Magnetic Resonance Imaging (NCD 220.2)
|
|
|
- Lorin Whitehead
- 6 years ago
- Views:
Transcription
1 Policy Number Approved By UnitedHealthcare Medicare Committee Current Approval Date 05/14/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare Medicare Advantage Plans offered by UnitedHealthcare and its affiliates. You are responsible for submission of accurate claims. This reimbursement policy is intended to ensure that you are reimbursed based on the code or codes that correctly describe the health care services provided. UnitedHealthcare reimbursement policies use Current Procedural Terminology (CPT *), Centers for Medicare and Medicaid Services (CMS), or other coding guidelines. References to CPT or other sources are for definitional purposes only and do not imply any right to reimbursement. This reimbursement policy applies to all health care services billed on CMS 1500 forms and, when specified, to those billed on UB04 forms (CMS 1450). Coding methodology, industry-standard reimbursement logic, regulatory requirements, benefits design and other factors are considered in developing reimbursement policy. This information is intended to serve only as a general resource regarding UnitedHealthcare s reimbursement policy for the services described and is not intended to address every aspect of a reimbursement situation. Accordingly, UnitedHealthcare may use reasonable discretion in interpreting and applying this policy to health care services provided in a particular case. Further, the policy does not address all issues related to reimbursement for health care services provided to UnitedHealthcare enrollees. Other factors affecting reimbursement may supplement, modify or, in some cases, supersede this policy. These factors may include, but are not limited to: legislative mandates, the physician or other provider contracts, and/or the enrollee s benefit coverage documents. Finally, this policy may not be implemented exactly the same way on the different electronic claims processing systems used by UnitedHealthcare due to programming or other constraints; however, UnitedHealthcare strives to minimize these variations. UnitedHealthcare may modify this reimbursement policy at any time by publishing a new version of the policy on this Website. However, the information presented in this policy is accurate and current as of the date of publication. *CPT copyright 2010 (or such other date of publication of CPT) American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Table of Contents Application...2 Summary...2 Overview...2 Reimbursement Guidelines...3 MRI CPT/HCPCS Codes...5 MRA CPT/HCPCS Codes...8 Modifiers...9 Questions and Answers...9 References Included (but not limited to):...9 CMS NCD(s)...9 CMS LCD(s)...9 CMS Article...9 CMS Benefit Policy Manual...9 CMS Claims Processing Manual...9 CMS Transmittals...9 UnitedHealthcare Medicare Advantage Coverage Summaries...9 UnitedHealthcare Reimbursement Policies...9 MLN Matters...9 Others...9 History...9 Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 1
2 Application This reimbursement policy applies to services reported using the Health Insurance Claim Form CMS-1500 or its electronic equivalent or its successor form, and services reported using facility claim form CMS-1450 or its electronic equivalent or its successor form. This policy applies to all products, all network and non-network physicians, and other health care professionals. The HCPCS/CPT code(s) may be subject to Correct Coding Initiative (CCI) edits. This policy does not take precedence over CCI edits. Please refer to the CCI for correct coding guidelines and specific applicable code combinations prior to billing UnitedHealthcare. It is not enough to link the procedure code to a correct, payable ICD-9-CM diagnosis code. The diagnosis must be present for the procedure to be paid. Compliance with the provisions in this policy is subject to monitoring by pre-payment review and/or post-payment data analysis and subsequent medical review. The effective date of changes/additions/deletions to this policy is the committee meeting date unless otherwise indicated. CPT codes and descriptions are copyright 2010 American Medical Association (or such other date of publication of CPT). All rights reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DFARS restrictions apply to Government use. Fee schedules, relative value units, conversion factors, and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein. Current Dental Terminology (CDT), including procedure codes, nomenclature, descriptors, and other data contained therein, is copyright by the American Dental Association, 2002, All rights reserved. CDT is a registered trademark of the American Dental Association. Applicable FARS/DFARS apply. Summary Overview Magnetic Resonance Imaging (MRI), formerly called nuclear magnetic resonance (NMR), is a non-invasive method of graphically representing the distribution of water and other hydrogen-rich molecules in the human body. In contrast to conventional radiographs or computed tomography (CT) scans, in which the image is produced by x-ray beam attenuation by an object, MRI is capable of producing images by several techniques. In fact, various combinations of MRI image production methods may be employed to emphasize particular characteristics of the tissue or body part being examined. The basic elements by which MRI produces an image are the density of hydrogen nuclei in the object being examined, their motion, and the relaxation times, and the period of time required for the nuclei to return to their original states in the main, static magnetic field after being subjected to a brief additional magnetic field. These relaxation times reflect the physical-chemical properties of tissue and the molecular environment of its hydrogen nuclei. Only hydrogen atoms are present in human tissues in sufficient concentration for current use in clinical MRI. Magnetic Resonance Angiography (MRA) is a non-invasive diagnostic test that is an application of MRI. By analyzing the amount of energy released from tissues exposed to a strong magnetic field, MRA provides images of normal and diseased blood vessels, as well as visualization and quantification of blood flow through these vessels. Overall, MRI is a useful diagnostic imaging modality that is capable of demonstrating a wide variety of softtissue lesions with contrast resolution equal or superior to CT scanning in various parts of the body. Among the advantages of MRI are the absence of ionizing radiation and the ability to achieve high levels of tissue contrast resolution without injected iodinated radiological contrast agents. Recent advances in technology have resulted in development and Food and Drug Administration (FDA) approval of new paramagnetic contrast agents for MRI which allow even better visualization in some instances. Multislice imaging and the ability to image in multiple planes, especially sagittal and coronal, have provided flexibility not easily available with other modalities. Because cortical (outer layer) bone and metallic prostheses do not cause distortion of MR images, it has been possible to visualize certain lesions and body regions with greater certainty than has been possible with CT. The use of MRI on certain soft tissue structures for the purpose of detecting disruptive, neoplastic, degenerative, or inflammatory lesions has now become established in medical practice. Phase contrast (PC) and time-of-flight (TOF) are some of the available MRA techniques at the time these instructions are being issued. PC measures the difference between the phases of proton spins in tissue and blood and measures both the venous and arterial blood flow at any point in the cardiac cycle. TOF measures the difference between the amount of magnetization of tissue and blood and provides information on the structure of blood vessels, thus indirectly indicating blood flow. Two-dimensional (2D) and three-dimensional Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 2
3 (3D) images can be obtained using each method. Contrast-enhanced MRA (CE-MRA) involves blood flow imaging after the patient receives an intravenous injection of a contrast agent. Gadolinium, a non-ionic element, is the foundation of all contrast agents currently in use. Gadolinium affects the way in which tissues respond to magnetization, resulting in better visualization of structures when compared to un-enhanced studies. Unlike ionic (i.e., iodine-based) contrast agents used in conventional contrast angiography (CA), allergic reactions to gadolinium are extremely rare. Additionally, gadolinium does not cause the kidney failure occasionally seen with ionic contrast agents. Digital subtraction angiography (DSA) is a computer-augmented form of CA that obtains digital blood flow images as contrast agent courses through a blood vessel. The computer subtracts bone and other tissue from the image, thereby improving visualization of blood vessels. Physicians elect to use a specific MRA or CA technique based upon clinical information from each patient. The Centers for Medicare and Medicaid Services (CMS) issued a 2010 National Coverage Decision (NCD) that merged the Magnetic Resonance Angiography (MRA) NCD at section under the NCD for Magnetic Resonance Imaging (MRI) at section in Chapter 1 of Publication of the NCD Manual. In addition, a 2009 NCD removed a contraindication from C.2 of the NCD Manual concerning blood flow measurement. Currently, coverage is limited to MRI units that have received Food and Drug Administration (FDA) premarket approval, and such units must be operated within the parameters specified by the approval. Other uses of MRI for which CMS has not specifically indicated national coverage or national non-coverage are at the discretion of Medicare s local contractors. Reimbursement Guidelines Effective for claims with dates of service on and after July 7, 2011, UHC will deny MRI line items on facility and professional claims when billed with ICD-9 diagnosis code V45.01 if modifier KX is not also present on the line or the conditions are not met. Effective for claims with dates of service on and after February 24, 2011, Medicare will allow for coverage of MRI for beneficiaries with implanted PMs or cardioverter defibrillators (ICDs) for use in an MRI environment in a Medicare-approved clinical study. Claims for services in a Medicare-approved clinical study must be submitted to traditional Medicare for payment. Nationally Covered MRI and MRA Indications 1. MRI Although several uses of MRI are still considered investigational and some uses are clearly contraindicated, MRI is considered medically efficacious for a number of uses. Use the following descriptions as general guidelines or examples of what may be considered covered rather than as a restrictive list of specific covered indications. Coverage is limited to MRI units that have received FDA premarket approval, and such units must be operated within the parameters specified by the approval. In addition, the services must be reasonable and necessary for the diagnosis or treatment of the specific patient involved. a) Effective November 22, 1985, MRI is useful in examining the head, central nervous system, and spine. Multiple sclerosis can be diagnosed with MRI and the contents of the posterior fossa are visible. The inherent tissue contrast resolution of MRI makes it an appropriate standard diagnostic modality for general neuroradiology. b) Effective November 22, 1985, MRI can assist in the differential diagnosis of mediastinal and retroperitoneal masses, including abnormalities of the large vessels such as aneurysms and dissection. When a clinical need exists to visualize the parenchyma of solid organs to detect anatomic disruption or neoplasia, this can be accomplished in the liver, urogenital system, adrenals, and pelvic organs without the use of radiological contrast materials. When MRI is considered reasonable and necessary, the use of paramagnetic contrast materials may be covered as part of the study. MRI may also be used to detect and stage pelvic and retroperitoneal neoplasms and to evaluate disorders of cancellous bone and soft tissues. It may also be used in the detection of pericardial thickening. Primary and secondary bone neoplasm and aseptic necrosis can be detected at an early stage and monitored with MRI. Patients with metallic prostheses, especially of the hip, can be imaged in order to detect the early stages of infection of the bone to which the prosthesis is attached. c) Effective March 4, 1991, MRI with gating devices and surface coils, and gating devices that eliminate distorted images caused by cardiac and respiratory movement cycles are now considered state of the art techniques and may be covered. Surface and other specialty coils may also be covered, as they are used routinely for high resolution imaging where small limited regions of the body are studied. They produce Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 3
4 high signal-to-noise ratios resulting in images of enhanced anatomic detail. d) Effective March 22, 1994, MRI may also be covered to diagnose disc disease without regard to whether radiological imaging has been tried first to diagnose the problem. 2. MRA (MRI for Blood Flow) Currently covered indications include using MRA for specific conditions to evaluate flow in internal carotid vessels of the head and neck, peripheral arteries of lower extremities, abdomen and pelvis, and the chest. Coverage is limited to MRA units that have received FDA premarket approval, and such units must be operated within the parameters specified by the approval. In addition, the services must be reasonable and necessary for the diagnosis or treatment of the specific patient involved. a) Head and Neck Effective April 15, 2003, studies have proven that MRA is effective for evaluating flow in internal carotid vessels of the head and neck. However, not all potential applications of MRA have been shown to be reasonable and necessary. All of the following criteria must apply in order for Medicare to provide coverage for MRA of the head and neck: o MRA is used to evaluate the carotid arteries, the circle of Willis, the anterior, middle or posterior cerebral arteries, the vertebral or basilar arteries or the venous sinuses; o MRA is performed on patients with conditions of the head and neck for which surgery is anticipated and may be found to be appropriate based on the MRA. These conditions include, but are not limited to, tumor, aneurysms, vascular malformations, vascular occlusion or thrombosis. Within this broad category of disorders, medical necessity is the underlying determinant of the need for an MRA in specific diseases. The medical records should clearly justify and demonstrate the existence of medical necessity; and o MRA and CA are not expected to be performed on the same patient for diagnostic purposes prior to the application of anticipated therapy. Only one of these tests will be covered routinely unless the physician can demonstrate the medical need to perform both tests. b) Peripheral Arteries of Lower Extremities Effective April 15, 2003, studies have proven that MRA of peripheral arteries is useful in determining the presence and extent of peripheral vascular disease in lower extremities. This procedure is noninvasive and has been shown to find occult vessels in some patients for which those vessels were not apparent when CA was performed. Medicare will cover either MRA or CA to evaluate peripheral arteries of the lower extremities. However, both MRA and CA may be useful in some cases, such as: o A patient has had CA and this test was unable to identify a viable run-off vessel for bypass. When exploratory surgery is not believed to be a reasonable medical course of action for this patient, MRA may be performed to identify the viable runoff vessel; or o A patient has had MRA, but the results are inconclusive. c) Abdomen and Pelvis Pre-operative Evaluation of Patients Undergoing Elective Abdominal Aortic Aneurysm (AAA) Repair o Effective July 1, 1999, MRA is covered for pre-operative evaluation of patients undergoing elective AAA repair if the scientific evidence reveals MRA is considered comparable to CA in determining the extent of AAA, as well as in evaluating aortoiliac occlusion disease and renal artery pathology that may be necessary in the surgical planning of AAA repair. These studies also reveal that MRA could provide a net benefit to the patient. If preoperative CA is avoided, then patients are not exposed to the risks associated with invasive procedures, contrast media, end-organ damage, or arterial injury. Imaging the Renal Arteries and the Aortoiliac Arteries in the Absence of AAA or Aortic Dissection o Effective July 1, 2003, MRA coverage is expanded to include imaging the renal arteries and the aortoiliac arteries in the absence of AAA or aortic dissection. MRA should be obtained in those circumstances in which using MRA is expected to avoid obtaining CA, when physician history, physical examination, and standard assessment tools provide insufficient information for patient management, and obtaining an MRA has a high probability of positively affecting patient management. However, CA may be ordered after obtaining the results of an MRA in those rare instances where medical necessity is demonstrated. Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 4
5 d) Chest Diagnosis of Pulmonary Embolism o Current scientific data has shown that diagnostic pulmonary MRAs are improving due to recent developments such as faster imaging capabilities and gadolinium-enhancement. However, these advances in MRA are not significant enough to warrant replacement of pulmonary angiography in the diagnosis of pulmonary embolism for patients who have no contraindication to receiving intravenous iodinated contrast material. o Patients who are allergic to iodinated contrast material face a high risk of developing complications if they undergo pulmonary angiography or computed tomography angiography. Therefore, Medicare will cover MRA of the chest for diagnosing a suspected pulmonary embolism when it is contraindicated for the patient to receive intravascular iodinated contrast material. Evaluation of Thoracic Aortic Dissection and Aneurysm o Studies have shown that MRA of the chest has a high level of diagnostic accuracy for pre-operative and post-operative evaluation of aortic dissection of aneurysm. Depending on the clinical presentation, MRA may be used as an alternative to other non-invasive imaging technologies, such as transesophageal echocardiography and CT. Generally, Medicare will provide coverage only for MRA or for CA when used as a diagnostic test. However, if both MRA and CA of the chest are used, the physician must demonstrate the medical need for performing these tests. e) Cardiac MRA for Velocity Flow Mapping Cardiac velocity flow mapping is considered medically reasonable and necessary for quantitative assessment of the following, pre and post repair of the structural defects: o Magnitude of cardiac shunt fractions in patients with atrial septal defect, ventricular septal defect, or patient ductus arteriosus. o Valvular regurgation fractions in patients with valvular regurgitation or insufficiency o Grading of valvular, subvalvular (e.g., hypertrophic cardiomyopathy), supravalvular, or great vessel stenosis o Evaluation of differential flow of the pulmonary arteries in patients with Tetrology of Fallot Nationally Non-Covered Indications The MRI is not covered when the following patient-specific contraindications are present: MRI is not covered for patients with cardiac pacemakers or with metallic clips on vascular aneurysms unless the Medicare beneficiary meets the provisions of the following exceptions: Effective July 7, 2011, the contraindications will not apply to pacemakers when used according to the FDA-approved labeling in an MRI environment, or Effective February 24, 2011, CMS believes that the evidence is promising although not yet convincing that MRI will improve patient health outcomes if certain safeguards are in place to ensure that the exposure of the device to an MRI environment adversely affects neither the interpretation of the MRI result nor the proper functioning of the implanted device itself. CMS has determined that MRI of cortical bone and calcifications, and procedures involving spatial resolution of bone and calcifications, are not considered reasonable and necessary indications within the meaning of section 1862(a)(1)(A) of the Act, and are therefore non-covered. The following MRA services are considered not medically reasonable and necessary at this time: MRA of the spinal canal and contents MRA of the upper Extremities MRI CPT/HCPCS Codes Code Description Magnetic resonance (eg, proton) imaging, temporomandibular joint(s) Magnetic resonance (eg, proton) imaging, orbit, face, and/or neck; without contrast Magnetic resonance (eg, proton) imaging, orbit, face, and/or neck; with contrast Magnetic resonance (eg, proton) imaging, orbit, face, and/or neck; without contrast, followed by contrast and further sequences Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 5
6 70551 Magnetic resonance (eg, proton) imaging, brain (including brain stem); without contrast material Magnetic resonance (eg, proton) imaging, brain (including brain stem); with contrast Magnetic resonance (eg, proton) imaging, brain (including brain stem); without contrast material, followed by contrast and further sequences Magnetic resonance imaging, brain, functional MRI; including test selection and administration of repetitive body part movement and/or visual stimulation, not requiring physician or psychologist administration Magnetic resonance imaging, brain, functional MRI; requiring physician or psychologist administration of entire neurofunctional testing Magnetic resonance (eg, proton) imaging, brain (including brain stem and skull base), during open intracranial procedure (eg, to assess for residual tumor or residual vascular malformation); without contrast material Magnetic resonance (eg, proton) imaging, brain (including brain stem and skull base), during open intracranial procedure (eg, to assess for residual tumor or residual vascular malformation); with contrast Magnetic resonance (eg, proton) imaging, brain (including brain stem and skull base), during open intracranial procedure (eg, to assess for residual tumor or residual vascular malformation); without contrast, followed by contrast and further sequences Magnetic resonance (eg, proton) imaging, chest (eg, for evaluation of hilar and mediastinal lymphadenopathy); without contrast Magnetic resonance (eg, proton) imaging, chest (eg, for evaluation of hilar and mediastinal lymphadenopathy); with contrast Magnetic resonance (eg, proton) imaging, chest (eg, for evaluation of hilar and mediastinal lymphadenopathy); without contrast, followed by contrast and further sequences Magnetic resonance (eg, proton) imaging, spinal canal and contents, cervical; without contrast material Magnetic resonance (eg, proton) imaging, spinal canal and contents, cervical; with contrast Magnetic resonance (eg, proton) imaging, spinal canal and contents, thoracic; without contrast material Magnetic resonance (eg, proton) imaging, spinal canal and contents, thoracic; with contrast Magnetic resonance (eg, proton) imaging, spinal canal and contents, lumbar; without contrast material Magnetic resonance (eg, proton) imaging, spinal canal and contents, lumbar; with contrast Magnetic resonance (eg, proton) imaging, spinal canal and contents, without contrast material, followed by contrast and further sequences; cervical Magnetic resonance (eg, proton) imaging, spinal canal and contents, without contrast material, followed by contrast and further sequences; thoracic Magnetic resonance (eg, proton) imaging, spinal canal and contents, without contrast material, followed by contrast and further sequences; lumbar Magnetic resonance (eg, proton) imaging, pelvis; without contrast Magnetic resonance (eg, proton) imaging, pelvis; with contrast Magnetic resonance (eg, proton) imaging, pelvis; without contrast, followed by contrast and further sequences Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 6
7 73218 Magnetic resonance (eg, proton) imaging, upper extremity, other than joint; without contrast Magnetic resonance (eg, proton) imaging, upper extremity, other than joint; with contrast Magnetic resonance (eg, proton) imaging, upper extremity, other than joint; without contrast, followed by contrast and further sequences Magnetic resonance (eg, proton) imaging, any joint of upper extremity; without contrast Magnetic resonance (eg, proton) imaging, any joint of upper extremity; with contrast Magnetic resonance (eg, proton) imaging, any joint of upper extremity; without contrast, followed by contrast and further sequences Magnetic resonance (eg, proton) imaging, lower extremity other than joint; without contrast Magnetic resonance (eg, proton) imaging, lower extremity other than joint; with contrast Magnetic resonance (eg, proton) imaging, lower extremity other than joint; without contrast, followed by contrast and further sequences Magnetic resonance (eg, proton) imaging, any joint of lower extremity; without contrast material Magnetic resonance (eg, proton) imaging, any joint of lower extremity; with contrast Magnetic resonance (eg, proton) imaging, any joint of lower extremity; without contrast, followed by contrast and further sequences Magnetic resonance (eg, proton) imaging, abdomen; without contrast Magnetic resonance (eg, proton) imaging, abdomen; with contrast Magnetic resonance (eg, proton) imaging, abdomen; without contrast, followed by with contrast and further sequences Cardiac magnetic resonance imaging for morphology and function without contrast material; Cardiac magnetic resonance imaging for morphology and function without contrast material; with stress imaging Cardiac magnetic resonance imaging for morphology and function without contrast, followed by contrast and further sequences; Cardiac magnetic resonance imaging for morphology and function without contrast, followed by contrast and further sequences; with stress imaging Cardiac magnetic resonance imaging for velocity flow mapping (List separately in addition to code for primary procedure) Magnetic resonance imaging, breast, without and/or with contrast ; unilateral Magnetic resonance imaging, breast, without and/or with contrast ; bilateral C8903 Magnetic resonance imaging with contrast, breast; unilateral C8904 Magnetic resonance imaging without contrast, breast; unilateral C8905 Magnetic resonance imaging without contrast followed by with contrast, breast; unilateral C8906 Magnetic resonance imaging with contrast, breast; bilateral C8907 Magnetic resonance imaging without contrast, breast; bilateral C8908 Magnetic resonance imaging without contrast followed by with contrast, breast; bilateral Q9953 Injection, iron-based magnetic resonance contrast agent, per ml Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 7
8 MRA CPT/HCPCS Codes Code Description Magnetic resonance angiography, head; without contrast Magnetic resonance angiography, head; with contrast Magnetic resonance angiography, head; without contrast, followed by contrast and further sequences Magnetic resonance angiography, neck; without contrast Magnetic resonance angiography, neck; with contrast Magnetic resonance angiography, neck; without contrast, followed by contrast and further sequences Magnetic resonance angiography, chest (excluding myocardium), with or without contrast Magnetic resonance angiography, spinal canal and contents, with or without contrast (Not covered by Medicare in any payment system) Magnetic resonance angiography, pelvis, with or without contrast Magnetic resonance angiography, upper extremity, with or without contrast (Not covered by Medicare in any payment system) Magnetic resonance angiography, lower extremity, with or without contrast Magnetic resonance angiography, abdomen, with or without contrast Cardiac magnetic resonance imaging for velocity flow mapping (List separately in addition to code for primary procedure) C8900 Magnetic resonance angiography with contrast, abdomen C8901 Magnetic resonance angiography without contrast, abdomen C8902 Magnetic resonance angiography without contrast followed by with contrast, abdomen C8909 Magnetic resonance angiography with contrast, chest (excluding myocardium) C8910 Magnetic resonance angiography without contrast, chest (excluding myocardium) C8911 Magnetic resonance angiography without contrast followed by with contrast, chest (excluding myocardium) C8912 Magnetic resonance angiography with contrast, lower extremity C8913 Magnetic resonance angiography without contrast, lower extremity C8914 Magnetic resonance angiography without contrast followed by with contrast, lower extremity C8918 Magnetic resonance angiography with contrast, pelvis C8919 Magnetic resonance angiography without contrast, pelvis C8920 Magnetic resonance angiography without contrast followed by with contrast, pelvis C8931 Magnetic resonance angiography with contrast, spinal canal and contents C8932 Magnetic resonance angiography without contrast, spinal canal and contents C8933 Magnetic resonance angiography without contrast followed by with contrast, spinal canal and contents C8934 Magnetic resonance angiography with contrast, upper extremity C8935 Magnetic resonance angiography without contrast, upper extremity C8936 Magnetic resonance angiography without contrast followed by with contrast, upper extremity Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 8
9 Modifiers Code KX Description Requirements specified in the medical policy have been met Questions and Answers Q: Can a Medicare beneficiary have a MRI covered if they have an implanted permanent pacemaker (PMs)? A: Effective for claims with dates of service on or after July 7, 2011, CMS believes that the evidence is adequate to conclude that magnetic resonance imaging (MRI) improves health outcomes for Medicare beneficiaries with implanted permanent pacemakers (PMs) when the PMs are used according to the FDA-approved labeling for use in an MRI environment. Other contraindications that may be present in any given beneficiary would continue to apply in patients with PMs. These other contraindications are listed in section C.1 of the National Coverage Determinations (NCD) manual and referenced in CR Effective date: 07/07/2011 Implementation date: 09/26/2011. References Included (but not limited to): CMS NCD(s) NCD Magnetic Resonance Imaging Reference NCDs: NCD Magnetic Resonance Spectroscopy, NCD Magnetic Resonance Angiography CMS LCD(s) Numerous LCDs CMS Article One Article CMS Benefit Policy Manual Chapter 15; Interpreting Physician Determines a Different Diagnostic Test is Appropriate, 170 Inpatient Hospital or SNF Services Not Delivered Directly or Under Arrangement by the Provider CMS Claims Processing Manual Chapter 13; Magnetic Resonance Imaging (MRI) Procedures, Deep Brain Stimulation for Essential Tremor and Parkinson s Disease CMS Transmittals Transmittal 135, Change Request 7441, Dated 09/22/2011 (Magnetic Resonance Imaging (MRI) in Medicare Beneficiaries with FDA-Approved Implanted Permanent Pacemakerments (PMs) for use in an MRI Environ) Transmittal 2293, Change Request 7441, Dated 08/26/2011 (Magnetic Resonance Imaging (MRI) in Medicare Beneficiaries with FDA-Approved Implanted Permanent Pacemakers (PMs) for use in an MRI Environment) Transmittal 2307, Change Request 7441, Dated 09/22/2011 (Magnetic Resonance Imaging (MRI) in Medicare Beneficiaries with FDA-Approved Implanted Permanent Pacemakers (PMs) for use in an MRI Environment) UnitedHealthcare Medicare Advantage Coverage Summaries Radiologic Diagnostic Procedures UnitedHealthcare Reimbursement Policies Magnetic Resonance Spectroscopy MLN Matters Article MM6672, Magnetic Resonance Imaging (MRI) Article MM7441, Magnetic Resonance Imaging (MRI) in Medicare Beneficiaries with Food and Drug Administration (FDA)-Approved Implanted Permanent Pacemakers (PMs) for Use in the MRI Environment Others MLN Matters Fact Sheet, Medicare Coverage of Imaging Services CMS Medicare National Coverage Determinations Manual, Chapter 1, History Date Revisions 09/04/2014 Administrative updates Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 9
10 05/14/2014 Policy re-review presented to MRPC for approval Administrative updates 04/10/2013 Policy re-review presented to MRPC for approval 01/25/2012 Policy re-review presented to MRPC for approval Proprietary information of UnitedHealthcare. Copyright 2014 United HealthCare Services, Inc. Page 10
FOR CMS (MEDICARE) MEMBERS ONLY NATIONAL COVERAGE DETERMINATION (NCD) FOR MAGNETIC RESONANCE IMAGING:
 National Imaging Associates, Inc. Clinical guidelines SINUS MRI Original Date: November 2007 Page 1 of 5 CPT Codes: 70540, 70542, 70543 Last Review Date: July 2014 NCD 220.2 MRI Last Effective Date: July
National Imaging Associates, Inc. Clinical guidelines SINUS MRI Original Date: November 2007 Page 1 of 5 CPT Codes: 70540, 70542, 70543 Last Review Date: July 2014 NCD 220.2 MRI Last Effective Date: July
FOR CMS (MEDICARE) MEMBERS ONLY NATIONAL COVERAGE DETERMINATION (NCD) FOR MAGNETIC RESONANCE IMAGING:
 National Imaging Associates, Inc. Clinical guidelines BONE MARROW MRI Original Date: July 2008 Page 1 of 5 CPT Codes: 77084 Last Review Date: September 2014 NCD 220.2 MRI Last Effective Date: July 2011
National Imaging Associates, Inc. Clinical guidelines BONE MARROW MRI Original Date: July 2008 Page 1 of 5 CPT Codes: 77084 Last Review Date: September 2014 NCD 220.2 MRI Last Effective Date: July 2011
FOR CMS (MEDICARE) MEMBERS ONLY NATIONAL COVERAGE DETERMINATION (NCD) FOR MAGNETIC RESONANCE IMAGING:
 National Imaging Associates, Inc. Clinical guidelines TEMPOROMANDIBULAR JOINT (TMJ) MRI Original Date: May 23, 2003 Page 1 of 5 CPT Code: 70336 Last Review Date: May 2016 NCD 220.2 MRI Last Effective Date:
National Imaging Associates, Inc. Clinical guidelines TEMPOROMANDIBULAR JOINT (TMJ) MRI Original Date: May 23, 2003 Page 1 of 5 CPT Code: 70336 Last Review Date: May 2016 NCD 220.2 MRI Last Effective Date:
Ultrasound and Fluoroscopic Paravertebral Facet Joint Injections
 Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Pharmacogenomic Testing for Warfarin Response (NCD 90.1)
 Policy Number 90.1 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number 90.1 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Extracranial-Intracranial (EC-IC) Arterial Bypass Surgery (NCD 20.2)
 Policy Number 20.2 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 05/28/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number 20.2 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 05/28/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
MOLINA HEALTHCARE OF MICHIGAN PRIOR AUTHORIZATION / PRE-SERVICE REVIEW GUIDE IMAGING CODES REQUIRING PRIOR AUTHORIZATION EFFECTIVE 1/1/2014
 70336 MRI MRI, temporomandibular joint(s) 70450 CT/CTA CT, head or brain; without contrast material 70460 CT/CTA CT, head or brain; with contrast material(s) 70470 CT/CTA CT, head or brain; without contrast
70336 MRI MRI, temporomandibular joint(s) 70450 CT/CTA CT, head or brain; without contrast material 70460 CT/CTA CT, head or brain; with contrast material(s) 70470 CT/CTA CT, head or brain; without contrast
Carotid Sinus Nerve Stimulator (NCD 160.6)
 Policy Number Reimbursement Policy 160.6 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 05/28/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy
Policy Number Reimbursement Policy 160.6 Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 05/28/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy
MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE
 UnitedHealthcare Commercial Utilization Review Guideline MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE Guideline Number: URG-13.01 Effective Date: February 1, 2019 Table
UnitedHealthcare Commercial Utilization Review Guideline MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE Guideline Number: URG-13.01 Effective Date: February 1, 2019 Table
screening; including image post processing CT, heart; without contrast material; with Requires authorization
 0042T Cerebral perfusion analysis using CT; with ; including of parametric maps with determination of cerebral blood flow, cerebral blood volume, and mean transit time 74263 Computed tomographic (CT) colonography,
0042T Cerebral perfusion analysis using CT; with ; including of parametric maps with determination of cerebral blood flow, cerebral blood volume, and mean transit time 74263 Computed tomographic (CT) colonography,
Cigna - Prior Authorization Procedure List: Radiology & Cardiology
 Cigna - Prior Authorization Procedure List: Radiology & Cardiology Category CPT Code CPT Code Description 93451 Right heart catheterization 93452 Left heart catheterization 93453 Combined right and left
Cigna - Prior Authorization Procedure List: Radiology & Cardiology Category CPT Code CPT Code Description 93451 Right heart catheterization 93452 Left heart catheterization 93453 Combined right and left
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 70544 Magnetic resonance angiography, head; without contrast material(s) 70545 with contrast material(s)
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 70544 Magnetic resonance angiography, head; without contrast material(s) 70545 with contrast material(s)
Cigna - Prior Authorization Procedure List: Radiology & Cardiology
 Cigna - Prior Authorization Procedure List: Radiology & Cardiology Product Category CPT Code CPT Code Description Radiology MR 70336 MRI Temporomandibular Joint(s), (TMJ) Radiology CT 70450 CT Head or
Cigna - Prior Authorization Procedure List: Radiology & Cardiology Product Category CPT Code CPT Code Description Radiology MR 70336 MRI Temporomandibular Joint(s), (TMJ) Radiology CT 70450 CT Head or
Radiology Codes Requiring Authorization*
 70336 Magnetic resonance (eg, proton) imaging, temporomandibular joint(s) 70450 Computed tomography, head or brain; without contrast material 70460 Computed tomography, head or brain; with contrast material(s)
70336 Magnetic resonance (eg, proton) imaging, temporomandibular joint(s) 70450 Computed tomography, head or brain; without contrast material 70460 Computed tomography, head or brain; with contrast material(s)
Anthem Blue Cross and Blue Shield Virginia Advanced Imaging Procedures Requiring Precertification Revised 02/13/2013
 Anthem Blue Cross and Blue Shield Virginia Advanced Imaging Procedures Requiring Precertification Revised 02/13/2013 Modality and CT Head CTA Head: Cerebrovascular MRI Head MRA Head: Cerebrovascular Functional
Anthem Blue Cross and Blue Shield Virginia Advanced Imaging Procedures Requiring Precertification Revised 02/13/2013 Modality and CT Head CTA Head: Cerebrovascular MRI Head MRA Head: Cerebrovascular Functional
Medical Review Guidelines Magnetic Resonance Angiography
 Medical Review Guidelines Magnetic Resonance Angiography Medical Guideline Number: MRG2001-05 Effective Date: 2/13/01 Revised Date: 2/14/2006 OHCA Reference OAC 317:30-5-24. Radiology. (f) Magnetic Resonance
Medical Review Guidelines Magnetic Resonance Angiography Medical Guideline Number: MRG2001-05 Effective Date: 2/13/01 Revised Date: 2/14/2006 OHCA Reference OAC 317:30-5-24. Radiology. (f) Magnetic Resonance
Diagnostic Imaging Utilization Management and Consultation Management Programs Imaging Code Listing for Connecticut, Maine and New Hampshire
 Diagnostic Imaging Utilization Management and Consultation Management Programs Imaging Code Listing for Connecticut, Maine and New Hampshire The grid below contains the CPT * codes that are subject to
Diagnostic Imaging Utilization Management and Consultation Management Programs Imaging Code Listing for Connecticut, Maine and New Hampshire The grid below contains the CPT * codes that are subject to
FOR CMS (MEDICARE) MEMBERS ONLY NATIONAL COVERAGE DETERMINATION (NCD) FOR MAGNETIC RESONANCE IMAGING:
 National Imaging Associates, Inc. Clinical guidelines ORBIT MRI Original Date: September 1997 Page 1 of 7 CPT Codes: 70540, 70542, 70543 Last Review Date: August 2015 NCD 220.2 MRI Last Effective Date:
National Imaging Associates, Inc. Clinical guidelines ORBIT MRI Original Date: September 1997 Page 1 of 7 CPT Codes: 70540, 70542, 70543 Last Review Date: August 2015 NCD 220.2 MRI Last Effective Date:
High Tech Imaging Quick Reference Guide
 High Tech Imaging Quick Reference Guide 1 High Tech Imaging Authorizations may now be requested through our secure provider portal, BlueAccess. Getting Started Step 1: Log into BlueAccess from www.bcbst.com
High Tech Imaging Quick Reference Guide 1 High Tech Imaging Authorizations may now be requested through our secure provider portal, BlueAccess. Getting Started Step 1: Log into BlueAccess from www.bcbst.com
ADI Procedure Codes. August 2016 Revised April 2017 Page 1 of 7 ADI Procedure Codes
 Code Description 70450 CT Head without contrast 70460 CT Head with contrast 70470 CT Head with & without contrast 70480 CT Orbit, et al without contrast 70481 CT Orbit, et al with contrast 70482 CT Orbit,
Code Description 70450 CT Head without contrast 70460 CT Head with contrast 70470 CT Head with & without contrast 70480 CT Orbit, et al without contrast 70481 CT Orbit, et al with contrast 70482 CT Orbit,
05/02/ CPT Preauthorization Groupings Effective May 2, Computerized Tomography (CT) Abdomen 6. CPT Description SEGR CT01
 Computerized Tomography (CT) 6 & 101 5 Upper Extremity 11 Lower Extremity 12 Head 3 Orbit 1 Sinus 2 Neck 4 7 Cervical Spine 8 Thoracic Spine 9 Lumbar Spine 10 Colon 13 CPT Preauthorization Groupings CPT
Computerized Tomography (CT) 6 & 101 5 Upper Extremity 11 Lower Extremity 12 Head 3 Orbit 1 Sinus 2 Neck 4 7 Cervical Spine 8 Thoracic Spine 9 Lumbar Spine 10 Colon 13 CPT Preauthorization Groupings CPT
POSITRON EMISSION TOMOGRAPHY (PET)
 Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
2014 CPT Radiology Codes Requiring Review
 CT Head 1 70480 CT orbit, sella or posterior fossa; w/o contrast 1 CT Head 1 70481 CT orbit, sella or posterior fossa; with CT orbit, sella or posterior fossa; w/o contrast CT Head 1 70482 followed by
CT Head 1 70480 CT orbit, sella or posterior fossa; w/o contrast 1 CT Head 1 70481 CT orbit, sella or posterior fossa; with CT orbit, sella or posterior fossa; w/o contrast CT Head 1 70482 followed by
Codes Requiring Authorization from MedSolutions (MSI): Updated 3/2014
 s Requiring Authorization from MedSolutions (): Updated 3/2014 0042T Cerebral Perfusion Analysis using CT with contrast 0159T CAD, including computer algorithm analysis, BREAST MRI 0195T prepare interspace,
s Requiring Authorization from MedSolutions (): Updated 3/2014 0042T Cerebral Perfusion Analysis using CT with contrast 0159T CAD, including computer algorithm analysis, BREAST MRI 0195T prepare interspace,
Policy #: 291 Latest Review Date: February 2013
 Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
AIM 2014 CPT Radiology & Cardiac Codes Requiring Review
 AIM 2014 CPT Radiology & Cardiac Codes Requiring Review Modality Body Part CT Head 1 70480 CT orbit, sella or posterior fossa; w/o contrast 1 CT Head 1 70481 CT orbit, sella or posterior fossa; with CT
AIM 2014 CPT Radiology & Cardiac Codes Requiring Review Modality Body Part CT Head 1 70480 CT orbit, sella or posterior fossa; w/o contrast 1 CT Head 1 70481 CT orbit, sella or posterior fossa; with CT
2012 CPT Radiology Codes Requiring Review Blue Cross and Blue Shield of Louisiana
 2012 CPT Radiology Codes Requiring Review Blue Cross and Blue Shield of Louisiana CT Head 70480 CT orbit, sella or posterior fossa; w/o CT Head 70481 CT orbit, sella or posterior fossa; with CT Head 70482
2012 CPT Radiology Codes Requiring Review Blue Cross and Blue Shield of Louisiana CT Head 70480 CT orbit, sella or posterior fossa; w/o CT Head 70481 CT orbit, sella or posterior fossa; with CT Head 70482
AMERICAN IMAGING MANAGEMENT
 2012 CPT Codes Computerized Tomography (CT) CPT Description Abdomen 74150 CT abdomen; w/o 74160 CT abdomen; with 74170 CT abdomen; w/o followed by Chest 71250 CT thorax; w/o 71260 CT thorax; with 71270
2012 CPT Codes Computerized Tomography (CT) CPT Description Abdomen 74150 CT abdomen; w/o 74160 CT abdomen; with 74170 CT abdomen; w/o followed by Chest 71250 CT thorax; w/o 71260 CT thorax; with 71270
AMERICAN IMAGING MANAGEMENT
 2010 BCBS of Georgia CPT Codes With Grouper Numbers Computerized Tomography (CT) CPT Description Abdomen 74150 CT abdomen; w/o contrast 6 74160 CT abdomen; with contrast 74170 CT abdomen; w/o contrast
2010 BCBS of Georgia CPT Codes With Grouper Numbers Computerized Tomography (CT) CPT Description Abdomen 74150 CT abdomen; w/o contrast 6 74160 CT abdomen; with contrast 74170 CT abdomen; w/o contrast
Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft
 MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
Local Coverage Article for Chiropractic Services (A47798) Contractor Information. Article Information. Contractor Name. Contractor Numbers
 Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
BlueAdvantage SM. & BlueChoice SM Radiology Prior Authorization Program Code List CPT /HCPS
 BlueAdvantage SM & BlueChoice SM Radiology Prior Authorization Program Code List CPT /HCPS 70336 MRI TMJ 70450 CT Head Without Contrast 70460 CT Head With Contrast 70470 CT Head Without & With Contrast
BlueAdvantage SM & BlueChoice SM Radiology Prior Authorization Program Code List CPT /HCPS 70336 MRI TMJ 70450 CT Head Without Contrast 70460 CT Head With Contrast 70470 CT Head Without & With Contrast
Halaven (Eribulin Mesylate)
 Policy Number HAL02282012RP Approved By UnitedHealthcare Medicare Committee Current Approval Date 09/24/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number HAL02282012RP Approved By UnitedHealthcare Medicare Committee Current Approval Date 09/24/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Physical Medicine & Rehabilitation: Maximum Combined Frequency per Day Policy
 Policy Number Physical Medicine & Rehabilitation: Maximum Combined Frequency per Day Policy 2017R0101E Annual Approval Date 7/13/2016 Approved By Payment Policy Oversight Committee IMPORTANT NOTE ABOUT
Policy Number Physical Medicine & Rehabilitation: Maximum Combined Frequency per Day Policy 2017R0101E Annual Approval Date 7/13/2016 Approved By Payment Policy Oversight Committee IMPORTANT NOTE ABOUT
Intravenous Immune Globulin (IVIg)
 Policy Number Reimbursement Policy IVIG04272010RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 10/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This
Policy Number Reimbursement Policy IVIG04272010RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 10/08/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice)
 Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Diagnostic Imaging Prior Review Code List 2 nd Quarter 2018
 Computerized Tomography (CT) Abdomen 6 Abdomen/Pelvis Combination 101 Service 74150 CT abdomen; w/o 74160 CT abdomen; with 74170 CT abdomen; w/o followed by 74176 Computed tomography, abdomen and pelvis;
Computerized Tomography (CT) Abdomen 6 Abdomen/Pelvis Combination 101 Service 74150 CT abdomen; w/o 74160 CT abdomen; with 74170 CT abdomen; w/o followed by 74176 Computed tomography, abdomen and pelvis;
Jurisdiction New Mexico. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
Diagnostic Imaging Utilization Management and Consultation Management Programs Imaging Code Listing for Connecticut, Maine and New Hampshire
 Diagnostic Imaging Utilization Management and Consultation Management Programs Imaging Code Listing for Connecticut, Maine and New Hampshire The grid below contains the CPT * codes that are subject to
Diagnostic Imaging Utilization Management and Consultation Management Programs Imaging Code Listing for Connecticut, Maine and New Hampshire The grid below contains the CPT * codes that are subject to
Policy #: 222 Latest Review Date: March 2009
 Name of Policy: MRI Phase-Contrast Flow Measurement Policy #: 222 Latest Review Date: March 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Name of Policy: MRI Phase-Contrast Flow Measurement Policy #: 222 Latest Review Date: March 2009 Category: Radiology Policy Grade: Active Policy but no longer scheduled for regular literature reviews and
Jurisdiction Georgia. Retirement Date N/A
 If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
Clinical Appropriateness Guidelines: Advanced Imaging
 Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Imaging of Bone Marrow Blood Supply Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007 Last revised:
Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Imaging of Bone Marrow Blood Supply Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007 Last revised:
2018 Cerebrovascular Reimbursement Coding Fact Sheet
 The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
Lumify. Lumify reimbursement guide {D DOCX / 1
 Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1
 GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
HEALTHFIRST 2011 RADIOLOGY PROGRAM CODE LIST
 HEALTHFIRST 2011 RADIOLOGY PROGRAM CODE LIST Outpatient Radiology utilization call Carecore at 1-877-773-6964 Modality CPT CODE Description CT SCANS 70450 CT HEAD/BRAIN W/O CONTRAST CT SCANS 70460 CT HEAD/BRAIN
HEALTHFIRST 2011 RADIOLOGY PROGRAM CODE LIST Outpatient Radiology utilization call Carecore at 1-877-773-6964 Modality CPT CODE Description CT SCANS 70450 CT HEAD/BRAIN W/O CONTRAST CT SCANS 70460 CT HEAD/BRAIN
Professional Non Covered Codes Policy
 Policy Number 2018R7102I Professional Non Covered Codes Policy Annual Approval Date 7/12/2017 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY You are
Policy Number 2018R7102I Professional Non Covered Codes Policy Annual Approval Date 7/12/2017 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY You are
Cardiac MRI in ACHD What We. ACHD Patients
 Cardiac MRI in ACHD What We Have Learned to Apply to ACHD Patients Faris Al Mousily, MBChB, FAAC, FACC Consultant, Pediatric Cardiology, KFSH&RC/Jeddah Adjunct Faculty, Division of Pediatric Cardiology
Cardiac MRI in ACHD What We Have Learned to Apply to ACHD Patients Faris Al Mousily, MBChB, FAAC, FACC Consultant, Pediatric Cardiology, KFSH&RC/Jeddah Adjunct Faculty, Division of Pediatric Cardiology
Drug Testing Policy. Reimbursement Policy CMS Approved By. Policy Number. Annual Approval Date. Reimbursement Policy Oversight Committee
 Reimbursement Policy CMS 1500 Drug Testing Policy Policy Number 2017R6005A Annual Approval Date 05/10/2017 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY
Reimbursement Policy CMS 1500 Drug Testing Policy Policy Number 2017R6005A Annual Approval Date 05/10/2017 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY
LCD L B-type Natriuretic Peptide (BNP) Assays
 LCD L30559 - B-type Natriuretic Peptide (BNP) Assays Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302, 12401,
LCD L30559 - B-type Natriuretic Peptide (BNP) Assays Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302, 12401,
Contractor Number 03201
 Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Deborah K. Mann & Jennifer Bash. Coding Documentation and Education Managers
 Deborah K. Mann & Jennifer Bash Coding Documentation and Education Managers OBJECTIVES Review the basics of Diagnostic, CT, & MRI documentation Risk areas in radiology associated with Diagnostic, CT, &
Deborah K. Mann & Jennifer Bash Coding Documentation and Education Managers OBJECTIVES Review the basics of Diagnostic, CT, & MRI documentation Risk areas in radiology associated with Diagnostic, CT, &
Chapter 5 Section 1.1. Diagnostic Radiology (Diagnostic Imaging)
 Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) 1.0 CPT 1 PROCEDURE CODES 70010-72292, 73000-76499, 77071-77084,
Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) 1.0 CPT 1 PROCEDURE CODES 70010-72292, 73000-76499, 77071-77084,
Clinical Applications
 C H A P T E R 16 Clinical Applications In selecting pulse sequences and measurement parameters for a specific application, MRI allows the user tremendous flexibility to produce variations in contrast between
C H A P T E R 16 Clinical Applications In selecting pulse sequences and measurement parameters for a specific application, MRI allows the user tremendous flexibility to produce variations in contrast between
HIP RADIOLOGY PROGRAM CODE LISTS
 EFFECTIVE OCTOBER 1, 2012 70336 MAGNETIC RESONANCE IMAGING TMJ 70450 COMPUTED TOMOGRAPHY HEAD/BRAIN WITHOUT 70460 COMPUTED TOMOGRAPHY HEAD/BRAIN WITH 70470 COMPUTED TOMOGRAPHY HEAD/BRAIN WITHOUT AND WITH
EFFECTIVE OCTOBER 1, 2012 70336 MAGNETIC RESONANCE IMAGING TMJ 70450 COMPUTED TOMOGRAPHY HEAD/BRAIN WITHOUT 70460 COMPUTED TOMOGRAPHY HEAD/BRAIN WITH 70470 COMPUTED TOMOGRAPHY HEAD/BRAIN WITHOUT AND WITH
HIGHMARK Medicare Clinical Guidelines for Medical Necessity Review. Medicare - Pennsylvania
 HIGHMARK 2017 Medicare Clinical Guidelines for Medical Necessity Review Medicare - Pennsylvania Version: 1 Effective October 2016 2017 Magellan Health, Inc. Proprietary Page 1 of 31 Guidelines for Clinical
HIGHMARK 2017 Medicare Clinical Guidelines for Medical Necessity Review Medicare - Pennsylvania Version: 1 Effective October 2016 2017 Magellan Health, Inc. Proprietary Page 1 of 31 Guidelines for Clinical
LCD Information Document Information LCD ID Number L30046
 Local Coverage Determination (LCD): Pathology and Laboratory: B-type Natriuretic Peptide (BNP) Testing (L30046) LCD Information Document Information LCD ID Number L30046 LCD Title Pathology and Laboratory:
Local Coverage Determination (LCD): Pathology and Laboratory: B-type Natriuretic Peptide (BNP) Testing (L30046) LCD Information Document Information LCD ID Number L30046 LCD Title Pathology and Laboratory:
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
Inspire Medical Systems. Physician Billing Guide
 Inspire Medical Systems Physician Billing Guide 2019 Inspire Medical Systems Physician Billing Guide This Physician Billing Guide was developed to help providers correctly bill for Inspire Upper Airway
Inspire Medical Systems Physician Billing Guide 2019 Inspire Medical Systems Physician Billing Guide This Physician Billing Guide was developed to help providers correctly bill for Inspire Upper Airway
CMS Limitations Guide MRA Radiology Services
 CMS Limitations Guide MRA Radiology Services Starting July 1, 2008, CMS has placed numerous medical necessity limits on tests and procedures. This reference guide provides you with all of the latest changes.
CMS Limitations Guide MRA Radiology Services Starting July 1, 2008, CMS has placed numerous medical necessity limits on tests and procedures. This reference guide provides you with all of the latest changes.
Cardiac Imaging Tests
 Cardiac Imaging Tests http://www.medpagetoday.com/upload/2010/11/15/23347.jpg Standard imaging tests include echocardiography, chest x-ray, CT, MRI, and various radionuclide techniques. Standard CT and
Cardiac Imaging Tests http://www.medpagetoday.com/upload/2010/11/15/23347.jpg Standard imaging tests include echocardiography, chest x-ray, CT, MRI, and various radionuclide techniques. Standard CT and
Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians
 GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians January, 2013 www.gehealthcare.com/reimbursement This overview
GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Procedures 1 Performed by Emergency Medicine Physicians January, 2013 www.gehealthcare.com/reimbursement This overview
Drug Testing Policy. Reimbursement Policy CMS Approved By. Policy Number. Annual Approval Date. Reimbursement Policy Oversight Committee
 Reimbursement Policy CMS 1500 Drug Testing Policy Policy Number 2018R6005A Annual Approval Date 07/11/2018 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY
Reimbursement Policy CMS 1500 Drug Testing Policy Policy Number 2018R6005A Annual Approval Date 07/11/2018 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY
Clinical Policy: Digital Breast Tomosynthesis Reference Number: CP.MP.90
 Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
2019 MITRACLIP CODING AND PAYMENT GUIDE
 CLAIM 2019 MITRACLIP AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Hospital Rates: Effective October 1, 2018 Physician Rates: Effective January 1, 2019 References and Brief Summary 1 CLAIM
CLAIM 2019 MITRACLIP AND PAYMENT GUIDE MitraClip Transcatheter Mitral Valve Repair Hospital Rates: Effective October 1, 2018 Physician Rates: Effective January 1, 2019 References and Brief Summary 1 CLAIM
Drug Testing Policy. Approved By 05/10/2017. Application This reimbursement policy applies to UnitedHealthcare Community Plan Medicaid products.
 Policy Number 2017R6005B Annual Approval Date Drug Testing Policy 05/10/2017 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY You are responsible for
Policy Number 2017R6005B Annual Approval Date Drug Testing Policy 05/10/2017 Approved By Reimbursement Policy Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY You are responsible for
Erbitux (Cetuximab) UnitedHealthcare Medicare Reimbursement Policy Committee
 Policy Number ERB12012011RP Approved By UnitedHealthcare Medicare Committee Current Approval Date 09/10/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
Policy Number ERB12012011RP Approved By UnitedHealthcare Medicare Committee Current Approval Date 09/10/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare
ADVANCED CARDIOVASCULAR IMAGING. Medical Knowledge. Goals and Objectives PF EF MF LF Aspirational
 Medical Knowledge Goals and Objectives PF EF MF LF Aspirational Know the basic principles of magnetic resonance imaging (MRI) including the role of the magnetic fields and gradient coil systems, generation
Medical Knowledge Goals and Objectives PF EF MF LF Aspirational Know the basic principles of magnetic resonance imaging (MRI) including the role of the magnetic fields and gradient coil systems, generation
Local Coverage Determination for Colorectal Cancer Screening (L29796)
 Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Diagnostic and interventional venous procedures (lower extremity)
 2017 Coding and Medicare payment guide Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered
2017 Coding and Medicare payment guide Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered
Cardiovascular nuclear imaging employs non-invasive techniques to assess alterations in coronary artery flow, and ventricular function.
 National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
Chapter 5 Section 1.1
 Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) Copyright: CPT only 2006 American Medical Association (or such
Radiology Chapter 5 Section 1.1 Issue Date: March 7, 1986 Authority: 32 CFR 199.4(a), (b)(2)(x), (c)(2)(viii), (e)(14) and 32 CFR 199.6(d)(2) Copyright: CPT only 2006 American Medical Association (or such
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments
 CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
Head MRA/MRV studies of the head may be considered medically necessary for the following strongly suspected vascular diseases:
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: September 5, 2014 I. POLICY Head MRA/MRV studies of the head may be considered medically
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: September 5, 2014 I. POLICY Head MRA/MRV studies of the head may be considered medically
Icd 10 code for ct pelvis with contrast
 Icd 10 code for ct pelvis with contrast November 16, 2009. How to Code for CT Angiography. By Anthony McCallum, CPC, CCS, CIRCC, CPC-I Radiology Today Vol. 10 No. 18 P. 12. CT. procedure code and description
Icd 10 code for ct pelvis with contrast November 16, 2009. How to Code for CT Angiography. By Anthony McCallum, CPC, CCS, CIRCC, CPC-I Radiology Today Vol. 10 No. 18 P. 12. CT. procedure code and description
Drug Testing Policy. Approved By 06/14/2017
 Drug Testing Policy Policy Number Annual Approval Date 06/14/2017 Approved By Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare Medicare Advantage
Drug Testing Policy Policy Number Annual Approval Date 06/14/2017 Approved By Oversight Committee IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable to UnitedHealthcare Medicare Advantage
1Pulse sequences for non CE MRA
 MRI: Principles and Applications, Friday, 8.30 9.20 am Pulse sequences for non CE MRA S. I. Gonçalves, PhD Radiology Department University Hospital Coimbra Autumn Semester, 2011 1 Magnetic resonance angiography
MRI: Principles and Applications, Friday, 8.30 9.20 am Pulse sequences for non CE MRA S. I. Gonçalves, PhD Radiology Department University Hospital Coimbra Autumn Semester, 2011 1 Magnetic resonance angiography
CMS Limitations Guide - Radiology Services
 CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Assignable revenue codes: Explanation of services:
 COMPUTED TOMOGRAPHY Chest/Cardiac Assignable revenue codes: 0350 CT Scan General Classification 0351 CT Scan Head Scan 0352 CT Scan Body Scan 0359 CT Scan Other CT Scans Explanation of services: Known
COMPUTED TOMOGRAPHY Chest/Cardiac Assignable revenue codes: 0350 CT Scan General Classification 0351 CT Scan Head Scan 0352 CT Scan Body Scan 0359 CT Scan Other CT Scans Explanation of services: Known
RETIRED. Maximum Drug Dose Policy
 RETIRED DRUG POLICY Policy Number 2017D0034A Maximum Drug Dose Policy Annual Approval Date 3/1/2017 Approved By UnitedHealthcare National Pharmacy & Therapeutics Committee United Healthcare Community Plan
RETIRED DRUG POLICY Policy Number 2017D0034A Maximum Drug Dose Policy Annual Approval Date 3/1/2017 Approved By UnitedHealthcare National Pharmacy & Therapeutics Committee United Healthcare Community Plan
Icd 10 code for pre screening for mri
 Icd 10 code for pre screening for mri Free, official coding info for 2018 ICD-10-CM Z02.1 - includes detailed rules, notes, synonyms, ICD-9-CM conversion, index and annotation crosswalks, DRG. ICD-9-CM
Icd 10 code for pre screening for mri Free, official coding info for 2018 ICD-10-CM Z02.1 - includes detailed rules, notes, synonyms, ICD-9-CM conversion, index and annotation crosswalks, DRG. ICD-9-CM
Certification Review. Module 28. Medical Coding. Radiology
 Module 28 is the study of x-rays, using radiant energy and other imaging techniques, such as resonance imaging or ultrasound, to diagnose illnesses and diseases. Vocabulary Barium enema (BE): lower gastrointestinal
Module 28 is the study of x-rays, using radiant energy and other imaging techniques, such as resonance imaging or ultrasound, to diagnose illnesses and diseases. Vocabulary Barium enema (BE): lower gastrointestinal
Physical Therapy and Occupational Therapy Initial Evaluation and Reevaluation Reimbursement Policy. Approved By
 Policy Number Physical Therapy and Occupational Therapy Initial Evaluation and Reevaluation Reimbursement Policy 0044 Annual Approval Date 4/2017 Approved By Optum Reimbursement Committee Optum Quality
Policy Number Physical Therapy and Occupational Therapy Initial Evaluation and Reevaluation Reimbursement Policy 0044 Annual Approval Date 4/2017 Approved By Optum Reimbursement Committee Optum Quality
Clinical Appropriateness Guidelines: Advanced Imaging
 Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Quantitative CT (QCT) Bone Mineral Densitometry Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007
Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Quantitative CT (QCT) Bone Mineral Densitometry Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION. Original Date: October 2015 Page 1 of 5
 National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
National Imaging Associates, Inc. Clinical guidelines CARDIAC CATHETERIZATION -LEFT HEART CATHETERIZATION CPT Codes: 93451, 93452, 93453, 93454, 93455, 93456, 93457, 93458, 93459, 93460, 93461 LCD ID Number:
Electrical Stimulation Device Used for Cancer Treatment
 Electrical Stimulation Device Used for Cancer Treatment OPTUNE (NOVOTTF 100A SYSTEM) For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit
Electrical Stimulation Device Used for Cancer Treatment OPTUNE (NOVOTTF 100A SYSTEM) For any item to be covered by The Health Plan, it must: 1. Be eligible for a defined Medicare or The Health Plan benefit
CD Horizon Spire. CD Horizon Spire Z PHYSICIAN REIMBURSEMENT REIMBURSEMENT GUIDE. Spinal System and. Spinal System
 REIMBURSEMENT GUIDE CD Horizon Spire Spinal System and CD Horizon Spire Z Spinal System The CD Horizon Spire Plate is a posterior, single level, non-pedicle supplemental fixation device intended for use
REIMBURSEMENT GUIDE CD Horizon Spire Spinal System and CD Horizon Spire Z Spinal System The CD Horizon Spire Plate is a posterior, single level, non-pedicle supplemental fixation device intended for use
Product Name or Headline
 Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
POLICY AND PROCEDURE
 PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
CODING SHEET HYDROCEPHALUS REIMBURSEMENT. All Medicare information is current as of the time of printing.
 CODING SHEET HYDROCEPHALUS REIMBURSEMENT All Medicare information is current as of the January 2014 Hydrocephalus ing Coding Options Commonly Billed Codes for Physicians, Hospitals, and Ambulatory Surgery
CODING SHEET HYDROCEPHALUS REIMBURSEMENT All Medicare information is current as of the January 2014 Hydrocephalus ing Coding Options Commonly Billed Codes for Physicians, Hospitals, and Ambulatory Surgery
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 93875 Non-invasive physiologic studies of extracranial arteries, complete bilateral study (eg, periorbital
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 93875 Non-invasive physiologic studies of extracranial arteries, complete bilateral study (eg, periorbital
b. To facilitate the management decision of a patient with an equivocal stress test.
 National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
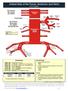
Arterial Map of the Thorax, Abdomen and Pelvis 2017 Edition
 Arterial Map of the Thorax, Abdomen and Pelvis Angiography 75605 (-26) Aortography, thoracic 75625 (-26) Aortography, abdominal by serialography 75630 (-26) Aortography, abdominal + bilat iliofemoral 75705
Arterial Map of the Thorax, Abdomen and Pelvis Angiography 75605 (-26) Aortography, thoracic 75625 (-26) Aortography, abdominal by serialography 75630 (-26) Aortography, abdominal + bilat iliofemoral 75705
CD Horizon Solera 5.5/6.0mm Fenestrated Screw Set
 REIMBURSEMENT GUIDE CD Horizon Solera 5.5/6.0mm Fenestrated Screw Set DEVICE DESCRIPTION The CD Horizon Solera 5.5/6.0mm Fenestrated Screw Set consists of a variety of cannulated multi-axial screws (MAS)
REIMBURSEMENT GUIDE CD Horizon Solera 5.5/6.0mm Fenestrated Screw Set DEVICE DESCRIPTION The CD Horizon Solera 5.5/6.0mm Fenestrated Screw Set consists of a variety of cannulated multi-axial screws (MAS)
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Texas. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
CT/MR Coder. Including CT, CTA, MRI and MRA Services. Prepared and Published By:
 CT/MR Coder 2018 Including CT, CTA, MRI and MRA Services Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN 55101 1-800-252-1578
CT/MR Coder 2018 Including CT, CTA, MRI and MRA Services Prepared and Published By: MedLearn Publishing, A Division of MedLearn Media, Inc. 445 Minnesota Street, Suite 514 St. Paul, MN 55101 1-800-252-1578
REIMBURSEMENT GUIDE. Sovereign. Spinal System
 REIMBURSEMENT GUIDE Sovereign Spinal System REIMBURSEMENT GUIDE The Sovereign Spinal System is indicated for use with autogenous bone graft in patients with degenerative disc disease The Sovereign Spinal
REIMBURSEMENT GUIDE Sovereign Spinal System REIMBURSEMENT GUIDE The Sovereign Spinal System is indicated for use with autogenous bone graft in patients with degenerative disc disease The Sovereign Spinal
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090)
 Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Description MRI, TMJ C T Head Without Contrast C T Head With Contrast C T Head Without & With Contrast
 s Requiring Prior Authorization for the Advanced Imaging 70336 MRI, TMJ 70450 C T Head Without Contrast 70460 C T Head With Contrast 70470 C T Head Without & With Contrast 70480 C T Orbit Without Contrast
s Requiring Prior Authorization for the Advanced Imaging 70336 MRI, TMJ 70450 C T Head Without Contrast 70460 C T Head With Contrast 70470 C T Head Without & With Contrast 70480 C T Orbit Without Contrast
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 71250 Computed tomography, thorax; without contrast material 71260 with contrast material(s) 71270 without
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 71250 Computed tomography, thorax; without contrast material 71260 with contrast material(s) 71270 without
