abstract n engl j med 374;18 nejm.org May 5,
|
|
|
- Derick Caldwell
- 6 years ago
- Views:
Transcription
1 The new england journal of medicine established in 1812 May 5, 2016 vol. 374 no. 18 Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest P.J. Kudenchuk, S.P. Brown, M. Daya, T. Rea, G. Nichol, L.J. Morrison, B. Leroux, C. Vaillancourt, L. Wittwer, C.W. Callaway, J. Christenson, D. Egan, J.P. Ornato, M.L. Weisfeldt, I.G. Stiell, A.H. Idris, T.P. Aufderheide, J.V. Dunford, M.R. Colella, G.M. Vilke, A.M. Brienza, P. Desvigne Nickens, P.C. Gray, R. Gray, N. Seals, R. Straight, and P. Dorian, for the Resuscitation Outcomes Consortium Investigators* abstract BACKGROUND Antiarrhythmic drugs are used commonly in out-of-hospital cardiac arrest for shockrefractory ventricular fibrillation or pulseless ventricular tachycardia, but without proven survival benefit. METHODS In this randomized, double-blind trial, we compared parenteral amiodarone, lidocaine, and saline placebo, along with standard care, in adults who had nontraumatic out-ofhospital cardiac arrest, shock-refractory ventricular fibrillation or pulseless ventricular tachycardia after at least one shock, and vascular access. Paramedics enrolled patients at 10 North American sites. The primary outcome was survival to hospital discharge; the secondary outcome was favorable neurologic function at discharge. The per-protocol (primary analysis) population included all randomly assigned participants who met eligibility criteria and received any dose of a trial drug and whose initial cardiac-arrest rhythm of ventricular fibrillation or pulseless ventricular tachycardia was refractory to shock. RESULTS In the per-protocol population, 3026 patients were randomly assigned to amiodarone (974), lidocaine (993), or placebo (1059); of those, 24.4%, 23.7%, and 21.0%, respectively, survived to hospital discharge. The difference in survival rate for amiodarone versus placebo was 3.2 percentage points (95% confidence interval [CI], 0.4 to 7.0; P = 0.08); for lidocaine versus placebo, 2.6 percentage points (95% CI, 1.0 to 6.3; P = 0.16); and for amiodarone versus lidocaine, 0.7 percentage points (95% CI, 3.2 to 4.7; P = 0.70). Neurologic outcome at discharge was similar in the three groups. There was heterogeneity of treatment effect with respect to whether the arrest was witnessed (P = 0.05); active drugs were associated with a survival rate that was significantly higher than the rate with placebo among patients with bystander-witnessed arrest but not among those with unwitnessed arrest. More amiodarone recipients required temporary cardiac pacing than did recipients of lidocaine or placebo. CONCLUSIONS Overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival or favorable neurologic outcome than the rate with placebo among patients with out-of-hospital cardiac arrest due to initial shock-refractory ventricular fibrillation or pulseless ventricular tachycardia. (Funded by the National Heart, Lung, and Blood Institute and others; ClinicalTrials.gov number, NCT ) The authors full names, academic degrees, and affiliations are listed in the Appendix. Address reprint requests to Dr. Kudenchuk at the Division of Cardiology, University of Washington, 1959 N.E. Pacific St., Box , Seattle, WA * A complete list of the Resuscitation Outcomes Consortium investigators is provided in the Supplementary Appendix, available at NEJM.org. This article was published on April 4, 2016, at NEJM.org. N Engl J Med 2016;374: DOI: /NEJMoa Copyright 2016 Massachusetts Medical Society. n engl j med 374;18 nejm.org May 5,
2 The new england journal of medicine Out-of-hospital cardiac arrest is responsible for more than 300,000 deaths each year in North America. 1 Many outof-hospital cardiac arrests are attributable to ventricular fibrillation or pulseless ventricular tachycardia. Although ventricular fibrillation or pulseless ventricular tachycardia is regarded as the most treatable presentation of out-of-hospital cardiac arrest because of its responsiveness to shock, 2 most defibrillation attempts do not result in sustained return of spontaneous circulation. 3 Ventricular fibrillation or pulseless ventricular tachycardia commonly persists or recurs after shock, and there is a significant inverse relationship between the duration of ventricular fibrillation or pulseless ventricular tachycardia, or the frequency of acute recurrences, and resuscitation outcome. 4-6 Amiodarone and lidocaine are used commonly to promote successful defibrillation of shockrefractory ventricular fibrillation or pulseless ventricular tachycardia and prevent recurrences. In controlled trials involving patients with outof-hospital cardiac arrest, those who received amiodarone were more likely than those who received placebo or lidocaine to have a return of spontaneous circulation and to survive to be admitted to the hospital, 7,8 but the effects of amiodarone on survival to hospital discharge or neurologic outcome remain uncertain. To address this knowledge gap, we compared the effects of amiodarone, lidocaine, and placebo on survival to hospital discharge after out-of-hospital cardiac arrest due to shock-refractory ventricular fibrillation or pulseless ventricular tachycardia. Methods Trial Conduct and Oversight The background and methods of the trial were described previously. 9 Paramedics from 55 emergency medical services (EMS) agencies enrolled patients with out-of-hospital cardiac arrest at 10 North American sites participating in the Resuscitation Outcomes Consortium (ROC). 10 The trial was conducted under exception from informed consent in emergency research in accordance with applicable regulatory requirements, oversight by the Food and Drug Administration and Health Canada, approval by institutional review boards in participating communities, and monitoring by an independent data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute (NHLBI). The trial was sponsored by the NHLBI, the Canadian Institutes of Health Research, and others (see the support statement at the end of the article). In addition, Baxter Healthcare provided the trial drugs without cost and tested the stability of these products over the trial duration but otherwise played no role in the trial. The investigators designed and conducted the trial; gathered, analyzed, and interpreted the data; wrote the manuscript draft (the first author); and made the decision to submit it for publication. The trial statisticians had full access to all trial data and take responsibility for their integrity, analytic accuracy, and completeness and for the fidelity of this report to the trial protocol, which is available with the full text of this article at NEJM.org. Patients The trial included patients 18 years of age or older with nontraumatic out-of-hospital cardiac arrest and shock-refractory ventricular fibrillation or pulseless ventricular tachycardia, defined as confirmed persistent (nonterminating) or recurrent (restarting after successful termination) ventricular fibrillation or pulseless ventricular tachycardia after one or more shocks anytime during resuscitation (inclusive of rhythms interpreted as being shockable by an automated external defibrillator). Eligible patients were also required to have intravenous or intraosseous vascular access. We excluded patients who had already received open-label intravenous lidocaine or amiodarone during resuscitation or had known hypersensitivity to these drugs. A complete list of trial inclusion and exclusion criteria is provided in the Supplementary Appendix, available at NEJM.org. The trial protocol specified that the primary analysis population (the per-protocol population) would include only those randomly assigned participants who actually met the eligibility criteria, who received any dose of a trial drug during shock-refractory ventricular fibrillation or pulseless ventricular tachycardia, and who were confirmed to have an initial (rather than secondary) cardiac-arrest rhythm of ventricular fibrillation or pulseless ventricular tachycardia. Analyses were also performed in all randomly assigned patients (the intention-to-treat population) n engl j med 374;18 nejm.org May 5, 2016
3 Amiodarone, Lidocaine, or Placebo in Cardiac Arrest Interventions The trial evaluated licensed parenteral preparations of lidocaine, normal saline and a recently approved Captisol-based formulation of amiodarone (Nexterone, Baxter Healthcare) that is designed to reduce hypotensive effects. 11,12 Trial drugs were packaged in identically appearing, sealed kits each having three identically formulated syringes. Each syringe held 3 ml of colorless fluid containing 150 mg of amiodarone (totaling 450 mg in the amiodarone kit), 60 mg of lidocaine (180 mg in the lidocaine kit), or normal saline. Kits and their syringe contents were indistinguishable except by numerical code and were randomly distributed to EMS providers in a ratio of 1:1:1. Randomization was performed in permuted blocks of concealed size and was stratified according to participating site and agency. Trial drugs were tested regularly for stability and were confirmed to maintain their integrity in the simulated climates of trial communities. 9 Treatment Protocol Patients with out-of-hospital cardiac arrest were treated in accordance with local EMS protocols that complied with American Heart Association (AHA) guidelines for advanced life support. 13 Some patients were coenrolled in a concurrent trial comparing continuous chest compressions with interrupted chest compressions during cardiopulmonary resuscitation (CPR). 14 After the failure of one or more shocks to terminate ventricular fibrillation or pulseless ventricular tachycardia or prevent its recurrence, eligible patients received a vasopressor and were then enrolled in the trial by the EMS personnel s act of opening a trial-drug kit whose masked contents (amiodarone, lidocaine, or placebo) determined the patient s random assignment (details are provided in the Supplementary Appendix). Patients, investigators, and trial personnel were unaware of the trial-drug assignments. The initial dose of a trial drug, approximating current clinical practice, consisted of two syringes (one syringe if the estimated body weight was <100 lb [45.4 kg]) that were administered by rapid bolus. 10,15,16 If ventricular fibrillation or pulseless ventricular tachycardia persisted after the initial dose of the trial drug, standard resuscitation measures, and additional shocks, a supplemental dose (one syringe) of the same drug was administered. Thereafter, standard interventions for advanced life support ensued according to local practice, excluding open-label lidocaine or amiodarone before hospitalization. Post Cardiac Arrest Care All trial interventions were completed before hospital arrival. On arrival, hospital care providers were notified of the patient s enrollment in the trial and encouraged to provide usual post cardiac arrest care in accordance with published AHA guidelines, 17 including open-label amiodarone or lidocaine if necessary. Components of hospital care were monitored but were not standardized by the trial protocol, and their performance was reported back to hospitals periodically. A patient s trial-drug assignment was not disclosed to care providers, investigators, or site personnel unless emergency unblinding was requested, and then only to the treating physicians. Data Collection and Outcomes Data from prehospital patient care records, CPRprocess measures, and data from hospital medical records were collected as described in the Supplementary Appendix. The primary outcome of the trial was survival to hospital discharge. The main aim was to compare survival in amiodarone recipients versus placebo recipients, with secondary comparisons of survival in lidocaine recipients versus placebo recipients and in amiodarone recipients versus lidocaine recipients. The secondary outcome was survival with favorable neurologic status at discharge, defined as a score on the modified Rankin scale (range, 0 [no symptoms] to 6 [death]) of 3 or less, indicating the ability to conduct daily activities independently or with minimal assistance. 18 These outcomes were determined in both the perprotocol population (the primary analysis) and in the intention-to-treat population. Mechanistic outcomes that were assessed for exploratory purposes included the number of defibrillation shocks administered after receipt of the trial drug, return of spontaneous circulation at hospital arrival, hospital admission, hospital treatments, and time to withdrawal of lifesustaining treatments. Prespecified subgroups were defined according to status with respect to witnessing of the cardiac arrest (witnessed by EMS, witnessed by bystander, or unwitnessed), receipt of bystander-initiated CPR (yes or no), n engl j med 374;18 nejm.org May 5,
4 The new england journal of medicine location of the arrest (public or private), time to trial-drug administration (<15 or 15 minutes), route of trial-drug administration (intravenous or intraosseous), treatment group in the concurrent trial of continuous or interrupted chest compressions during CPR, baseline survival rate at the trial site (in quartiles), and EMS drug-administration practice (see the Supplementary Appendix). Adverse events were considered to be drugrelated if they were reported previously with these medications 19,20 (e.g., anaphylaxis, thrombophlebitis requiring therapeutic intervention, clinical seizure activity, and bradycardia requiring temporary cardiac pacing) and if they occurred within 24 hours after trial-drug administration. Serious or unexpected adverse events attributable to trial interventions 21 and complications related to vascular access were also assessed. Other adverse events such as pulmonary edema, hypotension, or pneumonia, which are common after out-of-hospital cardiac arrest, were monitored but were not considered to be drugrelated unless imbalanced between trial groups. Statistical Analysis We estimated that a sample size of 3000 in the per-protocol population (1000 patients per group) would provide 90% power to detect an absolute difference of 6.3 percentage points in the rate of survival to hospital discharge between the amiodarone group and the placebo group (29.7% vs. 23.4%). The baseline survival rate was estimated from patients with a first recorded rhythm of ventricular fibrillation or pulseless ventricular tachycardia who received at least two shocks in previous ROC trials. 22,23 The projected difference in survival with amiodarone was estimated from a previous trial database 7 and was the comparison for which this trial was powered. Survival was evaluated across groups with the use of the z-test for comparison of binomial proportions with pooled variance, with a onesided significance level of for comparisons between an active drug and placebo (based on the monitoring plan of the trial) and a two-sided significance level of 0.05 when comparing amiodarone with lidocaine. 9 All comparisons in this report, including testing interactions, were recalculated as two-sided with P values of less than or equal to 0.05 considered to indicate statistical significance (as most comparisons were initially performed), which did not substantially change the results. Figure 1 (facing page). Screening and Randomization. The inclusion and exclusion of patients depended on their clinical and cardiac-rhythm characteristics at the time of potential trial-drug receipt. Of 46 patients in protected populations who were determined to be ineligible before enrollment, 33 were children, 12 were prisoners, and 1 was pregnant. Of 5 patients who had other reasons for ineligibility, 3 had exsanguination and 2 had a history of allergy to amiodarone or lidocaine. Circumstantial issues that were reasons for nonenrollment included safety concerns at the scene, arrival at the hospital before the trial-drug kit was opened, debatable asystole, shocks from an implanted defibrillator, infiltration or loss of intravenous (IV) access, and a need for extrication of the patient. The intention-to-treat population excluded patients in protected populations. There were 8 such patients (6 children and 2 prisoners) who were enrolled; 3 were assigned to amiodarone, 1 to lidocaine, and 4 to placebo. The intention-to-treat population included all patients for whom a trial-drug kit was opened, regardless of their eligibility, initial cardiac-arrest rhythm, or actual receipt of the trial drug. A total of 1627 patients in the intentionto-treat population were excluded from the per-protocol population (Table S1 in the Supplementary Appendix). The per-protocol population was composed of randomly assigned, trial-eligible patients whose initial cardiacarrest rhythm was ventricular fibrillation or pulseless ventricular tachycardia (VF/VT), who continued to have shock-refractory VF/VT, and who received any dose of a trial drug. EMS denotes emergency medical services. The data and safety monitoring board performed interim reviews twice a year with the use of group sequential methods with formal stopping boundaries; final differences and 95% confidence intervals for the primary outcome were adjusted accordingly. 9 Apart from this, there were no adjustments for multiple comparisons. Results Trial Populations The trial began on May 7, 2012 and completed enrollment on October 25, Of 37,889 patients with nontraumatic out-of-hospital cardiac arrest, 7051 (18.6%) had shock-refractory ventricular fibrillation or pulseless ventricular tachycardia at some time and were potentially eligible for enrollment in the trial (Fig. 1). The intentionto-treat population of 4653 patients was composed of 4667 with opened drug kits, excluding 6 with an unknown trial-drug assignment and 8 in protected populations. Of these, 3026 comprised the per-protocol population of trial-eligible patients with out-of-hospital cardiac arrest and initial shock-refractory ventricular fibrillation or 1714 n engl j med 374;18 nejm.org May 5, 2016
5 Amiodarone, Lidocaine, or Placebo in Cardiac Arrest 37,889 Patients with out-of-hospital cardiac arrest were assessed for eligibility 30,838 (81.4%) Were ineligible 30,487 Did not have shockrefractory VF/VT 187 Did not have vascular access 75 Had received IV amiodarone or lidocaine previously 46 Were in protected populations 19 Had advance directive 19 Did not receive advanced life support 5 Had other reasons 7051 (18.6%) Were potentially eligible 2384 (6.3%) Were excluded 602 Had protocol violations 150 Received care from EMS personnel who did not have trial-drug kit at scene 1318 Had VF/VT terminated 275 Had circumstantial issues 39 Had unknown reasons 4667 (12.3%) Had trial-drug kit that was opened by EMS and thereby underwent randomization 14 Were excluded 6 Had an unknown trial-drug assignment 8 Were in protected populations 4653 Were included in the intention-to-treat population 1539 In the intention-to-treat population were assigned to receive amiodarone 1541 In the intention-to-treat population were assigned to receive lidocaine 1573 In the intention-to-treat population were assigned to receive placebo 565 (36.7%) Were excluded from per-protocol population 548 (35.6%) Were excluded from per-protocol population 514 (32.7%) Were excluded from per-protocol population 974 (63.3%) In the per-protocol population received amiodarone 993 (64.4%) In the per-protocol population received lidocaine 1059 (67.3%) In the per-protocol population received placebo 4 Had unknown outcome 8 Had unknown outcome 3 Had unknown outcome 970 (99.6%) Were included in primary analysis 985 (99.2%) Were included in primary analysis 1056 (99.7%) Were included in primary analysis pulseless ventricular tachycardia who were randomly assigned recipients of amiodarone (974 patients), lidocaine (993), or placebo (1059), excluding 1627 who did not meet the per-protocol criteria (Table S1 in the Supplementary Appendix). Emergency unblinding of the trial-drug assign- n engl j med 374;18 nejm.org May 5,
6 The new england journal of medicine Table 1. Prerandomization Characteristics of the Per-Protocol Population.* Characteristic Amiodarone (N = 974) Lidocaine (N = 993) Placebo (N = 1059) Age yr 63.7± ± ±14.6 Male sex no./total no. (%) 762/973 (78.3) 816/993 (82.2) 844/1059 (79.7) Cardiac arrest occurred in public location no./total no. (%) 303/974 (31.1) 312/993 (31.4) 316/1056 (29.9) Cardiac arrest witnessed no./total no. (%) By EMS 57/950 (6.0) 44/965 (4.6) 54/1027 (5.3) By bystander 621/950 (65.4) 636/965 (65.9) 687/1027 (66.9) Bystander-initiated PAD shock no./total no. (%) 62/905 (6.9) 51/927 (5.5) 57/988 (5.8) Bystander-initiated CPR no./total no. (%) 556/905 (61.4) 549/927 (59.2) 595/988 (60.2) Time from initial call To first arrival of EMS min 5.8± ± ±2.6 To first arrival of EMS 4 min no./total no. (%) 209/973 (21.5) 237/992 (23.9) 244/1058 (23.1) To first arrival of ALS min 8.0± ± ±4.6 Trial site no. (%) A 104 (10.7) 97 (9.8) 92 (8.7) B 154 (15.8) 158 (15.9) 162 (15.3) C 74 (7.6) 77 (7.8) 80 (7.6) D 59 (6.1) 60 (6.0) 66 (6.2) E 4 (0.4) 8 (0.8) 7 (0.7) F 215 (22.1) 223 (22.5) 260 (24.6) G 21 (2.2) 14 (1.4) 15 (1.4) H 163 (16.7) 149 (15.0) 169 (16.0) I 63 (6.5) 85 (8.6) 80 (7.6) J 117 (12.0) 122 (12.3) 128 (12.1) * Plus minus values are means ±SD. No baseline factors varied significantly according to trial group (P>0.05). ALS denotes advanced life support, CPR cardiopulmonary resuscitation, EMS emergency medical services, and PAD public-access defibrillation. Initial call refers to the initial notification of an occurrence of cardiac arrest to an emergency call center. ment was requested in 24 patients (0.8%) and was proportionately similar across trial groups. The baseline patient characteristics and event characteristics in the per-protocol population were well balanced across trial groups (Tables 1 and 2). The first dose of the trial drugs was given a mean (±SD) of 19.3±7.4 minutes after the initial call to EMS and after a median of three shocks (interquartile range, two to four) had been administered. Outcomes Outcomes were available for 99.5% of all patients in the per-protocol population (Fig. 1). Among amiodarone recipients in the per-protocol population, 237 (24.4%) survived to hospital discharge (the primary outcome), as compared to 233 (23.7%) who received lidocaine and 222 (21.0%) who received placebo. The absolute risk difference for the primary comparison of amiodarone versus placebo was 3.2 percentage points (95% confidence interval [CI], 0.4 to 7.0; P = 0.08). For the secondary comparison of lidocaine versus placebo, the risk difference was 2.6 percentage points (95% CI, 1.0 to 6.3; P = 0.16), and for the secondary comparison of amiodarone versus lidocaine, it was 0.7 percentage points (95% CI, 3.2 to 4.7; P = 0.70) (Table 3) n engl j med 374;18 nejm.org May 5, 2016
7 Amiodarone, Lidocaine, or Placebo in Cardiac Arrest Table 2. Event Characteristics and Treatments Received in the Per-Protocol Population.* Characteristic Time from initial call to first dose of trial drug in patients with non EMS-witnessed cardiac arrest min Time from cardiac arrest to first dose of trial drug in patients with EMS-witnessed arrest min Amiodarone (N = 974) Lidocaine (N = 993) Placebo (N = 1059) Overall P Value 19.3± ± ± ± ± ± Time from initial call to first dose of epinephrine min 16.1± ± ± Trial drug given through intraosseous access no./total no. (%) 212/974 (21.8) 220/991 (22.2) 229/1054 (21.7) 0.96 Syringes of trial drug given no./total no. (%) < syringes 621/967 (64.2) 594/981 (60.6) 758/1051 (72.1) 2 syringes 327/967 (33.8) 368/981 (37.5) 273/1051 (26.0) 1 syringe 19/967 (2.0) 19/981 (1.9) 20/1051 (1.9) Prehospital advanced airway management successful no. (%) 819 (84.1) 854 (86.0) 893 (84.3) 0.45 CPR-process measures in first 10 min after pad placement Pre-shock pause sec 10.2± ± ± Post-shock pause sec 5.1± ± ± Compression rate/min 110.3± ± ± Compression depth mm 50.9± ± ± CPR fraction 0.83± ± ± No. of EMS shocks median (IQR) 5 (3 7) 5 (3 7) 6 (4 9) <0.001 No. of shocks before first dose of trial drug median (IQR) 3 (2 4) 3 (2 4) 3 (2 4) 0.73 No. of EMS shocks after first dose of trial drug median (IQR) 2 (1 4) 2 (1 3) 3 (1 6) <0.001 Prehospital drugs administered Epinephrine no. (%) 961 (98.7) 981 (98.8) 1046 (98.8) 1.00 Vasopressin no./total no. (%) 67/974 (6.9) 60/992 (6.0) 55/1059 (5.2) 0.28 Bicarbonate no. (%) 271 (27.8) 258 (26.0) 308 (29.1) 0.29 Atropine no./total no. (%) 52/974 (5.3) 43/992 (4.3) 33/1059 (3.1) 0.04 Beta-blocker no./total no. (%) 6/974 (0.6) 2/992 (0.2) 10/1059 (0.9) 0.09 Open-label lidocaine no./total no. (%) 4/974 (0.4) 6/992 (0.6) 13/1059 (1.2) 0.08 Open-label amiodarone no./total no. (%) 7/974 (0.7) 13/992 (1.3) 15/1059 (1.4) 0.29 Procainamide no./total no. (%) 67/974 (6.9) 57/992 (5.7) 92/1059 (8.7) 0.03 Magnesium no./total no. (%) 78/974 (8.0) 68/992 (6.8) 119/1059 (11.2) Coenrollment in CPR trial no./total no. (%) 0.95 Received continuous chest compressions 234/973 (24.0) 249/993 (25.1) 253/1059 (23.9) Received interrupted chest compressions 264/973 (27.1) 259/993 (26.1) 290/1059 (27.4) Were not enrolled in CPR trial 475/973 (48.8) 485/993 (48.8) 516/1059 (48.7) * IQR denotes interquartile range. Data are based on the initial dose of the trial drug. Shown is the mean pause with respect to the first three shocks. The CPR fraction is the proportion of each minute in which compressions are given. In this randomized trial involving patients with out-of-hospital cardiac arrest, one group received continuous chest compressions with positive-pressure ventilation, and the other group received compressions that were interrupted for ventilations at a ratio of 30 compressions to two ventilations. n engl j med 374;18 nejm.org May 5,
8 The new england journal of medicine Table 3. Outcomes According to Trial Group in the Per-Protocol Population.* Outcome Amiodarone (N = 974) Lidocaine (N = 993) Placebo (N = 1059) Amiodarone vs. Placebo Lidocaine vs. Placebo Amiodarone vs. Lidocaine Difference (95% CI) P Value Difference (95% CI) P Value Difference (95% CI) P Value percentage points percentage points percentage points Primary outcome: survival to discharge no./total no. (%) Secondary outcome: modified Rankin score 3 no./total no. (%) Mechanistic (exploratory) outcomes Return of spontaneous circulation at ED arrival no./total no. (%) 237/970 (24.4) 233/985 (23.7) 222/1056 (21.0) 3.2 ( 0.4 to 7.0) 182/967 (18.8) 172/984 (17.5) 175/1055 (16.6) 2.2 ( 1.1 to 5.6) 350/974 (35.9) 396/992 (39.9) 366/1059 (34.6) 1.4 ( 2.8 to 5.5) Admitted to hospital no. (%) 445 (45.7) 467 (47.0) 420 (39.7) 6.0 (1.7 to 10.3) Modified Rankin score in all patients 5.0± ± ± ( 0.30 to 0.02) Modified Rankin score in survivors 2.0± ± ±2.6 Distribution of modified Rankin scores no./total no. (%) 0 60/966 (6.2) 49/981 (5.0) 55/1053 (5.2) 1 47/966 (4.9) 37/981 (3.8) 39/1053 (3.7) 2 41/966 (4.2) 46/981 (4.7) 40/1053 (3.8) 3 34/966 (3.5) 37/981 (3.8) 41/1053 (3.9) 4 31/966 (3.2) 36/981 (3.7) 27/1053 (2.6) 5 21/966 (2.2) 24/981 (2.4) 18/1053 (1.7) 6 732/966 (75.8) 752/981 (76.7) 833/1053 (79.1) ( 1.0 to 6.3) ( 2.4 to 4.2) (1.2 to 9.5) (3.1 to 11.6) ( 0.22 to 0.10) ( 3.2 to 4.7) ( 2.1 to 4.8) ( 8.3 to 0.3) < ( 5.7 to 3.1) ( 0.24 to 0.08) * CI denotes confidence interval, and ED emergency department. The difference and 95% CI were adjusted for sequential monitoring. Scores on the modified Rankin scale range from 0 (no symptoms) to 6 (death). A score of 3 or less indicates the ability to conduct daily activities independently or with minimal assistance n engl j med 374;18 nejm.org May 5, 2016
9 Amiodarone, Lidocaine, or Placebo in Cardiac Arrest Table 4. Adverse Events in the Per-Protocol Population.* Event Amiodarone (N = 974) Lidocaine (N = 993) Placebo (N = 1059) Overall P Value number (percent) Thrombophlebitis within 24 hr 1 (0.1) 3 (0.3) 2 (0.2) 0.61 Anaphylaxis within 24 hr NA Clinical seizure activity within 24 hr 31 (3.2) 51 (5.1) 39 (3.7) 0.07 Temporary cardiac pacing within 24 hr 48 (4.9) 32 (3.2) 29 (2.7) 0.02 Complications of intravenous or intraosseous access 2 (0.2) 0 2 (0.2) 0.37 within 24 hr Any nonfatal serious adverse event within 24 hr 11 (1.1) 12 (1.2) 4 (0.4) 0.09 Any nonfatal adverse event within 24 hr 81 (8.3) 84 (8.5) 69 (6.5) 0.18 Death before hospital discharge 733 (75.3) 752 (75.7) 834 (78.8) 0.16 Any adverse event within 24 hr or death before hospital discharge 763 (78.3) 775 (78.0) 851 (80.4) 0.20 * Adverse events were defined as drug-related if they occurred in the first 24 hours after randomization. NA denotes not applicable. Excluded were patients in whom pacing was initiated before the trial drug was given. Nonfatal serious adverse events were defined as events that were life-threatening or potentially resulting in prolonged hospitalization or disability, as designated by a trial site, excluding death. Shown is the total number of patients (some patients may have had >1 event). Rates of survival with favorable neurologic status (the secondary outcome) were similar in the amiodarone group (182 patients [18.8%]), lidocaine group (172 [17.5%]), and placebo group (175 [16.6%]). The risk difference for the secondary outcome for amiodarone versus placebo was 2.2 percentage points (95% CI, 1.1 to 5.6; P = 0.19); for lidocaine versus placebo, 0.9 percentage points (95% CI, 2.4 to 4.2; P = 0.59); and for amiodarone versus lidocaine, 1.3 percentage points (95% CI, 2.1 to 4.8; P = 0.44). Subgroups There was heterogeneity of treatment effect with respect to whether or not the out-of-hospital cardiac arrest was witnessed (P = 0.05 for interaction); active drugs were associated with a higher rate of survival to hospital discharge than the rate with placebo among patients with witnessed out-of-hospital cardiac arrest (Table S2 in the Supplementary Appendix). Among 1934 patients with bystander-witnessed arrest, the survival rate was higher with amiodarone (27.7%) or lidocaine (27.8%) than with placebo (22.7%). This absolute risk difference was significant for amiodarone versus placebo (5.0 percentage points; 95% CI, 0.3 to 9.7; P = 0.04) and for lidocaine versus placebo (5.2 percentage points; 95% CI, 0.5 to 9.9; P = 0.03), but did not differ significantly between amiodarone and lidocaine ( 0.1 percentage points; 95% CI, 5.1 to 4.9; P = 0.97). The survival rate was also higher among amiodarone recipients than placebo recipients with EMS-witnessed arrest, a risk difference of 21.9 percentage points (95% CI, 5.8 to 38.0; P = 0.01). Conversely, among 839 patients in whom out-ofhospital cardiac arrest was unwitnessed, survival did not differ significantly between trial groups. No other significant interaction with treatment was found in other prespecified subgroups. Mechanistic Outcomes After randomization, placebo recipients were more likely to require an additional dose of blinded trial drug than recipients of amiodarone or lidocaine, and they received a greater number of subsequent shocks and other rhythm-control medications (Table 2). More lidocaine recipients than placebo recipients had sustained return of spontaneous circulation on hospital arrival (Table 3). Patients were more likely to survive to hospital admission after receipt of amiodarone or lidocaine than after receipt of placebo. Fewer recipients of amiodarone or lidocaine than of placebo required CPR during hospitalization (Table S3 in the Supplementary Appendix). Use n engl j med 374;18 nejm.org May 5,
10 The new england journal of medicine of open-label antiarrhythmic drugs (particularly open-label amiodarone) during the first 24 hours of hospitalization was also less common in the amiodarone group than in the lidocaine or placebo groups. Adverse Events In the per-protocol population, the overall frequency of prespecified drug-related adverse events did not differ significantly among patients who received amiodarone, lidocaine, or placebo, nor did serious adverse events (Table 4, and Table S4 in the Supplementary Appendix). There was a greater need for temporary cardiac pacing after receipt of amiodarone (4.9%) than after receipt of lidocaine (3.2%) or placebo (2.7%). Intention-to-Treat Population Patients enrolled in the intention-to-treat population had balanced baseline characteristics across trial groups (Table S5 in the Supplementary Appendix). There were no significant differences between the trial groups in the rates of the primary and secondary outcomes (Table S6 in the Supplementary Appendix). There were also no significant differences between the trial groups in the rates of drug-related adverse events or serious adverse events (Tables S7 and S8 in the Supplementary Appendix). Discussion In this randomized, double-blind, placebo-controlled, prehospital trial, we found that treatment with amiodarone or lidocaine did not result in a significantly higher rate of survival to hospital discharge or favorable neurologic outcome at discharge than the rate with placebo after out-of-hospital cardiac arrest caused by shock-refractory initial ventricular fibrillation or pulseless ventricular tachycardia. There were also no significant differences in these outcomes between amiodarone and lidocaine. Two previous small, randomized trials showed significantly higher rates of return of spontaneous circulation and hospital admission with amiodarone than with placebo or lidocaine after shock-refractory out-of-hospital cardiac arrest. 7,8 The current trial, which was larger and performed in the context of well-executed CPR, showed similar benefits with respect to shortterm outcomes, but with both drugs. The time to treatment with these drugs was typically late across all the trials, averaging 19 minutes from the initial call to EMS in this trial and 21 to 25 minutes in the others. 7,8 Such delays may attenuate the effectiveness of antiarrhythmic interventions as patients progress to the metabolic phase of out-of-hospital cardiac arrest, when cellular injury and physiological derangements may be irreversible despite restored circulation. 24 Our results could be interpreted in several ways. First, antiarrhythmic drugs may simply be ineffective in this population because they lack antiarrhythmic or restorative effects on circulation. This explanation seems unlikely, given that both amiodarone and lidocaine facilitated termination of ongoing or recurrent ventricular fibrillation or pulseless ventricular tachycardia with fewer shocks than placebo, were associated with higher rates of hospital admission, and resulted in a lesser need for CPR or antiarrhythmic therapies during hospitalization, which could even be taken as potential mechanisms for improved survival. Drug-related adverse events could also have mitigated survival. This too seems unlikely, because no significant betweengroup differences were observed in the frequency of adverse events. Conversely, because hospital care was not standardized, treatment imbalances between trial groups might have attenuated the survival benefit from amiodarone or lidocaine. However, the trial was randomized and blinded throughout, and the frequency of coronary catheterization, therapeutic hypothermia, and withdrawal of life-sustaining treatments did not differ significantly across trial groups. The effectiveness of active treatment could also depend on physiological conditions, timing, and patient characteristics. We observed an interaction of treatment with the witnessed status of out-of-hospital cardiac arrest, which is often taken as a surrogate for early recognition of cardiac arrest, a short interval between the patient s collapse from cardiac arrest and the initiation of treatment, and a greater likelihood of therapeutic responsiveness. Though prespecified, this subgroup analysis was performed in the context of an insignificant difference for the overall analysis, and the P value for heterogeneity in this subgroup analysis was not adjusted for the number of subgroup comparisons. Nonetheless, the suggestion that survival was improved by drug treatment in patients with witnessed out-of n engl j med 374;18 nejm.org May 5, 2016
11 Amiodarone, Lidocaine, or Placebo in Cardiac Arrest hospital cardiac arrest, without evidence of harm in those with unwitnessed arrest, merits thoughtful consideration. Finally, the point estimates of the survival rates in the placebo group and the amiodarone group differed less than anticipated when the trial was designed, which suggests that the trial may have been underpowered. If amiodarone has a true treatment effect of 3 percentage points, approximately 9000 patients across the three trial groups would be needed to establish this difference in outcome with 90% power. Though seemingly small, a confirmed overall difference of 3 percentage points in survival with drug therapy would mean that 1800 additional lives could be saved each year in North America alone after out-of-hospital cardiac arrest. Several limitations of this trial should be considered. Selection bias could have influenced trial enrollment. However, reasons for nonenrollment were systematically tracked, and questionable instances of exclusion were numerically small. The trial tested only one administration strategy without active-treatment crossover; other strategies may produce different results. Last, enrollment of patients whose condition at randomization afforded little or no chance of survival irrespective of treatment may have diluted the presence of a more robust treatment effect in others, resulting in a smaller overall benefit than had eligibility been more selective. 25 In conclusion, in this randomized trial, we found that overall neither amiodarone nor lidocaine resulted in a significantly higher rate of survival to hospital discharge or favorable neurologic outcome than the rate with placebo among patients with out-of-hospital cardiac arrest due to initial shock-refractory ventricular fibrillation or pulseless ventricular tachycardia. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute (NHLBI) or the National Institutes of Health. The Resuscitation Outcomes Consortium (ROC) was supported by the NHLBI through a series of cooperative agreements with nine regional clinical centers (spanning 10 North American communities) and one data coordinating center (5U01 HL077863, with the University of Washington Data Coordinating Center; HL077866, with the Medical College of Wisconsin; HL077867, with the University of Washington; HL077871, with the University of Pittsburgh; HL077872, with St. Michael s Hospital; HL077873, with Oregon Health and Science University; HL077881, with the University of Alabama at Birmingham; HL077885, with the Ottawa Hospital Research Institute; HL077887, with the University of Texas Southwestern Medical Center; and HL077908, with the University of California San Diego). Additional funding was provided by U.S. Army Medical Research and Materiel Command, the Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart and Stroke Foundation of Canada, and the American Heart Association. Two authors are employees of the NHLBI; they helped in the design and conduct of the trial, data analysis and interpretation, and revision of the manuscript. The NHLBI, as the trial funder, also appointed members of the protocol review committee of the ROC and members of the data and safety monitoring board of this trial but otherwise played no role in its conduct. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. We thank the nurse coordinators, research personnel, and especially the paramedic prehospital care providers across the ROC, whose enthusiasm, professionalism, and dedication to advancing patient care and gaining new knowledge to save lives were the foundation for this endeavor. Appendix The authors full names and academic degrees are as follows: Peter J. Kudenchuk, M.D., Siobhan P. Brown, Ph.D., Mohamud Daya, M.D., Thomas Rea, M.D., M.P.H., Graham Nichol, M.D., M.P.H., Laurie J. Morrison, M.D., Brian Leroux, Ph.D., Christian Vaillancourt, M.D., Lynn Wittwer, M.D., Clifton W. Callaway, M.D., Ph.D., James Christenson, M.D., Debra Egan, M.Sc., M.P.H., Joseph P. Ornato, M.D., Myron L. Weisfeldt, M.D., Ian G. Stiell, M.D., Ahamed H. Idris, M.D., Tom P. Aufderheide, M.D., James V. Dunford, M.D., M. Riccardo Colella, D.O., M.P.H., Gary M. Vilke, M.D., Ashley M. Brienza, B.S., Patrice Desvigne Nickens, M.D., Pamela C. Gray, NREMT-P, Randal Gray, M.Ed., NREMT-P, Norman Seals, B.S., Ron Straight, M.Ed., and Paul Dorian, M.D. The authors affiliations are as follows: the Department of Medicine (P.J.K., T.R., G.N.) and Division of Cardiology (P.J.K.), University of Washington, the King County Emergency Medical Services, Public Health (P.J.K., T.R.), the Department of Biostatistics, University of Washington Clinical Trial Center (S.P.B., G.N., B.L.), and University of Washington Harborview Center for Prehospital Emergency Care (G.N.), Seattle, and Clark County Emergency Medical Services, Vancouver (L.W.) all in Washington; the Department of Emergency Medicine, Oregon Health and Science University, Portland (M.D.); Rescu, Li Ka Shing Knowledge Institute, St. Michael s Hospital (L.J.M., P.D.), and the Divisions of Emergency Medicine (L.J.M.) and Cardiology (P.D.), Department of Medicine, University of Toronto, Toronto, the Department of Emergency Medicine, Ottawa Hospital Research Institute, University of Ottawa, Ottawa (C.V., I.G.S.), and the Department of Emergency Medicine, Providence Health Care Research Institute, University of British Columbia Faculty of Medicine (J.C.), and Providence Health Care Research Institute and British Columbia Emergency Health Services (R.S.), Vancouver all in Canada; University of Pittsburgh, Pittsburgh (C.W.C., A.M.B.); National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda (D.E., P.D.- N.), and Johns Hopkins University, Baltimore (M.L.W.) both in Maryland; the Department of Emergency Medicine, Virginia Commonwealth University, Richmond (J.P.O.); the Departments of Emergency Medicine and Internal Medicine, University of Texas Southwestern Medical Center (A.H.I.), and Dallas Fire Rescue Department (N.S.) both in Dallas; the Departments of Emergency Medicine (T.P.A.) and Pediatrics (M.R.C.), Medical College of Wisconsin, Milwaukee; the Department of Emergency Medicine, University of California San Diego (J.V.D., G.M.V.), and San Diego Fire Rescue Department (J.V.D.) both in San Diego; and University of Alabama at Birmingham, Birmingham (P.C.G., R.G.). n engl j med 374;18 nejm.org May 5,
12 Amiodarone, Lidocaine, or Placebo in Cardiac Arrest References 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics 2015 update: a report from the American Heart Association. Circulation 2015; 131(4): e Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of outof-hospital ventricular fibrillation, JAMA 2002; 288: Kudenchuk PJ, Cobb LA, Copass MK, Olsufka M, Maynard C, Nichol G. Transthoracic Incremental Monophasic versus Biphasic Defibrillation by Emergency Responders (TIMBER): a randomized comparison of monophasic with biphasic waveform ascending energy defibrillation for the resuscitation of out-of-hospital cardiac arrest due to ventricular fibrillation. Circulation 2006; 114: van Alem AP, Post J, Koster RW. VF recurrence: characteristics and patient outcome in out-of-hospital cardiac arrest. Resuscitation 2003; 59: Eilevstjønn J, Kramer-Johansen J, Sunde K. Shock outcome is related to prior rhythm and duration of ventricular fibrillation. Resuscitation 2007; 75: Berdowski J, ten Haaf M, Tijssen JGP, Chapman FW, Koster RW. Time in recurrent ventricular fibrillation and survival after out-of-hospital cardiac arrest. Circulation 2010; 122: Kudenchuk PJ, Cobb LA, Copass MK, et al. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med 1999; 341: Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med 2002; 346: Kudenchuk PJ, Brown SP, Daya M, et al. Resuscitation Outcomes Consortium Amiodarone, Lidocaine or Placebo Study (ROC-ALPS): rationale and methodology behind an out-of-hospital cardiac arrest antiarrhythmic drug trial. Am Heart J 2014; 167(5): e Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of Emergency Medical Service systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care 2007; 11: Cushing DJ, Kowey PR, Cooper WD, Massey BW, Gralinski MR, Lipicky RJ. PM101: a cyclodextrin-based intravenous formulation of amiodarone devoid of adverse hemodynamic effects. Eur J Pharmacol 2009; 607: Souney PF, Cooper WD, Cushing DJ. PM101: intravenous amiodarone formulation changes can improve medication safety. Expert Opin Drug Saf 2010; 9: Neumar RW, Otto CW, Link MS, et al. Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122: Suppl 3: S Nichol G, Leroux B, Wang H, et al. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med 2015; 373: American Society of Health-System Pharmacists. AHFS drug information. Bethesda, MD: American Hospital Formulary Service, 2001: Glover BM, Brown SP, Morrison L, et al. Wide variability in drug use in outof-hospital cardiac arrest: a report from the resuscitation outcomes consortium. Resuscitation 2012; 83: Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008; 118: Reynolds JC, Frisch A, Rittenberger JC, Callaway CW. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: when should we change to novel therapies? Circulation 2013; 128: Collinsworth KA, Kalman SM, Harrison DC. The clinical pharmacology of lidocaine as an antiarrhythymic drug. Circulation 1974; 50: Marinelli A, Capucci A. Amiodarone (Nexterone) injection for the treatment and prophylaxis of frequently recurring ventricular fibrillation. Expert Opin Pharmacother 2012; 13: Food and Drug Administration. What is a serious adverse event? 2016 ( safety/ medwatch/ howtoreport/ ucm htm). 22. Aufderheide TP, Nichol G, Rea TD, et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N Engl J Med 2011; 365: Stiell IG, Nichol G, Leroux BG, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med 2011; 365: Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase timesensitive model. JAMA 2002; 288: Kreutziger J, Wenzel V. Overcoming frustration about neutral clinical studies in cardiopulmonary resuscitation. Resuscitation 2009; 80: Copyright 2016 Massachusetts Medical Society. receive immediate notification when an article is published online first To be notified by when Journal articles are published Online First, sign up at NEJM.org n engl j med 374;18 nejm.org May 5, 2016
Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest
 Original Article Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest P.J. Kudenchuk, S.P. Brown, M. Daya, T. Rea, G. Nichol, L.J. Morrison, B. Leroux, C. Vaillancourt, L. Wittwer, C.W.
Original Article Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest P.J. Kudenchuk, S.P. Brown, M. Daya, T. Rea, G. Nichol, L.J. Morrison, B. Leroux, C. Vaillancourt, L. Wittwer, C.W.
GETTING TO THE HEART OF THE MATTER. Ritu Sahni, MD, MPH Lake Oswego Fire Department Washington County EMS Clackamas County EMS
 GETTING TO THE HEART OF THE MATTER Ritu Sahni, MD, MPH Lake Oswego Fire Department Washington County EMS Clackamas County EMS TAKE HOME POINTS CPR is the most important thing Train like we fight Measure
GETTING TO THE HEART OF THE MATTER Ritu Sahni, MD, MPH Lake Oswego Fire Department Washington County EMS Clackamas County EMS TAKE HOME POINTS CPR is the most important thing Train like we fight Measure
The ARREST Trial: Amiodarone for Resuscitation After Out-of-Hospital Cardiac Arrest Due to Ventricular Fibrillation
 The ARREST Trial: Amiodarone for Resuscitation After Out-of-Hospital Cardiac Arrest Due to Ventricular Fibrillation Introduction The ARREST (Amiodarone in out-of-hospital Resuscitation of REfractory Sustained
The ARREST Trial: Amiodarone for Resuscitation After Out-of-Hospital Cardiac Arrest Due to Ventricular Fibrillation Introduction The ARREST (Amiodarone in out-of-hospital Resuscitation of REfractory Sustained
journal of medicine The new england Trial of Continuous or Interrupted Chest Compressions during CPR abstract
 The new england journal of medicine established in 1812 December 3, 2015 vol. 373 no. 23 Trial of Continuous or Interrupted Chest Compressions during CPR Graham Nichol, M.D., M.P.H., Brian Leroux, Ph.D.,
The new england journal of medicine established in 1812 December 3, 2015 vol. 373 no. 23 Trial of Continuous or Interrupted Chest Compressions during CPR Graham Nichol, M.D., M.P.H., Brian Leroux, Ph.D.,
Trial of Continuous or Interrupted Chest Compressions during CPR
 Original Article Trial of Continuous or Interrupted Chest Compressions during CPR Graham Nichol, M.D., M.P.H., Brian Leroux, Ph.D., Henry Wang, M.D., Clifton W. Callaway, M.D., Ph.D., George Sopko, M.D.,
Original Article Trial of Continuous or Interrupted Chest Compressions during CPR Graham Nichol, M.D., M.P.H., Brian Leroux, Ph.D., Henry Wang, M.D., Clifton W. Callaway, M.D., Ph.D., George Sopko, M.D.,
PROBLEM: Shock refractory VF/pVT BACKGROUND: Both in 2015 CoSTR. Amiodarone favoured.
 Question Should AMIODARONE vs LIDOCAINE be used for adults with shock refractory VF/pVT PROBLEM: Shock refractory VF/pVT BACKGROUND: Both in 2015 CoSTR. Amiodarone favoured. OPTION: AMIODARONE plus standard
Question Should AMIODARONE vs LIDOCAINE be used for adults with shock refractory VF/pVT PROBLEM: Shock refractory VF/pVT BACKGROUND: Both in 2015 CoSTR. Amiodarone favoured. OPTION: AMIODARONE plus standard
Manual Defibrillation. CPR AGE: 18 years LOA: Altered HR: N/A RR: N/A SBP: N/A Other: N/A
 ROC AMIODARONE, LIDOCAINE OR PLACEBO FOR OUT OF HOSPITAL CARDIAC ARREST DUE TO VENTRICULAR FIBRILLATION OR TACHYCARDIA (ALPS) STUDY: MEDICAL CARDIAC ARREST MEDICAL DIRECTIVE An Advanced Care Paramedic
ROC AMIODARONE, LIDOCAINE OR PLACEBO FOR OUT OF HOSPITAL CARDIAC ARREST DUE TO VENTRICULAR FIBRILLATION OR TACHYCARDIA (ALPS) STUDY: MEDICAL CARDIAC ARREST MEDICAL DIRECTIVE An Advanced Care Paramedic
Ventricular Tachyarrhythmias after Cardiac Arrest in Public versus at Home
 original article Ventricular Tachyarrhythmias after Cardiac Arrest in Public versus at Myron L. Weisfeldt, M.D., Siobhan Everson-Stewart, Ph.D., Colleen Sitlani, M.S., Thomas Rea, M.D., Tom P. Aufderheide,
original article Ventricular Tachyarrhythmias after Cardiac Arrest in Public versus at Myron L. Weisfeldt, M.D., Siobhan Everson-Stewart, Ph.D., Colleen Sitlani, M.S., Thomas Rea, M.D., Tom P. Aufderheide,
A BS TR AC T. n engl j med 365;9 nejm.org september 1,
 The new england journal of medicine established in 1812 september 1, 2011 vol. 365 no. 9 Early versus Later Rhythm Analysis in Patients with Out-of-Hospital Cardiac Arrest Ian G. Stiell, M.D., Graham Nichol,
The new england journal of medicine established in 1812 september 1, 2011 vol. 365 no. 9 Early versus Later Rhythm Analysis in Patients with Out-of-Hospital Cardiac Arrest Ian G. Stiell, M.D., Graham Nichol,
ROC ALPS. Amiodarone, Lidocaine, or Placebo Study
 ROC ALPS Amiodarone, Lidocaine, or Placebo Study Learning Objectives Understand the rationale for antiarrhythmic use in out-of-hospital cardiac arrest Understand how to carry out the ROC ALPS study protocol
ROC ALPS Amiodarone, Lidocaine, or Placebo Study Learning Objectives Understand the rationale for antiarrhythmic use in out-of-hospital cardiac arrest Understand how to carry out the ROC ALPS study protocol
Resuscitation Outcomes Consortium: Overview and Update
 Resuscitation Outcomes Consortium: Overview and Update RESUSCITATION OUTCOMES CONSORTIUM OPALS PRG Annual Meeting Ottawa 2006 Resuscitation Outcomes Consortium: Overview Mandate and Overview Partners Project
Resuscitation Outcomes Consortium: Overview and Update RESUSCITATION OUTCOMES CONSORTIUM OPALS PRG Annual Meeting Ottawa 2006 Resuscitation Outcomes Consortium: Overview Mandate and Overview Partners Project
The New England Journal of Medicine AMIODARONE AS COMPARED WITH LIDOCAINE FOR SHOCK-RESISTANT VENTRICULAR FIBRILLATION AND AIALA BARR, PH.D.
 AMIODARONE AS COMPARED WITH LIDOCAINE FOR SHOCK-RESISTANT VENTRICULAR FIBRILLATION PAUL DORIAN, M.D., DAN CASS, M.D., BRIAN SCHWARTZ, M.D., RICHARD COOPER, M.D., ROBERT GELAZNIKAS, B.SC., AND AIALA BARR,
AMIODARONE AS COMPARED WITH LIDOCAINE FOR SHOCK-RESISTANT VENTRICULAR FIBRILLATION PAUL DORIAN, M.D., DAN CASS, M.D., BRIAN SCHWARTZ, M.D., RICHARD COOPER, M.D., ROBERT GELAZNIKAS, B.SC., AND AIALA BARR,
Resuscitation 83 (2012) Contents lists available at SciVerse ScienceDirect. Resuscitation
 Resuscitation 83 (2012) 1324 1330 Contents lists available at SciVerse ScienceDirect Resuscitation j ourna l h o me pag e: www. elsevier.com/locate/resuscitation Clinical Paper Wide variability in drug
Resuscitation 83 (2012) 1324 1330 Contents lists available at SciVerse ScienceDirect Resuscitation j ourna l h o me pag e: www. elsevier.com/locate/resuscitation Clinical Paper Wide variability in drug
Cardiac Arrest January 2017 CPR /3/ Day to Survival Propensity Matched
 Cardiac Arrest January 217 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN CPR 217 Used data based on protocol that
Cardiac Arrest January 217 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN CPR 217 Used data based on protocol that
CARDIAC ARREST RESEARCH STUDIES. Frequently Asked Questions. Why are these studies being conducted in Milwaukee County?
 CARDIAC ARREST RESEARCH STUDIES Frequently Asked Questions Why are these studies being conducted in Milwaukee County? For the past 20-30 years, Milwaukee County Emergency Medical Services (EMS) System
CARDIAC ARREST RESEARCH STUDIES Frequently Asked Questions Why are these studies being conducted in Milwaukee County? For the past 20-30 years, Milwaukee County Emergency Medical Services (EMS) System
The 2015 BLS & ACLS Guideline Updates What Does the Future Hold?
 The 2015 BLS & ACLS Guideline Updates What Does the Future Hold? Greater Kansas City Chapter Of AACN 2016 Visions Critical Care Conference Nicole Kupchik RN, MN, CCNS, CCRN, PCCN, CMC Independent CNS/Staff
The 2015 BLS & ACLS Guideline Updates What Does the Future Hold? Greater Kansas City Chapter Of AACN 2016 Visions Critical Care Conference Nicole Kupchik RN, MN, CCNS, CCRN, PCCN, CMC Independent CNS/Staff
The Pragmatic Airway Resuscitation Trial
 The Pragmatic Airway Resuscitation Trial Henry E. Wang, MD, MS Professor and Vice Chair for Research Department of Emergency Medicine The University of Texas Health Science Center at Houston McGovern Medical
The Pragmatic Airway Resuscitation Trial Henry E. Wang, MD, MS Professor and Vice Chair for Research Department of Emergency Medicine The University of Texas Health Science Center at Houston McGovern Medical
Management of Cardiac Arrest Based on : 2010 American Heart Association Guidelines
 Management of Cardiac Arrest Based on : 2010 American Heart Association Guidelines www.circ.ahajournals.org Elham Pishbin. M.D Assistant Professor of Emergency Medicine MUMS C H E S Advanced Life Support
Management of Cardiac Arrest Based on : 2010 American Heart Association Guidelines www.circ.ahajournals.org Elham Pishbin. M.D Assistant Professor of Emergency Medicine MUMS C H E S Advanced Life Support
CARDIOPULMONARY RESUSCITATION QUALITY: WIDESPREAD VARIATION IN DATA INTERVALS USED FOR ANALYSIS
 Accepted manuscript of: Talikowska, M. and Tohira, H. and Bailey, P. and Finn, J. 2016. Cardiopulmonary resuscitation quality: Widespread variation in data intervals used for analysis. Resuscitation. 102:
Accepted manuscript of: Talikowska, M. and Tohira, H. and Bailey, P. and Finn, J. 2016. Cardiopulmonary resuscitation quality: Widespread variation in data intervals used for analysis. Resuscitation. 102:
Emergency Cardiac Care Guidelines 2015
 Emergency Cardiac Care Guidelines 2015 VACEP 2016 William Brady, MD University of Virginia Guidelines 2015 Basic Life Support & Advanced Cardiac Life Support Acute Coronary Syndrome Pediatric Advanced
Emergency Cardiac Care Guidelines 2015 VACEP 2016 William Brady, MD University of Virginia Guidelines 2015 Basic Life Support & Advanced Cardiac Life Support Acute Coronary Syndrome Pediatric Advanced
Out-of-hospital cardiac arrest is a leading cause of premature. Resuscitation Science
 Resuscitation Science Chest Compression Fraction Determines Survival in Patients With Out-of-Hospital Ventricular Fibrillation Jim Christenson, MD; Douglas Andrusiek, MSc; Siobhan Everson-Stewart, MS;
Resuscitation Science Chest Compression Fraction Determines Survival in Patients With Out-of-Hospital Ventricular Fibrillation Jim Christenson, MD; Douglas Andrusiek, MSc; Siobhan Everson-Stewart, MS;
Epinephrine Cardiovascular Emergencies Symposium 2018
 Epinephrine Cardiovascular Emergencies Symposium 218 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN High Quality
Epinephrine Cardiovascular Emergencies Symposium 218 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN High Quality
Title: European Resuscitation Council Guidelines for Resuscitation: 2018 Update Antiarrhythmic drugs for cardiac arrest
 Accepted Manuscript Title: European Resuscitation Council Guidelines for Resuscitation: 2018 Update Antiarrhythmic drugs for cardiac arrest Authors: Jasmeet Soar, Gavin D. Perkins, Ian Maconochie, Bernd
Accepted Manuscript Title: European Resuscitation Council Guidelines for Resuscitation: 2018 Update Antiarrhythmic drugs for cardiac arrest Authors: Jasmeet Soar, Gavin D. Perkins, Ian Maconochie, Bernd
in Cardiac Arrest Management Sean Kivlehan, MD, MPH May 2014
 in Cardiac Arrest Management Sean Kivlehan, MD, MPH May 2014 1. Capnography 2. Compressions 3. CPR Devices 4. Hypothermia 5. Access 6. Medications Outline Capnography & Termination Significantly Associated
in Cardiac Arrest Management Sean Kivlehan, MD, MPH May 2014 1. Capnography 2. Compressions 3. CPR Devices 4. Hypothermia 5. Access 6. Medications Outline Capnography & Termination Significantly Associated
IMPACT OF THE 2005 AHA GUIDELINES ON RESUSCITATION OUTCOMES Ronna Zaremski, RN, MSN, CCRN
 Page 1 of 9 IMPACT OF THE 2005 AHA GUIDELINES ON RESUSCITATION OUTCOMES Ronna Zaremski, RN, MSN, CCRN Introduction Recommendations for the management of cardiac arrest have been developed, refined, and
Page 1 of 9 IMPACT OF THE 2005 AHA GUIDELINES ON RESUSCITATION OUTCOMES Ronna Zaremski, RN, MSN, CCRN Introduction Recommendations for the management of cardiac arrest have been developed, refined, and
Evidence for Lidocaine and Amiodarone in Cardiac Arrest Due to VF/Pulseless VT
 Evidence for Lidocaine and Amiodarone in Cardiac Arrest Due to VF/Pulseless VT Introduction Evidence supporting the use of lidocaine and amiodarone for advanced cardiac life support was considered by international
Evidence for Lidocaine and Amiodarone in Cardiac Arrest Due to VF/Pulseless VT Introduction Evidence supporting the use of lidocaine and amiodarone for advanced cardiac life support was considered by international
Update on Sudden Cardiac Death and Resuscitation
 Update on Sudden Cardiac Death and Resuscitation Ashish R. Panchal, MD, PhD Medical Director Center for Emergency Medical Services Assistant Professor Clinical Department of Emergency Medicine The Ohio
Update on Sudden Cardiac Death and Resuscitation Ashish R. Panchal, MD, PhD Medical Director Center for Emergency Medical Services Assistant Professor Clinical Department of Emergency Medicine The Ohio
Classification of Cardiopulmonary Resuscitation Chest Compression Patterns: Manual Versus Automated Approaches
 ORIGINAL CONTRIBUTION Classification of Cardiopulmonary Resuscitation Chest Compression Patterns: Manual Versus Automated Approaches Henry E. Wang, MD, MS, Robert H. Schmicker, MS, Heather Herren, RN,
ORIGINAL CONTRIBUTION Classification of Cardiopulmonary Resuscitation Chest Compression Patterns: Manual Versus Automated Approaches Henry E. Wang, MD, MS, Robert H. Schmicker, MS, Heather Herren, RN,
Supplementary Online Content
 Supplementary Online Content Hasegawa K, Hiraide A, Chang Y, Brown DFM. Association of prehospital advancied airway management with neurologic outcome and survival in patients with out-of-hospital cardiac
Supplementary Online Content Hasegawa K, Hiraide A, Chang Y, Brown DFM. Association of prehospital advancied airway management with neurologic outcome and survival in patients with out-of-hospital cardiac
Advanced Resuscitation - Adult
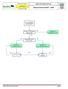 C02A Resuscitation 2017-03-23 17 years & older Office of the Medical Director Advanced Resuscitation - Adult Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
C02A Resuscitation 2017-03-23 17 years & older Office of the Medical Director Advanced Resuscitation - Adult Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
SHOCKING UPDATES IN ACUTE CARDIAC LIFE SUPPORT (ACLS)
 SHOCKING UPDATES IN ACUTE CARDIAC LIFE SUPPORT (ACLS) Reagan Collins, PharmD, BCCCP Clinical Pharmacy Specialist in Critical Care and Nutrition Support The University of Texas MD Anderson Cancer Center
SHOCKING UPDATES IN ACUTE CARDIAC LIFE SUPPORT (ACLS) Reagan Collins, PharmD, BCCCP Clinical Pharmacy Specialist in Critical Care and Nutrition Support The University of Texas MD Anderson Cancer Center
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes
2015 AHA Guidelines: Pediatric Updates
 2015 AHA Guidelines: Pediatric Updates Advances in Pediatric Emergency Medicine December 9, 2016 Karen O Connell, MD, MEd Associate Professor of Pediatrics and Emergency Medicine Emergency Medicine and
2015 AHA Guidelines: Pediatric Updates Advances in Pediatric Emergency Medicine December 9, 2016 Karen O Connell, MD, MEd Associate Professor of Pediatrics and Emergency Medicine Emergency Medicine and
THE FOLLOWING QUESTIONS RELATE TO THE RESUSCITATION COUNCIL (UK) RESUSCITATION GUIDELINES 2005
 THE FOLLOWING QUESTIONS RELATE TO THE RESUSCITATION COUNCIL (UK) RESUSCITATION GUIDELINES 2005 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care
THE FOLLOWING QUESTIONS RELATE TO THE RESUSCITATION COUNCIL (UK) RESUSCITATION GUIDELINES 2005 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care
Over the last 3 decades, advances in the understanding of
 Temporal Trends in Sudden Cardiac Arrest A 25-Year Emergency Medical Services Perspective Thomas D. Rea, MD, MPH; Mickey S. Eisenberg, MD, PhD; Linda J. Becker, MA; John A. Murray, MD; Thomas Hearne, PhD
Temporal Trends in Sudden Cardiac Arrest A 25-Year Emergency Medical Services Perspective Thomas D. Rea, MD, MPH; Mickey S. Eisenberg, MD, PhD; Linda J. Becker, MA; John A. Murray, MD; Thomas Hearne, PhD
Lesson learnt from big trials. Sung Phil Chung, MD Gangnam Severance Hospital, Yonsei Univ.
 Lesson learnt from big trials Sung Phil Chung, MD Gangnam Severance Hospital, Yonsei Univ. Trend of cardiac arrest research 1400 1200 1000 800 600 400 200 0 2008 2009 2010 2011 2012 2013 2014 2015 2016
Lesson learnt from big trials Sung Phil Chung, MD Gangnam Severance Hospital, Yonsei Univ. Trend of cardiac arrest research 1400 1200 1000 800 600 400 200 0 2008 2009 2010 2011 2012 2013 2014 2015 2016
CPR What Works, What Doesn t
 Resuscitation 2017 ECMO and ECLS April 1, 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Circulation 2013;128:417-35
Resuscitation 2017 ECMO and ECLS April 1, 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Circulation 2013;128:417-35
Science Behind Resuscitation. Vic Parwani, MD ED Medical Director CarolinaEast Health System August 6 th, 2013
 Science Behind Resuscitation Vic Parwani, MD ED Medical Director CarolinaEast Health System August 6 th, 2013 Conflict of Interest No Financial or Industrial Conflicts Slides: Drs. Nelson, Cole and Larabee
Science Behind Resuscitation Vic Parwani, MD ED Medical Director CarolinaEast Health System August 6 th, 2013 Conflict of Interest No Financial or Industrial Conflicts Slides: Drs. Nelson, Cole and Larabee
Most Important EMS Articles EAGLES 2017
 Most Important EMS Articles EAGLES 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Overview Best antiarrhythmic
Most Important EMS Articles EAGLES 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Overview Best antiarrhythmic
HigHligHts of the 2018 Focused In 2015 Updates to the American Heart Association Guidelines for CPR and ECC: Advanced Cardiovascular Life
 Highlights of the 2018 Focused Updates to the American Heart Association Guidelines for CPR and ECC: Advanced Cardiovascular Life Support and Pediatric Advanced Life Support - Heart and Stroke Foundation
Highlights of the 2018 Focused Updates to the American Heart Association Guidelines for CPR and ECC: Advanced Cardiovascular Life Support and Pediatric Advanced Life Support - Heart and Stroke Foundation
New ACLS/Post Arrest Guidelines: For Everyone? Laurie Morrison, Li Ka Shing, Knowledge Institute, St Michael s Hospital, University of Toronto
 New ACLS/Post Arrest Guidelines: For Everyone? Laurie Morrison, Li Ka Shing, Knowledge Institute, St Michael s Hospital, University of Toronto COI Declaration Industry and ROC ALS Taskforce ILCOR Author
New ACLS/Post Arrest Guidelines: For Everyone? Laurie Morrison, Li Ka Shing, Knowledge Institute, St Michael s Hospital, University of Toronto COI Declaration Industry and ROC ALS Taskforce ILCOR Author
Disclosure. Co-investigators 1/23/2015
 The impact of chest compression fraction on clinical outcomes from shockable out-of-hospital cardiac arrest during the ROC PRIMED trial Sheldon Cheskes, MD CCFP(EM) FCFP Medical Director, Sunnybrook Centre
The impact of chest compression fraction on clinical outcomes from shockable out-of-hospital cardiac arrest during the ROC PRIMED trial Sheldon Cheskes, MD CCFP(EM) FCFP Medical Director, Sunnybrook Centre
ROC PRIMED Questions and Answers
 ROC PRIMED Questions and Answers 1) What is the ROC PRIMED study? ROC PRIMED stands for the Resuscitation Outcomes Consortium Prehospital Resuscitation using an IMpedance valve and Early versus Delayed
ROC PRIMED Questions and Answers 1) What is the ROC PRIMED study? ROC PRIMED stands for the Resuscitation Outcomes Consortium Prehospital Resuscitation using an IMpedance valve and Early versus Delayed
Most Important EMS Articles EAGLES 2017
 Most Important EMS Articles EAGLES 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Overview Best antiarrhythmic
Most Important EMS Articles EAGLES 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Overview Best antiarrhythmic
Advanced Resuscitation - Child
 C02C Resuscitation 2017-03-23 1 up to 10 years Office of the Medical Director Advanced Resuscitation - Child Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
C02C Resuscitation 2017-03-23 1 up to 10 years Office of the Medical Director Advanced Resuscitation - Child Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
Update on Sudden Cardiac Death and Resuscitation
 Update on Sudden Cardiac Death and Resuscitation Ashish R. Panchal, MD, PhD Medical Director Center for Emergency Medical Services Assistant Professor Clinical Department of Emergency Medicine The Ohio
Update on Sudden Cardiac Death and Resuscitation Ashish R. Panchal, MD, PhD Medical Director Center for Emergency Medical Services Assistant Professor Clinical Department of Emergency Medicine The Ohio
But unfortunately, the first sign of cardiovascular disease is often the last. Chest-Compression-Only Resuscitation Gordon A.
 THE UNIVERSITY OF ARIZONA Sarver Heart Center 1 THE UNIVERSITY OF ARIZONA Sarver Heart Center 2 But unfortunately, the first sign of cardiovascular disease is often the last 3 4 1 5 6 7 8 2 Risk of Cardiac
THE UNIVERSITY OF ARIZONA Sarver Heart Center 1 THE UNIVERSITY OF ARIZONA Sarver Heart Center 2 But unfortunately, the first sign of cardiovascular disease is often the last 3 4 1 5 6 7 8 2 Risk of Cardiac
JUST SAY NO TO DRUGS?
 JUST SAY NO TO DRUGS? THE EVIDENCE BEHIND MEDICATIONS USED IN CARDIAC RESUSCITATION NTI 2014 CLASS CODE 148 Nicole Kupchik RN, MN, CCNS, CCRN, PCCN Objectives 1. Discuss the historical evidence supporting
JUST SAY NO TO DRUGS? THE EVIDENCE BEHIND MEDICATIONS USED IN CARDIAC RESUSCITATION NTI 2014 CLASS CODE 148 Nicole Kupchik RN, MN, CCNS, CCRN, PCCN Objectives 1. Discuss the historical evidence supporting
Advanced Resuscitation - Adolescent
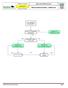 C02B Resuscitation 2017-03-23 10 up to 17 years Office of the Medical Director Advanced Resuscitation - Adolescent Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia
C02B Resuscitation 2017-03-23 10 up to 17 years Office of the Medical Director Advanced Resuscitation - Adolescent Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia
Resuscitation 82 (2011) Contents lists available at ScienceDirect. Resuscitation. journal homepage:
 Resuscitation 82 (2011) 277 284 Contents lists available at ScienceDirect Resuscitation journal homepage: www.elsevier.com/locate/resuscitation Clinical paper Variation in out-of-hospital cardiac arrest
Resuscitation 82 (2011) 277 284 Contents lists available at ScienceDirect Resuscitation journal homepage: www.elsevier.com/locate/resuscitation Clinical paper Variation in out-of-hospital cardiac arrest
Michigan Pediatric Cardiac Protocols. Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS
 Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Michigan Pediatric Cardiac Protocols. Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS
 Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
OTHER FEATURES SMART CPR
 SMART CPR Philips has augmented the HeartStart AED s well proven patient analysis logic with SMART CPR, a feature that provides a tool for Medical Directors and Administrators to implement existing or
SMART CPR Philips has augmented the HeartStart AED s well proven patient analysis logic with SMART CPR, a feature that provides a tool for Medical Directors and Administrators to implement existing or
Johnson County Emergency Medical Services Page 23
 Non-resuscitation Situations: Resuscitation should not be initiated in the following situations: Prolonged arrest as evidenced by lividity in dependent parts, rigor mortis, tissue decomposition, or generalized
Non-resuscitation Situations: Resuscitation should not be initiated in the following situations: Prolonged arrest as evidenced by lividity in dependent parts, rigor mortis, tissue decomposition, or generalized
ADVANCED LIFE SUPPORT
 ANSWERS IN ITALICS WITH REFERENCES 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care professionals equipped with a manual defibrillator, the providers
ANSWERS IN ITALICS WITH REFERENCES 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care professionals equipped with a manual defibrillator, the providers
CPR with Chest Compression Alone or with Rescue Breathing
 original article CPR with Chest Compression Alone or with Rescue Breathing Thomas D. Rea, M.D., Carol Fahrenbruch, M.S.P.H., Linda Culley, B.A., Rachael T. Donohoe, Ph.D., Cindy Hambly, E.M.T., Jennifer
original article CPR with Chest Compression Alone or with Rescue Breathing Thomas D. Rea, M.D., Carol Fahrenbruch, M.S.P.H., Linda Culley, B.A., Rachael T. Donohoe, Ph.D., Cindy Hambly, E.M.T., Jennifer
ANZCOR Guideline 11.2 Protocols for Adult Advanced Life Support
 ANZCOR Guideline 11.2 Protocols for Adult Advanced Life Support Summary Who does this guideline apply to? This guideline applies to adults who require advanced life support (ALS). Who is the audience for
ANZCOR Guideline 11.2 Protocols for Adult Advanced Life Support Summary Who does this guideline apply to? This guideline applies to adults who require advanced life support (ALS). Who is the audience for
Circulation. 2011;124:58-66; originally published online June 20, 2011; doi: /CIRCULATIONAHA
 Perishock Pause : An Independent Predictor of Survival From Out-of-Hospital Shockable Cardiac Arrest Sheldon Cheskes, Robert H. Schmicker, Jim Christenson, David D. Salcido, Tom Rea, Judy Powell, Dana
Perishock Pause : An Independent Predictor of Survival From Out-of-Hospital Shockable Cardiac Arrest Sheldon Cheskes, Robert H. Schmicker, Jim Christenson, David D. Salcido, Tom Rea, Judy Powell, Dana
AED Therapy for Sudden Cardiac Arrest: Focus on Exercise Facilities
 AED Therapy for Sudden Cardiac Arrest: Focus on Exercise Facilities Richard L. Page, M.D. University of Wisconsin School of Medicine and Public Health Disclosures I have no conflict of interest related
AED Therapy for Sudden Cardiac Arrest: Focus on Exercise Facilities Richard L. Page, M.D. University of Wisconsin School of Medicine and Public Health Disclosures I have no conflict of interest related
JUST SAY NO? THE LATEST LOOK AT ACLS MEDICATIONS BRIDGETTE SVANCAREK, MD
 JUST SAY NO? THE LATEST LOOK AT ACLS MEDICATIONS BRIDGETTE SVANCAREK, MD OBJECTIVES Review the progression of the American Heart Association s ACLS cardiac arrest medication guidelines Identify the latest
JUST SAY NO? THE LATEST LOOK AT ACLS MEDICATIONS BRIDGETTE SVANCAREK, MD OBJECTIVES Review the progression of the American Heart Association s ACLS cardiac arrest medication guidelines Identify the latest
WORKSHEET for Evidence-Based Review of Science for Veterinary CPCR
 RECOVER 2011 1 of 6 WORKSHEET for Evidence-Based Review of Science for Veterinary CPCR 1. Basic Demographics Worksheet author(s) James Barr Mailing address: 4474 TAMU Texas A&M University College Station,
RECOVER 2011 1 of 6 WORKSHEET for Evidence-Based Review of Science for Veterinary CPCR 1. Basic Demographics Worksheet author(s) James Barr Mailing address: 4474 TAMU Texas A&M University College Station,
Code Talkers NONE. Disclosures Brady & Slovis. Lay Provider Care. Cardiac Arrest 2017 Resuscitation & Post-arrest Management
 X 10/27/2017 Code Talkers 2017 Cardiac Arrest 2017 Resuscitation & Post-arrest Management What makes sense - & doesn t - in cardiac arrest management William Brady, MD University of Virginia Corey Slovis,
X 10/27/2017 Code Talkers 2017 Cardiac Arrest 2017 Resuscitation & Post-arrest Management What makes sense - & doesn t - in cardiac arrest management William Brady, MD University of Virginia Corey Slovis,
Role of Non-Implantable Defibrillators in the Management of Patients at High Risk for Sudden Cardiac Death
 Role of Non-Implantable Defibrillators in the Management of Patients at High Risk for Sudden Cardiac Death 29 October 2011 Update in Electrocardiography and Arrhythmias Zian H. Tseng, M.D., M.A.S. Associate
Role of Non-Implantable Defibrillators in the Management of Patients at High Risk for Sudden Cardiac Death 29 October 2011 Update in Electrocardiography and Arrhythmias Zian H. Tseng, M.D., M.A.S. Associate
Chain of Survival. Highlights of 2010 American Heart Guidelines CPR
 Highlights of 2010 American Heart Guidelines CPR Compressions rate of at least 100/min. allow for complete chest recoil Adult CPR depth of at least 2 inches Child/Infant CPR depth of 1/3 anterior/posterior
Highlights of 2010 American Heart Guidelines CPR Compressions rate of at least 100/min. allow for complete chest recoil Adult CPR depth of at least 2 inches Child/Infant CPR depth of 1/3 anterior/posterior
Antiarrhythmic drugs for out-of-hospital cardiac arrest with refractory ventricular fibrillation
 Tagami et al. Critical Care (2017) 21:59 DOI 10.1186/s13054-017-1639-8 REVIEW Antiarrhythmic drugs for out-of-hospital cardiac arrest with refractory ventricular fibrillation Takashi Tagami 1,2*, Hideo
Tagami et al. Critical Care (2017) 21:59 DOI 10.1186/s13054-017-1639-8 REVIEW Antiarrhythmic drugs for out-of-hospital cardiac arrest with refractory ventricular fibrillation Takashi Tagami 1,2*, Hideo
POTENTIAL UTILITY OF NEAR-INFRARED SPECTROSCOPY IN OUT-OF-HOSPITAL CARDIAC ARREST: AN ILLUSTRATIVE CASE SERIES
 POTENTIAL UTILITY OF NEAR-INFRARED SPECTROSCOPY IN OUT-OF-HOSPITAL CARDIAC ARREST: AN ILLUSTRATIVE CASE SERIES Adam Frisch, MD, Brian P. Suffoletto, MD, MS, Rachel Frank, EMT, Christian Martin-Gill, MD,
POTENTIAL UTILITY OF NEAR-INFRARED SPECTROSCOPY IN OUT-OF-HOSPITAL CARDIAC ARREST: AN ILLUSTRATIVE CASE SERIES Adam Frisch, MD, Brian P. Suffoletto, MD, MS, Rachel Frank, EMT, Christian Martin-Gill, MD,
Stayin Alive: Pediatric Advanced Life Support (PALS) Updated Guidelines
 Stayin Alive: Pediatric Advanced Life Support (PALS) Updated Guidelines Margaret Oates, PharmD, BCPPS Pediatric Critical Care Specialist GSHP Summer Meeting July 16, 2016 Disclosures I have nothing to
Stayin Alive: Pediatric Advanced Life Support (PALS) Updated Guidelines Margaret Oates, PharmD, BCPPS Pediatric Critical Care Specialist GSHP Summer Meeting July 16, 2016 Disclosures I have nothing to
Compression-Only CPR or Standard CPR in Out-of-Hospital Cardiac Arrest
 The new england journal of medicine original article Compression-Only or Standard in Out-of-Hospital Cardiac Arrest Leif Svensson, M.D., Ph.D., Katarina Bohm, R.N., Ph.D., Maaret Castrèn, M.D., Ph.D.,
The new england journal of medicine original article Compression-Only or Standard in Out-of-Hospital Cardiac Arrest Leif Svensson, M.D., Ph.D., Katarina Bohm, R.N., Ph.D., Maaret Castrèn, M.D., Ph.D.,
Adult Advanced Cardiovascular Life Support 2015 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular
 Adult Advanced Cardiovascular Life Support 2015 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care 1 DR. Alireza Abootalebi Assistant Professor Of
Adult Advanced Cardiovascular Life Support 2015 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care 1 DR. Alireza Abootalebi Assistant Professor Of
Emergency Medicine Research: Creating Evidence to Improve Safety and Effectiveness of ED Patient Care
 Emergency Medicine Research: Creating Evidence to Improve Safety and Effectiveness of ED Patient Care Dr Eric Clark MD, FRCPC Department of Emergency Medicine University of Ottawa Canada No Conflicts of
Emergency Medicine Research: Creating Evidence to Improve Safety and Effectiveness of ED Patient Care Dr Eric Clark MD, FRCPC Department of Emergency Medicine University of Ottawa Canada No Conflicts of
Portage County EMS Annual Skills Labs
 Portage County EMS Annual Skills Labs Scope: Provide skills labs for all Emergency Medical Responders and First Response EMTs to assure proficiency of skills and satisfy the Wisconsin State approved Operational
Portage County EMS Annual Skills Labs Scope: Provide skills labs for all Emergency Medical Responders and First Response EMTs to assure proficiency of skills and satisfy the Wisconsin State approved Operational
Lessons Learned From Cardiac Resuscitation Research: What Matters at the Bedside?
 Lessons Learned From Cardiac Resuscitation Research: What Matters at the Bedside? JILL LEY, MS, RN, CNS, FAAN CLINICAL NURSE SPECIALIST SURGICAL SERVICES CALIFORNIA PACIFIC MEDICAL CENTER CLINICAL PROFESSOR,
Lessons Learned From Cardiac Resuscitation Research: What Matters at the Bedside? JILL LEY, MS, RN, CNS, FAAN CLINICAL NURSE SPECIALIST SURGICAL SERVICES CALIFORNIA PACIFIC MEDICAL CENTER CLINICAL PROFESSOR,
Improving Outcome from In-Hospital Cardiac Arrest
 Improving Outcome from In-Hospital Cardiac Arrest National Teaching Institute San Diego, CA Nicole Kupchik RN, MN, CCNS, CCRN, PCCN, CMC Independent CNS/Staff Nurse Objectives 1. Discuss the AHA in-hospital
Improving Outcome from In-Hospital Cardiac Arrest National Teaching Institute San Diego, CA Nicole Kupchik RN, MN, CCNS, CCRN, PCCN, CMC Independent CNS/Staff Nurse Objectives 1. Discuss the AHA in-hospital
5 Key EMS Articles for 2012
 5 Key EMS Articles for 2012 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN 5 Key Topics Cardiac Arrest Trauma
5 Key EMS Articles for 2012 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN 5 Key Topics Cardiac Arrest Trauma
2015 Interim Training Materials
 2015 Interim Training Materials ACLS Manual and ACLS EP Manual Comparison Chart Assessment sequence Manual, Part 2: The Systematic Approach, and Part BLS Changes The HCP should check for response while
2015 Interim Training Materials ACLS Manual and ACLS EP Manual Comparison Chart Assessment sequence Manual, Part 2: The Systematic Approach, and Part BLS Changes The HCP should check for response while
University of Washington. From the SelectedWorks of Kent M Koprowicz
 University of Washington From the SelectedWorks of Kent M Koprowicz 2007 Site variation in EMS Treatment, Transport and Survival in relation to Restoration of Spontaneous Circulation (ROSC) for Adult Out-of-Hospital
University of Washington From the SelectedWorks of Kent M Koprowicz 2007 Site variation in EMS Treatment, Transport and Survival in relation to Restoration of Spontaneous Circulation (ROSC) for Adult Out-of-Hospital
The evidence behind ACLS: the importance of good BLS
 The evidence behind ACLS: the importance of good BLS Benjamin S. Abella, MD, MPhil, FACEP CRS Center for Resuscitation Science Clinical Research Director Center for Resuscitation Science Vice Chair of
The evidence behind ACLS: the importance of good BLS Benjamin S. Abella, MD, MPhil, FACEP CRS Center for Resuscitation Science Clinical Research Director Center for Resuscitation Science Vice Chair of
CLINICAL RESEARCH STUDY
 CLINICAL RESEARCH STUDY The Effects of Sex on Out-of-Hospital Cardiac Arrest Outcomes Manabu Akahane, MD, PhD, a Toshio Ogawa, MSc, a Soichi Koike, MD, PhD, b Seizan Tanabe, MD, c Hiromasa Horiguchi, PhD,
CLINICAL RESEARCH STUDY The Effects of Sex on Out-of-Hospital Cardiac Arrest Outcomes Manabu Akahane, MD, PhD, a Toshio Ogawa, MSc, a Soichi Koike, MD, PhD, b Seizan Tanabe, MD, c Hiromasa Horiguchi, PhD,
What works? What doesn t? What s new? Terry M. Foster, RN
 What works? What doesn t? What s new? Terry M. Foster, RN 2016 Changes Updated every 5 years Last update was 2010 All recommendations have been heavily researched with studies involving large number of
What works? What doesn t? What s new? Terry M. Foster, RN 2016 Changes Updated every 5 years Last update was 2010 All recommendations have been heavily researched with studies involving large number of
ACLS/ACS Updates 2015
 ACLS/ACS Updates 2015 Advanced Cardiovascular Life Support by: Fareed Al Nozha, JBIM, ABIM, FKFSH&RC(Cardiology) Consultant Cardiologist Faculty, National CPR Committee, ACLS Program Head, SHA Dr Abdulhalim
ACLS/ACS Updates 2015 Advanced Cardiovascular Life Support by: Fareed Al Nozha, JBIM, ABIM, FKFSH&RC(Cardiology) Consultant Cardiologist Faculty, National CPR Committee, ACLS Program Head, SHA Dr Abdulhalim
Resuscitation Science. Relationship Between Chest Compression Rates and Outcomes From Cardiac Arrest
 Resuscitation Science Relationship Between Chest Compression Rates and Outcomes From Cardiac Arrest Ahamed H. Idris, MD; Danielle Guffey, BS; Tom P. Aufderheide, MD; Siobhan Brown, PhD; Laurie J. Morrison,
Resuscitation Science Relationship Between Chest Compression Rates and Outcomes From Cardiac Arrest Ahamed H. Idris, MD; Danielle Guffey, BS; Tom P. Aufderheide, MD; Siobhan Brown, PhD; Laurie J. Morrison,
2016 Top Papers in Critical Care
 2016 Top Papers in Critical Care Briana Witherspoon DNP, APRN, ACNP-BC Assistant Director of Advanced Practice, Neuroscience Assistant in Division of Critical Care, Department of Anesthesiology Neuroscience
2016 Top Papers in Critical Care Briana Witherspoon DNP, APRN, ACNP-BC Assistant Director of Advanced Practice, Neuroscience Assistant in Division of Critical Care, Department of Anesthesiology Neuroscience
Automated external defibrillators and survival after in-hospital cardiac arrest: early experience at an Australian teaching hospital
 Automated external defibrillators and survival after in-hospital cardiac arrest: early experience at an Australian teaching hospital Roger J Smith, Bernadette B Hickey and John D Santamaria Early defibrillation
Automated external defibrillators and survival after in-hospital cardiac arrest: early experience at an Australian teaching hospital Roger J Smith, Bernadette B Hickey and John D Santamaria Early defibrillation
Sooner to the Ballooner: Going Straight to the Cath Lab with Refractory VF/VT
 Sooner to the Ballooner: Going Straight to the Cath Lab with Refractory VF/VT Marc Conterato, MD, FACEP Office of the Medical Director NMAS and the HC EMS Council/Minnesota Resuscitation Consortium DISCLOSURE
Sooner to the Ballooner: Going Straight to the Cath Lab with Refractory VF/VT Marc Conterato, MD, FACEP Office of the Medical Director NMAS and the HC EMS Council/Minnesota Resuscitation Consortium DISCLOSURE
ANZCOR Guideline 11.1 Introduction to Advanced Life Support
 ANZCOR Guideline 11.1 Introduction to Advanced Life Support Who does this guideline apply to? Summary This guideline applies to adults who require advanced life support. Who is the audience for this guideline?
ANZCOR Guideline 11.1 Introduction to Advanced Life Support Who does this guideline apply to? Summary This guideline applies to adults who require advanced life support. Who is the audience for this guideline?
Regionalization of Post-Cardiac Arrest Care
 Regionalization of Post-Cardiac Arrest Care David A. Pearson, MD, FACEP, FAAEM Department of Emergency Medicine Disclosures I have no financial interest, arrangement, or affiliations and no commercial
Regionalization of Post-Cardiac Arrest Care David A. Pearson, MD, FACEP, FAAEM Department of Emergency Medicine Disclosures I have no financial interest, arrangement, or affiliations and no commercial
Objectives: This presentation will help you to:
 emergency Drugs Objectives: This presentation will help you to: Five rights for medication administration Recognize different cardiac arrhythmias and determine the common drugs used for each one List the
emergency Drugs Objectives: This presentation will help you to: Five rights for medication administration Recognize different cardiac arrhythmias and determine the common drugs used for each one List the
Bone Voyage Administrating ALS Medications via the Intra- Osseous Route. Jon Jui MD, MPH
 Bone Voyage Administrating ALS Medications via the Intra- Osseous Route Jon Jui MD, MPH Do ALS Medications lead to Improved Survival in Out of Hospital Cardiac Arrest? Intravenous drug administration during
Bone Voyage Administrating ALS Medications via the Intra- Osseous Route Jon Jui MD, MPH Do ALS Medications lead to Improved Survival in Out of Hospital Cardiac Arrest? Intravenous drug administration during
Prehospital Care Monograph
 Prehospital Care Monograph Amiodarone (Cordarone) City of Pittsburgh Bureau of Emergency Medical Services And Medical Direction Committee Center for Emergency Medicine Of Western Pennsylvania 1 This monograph
Prehospital Care Monograph Amiodarone (Cordarone) City of Pittsburgh Bureau of Emergency Medical Services And Medical Direction Committee Center for Emergency Medicine Of Western Pennsylvania 1 This monograph
18% Survival from In-Hospital Cardiac Arrest Ways we can do better! National Teaching Institute Denver, CO Class Code: 149 A
 18% Survival from In-Hospital Cardiac Arrest Ways we can do better! National Teaching Institute Denver, CO Class Code: 149 A Nicole Kupchik RN, MN, CCNS, CCRN, PCCN Independent CNS/Staff Nurse Objectives
18% Survival from In-Hospital Cardiac Arrest Ways we can do better! National Teaching Institute Denver, CO Class Code: 149 A Nicole Kupchik RN, MN, CCNS, CCRN, PCCN Independent CNS/Staff Nurse Objectives
SUMMARY OF MAJOR CHANGES 2010 AHA GUIDELINES FOR CPR & ECC
 SUMMARY OF MAJOR CHANGES 2010 AHA GUIDELINES FOR CPR & ECC The following is a summary of the key issues and changes in the AHA 2010 Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiac
SUMMARY OF MAJOR CHANGES 2010 AHA GUIDELINES FOR CPR & ECC The following is a summary of the key issues and changes in the AHA 2010 Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiac
Beth Cetanyan, RN AHA RF Aka The GURU
 * Beth Cetanyan, RN AHA RF Aka The GURU *Discuss common causes of Pediatric CA *Review current PALS Guidelines *Through case presentations and discussion, become more comfortable and confident in providing
* Beth Cetanyan, RN AHA RF Aka The GURU *Discuss common causes of Pediatric CA *Review current PALS Guidelines *Through case presentations and discussion, become more comfortable and confident in providing
Early Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac Arrest
 Original Article Early Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac Arrest Ingela Hasselqvist Ax, R.N., Gabriel Riva, M.D., Johan Herlitz, M.D., Ph.D., Mårten Rosenqvist, M.D., Ph.D., Jacob
Original Article Early Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac Arrest Ingela Hasselqvist Ax, R.N., Gabriel Riva, M.D., Johan Herlitz, M.D., Ph.D., Mårten Rosenqvist, M.D., Ph.D., Jacob
RESEARCH. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, clusterrandomised
 Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, clusterrandomised trial David Hostler, research associate professor of emergency medicine, 1 Siobhan Everson-Stewart,
Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, clusterrandomised trial David Hostler, research associate professor of emergency medicine, 1 Siobhan Everson-Stewart,
ACLS. Advanced Cardiac Life Support Practice Test Questions. 1. The following is included in the ACLS Survey?
 1. The following is included in the ACLS Survey? a. Airway, Breathing, Circulation, Differential Diagnosis b. Airway, Breathing, Circulation, Defibrillation c. Assessment, Breathing, Circulation, Defibrillation
1. The following is included in the ACLS Survey? a. Airway, Breathing, Circulation, Differential Diagnosis b. Airway, Breathing, Circulation, Defibrillation c. Assessment, Breathing, Circulation, Defibrillation
Science Behind CPR Update from Darrell Nelson, MD, FACEP Emergency Medicine Wake Forest University Health Sciences
 Science Behind CPR Update from 2010 Darrell Nelson, MD, FACEP Emergency Medicine Wake Forest University Health Sciences FRAMING THE DISCUSSION NO ONE SURVIVES CARDIAC ARREST, EXCEPT ON TV Conflicts of
Science Behind CPR Update from 2010 Darrell Nelson, MD, FACEP Emergency Medicine Wake Forest University Health Sciences FRAMING THE DISCUSSION NO ONE SURVIVES CARDIAC ARREST, EXCEPT ON TV Conflicts of
Sudden Cardiac Arrest
 Sudden Cardiac Arrest Amit Sharma, MD, FACP, FACC Interventional Cardiologist Rockledge Regional Medical Center Assistant Professor of Medicine University of Central Florida Disclosures No relevant financial
Sudden Cardiac Arrest Amit Sharma, MD, FACP, FACC Interventional Cardiologist Rockledge Regional Medical Center Assistant Professor of Medicine University of Central Florida Disclosures No relevant financial
DSED: Is It Real? Brent Myers, MD MPH FACEP CMO and EVP of Medical Operations, Evolution Health Associate CMO, American Medical Response
 DSED: Is It Real? Brent Myers, MD MPH FACEP CMO and EVP of Medical Operations, Evolution Health Associate CMO, American Medical Response However beautiful the strategy, you should occasionally look at
DSED: Is It Real? Brent Myers, MD MPH FACEP CMO and EVP of Medical Operations, Evolution Health Associate CMO, American Medical Response However beautiful the strategy, you should occasionally look at
Consensus Paper on Out-of-Hospital Cardiac Arrest in England
 Consensus Paper on Out-of-Hospital Cardiac Arrest in England Date: 16 th October 2014 Revision Date: 16 th October 2015 Introduction The purpose of this paper is to bring some clarity to the analysis of
Consensus Paper on Out-of-Hospital Cardiac Arrest in England Date: 16 th October 2014 Revision Date: 16 th October 2015 Introduction The purpose of this paper is to bring some clarity to the analysis of
MORE HEARTBEAT THAN A. Enhanced CPR. Better neurologic outcomes. Steve Dunn, Ph.D., Professor, University of Wisconsin Oshkosh
 MORE THAN A HEARTBEAT Enhanced CPR. Better neurologic outcomes. Steve Dunn, Ph.D., Professor, University of Wisconsin Oshkosh Thanks to the ResQPOD and a dedicated EMS team, Steve survived sudden cardiac
MORE THAN A HEARTBEAT Enhanced CPR. Better neurologic outcomes. Steve Dunn, Ph.D., Professor, University of Wisconsin Oshkosh Thanks to the ResQPOD and a dedicated EMS team, Steve survived sudden cardiac
