sungkyunkwan University
Optimal Management of GERD with Dexlansoprazole - Extended plasma concentration and dosing flexibility with a dual delayed release PPI Jun Heng Lee, M.D. Samsung Medical Center, Sungkyunkwan University
July 19, Division of Dockets Management Food and Drug Administration 5630 Fishers Lane Room 1061, HFA-305 Rockville, Maryland 20852
 July 19, 2017 Division of Dockets Management Food and Drug Administration 5630 Fishers Lane Room 1061, HFA-305 Rockville, Maryland 20852 Re: Comments on Citizen s Petition #FDA-2017-P-2733 Herein, the
July 19, 2017 Division of Dockets Management Food and Drug Administration 5630 Fishers Lane Room 1061, HFA-305 Rockville, Maryland 20852 Re: Comments on Citizen s Petition #FDA-2017-P-2733 Herein, the
Meta-analysis: the efficacy of over-the-counter gastro-oesophageal reflux disease therapies
 Alimentary Pharmacology & Therapeutics Meta-analysis: the efficacy of over-the-counter gastro-oesophageal reflux disease therapies T. TRAN*, A. M. LOWRY &H.B.EL-SERAG* *The Sections of Health Services
Alimentary Pharmacology & Therapeutics Meta-analysis: the efficacy of over-the-counter gastro-oesophageal reflux disease therapies T. TRAN*, A. M. LOWRY &H.B.EL-SERAG* *The Sections of Health Services
Assessment of reflux symptom severity: methodological options and their attributes
 iv28 Assessment of reflux symptom severity: methodological options and their attributes P Bytzer... Despite major advances in our understanding of reflux disease, the management of this disorder still
iv28 Assessment of reflux symptom severity: methodological options and their attributes P Bytzer... Despite major advances in our understanding of reflux disease, the management of this disorder still
ORIGINAL ARTICLES ALIMENTARY TRACT
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:612 619 ORIGINAL ARTICLES ALIMENTARY TRACT Regurgitation Is Less Responsive to Acid Suppression Than Heartburn in Patients With Gastroesophageal Reflux
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2012;10:612 619 ORIGINAL ARTICLES ALIMENTARY TRACT Regurgitation Is Less Responsive to Acid Suppression Than Heartburn in Patients With Gastroesophageal Reflux
1. The proposed strength, quantity, dosage form, dose and route of administration of the medicine including indication
 Investigation 10mg 1. The proposed strength, quantity, dosage form, dose and route of administration of the medicine including indication Blister packs of 14 Solid dose form for oral administration. It
Investigation 10mg 1. The proposed strength, quantity, dosage form, dose and route of administration of the medicine including indication Blister packs of 14 Solid dose form for oral administration. It
Proton Pump Inhibitors:
 Proton Pump Inhibitors: How bad could they be? Andrea Flanagan, Pharm.D. Iowa City VA Medical Center PGY-1 Pharmacy Resident Objectives for Pharmacists At the end of this presentation PHARMACISTS should
Proton Pump Inhibitors: How bad could they be? Andrea Flanagan, Pharm.D. Iowa City VA Medical Center PGY-1 Pharmacy Resident Objectives for Pharmacists At the end of this presentation PHARMACISTS should
Nimish Vakil 1*, Anna Niklasson 2, Hans Denison 2 and Anna Rydén 2
 Vakil et al. BMC Gastroenterology 2014, 14:177 RESEARCH ARTICLE Open Access Symptom profile in partial responders to a proton pump inhibitor compared with treatment-naïve patients with gastroesophageal
Vakil et al. BMC Gastroenterology 2014, 14:177 RESEARCH ARTICLE Open Access Symptom profile in partial responders to a proton pump inhibitor compared with treatment-naïve patients with gastroesophageal
Review article: alternative approaches to the long-term management of GERD
 Aliment Pharmacol Ther 2005; 22 (Suppl. 3): 39 44. Review article: alternative approaches to the long-term management of GERD M. B. FENNERTY Division of Gastroenterology, Oregon Health and Science University,
Aliment Pharmacol Ther 2005; 22 (Suppl. 3): 39 44. Review article: alternative approaches to the long-term management of GERD M. B. FENNERTY Division of Gastroenterology, Oregon Health and Science University,
Alimentary Pharmacology & Therapeutics SUMMARY
 Alimentary Pharmacology & Therapeutics Comparison of the effects of immediate-release omeprazole oral suspension, delayed-release lansoprazole capsules and delayedrelease esomeprazole capsules on nocturnal
Alimentary Pharmacology & Therapeutics Comparison of the effects of immediate-release omeprazole oral suspension, delayed-release lansoprazole capsules and delayedrelease esomeprazole capsules on nocturnal
COMPUS OPTIMAL THERAPY REPORT. Supporting Informed Decisions. À l appui des décisions éclairées
 OPTIMAL THERAPY REPORT COMPUS Volume 1, Issue 5 March 2007 Current Practice Analysis Report for the Prescribing and Use of Proton Pump Inhibitors (PPIs) Supporting Informed Decisions À l appui des décisions
OPTIMAL THERAPY REPORT COMPUS Volume 1, Issue 5 March 2007 Current Practice Analysis Report for the Prescribing and Use of Proton Pump Inhibitors (PPIs) Supporting Informed Decisions À l appui des décisions
Introduction ORIGINAL ARTICLE. Vijayalakshmi S. Pratha 1, Thomas McGraw 2 & William Tobin 3. Abstract
 ORIGINAL ARTICLE A randomized, crossover pharmacodynamic study of immediate-release omeprazole/sodium bicarbonate and delayed-release lansoprazole in healthy adult volunteers Vijayalakshmi S. Pratha 1,
ORIGINAL ARTICLE A randomized, crossover pharmacodynamic study of immediate-release omeprazole/sodium bicarbonate and delayed-release lansoprazole in healthy adult volunteers Vijayalakshmi S. Pratha 1,
Relieving Frequent Heartburn Day Through Night
 Page 1 Relieving Frequent Heartburn Day Through Night Relieving Frequent Heartburn Day Through Night David Metz, MD Professor of Medicine University of Pennsylvania School of Medicine Philadelphia, PA
Page 1 Relieving Frequent Heartburn Day Through Night Relieving Frequent Heartburn Day Through Night David Metz, MD Professor of Medicine University of Pennsylvania School of Medicine Philadelphia, PA
Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial
 Alimentary Pharmacology & Therapeutics Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial E. BJÖRNSSON, H. ABRAHAMSSON, M. SIMRÉN, N. MATTSSON,
Alimentary Pharmacology & Therapeutics Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial E. BJÖRNSSON, H. ABRAHAMSSON, M. SIMRÉN, N. MATTSSON,
Source of effectiveness data The effectiveness data were derived from a review and synthesis of completed studies.
 Economic evaluation of maintenance therapies for reflux esophagitis: comparison between step-up therapies and step-down therapies Sugano K, Kobayashi M Record Status This is a critical abstract of an economic
Economic evaluation of maintenance therapies for reflux esophagitis: comparison between step-up therapies and step-down therapies Sugano K, Kobayashi M Record Status This is a critical abstract of an economic
Proton Pump Inhibitors (PPIs) (Sherwood Employer Group)
 Proton Pump Inhibitors (PPIs) (Sherwood Employer Group) BCBSKS will review Prior Authorization requests Prior Authorization Form: https://www.bcbsks.com/customerservice/forms/pdf/priorauth-6058ks-st-ippi.pdf
Proton Pump Inhibitors (PPIs) (Sherwood Employer Group) BCBSKS will review Prior Authorization requests Prior Authorization Form: https://www.bcbsks.com/customerservice/forms/pdf/priorauth-6058ks-st-ippi.pdf
Aim: To perform a systematic review on the efficacy of intermittent and on-demand therapy with either histamine. reflux disease patients.
 Aliment Pharmacol Ther 2005; 21: 1299 1312. doi: 10.1111/j.1365-2036.2005.02490.x Systematic review: the efficacy of intermittent and on-demand therapy with histamine H 2 -receptor antagonists or proton
Aliment Pharmacol Ther 2005; 21: 1299 1312. doi: 10.1111/j.1365-2036.2005.02490.x Systematic review: the efficacy of intermittent and on-demand therapy with histamine H 2 -receptor antagonists or proton
OTC PPI Therapy in Frequent Heartburn
 Page 1 Gate Keeper to the Appropriate Use of OTC PPI Therapy for Conflicts of Interest Gatekeeper to the Appropriate Use of OTC PPI Therapy in Colin W. Howden, MD, FRCP (Glasg), FACP, FACG, FCP Professor
Page 1 Gate Keeper to the Appropriate Use of OTC PPI Therapy for Conflicts of Interest Gatekeeper to the Appropriate Use of OTC PPI Therapy in Colin W. Howden, MD, FRCP (Glasg), FACP, FACG, FCP Professor
Intragastric ph With Oral vs Intravenous Bolus Plus Infusion Proton- Pump Inhibitor Therapy in Patients With Bleeding Ulcers
 Intragastric ph With Oral vs Intravenous Bolus Plus Infusion Proton- Pump Inhibitor Therapy in Patients With Bleeding Ulcers LOREN LAINE, ABBID SHAH, and SHAHROOZ BEMANIAN Division of Gastrointestinal
Intragastric ph With Oral vs Intravenous Bolus Plus Infusion Proton- Pump Inhibitor Therapy in Patients With Bleeding Ulcers LOREN LAINE, ABBID SHAH, and SHAHROOZ BEMANIAN Division of Gastrointestinal
Refractory GERD. Kenneth R. DeVault, MD, FACG President American College of Gastroenterology Chair Department of Medicine Mayo Clinic Florida
 Refractory GERD Kenneth R. DeVault, MD, FACG President American College of Gastroenterology Chair Department of Medicine Mayo Clinic Florida Objectives Define the terminology associated with refractory
Refractory GERD Kenneth R. DeVault, MD, FACG President American College of Gastroenterology Chair Department of Medicine Mayo Clinic Florida Objectives Define the terminology associated with refractory
Measuring and Evaluating Indicators of Appropriate Prescribing in Older. Populations
 HRB PhD Scholar Division of Population Health Sciences RCSI Measuring and Evaluating Indicators of Appropriate Prescribing in Older Cost-Effective Proton Pump Populations Potential Strategies for more
HRB PhD Scholar Division of Population Health Sciences RCSI Measuring and Evaluating Indicators of Appropriate Prescribing in Older Cost-Effective Proton Pump Populations Potential Strategies for more
Review article: management of mild and severe gastro-oesophageal reflux disease
 Aliment Pharmacol Ther 2003; 17 (Suppl. 2): 52 56. Review article: management of mild and severe gastro-oesophageal reflux disease G. N. J. TYTGAT Department of Gastroenterology and Hepatology, Academic
Aliment Pharmacol Ther 2003; 17 (Suppl. 2): 52 56. Review article: management of mild and severe gastro-oesophageal reflux disease G. N. J. TYTGAT Department of Gastroenterology and Hepatology, Academic
TBURN TBURN BURN ARTBURN EARTBURN EART HEARTBURN: HOW TO GET IT OFF YOUR CHEST
 TBURN BURN TBURN ARTBURN. EARTBURN EART N EARTBURN HEARTBURN: HOW TO GET IT OFF YOUR CHEST Do you sometimes wake up at night with a sharp, burning sensation in your chest? Does this sometimes happen during
TBURN BURN TBURN ARTBURN. EARTBURN EART N EARTBURN HEARTBURN: HOW TO GET IT OFF YOUR CHEST Do you sometimes wake up at night with a sharp, burning sensation in your chest? Does this sometimes happen during
CYP2C19-Proton Pump Inhibitors
 CYP2C19-Proton Pump Inhibitors Cameron Thomas, Pharm.D. PGY2 Clinical Pharmacogenetics Resident St. Jude Children s Research Hospital February 1, 2018 Objectives: CYP2C19-PPI Implementation Review the
CYP2C19-Proton Pump Inhibitors Cameron Thomas, Pharm.D. PGY2 Clinical Pharmacogenetics Resident St. Jude Children s Research Hospital February 1, 2018 Objectives: CYP2C19-PPI Implementation Review the
Difference between omeprazole and omeprazole delayed release
 Cari untuk: Cari Cari Difference between omeprazole and omeprazole delayed release 7-2-2018 Easy to read patient leaflet for Omeprazole Delayed-Release Capsules. Includes indications, proper use, special
Cari untuk: Cari Cari Difference between omeprazole and omeprazole delayed release 7-2-2018 Easy to read patient leaflet for Omeprazole Delayed-Release Capsules. Includes indications, proper use, special
Esomeprazole Pharmacokinetics in Patients with Cirrhosis and Healthy Controls ABSTRACT
 Original Article 21 Esomeprazole Pharmacokinetics in Patients with Cirrhosis and Healthy Controls Kanlayanee Archasantisuk, M.Sc.* Sombat Treeprasertsuk, M.D., M.Sc.** Mayyuree Tantisira, Ph.D.** Arun
Original Article 21 Esomeprazole Pharmacokinetics in Patients with Cirrhosis and Healthy Controls Kanlayanee Archasantisuk, M.Sc.* Sombat Treeprasertsuk, M.D., M.Sc.** Mayyuree Tantisira, Ph.D.** Arun
Proton Pump Inhibitors. Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.01 Subject: Proton Pump Inhibitors Page: 1 of 6 Last Review Date: June 24, 2015 Proton Pump Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.01 Subject: Proton Pump Inhibitors Page: 1 of 6 Last Review Date: June 24, 2015 Proton Pump Inhibitors
Policy Evaluation: Proton Pump Inhibitors (PPIs)
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Treatment with PPIs for Patients with GERD Symptoms
 9. Treatment with PPIs for Patients with GERD Symptoms How long should you consider continuing treatment with PPIs for patients with gastroesophageal reflux disease (GERD) symptoms? Dr. H. Ayad Pinawa,
9. Treatment with PPIs for Patients with GERD Symptoms How long should you consider continuing treatment with PPIs for patients with gastroesophageal reflux disease (GERD) symptoms? Dr. H. Ayad Pinawa,
FARMACI E ALTE VIE DIGESTIVE NELL ANZIANO: UTILITÀ E LIMITI
 FARMACI E ALTE VIE DIGESTIVE NELL ANZIANO: UTILITÀ E LIMITI Edoardo V. Savarino, MD, PhD Professor of Gastroenterology Department of Surgery, Oncology and Gastroenterology University of Padua Italy COMMON
FARMACI E ALTE VIE DIGESTIVE NELL ANZIANO: UTILITÀ E LIMITI Edoardo V. Savarino, MD, PhD Professor of Gastroenterology Department of Surgery, Oncology and Gastroenterology University of Padua Italy COMMON
COMPUS OPTIMAL THERAPY REPORT. Supporting Informed Decisions. À l appui des décisions éclairées
 OPTIMAL THERAPY REPORT COMPUS Volume 1, Issue 6 March 2007 Gap Analysis Report for the Prescribing and Use of Proton Pump Inhibitors (PPIs) Supporting Informed Decisions À l appui des décisions éclairées
OPTIMAL THERAPY REPORT COMPUS Volume 1, Issue 6 March 2007 Gap Analysis Report for the Prescribing and Use of Proton Pump Inhibitors (PPIs) Supporting Informed Decisions À l appui des décisions éclairées
MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
Reflux of gastric contents, particularly acid, into the esophagus
 Heartburn Reflux of gastric contents, particularly acid, into the esophagus Patient assessment with GERD 1-signs and symptoms The hallmark of typical symptom of GERD is heartburn (restrosternal),acid regurgitation,
Heartburn Reflux of gastric contents, particularly acid, into the esophagus Patient assessment with GERD 1-signs and symptoms The hallmark of typical symptom of GERD is heartburn (restrosternal),acid regurgitation,
PPIs: Good or Bad? 1. Basics of PPIs. Gastric Acid Basics. Outline. Gastric Acid Basics. Proton Pump Inhibitors (PPI)
 Outline Quick basics on Proton Pump Inhibitors (PPIs) PPIs: Good or Bad? What are potential risks of PPI therapy? How to approach your patients American Gastroenterology Association (AGA) recommendations
Outline Quick basics on Proton Pump Inhibitors (PPIs) PPIs: Good or Bad? What are potential risks of PPI therapy? How to approach your patients American Gastroenterology Association (AGA) recommendations
SUMMARY INTRODUCTION. Aliment Pharmacol Ther 2000; 14: 1595±1603. Accepted for publication 14 August 2000
 Aliment Pharmacol Ther 2000; 14: 1595±1603. Omeprazole 40 mg once a day is equally effective as lansoprazole 30 mg twice a day in symptom control of patients with gastro-oesophageal re ux disease (GERD)
Aliment Pharmacol Ther 2000; 14: 1595±1603. Omeprazole 40 mg once a day is equally effective as lansoprazole 30 mg twice a day in symptom control of patients with gastro-oesophageal re ux disease (GERD)
Rpts. GENERAL General Schedule (Code GE)
 Pantoprazole 20mg Name, Restriction, Manner of administration and form Pantoprazole 20mg enteric tablet, 30 (8399C) Gastro-oesophageal reflux disease Name, Restriction, Manner of administration and form
Pantoprazole 20mg Name, Restriction, Manner of administration and form Pantoprazole 20mg enteric tablet, 30 (8399C) Gastro-oesophageal reflux disease Name, Restriction, Manner of administration and form
ACID REFLUX & GERD: The Unsettling Reality in Canada
 ACID REFLUX & GERD: The Unsettling Reality in Canada gerd fact 1 see page 8 Canadian Society of Intestinal Research On average, ARD patients wait over two years before seeking care 1. 1 gerd fact 2 see
ACID REFLUX & GERD: The Unsettling Reality in Canada gerd fact 1 see page 8 Canadian Society of Intestinal Research On average, ARD patients wait over two years before seeking care 1. 1 gerd fact 2 see
Hold the Wrap! There is so much more to be done!
 Hold the Wrap! There is so much more to be done! (Well, a few things that can be done.) (Well, not all that much, really ) (But Blair has never killed anyone with a PPI!) Nicholas Shaheen, MD, MPH Center
Hold the Wrap! There is so much more to be done! (Well, a few things that can be done.) (Well, not all that much, really ) (But Blair has never killed anyone with a PPI!) Nicholas Shaheen, MD, MPH Center
June By: Reza Gholami
 ACG/CAG guideline on Management of Dyspepsia June 2017 By: Reza Gholami DEFINITION OF DYSPEPSIA AND SCOPE OF THE GUIDELINE Dyspepsia was originally defined as any symptoms referable to the upper gastrointestinal
ACG/CAG guideline on Management of Dyspepsia June 2017 By: Reza Gholami DEFINITION OF DYSPEPSIA AND SCOPE OF THE GUIDELINE Dyspepsia was originally defined as any symptoms referable to the upper gastrointestinal
compared with ranitidine
 1458 Gut 1993; 34:1458-1462 CLINICAL TRIAL Department of Therapeutics, University Hospital, Nottingham C J Hawkey R G Long Rotherham District General Hospital, Rotherham K D Bardhan Ninewelis Hospital,
1458 Gut 1993; 34:1458-1462 CLINICAL TRIAL Department of Therapeutics, University Hospital, Nottingham C J Hawkey R G Long Rotherham District General Hospital, Rotherham K D Bardhan Ninewelis Hospital,
A C A D E M I C D E TA I L I N G C H O O S I N G W I S E LY C O N F E R E N C E O C T 2 1, PA M M C L E A N - V E Y S E Y B S C P H A R M D R
 PPI DEPRESCRIBING Canadian Deprescribing Network (CaDeN) goals are to: Reduce harm by raising awareness and cutting risky prescriptions for seniors by 50% by 2020. Promote health by ensuring access to
PPI DEPRESCRIBING Canadian Deprescribing Network (CaDeN) goals are to: Reduce harm by raising awareness and cutting risky prescriptions for seniors by 50% by 2020. Promote health by ensuring access to
Functional Heartburn and Dyspepsia
 Functional Heartburn and Dyspepsia Nicholas Shaheen, MD, MPH Center for Esophageal Diseases and Swallowing University of North Carolina Objectives Understand the means of diagnosing functional heartburn
Functional Heartburn and Dyspepsia Nicholas Shaheen, MD, MPH Center for Esophageal Diseases and Swallowing University of North Carolina Objectives Understand the means of diagnosing functional heartburn
Losec & Losec Extra Tablets
 Proposal for Reclassification of Losec & Losec Extra Tablets Omeprazole 10 mg & 20 mg Extension of Maximum Pack Size to 28 Tablets INDEX Page PART A 2 PART B 14 Safety Profile 15 Risk of Masking Serious
Proposal for Reclassification of Losec & Losec Extra Tablets Omeprazole 10 mg & 20 mg Extension of Maximum Pack Size to 28 Tablets INDEX Page PART A 2 PART B 14 Safety Profile 15 Risk of Masking Serious
Burning Issues in Gastroesophageal Reflux Disease (GERD)
 3:45 4:45pm Burning Issues in GERD SPEAKER Prateek Sharma, MD, FACG, FACP Presenter Disclosure Information The following relationships exist related to this presentation: Prateek Sharma, MD, FACG, FACP,
3:45 4:45pm Burning Issues in GERD SPEAKER Prateek Sharma, MD, FACG, FACP Presenter Disclosure Information The following relationships exist related to this presentation: Prateek Sharma, MD, FACG, FACP,
Rabeprazole is superior to omeprazole for the inhibition of peptone meal-stimulated gastric acid secretion in Helicobacter pylori-negative subjects
 Aliment Pharmacol Ther 2003; 17: 1109 1114. doi: 10.1046/j.0269-2813.2003.01573.x Rabeprazole is superior to omeprazole for the inhibition of peptone meal-stimulated gastric acid secretion in Helicobacter
Aliment Pharmacol Ther 2003; 17: 1109 1114. doi: 10.1046/j.0269-2813.2003.01573.x Rabeprazole is superior to omeprazole for the inhibition of peptone meal-stimulated gastric acid secretion in Helicobacter
GASTROESOPHAGEAL reflux
 ORIGINAL INVESTIGATION Lansoprazole Compared With Ranitidine for the Treatment of Nonerosive Gastroesophageal Reflux Disease Joel E. Richter, MD; Donald R. Campbell, MD; Peter J. Kahrilas, MD; Bidan Huang,
ORIGINAL INVESTIGATION Lansoprazole Compared With Ranitidine for the Treatment of Nonerosive Gastroesophageal Reflux Disease Joel E. Richter, MD; Donald R. Campbell, MD; Peter J. Kahrilas, MD; Bidan Huang,
Gastroesophageal reflux disease. The case for improving patient education in primary care
 Original Research Gastroesophageal reflux disease: The case for improving patient education in primary care This study reveals that something as simple as knowing when to take GERD medication is compromised
Original Research Gastroesophageal reflux disease: The case for improving patient education in primary care This study reveals that something as simple as knowing when to take GERD medication is compromised
NEGATIVE ENDOSCOPY, What is the Diagnosis and Treatment?
 NEGATIVE ENDOSCOPY, PPI REFRACTORY REFLUX: What is the Diagnosis and Treatment? Michael F. Vaezi, MD, PhD, MSc, FACG Professor of Medicine Clinical Director Division of Gastroenterology, Hepatology and
NEGATIVE ENDOSCOPY, PPI REFRACTORY REFLUX: What is the Diagnosis and Treatment? Michael F. Vaezi, MD, PhD, MSc, FACG Professor of Medicine Clinical Director Division of Gastroenterology, Hepatology and
754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011
 754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011 Brief Report Characterization of Abdominal Pain During Methylnaltrexone Treatment of Opioid-Induced Constipation in Advanced Illness:
754 Journal of Pain and Symptom Management Vol. 42 No. 5 November 2011 Brief Report Characterization of Abdominal Pain During Methylnaltrexone Treatment of Opioid-Induced Constipation in Advanced Illness:
Laryngopharyngeal Reflux
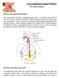 Laryngopharyngeal Reflux The Silent Reflux What is Laryngopharyngeal Reflux? Also called Reflux laryngitis, laryngopharyngeal reflux is a condition where the acid from the stomach reaches the voicebox
Laryngopharyngeal Reflux The Silent Reflux What is Laryngopharyngeal Reflux? Also called Reflux laryngitis, laryngopharyngeal reflux is a condition where the acid from the stomach reaches the voicebox
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 PROTON PUMP INHIBITORS, NON-PREFERRED FORMS: ACIPHEX (rabeprazole sodium EC) oral tablet ACIPHEX SPRINKLE (rabeprazole sodium DR) oral capsule ESOMEPRAZOLE STRONTIUM (esomeprazole strontium DR) oral capsule
PROTON PUMP INHIBITORS, NON-PREFERRED FORMS: ACIPHEX (rabeprazole sodium EC) oral tablet ACIPHEX SPRINKLE (rabeprazole sodium DR) oral capsule ESOMEPRAZOLE STRONTIUM (esomeprazole strontium DR) oral capsule
Proton Pump Inhibitors Drug Class Prior Authorization Protocol
 Proton Pump Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: November 15, 2017 Effective Date: January 1, 2018 This policy has been developed through review
Proton Pump Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: November 15, 2017 Effective Date: January 1, 2018 This policy has been developed through review
Pthaigastro.org. Evolution of antisecretory agents. History. Antacids and anticholinergic drugs
 Evolution of antisecretory agents uthapong Ukarapol, MD. Division of Gastroenterology Chiang Mai University istory 1823- Prout discovered gastric hydrochloric acid 1875- eidenhain and 1893- Golgi identified
Evolution of antisecretory agents uthapong Ukarapol, MD. Division of Gastroenterology Chiang Mai University istory 1823- Prout discovered gastric hydrochloric acid 1875- eidenhain and 1893- Golgi identified
GERD DIAGNOSIS & TREATMENT DISCLOSURES 4/18/2018
 GERD DIAGNOSIS & TREATMENT Subhash Chandra MBBS Assistant Professor CHI Health Clinic Gastroenterology Creighton University, School of Medicine April 28, 2018 DISCLOSURES None 1 OBJECTIVES Review update
GERD DIAGNOSIS & TREATMENT Subhash Chandra MBBS Assistant Professor CHI Health Clinic Gastroenterology Creighton University, School of Medicine April 28, 2018 DISCLOSURES None 1 OBJECTIVES Review update
Dexlansoprazole for Heartburn Relief in Adolescents with Symptomatic, Nonerosive Gastro-esophageal Reflux Disease
 Dig Dis Sci (2017) 62:3059 3068 DOI 10.1007/s10620-017-4743-3 ORIGINAL ARTICLE Dexlansoprazole for Heartburn Relief in Adolescents with Symptomatic, Nonerosive Gastro-esophageal Reflux Disease Benjamin
Dig Dis Sci (2017) 62:3059 3068 DOI 10.1007/s10620-017-4743-3 ORIGINAL ARTICLE Dexlansoprazole for Heartburn Relief in Adolescents with Symptomatic, Nonerosive Gastro-esophageal Reflux Disease Benjamin
ORIGINAL ARTICLES ALIMENTARY TRACT. A Randomized, Comparative Study of Three Doses of AZD0865 and Esomeprazole for Healing of Reflux Esophagitis
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2007;5:1385 1391 ORIGINAL ARTICLES ALIMENTARY TRACT A Randomized, Comparative Study of Three Doses of AZD0865 and Esomeprazole for Healing of Reflux Esophagitis
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2007;5:1385 1391 ORIGINAL ARTICLES ALIMENTARY TRACT A Randomized, Comparative Study of Three Doses of AZD0865 and Esomeprazole for Healing of Reflux Esophagitis
