MedDRA Coding Quality: How to Avoid Common Pitfalls
|
|
|
- Donald Tucker
- 6 years ago
- Views:
Transcription
1 MedDRA Coding Quality: How to Avoid Common Pitfalls Patricia Mozzicato, MD Chief Medical Officer MedDRA MSSO Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not be attributed to Drug Information Association, Inc. ( DIA ), its directors, officers, employees, volunteers, members, chapters, councils, Special Interest Area Communities or affiliates, or any organization with which the presenter is employed or affiliated. These PowerPoint slides are the intellectual property of the individual presenter and are protected under the copyright laws of the United States of America and other countries. Used by permission. All rights reserved. Drug Information Association, Drug Information Association Inc., DIA and DIA logo are registered trademarks. All other trademarks are the property of their respective owners. 2 1
2 Data Quality in Clinical Development Highly regulated environment with strong emphasis on safety surveillance and data quality Applies to clinical trials and post-marketing arena Increasing harmonization of safety reporting regulations globally 3 What is Meant by Good Quality Data? Complete Accurate Diagnosis supported by appropriate investigations Causality assessment for adverse events 4 2
3 Coding of Clinical Trial Data Most data entered on Case Report Forms are coded in some form Facilitates storage, retrieval, analysis, and presentation of data Some coding is performed by investigators at point of data entry For example, numeric codes for severity of adverse event: 1= mild, 2= moderate, etc. Other coding of text data is performed by sponsor company after data collection Accuracy of initial coding determines accuracy of analysis 5 Quality of Input = Quality of Output IN OUT 6 3
4 MedDRA Definition MedDRA is a clinically-validated international medical terminology used by regulatory authorities and the regulated biopharmaceutical industry. The terminology is used through the entire regulatory process, from pre-marketing to postmarketing, and for data entry, retrieval, evaluation, and presentation. 7 Key Features of MedDRA Structure facilitates data analysis and reporting and electronic communication Large terminology with >69,000 terms at lowest level allows greater specificity Approx. 19,000 Preferred Terms (PTs), each representing a unique medical concept Used for coding adverse events, signs and symptoms, procedures, investigations, indications, medical and social histories, medication errors and product quality issues 8 4
5 MedDRA Structure SOC = Cardiac disorders HLGT = Cardiac arrhythmias HLT = Rate and rhythm disorders NEC LLT Arrhythmia NOS LLT Arrhythmia PT = Arrhythmia LLT (Non-current) Other specified cardiac dysrhythmias LLT Dysrhythmias 9 MedDRA Structure (cont) 10 5
6 Problems With Coding Data Appropriate coding requires clear initial data What is clear to investigator at point of data entry may be unclear to sponsor at point of data coding Sponsor must only code reported verbatim term; not permitted to interpret or draw information from other sources Example: Ambiguous information Congestion (nasal, liver, sinus, pulmonary?) Cramp (muscle, menstrual, abdominal?) Pain (pain where?) 11 Problems With Coding Data (cont) Example: Ambiguous abbreviations MI (myocardial infarction or mitral incompetence?) GU pain (gastric ulcer pain or genito-urinary pain?) Decreased BS (breath sounds, bowel sounds or blood sugar?) Exercise caution with abbreviations that could be misinterpreted ECG, COPD, HIV are examples of standard abbreviations 12 6
7 Problems With Coding Data (cont) Example: Vague information Patient felt fuzzy, weird, experienced every adverse event Try to use accepted medical terminology Example: Non-specific information Left wrist edema (coded as LLT Peripheral edema) More specific - Injection site edema left wrist (coded as LLT Injection site edema) 13 Problems With Coding Data (cont) Death, hospitalization, and disability are outcomes and are not usually considered to be adverse events Provide details of underlying event, if known Examples: Death due to myocardial infarction (Coded as LLT Myocardial infarction with death captured as outcome) Hospitalization due to congestive heart failure (Coded as Congestive heart failure with hospitalization captured as outcome) 14 7
8 Problems With Coding Data (cont) Example: Ambiguous laboratory data Glucose of 40 (Source of specimen - blood, urine, CSF? What units?) Would have to code as LLT Glucose abnormal if additional clarification is not obtained Example: Conflicting laboratory data Hyperkalemia with serum potassium of 1.6 meq/l Would have to code as LLT Serum potassium abnormal If using numeric values, provide units and reference range. Be specific about specimen source and diagnostic result/clinical diagnosis. 15 Problems With Coding Data (cont) Example: Combination terms Diarrhea, nausea and vomiting Try to avoid combination terms these will have to be split into three individual terms Diarrhea Nausea Vomiting 16 8
9 Reporting a Specific Diagnosis Where possible, report most important medical event or specific diagnosis rather than individual signs and symptoms Can provide provisional diagnosis, e.g., possible, presumed, rule out Accuracy is important in preventing dilution of safety signals or generating false signals SIGNS and SYMPTOMS DIAGNOSIS Chest pain, dyspnea, diaphoresis, ECG changes Myocardial infarction 17 Benefits of Quality Data Accuracy in diagnosis is important for detection and evaluation of safety signals Accurate and timely information on issues that affect conduct of clinical trial and affect patient safety Improved communication among sponsors, investigators, and regulatory agencies about medicinal products Ensures accuracy of information about product including investigators brochures and prescribing information Benefits medical professionals and patients 18 9
10 How to Achieve Quality in MedDRA Coding Quality assurance steps Coding conventions Synonym lists 19 Quality Assurance Steps Coding QA reports Human oversight of automated coding results E.g., Adrenal insufficiency secondary to chronic traditional medication intake autoencoded as LLT Secondary adrenal insufficiency Secondary adrenal insufficiency is based on lack of ACTH or CRH Better term is LLT Adrenal insufficiency 20 10
11 Quality Assurance Steps (cont) Qualification of coder/review staff Errors in MedDRA should be addressed by submission of Change Requests to MSSO; no ad hoc structural alterations to MedDRA 21 MedDRA Coding Conventions Differences in medical aptitude of coders Consistency concerns (many more choices to manually code terms in MedDRA compared to older terminologies) Even with an autoencoder, may still need manual coding Should be consistent with ICH s MedDRA Term Selection: Points to Consider document 22 11
12 Synonym Lists Can be derived from existing term lists or directly from verbatims For recurring, but unusual, verbatims onetime assignment to a MedDRA term Enforces consistency by limiting choices once MedDRA term is assigned Increases likelihood of autoencoding hit Natural outgrowth of a legacy data conversion Maintenance required 23 In Summary Quality of initial data and quality of coding ultimately affect quality of analysis Be aware of potential pitfalls in coding with MedDRA There are ways to address quality issues such as coding conventions, etc
13 Quality Data IN OUT 25 Thank You 13
Seeing Chickens at Window Recording Adverse Events and GeneratingQuality Data. Margaret Band, Clinical Trial Manager, TCTU
 Seeing Chickens at Window Recording Adverse Events and GeneratingQuality Data Margaret Band, Clinical Trial Manager, TCTU Adverse Event Reporting Monitoring of adverse events (AEs) is critical to the patient
Seeing Chickens at Window Recording Adverse Events and GeneratingQuality Data Margaret Band, Clinical Trial Manager, TCTU Adverse Event Reporting Monitoring of adverse events (AEs) is critical to the patient
Preview of New MedDRA Hierarchy for Medication Errors Coding and Retrieval Considerations
 Preview of New MedDRA Hierarchy for Medication Errors Coding and Retrieval Considerations Judy Harrison, M.D. Chief Medical Officer MedDRA MSSO Information Day on Medication Errors 20 October 2016 London,
Preview of New MedDRA Hierarchy for Medication Errors Coding and Retrieval Considerations Judy Harrison, M.D. Chief Medical Officer MedDRA MSSO Information Day on Medication Errors 20 October 2016 London,
Data retrieval using the new SMQ Medication Errors
 Data retrieval using the new SMQ Medication Errors Christina Winter Medical Director Safety Evaluation and Risk Management, GSK Information Day on Medication Errors 20 October 2016 London, UK Disclaimer
Data retrieval using the new SMQ Medication Errors Christina Winter Medical Director Safety Evaluation and Risk Management, GSK Information Day on Medication Errors 20 October 2016 London, UK Disclaimer
MedDRA Overview A Standardized Terminology
 MedDRA Overview A Standardized Terminology Patrick Revelle Director, MedDRA MSSO 6 May 2010 MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations
MedDRA Overview A Standardized Terminology Patrick Revelle Director, MedDRA MSSO 6 May 2010 MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations
MedDRA Basic Concept
 MedDRA Basic Concept Pansie Zhang MSD R&D (China) Co., Ltd 23Apr2015 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not
MedDRA Basic Concept Pansie Zhang MSD R&D (China) Co., Ltd 23Apr2015 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not
MedDRA Coding and Versioning
 MedDRA Coding and Versioning 4th ANNUAL INNOVATIONS IN CLINICAL DATA MANAGEMENT Alexandria, VA. 27-28 October 2016 Anna Zhao-Wong, MD, PhD Deputy Director of MedDRA MSSO anna.zhao-wong@meddra.org Agenda
MedDRA Coding and Versioning 4th ANNUAL INNOVATIONS IN CLINICAL DATA MANAGEMENT Alexandria, VA. 27-28 October 2016 Anna Zhao-Wong, MD, PhD Deputy Director of MedDRA MSSO anna.zhao-wong@meddra.org Agenda
Coding with MedDRA 4/22/2015
 Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The activities
Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The activities
Coding with MedDRA 3/6/2019
 Coding with MedDRA MedDRA was developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The activities of the MedDRA
Coding with MedDRA MedDRA was developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The activities of the MedDRA
Other EU Activities Contributing to Harmonization of Labeling
 Other EU Activities Contributing to Harmonization of Labeling Dr Laurent Brassart European Medicines Agency Medical Information Sector DIA Labeling Harmonisation 2011 Workshop October 13-14. 2011 Disclaimer
Other EU Activities Contributing to Harmonization of Labeling Dr Laurent Brassart European Medicines Agency Medical Information Sector DIA Labeling Harmonisation 2011 Workshop October 13-14. 2011 Disclaimer
MedDRA - International standard for coding safety information. The Golden Triangle
 MedDRA - International standard for coding safety information International Pharmacovigilance Conference Moscow, Russia 10 October 2018 Anna Zhao-Wong, MD, PhD MedDRA MSSO The Golden Triangle (MedDRA)
MedDRA - International standard for coding safety information International Pharmacovigilance Conference Moscow, Russia 10 October 2018 Anna Zhao-Wong, MD, PhD MedDRA MSSO The Golden Triangle (MedDRA)
CTCAE & Source Documentation. Elizabeth Ness, RN, MS Director, Office of Education and Compliance Center for Cancer Research
 CTCAE & Source Documentation Elizabeth Ness, RN, MS Director, Office of Education and Compliance Center for Cancer Research Agenda 1.Definition of AE 2.AE standards 3.AE documentation 4.AE and data quality
CTCAE & Source Documentation Elizabeth Ness, RN, MS Director, Office of Education and Compliance Center for Cancer Research Agenda 1.Definition of AE 2.AE standards 3.AE documentation 4.AE and data quality
Advanced MedDRA Coding
 Advanced MedDRA Coding MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The activities
Advanced MedDRA Coding MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The activities
GDUFA: 2 ½ years later Impact & Importance
 GDUFA: 2 ½ years later Impact & Importance Georgia Hizon Manager, Regulatory Publications Fresenius Kabi USA, LLC Disclaimer The views and opinions expressed in the following PowerPoint slides are those
GDUFA: 2 ½ years later Impact & Importance Georgia Hizon Manager, Regulatory Publications Fresenius Kabi USA, LLC Disclaimer The views and opinions expressed in the following PowerPoint slides are those
Regulatory Experience in Reviewing CV Safety for Diabetes
 Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
Coding with MedDRA. MedDRA trademark is owned by IFPMA on behalf of ICH
 Coding with MedDRA MedDRA trademark is owned by IFPMA on behalf of ICH MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration
Coding with MedDRA MedDRA trademark is owned by IFPMA on behalf of ICH MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration
Coding with MedDRA. of the MedDRA Maintenance and Support Services Organization (MSSO) are overseen by an ICH MedDRA
 Coding with MedDRA MedDRA trademark is owned by IFPMA on behalf of ICH MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration
Coding with MedDRA MedDRA trademark is owned by IFPMA on behalf of ICH MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration
Coding with MedDRA 3/2/2017
 Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The activities
Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The activities
Overview of the MedDRA Data Retrieval and Presentation: Points to Consider Document including Standardised MedDRA Queries (SMQs)
 Overview of the MedDRA Data Retrieval and Presentation: Points to Consider Document including Standardised MedDRA Queries (SMQs) Judy Harrison Chief Medical Officer MedDRA MSSO 22 October 2013 MedDRA Data
Overview of the MedDRA Data Retrieval and Presentation: Points to Consider Document including Standardised MedDRA Queries (SMQs) Judy Harrison Chief Medical Officer MedDRA MSSO 22 October 2013 MedDRA Data
MedDRA TERM SELECTION: POINTS TO CONSIDER
 MedDRA TERM SELECTION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users Release 4.13 Based on MedDRA Version 20.0 1 March 2017 Disclaimer and Copyright Notice This document is protected by copyright
MedDRA TERM SELECTION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users Release 4.13 Based on MedDRA Version 20.0 1 March 2017 Disclaimer and Copyright Notice This document is protected by copyright
MedDRA TERM SELECTION: POINTS TO CONSIDER
 MedDRA TERM SELECTION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users Release 4.4 Based on MedDRA Version 15.1 1 October 2012 Disclaimer and Copyright Notice This document is protected by copyright
MedDRA TERM SELECTION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users Release 4.4 Based on MedDRA Version 15.1 1 October 2012 Disclaimer and Copyright Notice This document is protected by copyright
MedDRA TERM SELECTION: POINTS TO CONSIDER
 MedDRA TERM SELECTION: POINTS TO CONSIDER Release 3.10 Based on MedDRA Version 11.0 ICH-Endorsed Guide for MedDRA Users Application to Adverse Drug Reactions /Adverse Events & Medical and Social History
MedDRA TERM SELECTION: POINTS TO CONSIDER Release 3.10 Based on MedDRA Version 11.0 ICH-Endorsed Guide for MedDRA Users Application to Adverse Drug Reactions /Adverse Events & Medical and Social History
Disclaimer. The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not be
 Ending the Myths: Best Practice in Trial Conduct in Latin America Katie Margules Global Vice President Alliance Management Covance Disclaimer The views and opinions expressed in the following PowerPoint
Ending the Myths: Best Practice in Trial Conduct in Latin America Katie Margules Global Vice President Alliance Management Covance Disclaimer The views and opinions expressed in the following PowerPoint
Global Pediatric Development: We Are Making Progress. PMDA Perspective. Junko Sato, PhD PMDA
 Global Pediatric Development: We Are Making Progress PMDA Perspective Junko Sato, PhD PMDA Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
Global Pediatric Development: We Are Making Progress PMDA Perspective Junko Sato, PhD PMDA Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
The Telemetric and Holter ECG Warehouse (THEW) Initiative
 The Telemetric and Holter ECG Warehouse (THEW) Initiative Jean-Philippe Couderc, PhD THEW HRFUP- Cardiology University of Rochester Medical Center, NY Mission Statement The objective of the Telemetric
The Telemetric and Holter ECG Warehouse (THEW) Initiative Jean-Philippe Couderc, PhD THEW HRFUP- Cardiology University of Rochester Medical Center, NY Mission Statement The objective of the Telemetric
MedDRA Term Selection: Latest activities of the Points to Consider Working Group
 MedDRA Term Selection: Latest activities of the Points to Consider Working Group Patrick Revelle MedDRA MSSO 23 May 2013 Overview The new EU pharmacovigilance legislation broadened the definition of an
MedDRA Term Selection: Latest activities of the Points to Consider Working Group Patrick Revelle MedDRA MSSO 23 May 2013 Overview The new EU pharmacovigilance legislation broadened the definition of an
Disclaimer. Statistical Aspects of Revision of CHMP Bioequivalence Guidelines. David Brown MHRA
 Statistical Aspects of Revision of CHMP Bioequivalence Guidelines David Brown MHRA 1 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
Statistical Aspects of Revision of CHMP Bioequivalence Guidelines David Brown MHRA 1 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter
Summary of Changes to. MedDRA TERM SELECTION: POINTS TO CONSIDER
 Summary of Changes to MedDRA TERM SELECTION: POINTS TO CONSIDER Release 3.8 Based on MedDRA Version 10.0 ICH-Endorsed Guide for MedDRA Users Application to Adverse Drug Reactions /Adverse Events & Medical
Summary of Changes to MedDRA TERM SELECTION: POINTS TO CONSIDER Release 3.8 Based on MedDRA Version 10.0 ICH-Endorsed Guide for MedDRA Users Application to Adverse Drug Reactions /Adverse Events & Medical
9/20/2011. Impact of EU Paediatric Regulation on drug development and Marketing authorisations Industry experience
 Impact of EU Paediatric Regulation on drug development and Marketing authorisations Industry experience Judith Creba Head EU Liaison and Policy, DRA Novartis Pharma AG, Switzerland Disclaimer The views
Impact of EU Paediatric Regulation on drug development and Marketing authorisations Industry experience Judith Creba Head EU Liaison and Policy, DRA Novartis Pharma AG, Switzerland Disclaimer The views
MedDRA TERM SELECTION: POINTS TO CONSIDER
 MedDRA TERM SELECTION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users Release 4.8 Based on MedDRA Version 17.1 1 September 2014 Disclaimer and Copyright Notice This document is protected by copyright
MedDRA TERM SELECTION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users Release 4.8 Based on MedDRA Version 17.1 1 September 2014 Disclaimer and Copyright Notice This document is protected by copyright
MedDRA Coding/ AE Log Item 1 Refresher. ASPIRE Protocol Team Meeting February 10, 2013
 MedDRA Coding/ AE Log Item 1 Refresher ASPIRE Protocol Team Meeting February 10, 2013 MedDRA Coding Overview MedDRA: standardized dictionary of medical terminology Results from ICH initiative to standardize
MedDRA Coding/ AE Log Item 1 Refresher ASPIRE Protocol Team Meeting February 10, 2013 MedDRA Coding Overview MedDRA: standardized dictionary of medical terminology Results from ICH initiative to standardize
Introduction to MedDRA
 Introduction to MedDRA MedDRA trademark is owned by IFPMA on behalf of ICH MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration
Introduction to MedDRA MedDRA trademark is owned by IFPMA on behalf of ICH MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration
Web-based epro: Validation, Equivalence, and Data Quality. Brian Tiplady
 Web-based epro: Validation, Equivalence, and Data Quality Brian Tiplady Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not
Web-based epro: Validation, Equivalence, and Data Quality Brian Tiplady Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not
Safety Data Analysis and Query Creation with MedDRA
 Safety Data Analysis and Query Creation with MedDRA Anne Kehely & Eva Rump Eli Lilly/MSSO 24th Annual EuroMeeting 26-28 March 2012 Copenhagen, Denmark Insert your logo in this area then delete this text
Safety Data Analysis and Query Creation with MedDRA Anne Kehely & Eva Rump Eli Lilly/MSSO 24th Annual EuroMeeting 26-28 March 2012 Copenhagen, Denmark Insert your logo in this area then delete this text
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER
 MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users on Data Output Release 3.6 Based on MedDRA Version 16.1 1 October 2013 Disclaimer and Copyright Notice This
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users on Data Output Release 3.6 Based on MedDRA Version 16.1 1 October 2013 Disclaimer and Copyright Notice This
3/31/2009. Phase 0 Microdosing Studies with Renin Inhibitors. Disclaimer. Overview of Today s Presentation. J. Chris Jensen Consultant
 Phase 0 Microdosing Studies with Renin Inhibitors J. Chris Jensen Consultant Speedel Holding Ltd. Basel, Switzerland Disclaimer The views and opinions expressed in the following PowerPoint slides are those
Phase 0 Microdosing Studies with Renin Inhibitors J. Chris Jensen Consultant Speedel Holding Ltd. Basel, Switzerland Disclaimer The views and opinions expressed in the following PowerPoint slides are those
Healthcare Professional Information & Patient Information Leaflet (PL)
 New concept for the SmPC/ Healthcare Professional Information & Patient Information Leaflet (PL) Fiona Reekie Director Global Regulatory Affairs Johnson & Johnson Pharmaceuticals Group Disclaimer The views
New concept for the SmPC/ Healthcare Professional Information & Patient Information Leaflet (PL) Fiona Reekie Director Global Regulatory Affairs Johnson & Johnson Pharmaceuticals Group Disclaimer The views
Instruct patient and caregivers: Need for constant monitoring Potential complications of drug therapy
 Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
Assessment Prior to administration: Assess patient for chest pain, dysrhythmias, and vital signs (initially and throughout therapy) Obtain complete medical history, including allergies, especially heart
PMDA Perspectives on Oncology Panel. REIKO YANAGIHARA, Ph.D. Office of In Vitro Diagnostics PMDA
 15th DIA Japan Annual Meeting 2018 Promoting Better Collaboration to Drive Global Health and Innovation in an Era of Medical and Scientific Transformation November 11-13, 2018 Tokyo Big Sight PMDA Perspectives
15th DIA Japan Annual Meeting 2018 Promoting Better Collaboration to Drive Global Health and Innovation in an Era of Medical and Scientific Transformation November 11-13, 2018 Tokyo Big Sight PMDA Perspectives
Coding with MedDRA 1
 Coding with Coding with MedDRA 1 4/3/2015 Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals
Coding with Coding with MedDRA 1 4/3/2015 Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals
So, My FitBit is Clinical Trial Grade Right? Keith Wenzel Senior Director, Solution Incubator PAREXEL International
 So, My FitBit is Clinical Trial Grade Right? Keith Wenzel Senior Director, Solution Incubator PAREXEL International 1 Disclaimer The views and opinions expressed in the following PowerPoint slides are
So, My FitBit is Clinical Trial Grade Right? Keith Wenzel Senior Director, Solution Incubator PAREXEL International 1 Disclaimer The views and opinions expressed in the following PowerPoint slides are
Coding with Confidence
 Coding with Confidence Hilary Vass (Global Clinical Dictionary Manager AstraZeneca UK Limited) Tomás Moraleda (Medical Officer MSSO) 25th Annual EuroMeeting 4-6 March 2013 RAI, Amsterdam Netherlands Disclaimer
Coding with Confidence Hilary Vass (Global Clinical Dictionary Manager AstraZeneca UK Limited) Tomás Moraleda (Medical Officer MSSO) 25th Annual EuroMeeting 4-6 March 2013 RAI, Amsterdam Netherlands Disclaimer
Disclaimer. Dialogue with a Patient 3/18/2016
 Medical Affairs and Scientific Communications 2016 Annual Forum Dialogue with a Patient Bill Wilkins, Co-Founder, Executive Director, Wilkins Parkinsons Foundation March 21-23 Kissimmee, FL Disclaimer
Medical Affairs and Scientific Communications 2016 Annual Forum Dialogue with a Patient Bill Wilkins, Co-Founder, Executive Director, Wilkins Parkinsons Foundation March 21-23 Kissimmee, FL Disclaimer
Data Analysis and Query Building with MedDRA
 Data Analysis and Query Building with MedDRA MedDRA was developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The
Data Analysis and Query Building with MedDRA MedDRA was developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER
 MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER Release draft Based on MedDRA version 7.1 ICH-Endorsed Guide for MedDRA Users on Data Output 18 November 2004 Copyright ICH Secretariat (c/o IFPMA)
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER Release draft Based on MedDRA version 7.1 ICH-Endorsed Guide for MedDRA Users on Data Output 18 November 2004 Copyright ICH Secretariat (c/o IFPMA)
Coding with MedDRA 1
 Coding with Coding with MedDRA 1 3/9/2017 Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals
Coding with Coding with MedDRA 1 3/9/2017 Coding with MedDRA MedDRA was developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals
What s New MedDRA Version March
 c What s New MedDRA Version 20.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA trademark is owned by IFPMA on behalf of ICH. Disclaimer and Copyright Notice This document is protected by copyright and may be
c What s New MedDRA Version 20.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA trademark is owned by IFPMA on behalf of ICH. Disclaimer and Copyright Notice This document is protected by copyright and may be
What s New MedDRA Version March
 C What s New MedDRA Version 21.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA trademark is registered by IFPMA on behalf of ICH. Disclaimer and Copyright Notice This document is protected by copyright and
C What s New MedDRA Version 21.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA trademark is registered by IFPMA on behalf of ICH. Disclaimer and Copyright Notice This document is protected by copyright and
Cardiology. Objectives. Chapter
 1:44 M age 1121 Chapter Cardiology Objectives art 1: Cardiovascular natomy and hysiology, ECG Monitoring, and Dysrhythmia nalysis (begins on p. 1127) fter reading art 1 of this chapter, you should be able
1:44 M age 1121 Chapter Cardiology Objectives art 1: Cardiovascular natomy and hysiology, ECG Monitoring, and Dysrhythmia nalysis (begins on p. 1127) fter reading art 1 of this chapter, you should be able
Safety Assessment in Clinical Trials and Beyond
 Safety Assessment in Clinical Trials and Beyond Yuliya Yasinskaya, MD Medical Team Leader Division of Anti-Infective Products Center for Drug Evaluation and Research FDA Clinical Investigator Training
Safety Assessment in Clinical Trials and Beyond Yuliya Yasinskaya, MD Medical Team Leader Division of Anti-Infective Products Center for Drug Evaluation and Research FDA Clinical Investigator Training
What Medical Writers Need to Know About MedDRA
 What Medical Writers Need to Know About MedDRA Expert Seminar Series Session 2 40 th EMWA Conference, Dublin 6 May 2015 Judy Harrison, M.D. Chief Medical Officer, MedDRA MSSO MedDRA was developed under
What Medical Writers Need to Know About MedDRA Expert Seminar Series Session 2 40 th EMWA Conference, Dublin 6 May 2015 Judy Harrison, M.D. Chief Medical Officer, MedDRA MSSO MedDRA was developed under
Food can serve as a non pharmacological control in thorough cardiac safety studies
 Food can serve as a non pharmacological control in thorough cardiac safety studies Jorg Taubel MD FFPM Washington 14 th April 2011 Disclaimer The views and opinions expressed in the following PowerPoint
Food can serve as a non pharmacological control in thorough cardiac safety studies Jorg Taubel MD FFPM Washington 14 th April 2011 Disclaimer The views and opinions expressed in the following PowerPoint
Regulatory scientific significance of Japan s ADR relief system
 Regulatory scientific significance of Japan s ADR relief system Toshi TOMINGA, Ph.D. Associate Executive Director Pharmaceuticals and Medical Devices Agency (PMDA) 1 Disclaimer The views and opinions expressed
Regulatory scientific significance of Japan s ADR relief system Toshi TOMINGA, Ph.D. Associate Executive Director Pharmaceuticals and Medical Devices Agency (PMDA) 1 Disclaimer The views and opinions expressed
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER
 MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users on Data Output Release 3.14 Based on MedDRA Version 20.1 1 September 2017 Disclaimer and Copyright Notice
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER ICH-Endorsed Guide for MedDRA Users on Data Output Release 3.14 Based on MedDRA Version 20.1 1 September 2017 Disclaimer and Copyright Notice
Use of MedDRA in Special Situations
 Use of MedDRA in Special Situations Patrick Revelle MedDRA MSSO 13 March 2012 Overview Existing terms in MedDRA for off-label use, abuse, misuse, overdose, and medication errors Current coding recommendations
Use of MedDRA in Special Situations Patrick Revelle MedDRA MSSO 13 March 2012 Overview Existing terms in MedDRA for off-label use, abuse, misuse, overdose, and medication errors Current coding recommendations
Summary of Changes to. MedDRA TERM SELECTION: POINTS TO CONSIDER
 Summary of Changes to MedDRA TERM SELECTION: POINTS TO CONSIDER Release 3.12 Based on MedDRA Version 12.0 ICH-Endorsed Guide for MedDRA Users Application to Adverse Drug Reactions /Adverse Events & Medical
Summary of Changes to MedDRA TERM SELECTION: POINTS TO CONSIDER Release 3.12 Based on MedDRA Version 12.0 ICH-Endorsed Guide for MedDRA Users Application to Adverse Drug Reactions /Adverse Events & Medical
Building Higher Order Thinking Skills in Tomorrow s Health Care Professionals A Quality Enhancement Plan for the GSBS
 Building Higher Order Thinking Skills in Tomorrow s Health Care Professionals A Quality Enhancement Plan for the GSBS Focus of the QEP at UNTHSC: To improve students Higher Order Thinking (HOT) skills
Building Higher Order Thinking Skills in Tomorrow s Health Care Professionals A Quality Enhancement Plan for the GSBS Focus of the QEP at UNTHSC: To improve students Higher Order Thinking (HOT) skills
Data Analysis and Query Building with MedDRA
 Data Analysis and Query Building with MedDRA MedDRA was developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The
Data Analysis and Query Building with MedDRA MedDRA was developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The
What s New MedDRA Version 13.1
 What s New MedDRA Version 13.1 Acknowledgements ACKNOWLEDGEMENTS MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Adobe is a registered
What s New MedDRA Version 13.1 Acknowledgements ACKNOWLEDGEMENTS MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Adobe is a registered
The Adverse Reactions and Interactions
 The Adverse Reactions and Interactions Section of a CCDS Leander Fontaine Barbara Lachm Bethesda, October 2011 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of
The Adverse Reactions and Interactions Section of a CCDS Leander Fontaine Barbara Lachm Bethesda, October 2011 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of
Electrocardiographic Markers Associated with Sotalol-induced Torsades de Pointes
 Electrocardiographic Markers Associated with Sotalol-induced Torsades de Pointes Based on Data from the Telemetric and Holter ECG Warehouse (THEW) Initiative Jean-PhiIippe Couderc, PhD Center for Quantitative
Electrocardiographic Markers Associated with Sotalol-induced Torsades de Pointes Based on Data from the Telemetric and Holter ECG Warehouse (THEW) Initiative Jean-PhiIippe Couderc, PhD Center for Quantitative
10/27/2011. Challenges in Anti-diabetic Global Drug Development A Shift from Surrogate to Outcome Trials. Disclaimer
 Challenges in Anti-diabetic Global Drug Development A Shift from Surrogate to Outcome Trials Eckhard Leifke, MD PhD Senior Medical Director Takeda Global Research Development Center, Inc. Presentation
Challenges in Anti-diabetic Global Drug Development A Shift from Surrogate to Outcome Trials Eckhard Leifke, MD PhD Senior Medical Director Takeda Global Research Development Center, Inc. Presentation
Nursing Process Focus: Patients Receiving Dextran 40 (Gentran 40)
 Assess for presence/history of hypovolemia, shock, venous thrombosis. Assess vital signs: Hypovolemic shock secondary to surgery, burns, hemorrhage, other serious condition PT and PTT abnormalities Venous
Assess for presence/history of hypovolemia, shock, venous thrombosis. Assess vital signs: Hypovolemic shock secondary to surgery, burns, hemorrhage, other serious condition PT and PTT abnormalities Venous
Conducting Observational studies in Latin America: Academia, Ethical Committees & Pharmaceutic Industry
 Conducting Observational studies in Latin America: Academia, Ethical Committees & Pharmaceutic Industry Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual
Conducting Observational studies in Latin America: Academia, Ethical Committees & Pharmaceutic Industry Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual
Centralized Procedure
 Centralized Procedure CHMP/EMA Overview of CHMP Operations Anthony Humphreys Head of Regulatory, Procedural and Committee Support DIA European Regulatory Affairs Forum 01-02 June 2010 London, UK Disclaimer
Centralized Procedure CHMP/EMA Overview of CHMP Operations Anthony Humphreys Head of Regulatory, Procedural and Committee Support DIA European Regulatory Affairs Forum 01-02 June 2010 London, UK Disclaimer
Coding with MedDRA. Course Overview
 Coding with MedDRA MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Course Overview MedDRA background MedDRA s structure, scope,
Coding with MedDRA MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Course Overview MedDRA background MedDRA s structure, scope,
Heart Disorders. Cardiovascular Disorders (Part B-1) Module 5 -Chapter 8. Overview Heart Disorders Vascular Disorders
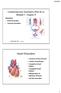 Cardiovascular Disorders (Part B-1) Module 5 -Chapter 8 Overview Heart Disorders Vascular Disorders Susie Turner, MD 1/7/13 Heart Disorders Coronary Artery Disease Cardiac Arrhythmias Congestive Heart
Cardiovascular Disorders (Part B-1) Module 5 -Chapter 8 Overview Heart Disorders Vascular Disorders Susie Turner, MD 1/7/13 Heart Disorders Coronary Artery Disease Cardiac Arrhythmias Congestive Heart
NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults
 NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults Co-ordinators: Medicines Information Pharmacist Consultation Group: See relevant page in guidance Approver: Medicine Guidelines
NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults Co-ordinators: Medicines Information Pharmacist Consultation Group: See relevant page in guidance Approver: Medicine Guidelines
Pharmacy Prep. Qualifying Pharmacy Review
 Pharmacy Prep 2014 Misbah Biabani, Ph.D Director, Tips Review Centres 5460 Yonge St. Suites 209 & 210 Toronto ON M2N 6K7, Canada Luay Petros, R.Ph Pharmacy Manager, Wal-Mart, Canada 1 Disclaimer Your use
Pharmacy Prep 2014 Misbah Biabani, Ph.D Director, Tips Review Centres 5460 Yonge St. Suites 209 & 210 Toronto ON M2N 6K7, Canada Luay Petros, R.Ph Pharmacy Manager, Wal-Mart, Canada 1 Disclaimer Your use
Pre-marketing Assessment of Drug Safety
 Pre-marketing Assessment of Drug Safety Judith A. Racoosin, MD, MPH Safety Team Leader Division of Neurology Products/Division of Psychiatry Products (formerly the Division of Neuropharmacological Drug
Pre-marketing Assessment of Drug Safety Judith A. Racoosin, MD, MPH Safety Team Leader Division of Neurology Products/Division of Psychiatry Products (formerly the Division of Neuropharmacological Drug
MedDRA 13.0 Review. General Remarks. Version 13.0 Changes Overview. Changes in a Nutshell. Changes Highlights. MedDRA Version Changes
 MedDRA 13.0 Review MedDRA Version Changes General Remarks MedDRA Version 13.0 Specific Changes Version 13.0 Changes Overview Changes in a Nutshell Changes Highlights MedDRA Version 13.0 Terminology Frequency
MedDRA 13.0 Review MedDRA Version Changes General Remarks MedDRA Version 13.0 Specific Changes Version 13.0 Changes Overview Changes in a Nutshell Changes Highlights MedDRA Version 13.0 Terminology Frequency
EKG Competency for Agency
 EKG Competency for Agency Name: Date: Agency: 1. The upper chambers of the heart are known as the: a. Atria b. Ventricles c. Mitral Valve d. Aortic Valve 2. The lower chambers of the heart are known as
EKG Competency for Agency Name: Date: Agency: 1. The upper chambers of the heart are known as the: a. Atria b. Ventricles c. Mitral Valve d. Aortic Valve 2. The lower chambers of the heart are known as
Bioequivalence Requirements: USA and EU
 Bioequivalence Requirements: USA and EU Dr. Nicholas Cappuccino Chair, IGPA Science Committee Global Head of Quality, Dr. Reddy s Laboratories Ltd. 15 th Annual IGPA Conference Kyoto, Japan December 6,
Bioequivalence Requirements: USA and EU Dr. Nicholas Cappuccino Chair, IGPA Science Committee Global Head of Quality, Dr. Reddy s Laboratories Ltd. 15 th Annual IGPA Conference Kyoto, Japan December 6,
Terminologies for recording of ADR:s
 Terminologies for recording of ADR:s 1 Adverse reaction terms? Dizziness SOB. feeling hot.? dyspnoea lightheadedness.? breath shortness. evidence of VEBS on 2 ECG Concepts Terminology collection of accepted
Terminologies for recording of ADR:s 1 Adverse reaction terms? Dizziness SOB. feeling hot.? dyspnoea lightheadedness.? breath shortness. evidence of VEBS on 2 ECG Concepts Terminology collection of accepted
SYNOPSIS. Date 15 June 2004
 Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Implemented MedDRA Version 22.0 Complex Changes (October 2018)
 Implemented MedDRA Version 22.0 Complex Changes (October 2018) MedDRA User Proposed Requests: 1. Move HLT Cardiac disorders NEC from HLGT Cardiac disorder signs and symptoms to new HLGT Cardiac disorders
Implemented MedDRA Version 22.0 Complex Changes (October 2018) MedDRA User Proposed Requests: 1. Move HLT Cardiac disorders NEC from HLGT Cardiac disorder signs and symptoms to new HLGT Cardiac disorders
What s New MedDRA Version 14.0
 What s New MedDRA Version 14.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Adobe is a registered
What s New MedDRA Version 14.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA). Adobe is a registered
What s New MedDRA Version MSSO-DI March 2016
 c What s New MedDRA Version 19.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA trademark is owned by IFPMA on behalf of ICH. Disclaimer and Copyright Notice This document is protected by copyright and may be
c What s New MedDRA Version 19.0 Acknowledgements ACKNOWLEDGEMENTS MedDRA trademark is owned by IFPMA on behalf of ICH. Disclaimer and Copyright Notice This document is protected by copyright and may be
CMS Limitations Guide - Cardiovascular Services
 CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Electrocardiography for Healthcare Professionals
 Electrocardiography for Healthcare Professionals Kathryn A. Booth Thomas O Brien Chapter 5: Rhythm Strip Interpretation and Sinus Rhythms Learning Outcomes 5.1 Explain the process of evaluating ECG tracings
Electrocardiography for Healthcare Professionals Kathryn A. Booth Thomas O Brien Chapter 5: Rhythm Strip Interpretation and Sinus Rhythms Learning Outcomes 5.1 Explain the process of evaluating ECG tracings
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Review 4 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The level of EMS training that emphasizes activation of the EMS system and providing
Exam Review 4 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) The level of EMS training that emphasizes activation of the EMS system and providing
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Alzheimer's disease is the most common form of dementia, a general term for memory loss and other intellectual abilities serious
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Alzheimer's disease is the most common form of dementia, a general term for memory loss and other intellectual abilities serious
Presenter: Steven Brust, HCS-D, HCS-H Product Manager, Home Health Coding Center
 Presenter: Steven Brust, HCS-D, HCS-H Product Manager, Home Health Coding Center Pinpoint & properly assign the appropriate heart failure codes Left- vs. Right-sided Left ventricular failure (LVF) may
Presenter: Steven Brust, HCS-D, HCS-H Product Manager, Home Health Coding Center Pinpoint & properly assign the appropriate heart failure codes Left- vs. Right-sided Left ventricular failure (LVF) may
A COPD medication delivery device option: an overview of the NEOHALER
 A COPD medication delivery device option: an overview of the NEOHALER 2017 Sunovion Pharmaceuticals Inc. All rights reserved 9/17 RESP019-17 Indication and Boxed Warning INDICATION ARCAPTA NEOHALER (indacaterol)
A COPD medication delivery device option: an overview of the NEOHALER 2017 Sunovion Pharmaceuticals Inc. All rights reserved 9/17 RESP019-17 Indication and Boxed Warning INDICATION ARCAPTA NEOHALER (indacaterol)
12.1 Apply Your Knowledge How long does an ambulatory monitor typically remain on a patient?
 1 2 3 4 5 6 7 Learning Outcomes (Cont d) 12.4 Educate the patient about ambulatory monitoring. 12.5 Prepare a patient for application of an ambulatory monitor. 12.6 Describe the procedure for applying
1 2 3 4 5 6 7 Learning Outcomes (Cont d) 12.4 Educate the patient about ambulatory monitoring. 12.5 Prepare a patient for application of an ambulatory monitor. 12.6 Describe the procedure for applying
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Focus On Signs and Symptoms
 Focus On Signs and Symptoms Decreasing Primary Care Access Help Your Patients with Their Insurance Benefits Risk Management and Reimbursement Align Don t Forget to Document Abnormal Vitals and Labs Document
Focus On Signs and Symptoms Decreasing Primary Care Access Help Your Patients with Their Insurance Benefits Risk Management and Reimbursement Align Don t Forget to Document Abnormal Vitals and Labs Document
Farmakovijilans ve MedDRA Pharmacovigilance and MedDRA FARMAKOVİJİLANS ve FARMAKOVİJİLANS UYGULAMALARI EKİM Dr.
 Farmakovijilans ve MedDRA Pharmacovigilance and MedDRA FARMAKOVİJİLANS ve FARMAKOVİJİLANS UYGULAMALARI 23-24 EKİM 2003 Dr. Tomás Moraleda Agenda 1. What is MedDRA? 2. Conditions before MedDRA 3. MedDRA
Farmakovijilans ve MedDRA Pharmacovigilance and MedDRA FARMAKOVİJİLANS ve FARMAKOVİJİLANS UYGULAMALARI 23-24 EKİM 2003 Dr. Tomás Moraleda Agenda 1. What is MedDRA? 2. Conditions before MedDRA 3. MedDRA
Acid Base Balance by: Susan Mberenga RN, BSN, MSN
 Acid Base Balance by: Susan Mberenga RN, BSN, MSN Acid Base Balance Refers to hydrogen ions as measured by ph Normal range: 7.35-7.45 Acidosis/acidemia: ph is less than 7.35 Alkalosis/alkalemia: ph is
Acid Base Balance by: Susan Mberenga RN, BSN, MSN Acid Base Balance Refers to hydrogen ions as measured by ph Normal range: 7.35-7.45 Acidosis/acidemia: ph is less than 7.35 Alkalosis/alkalemia: ph is
Best practices in VeDDRA coding
 Best practices in VeDDRA coding 27 November 2013 Presented by: Giles Davis Chair of PhVWP-V VeDDRA sub-group An agency of the European Union Veterinary Dictionary for Drug Related Affairs SOC = System
Best practices in VeDDRA coding 27 November 2013 Presented by: Giles Davis Chair of PhVWP-V VeDDRA sub-group An agency of the European Union Veterinary Dictionary for Drug Related Affairs SOC = System
CHAPTER 4 POST-MARKETING SURVEILLANCE OF DRUGS
 CHAPTER 4 POST-MARKETING SURVEILLANCE OF DRUGS Post-marketing surveillance (PMS) to assure the quality, efficacy and safety of drugs after they go on the market and to establish proper methods of use of
CHAPTER 4 POST-MARKETING SURVEILLANCE OF DRUGS Post-marketing surveillance (PMS) to assure the quality, efficacy and safety of drugs after they go on the market and to establish proper methods of use of
Introductory Guide MedDRA Version 15.0
 Introductory Guide MedDRA Version 15.0 Acknowledgements Notice to Reader This Introductory Guide is written in English and is intended only for use with the English version of MedDRA. Additional Introductory
Introductory Guide MedDRA Version 15.0 Acknowledgements Notice to Reader This Introductory Guide is written in English and is intended only for use with the English version of MedDRA. Additional Introductory
Supplement Table 1. Definitions for Causes of Death
 Supplement Table 1. Definitions for Causes of Death 3. Cause of Death: To record the primary cause of death. Record only one answer. Classify cause of death as one of the following: 3.1 Cardiac: Death
Supplement Table 1. Definitions for Causes of Death 3. Cause of Death: To record the primary cause of death. Record only one answer. Classify cause of death as one of the following: 3.1 Cardiac: Death
DRUG TESTING IN PAIN MANAGEMENT AND SUBSTANCE USE DISORDER(S) TREATMENT
 TREATMENT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
TREATMENT Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
Introductory Guide MedDRA Version 18.1
 Introductory Guide MedDRA Version 18.1 Notice to Reader Notice to Reader This Introductory Guide is written in English and is intended only for use with the English version of MedDRA. Additional Introductory
Introductory Guide MedDRA Version 18.1 Notice to Reader Notice to Reader This Introductory Guide is written in English and is intended only for use with the English version of MedDRA. Additional Introductory
DIA Oligonucleotide-Based Therapeutics Conference
 DIA Oligonucleotide-Based Therapeutics Conference North Bethesda MD October 25-27 Translating PD Biomarkers From Preclinical Studies to Clinical Trials: MRG-201, an Oligonucleotide Mimic of mir- 29, Inhibits
DIA Oligonucleotide-Based Therapeutics Conference North Bethesda MD October 25-27 Translating PD Biomarkers From Preclinical Studies to Clinical Trials: MRG-201, an Oligonucleotide Mimic of mir- 29, Inhibits
Risk Adjustment Documentation & Coding Improvement Reference Information for 2017
 Risk Adjustment Documentation & Coding Improvement Reference Information for 2017 In today s quality and patient-centered health care environment, the importance of accurate, specific and thorough medical
Risk Adjustment Documentation & Coding Improvement Reference Information for 2017 In today s quality and patient-centered health care environment, the importance of accurate, specific and thorough medical
Introductory Guide MedDRA Version 14.0
 Introductory Guide MedDRA Version 14.0 Notice to Reader Notice to Reader This Introductory Guide is written in English and is intended only for use with the English version of MedDRA. Additional Introductory
Introductory Guide MedDRA Version 14.0 Notice to Reader Notice to Reader This Introductory Guide is written in English and is intended only for use with the English version of MedDRA. Additional Introductory
Evaluating Exam Review Book and Guide
 Pharmacy Prep Evaluating Exam Review Book and Guide Misbah Biabani, Ph.D Director Toronto Institute of Pharmaceutical Sciences (TIPS) Inc. Toronto, ON M2N 6K7 Pharmacy Prep Professional Exams Preparation
Pharmacy Prep Evaluating Exam Review Book and Guide Misbah Biabani, Ph.D Director Toronto Institute of Pharmaceutical Sciences (TIPS) Inc. Toronto, ON M2N 6K7 Pharmacy Prep Professional Exams Preparation
HOMES AND SENIORS SERVICES. APPROVAL DATE: February 2011 REVISION DATE: January 2015; July 2018
 POLICY: Page 1 of 6 A resident requiring enteral (tube) feeding as a sole source or adjunctive nutrition support have access to a comprehensive enteral feeding program and receive appropriate support from
POLICY: Page 1 of 6 A resident requiring enteral (tube) feeding as a sole source or adjunctive nutrition support have access to a comprehensive enteral feeding program and receive appropriate support from
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER
 MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER Release 1.4 Based on MedDRA Version 10.1 ICH-Endorsed Guide for MedDRA Users on Data Output 12 September 2007 Copyright ICH Secretariat (c/o IFPMA)
MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER Release 1.4 Based on MedDRA Version 10.1 ICH-Endorsed Guide for MedDRA Users on Data Output 12 September 2007 Copyright ICH Secretariat (c/o IFPMA)
