Local Coverage Determination (LCD) for Noninvasive Cerebrovascular Studies (L28283)
|
|
|
- Marjorie McKenzie
- 6 years ago
- Views:
Transcription
1 Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms Help Print Back to Local Coverage Determinations (LCDs) for Palmetto GBA (01192, MAC - Part B) Local Coverage Determination (LCD) for Noninvasive Cerebrovascular Studies (L28283) Select the Print Record, Add to Basket or Record buttons to print the record, to add it to your basket or to the record. Section Navigation Select Section Go Contractor Information Contractor Name Palmetto GBA Contractor Number Contractor Type MAC - Part B Back to Top LCD Information Document Information LCD ID Number L28283 LCD Title Noninvasive Cerebrovascular Studies Contractor's Determination Number J1B L AMA CPT/ADA CDT Copyright Statement CPT only copyright American Medical Association. All Rights Reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DFARS Apply to Government Use. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or Primary Geographic Jurisdiction California - Southern Oversight Region Region X Original Determination Effective Date For services performed on or after 09/02/2008 Original Determination Ending Date Revision Effective Date For services performed on or after 01/01/2012
2 dispense medical services. The AMA assumes no liability for data contained or not contained herein. The Code on Dental Procedures and Nomenclature (Code) is published in Current Dental Terminology (CDT). Copyright American Dental Association. All rights reserved. CDT and CDT-2010 are trademarks of the American Dental Association. Revision Ending Date CMS National Coverage Policy Title XVIII of the Social Security Act, 1862(a)(1)(A) allows coverage and payment for only those services that are considered to be medically reasonable and necessary. Title XVIII of the Social Security Act, 1833(e) prohibits Medicare payment for any claim, which lacks the necessary information to process the claim. CMS Manual System, Pub , Medicare National Coverage Determinations Manual, Chapter 1, Part 1, Plethysmography. CMS Manual System, Pub , Medicare National Coverage Determinations Manual, Chapter 1, Part 1, Noninvasive Tests of Carotid Function. CMS Manual System, Pub , Medicare National Coverage Determinations Manual, Chapter 1, Part 4, Ultrasound Diagnostic Procedures. CMS Manual System, Pub , Medicare National Coverage Determinations Manual, Chapter 1, Part 4, Thermography. CMS Manual System, Pub , Medicare Benefit Policy Manual, Chapter 15, Definitions (treating physician, treating practitioner, testing facility and order. Indications and Limitations of Coverage and/or Medical Necessity Noninvasive cerebrovascular arterial studies are used to identify possible problems in structure or flow of the carotid artery and other cerebrovasculature. There are a wide variety of these tests that measure various anatomical and physiological aspects of carotid function. In vascular ultrasound, a transducer directs high-frequency sound waves through layers of tissue of the artery or vein being tested. When these waves strike red blood cells (RBCs) moving through the bloodstream, their frequency changes in proportion to the flow velocity of the RBCs. Recording of these waves permits detection of arterial and venous obstruction. Duplex Scan This procedure combines high resolution B-mode real-time imaging with Doppler ultrasound and spectral analysis. The scan provides anatomic and hemodynamic information regarding the cervical carotid arteries. Data regarding percent stenosis and characterization of atheromatous plaque are provided. Color-flow Doppler is used to enhance conventional data acquisition. Physiologic Studies This term implies functional measurement procedures including Doppler ultrasound studies, ocular pneumoplethysmography, blood pressure measurement, transcutaneous oxygen tension measurements and/or plethysmography. A complete study includes pressure measurements and an additional physiologic technique (e.g., Doppler waveforms
3 or plethysmography). Plethysmography implies volume measurement procedures including air, impedance or strain gauge methods. Transcranial Doppler Pulsed Doppler ultrasound is used to interrogate the intracranial vasculature of the Circle of Willis. Its value has been established in detecting severe stenoses in the major intracranial arteries, assessing patterns and extent of collateral circulation in patients with known regions of severe stenosis or occlusion and evaluating and following patients with vasoconstriction particularly after subarachnoid hemorrhage. "The use of a simple hand-held or other Doppler device that does not produce hard copy output, or that produces a record that does not permit analysis of bidirectional vascular flow, is considered to be part of the physical examination of the vascular system and is not separately reported." (End of Quote) (CPT 2007, p 398) Noninvasive vascular studies include the patient care required to perform the studies, supervision of the studies, and interpretation of study results, with copies for patient records of hard copy output with analysis of all data, including bi-directional vascular flow or imaging when provided. Indications for CPT Codes 93875, and for Cerebrovascular Evaluation: 1. Cervical bruits. 2. Amaurosis fugax. 3. Focal cerebral or ocular transient ischemic attacks (i.e., localizing symptoms, weakness of one side of the face, slurred speech, weakness of a limb). Visual transient ischemic attacks are defined as retinal or hemispheric visual field deficits and not temporary blurred vision. 4. Drop attack or syncope is a rare indication primarily seen with vertebrobasilar or bilateral carotid artery disease. Incoordination or limb dysfunction should be grouped with unilateral weakness of the face or extremities. 5. Subclavian steal syndrome. 6. Blunt neck trauma. 7. Follow-up after a carotid endarterectomy. 8. Re-evaluation of existing carotid stenosis. 9. Evaluation of pulsatile neck mass. 10. Preoperative evaluation of patients scheduled for major cardiovascular surgical procedures. 11. Evaluation of nonhemispheric or unexplained neurologic symptoms. 12. Retinal arterial emboli. 13. Evaluation of suspected dissection Indications considered investigational for TCD (Transcranial Doppler) (CPT Code and 93888) include: 1. Migraine or headaches. 2. Dizziness not associated with localizing symptoms. While ICD-9-CM is listed as a covered diagnosis code, it is important to note that dizziness and giddiness alone are not usual indications unless associated with other localizing signs and symptoms. Episodic dizziness with symptoms typical of transient ischemic attacks may indicate reasonableness and necessity, especially when other more common sources (e.g., postural hypotension or transiently decreased cardiac output as demonstrated by cardiac event monitoring) have been previously excluded. 3. Monitoring during carotid endarterectomy, cardiopulmonary bypass and other cerebrovascular and cardiovascular intervention and surgical procedures. 4. Evaluation of patients with dilated vasculopathies such as fusiform aneurysms. 5. Assessing auto-regulation, physiologic and pharmacological responses of cerebral
4 arteries. 6. Evaluating children with various vasculopathies such as sickle cell disease, moya-moya and neurofibromatosis. 7. As an aid to differentiate vertebrobasilar from carotid symptoms. TCD studies (CPT Codes or 93888) may be necessary for: 1. Detection and evaluation of the hemodynamic effects of severe stenosis or occlusion of the extracranial (greater than or equal to 60% diameter reduction) and major basal intracranial arteries (greater than or equal to 50% diameter reduction). 2. Assessing patterns and extent of collateral circulation in patient with known region of severe stenosis or occlusion. 3. Detection and serial evaluation of cerebral vasospasm complicating subarachnoid hemorrhage. 4. Evaluation of invasive therapeutic interventions for cerebral malformations. 5. Evaluation of intracranial hemodynamic abnormalities in patients with suspected brain death. 6. Evaluation of cerebral embolization. 7. Detecting arteriovenous malformation and studying their supply arteries and flow pattern. While TCD is indicated for the evaluation of intracranial hemodynamic abnormalities in patients with suspected brain death, it would be expected that CPT Code (EEG for cerebral death evaluation) will be primarily used in the diagnosis of brain death. Indications not reasonable and necessary for TCD include: 1. Evaluation of brain tumors. 2. Assessment of familial and degenerative disease of the cerebrum, brainstem, cerebellum, basal ganglia and motor neurons. 3. Evaluation of infectious and inflammatory conditions. 4. Psychiatric disorder. 5. Epilepsy. 6. Routine evaluation of cerebrovascular symptoms and signs. Noninvasive studies are reasonable and necessary only if the outcome will potentially impact the clinical course of the patient. For example, if a patient is (or is not) going to proceed on to other diagnostic and/or therapeutic procedures regardless of the outcome of the non-invasive vascular procedures, the studies are not medically necessary. That is, if it is obvious from the findings of the history and physical examination that the patient is going to proceed to angiography, then non-invasive vascular studies are not medically necessary. Recommendations For Follow-up Studies (CPT Codes through 93888): 1. Stenosis of 20-50% (diameter reduction), annual study. 2. Stenosis of 50-79% every six months. 3. Stenosis of 80-99% surgery is usually recommended. 4. After carotid endarterectomy, repeat examinations are allowed at six weeks, six months, 12 months and yearly thereafter. Post operatively follow-up studies should be unilateral unless signs and symptoms provide indications for a bilateral procedure. The following are not acceptable methods for reimbursement: Thermography, mechanical oscillometry, inductance plethysmography, capacitance plethysmography, photoelectric plethysmography, pulse-delay oculoplethysmography, carotid phonoangiography and other forms of bruit analysis are included in the reimbursement for the office visit. Also, periorbital photoplethysmography and light reflection rheography are not covered services
5 because of lack of documentation in the current literature for reasonableness and necessity. All other codes not listed in the "ICD-9-CM Codes That Support Medical Necessity" section will be denied without additional information to warrant reasonableness and necessity. Studies will be denied if they are determined to be screening studies, were duplicative of other vascular studies or were not needed to make management decisions. Compliance with the provisions in this policy is subject to monitoring by post payment data analysis and subsequent medical review. Back to Top Coding Information Bill Type Codes: Contractors may specify Bill Types to help providers identify those Bill Types typically used to report this service. Absence of a Bill Type does not guarantee that the policy does not apply to that Bill Type. Complete absence of all Bill Types indicates that coverage is not influenced by Bill Type and the policy should be assumed to apply equally to all claims. 999x Not Applicable Revenue Codes: Contractors may specify Revenue Codes to help providers identify those Revenue Codes typically used to report this service. In most instances Revenue Codes are purely advisory; unless specified in the policy services reported under other Revenue Codes are equally subject to this coverage determination. Complete absence of all Revenue Codes indicates that coverage is not influenced by Revenue Code and the policy should be assumed to apply equally to all Revenue Codes Not Applicable CPT/HCPCS Codes DUPLEX SCAN OF EXTRACRANIAL ARTERIES; COMPLETE BILATERAL STUDY DUPLEX SCAN OF EXTRACRANIAL ARTERIES; UNILATERAL OR LIMITED STUDY TRANSCRANIAL DOPPLER STUDY OF THE INTRACRANIAL ARTERIES; COMPLETE STUDY TRANSCRANIAL DOPPLER STUDY OF THE INTRACRANIAL ARTERIES; LIMITED STUDY ICD-9 Codes that Support Medical Necessity
6 These are the only covered ICD-9-CM Codes That Support Medical Necessity: Group 1 Only one of the following diagnoses is required: DELIRIUM DUE TO CONDITIONS CLASSIFIED ELSEWHERE UNSPECIFIED TRANSIENT MENTAL DISORDER IN CONDITIONS CLASSIFIED ELSEWHERE OTHER CEREBELLAR ATAXIA FLACCID HEMIPLEGIA AND HEMIPARESIS AFFECTING UNSPECIFIED SIDE FLACCID HEMIPLEGIA AND HEMIPARESIS AFFECTING DOMINANT SIDE FLACCID HEMIPLEGIA AND HEMIPARESIS AFFECTING NONDOMINANT SIDE SPASTIC HEMIPLEGIA AND HEMIPARESIS AFFECTING UNSPECIFIED SIDE SPASTIC HEMIPLEGIA AND HEMIPARESIS AFFECTING DOMINANT SIDE SPASTIC HEMIPLEGIA AND HEMIPARESIS AFFECTING NONDOMINANT SIDE OTHER SPECIFIED HEMIPLEGIA AND HEMIPARESIS AFFECTING UNSPECIFIED SIDE OTHER SPECIFIED HEMIPLEGIA AND HEMIPARESIS AFFECTING DOMINANT SIDE OTHER SPECIFIED HEMIPLEGIA AND HEMIPARESIS AFFECTING NONDOMINANT SIDE UNSPECIFIED HEMIPLEGIA AND HEMIPARESIS AFFECTING UNSPECIFIED SIDE UNSPECIFIED HEMIPLEGIA AND HEMIPARESIS AFFECTING DOMINANT SIDE UNSPECIFIED HEMIPLEGIA AND HEMIPARESIS AFFECTING NONDOMINANT SIDE QUADRIPLEGIA UNSPECIFIED QUADRIPLEGIA C1-C4 COMPLETE QUADRIPLEGIA C1-C4 INCOMPLETE QUADRIPLEGIA C5-C7 COMPLETE
7 QUADRIPLEGIA C5-C7 INCOMPLETE OTHER QUADRIPLEGIA PARAPLEGIA DIPLEGIA OF UPPER LIMBS MONOPLEGIA OF LOWER LIMB AFFECTING UNSPECIFIED SIDE MONOPLEGIA OF LOWER LIMB AFFECTING DOMINANT SIDE MONOPLEGIA OF LOWER LIMB AFFECTING NONDOMINANT SIDE MONOPLEGIA OF UPPER LIMB AFFECTING UNSPECIFIED SIDE MONOPLEGIA OF UPPER LIMB AFFECTING DOMINANT SIDE MONOPLEGIA OF UPPER LIMB AFFECTING NONDOMINANT SDE UNSPECIFIED MONOPLEGIA LOCKED-IN STATE OTHER SPECIFIED PARALYTIC SYNDROME PARALYSIS UNSPECIFIED MIGRAINE WITH AURA, WITH INTRACTABLE MIGRAINE, SO STATED, WITHOUT MENTION OF STATUS MIGRAINOSUS VARIANTS OF MIGRAINE, NOT ELSEWHERE CLASSIFIED, WITHOUT MENTION OF INTRACTABLE MIGRAINE WITHOUT MENTION OF STATUS MIGRAINOSUS VARIANTS OF MIGRAINE, NOT ELSEWHERE CLASSIFIED, WITH INTRACTABLE MIGRAINE, SO STATED, WITHOUT MENTION OF STATUS MIGRAINOSUS OTHER FORMS OF MIGRAINE, WITHOUT MENTION OF INTRACTABLE MIGRAINE WITHOUT MENTION OF STATUS MIGRAINOSUS OTHER FORMS OF MIGRAINE, WITH INTRACTABLE MIGRAINE, SO STATED, WITHOUT MENTION OF STATUS MIGRAINOSUS ANOXIC BRAIN DAMAGE CEREBRAL EDEMA OTHER CONDITIONS OF BRAIN BACKGROUND RETINOPATHY UNSPECIFIED HYPERTENSIVE RETINOPATHY
8 EXUDATIVE RETINOPATHY CHANGES IN VASCULAR APPEARANCE OF RETINA RETINAL MICROANEURYSMS NOS RETINAL TELANGIECTASIA RETINAL NEOVASCULARIZATION NOS OTHER INTRARETINAL MICROVASCULAR ABNORMALITIES RETINAL VASCULITIS RETINAL VASCULAR OCCLUSION UNSPECIFIED CENTRAL RETINAL ARTERY OCCLUSION RETINAL ARTERIAL BRANCH OCCLUSION PARTIAL RETINAL ARTERIAL OCCLUSION TRANSIENT RETINAL ARTERIAL OCCLUSION CENTRAL RETINAL VEIN OCCLUSION VENOUS TRIBUTARY (BRANCH) OCCLUSION OF RETINA VENOUS ENGORGEMENT OF RETINA RETINAL ISCHEMIA AMBLYOPIA UNSPECIFIED STRABISMIC AMBLYOPIA DEPRIVATION AMBLYOPIA REFRACTIVE AMBLYOPIA SUBJECTIVE VISUAL DISTURBANCE UNSPECIFIED SUDDEN VISUAL LOSS TRANSIENT VISUAL LOSS VISUAL DISCOMFORT VISUAL DISTORTIONS OF SHAPE AND SIZE OTHER VISUAL DISTORTIONS AND ENTOPTIC PHENOMENA PSYCHOPHYSICAL VISUAL DISTURBANCES
9 368.2 DIPLOPIA BINOCULAR VISION DISORDER UNSPECIFIED SUPPRESSION OF BINOCULAR VISION SIMULTANEOUS VISUAL PERCEPTION WITHOUT FUSION FUSION WITH DEFECTIVE STEREOPSIS ABNORMAL RETINAL CORRESPONDENCE VISUAL FIELD DEFECT UNSPECIFIED SCOTOMA INVOLVING CENTRAL AREA SCOTOMA OF BLIND SPOT AREA SECTOR OR ARCUATE VISUAL FIELD DEFECTS OTHER LOCALIZED VISUAL FIELD DEFECT GENERALIZED VISUAL FIELD CONTRACTION OR CONSTRICTION HOMONYMOUS BILATERAL FIELD DEFECTS HETERONYMOUS BILATERAL FIELD DEFECTS PROTAN DEFECT DEUTAN DEFECT TRITAN DEFECT ACHROMATOPSIA ACQUIRED COLOR VISION DEFICIENCIES OTHER COLOR VISION DEFICIENCIES NIGHT BLINDNESS UNSPECIFIED CONGENITAL NIGHT BLINDNESS ACQUIRED NIGHT BLINDNESS ABNORMAL DARK ADAPTATION CURVE OTHER NIGHT BLINDNESS OTHER SPECIFIED VISUAL DISTURBANCES UNSPECIFIED VISUAL DISTURBANCE
10 ONE EYE: TOTAL VISION IMPAIRMENT; OTHER EYE: NOT SPECIFIED ONE EYE: TOTAL VISION IMPAIRMENT; OTHER EYE: NEAR-NORMAL VISION ONE EYE: TOTAL VISION IMPAIRMENT; OTHER EYE: NORMAL VISION ONE EYE: NEAR-TOTAL VISION IMPAIRMENT; OTHER EYE: VISION NOT SPECIFIED ONE EYE: NEAR-TOTAL VISION IMPAIRMENT; OTHER EYE: NEAR-NORMAL VISION ONE EYE: NEAR-TOTAL VISION IMPAIRMENT; OTHER EYE: NORMAL VISION UNQUALIFIED VISUAL LOSS ONE EYE UNSPECIFIED VISUAL LOSS PARALYTIC STRABISMUS UNSPECIFIED THIRD OR OCULOMOTOR NERVE PALSY PARTIAL THIRD OR OCULOMOTOR NERVE PALSY TOTAL FOURTH OR TROCHLEAR NERVE PALSY SIXTH OR ABDUCENS NERVE PALSY EXTERNAL OPHTHALMOPLEGIA TOTAL OPHTHALMOPLEGIA NYSTAGMUS UNSPECIFIED LATENT NYSTAGMUS VISUAL DEPRIVATION NYSTAGMUS NYSTAGMUS ASSOCIATED WITH DISORDERS OF THE VESTIBULAR SYSTEM DISSOCIATED NYSTAGMUS OTHER FORMS OF NYSTAGMUS DEFICIENCIES OF SACCADIC EYE MOVEMENTS DEFICIENCIES OF SMOOTH PURSUIT MOVEMENTS OTHER IRREGULARITIES OF EYE MOVEMENTS VERTIGO OF CENTRAL ORIGIN
11 TRANSIENT ISCHEMIC DEAFNESS SUBJECTIVE TINNITUS SENSORINEURAL HEARING LOSS UNSPECIFIED CENTRAL HEARING LOSS SENSORINEURAL HEARING LOSS, UNILATERAL SENSORINEURAL HEARING LOSS, ASYMMETRICAL SENSORINEURAL HEARING LOSS, BILATERAL OTHER SPECIFIED FORMS OF HEARING LOSS 430 SUBARACHNOID HEMORRHAGE 431 INTRACEREBRAL HEMORRHAGE NONTRAUMATIC EXTRADURAL HEMORRHAGE SUBDURAL HEMORRHAGE UNSPECIFIED INTRACRANIAL HEMORRHAGE OCCLUSION AND STENOSIS OF BASILAR ARTERY WITHOUT CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF BASILAR ARTERY WITH CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF CAROTID ARTERY WITHOUT CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF CAROTID ARTERY WITH CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF VERTEBRAL ARTERY WITHOUT CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF VERTEBRAL ARTERY WITH CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF MULTIPLE AND BILATERAL PRECEREBRAL ARTERIES WITHOUT CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF MULTIPLE AND BILATERAL PRECEREBRAL ARTERIES WITH CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF OTHER SPECIFIED PRECEREBRAL ARTERY WITHOUT CEREBRAL INFARCTION
12 OCCLUSION AND STENOSIS OF OTHER SPECIFIED PRECEREBRAL ARTERY WITH CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF UNSPECIFIED PRECEREBRAL ARTERY WITHOUT CEREBRAL INFARCTION OCCLUSION AND STENOSIS OF UNSPECIFIED PRECEREBRAL ARTERY WITH CEREBRAL INFARCTION CEREBRAL THROMBOSIS WITHOUT CEREBRAL INFARCTION CEREBRAL THROMBOSIS WITH CEREBRAL INFARCTION CEREBRAL EMBOLISM WITHOUT CEREBRAL INFARCTION CEREBRAL EMBOLISM WITH CEREBRAL INFARCTION CEREBRAL ARTERY OCCLUSION UNSPECIFIED WITHOUT CEREBRAL INFARCTION CEREBRAL ARTERY OCCLUSION UNSPECIFIED WITH CEREBRAL INFARCTION BASILAR ARTERY SYNDROME VERTEBRAL ARTERY SYNDROME SUBCLAVIAN STEAL SYNDROME VERTEBROBASILAR ARTERY SYNDROME OTHER SPECIFIED TRANSIENT CEREBRAL ISCHEMIAS UNSPECIFIED TRANSIENT CEREBRAL ISCHEMIA 436 ACUTE BUT ILL-DEFINED CEREBROVASCULAR DISEASE CEREBRAL ATHEROSCLEROSIS OTHER GENERALIZED ISCHEMIC CEREBROVASCULAR DISEASE HYPERTENSIVE ENCEPHALOPATHY CEREBRAL ANEURYSM NONRUPTURED CEREBRAL ARTERITIS MOYAMOYA DISEASE NONPYOGENIC THROMBOSIS OF INTRACRANIAL VENOUS SINUS TRANSIENT GLOBAL AMNESIA
13 437.8 OTHER ILL-DEFINED CEREBROVASCULAR DISEASE UNSPECIFIED CEREBROVASCULAR DISEASE COGNITIVE DEFICITS SPEECH AND LANGUAGE DEFICIT UNSPECIFIED APHASIA DYSPHASIA LATE EFFECTS OF CEREBROVASCULAR DISEASE, DYSARTHRIA OTHER SPEECH AND LANGUAGE DEFICITS HEMIPLEGIA AFFECTING UNSPECIFIED SIDE HEMIPLEGIA AFFECTING DOMINANT SIDE HEMIPLEGIA AFFECTING NONDOMINANT SIDE MONOPLEGIA OF UPPER LIMB AFFECTING UNSPECIFIED SIDE MONOPLEGIA OF UPPER LIMB AFFECTING DOMINANT SIDE MONOPLEGIA OF UPPER LIMB AFFECTING NONDOMINANT SIDE MONOPLEGIA OF LOWER LIMB AFFECTING UNSPECIFIED SIDE MONOPLEGIA OF LOWER LIMB AFFECTING DOMINANT SIDE MONOPLEGIA OF LOWER LIMB AFFECTING NONDOMINANT SIDE OTHER PARALYTIC SYNDROME AFFECTING UNSPECIFIED SIDE OTHER PARALYTIC SYNDROME AFFECTING DOMINANT SIDE OTHER PARALYTIC SYNDROME AFFECTING NONDOMINANT SIDE OTHER PARALYTIC SYNDROME BILATERAL ALTERATIONS OF SENSATIONS DISTURBANCES OF VISION APRAXIA CEREBROVASCULAR DISEASE DYSPHAGIA CEREBROVASCULAR DISEASE FACIAL WEAKNESS ATAXIA
14 VERTIGO * OTHER LATE EFFECTS OF CEREBROVASCULAR DISEASE UNSPECIFIED LATE EFFECTS OF CEREBROVASCULAR DISEASE ANEURYSM OF ARTERY OF NECK ANEURYSM OF SUBCLAVIAN ARTERY DISSECTION OF CAROTID ARTERY DISSECTION OF VERTEBRAL ARTERY POLYARTERITIS NODOSA ACUTE FEBRILE MUCOCUTANEOUS LYMPH NODE SYNDROME (MCLS) HYPERSENSITIVITY ANGIITIS UNSPECIFIED GOODPASTURE'S SYNDROME OTHER SPECIFIED HYPERSENSITIVITY ANGIITIS LETHAL MIDLINE GRANULOMA WEGENER'S GRANULOMATOSIS GIANT CELL ARTERITIS THROMBOTIC MICROANGIOPATHY TAKAYASU'S DISEASE STRICTURE OF ARTERY RUPTURE OF ARTERY ARTERITIS UNSPECIFIED OTHER SPECIFIED DISORDERS OF ARTERIES AND ARTERIOLES UNSPECIFIED DISORDERS OF ARTERIES AND ARTERIOLES UNSPECIFIED CIRCULATORY SYSTEM DISORDER CONGENITAL ANOMALIES OF CEREBROVASCULAR SYSTEM TRANSIENT ALTERATION OF AWARENESS SYNCOPE AND COLLAPSE 780.4* DIZZINESS AND GIDDINESS
15 MEMORY LOSS EARLY SATIETY EXCESSIVE CRYING OF CHILD, ADOLESCENT, OR ADULT OTHER GENERAL SYMPTOMS ABNORMALITY OF GAIT LACK OF COORDINATION TRANSIENT PARALYSIS OF LIMB FACIAL WEAKNESS OTHER SYMPTOMS INVOLVING NERVOUS AND MUSCULOSKELETAL SYSTEMS DISTURBANCE OF SKIN SENSATION SWELLING MASS OR LUMP IN HEAD AND NECK APHASIA VOICE AND RESONANCE DISORDER, UNSPECIFIED DYSARTHRIA FLUENCY DISORDER IN CONDITIONS CLASSIFIED ELSEWHERE OTHER SPEECH DISTURBANCE OTHER SYMPTOMS INVOLVING CARDIOVASCULAR SYSTEM * DYSPHAGIA, UNSPECIFIED * DYSPHAGIA, ORAL PHASE * DYSPHAGIA, OROPHARYNGEAL PHASE * DYSPHAGIA, PHARYNGEAL PHASE * DYSPHAGIA, PHARYNGOESOPHAGEAL PHASE * OTHER DYSPHAGIA INJURY TO CAROTID ARTERY UNSPECIFIED INJURY TO COMMON CAROTID ARTERY INJURY TO EXTERNAL CAROTID ARTERY
16 INJURY TO INTERNAL CAROTID ARTERY INJURY TO INTERNAL JUGULAR VEIN INJURY TO EXTERNAL JUGULAR VEIN INJURY TO MULTIPLE BLOOD VESSELS OF HEAD AND NECK INJURY TO OTHER SPECIFIED BLOOD VESSELS OF HEAD AND NECK INJURY TO UNSPECIFIED BLOOD VESSEL OF HEAD AND NECK INJURY TO INNOMINATE AND SUBCLAVIAN ARTERIES TRAUMATIC SHOCK OTHER AND UNSPECIFIED INJURY TO FACE AND NECK MECHANICAL COMPLICATIONS OF UNSPECIFIED CARDIAC DEVICE IMPLANT AND GRAFT MECHANICAL COMPLICATION DUE TO CARDIAC PACEMAKER (ELECTRODE) MECHANICAL COMPLICATION DUE TO HEART VALVE PROSTHESIS MECHANICAL COMPLICATION OF OTHER VASCULAR DEVICE IMPLANT AND GRAFT MECHANICAL COMPLICATION OF NERVOUS SYSTEM DEVICE IMPLANT AND GRAFT MECHANICAL COMPLICATION OF PROSTHETIC GRAFT OF OTHER TISSUE NOT ELSEWHERE CLASSIFIED MECHANICAL COMPLICATION OF OTHER IMPLANT AND INTERNAL DEVICE NOT ELSEWHERE CLASSIFIED INFECTION AND INFLAMMATORY REACTION DUE TO UNSPECIFIED DEVICE IMPLANT AND GRAFT INFECTION AND INFLAMMATORY REACTION DUE TO CARDIAC DEVICE IMPLANT AND GRAFT INFECTION AND INFLAMMATORY REACTION DUE TO OTHER VASCULAR DEVICE IMPLANT AND GRAFT INFECTION AND INFLAMMATORY REACTION DUE TO NERVOUS SYSTEM DEVICE IMPLANT AND GRAFT INFECTION AND INFLAMMATORY REACTION DUE TO INDWELLING URINARY CATHETER INFECTION AND INFLAMMATORY REACTION DUE TO OTHER GENITOURINARY DEVICE IMPLANT AND GRAFT
17 INFECTION AND INFLAMMATORY REACTION DUE TO INTERNAL JOINT PROSTHESIS INFECTION AND INFLAMMATORY REACTION DUE TO OTHER INTERNAL ORTHOPEDIC DEVICE IMPLANT AND GRAFT INFECTION AND INFLAMMATORY REACTION DUE TO PERITONEAL DIALYSIS CATHETER INFECTION AND INFLAMMATORY REACTION DUE TO OTHER INTERNAL PROSTHETIC DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO UNSPECIFIED DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO HEART VALVE PROSTHESIS OTHER COMPLICATIONS DUE TO OTHER CARDIAC DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO RENAL DIALYSIS DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO OTHER VASCULAR DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO NERVOUS SYSTEM DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO GENITOURINARY DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO INTERNAL JOINT PROSTHESIS OTHER COMPLICATIONS DUE TO OTHER INTERNAL ORTHOPEDIC DEVICE IMPLANT AND GRAFT OTHER COMPLICATIONS DUE TO OTHER INTERNAL PROSTHETIC DEVICE IMPLANT AND GRAFT NERVOUS SYSTEM COMPLICATION UNSPECIFIED CENTRAL NERVOUS SYSTEM COMPLICATION IATROGENIC CEREBROVASCULAR INFARCTION OR HEMORRHAGE OTHER NERVOUS SYSTEM COMPLICATIONS HEMORRHAGE COMPLICATING A PROCEDURE HEMATOMA COMPLICATING A PROCEDURE SEROMA COMPLICATING A PROCEDURE
18 998.2 ACCIDENTAL PUNCTURE OR LACERATION DURING A PROCEDURE NOT ELSEWHERE CLASSIFIED DISRUPTION OF WOUND, UNSPECIFIED DISRUPTION OF INTERNAL OPERATION (SURGICAL) WOUND DISRUPTION OF EXTERNAL OPERATION (SURGICAL) WOUND DISRUPTION OF TRAUMATIC INJURY WOUND REPAIR FOREIGN BODY ACCIDENTALLY LEFT DURING A PROCEDURE NOT ELSEWHERE CLASSIFIED INFECTED POSTOPERATIVE SEROMA OTHER POSTOPERATIVE INFECTION PERSISTENT POSTOPERATIVE FISTULA NOT ELSEWHERE CLASSIFIED ACUTE REACTION TO FOREIGN SUBSTANCE ACCIDENTALLY LEFT DURING A PROCEDURE NOT ELSEWHERE CLASSIFIED EMPHYSEMA (SUBCUTANEOUS) (SURGICAL) RESULTING FROM PROCEDURE CATARACT FRAGMENTS IN EYE FOLLOWING CATARACT SURGERY NON-HEALING SURGICAL WOUND V12.54* V58.73 OTHER SPECIFIED COMPLICATIONS OF PROCEDURES NOT ELSEWHERE CLASSIFIED UNSPECIFIED COMPLICATION OF PROCEDURE NOT ELSEWHERE CLASSIFIED PERSONAL HISTORY OF TRANSIENT ISCHEMIC ATTACK (TIA), AND CEREBRAL INFARCTION WITHOUT RESIDUAL DEFICITS AFTERCARE FOLLOWING SURGERY OF THE CIRCULATORY SYSTEM NOT ELSEWHERE CLASSIFIED V67.00 FOLLOW-UP EXAMINATION FOLLOWING UNSPECIFIED SURGERY * While ICD-9-CM is listed as a covered diagnosis code, it is important to note that dizziness and giddiness alone are not usual indications unless associated with other localizing signs and symptoms. Group 2 Two diagnoses are required: Either V72.81 or V72.83
19 Plus one of the following diagnoses: * * * * * * * * * * * * * * * * * * ACUTE MYOCARDIAL INFARCTION OF ANTEROLATERAL WALL EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF ANTEROLATERAL WALL INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF ANTEROLATERAL WALL SUBSEQUENT EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF OTHER ANTERIOR WALL EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF OTHER ANTERIOR WALL INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF OTHER ANTERIOR WALL SUBSEQUENT EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF INFEROLATERAL WALL EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF INFEROLATERAL WALL INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF INFEROLATERAL WALL SUBSEQUENT EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF INFEROPOSTERIOR WALL EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF INFEROPOSTERIOR WALL INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF INFEROPOSTERIOR WALL SUBSEQUENT EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF OTHER INFERIOR WALL EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF OTHER INFERIOR WALL INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF OTHER INFERIOR WALL SUBSEQUENT EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF OTHER LATERAL WALL EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF OTHER LATERAL WALL INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF OTHER LATERAL WALL SUBSEQUENT EPISODE OF CARE
20 410.60* TRUE POSTERIOR WALL INFARCTION EPISODE OF CARE UNSPECIFIED * TRUE POSTERIOR WALL INFARCTION INITIAL EPISODE OF CARE * TRUE POSTERIOR WALL INFARCTION SUBSEQUENT EPISODE OF CARE * SUBENDOCARDIAL INFARCTION EPISODE OF CARE UNSPECIFIED * SUBENDOCARDIAL INFARCTION INITIAL EPISODE OF CARE * SUBENDOCARDIAL INFARCTION SUBSEQUENT EPISODE OF CARE * * * * * * ACUTE MYOCARDIAL INFARCTION OF OTHER SPECIFIED SITES EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF OTHER SPECIFIED SITES INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF OTHER SPECIFIED SITES SUBSEQUENT EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF UNSPECIFIED SITE EPISODE OF CARE UNSPECIFIED ACUTE MYOCARDIAL INFARCTION OF UNSPECIFIED SITE INITIAL EPISODE OF CARE ACUTE MYOCARDIAL INFARCTION OF UNSPECIFIED SITE SUBSEQUENT EPISODE OF CARE 411.0* POSTMYOCARDIAL INFARCTION SYNDROME 411.1* INTERMEDIATE CORONARY SYNDROME * ACUTE CORONARY OCCLUSION WITHOUT MYOCARDIAL INFARCTION * OTHER ACUTE AND SUBACUTE FORMS OF ISCHEMIC HEART DISEASE OTHER 412* OLD MYOCARDIAL INFARCTION 413.0* ANGINA DECUBITUS 413.1* PRINZMETAL ANGINA 413.9* OTHER AND UNSPECIFIED ANGINA PECTORIS * CORONARY ATHEROSCLEROSIS OF UNSPECIFIED TYPE OF VESSEL NATIVE OR GRAFT * CORONARY ATHEROSCLEROSIS OF NATIVE CORONARY ARTERY * CORONARY ATHEROSCLEROSIS OF AUTOLOGOUS VEIN BYPASS GRAFT
21 414.03* CORONARY ATHEROSCLEROSIS OF NONAUTOLOGOUS BIOLOGICAL BYPASS GRAFT * CORONARY ATHEROSCLEROSIS OF ARTERY BYPASS GRAFT * CORONARY ATHEROSCLEROSIS OF UNSPECIFIED BYPASS GRAFT * * CORONARY ATHEROSCLEROSIS OF NATIVE CORONARY ARTERY OF TRANSPLANTED HEART CORONARY ATHEROSCLEROSIS OF BYPASS GRAFT (ARTERY) (VEIN) OF TRANSPLANTED HEART * ANEURYSM OF HEART (WALL) * ANEURYSM OF CORONARY VESSELS * DISSECTION OF CORONARY ARTERY * OTHER ANEURYSM OF HEART 414.2* CHRONIC TOTAL OCCLUSION OF CORONARY ARTERY 414.3* CORONARY ATHEROSCLEROSIS DUE TO LIPID RICH PLAQUE 414.8* OTHER SPECIFIED FORMS OF CHRONIC ISCHEMIC HEART DISEASE 414.9* CHRONIC ISCHEMIC HEART DISEASE UNSPECIFIED 440.0* ATHEROSCLEROSIS OF AORTA 440.1* ATHEROSCLEROSIS OF RENAL ARTERY * * * * * ATHEROSCLEROSIS OF NATIVE ARTERIES OF THE EXTREMITIES UNSPECIFIED ATHEROSCLEROSIS OF NATIVE ARTERIES OF THE EXTREMITIES WITH INTERMITTENT CLAUDICATION ATHEROSCLEROSIS OF NATIVE ARTERIES OF THE EXTREMITIES WITH REST PAIN ATHEROSCLEROSIS OF NATIVE ARTERIES OF THE EXTREMITIES WITH ULCERATION ATHEROSCLEROSIS OF NATIVE ARTERIES OF THE EXTREMITIES WITH GANGRENE * OTHER ATHEROSCLEROSIS OF NATIVE ARTERIES OF THE EXTREMITIES * ATHEROSCLEROSIS OF UNSPECIFIED BYPASS GRAFT OF THE EXTREMITIES
22 440.31* * ATHEROSCLEROSIS OF AUTOLOGOUS VEIN BYPASS GRAFT OF THE EXTREMITIES ATHEROSCLEROSIS OF NONAUTOLOGOUS BIOLOGICAL BYPASS GRAFT OF THE EXTREMITIES 440.4* CHRONIC TOTAL OCCLUSION OF ARTERY OF THE EXTREMITIES V72.81* V72.83* PRE-OPERATIVE CARDIOVASCULAR EXAMINATION OTHER SPECIFIED PRE-OPERATIVE EXAMINATION *Note: Two ICD-9-CM Codes are required for payment. Either V72.81 or V72.83 plus any of , 440.0, 440.1, , , Diagnoses that Support Medical Necessity All diagnoses listed in ICD-9-CM Codes That Support Medical Necessity above. ICD-9 Codes that DO NOT Support Medical Necessity All diagnoses not listed in ICD-9-CM Codes That Support Medical Necessity above. ICD-9 Codes that DO NOT Support Medical Necessity Asterisk Explanation Diagnoses that DO NOT Support Medical Necessity All diagnoses not listed in ICD-9-CM Codes That Support Medical Necessity above. Back to Top General Information Documentations Requirements The provider must ensure documentation showing reasonableness and necessity of the procedures are kept on file and made available upon request by the Medicare carrier. When using syncope as an indication, it is necessary to document that other more common causes have been ruled out. The accuracy of noninvasive vascular diagnostic studies depends on the knowledge, skills and experience of the technologist and physician performing and interpreting the study. It is recommended that noninvasive vascular studies either be rendered in a physician's office by/or under the direct supervision of persons credentialed in the specific type of procedure being performed or performed in laboratories accredited in the specific type of evaluation. Noninvasive vascular studies done in an IDTF facility or vascular laboratory are subject to the rules and regulations governing the facility. This A/B MAC is not a credentialing body; therefore, this LCD will recommend certification, but not recommend certifying bodies. The HCPCS/CPT code(s) may be subject to Correct Coding Initiative (CCI) edits. This policy does not take precedence over CCI edits. Please refer to the CCI for correct coding guidelines and specific applicable code combinations prior to billing Medicare.
23 When the documentation does not meet the criteria for the service rendered or the documentation does not establish the medical necessity for the services, such services will be denied as not reasonable and necessary. When requesting a written redetermination (formerly appeal), providers must include all relevant documentation with the request. Appendices Utilization Guidelines Sources of Information and Basis for Decision 1. Drader KS, Herrick IA. Carotid Endarterectomy: Monitoring and Its Effect on Outcome. Anesthesiology Clinics of North America. Sep 1997;15(3): Endarterectomy for Asymptomatic Carotid Artery Stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. May 1995;273(18): ICAVL Essentials and Standards for Accreditation in Noninvasive Vascular Testing; Part II, Vascular Laboratory Operations, Cerebrovascular Testing Illustrated Guide to Diagnostic Tests. 2nd ed. Springhouse Corporation. 1998:779-84, Carrier Medical Directors and Consultants NOTE: Some of the websites used to create this policy may no longer be available. Advisory Committee Meeting Notes This policy does not reflect the sole opinion of the contractor or Contractor Medical Director. Although the final decision rests with the contractor, this policy was developed in cooperation with advisory groups, which include representatives from the affected provider community. Contractor Advisory Committee meeting dates: California - Hawaii - Nevada - Start Date of Comment Period End Date of Comment Period Start Date of Notice Period 06/16/2008 Revision History Number Revision #9 Revision History Explanation Revision #9, effective for dates of service on or after 01/01/2012 Revision made: CPT/HCPCS Codes removed invalid CPT code This revision is the result of the 2012 Annual CPT/HCPCS Update. Revision #8, effective for dates of service on or after 10/01/2011 Revisions made: Under ICD-9 Codes that Support Medical Necessity deleted The descriptors for , & were revised. This LCD is being revised due to the annual FY 2012 ICD-9-CM code update. Revision #7, effective for dates of service on or after 10/01/2010
24 Revision made: Under ICD-9 codes that Support Medical Necessity the following ICD-9 code was added to this LCD: This change was the result of Medicare Contractor Annual Update of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) CR 7006, Transmittal Revision #6, effective for dates of service on or after 6/24/2010 Revision made: Under Sources of Information and Basis for Decision authors names Drader KS,and Herrick IA. were added to the article "Carotid Endarterectomy: Monitoring and Its Effect on Outcome." Added the complete title to the Endarterectomy fo Asymptomatic Carotid Artery Stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study and the Month it was published in JAMA May Removed two citations as these were unable to be located Non-Invasive Vascular Diagnostic Studies, Physician's Procedural Terminology. American Medical Association, 1999:368 and TCD During Carotid Endarterectomy, as the website was no longer valid. Revision #5 effective for dates of service on or after 10/01/2009. Revisions made: Under "ICD-9 Codes that Support Medical Necessity", the following ICD- 9 codes were added to support the medical necessity for CPT codes 93875, 93880, 93882, and 93888: was expanded and only was added, , was expanded to and ICD-9 code descriptor was revised. These changes are per CMS Manual System, Publication , Medicare Claims Processing, Chapter 23, 10.2; CR 6520, Transmittal 1770, dated July 10, 2009; Annual Update of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Revision #4, effective for dates of service on or after 07/09/2009 Revisions made: Under "CMS Coverage Policy" added reference Pub , Medicare Benefit Policy Manual Chapter 15, Under Sources of Information and Basis for Decision removed extended page numbers under JAMA citation and Illustrated Guide to Diagnostic Tests citation. Revision #3, 02/26/2009 This LCD is being revised to implement the streamlining of the Part B LCDs per the published article Palmetto Team to Streamline Part B LCDs in Jurisdiction 1 (J1). This article can be viewed at by searching for the above article name. This revision will become effective on 02/26/2009. Revision #2, 10/01/2008 This LCD is being revised due to the annual FY 2009 ICD-9-CM code update. Under CMS National Coverage Policy deleted verbiage. Under ICD-9 Codes That Support Medical Necessity-Group 1 added ICD-9 codes and The verbiage was revised for ICD-9 codes , , , , and under Group 1. Added ICD-9 code under ICD-9 Codes That Support Medical Necessity- Group 2. The paragraphs regarding the 2008 ICD-9-CM updates were removed due to completion of those updates. Under Documentation Requirements removed duplicative SSA citation. Under Sources of Information and Basis for Decision the references were placed in the AMA citation format. This revision becomes effective 10/01/2008. Revision #1, 09/02/2008 This LCD is being revised to add Bill Type 999X because the automated system transcription process was incomplete. 08/08/ This policy was updated by the ICD Annual Update. 08/27/ This policy was updated by the ICD Annual Update.
25 11/21/ The following CPT/HCPCS codes were deleted: was deleted from Group 1 Reason for Change Maintenance (annual review with new changes, formatting, etc.) Other Related Documents This LCD has no Related Documents. LCD Attachments There are no attachments for this LCD. Back to Top All Versions Updated on 04/13/2012 with effective dates 01/01/ N/A Updated on 11/23/2011 with effective dates 01/01/ N/A Updated on 09/14/2011 with effective dates 10/01/ /31/2011 Updated on 06/16/2011 with effective dates 10/01/ /30/2011 Updated on 09/10/2010 with effective dates 10/01/ N/A Some older versions have been archived. Please visit the MCD Archive Site them. to retrieve Read the LCD Disclaimer Back to Top Department of Health & Human Services Medicare.gov USA.gov Web Policies & Important Links Privacy Policy Freedom of Information Act No Fear Act Centers for Medicare & Medicaid Services, 7500 Security Boulevard Baltimore, MD
CMS Limitations Guide - Radiology Services
 CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Any vascular studies performed should be as a result of, or to complement, a thorough patient evaluation and neurological examination.
 National Imaging Associates, Inc. Clinical guidelines NON-INVASIVE CEREBROVASCULAR ARTERIALS TUDIES Original Date: October 2015 Page 1 of 8 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes: Please refer to page
National Imaging Associates, Inc. Clinical guidelines NON-INVASIVE CEREBROVASCULAR ARTERIALS TUDIES Original Date: October 2015 Page 1 of 8 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes: Please refer to page
Contractor Information. LCD Information. Local Coverage Determination (LCD): Magnetic Resonance Angiography (L34424) Document Information
 Local Coverage Determination (LCD): Magnetic Resonance Angiography (L34424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Magnetic Resonance Angiography (L34424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 93875 Non-invasive physiologic studies of extracranial arteries, complete bilateral study (eg, periorbital
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 93875 Non-invasive physiologic studies of extracranial arteries, complete bilateral study (eg, periorbital
CMS Limitations Guide MRA Radiology Services
 CMS Limitations Guide MRA Radiology Services Starting July 1, 2008, CMS has placed numerous medical necessity limits on tests and procedures. This reference guide provides you with all of the latest changes.
CMS Limitations Guide MRA Radiology Services Starting July 1, 2008, CMS has placed numerous medical necessity limits on tests and procedures. This reference guide provides you with all of the latest changes.
Medical Review Guidelines Magnetic Resonance Angiography
 Medical Review Guidelines Magnetic Resonance Angiography Medical Guideline Number: MRG2001-05 Effective Date: 2/13/01 Revised Date: 2/14/2006 OHCA Reference OAC 317:30-5-24. Radiology. (f) Magnetic Resonance
Medical Review Guidelines Magnetic Resonance Angiography Medical Guideline Number: MRG2001-05 Effective Date: 2/13/01 Revised Date: 2/14/2006 OHCA Reference OAC 317:30-5-24. Radiology. (f) Magnetic Resonance
Local Coverage Determination (LCD) for Endoscopic Treatment of GERD (L28256)
 Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Contractor Information. LCD Information. Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Document Information
 Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
FLORIDA MEDICARE PART B LOCAL MEDICAL REVIEW POLICY
 FLORIDA MEDICARE PART B LOCAL MEDICAL REVIEW POLICY CPT/HCPCS Codes 93925 Duplex scan of lower extremity arteries or arterial bypass grafts; complete bilateral study 93926 unilateral or limited study Policy
FLORIDA MEDICARE PART B LOCAL MEDICAL REVIEW POLICY CPT/HCPCS Codes 93925 Duplex scan of lower extremity arteries or arterial bypass grafts; complete bilateral study 93926 unilateral or limited study Policy
Local Coverage Determination for Colorectal Cancer Screening (L29796)
 Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
LCD Information Document Information LCD ID Number L30046
 Local Coverage Determination (LCD): Pathology and Laboratory: B-type Natriuretic Peptide (BNP) Testing (L30046) LCD Information Document Information LCD ID Number L30046 LCD Title Pathology and Laboratory:
Local Coverage Determination (LCD): Pathology and Laboratory: B-type Natriuretic Peptide (BNP) Testing (L30046) LCD Information Document Information LCD ID Number L30046 LCD Title Pathology and Laboratory:
Local Coverage Determination (LCD) for Noninvasive Peripheral Arterial Studies (L28284)
 Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Search Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & Education People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Nicolas Bianchi M.D. May 15th, 2012
 Nicolas Bianchi M.D. May 15th, 2012 New concepts in TIA Differential Diagnosis Stroke Syndromes To learn the new definitions and concepts on TIA as a condition of high risk for stroke. To recognize the
Nicolas Bianchi M.D. May 15th, 2012 New concepts in TIA Differential Diagnosis Stroke Syndromes To learn the new definitions and concepts on TIA as a condition of high risk for stroke. To recognize the
LCD L B-type Natriuretic Peptide (BNP) Assays
 LCD L30559 - B-type Natriuretic Peptide (BNP) Assays Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302, 12401,
LCD L30559 - B-type Natriuretic Peptide (BNP) Assays Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302, 12401,
Guidelines for Ultrasound Surveillance
 Guidelines for Ultrasound Surveillance Carotid & Lower Extremity by Ian Hamilton, Jr, MD, MBA, RPVI, FACS Corporate Medical Director BlueCross BlueShield of Tennessee guidelines for ultrasound surveillance
Guidelines for Ultrasound Surveillance Carotid & Lower Extremity by Ian Hamilton, Jr, MD, MBA, RPVI, FACS Corporate Medical Director BlueCross BlueShield of Tennessee guidelines for ultrasound surveillance
Jurisdiction Georgia. Retirement Date N/A
 If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): Surgery: Injections of the Spinal Canal (L32112) Contractor Information
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090)
 Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Noninvasive Extracranial Arterial Studies
 Noninvasive Extracranial Arterial Studies Noridian Healthcare Solutions, LLC Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the current policies
Noninvasive Extracranial Arterial Studies Noridian Healthcare Solutions, LLC Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the current policies
Lnformation Coverage Guidance
 Lnformation Coverage Guidance Coverage Indications, Limitations, and/or Medical Necessity Abstract: B-type natriuretic peptide (BNP) is a cardiac neurohormone produced mainly in the left ventricle. It
Lnformation Coverage Guidance Coverage Indications, Limitations, and/or Medical Necessity Abstract: B-type natriuretic peptide (BNP) is a cardiac neurohormone produced mainly in the left ventricle. It
Local Coverage Determination for Hospice - Liver Disease (L31536)
 Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Local Coverage Determination for Hospice Alzheimer's Disease &Related Disorders (L31539)
 Page 1 of 6 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Page 1 of 6 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice)
 Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Local Coverage Determination (LCD) for Chiropractic Services (L34816) (Posted for Notice) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 04911, 07101, 07102, 07201,
Jurisdiction New Mexico. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
Local Coverage Determination (LCD): Chiropractic Services (L34816) Contractor Information Contractor Name Novitas Solutions, Inc. opens in new Contract Number 04212 Contract Type A and B MAC J - H LCD
12102, 12202, 12302, 12501, 12301, 12201, 12401, 12402, 12101, 12502, 12901
 https://www.novitas-solutions.com/policy/mac-ab/l30827-r5.html LCD L30827 - Non-Invasive Peripheral Arterial Studies Print Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s):
https://www.novitas-solutions.com/policy/mac-ab/l30827-r5.html LCD L30827 - Non-Invasive Peripheral Arterial Studies Print Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s):
The determination of eligible population for this measure requires administrative claims data.
 Overuse of Imaging Measure 6: Ratio of Magnetic Resonance Imaging Scans to Computed Tomography Scans for the Evaluation of Children with Atraumatic Headache Description This measure assesses the ratio
Overuse of Imaging Measure 6: Ratio of Magnetic Resonance Imaging Scans to Computed Tomography Scans for the Evaluation of Children with Atraumatic Headache Description This measure assesses the ratio
Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1
 GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
GE Healthcare Reimbursement Information for Diagnostic Ultrasound and Ultrasound-guided Vascular Procedures 1 January, 2013 www.gehealthcare.com/reimbursement This overview addresses coding, coverage,
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 70544 Magnetic resonance angiography, head; without contrast material(s) 70545 with contrast material(s)
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 70544 Magnetic resonance angiography, head; without contrast material(s) 70545 with contrast material(s)
Contractor Name: Novitas Solutions, Inc. Contractor Number: Contractor Type: MAC B. LCD ID Number: L34834 Status: A-Approved
 LCD for Blood Glucose Monitoring in a Skilled Nursing Facility (SNF) (L34834) Contractor Name: Novitas Solutions, Inc. Contractor Number: 12502 Contractor Type: MAC B LCD ID Number: L34834 Status: A-Approved
LCD for Blood Glucose Monitoring in a Skilled Nursing Facility (SNF) (L34834) Contractor Name: Novitas Solutions, Inc. Contractor Number: 12502 Contractor Type: MAC B LCD ID Number: L34834 Status: A-Approved
Physician s Vascular Interpretation Examination Content Outline
 Physician s Vascular Interpretation Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 6 Cerebrovascular Abdominal Peripheral Arterial - Duplex Imaging Peripheral Arterial
Physician s Vascular Interpretation Examination Content Outline (Outline Summary) # Domain Subdomain Percentage 1 2 3 4 5 6 Cerebrovascular Abdominal Peripheral Arterial - Duplex Imaging Peripheral Arterial
Local Coverage Article for Chiropractic Services (A47798) Contractor Information. Article Information. Contractor Name. Contractor Numbers
 Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
Local Coverage Article for Chiropractic Services (A47798) Print Contractor Information Contractor Name Novitas Solutions, Inc. Contractor Numbers 12501, 12502, 12101, 12102, 12201, 12202, 12301, 12302,
Local Coverage Determination (LCD): RAST Type Tests ( L30524 )
 Page 2 of 6 Local Coverage Determination (LCD): RAST Type Tests ( L30524 ) Contractor Information Contractor Name Novitas Solutions, Inc. Contract Number 12502 Contract Type A and B MAC LCD Information
Page 2 of 6 Local Coverage Determination (LCD): RAST Type Tests ( L30524 ) Contractor Information Contractor Name Novitas Solutions, Inc. Contract Number 12502 Contract Type A and B MAC LCD Information
Local Coverage Determination for Hospice The Adult Failure To Thrive Syndrome (L31541)
 Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Page 1 of 5 Centers for Medicare & Medicaid Services Print Message: If you are experiencing issues printing this page, then please click Return to Previous Page and select the 'Need a PDF?' button. You
Contractor Number Oversight Region Region IV
 Local Coverage Determination (LCD) for Hospice - Renal Care (L31538) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11004 Contractor Type HHH MAC LCD Information
Local Coverage Determination (LCD) for Hospice - Renal Care (L31538) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11004 Contractor Type HHH MAC LCD Information
Corporate Medical Policy
 Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Contractor Information. LCD Information. Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Document Information
 Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information Contract Number
Local Coverage Determination (LCD): Hospice - Neurological Conditions (L31537) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information Contract Number
ICD-9 to ICD-10 Crosswalk Adult Codes
 ICD- to ICD- Crosswalk Adult Codes On October 1, 2015, the Centers for Medicare & Medicaid Services (CMS) transitioned to the new International Classification of Diseases, th Revision System (ICD-), which
ICD- to ICD- Crosswalk Adult Codes On October 1, 2015, the Centers for Medicare & Medicaid Services (CMS) transitioned to the new International Classification of Diseases, th Revision System (ICD-), which
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 Local Coverage Determination (LCD): Drugs and Biologicals: Botulinum Toxins (L34253) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Drugs and Biologicals: Botulinum Toxins (L34253) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
2018 Diagnosis Coding Fact Sheet
 The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
The information contained in this document is provided for informational purposes only and represents no statement, promise, or guarantee by Cordis Corporation concerning levels of reimbursement, payment,
Clinical Policy: Cardiac Biomarker Testing for Acute Myocardial Infarction Reference Number: CP.MP.156
 Clinical Policy: Reference Number: CP.MP.156 Effective Date: 12/17 Last Review Date: 12/17 See Important Reminder at the end of this policy for important regulatory and legal information. Description The
Clinical Policy: Reference Number: CP.MP.156 Effective Date: 12/17 Last Review Date: 12/17 See Important Reminder at the end of this policy for important regulatory and legal information. Description The
Contract Number
 Source: Part A - Novitas MAC - JL Chapter: Subject: Magnetic Resonance Angiography (MRA) Version: 2015-10-01 - Contractor Name Novitas Solutions, Inc. Contract Number 12101 12201 12301 12401 12501 12901
Source: Part A - Novitas MAC - JL Chapter: Subject: Magnetic Resonance Angiography (MRA) Version: 2015-10-01 - Contractor Name Novitas Solutions, Inc. Contract Number 12101 12201 12301 12401 12501 12901
ICD 10 CM Coding and Documentation
 ICD 10 CM Coding and Documentation Adult Day Health Care Council Karen L. Fabrizio, RHIA, CHTS CP, CPRA April 10, 2014 Presented by: Karen Fabrizio, RHIA CHTS CP CPRA is an AHIMA Approved ICD 10 CM/PCS
ICD 10 CM Coding and Documentation Adult Day Health Care Council Karen L. Fabrizio, RHIA, CHTS CP, CPRA April 10, 2014 Presented by: Karen Fabrizio, RHIA CHTS CP CPRA is an AHIMA Approved ICD 10 CM/PCS
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Circulating Tumor Cell Marker Assays (L35096) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
b. To facilitate the management decision of a patient with an equivocal stress test.
 National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
National Imaging Associates, Inc. Clinical guidelines EBCT HEART CT & HEART CT CONGENITAL CCTA CPT4 Codes: 75571 EBCT 75572, 75573 Heart CT & Heart CT Congenital 75574 - CCTA LCD ID Number: L33559 J K
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments
 CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
CY2017 Hospital Outpatient: Vascular Procedure APCs and Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) In CY2015 and in an effort to help pay providers for quality, not
HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM
 REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
Contractor Information. LCD Information. Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426)
 Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
/ / / / / / Hospital Abstraction: Stroke/TIA. Participant ID: Hospital Code: Multi-Ethnic Study of Atherosclerosis
 Multi-Ethnic Study of Atherosclerosis Participant ID: Hospital Code: Hospital Abstraction: Stroke/TIA History and Hospital Record 1. Was the participant hospitalized as an immediate consequence of this
Multi-Ethnic Study of Atherosclerosis Participant ID: Hospital Code: Hospital Abstraction: Stroke/TIA History and Hospital Record 1. Was the participant hospitalized as an immediate consequence of this
Student Outline. Improving Transportation Safety: Commercial Driver Medical Examiner Training CHAPTER 1. General FMCSA Information
 Student Outline CHAPTER 1 General FMCSA Information FMCSA Mission Statement / Dedicated to Safety / NRCME Important Definitions Regulations Vs. Medical Guidelines Privacy and the Medical Examination 13
Student Outline CHAPTER 1 General FMCSA Information FMCSA Mission Statement / Dedicated to Safety / NRCME Important Definitions Regulations Vs. Medical Guidelines Privacy and the Medical Examination 13
Maya S. Safarova MD, PhD Carin Y. Smith Iftikhar J. Kullo MD
 CODES SUPPLEMENTARY MATERIAL TEAM (Mayo Clinic, Rochester, MN) Maya S. Safarova MD, PhD Safarova.Mayya@mayo.edu Carin Y. Smith Smith.Carin@mayo.edu Iftikhar J. Kullo MD Kullo.Iftikhar@mayo.edu Updated
CODES SUPPLEMENTARY MATERIAL TEAM (Mayo Clinic, Rochester, MN) Maya S. Safarova MD, PhD Safarova.Mayya@mayo.edu Carin Y. Smith Smith.Carin@mayo.edu Iftikhar J. Kullo MD Kullo.Iftikhar@mayo.edu Updated
Contractor Information. LCD Information. Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539)
 Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information
Local Coverage Determination (LCD): Hospice Alzheimer's Disease & Related Disorders (L31539) Contractor Information Contractor Name Palmetto GBA opens in new window LCD Information Document Information
Stroke School for Internists Part 1
 Stroke School for Internists Part 1 November 4, 2017 Dr. Albert Jin Dr. Gurpreet Jaswal Disclosures I receive a stipend for my role as Medical Director of the Stroke Network of SEO I have no commercial
Stroke School for Internists Part 1 November 4, 2017 Dr. Albert Jin Dr. Gurpreet Jaswal Disclosures I receive a stipend for my role as Medical Director of the Stroke Network of SEO I have no commercial
SAMPLE. Laboratory Services. An essential coding, billing, and reimbursement resource for laboratory and pathology services ICD-10
 Coding and Payment Guide www.optumcoding.com Laboratory Services An essential coding, billing, and reimbursement resource for laboratory and pathology services 2017 ICD-10 A full suite of resources including
Coding and Payment Guide www.optumcoding.com Laboratory Services An essential coding, billing, and reimbursement resource for laboratory and pathology services 2017 ICD-10 A full suite of resources including
Overview of Stroke: Etiologies, Demographics, Syndromes, and Outcomes. Alex Abou-Chebl, MD, FSVIN Medical Director, Stroke Baptist Health Louisville
 Overview of Stroke: Etiologies, Demographics, Syndromes, and Outcomes Alex Abou-Chebl, MD, FSVIN Medical Director, Stroke Baptist Health Louisville Disclosure Statement of Financial Interest Within the
Overview of Stroke: Etiologies, Demographics, Syndromes, and Outcomes Alex Abou-Chebl, MD, FSVIN Medical Director, Stroke Baptist Health Louisville Disclosure Statement of Financial Interest Within the
CMS Limitations Guide - Cardiovascular Services
 CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Cerebrovascular Disorders. Blood, Brain, and Energy. Blood Supply to the Brain 2/14/11
 Cerebrovascular Disorders Blood, Brain, and Energy 20% of body s oxygen usage No oxygen/glucose reserves Hypoxia - reduced oxygen Anoxia - Absence of oxygen supply Cell death can occur in as little as
Cerebrovascular Disorders Blood, Brain, and Energy 20% of body s oxygen usage No oxygen/glucose reserves Hypoxia - reduced oxygen Anoxia - Absence of oxygen supply Cell death can occur in as little as
Cancer and Heart/Stroke
 Cancer and Heart/Stroke A plan providing cash benefits to help pay for out-of-pocket costs associated with a cancer, heart attack or stroke diagnosis National General Accident and Health markets products
Cancer and Heart/Stroke A plan providing cash benefits to help pay for out-of-pocket costs associated with a cancer, heart attack or stroke diagnosis National General Accident and Health markets products
2019 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule
 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
Contractor Information. LCD Information. Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426)
 Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
Cardiothoracic and Cardiothoracic Surgery ICD-10-CM 2014: Reference Mapping Card
 2014: Reference Mapping Card 162.3 Malignant neoplasm upper lobe lung 162.5 Malignant neoplasm lower lobe lung 162.9 lung/bronchus 396.2 396.3 Mitral insufficiency, aortic stenosis Mitral aortic valve
2014: Reference Mapping Card 162.3 Malignant neoplasm upper lobe lung 162.5 Malignant neoplasm lower lobe lung 162.9 lung/bronchus 396.2 396.3 Mitral insufficiency, aortic stenosis Mitral aortic valve
Documentation for the IRF Provider
 Documentation for the IRF Provider Timothy N. Brundage, MD, CCDS Certified Clinical Documentation Specialist DrBrundage@gmail.com 1 Medicare controls the ball field If you want to play ball, you have to
Documentation for the IRF Provider Timothy N. Brundage, MD, CCDS Certified Clinical Documentation Specialist DrBrundage@gmail.com 1 Medicare controls the ball field If you want to play ball, you have to
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
Emergency Department Stroke Registry Indicator Specifications 2018 Report Year (07/01/2017 to 06/30/2018 Discharge Dates)
 2018 Report Year (07/01/2017 to 06/30/2018 Discharge Dates) Summary of Changes I62.9 added to hemorrhagic stroke ICD-10-CM diagnosis code list (table 3) Measure Description Methodology Rationale Measurement
2018 Report Year (07/01/2017 to 06/30/2018 Discharge Dates) Summary of Changes I62.9 added to hemorrhagic stroke ICD-10-CM diagnosis code list (table 3) Measure Description Methodology Rationale Measurement
CVA. Alison Atwater PA-C
 CVA Alison Atwater PA-C Types of CVAs Ischemic strokes 80% of strokes 2/3 are thrombotic 1/3 are embolic emboli from the heart or arteries feeding the brain such as carotids, vertebral and basilar etc
CVA Alison Atwater PA-C Types of CVAs Ischemic strokes 80% of strokes 2/3 are thrombotic 1/3 are embolic emboli from the heart or arteries feeding the brain such as carotids, vertebral and basilar etc
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 70450 Computed tomography, head or brain; without contrast material 70460 with contrast material(s) 70470
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 70450 Computed tomography, head or brain; without contrast material 70460 with contrast material(s) 70470
Cerebral Vascular Diseases. Nabila Hamdi MD, PhD
 Cerebral Vascular Diseases Nabila Hamdi MD, PhD Outline I. Stroke statistics II. Cerebral circulation III. Clinical symptoms of stroke IV. Pathogenesis of cerebral infarcts (Stroke) 1. Ischemic - Thrombotic
Cerebral Vascular Diseases Nabila Hamdi MD, PhD Outline I. Stroke statistics II. Cerebral circulation III. Clinical symptoms of stroke IV. Pathogenesis of cerebral infarcts (Stroke) 1. Ischemic - Thrombotic
PTA 106 Unit 1 Lecture 3
 PTA 106 Unit 1 Lecture 3 The Basics Arteries: Carry blood away from the heart toward tissues. They typically have thicker vessels walls to handle increased pressure. Contain internal and external elastic
PTA 106 Unit 1 Lecture 3 The Basics Arteries: Carry blood away from the heart toward tissues. They typically have thicker vessels walls to handle increased pressure. Contain internal and external elastic
Subclavian artery Stenting
 Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Cardiovascular nuclear imaging employs non-invasive techniques to assess alterations in coronary artery flow, and ventricular function.
 National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
Local Coverage Determination (LCD): Scanning Computerized Ophthalmic Diagnostic Imaging (SCODI) (L34431)
 Local Coverage Determination (LCD): Scanning Computerized Ophthalmic Diagnostic Imaging (SCODI) (L34431) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
Local Coverage Determination (LCD): Scanning Computerized Ophthalmic Diagnostic Imaging (SCODI) (L34431) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
2015 Facility and Physician Billing Guide Heart Valve Technologies
 2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Texas. Retirement Date N/A
 Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Chiropractic Services (L35424) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483)
 moldx: Chromosome 1p/19q deletion analysis (DL36483) Page 1 of 8 PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483) Close Section Navigation
moldx: Chromosome 1p/19q deletion analysis (DL36483) Page 1 of 8 PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Chromosome 1p/19q deletion analysis (DL36483) Close Section Navigation
POLICY AND PROCEDURE
 PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
PAGE: Page 1 of 8 SCOPE: This policy applies to any provider furnishing services represented by Category III CPT codes. PURPOSE & IMPORTANT REMINDER: This policy is current at the time of publication.
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.049.MH Visually Evoked Response Test This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar CareFirst
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.049.MH Visually Evoked Response Test This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar CareFirst
Ultrasound and Fluoroscopic Paravertebral Facet Joint Injections
 Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Policy Number FAC06222011RP Ultrasound and Fluoroscopic Approved By UnitedHealthcare Medicare Committee Current Approval Date 06/25/2014 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
MolDX: HLA-DQB1*06:02 Testing for Narcolepsy
 MolDX: HLA-DQB1*06:02 Testing for Narcolepsy CGS Administrators, LLC Jump to Section... Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the
MolDX: HLA-DQB1*06:02 Testing for Narcolepsy CGS Administrators, LLC Jump to Section... Please Note: This is a Proposed LCD. Proposed LCDs are works in progress and not necessarily a reflection of the
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Oregon. Retirement Date N/A
 Local Coverage Determination (LCD): MolDX: GeneSight Assay for Refractory Depression (L36324) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Local Coverage Determination (LCD): MolDX: GeneSight Assay for Refractory Depression (L36324) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Key Clinical Concepts
 Cerebrovascular Review and General Vascular Syndromes, Including Those That Impact Dizziness Key Clinical Concepts Basic Review of Cerebrovascular Circulation Circulation to the brain is divided into anterior
Cerebrovascular Review and General Vascular Syndromes, Including Those That Impact Dizziness Key Clinical Concepts Basic Review of Cerebrovascular Circulation Circulation to the brain is divided into anterior
Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft
 MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
MEDICAL Reimbursement Guide Zenith Fenestrated AAA Endovascular Graft Disclaimer: The information provided herein reflects Cook s understanding of the procedure(s) and/or device(s) from sources that may
Icd 10 code for subclavian artery disease
 Icd 10 code for subclavian artery disease The Borg System is 100 % Icd 10 code for subclavian artery disease Icd 10 code for subclavian artery disease -- Sometimes it turns violent would help bridge that
Icd 10 code for subclavian artery disease The Borg System is 100 % Icd 10 code for subclavian artery disease Icd 10 code for subclavian artery disease -- Sometimes it turns violent would help bridge that
Stroke/TIA. Tom Bedwell
 Stroke/TIA Tom Bedwell tab1g11@soton.ac.uk The Plan Definitions Anatomy Recap Aetiology Pathology Syndromes Brocas / Wernickes Investigations Management Prevention & Prognosis TIAs Key Definitions Transient
Stroke/TIA Tom Bedwell tab1g11@soton.ac.uk The Plan Definitions Anatomy Recap Aetiology Pathology Syndromes Brocas / Wernickes Investigations Management Prevention & Prognosis TIAs Key Definitions Transient
Premier Health Plan considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL MP.091.PH - Intravascular Ultrasound for Coronary Vessels This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Premier Health Plan POLICY AND PROCEDURE MANUAL MP.091.PH - Intravascular Ultrasound for Coronary Vessels This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Vascular Disorders. Nervous System Disorders (Part B-1) Module 8 -Chapter 14. Cerebrovascular disease S/S 1/9/2013
 Nervous System Disorders (Part B-1) Module 8 -Chapter 14 Overview ACUTE NEUROLOGIC DISORDERS Vascular Disorders Infections/Inflammation/Toxins Metabolic, Endocrinologic, Nutritional, Toxic Neoplastic Traumatic
Nervous System Disorders (Part B-1) Module 8 -Chapter 14 Overview ACUTE NEUROLOGIC DISORDERS Vascular Disorders Infections/Inflammation/Toxins Metabolic, Endocrinologic, Nutritional, Toxic Neoplastic Traumatic
ICD-9 to ICD-10 Conversion Sample for Physical, Occupational and Speech Therapy - SNF Setting
 1 ICD-9 to ICD-10 Conversion Sample for Physical, Occupational and Speech Therapy - SNF Setting ICD-9 Code ICD-9 Description New ICD-10 New ICD-10 Description DM with Neuropathy E11.21 Type 2 diabetes
1 ICD-9 to ICD-10 Conversion Sample for Physical, Occupational and Speech Therapy - SNF Setting ICD-9 Code ICD-9 Description New ICD-10 New ICD-10 Description DM with Neuropathy E11.21 Type 2 diabetes
2015 ARDMS Physicians Vascular Interpretation Job Task Analysis Summary Report
 P a g e 1 2015 ARDMS Physicians Vascular Interpretation Job Task Analysis Summary Report American Registry for Diagnostic Medical Sonography (ARDMS) P a g e 2 Table of Contents ABOUT THE REPORT... 3 METHODOLOGY...
P a g e 1 2015 ARDMS Physicians Vascular Interpretation Job Task Analysis Summary Report American Registry for Diagnostic Medical Sonography (ARDMS) P a g e 2 Table of Contents ABOUT THE REPORT... 3 METHODOLOGY...
Head MRA/MRV studies of the head may be considered medically necessary for the following strongly suspected vascular diseases:
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: September 5, 2014 I. POLICY Head MRA/MRV studies of the head may be considered medically
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: September 5, 2014 I. POLICY Head MRA/MRV studies of the head may be considered medically
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION
 FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 71250 Computed tomography, thorax; without contrast material 71260 with contrast material(s) 71270 without
FIRST COAST SERVICE OPTIONS FLORIDA MEDICARE PART B LOCAL COVERAGE DETERMINATION CPT/HCPCS Codes 71250 Computed tomography, thorax; without contrast material 71260 with contrast material(s) 71270 without
Policies and Statements D16. Intracranial Cerebrovascular Ultrasound
 Policies and Statements D16 Intracranial Cerebrovascular Ultrasound SECTION 1: INSTRUMENTATION Policies and Statements D16 Intracranial Cerebrovascular Ultrasound May 2006 (Reaffirmed July 2007) Essential
Policies and Statements D16 Intracranial Cerebrovascular Ultrasound SECTION 1: INSTRUMENTATION Policies and Statements D16 Intracranial Cerebrovascular Ultrasound May 2006 (Reaffirmed July 2007) Essential
Lipids Testing
 Previously Listed as Edit 12 190.23 - Lipids Testing Lipoproteins are a class of heterogeneous particles of varying sizes and densities containing lipid and protein. These lipoproteins include cholesterol
Previously Listed as Edit 12 190.23 - Lipids Testing Lipoproteins are a class of heterogeneous particles of varying sizes and densities containing lipid and protein. These lipoproteins include cholesterol
Diagnostic and interventional venous procedures (lower extremity)
 Coding and Medicare national payment guide 2018 Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips is gathered
Coding and Medicare national payment guide 2018 Diagnostic and interventional venous procedures (lower extremity) All coding, coverage, billing and payment information provided herein by Philips is gathered
CPT Code Details
 CPT Code 93925 Details Code Descriptor Duplex scan of lower extremity arteries or arterial bypass grafts; complete bilateral study Lay Term The provider performs a duplex ultrasound scan of the lower extremity
CPT Code 93925 Details Code Descriptor Duplex scan of lower extremity arteries or arterial bypass grafts; complete bilateral study Lay Term The provider performs a duplex ultrasound scan of the lower extremity
Cardiovascular nuclear imaging employs non-invasive techniques to assess alterations in coronary artery flow, and ventricular function.
 National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA Original Date: October 2015 Page 1 of 9 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes:
National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA Original Date: October 2015 Page 1 of 9 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes:
Cardiology/Cardiothoracic
 Cardiology/Cardiothoracic ICD-9-CM to ICD-10-CM Code Mapper 800-334-5724 www.contexomedia.com 2013 ICD-9-CM 272.0 Pure hypercholesterolemia 272.2 Mixed hyperlipidemia 272.4 Other and hyperlipidemia 278.00
Cardiology/Cardiothoracic ICD-9-CM to ICD-10-CM Code Mapper 800-334-5724 www.contexomedia.com 2013 ICD-9-CM 272.0 Pure hypercholesterolemia 272.2 Mixed hyperlipidemia 272.4 Other and hyperlipidemia 278.00
Louisiana Policy Notice July 2001
 Louisiana Policy Notice 2001-02 July 2001 The Contractor Advisory Committee in Louisiana met in April and has reviewed and accepted several policies which were HCFA directed. Implementation dates appear
Louisiana Policy Notice 2001-02 July 2001 The Contractor Advisory Committee in Louisiana met in April and has reviewed and accepted several policies which were HCFA directed. Implementation dates appear
Ultrasound Imaging of The Posterior Circulation
 Ultrasound Imaging of The Posterior Circulation Michigan Sonographers Society 2 Nd Annual Fall Vascular Conference Larry N. Raber RDMS-RVT Clinical Manager General Ultrasound/Neurovascular Laboratory Cleveland
Ultrasound Imaging of The Posterior Circulation Michigan Sonographers Society 2 Nd Annual Fall Vascular Conference Larry N. Raber RDMS-RVT Clinical Manager General Ultrasound/Neurovascular Laboratory Cleveland
Arterial Map of the Thorax, Abdomen and Pelvis 2017 Edition
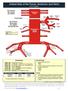 Arterial Map of the Thorax, Abdomen and Pelvis Angiography 75605 (-26) Aortography, thoracic 75625 (-26) Aortography, abdominal by serialography 75630 (-26) Aortography, abdominal + bilat iliofemoral 75705
Arterial Map of the Thorax, Abdomen and Pelvis Angiography 75605 (-26) Aortography, thoracic 75625 (-26) Aortography, abdominal by serialography 75630 (-26) Aortography, abdominal + bilat iliofemoral 75705
Cancer and Heart/Stroke
 Cancer and Heart/Stroke A plan providing cash benefits to help pay for out-of-pocket costs associated with a cancer, heart attack or stroke diagnosis National General Accident and Health markets products
Cancer and Heart/Stroke A plan providing cash benefits to help pay for out-of-pocket costs associated with a cancer, heart attack or stroke diagnosis National General Accident and Health markets products
Cancer and Heart/Stroke
 Cancer and Heart/Stroke A plan providing cash benefits to help pay for out-of-pocket costs associated with a cancer, heart attack or stroke diagnosis National General Accident and Health markets products
Cancer and Heart/Stroke A plan providing cash benefits to help pay for out-of-pocket costs associated with a cancer, heart attack or stroke diagnosis National General Accident and Health markets products
