Not only...but also : factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus
|
|
|
- Melvyn Shelton
- 5 years ago
- Views:
Transcription
1 Rheumatology 2005;44: Advance Access publication 18 October 2005 Review doi: /rheumatology/kei142 Michael Mason Prize Essay Not only...but also : factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus I. N. Bruce Premature coronary heart disease (CHD) has emerged as a major cause of morbidity and mortality in patients with systemic lupus erythematosus (SLE). Overall SLE patients have a 5 6-fold increased risk of CHD and this excess risk is especially pronounced in younger women where the excess risk may be `50-fold. Studies from our group and others have also demonstrated that SLE patients have a higher prevalence of subclinical atherosclerosis compared with controls, with approximately 30% having evidence of subclinical involvement. It is important to consider what factors may underlie this excess risk. We have found that certain classic risk factors, i.e. hypertension and diabetes mellitus, are more prevalent in SLE and that persistent hypercholesterolaemia independently predicts patients who will develop CHD. These risk factors alone do not completely explain the excess risk observed, and after adjusting for classic risk factors SLE remains independently associated with both clinical and subclinical outcomes. Certain other metabolic changes also occur more frequently in SLE, namely premature menopause, renal impairment, high triglycerides and higher plasma homocysteine. In addition, insulin resistance is more pronounced in patients with SLE, and approximately 18% have the metabolic syndrome. It is also increasingly accepted that atherosclerosis is a chronic inflammatory condition, and in SLE systemic complement activation as well as immune complex formation can result in changes that promote the development of atheroma. Similarly, autoantibody production, especially antibodies directed against lipoprotein subtypes and those in the antiphospholipid (APLA) family, are gaining increasing attention. The role of the latter are particularly controversial as different subtypes have been shown to both promote and protect against atherogenesis. In a study looking at carotid plaque in SLE, we found that APLA was independently associated with the presence of plaque; this study also found that patients with plaque had higher white cell counts, suggesting ongoing chronic inflammation. We have also noted a negative correlation between activation of transforming growth factor beta-1 and carotid intima-medial thickness. This cytokine, which is known to be a potent anti-inflammatory molecule, has also been shown to be protective against atherogenesis. With regard to therapy, steroids may be a true doubleedged sword, with low doses exerting a beneficial anti-inflammatory role whereas higher doses may be detrimental through exacerbation of metabolic risk factors. In contrast, we have found that antimalarials have a beneficial effect on lipids especially when co-prescribed with steroids, and this, along with anti-inflammatory and proposed antiplatelet effects, may confer protection against CHD in lupus. The risk of premature CHD in SLE is therefore mediated by a number of factors that involve not only classic risk factors but also a range of factors associated with SLE itself. Preventative strategies will therefore need to address all potential risk factors of relevance. A more through understanding of the interplay between autoimmunity and atherogenesis should be possible by the study of SLE, and this may not only benefit lupus patients but also may have implications for our understanding of atherosclerosis in general. In 1976, Urowitz first proposed what he described as the bimodal mortality pattern of systemic lupus erythematosus (SLE) [1]. This was based on the observation that in SLE deaths early in the disease course were due to active SLE or associated complications such as sepsis. In late SLE (45 yr after diagnosis), deaths were frequently associated with atherosclerotic complications. In the almost 30 years since this original observation many other groups have confirmed the association of SLE and premature atherosclerotic disease [2, 3]. More recently two further developments have occurred that have intensified the The University of Manchester, Rheumatism Research Centre, Central Manchester and Manchester Children s University Hospital Trust, Oxford Road, Manchester M13 9WL, UK. Submitted 30 April 2005; accepted 2 September Correspondence to: I. N. Bruce. ian.bruce@manchester.ac.uk 1492 ß The Author Published by Oxford University Press on behalf of the British Society for Rheumatology. All rights reserved. For Permissions, please journals.permissions@oxfordjournals.org
2 Factors contributing to accelerated atherosclerosis and premature CHD in SLE 1493 interest in this association. Firstly, in other rheumatic diseases, most notably rheumatoid arthritis, a similar association has been confirmed [4]. Secondly, atherosclerosis in the general population has been recognized to have a significant inflammatory component to its pathogenesis [5]. As such the study of the process of atherogenesis in SLE may provide important lessons for other rheumatic diseases as well as enhancing our understanding of atherogenesis in the general population. The purpose of this review is to summarize the evidence to date regarding the risk of atherosclerosis and coronary heart disease (CHD) in SLE. It will also consider what is known about factors that may contribute to the risk of CHD in lupus, in particular how not only classic risk factors but also SLE-related factors influence the atherogenic process. Evidence for an increased risk of atherosclerosis and CHD in lupus Clinical studies Several groups have reported the prevalence of clinical CHD, either myocardial infarction (MI) or angina, in their lupus cohorts. In prospective cohorts, where incident cases are recorded, the prevalence has been 6 10% [2, 6, 7] and the estimated incidence of new CHD events in patients with SLE is approximately % per annum [7, 8]. Comparing this with the general population, Manzi et al. [2] utilized data from the Pittsburgh lupus cohort and compared them with data from women followed in the Framingham Offspring Study, a large community-based study of CHD [2]. Overall, women with SLE had a 5 6-fold increased risk of CHD. Of particular note, women with SLE aged yr were 52 times more likely to develop CHD. Similarly, employing data from the California Discharge Database, Ward [3] found that women with SLE aged yr were more likely than age-matched controls to have a hospital admission due to MI [odds ratio (OR) 2.27; 95% confidence interval (CI) ], congestive heart failure (OR 3.8; 95% CI ) and stroke (OR 2.05; 95% CI ). Extrapolating from the population prevalence of SLE, he estimated that overall MI, heart failure and stroke were 8.5, 13.2 and 10.1 times more likely in women with SLE. This excess risk has also been confirmed in the UK. In a case control study from the UK General Practice Research Database (GPRD), Fischer et al. [9] found that in patients presenting with their first MI the adjusted odds ratio (adjusted for classic risk factors, previous angina and kidney disease) for a diagnosis of SLE was 2.67 (95% CI ). In cases aged <70 yr the adjusted OR was 3.47 (95% CI ). These studies emphasize the high risk of CHD associated with lupus, and the absolute risk in SLE exceeds that of other conditions such as diabetes mellitus [10]. The immediate question that follows, therefore, is whether the increased risk of CHD relates to a higher prevalence of underlying atherosclerotic disease or whether it is mainly caused by more events occurring in this population due to, for example, increased thrombotic risk. One way to do address this is to study the presence of subclinical atherosclerosis. Subclinical disease In a review of autopsies performed in patients with SLE, Abu-Shakra et al. [11] found that, regardless of the actual cause of death, 52% of SLE patients had significant evidence of generalized atherosclerosis at the time of death. Bulkley and Roberts [12] had also previously noted that 42% of SLE patients who had received steroid therapy for 41 yr had significant atherosclerotic plaque formation in at least one coronary vessel and half of these had a history of MI. More recently, several techniques have been employed by our group and others in order to determine the scale of this problem in cross-sectional studies of SLE patients. In the Toronto lupus cohort, we studied subclinical coronary disease using SPECT dual-isotope myocardial perfusion imaging (DIMPI) [13]. In a previous study of 26 patients with SLE using exercise thallium-201 scintigraphy, Hosenpud et al. [14] had found segmental perfusion abnormalities in 10 (46%). Since that study several advances in nuclear cardiac imaging have occurred. The dual-isotope technique has a better negative predictive value as well as better image resolution, especially in women. Of 130 patients studied, 52 (40%) had perfusion abnormalities. Reversible defects consistent with ischaemia constituted 47 (90.4%) of the abnormalities. Fourteen (27%) also had a fixed defect consistent with previous myocardial necrosis [13]. These defects were seen in the distribution of the epicardial coronary vessel supply rather than diffuse abnormalities suggestive of small vessel disease. This uncontrolled study therefore suggested a high prevalence of coronary vessel abnormalities in SLE. A similar prevalence of nuclear perfusion abnormalities was also confirmed by Sella et al. [15]. A high percentage of these patients also had significant associated angiographic abnormalities, especially in the presence of additional risk factors for CHD [16]. Controlled studies are of course necessary to confirm that the prevalence of subclinical disease is actually higher in SLE. Roman et al. [17] recently published data comparing the prevalence of carotid plaque (detected using B-mode ultrasound) in a cohort of women with SLE with that seen in a control population. Overall SLE patients had a significantly higher prevalence of plaque (37 vs 15%, P < 0.001). We have recently studied 200 women with SLE in the northwest of England and 100 controls recruited on a best friend system. Our study found that, overall, 29% of SLE patients had carotid plaque. The prevalence was significantly higher in SLE than controls aged <55 yr [18] (Fig. 1). Endothelial dysfunction represents an even earlier stage in the process of atherosclerosis. It is a generalized phenomenon, and changes in the brachial artery correlate well with coronary artery endothelial responses. Doppler ultrasound of the brachial artery is used to measure the percentage increase in arterial diameter induced by changes in blood flow through the artery [flow-mediated dilatation (FMD)]. This is an endothelium-dependent process. The percentage change in arterial diameter induced by sublingual glyceryl trinitrate is then used to assess endothelium-independent arterial responses. Endothelial function is impaired in SLE compared with controls [19, 20]. Using a cut-off value of 4.5% to define endothelial dysfunction, we found that 54.8% of SLE patients, compared with 26.3% of controls, had an FMD 4.5% (P ¼ 0.04) (Fig. 1). Using several different imaging modalities, the prevalence of early and subclinical atherosclerotic disease in patients with SLE is increased compared with controls. This supports the hypothesis that the increased risk of CHD events is at least in part due to a higher background burden of atherosclerosis. These studies also confirm the accelerated nature of atherosclerosis in SLE as the burden is higher that that seen in agematched women, especially in younger age groups. Table 1 summarizes several published studies looking at subclinical atherosclerosis in SLE. Remarkably, this phenomenon has been observed in many different countries and healthcare settings, confirming that this is a problem of global relevance. Such studies have also begun to shed light on the factors that may contribute to this process. The importance of classic CHD risk factors in SLE patients It is well established in population studies that certain risk factors are associated with the future development of CHD.
3 1494 I. N. Bruce 60 P=0.04 P<0.01 % prevalence of subclinical disease Controls SLE Controls SLE A: Endothelial dysfunction Many of these have been elucidated through large communitybased cohorts such as the Framingham Study in the USA. These so-called classic CHD risk factors include irreversible factors such as age, family history and gender, as well as potentially modifiable factors such as cholesterol and its subfractions, hypertension, diabetes mellitus, and smoking [21]. As a first step it is important to determine whether SLE patients have more classic risk factors than expected in the general population and also what impact these risk factors have on the development of future CHD in SLE. These are two separate but linked questions and they require different study approaches to answer them. Do SLE patients have more classic risk factors? Petri et al. [6] reported an initial cross-sectional study in which they assessed the prevalence of certain classic CHD risk factors in the Baltimore SLE cohort. Fifty-three per cent of SLE patients had at least three CHD risk factors. A sedentary lifestyle (70%) as well as obesity (56%) and hypercholesterolaemia (56%) were particularly prevalent. In two smaller cross-sectional studies, Borba et al. [22, 23] also found that, compared with controls, patients with SLE had lower high-density lipoprotein (HDL) cholesterol, as well as higher very low-density lipoprotein (VLDL) cholesterol, triglycerides and lipoprotein(a) concentrations. More recently we undertook a large cohort control study B: Carotid plaque FIG. 1. Comparison of the prevalence of subclinical atherosclerosis in patients with SLE compared with healthy controls using two different methods of assessment in the Manchester cohort. Patients with SLE had a significantly higher prevalence of both endothelial dysfunction (A) and carotid plaque development (B). TABLE 1. Summary of the prevalence of subclinical atherosclerotic disease in selected lupus cohorts according to country of origin and technique applied Author Country Outcome measure Number with subclinical disease Proportion with subclinical disease Selzer et al. [40] USA Carotid Plaque 68/214 32% Roman et al. [17] USA Carotid Plaque 73/197 37% Doria et al. [32] Italy Carotid Plaque 22/78 17% Ahmad et al. [18] UK Carotid Plaque 58/200 29% Bruce et al. [13] Canada Myocardial perfusion defects 49/129 38% Sella et al. [15] Brazil Myocardial perfusion defects 23/82 28% Asanuma et al. [85] USA Coronary calcification 20/65 31% Manger et al. [53] Germany Coronary calcification 21/75 28% Theodoridou et al. [69] UK Ankle Brachial Pressure index 34/91 37% El-Magadmi et al. [20] UK Endothelial dysfunction 34/62 55% TABLE 2. Metabolic risk factors found to be significantly different in a cohort control study of 235 patients with SLE compared with a primary care population (after Bruce et al. [24]) Risk factor SLE (%) Controls (%) P Hypertension <0.01 Diabetes mellitus 5 1 <0.01 Post-menopause <0.01 Elevated creatinine 9 0 <0.01 Sedentary lifestyle 15 9 <0.05 Waist:hip ratio 0.8 (0.06) 0.78 (0.06) <0.01 VLDL cholesterol (mmol/l) 0.45 (0.34) 0.38 (0.27) <0.05 Triglycerides (mmol/l) 1.4 (0.88) 1.2 (0.67) <0.05 Homocysteine 415 mmol/l <0.01 All results expressed as % with the abnormality except waist:hip ratio, VLDL cholesterol and triglycerides where mean (S.D.) values are shown. comparing consecutive SLE patients with women attending a local family practice for an annual physical examination [24]. Overall 235 patients and controls were studied. The significant results are summarized in Table 2. Women with SLE were more likely to have hypertension and diabetes mellitus. In addition they had a more sedentary lifestyle and higher VLDL cholesterol and triglycerides. Another important observation was that
4 Factors contributing to accelerated atherosclerosis and premature CHD in SLE 1495 TABLE 3. Summary of risk factors associated with the development of clinical coronary heart disease in selected studies despite the mean age of the groups being the same, 38% of SLE patients compared with 19% of controls were post-menopausal [24]. This is because SLE patients experience the menopause on average 3 4 yr earlier. This has clear implications for CHD risk given the pre-menopausal protection generally observed in women as well as the lack of any cardioprotective role for hormone replacement therapy. The other practical implication of this study stems from the observation that the estimated 10-yr risk of CHD, using the Framingham risk equation, was no different in SLE and as such it did not reflect the 5 8-fold excess risk already noted. Relying on such estimates of risk assessment alone will therefore underestimate the true CHD risk in SLE several-fold. Do classic risk factors have any impact on CHD risk in SLE? Several groups have undertaken case control studies to identify factors associated with clinical CHD in SLE. Almost every study has noted that classic risk factors are associated with the development of atherosclerotic disease [2, 25 27]. Owing to the small numbers, their power is, however, limited to identifying all but the most notable risk factors. Despite this limitation, two factors have been consistently identified across the majority of studies, namely hypercholesterolaemia and older age at SLE diagnosis. Other factors have also been identified such as increasing age and/or disease duration, hypertension and smoking history (Table 3). The impact of total cholesterol on future CHD risk has also been addressed by our group in a cohort study [8]. An inception cohort of newly diagnosed SLE patients were identified, all of whom had total cholesterol measured as part of their routine biochemical analysis during a time period where lipid-lowering therapy was not routine medical practice. Over a yr follow-up, persistently elevated total cholesterol 45.2 mmol/l independently predicted future CHD events. In patients with persistent hypercholesterolaemia, 24% developed a new CHD event compared with 3% of those who had consistently normal cholesterol [8]. More recently, the UK GPRD study also noted that in addition to SLE being associated with an increased risk of first MI, the combination of SLE and hyperlipidaemia carried a much higher odds ratio for MI (OR 18.24, 95% CI ) [9]. In the general population, however, it is recognized that classic risk factors alone do not fully explain CHD risk. Patients with SLE have been found to have on average one less classic CHD risk factor at the time of their event compared with other populations with premature CHD [28]. Esdaile et al. [7] identified patients at their first visit to one of two lupus clinics in Montreal. At the baseline visit, all patients had a risk factor profile performed, and from this Toronto, Canada [25] Baltimore, USA [26] Pittsburgh, USA [2] Stockholm, Sweden [27] Older age at diagnosis of SLE Longer disease duration Hypercholesterolaemia Hypertension Hypertriglyceridaemia Steroid use Longer duration of use Longer duration use Total cumulative dose Other metabolic factors Diabetes mellitus Obesity Post-menopausal "VLDL cholesterol a, "lipoprotein(a), #HDL b, "homocysteine Other lupus factors Pericarditis, myocarditis, congestive heart failure Older age at clinic entry a Very low-density lipoprotein, b high-density lipoprotein, c malondialdehyde-modified low-density lipoprotein. Lupus anticoagulant, anti-mda-ldl antibodies c, "ESR, "CRP, "orosomucoid, "1-antitrypsin their 10-yr CHD and stroke risks were estimated. After adjustment for the baseline Framingham risk estimates, patients with SLE still had a fold excess risk of cardiovascular events. Bessant et al. [29] also confirmed that SLE patients had significantly more strokes and CHD events than predicted by these risk equations. Importantly, subclinical disease also follows this pattern. Studies looking at carotid plaque and endothelial dysfunction have both shown that, after adjustment for classic risk factors, SLE remains associated with an excess burden of early atherosclerosis [17, 20]. Therefore, patients with SLE have a higher prevalence of certain classic risk factors. As in the general population, exposure to such risk factors increases the risk of CHD. However, even after accounting for known classic risk factors, patients with SLE still have an excess risk of CHD, suggesting that SLE patients are set with a higher baseline risk than the general population (Fig. 2). Some of the additional factors identified in the Toronto Risk Factor Study, such as premature menopause, high triglycerides and hyperhomocysteinaemia, may contribute to this [24]. More intriguingly, however, other SLE-associated factors may also play a major role. Which SLE-related factors may be important to atherosclerosis risk? Steroid therapy Bulkley and Robert s original study [12] proposed that exposure to steroid therapy for more that 1 yr was associated with atherosclerosis observed at post-mortem examination [12]. Intuitively steroids should have an important atherogenic role. Steroid therapy is known to adversely affect many metabolic factors such as body fat distribution, blood pressure and glucose metabolism. Steroids, on the other hand, may also be of benefit mainly due to their anti-inflammatory effects. Intriguingly, Roman et al. [17] found that patients with carotid plaque tended to have been exposed to less total steroid therapy, suggesting that the underlying inflammatory disease may be less adequately controlled. Also, in the context of SLE, steroids are a marker of the severity of the underlying inflammatory disease state and/or the organ distribution of clinical features. As such it is difficult to decide whether to attribute any possible association with the drug or the disease phenotype. An additional problem is that the benefit/harm of steroids may be dose-related. MacGregor et al. found that with regard to lipid profiles in SLE patients, a dose of <10 mg daily did not adversely affect lipid levels whereas a daily dose of 410 mg increased triglycerides and apolipoprotein B concentrations [30]. Similarly, Petri et al. [6] found that doses of prednisone 410 mg/day were associated with hypercholesterolaemia. Other studies have also found that higher steroid doses are associated with hypercholesterolaemia [8, 31]. The final
5 1496 I. N. Bruce Risk of CHD Low Traditional risk factors SLE General population High FIG. 2. Overall summary of the influence of classic risk factors for CHD in SLE compared with the general population. In both, the risk of CHD is increased by increased exposure to classic risk factors. However, SLE patients also have a higher baseline risk that is probably explained by additional factors relating to the inflammatory and autoimmune nature of SLE. (Reproduced from Wajed et al. Rheumatology 2004;43:7 12.) problem is that in studies attempting to look at steroid exposure in SLE, authors tend to use a variety of different variables, e.g. duration of use, cumulative dose, highest dose and/or mean daily steroid dose as well as the development at any time of any Cushingoid features. Which, if any, of these provide the best summary of steroid exposure remains far from clear. With these caveats in mind, a number of groups have found associations between increased exposure to steroids and the development of clinical and subclinical atherosclerosis [2, 26, 32]. Other studies have also compared risk factors in patients before and after initiating steroid therapy. Using this approach, steroid therapy in SLE has been found to improve HDL subfractions (which may be atheroprotective); other changes associated with initiating steroid therapy have included increased total cholesterol, VLDL cholesterol and LDL cholesterol [33, 34]. Levels of triglycerides are also elevated in adult patients on steroid therapy [30, 33]. Overall, the balance of evidence to date suggests that increased exposure to steroids is likely to adversely affect cardiovascular risk. However, steroid therapy may truly be a double-edged sword and an individual s own metabolic and anti-inflammatory response to steroids may influence whether there is net benefit or harm to them with respect to cardiovascular risk. The contribution of inflammatory factors to CHD risk in SLE It is increasingly accepted that atherosclerosis can be considered to have a significant chronic inflammatory component [5]. Lesions are characterized from an early stage by infiltration of monocyte-derived macrophages and lymphocyte populations, particularly T cells [35]. This infiltration is facilitated by chemoattractant molecules such as monocyte chemotactic protein-1 (MCP-1), and endothelial expression of adhesion molecules. Within the subendothelial space, macrophages ingest altered lipids and transform into the lipid-laden foam cells characteristic of atherosclerotic plaques [5]. Inflammatory mechanisms also appear to contribute to late instability and rupture of plaques. In particular, a subset of interferon (IFN)-secreting CD4þ CD28-null T cells are found in the shoulder area of atherosclerotic plaques. These contribute to reduced collagen synthesis and weakening of the plaques fibrous cap and this, in conjunction with production of matrix metalloproteinases, destabilizes the plaque facilitating fissuring and rupture [36, 37]. As well as a growing body of basic scientific evidence, there is also intense interest in markers of the acute phase response as predictors of future coronary events in population studies. A nested case control study of post-menopausal women found that high-sensitivity C-reactive protein (hs-crp) was the strongest univariate predictor of future cardiovascular risk, and in multivariate analysis, hs-crp independently predicted events with a relative risk (RR) of 1.5 (95%CI ) similar to that of the total:hdl cholesterol ratio (RR 1.4; 95% CI ) [38]. Indeed, hs-crp seems to identify additional patients at increased risk of cardiovascular disease over and above those who can be identified by using conventional risk factors alone [39]. Systemic lupus erythematosus represents the classic model of a chronic immune complex-mediated inflammatory disease of blood vessels. Studies that have investigated the occurrence of clinical or subclinical atherosclerosis have demonstrated that certain inflammatory features of the disease as well as markers of chronic inflammation are associated with the development of atherosclerosis in SLE. For example, in a study of the influence of hyperlipidaemia on coronary risk in the Toronto cohort, a multivariate model also found that previous inflammatory lung involvement also predicted coronary events [8]. Similarly, Hosenpud et al. [14] noted increased myocardial perfusion defects in patients with a previous history of pericarditis, and using a similar imaging modality Sella et al. [15] noted that digital vasculitis was also associated with abnormal myocardial perfusion. Several studies have also suggested that elevations in CRP, C3 complement and an increased white cell count may also be associated with clinical or subclinical atherosclerosis [27, 40]. There are several mechanisms of relevance to lupus that seem to be pertinent to the development of atherosclerosis. Firstly, the inflammatory response in lupus is associated with deposition of immune complex in target tissues as well as systemic complement activation. Complement activation appears to play an important role in the development and progression of atherosclerotic plaques by stimulating endothelial cell activation and enhancing recruitment of leucocytes to inflammatory sites [37]. In addition, the terminal complement complex C5b-9 induces the rapid early release of MCP-1, and later the release of IL-6, from vascular smooth muscle cells, both of which promote further accumulation of monocytes and perpetuation of the inflammatory response within lesions [41]. Interestingly, serum from patients with SLE stimulated a fold increase uptake of cholesterol by smooth muscle cells [42]. A similar effect could be achieved by isolating circulating immune complexes from lupus serum, and this increased uptake correlated with the LDL content of the immune complexes [42]. Immune complex bound to C1q via the C1q receptor formation has also been shown to inhibit the enzyme cholesterol 27-hydroxylase, which is expressed in arterial endothelium and monocyte/macrophage cell lines [43]. This enzyme catalyses the hydroxylation of cholesterol to 27- hydroxycholesterol, which is more water soluble and more easily removed from the arterial wall. This process contributes to reverse cholesterol transport and protects against accumulation of lipid and development of plaque [44]. Finally, immune complexes also stimulate endothelial cells to express vascular cell adhesion molecule 1 (VCAM-1), an adhesion molecule known to be up-regulated in patients with SLE, which can promote recruitment of monocytes to the arterial wall [45]. Another cytokine that may be of relevance in SLE is transforming growth factor beta-1 (TGF-1). This is the most potent naturally occurring immunosuppressant, it is produced by all cells of the immune system and plays a fundamental role in controlling proliferation and the fate of cells through apoptosis [46]. Transforming growth factor beta-1 knockout mice rapidly develop a lupus-like illness resulting in early death at 3 4 weeks of age [47]. In addition, when fed a lipid-rich diet, mice heterozygous for TGF-1 expression develop marked deposition
6 Factors contributing to accelerated atherosclerosis and premature CHD in SLE 1497 Carotid IMT (cm) n = 32 r = P< TGF b-1 activation index FIG. 3. Carotid artery intima-medial thickness (IMT) was correlated with a TGF-1 activation index in SLE patients using Pearson s correlation. A significant inverse relationship was identified for SLE patients with low TGF-1 activation index being associated with increased carotid IMT. of lipid in their arterial wall compared with wild-type mice [48]. Active TGF-1 therefore appears to be protective against atherosclerosis by inhibiting proliferation of smooth muscle and endothelial cells. We have recently studied activation of TGF-1 using a novel in vitro assay in patients with lupus compared with healthy controls. The TGF-1 activation index was not significantly different in patients compared with controls. The activation index in patients with lupus did, however, show a significant inverse correlation with carotid intima-medial thickness (M. Jackson et al., submitted) (Fig. 3). It also inversely correlated with total cholesterol and a higher Systemic Lupus International Collaborating Clinics (SLICC) damage index. This preliminary study suggests that an impaired ability to activate TGF-1 in the peripheral circulation may be associated with increased early markers of atherosclerosis in the context of lupus, and additional studies are required to further examine this relationship (M. Jackson et al., submitted). Inflammation in SLE also contributes to the typical dyslipidaemia associated with lupus. This is characterized by elevations of VLDL cholesterol, LDL cholesterol and triglycerides as well as a reduced HDL cholesterol [22]. Inflammation may contribute to this pattern by IL-6-mediated inhibition of lipoprotein lipase (LPL), the enzyme central to metabolism of VLDL to LDL. In support of this, patients with untreated SLE have an impaired ability to clear chylomicrons and this is associated with reduced LPL activity [49]. More recently, Reichlin et al. [50] found that serum reactivity with LPL in 47% of patients with SLE and anti-lpl activity correlated strongly with serum triglycerides. These two complementary mechanisms may therefore exacerbate atherogenic lipid profiles in SLE. Elevated triglycerides have also been found to be a common abnormality in patients with SLE [22, 24, 51]. In the context of the dyslipoproteinaemia described, two other important changes occur which may contribute further to atherogenesis. Firstly, this pattern of lipid abnormalities is frequently associated with the generation of small dense LDL molecules, and it is this subgroup of LDL that is most susceptible to oxidation. Nuttall et al. [51] reported that there is an increased frequency of small dense LDL subfractions in lupus patients and these changes were associated with increased markers of oxidant stress. Interestingly, they also found a moderately raised CRP in this group, suggesting that inflammation and a pro-oxidant state occur together in the context of SLE. Oxidized LDL is more readily retained within the subendothelial space and contributes to formation of foam cells. In addition, oxidatively modified LDL provokes an antibody response that in turn will result in local immune complex formation. The other component of this lipid profile of relevance is low HDL. The HDL molecule is believed to be centrally involved in reverse cholesterol transport and contributes to the clearance of cholesterol from the periphery. Reduced HDL is an important cardiovascular risk factor in the general population, and again we and others have found that low HDL concentrations may be associated with clinical or subclinical atherosclerosis in SLE [27, 52, 53]. In addition to its role in reverse cholesterol transport, the HDL molecule has several other important biological roles, one of which is its antioxidant capacity associated with the enzyme paraoxonase-1 (PON-1). Paraoxonase-1 physiologically accounts for the antioxidant capacity of HDL. Patients with SLE have been found to have reduced PON-1 activity which reflected total serum antioxidant capacity and was inversely correlated with anti-hdl antibody titres [54]. Therefore, inflammation may inhibit the generation of HDL as part of the inflammatory dyslipoproteinaemia. In addition, patients with SLE may have antibodies against HDL or its main component apolipoprotein-a1 (Apo-A1) [54, 55]. As a result, the protective effects of HDL will be diminished and the significant antioxidant capacity of this molecule may be reduced through reduction in PON-1 activity. In the context of SLE, therefore, the antioxidant capacity of the serum is reduced and more oxidation-prone small dense LDL particles are generated. Do antiphospholipid antibodies also promote atherogenesis in SLE? The antiphospholipid syndrome (APS) describes the clinical occurrence of vascular thrombotic events or recurrent miscarriages with either antiphospholipid antibodies (APLA) and/or the lupus anticoagulant (LAC). This syndrome was first delineated in patients with SLE and clearly puts thrombotic risk in a central position within lupus [56]. Given that arterial thrombosis, e.g. stroke and MI, may be features of APS, an association between APLA and the presence of clinical vascular disease would not be surprising. Indeed, it could be argued that thrombosis associated with APLA may occur within blood vessels that are relatively histologically normal [57]. In the context of patients with an increased prevalence of atherosclerotic plaque, it could therefore be argued that APLA would make these patients more likely to experience a clinical event than those without these markers. Interestingly, this has been demonstrated in the general population. In a nested case control study from the Helsinki Heart Study, Vaarala et al. [58] found that at baseline the presence of anticardiolipin antibodies (ACLA) was greater in patients who developed MI during the trial. Wu et al. [59] also confirmed the predictive value of ACLA in men for future MI up to 20 yr after the antibody test was found to be positive. What is most intriguing is whether APLA or subgroups of these antibodies may actually contribute to atherogenesis. Those SLE patients with IgG ACLA have significantly lower total and HDL cholesterol as well as apolipoprotein A-1 concentrations compared than patients negative for ACLA [60]. This effect was seen in patients not taking steroids and was independent of inflammatory disease activity levels [60]. It was suggested that certain antibody subgroups may be directed against Apo A-1, and indeed such antibodies are detectable and are associated with low HDL as well as subclinical carotid atherosclerosis [55, 61]. Further work has also found that there is significant cross-reactivity between anticardiolipin, anti-hdl and anti-apo A-1 IgG antibodies in SLE and APS patients [54]. Anticardiolipin antibodies also cross-react with antibodies to oxidized LDL and there is a high prevalence of antibodies to malondiadehyde-modified lipoprotein(a) in patients in primary and secondary APS [62, 63].
7 1498 I. N. Bruce Therefore, ACLA represent a family of antibodies with varying antigenic specificities and these may in part reflect underlying oxidant stress. Certain APLA may also contribute to the development of atherosclerotic lesions. One proposed physiological function of the beta-2 glycoprotein-1 ( 2 -GP1) molecule is to prevent uptake of oxidized LDL by macrophages via scavenger receptors. In the presence of anti- 2 -GP1 antibodies, however, the complex formed enables uptake of oxidized LDL by the Fc receptor on macrophages thus facilitating formation of foam cells [64]. More recently, in a study of 127 patients with lupus and/or APS (82 of them had APS), circulating 2 -GP1 oxidized LDL complexes were detected in 57% of patients [65]. These complexes were significantly associated with a history of arterial thrombosis, especially in the presence of anti- 2 -GP1-oxLig-1 IgG antibodies, suggesting that these antibodies contribute to the development of arterial disease [65]. Clinical studies have been somewhat divided as to whether this family of antibodies is associated with development of atherosclerosis. Svenungsson et al. [27] found that patients with SLE and a history of cardiovascular events were more likely to have a positive lupus anticoagulant. Several studies have found no clear association of APLA/LAC with subclinical atherosclerosis [17, 40]. However, in a prospective study assessing carotid intima-medial thickness (IMT), Doria et al. [32] found that over a 5-yr follow-up period the presence of antibodies to oxidized palmitoyl-arachidonoyl phosphocholine (oxpapc) were associated with higher IMT. Similarly, we have found in a cross-sectional study of 200 Caucasian women in the northwest of England that the presence of APLA and/or LAC was a significant and independent factor associated with carotid plaque formation [18]. It is worth pointing out that in animal studies certain subgroups of these antibodies may actually be protective against the development of atherosclerosis [66]. This is clearly an exciting and controversial area and further long-term protective studies are necessary to determine the exact role, if any, that the APLA family of antibodies play in atherogenesis, atheroprotection and precipitation of arterial events in SLE. Renal disease In addition to the previously mentioned organ distribution of lupus associated with cardiovascular disease, renal disease specifically has also been the subject of several studies. Nephrotic syndrome and excess proteinuria are associated with an adverse lipid profile [31] as well as a prothrombotic risk, which may of course contribute to the development of clinical CHD [67, 68]. Several studies have found that a history of significant proteinuria is associated with subclinical atherosclerosis in SLE [53, 69]. In addition to proteinuria, renal impairment is also an important (and a potentially underestimated) factor in SLE. Elevated creatinine is, not surprisingly, more prevalent in SLE than in an age-matched control population [24] and a history of renal disease or an elevated serum creatinine can be associated with early atherosclerosis [32, 40]. It is generally accepted that measurement of the serum creatinine in isolation does not accurately estimate levels of renal function and therefore there is an increasing trend to use mathematical modelling to estimate the glomerular filtration rate (egfr). Impairment of renal function even within the mild end of the range estimated by such equations is an important and significant risk factor for cardiovascular events and mortality [70]. In our cross-sectional cohort we found a significant association of egfr and the presence of carotid plaque. This remained significant in a multivariate model that examined patients free of clinical cardiovascular events at the time of study (Y. Ahmad, personal communication). Given the importance of chronic renal disease as a risk factor for cardiovascular disease in the whole population, use of the egfr in the long-term follow-up of patients with lupus will assume increasing significance and undoubtedly explains part of the excess cardiovascular risk observed in these patients. Insulin resistance Insulin resistance has been investigated as a potential CHD risk factor in the general population. It can be defined as the reduced ability of insulin to stimulate glucose uptake in skeletal muscle and fat cells, and to inhibit lipolysis of adipose tissue [71]. The insulin resistance cluster of risk factors is associated with an increased risk of CHD, particularly in women [72]. The metabolic syndrome has now been recognized by the Adult Treatment Panel 3 guidelines of The National Cholesterol Education Programme as important in identifying people at high risk of CHD to whom more intensive risk factor management should be aimed [21]. We have recently studied a group of non-diabetic Caucasian women with SLE. Compared with controls, patients with lupus had higher fasting plasma insulin levels as well as reduced insulin sensitivity and higher beta cell secretory function [73]. Even after exclusion of patients on corticosteroids, insulin sensitivity was lower in SLE. Interestingly, there was only a weak non-significant correlation between insulin sensitivity and current or recent steroid exposure, and there was no significant association with levels of disease activity. In SLE, insulin sensitivity showed a negative correlation with oxidized LDL (r ¼ 0.57, P<0.01). In a larger cross-sectional study we also found that 18% of lupus patients studied satisfied the criteria for the metabolic syndrome [73]. These results confirm the recent study of paediatric and adolescent SLE patients, where, again, higher fasting insulin levels were found [74]. The degree of insulin resistance we noted in SLE patients is similar to that seen in newly diagnosed type 2 diabetics and in women with polycystic ovary syndrome [75]. Hyperinsulinaemia and insulin resistance may therefore play a pivotal role in atherogenesis in lupus, either independently or via its association with other metabolic risk factors and increased oxidative stress. The potential beneficial effects of antimalarial drugs Antimalarial (AM) drugs may have several important advantages in regard to atherosclerotic risk in lupus. These agents have a key role in treating mild to moderate SLE and are of particular benefit in the treatment of cutaneous and musculoskeletal features. As such, their anti-inflammatory role would be expected to control low-grade disease activity. Petri et al. [76] found that the use of hydroxychloroquine was associated with significantly lower total cholesterol concentrations. Wallace et al. [77] also confirmed that AM therapy was associated with lower total cholesterol, LDL cholesterol and triglycerides in patients with rheumatoid arthritis (RA) and SLE on steroid therapy. In a study that prospectively assessed several groups of patients with SLE either starting or stopping anti-malarial therapy, Rahman et al. [78] noted that AM monotherapy did not significantly alter total cholesterol concentrations over a 6-month period. In contrast, co-prescription of AMs along with steroid therapy was associated with an approximately 9 11% reduction in total cholesterol compared with patients taking a prolonged stable dose of steroids. Underlying their effect on total cholesterol concentrations, AMs significantly reduce VLDL and LDL cholesterol [79]. They also appear to increase levels of HDL cholesterol when given alone or concomitantly with steroids, and in the presence of steroids triglyceride concentrations have also been found to be lower in patients co-prescribed an antimalarial [80].
8 Factors contributing to accelerated atherosclerosis and premature CHD in SLE 1499 As well as contributing to a more favourable lipid profile in SLE patients, AMs may also have anticoagulant properties. Hydroxychloroquine sulphate was suggested as a preventative therapy for thromboembolic complications in post-operative patients [81]. The exact mechanism for this effect is unclear; however, in vitro studies suggest that hydroxychloroquine may inhibit platelet aggregation [82]. More recently, hydroxychloroquine has been shown to inhibit, in a dose-dependent manner, platelet activation induced by exposure to APL antibodies [83]. The third potentially important mechanism of action is the observation that AMs prolong the half-life of the active insulin receptor complex through inhibition of insulin disassociation from its receptor [84]. As a result, patients on antimalarials tend to have lower fasting blood glucose concentrations (El-Magadmi, personal communication and [85]). Summary and implications for preventative strategy in SLE patients Studies in lupus over the past 30 years have confirmed the initial observation that patients with SLE are at increased risk of premature CHD [1]. In certain age groups, the risk associated with lupus may be 450 times that of an age-matched population [2]. A number of studies have also demonstrated a high burden of subclinical atherosclerosis in this population, especially in younger age groups [17, 86]. Therefore, the excess risk of CHD events in lupus is due not only to the increased thrombotic risk associated with lupus but also to the underlying accelerated atherosclerosis. The observation that this excess risk cannot be fully explained by the presence of classic coronary risk factors could be misconstrued as suggesting that these factors have little impact on coronary risk in lupus. However, as this review has demonstrated, patients with SLE have an increased prevalence of several key classic risk factors such as hypertension and diabetes mellitus, and classic risk factors play an important role in the development of atherosclerosis. The observation that these classic risk factors fail to fully explain atherosclerosis in lupus is no surprise as this is also true in the general population. The pathogenesis of atherosclerosis in SLE therefore not only involves classic risk factors but also involves a range of additional factors. Our work and that of other investigators has identified additional factors at play in patients with SLE (Table 4). These include certain lifestyle and metabolic abnormalities such as premature menopause, sedentary lifestyle, elevated VLDL cholesterol and triglycerides as well as insulin resistance and chronic renal impairment. Other specific lupus factors, including chronic inflammation, antiphospholipid antibodies and chronic exposure to steroid therapy, are all likely to play a major role (Fig. 4). These factors can directly influence the development of atherosclerosis through a variety of mechanisms such as immune complex generation, complement activation, changes in TGF-1 activation and alteration of the oxidant antioxidant balance locally within the vessel wall. Large-scale prospective studies are now necessary in order to study the relative influence of these factors in more detail. In the meantime, we have adopted a proactive approach to management of classic risk factors in our own lupus cohort [87]. This was driven by the observation that while there is general awareness of this clinical problem in SLE, physicians managing SLE patients do not address risk factors particularly well [88, 89]. Our own ideal targets for certain risk factors have been based on the notion that SLE, like diabetes, can be viewed as a CHD-equivalent condition. It therefore warrants stringent control of classic risk factors such as LDL cholesterol, blood pressure, body weight etc. (Table 5). In addition, we believe that certain subgroups of patients with SLE may warrant treatment with ACE inhibitors for their cardioprotective effects and aspirin prophylaxis should be considered for the majority TABLE 4. Classic and lupus-related risk factors associated with the development of carotid artery plaque in three recent studies. All markers significant in a univariate analysis are included Risk factor Selzer et al. [40] Roman et al. [17] Ahmad et al.* Older age Older age at diagnosis Longer disease duration Post-menopausal "Body mass index "Systolic blood pressure "Diastolic blood pressure "Cholesterol "Triglycerides - Diabetes mellitus Smoking "Homocysteine Steroid use Longer duration Lower mean daily dose Longer duration Cyclophosphamide use Lower use Hydroxychloroquine use Lower use Azathioprine use Higher use Lower SLE disease activity "CRP Higher white cell count "SLICC damage index Prevalent coronary heart disease Renal impairment Pulmonary hypertension Previous lung involvement Anticardiolipin antibodies Lower prevalence Higher prevalence Anti-RNP antibodies Lower prevalence Anti-Sm antibodies Lower prevalence Antidepressant use *Ahmad Y, personal communication (and [18]). Classic risk factors Vascular inflammation Lupus-associated Metabolic factors Accelerated Atherosclerosis +/ Antiphospholipid antibodies Premature CHD FIG. 4. Summary of the factors that we propose contribute to the development of premature coronary heart disease (CHD) in SLE. Accelerated atherosclerosis is the key underlying pathological process. Classic risk factors and additional lupus-associated metabolic changes such as premature menopause, renal impairment and increased triglycerides are all likely to contribute to atherogenesis. In addition, chronic vascular inflammatory mechanisms, e.g. immune complex formation and impaired TGF-1 activation, will accelerate the atherosclerotic process. Antiphospholipid antibodies may precipitate thrombotic events but certain subgroups of these antibodies may either contribute to, or protect against, plaque development.
Lupus as a risk factor for cardiovascular disease
 Lupus as a risk factor for cardiovascular disease SØREN JACOBSEN Department Rheumatology, Rigshospitalet Søren Jacobsen Main sponsors: Gigtforeningen Novo Nordisk Fonden Rigshospitalet Disclaimer: Novo
Lupus as a risk factor for cardiovascular disease SØREN JACOBSEN Department Rheumatology, Rigshospitalet Søren Jacobsen Main sponsors: Gigtforeningen Novo Nordisk Fonden Rigshospitalet Disclaimer: Novo
PATIENTS AND METHODS:
 BACKGROUND: Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by erosive synovitis that involves peripheral joints and implicates an important influence in the quality
BACKGROUND: Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by erosive synovitis that involves peripheral joints and implicates an important influence in the quality
Dyslipidemia Endothelial dysfunction Free radicals Immunologic
 ATHEROSCLEROSIS Hossein Mehrani Professor of Clinical Biochemistry Definition Atherosclerosis: Is a chronic inflammatory process characterized by plaque formation within the vessel wall of arteries and
ATHEROSCLEROSIS Hossein Mehrani Professor of Clinical Biochemistry Definition Atherosclerosis: Is a chronic inflammatory process characterized by plaque formation within the vessel wall of arteries and
KEY COMPONENTS. Metabolic Risk Cardiovascular Risk Vascular Inflammation Markers
 CardioMetabolic Risk Poor blood sugar regulation and unhealthy triglyceride and lipoprotein levels often present long before the diagnosis of type 2 Diabetes. SpectraCell s CardioMetabolic and Pre-Diabetes
CardioMetabolic Risk Poor blood sugar regulation and unhealthy triglyceride and lipoprotein levels often present long before the diagnosis of type 2 Diabetes. SpectraCell s CardioMetabolic and Pre-Diabetes
Arteriosclerosis & Atherosclerosis
 Arteriosclerosis & Atherosclerosis Arteriosclerosis = hardening of arteries = arterial wall thickening + loss of elasticity 3 types: -Arteriolosclerosis -Monckeberg medial sclerosis -Atherosclerosis Arteriosclerosis,
Arteriosclerosis & Atherosclerosis Arteriosclerosis = hardening of arteries = arterial wall thickening + loss of elasticity 3 types: -Arteriolosclerosis -Monckeberg medial sclerosis -Atherosclerosis Arteriosclerosis,
Pathophysiology of Lipid Disorders
 Pathophysiology of Lipid Disorders Henry Ginsberg, M.D. Division of Preventive Medicine and Nutrition CHD in the United States CHD is the single largest killer of men and women 12 million have history
Pathophysiology of Lipid Disorders Henry Ginsberg, M.D. Division of Preventive Medicine and Nutrition CHD in the United States CHD is the single largest killer of men and women 12 million have history
The 10 th International & 15 th National Congress on Quality Improvement in Clinical Laboratories
 The 10 th International & 15 th National Congress on Quality Improvement in Clinical Laboratories Cardiac biomarkers in atherosclerosis Najma Asadi MD-APCP Ross and Colleagues in 1973: Response to Injury
The 10 th International & 15 th National Congress on Quality Improvement in Clinical Laboratories Cardiac biomarkers in atherosclerosis Najma Asadi MD-APCP Ross and Colleagues in 1973: Response to Injury
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD
 Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
Role of apolipoprotein B-containing lipoproteins in the development of atherosclerosis Jan Borén MD, PhD Our laboratory focuses on the role of apolipoprotein (apo) B- containing lipoproteins in normal
THE CARDIOVASCULAR INFLAMMATORY CONTINUUM DR AB MAHARAJ
 THE CARDIOVASCULAR INFLAMMATORY CONTINUUM DR AB MAHARAJ Disclosures: On National Advisory Boards of: (1) Pfizer Pharmaceuticals (2) MSD (3) Roche Pharmaceuticals (4) Abbott International: AfME Rheumatology
THE CARDIOVASCULAR INFLAMMATORY CONTINUUM DR AB MAHARAJ Disclosures: On National Advisory Boards of: (1) Pfizer Pharmaceuticals (2) MSD (3) Roche Pharmaceuticals (4) Abbott International: AfME Rheumatology
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones?
 1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
1Why lipids cannot be transported in blood alone? 2How we transport Fatty acids and steroid hormones? 3How are dietary lipids transported? 4How lipids synthesized in the liver are transported? 5 Lipoprotien
Part 1 Risk Factors and Atherosclerosis. LO1. Define the Different Forms of CVD
 Week 3: Cardiovascular Disease Learning Outcomes: 1. Define the difference forms of CVD 2. Describe the various risk factors of CVD 3. Describe atherosclerosis and its stages 4. Describe the role of oxidation,
Week 3: Cardiovascular Disease Learning Outcomes: 1. Define the difference forms of CVD 2. Describe the various risk factors of CVD 3. Describe atherosclerosis and its stages 4. Describe the role of oxidation,
Lipoprotein Particle Profile
 Lipoprotein Particle Profile 50% of people at risk for HEART DISEASE are not identified by routine testing. Why is LPP Testing The Most Comprehensive Risk Assessment? u Provides much more accurate cardiovascular
Lipoprotein Particle Profile 50% of people at risk for HEART DISEASE are not identified by routine testing. Why is LPP Testing The Most Comprehensive Risk Assessment? u Provides much more accurate cardiovascular
Acute coronary syndrome. Dr LM Murray Chemical Pathology Block SA
 Acute coronary syndrome Dr LM Murray Chemical Pathology Block SA13-2014 Acute myocardial infarction (MI) MI is still the leading cause of death in many countries It is characterized by severe chest pain,
Acute coronary syndrome Dr LM Murray Chemical Pathology Block SA13-2014 Acute myocardial infarction (MI) MI is still the leading cause of death in many countries It is characterized by severe chest pain,
HIGH LDL CHOLESTEROL IS NOT AN INDEPENDENT RISK FACTOR FOR HEART ATTACKS AND STROKES
 HIGH LDL CHOLESTEROL IS NOT AN INDEPENDENT RISK FACTOR FOR HEART ATTACKS AND STROKES A study published in the British Medical Journal shows that not only is high LDL cholesterol not a risk factor for all-caused
HIGH LDL CHOLESTEROL IS NOT AN INDEPENDENT RISK FACTOR FOR HEART ATTACKS AND STROKES A study published in the British Medical Journal shows that not only is high LDL cholesterol not a risk factor for all-caused
Mandana Nikpour 1,2, Murray B Urowitz 1*, Dominique Ibanez 1, Paula J Harvey 3 and Dafna D Gladman 1. Abstract
 RESEARCH ARTICLE Open Access Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective
RESEARCH ARTICLE Open Access Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective
ROLE OF INFLAMMATION IN HYPERTENSION. Dr Barasa FA Physician Cardiologist Eldoret
 ROLE OF INFLAMMATION IN HYPERTENSION Dr Barasa FA Physician Cardiologist Eldoret Outline Inflammation in CVDs the evidence Basic Science in Cardiovascular inflammation: The Main players Inflammation as
ROLE OF INFLAMMATION IN HYPERTENSION Dr Barasa FA Physician Cardiologist Eldoret Outline Inflammation in CVDs the evidence Basic Science in Cardiovascular inflammation: The Main players Inflammation as
Tracking a Killer Molecule
 Tracking a Killer Molecule Mercodia Oxidized LDL ELISA www.mercodia.com Mercodia Oxidized LDL ELISA products Product Catalog No Kit size Oxidized LDL ELISA 10-1143-01 96 wells Oxidized LDL competitive
Tracking a Killer Molecule Mercodia Oxidized LDL ELISA www.mercodia.com Mercodia Oxidized LDL ELISA products Product Catalog No Kit size Oxidized LDL ELISA 10-1143-01 96 wells Oxidized LDL competitive
Systemic Lupus Erythematosus. An Independent Risk Factor for Endothelial Dysfunction in Women
 Systemic Lupus Erythematosus An Independent Risk Factor for Endothelial Dysfunction in Women Masoud El-Magadmi, MB; Helena Bodill, MSc; Yasmeen Ahmad, MB, MRCP; Paul N. Durrington, MD, F Med Sci; Michael
Systemic Lupus Erythematosus An Independent Risk Factor for Endothelial Dysfunction in Women Masoud El-Magadmi, MB; Helena Bodill, MSc; Yasmeen Ahmad, MB, MRCP; Paul N. Durrington, MD, F Med Sci; Michael
Complications of Diabetes mellitus. Dr Bill Young 16 March 2015
 Complications of Diabetes mellitus Dr Bill Young 16 March 2015 Complications of diabetes Multi-organ involvement 2 The extent of diabetes complications At diagnosis as many as 50% of patients may have
Complications of Diabetes mellitus Dr Bill Young 16 March 2015 Complications of diabetes Multi-organ involvement 2 The extent of diabetes complications At diagnosis as many as 50% of patients may have
Guidelines on cardiovascular risk assessment and management
 European Heart Journal Supplements (2005) 7 (Supplement L), L5 L10 doi:10.1093/eurheartj/sui079 Guidelines on cardiovascular risk assessment and management David A. Wood 1,2 * 1 Cardiovascular Medicine
European Heart Journal Supplements (2005) 7 (Supplement L), L5 L10 doi:10.1093/eurheartj/sui079 Guidelines on cardiovascular risk assessment and management David A. Wood 1,2 * 1 Cardiovascular Medicine
Identifying and Quantifying Dynamic Risk Factors for Coronary Artery Disease in Systemic Lupus Erythematosus
 Identifying and Quantifying Dynamic Risk Factors for Coronary Artery Disease in Systemic Lupus Erythematosus by Mandana Nikpour A thesis submitted in conformity with the requirements for the degree of
Identifying and Quantifying Dynamic Risk Factors for Coronary Artery Disease in Systemic Lupus Erythematosus by Mandana Nikpour A thesis submitted in conformity with the requirements for the degree of
Lipid/Lipoprotein Structure and Metabolism (Overview)
 Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
Lipid/Lipoprotein Structure and Metabolism (Overview) Philip Barter President, International Atherosclerosis Society Centre for Vascular Research University of New South Wales Sydney, Australia Disclosures
Ischemic Heart and Cerebrovascular Disease. Harold E. Lebovitz, MD, FACE Kathmandu November 2010
 Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
Ischemic Heart and Cerebrovascular Disease Harold E. Lebovitz, MD, FACE Kathmandu November 2010 Relationships Between Diabetes and Ischemic Heart Disease Risk of Cardiovascular Disease in Different Categories
Demir Baykal,MD,FACC,FASE Diplomat ABCL,SCCT Gwinnett Consultants in Cardiology Medical Software,LLC
 Demir Baykal,MD,FACC,FASE Diplomat ABCL,SCCT Gwinnett Consultants in Cardiology Medical Software,LLC 1950-1979 1980-1992 Gabriel, S. et al., Arthritis Rheum 1999;42:46-50. 1. Pericarditis 2. Myocarditis
Demir Baykal,MD,FACC,FASE Diplomat ABCL,SCCT Gwinnett Consultants in Cardiology Medical Software,LLC 1950-1979 1980-1992 Gabriel, S. et al., Arthritis Rheum 1999;42:46-50. 1. Pericarditis 2. Myocarditis
CIC Edizioni Internazionali. Cardio-metabolic comorbidities in rheumatoid arthritis and SLE. Review. 120 Clinical Dermatology 2013; 1 (2):
 Review Cardio-metabolic comorbidities in rheumatoid arthritis and SLE Andrea Doria Division of Rheumatology University of Padua, Italy Address for correspondence: Division of Rheumatology University of
Review Cardio-metabolic comorbidities in rheumatoid arthritis and SLE Andrea Doria Division of Rheumatology University of Padua, Italy Address for correspondence: Division of Rheumatology University of
Familial hypercholesterolaemia in children and adolescents
 Familial hypercholesterolaemia in children and adolescents Rationale and recommendations for early identification and treatment European Atherosclerosis Society Consensus Panel Slide deck adapted from:
Familial hypercholesterolaemia in children and adolescents Rationale and recommendations for early identification and treatment European Atherosclerosis Society Consensus Panel Slide deck adapted from:
Lupus and the heart. Lupus Foundation of America
 Lupus and the heart Lupus Foundation of America Teleconference FEB 2015 Premature Atherosclerotic ti Cardiovascular Disease in Systemic Lupus Erythematosus Joan M. Von Feldt, MD, MSEd Professor of Medicine
Lupus and the heart Lupus Foundation of America Teleconference FEB 2015 Premature Atherosclerotic ti Cardiovascular Disease in Systemic Lupus Erythematosus Joan M. Von Feldt, MD, MSEd Professor of Medicine
The New Gold Standard for Lipoprotein Analysis. Advanced Testing for Cardiovascular Risk
 The New Gold Standard for Lipoprotein Analysis Advanced Testing for Cardiovascular Risk Evolution of Lipoprotein Testing The Lipid Panel Total Cholesterol = VLDL + LDL + HDL Evolution of Lipoprotein Testing
The New Gold Standard for Lipoprotein Analysis Advanced Testing for Cardiovascular Risk Evolution of Lipoprotein Testing The Lipid Panel Total Cholesterol = VLDL + LDL + HDL Evolution of Lipoprotein Testing
 Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Glossary For TheFatNurse s For All Ages Series Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Apolipoprotein
Hans Strijdom SA Heart Meeting November 2017
 Hans Strijdom SA Heart Meeting November 2017 HIV-infection and ART, but not high sensitivity CRP, are associated with markers of vascular function: Results from the Western Cape cohort of the EndoAfrica
Hans Strijdom SA Heart Meeting November 2017 HIV-infection and ART, but not high sensitivity CRP, are associated with markers of vascular function: Results from the Western Cape cohort of the EndoAfrica
Accelerated Atherosclerosis in Autoimmune Rheumatic Diseases Prof. Andrea Doria
 Accelerated Atherosclerosis Andrea Doria Division of Rheumatology University of Padova 1 Endothelial Dysfunction Fatty-Streak Formation Endothelial Leukocyte Endothelial Leukocyte permeability migration
Accelerated Atherosclerosis Andrea Doria Division of Rheumatology University of Padova 1 Endothelial Dysfunction Fatty-Streak Formation Endothelial Leukocyte Endothelial Leukocyte permeability migration
CRP and fibrinogen imply clinical outcome of patients with type-2 diabetes. and coronary artery disease
 CRP and fibrinogen imply clinical outcome of patients with type-2 diabetes and coronary artery disease Marijan Bosevski 1, *, Golubinka Bosevska 1, Lily Stojanovska 2, Vasso Apostolopoulos 2, * 1 University
CRP and fibrinogen imply clinical outcome of patients with type-2 diabetes and coronary artery disease Marijan Bosevski 1, *, Golubinka Bosevska 1, Lily Stojanovska 2, Vasso Apostolopoulos 2, * 1 University
 Glossary For TheFatNurse s For All Ages Series Apolipoprotein B (APOB or ApoB) are the primary apolipoproteins of chylomicrons and low-density lipoproteins (LDL - known commonly by the misnomer "bad cholesterol"
Glossary For TheFatNurse s For All Ages Series Apolipoprotein B (APOB or ApoB) are the primary apolipoproteins of chylomicrons and low-density lipoproteins (LDL - known commonly by the misnomer "bad cholesterol"
Seminars in Arthritis and Rheumatism
 Seminars in Arthritis and Rheumatism 43 (2013) 77 95 Contents lists available at ScienceDirect Seminars in Arthritis and Rheumatism journal homepage: www.elsevier.com/locate/semarthrit Systemic Lupus Erythematosus
Seminars in Arthritis and Rheumatism 43 (2013) 77 95 Contents lists available at ScienceDirect Seminars in Arthritis and Rheumatism journal homepage: www.elsevier.com/locate/semarthrit Systemic Lupus Erythematosus
Cho et al., 2009 Journal of Cardiology (2009), 54:
 Endothelial Dysfunction, Increased Carotid Artery Intima-media Thickness and Pulse Wave Velocity, and Increased Level of Inflammatory Markers are Associated with Variant Angina Cho et al., 2009 Journal
Endothelial Dysfunction, Increased Carotid Artery Intima-media Thickness and Pulse Wave Velocity, and Increased Level of Inflammatory Markers are Associated with Variant Angina Cho et al., 2009 Journal
Marshall Tulloch-Reid, MD, MPhil, DSc, FACE Epidemiology Research Unit Tropical Medicine Research Institute The University of the West Indies, Mona,
 Marshall Tulloch-Reid, MD, MPhil, DSc, FACE Epidemiology Research Unit Tropical Medicine Research Institute The University of the West Indies, Mona, Jamaica At the end of this presentation the participant
Marshall Tulloch-Reid, MD, MPhil, DSc, FACE Epidemiology Research Unit Tropical Medicine Research Institute The University of the West Indies, Mona, Jamaica At the end of this presentation the participant
1. Which one of the following patients does not need to be screened for hyperlipidemia:
 Questions: 1. Which one of the following patients does not need to be screened for hyperlipidemia: a) Diabetes mellitus b) Hypertension c) Family history of premature coronary disease (first degree relatives:
Questions: 1. Which one of the following patients does not need to be screened for hyperlipidemia: a) Diabetes mellitus b) Hypertension c) Family history of premature coronary disease (first degree relatives:
Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors
 Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors Carmine Pizzi 1 ; Lamberto Manzoli 2, Stefano Mancini 3 ; Gigliola Bedetti
Autonomic nervous system, inflammation and preclinical carotid atherosclerosis in depressed subjects with coronary risk factors Carmine Pizzi 1 ; Lamberto Manzoli 2, Stefano Mancini 3 ; Gigliola Bedetti
Assessment for Early Cardiovascular Risk in Pediatric Rheumatic Disease
 Assessment for Early Cardiovascular Risk in Pediatric Rheumatic Disease by Pascal Norman Tyrrell A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Institute
Assessment for Early Cardiovascular Risk in Pediatric Rheumatic Disease by Pascal Norman Tyrrell A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Institute
STABILITY Stabilization of Atherosclerotic plaque By Initiation of darapladib TherapY. Harvey D White on behalf of The STABILITY Investigators
 STABILITY Stabilization of Atherosclerotic plaque By Initiation of darapladib TherapY Harvey D White on behalf of The STABILITY Investigators Lipoprotein- associated Phospholipase A 2 (Lp-PLA 2 ) activity:
STABILITY Stabilization of Atherosclerotic plaque By Initiation of darapladib TherapY Harvey D White on behalf of The STABILITY Investigators Lipoprotein- associated Phospholipase A 2 (Lp-PLA 2 ) activity:
Pathology of Coronary Artery Disease
 Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
Pathology of Coronary Artery Disease Seth J. Kligerman, MD Pathology of Coronary Artery Disease Seth Kligerman, MD Assistant Professor Medical Director of MRI University of Maryland Department of Radiology
Supplemental Figures and Legends
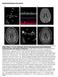 Supplemental Figures and Legends Online Figure 1. 18 year old female with SLE and acute homonymous hemianopsia, confusional state, and cognitive dysfunction. A. This TEE four chamber view demonstrates
Supplemental Figures and Legends Online Figure 1. 18 year old female with SLE and acute homonymous hemianopsia, confusional state, and cognitive dysfunction. A. This TEE four chamber view demonstrates
Demir Baykal,MD,FACC,FASE Diplomat ABCL,SCCT Gwinnett Consultants in Cardiology Medical Software,LLC
 Demir Baykal,MD,FACC,FASE Diplomat ABCL,SCCT Gwinnett Consultants in Cardiology Medical Software,LLC 1950-1979 1980-1992 Gabriel, S. et al., Arthritis Rheum 1999;42:46-50. 1 1. Pericarditis 2. Myocarditis
Demir Baykal,MD,FACC,FASE Diplomat ABCL,SCCT Gwinnett Consultants in Cardiology Medical Software,LLC 1950-1979 1980-1992 Gabriel, S. et al., Arthritis Rheum 1999;42:46-50. 1 1. Pericarditis 2. Myocarditis
Inflammation and and Heart Heart Disease in Women Inflammation and Heart Disease
 Inflammation and Heart Disease in Women Inflammation and Heart Disease What is the link between een inflammation and atherosclerotic disease? What is the role of biomarkers in predicting cardiovascular
Inflammation and Heart Disease in Women Inflammation and Heart Disease What is the link between een inflammation and atherosclerotic disease? What is the role of biomarkers in predicting cardiovascular
ATHEROSCLEROSIS. Secondary changes are found in other coats of the vessel wall.
 ATHEROSCLEROSIS Atherosclerosis Atherosclerosis is a disease process affecting the intima of the aorta and large and medium arteries, taking the form of focal thickening or plaques of fibrous tissue and
ATHEROSCLEROSIS Atherosclerosis Atherosclerosis is a disease process affecting the intima of the aorta and large and medium arteries, taking the form of focal thickening or plaques of fibrous tissue and
CLINICAL OUTCOME Vs SURROGATE MARKER
 CLINICAL OUTCOME Vs SURROGATE MARKER Statin Real Experience Dr. Mostafa Sherif Senior Medical Manager Pfizer Egypt & Sudan Objective Difference between Clinical outcome and surrogate marker Proper Clinical
CLINICAL OUTCOME Vs SURROGATE MARKER Statin Real Experience Dr. Mostafa Sherif Senior Medical Manager Pfizer Egypt & Sudan Objective Difference between Clinical outcome and surrogate marker Proper Clinical
Health and Disease of the Cardiovascular system
 1 Health and Disease of the Cardiovascular system DR CHRIS MOORE Instructions 2 USE THE ARROWS TO NAVIGATE, OR TAP OUTLINE AT THE TOP TO BRING DOWN A SLIDE MENU Click these where you see them to zoom or
1 Health and Disease of the Cardiovascular system DR CHRIS MOORE Instructions 2 USE THE ARROWS TO NAVIGATE, OR TAP OUTLINE AT THE TOP TO BRING DOWN A SLIDE MENU Click these where you see them to zoom or
Chapter 18. Diet and Health
 Chapter 18 Diet and Health Risk Factors and Chronic Diseases Interrelationships among Chronic Diseases Chronic Disease Heart Disease and Stroke Hypertension Cancer Diabetes The Formation of Plaques in
Chapter 18 Diet and Health Risk Factors and Chronic Diseases Interrelationships among Chronic Diseases Chronic Disease Heart Disease and Stroke Hypertension Cancer Diabetes The Formation of Plaques in
Plasma fibrinogen level, BMI and lipid profile in type 2 diabetes mellitus with hypertension
 World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
ATHEROSCLEROSIS زيد ثامر جابر. Zaid. Th. Jaber
 ATHEROSCLEROSIS زيد ثامر جابر Zaid. Th. Jaber Objectives 1- Review the normal histological features of blood vessels walls. 2-define the atherosclerosis. 3- display the risk factors of atherosclerosis.
ATHEROSCLEROSIS زيد ثامر جابر Zaid. Th. Jaber Objectives 1- Review the normal histological features of blood vessels walls. 2-define the atherosclerosis. 3- display the risk factors of atherosclerosis.
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice
 Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
Leptin deficiency suppresses progression of atherosclerosis in apoe-deficient mice Atherosclerosis, 2007 Chiba T, Shinozaki S, Nakazawa T, et al. Present by Sudaporn Pummoung Apolipoprotein E (apoe( apoe)
High density lipoprotein metabolism
 High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
High density lipoprotein metabolism Lipoprotein classes and atherosclerosis Chylomicrons, VLDL, and their catabolic remnants Pro-atherogenic LDL HDL Anti-atherogenic Plasma lipid transport Liver VLDL FC
John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam
 Latest Insights from the JUPITER Study John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam Inflammation, hscrp, and Vascular Prevention
Latest Insights from the JUPITER Study John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam Inflammation, hscrp, and Vascular Prevention
Clinical Trial Synopsis TL-OPI-518, NCT#
 Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Clinical Trial Synopsis, NCT# 00225264 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl vs Glimepiride
Term-End Examination December, 2009 MCC-006 : CARDIOVASCULAR EPIDEMIOLOGY
 MCC-006 POST GRADUATE DIPLOMA IN CLINICAL CARDIOLOGY (PGDCC) 00269 Term-End Examination December, 2009 MCC-006 : CARDIOVASCULAR EPIDEMIOLOGY Time : 2 hours Maximum Marks : 60 Note : There will be multiple
MCC-006 POST GRADUATE DIPLOMA IN CLINICAL CARDIOLOGY (PGDCC) 00269 Term-End Examination December, 2009 MCC-006 : CARDIOVASCULAR EPIDEMIOLOGY Time : 2 hours Maximum Marks : 60 Note : There will be multiple
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry
 Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Lipoproteins Metabolism Reference: Campbell Biochemistry and Lippincott s Biochemistry Learning Objectives 1. Define lipoproteins and explain the rationale of their formation in blood. 2. List different
Subclinical atherosclerosis in systemic lupus erythematosus (SLE): the relative contribution of classic risk factors and the lupus phenotype
 Rheumatology 2007;46:983 988 Advance Access publication 23 March 2007 doi:10.1093/rheumatology/kem002 Subclinical atherosclerosis in systemic lupus erythematosus (SLE): the relative contribution of classic
Rheumatology 2007;46:983 988 Advance Access publication 23 March 2007 doi:10.1093/rheumatology/kem002 Subclinical atherosclerosis in systemic lupus erythematosus (SLE): the relative contribution of classic
Classification of Endothelial Dysfunction. Stefano Taddei Department of Internal Medicine University of Pisa, Italy
 Classification of Endothelial Dysfunction Stefano Taddei Department of Internal Medicine University of Pisa, Italy Pathogenesis of atherosclerosis from endothelial dysfunction to clinical disease endothelial
Classification of Endothelial Dysfunction Stefano Taddei Department of Internal Medicine University of Pisa, Italy Pathogenesis of atherosclerosis from endothelial dysfunction to clinical disease endothelial
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 6 October 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 6 October 2010 CRESTOR 5 mg, film-coated tablet B/30 (CIP code: 369 853-8) B/90 (CIP code: 391 690-0) CRESTOR 10 mg,
A Study of Atherosclerosis in Systemic Vasculitis
 Original Article A Study of Atherosclerosis in Systemic Vasculitis Silas Supragya Nelson, Sonjjay Pande, Avadhesh Pratap Singh Kushwah 3 Assistant Professor in the Department of Medicine, NSCB Medical
Original Article A Study of Atherosclerosis in Systemic Vasculitis Silas Supragya Nelson, Sonjjay Pande, Avadhesh Pratap Singh Kushwah 3 Assistant Professor in the Department of Medicine, NSCB Medical
5. THE ROLE OF LIPIDS IN THE DEVELOPMENT OF ATHEROSCLEROSIS AND CORONARY HEART DISEASE: GUIDELINES FOR DIAGNOSIS AND TREATMENT
 5. THE ROLE OF LIPIDS IN THE DEVELOPMENT OF ATHEROSCLEROSIS AND CORONARY HEART DISEASE: GUIDELINES FOR DIAGNOSIS AND TREATMENT Prof. Victor Blaton, Ph.D. Department of Clinical Chemistry, Hospital AZ Sint-Jan
5. THE ROLE OF LIPIDS IN THE DEVELOPMENT OF ATHEROSCLEROSIS AND CORONARY HEART DISEASE: GUIDELINES FOR DIAGNOSIS AND TREATMENT Prof. Victor Blaton, Ph.D. Department of Clinical Chemistry, Hospital AZ Sint-Jan
Thematic review series: The Immune System and Atherogenesis
 thematic review Thematic review series: The Immune System and Atherogenesis Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? Alan Chait, 1 Chang Yeop Han, John
thematic review Thematic review series: The Immune System and Atherogenesis Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? Alan Chait, 1 Chang Yeop Han, John
Update On Diabetic Dyslipidemia: Who Should Be Treated With A Fibrate After ACCORD-LIPID?
 Update On Diabetic Dyslipidemia: Who Should Be Treated With A Fibrate After ACCORD-LIPID? Karen Aspry, MD, MS, ABCL, FACC Assistant Clinical Professor of Medicine Warren Alpert Medical School of Brown
Update On Diabetic Dyslipidemia: Who Should Be Treated With A Fibrate After ACCORD-LIPID? Karen Aspry, MD, MS, ABCL, FACC Assistant Clinical Professor of Medicine Warren Alpert Medical School of Brown
SUMMARY. Introduction. Study design. Summary
 SUMMARY Introduction The global prevalence of type 2 diabetes is increasing rapidly and nowadays affects almost 250 million people. Cardiovascular disease is the most prevalent complication of type 2 diabetes,
SUMMARY Introduction The global prevalence of type 2 diabetes is increasing rapidly and nowadays affects almost 250 million people. Cardiovascular disease is the most prevalent complication of type 2 diabetes,
 Five chapters 1. What is CVD prevention 2. Why is CVD prevention needed 3. Who needs CVD prevention 4. How is CVD prevention applied 5. Where should CVD prevention be offered Shorter, more adapted to clinical
Five chapters 1. What is CVD prevention 2. Why is CVD prevention needed 3. Who needs CVD prevention 4. How is CVD prevention applied 5. Where should CVD prevention be offered Shorter, more adapted to clinical
Coronary Artery Calcification
 Coronary Artery Calcification Julianna M. Czum, MD OBJECTIVES CORONARY ARTERY CALCIFICATION Julianna M. Czum, MD Dartmouth-Hitchcock Medical Center 1. To review the clinical significance of coronary heart
Coronary Artery Calcification Julianna M. Czum, MD OBJECTIVES CORONARY ARTERY CALCIFICATION Julianna M. Czum, MD Dartmouth-Hitchcock Medical Center 1. To review the clinical significance of coronary heart
Chapter (5) Etiology of Low HDL- Cholesterol
 Chapter (5) Etiology of Low HDL- Cholesterol The aim of this chapter is to summarize the different etiological factors mainly the role of life-style and different disease conditions contributing to the
Chapter (5) Etiology of Low HDL- Cholesterol The aim of this chapter is to summarize the different etiological factors mainly the role of life-style and different disease conditions contributing to the
Mechanisms of Action for Arsenic in Cardiovascular Toxicity and Implications for Risk Assessment
 Mechanisms of Action for Arsenic in Cardiovascular Toxicity and Implications for Risk Assessment Mandeep Sidhu, MD, MBA, FACC Assistant Professor of Medicine Division of Cardiology Albany Medical College
Mechanisms of Action for Arsenic in Cardiovascular Toxicity and Implications for Risk Assessment Mandeep Sidhu, MD, MBA, FACC Assistant Professor of Medicine Division of Cardiology Albany Medical College
JUPITER NEJM Poll. Panel Discussion: Literature that Should Have an Impact on our Practice: The JUPITER Study
 Panel Discussion: Literature that Should Have an Impact on our Practice: The Study Kaiser COAST 11 th Annual Conference Maui, August 2009 Robert Blumberg, MD, FACC Ralph Brindis, MD, MPH, FACC Primary
Panel Discussion: Literature that Should Have an Impact on our Practice: The Study Kaiser COAST 11 th Annual Conference Maui, August 2009 Robert Blumberg, MD, FACC Ralph Brindis, MD, MPH, FACC Primary
Low-density lipoprotein as the key factor in atherogenesis too high, too long, or both
 Low-density lipoprotein as the key factor in atherogenesis too high, too long, or both Lluís Masana Vascular Medicine and Metabolism Unit. Sant Joan University Hospital. IISPV. CIBERDEM Rovira i Virgili
Low-density lipoprotein as the key factor in atherogenesis too high, too long, or both Lluís Masana Vascular Medicine and Metabolism Unit. Sant Joan University Hospital. IISPV. CIBERDEM Rovira i Virgili
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL
 Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
Behind LDL: The Metabolism of ApoB, the Essential Apolipoprotein in LDL and VLDL Sung-Joon Lee, PhD Division of Food Science Institute of Biomedical Science and Safety Korea University Composition of Lipoproteins:
Table S1. Read and ICD 10 diagnosis codes for polymyalgia rheumatica and giant cell arteritis
 SUPPLEMENTARY MATERIAL TEXT Text S1. Multiple imputation TABLES Table S1. Read and ICD 10 diagnosis codes for polymyalgia rheumatica and giant cell arteritis Table S2. List of drugs included as immunosuppressant
SUPPLEMENTARY MATERIAL TEXT Text S1. Multiple imputation TABLES Table S1. Read and ICD 10 diagnosis codes for polymyalgia rheumatica and giant cell arteritis Table S2. List of drugs included as immunosuppressant
ΛΟΙΜΩΞΗ HIV. Ιγνάτιος Οικονομίδης,MD,FESC Β Πανεπιστημιακή Καρδιολογική
 ΛΟΙΜΩΞΗ HIV Ιγνάτιος Οικονομίδης,MD,FESC Β Πανεπιστημιακή Καρδιολογική Κλινική, Νοσοκομείο «Αττικόν» Infection Acute Myocardial Infarction p er 100,000 HIV/AIDS discharg es 800 700 600 500 400 300 200
ΛΟΙΜΩΞΗ HIV Ιγνάτιος Οικονομίδης,MD,FESC Β Πανεπιστημιακή Καρδιολογική Κλινική, Νοσοκομείο «Αττικόν» Infection Acute Myocardial Infarction p er 100,000 HIV/AIDS discharg es 800 700 600 500 400 300 200
Normal blood vessels A= artery V= vein
 Normal blood vessels A= artery V= vein Artery (A) versus vein (V) ARTERIOSCLEROSIS Arteriosclerosis ="hardening of the arteries" arterial wall thickening and loss of elasticity. Three patterns are recognized,
Normal blood vessels A= artery V= vein Artery (A) versus vein (V) ARTERIOSCLEROSIS Arteriosclerosis ="hardening of the arteries" arterial wall thickening and loss of elasticity. Three patterns are recognized,
Review of guidelines for management of dyslipidemia in diabetic patients
 2012 international Conference on Diabetes and metabolism (ICDM) Review of guidelines for management of dyslipidemia in diabetic patients Nan Hee Kim, MD, PhD Department of Internal Medicine, Korea University
2012 international Conference on Diabetes and metabolism (ICDM) Review of guidelines for management of dyslipidemia in diabetic patients Nan Hee Kim, MD, PhD Department of Internal Medicine, Korea University
The Role of Vitamin D in Heart Disease. Janet Long, MSN, ACNP, CLS, FAHA, FNLA Cardiovascular Institute Rhode Island Hospital and The Miriam Hospital
 The Role of Vitamin D in Heart Disease Janet Long, MSN, ACNP, CLS, FAHA, FNLA Cardiovascular Institute Rhode Island Hospital and The Miriam Hospital None Conflict of Interest What is Vitamin D Produced
The Role of Vitamin D in Heart Disease Janet Long, MSN, ACNP, CLS, FAHA, FNLA Cardiovascular Institute Rhode Island Hospital and The Miriam Hospital None Conflict of Interest What is Vitamin D Produced
Plasma lipoproteins & atherosclerosis by. Prof.Dr. Maha M. Sallam
 Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
Biochemistry Department Plasma lipoproteins & atherosclerosis by Prof.Dr. Maha M. Sallam 1 1. Recognize structures,types and role of lipoproteins in blood (Chylomicrons, VLDL, LDL and HDL). 2. Explain
13/09/2012. Dietary fatty acids. Triglyceride. Phospholipids:
 CARDIOVASCULAR DISEASES (CVD) and NUTRITION Major cause of morbidity & mortality in Canada & other developed countries e.g., majority of approved health claims on food labels relate to lowering CVD Relation
CARDIOVASCULAR DISEASES (CVD) and NUTRITION Major cause of morbidity & mortality in Canada & other developed countries e.g., majority of approved health claims on food labels relate to lowering CVD Relation
C-Reactive Protein and Your Heart
 C-Reactive Protein and Your Heart By: James L. Holly, MD Inflammation is the process by which the body responds to injury. Laboratory evidence and findings at autopsy studies suggest that the inflammatory
C-Reactive Protein and Your Heart By: James L. Holly, MD Inflammation is the process by which the body responds to injury. Laboratory evidence and findings at autopsy studies suggest that the inflammatory
Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease. Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.
 Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.Κιλκίς Primary CVD Prevention A co-ordinated set of actions,
Antiplatelet Therapy in Primary CVD Prevention and Stable Coronary Artery Disease Καρακώστας Γεώργιος Διευθυντής Καρδιολογικής Κλινικής, Γ.Ν.Κιλκίς Primary CVD Prevention A co-ordinated set of actions,
Choosing Study Outcomes that Reflect Cardiovascular Disease: From Biomarkers to Burden of Disease. Greg Wellenius Joel Kaufman
 Choosing Study Outcomes that Reflect Cardiovascular Disease: From Biomarkers to Burden of Disease Greg Wellenius Joel Kaufman Framework for Choosing Subclinical Outcomes To Study What clinical outcomes
Choosing Study Outcomes that Reflect Cardiovascular Disease: From Biomarkers to Burden of Disease Greg Wellenius Joel Kaufman Framework for Choosing Subclinical Outcomes To Study What clinical outcomes
Disclosures. Background 1 What is Known MENOPAUSE, ESTROGENS, AND LIPOPROTEIN PARTICLES. Background 2 What is Not Known 10/2/2017
 Disclosures MENOPAUSE, ESTROGENS, AND LIPOPROTEIN PARTICLES Grants: NIH, Quest Diagnostics Consultant: Quest Diagnostics Merck Global Atherosclerosis Advisory Board Ronald M. Krauss, Children s Hospital
Disclosures MENOPAUSE, ESTROGENS, AND LIPOPROTEIN PARTICLES Grants: NIH, Quest Diagnostics Consultant: Quest Diagnostics Merck Global Atherosclerosis Advisory Board Ronald M. Krauss, Children s Hospital
HDL and Arterial Wall
 JIFA January 31th 2014 HDL and Arterial Wall P-J TOUBOUL INSERM698 Bichat University Conflict of Interest M Ath intellectual property owner Involvement in R & D for atherosclerosis software developments
JIFA January 31th 2014 HDL and Arterial Wall P-J TOUBOUL INSERM698 Bichat University Conflict of Interest M Ath intellectual property owner Involvement in R & D for atherosclerosis software developments
Role of dyslipidaemia in cardiovascular diseases and the therapeutic benefits by the appropriate drugs Banoo H
 Role of dyslipidaemia in cardiovascular diseases and the therapeutic benefits by the appropriate drugs Banoo H Introduction Coronary artery disease is an important cause of mortality and mor- bidity in
Role of dyslipidaemia in cardiovascular diseases and the therapeutic benefits by the appropriate drugs Banoo H Introduction Coronary artery disease is an important cause of mortality and mor- bidity in
Dyslipidaemia. Is there any new information? Dr. A.R.M. Saifuddin Ekram
 Dyslipidaemia Is there any new information? Dr. A.R.M. Saifuddin Ekram PhD,FACP,FCPS(Medicine) Professor(c.c.) & Head Department of Medicine Rajshahi Medical College Rajshahi-6000 New features of ATP III
Dyslipidaemia Is there any new information? Dr. A.R.M. Saifuddin Ekram PhD,FACP,FCPS(Medicine) Professor(c.c.) & Head Department of Medicine Rajshahi Medical College Rajshahi-6000 New features of ATP III
The aorta is an integral part of the cardiovascular system and should not be considered as just a conduit for blood supply from the heart to the
 The aorta is an integral part of the cardiovascular system and should not be considered as just a conduit for blood supply from the heart to the limbs and major organs. A range of important pathologies
The aorta is an integral part of the cardiovascular system and should not be considered as just a conduit for blood supply from the heart to the limbs and major organs. A range of important pathologies
The changes of serum BDNF, blood lipid and PCI in the elderly patients with coronary heart disease complicated with diabetes mellitus
 184 Journal of Hainan Medical University 2016; 22(16): 184-188 Journal of Hainan Medical University http://www.hnykdxxb.com/ The changes of serum BDNF, blood lipid and PCI in the elderly patients with
184 Journal of Hainan Medical University 2016; 22(16): 184-188 Journal of Hainan Medical University http://www.hnykdxxb.com/ The changes of serum BDNF, blood lipid and PCI in the elderly patients with
THE CLINICAL BIOCHEMISTRY OF LIPID DISORDERS
 THE CLINICAL BIOCHEMISTRY OF LIPID DISORDERS Hormonal regulation INSULIN lipid synthesis, lipolysis CORTISOL lipolysis GLUCAGON lipolysis GROWTH HORMONE lipolysis CATECHOLAMINES lipolysis LEPTIN catabolism
THE CLINICAL BIOCHEMISTRY OF LIPID DISORDERS Hormonal regulation INSULIN lipid synthesis, lipolysis CORTISOL lipolysis GLUCAGON lipolysis GROWTH HORMONE lipolysis CATECHOLAMINES lipolysis LEPTIN catabolism
Andrew Cohen, MD and Neil S. Skolnik, MD INTRODUCTION
 2 Hyperlipidemia Andrew Cohen, MD and Neil S. Skolnik, MD CONTENTS INTRODUCTION RISK CATEGORIES AND TARGET LDL-CHOLESTEROL TREATMENT OF LDL-CHOLESTEROL SPECIAL CONSIDERATIONS OLDER AND YOUNGER ADULTS ADDITIONAL
2 Hyperlipidemia Andrew Cohen, MD and Neil S. Skolnik, MD CONTENTS INTRODUCTION RISK CATEGORIES AND TARGET LDL-CHOLESTEROL TREATMENT OF LDL-CHOLESTEROL SPECIAL CONSIDERATIONS OLDER AND YOUNGER ADULTS ADDITIONAL
DYSLIPIDEMIA IN SYSTEMIC LUPUS ERYTHEMATOSUS. Leong Keng Hong
 DYSLIPIDEMIA IN SYSTEMIC LUPUS ERYTHEMATOSUS Leong Keng Hong MBBS, M,Med (Internal Medicine),MRCP (UK) FRCP (Edinburgh), FAMS (Rheumatology) A THESIS SUBMITTED FOR THE DOCTORATE OF MEDICINE DEPARTMENT
DYSLIPIDEMIA IN SYSTEMIC LUPUS ERYTHEMATOSUS Leong Keng Hong MBBS, M,Med (Internal Medicine),MRCP (UK) FRCP (Edinburgh), FAMS (Rheumatology) A THESIS SUBMITTED FOR THE DOCTORATE OF MEDICINE DEPARTMENT
Diabetic Dyslipidemia
 Diabetic Dyslipidemia Dr R V S N Sarma, M.D., (Internal Medicine), M.Sc., (Canada), Consultant Physician Cardiovascular disease (CVD) is a significant cause of illness, disability, and death among individuals
Diabetic Dyslipidemia Dr R V S N Sarma, M.D., (Internal Medicine), M.Sc., (Canada), Consultant Physician Cardiovascular disease (CVD) is a significant cause of illness, disability, and death among individuals
Novel Markers of Arterial Dysfunction
 혈관연구회창립심포지움, 3 월 3 일, 2005 Novel Markers of Arterial Dysfunction Kwang Kon Koh, MD, FACC, FAHA Cardiology Gachon Medical School Incheon, Korea Atherosclerosis: A progressive process PHASE I: Initiation
혈관연구회창립심포지움, 3 월 3 일, 2005 Novel Markers of Arterial Dysfunction Kwang Kon Koh, MD, FACC, FAHA Cardiology Gachon Medical School Incheon, Korea Atherosclerosis: A progressive process PHASE I: Initiation
9/25/2013 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
Annals of RSCB Vol. XVI, Issue 1
 THE STUDY OF PROTHROBOTIC STATE IN PATIENTS WITH TYPE 2 DIABETES MELLITUS AND CARDIOVASCULAR DISEASES Oana Bădulescu 1, Codruţa Bădescu 2, Manuela Ciocoiu 1, Magda Bădescu 1 1 DEPARTMENT OF PATHOPHYSIOLOGY;
THE STUDY OF PROTHROBOTIC STATE IN PATIENTS WITH TYPE 2 DIABETES MELLITUS AND CARDIOVASCULAR DISEASES Oana Bădulescu 1, Codruţa Bădescu 2, Manuela Ciocoiu 1, Magda Bădescu 1 1 DEPARTMENT OF PATHOPHYSIOLOGY;
Changing lipid-lowering guidelines: whom to treat and how low to go
 European Heart Journal Supplements (2005) 7 (Supplement A), A12 A19 doi:10.1093/eurheartj/sui003 Changing lipid-lowering guidelines: whom to treat and how low to go C.M. Ballantyne Section of Atherosclerosis,
European Heart Journal Supplements (2005) 7 (Supplement A), A12 A19 doi:10.1093/eurheartj/sui003 Changing lipid-lowering guidelines: whom to treat and how low to go C.M. Ballantyne Section of Atherosclerosis,
Manifestation of Antiphospholipid Syndrome among Saudi patients :examining the applicability of sapporo Criteria
 Manifestation of Antiphospholipid Syndrome among Saudi patients :examining the applicability of sapporo Criteria Farjah H AlGahtani Associate professor,md,mph Leukemia,Lymphoma in adolescent,thromboembolic
Manifestation of Antiphospholipid Syndrome among Saudi patients :examining the applicability of sapporo Criteria Farjah H AlGahtani Associate professor,md,mph Leukemia,Lymphoma in adolescent,thromboembolic
REAGENTS. RANDOX sdldl CHOLESTEROL (sdldl-c) SIZE MATTERS: THE TRUE WEIGHT OF RISK IN LIPID PROFILING
 REAGENTS RANDOX sdldl CHOLESTEROL (sdldl-c) SIZE MATTERS: THE TRUE WEIGHT OF RISK IN LIPID PROFILING Randox sdldl Cholesterol (sdldl-c) Size Matters: The True Wight of Risk in Lipid Profiling 1. BACKGROUND
REAGENTS RANDOX sdldl CHOLESTEROL (sdldl-c) SIZE MATTERS: THE TRUE WEIGHT OF RISK IN LIPID PROFILING Randox sdldl Cholesterol (sdldl-c) Size Matters: The True Wight of Risk in Lipid Profiling 1. BACKGROUND
PREVENTION OF CARDIOVASCULAR DISEASE IN WOMEN
 1980 to 2000: Death rate fell from: 542.9 to 266.8 per 100K men 263.3 to 134.4 per 100K women 341,745 fewer deaths from CHD in 2000 Ford ES, NEJM, 2007 47% from CHD treatments, 44% from risk factor modification
1980 to 2000: Death rate fell from: 542.9 to 266.8 per 100K men 263.3 to 134.4 per 100K women 341,745 fewer deaths from CHD in 2000 Ford ES, NEJM, 2007 47% from CHD treatments, 44% from risk factor modification
Low-density lipoproteins cause atherosclerotic cardiovascular disease (ASCVD) 1. Evidence from genetic, epidemiologic and clinical studies
 Low-density lipoproteins cause atherosclerotic cardiovascular disease (ASCVD) 1. Evidence from genetic, epidemiologic and clinical studies A Consensus Statement from the European Atherosclerosis Society
Low-density lipoproteins cause atherosclerotic cardiovascular disease (ASCVD) 1. Evidence from genetic, epidemiologic and clinical studies A Consensus Statement from the European Atherosclerosis Society
