The direct thrombin inhibitor melagatran/ximelagatran
|
|
|
- Paul Dustin Banks
- 6 years ago
- Views:
Transcription
1 The direct thrombin inhibitor melagatran/ximelagatran Timothy A Brighton In Australia, warfarin sodium is the principal oral anticoagulant prescribed for patients with arterial and venous thromboembolic disease. Although effective, warfarin has a narrow therapeutic window, with significant risks of haemorrhage at therapeutic concentrations. It has limitations in clinical practice, including the need for individualised dosing and frequent INR (international normalised ratio) measurements. Here, I will summarise the pharmacology and give an overview of the clinical trial results of a new oral anticoagulant drug, ximelagatran. The Medical Its development Journal of Australia began in ISSN: the laboratories of Astra in Sweden in 1985 with the deliberate targeting of thrombin by 729X 18 October newly synthesised small-molecular-weight direct inhibitors. Melagatran, a dipeptide, was one of three compounds developed. The Medical Journal of Australia 2004 Melagatran New Drugs, is a parenteral Old Drugsagent with low oral bioavailability. By contrast, ximelagatran, an oral prodrug without direct antithrombin activity, is rapidly converted to melagatran in vivo. 1 Overview of the coagulation pathways, highlighting current and selected new antithrombotic agents ABSTRACT Melagatran is a synthetic, small-peptide direct thrombin inhibitor with anticoagulant activity. Ximelagatran, an oral prodrug, undergoes rapid enzymatic conversion to melagatran. Melagatran has rapid onset of action, fixed twice-daily dosing, stable absorption, apparent low potential for medication interactions, and no requirement for monitoring drug levels or dose adjustment. There is no specific antidote, but the drug has a short plasma elimination half-life (about 4 hours). In clinical studies, melagatran/ximelagatran is not inferior to warfarin for stroke prevention in patients with non-valvular atrial fibrillation, to heparin warfarin for acute treatment and extended secondary prevention of deep vein thrombosis, and superior to warfarin for prevention of venous thromboembolism after major orthopaedic surgery. Major bleeding with melagatran/ximelagatran occurred at rates similar to those in patients treated with warfarin. 6% 12% of patients taking ximelagatran develop asymptomatic elevated liver enzyme levels (predominantly alanine aminotransferase) after 1 6 months of therapy; this usually resolves with cessation of therapy. Less than 1% of patients develop abnormal liver function while taking ximelagatran; this rarely persists or develops into clinical illness. MJA 2004; 181: Ximelagatran is approved in several European countries for shortterm orthopaedic prophylaxis. It is under regulatory review in the United States for prophylaxis, stroke prevention and treatment of deep vein thrombosis. In Australia, ximelagatran is not yet approved for clinical use outside clinical trials. Department of Haematology, St George Hospital, Sydney, NSW. Timothy A Brighton, FRACP, FRCPA, MD, Haematologist, and Senior Lecturer, St George Clinical School, University of New South Wales. Reprints will not be available from the author. Correspondence: Dr Timothy A Brighton, Department of Haematology, St George Hospital, Gray Street, Kogarah, NSW t.brighton@unsw.edu.au Properties of ximelagatran Coagulation is initiated by the tissue-factor-dependent activation of factor VII (see Box 1). Key events in the coagulation cascade are the activation of platelets, the generation of activated factor X, and the subsequent activation of prothrombin to thrombin by the prothrombinase complex. Thrombin is the key enzyme cleaving fibrinogen to produce fibrin clot. Thrombin has many other activities, including a potent positive feedback by activating factors V and VIII, further activation of platelets, stimulation of anticoagulation mechanisms, and promotion of clot dissolution and healing. A number of anticoagulant drugs targeting activated factor X or thrombin are currently marketed or in advanced stages of clinical testing (Box 1). Ximelagatran has many desirable properties as an anticoagulant when compared with warfarin sodium (see Box 2). 1 Melagatran is a potent, rapidly binding, competitive and reversible direct inhibitor of soluble and clot-bound thrombin. Oral ximelagatran has stable bioavailability and is rapidly converted to melagatran, with peak plasma melagatran concentrations hours after oral 432 MJA Volume 181 Number 8 18 October 2004
2 dosing. Absorption is minimally influenced by food and other medications. Melagatran has no significant protein binding, is not significantly metabolised, and 80% is excreted renally over 24 hours. The half-life of melagatran is relatively short at hours in young, healthy people, necessitating twice-daily dosing. Because of the age-related decrease in renal function, the plasma half-life of melagatran increases with patient age; its half-life in a typical 70- year-old patient is 4 5 hours. In patients with severe renal impairment, the plasma half-life of melagatran is significantly longer. 2 The impact of renal impairment on dosing and adverse effects needs to be explored in greater detail, although there was no significant accumulation with repeated dosing in healthy young or elderly individuals. Doses of melagatran/ximelagatran have varied in different clinical indications. Maximal plasma concentration of 600 nmol/l is achieved after 5 mg subcutaneous melagatran or 60 mg of oral ximelagatran. Ximelagatran administration at therapeutic doses results in significant prolongations of clotting times (eg, activated partial thromboplastin time, prothrombin time and thrombin time). In clinical studies, over patients have received melagatran/ximelagatran and no observed interactions with concomitant medications have been noted. Preliminary studies of possible interactions of melagatran/ximelagatran with alcohol or specific medications (including nifedipine, diazepam, diclofenac, acetylsalicylic acid, digoxin and statins) have found none, as melagatran/ ximelagatran metabolism is independent of CYP450 enzymes (abstract presentations only). One study found that concomitant ximelagatran and erythromycin increased maximal plasma concentration of melagatran in healthy volunteers by almost twofold. Excluding haemorrhage (discussed below), melagatran/ximelagatran has been remarkably free of significant adverse effects. Most attention has focused on the dose-dependent increase in liver enzyme levels (predominantly alanine aminotransferase [ALT]), which is usually asymptomatic and occurs in 6% 12% of patients. This biochemical abnormality typically develops between the first and sixth month of therapy, but rarely after 6 months. The proportion of patients with ximelagatran-induced increased liver enzyme concentrations who become jaundiced or develop symptoms attributable to liver dysfunction appears to be small (< 1% 2%), but precise details have not yet been published. All patients taking melagatran/ximelagtran require monthly liver function testing for the first 6 months. Therapy should be ceased if the ALT concentration exceeds five times the upper limit of normal at any time. More frequent monitoring is required in patients in whom ALT concentrations increase to 3 5 times the upper limit of normal. The biochemical abnormality usually (> 95% of occasions) completely resolves with either continuation or discontinuation of the medication. The mechanism of the liver enzyme abnormality remains unclear. The importance of concurrent liver disease and concomitant use of other liver toxins remains to be determined. Clinical studies of melagatran/ximelagatran The clinical efficacy and safety of melagatran/ximelagatran has been assessed in an expansive clinical study program. These have focused on stroke prevention in non-valvular atrial fibrillation, prophylaxis of vein thrombosis after orthopaedic surgery, acute therapy of vein thrombosis, secondary prevention of recurrent vein thrombosis, and acute coronary syndromes. In this article ximelagatran refers to the combination of melagatran and ximelagatran unless otherwise specified. 2 Comparison of melagatran/ximelagatran and warfarin sodium Property Warfarin sodium Melagatran/ximelagatran Mechanism of action Reduced synthesis of functional prothrombin and other clotting factors Direct competitive and reversible inhibition of soluble and clot-bound thrombin Rapid onset of action No Yes Effective anticoagulant Yes Yes (not inferior to well-controlled warfarin therapy in most studies) Risk of haemorrhage Significant Equivalent to warfarin in most studies Route of administration Oral, once daily Oral, twice daily Stable, predictable pharmacokinetics No Yes Interactions with diet and alcohol Clinically significant No Interactions with other medications Many Low potential Dosing Individualised to each patient and a target INR Fixed dosing dependent on indication Monitoring International normalised ratio (INR) every 1 2 weeks No monitoring of anticoagulant level. Monthly liver enzyme tests for first 6 months Dose adjustments Frequent No Use in severe liver disease Problematic Unknown (such patients excluded from studies) Use in severe renal disease Yes No. Melagatran is excreted renally, and patients with renal disease were excluded from clinical studies Reversibility after cessation Antidote Slow elimination and reversal of antithrombotic effect Rapid reversal with plasma or factor replacement Slow reversal with vitamin K Drug cost Cheap Unknown Reversal of thrombin inhibition dependent on plasma concentration and elimination half-life (approx. 4 h) None available. Plasma or factor replacement not effective. Possible removal by dialysis MJA Volume 181 Number 8 18 October
3 3 Summary of phase III studies of ximelagatran versus warfarin for stroke prevention in non-valvular atrial fibrillation* Study SPORTIF III SPORTIF V Design RCT open-label RCT double-blind Study interventions Ximelagatran 36 mg twice daily 36 mg twice daily Warfarin Target INR, 2 3 Target INR, 2 3 Number of patients Ximelagatran Warfarin Absolute risk of stroke + systemic embolism Ximelagatran 1.6% 1.6% Warfarin 2.3% 1.2% Absolute risk of fatal or major bleeding Ximelagatran 1.3% 2.4% Warfarin 1.8% 3.1% % of patients with ALT level > 3 times upper limit of normal Ximelagatran 6% 6% Warfarin 0.8% 1.0% In SPORTIF III, medication discontinuation rates were 3.2% for patients taking ximelagatran, and 0.3% for those taking warfarin. Discontinuation rates were not published for SPORTIF IV. INR = international normalised ratio. ALT = alanine aminotransferase. * Patients were at moderate risk of stroke or systemic embolism as defined by at least one additional risk factor for stroke, including previous stroke, previous systemic embolism, age > 65 years, hypertension, diabetes, coronary artery disease, or left ventricular dysfunction (as defined by a left ventricular ejection fraction < 40%), or symptomatic congestive heart failure within 3 months. Difference between ximelagatran and warfarin not significant. Difference between ximelagatran and control therapy significant at P <0.05. Stroke prevention in atrial fibrillation Non-valvular atrial fibrillation (NVAF) affects 6% of people over the age of 65 years and about 10% of those over 80. Patients with NVAF have an absolute risk of stroke and systemic embolism of around 5%, which varies with age and other risk factors. Clinical studies have conclusively shown that warfarin, given to achieve a target INR of 2 3, is highly effective at preventing stroke (risk reduction of 62% in all risk groups), but at a cost of around 1% per year of fatal haemorrhage. Despite the published efficacy of warfarin, studies consistently show that only 20% 40% of eligible patients actually receive warfarin. 4 Ximelagatran has been evaluated in two dose-finding exploratory studies (SPORTIF II 5 and SPORTIF IV [unpublished]) and two phase III clinical studies (SPORTIF III 6 and SPORTIF V 7 ). The SPORTIF III and V studies recruited over 5000 patients with NVAF at moderate risk of stroke or systemic embolism (see Box 3). Their objective was to establish that fixed-dose ximelagatran (36 mg twice daily) was not inferior to warfarin (target INR, 2 3). 8 Absolute rates of stroke and systemic embolism for patients treated with warfarin and ximelagatran were equivalent. Rates of major or fatal bleeding were not significantly different, although there was a small but significant excess of total bleeding in patients treated with warfarin. Abnormal ALT concentrations (above three times upper limit of normal) occurred in 1% of patients treated with warfarin and 6% of those treated with ximelagatran. These studies concluded that ximelagatran (36 mg twice daily) is not inferior to warfarin (target INR, 2 3) for preventing stroke, with no difference in significant bleeding rates (E2). (Levels of evidence are explained in a National Health and Medical Research Council publication. 24 ) Prophylaxis for venous thromboembolism after major orthopaedic surgery Without prophylaxis, arthroplasty of the knee and hip carries unacceptable risks of postoperative vein thrombosis and death from pulmonary embolism. 9 Standard prophylaxis includes early mobilisation, compression stockings and once-daily injections of low-molecular-weight heparin (LMWH) for 5 10 days. A proportion of patients undergoing knee-replacement surgery receive prophylaxis with warfarin rather than LMWH. With this prophylaxis 1% 2% of patients will still develop symptomatic deep vein thrombosis (DVT) or pulmonary embolism (PE), with fatality rates of 0.1% 0.5% over the 6-week postoperative period. Most of these clinical events occur out of hospital. Recent studies of prolonged prophylaxis with LMWH for 4 6 weeks postoperatively significantly reduce the incidence of symptomatic venous thromboembolism. 10 The high rates of venous thromboembolism after major orthopaedic surgery provide an opportunity for more effective anticoagulants to improve clinical outcomes. However, clinical studies based on symptomatic events alone are difficult to perform, as the number of participants needed to achieve adequate statistical power is immense. Most studies use bilateral contrast venography performed at 7 14 days after surgery as a validated surrogate outcome. With standard prophylaxis, such venography typically finds total DVT rates of 15% after hip replacement and 30% after knee replacement, and proximal DVT rates of about 6% in both groups. Recent studies incorporating venographic endpoints have shown impressive superiority of fondaparinux over standard LMWH prophylaxis. 11 Ximelagatran has been extensively studied after joint-replacement surgery. The dose-ranging pilot study (METHRO I 12 ) and phase II study (METHRO II 13 ) found an overall dose-dependent decrease in total and proximal DVT on venography and/or symptomatic pulmonary embolism with increasing doses of ximelagatran. The phase III studies of ximelagatran are summarised in Box 4. No difference in the rates of proximal asymptomatic DVT on venography, symptomatic vein thrombosis or major bleeding were shown between patients treated with ximelagatran and enoxaparin Total rates of DVT on venography were significantly reduced in patients treated with ximelagatran compared with those treated with enoxaparin in the EXPRESS study 15 and those treated with warfarin in the EXULT studies. 16,17 These studies concluded that ximelagatran is an effective agent for preventing venous thromboembolism after major orthopaedic surgery (E2). Ximelagatran has recently been approved in 13 European countries for orthopaedic prophylaxis. Therapy for vein thrombosis Acute treatment of acute venous thromboembolism, either DVT or PE, requires initial anticoagulation with LMWH and subsequent warfarin therapy in most patients. Most patients can be treated safely out of hospital. Warfarin therapy is usually initiated within hours, but therapeutic anticoagulation (as measured by INR) is not achieved for about 1 week. Heparin and warfarin should be given concurrently for at least 5 days, and heparin 434 MJA Volume 181 Number 8 18 October 2004
4 4 Summary of phase III studies of ximelagatran for prophylaxis against vein thrombosis after major orthopaedic surgery Study METHRO III EXPRESS EXULT A EXULT B Design Randomised double blind Randomised double blind Type of surgery Hip and knee replacement Knee replacement Study interventions Control Enoxaparin (40 mg) 12 h before surgery; then once daily after surgery for 8 11 days Warfarin initiated evening after surgery and adjusted to INR 2.5 Ximelagatran Melagatran 3 mg 4 8 h after surgery; then ximelagatran 24 mg twice daily for 8 11 days Melagatran 2 mg at knife-toskin; melagatran 3 mg 8 h after surgery, then ximelagatran 24 mg twice daily for 8 11 days Ximelagatran 24 mg or 36 mg initiated 12 h after surgery; then twice daily for 7 12 days Ximelagatran 36 mg twice daily initiated morning after surgery for 7 12 days Numbers of patients Ximelagatran (control) 1399 (1389)* 1410 (1425)* 614 (24mg) 1151 (1148)* 629 (36 mg) (608)* Total venous thromboembolism Ximelagatran (control) 31.0% (27.3%)* 20.3% (26.6%) 24.9% (24 mg) 20.3% (36 mg) (27.6%) 22.5% (31.9%) Major venous thromboembolism Ximelagatran (control) 5.7% (6.2%)* 2.3% (6.3%) 2.5% (24 mg) 3.9% (4.1%)* 2.7% (36 mg) (4.1%)* Fatal or major bleeding Ximelagatran (control) 1.6% (1.7%)* 3.3% (1.2%)* 0.8% (0.7%)* 1.0% (0.4%)* INR = international normalised ratio. Total venous thromboembolism = Distal plus proximal deep vein thrombosis on venography plus fatal or non-fatal pulmonary embolism plus death from any cause during treatment period. Major venous thromboembolism = Proximal deep vein thrombosis on venography plus fatal or non-fatal pulmonary embolism plus unexplained death during treatment period where pulmonary embolism could not be excluded. * Difference between ximelagatran and control therapy not significant. Difference between ximelagatran and control therapy significant at P <0.05. treatment should not be ceased until INR measurements have been in the target range of 2 3 for 2 consecutive days. The optimal duration of warfarin therapy is uncertain. In most patients with their first provoked (eg, postoperative) DVT or PE, 3 6 months is adequate. Patients with a first unprovoked proximal DVT or PE usually receive 6 months of warfarin therapy. Patients with unprovoked DVT or PE have a high rate of recurrent vein thrombosis (about 1 in 3 over 10 years). A number of studies have confirmed that extending warfarin therapy (target INR, 2 3) beyond 3 6 months is highly ( 95%) effective for preventing recurrent thrombosis. Unfortunately, the unpredictable risk of major or fatal haemorrhage all but eliminates the benefit for extended warfarin therapy. Extended warfarin therapy is generally reserved for patients with first unprovoked DVT/PE and significant thrombophilia (eg, malignant disease) or recurrent vein thrombosis. Ximelagatran has been compared with standard therapy for acute treatment of DVT with or without asymptomatic PE (THRIVE I, 18 THRIVE II [unpublished], THRIVE IV 19 and THRIVE V 20 ), and with placebo for extended secondary prophylaxis (THRIVE III 21 ). The details of these studies are summarised in Box 5. The combined results of the THRIVE II and V studies found that ximelagatran (36 mg twice daily for 6 months) was not inferior to standard therapy (enoxaparin and warfarin [targeted INR, 2 3] for 6 months) (E2). 20 There was no difference in major haemorrhage or all-cause mortality between patients treated with ximelagatran or warfarin. In the THRIVE III study of extended prophylaxis, 1233 patients were randomly assigned in a double-blind fashion to receive placebo or ximelagatran (24 mg twice daily) for 18 months after completing 6 months of anticoagulation therapy for acute DVT or PE. 21 The cumulative risk of symptomatic recurrent vein thrombosis was 2.8% in patients treated with ximelagatran and 12.6% in those taking placebo (risk reduction, 0.84; 95% CI, ; P < 0.001). Major haemorrhage occurred in 6 of 612 patients treated with ximelagatran and 7 of 611 patients given placebo. While ximelagatran is clearly superior to placebo for secondary prophylaxis (E2), identical results are obtained when placebo and warfarin therapy are compared. The haemorrhagic potential of ximelagatran compared with warfarin in this setting remains to be clarified. Acute coronary syndromes Recent studies have shown that warfarin, or warfarin combined with low-dose aspirin, is effective in patients with acute coronary disease for preventing recurrent ischaemia, reinfarction and death. 22 A single phase II study, the ESTEEM study, compared (in patients all taking 160 mg aspirin once daily) placebo with varying doses of ximelagatran (24, 36, 48 or 60 mg twice daily). 23 Overall, there was a 20% reduction in a composite endpoint of recurrent non-fatal myocardial infarction, recurrent ischaemia or death from any cause in patients treated with ximelagatran. Major bleeding occurred in 1.8% of the combined ximelagatran group and 0.9% of patients treated with aspirin only (difference not statistically significant). These results are equivalent to those seen with warfarin or warfarin combined with aspirin, 21 but more studies are required before firm conclusions can be reached (E3). MJA Volume 181 Number 8 18 October
5 5 Summary of phase III studies of ximelagatran for treatment of deep vein thrombosis Study THRIVE II and IV THRIVE III Design Randomised double-blind Randomised double-blind Indication Acute therapy for proximal DVT Extended secondary prevention of DVT Interventions Control Enoxaparin + warfarin (INR, 2 3) Placebo for 18 months for 6 months Ximelagatran 36 mg twice daily for 6 months 24 mg twice daily for 18 months Number of patients Ximelagatran (control) 1240 (1249)* 612 (611)* Recurrent symptomatic vein thrombosis Ximelagatran (control) 2.1% (2.0%)* 2.8% (12.6%) Deaths Ximelagatran (control) 2.3% (3.4%)* 1.1% (1.4%)* Fatal or major bleeding Ximelagatran (control) 1.3% (2.2%)* 1.1% (1.3%)* % of patients with ALT level > 3 times upper limit of normal Ximelagatran (control) 9.6% (2.0%)* 6.0% (1.0%)* In THRIVE III, 2.1% of patients taking ximelagatran discontinued their medication. Discontinuation rates were not published for the placebo in THRIVE III, or for ximelagatran or the control in THRIVE II and IV. DVT = Deep vein thrombosis. INR = international normalised ratio. * Difference between ximelagatran and control therapy not significant. Difference between ximelagatran and control therapy in cumulative symptomatic recurrent events at 18 months significant at P < Conclusion The clinical studies of ximelagatran confirm that it is an effective antithrombotic agent in stroke prevention in non-valvular atrial fibrillation (E2), prevention of vein thrombosis after major orthopaedic surgery (E2), acute therapy of DVT (E2) and extended prevention of recurrent vein thrombosis (E2), and possibly in preventing recurrent ischaemia after acute myocardial infarction (E3). In most indications, ximelagatran was as effective as standard therapy and had equivalent propensity to cause bleeding. In major arthroplasty ximelagatran was more effective than standard therapy as prophylaxis against vein thrombosis. Important information for patients is shown in Box 6. Patients taking ximelagatran will require monthly blood tests for liver biochemistry for 6 months. Some 6% 12% of patients treated with ximelagatran will develop asymptomatic, but significantly abnormal, ALT levels, leading to discontinuation of ximelagatran therapy in about half of cases. While serious or symptomatic liver toxicity occurred very infrequently in the clinical studies, questions remain about the mechanism of toxicity and implications for wider community use. A number of important patient groups have been excluded from the clinical evaluation of ximelagatran (pregnant and lactating women, and patients with a serum creatinine level > 150 µmol/l, abnormal liver enzyme levels, or symptomatic pulmonary embolism). Further study in patients with varying degrees of renal or liver dysfunction is required. 6 Important messages for patients Ximelagatran is a new oral anticoagulant drug with similar effects to those of warfarin. Use of ximelagatran does not require frequent blood testing to check the dose, but monthly blood tests will be required to monitor liver function. Ximelagatran has low potential for interactions with diet and other medications. Ximelagtran is not yet marketed in Australia. A number of new anticoagulant medications will emerge over the next 5 10 years for patients with thrombotic disease. For example, in orthopaedic surgery, new agents or an extension of standard prophylaxis with LMWH for 4 weeks has already been established as significantly superior to standard 7 10-day postoperative prophylaxis. Nevertheless, ximelagatran is an exciting new oral antithrombotic drug which it is hoped will benefit many patients with venous and arterial thromboembolic disease. Competing interests The author receives honoraria as an occasional speaker, clinical investigator and consultant for AstraZeneca, Aventis and Pfizer (formerly Pharmacia) and Sanofi-Synthelabo. Addendum The Cardiovascular and Renal Drug Advisory Committee of the Food and Drug Administration (US) at its 10 September, 2004 meeting voted against recommending ximelagatran for both long-term medications of prevention of stroke and other thrombo-embolic complications associated with atrial fibrillation and secondary prevention of venous thrombo-embolism after standard treatment for an acute episode along with short term prevention of VTE in patients undergoing knee replacement. The Committee members were concerned about the high risk of liver toxicity in long term indications. Overall, the Committee members agreed that ximelagatran demonstrated efficacy in clinical studies and would be an important alternative to warfarin if safety issues could be resolved. Based on currently available data, it is not possible to identify patients at risk of developing severe liver toxicity after being exposed to ximelagatran. References 1 Gustafsson D, Elg M. The pharmacodynamics and pharmacokinetics of the oral direct thrombin inhibitor ximelagatran and its active metabolite melagatran: a mini-review. Thrombosis Res 2003; 109: S9-S16. 2 Eriksson UG, Johansson S, Attman P, et al. Influence of severe renal impairment on the pharmacokinetics and pharmacodynamics of oral ximelagatran and subcutaneous melagatran. Clin Pharmacokinet 2003; 42: Dorani H, Schützer K, Sarich T, et al. The pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor ximelagatran when administered with erythromycin. Poster PO08, presented at the 18th International Congress on Thrombosis. Available at: (accessed Aug 2004). 4 Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med 2000; 160: Petersen P, Grind M, Adler J. Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. SPORTIF II: a doseguiding, tolerability, and safety study. J Am Coll Cardiol 2003; 41: Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPOR- TIF III): randomised controlled trial. Lancet 2003; 362: Stroke prevention using the oral direct thrombin inhibitor ximelagatran in patients with nonvalvular atrial fibrillation (SPORTIF V). Circulation 2003; 108: Halperin JL. Ximelagatran compared with warfarin for prevention of thromboembolism in patients with nonvalvular atrial fibrillation: Rationale, objectives, and design of a pair of clinical studies and baseline patient characteristics (SPORTIF III and V). Am Heart J 2003; 146 : MJA Volume 181 Number 8 18 October 2004
6 9 Geerts WH, Heit JA, Clagett GP, et al Prevention of venous thromboembolism. Chest 2001; 119: 132S-175S. 10 Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet 2001; 358: Bounameaux H, Perneger T. Fonfaparinux: a new synthetic pentasaccharide for thrombosis prevention [Commentary]. Lancet 2002; 359: Eriksson BI, Arfwidsson A-C, Frison L, et al. A dose-ranging study of the oral direct thrombin inhibitor, ximelagatran, and its subcutaneous form, melagatran, compared with dalteparin in the prophylaxis of thromboembolism after hip or knee replacement. METHRO I. Thromb Haemost 2002; 87: Eriksson BI, Bergqvist D, Kalebo P, et al. Ximelagatran and melagatran compared with dalteparin for prevention of venous thromboembolism after total hip or knee replacement: the METHRO II randomised trial. Lancet 2002; 360: Eriksson BI, Agnelli G, Cohen AT, et al; METHRO III Study Group. Direct thrombin inhibitor melagatran followed by oral ximelagatran in comparison with enoxaparin for prevention of venous thromboembolism after total hip or knee replacement. Thromb Haemost 2003; 89: Eriksson BI, Agnelli G, Cohen AT, et al. The direct thrombin inhibitor melagatran followed by oral ximelagatran compared with enoxaparin for the prevention of venous thromboembolism after total hip or knee replacement: the EXPRESS study. J Thromb Haemost 2003; 1: Francis CW, Berkowitz SD, Comp PC, et al. Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement (Exult A study). N Engl J Med 2003; 349: Colwell CW, Berkowitz SD, Comp PC, et al. Randomised double-blind comparison of ximelagatran, an oral direct thrombin inhibitor, and warfarin to prevent venous thromboembolism after total knee replacement (Exult B study). Blood 2003; 102: 14a. 18 Eriksson H, Wahlander K, Gustafsson D, et al. A randomised, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. J Thromb Haemost 2003; 1: Walhander K, Lapidus L, Olsson CG, et al. Pharmacokinetics, pharmacodynamics and clinical effects of the oral direct thrombin inhibitor ximelagatran in acute treatment of patients with pulmonary embolism and deep vein thrombosis. Thromb Res 2002; 107: Francis CW, Ginsberg JS, Berkowitz SD, et al. Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current standard therapy for acute, symptomatic deep vein thrombosis, with or without pulmonary embolism: The THRIVE Treatment Study. Blood 2003; 102: 6a. 21 Schulman S, Wahlander K, Lundstrom T, et al. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran (THRIVE III). N Engl J Med 2003; 349: Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002; 347: Wallentin L, Wilcox RG, Weaver WD, et al. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomised controlled trial. Lancet 2003; 362: National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, AusInfo, (Received 25 Mar 2004, accepted 26 Jul 2004) MJA Volume 181 Number 8 18 October
For decades, the standards of antithrombotic therapy have
 PHARMACOLOGY NOTES Ximelagatran (Exanta): alternative to? CHRISTY VAUGHAN, PHARMD For decades, the standards of antithrombotic therapy have been heparin and coumarin compounds, primarily (1). Other newer
PHARMACOLOGY NOTES Ximelagatran (Exanta): alternative to? CHRISTY VAUGHAN, PHARMD For decades, the standards of antithrombotic therapy have been heparin and coumarin compounds, primarily (1). Other newer
Ximelagatran, the Oral Anticoagulant of the Future An Evidence Based Review
 QATAR MEDICAL JOURNAL VOL. 14 / NO. 2 / NOVEMBER 2005 REVIEW Ximelagatran, the Oral Anticoagulant of the Future An Evidence Based Review *Salam A.,**A1 Homsi U.,*Gehani A.A. *Cardiology and Cardiovascular
QATAR MEDICAL JOURNAL VOL. 14 / NO. 2 / NOVEMBER 2005 REVIEW Ximelagatran, the Oral Anticoagulant of the Future An Evidence Based Review *Salam A.,**A1 Homsi U.,*Gehani A.A. *Cardiology and Cardiovascular
Results from RE-COVER RE-COVER II RE-MEDY RE-SONATE EXECUTIVE SUMMARY
 Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and the prevention of recurrent DVT and PE Results from
Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and the prevention of recurrent DVT and PE Results from
Outcome Paper Option#3 Final Paper
 Outcome Paper Option#3 Final Paper Evaluation of Ximelagatran in Comparison to: Warfarin in Atrial Fibrillation, Placebo in Post DVT Patients, Either Warfarin or Enoxaparin in Joint Replacement. Christine
Outcome Paper Option#3 Final Paper Evaluation of Ximelagatran in Comparison to: Warfarin in Atrial Fibrillation, Placebo in Post DVT Patients, Either Warfarin or Enoxaparin in Joint Replacement. Christine
Chapter 1 Introduction
 Chapter 1 Introduction There are several disorders which carry an increased risk of thrombosis, clots that interfere with normal circulation, including: venous thromboembolism (VTE), comprising both deep
Chapter 1 Introduction There are several disorders which carry an increased risk of thrombosis, clots that interfere with normal circulation, including: venous thromboembolism (VTE), comprising both deep
FACTOR Xa AND PAR-1 BLOCKER : ATLAS-2, APPRAISE-2 & TRACER TRIALS
 New Horizons In Atherothrombosis Treatment 2012 순환기춘계학술대회 FACTOR Xa AND PAR-1 BLOCKER : ATLAS-2, APPRAISE-2 & TRACER TRIALS Division of Cardiology, Jeonbuk National University Medical School Jei Keon Chae,
New Horizons In Atherothrombosis Treatment 2012 순환기춘계학술대회 FACTOR Xa AND PAR-1 BLOCKER : ATLAS-2, APPRAISE-2 & TRACER TRIALS Division of Cardiology, Jeonbuk National University Medical School Jei Keon Chae,
What s new with DOACs? Defining place in therapy for edoxaban &
 What s new with DOACs? Defining place in therapy for edoxaban & Use of DOACs in cardioversion Caitlin M. Gibson, PharmD, BCPS Assistant Professor, Department of Pharmacotherapy University of North Texas
What s new with DOACs? Defining place in therapy for edoxaban & Use of DOACs in cardioversion Caitlin M. Gibson, PharmD, BCPS Assistant Professor, Department of Pharmacotherapy University of North Texas
DEEP VEIN THROMBOSIS (DVT): TREATMENT
 DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
DEEP VEIN THROMBOSIS (DVT): TREATMENT OBJECTIVE: To provide an evidence-based approach to treatment of patients presenting with deep vein thrombosis (DVT). BACKGROUND: An estimated 45,000 patients in Canada
Indications of Anticoagulants; Which Agent to Use for Your Patient? Marc Carrier MD MSc FRCPC Thrombosis Program Ottawa Hospital Research Institute
 Indications of Anticoagulants; Which Agent to Use for Your Patient? Marc Carrier MD MSc FRCPC Thrombosis Program Ottawa Hospital Research Institute Disclosures Research Support/P.I. Employee Leo Pharma
Indications of Anticoagulants; Which Agent to Use for Your Patient? Marc Carrier MD MSc FRCPC Thrombosis Program Ottawa Hospital Research Institute Disclosures Research Support/P.I. Employee Leo Pharma
ADVANCES IN ANTICOAGULATION
 ADVANCES IN ANTICOAGULATION The Clinicians Perspective Claudine M. Lewis Cardiologist OUTLINE Indications for anticoagulants Review - Physiology of Hemostasis Types of anticoagulants New anticoagulants
ADVANCES IN ANTICOAGULATION The Clinicians Perspective Claudine M. Lewis Cardiologist OUTLINE Indications for anticoagulants Review - Physiology of Hemostasis Types of anticoagulants New anticoagulants
New Oral Anticoagulant Drugs in the Prevention of DVT
 New Oral Anticoagulant Drugs in the Prevention of DVT Targets for Anticoagulants ORAL DIRECT VKAs inhibit the hepatic synthesis of several coagulation factors Rivaroxaban Apixaban Edoxaban Betrixaban X
New Oral Anticoagulant Drugs in the Prevention of DVT Targets for Anticoagulants ORAL DIRECT VKAs inhibit the hepatic synthesis of several coagulation factors Rivaroxaban Apixaban Edoxaban Betrixaban X
DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE)
 DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE) Introduction VTE (DVT/PE) is an important complication in hospitalized patients Hospitalization for acute medical illness
DVT PROPHYLAXIS IN HOSPITALIZED MEDICAL PATIENTS SAURABH MAJI SR (PULMONARY,MEDICINE) Introduction VTE (DVT/PE) is an important complication in hospitalized patients Hospitalization for acute medical illness
Direct Oral Anticoagulants (DOACs). Dr GM Benson Director NI Haemophilia Comprehensive Care Centre and Thrombosis Unit BHSCT
 Direct Oral Anticoagulants (DOACs). Dr GM Benson Director NI Haemophilia Comprehensive Care Centre and Thrombosis Unit BHSCT OAC WARFARIN Gold standard DABIGATRAN RIVAROXABAN APIXABAN EDOXABAN BETRIXABAN
Direct Oral Anticoagulants (DOACs). Dr GM Benson Director NI Haemophilia Comprehensive Care Centre and Thrombosis Unit BHSCT OAC WARFARIN Gold standard DABIGATRAN RIVAROXABAN APIXABAN EDOXABAN BETRIXABAN
New Anticoagulants Therapies
 New Anticoagulants Therapies Rachel P. Rosovsky, MD, MPH October 22, 2015 Conflicts of Interest No disclosures 2 Agenda 3 Historical perspective Novel oral anticoagulants Stats Trials Approval Concerns/Limitations
New Anticoagulants Therapies Rachel P. Rosovsky, MD, MPH October 22, 2015 Conflicts of Interest No disclosures 2 Agenda 3 Historical perspective Novel oral anticoagulants Stats Trials Approval Concerns/Limitations
Treatment Options and How They Work
 Treatment Options and How They Work Robin Offord Director of Clinical Pharmacy UCL Hospitals NHS Foundation Trust robin.offord@uclh.nhs.uk Introducing the term anticoagulant... What they do Inhibit the
Treatment Options and How They Work Robin Offord Director of Clinical Pharmacy UCL Hospitals NHS Foundation Trust robin.offord@uclh.nhs.uk Introducing the term anticoagulant... What they do Inhibit the
Results from Hokusai-VTE presented during ESC Congress 2013 Hot Line session and published in the New England Journal of Medicine
 Press Release Daiichi Sankyo s Once-Daily Edoxaban Shows Comparable Efficacy and Superiority for the Principal Safety Endpoint Compared to Warfarin in a Phase 3 Study for the Treatment of Symptomatic VTE
Press Release Daiichi Sankyo s Once-Daily Edoxaban Shows Comparable Efficacy and Superiority for the Principal Safety Endpoint Compared to Warfarin in a Phase 3 Study for the Treatment of Symptomatic VTE
3/19/2012. What is the indication for anticoagulation? Has the patient previously been on warfarin? If so, what % of the time was the INR therapeutic?
 Abigail E. Miller, PharmD, BCPS Clinical Specialist, Cardiology University of North Carolina Hospitals I have no personal financial relationships with the manufacturers of the products to disclose. Boehringer
Abigail E. Miller, PharmD, BCPS Clinical Specialist, Cardiology University of North Carolina Hospitals I have no personal financial relationships with the manufacturers of the products to disclose. Boehringer
Update on Oral Anticoagulants. Dr. Miten R. Patel Cancer Specialists of North Florida Cell
 Update on Oral Anticoagulants Dr. Miten R. Patel Cancer Specialists of North Florida Cell 904-451-9820 Email miten.patel@csnf.us Overview Highlights of the 4 new approved oral anticoagulants Results from
Update on Oral Anticoagulants Dr. Miten R. Patel Cancer Specialists of North Florida Cell 904-451-9820 Email miten.patel@csnf.us Overview Highlights of the 4 new approved oral anticoagulants Results from
UPDATE ON TREATMENT OF ACUTE VENOUS THROMBOSIS
 UPDATE ON TREATMENT OF ACUTE VENOUS THROMBOSIS Armando Mansilha MD, PhD, FEBVS 16 th National Congress of the Italian Society of Vascular and Endovascular Surgery Bologna, 2017 Disclosure I have the following
UPDATE ON TREATMENT OF ACUTE VENOUS THROMBOSIS Armando Mansilha MD, PhD, FEBVS 16 th National Congress of the Italian Society of Vascular and Endovascular Surgery Bologna, 2017 Disclosure I have the following
New Oral Anticoagulants in treatment of VTE, PE DR.AMR HANAFY (LECTURER OF CARDIOLOGY ) ASWAN UNIVERSITY
 New Oral Anticoagulants in treatment of VTE, PE DR.AMR HANAFY (LECTURER OF CARDIOLOGY ) ASWAN UNIVERSITY Fact VTE is deadly! It nibbles after it bites! The 30-day mortality rates for first-time DVT or
New Oral Anticoagulants in treatment of VTE, PE DR.AMR HANAFY (LECTURER OF CARDIOLOGY ) ASWAN UNIVERSITY Fact VTE is deadly! It nibbles after it bites! The 30-day mortality rates for first-time DVT or
KCS Congress: Impact through collaboration
 Stroke Prevention in Atrial Fibrillation (SPAF) in Kenya Elijah N. Ogola FACC University of Nairobi Kenya Cardiac Society Annual Scientific Congress Mombasa 28 th June 1 st July 2017 KCS Congress: Impact
Stroke Prevention in Atrial Fibrillation (SPAF) in Kenya Elijah N. Ogola FACC University of Nairobi Kenya Cardiac Society Annual Scientific Congress Mombasa 28 th June 1 st July 2017 KCS Congress: Impact
Pradaxa (dabigatran)
 Pradaxa (dabigatran) Policy Number: 5.01.574 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Pradaxa
Pradaxa (dabigatran) Policy Number: 5.01.574 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Pradaxa
Mabel Labrada, MD Miami VA Medical Center
 Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Mabel Labrada, MD Miami VA Medical Center *1-Treatment for acute DVT with underlying malignancy is for 3 months. *2-Treatment of provoked acute proximal DVT can be stopped after 3months of treatment and
Results from RE-LY and RELY-ABLE
 Results from RE-LY and RELY-ABLE Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in longterm stroke prevention EXECUTIVE SUMMARY Dabigatran etexilate (Pradaxa ) has shown a consistent
Results from RE-LY and RELY-ABLE Assessment of the safety and efficacy of dabigatran etexilate (Pradaxa ) in longterm stroke prevention EXECUTIVE SUMMARY Dabigatran etexilate (Pradaxa ) has shown a consistent
Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales
 Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales Merimbula, 6 th November 2010 University of Sydney Department of Surgery Westmead Hospital
Prevention and management of deep venous thrombosis (DVT) John Fletcher Wound Care Association of New South Wales Merimbula, 6 th November 2010 University of Sydney Department of Surgery Westmead Hospital
Title: Low Molecular Weight Heparins (LMWH), fondaparinux (Arixtra)
 Origination: 03/29/05 Revised: 09/01/10 Annual Review: 11/20/13 Purpose: To provide guidelines and criteria for the review and decision determination of requests for medications that requires prior authorization.
Origination: 03/29/05 Revised: 09/01/10 Annual Review: 11/20/13 Purpose: To provide guidelines and criteria for the review and decision determination of requests for medications that requires prior authorization.
Aspirin as Venous Thromboprophylaxis
 Canadian Society of Internal Medicine Nov 2, 2017 Aspirin as Venous Thromboprophylaxis Bill Geerts, MD, FRCPC Thromboembolism Consultant, Sunnybrook HSC Professor of Medicine, University of Toronto Disclosures
Canadian Society of Internal Medicine Nov 2, 2017 Aspirin as Venous Thromboprophylaxis Bill Geerts, MD, FRCPC Thromboembolism Consultant, Sunnybrook HSC Professor of Medicine, University of Toronto Disclosures
VTE Management in Surgical Patients: Optimizing Prophylaxis Strategies
 VTE Management in Surgical Patients: Optimizing Prophylaxis Strategies VTE in Surgical Patients: Recognizing the Patients at Risk Pathogenesis of thrombosis: Virchow s triad and VTE Risk Hypercoagulability
VTE Management in Surgical Patients: Optimizing Prophylaxis Strategies VTE in Surgical Patients: Recognizing the Patients at Risk Pathogenesis of thrombosis: Virchow s triad and VTE Risk Hypercoagulability
Low molecular weight heparins and heparinoids
 NEW DRUGS, OLD DRUGS Low molecular weight heparins and heparinoids John W Eikelboom and Graeme J Hankey UNFRACTIONATED HEPARIN has been used in clinical practice for more than 50 years and is established
NEW DRUGS, OLD DRUGS Low molecular weight heparins and heparinoids John W Eikelboom and Graeme J Hankey UNFRACTIONATED HEPARIN has been used in clinical practice for more than 50 years and is established
Xarelto (rivaroxaban) Prescriber Guide
 Xarelto (rivaroxaban) Prescriber Guide October 2018 PP-XAR-IE-0031 Contents Patient Alert Card 4 Dosing Recommendations 4 Stroke prevention in adult patients with non-valvular atrial fibrillation 4 Patients
Xarelto (rivaroxaban) Prescriber Guide October 2018 PP-XAR-IE-0031 Contents Patient Alert Card 4 Dosing Recommendations 4 Stroke prevention in adult patients with non-valvular atrial fibrillation 4 Patients
Debate: New Generation Anti-Coagulation Agents are a Better Choice than Warfarin in the Management of AF
 Debate: New Generation Anti-Coagulation Agents are a Better Choice than Warfarin in the Management of AF Bradley P. Knight, MD Director of Cardiac Electrophysiology Bluhm Cardiovascular Institute Northwestern
Debate: New Generation Anti-Coagulation Agents are a Better Choice than Warfarin in the Management of AF Bradley P. Knight, MD Director of Cardiac Electrophysiology Bluhm Cardiovascular Institute Northwestern
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 15 November 2007 CPMP/EWP/707/98 Rev.1 corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR PROPHYLAXIS
European Medicines Agency London, 15 November 2007 CPMP/EWP/707/98 Rev.1 corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR PROPHYLAXIS
Venous Thromboembolism Prophylaxis - Why Should We Care? Harry Gibbs FRACP FCSANZ Vascular Physician The Alfred Hospital
 Venous Thromboembolism Prophylaxis - Why Should We Care? Harry Gibbs FRACP FCSANZ Vascular Physician The Alfred Hospital VTE is common and dangerous 5 VTE is Common VTE Incidence: 1.5 / 1000 per year
Venous Thromboembolism Prophylaxis - Why Should We Care? Harry Gibbs FRACP FCSANZ Vascular Physician The Alfred Hospital VTE is common and dangerous 5 VTE is Common VTE Incidence: 1.5 / 1000 per year
Oral Anticoagulation Drug Class Prior Authorization Protocol
 Oral Anticoagulation Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Oral Anticoagulation Drug Class Prior Authorization Protocol Line of Business: Medicaid P & T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Fatal P.E. Historic 1-2% Current %
 Dr. (Prof.) Anil Arora MS (Ortho) DNB (Ortho) Dip SIROT (USA) FAPOA (Korea), FIGOF (Germany), FJOA (Japan) Commonwealth Fellow Joint Replacement (Royal National Orthopaedic Hospital, London, UK) Senior
Dr. (Prof.) Anil Arora MS (Ortho) DNB (Ortho) Dip SIROT (USA) FAPOA (Korea), FIGOF (Germany), FJOA (Japan) Commonwealth Fellow Joint Replacement (Royal National Orthopaedic Hospital, London, UK) Senior
Apixaban for stroke prevention in atrial fibrillation. August 2010
 Apixaban for stroke prevention in atrial fibrillation August 2010 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Apixaban for stroke prevention in atrial fibrillation August 2010 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC
 Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC DEBATE: DOAC vs Good Old Warfarin André Roussin MD, FRCP, CSPQ CHUM and ICM/MHI Associate professor University of Montreal A. Roussin
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC DEBATE: DOAC vs Good Old Warfarin André Roussin MD, FRCP, CSPQ CHUM and ICM/MHI Associate professor University of Montreal A. Roussin
Venous Thromboembolism Prophylaxis After Major Orthopaedic Surgery: A Pooled Analysis of Randomized Controlled Trials
 Winner of the AAHKS Award Venous Thromboembolism Prophylaxis After Major Orthopaedic Surgery: A Pooled Analysis of Randomized Controlled Trials Greg A. Brown, MD, PhD The Journal of Arthroplasty Vol. 24
Winner of the AAHKS Award Venous Thromboembolism Prophylaxis After Major Orthopaedic Surgery: A Pooled Analysis of Randomized Controlled Trials Greg A. Brown, MD, PhD The Journal of Arthroplasty Vol. 24
Anticoagulation for prevention of venous thromboembolism
 Anticoagulation for prevention of venous thromboembolism Original article by: Michael Tam Note: updated in June 2009 with the eighth edition (from the seventh) evidence-based clinical practice guidelines
Anticoagulation for prevention of venous thromboembolism Original article by: Michael Tam Note: updated in June 2009 with the eighth edition (from the seventh) evidence-based clinical practice guidelines
Family Medicine Clinical Pharmacy Forum Vol. 4, Issue 5 (September/October 2008)
 1 Family Medicine Clinical Pharmacy Forum Vol. 4, Issue 5 (September/October 2008) Family Medicine Clinical Pharmacy Forum is a brief bi-monthly publication from the Family Medicine clinical pharmacists
1 Family Medicine Clinical Pharmacy Forum Vol. 4, Issue 5 (September/October 2008) Family Medicine Clinical Pharmacy Forum is a brief bi-monthly publication from the Family Medicine clinical pharmacists
Daiichi Sankyo s Once-Daily Lixiana
 Daiichi Sankyo s Once-Daily Lixiana (edoxaban) Receives Positive CHMP Opinion for the Prevention of Stroke and Systemic Embolism in Non-Valvular Atrial Fibrillation and for the Treatment and Prevention
Daiichi Sankyo s Once-Daily Lixiana (edoxaban) Receives Positive CHMP Opinion for the Prevention of Stroke and Systemic Embolism in Non-Valvular Atrial Fibrillation and for the Treatment and Prevention
Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital Foundation Trust
 MANAGEMENT OF PATIENTS WITH DEEP VEIN THROMBOSIS (DVT) IN THE COMMUNITY SETTING & ANTICOAGULATION CLINICS THE PAST, PRESENT AND THE FUTURE Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital
MANAGEMENT OF PATIENTS WITH DEEP VEIN THROMBOSIS (DVT) IN THE COMMUNITY SETTING & ANTICOAGULATION CLINICS THE PAST, PRESENT AND THE FUTURE Dr. Riaz JanMohamed Consultant Haematologist The Hillingdon Hospital
Updates in Management of Venous Thromboembolic Disease
 Updates in Management of Venous Thromboembolic Disease November 7 th 2018 UHN Emergency Conference Susan Jenkins RN(EC) NP-Adult Thrombosis and Hemostasis Program University Health Network Disclosures
Updates in Management of Venous Thromboembolic Disease November 7 th 2018 UHN Emergency Conference Susan Jenkins RN(EC) NP-Adult Thrombosis and Hemostasis Program University Health Network Disclosures
Clinical Policy: Dalteparin (Fragmin) Reference Number: ERX.SPA.207 Effective Date:
 Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Fragmin) Reference Number: ERX.SPA.207 Effective Date: 01.11.17 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Ximelagatran: Oral Direct Thrombin Inhibition as Anticoagulant Therapy in Atrial Fibrillation
 Journal of the American College of Cardiology Vol. 45, No. 1, 2005 2005 by the American College of Cardiology Foundation ISSN 0735-1097/05/$30.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2004.09.049
Journal of the American College of Cardiology Vol. 45, No. 1, 2005 2005 by the American College of Cardiology Foundation ISSN 0735-1097/05/$30.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2004.09.049
Technology appraisal guidance Published: 17 December 2014 nice.org.uk/guidance/ta327
 Dabigatran an etexilate for the treatment and secondary prevention ention of deep vein thrombosis and/or pulmonary embolism Technology appraisal guidance Published: 17 December 2014 nice.org.uk/guidance/ta327
Dabigatran an etexilate for the treatment and secondary prevention ention of deep vein thrombosis and/or pulmonary embolism Technology appraisal guidance Published: 17 December 2014 nice.org.uk/guidance/ta327
Clinical issues which drug for which patient
 Anticoagulants - a matter of heart! Towards a bright future? Clinical issues which drug for which patient Sabine Eichinger Dept. of Medicine I Medical University of Vienna/Austria Conflicts of interest
Anticoagulants - a matter of heart! Towards a bright future? Clinical issues which drug for which patient Sabine Eichinger Dept. of Medicine I Medical University of Vienna/Austria Conflicts of interest
Drug Class Review Newer Oral Anticoagulant Drugs
 Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Drug Class Review Newer Oral Anticoagulant Drugs Final Original Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Understanding thrombosis in venous thromboembolism. João Morais Head of Cardiology Division and Research Centre Leiria Hospital Centre Portugal
 Understanding thrombosis in venous thromboembolism João Morais Head of Cardiology Division and Research Centre Leiria Hospital Centre Portugal Disclosures João Morais On the last year JM received honoraria
Understanding thrombosis in venous thromboembolism João Morais Head of Cardiology Division and Research Centre Leiria Hospital Centre Portugal Disclosures João Morais On the last year JM received honoraria
EXTENDING VTE PROPHYLAXIS IN ACUTELY ILL MEDICAL PATIENTS
 EXTENDING VTE PROPHYLAXIS IN ACUTELY ILL MEDICAL PATIENTS Samuel Z. Goldhaber, MD Director, VTE Research Group Cardiovascular Division Brigham and Women s Hospital Professor of Medicine Harvard Medical
EXTENDING VTE PROPHYLAXIS IN ACUTELY ILL MEDICAL PATIENTS Samuel Z. Goldhaber, MD Director, VTE Research Group Cardiovascular Division Brigham and Women s Hospital Professor of Medicine Harvard Medical
Xarelto (rivaroxaban)
 Xarelto (rivaroxaban) Policy Number: 5.01.575 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Xarelto
Xarelto (rivaroxaban) Policy Number: 5.01.575 Last Review: 7/2018 Origination: 6/2014 Next Review: 7/2019 LoB: ACA Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Xarelto
DVT - initial management NSCCG
 Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Background information Information resources for patients and carers Updates to this care map Synonyms Below knee DVT and bleeding risks Patient with confirmed DVT Scan confirms superficial thrombophlebitis
Is Oral Rivaroxaban Safe and Effective in the Treatment of Patients with Symptomatic DVT?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 1-1-2013 Is Oral Rivaroxaban Safe and Effective
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 1-1-2013 Is Oral Rivaroxaban Safe and Effective
New Antithrombotic and Antiplatelet Drugs in CAD : (Factor Xa inhibitors, Direct Thrombin inhibitors and Prasugrel)
 New Antithrombotic and Antiplatelet Drugs in CAD : (Factor Xa inhibitors, Direct Thrombin inhibitors and Prasugrel) Limitations and Advantages of UFH and LMWH Biological limitations of UFH : 1. immune-mediated
New Antithrombotic and Antiplatelet Drugs in CAD : (Factor Xa inhibitors, Direct Thrombin inhibitors and Prasugrel) Limitations and Advantages of UFH and LMWH Biological limitations of UFH : 1. immune-mediated
Novel oral anticoagulants
 REVIEW ARTICLE Novel oral anticoagulants C. W. Khoo, K.-H. Tay, E. Shantsila, G. Y. H. Lip University Department of Medicine, City Hospital, Birmingham, England, UK Correspondence to: Prof. Gregory Y.
REVIEW ARTICLE Novel oral anticoagulants C. W. Khoo, K.-H. Tay, E. Shantsila, G. Y. H. Lip University Department of Medicine, City Hospital, Birmingham, England, UK Correspondence to: Prof. Gregory Y.
New Aspects in the Diagnosis and Treatment of Atrial Fibrillation: Antithrombotic Therapy
 New Aspects in the Diagnosis and Treatment of Atrial Fibrillation: Antithrombotic Therapy Hans-Christoph Diener Department of Neurology and Stroke Center University Hospital Essen Germany Conflict of Interest
New Aspects in the Diagnosis and Treatment of Atrial Fibrillation: Antithrombotic Therapy Hans-Christoph Diener Department of Neurology and Stroke Center University Hospital Essen Germany Conflict of Interest
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H
 Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
National Institute for Health and Clinical Excellence Health Technology Appraisal
 National Institute for Health and Clinical Excellence Health Technology Appraisal Rivaroxaban for the prevention of venous thromboembolism after elective orthopaedic surgery of the lower limbs Comment
National Institute for Health and Clinical Excellence Health Technology Appraisal Rivaroxaban for the prevention of venous thromboembolism after elective orthopaedic surgery of the lower limbs Comment
Non commercial use only. The treatment of venous thromboembolism with new oral anticoagulants. Background
 Italian Journal of Medicine 2013; volume 7(s8):29-35 The treatment of venous thromboembolism with new oral anticoagulants Davide Imberti AUSL Piacenza, Italy ABSTRACT Traditional anticoagulants, such as
Italian Journal of Medicine 2013; volume 7(s8):29-35 The treatment of venous thromboembolism with new oral anticoagulants Davide Imberti AUSL Piacenza, Italy ABSTRACT Traditional anticoagulants, such as
Updates in Anticoagulation for Atrial Fibrillation and Venous Thromboembolism
 Disclosures Updates in Anticoagulation for Atrial Fibrillation and Venous Thromboembolism No financial conflicts of interest Member of the ABIM Focused- Practice in Hospital Medicine Self Examination Process
Disclosures Updates in Anticoagulation for Atrial Fibrillation and Venous Thromboembolism No financial conflicts of interest Member of the ABIM Focused- Practice in Hospital Medicine Self Examination Process
CANCER ASSOCIATED THROMBOSIS. Pankaj Handa Department of General Medicine Tan Tock Seng Hospital
 CANCER ASSOCIATED THROMBOSIS Pankaj Handa Department of General Medicine Tan Tock Seng Hospital My Talk Today 1.Introduction 2. Are All Cancer Patients at Risk of VTE? 3. Should All VTE Patients Be Screened
CANCER ASSOCIATED THROMBOSIS Pankaj Handa Department of General Medicine Tan Tock Seng Hospital My Talk Today 1.Introduction 2. Are All Cancer Patients at Risk of VTE? 3. Should All VTE Patients Be Screened
TITLE: Acetylsalicylic Acid for Venous Thromboembolism Prophylaxis: A Review of Clinical Evidence, Benefits and Harms
 TITLE: Acetylsalicylic Acid for Venous Thromboembolism Prophylaxis: A Review of Clinical Evidence, Benefits and Harms DATE: 23 August 2011 CONTEXT AND POLICY ISSUES: Thromboembolism occurs when a blood
TITLE: Acetylsalicylic Acid for Venous Thromboembolism Prophylaxis: A Review of Clinical Evidence, Benefits and Harms DATE: 23 August 2011 CONTEXT AND POLICY ISSUES: Thromboembolism occurs when a blood
Bayer s Rivaroxaban Demonstrated Superior Protection Against Recurrent Venous Thromboembolism Compared with Aspirin in EINSTEIN CHOICE Study
 News Release Not intended for U.S. and UK Media Bayer AG Communications and Public Affairs 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com New Late-Breaking Study Data Presented at ACC.17:
News Release Not intended for U.S. and UK Media Bayer AG Communications and Public Affairs 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com New Late-Breaking Study Data Presented at ACC.17:
Challenges in Anticoagulation and Thromboembolism
 Challenges in Anticoagulation and Thromboembolism Ethan Cumbler M.D. Assistant Professor of Medicine Hospitalist Medicine Section University of Colorado Denver May 2010 No Conflicts of Interest Objectives
Challenges in Anticoagulation and Thromboembolism Ethan Cumbler M.D. Assistant Professor of Medicine Hospitalist Medicine Section University of Colorado Denver May 2010 No Conflicts of Interest Objectives
DOACs in CAT. Fellow: Shweta Jain, MD Faculty Discussant: David Garcia, MD
 DOACs in CAT Fellow: Shweta Jain, MD Faculty Discussant: David Garcia, MD Case 65 year old post menopausal female Left breast lesion Oct 2015 Biopsy Invasive ductal carcinoma Lumpectomy with SNB- pt1cno
DOACs in CAT Fellow: Shweta Jain, MD Faculty Discussant: David Garcia, MD Case 65 year old post menopausal female Left breast lesion Oct 2015 Biopsy Invasive ductal carcinoma Lumpectomy with SNB- pt1cno
Oral rivaroxaban versus standard therapy for the acute and continued treatment of symptomatic deep vein thrombosis. The EINSTEIN DVT study.
 Oral rivaroxaban versus standard therapy for the acute and continued treatment of symptomatic deep vein thrombosis. The EINSTEIN DVT study Comments Harald Darius, Berlin Disclosures for Harald Darius Research
Oral rivaroxaban versus standard therapy for the acute and continued treatment of symptomatic deep vein thrombosis. The EINSTEIN DVT study Comments Harald Darius, Berlin Disclosures for Harald Darius Research
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Lessons from recent antithrombotic studies and trials in atrial fibrillation
 Lessons from recent antithrombotic studies and trials in atrial fibrillation Thromboembolism cause of stroke in AF Lars Wallentin Uppsala Clinical Research Centre (UCR) Uppsala Disclosures for Lars Wallentin
Lessons from recent antithrombotic studies and trials in atrial fibrillation Thromboembolism cause of stroke in AF Lars Wallentin Uppsala Clinical Research Centre (UCR) Uppsala Disclosures for Lars Wallentin
Edoxaban: a new oral direct factor Xa inhibitor for the prevention and treatment of thromboembolic disorders
 Edoxaban: a new oral direct factor Xa inhibitor for the prevention and treatment of thromboembolic disorders Anticoagulants have a key role in the treatment of arterial and venous thromboembolic disorders.
Edoxaban: a new oral direct factor Xa inhibitor for the prevention and treatment of thromboembolic disorders Anticoagulants have a key role in the treatment of arterial and venous thromboembolic disorders.
NEW/NOVEL ORAL ANTICOAGULANTS (NOACS): COMPARISON AND FREQUENTLY ASKED QUESTIONS
 NEW/NOVEL ORAL ANTICOAGULANTS (NOACS): COMPARISON AND FREQUENTLY ASKED QUESTIONS OBJECTIVES: To provide a comparison of the new/novel oral anticoagulants (NOACs) currently available in Canada. To address
NEW/NOVEL ORAL ANTICOAGULANTS (NOACS): COMPARISON AND FREQUENTLY ASKED QUESTIONS OBJECTIVES: To provide a comparison of the new/novel oral anticoagulants (NOACs) currently available in Canada. To address
Venous Thrombo-Embolism. John de Vos Consultant Haematologist RSCH
 Venous Thrombo-Embolism John de Vos Consultant Haematologist RSCH overview The statistics Pathogenesis Prophylaxis Treatment Agent Duration Incidental VTE Recurrence of VTE IVC filters CVC related thrombosis
Venous Thrombo-Embolism John de Vos Consultant Haematologist RSCH overview The statistics Pathogenesis Prophylaxis Treatment Agent Duration Incidental VTE Recurrence of VTE IVC filters CVC related thrombosis
CADTH Rapid Response Report: ASA for Venous Thromboembolism Prophylaxis: Evidence for Clinical Benefit and Harm
 CADTH Rapid Response Report: ASA for Venous Thromboembolism Prophylaxis: Evidence for Clinical Benefit and Harm P. Timothy Pollak, MD, PhD University of Calgary Rocky Mountain/ACP Internal Medicine Meeting,
CADTH Rapid Response Report: ASA for Venous Thromboembolism Prophylaxis: Evidence for Clinical Benefit and Harm P. Timothy Pollak, MD, PhD University of Calgary Rocky Mountain/ACP Internal Medicine Meeting,
Anticoagulation Therapy in LTC
 Anticoagulation Therapy in LTC By: Cynthia Leung, RPh, BScPhm, PharmD. Clinical Consultant Pharmacist MediSystem Pharmacy Jun 11, 2013 Agenda Stroke and Bleeding Risk Assessment Review of Oral Anticoagulation
Anticoagulation Therapy in LTC By: Cynthia Leung, RPh, BScPhm, PharmD. Clinical Consultant Pharmacist MediSystem Pharmacy Jun 11, 2013 Agenda Stroke and Bleeding Risk Assessment Review of Oral Anticoagulation
Venous Thromboembolism Prophylaxis: Checked!
 Venous Thromboembolism Prophylaxis: Checked! William Geerts, MD, FRCPC Director, Thromboembolism Program, Sunnybrook HSC Professor of Medicine, University of Toronto National Lead, VTE Prevention, Safer
Venous Thromboembolism Prophylaxis: Checked! William Geerts, MD, FRCPC Director, Thromboembolism Program, Sunnybrook HSC Professor of Medicine, University of Toronto National Lead, VTE Prevention, Safer
Drug Class Monograph
 Drug Class Monograph Class: Oral Anticoagulants Drug: Coumadin (warfarin), Eliquis (apixaban), Pradaxa (dabigatran), Savaysa (edoxaban), arelto (rivaroxaban) Formulary Medications: Eliquis (apixaban),
Drug Class Monograph Class: Oral Anticoagulants Drug: Coumadin (warfarin), Eliquis (apixaban), Pradaxa (dabigatran), Savaysa (edoxaban), arelto (rivaroxaban) Formulary Medications: Eliquis (apixaban),
Joshua D. Lenchus, DO, RPh, FACP, SFHM Associate Professor of Medicine and Anesthesiology University of Miami Miller School of Medicine
 Joshua D. Lenchus, DO, RPh, FACP, SFHM Associate Professor of Medicine and Anesthesiology University of Miami Miller School of Medicine Antithrombotics Antiplatelets Aspirin Ticlopidine Prasugrel Dipyridamole
Joshua D. Lenchus, DO, RPh, FACP, SFHM Associate Professor of Medicine and Anesthesiology University of Miami Miller School of Medicine Antithrombotics Antiplatelets Aspirin Ticlopidine Prasugrel Dipyridamole
Clinical Policy: Dabigatran (Pradaxa) Reference Number: CP.PMN.49 Effective Date: Last Review Date: 05.18
 Clinical Policy: (Pradaxa) Reference Number: CP.PMN.49 Effective Date: 05.01.12 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Pradaxa) Reference Number: CP.PMN.49 Effective Date: 05.01.12 Last Review Date: 05.18 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
With All the New Drugs, This is How I Treat Acute DVT and Superficial Phlebitis
 BRIGHAM AND WOMEN S HOSPITAL With All the New Drugs, This is How I Treat Acute DVT and Superficial Phlebitis Gregory Piazza, MD, MS Division of Cardiovascular Medicine Brigham and Women s Hospital April
BRIGHAM AND WOMEN S HOSPITAL With All the New Drugs, This is How I Treat Acute DVT and Superficial Phlebitis Gregory Piazza, MD, MS Division of Cardiovascular Medicine Brigham and Women s Hospital April
Medical Patients: A Population at Risk
 Case Vignette A 68-year-old woman with obesity was admitted to the Medical Service with COPD and pneumonia and was treated with oral corticosteroids, bronchodilators, and antibiotics. She responded well
Case Vignette A 68-year-old woman with obesity was admitted to the Medical Service with COPD and pneumonia and was treated with oral corticosteroids, bronchodilators, and antibiotics. She responded well
Reversal of Novel Oral Anticoagulants. Angelina The, MD March 22, 2016
 Reversal of Novel Oral Anticoagulants Angelina The, MD March 22, 2016 Argatroban Bivalirudin Enoxaparin Lepirudin Heparin Dabigatran Apixaban 1939 1954 1998 2000 1999 2001 10/2010 7/2011 12/2012 1/2015
Reversal of Novel Oral Anticoagulants Angelina The, MD March 22, 2016 Argatroban Bivalirudin Enoxaparin Lepirudin Heparin Dabigatran Apixaban 1939 1954 1998 2000 1999 2001 10/2010 7/2011 12/2012 1/2015
24 mg tablets. Summary of product characteristics Exanta. Elderly In patients over 75 years of age there is limited clinical
 Summary of product characteristics Exanta 24 mg tablets 1. NAME OF THE MEDICINAL PRODUCT Exanta 24 mg, film-coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 24 mg ximelagatran.
Summary of product characteristics Exanta 24 mg tablets 1. NAME OF THE MEDICINAL PRODUCT Exanta 24 mg, film-coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 24 mg ximelagatran.
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
Prevention and treatment of venous thromboembolic disease
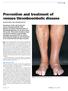 REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
REVIEW Prevention and treatment of venous thromboembolic disease SUSAN McNEILL AND CATHERINE BAGOT Awareness of the risk factors for venous thromboembolic (VTE) disease and timely administration of thromboprophylaxis
Bath, Philip M.W. and England, Timothy J. (2009) Thighlength compression stockings and DVT after stroke. Lancet. ISSN (In Press)
 Bath, Philip M.W. and England, Timothy J. (2009) Thighlength compression stockings and DVT after stroke. Lancet. ISSN 0140-6736 (In Press) Access from the University of Nottingham repository: http://eprints.nottingham.ac.uk/1087/1/lancet_clots_1_20090522_4.pdf
Bath, Philip M.W. and England, Timothy J. (2009) Thighlength compression stockings and DVT after stroke. Lancet. ISSN 0140-6736 (In Press) Access from the University of Nottingham repository: http://eprints.nottingham.ac.uk/1087/1/lancet_clots_1_20090522_4.pdf
Anticoagulation. MPharm Programme & OSPAP Programme. Tania Jones Senior Lecturer in Pharmacy Practice & Therapeutics
 MPharm Programme & OSPAP Programme Anticoagulation Tania Jones Senior Lecturer in Pharmacy Practice & Therapeutics tania.jones@sunderland.ac.uk Lecture MPHM13 / MPHM14 2017-2018 MPHM13 & MPHM14 Objectives
MPharm Programme & OSPAP Programme Anticoagulation Tania Jones Senior Lecturer in Pharmacy Practice & Therapeutics tania.jones@sunderland.ac.uk Lecture MPHM13 / MPHM14 2017-2018 MPHM13 & MPHM14 Objectives
Rivaroxaban (Xarelto) in the management of stroke and DVT
 Rivaroxaban (Xarelto) in the management of stroke and DVT Steve Chaplin MSc, MRPharmS, Victoria Haunton BM, DGM, MRCP, Thompson Robinson BMedSci, MD, FRCP and Catherine Bagot MD, FRCPath KEY POINTS is
Rivaroxaban (Xarelto) in the management of stroke and DVT Steve Chaplin MSc, MRPharmS, Victoria Haunton BM, DGM, MRCP, Thompson Robinson BMedSci, MD, FRCP and Catherine Bagot MD, FRCPath KEY POINTS is
Venous thrombosis is common and often occurs spontaneously, but it also frequently accompanies medical and surgical conditions, both in the community
 Venous Thrombosis Venous Thrombosis It occurs mainly in the deep veins of the leg (deep vein thrombosis, DVT), from which parts of the clot frequently embolize to the lungs (pulmonary embolism, PE). Fewer
Venous Thrombosis Venous Thrombosis It occurs mainly in the deep veins of the leg (deep vein thrombosis, DVT), from which parts of the clot frequently embolize to the lungs (pulmonary embolism, PE). Fewer
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised
 Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Effect of fondaparinux 2.5 mg once daily on mortality: a meta-analysis of phase III randomized trials of venous thromboembolism prevention
 European Heart Journal Supplements (2008) 10 (Supplement C), C8 C13 doi:10.1093/eurheartj/sun004 Effect of fondaparinux 2.5 mg once daily on mortality: a meta-analysis of phase III randomized trials of
European Heart Journal Supplements (2008) 10 (Supplement C), C8 C13 doi:10.1093/eurheartj/sun004 Effect of fondaparinux 2.5 mg once daily on mortality: a meta-analysis of phase III randomized trials of
The clinical relevance of AMPLIFY programme
 Venice October 16th 2015 The clinical relevance of AMPLIFY programme Francesco Dentali Department of Clinical Medicine Insubria University Varese Disclosures Bayer Bristol-Myers Squibb/Pfizer Boehringer
Venice October 16th 2015 The clinical relevance of AMPLIFY programme Francesco Dentali Department of Clinical Medicine Insubria University Varese Disclosures Bayer Bristol-Myers Squibb/Pfizer Boehringer
New drugs for anticoagulation so much choice, how do they compare? Dr Patrick Kesteven Newcastle
 New drugs for anticoagulation so much choice, how do they compare? Dr Patrick Kesteven Newcastle CONCLUSIONS 1. Arrival of new anticoagulants is a Good Thing. CONCLUSIONS 1. Arrival of new anticoagulants
New drugs for anticoagulation so much choice, how do they compare? Dr Patrick Kesteven Newcastle CONCLUSIONS 1. Arrival of new anticoagulants is a Good Thing. CONCLUSIONS 1. Arrival of new anticoagulants
New Antithrombotic Agents DISCLOSURE
 New Antithrombotic Agents DISCLOSURE Relevant Financial Relationship(s) Speaker Bureau None Research Alexion (PNH) delought@ohsu.edu Tom DeLoughery, MD FACP FAWM Oregon Health and Sciences University What
New Antithrombotic Agents DISCLOSURE Relevant Financial Relationship(s) Speaker Bureau None Research Alexion (PNH) delought@ohsu.edu Tom DeLoughery, MD FACP FAWM Oregon Health and Sciences University What
Comparison of Ximelagatran with Warfarin for the Prevention of Venous Thromboembolism after Total Knee Replacement
 The new england journal of medicine original article Comparison of Ximelagatran with Warfarin for the Prevention of Venous Thromboembolism after Total Knee Replacement Charles W. Francis, M.D., Scott D.
The new england journal of medicine original article Comparison of Ximelagatran with Warfarin for the Prevention of Venous Thromboembolism after Total Knee Replacement Charles W. Francis, M.D., Scott D.
Pharmacy Prior Authorization
 Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Anticoagulant Injectable (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Pharmacy Prior Authorization AETA BETTER HEALTH EW JERSE (MEDICAID) Anticoagulant Injectable (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review
Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of deep vein thrombosis (Review)
 Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of deep vein thrombosis (Review) Robertson L, Kesteven P, McCaslin JE This is a reprint of a Cochrane review, prepared and
Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of deep vein thrombosis (Review) Robertson L, Kesteven P, McCaslin JE This is a reprint of a Cochrane review, prepared and
Do s and Don t of DOACs DISCLOSURE
 Do s and Don t of DOACs Tom DeLoughery, MD MACP FAWM Oregon Health and Sciences University DISCLOSURE Relevant Financial Relationship(s) Speaker Bureau - None Consultant/Research none Content Expert: Elsevier
Do s and Don t of DOACs Tom DeLoughery, MD MACP FAWM Oregon Health and Sciences University DISCLOSURE Relevant Financial Relationship(s) Speaker Bureau - None Consultant/Research none Content Expert: Elsevier
Deep vein thrombosis: diagnosis, prevention and treatment
 Deep vein thrombosis: diagnosis, prevention and treatment Catherine Bagot BSc, MD, MRCP, FRCPath and Campbell Tait BSc, FRCP, FRCPath Deep vein thrombosis can lead to significant morbidity and has well-recognised
Deep vein thrombosis: diagnosis, prevention and treatment Catherine Bagot BSc, MD, MRCP, FRCPath and Campbell Tait BSc, FRCP, FRCPath Deep vein thrombosis can lead to significant morbidity and has well-recognised
DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients. David Liff MD Oklahoma Heart Institute Vascular Center
 DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients David Liff MD Oklahoma Heart Institute Vascular Center Overview Pathophysiology of DVT Epidemiology and risk factors for DVT in the
DVT Pathophysiology and Prophylaxis in Medically Hospitalized Patients David Liff MD Oklahoma Heart Institute Vascular Center Overview Pathophysiology of DVT Epidemiology and risk factors for DVT in the
Original Policy Date
 MP 5.01.15 Newer Oral Anticoagulants Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return to Medical
MP 5.01.15 Newer Oral Anticoagulants Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return to Medical
