Commissioning Policy: Targeted Therapies for use in Pulmonary Hypertension in Adults
|
|
|
- Erik Craig
- 6 years ago
- Views:
Transcription
1 Commissioning Policy: Targeted Therapies for use in Pulmonary Hypertension in Adults Reference: NHS England A11/P/c
2 NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information Nursing Trans. & Corp. Ops. Commissioning Strategy Finance Publications Gateway Reference: Document Purpose Document Name Author Publication Date Target Audience Policy A11/P/c Targeted Therapies for use in Pulmonary Hypertension in Adults Specialised Commissioning Team, NHS England July 2015 Local Team Assistant Directors of Specialised Commissioning; Regional Team IFR Leads; Finance Leads; Local Team Pharmacists; Chairs of Clinical Reference Groups; Members of Clinical Reference Groups and registered stakeholders; Acute Trust Chief Executives; Acute Trust Medical Directors; Acute Trust Chief Pharmacists Additional Circulation List Description Cross Reference Superseded Docs (if applicable) Action Required Regional Medical Directors; Regional Directors of Specialised Commissioning; Regional Clinical Directors of Specialised Commissioning; Regional Directors of Nursing NHS England will routinely commission this specialised treatment in accordance with the criteria described in this policy Timing / Deadlines (if applicable) Contact Details for further information By 00 January 1900 jeremyglyde@nhs.net for policy issues 0 Document Status This is a controlled document. Whilst this document may be printed, the electronic version posted on the intranet is the controlled copy. Any printed copies of this document are not controlled. As a controlled document, this document should not be saved onto local or network drives but should always be accessed from the intranet. NB: The National Health Service Commissioning Board was established on 1 October 2012 as an executive non-departmental public body. Since 1 April 2013, the National Health Service Commissioning Board has used the name NHS England for operational purposes. 2
3 Contents 1 Executive Summary... 4 Policy Statement... 4 Equality Statement... 4 Plain Language Summary Introduction Definitions Aims and Objectives Epidemiology and Needs Assessment Evidence Base Rationale behind the Policy Statement Criteria for Commissioning Patient Pathway Governance Arrangements Mechanism for Funding Audit Requirements Documents which have informed this Policy Links to other Policies Date of Review References
4 1 Executive Summary Policy Statement NHS England will commission the treatments outlined in this policy in accordance with the criteria outlined in this document. In creating this policy NHS England has reviewed this clinical condition and the options for its treatment. It has considered the place of this treatment in current clinical practice, whether scientific research has shown the treatment to be of benefit to patients, (including how any benefit is balanced against possible risks) and whether its use represents the best use of NHS resources. This policy document outlines the arrangements for funding of this treatment for the population in England. Equality Statement NHS England has a duty to have regard to the need to reduce health inequalities in access to health services and health outcomes achieved as enshrined in the Health and Social Care Act NHS England is committed to fulfilling this duty as to equality of access and to avoiding unlawful discrimination on the grounds of age, gender, disability (including learning disability), gender reassignment, marriage and civil partnership, pregnancy and maternity, race, religion or belief, gender or sexual orientation. In carrying out its functions, NHS England will have due regard to the different needs of protected equality groups, in line with the Equality Act This document is compliant with the NHS Constitution and the Human Rights Act This applies to all activities for which NHS England is responsible, including policy development, review and implementation. Plain Language Summary Pulmonary hypertension (often shortened to PH) is a serious condition where the blood pressure in the pulmonary arteries is high. This causes progressive damage to the heart and lungs. 4
5 There are many different treatments available for PH. These treatments can improve the symptoms of PH and therefore improve quality of life. Some can slow the progression of PH and can also help reverse damage to the heart and lungs. Treatment for PH can be split into three categories, conventional therapy such as diuretics, targeted therapy and surgery. Many people with PH are treated with both conventional and targeted therapies, although this can be different for different people. Some people with PH may need surgery. How PH is treated will depend on a number of things, for example how severe the PH is or what type of PH the patient has. This policy describes the use of targeted therapies for adult patients with pulmonary hypertension currently funded by NHS England. 2 Introduction Pulmonary arterial hypertension (PAH) is a rare and debilitating chronic disease of the pulmonary vasculature, characterised by vascular proliferation and remodelling of the small pulmonary arteries (Galiè N et al., 2009). This results in a progressive increase in pulmonary vascular resistance (PVR) which can ultimately lead to right heart failure and premature death (Galiè N et al., 2009). PAH is defined as an increase in mean pulmonary arterial pressure (mpap) 25 mmhg (assessed by right heart catheterization [RHC]), a pulmonary wedge pressure of 15 mmhg and normal or reduced cardiac output (Galiè N et al., 2009). PAH can be classified into five etiological subgroups including; idiopathic, heritable, drug and toxin induced, associated, and persistent pulmonary hypertension of the newborn (Simonneau et al., 2009). In addition, PAH is typically scored on the basis of the severity of PAH-specific symptoms into four different World Health Organisation (WHO) functional classes (FC) (Rubin, 2004). This system allows clinicians to make accurate differential diagnoses among diseases that demonstrate similarities in clinical presentation and pathophysiology, and helps to guide their decisions regarding appropriate treatment (Table 1). 5
6 There is currently no cure for PAH, other than lung transplantation (Galiè N et al., 2009). Updated Classification of Pulmonary Hypertension 1. Pulmonary arterial hypertension 1.1 Idiopathic PAH 1.2 Heritable PAH BMPR ALK-1, ENG, SMAD9, CAV1, KCNK Unknown 1.3 Drug and toxin induced 1.4 Associated with: Connective tissue disease HIV infection Portal hypertension Congenital heart diseases Schistosomiasis1 Pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis1. Persistent pulmonary hypertension of the newborn (PPHN). 2. Pulmonary hypertension due to left heart disease 2.1 Left ventricular systolic dysfunction 2.2 Left ventricular diastolic dysfunction 2.3 Valvular disease 2.4 Congenital/acquired left heart inflow/outflow tract obstruction and congenital cardiomyopathies. 3. Pulmonary hypertension due to lung diseases and/or hypoxia 3.1 Chronic obstructive pulmonary disease 3.2 Interstitial lung disease 3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern 3.4 Sleepdisordered breathing 3.5 Alveolar hypoventilation disorders 3.6 Chronic exposure to high altitude 3.7 Developmental lung diseases. 4. Chronic thromboembolic pulmonary hypertension (CTEPH) 5. Pulmonary hypertension with unclear multifactorial mechanisms 5.1 Hematologic disorders: chronic hemolytic anemia, myeloproliferative disorders, splenectomy 5.2 Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis 5.3 Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders 5.4 Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure, segmental PH. Taken from Simonneau G et al (2013). 3 Definitions Pulmonary hypertension (PH) is a rare disorder of the blood vessels in the lung, characterised by raised pressure in the pulmonary artery, which results in a range of symptoms and may be life threatening. 6
7 PH is defined as an increase in mean pulmonary artery pressure (PAP) of 25mmHg or greater at rest as assessed by right heart catheterisation. A definition of PH on exercise (as a mean PAP >30mm Hg) is not supported by published data. PH can be found in a diverse range of clinical conditions, including connective tissue disease, congenital heart diseases, chronic pulmonary thromboembolism, sickle cell disease, HIV infection, use of an appetite suppressant, and liver disease. Pulmonary arterial hypertension (PAH) is a clinical condition characterised by the presence of pre-capillary PH in the absence of other causes of pre-capillary PH such as lung disease, chronic thromboembolism, or other rare causes. If the cause is unknown then it is referred to as idiopathic pulmonary arterial hypertension (IPAH). IPAH can occur sporadically or may be familial. Chronic thromboembolic pulmonary hypertension (CTEPH), is a form of pulmonary hypertension (PH), categorized by the WHO as Group 4 PH. It is defined as mean pulmonary arterial pressure 25 mmhg and pulmonary capillary wedge pressure 15mmHg in the presence of multiple chronic/organized occlusive thrombi/emboli in the elastic pulmonary arteries (main, lobar, segmental, sub-segmental) after at least three months of effective anticoagulation. Functional class Assessment of WHO Functional Class (FC) is an important predictor of survival, despite large inter-observer variation in its measurement. Table 2 describes the characteristics of the four classes. In untreated patients with IPAH or heritable PAH, historical data suggests a median survival of 6 months in patients in WHO-FC IV, 2.5 years for those in WHO-FC III, and 6 years for WHO-FC I and II. 1 It is expected that defining a patient s functional class will be a multidisciplinary team decision. 7
8 4 Aims and Objectives This policy aims to provide criteria against which treatments for PAH and CTEPH will be commissioned. In addition, it includes the inclusion of a new class of drug riociguat which is a soluble guanulate cyclase stimulator. The objectives of the policy amendment and evidence review are to only consider patients with pulmonary hypertension due to CTEPH. The treatment for PAH remains unchanged within the policy. 5 Epidemiology and Needs Assessment The estimated annual incidence of diagnosed PAH in the general population ranges from 0.9 to 7.6 cases per million persons, while the prevalence of diagnosed PAH in the general population is between 6.6 and 26 cases per million persons (Frost et al., 2013, Humbert et al., 2006, Peacock et al., 2007, Ling et al., 2012, Hurdman et al., 2012). Incidence and prevalence rates may be underestimated as a result of mis and/or undiagnosed patients (Frost et al., 2013). Natural history PAH is a progressive illness; if not diagnosed early and/or left untreated, patient condition can deteriorate rapidly, leading to premature mortality in all aetiologies 8
9 (Gomberg-Maitland, 2011). Figure 1 below demonstrates the progression of PAH over time, as measured by hemodynamic parameters (ref adapted from Harrison s Principles of Internal Medicine, 14th ed). As shown, the disease can progress even while the patient remains asymptomatic or exhibits only non-specific and subtle signs of PAH. In addition, mildly symptomatic patients may already have impaired hemodynamics, including elevated PVR and pulmonary arterial pressure (PAP). The predominant symptom of PAH is dyspnoea on exertion, and most patients present with this symptom (Galiè N et al., 2009, McLaughlin et al., 2009b, McGoon and Kane, 2009). Approximately one-third of PAH patients also experience angina during the course of the disease, and syncope occurs in a similar proportion of patients (McGoon and Kane, 2009). Patients with PAH are prone to contract pneumonia, the cause of death in 7% of cases (Galiè N et al., 2009). With progression to decompensated right heart failure, patients develop fluid retention that leads to increased central venous pressure, abdominal organ (e.g., hepatic) congestion, peripheral oedema and ascites (Galiè N et al., 2009); (McGoon and Kane, 2009). It is important to recognise that riociguat is the only drug to meet its end point positively in a randomised placebo controlled double blind trial. 9
10 6 Evidence Base The evidence for PAH treatments approved before April 2013 were reviewed by the former Yorkshire and Humber Specialised Commissioning Group (SCG) on behalf of the ten former SCGs in England. This review supported the approval of the National PAH Guidelines published in 2011 and subsequent adoption by NHS England as a policy statement. The policy was further amended to include the addition of macitentan, a new endothelin receptor antagonist in May This updated policy includes the addition of riociguat, a new drug from a class of treatments termed Soluble Guanylate Cyclase Stimulators (SGCS) therapies for approval within the policy for the treatment of patients with Chronic Thromboembolic Pulmonary Hypertension (CTEPH). Macitentan is a dual endothelin A/B (ET A /ET B ) receptor antagonist.(iglarz et al., 2008) It is fundamentally different from the other ERAs currently approved for PAH therapy namely, the dual ET A /ET B receptor antagonist, bosentan, and the ET A - selective antagonist, ambrisentan.(bolli et al, 2012) Clinical efficacy has been documented in one randomised, double-blind, placebocontrolled Phase III trial (Pulido et al., 2013). This study (SERAPHIN) is the largest and longest randomised trial conducted to date in PAH, using the recommended primary outcome of morbidity and mortality (McLaughlin et al., 2009c). Existing oral PAH therapies have been approved on the basis of small, randomised clinical trials with short follow-up periods and with primary outcomes that do not reflect clinical events indicative of true disease progression. Macitentan is the first PAH-specific agent shown to have an impact on clinical outcomes in an event-driven, long-term randomised controlled trial (RCT). SERAPHIN demonstrated a significant and clinically relevant 45% reduction in the risk of morbidity and mortality outcomes with macitentan versus placebo (p<0.001). This effect was established early and sustained over a median treatment duration of more than two years. Importantly, macitentan significantly reduced the risk of morbidity and mortality in patients treated with PAH-specific background therapy, 10
11 consisting mainly (96%) of phosphodiesterase-5 (PDE-5) inhibitors (38% reduction, p=0.009), as well as in treatment-naive patients. A significant reduction was also demonstrated in the risk of death or hospitalisation due to PAH (50%, p<0.001), as well as the frequency of (55%, p<0.001) and length of stay for PAH-related hospitalisations (52% reduction, p=0.04) (Actelion Pharmaceuticals Ltd, 2012). In addition, treatment with macitentan led to improvements in short-term endpoints, including six-minute walk distance (6MWD) and WHO FC (Pulido et al., 2013). Treatment with macitentan also showed significant and clinically relevant improvements in health-related quality of life (HRQoL) assessed using the short form 36 (SF-36) questionnaire. While it is not possible to compare the risk of morbidity and mortality between macitentan and other endothelin receptor antagonists (ERAs), a comparison based on short-term endpoints showed no significant differences. Changes in short-term endpoints are, however, not predictive of long-term outcomes. Therefore, macitentan is the only ERA which has demonstrated both short- and longterm benefits in an RCT. Consequently, macitentan is the only PAH-specific therapy with a licence for long-term treatment. Treatment with macitentan was well tolerated, with a similar overall incidence of treatment-emergent adverse events (TEAEs) to placebo. The majority of AEs in the trial were mild-to-moderate in intensity, with nasopharyngitis, headache and anaemia being reported most frequently. A significant safety concern associated with ERAs has been their potential for causing liver injury. The incidence of liver disorders and abnormal liver function-related AEs with macitentan was similar to that observed with placebo, as was the risk of oedema (Pulido et al., 2013). Riociguat: the evidence for riociguat for the treatment of inoperable/residual CTEPH, data was gathered from published literature. Articles were retrieved by electronic search strategy. Searches were carried out in MEDLINE, Cochrane, NHS Evidence and CRD databases. The search terms used were Riociguat, Adempas, Sildenafil, Viagra, Tadalafil Cialis, Pulmonary hypertension, Pulmonary arterial hypertension, Chronic thromboembolic pulmonary hypertension and their combinations. 11
12 Inclusion criteria Articles published between January 2000 and July 2014 Articles in the English language Phase 3 Randomised control trials reporting clinical efficacy and safety Any study designs reporting on cost-effectiveness One study was found which met the inclusion criteria, reporting on the clinical effectiveness and safety of Riociguat for CTEPH- see Table 1. Table 1: Riociguat for CTEPH Study type RCT Reference Ghofrani et al 2013b Clinical Effectiveness of Riociguat in treatment of inoperable/residual CTEPH A phase 3 multi centre, double blinded RCT which included 261 patients chronic thromboembolic pulmonary hypertension that was adjudicated to be technically inoperable (72%) or if they had persistent or recurrent pulmonary hypertension after undergoing pulmonary endarterectomy (28%) and if they had: a pulmonary vascular resistance greater than 300 dyn sec cm 5, a mean pulmonary-artery pressure of at least 25 mm Hg, and a 6 minute walk distance (MWD) of 150 to 450 m. Patients were excluded if they had received an endothelin-receptor antagonist, prostacyclin analogue, PDE5i, or nitric oxide donor within the 3 months before study entry. Majority of patients were in WHO functional class II or III (31% and 64% respectively). 72% of patients had inoperable chronic thromboembolic pulmonary hypertension whilst (28%) had postoperative persistent or recurrent pulmonary hypertension. Majority of patients were in WHO functional class II or III (31% and 64% respectively). Patients received placebo or individually adjusted to a maximum of 2.5 mg Riociguat 3 times daily At 16 weeks, the primary end point of 6MWD, in the Riociguat group was reported to be increased by 39 m compared with a 6 m decrease in the placebo group (p < 12
13 0.001) from a baseline of 342 m, mean difference of 46 m; 95% confidence interval [CI], 25 to 67; P<0.001). This effect was seen in both patients with inoperable and residual CTEPH post-pea although a greater increase was seen in the inoperable group (54 vs 26 m). Increases in primary outcome reported (mean (±SD) an increase of 63±64 m over the baseline distance in CHEST-1 during the first 12 weeks of the long-term extension study (CHEST-II, n=129). Riociguat was also associated with significant improvements in the secondary endpoints of PVR, NTproBNP and WHO functional class at 16 weeks. Safety of Riociguat in treatment of inoperable/residual CTEPH Overall, riociguat was well tolerated in the CHEST- I study. Drug related serious adverse events in the riociguat group included syncope in three patients (2%) and gastritis, acute renal failure, and hypotension in one patient each (1%); in the placebo group, syncope and trauma occurred in one patient each (1%). A case of acute renal failure was considered by the investigator to be related to the study drug. Cost Effectiveness of Riociguat in treatment of inoperable/residual CTEPH No cost effectiveness studies related to use of Riociguat in CTEPH were found. Clinical Effectiveness of Riociguat in treatment of potentially operable CTEPH No studies related to use of Riociguat in potentially operable CTEPH were found. Safety of Riociguat in treatment of potentially operable CTEPH No studies related to use of Riociguat in potentially operable CTEPH were found. 7 Rationale behind the Policy Statement This policy updates the existing NHS England clinical commissioning policy A11/P/b to include riociguat as a treatment for patients with CTEPH. Where used as a treatment alternative, riociguat is being made available at equivalent costs to other agents through a commercial in confidence discount. 13
14 Riociguat is the only licensed drug where evidence has shown that patients receiving this drug, benefit from a better quality of life and improved exercise tolerance. 8 Criteria for Commissioning This policy has been agreed on the basis of NHS England s understanding of the likely price of care associated with enacting the policy for all patients for whom NHS England has funding responsibility, as at the time of the policy s adoption. Should these prices materially change, and in particular should they increase, NHS England may need to review whether the policy remains affordable and may need to make revisions to the published policy. Included patient populations Treatment with disease-targeted medicines will be routinely commissioned for adults, assessed as in WHO-FC III or IV, in one of the following clinical classifications: Group 1 Pulmonary arterial hypertension (PAH) Group 1* Pulmonary veno-occlusive disease (PVOD)and/or pulmonary capillary haemangiomatosis (PCH) Group 4 Inoperable chronic thromboembolic pulmonary hypertension (CTEPH) [NB This includes patients with potentially operable disease who refuse surgery or who are waiting for acceptance for surgery] Treatment will also be routinely commissioned for patients with chronic renal failure on dialysis or sarcoidosis associated with pulmonary hypertension, and for women who are pregnant (see below), who fall within one of the allowed classes described above. Excluded patient populations Treatment with disease-targeted medicines will not be routinely commissioned for patients in the following groups unless described otherwise above: 14
15 WHO-FC I or II (except for those patients who meet the clinical criteria listed in the Clinical Commissioning Policy A11/P/a: Targeted Therapies for Pulmonary Hypertension Functional Class II). Patients in clinical classifications 2 and 3 (Table 2): the use of targeted therapies for patients in these classifications is not recommended until robust data are available. PH with unclear or multifactorial mechanisms (clinical classification group 5): there is no robust evidence to support treatment with targeted therapies in these patient groups. NB: treatment for patients with chronic renal impairment on dialysis and those with sarcoidosis is routinely commissioned. 1.1 Approved medicines Four types of medicine may be used under this policy. However, due to differences between some products within each class, particularly in price (see Table 1), they are commissioned by individual product rather than by type, unless stated otherwise. Doses above those specified (e.g. bosentan 250mg twice daily) will not be routinely funded. 1) Phosphodiesterase type 5 inhibitors (PDE5I) a) Sildenafil (oral) As generic sildenafil tablets (unlicensed indication): for dose escalation mg three times daily As Revatio tablets: for use only at licensed dose of 20mg three times daily b) Tadalafil (oral) tablets: for use only at licensed dose of 40mg once daily 2) Endothelin receptor antagonists (ERA) (NB commissioned by type) a) Bosentan (oral) tablets: 62.5mg 125mg twice daily b) Ambrisentan (oral) tablets: 5-10mg once daily c) Macitentan* (oral) tablets: 10mg once daily *Only when purchased at the commercial in confidence discount 15
16 Soluble Guanylate Cyclase Stimulators (SCGS) a) Riociguat* (oral) tablets : dose as per titration usually 2.5mg three times daily *Only when purchased at the commercial in confidence discount 3) Prostanoids a) Epoprostenol (intravenous): dose titrated to response b) Iloprost (nebulised): 5micrograms up to 9-times daily c) Iloprost (intravenous, unlicensed product): dose titrated to response Treprostinil will not be routinely commissioned for new patients but funding will continue for patients already established under the terms of the national policy. Treprostinil is a prostanoid that may be administered by sub-cutaneous or intravenous routes (unlicensed product). While it may have a role in the subcutaneous treatment of very small numbers of seriously ill patients with PH who are not suitable for nebulised or intravenous administration of a prostanoid, commissioners believe that, at around 3-4 times the price of equivalent alternatives, its new pricing structure cannot be justified. Table 1: Approximate annual treatment costs at recommended doses Cost without homecare* Sildenafil (generic) Sildenafil (Revatio) 5,356 Tadalafil 6,400 Bosentan 23,500 Ambrisentan Circa 23,500 Macitentan (excluding discount) 27,672 Riociguat (excluding discount) 26,000 IV Iloprost (including pump) 39,000 Nebulised Iloprost (Ventafee) 32,200 Epoprostenol (including pump) Circa 35,000 Trepostinil (including pump) Circa 120,000 *Prices will vary depending on local agreements if delivered via homecare Treatment should be initiated and, where appropriate, escalated with the least expensive suitable product. Doses higher than those specified above will not be routinely funded. 16
17 Approved regimens Pulmonary Arterial Hypertension First line therapy Monotherapy with an oral PDE5I will be routinely commissioned as first line therapy. Where a PDE5I is not clinically appropriate, an ERA may be substituted. The choice of medicine is subject to clinical discretion bearing in mind relative safety, evidence of efficacy, and cost of treatment. Monotherapy with a prostanoid will be routinely commissioned for patients at WHO FC-IV with clinical classification 1 or 1* (see table 2). Second line therapy Patients who have failed to respond to a trial of therapy of adequate dose and duration (typically 8-12 weeks treatment), or failed to tolerate one of the oral therapies should be switched to an alternative oral product as monotherapy. Patients who have initially responded to first-line therapy but then deteriorated despite dose escalation (if appropriate) may be considered for dual therapy (see below). Patients who have had a suboptimal response to first-line therapy (with dose escalation where appropriate) may be considered for dual therapy. A prostanoid will be routinely commissioned for patients with clinical classification 1 and 1* (see table 2) with WHO FC-III who have failed to respond adequately or tolerate dual therapy with an oral PDE5I and an oral ERA. [NB In exceptional cases, where an acutely unwell patient requires inpatient treatment, monotherapy with a prostanoid may be initiated as an alternative to dual therapy]. A prostanoid will not be routinely commissioned for use in patients with other clinical classification Dual therapy Dual therapy will only be funded in combinations involving a PDE5I unless there are exceptional circumstances. 17
18 Dual therapy will be commissioned for patients with progressive disease: who have failed to respond to 1st and 2nd-line monotherapy. who have initially responded to monotherapy but subsequently deteriorated despite dose escalation (if appropriate). who have had a suboptimal response to monotherapy (with dose escalation, where appropriate) In exceptional cases, where a patient is acutely unwell and hospitalised, the progression to dual therapy may be accelerated. Triple therapy Triple therapy will be routinely commissioned only for patients who have been formally assessed by a transplant centre and accepted as a suitable candidate. Pregnancy PH in pregnancy is associated with high mortality. Disease-targeted therapies may be used alone, or in combination, according to clinical signs and symptoms at the discretion of the treating clinician, irrespective of functional class. Exceptional Cases Pre-agreed exceptions for dual therapy in combination, not involving a PDE5I, are: Dual therapy: as a bridge for a patient switching from one mono-therapy to an alternative mono-therapy (up to a maximum of 12 weeks) Dual therapy: for patients who have been listed for the following surgery: Heart-lung transplantation Double Lung transplantation Thrombo-endarterectomy (in patients with chronic thrombo-embolic disease) Continuation of existing treatments (including a prostanoid) for patients making the transition from children s services to adult services where it 18
19 would be inappropriate to change treatments only to comply with the commissioning policy. Continuation of existing treatments (including a prostanoid) for adult patients (i.e. started prior to the policy being agreed) which are not in accordance with the commissioning policy is permitted until the patient and their clinician consider it appropriate to stop. Clinical Trials NHS England will not pick up the funding of patients exiting clinical trials funded by the pharmaceutical industry, extended access programs or compassionate funding programs unless prior arrangements have been made. It is seen as the responsibility of those initiating therapy, and manufacturers sponsoring trials, to ensure that there is either an exit strategy or that ongoing treatment is provided. Patients should be fully informed of these arrangements. Approved regimens Inoperable / Residual Chronic Thromboembolic Pulmonary Hypertension. A. Patients with potentially operable CTEPH in WHO FC III or IV. who have: significant RV impairment and/or significant comorbidities and/or evidence of deterioration whilst awaiting decision for pulmonary endarterectomy may also be considered for sildenafil. Where sildenafil is not clinically appropriate, tadalafill or an ERA may be substituted. Triple therapy including riociguat will not be commissioned. B. Patients in WHO FC II, III or IV unsuitable for surgery either due to comorbidity or patient choice First line therapy Monotherapy with an sildenafil will be routinely commissioned. 19
20 Where sildenafil is not clinically appropriate, an ERA or riociguat may be substituted. Second line therapy Patients who have failed to respond to a trial of therapy of adequate dose and duration (typically 12 weeks treatment), or failed to tolerate one of the oral therapies should be switched to an alternative oral product (riociguat) as monotherapy. Operable patients initially declining surgery who fail to respond to medical treatment will be advised to revisit the surgical option. Dual therapy / Triple therapy Dual therapy with addition of ERA or prostanoid to sildenafil will be considered in patients who have failed to respond to a trial of therapy of adequate dose and duration (typically 12 weeks treatment). Dual therapy involving riociguat + ERA or prostanoid will not be commissioned. Triple therapy will not be routinely commissioned for CTEPH patients. The exception is to consider sildenafil, ERA and a prostenoid but only for patients as listed below: Patients with significant inoperable disease felt to be behaving in a similar manner to IPAH Patients who have been formally assessed by a transplant centre and accepted as a suitable candidate. Triple therapy including riociguat will not be commissioned. C. Patients in WHO FC II, III or IV unsuitable for surgery either due to anatomical distribution of disease or residual CTEPH post PE (The phenotype of patient in the CHEST study) First line therapy Monotherapy with oral riociguat will be routinely commissioned. 20
21 Where riociguat is not appropriate (either clinically or due to titration logistics), sildenafil may be substituted. Where both riociguat and sildenafil are not clinically appropriate, tadalafill or an ERA may be substituted. Second line therapy Patients who have failed to respond to a trial of therapy of adequate dose and duration (typically 12 weeks treatment), or failed to tolerate riociguat should be switched to (sildenafil) as monotherapy. Dual therapy / Triple therapy Dual therapy with addition of ERA or prostanoid to sildenafil will be considered in patients who have failed to respond to a trial of therapy of adequate dose and duration (typically 12 weeks treatment). Dual therapy involving riociguat + ERA or prostanoid will not be commissioned. Triple therapy compromising of sildenafil, + ERA + prostanoid will not be routinely commissioned for CTEPH patients except : Patients with significant inoperable disease felt to be behaving in a similar manner to IPAH Patients who have been formally assessed by a transplant centre and accepted as a suitable candidate. Triple therapy including riociguat will not be commissioned. Pregnancy CTEPH in pregnancy is associated with high mortality. Disease-targeted therapies may be used alone, in non-operable cases or in combination, according to clinical signs and symptoms at the discretion of the treating clinician, irrespective of functional class, as above. CTEPH is extremely rare in paediatric practice. 21
22 Cases not addressed above Pre-agreed exceptions for dual therapy in combination, not involving sildenafil, are: Continuation of existing treatments (including a prostanoid) for patients making the transition from children s services to adult services where it would be inappropriate to change treatments only to comply with the commissioning policy. Continuation of existing treatments (including a prostanoid) for adult patients (i.e. started prior to the policy being agreed) which are not in accordance with the commissioning policy is permitted until the patient and their clinician consider it appropriate to stop. Patient Stopping Criteria The continued use of target therapies will be reviewed on a regular basis. The key factors influencing the cessation of treatment will be:- Successful surgery Clinically relevant side-effects e.g. liver function Poor/no response to treatment No other active treatment option available Drug therapies may also be withdrawn at the end of life phase. 9 Patient Pathway Please refer to the service specifications relating to Pulmonary Hypertension Centres (Adult) and Pulmonary Hypertension Shared Care (Adult). 10 Governance Arrangements Six centres are designated to provide pulmonary hypertension services for adults. The centres offer investigation and treatment of patients with idiopathic pulmonary hypertension, pulmonary hypertension complicating other diseases and assessment 22
23 of response to treatment. The centres and staff also provide support for patients and their families. Importantly, only the designated centres are able to initiate treatment with a disease-targeted medicine under this policy. In some circumstances, explicit and formalised shared-care agreements may be made by the designated centres with other specialist centres to prescribe disease-targeted therapies. However, nonspecialist clinicians and General Practitioners should not be asked to routinely prescribe these medicines since they are not able to submit information to the national database. Where a patient is started on a disease-targeted therapy, their GP should be informed and alerted to any potential for unwanted effects, including interactions with other medicines. A service specification for Pulmonary Hypertension has been published by NHS England including standards for the delivery of care. The designated Adult centres are: NB Great Ormond Street Hospital is a designated Highly Specialised Service to provide pulmonary hypertension services for children. 11 Mechanism for Funding 23
24 The treatments as defined in this policy are routinely commissioned by NHS England. 12 Audit Requirements Each centre will need to provide commissioners with a monthly monitoring statement covering the following fields: ID number Patient Initials NHS number PCT/SCG codes Drug and dose Notification of changes to drugs and dosage Takeoff date Reason for takeoff Monthly cost Annual cost Survival Quality of Life estimate (emphasis 10) Absolute 6 minute walk 13 Documents which have informed this Policy See references 14 Links to other Policies Clinical Commissioning Policy A11/P/a: Targeted Therapies for Pulmonary Hypertension Functional Class II. This policy follows the principles set out in the ethical framework that govern the commissioning of NHS healthcare and those policies dealing with the approach to 24
25 experimental treatments and processes for the management of individual funding requests (IFR). 15 Date of Review This policy will be reviewed in October 2017 unless information is received which indicates that the proposed review date should be brought forward or delayed. References 1. The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal (2009) 30, Available: Guidelines Documents/guidelines-PH-FT.pdf 2. Humbert M, et al, Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med;173: Peacock AJ, et al, An epidemiological study of pulmonary arterial hypertension. Eur Respir J; 30: Galie, N., et al, R. J., PULMONARY ARTERIAL, H. & RESPONSE TO TADALAFIL STUDY, G Tadalafil therapy for pulmonary arterial hypertension. Circulation, 119, Simonneau, G., et a, l Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol, 54, S Rubin, L. J Introduction*: Diagnosis and management of pulmonary arterial hypertension: accp evidence-based clinical practice guidelines. CHEST Journal, 126, 7S-10S. 7. Frost, A. E., et al, Demographics and outcomes of patients diagnosed with pulmonary hypertension with pulmonary capillary wedge pressures 16 to 18 mm Hg: insights from the REVEAL Registry. Chest, 143, Humbert, M., et al, Pulmonary arterial hypertension in France: results from a national registry. American journal of respiratory and critical care medicine, 173,
26 9. Peacock, A. J., et al, An epidemiological study of pulmonary arterial hypertension. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology, 30, Ling, Y., et al, Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med, 186, Hurdman, J., et al, Pulmonary hypertension in COPD: results from the ASPIRE registry. European Respiratory Journal. 12. Gomberg-Maitland, M Naming and understanding rare diseases: International Classification of Diseases coding and the epidemiologic designations of idiopathic pulmonary arterial hypertension. Chest, 139, McLaughlin, V. V., et al, 2009b. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association Developed in Collaboration With the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. Journal of the American College of Cardiology, 53, McGoon, M. D. & Kane, G. C Pulmonary hypertension: diagnosis and management. Mayo Clin Proc, 84, Iglarz, M., et al, Pharmacology of macitentan, an orally active tissuetargeting dual endothelin receptor antagonist. J Pharmacol Exp Ther, 327, Bolli, M. H., et al, The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo- 2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-p ropylsulfamide (Macitentan), an orally active, potent dual endothelin receptor antagonist. J Med Chem, 55, Pulido, T., et al, Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med, 369, Mclaughlin, V. V., et al, 2009c. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol, 54, S
27 19. Cannon JE, Pepke-Zaba J. Riociguat for pulmonary hypertension. Expert Rev Clin Pharmacol May;7(3): doi: / Epub 2014 Mar Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ; PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013a Jul 25;369(4): Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C; CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013b Jul 25;369(4):
28 28
Clinical Commissioning Policy: National policy for targeted therapies for the treatment of pulmonary hypertension in adults
 Clinical Commissioning Policy: National policy for targeted therapies for the treatment of pulmonary hypertension in adults Reference: NHS England A11/P/c 1 Clinical Commissioning Policy: National policy
Clinical Commissioning Policy: National policy for targeted therapies for the treatment of pulmonary hypertension in adults Reference: NHS England A11/P/c 1 Clinical Commissioning Policy: National policy
22nd Annual Heart Failure 2018 an Update on Therapy. Pulmonary Arterial Hypertension: Contemporary Approach to Treatment
 22nd Annual Heart Failure 2018 an Update on Therapy Pulmonary Arterial Hypertension: Contemporary Approach to Treatment Ronald J. Oudiz, MD, FACP, FACC, FCCP Professor of Medicine The David Geffen School
22nd Annual Heart Failure 2018 an Update on Therapy Pulmonary Arterial Hypertension: Contemporary Approach to Treatment Ronald J. Oudiz, MD, FACP, FACC, FCCP Professor of Medicine The David Geffen School
Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England A11X04/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England A11X04/01 Information Reader Box (IRB) to be inserted on inside front cover
Effective Strategies and Clinical Updates in Pulmonary Arterial Hypertension
 Effective Strategies and Clinical Updates in Pulmonary Arterial Hypertension Hap Farber Director, Pulmonary Hypertension Center Boston University School of Medicine Disclosures 1) Honoria: Actelion, Gilead,
Effective Strategies and Clinical Updates in Pulmonary Arterial Hypertension Hap Farber Director, Pulmonary Hypertension Center Boston University School of Medicine Disclosures 1) Honoria: Actelion, Gilead,
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
ADCIRCA (tadalafil) The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Pulmonary arterial hypertension is a rare disorder of the pulmonary arteries in which the pulmonary arterial pressure rises above normal levels in the absence
RATIONALE FOR INCLUSION IN PA PROGRAM Background Pulmonary arterial hypertension is a rare disorder of the pulmonary arteries in which the pulmonary arterial pressure rises above normal levels in the absence
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.10 Subject: Uptravi Page: 1 of 6 Last Review Date: September 15, 2017 Uptravi Description Uptravi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.10 Subject: Uptravi Page: 1 of 6 Last Review Date: September 15, 2017 Uptravi Description Uptravi
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.17 Subject: Remodulin Page: 1 of 5 Last Review Date: June 24, 2016 Remodulin Description Remodulin
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.17 Subject: Remodulin Page: 1 of 5 Last Review Date: June 24, 2016 Remodulin Description Remodulin
REVATIO (sildenafil)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Pulmonary arterial hypertension is a rare disorder of the pulmonary arteries in which the pulmonary arterial pressure rises above normal levels in the absence
RATIONALE FOR INCLUSION IN PA PROGRAM Background Pulmonary arterial hypertension is a rare disorder of the pulmonary arteries in which the pulmonary arterial pressure rises above normal levels in the absence
PVDOMICS. Study Introduction. Kristin Highland, MD Gerald Beck, PhD. NHLBI Pulmonary Vascular Disease Phenomics Program
 PVDOMICS Study Introduction Kristin Highland, MD Gerald Beck, PhD NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
PVDOMICS Study Introduction Kristin Highland, MD Gerald Beck, PhD NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
Update in Pulmonary Arterial Hypertension
 Update in Pulmonary Arterial Hypertension Michael J Sanley, MD April 12, 2018 Disclosures I have nothing to disclose 2 1 Case Presentation 67 yo male with atrial fibrillation, CLL on IVIG, presents with
Update in Pulmonary Arterial Hypertension Michael J Sanley, MD April 12, 2018 Disclosures I have nothing to disclose 2 1 Case Presentation 67 yo male with atrial fibrillation, CLL on IVIG, presents with
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.16 Subject: Letairis Page: 1 of 6 Last Review Date: June 24, 2016 Letairis Description Letairis (ambrisentan)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.16 Subject: Letairis Page: 1 of 6 Last Review Date: June 24, 2016 Letairis Description Letairis (ambrisentan)
PDE5 INHIBITOR POWDERS Sildenafil powder, Tadalafil powder
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Sildenafil and Tadalafil are marketed as Revatio and Adcirca for pulmonary arterial hypertension. This is a rare disorder of the pulmonary arteries in which
RATIONALE FOR INCLUSION IN PA PROGRAM Background Sildenafil and Tadalafil are marketed as Revatio and Adcirca for pulmonary arterial hypertension. This is a rare disorder of the pulmonary arteries in which
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.15 Subject: Flolan Veletri Page: 1 of 5 Last Review Date: September 15, 2017 Flolan Veletri Description
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.15 Subject: Flolan Veletri Page: 1 of 5 Last Review Date: September 15, 2017 Flolan Veletri Description
National Horizon Scanning Centre. Tadalafil for pulmonary arterial hypertension. October 2007
 Tadalafil for pulmonary arterial hypertension October 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Tadalafil for pulmonary arterial hypertension October 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.13 Section: Prescription Drugs Effective Date: July 1 2016 Subject: Tyvaso Page: 1 of 4 Last Review
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.13 Section: Prescription Drugs Effective Date: July 1 2016 Subject: Tyvaso Page: 1 of 4 Last Review
Real-world experience with riociguat in CTEPH
 Real-world experience with riociguat in CTEPH Matthias Held Center of Pulmonary Hypertension and Pulmonary Vascular Disease, Medical Mission Hospital, Würzburg, Germany Tuesday, 29 September ERS International
Real-world experience with riociguat in CTEPH Matthias Held Center of Pulmonary Hypertension and Pulmonary Vascular Disease, Medical Mission Hospital, Würzburg, Germany Tuesday, 29 September ERS International
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.23 Subject: Sildenafil Citrate Powder Page: 1 of 6 Last Review Date: September 15, 2017 Sildenafil
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.23 Subject: Sildenafil Citrate Powder Page: 1 of 6 Last Review Date: September 15, 2017 Sildenafil
PVDOMICS. Study Introduction. Kristin Highland, MD Gerald Beck, PhD. NHLBI Pulmonary Vascular Disease Phenomics Program
 PVDOMICS Study Introduction Kristin Highland, MD Gerald Beck, PhD NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
PVDOMICS Study Introduction Kristin Highland, MD Gerald Beck, PhD NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.21 Subject: Orenitram Page: 1 of 6 Last Review Date: June 24, 2016 Orenitram Description Orenitram
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.40.21 Subject: Orenitram Page: 1 of 6 Last Review Date: June 24, 2016 Orenitram Description Orenitram
Recruitment and Consenting
 1 Recruitment and Consenting MOP Chapter 4 NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health with support from
1 Recruitment and Consenting MOP Chapter 4 NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health with support from
Riociguat for chronic thromboembolic pulmonary hypertension
 Riociguat for chronic thromboembolic pulmonary hypertension This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Riociguat for chronic thromboembolic pulmonary hypertension This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Chronic Thromboembolic Pulmonary Hypertention CTEPH
 Chronic Thromboembolic Pulmonary Hypertention CTEPH Medical Management Otto Schoch, Prof. Dr. Klinik für Pneumologie und Schlafmedizin Kantonsspital St.Gallen CTEPH: Medical Management Diagnostic aspects
Chronic Thromboembolic Pulmonary Hypertention CTEPH Medical Management Otto Schoch, Prof. Dr. Klinik für Pneumologie und Schlafmedizin Kantonsspital St.Gallen CTEPH: Medical Management Diagnostic aspects
The World Health Organization (WHO) has classified pulmonary hypertension into five different groups: (2)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Tracleer Page: 1 of 6 Last Review Date: September 15, 2017 Tracleer Description Tracleer (bosentan)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Tracleer Page: 1 of 6 Last Review Date: September 15, 2017 Tracleer Description Tracleer (bosentan)
Clinical Commissioning Policy: Selexipag for treating pulmonary arterial hypertension (all ages)
 Clinical Commissioning Policy: Selexipag for treating pulmonary arterial hypertension (all ages) NHS England Reference: 170065P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Selexipag for treating pulmonary arterial hypertension (all ages) NHS England Reference: 170065P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Oral Therapies for Pulmonary Arterial Hypertension
 Oral Therapies for Pulmonary Arterial Hypertension Leslie Wooten, PharmD PGY2 Internal Medicine Pharmacy Resident University of Cincinnati Medical Center April 30 th, 2018 Objectives Pharmacist Objectives
Oral Therapies for Pulmonary Arterial Hypertension Leslie Wooten, PharmD PGY2 Internal Medicine Pharmacy Resident University of Cincinnati Medical Center April 30 th, 2018 Objectives Pharmacist Objectives
Pulmonary arterial hypertension. Pulmonary arterial hypertension: newer therapies. Definition of PH 12/18/16. WHO Group classification of PH
 Pulmonary arterial hypertension Pulmonary arterial hypertension: newer therapies Ramona L. Doyle, MD Clinical Professor of Medicine, UCSF Attending Physician UCSF PH Clinic Definition and classification
Pulmonary arterial hypertension Pulmonary arterial hypertension: newer therapies Ramona L. Doyle, MD Clinical Professor of Medicine, UCSF Attending Physician UCSF PH Clinic Definition and classification
Scottish Medicines Consortium
 Scottish Medicines Consortium sildenafil, 20mg (as citrate) tablets (Revatio ) No. (596/10) Pfizer Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Scottish Medicines Consortium sildenafil, 20mg (as citrate) tablets (Revatio ) No. (596/10) Pfizer Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Therapeutic approaches in P(A)H and the new ESC Guidelines
 Therapeutic approaches in P(A)H and the new ESC Guidelines Jean-Luc Vachiéry, FESC Head Pulmonary Vascular Diseases and Heart Failure Clinic Hôpital Universitaire Erasme Université Libre de Bruxelles Belgium
Therapeutic approaches in P(A)H and the new ESC Guidelines Jean-Luc Vachiéry, FESC Head Pulmonary Vascular Diseases and Heart Failure Clinic Hôpital Universitaire Erasme Université Libre de Bruxelles Belgium
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal. Drugs for the treatment of pulmonary arterial hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Draft remit / appraisal objective: Draft scope To appraise the clinical and cost effectiveness
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Draft remit / appraisal objective: Draft scope To appraise the clinical and cost effectiveness
Pulmonary Hypertension in 2012
 Pulmonary Hypertension in 2012 Evan Brittain, MD December 7, 2012 Kingston, Jamaica VanderbiltHeart.com Disclosures None VanderbiltHeart.com Outline Definition and Classification of PH Hemodynamics of
Pulmonary Hypertension in 2012 Evan Brittain, MD December 7, 2012 Kingston, Jamaica VanderbiltHeart.com Disclosures None VanderbiltHeart.com Outline Definition and Classification of PH Hemodynamics of
Where are we now in the longterm. of PAH and CTEPH? Hits and misses of medical treatment. Hap Farber Boston University School of Medicine, Boston, USA
 Where are we now in the longterm management of PAH and CTEPH? Hits and misses of medical treatment Hap Farber Boston University School of Medicine, Boston, USA Monday, 28 September ERS International Congress
Where are we now in the longterm management of PAH and CTEPH? Hits and misses of medical treatment Hap Farber Boston University School of Medicine, Boston, USA Monday, 28 September ERS International Congress
MACITENTAN DEVELOPMENT IN CHILDREN WITH PULMONARY HYPERTENSION (PAH)
 MACITENTAN DEVELOPMENT IN CHILDREN WITH PULMONARY HYPERTENSION (PAH) ORPHAN DRUG AND RARE DISEASE 11 MAY 2017 Catherine Lesage, MD, Pediatrics Program Head, Actelion Copyright AGENDA Pulmonary Arterial
MACITENTAN DEVELOPMENT IN CHILDREN WITH PULMONARY HYPERTENSION (PAH) ORPHAN DRUG AND RARE DISEASE 11 MAY 2017 Catherine Lesage, MD, Pediatrics Program Head, Actelion Copyright AGENDA Pulmonary Arterial
Approach to Pulmonary Hypertension in the Hospital
 Approach to Pulmonary Hypertension in the Hospital Todd M Bull MD Professor of Medicine Director Pulmonary Vascular Disease Center Director Center for Lungs and Breathing Division of Pulmonary Sciences
Approach to Pulmonary Hypertension in the Hospital Todd M Bull MD Professor of Medicine Director Pulmonary Vascular Disease Center Director Center for Lungs and Breathing Division of Pulmonary Sciences
Updates in Pulmonary Hypertension Pharmacotherapy. Ziad Sadik PharmD BCPS
 Updates in Pulmonary Hypertension Pharmacotherapy Ziad Sadik PharmD BCPS Disclosure Information I have no financial relationship to disclose AND I will not discuss off label use and/or investigational
Updates in Pulmonary Hypertension Pharmacotherapy Ziad Sadik PharmD BCPS Disclosure Information I have no financial relationship to disclose AND I will not discuss off label use and/or investigational
ADVANCED THERAPIES FOR PHARMACOLOGICAL TREATMENT OF PULMONARY HYPERTENSION
 Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-107 Effective Date: 04/21/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-107 Effective Date: 04/21/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Pharmacy Management Drug Policy
 SUBJECT: Pulmonary Arterial Hypertension (PAH) POLICY NUMBER: Pharmacy-42 Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed
SUBJECT: Pulmonary Arterial Hypertension (PAH) POLICY NUMBER: Pharmacy-42 Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed
Pulmonary Hypertension. Murali Chakinala, M.D. Washington University School of Medicine
 Pulmonary Hypertension Murali Chakinala, M.D. Washington University School of Medicine Pulmonary Circulation Alveolar Capillary relationship Pulmonary Circulation High flow, low resistance PVR ~1/15 of
Pulmonary Hypertension Murali Chakinala, M.D. Washington University School of Medicine Pulmonary Circulation Alveolar Capillary relationship Pulmonary Circulation High flow, low resistance PVR ~1/15 of
Anjali Vaidya, MD, FACC, FASE, FACP Associate Director, Pulmonary Hypertension, Right Heart Failure, and Pulmonary Thromboendarterectomy Program
 Anjali Vaidya, MD, FACC, FASE, FACP Associate Director, Pulmonary Hypertension, Right Heart Failure, and Pulmonary Thromboendarterectomy Program Advanced Heart Failure & Cardiac Transplant Temple University
Anjali Vaidya, MD, FACC, FASE, FACP Associate Director, Pulmonary Hypertension, Right Heart Failure, and Pulmonary Thromboendarterectomy Program Advanced Heart Failure & Cardiac Transplant Temple University
Clinical Policy: Macitentan (Opsumit) Reference Number: ERX.SPMN.88
 Clinical Policy: (Opsumit) Reference Number: ERX.SPMN.88 Effective Date: 07/16 Last Review Date: 06/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Opsumit) Reference Number: ERX.SPMN.88 Effective Date: 07/16 Last Review Date: 06/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
National Horizon Scanning Centre. Oral and inhaled treprostinil for pulmonary arterial hypertension: NYHA class III. April 2008
 Oral and inhaled treprostinil for pulmonary arterial hypertension: NYHA class April 2008 This technology summary is based on information available at the time of research and a limited literature search.
Oral and inhaled treprostinil for pulmonary arterial hypertension: NYHA class April 2008 This technology summary is based on information available at the time of research and a limited literature search.
Pharmacy Management Drug Policy
 SUBJECT: POLICY NUMBER: PHARMACY-42 EFFECTIVE DATE: 6/2005 LAST REVIEW DATE: 4/19/2018 If the member s subscriber contract excludes coverage for a specific service or prescription drug, it is not covered
SUBJECT: POLICY NUMBER: PHARMACY-42 EFFECTIVE DATE: 6/2005 LAST REVIEW DATE: 4/19/2018 If the member s subscriber contract excludes coverage for a specific service or prescription drug, it is not covered
Acute Vasodilator Testing in Pulmonary Hypertension: What, When, and How?
 Acute Vasodilator Testing in Pulmonary Hypertension: What, When, and How? Teresa De Marco, MD University of California, San Francisco Disclosures: Grants/Research: United Therapeutics, Lung Biotechnology,
Acute Vasodilator Testing in Pulmonary Hypertension: What, When, and How? Teresa De Marco, MD University of California, San Francisco Disclosures: Grants/Research: United Therapeutics, Lung Biotechnology,
Progress in PAH. Gerald Simonneau
 Progress in PAH Gerald Simonneau National Reference center for Pulmonary Hypertension Bicetre University Hospital, INSERM U 999 Paris-Sud University Le Kremlin Bicêtre France Clinical Classification of
Progress in PAH Gerald Simonneau National Reference center for Pulmonary Hypertension Bicetre University Hospital, INSERM U 999 Paris-Sud University Le Kremlin Bicêtre France Clinical Classification of
Paediatric PAH in the current era
 Paediatric PAH in the current era Dunbar Ivy, MD The Children s Hospital Heart Institute University of Colorado School of Medicine Paediatric PAH in the current era & A Gap Analysis Dunbar Ivy, MD The
Paediatric PAH in the current era Dunbar Ivy, MD The Children s Hospital Heart Institute University of Colorado School of Medicine Paediatric PAH in the current era & A Gap Analysis Dunbar Ivy, MD The
PULMONARY HYPERTENSION RESPIRATORY & CRITICAL CARE CONFERENCE APRIL 21, 2016 LAURA G. HOOPER
 PULMONARY HYPERTENSION RESPIRATORY & CRITICAL CARE CONFERENCE APRIL 21, 2016 LAURA G. HOOPER OUTLINE Brief review of WHO Group Classification Scheme Subgroups we ll focus on: WHO Group I Pulmonary Arterial
PULMONARY HYPERTENSION RESPIRATORY & CRITICAL CARE CONFERENCE APRIL 21, 2016 LAURA G. HOOPER OUTLINE Brief review of WHO Group Classification Scheme Subgroups we ll focus on: WHO Group I Pulmonary Arterial
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency London, 18 December 2008 Doc. Ref. EMEA/CHMP/EWP/356954/2008 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT CHMP GUIDELINE ON THE CLINICAL INVESTIGATIONS OF MEDICINAL
European Medicines Agency London, 18 December 2008 Doc. Ref. EMEA/CHMP/EWP/356954/2008 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT CHMP GUIDELINE ON THE CLINICAL INVESTIGATIONS OF MEDICINAL
ELIGIBILITY CRITERIA FOR PULMONARY ARTERIAL HYPERTENSION THERAPY
 ELIGIBILITY CRITERIA FOR PULMONARY ARTERIAL HYPERTENSION THERAPY Contents Eligibility criteria for Pulmonary Arterial Hypertension therapy...2-6 Initial Application for funding of Pulmonary Arterial Hypertension
ELIGIBILITY CRITERIA FOR PULMONARY ARTERIAL HYPERTENSION THERAPY Contents Eligibility criteria for Pulmonary Arterial Hypertension therapy...2-6 Initial Application for funding of Pulmonary Arterial Hypertension
Corporate Medical Policy
 Corporate Medical Policy Pulmonary Hypertension, Drug Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pulmonary_hypertension_drug_management 06/1998 3/2018 3/2019 3/2018
Corporate Medical Policy Pulmonary Hypertension, Drug Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pulmonary_hypertension_drug_management 06/1998 3/2018 3/2019 3/2018
Pulmonary Hypertension: Clinical Features & Recent Advances
 Pulmonary Hypertension: Clinical Features & Recent Advances Lisa J. Rose-Jones, MD Assistant Professor of Medicine, Division of Cardiology Advanced Heart Failure/Cardiac Transplantation & Pulmonary Hypertension
Pulmonary Hypertension: Clinical Features & Recent Advances Lisa J. Rose-Jones, MD Assistant Professor of Medicine, Division of Cardiology Advanced Heart Failure/Cardiac Transplantation & Pulmonary Hypertension
Sildenafil Citrate Powder. Sildenafil citrate powder. Description. Section: Prescription Drugs Effective Date: January 1, 2016
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.06.15 Subject: Sildenafil Citrate Powder Page: 1 of 6 Last Review Date: December 3, 2015 Sildenafil Citrate
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.06.15 Subject: Sildenafil Citrate Powder Page: 1 of 6 Last Review Date: December 3, 2015 Sildenafil Citrate
Combination therapy in the treatment of pulmonary arterial hypertension 2015 update
 Journal of Rare Cardiovascular Diseases 2015; 2 (4): 103 107 www.jrcd.eu REVIEW ARTICLE Rare diseases of pulmonary circulation Combination therapy in the treatment of pulmonary arterial hypertension 2015
Journal of Rare Cardiovascular Diseases 2015; 2 (4): 103 107 www.jrcd.eu REVIEW ARTICLE Rare diseases of pulmonary circulation Combination therapy in the treatment of pulmonary arterial hypertension 2015
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult)
 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
1. Phosphodiesterase Type 5 Enzyme Inhibitors: Sildenafil (Revatio), Tadalafil (Adcirca)
 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutic
This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the IEHP Pharmacy and Therapeutic
Teaching Round Claudio Sartori
 Teaching Round 14.03.2017 Claudio Sartori Cas clinique Femme 47 ans, connue pour un BPCO, asthénie, douleurs thoraciques, dyspnée à l effort, œdèmes membres inférieurs, deux syncopes. Tabac, BMI 31 kg/m2
Teaching Round 14.03.2017 Claudio Sartori Cas clinique Femme 47 ans, connue pour un BPCO, asthénie, douleurs thoraciques, dyspnée à l effort, œdèmes membres inférieurs, deux syncopes. Tabac, BMI 31 kg/m2
Commissioning Brief - Background Information. Exercise training for people with pulmonary hypertension
 Commissioning Brief - Background Information HTA no 17/129 Exercise training for people with pulmonary hypertension This background document provides further information to support applicants for this
Commissioning Brief - Background Information HTA no 17/129 Exercise training for people with pulmonary hypertension This background document provides further information to support applicants for this
Pulmonary Arterial Hypertension (PAH): Emerging Therapeutic Strategies
 Pulmonary Arterial Hypertension (PAH): Emerging Therapeutic Strategies Nick H. Kim, M.D. Clinical Professor of Medicine Director, Pulmonary Vascular Medicine Clinical Service Chief, PCCSM La Jolla Pulmonary,
Pulmonary Arterial Hypertension (PAH): Emerging Therapeutic Strategies Nick H. Kim, M.D. Clinical Professor of Medicine Director, Pulmonary Vascular Medicine Clinical Service Chief, PCCSM La Jolla Pulmonary,
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents
 Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
Pulmonary Hypertension Perioperative Management
 Pulmonary Hypertension Perioperative Management Bruce J Leone, MD Professor of Anesthesiology Chief, Neuroanesthesiology Vice Chair for Academic Affairs Mayo Clinic Jacksonville, Florida Introduction Definition
Pulmonary Hypertension Perioperative Management Bruce J Leone, MD Professor of Anesthesiology Chief, Neuroanesthesiology Vice Chair for Academic Affairs Mayo Clinic Jacksonville, Florida Introduction Definition
PULMONARY HYPERTENSION
 PULMONARY HYPERTENSION REVIEW & UPDATE Olga M. Fortenko, M.D. Pulmonary & Critical Care Medicine Pulmonary Vascular Diseases Sequoia Hospital 650-216-9000 Olga.Fortenko@dignityhealth.org Disclosures None
PULMONARY HYPERTENSION REVIEW & UPDATE Olga M. Fortenko, M.D. Pulmonary & Critical Care Medicine Pulmonary Vascular Diseases Sequoia Hospital 650-216-9000 Olga.Fortenko@dignityhealth.org Disclosures None
Pulmonary Arterial Hypertension: The Approach to Management in 2019
 Pulmonary Arterial Hypertension: The Approach to Management in 2019 Munir S. Janmohamed M.D. FACC Medical Director Mechanical Circulatory Support/Heart Failure Program Mercy General Hospital/Mercy Medical
Pulmonary Arterial Hypertension: The Approach to Management in 2019 Munir S. Janmohamed M.D. FACC Medical Director Mechanical Circulatory Support/Heart Failure Program Mercy General Hospital/Mercy Medical
Pulmonary Hypertension and Left Heart Disease: What s good for the goose is not necessary good for the gander
 Pulmonary Hypertension and Left Heart Disease: What s good for the goose is not necessary good for the gander Jacqueline Fearon-Clarke, MA, ACNP-BC Heart Failure and Pulmonary Hypertension Nurse Practitioner
Pulmonary Hypertension and Left Heart Disease: What s good for the goose is not necessary good for the gander Jacqueline Fearon-Clarke, MA, ACNP-BC Heart Failure and Pulmonary Hypertension Nurse Practitioner
2017 UnitedHealthcare Services, Inc.
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2020-10 Program Prior Authorization/Medical Necessity PAH Agents Medication Adcirca (tadalafil), Adempas (riociguat), Letairis
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 2020-10 Program Prior Authorization/Medical Necessity PAH Agents Medication Adcirca (tadalafil), Adempas (riociguat), Letairis
Pulmonary Hypertension: Another Use for Viagra
 Pulmonary Hypertension: Another Use for Viagra Kathleen Tong, MD Director, Heart Failure Program Assistant Clinical Professor University of California, Davis Disclosures I have no financial conflicts A
Pulmonary Hypertension: Another Use for Viagra Kathleen Tong, MD Director, Heart Failure Program Assistant Clinical Professor University of California, Davis Disclosures I have no financial conflicts A
Pulmonary Hypertension: When to Initiate Advanced Therapy. Jonathan D. Rich, MD Associate Professor of Medicine Northwestern University
 Pulmonary Hypertension: When to Initiate Advanced Therapy Jonathan D. Rich, MD Associate Professor of Medicine Northwestern University Disclosures Medtronic, Abbott: Consultant Hemodynamic Definition of
Pulmonary Hypertension: When to Initiate Advanced Therapy Jonathan D. Rich, MD Associate Professor of Medicine Northwestern University Disclosures Medtronic, Abbott: Consultant Hemodynamic Definition of
The Case of Marco Nazzareno Galiè, M.D.
 The Case of Marco Nazzareno Galiè, M.D. DIMES Disclosures Consulting fees and research support from Actelion Pharmaceuticals Ltd, Bayer HealthCare, Eli Lilly and Co, GlaxoSmithKline and Pfizer Ltd Clinical
The Case of Marco Nazzareno Galiè, M.D. DIMES Disclosures Consulting fees and research support from Actelion Pharmaceuticals Ltd, Bayer HealthCare, Eli Lilly and Co, GlaxoSmithKline and Pfizer Ltd Clinical
Class Update with New Drug Evaluation: Drugs for Pulmonary Arterial Hypertension
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
THERAPEUTICS IN PULMONARY ARTERIAL HYPERTENSION Evidences & Guidelines
 THERAPEUTICS IN PULMONARY ARTERIAL HYPERTENSION Evidences & Guidelines Vu Nang Phuc, MD Dinh Duc Huy, MD Pham Nguyen Vinh, MD, PhD, FACC Tam Duc Cardiology Hospital Faculty Disclosure No conflict of interest
THERAPEUTICS IN PULMONARY ARTERIAL HYPERTENSION Evidences & Guidelines Vu Nang Phuc, MD Dinh Duc Huy, MD Pham Nguyen Vinh, MD, PhD, FACC Tam Duc Cardiology Hospital Faculty Disclosure No conflict of interest
Image: Patrick J. Lynch, Wikimedia Commons
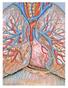 Image: Patrick J. Lynch, Wikimedia Commons Victor J. Samillan 1,2 Andrew J. Peacock 1 1 Scottish Pulmonary Vascular Unit, Golden Jubilee National Hospital, Glasgow, UK 2 Dept of Human Physiology, Medical
Image: Patrick J. Lynch, Wikimedia Commons Victor J. Samillan 1,2 Andrew J. Peacock 1 1 Scottish Pulmonary Vascular Unit, Golden Jubilee National Hospital, Glasgow, UK 2 Dept of Human Physiology, Medical
Clinical Commissioning Policy Proposition: Pasireotide: An injectable medical therapy for the treatment of Cushing's disease
 Clinical Commissioning Policy Proposition: Pasireotide: An injectable medical therapy for the treatment of Cushing's disease Information Reader Box (IRB) to be inserted on inside front cover for documents
Clinical Commissioning Policy Proposition: Pasireotide: An injectable medical therapy for the treatment of Cushing's disease Information Reader Box (IRB) to be inserted on inside front cover for documents
Clinical Policy: Ambrisentan (Letairis) Reference Number: ERX.SPMN.84 Effective Date: 07/16
 Clinical Policy: (Letairis) Reference Number: ERX.SPMN.84 Effective Date: 07/16 Last Review Date: 06/16 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Letairis) Reference Number: ERX.SPMN.84 Effective Date: 07/16 Last Review Date: 06/16 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review
 Pharmacy Medical Necessity Guidelines: Effective: July 11, 2017 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED) Benefit
Pharmacy Medical Necessity Guidelines: Effective: July 11, 2017 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review Pharmacy (RX) or Medical (MED) Benefit
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex
 Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages)
 Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Pharmacy Coverage Guidelines are subject to change as new information becomes available.
 SILDENAFIL oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline
SILDENAFIL oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline
Advanced Therapies for Pharmacological Treatment of Pulmonary Arterial Hypertension
 MEDICAL POLICY 5.01.522 Advanced Therapies for Pharmacological Treatment of Pulmonary Arterial Hypertension BCBSA Ref. Policy: 5.01.09* Effective Date: Dec. 1, 2017 Last Revised: Nov. 21, 2017 Replaces:
MEDICAL POLICY 5.01.522 Advanced Therapies for Pharmacological Treatment of Pulmonary Arterial Hypertension BCBSA Ref. Policy: 5.01.09* Effective Date: Dec. 1, 2017 Last Revised: Nov. 21, 2017 Replaces:
Therapy Update: ERAs. Review of Mechanism. Disclosure Statements. Outline. Disclosure: Research support from United Therapeutics
 1 Therapy Update: ERAs Disclosure Statements Disclosure: Research support from United Therapeutics Most of the medications discussed in this presentation are off-label usage Nidhy Varghese, MD Pulmonary
1 Therapy Update: ERAs Disclosure Statements Disclosure: Research support from United Therapeutics Most of the medications discussed in this presentation are off-label usage Nidhy Varghese, MD Pulmonary
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION Macitentan (Opsumit Actelion Pharmaceuticals Canada Inc.) Indication: Pulmonary Arterial Hypertension Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that
CDEC FINAL RECOMMENDATION Macitentan (Opsumit Actelion Pharmaceuticals Canada Inc.) Indication: Pulmonary Arterial Hypertension Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that
PULMONARY ARTERIAL HYPERTENSION AGENTS
 Approvable Criteria: PULMONARY ARTERIAL HYPERTENSION AGENTS Brand Name Generic Name Length of Authorization Adcirca tadalafil Calendar Year Adempas riociguat Calendar Year Flolan epoprostenol sodium Calendar
Approvable Criteria: PULMONARY ARTERIAL HYPERTENSION AGENTS Brand Name Generic Name Length of Authorization Adcirca tadalafil Calendar Year Adempas riociguat Calendar Year Flolan epoprostenol sodium Calendar
Referral Forms for TYVASO and REMODULIN
 Referral Forms for TYVASO and REMODULIN HOW TO GET STARTED Tyvaso and Remodulin are available only through select Specialty Pharmacy Services (SPS) providers. Follow these 5 simple steps to complete each
Referral Forms for TYVASO and REMODULIN HOW TO GET STARTED Tyvaso and Remodulin are available only through select Specialty Pharmacy Services (SPS) providers. Follow these 5 simple steps to complete each
Pharmacy Medical Necessity Guidelines: Pulmonary Hypertension Medications
 Pharmacy Medical Necessity Guidelines: Pulmonary Hypertension Medications Effective: January 15, 2018 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review
Pharmacy Medical Necessity Guidelines: Pulmonary Hypertension Medications Effective: January 15, 2018 Prior Authorization Required Type of Review Care Management Not Covered Type of Review Clinical Review
4/14/2010. Pulmonary Hypertension: An Update. Tim Williamson, MD, FCCP. University of Kansas Hospital. Normal Physiology
 Pulmonary Hypertension: An Update Tim Williamson, MD, FCCP Director, Pulmonary Vascular Program University of Kansas Hospital Normal Physiology 1 Pulmonary Perfusion 101 High Pressure Low Pressure Pulmonary
Pulmonary Hypertension: An Update Tim Williamson, MD, FCCP Director, Pulmonary Vascular Program University of Kansas Hospital Normal Physiology 1 Pulmonary Perfusion 101 High Pressure Low Pressure Pulmonary
Abstract Book. Inspiring Approaches to Clinical Challenges in Pulmonary Hypertension
 Inspiring Approaches to Clinical Challenges in Pulmonary Hypertension Abstract Book A symposium sponsored by Bayer Schering Pharma at ERS 2008 Annual Congress www.ventavis.com Welcome Dear Colleague, It
Inspiring Approaches to Clinical Challenges in Pulmonary Hypertension Abstract Book A symposium sponsored by Bayer Schering Pharma at ERS 2008 Annual Congress www.ventavis.com Welcome Dear Colleague, It
Pulmonary Hypertension Drugs
 Pulmonary Hypertension Drugs Policy Number: Original Effective Date: MM.04.028 10/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/22/2015 Section: Prescription Drugs
Pulmonary Hypertension Drugs Policy Number: Original Effective Date: MM.04.028 10/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/22/2015 Section: Prescription Drugs
Insert heading depending line on length; please delete delete. length; please delete other cover options once
 Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
Disclosures. Inhaled Therapy in Pediatric Pulmonary Hypertension. Inhaled Prostacyclin: Rationale. Outline
 Disclosures Inhaled Therapy in Pediatric Pulmonary Hypertension The University of Colorado receives fees for Dr Ivy to be a consultant for Actelion, Gilead, Lilly, Pfizer, and United Therapeutics Dunbar
Disclosures Inhaled Therapy in Pediatric Pulmonary Hypertension The University of Colorado receives fees for Dr Ivy to be a consultant for Actelion, Gilead, Lilly, Pfizer, and United Therapeutics Dunbar
Drug Class Monograph. Policy/Criteria:
 Drug Class Monograph Class: Pulmonary Arterial Hypertension Agents Drugs: Adcirca (tadalafil), Adempas (riociguat), Flolan (epoprostenol), Letairis (ambrisentan), Opsumit (macitentan), Orenitram (treprostinil),
Drug Class Monograph Class: Pulmonary Arterial Hypertension Agents Drugs: Adcirca (tadalafil), Adempas (riociguat), Flolan (epoprostenol), Letairis (ambrisentan), Opsumit (macitentan), Orenitram (treprostinil),
Role of Combination PAH Therapies
 Role of Combination PAH Therapies Ronald J. Oudiz, MD, FACP, FACC Associate Professor of Medicine, David Geffen School of Medicine at UCLA Director, Liu Center for Pulmonary Hypertension Los Angeles Biomedical
Role of Combination PAH Therapies Ronald J. Oudiz, MD, FACP, FACC Associate Professor of Medicine, David Geffen School of Medicine at UCLA Director, Liu Center for Pulmonary Hypertension Los Angeles Biomedical
ACCP PAH Medical Therapy Guidelines: 2007 Update. David Badesch, MD University of Colorado School of Medicine Denver, CO
 ACCP PAH Medical Therapy Guidelines: 2007 Update David Badesch, MD University of Colorado School of Medicine Denver, CO Disclosure of Commercial Interest Dr. Badesch has received grant/research support
ACCP PAH Medical Therapy Guidelines: 2007 Update David Badesch, MD University of Colorado School of Medicine Denver, CO Disclosure of Commercial Interest Dr. Badesch has received grant/research support
*Division of Pulmonary, Sleep, and Critical Care Medicine, Rhode Island Hospital, Alpert Medical School of Brown University, Providence, RI, USA
 The Relationship between NO Pathway Biomarkers and Response to Riociguat in the RESPITE Study of Patients with PAH Not Reaching Treatment Goals with Phosphodiesterase 5 Inhibitors James R Klinger,* Raymond
The Relationship between NO Pathway Biomarkers and Response to Riociguat in the RESPITE Study of Patients with PAH Not Reaching Treatment Goals with Phosphodiesterase 5 Inhibitors James R Klinger,* Raymond
Pulmonary Hypertension. Pulmonary Arterial Hypertension Diagnosis, Impact and Outcomes
 Pulmonary Hypertension Pulmonary Arterial Hypertension Diagnosis, Impact and Outcomes Pulmonary Arterial Hypertension Disease of small pulmonary arteries Characteristic changes Medial hypertrophy Intimal
Pulmonary Hypertension Pulmonary Arterial Hypertension Diagnosis, Impact and Outcomes Pulmonary Arterial Hypertension Disease of small pulmonary arteries Characteristic changes Medial hypertrophy Intimal
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Adcirca) Reference Number: HIM.PA.SP23 Effective Date: 05/17 Last Review Date: Line of Business: Health Insurance Marketplace Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Adcirca) Reference Number: HIM.PA.SP23 Effective Date: 05/17 Last Review Date: Line of Business: Health Insurance Marketplace Coding Implications Revision Log See Important Reminder at
Does Tadalafil Improve Exercise Capacitance in Patients over 12 Years Old with Pulmonary Hypertension?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2012 Does Tadalafil Improve Exercise Capacitance
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2012 Does Tadalafil Improve Exercise Capacitance
Pharmacy Management Drug Policy
 SUBJECT: POLICY NUMBER: PHARMACY-42 EFFECTIVE DATE: 6/2005 LAST REVIEW DATE: 10/1/2018 If the member s subscriber contract excludes coverage for a specific service or prescription drug, it is not covered
SUBJECT: POLICY NUMBER: PHARMACY-42 EFFECTIVE DATE: 6/2005 LAST REVIEW DATE: 10/1/2018 If the member s subscriber contract excludes coverage for a specific service or prescription drug, it is not covered
Pulmonary Arterial Hypertension Drug Prior Authorization Protocol
 Pulmonary Arterial Hypertension Drug Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Pulmonary Arterial Hypertension Drug Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Anjali Vaidya, MD, FACC, FASE, FACP Associate Director, Pulmonary Hypertension, Right Heart Failure, Pulmonary Thromboendarterectomy Program Advanced
 Anjali Vaidya, MD, FACC, FASE, FACP Associate Director, Pulmonary Hypertension, Right Heart Failure, Pulmonary Thromboendarterectomy Program Advanced Heart Failure & Cardiac Transplant Temple University
Anjali Vaidya, MD, FACC, FASE, FACP Associate Director, Pulmonary Hypertension, Right Heart Failure, Pulmonary Thromboendarterectomy Program Advanced Heart Failure & Cardiac Transplant Temple University
Recent Treatment of Pulmonary Artery Hypertension. Cardiology Division Yonsei University College of Medicine
 Recent Treatment of Pulmonary Artery Hypertension Cardiology Division Yonsei University College of Medicine Definition Raised Pulmonary arterial pressure (PAP) WHO criteria : spap>40 mmhg NIH Criteria
Recent Treatment of Pulmonary Artery Hypertension Cardiology Division Yonsei University College of Medicine Definition Raised Pulmonary arterial pressure (PAP) WHO criteria : spap>40 mmhg NIH Criteria
