The technology described in this briefing is the Zio Service. It is a remote cardiac monitoring system used for detecting cardiac arrhythmias.
|
|
|
- Ross Wheeler
- 6 years ago
- Views:
Transcription
1 pat hways Zio Service for detecting cardiac arrhythmias Medtech innovation briefing Published: 28 March 20 nice.org.uk/guidance/mib101 Summary The technology described in this briefing is the Zio Service. It is a remote cardiac monitoring system used for detecting cardiac arrhythmias. The innovative aspects are that this device can collect continuous cardiac monitoring data for up to 14 days through an adhesive biosensor patch (the Zio Patch). At the end of the monitoring period, the user posts the Zio Patch to the manufacturer, who produces a report for clinicians (the Zio Report). The Zio Patch is non-invasive, water resistant has no leads or wires so people may find it easier to wear than other ambulatory monitors. The intended place in therapy would be instead of current methods of cardiac event detection, such as Holter monitoring or event recording in people suspected of having cardiac arrhythmias. The main points from the evidence summarised in this briefing are from 6 studies based outside the UK: 2 comparative studies (n=220), 2 non-comparative prospective studies (n=249) 2 large non-comparative retrospective studies (n=149,205) on adult patients in a home setting. Of the 2 comparative studies, 1 reported that the Zio Service detected more arrhythmia events than Holter monitoring in the first 24 hours the other reported excellent agreement between the 2 methods. Both studies found that using the Zio Service over 14 days detected more arrhythmia events than Holter monitoring over 24 hours. Key uncertainties around the evidence or technology include that there is no evidence comparing the Zio Service with other monitoring devices that have a similar monitoring period. NICE 20. All rights reserved. Subject to Notice of rights ( Page 1 of
2 The list price of the Zio Service is around 800 per unit (exclusive of VAT). This includes the patch, the data analysis the report. The resource impact would be increased monitoring costs compared with 24-hour Holter monitoring, but this could be offset if Zio Patch were shown to increase early correct diagnoses reduce repeat monitoring admissions. The technology The Zio Service (irhythm Technologies) is designed to detect cardiac arrhythmias is comprised of the Zio Patch, an adhesive patch with a 1-lead ambulatory electrocardiogram (ECG), the Zio Report, a summary of the recorded data that have been analysed. The Zio Patch is a lightweight water-resistant ECG monitor that has no external leads or wires. The patch is stuck on the person's left upper chest can record a continuous beat-to-beat ECG for up to 14 days. Each Zio Patch is intended for single-patient use. Wearers can carry on with their usual daily activities during monitoring. When they feel a symptom, the wearer can press a trigger button on the device that highlights the recording 45 seconds before after the button was pressed. The wearer is also asked to keep a paperbased log in which they write down any symptomatic events that happen during the 14-day monitoring period, as well as information on what they were doing the conditions at the time. This allows for a symptom-rhythm correlation to be included in the final report. After the monitoring period, the wearer removes the Zio Patch sends it to the company by Freepost through the Royal Mail. The recordings are analysed by the company, using proprietary machine-learned algorithms (Zio ECG Utilization Service System [ZEUS System]), a report is produced. The report describes all cardiac arrhythmia events over the total wear time the analysis by the ZEUS system, it is reviewed by a certified cardiac technician. This Zio Report is sent electronically to the ordering clinician through irhythm's secure web-based portal. The full ECG data can be sent to the clinician on request. Patient data are otherwise only used by the company in a de-identified form for quality reporting system improvements. Parts of the analysis are done in the UK the US. The manufacturer states that transmission, processing storage of all data complies with relevant EU UK legislation. There are no patient identifiers in or on the Zio Patch data cannot be accessed if the Zio Patch were to be physically intercepted. irhythm uses security measures to prevent inappropriate access to or manipulation of the data. NICE 20. All rights reserved. Subject to Notice of rights ( Page 2 of
3 Innovations The Zio Service provides a continuous recording of ambulatory cardiac monitoring for up to 14 days. The wearer can go about their normal daily activities during monitoring, including showering or bathing because the device is water resistant. The Zio Service can be used for a longer monitoring period than a stard Holter monitor, which can be up to 7 days but usually for 24 to 48 hours. The Zio Patch has no external leads or wires this is intended to reduce noise artefacts in the data. It can be worn under clothing, so may be more discreet than Holter monitors, which are generally worn in a pouch around the waist or neck, or carried in a pocket. The Zio Service uses machine-learned analytics in the form of proprietary software to create the report that is delivered to the clinician. This is intended to reduce the time needed for NHS staff to analyse the continuous monitoring data. Current NHS pathway The current methods of arrhythmia detection (NHS Choices) are: 12-lead ECG 24- to 48-hour or 7-day continuous ambulatory monitoring, including using Holter monitors long-term continuous monitoring for up to 30 days external loop recorders insertable loop recorders, which can record events for up to 3 years for arrhythmias that occur sometimes months apart. The Holter monitor is the method most commonly used in the NHS for detecting atrial fibrillation. Holter monitors continuously record the heart rhythm using several electrode patches, which are stuck on the user's chest. These electrodes detect record electrical signals produced by each heartbeat, are connected by wires to a portable recording machine. The user can press a button on the front of the recording machine at specific times, such as when having symptoms, going to bed or taking medication. These points can then be easily found in the continuous monitoring data. Holter monitoring is used for 24 to 48 hours for people who have regular symptoms, or can be used for up to 7 days for people with symptoms that happen less often, such as if they only have arrhythmia every 3 to 4 days. Results are analysed by the user's clinician. NICE 20. All rights reserved. Subject to Notice of rights ( Page 3 of
4 The NICE guidelines on managing atrial fibrillation transient loss of consciousness ('blackouts') in over 16s recommend a 12-lead ECG for the first assessment. If further assessment of possible cardiac arrhythmia is needed, ambulatory ECG monitoring is recommended for 24 or 48 hours. The choice of monitor depends on symptoms symptom frequency includes Holter monitoring external or implantable event recorders. If the first 24- to 48-hour Holter monitor test does not give a clear diagnosis, people are referred for further investigations. This can include event recording for up to 7 days or admission to hospital for more invasive options, such as fitting an implantable loop recorder. Estimates of the diagnostic yield for 24-hour Holter monitoring vary. Barbeito Caamano et al. (2016) reported that syncope was only diagnosed in 4% of people in a cohort monitored for 24 hours. De Asmundis et al. (2014) reported that Holter monitoring led to a diagnosis of any arrhythmia in 1.8% of people monitored for 24 hours, whereas Kinlay et al. (1996) reported a diagnosis rate of 35% after 48-hour Holter monitoring. The Zio Service would be used for monitoring over 14 days as well as, or instead of, 24- to 48-hour 7-day Holter monitoring or event recording. NICE is aware of the following CE-marked devices that appear to fulfil a similar function to Zio Service: Bardy 7-day patch (Cardiologic) SEEQ MCT monitor (Medtronic) Population, setting intended user The Zio Service would be used in a home setting for people with suspected cardiac arrhythmias. The device can be used for people with suspected arrhythmias unexplained loss of consciousness. The Zio Service would be used instead of Holter monitoring or event recording when continuous monitoring needs to be extended for up to 14 days. A clinician would prescribe monitoring with the Zio Service, most likely a cardiologist in secondary care or possibly a GP in primary care. The Zio Patch is intended to be applied to the person by the clinician, or by a cardiac physiologist or healthcare support worker in hospital, or a nurse or healthcare support worker in primary care. Occasionally, it may be applied by the person themselves if the clinician thinks that this is appropriate. After the monitoring period, the person NICE 20. All rights reserved. Subject to Notice of rights ( Page 4 of
5 sends the Zio Patch by Freepost through the Royal Mail to irhythm for data analysis production of the report. The manufacturer states that minimal training is needed to apply use the Zio Patch that full instructions are provided in the user manual. The chest area may sometimes need to be shaved before applying the device. irhythm states that the clinician will not need additional training to interpret the Zio Report, because it is clear complete. Very occasionally, such as with paroxysmal atrial fibrillation, monitoring for more than 14 days may be needed because events are very intermittent. In this situation 2 patches would be worn in succession. Costs The manufacturer has given an example list price of 800 per unit for the Zio Service. In practice, prices will vary depending on the procurement volume arrangements for each hospital. This single price includes the cost of the Zio Patch, the data analysis, the clinical report for one 14-day monitoring period for a single patient. A specialist commentator provided the following costs (excluding equipment costs) for 24-hour Holter monitoring, per patient: monitoring interpretation of results: without overheads (defined as staff, heating computer use) with overheads monitoring no interpretation of results: 39. without overheads with overheads. An example of a reusable Holter monitor is the Spacelabs LifeCard CF Holter recorder, which has a list price of 1, (NHS Supply Chain). Resource consequences The Zio Service is currently available in 6 NHS trusts. NICE 20. All rights reserved. Subject to Notice of rights ( Page 5 of
6 The purchase price of the Zio Service may be lower than that of a Holter monitor, but the cost of 24-hour Holter monitoring per patient would be lower. Cost savings could be generated if using the Zio Service reduced the need for repeated or prolonged Holter or event monitoring. The Zio Service offers 14-day monitoring, which may improve the detection of infrequent arrhythmias compared with 24- to 48-hour or 7-day Holter monitoring. This could save any costs that could be avoided by having a correct diagnosis, including repeat hospital inpatient outpatient admissions related to complications such as syncope, chest pain, stroke or transient ischaemic attack. The costs of applying the Zio Patch to the patient are minimal. It is simple to use can be removed without medical supervision. People having Holter monitoring must visit the cardiologist to return the monitor after the recording period, whereas the Zio Service is returned to irhythm by post, which may be more convenient for people. Regulatory information The Zio Service was CE marked as a Class IIa device in December A search of the Medicines Healthcare products Regulatory Agency website revealed that no manufacturer field safety notices or medical device alerts have been issued for this technology. Equality considerations NICE is committed to promoting equality, eliminating unlawful discrimination fostering good relations between people with particular protected characteristics others. In producing guidance advice, NICE aims to comply fully with all legal obligations to: promote race disability equality equality of opportunity between men women, eliminate unlawful discrimination on grounds of race, disability, age, sex, gender reassignment, marriage civil partnership, pregnancy maternity (including women post-delivery), sexual orientation, religion or belief (these are protected characteristics under the Equality Act 2010). Cardiac arrhythmias can develop in people of any age, but are more common in people over 60 years. Women tend to be at higher risk of certain arrhythmias, including atrioventricular nodal tachycardia, whereas men are 3 times more likely to develop atrial fibrillation at any age. However, of those people who develop atrial fibrillation, women have a much higher incidence of morbidity mortality. Age sex are protected characteristics under the Equality Act. People whose first language is not English or who cannot write may not be able to give written information on their symptoms while using the Zio Service. NICE 20. All rights reserved. Subject to Notice of rights ( Page 6 of
7 Clinical technical evidence A literature search was carried out for this briefing in accordance with the interim process methods statement. This briefing includes the most relevant or best available published evidence relating to the clinical effectiveness of the technology. Further information about how the evidence for this briefing was selected is available on request by contacting mibs@nice.org.uk. Published evidence Six studies are summarised in this briefing with a total of 149,674 participants. Two of these studies were within-subject comparative studies comparing ambulatory 14-day Zio Service monitoring with 24-hour stard care Holter monitoring (n=220), 2 were non-comparative prospective studies (n=249) 2 were very large, non-comparative retrospective studies (n=149,205). Table 1 summarises the clinical evidence as well as its strengths limitations. Overall assessment of the evidence There is limited evidence on the effectiveness of the Zio Service compared with stard care. The 2 available comparative studies do not compare Zio Service with a monitoring device that uses a similar monitoring period, instead comparing 14-day Zio Service monitoring with 24-hour Holter monitoring. The sizes of the 2 comparative studies on Zio Service are relatively small with a total of 220 people included. Although these studies were carried out in the US, they are likely to be relevant to the current NHS care pathway because they compared Zio Service with 24-hour Holter monitoring, which is considered stard care in the NHS. The 2 very large retrospective studies are less informative because they were non-comparative retrospective. More studies comparing the Zio Service with ambulatory electrocardiogram (ECG) including Holter event monitoring over 7 days or longer would be useful to determine its clinical cost effectiveness in the NHS. Two studies, which will compare the Zio Service with stard monitoring in a UK cohort, are currently in progress. Table 1 Summary of included studies Barrett et al. (2014) NICE 20. All rights reserved. Subject to Notice of rights ( Page 7 of
8 Study size, design location Prospective within-subject comparison of 24-hour Holter monitoring 14-day Zio Patch monitoring. Evaluation of cardiac arrhythmias in 146 adult outpatients (after loss to follow-up). US. Intervention comparator Key outcomes The Zio Patch compared with the Holter monitor. All patients wore both devices for the first 24 hours then continued with Zio Patch. During the first 24 hours of simultaneous monitoring, the Holter monitor detected statistically significantly more events than the Zio Patch. All the clinically significant arrhythmias that were undetected by the Zio Patch were later detected during prolonged monitoring with the patch. The Zio Patch detected statistically significantly more arrhythmia events than the Holter monitor over the total wear time. Participants found the Zio Patch more comfortable to wear preferred it to the Holter monitor. Median wear time for the Zio Patch was 11.1 days (no reasons were given for the shorter than expected wear time). Strengths limitations Some of the patients had pre-existing arrhythmias were referred for reasons other than symptomatic arrhythmias. The manufacturer, irhythm, partly funded the study. Rosenberg et al. (2013) Study size, design location Prospective within-subject comparison of 24-hour Holter monitoring 14-day Zio Patch monitoring. Evaluation of 74 adults with paroxysmal atrial fibrillation (after loss to follow-up). US. Intervention comparator Monitoring using the Zio Patch compared with the Holter monitor. All patients had both types of monitoring. NICE 20. All rights reserved. Subject to Notice of rights ( Page 8 of
9 Key outcomes During the first 24 hours of simultaneous monitoring, there was excellent agreement between both devices for identifying AF events AF burden. Because of a longer monitoring time, AF episodes were detected in statistically significantly more patients with the Zio Patch compared with the Holter monitor. AF events were identified in 18 more participants the documented pattern of symptoms changed in 21 more participants using the Zio Patch than with the Holter. Clinical management of the condition changed as a result of the Zio Patch in 28.4% of patients. Less than half of the self-reported symptoms (using the button press on the Zio Patch) correlated with arrhythmia events. Median time to detection of first event with Zio Patch was 3.7 days, with 90% detected by day 7. Mean wear time for Zio Patch was 10.8 days. Strengths limitations Pilot study with a relatively small sample size. The manufacturer, irhythm, supported the study with a restricted research grant. Schreiber et al. (2014) Study size, design location Intervention comparator Key outcomes Prospective, multicentre observational study of 4 adult participants using the Zio Patch. US. The Zio Patch; no comparator. Ninety-eight arrhythmia events were detected over a median wear time of 6.9 days. Intervention duration was only 7 days. Of the patients who pressed the event button, 53% did not actually have an arrhythmia at the time. Median time-to-first event was 1.0 days. Median time-to-first symptomatic event was 1.5 days. Median time to event for some types of arrhythmia was 5.8 days. Overall diagnostic yield was 63.2%. NICE 20. All rights reserved. Subject to Notice of rights ( Page 9 of
10 Strengths limitations All of the Zio Patches were returned for analysis, suggesting that all of the patients used them as advised. There was no statistical analysis of the data. Enrolment was based on clinician judgement alone because it was an observational study. There was no romised comparison with stard care. The duration of monitoring was short compared with other studies. Clinical history data on pre-existing cardiac abnormalities were lacking. Solomon et al. (2016) Study size, design location Intervention comparator Key outcomes Retrospective cross-sectional analysis of 122,454 adult patients using the Zio Service. US. The Zio Service; no comparator. High-risk arrhythmia events were detected in 20,685 (21.7%) records. For ventricular arrhythmias, only 52.5% were detected in the first 24 hours 92.9% were identified by day 7. The trend for bradyarrhythmia results was similar. The differences in diagnostic yield between 2 7 days for both ventricular arrhythmias bradyarrhythmias were statistically significant. Median time-to-first event was between hours depending on type of arrhythmia. Mean wear time was 9.6 days. Strengths limitations The sample was very large. There was no follow-up after the first monitoring period. The investigators did not have the symptomatic self-report data so could not correlate symptomatic triggers with true arrhythmias. A quarter of patients did not wear their devices for at least 7 days, which limited the amount of data for analysis. The manufacturer, irhythm, sponsored the study. Turakhia et al. (2013) NICE 20. All rights reserved. Subject to Notice of rights ( Page 10 of
11 Study size, design location Intervention comparator Key outcomes Retrospective cross-sectional study of 26,751 adult patients having monitoring using the Zio Patch. Single centre in US. The Zio Patch; no comparator. Single multiple arrhythmias were detected in 16,142 (60.3%) of patients. The overall mean time taken to detect the first arrhythmia was 1.7 days, with some specific arrhythmias taking longer to detect (mean 3.4 days). Mean wear time was 7.6 days. Strengths limitations The sample was large. Some parameters were not analysed statistically, for example, whether the diagnostic yields were different between early late monitoring. Patients' clinical backgrounds differed. Some of the arrhythmias detected may not have been clinically significant because of their short duration, which could have skewed the results. The manufacturer, irhythm, supported the study through a grant. The mean wear time was short compared with other studies, possibly because of clinicians prescribing different monitoring durations. Turakhia et al. (2015) Study size, design location Intervention comparators Single-centre prospective screening study of 75 male adult patients. US. The Zio Patch; no comparator. NICE 20. All rights reserved. Subject to Notice of rights ( Page 11 of
12 Key outcomes Any arrhythmia of more than 8 beats was detected in 36 (48%) of participants; 35% of them had the first arrhythmia after 48 hours of monitoring. AF was detected in 4 participants, with 3 of them having their longest episode after 48 hours of monitoring. Over a third of participants had an arrhythmia other than AF more than 48 hours after monitoring started. Mean wear time was 13 days. Strengths limitations Procedures were more likely to be stardised because the study was done at a single centre. Participants wore the device for a reasonable length of time, which increased available data. Used an all-male sample, which is not representative of the population as a whole could have biased the results. Some parameters were not analysed statistically, for example, whether the diagnostic yields were different between early late monitoring. Abbreviation: AF, atrial fibrillation. Recent ongoing studies NCT Comparison of continuous sternal ECG patch monitors (Carnation Zio) trial. Trial is enrolling participants by invitation only. Estimated completion date was January 20. NCT ELR monitoring against permanent pacemaker in atrial fibrillation (REMAP-AF). UK-based trial but not yet open for recruitment. The manufacturer has advised that the estimated completion date is June 20. NCT Diagnostic yield of an ambulatory patch monitor in unexplained emergency department syncope: a pilot study (PATCH-ED). UK-based trial currently recruiting participants. Estimated completion date is December 20. Real-world heart monitoring strategy, evaluation, treatment patterns health metrics in atrial fibrillation (RHYTHM Study). US-based trial. Estimated completion date of Aim 1 is May 20 Aims 2 3 is October 20. NICE 20. All rights reserved. Subject to Notice of rights ( Page 12 of
13 Romised clinical trial of early prolonged ambulatory cardiac monitoring after stroke (EPACS). UK-based trial. Results presented at the International Stroke Conference in February 20. Enrolment completed. CCG trial of innovative alternative palpitation pathway. UK-based trial with the Ealing Clinical Commissioning Group. Estimated completion date is June 20. Specialist commentator comments Comments on this technology were invited from clinical experts working in the field relevant patient organisations. The comments received are individual opinions do not represent NICE's view. Two out of 3 specialist commentators were familiar with or had used this technology before. One stated that they were involved in an ongoing clinical trial using the device another that it was used regularly in their private practice. Level of innovation Two commentators agreed that the Zio Service is very innovative. One stated that people tolerate it much better than other monitoring devices another noted that it is beneficial for people who have transient loss of consciousness during arrhythmia because they cannot actively record symptoms as needed with some mobile apps. The third commentator stated that although it is a novel concept design, extended monitoring for up to 7 days is readily available in the NHS has been for many years. One commentator stated that the Zio Patch's ease of use waterproof design are an improvement on other traditional recorders that the report produced is excellent with an easyto-read layout. The commentator also recommended showing people how to apply the patch, but has previously sent patients the patch to apply themselves they did not have any problems doing this. Another commentator stated that no training on applying the patch is needed, only on how to send it for analysis. A third believed that older people could struggle to correctly position the device, but that it is quite simple for those who have been appropriately trained. Potential patient impact All commentators agreed that the Zio Service could have benefits for people. One commented that the device can be applied during consultation, avoiding an extra session or outpatient appointment to have a monitor fitted. Also, there is less chance of a Zio Patch failing than a Holter monitor. NICE 20. All rights reserved. Subject to Notice of rights ( Page 13 of
14 Another commentator noted that Holter monitors can be affected by motion artefacts the leads can get damaged, which can prevent accurate monitoring. The Zio Patch is beneficial for all people because it is leadless more robust, but also particularly for children, for people with mental health or cognitive problems. All 3 of the commentators noted that because the Zio Service can be used for longer than the stard 24- to 48-hour monitoring period, it can help to detect arrhythmias that occur after this period. One commentator felt that Zio Service could also be useful in quantifying atrial fibrillation burden stratifying the risk of stroke. It is likely to prevent some people from having implantable devices that are not needed, which could reduce discomfort infection risk. One commentator suggested that this device could result in earlier diagnosis another that it may reduce the number of hospital visits for patients because they can return the monitor by post. One commentator also mentioned that there is less risk of skin reactions because of fewer electrodes being attached to the patient less repeat monitoring, which could mean that people wear it for the full monitoring period. Potential system impact One commentator stated that using the Zio Patch could increase the efficiency of screening patients for arrhythmias, in theory, replace a consultation in secondary or tertiary care if the results showed that the symptoms were not serious or could be managed in primary care. But, because an external agency was producing the reports, clinicians would be unaware if data had been missed unless the full dataset was disclosed. Two commentators agreed that having the data analysed externally could free up clinical staff to be used elsewhere. A third commentator thought that the device may reduce admissions because it can easily be applied in an emergency department rather than needing to admit someone for observation. But, the report would need to be interpreted by a senior member of the clinical team, probably a consultant cardiologist, to decide future management. All commentators agreed that minimal changes in facilities or infrastructure would be needed if the Zio Service was adopted in stard practice, with only storage of return boxes typical IT infrastructure needed. Two of the 3 commentators felt that it would be unlikely to generate cost savings for the NHS because the device has a significant cost for each patient. One commented that it might be cost effective in a specific subset of patients as an adjunct rather than a replacement for current methods of monitoring. The third commentator believed it could be cost effective because of NICE 20. All rights reserved. Subject to Notice of rights ( Page 14 of
15 reduced need for repeat monitoring, admissions or implantable event recording devices associated costs, but that savings would depend on the cost of the device to the NHS. One commentator pointed out that implantable loop recorders are more expensive, but offer 18 to 24 months of monitoring, which might be more cost effective for certain patients, that it would be useful to determine their comparative effectiveness with the Zio Service in a romised controlled trial. General comments One commentator noted that other similar products are available felt that these should be considered alongside the Zio Service. Another commentator warned against clinicians recommending this kind of monitoring service unnecessarily instead of taking a full clinical history to inform decision-making. Clinicians should also be aware that extended monitoring could pick up unexplained symptoms with unknown significance consideration is needed on how to deal with this. Patient organisation comments The Arrhythmia Alliance, the Atrial Fibrillation Association the Syncope Trust And Reflex Anoxic Seizures (STARS) Charity were all asked to comment on this briefing. Their responses are summarised below. All 3 patient organisations agreed that the Zio Service would allow people to be monitored at home because the patch is very discreet lets wearers carry on with their normal day-to-day life. One organisation stated that during Holter monitoring, some people with syncope will not have a syncopal attack because their daily activities are limited. The added benefit of the patch being water resistant means that wearers can still exercise shower. The Zio Service could improve patient outcomes by capturing symptoms that may be missed during 24-hour Holter monitoring, resulting in earlier correct diagnosis. The Atrial Fibrillation Association noted that atrial fibrillation is often intermittent, so prolonged monitoring with the Zio Service could increase the chance of capturing an event reduce the delay in diagnosis. It could also reduce strokes related to atrial fibrillation the misdiagnosis of epilepsy in people with unexplained loss of consciousness. In relation to children, the STARS charity commented that because the Zio Patch is small its application is similar to putting on a plaster, children are less scared of the device than of traditional monitors. NICE 20. All rights reserved. Subject to Notice of rights ( Page 15 of
16 None of the patient organisations reported negative feedback from people who had used the Zio Service. The Arrhythmia Alliance noted that wearers sometimes have to order a second patch if there are no symptoms in the first 14 days. This is beneficial because it could avoid the need for a further more invasive procedure. All the organisations agreed that because of its ease of use minimal training need, it is better than current methods for detecting arrhythmias. They also thought that using the Zio Service could be cost saving for the NHS because it could reduce hospital visits long waiting times for monitoring equipment, avoid the need for large pieces of equipment usually used to diagnose arrhythmias. All of the patient organisations would recommend the Zio Service. Specialist commentators Shane Exton, Lead Cardiac Physiologist/Clinical Scientist for Non-Invasive Cardiac Diagnostics, University Hospital Wales, Cardiff Vale University Health Board. No conflicts of interest declared. Dr Stephen Murray, Cardiologist, Freeman Hospital, Newcastle, The Newcastle upon Tyne Hospitals NHS Foundation Trust. No conflicts of interest declared. Dr Matthew Reed, Consultant in Emergency Medicine, Royal Infirmary of Edinburgh, NHS Lothian. Chief investigator on the PATCH-ED study investigating Zio Patch. irhythm is only supplying the device has no input on the study design, process or reporting. Patient organisations The following patient organisations contributed to this briefing: Arrhythmia Alliance Atrial Fibrillation Association Syncope Trust And Reflex Anoxic Seizures (STARS) Development elopment of this briefing This briefing was developed for NICE by Cedar. The interim process methods statement sets out the process NICE uses to select topics, how the briefings are developed, quality-assured approved for publication. NICE 20. All rights reserved. Subject to Notice of rights ( Page 16 of
17 ISBN: NICE 20. All rights reserved. Subject to Notice of rights ( Page of
The innovative aspects are that the test automatically generates a report on ALK status that any clinician can interpret.
 pat hways HTG EdgeSeq ALKPlus Assay EU for ALK status testing in non-small-cell lung cancer Medtech innovation briefing Published: 7 November 2017 nice.org.uk/guidance/mib128 Summary The technology described
pat hways HTG EdgeSeq ALKPlus Assay EU for ALK status testing in non-small-cell lung cancer Medtech innovation briefing Published: 7 November 2017 nice.org.uk/guidance/mib128 Summary The technology described
Key uncertainties around the evidence or technology are that the test has only been validated in biochemical studies.
 pat hways PredictSure-IBD for inflammatory bowel disease prognosis Medtech innovation briefing Published: 25 March 2019 nice.org.uk/guidance/mib178 Summary The technology described in this briefing is
pat hways PredictSure-IBD for inflammatory bowel disease prognosis Medtech innovation briefing Published: 25 March 2019 nice.org.uk/guidance/mib178 Summary The technology described in this briefing is
pat hways Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87
 pat hways CytoSorb therapy for sepsis Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87 Summary The technology described in this briefing is CytoSorb therapy. It is an
pat hways CytoSorb therapy for sepsis Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87 Summary The technology described in this briefing is CytoSorb therapy. It is an
GDm-Health is free to download and use. Its use may result in efficiency savings from reducing face-to-face clinic appointments.
 pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
The innovative aspects are that ORA G3 is currently the only device capable of measuring corneal hysteresis.
 pat hways ORA G3 to measure corneal hysteresis Medtech innovation briefing Published: 18 June 2018 nice.org.uk/guidance/mib150 Summary The technology described in this briefing is Ocular Response Analyzer
pat hways ORA G3 to measure corneal hysteresis Medtech innovation briefing Published: 18 June 2018 nice.org.uk/guidance/mib150 Summary The technology described in this briefing is Ocular Response Analyzer
The innovative aspect is that it detects bladder cancer based on the novel biomarker, minichromosome maintenance complex component 5 (MCM5).
 pat hways ADXBLADDER for detecting bladder cancer Medtech innovation briefing Published: 12 April 2019 nice.org.uk/guidance/mib180 Summary The technology described in this briefing is ADXBLADDER. It is
pat hways ADXBLADDER for detecting bladder cancer Medtech innovation briefing Published: 12 April 2019 nice.org.uk/guidance/mib180 Summary The technology described in this briefing is ADXBLADDER. It is
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Single-use Ambulatory Electrocardiographic Monitors (e.g., Zio Patch) MP-076-MD-DE Medical Management Provider Notice Date:
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Single-use Ambulatory Electrocardiographic Monitors (e.g., Zio Patch) MP-076-MD-DE Medical Management Provider Notice Date:
pat hways Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76
 pat hways Axxent brachytherapy system for early stage breast cancer Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76 Summary The technology described in this briefing is
pat hways Axxent brachytherapy system for early stage breast cancer Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76 Summary The technology described in this briefing is
pat hways Medtech innovation briefing Published: 16 February 2018 nice.org.uk/guidance/mib141
 pat hways Reveal eal LINQ insertable cardiac monitor to detect atrial fibrillation after cryptogenic stroke Medtech innovation briefing Published: 16 February 2018 nice.org.uk/guidance/mib141 Summary The
pat hways Reveal eal LINQ insertable cardiac monitor to detect atrial fibrillation after cryptogenic stroke Medtech innovation briefing Published: 16 February 2018 nice.org.uk/guidance/mib141 Summary The
WHAT CAN I DO ABOUT ATRIAL FIBRILLATION? Finding answers about atrial fibrillation
 WHAT CAN I DO ABOUT ATRIAL FIBRILLATION? Finding answers about atrial fibrillation DEBBIE S STORY With a full schedule of entertaining friends, dancing, working as a nurse and babysitting her granddog,
WHAT CAN I DO ABOUT ATRIAL FIBRILLATION? Finding answers about atrial fibrillation DEBBIE S STORY With a full schedule of entertaining friends, dancing, working as a nurse and babysitting her granddog,
pat hways Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77
 pat hways OSNA for colon cancer staging Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77 Summary The technology described in this briefing is the OSNA in vitro diagnostic
pat hways OSNA for colon cancer staging Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77 Summary The technology described in this briefing is the OSNA in vitro diagnostic
A SIMPLE SOLUTION FOR CARDIAC MONITORING
 A SIMPLE SOLUTION FOR CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry System Actual size. NOW THERE S AN EXTERNAL HEART MONITOR THAT S AS SIMPLE TO WEAR AS A BANDAGE It s called the SEEQ Mobile Cardiac
A SIMPLE SOLUTION FOR CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry System Actual size. NOW THERE S AN EXTERNAL HEART MONITOR THAT S AS SIMPLE TO WEAR AS A BANDAGE It s called the SEEQ Mobile Cardiac
WHY AM I FAINTING? Finding answers about unexplained fainting
 WHY AM I FAINTING? Finding answers about unexplained fainting PERSONAL STORIES For about 15 years, Lloyd s friends and family thought he was clumsy. Each spring, he seemed to have an unusual accident.
WHY AM I FAINTING? Finding answers about unexplained fainting PERSONAL STORIES For about 15 years, Lloyd s friends and family thought he was clumsy. Each spring, he seemed to have an unusual accident.
Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy
 Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy File Name: Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry File Code: UM.SPSVC.13 Origination: 10/2015 Last
Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy File Name: Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry File Code: UM.SPSVC.13 Origination: 10/2015 Last
pat hways Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157
 pat hways AlignRT in breast cancer radiotherapy Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157 Summary The technology described in this briefing is the AlignRT patient
pat hways AlignRT in breast cancer radiotherapy Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157 Summary The technology described in this briefing is the AlignRT patient
The technology described in this briefing is minimally invasive percutaneous nephrolitholapaxy medium (MIP-M). It is used to remove kidney stones.
 pat hways Minimally invasive percutaneous nephrolitholapaxy medium (MIP-M) for removing kidney stones Medtech innovation briefing Published: 26 January 2018 nice.org.uk/guidance/mib8 Summary The technology
pat hways Minimally invasive percutaneous nephrolitholapaxy medium (MIP-M) for removing kidney stones Medtech innovation briefing Published: 26 January 2018 nice.org.uk/guidance/mib8 Summary The technology
Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
Specialised Services Policy Position PP151
 Specialised Services Policy Position PP151 Complex Devices: Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for arrhythmias and heart failure January 2019 Version 1.0 Document
Specialised Services Policy Position PP151 Complex Devices: Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for arrhythmias and heart failure January 2019 Version 1.0 Document
Atrial fibrillation. Understanding NICE guidance
 Understanding NICE guidance Information for people who use NHS services Atrial fibrillation NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases and the treatments
Understanding NICE guidance Information for people who use NHS services Atrial fibrillation NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases and the treatments
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
UNLOCK THE ANSWER. The Reveal LINQ TM System lets your physician learn about your heart while you live your life.
 UNLOCK THE ANSWER The Reveal LINQ TM System lets your physician learn about your heart while you live your life. WHY LONG-TERM MONITORING MAY BE RIGHT FOR YOU Accurate information is the key to better
UNLOCK THE ANSWER The Reveal LINQ TM System lets your physician learn about your heart while you live your life. WHY LONG-TERM MONITORING MAY BE RIGHT FOR YOU Accurate information is the key to better
The technology described in this briefing is Promonitor. It is used to monitor response to biologic therapies.
 pat hways Promonitor for monitoring response to biologics in rheumatoid arthritis Medtech innovation briefing Published: 27 October 2017 nice.org.uk/guidance/mib126 Summary The technology described in
pat hways Promonitor for monitoring response to biologics in rheumatoid arthritis Medtech innovation briefing Published: 27 October 2017 nice.org.uk/guidance/mib126 Summary The technology described in
Patient Information Sheet Opportunistic Detection of Atrial Fibrillation in Ambulatory Blood Pressure Monitoring
 Patient Information Sheet Opportunistic Detection of Atrial Fibrillation in Ambulatory Blood Pressure Monitoring We'd like to invite you to take part in our research study. Before you decide if you would
Patient Information Sheet Opportunistic Detection of Atrial Fibrillation in Ambulatory Blood Pressure Monitoring We'd like to invite you to take part in our research study. Before you decide if you would
Diagnostic tests for syncope
 Diagnostic tests for syncope Working together with individuals, families and medical professionals to offer support and information on syncope and reflex anoxic seizures www.stars.org.uk Registered Charity
Diagnostic tests for syncope Working together with individuals, families and medical professionals to offer support and information on syncope and reflex anoxic seizures www.stars.org.uk Registered Charity
Arrhythmia Care in the DGH What Still Needs to be Done? Dr. Sundeep Puri Consultant Cardiologist
 Arrhythmia Care in the DGH What Still Needs to be Done? Dr. Sundeep Puri Consultant Cardiologist LOTS!!! This presentation confines itself to the situation in the North West. The views expressed are my
Arrhythmia Care in the DGH What Still Needs to be Done? Dr. Sundeep Puri Consultant Cardiologist LOTS!!! This presentation confines itself to the situation in the North West. The views expressed are my
Zio System Publications. Peer-reviewed publications demonstrating the clinical validity and utility of the Zio system
 Zio System Publications Peer-reviewed publications demonstrating the clinical validity and utility of the Zio system Contents Electrocardiographic Responses to Deer Hunting in Men and Women. Wilderness
Zio System Publications Peer-reviewed publications demonstrating the clinical validity and utility of the Zio system Contents Electrocardiographic Responses to Deer Hunting in Men and Women. Wilderness
Specialised Services Policy: CP23 Vagal Nerve Stimulation
 Specialised Services Policy: CP23 Vagal Nerve Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning and Performance
Specialised Services Policy: CP23 Vagal Nerve Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning and Performance
Waterproof quality IP X4
 Actual size Thanks to its high level of miniaturisation, the R.Test Evolution 3 is compact and lightweight (45 g. batteries included), for total discretion. Its exceptional design ensures high patient
Actual size Thanks to its high level of miniaturisation, the R.Test Evolution 3 is compact and lightweight (45 g. batteries included), for total discretion. Its exceptional design ensures high patient
Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41
 Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
pat hways Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120
 pat hways Caris Molecular Intelligence for guiding cancer treatment Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120 Summary The technology described in this briefing
pat hways Caris Molecular Intelligence for guiding cancer treatment Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120 Summary The technology described in this briefing
CONFIRM Rx INSERTABLE CARDIAC MONITOR CONTINUOUS HEART MONITORING LIVE LIFE WITHOUT SKIPPING A BEAT
 CONFIRM Rx INSERTABLE CARDIAC MONITOR CONTINUOUS HEART MONITORING LIVE LIFE WITHOUT SKIPPING A BEAT FIND YOUR RHYTHM DON T IGNORE THE SYMPTOMS OF AN IRREGULAR HEARTBEAT 7.1 MILLION Up to 7.1 million people
CONFIRM Rx INSERTABLE CARDIAC MONITOR CONTINUOUS HEART MONITORING LIVE LIFE WITHOUT SKIPPING A BEAT FIND YOUR RHYTHM DON T IGNORE THE SYMPTOMS OF AN IRREGULAR HEARTBEAT 7.1 MILLION Up to 7.1 million people
MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection
 POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0039 ORIGINAL EFFECTIVE: 10/01/11 LAST REVIEW: 12/12/17 MEDICAL POLICY Cardiac Event Monitors/ Cardiac Event Detection GUIDELINES This policy does not certify benefits or authorization of benefits,
Diagnostic tests for syncope
 Diagnostic tests for syncope Working together with individuals, families and medical professionals to offer support and information on syncope and reflex anoxic seizures www.stars.org.uk Registered Charity
Diagnostic tests for syncope Working together with individuals, families and medical professionals to offer support and information on syncope and reflex anoxic seizures www.stars.org.uk Registered Charity
Patient Resources: Syncope
 Patient Resources: Syncope Overview Syncope is the medical term for fainting or loss of consciousness. Fainting can occur for a few different reasons. The autonomic (involuntary) nervous system helps to
Patient Resources: Syncope Overview Syncope is the medical term for fainting or loss of consciousness. Fainting can occur for a few different reasons. The autonomic (involuntary) nervous system helps to
Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180)
 Putting NICE guidance into practice Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180) Published: June 2014 This costing report accompanies the clinical
Putting NICE guidance into practice Costing Report: atrial fibrillation Implementing the NICE guideline on atrial fibrillation (CG180) Published: June 2014 This costing report accompanies the clinical
The cost of the Axonics sacral neuromodulation system is 475 for the trial phase, 9,210 for a
 pat hways Axonics sacral al neuromodulation system for overactive bladder and faecal incontinence Medtech innovation briefing Published: 10 December 2018 nice.org.uk/guidance/mib164 Summary The technology
pat hways Axonics sacral al neuromodulation system for overactive bladder and faecal incontinence Medtech innovation briefing Published: 10 December 2018 nice.org.uk/guidance/mib164 Summary The technology
November 2016 POWERFULLY SIMPLE CARDIAC MONITORING. SEEQ External Cardiac Monitor (ECM) System. From the leaders who brought you Reveal LINQ ICM
 November 2016 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ External Cardiac Monitor (ECM) System From the leaders who brought you Reveal LINQ ICM TRADITIONAL HOLTER MONITORING TYPICALLY 1-2 DAYS NOT LONG
November 2016 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ External Cardiac Monitor (ECM) System From the leaders who brought you Reveal LINQ ICM TRADITIONAL HOLTER MONITORING TYPICALLY 1-2 DAYS NOT LONG
The technology described in this briefing is Permacol, a collagen paste that is injected into anal fistulae.
 pat hways Permacol for treating anal fistulae Medtech innovation briefing Published: 23 May 2017 nice.org.uk/guidance/mib105 Summary The technology described in this briefing is Permacol, a collagen paste
pat hways Permacol for treating anal fistulae Medtech innovation briefing Published: 23 May 2017 nice.org.uk/guidance/mib105 Summary The technology described in this briefing is Permacol, a collagen paste
GOVERNING BODY REPORT
 GOVERNING BODY REPORT DATE OF MEETING: 20th September 2012 TITLE OF REPORT: KEY MESSAGES: NHS West Cheshire Clinical Commissioning Group has identified heart disease as one of its six strategic clinical
GOVERNING BODY REPORT DATE OF MEETING: 20th September 2012 TITLE OF REPORT: KEY MESSAGES: NHS West Cheshire Clinical Commissioning Group has identified heart disease as one of its six strategic clinical
2009 CPT Codes for Cardiac Device Monitoring
 2009 CPT Codes for Cardiac Device Monitoring December 2008 Notices Current Procedural Terminology (CPT ) is copyright 2008 American Medical Association. All Rights reserved. No fee schedules, basic units,
2009 CPT Codes for Cardiac Device Monitoring December 2008 Notices Current Procedural Terminology (CPT ) is copyright 2008 American Medical Association. All Rights reserved. No fee schedules, basic units,
Cardiology Testing. What is an echocardiogram ( echo )?
 Cardiology Testing What is an echocardiogram ( echo )? An echo is an ultrasound test used to evaluate the heart s anatomy/blood vessels, blood flow, heart muscle function and pressures within the heart.
Cardiology Testing What is an echocardiogram ( echo )? An echo is an ultrasound test used to evaluate the heart s anatomy/blood vessels, blood flow, heart muscle function and pressures within the heart.
Low back pain and sciatica in over 16s NICE quality standard
 March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
NHS. Implantable cardioverter defibrillators (ICDs) for arrhythmias. National Institute for Health and Clinical Excellence. Issue date: January 2006
 NHS National Institute for Health and Clinical Excellence Issue date: January 2006 Implantable cardioverter defibrillators (ICDs) for arrhythmias Understanding NICE guidance information for people with
NHS National Institute for Health and Clinical Excellence Issue date: January 2006 Implantable cardioverter defibrillators (ICDs) for arrhythmias Understanding NICE guidance information for people with
Patient Resources: Arrhythmias and Congenital Heart Disease
 Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Patient Resources: Arrhythmias and Congenital Heart Disease Overview Arrhythmias (abnormal heart rhythms) can develop in patients with congenital heart disease (CHD) due to thickening/weakening of their
Economic Impact Evaluation Case Study: AliveCor Kardia Mobile
 NHS Innovation Accelerator Economic Impact Evaluation Case Study: AliveCor Kardia Mobile 1. BACKGROUND The AliveCor Kardia Mobile ECG (Kardia Mobile) is a portable single-channel cardiac event recorder
NHS Innovation Accelerator Economic Impact Evaluation Case Study: AliveCor Kardia Mobile 1. BACKGROUND The AliveCor Kardia Mobile ECG (Kardia Mobile) is a portable single-channel cardiac event recorder
Understanding our advice ~ December The use of troponin testing in acute coronary syndromes
 Understanding our advice ~ December 2003 The use of troponin testing in acute coronary syndromes The use of troponin testing in acute coronary syndromes Purpose of this document NHS Quality Improvement
Understanding our advice ~ December 2003 The use of troponin testing in acute coronary syndromes The use of troponin testing in acute coronary syndromes Purpose of this document NHS Quality Improvement
Ambulatory Cardiac Monitoring Modalities
 Ambulatory Cardiac Monitoring Modalities NAVIGATING THE CHOICES MKT0390.03 LEARNING OBJECTIVES Identify the Clinical Applications for Each Modality Define and Distinguish Between the Various Monitoring
Ambulatory Cardiac Monitoring Modalities NAVIGATING THE CHOICES MKT0390.03 LEARNING OBJECTIVES Identify the Clinical Applications for Each Modality Define and Distinguish Between the Various Monitoring
Deep brain stimulation for difficultto-treat
 Issue date January 2012 Understanding NICE guidance Information for people who use NHS services Deep brain stimulation for difficultto-treat epilepsy NICE interventional procedures guidance advises the
Issue date January 2012 Understanding NICE guidance Information for people who use NHS services Deep brain stimulation for difficultto-treat epilepsy NICE interventional procedures guidance advises the
pat hways Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147
 pat hways AlignRT for intracranial anial stereotactic radiosurgery Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147 Summary The technology and indication described in this
pat hways AlignRT for intracranial anial stereotactic radiosurgery Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147 Summary The technology and indication described in this
Specialised Services Policy:
 Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
pat hways Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166
 pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
Delivering first class mental health care services in south west London Committed to care, creating communities
 Estates Modernisation Programme Delivering first class mental health care services in south west London Committed to care, creating communities Contents Introduction to the Estates Modernisation Programme
Estates Modernisation Programme Delivering first class mental health care services in south west London Committed to care, creating communities Contents Introduction to the Estates Modernisation Programme
The technology described in this briefing is Arctic Sun It is used for therapeutic body temperature management.
 pat hways Arctic Sun 5000 for therapeutic hypothermia after cardiac arrest Medtech innovation briefing Published: 18 July 2017 nice.org.uk/guidance/mib112 Summary The technology described in this briefing
pat hways Arctic Sun 5000 for therapeutic hypothermia after cardiac arrest Medtech innovation briefing Published: 18 July 2017 nice.org.uk/guidance/mib112 Summary The technology described in this briefing
Which ECG is Right for You?
 Which ECG is Right for You? Working together to improve the diagnosis, treatment and quality of life for all those affected by arrhythmias www.heartrhythmalliance.org Registered Charity No. 1107496 Glossary
Which ECG is Right for You? Working together to improve the diagnosis, treatment and quality of life for all those affected by arrhythmias www.heartrhythmalliance.org Registered Charity No. 1107496 Glossary
IS THERE A LINK BETWEEN ATRIAL FIBRILLATION AND MY STROKE? Finding answers about cryptogenic stroke
 IS THERE A LINK BETWEEN ATRIAL FIBRILLATION AND MY STROKE? Finding answers about cryptogenic stroke UNDERSTANDING WHAT CAUSED YOUR STROKE IS VERY IMPORTANT A stroke happens when a blood vessel in the brain
IS THERE A LINK BETWEEN ATRIAL FIBRILLATION AND MY STROKE? Finding answers about cryptogenic stroke UNDERSTANDING WHAT CAUSED YOUR STROKE IS VERY IMPORTANT A stroke happens when a blood vessel in the brain
Cardiac Resynchronisation Therapy Defibrillator (CRT-D)
 Patient information Cardiac Resynchronisation Therapy Defibrillator (CRT-D) i Important information for all patients requiring resynchronisation therapy. Golden Jubilee National Hospital Agamemnon Street
Patient information Cardiac Resynchronisation Therapy Defibrillator (CRT-D) i Important information for all patients requiring resynchronisation therapy. Golden Jubilee National Hospital Agamemnon Street
About atrial fibrillation (AFib) Atrial Fibrillation (AFib) What is AFib? What s the danger? Who gets AFib?
 Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
Understanding AFib Atrial Fibrillation (AFib) About AFib 3 How Your Heart Works 4 Types of AFib 5 Symptoms 5 Risk Factors 5 How is AFib Diagnosed? 6 Treatment 6 What to Ask Your Doctor 7 A normal heartbeat
A Streamlined Approach to Atrial Fibrillation Screening
 A Streamlined Approach to Atrial Fibrillation Screening Partnership between the Cardiac Rhythm Management Team & Department of Stroke Medicine 1 Dowds J, 1 Britton J, 1 McNair W, 1 Turkington L, 1 Curry
A Streamlined Approach to Atrial Fibrillation Screening Partnership between the Cardiac Rhythm Management Team & Department of Stroke Medicine 1 Dowds J, 1 Britton J, 1 McNair W, 1 Turkington L, 1 Curry
Implantable Loop Recorder
 Implantable Loop Recorder Working together to improve the diagnosis, treatment and quality of life for all those affected by arrhythmias www.heartrhythmalliance.org Non-profit organization 501(c)(3) Glossary
Implantable Loop Recorder Working together to improve the diagnosis, treatment and quality of life for all those affected by arrhythmias www.heartrhythmalliance.org Non-profit organization 501(c)(3) Glossary
Cardiology. Ambulatory ECG
 Cardiology Ambulatory ECG Cardiology services are provided in the Specialist Outpatient Clinic, Emergency Department, Intensive Care Unit and as an inpatient service. A full spectrum of care is provided
Cardiology Ambulatory ECG Cardiology services are provided in the Specialist Outpatient Clinic, Emergency Department, Intensive Care Unit and as an inpatient service. A full spectrum of care is provided
OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices
 OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices Ontario Health Technology Advisory Committee January 2012 Issue Background The Ontario
OHTAC Recommendation: Internet- Based Device-Assisted Remote Monitoring of Cardiovascular Implantable Electronic Devices Ontario Health Technology Advisory Committee January 2012 Issue Background The Ontario
Draft Falls Prevention Strategy
 Cheshire West & Chester Council Draft Falls Prevention Strategy 2017-2020 Visit: cheshirewestandchester.gov.uk Visit: cheshirewestandchester.gov.uk 02 Cheshire West and Chester Council Draft Falls Prevention
Cheshire West & Chester Council Draft Falls Prevention Strategy 2017-2020 Visit: cheshirewestandchester.gov.uk Visit: cheshirewestandchester.gov.uk 02 Cheshire West and Chester Council Draft Falls Prevention
Catheter Ablation for Supra-ventricular Tachycardia
 Additional Notes Our mission To provide the excellent care we would expect for our families. The Royal Bournemouth Hospital, Castle Lane East, Bournemouth, Dorset, BH7 7DW The Bournemouth Hospital Charity
Additional Notes Our mission To provide the excellent care we would expect for our families. The Royal Bournemouth Hospital, Castle Lane East, Bournemouth, Dorset, BH7 7DW The Bournemouth Hospital Charity
Information for patients, parents and guardians. Your child s doctor has recommended that your child has a procedure called an ablation.
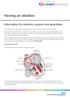 Having an ablation Information for patients, parents and guardians Your child s doctor has recommended that your child has a procedure called an ablation. An ablation is a treatment for an abnormal heartbeat.
Having an ablation Information for patients, parents and guardians Your child s doctor has recommended that your child has a procedure called an ablation. An ablation is a treatment for an abnormal heartbeat.
pat hways Medtech innovation briefing Published: 4 December 2017 nice.org.uk/guidance/mib132
 pat hways Point-of-care and home faecal calprotectin tests for monitoring treatment response in inflammatory bowel disease Medtech innovation briefing Published: 4 December 2017 nice.org.uk/guidance/mib132
pat hways Point-of-care and home faecal calprotectin tests for monitoring treatment response in inflammatory bowel disease Medtech innovation briefing Published: 4 December 2017 nice.org.uk/guidance/mib132
Exercise tolerance testing. Information for patients Sheffield Teaching Hospitals
 Exercise tolerance testing Information for patients Sheffield Teaching Hospitals page 2 of 8 What is an exercise tolerance test? An exercise tolerance test is also known as an exercise ECG or stress test.
Exercise tolerance testing Information for patients Sheffield Teaching Hospitals page 2 of 8 What is an exercise tolerance test? An exercise tolerance test is also known as an exercise ECG or stress test.
Community Cardiology Service - Reinventing Care
 Cardiology Community Cardiology Service - Reinventing Care Dr Dilsher Singh Clinical Cardiology Lead Connected care Partnership Background Evidence of Need Cardiology accounts for high volumes of OP activity
Cardiology Community Cardiology Service - Reinventing Care Dr Dilsher Singh Clinical Cardiology Lead Connected care Partnership Background Evidence of Need Cardiology accounts for high volumes of OP activity
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE SCOPE. Clinical guideline for the management of atrial fibrillation
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Clinical guideline for the management of atrial fibrillation 1.1 Short title Atrial fibrillation 2 Background a) The National Institute
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Clinical guideline for the management of atrial fibrillation 1.1 Short title Atrial fibrillation 2 Background a) The National Institute
Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry
 Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry Policy Number: 2.02.08 Last Review: 9/2018 Origination: 11/2002 Next Review: 9/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry Policy Number: 2.02.08 Last Review: 9/2018 Origination: 11/2002 Next Review: 9/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Transient loss of consciousness
 Issue date: August 2010 Transient loss of consciousness Transient loss of consciousness ( blackouts ) management in adults and young people Developed by the National Clinical Guideline Centre Transient
Issue date: August 2010 Transient loss of consciousness Transient loss of consciousness ( blackouts ) management in adults and young people Developed by the National Clinical Guideline Centre Transient
NICE Action Plan 6/13 Transient loss of consciousness ('blackouts') management in adults and young people NICE CG 109 December 2013
 NICE Action Plan 6/13 Transient loss of consciousness ('blackouts') management in adults and young people NICE CG 109 December 2013 Title: Prepared by: Presented by: Main aim: Recommendations: Previous
NICE Action Plan 6/13 Transient loss of consciousness ('blackouts') management in adults and young people NICE CG 109 December 2013 Title: Prepared by: Presented by: Main aim: Recommendations: Previous
POWERFULLY SIMPLE CARDIAC MONITORING
 POWERFULLY SIMPLE CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry (MCT) System From the leaders who brought you Reveal LINQ ICM MEDTRONIC CARDIAC DIAGNOSTIC & MONITORING SYSTEMS TRANSFORM Transform your
POWERFULLY SIMPLE CARDIAC MONITORING SEEQ Mobile Cardiac Telemetry (MCT) System From the leaders who brought you Reveal LINQ ICM MEDTRONIC CARDIAC DIAGNOSTIC & MONITORING SYSTEMS TRANSFORM Transform your
ATRIAL FIBRILLATION ANSWERS. A Patient Education Handbook on Electrophysiology
 ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
ATRIAL FIBRILLATION ANSWERS A Patient Education Handbook on Electrophysiology MY LIFE HAS TAKEN A TURN FOR THE BETTER. -Emie Bishop ARRHYTHMIAANSWERS.COM AF ANSWERS. A PATIENT EDUCATION HANDBOOK ON ELECTROPHYSIOLOGY
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure (review
Cardiology Services Bon Secours Hospital. Mary Buckley Staff Nurse Cardiology
 Cardiology Services Bon Secours Hospital Mary Buckley Staff Nurse Cardiology Overview Philosophy Cardiology Team Referral Criteria Electrocardiograph (ECG) 24/48 Hour Holter Monitor Event Monitors 24 Hour
Cardiology Services Bon Secours Hospital Mary Buckley Staff Nurse Cardiology Overview Philosophy Cardiology Team Referral Criteria Electrocardiograph (ECG) 24/48 Hour Holter Monitor Event Monitors 24 Hour
ICD Implantation Patient Information
 Melbourne Heart Rhythm ICD Implantation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers (the right atrium
Melbourne Heart Rhythm ICD Implantation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers (the right atrium
Putting NICE guidance into practice. Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80)
 Putting NICE guidance into practice Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80) Published: November 2017 Summary This report focuses on the recommendations
Putting NICE guidance into practice Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80) Published: November 2017 Summary This report focuses on the recommendations
FRAILTY PATIENT FOCUS GROUP
 FRAILTY PATIENT FOCUS GROUP Community House, Bromley 28 November 2016-10am to 12noon In attendance: 7 Patient and Healthwatch representatives: 4 CCG representatives: Dr Ruchira Paranjape went through the
FRAILTY PATIENT FOCUS GROUP Community House, Bromley 28 November 2016-10am to 12noon In attendance: 7 Patient and Healthwatch representatives: 4 CCG representatives: Dr Ruchira Paranjape went through the
Specialised Services Commissioning Policy: CP34 Circumcision for children
 Specialised Services Commissioning Policy: CP34 Circumcision for children March 2019 Version 3.0 Document information Document purpose Document name Author Policy Circumcision for Children Welsh Health
Specialised Services Commissioning Policy: CP34 Circumcision for children March 2019 Version 3.0 Document information Document purpose Document name Author Policy Circumcision for Children Welsh Health
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES
 UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
UNDERSTANDING ELECTROPHYSIOLOGY STUDIES Testing and Treating Your Heart s Electrical System A Problem with Your Heart Rhythm The speed and pattern of a heartbeat is called the heart rhythm. The rhythm
abcdefghijklmnopqrstu
 Chief Medical Officer Directorate Chief Medical Officer and Secretariat Division abcdefghijklmnopqrstu T: 0131-244 2399 F: 0131-244 2989 E: sandra.falconer@scotland.gsi.gov.uk NHS Board Medical and Nursing
Chief Medical Officer Directorate Chief Medical Officer and Secretariat Division abcdefghijklmnopqrstu T: 0131-244 2399 F: 0131-244 2989 E: sandra.falconer@scotland.gsi.gov.uk NHS Board Medical and Nursing
This leaflet can be made available in other formats including large print, CD and Braille and in languages other than English, upon request.
 Information for patients This leaflet can be made available in other formats including large print, CD and Braille and in languages other than English, upon request. This leaflet tells you about acupuncture
Information for patients This leaflet can be made available in other formats including large print, CD and Braille and in languages other than English, upon request. This leaflet tells you about acupuncture
That the Single Commissioning Board supports the project outlined in this report and proceeds as described.
 Report to: SINGLE COMMISSIONING BOARD Date: 26 September 2017 Officer of Single Commissioning Board Subject: Report Summary: Recommendations: Jessica Williams Interim Director of Commissioning ATRIAL FIBRILLATION
Report to: SINGLE COMMISSIONING BOARD Date: 26 September 2017 Officer of Single Commissioning Board Subject: Report Summary: Recommendations: Jessica Williams Interim Director of Commissioning ATRIAL FIBRILLATION
The Blackouts Checklist i
 The Blackouts Checklist i The Blackouts Checklist key aim is to help you and your doctor reach the correct diagnosis for any unexplained loss of consciousness (blackout). The Checklist gives you information
The Blackouts Checklist i The Blackouts Checklist key aim is to help you and your doctor reach the correct diagnosis for any unexplained loss of consciousness (blackout). The Checklist gives you information
Joint Mental Health Commissioning Strategy for Adults
 Joint Mental Health Commissioning Strategy for Adults 2014-2019 Summary Developed in partnership with: NHS Ipswich and East Suffolk CCG, NHS West Suffolk CCG, Suffolk Constabulary and Suffolk County Council
Joint Mental Health Commissioning Strategy for Adults 2014-2019 Summary Developed in partnership with: NHS Ipswich and East Suffolk CCG, NHS West Suffolk CCG, Suffolk Constabulary and Suffolk County Council
British Society for Dermatological Surgery (BSDS) & British Heart Rhythm Society (BHRS) Guidance on Implanted Devices & Dermatological Surgery
 British Society for Dermatological Surgery (BSDS) & British Heart Rhythm Society (BHRS) Guidance on Implanted Devices & Dermatological Surgery Contents Page 1. Introduction 2. Summary & PPM 3. ICD & ILR
British Society for Dermatological Surgery (BSDS) & British Heart Rhythm Society (BHRS) Guidance on Implanted Devices & Dermatological Surgery Contents Page 1. Introduction 2. Summary & PPM 3. ICD & ILR
Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181)
 Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Direct Current Cardioversion (DCCV)
 PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is a This is a procedure carried out under a brief anaesthetic, which uses a
PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is a This is a procedure carried out under a brief anaesthetic, which uses a
Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory electrocardiographic monitoring
 Solomon et al. BMC Cardiovascular Disorders (2016) 16:35 DOI 10.1186/s12872-016-0210-x RESEARCH ARTICLE Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory
Solomon et al. BMC Cardiovascular Disorders (2016) 16:35 DOI 10.1186/s12872-016-0210-x RESEARCH ARTICLE Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory
The Fainting Checklist
 Take Fainting to Heart There is no such thing as a simple faint The Fainting Checklist BMA Patient Information Awards www.stars-international.org Registered Charity No. 1084898 Registered Non-Profit 501(c)(3)
Take Fainting to Heart There is no such thing as a simple faint The Fainting Checklist BMA Patient Information Awards www.stars-international.org Registered Charity No. 1084898 Registered Non-Profit 501(c)(3)
Kadlec Regional Medical Center Cardiac Electrophysiology
 Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
Definition of electrophysiology study and ablation Kadlec Regional Medical Center Cardiac Electrophysiology Electrophysiology Study and Ablation An electrophysiology, or EP, study is a test of the heart
Clinical Commissioning Policy Proposition: Robotic assisted lung resection for primary lung cancer
 Clinical Commissioning Policy Proposition: Robotic assisted lung resection for primary lung cancer Reference: NHS England B10X03/01 Information Reader Box (IRB) to be inserted on inside front cover for
Clinical Commissioning Policy Proposition: Robotic assisted lung resection for primary lung cancer Reference: NHS England B10X03/01 Information Reader Box (IRB) to be inserted on inside front cover for
12.1 Apply Your Knowledge How long does an ambulatory monitor typically remain on a patient?
 1 2 3 4 5 6 7 Learning Outcomes (Cont d) 12.4 Educate the patient about ambulatory monitoring. 12.5 Prepare a patient for application of an ambulatory monitor. 12.6 Describe the procedure for applying
1 2 3 4 5 6 7 Learning Outcomes (Cont d) 12.4 Educate the patient about ambulatory monitoring. 12.5 Prepare a patient for application of an ambulatory monitor. 12.6 Describe the procedure for applying
Peri-operative management of pacemakers and implantable cardiac defibrillators
 Guideline Title: Peri-operative management of patients fitted with Permanent Pacemakers (PPMs) and Implantable Cardioverter Defibrillators (ICDs). Author(s): Jessica Osman (Chief Pacing Physiologist),
Guideline Title: Peri-operative management of patients fitted with Permanent Pacemakers (PPMs) and Implantable Cardioverter Defibrillators (ICDs). Author(s): Jessica Osman (Chief Pacing Physiologist),
Electrocardiography for Healthcare Professionals
 Electrocardiography for Healthcare Professionals Kathryn A. Booth Thomas O Brien Chapter 12: Ambulatory Monitoring 1 Learning Outcomes 12.1 Identify the types of ambulatory monitors and their functions.
Electrocardiography for Healthcare Professionals Kathryn A. Booth Thomas O Brien Chapter 12: Ambulatory Monitoring 1 Learning Outcomes 12.1 Identify the types of ambulatory monitors and their functions.
What to expect when having a pacemaker implantation
 What to expect when having a pacemaker implantation Cardiology Department Patient information leaflet What is a pacemaker? A pacemaker is a small metal device which contains an electronic circuit and a
What to expect when having a pacemaker implantation Cardiology Department Patient information leaflet What is a pacemaker? A pacemaker is a small metal device which contains an electronic circuit and a
Treating atrial fibrillation using heat energy delivered to the outside of the heart through a thin tube
 Issue date March 2009 Understanding NICE guidance Information for people who use NHS services NICE interventional procedures guidance advises the NHS on when and how new procedures can be used in clinical
Issue date March 2009 Understanding NICE guidance Information for people who use NHS services NICE interventional procedures guidance advises the NHS on when and how new procedures can be used in clinical
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy
 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Electroconvulsive Therapy (ECT) Patient Information Leaflet
 Further information about the content, reference sources or production of this leaflet can be obtained from the Patient Information Centre. This information can be made available in a range of formats
Further information about the content, reference sources or production of this leaflet can be obtained from the Patient Information Centre. This information can be made available in a range of formats
Service Specification & Contract Intermediate Stop Smoking Service & Voucher fulfilment - Pharmacy Newcastle
 Service Specification & Contract Intermediate Stop Smoking Service & Voucher fulfilment - Pharmacy Newcastle Contents 1. Agreement period 2. Scope 3. Targets 4. Service outline 5. Support for clients using
Service Specification & Contract Intermediate Stop Smoking Service & Voucher fulfilment - Pharmacy Newcastle Contents 1. Agreement period 2. Scope 3. Targets 4. Service outline 5. Support for clients using
