Diagnostics Assessment Programme. Final scope
|
|
|
- Elijah Perkins
- 5 years ago
- Views:
Transcription
1 Diagnostics Assessment Programme Self-monitoring coagulometers (CoaguChek XS system, INRatio2 PT/INR monitor and ProTime Microcoagulation system), for selftesting or self-managing coagulation status in people for whom long-term vitamin K antagonist therapy is intended Final scope June Introduction The Coaguchek XS system is manufactured by Roche Diagnostics. The Medical Technologies Advisory Committee identified the Coaguchek XS system as potentially suitable for evaluation by the Diagnostics Assessment Programme on the basis of a briefing note. The final scope was informed by the scoping workshop held on 30 th April 2013 and by the assessment subgroup meeting held on 21 st May A glossary of terms is provided in appendix A. 2 Description of the technologies This section describes the properties of the diagnostic technologies based on information provided to NICE from the manufacturers and on information available in the public domain. NICE has not carried out an independent evaluation of this description. Final scope June of 18
2 2.1 Purpose of the medical technologies The point of care coagulometers are designed to monitor the clotting tendency of blood in people on long-term vitamin K antagonist therapy, such as those with atrial fibrillation or artificial heart valves who are at risk of thrombosis. The tests allow monitoring by two different methods of care: self-testing and selfmanaging. Both methods are based on the international normalised ratio (INR) which is a standardised unit for measuring the time it takes for blood to clot. Self-testing refers to the user performing the INR test themselves and then contacting their health professional with the reading to receive advice on any change to the dosage of the anticoagulant that may be required. Selfmanaging refers to the user performing the INR test themselves and then self adjusting the dosage of their anticoagulant medication by following an agreed care protocol. Together, these methods of care are referred to as selfmonitoring. The use of these coagulometers may reduce the frequency of visits to hospital for patients and enable them to be monitored more regularly. This may improve health outcomes for patients by enabling the dose of therapy to be adjusted more accurately, thereby avoiding adverse events that can result from an over- or under-dose of long-term vitamin K antagonist therapy, such as stroke and major haemorrhage. 2.2 Product properties Coaguchek XS system (Roche Diagnostics) The Coaguchek XS system comprises a meter and specifically designed test strips which can analyse a blood sample (fresh capillary blood or fresh untreated whole venous blood) and calculate the prothrombin time (PT) and the international normalised ratio (INR). These measures indicate the rate at which the blood clots. If the INR is too low, there is a higher risk of blood clots which can lead to a heart attack or a stroke. If the INR is too high, there is a Final scope June of 18
3 higher risk of bleeding which in severe cases can be gastrointestinal or intracerebral bleeding. A code chip, which contains calibration data and the expiry date of the test strips, is inserted into the meter before it is switched on. Once the device is switched on, a test strip is inserted and the blood sample is applied. The test result is displayed approximately 1 minute after application of the sample and the monitor automatically stores the result in memory. The user is guided through the process by on-screen graphical instructions. The CoaguChek test strip contains a lyophilized reagent consisting of thromboplastin and a peptide substrate. When a blood sample is applied, thromboplastin activates coagulation, which leads to the formation of thrombin. At the same time the meter starts to measure the time. The enzyme thrombin cleaves the peptide substrate, generating an electrochemical signal. Depending on the time elapsed when it first appears, this signal is then converted by means of an algorithm into customary coagulation units and the result is displayed on the screen. This can be displayed as prothrombin time in seconds, Quick value, or INR. The CoaguChek XS system has a number of in-built quality control functions including checks of the electric components when switched on, the test strip temperature during testing, and checks on the test strip batch such as the expiry date and quality of each strip. The CoaguChek meter is supplied with 4 x AAA batteries, a CoaguChek Softclix finger pricker and 20 Softclix XL lancets, 6 test strips, a user manual and carry case. The system can carry out about 60 tests per set of batteries. The meter is 138 mm x 78 mm x 28 mm and weighs 127 g (without batteries) InRatio2 PT/INR Monitor (Alere) The INRatio2 PT/INR Monitor performs a modified version of the one-stage Prothrombin Time test using a recombinant human thromboplastin reagent. Final scope June of 18
4 The clot formed in the reaction is detected by the change in the electrical impedance of the sample during the coagulation process. The system consists of a monitor and disposable test strips. The monitor provides a user interface, heats the test strip to the appropriate reaction temperature, measures the impedance of blood samples, and calculates and reports PT and INR results. Instructions and test results on displayed on an LCD. The monitor can store the results so that past test results can be reviewed. The test strip comprises 2 layers of transparent plastic laminated to each other which contain 1 sample well, 3 clot cells, and narrow channels connecting the sample well and the clot cells. The top side of the bottom layer is printed with three pairs of silver electrodes, one pair per cell, that start from inside the clot cells to the end of the strip where they are connected to the monitor main circuitry via a strip connector. The INRatio2 PT/INR Monitor analyses fresh capillary blood and when the blood sample is applied to the sample well, it is drawn through the narrow channels by capillary action to the clot cells where the impedance of the sample is measured by the monitor via the electrodes. Clot cells have reagents applied and the reagents are different for each channel. One channel contains the thromboplastin reagent for the PT test. The other two channels contain reagents that produce a low and high control time, regardless of the clotting time of the sample. Initially, the electrode impedance is infinite but drops to a minimum value when the blood sample fills the clot cells. The time when this initial minimum impedance is achieved is registered by the monitor as the start of the coagulation (T = 0). As the reaction progresses, the sample impedance increases to a maximum and then gradually drops as the clotting proceeds. The elapsed time, in seconds, from T = 0 until the clotting endpoint is reached is the PT time. The monitor software calculates the INR of the sample using PT time and calibration coefficients. Final scope June of 18
5 The Alere InRatio monitor performs a self-test when it is turned on and each test strip has a code which is accepted by the monitor if the strip code is in the correct format. The monitor uses four AA batteries or a Mains adapter as a power source, and can interface a printer or computer via the RS232 serial communication ProTime Microcoagulation System (International Technidyne Corporation (ITC)) The ProTime Microcoagulation System is designed for measuring prothrombin time and International Normalised Ratio. The test is performed in a cuvette which contains the reagents. Two different cuvettes are available depending on the amount of blood that needs to be collected and tested: the standard ProTime cuvette and the ProTime3 cuvette. The standard ProTime cuvette has five micro-channels, which contain the dried reagents required to perform triplicate testing of the PT assay and two levels of controls. The ProTime3 cuvette has three functional micro-channels: two micro-channels perform the controls, and one micro-channel performs the PT test. The standard ProTime cuvette is designed to hold 65μL of blood (approximately 3 drops) to fill all five micro-channels. The ProTime3 cuvette collects 27 μl of blood (approximately 1 large drop of fresh capillary blood or venous whole blood) to fill the three micro-channels. The instrument draws the precise volume of blood into the micro-channels of each cuvette, which contain dried thromboplastin, stabilisers and buffers. An array of LEDs detects the motion of sample and reagent mixtures as they move through a precision restriction in each channel. The blood is pumped back and forth until a clot forms, obstructing the channel and slowing the flow of blood. The instrument detects the clot when the blood movement decreases below a predetermined rate. The ProTime instrument and cuvettes are pre-calibrated so no calibration is necessary. Final scope June of 18
6 3 Target conditions There are a number of conditions which can result in people having a higher risk of thrombosis and consequently, receiving long term vitamin K antagonist therapy. These conditions include atrial fibrillation, heart valve disease and venous thromboembolism. 3.1 Atrial fibrillation Atrial fibrillation is the most common heart arrhythmia and affects around 800,000 people in the UK. It can affect adults of any age but it is more common in older people: 0.5% of people aged years; around 8% of people aged over 65 years. Atrial fibrillation is also more common in men than women, and is more common in people with other conditions, such as high blood pressure, atherosclerosis, or heart valve problems. Approximately 47% of people with atrial fibrillation currently receive anticoagulation therapy, such as warfarin. It is estimated that a further 30% of people with atrial fibrillation could receive this therapy but currently do not. People with atrial fibrillation are at a 5-6 times greater risk of stroke, with 12,500 strokes directly attributable to atrial fibrillation every year in the UK. Treatment with warfarin reduces this risk by 50 70%. 3.2 Heart valve disease Valve disease can affect blood flow through the heart in two ways; valve stenosis, where the valve does not open fully, and valve regurgitation (or incompetence) where the valve does not close properly, allowing blood to leak backwards. Disease can occur in any of the four heart valves, although disorders of the aortic and mitral valves are more serious. The main causes of heart valve disease are congenital heart disease and other diseases such as rheumatic fever, lupus, cardiomyopathy or endocarditis. Aortic stenosis is the most common type of valve disease and it affects one in 20 adults over the age of 65 years in the UK. Final scope June of 18
7 Data from the UK heart valve registry (UKHVR) indicate that approximately 0.2% of the UK population has prosthetic heart valves. Around 6,500 adult heart valve replacements (using mechanical or biological valves) are carried out each year, of which around 5,000 are aortic valve replacements. Patients with mechanical heart valves (and some patients with prosthetic valves) are susceptible to thromboembolism and need lifelong anticoagulant therapy. 3.3 Venous thromboembolism Venous thromboembolism (VTE) is a condition in which a blood clot (a thrombus) forms in a vein and then dislodges to travel in the blood (an embolus). A venous thrombus most commonly occurs in the deep veins of the legs or pelvis; this is then called a deep vein thrombosis (DVT). Blood flow through the affected vein can be limited by the clot, and it can cause swelling and pain in the leg. If it dislodges and travels to the lungs, to the pulmonary arteries, it is called a pulmonary embolism (PE), which in some cases may be fatal. Major risk factors for VTE include a prior history of DVT, age over 60 years, surgery, obesity, prolonged travel, acute medical illness, cancer, immobility, thrombophilia (an abnormal tendency for the blood to clot) and pregnancy. It has been estimated that every year 25,000 people in the UK die from preventable hospital-acquired VTE and that it causes over 500,000 deaths in Europe. Non-fatal VTE is also important as it can cause serious longer-term conditions such as post-thrombotic syndrome (PTS) and chronic thromboembolic pulmonary hypertension (CTEPH). PTS is a chronic condition characterised by symptoms and signs which develop after DVT due to damage to the deep veins and their valves. Its manifestations range from minor skin changes, pain or swelling, to established leg ulceration. It affects 20%-40% of patients after DVT of the lower limb, can be debilitating to patients, and have a significant impact on their quality of life. CTEPH is less common and is caused by obstruction of the pulmonary arteries due to PE. Final scope June of 18
8 This puts excessive pressure on the heart which can be harmful for some patients, causing heart failure. The current standard practice for the treatment of VTE is anticoagulation. There is a wide variation in practice, but patients are usually given a brief course of heparin treatment initially while they start on a 3 6 month course of warfarin. Patients who have had recurrent VTE or who are at high risk of recurrence may be given indefinite treatment with anticoagulants to prevent further VTE episodes (NICE CG144, 2012). 3.4 Patient issues and preferences The time and cost of attending an anticoagulation clinic is a significant burden for people on long-term oral anticoagulation therapy. It impacts significantly on both their working life and family life. Patients reported that warfarin clearly interacted with variables such as their diet, medication and lifestyle, and selfmonitoring allowed these variables to be incorporated into their treatment more effectively. Patients also reported that self-monitoring gave them greater understanding and ownership of their condition, and that one significant benefit is that it allows them to travel ( There is also a significant amount of anxiety for patients associated with waiting for their results from a coagulation test and in continuing normal daily activities without knowing their risk of a bleed or clot. 3.5 Diagnostic pathway Guidelines on oral anticoagulation with warfarin, published by the British Committee for Standards in Haematology (Keeling et al., 2011) outline the process for INR monitoring for those receiving warfarin. The guidelines state that INR can be measured effectively in venous or capillary blood samples, which are easier to obtain but slightly less accurate. People being tested should receive a written copy of their INR result including any necessary dose adjustments and a date for the next check. Final scope June of 18
9 The guidelines state that the INR should be measured: daily, or on alternate days, until it is within the therapeutic range (usually between 2.0 and 3.0, ideally 2.5) on two consecutive occasions then, twice weekly for 1 2 weeks, followed by weekly measurements until the INR is stable within the therapeutic range thereafter, depending on the stability of the INR, at longer intervals (for example, up to every 12 weeks, if agreed locally). More frequent monitoring of the INR is recommended for patients at risk of overcoagulation or bleeding, or those having problems adhering to treatment. Intravenous drug users, and people with hepatitis B, hepatitis C, or HIV, may be referred to a specialist clinic according to local arrangements. INR monitoring can be managed by local anticoagulant clinics in primary care, but often clinics are based in secondary care, requiring travel to hospital. The NICE anticoagulation commissioning guide (2007) states that anticoagulation therapy services can be delivered in a number of different ways, and that mixed models of provision may be required across a local health economy. This could include full service provision in secondary or primary care, shared provision, domiciliary provision or self-management. Services may be managed by a range of healthcare professionals including nurses, pharmacists and general practitioners. The NICE clinical guideline on atrial fibrillation recommends that selfmonitoring of INR should be considered for patients with atrial fibrillation receiving long-term anticoagulation, if they prefer this form of testing and if the following criteria are met: the patient (or a designated carer) is both physically and cognitively able to perform the self-monitoring test Final scope June of 18
10 an adequate supportive educational programme is in place to train patients and/or carers the patient s ability to self-manage is regularly reviewed the equipment for self-monitoring is regularly checked via a quality control programme. The guidelines on valve disease also state that appropriate education and training to allow patient self-management of anticoagulation should be provided, where possible. 4 Scope of the evaluation Table 1: Scope of the evaluation Decision question Populations Intervention Are self-monitoring coagulometers cost effective if used for self-testing or self-managing coagulation status in people for whom long term vitamin K antagonist therapy is intended, in the NHS? People for whom long term vitamin K antagonist therapy is intended For self testing or self management: Coaguchek XS system (Roche Diagnostics) Alternative interventions For self testing or self management: INRatio2 RT/INR monitor (Alere) ProTime Microcoagulation system (International Technidyne Corporation (ITC)) Comparator INR testing in primary care using laboratory analysers INR testing in primary care using point of care tests INR testing in secondary care using laboratory Final scope June of 18
11 analysers INR testing in secondary care using point of care tests Healthcare setting Outcomes Self-use (self testing or self management) supervised by primary or secondary care Intermediate measures for consideration may include: INR values Test failure rate Time to test result Patient adherence to testing schedule Patient compliance with treatment Frequency of testing Frequency of visits to primary or secondary care clinics Time in therapeutic range (TTR) Clinical outcomes for consideration may include: Patient anxiety associated with waiting time for results and not knowing their current coagulation status and risk Number of bleeds or blood clots Morbidity and mortality from testing, an underor over-dose of vitamin K antagonist therapy, and treatment of bleeding or clots and associated sequelae. Adverse events from testing, false test results and vitamin K antagonist therapy Health related quality of life Costs will be considered from an NHS and Personal Social Services perspective. Costs for consideration Final scope June of 18
12 may include: Costs of equipment, reagents and consumables Costs of staff and training of staff Costs of training users or carers Maintenance of equipment Consumption of vitamin K antagonist therapy Medical costs arising from on-going monitoring and treatment with vitamin K antagonist therapy Medical costs arising from adverse events associated with testing and treatment The cost-effectiveness of interventions should be expressed in terms of incremental cost per qualityadjusted life year. Time horizon Life time 5 Modelling approach The aim and structure of the economic model will depend upon the final scope. 5.1 Existing models One published Health Technology (HTA) report considered the clinical effectiveness and cost effectiveness of different models of managing longterm oral anticoagulation therapy (Connock et al., 2007). It reported that selfmonitoring was clinically effective and safe in patients receiving long-term oral anticoagulation therapy. However, the economic evaluation showed that selfmanagement was more expensive than routine care in a specialised UK anticoagulation clinic and suggested that self-monitoring was unlikely to be costeffective. Two HTA reports, one based in Ontario (2009) and one based in Final scope June of 18
13 Belgium (Gailly et al., 2009), both reported that self-management was more cost effective than routine care. 5.2 Modelling possibilities The issues that will need to be addressed, through modelling if necessary, include the short term outcomes from unstable INR values, side-effects of INR testing and the long-term outcomes associated with receiving long-term vitamin K antagonist therapy. The effect of Coagucheck XS testing on increased or reduced time in therapeutic range compared with current standard practice should be assessed. The potential variations in test accuracy and frequency of testing will need to be determined and modelled to identify resulting changes in final health outcomes. Assumptions about the size of the population eligible for self-monitoring will also have to be made with regard to the type of anticoagulation therapy being received and the ability of patients to self-monitor (both physically and cognitively). 6 Equality issues NICE is committed to promoting equality of opportunity, eliminating unlawful discrimination and fostering good relations between people with particular protected characteristics and others. Access to the device may be of particular benefit to groups receiving longterm vitamin K anatgonist therapy, such as children and young adults in education or older people, who may find travel to clinics for INR testing difficult or demanding. In addition, access to the device may benefit some people with disabilities who may find travel to clinics for INR testing difficult or demanding. Users must be physically and cognitively able to perform the self-monitoring test correctly. Atrial fibrillation and valve disease is more common in older people. Final scope June of 18
14 7 Implementation issues Patients must be assessed to determine they are both physically and cognitively able to perform self-monitoring correctly. Training and educational tools are needed to teach patients about self-monitoring. A patient s ability to self-monitor must be regularly reviewed. A quality control programme is needed to ensure equipment is regularly checked. Implementation of self-monitoring may require significant changes to be made to current services. Some services may no longer be needed. Final scope June of 18
15 Appendix A Glossary of terms International normalised ratio (INR): Globally recommended unit for measuring thromboplastin time, which renders different measurements comparable despite the different thromboplastins used. It is calculated as: INR = (Patient's PT / Normal mean PT) ISI For example: The PT of a patient receiving oral anticoagulant is 64 seconds (= 18% Quick). The prothrombin time of a normal plasma is 22 seconds (= 100% Quick). The ISI of the thromboplastin used is Substituting this value in the formula above gives the following INR: (64) / (22) 0.93 = 2.7 INR This signifies a coagulation time that is 2.7 times longer than the standard. The longer the patient's coagulation time, the higher the INR. Prothrombin time (PT): Time (in seconds) taken for a blood sample to clot in the presence of added thromboplastin. Final scope June of 18
16 Appendix B Related NICE guidance and pathways Related guidance Venous thromboembolic diseases: NICE clinical guideline CG144 (2012). Available from: Date for review: Not stated Venous thromboembolism - reducing the risk: NICE clinical guideline CG92 (2010). Available from: Date for review: Oct 2012 (the review decision consultation is currently ongoing) Anticoagulation therapy service commissioning guide: NICE commissioning guide (2007). Available from: ationtherapyservice/anticoagulationtherapyservice.jsp Stroke: NICE clinical guideline CG68 (2008). Available from: Date for review: April 2012 (Decision: the guideline should not be updated) Atrial fibrillation: NICE clinical guideline CG36 (2006). Available from: Date for review: August 2011 (Decision: the guideline is currently being updated) Pathways The self-monitoring coagulometers guidance will be included in several NICE pathways, for example: Chronic heart failure (published), Stroke pathway (published), Venous thromboembolism pathway (published), Atrial fibrillation pathway (draft), Familial hypercholesterolaemia pathway (draft), Myocardial infarction secondary prevention (draft). Final scope June of 18
17 In some of the these pathways, it may be appropriate to include the full recommendations of the guidance, in others it will only be necessary to give a link to the guidance. Final scope June of 18
18 Appendix C References Connock et al. (2007) Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling NIHR Health Technology Assessment Programme 11:38 Gailly, J, Gerkens, S, Van Den Bruel, A, Devriese, S, Obyn, C, and Cleemput, I. Use of point-of care devices in patients with oral anticoagulation: a Health Technology Assessment. KCE reports 117C Belgian Health Care Knowledge Centre. Keeling D, Baglin T, Tait C et al. (2011) Guidelines on oral anticoagulation with warfarin - fourth edition British Committee for Standards in Haematology Ontario Health Technology Assessment. Point-of-Care International Normalized Ratio (INR) Monitoring Devices for Patients on Long-term Oral Anticoagulation Therapy: an evidence based analysis. 9;12:2009. Medical Advisory Secretariat Venous thromboembolic diseases: NICE clinical guideline CG144 (2012). Available from: Final scope June of 18
Diagnostics guidance Published: 24 September 2014 nice.org.uk/guidance/dg14
 Atrial fibrillation and heart valve disease: self-monitoring coagulation status using point-of-care coagulometers (the CoaguChek XS system) Diagnostics guidance Published: 24 September 2014 nice.org.uk/guidance/dg14
Atrial fibrillation and heart valve disease: self-monitoring coagulation status using point-of-care coagulometers (the CoaguChek XS system) Diagnostics guidance Published: 24 September 2014 nice.org.uk/guidance/dg14
Costing statement: Atrial fibrillation and heart valve disease: selfmonitoring coagulation status using point-of-care coagulometers (the CoaguChek XS)
 Putting NICE guidance into practice Costing statement: Atrial fibrillation and heart valve disease: selfmonitoring coagulation status using point-of-care coagulometers (the CoaguChek XS) Published: September
Putting NICE guidance into practice Costing statement: Atrial fibrillation and heart valve disease: selfmonitoring coagulation status using point-of-care coagulometers (the CoaguChek XS) Published: September
DIAGNOSTICS ASSESSMENT PROGRAMME
 THEME: EFFECTIVENESS OF INRatio2 PT/INR MONITOR Comment Name and number organisation 1. Consultee 10: Alere Section number Section1 Comment We do not agree that the INRatio2 PT/ INR monitor is only recommended
THEME: EFFECTIVENESS OF INRatio2 PT/INR MONITOR Comment Name and number organisation 1. Consultee 10: Alere Section number Section1 Comment We do not agree that the INRatio2 PT/ INR monitor is only recommended
Point of Care Testing for INR using CoaguChek XS Plus
 Point of Care Testing, Pathology Page 1 of 18 Point of Care Testing for INR using CoaguChek XS Plus EDITION No 1.4 DATE OF ISSUE Feb 2014 REVIEW INTERVAL AUTHOR LOCATION OF COPIES 3 YEARS D O Neill 1.
Point of Care Testing, Pathology Page 1 of 18 Point of Care Testing for INR using CoaguChek XS Plus EDITION No 1.4 DATE OF ISSUE Feb 2014 REVIEW INTERVAL AUTHOR LOCATION OF COPIES 3 YEARS D O Neill 1.
A guide to anticoagulation management and self-testing
 A guide to management and self-testing Understanding If you re reading this, you, or someone you care about, may be on oral therapy, usually treated with a vitamin K antagonist (VKA). VKAs, such as warfarin,
A guide to management and self-testing Understanding If you re reading this, you, or someone you care about, may be on oral therapy, usually treated with a vitamin K antagonist (VKA). VKAs, such as warfarin,
I know my value CoaguChek XS Pro system
 I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
Additional cost-effectiveness analysis scenarios prepared for the Diagnostic Advisory Committee. Produced by the Aberdeen HTA group
 The clinical and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving
The clinical and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving
pat hways Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16
 pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
pat hways Anticoagulants, including non-vitamin K antagonist oral anticoagulants (NOACs) Key therapeutic topic Published: 26 February 2016 nice.org.uk/guidance/ktt16 Options for local implementation NICE
MEDICAL POLICY SUBJECT: HOME PROTHROMBIN TIME MONITORING DEVICE. POLICY NUMBER: CATEGORY: Equipment/Supplies
 MEDICAL POLICY SUBJECT: HOME PROTHROMBIN TIME 06/23/16, 6/22/17 PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
MEDICAL POLICY SUBJECT: HOME PROTHROMBIN TIME 06/23/16, 6/22/17 PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
Updates in Medical Management of Pulmonary Embolism and Deep Vein Thrombosis. By: Justin Youtsey, Elliott Reiff, William Montgomery, Grant Finlan
 Updates in Medical Management of Pulmonary Embolism and Deep Vein Thrombosis By: Justin Youtsey, Elliott Reiff, William Montgomery, Grant Finlan Objectives Describe the prevalence of PE and DVT as it relates
Updates in Medical Management of Pulmonary Embolism and Deep Vein Thrombosis By: Justin Youtsey, Elliott Reiff, William Montgomery, Grant Finlan Objectives Describe the prevalence of PE and DVT as it relates
Venous Thromboembolism Prophylaxis
 Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Approved by: Venous Thromboembolism Prophylaxis Vice President and Chief Medical Officer; and Vice President and Chief Operating Officer Corporate Policy & Procedures Manual Number: Date Approved January
Primary Care practice clinics within the Edmonton Southside Primary Care Network.
 INR Monitoring and Warfarin Dose Adjustment Last Review: November 2016 Intervention(s) and/or Procedure: Registered Nurses (RNs) adjust warfarin dosage according to individual patient International Normalized
INR Monitoring and Warfarin Dose Adjustment Last Review: November 2016 Intervention(s) and/or Procedure: Registered Nurses (RNs) adjust warfarin dosage according to individual patient International Normalized
Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
North East Essex Medicines Management Committee
 Colchester Hospital University NHS Foundation Trust North East Essex Clinical Commissioning Group North East Essex Medicines Management Committee ORAL ANTICOAGULANT (Vit K antagonist only) MANAGEMENT GUIDELINES
Colchester Hospital University NHS Foundation Trust North East Essex Clinical Commissioning Group North East Essex Medicines Management Committee ORAL ANTICOAGULANT (Vit K antagonist only) MANAGEMENT GUIDELINES
Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital
 Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
I know my value. Be an active part of your anticoagulation therapy with INR self-monitoring
 I know my value Be an active part of your anticoagulation therapy with INR self-monitoring INR* self-monitoring needs only a drop of blood, is easy, fast and decreases the possibility Be involved in your
I know my value Be an active part of your anticoagulation therapy with INR self-monitoring INR* self-monitoring needs only a drop of blood, is easy, fast and decreases the possibility Be involved in your
DVT - initial management NSCCG
 Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Mabel Labrada, MD Miami VA Medical Center
 Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Technology appraisal guidance Published: 25 July 2012 nice.org.uk/guidance/ta261
 Rivaroxaban for the treatment of deep vein thrombosis and prevention ention of recurrent deep vein thrombosis and pulmonary embolism Technology appraisal guidance Published: 25 July 2012 nice.org.uk/guidance/ta261
Rivaroxaban for the treatment of deep vein thrombosis and prevention ention of recurrent deep vein thrombosis and pulmonary embolism Technology appraisal guidance Published: 25 July 2012 nice.org.uk/guidance/ta261
Rhona Maclean
 An early evaluation of the impact of the North Trent policy regarding the use of Non-Vitamin K antagonists for SPAF in a secondary care anticoagulation clinic Rhona Maclean Rhona.maclean@sth.nhs.uk Risk
An early evaluation of the impact of the North Trent policy regarding the use of Non-Vitamin K antagonists for SPAF in a secondary care anticoagulation clinic Rhona Maclean Rhona.maclean@sth.nhs.uk Risk
Warfarin - Introduction for New Users
 Warfarin - Introduction for New Users Introduction Blood clots are frequent in patients who have diseases of the blood vessels or heart. Blood clots may pose a dangerous threat to some people, as they
Warfarin - Introduction for New Users Introduction Blood clots are frequent in patients who have diseases of the blood vessels or heart. Blood clots may pose a dangerous threat to some people, as they
Evaluation of the Precision and Accuracy of the INRatio 2 Prothrombin Time (PT) Monitoring System
 Evaluation of the Precision and Accuracy of the INRatio 2 Prothrombin Time (PT) Monitoring System Grace DeSantis, PhD Lisa Croner, PhD Michealle Havenhill, BA Shari Kipp, BSc (Honours) MBA Inverness Medical,
Evaluation of the Precision and Accuracy of the INRatio 2 Prothrombin Time (PT) Monitoring System Grace DeSantis, PhD Lisa Croner, PhD Michealle Havenhill, BA Shari Kipp, BSc (Honours) MBA Inverness Medical,
Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared Care Protocol for the Prescription and Supply of Low Molecular Weight Heparins Version 5.2 Version: 5.2 Authorised by: Joint Medicines
Alere INRatio 2 METHOD AND SAMPLE COLLECTION
 Alere INRatio 2 METHOD AND SAMPLE COLLECTION 1. PURPOSE AND SCOPE The purpose of this document is to describe the procedure for performing an INR test using the Alere INRatio2 analyser. The INRatio2 meter
Alere INRatio 2 METHOD AND SAMPLE COLLECTION 1. PURPOSE AND SCOPE The purpose of this document is to describe the procedure for performing an INR test using the Alere INRatio2 analyser. The INRatio2 meter
Internal Quality Control in the Haemostasis laboratory. Dr Steve Kitchen Sheffield Haemophilia and Thrombosis centre & UK NEQAS Blood Coagulation
 Internal Quality Control in the Haemostasis laboratory Dr Steve Kitchen Sheffield Haemophilia and Thrombosis centre & UK NEQAS Blood Coagulation Why do we need Quality control? Philadelphia Enquirer Aug
Internal Quality Control in the Haemostasis laboratory Dr Steve Kitchen Sheffield Haemophilia and Thrombosis centre & UK NEQAS Blood Coagulation Why do we need Quality control? Philadelphia Enquirer Aug
Apixaban for stroke prevention in atrial fibrillation. August 2010
 Apixaban for stroke prevention in atrial fibrillation August 2010 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Apixaban for stroke prevention in atrial fibrillation August 2010 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Bassett Healthcare Clinical Laboratory
 Therapeutic Drug Level Collection Guidelines Anti-epileptic drugs (carbamazepine, phenobarbital, phenytoin, primidone, valproic acid) Consider collecting after steady state conditions are reached, i.e.
Therapeutic Drug Level Collection Guidelines Anti-epileptic drugs (carbamazepine, phenobarbital, phenytoin, primidone, valproic acid) Consider collecting after steady state conditions are reached, i.e.
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
How to prevent blood clots whilst in hospital and after your return home
 How to prevent blood clots whilst in hospital and after your return home Patient information WHAT What IS is DEEP deep VEIN vein THROMBOSIS? thrombosis? Deep Vein Thrombosis DVT is a blood clot within
How to prevent blood clots whilst in hospital and after your return home Patient information WHAT What IS is DEEP deep VEIN vein THROMBOSIS? thrombosis? Deep Vein Thrombosis DVT is a blood clot within
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Clinical Medical Policy Department Clinical Affairs Division DESCRIPTION
 Prothrombin Time/International Normalized Ratio (PT/INR) Monitor for Home Anticoagulation Management [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr.
Prothrombin Time/International Normalized Ratio (PT/INR) Monitor for Home Anticoagulation Management [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr.
Anticoagulants. Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT
 Haemostasis Thrombosis Phases Endogenous anticoagulants Stopping blood loss Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT Vascular Platelet
Haemostasis Thrombosis Phases Endogenous anticoagulants Stopping blood loss Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT Vascular Platelet
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Thank you for agreeing to give us a statement on your organisation s view of the technology and the way
Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Thank you for agreeing to give us a statement on your organisation s view of the technology and the way
Technology appraisal guidance Published: 26 June 2013 nice.org.uk/guidance/ta287
 Rivaroxaban for treating pulmonary embolism and preventing enting recurrent venous thromboembolism Technology appraisal guidance Published: 26 June 2013 nice.org.uk/guidance/ta287 NICE 2018. All rights
Rivaroxaban for treating pulmonary embolism and preventing enting recurrent venous thromboembolism Technology appraisal guidance Published: 26 June 2013 nice.org.uk/guidance/ta287 NICE 2018. All rights
Deep Vein Thrombosis
 Deep Vein Thrombosis from NHS (UK) guidelines Introduction Deep vein thrombosis (DVT) is a blood clot in one of the deep veins in the body. Blood clots that develop in a vein are also known as venous thrombosis.
Deep Vein Thrombosis from NHS (UK) guidelines Introduction Deep vein thrombosis (DVT) is a blood clot in one of the deep veins in the body. Blood clots that develop in a vein are also known as venous thrombosis.
Warfarin Guidelines for Primary Care
 Warfarin Guidelines for Primary Care Produced by NECS Medicines Optimisation Team Date Produced: March 2014 Review Date: March 2016 Date Approved by: South Tees CCG March 2014 Version: 1.1 This guideline
Warfarin Guidelines for Primary Care Produced by NECS Medicines Optimisation Team Date Produced: March 2014 Review Date: March 2016 Date Approved by: South Tees CCG March 2014 Version: 1.1 This guideline
References: Murtagh J. Murtagh s General Practice. 5 th edn. Sydney: McGraw Hill; 2010.
 This presentation is designed to be delivered to people who work in a health care setting; such as nurses, carers and other nursing home staff. If you are an accredited pharmacist, you can use this presentation
This presentation is designed to be delivered to people who work in a health care setting; such as nurses, carers and other nursing home staff. If you are an accredited pharmacist, you can use this presentation
HEART HEALTH WEEK 2 SUPPLEMENT. A Beginner s Guide to Cardiovascular Disease ATHEROSCLEROSIS. Fatty deposits can narrow and harden the artery
 WEEK 2 SUPPLEMENT HEART HEALTH A Beginner s Guide to Cardiovascular Disease ATHEROSCLEROSIS FIGURE 1 Atherosclerosis is an inflammatory process where cholesterol is deposited in the wall of arteries and
WEEK 2 SUPPLEMENT HEART HEALTH A Beginner s Guide to Cardiovascular Disease ATHEROSCLEROSIS FIGURE 1 Atherosclerosis is an inflammatory process where cholesterol is deposited in the wall of arteries and
Venous Thromboembolism (VTE)
 Venous Thromboembolism (VTE) Nursing A guide for patients and carers Contents Why do blood clots form in veins?... 1 How common is a deep vein thrombosis (DVT) or pulmonary embolus (PE)?... 2 How are DVTs/
Venous Thromboembolism (VTE) Nursing A guide for patients and carers Contents Why do blood clots form in veins?... 1 How common is a deep vein thrombosis (DVT) or pulmonary embolus (PE)?... 2 How are DVTs/
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
requesting information regarding atrial fibrillation in NHS Ashford Clinical Commissioning Group
 October 2015 Our Ref: FOI.15.ASH0149 requesting information regarding atrial fibrillation in NHS Ashford Clinical Commissioning Group Original Request Survey attached. Question 1: Between 1 July 2014 and
October 2015 Our Ref: FOI.15.ASH0149 requesting information regarding atrial fibrillation in NHS Ashford Clinical Commissioning Group Original Request Survey attached. Question 1: Between 1 July 2014 and
Rivaroxaban film coated tablets are available in 2 strengths for this indication: 15mg and 20mg.
 Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
Primary Care Prescriber Information RIVAROXABAN (XARELTO ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Rivaroxaban is a non-vitamin K antagonist
Inhixa (Enoxaparin Sodium)
 Inhixa (Enoxaparin Sodium) P R E V ENTIS SAFETY D E V I C E P R E V E N T I S I S A N AU TO M AT I C N E E D L E S H I E L D I N G S Y S T E M, W H I C H H A S A C O V E R T H AT E X T E N D S O V E R
Inhixa (Enoxaparin Sodium) P R E V ENTIS SAFETY D E V I C E P R E V E N T I S I S A N AU TO M AT I C N E E D L E S H I E L D I N G S Y S T E M, W H I C H H A S A C O V E R T H AT E X T E N D S O V E R
Slide 1: Perioperative Management of Anticoagulation
 Perioperative Management of Anticoagulation by Steven L. Cohn, MD, FACP Director, Medical Consultation Service, Kings County Hospital Center, Clinical Professor of Medicine, SUNY Downstate, Brooklyn, NY
Perioperative Management of Anticoagulation by Steven L. Cohn, MD, FACP Director, Medical Consultation Service, Kings County Hospital Center, Clinical Professor of Medicine, SUNY Downstate, Brooklyn, NY
Venous thromboembolic diseases: diagnosis, management and thrombophilia testing (2012) NICE guideline CG144
 Venous thromboembolic diseases: diagnosis, management and thrombophilia testing (2012) NICE guideline CG144 Appendix A: Summary of new evidence from Summary of evidence from previous year Diagnosis Diagnostic
Venous thromboembolic diseases: diagnosis, management and thrombophilia testing (2012) NICE guideline CG144 Appendix A: Summary of new evidence from Summary of evidence from previous year Diagnosis Diagnostic
Challenges in Anticoagulation and Thromboembolism
 Challenges in Anticoagulation and Thromboembolism Ethan Cumbler M.D. Assistant Professor of Medicine Hospitalist Medicine Section University of Colorado Denver May 2010 No Conflicts of Interest Objectives
Challenges in Anticoagulation and Thromboembolism Ethan Cumbler M.D. Assistant Professor of Medicine Hospitalist Medicine Section University of Colorado Denver May 2010 No Conflicts of Interest Objectives
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
National Institute for Health and Clinical Excellence. Single Technology Appraisal (STA)
 National Institute for Health and Clinical Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial Response
National Institute for Health and Clinical Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial Response
Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism
 Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Edoxaban is a non-vitamin K antagonist oral
Primary Care Prescriber Information EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism INDICATION Edoxaban is a non-vitamin K antagonist oral
Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital Foundation Trust
 MANAGEMENT OF PATIENTS WITH DEEP VEIN THROMBOSIS (DVT) IN THE COMMUNITY SETTING & ANTICOAGULATION CLINICS THE PAST, PRESENT AND THE FUTURE Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital
MANAGEMENT OF PATIENTS WITH DEEP VEIN THROMBOSIS (DVT) IN THE COMMUNITY SETTING & ANTICOAGULATION CLINICS THE PAST, PRESENT AND THE FUTURE Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital
INTRODUCTION Indication and Licensing
 City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
NOTE: Deep Vein Thrombosis (DVT) Risk Factors
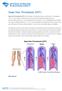 Deep Vein Thrombosis (DVT) Deep Vein Thrombosis (DVT) is the formation of a blood clot, known as a thrombus, in the deep leg vein. It is a very serious condition that can cause permanent damage to the
Deep Vein Thrombosis (DVT) Deep Vein Thrombosis (DVT) is the formation of a blood clot, known as a thrombus, in the deep leg vein. It is a very serious condition that can cause permanent damage to the
Technology appraisal guidance Published: 17 December 2014 nice.org.uk/guidance/ta327
 Dabigatran an etexilate for the treatment and secondary prevention ention of deep vein thrombosis and/or pulmonary embolism Technology appraisal guidance Published: 17 December 2014 nice.org.uk/guidance/ta327
Dabigatran an etexilate for the treatment and secondary prevention ention of deep vein thrombosis and/or pulmonary embolism Technology appraisal guidance Published: 17 December 2014 nice.org.uk/guidance/ta327
Drug Class Review Newer Oral Anticoagulant Drugs
 Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Bayer Pharma AG Berlin Germany Tel News Release. Not intended for U.S. and UK Media
 News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the
News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the
DRUG NAME: EDOXABAN (LIXIANA ) Transfer of Care document Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism
 City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism
City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: EDOXABAN (LIXIANA ) Treatment of acute venous thromboembolism and prevention of recurrent venous thromboembolism
Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients
 Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients What is a deep vein thrombosis (DVT)? A DVT is a blood clot that forms within a vein deep in the leg but can
Deep vein thrombosis (DVT) and pulmonary embolism (PE) advice for ophthalmic surgery patients What is a deep vein thrombosis (DVT)? A DVT is a blood clot that forms within a vein deep in the leg but can
Question 1: Between 1 July 2014 and 30 June 2015, in the area covered by your CCG:
 Atrial Fibrillation in Your Area Question 1: Between 1 July 2014 and 30 June 2015, in the area covered by your CCG: a) What was the prevalence of atrial fibrillation (AF)? 6636 (as of 22/10/2015) 2.1%
Atrial Fibrillation in Your Area Question 1: Between 1 July 2014 and 30 June 2015, in the area covered by your CCG: a) What was the prevalence of atrial fibrillation (AF)? 6636 (as of 22/10/2015) 2.1%
NEW/NOVEL ORAL ANTICOAGULANTS (NOACS): COMPARISON AND FREQUENTLY ASKED QUESTIONS
 NEW/NOVEL ORAL ANTICOAGULANTS (NOACS): COMPARISON AND FREQUENTLY ASKED QUESTIONS OBJECTIVES: To provide a comparison of the new/novel oral anticoagulants (NOACs) currently available in Canada. To address
NEW/NOVEL ORAL ANTICOAGULANTS (NOACS): COMPARISON AND FREQUENTLY ASKED QUESTIONS OBJECTIVES: To provide a comparison of the new/novel oral anticoagulants (NOACs) currently available in Canada. To address
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Guideline scope Stroke and transient ischaemic attack in over 16s: diagnosis and initial management (update)
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Stroke and transient ischaemic attack in over s: diagnosis and initial management (update) 0 0 This will update the NICE on stroke and
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Stroke and transient ischaemic attack in over s: diagnosis and initial management (update) 0 0 This will update the NICE on stroke and
Dr Shikha Chattree Haematology Consultant Sunderland Royal infirmary
 Dr Shikha Chattree Haematology Consultant Sunderland Royal infirmary Increasing use of Novel Oral Anticoagulants (NOACs) in the management of prophylaxis and management of venous thromboembolism and in
Dr Shikha Chattree Haematology Consultant Sunderland Royal infirmary Increasing use of Novel Oral Anticoagulants (NOACs) in the management of prophylaxis and management of venous thromboembolism and in
Patient Information. Preventing and treating blood clots
 Patient Information Preventing and treating blood clots 1_Clexane_Patient_Booklet_AW05.indd 1 8/04/2016 11:26 a The information provided in this document is for patients prescribed CLEXANE, and should
Patient Information Preventing and treating blood clots 1_Clexane_Patient_Booklet_AW05.indd 1 8/04/2016 11:26 a The information provided in this document is for patients prescribed CLEXANE, and should
2 Summary of NICE TA 249: Atrial fibrillation - Dabigatran Etexilate
 Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Service Notification in response to DHSSPS endorsed NICE Technology Appraisals NICE TA 249: Atrial fibrillation - Dabigatran Etexilate 1 Name of Commissioning Team Long Term Conditions Commissioning Team
Bayer AG Investor Relations Leverkusen Germany Investor News. Not intended for U.S. and UK Media
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer Initiates Xarelto (Rivaroxaban) Study in Patients with Non- Valvular Atrial
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer Initiates Xarelto (Rivaroxaban) Study in Patients with Non- Valvular Atrial
DOAC and NOAC are terms for a novel class of directly acting oral anticoagulant drugs including Rivaroxaban, Apixaban, Edoxaban, and Dabigatran.
 Guideline for Patients on Direct Oral Anticoagulant Therapy Requiring Urgent Surgery for Hip Fracture Trust Ref:C10/2017 1. Introduction This guideline is for the clinical management of patients on direct
Guideline for Patients on Direct Oral Anticoagulant Therapy Requiring Urgent Surgery for Hip Fracture Trust Ref:C10/2017 1. Introduction This guideline is for the clinical management of patients on direct
2017 Bryan Health Primary Care Conference. Dale Hansen MD Bryan Heart 5/20/17
 2017 Bryan Health Primary Care Conference Dale Hansen MD Bryan Heart 5/20/17 I have no financial disclosures or conflicts of interest Bridging Anticoagulation Primum Non Nocere 67 y.o. male with mechanical
2017 Bryan Health Primary Care Conference Dale Hansen MD Bryan Heart 5/20/17 I have no financial disclosures or conflicts of interest Bridging Anticoagulation Primum Non Nocere 67 y.o. male with mechanical
Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354)
 Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354) This document supports the
Rationale for Initiation, Continuation and Discontinuation (RICaD) Edoxaban Treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism (NICE TA354) This document supports the
2.5 Other Hematology Consult:
 The Warfarin Order Sheet has been approved by the P & T committee to be implemented by pharmacists. These orders are not used to treat patients with serious hemorrhagic complications. WARFARIN TARGET INR
The Warfarin Order Sheet has been approved by the P & T committee to be implemented by pharmacists. These orders are not used to treat patients with serious hemorrhagic complications. WARFARIN TARGET INR
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 11.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Appendix IV - Prescribing Guidance for Apixaban
 Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
Appendix IV - Prescribing Guidance for Apixaban Patient Factors Dose of Apixaban If your patient has any of the following MAJOR risk factors: Hypersensitivity to the active substance or to any of the excipients
Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the Secondary Prevention of Myo
 Xarelto (Rivaroxaban) Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the Secondary Prevention of Myocardial Infarction and Death in Patients with Coronary or Peripheral Artery
Xarelto (Rivaroxaban) Landmark Phase III Study of Bayer s Xarelto (Rivaroxaban) Initiated for the Secondary Prevention of Myocardial Infarction and Death in Patients with Coronary or Peripheral Artery
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised
 Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
How long to continue anticoagulation after DVT?
 How long to continue anticoagulation after DVT? Dr. Nihar Ranjan Pradhan M.S., DNB (Vascular Surgery), FVES(UK) Consultant Vascular Surgeon Apollo Hospital, Jubilee Hills, Hyderabad (Formerly Faculty in
How long to continue anticoagulation after DVT? Dr. Nihar Ranjan Pradhan M.S., DNB (Vascular Surgery), FVES(UK) Consultant Vascular Surgeon Apollo Hospital, Jubilee Hills, Hyderabad (Formerly Faculty in
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM
 PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
PREVENTION AND TREATMENT OF VENOUS THROMBOEMBOLISM International Consensus Statement 2013 Guidelines According to Scientific Evidence Developed under the auspices of the: Cardiovascular Disease Educational
A Brief Guide Treatment and Prevention
 A Brief Guide Treatment and Prevention Deep Vein Thrombosis and Pulmonary Embolism 08/18 Dear reader, This brochure provides you with information about deep vein thrombosis and pulmonary embolism. This
A Brief Guide Treatment and Prevention Deep Vein Thrombosis and Pulmonary Embolism 08/18 Dear reader, This brochure provides you with information about deep vein thrombosis and pulmonary embolism. This
Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta354
 Edoxaban for treating and for preventingenting deep vein thrombosis and pulmonary embolism Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta354 NICE 2017. All rights reserved.
Edoxaban for treating and for preventingenting deep vein thrombosis and pulmonary embolism Technology appraisal guidance Published: 26 August 2015 nice.org.uk/guidance/ta354 NICE 2017. All rights reserved.
Clinical Policy: Dalteparin (Fragmin) Reference Number: CP.PHAR.225
 Clinical Policy: (Fragmin) Reference Number: CP.PHAR.225 Effective Date: 05/16 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Fragmin) Reference Number: CP.PHAR.225 Effective Date: 05/16 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Diagnostic Technology: Point-of-Care International Normalised Ratio coagulometers for self-management of oral anticoagulation.
 Horizon Scan Report 0012 20 December 2010 Diagnostic Technology: Point-of-Care International Normalised Ratio coagulometers for self-management of oral anticoagulation. Clinical Question: In patients who
Horizon Scan Report 0012 20 December 2010 Diagnostic Technology: Point-of-Care International Normalised Ratio coagulometers for self-management of oral anticoagulation. Clinical Question: In patients who
Testing for factor V Leiden in patients with pulmonary or venous thromboembolism: a costeffectiveness
 Testing for factor V Leiden in patients with pulmonary or venous thromboembolism: a costeffectiveness analysis Eckman M H, Singh S K, Erban J K, Kao G Record Status This is a critical abstract of an economic
Testing for factor V Leiden in patients with pulmonary or venous thromboembolism: a costeffectiveness analysis Eckman M H, Singh S K, Erban J K, Kao G Record Status This is a critical abstract of an economic
Guideline scope Subarachnoid haemorrhage caused by a ruptured aneurysm: diagnosis and management
 0 0 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Subarachnoid haemorrhage caused by a ruptured aneurysm: diagnosis and management The Department of Health and Social Care in England
0 0 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Subarachnoid haemorrhage caused by a ruptured aneurysm: diagnosis and management The Department of Health and Social Care in England
CADTH Therapeutic Review
 Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Therapeutic Review August 2012 Volume 1, Issue 1A Antithrombotic Therapy for
Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Therapeutic Review August 2012 Volume 1, Issue 1A Antithrombotic Therapy for
Roche CoaguChek XS Plus INR
 Page 1 of 8 Roche CoaguChek XS Plus INR Edition No: 6.0 Author: Paul Simpson Operative Date: October 2016 Review Date: October 2017 Location: Country Health SA Integrated Cardiovascular Clinical Network
Page 1 of 8 Roche CoaguChek XS Plus INR Edition No: 6.0 Author: Paul Simpson Operative Date: October 2016 Review Date: October 2017 Location: Country Health SA Integrated Cardiovascular Clinical Network
Venous Thrombo-Embolism (VTE)
 Venous Thrombo-Embolism (VTE) Information for service users and carers RDaSH leading the way with care Older People s Mental Health Services Reducing risk of unwanted blood clots whilst in hospital About
Venous Thrombo-Embolism (VTE) Information for service users and carers RDaSH leading the way with care Older People s Mental Health Services Reducing risk of unwanted blood clots whilst in hospital About
Edoxaban Switch Programme - Frequently Asked Questions
 Edoxaban Switch Programme - Frequently Asked Questions What should I tell patients? NHS Tayside is reviewing all patients currently receiving a Direct Oral Anticoagulant (DOAC) for stroke prevention in
Edoxaban Switch Programme - Frequently Asked Questions What should I tell patients? NHS Tayside is reviewing all patients currently receiving a Direct Oral Anticoagulant (DOAC) for stroke prevention in
Appendix 3 PCC Warfarin Reversal
 Appendix 3 PCC Warfarin Reversal Reversal of Warfarin and Analogues 1. Principle of Procedure Guidelines for the Reversal of Oral-anticoagulation in the Event of Life Threatening Haemorrhage Prothrombin
Appendix 3 PCC Warfarin Reversal Reversal of Warfarin and Analogues 1. Principle of Procedure Guidelines for the Reversal of Oral-anticoagulation in the Event of Life Threatening Haemorrhage Prothrombin
Misunderstandings of Venous thromboembolism prophylaxis
 Misunderstandings of Venous thromboembolism prophylaxis Veerendra Chadachan Senior Consultant Dept of General Medicine (Vascular Medicine and Hypertension) Tan Tock Seng Hospital, Singapore Case scenario
Misunderstandings of Venous thromboembolism prophylaxis Veerendra Chadachan Senior Consultant Dept of General Medicine (Vascular Medicine and Hypertension) Tan Tock Seng Hospital, Singapore Case scenario
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Briefing paper QOF indicator area: Primary prevention of CVD Potential output:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Briefing paper QOF indicator area: Primary prevention of CVD Potential output:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Μακροχρόνια παρακολούθηση ασθενών με πνευμονική εμβολή
 Μακροχρόνια παρακολούθηση ασθενών με πνευμονική εμβολή Ευφροσύνη Δ. Μάναλη Λέκτορας Β Πανεπιστημιακή Πνευμονολογική Κλινική ΓΝΑ «Αττικόν» Εθνικό και Καποδιστριακό Πανεπιστήμιο Αθηνών Existing guidelines
Μακροχρόνια παρακολούθηση ασθενών με πνευμονική εμβολή Ευφροσύνη Δ. Μάναλη Λέκτορας Β Πανεπιστημιακή Πνευμονολογική Κλινική ΓΝΑ «Αττικόν» Εθνικό και Καποδιστριακό Πανεπιστήμιο Αθηνών Existing guidelines
Atrial fibrillation. Understanding NICE guidance
 Understanding NICE guidance Information for people who use NHS services Atrial fibrillation NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases and the treatments
Understanding NICE guidance Information for people who use NHS services Atrial fibrillation NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases and the treatments
To prevent blood clots after hip or knee replacement surgery This booklet contains information for those who have been prescribed ELIQUIS (apixaban)
 To prevent blood clots after hip or knee replacement surgery This booklet contains information for those who have been prescribed ELIQUIS (apixaban) after hip or knee replacement surgery Always read the
To prevent blood clots after hip or knee replacement surgery This booklet contains information for those who have been prescribed ELIQUIS (apixaban) after hip or knee replacement surgery Always read the
Confirmed blood clot
 n The Leeds Teaching Hospitals NHS Trust Confirmed blood clot (Deep vein thrombosis and pulmonary embolism) Information for patients Please read this leaflet carefully. It will give you information about
n The Leeds Teaching Hospitals NHS Trust Confirmed blood clot (Deep vein thrombosis and pulmonary embolism) Information for patients Please read this leaflet carefully. It will give you information about
Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180)
 Putting NICE guidance into practice Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180) Published: June 2014 This costing report accompanies the clinical
Putting NICE guidance into practice Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180) Published: June 2014 This costing report accompanies the clinical
National Institute for Health and Clinical Excellence Health Technology Appraisal
 National Institute for Health and Clinical Excellence Health Technology Appraisal Rivaroxaban for the prevention of venous thromboembolism after elective orthopaedic surgery of the lower limbs Comment
National Institute for Health and Clinical Excellence Health Technology Appraisal Rivaroxaban for the prevention of venous thromboembolism after elective orthopaedic surgery of the lower limbs Comment
Guideline scope Hypertension in adults (update)
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Hypertension in adults (update) This guideline will update the NICE guideline on hypertension in adults (CG127). The guideline will be
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Guideline scope Hypertension in adults (update) This guideline will update the NICE guideline on hypertension in adults (CG127). The guideline will be
Alison Tennant MRPharmS MPH Head of Service Improvement and Quality Dudley CCG
 Alison Tennant MRPharmS MPH Head of Service Improvement and Quality Dudley CCG Commissioning is the set of linked activities required to assess the healthcare needs of a population, specify the services
Alison Tennant MRPharmS MPH Head of Service Improvement and Quality Dudley CCG Commissioning is the set of linked activities required to assess the healthcare needs of a population, specify the services
