PHARMACEUTICAL FORM AND AMOUNT OF
|
|
|
- Delphia Ray
- 5 years ago
- Views:
Transcription
1 10-14 Rapilysin Actavis Reteplase COMPOSITION Vial containing dried substance Active ingredient: reteplase (rnn) Excipients: sucrose, phosphoric acid, dipotassium hydrogen phosphate, tranexamic acid, polysorbate 80. Prefilled syringe containing solvent 10 ml water for injections. PHARMACEUTICAL FORM AND AMOUNT OF ACTIVE INGREDIENT PER UNIT Dried substance and solvent for the preparation of a solution for injection. Vial containing 10 U. The strength of reteplase is expressed in units (U) based on a reference standard specific to reteplase which are not comparable with the units used for other thrombolytic agents. INDICATIONS AND POTENTIAL USES Thrombolytic treatment in acute myocardial infarction (within 12 h following the onset of symptoms). DOSAGE AND ADMINISTRATION Normal dosage The reteplase therapy should be administered as soon as possible after the onset of the symptoms of acute myocardial infarction. Reteplase is administered as two bolus injections (10 U + 10 U). Each bolus is administered as a slow intravenous injection (within 2 min). The second bolus injection is administered 30 min after the first. The boluses are injected via an intravenous access. Inadvertent paravenous injection must be avoided. The reteplase supplied in the vial is in a dried form. The lyophilizate is dissolved using the contents of the accompanying prefilled syringe. The reconstituted solution must be used immediately. After reconstitution, the solution must be visually checked. The solution should only be used if it is clear and colourless. If the solution is not clear and colourless, it must be discarded. Concomitant treatment with heparin and acetylsalicylic acid: Concomitant treatment with heparin and acetylsalicylic acid should be carried out before and after reteplase therapy, in order to avoid the risk of rethrombosis. The recommended dose of heparin is 5000 IU as a bolus injection, followed by an infusion of 1000 IU per h after the second reteplase bolus. Heparin should be administered for at least 24 h, the target aptt values being around 1.5 to 2 times the upper limit of the normal range. Acetylsalicylic acid should be administered from the start of the thrombolytic therapy and be continued at least until the patient s discharge from hospital. The initial dosage is mg; the dosage is then reduced to mg per day (see Interactions ). Heparin and Rapilysin are incompatible if combined in solution. Other incompatibilities may also exist. No other medication should be added to the injection solution (see below and the Incompatibilities section). Rapilysin should preferably be injected via an intravenous access reserved for this medicinal product. No other products should be injected via the access reserved for Rapilysin, before, during, or after the injection of Rapilysin. This applies to all products, including heparin and acetylsalicylic acid. In patients who have only one intravenous access for all medicines, this access (including the Y-connector) must be flushed thoroughly with 0.9% saline or 5% glucose solution before and after injection of Rapilysin. Special dosage instructions Patients with hepatic or renal impairment: There are no empirical clinical data available on the use of reteplase in patients with severe disturbances of liver or kidney function. ACTAVIS-RAPILYSIN - p.1/6
2 ACTAVIS-RAPILYSIN - p.2/6 Children: There are no empirical data available on the use of reteplase in children. Readministration There are no empirical data available concerning readministration of reteplase. No anaphylactic reactions have so far been observed during treatment with reteplase. This accords with the observation that reteplase does not induce antibody formation. If an anaphylactoid reaction occurs, the injection should be stopped immediately and appropriate therapy initiated. CONTRAINDICATIONS Hypersensitivity to any of the constituents. Since thrombolysis therapy increases the risk of bleeding, reteplase must not be used in the following circumstances: - within 3 months of severe bleeding, severe trauma, or major surgery, e.g. coronary bypass, obstetric delivery, organ biopsy, and previous puncture of non-compressible vessels - history of cerebrovascular events - recent intracranial or intraspinal surgery or trauma (within the last 3 months) - intracranial neoplasia, ateriovenous malformations or aneurysms - known hemorrhagic diathesis - severe, uncontrolled hypertension - active peptic ulcers - portal hypertension (esophageal varices) - other known contraindications to fibrinolytic therapy, e.g. acute pancreatitis, acute pericarditis, bacterial endocarditis, hemorrhagic retinopathy, e.g. in diabetes mellitus, or patients simultaneously receiving oral anticoagulants, e.g. phenprocoumon. WARNINGS AND PRECAUTIONS Bleeding The most common complication encountered during reteplase therapy is bleeding. Concomitant heparin therapy can increase the risk of bleeding. Because of the fibrinolytic effects of reteplase, bleeding from puncture sites can occur. Therefore, in patients receiving thrombolytic therapy, it is important to keep an eye on possible bleeding sites (e.g. catheters, arterial or venous puncture sites, other cutdown and puncture sites). The use of rigid catheters should be avoided for 3 days in patients receiving reteplase therapy, as should intramuscular injections and non-essential procedures. The risk of bleeding is increased for up to 3 days. In the event of severe bleeding (which cannot be controlled by the application of local pressure) any concomitant heparin therapy must be discontinued immediately. Furthermore, if the first bolus injection of reteplase is followed by severe bleeding, the second bolus injection should not be administered. (For emergency measures, see the Overdosage section). Any patient being considered for reteplase therapy should be carefully investigated. In the following cases reteplase therapy can carry increased risks, so a careful evaluation of the risks and benefits is required: - cerebrovascular diseases - recent gastrointestinal or urogenital bleeding (within the last 10 days) - intensive (traumatic) cardiac massage - hypertension: systolic BP 180 mm Hg and/or diastolic BP 110 mm Hg - high probability of a left-heart thrombus, e.g. in mitral stenosis with atrial fibrillation - hemostatic disturbances due to severe liver or kidney disease - neoplasia with increased risk of bleeding - pregnancy - septic thrombophlebitis or occluded, infected arteriovenous fistula - advanced age (over 75 years) - any other circumstances in which bleeding represents a serious danger or in which the source of bleeding is difficult to reach. Arrhythmias Coronary thrombolysis may result in arrhythmias associated with reperfusion. These reperfusion arrhythmias do not differ from those that can occur in the course of acute myocardial infarction. They can be controlled with the usual antiarrhythmic measures. Antiarrhythmic intervention facilities should therefore be available when reteplase is administered.
3 ACTAVIS-RAPILYSIN - p.3/6 Seizures As with other thrombolytic agents, there have been isolated reports of convulsions. Ischemic or hemorrhagic cerebrovascular events may be contributory or underlying factors. INTERACTIONS There have been no systematic studies of the interactions of reteplase and other drugs commonly administered to patients with myocardial infarction. Retrospective analyses of clinical studies in which reteplase was administered alongside other medicines did not find any clinically relevant interactions in patients with acute myocardial infarction. Heparin, vitamin K antagonists, medicines that interfere with platelet function (e.g. acetylsalicylic acid, dipyridamole), and combinations of heparin and acetylsalicylic acid can increase the risk of bleeding if used before, during, or after reteplase therapy. PREGNANCY AND LACTATION There are no empirical data available on the use of reteplase during human pregnancy. Reteplase should not be used during pregnancy unless it is strictly necessary. In animal studies, dose-dependent apathy and bleeding occurred after higher doses in the parent animals, and bleeding of the genital tract resulted in abortions in pregnant rabbits given higher doses. UNDESIRABLE EFFECTS Frequencies: Very common (>1/10), common (>1/100, <1/10), uncommon (>1/1000, <1/100), rare (>1/10,000, <1/1000), very rare (<1/10,000). Blood and lymph system Very common: bleeding, particularly from puncture sites [7.9% (0.9%)*]. Uncommon: gastrointestinal [2.9% (0.4%)*], urogenital [2.1% (0.1%)*], or gingival bleeding [1.8%*], retroperitoneal bleeding [0.2% (0.2%)*] or epistaxis [1.0%*], cerebral hemorrhage / hemorrhagic stroke [0.76%*]. Very rare: ocular bleeding and ecchymosis. * These frequencies (crude rates) were observed in 3288 patients treated with 10 U + 10 U reteplase. The figures in parentheses () represent the frequency of bleeding necessitating transfusion. Nervous system Very rare: convulsions, aphasia, speech disturbances, delirium, acute brain syndrome, agitation, confusion, depression, psychosis. Ischemic or hemorrhagic cerebrovascular events may be contributory or underlying factors. Cardiovascular system As with other thrombolytic agents, the following undesirable effects have been reported as sequelae of myocardial infarction and/or thrombolytic therapy: Common: recurrent ischemia / angina pectoris, hypotension and heart failure / pulmonary edema, Uncommon: disturbances of cardiac rhythm (e.g. AV block, atrial fibrillation /? utter, ventricular tachycardia / fibrillation, electromechanical dissociation (EMD)), cardiac arrest, cardiogenic shock, and reinfarction, Rare: mitral insufficiency, pulmonary embolism, other systemic embolisms / cerebral embolisms and ventricular septal defect. These cardiovascular side effects can be life threatening and prove fatal. General disturbances, and reactions at the administration site Rare: hypersensitivity reactions (e.g. allergic reactions). Very rare: serious anaphylactic / anaphylactoid reactions. In the case of reteplase there is no evidence that the hypersensitivity reactions are antibody-mediated. They are much more likely to be due to the fact that plasminogen activators can trigger non-specific activation of bradykinin and the complement cascade. OVERDOSAGE After an overdosage there is likely to be a drop in fibrinogen levels and an increase in the consumption of other hemostasiological parameters (e.g. coagulation factor V) and, subsequently, an increased risk of hemorrhage. Any concomitant heparin therapy should be stopped. In the event of substantial blood loss, it is recommended that fresh plasma or fresh blood be administered. Anti fibrinolytics (e.g. tranexamic acid, aprotinin) may be administered if required.
4 ACTAVIS-RAPILYSIN - p.4/6 PROPERTIES/EFFECTS ATC code: B01AD07 Action mechanism/pharmacodynamics Reteplase is a recombinant plasminogen activator which catalyses the formation of plasmin through cleavage of endogenous plasminogen. This plasminogenolysis occurs chiefly through interaction with fibrin, which is mediated via the kringle 2 domain of reteplase. Plasmin in turn breaks down the fibrin network of the thrombus, thereby exerting its thrombolytic action. Reteplase (10 U + 10 U) dose-dependently lowers plasma fibrinogen levels by about 60 to 80%. The fibrinogen levels return to normal within 2 days. As with other plasminogen activators, this is followed by a rebound phenomenon, during which fibrinogen levels reach a maximum within 9 days and remain elevated for up to 18 days. Decreased plasma levels of hemostasiological parameters (e.g. plasminogen, α2-antiplasmin, α2-macroglobulin) normalize within 1 to 3 days. Co-administration of acetylsalicylic acid and reteplase can lead to inhibition of platelet aggregation. This effect should therefore be taken into account if plasma fibrinogen levels are low. In vitro studies of human-plasma clots showed concentration-dependent lysis of the clots with reteplase. Animal studies found a dose-dependent thrombolytic effect. Clinical studies in patients with acute myocardial infarction showed earlier patency and higher patency rates after reteplase than after other registered thrombolytics. A large phase III comparative study showed that reteplase lowers the incidence of heart failure and acute myocardial infarction-associated mortality (primary end point). There is no evidence that reteplase has an antigenic effect. PHARMACOKINETICS Distribution After intravenous bolus injection of 10 U + 10 U in patients with acute myocardial infarction, reteplase antigen is distributed in plasma with a dominant half-life (t½) of 19 min and eliminated with a terminal half-life (t½) of 5.5 h. The plasma clearance rate of reteplase antigen is 121 ml/min; the volume of distribution (VD) is 35 l. Metabolism Only small amounts of reteplase were immunologically detectable in the urine. Experiments in rats indicate that active uptake and lysosomal degradation take place mainly in the liver and the kidneys. Additional in vitro studies performed using a humanplasma medium suggest that inhibition due to complexation contributes to the inactivation of reteplase in plasma. The clearance rate for the functionally active reteplase molecule is 283 ml/min, which thus exceeds the clearance rate for reteplase antigen. Elimination At therapeutic doses the effective half-life of reteplase activity is min. It is not known whether reteplase is excreted in breast milk. Pharmacokinetics in special patient populations Pharmacokinetic behaviour in cases of hepatic and renal impairment has not yet been investigated. PRECLINICAL DATA Acute toxicity studies were carried out in rats, rabbits, and monkeys. Subacute toxicity studies were performed in rats, dogs, and monkeys. The main acute symptom after administration of single high doses of reteplase to rats and rabbits was transient apathy immediately after the injection. In cynomolgus monkeys, the sedative effect ranged from slight apathy to unconsciousness, caused by a reversible dose-dependent drop in blood pressure. There was increased local hemorrhage at the injection site. Subacute toxicity studies did not reveal any unexpected side effects. In dogs, repeated administration of the human peptide reteplase led to immunological allergic reactions. Genotoxicity of reteplase was excluded by a comprehensive battery of tests with various genetic end points, both in vitro and in vivo. Reproductive toxicity studies were performed in rats (fertility and embryofetotoxicity studies including a
5 ACTAVIS-RAPILYSIN - p.5/6 littering phase) and in rabbits (embryofetotoxicity study, dose-range finding only). In rats, a species which is insensitive to the pharmacological effects of reteplase, there were no detectable adverse effects on fertility, embryofetal development, or the newborn offspring. In rabbits vaginal bleeding and abortions possibly connected with prolonged hemostasis were observed, but no fetal abnormalities. No preand postnatal toxicity studies of reteplase were performed. SPECIAL REMARKS Heparin and Rapilysin are incompatible if combined in solution. Other incompatibilities may also exist. No other medication should be added to the injection solution. No other products should be injected via the access reserved for Rapilysin, before, during, or after the injection of Rapilysin. This applies to all products, including heparin and acetylsalicylic acid, which should be injected before and after administration of reteplase, to avoid the risk of rethrombosis. In patients who have only one intravenous access for all medicines, this access (including the Y-connector) must be flushed thoroughly with 0.9% saline or 5% glucose solution before and after injection of Rapilysin. Shelf life The product must be used before the date indicated by EXP on the pack. Special precautions for storage Vial containing dried substance: Do not store above 30 C. Keep the container in the original pack in order to protect the contents from light. Instructions for use and handling a) Correctly prepared, the solution is physically and chemically stable for 8 h at 2-8 C. For microbiological reasons, the reconstituted product must be used immediately. b) Special instructions regarding the mode of use 1. Work under aseptic conditions. 2. Remove the plastic cap from the reteplase vial and disinfect the rubber closure with an alcohol wipe. 3. Take the reconstitution device out of the pack and remove the protective cap from the Luer attachment of the reconstitution device. 4. Take the 10 ml prefilled syringe with the Luer tip out of the pack. Remove the protective cap from the syringe. 5. Remove the protective cap from the spike of the reconstitution device. Insert the spike through the rubber closure into the vial of reteplase. Connect the syringe to the Luer attachment of the reconstitution device and inject the solvent (10 ml) into the reteplase vial. 6. With the spike of the reconstitution device (and the syringe) still attached to the vial, swirl the vial gently until the reteplase powder has completely dissolved. Do not shake. The prepared solution should be clear and colourless. 7. The reconstituted product is a clear, colourless solution. If the solution is not clear and colourless, it must be discarded. 8. Draw 10 ml of the reteplase solution into the syringe. The quantity left over in the vial is due to overage. Remove the syringe from the reconstitution device. The solution can now be administered. 9. Heparin and Rapilysin are incompatible if combined in solution. Other incompatibilities may also exist. No other medication should be added to the injection solution. PACKS 1 pack contains: 2 vials with powder for solution for injection 2 syringes with solvent 2 reconstitution devices and 2 needles 19 G 1 This is a medicament A medicament is a product which affects your health, and its consumption contrary to instructions is dangerous for you. Follow strictly the doctor s prescription, the method of use and the instructions of the pharmacist who sold the medicament. The doctor and the pharmacist are experts in medicine, its benefits and risks. Do not by yourself interrupt the period of treatment prescribed for you.
6 ACTAVIS-RAPILYSIN - p.6/6 Do not repeat the same prescription without consulting your doctor. Medicine: keep out of reach of children Latest revision date: July Marketing Authorization Holder: Actavis hf, Reykjavikurvegur 76-78, 220 Hafnarordur, Iceland Manufactured by: Roche Diagnostics GmbH, Mannheim, Germany Packaged and Released by: CENEXI, Fontenay-sous-bois, France.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Tenecteplase Boehringer Ingelheim Pharma KG 6,000 units Powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Tenecteplase Boehringer Ingelheim Pharma KG 6,000 units Powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE
RETAVASE (reteplase) for injection, for intravenous use Initial U.S. Approval: 1996
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RETAVASE safely and effectively. See full prescribing information for RETAVASE. RETAVASE (reteplase)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RETAVASE safely and effectively. See full prescribing information for RETAVASE. RETAVASE (reteplase)
NURSING DEPARTMENT CRITICAL CARE POLICY MANUAL CRITICAL CARE PROTOCOLS. ACTIVASE (t-pa) INFUSION PROTOCOL FOR ACUTE MYOCARDIAL INFARCTION
 NURSING DEPARTMENT CRITICAL CARE POLICY MANUAL CRITICAL CARE PROTOCOLS ACTIVASE (t-pa) FOR ACUTE MYOCARDIAL INFARCTION I. PURPOSE: A. To reduce the extent of myocardial infarction by lysing the clot in
NURSING DEPARTMENT CRITICAL CARE POLICY MANUAL CRITICAL CARE PROTOCOLS ACTIVASE (t-pa) FOR ACUTE MYOCARDIAL INFARCTION I. PURPOSE: A. To reduce the extent of myocardial infarction by lysing the clot in
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Metalyse 8,000 units powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Metalyse 8,000 units powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
CLINICAL GUIDELINES ID TAG
 Title: Author: Speciality/ Division: Directorate: Date Uploaded: Review Date: September 2019 CG ID TAG CG0423 CLINICAL GUIDELINES ID TAG Clinical Guideline for Alteplase in intra-arterial thrombolysis
Title: Author: Speciality/ Division: Directorate: Date Uploaded: Review Date: September 2019 CG ID TAG CG0423 CLINICAL GUIDELINES ID TAG Clinical Guideline for Alteplase in intra-arterial thrombolysis
P-RMS: LT/H/PSUR/0004/001
 Core Safety Profile Active substance: Dalteparine Pharmaceutical form(s)/strength: Solution for injection, 2500 I.U./0.2ml, 2500 I.U./ml, 5000 I.U./0.2ml, 7500 I.U./0.3ml, 7500 I.U./0.75ml, 10000 I.U./0.4ml,
Core Safety Profile Active substance: Dalteparine Pharmaceutical form(s)/strength: Solution for injection, 2500 I.U./0.2ml, 2500 I.U./ml, 5000 I.U./0.2ml, 7500 I.U./0.3ml, 7500 I.U./0.75ml, 10000 I.U./0.4ml,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Actilyse Powder and solvent for solution for injection and infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 vial with powder
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Actilyse Powder and solvent for solution for injection and infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 vial with powder
PACKAGE LEAFLET: INFORMATION FOR THE USER. Actilyse Cathflo 2 mg, poeder voor oplossing voor injectie en infusie. Alteplase
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Actilyse Cathflo 2 mg, poeder voor oplossing voor injectie en infusie Alteplase Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Actilyse Cathflo 2 mg, poeder voor oplossing voor injectie en infusie Alteplase Read all of this leaflet carefully before you start using this
ASSESS OPPORTUNITIES TO IDENTIFY MISSED ELIGIBLE PATIENTS KEY QUESTIONS TO CONSIDER What are the top reasons for non-treatment? Are patients excluded
 Identifying Missed Eligible AIS Patients Indication Activase (alteplase) is indicated for the treatment of acute ischemic stroke (AIS). Exclude intracranial hemorrhage as the primary cause of stroke signs
Identifying Missed Eligible AIS Patients Indication Activase (alteplase) is indicated for the treatment of acute ischemic stroke (AIS). Exclude intracranial hemorrhage as the primary cause of stroke signs
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
Package leaflet: Information for the patient. Trasylol 10,000 KIU/ml solution for injection or infusion. Aprotinin
 Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklonova 500 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Tranexamic acid 500 mg. For the full list of excipients,
METALYSE. (Tenecteplase)
 METALYSE (Tenecteplase) NAME OF THE MEDICINE METALYSE (tenecteplase) CAS Registry Number 191588-94-0 DESCRIPTION Tenecteplase is a tissue plasminogen activator (t-pa) produced by recombinant DNA technology,
METALYSE (Tenecteplase) NAME OF THE MEDICINE METALYSE (tenecteplase) CAS Registry Number 191588-94-0 DESCRIPTION Tenecteplase is a tissue plasminogen activator (t-pa) produced by recombinant DNA technology,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT 500 IU powder and solvent for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION is presented as a powder and solvent
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT 500 IU powder and solvent for solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION is presented as a powder and solvent
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN PROTHROMBIN COMPLEX PRODUCTS (CPMP/BPWG/3735/02)
 European Medicines Agency Human Medicines Evaluation Unit London, 21 October 2004 Corrigendum, 18 November 2004 CPMP/BPWG/3735/02 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN
European Medicines Agency Human Medicines Evaluation Unit London, 21 October 2004 Corrigendum, 18 November 2004 CPMP/BPWG/3735/02 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CORE SPC FOR HUMAN
Thrombolysis administration
 Thrombolysis administration Liz Mackey Stroke Nurse Practitioner Western Health Sunshine & Footscray Hospital, Melbourne Thanks ASNEN committee members Skye Coote, Acute Stroke Nurse, Eastern Health (slide
Thrombolysis administration Liz Mackey Stroke Nurse Practitioner Western Health Sunshine & Footscray Hospital, Melbourne Thanks ASNEN committee members Skye Coote, Acute Stroke Nurse, Eastern Health (slide
ADVERSE REACTIONS The most frequently occurring adverse reaction ( > 5%) is bleeding.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ACTIVASE safely and effectively. See full prescribing information for ACTIVASE. Activase (alteplase)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ACTIVASE safely and effectively. See full prescribing information for ACTIVASE. Activase (alteplase)
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Rapilysin 10 U powder and solvent for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 vial contains 10
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Rapilysin 10 U powder and solvent for solution for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 vial contains 10
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Medsamic 100 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: tranexamic acid 100 mg. For the
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Medsamic 100 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: tranexamic acid 100 mg. For the
Considering Activase (alteplase) for treatment of acute ischemic stroke
 Considering Activase (alteplase) for treatment of acute ischemic stroke A DISCUSSION GUIDE FOR PATIENTS AND CAREGIVERS Indication Activase (alteplase) is indicated for treating patients with acute ischemic
Considering Activase (alteplase) for treatment of acute ischemic stroke A DISCUSSION GUIDE FOR PATIENTS AND CAREGIVERS Indication Activase (alteplase) is indicated for treating patients with acute ischemic
POM. SUMMARY OF PRODUCT CHARACTERISTICS UK Specific
 (500 IU coagulation (human factor IX prothrombin per vial, powder complex) and solvent for infusion Human Prothrombin Complex) POM SUMMARY OF PRODUCT CHARACTERISTICS UK Specific Octapharma Limited The
(500 IU coagulation (human factor IX prothrombin per vial, powder complex) and solvent for infusion Human Prothrombin Complex) POM SUMMARY OF PRODUCT CHARACTERISTICS UK Specific Octapharma Limited The
Rasburicase 1.5 mg/ml powder and solvent for concentrate for solution for infusion. FASTURTEC
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
Inhixa (Enoxaparin Sodium)
 Inhixa (Enoxaparin Sodium) P R E V ENTIS SAFETY D E V I C E P R E V E N T I S I S A N AU TO M AT I C N E E D L E S H I E L D I N G S Y S T E M, W H I C H H A S A C O V E R T H AT E X T E N D S O V E R
Inhixa (Enoxaparin Sodium) P R E V ENTIS SAFETY D E V I C E P R E V E N T I S I S A N AU TO M AT I C N E E D L E S H I E L D I N G S Y S T E M, W H I C H H A S A C O V E R T H AT E X T E N D S O V E R
SUMMARY OF PRODUCT CHARACTERISTICS, LABELLING AND PACKAGE LEAFLET
 SUMMARY OF PRODUCT CHARACTERISTICS, LABELLING AND PACKAGE LEAFLET Version: February, 2019 1 SUMMARY OF PRODUCT CHARACTERISTICS Version: February, 2019 2 1. NAME OF THE MEDICINAL PRODUCT Actilyse 10 mg
SUMMARY OF PRODUCT CHARACTERISTICS, LABELLING AND PACKAGE LEAFLET Version: February, 2019 1 SUMMARY OF PRODUCT CHARACTERISTICS Version: February, 2019 2 1. NAME OF THE MEDICINAL PRODUCT Actilyse 10 mg
Activase (alteplase) for injection, for intravenous use Initial U.S. Approval: RECENT MAJOR CHANGES
 Guidelines for Achieving Door-to-Needle in 60 Minutes or Less Indication Activase (alteplase) is indicated for the treatment of acute ischemic stroke (AIS). Exclude intracranial hemorrhage as the primary
Guidelines for Achieving Door-to-Needle in 60 Minutes or Less Indication Activase (alteplase) is indicated for the treatment of acute ischemic stroke (AIS). Exclude intracranial hemorrhage as the primary
Other Components Protein C Protein S
 1. NAME OF THE MEDICINAL PRODUCT Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500 IU factor
1. NAME OF THE MEDICINAL PRODUCT Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500 IU factor
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
ANNEX III LABELLING AND PACKAGE LEAFLET
 ANNEX III LABELLING AND PACKAGE LEAFLET 1 B. PACKAGE LEAFLET 2 PACKAGE LEAFLET Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
ANNEX III LABELLING AND PACKAGE LEAFLET 1 B. PACKAGE LEAFLET 2 PACKAGE LEAFLET Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS ----------------------------------------------------------------------------------------------------------------- 1. NAME OF THE MEDICINAL PRODUCT Legalon SIL 528.5 mg
SUMMARY OF PRODUCT CHARACTERISTICS ----------------------------------------------------------------------------------------------------------------- 1. NAME OF THE MEDICINAL PRODUCT Legalon SIL 528.5 mg
Annex III. Summary of Product Characteristics and Package Leaflet
 Annex III Summary of Product Characteristics and Package Leaflet 11 SUMMARY OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLET 12 SUMMARY OF PRODUCT CHARACTERISTICS 13 1. NAME OF THE MEDICINAL PRODUCT Tranexamic
Annex III Summary of Product Characteristics and Package Leaflet 11 SUMMARY OF PRODUCT CHARACTERISTICS AND PACKAGE LEAFLET 12 SUMMARY OF PRODUCT CHARACTERISTICS 13 1. NAME OF THE MEDICINAL PRODUCT Tranexamic
DATA SHEET. Product Summary. 1. Trade Name of Medicinal Product. Protamine Sulphate Injection BP. 2. Qualitative and Quantitative Composition
 DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
Aminosteril N-Hepa 8%, solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 Title Page Information Aminosteril N-Hepa 8% November 2001 1 (7) 1. NAME OF THE MEDICINAL PRODUCT Aminosteril N-Hepa 8%, solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000 ml solution
Title Page Information Aminosteril N-Hepa 8% November 2001 1 (7) 1. NAME OF THE MEDICINAL PRODUCT Aminosteril N-Hepa 8%, solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000 ml solution
Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion.
 1. NAME OF THE MEDICINAL PRODUCT Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Protamine sulphate 1400 anti-heparin
1. NAME OF THE MEDICINAL PRODUCT Protamine sulphate LEO Pharma 1400 anti-heparin IU/ml solution for injection and infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Protamine sulphate 1400 anti-heparin
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
Management of Acute Myocardial Infarction
 Management of Acute Myocardial Infarction Prof. Hossam Kandil Professor of Cardiology Cairo University ST Elevation Acute Myocardial Infarction Aims Of Management Emergency care (Pre-hospital) Early care
Management of Acute Myocardial Infarction Prof. Hossam Kandil Professor of Cardiology Cairo University ST Elevation Acute Myocardial Infarction Aims Of Management Emergency care (Pre-hospital) Early care
BECLOT 500MG. INJECTION Composition : Each ml. contains : Tranexamic Acid I.P. 100mg.
 BECLOT 500MG. INJECTION Composition : Each ml. contains : Tranexamic Acid I.P. 100mg. DESCRIPTION Tranexamic acid is a synthetic analog of the amino acid lysine. It is used to treat or prevent excessive
BECLOT 500MG. INJECTION Composition : Each ml. contains : Tranexamic Acid I.P. 100mg. DESCRIPTION Tranexamic acid is a synthetic analog of the amino acid lysine. It is used to treat or prevent excessive
Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion.
 1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
PART III: CONSUMER INFORMATION
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION NiaStase RT (eptacog alfa, activated) Activated Recombinant Human Blood Coagulation Factor VII Room Temperature Stable This leaflet is Part III of
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION NiaStase RT (eptacog alfa, activated) Activated Recombinant Human Blood Coagulation Factor VII Room Temperature Stable This leaflet is Part III of
Administering Intravenous Alteplase (Tissue Plasminogen Activator [tpa])
![Administering Intravenous Alteplase (Tissue Plasminogen Activator [tpa]) Administering Intravenous Alteplase (Tissue Plasminogen Activator [tpa])](/thumbs/74/71168291.jpg) Administering Intravenous Alteplase (Tissue Plasminogen Activator [tpa]) Step 1: Eligibility---The eligibility criteria for patients with acute ischemic stroke within 3 hours of symptom onset include:
Administering Intravenous Alteplase (Tissue Plasminogen Activator [tpa]) Step 1: Eligibility---The eligibility criteria for patients with acute ischemic stroke within 3 hours of symptom onset include:
CLINICAL PHARMACOLOGY
 TNKase (Tenecteplase) DESCRIPTION TNKase (Tenecteplase) is a tissue plasminogen activator (tpa) produced by recombinant DNA technology using an established mammalian cell line (Chinese Hamster Ovary cells).
TNKase (Tenecteplase) DESCRIPTION TNKase (Tenecteplase) is a tissue plasminogen activator (tpa) produced by recombinant DNA technology using an established mammalian cell line (Chinese Hamster Ovary cells).
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fibrogammin 250 IU Powder and solvent for solution for injection or infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fibrogammin 250 IU Powder and solvent for solution for injection or infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient:
Cofact can be used for: The treatment of haemorrhages or the prevention of peri-operative haemorrhages as the result of
 Package leaflet: Information for the user Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500
Package leaflet: Information for the user Cofact 250 IU, human prothrombin complex, 250 IU factor IX per vial, powder and solvent for solution for injection. Cofact 500 IU, human prothrombin complex, 500
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS. Each ml solution for injection contains phenylephrine hydrochloride corresponding to 0.1 mg phenylephrine.
 1. NAME OF THE MEDICINAL PRODUCT Fenylefrin Abcur 0.05 mg/ml, solution for injection Fenylefrin Abcur 0.1 mg/ml, solution for injection SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE
1. NAME OF THE MEDICINAL PRODUCT Fenylefrin Abcur 0.05 mg/ml, solution for injection Fenylefrin Abcur 0.1 mg/ml, solution for injection SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE
Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein of which at least 95% is human albumin.
 1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
* Sections or subsections omitted from the Full Prescribing Information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ANDEXXA safely and effectively. See Full Prescribing Information for ANDEXXA. ANDEXXA (coagulation
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ANDEXXA safely and effectively. See Full Prescribing Information for ANDEXXA. ANDEXXA (coagulation
PATIENT / USER INFORMATION LEAFLET. Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human)
 PATIENT / USER INFORMATION LEAFLET Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human) Read all of this leaflet carefully before you start taking this medicine. Keep this
PATIENT / USER INFORMATION LEAFLET Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human) Read all of this leaflet carefully before you start taking this medicine. Keep this
The first 24 hours are critical when Activase (alteplase) is administered 1-3
 FOR YOUR ACUTE ISCHEMIC STROKE PATIENTS The first 24 hours are critical when Activase (alteplase) is administered 1-3 Close observation and frequent monitoring of patients for neurological changes, any
FOR YOUR ACUTE ISCHEMIC STROKE PATIENTS The first 24 hours are critical when Activase (alteplase) is administered 1-3 Close observation and frequent monitoring of patients for neurological changes, any
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
When the learner has completed this module, she/he will be able to:
 Thrombolytics and Myocardial Infarction WWW.RN.ORG Reviewed September 2017, Expires September 2019 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2017
Thrombolytics and Myocardial Infarction WWW.RN.ORG Reviewed September 2017, Expires September 2019 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2017
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklo-f 500 mg film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains tranexamic acid 500 mg For the full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cyklo-f 500 mg film coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains tranexamic acid 500 mg For the full
SUMMARY OF PRODUCT CHARACTERISTICS. A 2.5ml single-dose bottle containing IU Cholecalciferol (equivalent to 625 micrograms vitamin D 3 )
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fultium 25 000 IU Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION A 2.5ml single-dose bottle containing 25 000 IU Cholecalciferol
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fultium 25 000 IU Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION A 2.5ml single-dose bottle containing 25 000 IU Cholecalciferol
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 40 g/l is a solution containing 40 g/l (4%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 40 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 40 g/l is a solution containing 40 g/l (4%)
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alburex 5, 50 g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alburex 5 is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alburex 5, 50 g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Alburex 5 is a solution containing 50 g/l of total
METALYSE Tenecteplase
 METALYSE Tenecteplase Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Metalyse. It does not contain all the available information. It does not take
METALYSE Tenecteplase Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Metalyse. It does not contain all the available information. It does not take
Diagnosis and Management of Acute Myocardial Infarction
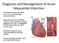 Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
SUMMARY OF PRODUCT CHARACTERISTICS
 MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
MUTUAL RECOGNITION PROCEDURE Page 1 of 5 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT, syrup 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of syrup contains 1 mg loratadine.
Package leaflet: Information for the user. ReoPro 2 mg/ml solution for injection or infusion. abciximab
 Package leaflet: Information for the user ReoPro 2 mg/ml solution for injection or infusion abciximab Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user ReoPro 2 mg/ml solution for injection or infusion abciximab Read all of this leaflet carefully before you start using this medicine because it contains important
Powder for reconstitution for use in central venous access devices
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Cathflo Activase (Alteplase) Powder for reconstitution for use in central venous access devices DESCRIPTION Cathflo Activase
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Cathflo Activase (Alteplase) Powder for reconstitution for use in central venous access devices DESCRIPTION Cathflo Activase
PRODUCT MONOGRAPH. (alteplase for injection) Lyophilized Powder for Injection - 50 mg and 100 mg. Fibrinolytic Agent. Product Monograph for
 PRODUCT MONOGRAPH Pr ACTIVASE rt-pa (alteplase for injection) Lyophilized Powder for Injection - 50 mg and 100 mg Fibrinolytic Agent Product Monograph for ACUTE ISCHEMIC STROKE INDICATION ONLY Distributed
PRODUCT MONOGRAPH Pr ACTIVASE rt-pa (alteplase for injection) Lyophilized Powder for Injection - 50 mg and 100 mg Fibrinolytic Agent Product Monograph for ACUTE ISCHEMIC STROKE INDICATION ONLY Distributed
Orgalutran 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
1 1. NAME OF THE MEDICINAL PRODUCT 0.25 mg/0.5 ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each pre-filled syringe contains 0.25 mg of ganirelix (INN) in 0.5 mg aqueous solution.
3. PHARMACEUTICAL FORM Solution for infusion. A clear, slightly viscous liquid; it is almost colourless, yellow, amber or green.
 1. NAME OF THE MEDICINAL PRODUCT Albutein 250 g/l, solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human albumin Albutein 250 g/l is a solution containing 250 g/l of total protein of
1. NAME OF THE MEDICINAL PRODUCT Albutein 250 g/l, solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human albumin Albutein 250 g/l is a solution containing 250 g/l of total protein of
PRESCRIBING INFORMATION. Dextrose Injection USP. (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher
 PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
BEAUMONT HOSPITAL. Beaumont Hospital Department of Nephrology and Renal Nursing ADMINISTRATION OF ACTILYSE. Guideline Number: 13. Guideline Version: B
 BEAUMONT HOSPITAL Beaumont Hospital Department of Nephrology and Renal Nursing Guideline Name: GUIDELINES FOR THE ADMINISTRATION OF ACTILYSE Guideline Number: 13 Guideline Version: B Developed By: Betty
BEAUMONT HOSPITAL Beaumont Hospital Department of Nephrology and Renal Nursing Guideline Name: GUIDELINES FOR THE ADMINISTRATION OF ACTILYSE Guideline Number: 13 Guideline Version: B Developed By: Betty
Annex I. Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s)
 Annex I Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s) 1 Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR(s) for alteplase
Annex I Scientific conclusions and grounds for the variation to the terms of the Marketing Authorisation(s) 1 Scientific conclusions Taking into account the PRAC Assessment Report on the PSUR(s) for alteplase
COMPANY CORE DATA SHEET CCDS (EDS/CORE/ENGLISH)
 COMPANY CORE DATA SHEET CCDS (EDS/CORE/ENGLISH) CLUVOT 250 IU/1250 IU Rev.: 08-MAY-2014 / First license CSL Behring Page 1 of 13 This medicinal product is subject to additional monitoring. This will allow
COMPANY CORE DATA SHEET CCDS (EDS/CORE/ENGLISH) CLUVOT 250 IU/1250 IU Rev.: 08-MAY-2014 / First license CSL Behring Page 1 of 13 This medicinal product is subject to additional monitoring. This will allow
ST Elevated Myocardial Infarction- Latest AHA recommendations
 ST Elevated Myocardial Infarction- Latest AHA recommendations Sherry Turner, DO, MPH, FACOEP Medical Director Emergency Services Wesley Medical Center The Problem 250,000 Americans each year 30% fail to
ST Elevated Myocardial Infarction- Latest AHA recommendations Sherry Turner, DO, MPH, FACOEP Medical Director Emergency Services Wesley Medical Center The Problem 250,000 Americans each year 30% fail to
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm Junior, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet of contains the following active
1.3.1 SPC, Labelling and Package Leaflet (Voluven Fresenius 6% Solution for Infusion)
 1.3.1 SPC, Labelling and Package Leaflet (Voluven Fresenius 6% Solution for Infusion) 1.3.1 SUMMARY OF PRODUCT CHARACTERISTICS This medicinal product is subject to additional monitoring. This will allow
1.3.1 SPC, Labelling and Package Leaflet (Voluven Fresenius 6% Solution for Infusion) 1.3.1 SUMMARY OF PRODUCT CHARACTERISTICS This medicinal product is subject to additional monitoring. This will allow
Online Supplementary Data. Country Number of centers Number of patients randomized
 A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
SUMMARY OF THE PRODUCT CHARACTERISTICS. The content of electrolyte ions per sachet when made up to 125 ml of solution.
 SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Molaxole powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains following active substances Macrogol
SUMMARY OF THE PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Molaxole powder for oral solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains following active substances Macrogol
New Zealand Datasheet
 New Zealand Datasheet Name of Medicine GlucaGen HypoKit Glucagon (rys) hydrochloride. Presentation White crystalline powder (which may appear more like a powdery tablet upon settling) and clear colourless
New Zealand Datasheet Name of Medicine GlucaGen HypoKit Glucagon (rys) hydrochloride. Presentation White crystalline powder (which may appear more like a powdery tablet upon settling) and clear colourless
SUMMARY OF PRODUCT CHARACTERISTICS. Flexbumin 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
Blood Thinner Agent. Done by: Meznah Al-mutairi Pharm.D Candidate PNU Collage of Pharmacy
 Blood Thinner Agent Done by: Meznah Al-mutairi Pharm.D Candidate PNU Collage of Pharmacy Outline: Blood thinner agent definition. anticoagulants drugs. Thrombolytics. Blood thinner agent Therapeutic interference
Blood Thinner Agent Done by: Meznah Al-mutairi Pharm.D Candidate PNU Collage of Pharmacy Outline: Blood thinner agent definition. anticoagulants drugs. Thrombolytics. Blood thinner agent Therapeutic interference
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
Package Insert. Cognitin
 Package Insert Cognitin Product Summary 1. Name of the medicinal product Cognitin 2. Qualitative and quantitative composition Each film coated tablet contains extract 60 mg 5mg 3. Pharmaceutical form Film
Package Insert Cognitin Product Summary 1. Name of the medicinal product Cognitin 2. Qualitative and quantitative composition Each film coated tablet contains extract 60 mg 5mg 3. Pharmaceutical form Film
Tranexamic acid. Hemanex 500 mg Capsule. Anti-fibrinolytic
 INSERT TEXT BioFemme Tranexamic acid Hemanex 500 mg Capsule Anti-fibrinolytic FORMULATION Each capsule contains: Tranexamic acid 500 mg PRODUCT DESCRIPTION Tranexamic acid (Hemanex) Size #0 hard gelatin
INSERT TEXT BioFemme Tranexamic acid Hemanex 500 mg Capsule Anti-fibrinolytic FORMULATION Each capsule contains: Tranexamic acid 500 mg PRODUCT DESCRIPTION Tranexamic acid (Hemanex) Size #0 hard gelatin
2. PRESCRIPTION STATUS/RESTRICTION OF SALES TO PHARMACIES ONLY 3. COMPOSITION OF THE MEDICINAL PRODUCT
 Title Page Product Information May 2000 1 (6) Product Nephrotect 1. NAME OF THE MEDICINAL PRODUCT Nephrotect 2. PRESCRIPTION STATUS/RESTRICTION OF SALES TO PHARMACIES ONLY For sale in pharmacies only 3.
Title Page Product Information May 2000 1 (6) Product Nephrotect 1. NAME OF THE MEDICINAL PRODUCT Nephrotect 2. PRESCRIPTION STATUS/RESTRICTION OF SALES TO PHARMACIES ONLY For sale in pharmacies only 3.
Updated Ischemic Stroke Guidelines นพ.ส ชาต หาญไชยพ บ ลย ก ล นายแพทย ทรงค ณว ฒ สาขาประสาทว ทยา สถาบ นประสาทว ทยา กรมการแพทย กระทรวงสาธารณส ข
 Updated Ischemic Stroke Guidelines นพ.ส ชาต หาญไชยพ บ ลย ก ล นายแพทย ทรงค ณว ฒ สาขาประสาทว ทยา สถาบ นประสาทว ทยา กรมการแพทย กระทรวงสาธารณส ข Emergency start at community level: Prehospital care Acute stroke
Updated Ischemic Stroke Guidelines นพ.ส ชาต หาญไชยพ บ ลย ก ล นายแพทย ทรงค ณว ฒ สาขาประสาทว ทยา สถาบ นประสาทว ทยา กรมการแพทย กระทรวงสาธารณส ข Emergency start at community level: Prehospital care Acute stroke
Package Insert. D-Bright
 Package Insert D-Bright Product Summary 1. Name of the medicinal product D-Bright 2. Qualitative and quantitative composition Each ml contains Cholecalciferol (Vitamin D3) IP 400 IU in a flavoured syrupy
Package Insert D-Bright Product Summary 1. Name of the medicinal product D-Bright 2. Qualitative and quantitative composition Each ml contains Cholecalciferol (Vitamin D3) IP 400 IU in a flavoured syrupy
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
Package leaflet: Information for the patient. NEGABAN 1g, powder for solution for injection or infusion Temocillin
 Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
After a stroke starts
 After a stroke starts What you need to know about clot-busting therapy What happens during a stroke When brain cells do not get the oxygen-carrying blood that they need to function, they begin to die.
After a stroke starts What you need to know about clot-busting therapy What happens during a stroke When brain cells do not get the oxygen-carrying blood that they need to function, they begin to die.
PRODUCT MONOGRAPH ACUTE MYOCARDIAL INFARCTION INDICATION ONLY
 PRODUCT MONOGRAPH Pr ACTIVASE rt-pa alteplase for injection Lyophilized powder for injection - 50 mg and 100 mg Fibrinolytic Agent ACUTE MYOCARDIAL INFARCTION INDICATION ONLY Hoffmann-La Roche Limited
PRODUCT MONOGRAPH Pr ACTIVASE rt-pa alteplase for injection Lyophilized powder for injection - 50 mg and 100 mg Fibrinolytic Agent ACUTE MYOCARDIAL INFARCTION INDICATION ONLY Hoffmann-La Roche Limited
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) CLUVOT 250 IU Rev.: 08-MAY-2014 / New license CSL Behring Page 1 of 10 Package leaflet: Information for the user Powder and solvent for solution for injection/infusion.
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) CLUVOT 250 IU Rev.: 08-MAY-2014 / New license CSL Behring Page 1 of 10 Package leaflet: Information for the user Powder and solvent for solution for injection/infusion.
PACKAGE LEAFLET: INFORMATION FOR THE USER. Variquel 1 mg powder and solvent for solution for injection Terlipressin acetate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Variquel 1 mg powder and solvent for solution for injection Terlipressin acetate Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER Variquel 1 mg powder and solvent for solution for injection Terlipressin acetate Read all of this leaflet carefully before you start using this medicine because
SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Lidokain Isdin 40 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g cream contains 40 mg lidocaine. Excipients: Propylene glycol
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Lidokain Isdin 40 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g cream contains 40 mg lidocaine. Excipients: Propylene glycol
Package leaflet: Information for the patient. Tranexamic Acid 100 mg/ml, Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic Acid 100 mg/ml, Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient Tranexamic Acid 100 mg/ml, Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT "Carbellon" Tablets. 2. Qualitative and Quantitative Composition Active Constituents: Activated Charcoal Ph.Eur. Magnesium Hydroxide Ph.Eur.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT "Carbellon" Tablets. 2. Qualitative and Quantitative Composition Active Constituents: Activated Charcoal Ph.Eur. Magnesium Hydroxide Ph.Eur.
Nitroglycerin and Heparin Drip Interfacility Protocols
 Nitroglycerin and Heparin Drip Interfacility Protocols EMS Protocol This protocol applies to nitroglycerin and Heparin drips that are initiated at the transferring facility prior to transport and are not
Nitroglycerin and Heparin Drip Interfacility Protocols EMS Protocol This protocol applies to nitroglycerin and Heparin drips that are initiated at the transferring facility prior to transport and are not
BIVASTAT Injection (Bivalirudin)
 Published on: 1 Aug 2014 BIVASTAT Injection (Bivalirudin) Compostion BIVASTAT Each vial contains: Bivalirudin.. 250 mg Excipients.q.s. Supplied with 5 ml Sterile Water for Injection, IP Dosage Form Sterile
Published on: 1 Aug 2014 BIVASTAT Injection (Bivalirudin) Compostion BIVASTAT Each vial contains: Bivalirudin.. 250 mg Excipients.q.s. Supplied with 5 ml Sterile Water for Injection, IP Dosage Form Sterile
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
Off-white, round, with faceted edges, scored on one side and bearing the inscription IK10 (10mg) or IK20 (20mg).
 1 TRADE NAME OF THE MEDICINAL PRODUCT Ikorel 10mg and 20mg Tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Nicorandil 10mg or 20mg For a full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM
1 TRADE NAME OF THE MEDICINAL PRODUCT Ikorel 10mg and 20mg Tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Nicorandil 10mg or 20mg For a full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM
SUMMARY OF PRODUCT CHARACTERISTICS
 Page 1/18 SUMMARY OF PRODUCT CHARACTERISTICS Page 2/18 1. NAME OF THE MEDICINAL PRODUCT < Product name > 100 mg/ml, solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 5 ml ampoule
Page 1/18 SUMMARY OF PRODUCT CHARACTERISTICS Page 2/18 1. NAME OF THE MEDICINAL PRODUCT < Product name > 100 mg/ml, solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 5 ml ampoule
SUMMARY OF PRODUCT CHARACTERISTICS. Medical conditions that require parenteral nutrition for supply of energy and essential fatty acids.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alphalipid 200 mg/ml emulsion for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000 ml of the emulsion contain: Soya-bean oil,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alphalipid 200 mg/ml emulsion for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000 ml of the emulsion contain: Soya-bean oil,
