2004 Annual Report. Addressing Unmet Cardiovascular Needs
|
|
|
- Barnard Arnold
- 5 years ago
- Views:
Transcription
1 2004 Annual Report Addressing Unmet Cardiovascular Needs
2 Perfan I.V. (i.v. enoximone) Enoximone Capsules Ambrisentan Darusentan Myogen Product Pipeline Potential Indication Pre-clinical Phase I Phase II Phase III Marketed Acute Decompensated Heart Failure Advanced Chronic Heart Failure Pulmonary Arterial Hypertension Resistant Systolic Hypertension Drug Discovery Heart Failure Research
3 Cardiovascular Disease The term cardiovascular disease is used to describe a continuum of clinical conditions resulting primarily from three underlying chronic diseases: atherosclerosis, hypertension and diabetes. These underlying diseases cause permanent damage to the heart, blood vessels and kidneys, leading to progressively debilitating clinical conditions such as chronic heart failure, angina, heart attack, stroke, systolic hypertension and chronic kidney disease. Cardiovascular disease is the leading cause of death and disability in the United States, accounting for 19% of all hospitalizations and over 60% of total mortality in The American Heart Association estimates that the total direct and indirect costs of cardiovascular disease in the United States will be approximately $394 billion in 2005, including $46 billion in costs for drugs and related medical durables and $140 billion in hospitalization and nursing home costs. Despite improved treatments and increased awareness of preventive measures, approximately 70 million people in the United States currently are living with one or more types of cardiovascular disease. Corporate Profile Myogen is a biopharmaceutical company focused on the discovery, development and commercialization of small molecule therapeutics for the treatment of cardiovascular disorders. Myogen currently markets one product, Perfan I.V., in Europe for the treatment of acute decompensated heart failure and has three oral product candidates in late-stage clinical development: enoximone for the treatment of patients with advanced chronic heart failure, ambrisentan for the treatment of patients with pulmonary arterial hypertension and darusentan for the treatment of patients with resistant systolic hypertension. Myogen, in partnership with Novartis, also conducts a comprehensive drug discovery research program focused on the development of disease-modifying drugs for the treatment of chronic heart failure and related cardiovascular disorders Accomplishments Initiated ARIES-1 & ARIES-2, the two pivotal Phase 3 trials evaluating ambrisentan in patients with pulmonary arterial hypertension (PAH). Completed EMOTE, a non-pivotal Phase 3 trial of enoximone capsules in 201 patients with advanced chronic heart failure, the preliminary results of which were reported in March, with detailed results presented at the annual meeting of the Heart Failure Society of America in September. Detailed results of the Phase 2 trial of ambrisentan in 64 patients with PAH presented at the American Thoracic Society annual meeting. Completed enrollment and drug treatment of 1,854 patients in ESSENTIAL I & II, the two pivotal Phase 3 trials evaluating enoximone capsules in patients with advanced chronic heart failure. Initiated the Phase 2b trial of darusentan in patients with resistant systolic hypertension. The U.S. Food and Drug Administration (FDA) granted ambrisentan orphan drug designation for the treatment of PAH. Completed a $60 million financing. 1
4 Addressing Unmet Cardiovascular Needs... Cardiovascular diseases are the number one killer of men and women. In the United States, cardiovascular diseases claim one victim every 34 seconds. To Our Shareholders: Cardiovascular diseases claim more lives each year than the next five leading causes of death combined. The cost in human life is immeasurable, as is the impact on patients families. The cost to our medical system, however, is measurable. In 2004, the estimated direct and indirect cost of cardiovascular disease was $368.4 billion. By comparison, in 2003, the cost of all cancers was $189 billion. Despite significant progress in past decades, there clearly remain significant unmet needs to be addressed in the treatment of cardiovascular diseases. Myogen was founded in 1996 with a mission to improve the treatment of cardiovascular disease. Many current therapies for cardiovascular disease do not adequately address the underlying molecular mechanisms of the disorders that make up the diseases. Our understanding of the biology of cardiovascular disease combined with our clinical development expertise has guided a strategy with two mutually supportive initiatives: Selectively in-license and acquire drugs in clinical development for which we have a unique ability to add value, and Leverage our understanding of cardiovascular disease at the molecular level in the discovery and development of disease modifying therapeutics. We have made significant progress under this strategy. We have in-licensed three separate compounds that are in late-stage clinical evaluation for three separate cardiovascular indications. We also have a drug discovery partnership with Novartis based on Myogen s proprietary drug targets and lead compounds and focused on the discovery, development and commercialization 2
5 of new therapeutics for the treatment of heart muscle disease was a productive and challenging year. We made significant progress in the development of our three product candidates enoximone, ambrisentan and darusentan as well as in our discovery research program. At the beginning of 2004, we announced five major corporate milestones for the year, all of which were achieved (see page one). An additional highlight was the completion of a $60 million financing in September. Despite our progress in 2004, the financial markets did not view these accomplishments positively, as is reflected in our valuation decline during However, our achievements last year have positioned Myogen for an exciting year in 2005 in which we expect to obtain results from four clinical trials. This year, we will learn how far we have progressed with Myogen s mission and what impact we may have on three significant cardiovascular disorders. Enoximone We are evaluating the chronic administration of enoximone capsules as a treatment for patients with advanced heart failure. ESSENTIAL I & ESSENTIAL II are our two pivotal Phase 3 trials of enoximone capsules in patients with Class III and IV chronic heart failure. We enrolled 1,854 patients in the trials between February 2002 and May Patient treatment was completed in November of last year with a mean treatment period for patients exceeding 18 months. All final patient visits were completed by January Collection of the trial data is progressing according to plan and we expect to report preliminary results of the trials in the middle of Ambrisentan At the beginning of 2004, we announced the initiation of patient enrollment in ARIES-1 & ARIES-2, the two pivotal Phase 3 trials evaluating ambrisentan in patients with pulmonary arterial hypertension. We expect to complete patient enrollment in ARIES-2 by the end of June 2005 and ARIES-1 in the fourth quarter of We plan to report preliminary results from each of the ARIES trials approximately six months following the completion of enrollment in each trial. Darusentan In July 2004, we initiated a Phase 2b randomized, double-blind, placebo-controlled 10-week clinical trial to evaluate the safety and efficacy of darusentan in patients with resistant systolic hypertension. We expect to complete the trial in the middle of 2005 and report trial results one to two months thereafter. If our clinical programs are successful and our product candidates receive regulatory approval, each of these product candidates has the potential to improve the treatment options available to the patients with these diseases. These improvements would represent only a step in the right direction for reducing the severity and pervasiveness of cardiovascular diseases. There is more work to be done. Declines in death rates from cardiovascular diseases are largely responsible for the recent major improvements in life expectancy. According to the Centers for Disease Control and Prevention/National Center for Health Statistics, if all forms of major cardiovascular disease were eliminated, life expectancy would rise by almost seven years. Since Myogen s founding eight years ago, we have worked to build an organization that was responsible for improving the quality of life of patients who suffer from debilitating cardiovascular disorders. In 2005, we will learn the results of our efforts thus far. I look forward to sharing our results with you during the year. Thank you for your continued support. J. William Freytag, Ph.D. President and Chief Executive Officer March 25,
6 Chronic Heart Failure In the United States, approximately five million patients live with chronic heart failure, with an additional 550,000 new cases reported each year. It is estimated that half of all individuals with chronic heart failure die within five years of diagnosis. Chronic heart failure is one of the largest health problems in the developed world, with annual direct and indirect healthcare costs in the U.S. alone exceeding $28 billion. 4
7 Chronic heart failure, also referred to as congestive heart failure, is a debilitating condition that occurs as a damaged heart becomes progressively less able to pump an adequate supply of blood throughout the body. This results in patients developing progressive symptoms of fatigue, shortness of breath (dyspnea), exercise limitation and fluid accumulation (edema). As the severity of chronic heart failure increases, patients develop worsening symptoms and acute decompensation resulting in periodic hospitalization, intensification of therapy and death. Chronic heart failure has several causes. It generally develops in individuals with a long history of poorly controlled high blood pressure, coronary artery disease or in those who have suffered some other heartdamaging event. Although medical therapy is improving, heart failure remains a major debilitating and progressive condition characterized by high mortality, frequent hospitalization and deteriorating quality of life. The severity of chronic heart failure is typically classified using a system established by the New York Heart Association that assesses the patient s degree of functional limitation based primarily on shortness of breath. This system is divided into four classes, I through IV, with Class IV being the most severe. Physicians use this We believe that individuals with advanced heart failure can benefit greatly from the availability of a product that can be used chronically on an outpatient basis to provide symptomatic relief, improve quality of life and reduce the frequency of hospitalizations by delaying episodes of acute decompensated heart failure. Enoximone, a positive inotrope, is a small molecule that inhibits type-3 phosphodiesterase (PDE-3), an enzyme that is present in the heart and plays an important regulatory role in cardiac function. Enoximone blocks the action of this enzyme, increasing the force of contraction of the heart, thereby Chronic Heart Failure What Is Myogen Doing? A Word from the Physician system to track patients disease progression and responses to therapies. Following diagnosis, patients with chronic heart failure are typically treated with multiple oral medications, including diuretics, digoxin, vasodilators, angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and beta-blockers. Diuretics reduce the fluid overload and edema. Digoxin acts to increase the force of cardiac contraction (inotropy). ACEIs and ARBs inhibit the renin-angiotensin-aldosterone hormone system (RAAS). Inhibition of the RAAS system has proven an important advance in the treatment of chronic heart failure, particularly the early stages, and has been demonstrated to improve patient symptoms and survival. Beta-blockers suppress the stress placed on the heart by increased levels of the hormones angiotensin and norepinephrine and have demonstrated an ability to increase patient survival time. Despite these important advances, over the last few years, clinical studies have demonstrated that further inhibition of the neurohormonal system with additional investigational drugs had not resulted in patient benefit. Thus there is a significant unmet medical need for therapies to treat the pump failure that typifies advanced chronic heart failure. increasing cardiac output. Enoximone also produces vasodilation and enhances ventricular filling, thereby further increasing cardiac efficiency. We are currently conducting Phase 3 clinical evaluation of low-dose oral enoximone in patients with advanced chronic heart failure. We plan to report preliminary results for the pivotal trials, ESSENTIAL I & II, in the middle of If our clinical program is successful and we receive regulatory approval, enoximone capsules would be the first oral inhibitor of PDE-3 to be commercialized for the treatment of advanced chronic heart failure. Heart failure used to be viewed as a relentlessly progressive disease associated with substantial disability and early mortality in all patients. With improved current and future therapies, heart failure may now be seen as a chronic disease syndrome associated with improved quality of life and longevity in most patients. William T. Abraham, M.D., Professor of Medicine, Chief, Division of Cardiovascular Medicine, The Ohio State University, Columbus, Ohio 5
8 Chronic Heart Failure: Research and Development Patients with chronic heart failure develop an enlargement of the heart called cardiac hypertrophy. The causes and effects of cardiac hypertrophy have been extensively documented, but the underlying molecular control mechanisms, or cardiac signaling pathways, remain poorly understood. 6
9 Understanding the Molecular Basis of Heart Failure We believe that the fundamental drivers of pathological remodeling of the heart (abnormal growth, shape and function of the heart) are increases in ventricular wall stress and neurohormonal and growth factor stimulation of cardiac muscle. These processes are set in motion by primary insults to the heart, including myocardial infarction (heart attack) and chronic high blood pressure. Wall stress and associated growth promoting stimuli lead to changes in heart Our scientists and academic collaborators affiliated with the University of Colorado and the University of Texas Southwestern Medical Center are focused on elucidating the underlying molecular mechanisms important in the progression of heart failure. Work conducted in Colorado in the laboratory of Dr. Michael Bristow, one of our founders and our Chief Medical Officer, is focused on identifying the set of fetal genes that are reactivated in chronic heart failure, understanding the consequences of their reactivation and discovering the means to control their expression. This work has led to the discovery of what we believe to be an important gene reactivation that occurs in the failing human heart, that appears to be responsible for weakening the contraction of the heart. Understanding cardiac signaling pathways is a central theme of the research of another scientific collaborator, Dr. Eric Olson at the University of Texas Southwestern Medical Center. This work has led to the discovery of several signaling pathways that appear to control cardiac hypertrophy. An essential component of our drug discovery strategy is to target the elements of gene expression regulation in the heart that are common to known cardiac remodeling and heart failure pathways. Of primary interest in this regard are the calcineurin, NFAT and What Is Myogen Doing? A Word from the Scientist muscle signaling pathways that ultimately produce pathological changes in gene expression in the heart. One of the characteristic changes that occurs in a failing heart is a change in gene expression wherein fetal genes that were turned off shortly after birth are reactivated in the disease process. Although this response may initially be beneficial to a patient with chronic heart failure, it becomes harmful as the disease progresses. MEF2 signaling pathways and their regulation by Class II histone deacetylases (HDACs), enzymes that repress gene transcription, and other regulatory proteins. NFAT is a transcription factor (controls gene expression) that is regulated by the enzyme calcineurin in the heart and other tissues. MEF2 is a transcription factor regulated by Class II HDACs. In addition, we, together with our collaborators, have discovered what we believe to be an important pathological role for Class I HDACs in pathological cardiac remodeling, and we have patented the use of HDAC inhibitors for treatment and prevention of cardiac disease. We have developed a series of high-throughput screening assays based on these discoveries and have identified several lead compounds that appear to inhibit cardiomyocyte hypertrophy and/or reverse abnormal fetal gene expression. These compounds are currently being studied in our laboratories in cell and animal assays to examine safety and efficacy and optimization of lead structures is underway. In October 2003, we established a research collaboration with the Novartis Institutes for BioMedical Research, Inc. to augment and accelerate the discovery of compounds that target the fundamental causes in chronic heart failure. Cardiac remodeling is associated with the activation of a pathological gene program that weakens cardiac performance. Thus, targeting the disease process at the level of gene expression represents a potentially powerful therapeutic approach. An understanding of the mechanistic underpinnings of heart failure represents an essential step toward developing novel therapeutics that will improve the quality of life and prolong survival of heart failure patients. Eric N. Olson, Professor and Chairman, Department of Molecular Biology, University of Texas Southwestern 7
10 Pulmonary Arterial Hypertension Approximately 60,000 individuals in the United States and 100,000 individuals in Europe live with pulmonary arterial hypertension (PAH), a highly debilitating disease of the lungs. The only known cure for PAH is lung transplantation. Despite recent advances in therapeutic options that have provided some symptomatic relief and improved survival, there remains an unmet need for additional pharmacologic alternatives for the treatment of PAH. 8
11 Pulmonary arterial hypertension (PAH) is a progressive and life-threatening lung condition characterized by increased pulmonary artery pressure and pulmonary vascular resistance leading to right ventricular heart failure and eventually death, if untreated. PAH can occur with no known underlying cause which is referred to as idiopathic PAH, or can be associated with connective tissue diseases like scleroderma, congenital heart defects, HIV infection, and other conditions. Patients with PAH develop symptoms of progressive fatigue, shortness of breath, edema and exertion limitation as the heart struggles to pump against the high pulmonary pressure. Historically, treatment has consisted of diuretics, digoxin and anticoagulants, and calcium channel blockers in select patients. As patients progress into more advanced stages of PAH, therapeutic options include continuous intravenous or subcutaneous infusion of prostacyclin, or more recently, inhaled prostacyclins. In 2002, an important advance occurred with the approval of an orally administered dual-selective endothelin receptor antagonist (ERA). We believe that a significant opportunity exists for a new class of ERAs that bind selectively to the ET A receptor in preference to the ET B receptor. Selective ET A antagonists are likely to block the negative effects of endothelin by preventing the harmful effects of vasoconstriction and cell proliferation, while preserving the beneficial effects of the ET B receptor. Ambrisentan is an oral ET A selective ERA. We are currently conducting two Phase 3 clinical trials of ambrisentan in patients with PAH. We believe the selectivity Pulmonary Arterial Hypertension What Is Myogen Doing? A Word from the Physician Endothelin is a small peptide hormone that is believed to play a critical role in the control of blood flow and cell growth. Elevated endothelin blood levels are associated with several cardiovascular disease conditions, including pulmonary hypertension, chronic kidney disease, coronary artery disease, hypertension and chronic heart failure. Therefore, it is believed that agents that block the detrimental effects of endothelin may provide significant benefits in the treatment of some of these conditions. There are two classes of endothelin receptors, ET A and ET B, which play significantly different roles in regulating blood vessel diameter. The binding of endothelin to ET A receptors located on vascular smooth muscle cells stimulates vasoconstriction, cell proliferation and hypertrophy. However, the binding of endothelin to ET B receptors located on the vascular endothelium causes vasodilation through the production of nitric oxide and prostacyclin. The activity of the ET B receptor is thought to be counter-regulatory, protecting against excessive vasoconstriction and proliferation, and is involved in the clearance of endothelin. and potency of ambrisentan may offer significant advantages over other ERAs, including enhanced and more durable efficacy, improved safety, once daily dosing and ease of use (alone or in combination with other therapies), all of which could position ambrisentan as the treatment of choice for PAH and potentially other cardiovascular disorders. The course of PAH is often one of steady deterioration and reduced life expectancy. Early detection and appropriate treatment of PAH may significantly improve patients lives and increase survival; therefore the primary goals of PAH treatment are to improve symptoms, including increasing a patient s exercise capacity, leading to improved quality of life and a better chance or survival. Lewis J. Rubin, M.D., Professor of Medicine, Director, Pulmonary Vascular Center, University of California, San Diego 9
12 RESISTANT SYSTOLIC HYPERTENSION Hypertension affects approximately 50 million individuals in the United States and approximately one billion worldwide. Untreated high blood pressure greatly increases the chance of heart attack, stroke and the progression of chronic kidney disease. Despite the availability and use of several classes of drugs to treat hypertension, a significant percentage of these patients do not achieve blood pressures within the recommended range, a condition referred to as resistant hypertension. 10
13 The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC7) defines resistant hypertension as the failure to achieve goal blood pressure in patients who are adhering to full doses of an appropriate three-drug regimen that includes a diuretic. According to JNC7, a systolic blood pressure of less than 140 mmhg and a diastolic blood pressure of less than 90 mmhg are recommended for patients with hypertension and no other serious conditions. For patients with serious conditions, such as diabetes and chronic kidney disease, target systolic and diastolic blood pressures are more stringent systolic of less than 130 mmhg and diastolic of less than 80 mmhg. Clinical studies in hypertension have shown that diastolic blood pressure can be controlled to a goal of We believe a considerable number of individuals with hypertension, especially those with diabetes or chronic kidney disease, are at risk for significant progressive cardiovascular and renal complications due primarily to elevated systolic blood pressure that is resistant to currently available therapies. As a result, we believe that there is a significant opportunity for a drug that is capable of lowering blood pressure in this resistant patient population, leading to the potential for enhanced patient outcomes. Darusentan, like Resistant Systolic Hypertension What Is Myogen Doing? A Word from the Physician 90 mmhg in approximately 90% of hypertensive patients. However, in these same studies, guidelinerecommended goals for systolic blood pressure were achieved in only 60% of patients, even when multidrug regimens were utilized. Clinical studies have also shown that hypertension in patients with diabetes or chronic kidney disease is consistently more difficult to manage, requiring treatment with a multi-drug regimen. Despite intensive, multi-drug therapy, however, only 50% of these patients reach standard blood pressure goals, with even fewer reaching the more stringent blood pressure goals now recommended by JNC7. Moreover, data from a recent study conducted in a hypertension specialist clinic revealed that more than half of the diabetic patients examined required treatment with three or more antihypertensive drugs and only 22% of the patients studied achieved systolic blood pressure of less than 130 mmhg. ambrisentan, is an ET A selective endothelin receptor antagonist (ERA) and has demonstrated a significant anti-hypertensive effect in patients with moderate essential hypertension. As an ERA, darusentan affects blood pressure by a mechanism different than other approved antihypertensive drugs. We are currently conducting a Phase 2b clinical evaluation of darusentan in patients with resistant systolic hypertension. We believe darusentan has the potential to be the first ERA available for treatment of resistant hypertension. Individuals with poorly controlled blood pressure are at significant risk for cardiovascular and cerebrovascular morbidity and mortality and represent a potentially substantial burden to the healthcare system. Setting appropriate blood pressure goals and working to meet them through aggressive anti-hypertensive treatment, with multiple agents, can reduce those risks. Henry R. Black, M.D., Charles J. and Margaret Roberts Professor and Chairman Department of Preventive Medicine, Rush University Medical Center 11
14 2005 Milestones Report ESSENTIAL I & II preliminary results in the middle of the year; Complete patient enrollment in ARIES-2 by the end of June; Complete the darusentan Phase 2b trial in resistant systolic hypertension in the middle of the year; Complete patient enrollment in ARIES-1 during the fourth quarter; and Report ARIES-2 preliminary results by the end of the year. Management Team J. William Freytag Ph.D. Michael R. Bristow M.D., Ph.D. Michael Gerber M.D. Richard Gorczynski Ph.D. John Julian Joseph Turner Andrew Dickinson 12
15 Corporate Information Board of Directors J. William Freytag, Ph.D. Chairman, President and Chief Executive Officer Michael R. Bristow, M.D., Ph.D. Chief Science and Medical Officer Kirk K. Calhoun Chairman Audit Committee Jerry T. Jackson Chairman Nominating and Corporate Governance Committee Daniel J. Mitchell Sequel Venture Partners Arnold L. Oronsky, Ph.D. InterWest Partners Michael J. Valentino Adams Respiratory Therapeutics Sigrid Van Bladel, Ph.D. New Enterprise Associates Management Team J. William Freytag, Ph.D. Chairman, President and Chief Executive Officer Michael Bristow, M.D., Ph.D. Chief Science and Medical Officer Michael Gerber, M.D. Senior Vice President, Clinical & Regulatory Affairs Richard Gorczynski, Ph.D. Senior Vice President, Research & Development John Julian Senior Vice President, Commercial Development Joseph Turner Senior Vice President, Finance & Administration and Chief Financial Officer Andrew Dickinson Vice President, General Counsel and Secretary Independent Auditors Ernst & Young LLP Denver, Colorado Legal Counsel Cooley Godward, LLP Broomfield, Colorado Corporate Headquarters 7575 W. 103rd Avenue Suite 102 Westminster, CO Transfer Agent and Registrar Communications concerning stock transfer requirements, lost certificates and change of address should be directed to Computershare Trust Company, Inc., 350 Indiana Street, Suite 800, Golden, CO 80401, , Stockholder Inquiries Inquiries from our stockholders and potential investors regarding our Company are always welcome. Please direct your requests for information to: Derek Cole Director, Investor Relations Myogen, Inc W. 103rd Avenue Suite 102 Westminster, Colorado USA Website Stock Listing Myogen, Inc. common stock is listed on the Nasdaq National Market under the ticker symbol MYOG. Annual Meeting The next annual meeting of stockholders will be held on May 11, 2005 at 9:00 a.m. (Mountain) at The Westin Westminster, Westminster Boulevard, Westminster, Colorado. designed by curran & connors, inc. / SAFE HARBOR STATEMENT This annual report contains forward-looking statements that involve significant risks and uncertainties, including the statements relating to reporting of preliminary results from the Company s pivotal Phase 3 trials of enoximone capsules and ambrisentan, completion of patient enrollment in the Company s pivotal Phase 3 trials of ambrisentan and completion of the Company s Phase 2b trial of darusentan. Actual results could differ materially from those projected and the Company cautions investors not to place undue reliance on the forward-looking statements contained in this report. Among other things, the Company s results may be affected by its effectiveness at managing its financial resources, its ability to successfully develop and market its current products, difficulties or delays in its clinical trials, difficulties or delays in manufacturing its products, and regulatory developments involving current and future products. Delays in clinical trials, whether caused by competition, adverse events, patient enrollment rates, regulatory issues or other factors, could adversely affect the Company s financial position and prospects. Results from earlier clinical trials are not necessarily predictive of future clinical results. Preliminary results may not be confirmed upon full analysis of the detailed results of a trial. If the Company s product candidates do not meet the safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Even if the Company s product candidates meet safety and efficacy endpoints, regulatory authorities may not approve them, or the Company may face post-approval problems that require the withdrawal of its product from the market. If our discovery research program is not successful, we may be unable to develop additional product candidates. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale back or discontinue one or more of its drug development or discovery research programs. Myogen is at an early stage of development and may not ever have any products that generate significant revenue. Additional risks and uncertainties relating to the Company and its business can be found in the Risk Factors section of Myogen s Form 10-K for the year ended December 31, 2004 and Myogen s periodic reports on Form 10-Q and Form 8-K. Myogen is providing the information contained in this report as of the date of the report and does not undertake any obligation to update any forward-looking statements as a result of new information, future events or otherwise.
16 7575 W. 103rd Avenue, Suite 102, Westminster, CO Tel: Fax:
Heart Failure (HF) Treatment
 Heart Failure (HF) Treatment Heart Failure (HF) Complex, progressive disorder. The heart is unable to pump sufficient blood to meet the needs of the body. Its cardinal symptoms are dyspnea, fatigue, and
Heart Failure (HF) Treatment Heart Failure (HF) Complex, progressive disorder. The heart is unable to pump sufficient blood to meet the needs of the body. Its cardinal symptoms are dyspnea, fatigue, and
Start of Phase IIIb Study with Bayer s Riociguat in PAH Patients Who Demonstrate an Insufficient Response to PDE-5 Inhibitors
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Pulmonary Arterial Hypertension (PAH): Start of Phase IIIb Study with Bayer
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Pulmonary Arterial Hypertension (PAH): Start of Phase IIIb Study with Bayer
2006 Annual Report. Anastomosis Made Simple
 2006 Annual Report R Anastomosis Made Simple Cardica designs and manufactures proprietary automated anastomosis systems used by surgeons to perform rapid, reliable and consistent connections, or anastomoses,
2006 Annual Report R Anastomosis Made Simple Cardica designs and manufactures proprietary automated anastomosis systems used by surgeons to perform rapid, reliable and consistent connections, or anastomoses,
Topic Page: congestive heart failure
 Topic Page: congestive heart failure Definition: congestive heart f ailure from Merriam-Webster's Collegiate(R) Dictionary (1930) : heart failure in which the heart is unable to maintain an adequate circulation
Topic Page: congestive heart failure Definition: congestive heart f ailure from Merriam-Webster's Collegiate(R) Dictionary (1930) : heart failure in which the heart is unable to maintain an adequate circulation
Recent Treatment of Pulmonary Artery Hypertension. Cardiology Division Yonsei University College of Medicine
 Recent Treatment of Pulmonary Artery Hypertension Cardiology Division Yonsei University College of Medicine Definition Raised Pulmonary arterial pressure (PAP) WHO criteria : spap>40 mmhg NIH Criteria
Recent Treatment of Pulmonary Artery Hypertension Cardiology Division Yonsei University College of Medicine Definition Raised Pulmonary arterial pressure (PAP) WHO criteria : spap>40 mmhg NIH Criteria
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal. Drugs for the treatment of pulmonary arterial hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Draft remit / appraisal objective: Draft scope To appraise the clinical and cost effectiveness
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Draft remit / appraisal objective: Draft scope To appraise the clinical and cost effectiveness
Phase 3 investigation of aprocitentan for resistant hypertension management. Investor Webcast June 2018
 Phase 3 investigation of aprocitentan for resistant hypertension management Investor Webcast June 2018 The following information contains certain forward-looking statements, relating to the company s business,
Phase 3 investigation of aprocitentan for resistant hypertension management Investor Webcast June 2018 The following information contains certain forward-looking statements, relating to the company s business,
Two-stage study designed to evaluate tolerability and efficacy of pracinostat combined with azacitidine in patients with high and very high risk MDS
 Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Pierre Legault CEO June 2, 2014
 April 2012 Pierre Legault CEO June 2, 2014 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
April 2012 Pierre Legault CEO June 2, 2014 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Theravance Announces Positive Results from Phase 1 and Phase 2 Clinical Studies with TD-1211 in Development for Opioid-Induced Constipation
 Theravance Announces Positive Results from Phase 1 and Phase 2 Clinical Studies with TD-1211 in Development for Opioid-Induced Constipation TD-1211 Achieves Primary and Secondary Endpoints SOUTH SAN FRANCISCO,
Theravance Announces Positive Results from Phase 1 and Phase 2 Clinical Studies with TD-1211 in Development for Opioid-Induced Constipation TD-1211 Achieves Primary and Secondary Endpoints SOUTH SAN FRANCISCO,
Heart Failure. Subjective SOB (shortness of breath) Peripheral edema. Orthopnea (2-3 pillows) PND (paroxysmal nocturnal dyspnea)
 Pharmacology I. Definitions A. Heart Failure (HF) Heart Failure Ezra Levy, Pharm.D. HF Results when one or both ventricles are unable to pump sufficient blood to meet the body s needs There are 2 types
Pharmacology I. Definitions A. Heart Failure (HF) Heart Failure Ezra Levy, Pharm.D. HF Results when one or both ventricles are unable to pump sufficient blood to meet the body s needs There are 2 types
Aerpio Reports Fourth Quarter and Full Year 2017 Financial Results and Provides Business Update
 Aerpio Reports Fourth Quarter and Full Year 2017 Financial Results and Provides Business Update TIME-2b Clinical Trial of AKB-9778 in Patients with Diabetic Retinopathy Remains on Track Conference Call
Aerpio Reports Fourth Quarter and Full Year 2017 Financial Results and Provides Business Update TIME-2b Clinical Trial of AKB-9778 in Patients with Diabetic Retinopathy Remains on Track Conference Call
ObsEva Reports Third Quarter 2018 Financial Results and Provides Business Update
 ObsEva Reports Third Quarter 2018 Financial Results and Provides Business Update IMPLANT 4 trial of nolasiban in IVF starting in Q4 2018, European MAA filing expected late 2019 24-week data from Phase
ObsEva Reports Third Quarter 2018 Financial Results and Provides Business Update IMPLANT 4 trial of nolasiban in IVF starting in Q4 2018, European MAA filing expected late 2019 24-week data from Phase
Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England A11X04/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England A11X04/01 Information Reader Box (IRB) to be inserted on inside front cover
Pipeline Preclinical Phase 1 Phase 2 Phase 3 BLA/NDA/MAA BIOMARIN:MATCHING PROVEN SCIENCE WITH PROVEN NEEDS
 Pipeline Preclinical Phase 1 Phase 2 Phase 3 BLA/NDA/MAA On The Market Naglazyme for MPS VI Aldurazyme for MPS I Orapred ODT for Inflammatory Conditions Phenoptin for PKU 6R-BH4 for Cardiovascular Indications
Pipeline Preclinical Phase 1 Phase 2 Phase 3 BLA/NDA/MAA On The Market Naglazyme for MPS VI Aldurazyme for MPS I Orapred ODT for Inflammatory Conditions Phenoptin for PKU 6R-BH4 for Cardiovascular Indications
Emisphere Technologies, Inc. Announces 2008 Second Quarter Financial Results
 August 11, 2008 Emisphere Technologies, Inc. Announces 2008 Second Quarter Financial Results Conference Call/Webcast to be Held Monday, August 11 at 10:00 AM EDT CEDAR KNOLLS, N.J., Aug 11, 2008 /PRNewswire-FirstCall
August 11, 2008 Emisphere Technologies, Inc. Announces 2008 Second Quarter Financial Results Conference Call/Webcast to be Held Monday, August 11 at 10:00 AM EDT CEDAR KNOLLS, N.J., Aug 11, 2008 /PRNewswire-FirstCall
National Horizon Scanning Centre. Tadalafil for pulmonary arterial hypertension. October 2007
 Tadalafil for pulmonary arterial hypertension October 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Tadalafil for pulmonary arterial hypertension October 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Broad and clinically important benefits beyond the initial registrational endpoints are now reported.
 Biohaven Announces Robust Clinical Data with Single Dose Rimegepant That Defines Acute and Durable Benefits to Patients: The First Oral CGRP Receptor Antagonist to Deliver Positive Data on Pain Freedom
Biohaven Announces Robust Clinical Data with Single Dose Rimegepant That Defines Acute and Durable Benefits to Patients: The First Oral CGRP Receptor Antagonist to Deliver Positive Data on Pain Freedom
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Abstract Book. Inspiring Approaches to Clinical Challenges in Pulmonary Hypertension
 Inspiring Approaches to Clinical Challenges in Pulmonary Hypertension Abstract Book A symposium sponsored by Bayer Schering Pharma at ERS 2008 Annual Congress www.ventavis.com Welcome Dear Colleague, It
Inspiring Approaches to Clinical Challenges in Pulmonary Hypertension Abstract Book A symposium sponsored by Bayer Schering Pharma at ERS 2008 Annual Congress www.ventavis.com Welcome Dear Colleague, It
ObsEva Reports First Quarter 2018 Financial Results and Provides Business Update
 ObsEva Reports First Quarter 2018 Financial Results and Provides Business Update -Key First Quarter 2018 Clinical Milestones Achieved Patient randomization completed in Phase 2b EDELWEISS trial of OBE2109
ObsEva Reports First Quarter 2018 Financial Results and Provides Business Update -Key First Quarter 2018 Clinical Milestones Achieved Patient randomization completed in Phase 2b EDELWEISS trial of OBE2109
Memory Pharmaceuticals Establishes Plans for Clinical Program for MEM 3454 in Schizophrenia. -Broadens Roche Nicotinic Alpha-7 Alliance-
 Memory Pharmaceuticals Establishes Plans for Clinical Program for MEM 3454 in Schizophrenia -Broadens Roche Nicotinic Alpha-7 Alliance- -Secures Additional $5M Debt Financing to Support Phase 2a Trial-
Memory Pharmaceuticals Establishes Plans for Clinical Program for MEM 3454 in Schizophrenia -Broadens Roche Nicotinic Alpha-7 Alliance- -Secures Additional $5M Debt Financing to Support Phase 2a Trial-
Interim report Second quarter 2017 and subsequent events (Unaudited)
 SERODUS ASA Interim report Second quarter 2017 and subsequent events (Unaudited) August 2017 2017-08-15 Q2-2017 report ABOUT SERODUS Serodus is a private biotech company with a focus on diabetic comorbidities
SERODUS ASA Interim report Second quarter 2017 and subsequent events (Unaudited) August 2017 2017-08-15 Q2-2017 report ABOUT SERODUS Serodus is a private biotech company with a focus on diabetic comorbidities
Heart Failure Update John Coyle, M.D.
 Heart Failure Update 2011 John Coyle, M.D. Causes of Heart Failure Anderson,B.Am Heart J 1993;126:632-40 It It is now well-established that at least one-half of the patients presenting with symptoms and
Heart Failure Update 2011 John Coyle, M.D. Causes of Heart Failure Anderson,B.Am Heart J 1993;126:632-40 It It is now well-established that at least one-half of the patients presenting with symptoms and
Savient's Pegloticase Data in Treatment-Failure Gout Patients Presented at 72nd Annual Meeting of the American College of Rheumatology Conference
 Savient's Pegloticase Data in Treatment-Failure Gout Patients Presented at 72nd Annual Meeting of the American College of Rheumatology Conference EAST BRUNSWICK, N.J., Oct 27, 2008 /PRNewswire-FirstCall
Savient's Pegloticase Data in Treatment-Failure Gout Patients Presented at 72nd Annual Meeting of the American College of Rheumatology Conference EAST BRUNSWICK, N.J., Oct 27, 2008 /PRNewswire-FirstCall
National Horizon Scanning Centre. Irbesartan (Aprovel) for heart failure with preserved systolic function. August 2008
 Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
Irbesartan (Aprovel) for heart failure with preserved systolic function August 2008 This technology summary is based on information available at the time of research and a limited literature search. It
Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome
 Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome Primary Endpoint Achieved - Statistically Significant Convulsive Seizure Reduction for ZX008
Zogenix Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of ZX008 in Dravet Syndrome Primary Endpoint Achieved - Statistically Significant Convulsive Seizure Reduction for ZX008
National Horizon Scanning Centre. Oral and inhaled treprostinil for pulmonary arterial hypertension: NYHA class III. April 2008
 Oral and inhaled treprostinil for pulmonary arterial hypertension: NYHA class April 2008 This technology summary is based on information available at the time of research and a limited literature search.
Oral and inhaled treprostinil for pulmonary arterial hypertension: NYHA class April 2008 This technology summary is based on information available at the time of research and a limited literature search.
Summary/Key Points Introduction
 Summary/Key Points Introduction Scope of Heart Failure (HF) o 6.5 million Americans 20 years of age have HF o 960,000 new cases of HF diagnosed annually o 5-year survival rate for HF is ~50% Classification
Summary/Key Points Introduction Scope of Heart Failure (HF) o 6.5 million Americans 20 years of age have HF o 960,000 new cases of HF diagnosed annually o 5-year survival rate for HF is ~50% Classification
M2 TEACHING UNDERSTANDING PHARMACOLOGY
 M2 TEACHING UNDERSTANDING PHARMACOLOGY USING CVS SYSTEM AS AN EXAMPLE NIGEL FONG 2 JAN 2014 TODAY S OBJECTIVE Pharmacology often seems like an endless list of mechanisms and side effects to memorize. To
M2 TEACHING UNDERSTANDING PHARMACOLOGY USING CVS SYSTEM AS AN EXAMPLE NIGEL FONG 2 JAN 2014 TODAY S OBJECTIVE Pharmacology often seems like an endless list of mechanisms and side effects to memorize. To
Redefining Relief 2014 ANNUAL REPORT
 Redefining Relief 2014 ANNUAL REPORT Phase 2b: A targeted approach to preventing migraines. A NTI-C GRP M ONOC LON AL A NTIB ODY ALD403 is our novel monoclonal antibody targeted to calcitonin gene-related
Redefining Relief 2014 ANNUAL REPORT Phase 2b: A targeted approach to preventing migraines. A NTI-C GRP M ONOC LON AL A NTIB ODY ALD403 is our novel monoclonal antibody targeted to calcitonin gene-related
Cohort A. Number of patients
 MyoKardia Announces Positive Results from Low- Dose Cohort of Phase 2 PIONEER-HCM Study of Mavacamten in Symptomatic, Obstructive Hypertrophic Cardiomyopathy Patients Met Primary Endpoint and Key Secondary
MyoKardia Announces Positive Results from Low- Dose Cohort of Phase 2 PIONEER-HCM Study of Mavacamten in Symptomatic, Obstructive Hypertrophic Cardiomyopathy Patients Met Primary Endpoint and Key Secondary
ObsEva Reports Third Quarter 2017 Financial Results and Provides Business Update
 ObsEva Reports Third Quarter 2017 Financial Results and Provides Business Update - All Three Development Compounds Progressing with Key Clinical Milestones Over the Next 12 Months - Geneva, Switzerland
ObsEva Reports Third Quarter 2017 Financial Results and Provides Business Update - All Three Development Compounds Progressing with Key Clinical Milestones Over the Next 12 Months - Geneva, Switzerland
Defined by Science Powered by Innovation Inspired by Patients
 CYTOKINETICS ANNUAL STOCKHOLDER MEETING MAY 22, 2013 1 Forward-Looking Statements 2 Statements in and/or made by our representatives in connection with this presentation regarding future events or our
CYTOKINETICS ANNUAL STOCKHOLDER MEETING MAY 22, 2013 1 Forward-Looking Statements 2 Statements in and/or made by our representatives in connection with this presentation regarding future events or our
Pioneering Precision Cardiovascular Medicine. (NASDAQ: MYOK) Corporate Presentation March 2018
 Pioneering Precision Cardiovascular Medicine (NASDAQ: MYOK) Corporate Presentation March 2018 Forward-Looking Statements Statements we make in this presentation may include statements which are not historical
Pioneering Precision Cardiovascular Medicine (NASDAQ: MYOK) Corporate Presentation March 2018 Forward-Looking Statements Statements we make in this presentation may include statements which are not historical
Incyte AR: THE DRIVE TO DISCOVER. THE EXPERIENCE TO DELIVER. INCYTE The Drive to Discover. The Experience to Deliver. INCYTE IS:
 Incyte THE DRIVE TO DISCOVER. THE EXPERIENCE TO DELIVER. INCYTE The Drive to Discover. The Experience to Deliver. INCYTE IS: Incyte Corporation Experimental Station Route 141 & Henry Clay Road, Building
Incyte THE DRIVE TO DISCOVER. THE EXPERIENCE TO DELIVER. INCYTE The Drive to Discover. The Experience to Deliver. INCYTE IS: Incyte Corporation Experimental Station Route 141 & Henry Clay Road, Building
UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam
 UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam as adjunctive treatment of partial-onset seizures in epilepsy Primary efficacy and safety data from the
UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam as adjunctive treatment of partial-onset seizures in epilepsy Primary efficacy and safety data from the
ADVANCED THERAPIES FOR PHARMACOLOGICAL TREATMENT OF PULMONARY HYPERTENSION
 Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-107 Effective Date: 04/21/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Status Active Medical and Behavioral Health Policy Section: Medicine Policy Number: II-107 Effective Date: 04/21/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Healthcare Implications of Achieving JNC 7 Blood Pressure Goals in Clinical Practice
 CONTINUING EDUCATION Healthcare Implications of Achieving JNC 7 Blood Pressure Goals in Clinical Practice GOAL To provide participants with current information about current blood pressure goals and effective
CONTINUING EDUCATION Healthcare Implications of Achieving JNC 7 Blood Pressure Goals in Clinical Practice GOAL To provide participants with current information about current blood pressure goals and effective
Antihypertensive drugs SUMMARY Made by: Lama Shatat
 Antihypertensive drugs SUMMARY Made by: Lama Shatat Diuretic Thiazide diuretics The loop diuretics Potassium-sparing Diuretics *Hydrochlorothiazide *Chlorthalidone *Furosemide *Torsemide *Bumetanide Aldosterone
Antihypertensive drugs SUMMARY Made by: Lama Shatat Diuretic Thiazide diuretics The loop diuretics Potassium-sparing Diuretics *Hydrochlorothiazide *Chlorthalidone *Furosemide *Torsemide *Bumetanide Aldosterone
Committed to Transforming the Treatment Paradigm for Migraine Prevention
 Committed to Transforming the Treatment Paradigm for Migraine Prevention 36th Annual J.P. Morgan Healthcare Conference January 8, 2018 Forward-Looking Statements This presentation and the accompanying
Committed to Transforming the Treatment Paradigm for Migraine Prevention 36th Annual J.P. Morgan Healthcare Conference January 8, 2018 Forward-Looking Statements This presentation and the accompanying
Corporate Presentation. October 2017
 AEPP Breakthrough technology for women s cancers Corporate Presentation October 2017 Oncolix 2017 1 Safe Harbor Certain of the statements and the projections set forth in this presentation constitute forward-looking
AEPP Breakthrough technology for women s cancers Corporate Presentation October 2017 Oncolix 2017 1 Safe Harbor Certain of the statements and the projections set forth in this presentation constitute forward-looking
Investor presentation. Bioshares Biotech Summit July 2017
 Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Investor presentation Bioshares Biotech Summit 2017 22 July 2017 1 Disclaimer Some of the information in this presentation may refer to Dimerix Limited ( Dimerix or the Company ) based on information available
Actelion provides an update on the progress towards launching Idorsia Key results for pipeline assets to be developed by Idorsia
 Page 1 of 7 Media Release 22 May 2017 Actelion provides an update on the progress towards launching Idorsia Key results for pipeline assets to be developed by Idorsia Positive dose-finding results with
Page 1 of 7 Media Release 22 May 2017 Actelion provides an update on the progress towards launching Idorsia Key results for pipeline assets to be developed by Idorsia Positive dose-finding results with
By Prof. Khaled El-Rabat
 What is The Optimum? By Prof. Khaled El-Rabat Professor of Cardiology - Benha Faculty of Medicine HT. Introduction Despite major worldwide efforts over recent decades directed at diagnosing and treating
What is The Optimum? By Prof. Khaled El-Rabat Professor of Cardiology - Benha Faculty of Medicine HT. Introduction Despite major worldwide efforts over recent decades directed at diagnosing and treating
- Linzagolix overall efficacy and safety maintained or improved at week 24
 Final results of ObsEva SA Phase 2b EDELWEISS trial of Linzagolix show sustained efficacy and Bone Mineral Density safety, for the treatment of endometriosis-associated pain - Linzagolix overall efficacy
Final results of ObsEva SA Phase 2b EDELWEISS trial of Linzagolix show sustained efficacy and Bone Mineral Density safety, for the treatment of endometriosis-associated pain - Linzagolix overall efficacy
34 th Annual J.P. Morgan Healthcare Conference
 JANUARY 2016 34 th Annual J.P. Morgan Healthcare Conference [ 1 ] Forward Looking Statements SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS In addition to historical information, this presentation contains
JANUARY 2016 34 th Annual J.P. Morgan Healthcare Conference [ 1 ] Forward Looking Statements SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS In addition to historical information, this presentation contains
FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF HER2-POSITIVE NODE-POSITIVE BREAST CANCER
 NEWS RELEASE Media Contact: Kimberly Ocampo (650) 467-0679 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Ajanta Horan (650) 467-1741 FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF
NEWS RELEASE Media Contact: Kimberly Ocampo (650) 467-0679 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Ajanta Horan (650) 467-1741 FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF
Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva )
 Press release 7 September 2017 Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva ) Achieved significant and clinically
Press release 7 September 2017 Verona Pharma Announces Positive Top-Line Data from Phase 2a Clinical Trial in COPD with RPL554 Dosed in Addition to Tiotropium (Spiriva ) Achieved significant and clinically
Definition of Congestive Heart Failure
 Heart Failure Definition of Congestive Heart Failure A clinical syndrome of signs & symptoms resulting from the heart s inability to supply adequate tissue perfusion. CHF Epidemiology Affects 4.7 million
Heart Failure Definition of Congestive Heart Failure A clinical syndrome of signs & symptoms resulting from the heart s inability to supply adequate tissue perfusion. CHF Epidemiology Affects 4.7 million
ASCEND Phase 2 Trial of AXS-05 in MDD Topline Results Conference Call
 NASDAQ: AXSM ASCEND Phase 2 Trial of in MDD Topline Results Conference Call January 7, 2019 Overview in MDD ASCEND Phase 2 Trial Topline Results Introduction Mark Jacobson, Senior Vice President, Operations
NASDAQ: AXSM ASCEND Phase 2 Trial of in MDD Topline Results Conference Call January 7, 2019 Overview in MDD ASCEND Phase 2 Trial Topline Results Introduction Mark Jacobson, Senior Vice President, Operations
Phase 3 Top-Line Results Show IBRANCE in Combination with Fulvestrant Meets Progression-Free Survival (PFS) Primary Endpoint
 For immediate release: April 15, 2015 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Announces PALOMA-3 Trial for IBRANCE (Palbociclib) Stopped Early Due
For immediate release: April 15, 2015 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Announces PALOMA-3 Trial for IBRANCE (Palbociclib) Stopped Early Due
JNC Evidence-Based Guidelines for the Management of High Blood Pressure in Adults
 JNC 8 2014 Evidence-Based Guidelines for the Management of High Blood Pressure in Adults Table of Contents Why Do We Treat Hypertension? Blood Pressure Treatment Goals Initial Therapy Strength of Recommendation
JNC 8 2014 Evidence-Based Guidelines for the Management of High Blood Pressure in Adults Table of Contents Why Do We Treat Hypertension? Blood Pressure Treatment Goals Initial Therapy Strength of Recommendation
Diastolic Heart Failure. Edwin Tulloch-Reid MBBS FACC Consultant Cardiologist Heart Institute of the Caribbean December 2012
 Diastolic Heart Failure Edwin Tulloch-Reid MBBS FACC Consultant Cardiologist Heart Institute of the Caribbean December 2012 Disclosures Have spoken for Merck, Sharpe and Dohme Sat on a physician advisory
Diastolic Heart Failure Edwin Tulloch-Reid MBBS FACC Consultant Cardiologist Heart Institute of the Caribbean December 2012 Disclosures Have spoken for Merck, Sharpe and Dohme Sat on a physician advisory
Chapter 10. Learning Objectives. Learning Objectives 9/11/2012. Congestive Heart Failure
 Chapter 10 Congestive Heart Failure Learning Objectives Explain concept of polypharmacy in treatment of congestive heart failure Explain function of diuretics Learning Objectives Discuss drugs used for
Chapter 10 Congestive Heart Failure Learning Objectives Explain concept of polypharmacy in treatment of congestive heart failure Explain function of diuretics Learning Objectives Discuss drugs used for
AVEO Oncology Announces Strategic Restructuring. AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET
 NEWS RELEASE FOR IMMEDIATE RELEASE AVEO Oncology Announces Strategic Restructuring AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET CAMBRIDGE, Mass., June 4, 2013 AVEO Oncology (NASDAQ: AVEO)
NEWS RELEASE FOR IMMEDIATE RELEASE AVEO Oncology Announces Strategic Restructuring AVEO to Host Conference Call Wednesday, June 5 at 8:30 a.m. ET CAMBRIDGE, Mass., June 4, 2013 AVEO Oncology (NASDAQ: AVEO)
Corporate Update January 2018
 Corporate Update January 2018 Tenax Strategic Update Specialty pharmaceutical company focused on search, development, and commercialization of drugs that address diseases with high unmet medical need Shift
Corporate Update January 2018 Tenax Strategic Update Specialty pharmaceutical company focused on search, development, and commercialization of drugs that address diseases with high unmet medical need Shift
Structure and organization of blood vessels
 The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
National Horizon Scanning Centre. Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation
 Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation August 2008 This technology summary is based on information available at the time of
Irbesartan (Aprovel) for prevention of cardiovascular complications in patients with persistent atrial fibrillation August 2008 This technology summary is based on information available at the time of
About X-Linked Hypophosphatemia (XLH)
 Ultragenyx and Kyowa Kirin Announce Publication of Phase 2 Study Results Demonstrating that Crysvita (burosumab) Improved Outcomes in Children with X-linked Hypophosphatemia in the New England Journal
Ultragenyx and Kyowa Kirin Announce Publication of Phase 2 Study Results Demonstrating that Crysvita (burosumab) Improved Outcomes in Children with X-linked Hypophosphatemia in the New England Journal
LCZ696 A First-in-Class Angiotensin Receptor Neprilysin Inhibitor
 The Angiotensin Receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction The Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection
The Angiotensin Receptor Neprilysin Inhibitor LCZ696 in Heart Failure with Preserved Ejection Fraction The Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection
Copyright 2011, 2007 by Mosby, Inc., an affiliate of Elsevier Inc. Normal Cardiac Anatomy
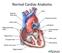 Mosby,, an affiliate of Elsevier Normal Cardiac Anatomy Impaired cardiac pumping Results in vasoconstriction & fluid retention Characterized by ventricular dysfunction, reduced exercise tolerance, diminished
Mosby,, an affiliate of Elsevier Normal Cardiac Anatomy Impaired cardiac pumping Results in vasoconstriction & fluid retention Characterized by ventricular dysfunction, reduced exercise tolerance, diminished
Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November 2010
 Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November Clinical trials in Paediatric Heart Failure List of participants: Michael Burch, Hugo Devlieger, Angeles Garcia, Daphne
Report on the Expert Group Meeting of Paediatric Heart Failure, London 29 November Clinical trials in Paediatric Heart Failure List of participants: Michael Burch, Hugo Devlieger, Angeles Garcia, Daphne
Novartis announces Phase III STRIVE data published in NEJM demonstrating significant and sustained efficacy of erenumab in migraine prevention
 Novartis International AG Novartis Global Communications CH-4002 Basel Switzerland http://www.novartis.com MEDIA RELEASE COMMUNIQUE AUX MEDIAS MEDIENMITTEILUNG Novartis announces Phase III STRIVE data
Novartis International AG Novartis Global Communications CH-4002 Basel Switzerland http://www.novartis.com MEDIA RELEASE COMMUNIQUE AUX MEDIAS MEDIENMITTEILUNG Novartis announces Phase III STRIVE data
First self-administered antibody therapy for HIV in late-stage clinical trials. CytoDyn Annual Meeting of Stockholders August 24, 2017
 First self-administered antibody therapy for HIV in late-stage clinical trials CytoDyn Annual Meeting of Stockholders August 24, 2017 (OTCQB: CYDY) www.cytodyn.com Forward-Looking Statements This presentation
First self-administered antibody therapy for HIV in late-stage clinical trials CytoDyn Annual Meeting of Stockholders August 24, 2017 (OTCQB: CYDY) www.cytodyn.com Forward-Looking Statements This presentation
HEART FAILURE PHARMACOLOGY. University of Hawai i Hilo Pre- Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D
 HEART FAILURE PHARMACOLOGY University of Hawai i Hilo Pre- Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 LEARNING OBJECTIVES Understand the effects of heart failure in the body
HEART FAILURE PHARMACOLOGY University of Hawai i Hilo Pre- Nursing Program NURS 203 General Pharmacology Danita Narciso Pharm D 1 LEARNING OBJECTIVES Understand the effects of heart failure in the body
Avenue Therapeutics, Inc. August 2016
 Avenue Therapeutics, Inc. August 2016 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning of the safe
Avenue Therapeutics, Inc. August 2016 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning of the safe
NEWS RELEASE Media Contact: Megan Pace Investor Contact: Kathee Littrell Patient Inquiries: Ajanta Horan
 NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
NEWS RELEASE Media Contact: Megan Pace 650-467-7334 Investor Contact: Kathee Littrell 650-225-1034 Patient Inquiries: Ajanta Horan 650-467-1741 GENENTECH RECEIVES COMPLETE RESPONSE LETTER FROM FDA FOR
Phase III ARISER trial data ASCO 2013 RENCAREX - Unique targeted antibody therapy for adjuvant treatment of non-metastatic clear cell renal cell
 Phase III ARISER trial data ASCO 2013 RENCAREX - Unique targeted antibody therapy for adjuvant treatment of non-metastatic clear cell renal cell carcinoma patients June 2013 Carbonic Anhydrase IX (CAIX):
Phase III ARISER trial data ASCO 2013 RENCAREX - Unique targeted antibody therapy for adjuvant treatment of non-metastatic clear cell renal cell carcinoma patients June 2013 Carbonic Anhydrase IX (CAIX):
Building a Stroke Portfolio. June 28, 2018
 Building a Stroke Portfolio June 28, 2018 1 Forward-Looking Statements This presentation contains forward-looking statements, including statements relating to: the potential benefits, safety and efficacy
Building a Stroke Portfolio June 28, 2018 1 Forward-Looking Statements This presentation contains forward-looking statements, including statements relating to: the potential benefits, safety and efficacy
Future Leaders in the Biotech Industry. Lee Bendekgey, SVP, CFO & General Counsel April 7, 2005
 Future Leaders in the Biotech Industry Lee Bendekgey, SVP, CFO & General Counsel April 7, 2005 Safe Harbor This presentation contains "forward-looking statements" regarding potential use of Nuvelo clinical
Future Leaders in the Biotech Industry Lee Bendekgey, SVP, CFO & General Counsel April 7, 2005 Safe Harbor This presentation contains "forward-looking statements" regarding potential use of Nuvelo clinical
Avenue Therapeutics, Inc. September 2016
 Avenue Therapeutics, Inc. September 2016 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning of the
Avenue Therapeutics, Inc. September 2016 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning of the
The Failing Heart in Primary Care
 The Failing Heart in Primary Care Hamid Ikram How fares the Heart Failure Epidemic? 4357 patients, 57% women, mean age 74 years HFSA 2010 Practice Guideline (3.1) Heart Failure Prevention A careful and
The Failing Heart in Primary Care Hamid Ikram How fares the Heart Failure Epidemic? 4357 patients, 57% women, mean age 74 years HFSA 2010 Practice Guideline (3.1) Heart Failure Prevention A careful and
Report for the fourth quarter of 2016 and subsequent events (Un-audited)
 SERODUS ASA Report for the fourth quarter of 2016 and subsequent events (Un-audited) Oslo, 27 February 2017 04-17 2016 Q4 report ABOUT SERODUS Serodus has been delisted from Oslo Axess and is now a private
SERODUS ASA Report for the fourth quarter of 2016 and subsequent events (Un-audited) Oslo, 27 February 2017 04-17 2016 Q4 report ABOUT SERODUS Serodus has been delisted from Oslo Axess and is now a private
MINNEAPOLIS September 12, 2012 Medtronic, Inc. (NYSE: MDT) today announced findings
 NEWS RELEASE Contacts: Wendy Dougherty Jeff Warren Public Relations Investor Relations 707-541-3004 763-505-2696 FOR IMMEDIATE RELEASE HEALTH-ECONOMIC ANALYSIS SUGGESTS MEDTRONIC SYMPLICITY RENAL DENERVATION
NEWS RELEASE Contacts: Wendy Dougherty Jeff Warren Public Relations Investor Relations 707-541-3004 763-505-2696 FOR IMMEDIATE RELEASE HEALTH-ECONOMIC ANALYSIS SUGGESTS MEDTRONIC SYMPLICITY RENAL DENERVATION
Scottish Medicines Consortium
 Scottish Medicines Consortium sildenafil, 20mg (as citrate) tablets (Revatio ) No. (596/10) Pfizer Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Scottish Medicines Consortium sildenafil, 20mg (as citrate) tablets (Revatio ) No. (596/10) Pfizer Ltd 15 January 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
A Patient s Guide to Understanding Pulmonary Arterial Hypertension in Systemic Sclerosis
 A Patient s Guide to Understanding Pulmonary Arterial Hypertension in Systemic Sclerosis Compared with the general population, patients with systemic sclerosis (also known as scleroderma) have a higher
A Patient s Guide to Understanding Pulmonary Arterial Hypertension in Systemic Sclerosis Compared with the general population, patients with systemic sclerosis (also known as scleroderma) have a higher
IRONWOOD AND FOREST ANNOUNCE POSITIVE LINACLOTIDE RESULTS FROM PHASE 3 TRIAL IN PATIENTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION
 FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
SPHERIX ANNOUNCES FIRST QUARTER 2010 FINANCIAL RESULTS
 SPHERIX Investor Relations Phone: (301) 897-2564 Email: info@spherix.com SPHERIX ANNOUNCES FIRST QUARTER 2010 FINANCIAL RESULTS BETHESDA, MD, May 21, 2010 - Spherix Incorporated (NASDAQ CM: SPEX), an innovator
SPHERIX Investor Relations Phone: (301) 897-2564 Email: info@spherix.com SPHERIX ANNOUNCES FIRST QUARTER 2010 FINANCIAL RESULTS BETHESDA, MD, May 21, 2010 - Spherix Incorporated (NASDAQ CM: SPEX), an innovator
REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS
 REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS IRVING, Texas August 8, 2018 Reata Pharmaceuticals, Inc. (Nasdaq: RETA), a clinical-stage
REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS IRVING, Texas August 8, 2018 Reata Pharmaceuticals, Inc. (Nasdaq: RETA), a clinical-stage
Oragenics Shareholder Update
 January 4, 2017 Oragenics Shareholder Update Advances Drug Development Programs Focused on Conditions with Significant Unmet Medical Needs TAMPA, Fla.--(BUSINESS WIRE)-- Oragenics, Inc. (NYSE MKT:OGEN.BC),
January 4, 2017 Oragenics Shareholder Update Advances Drug Development Programs Focused on Conditions with Significant Unmet Medical Needs TAMPA, Fla.--(BUSINESS WIRE)-- Oragenics, Inc. (NYSE MKT:OGEN.BC),
Advanced Care for Decompensated Heart Failure
 Advanced Care for Decompensated Heart Failure Sara Kalantari MD Assistant Professor of Medicine, University of Chicago Advanced Heart Failure, Mechanical Circulatory Support and Cardiac Transplantation
Advanced Care for Decompensated Heart Failure Sara Kalantari MD Assistant Professor of Medicine, University of Chicago Advanced Heart Failure, Mechanical Circulatory Support and Cardiac Transplantation
China Focused, Revenue Driven, Specialty Pharmaceutical Company
 China Focused, Revenue Driven, Specialty Pharmaceutical Company ANNUAL REPORT 2010 About SciClone SciClone Pharmaceuticals (NASDAQ: SCLN) is a revenue-generating, Chinacentric, specialty pharmaceutical
China Focused, Revenue Driven, Specialty Pharmaceutical Company ANNUAL REPORT 2010 About SciClone SciClone Pharmaceuticals (NASDAQ: SCLN) is a revenue-generating, Chinacentric, specialty pharmaceutical
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
Improving Transition of Care in Congestive Heart Failure. Mark J. Gloth, DO, MBA. Vice President, Chief Medical Officer HCR ManorCare
 Improving Transition of Care in Congestive Heart Failure Mark J. Gloth, DO, MBA. Vice President, Chief Medical Officer HCR ManorCare Heart Failure Fastest growing clinical cardiac disease in the United
Improving Transition of Care in Congestive Heart Failure Mark J. Gloth, DO, MBA. Vice President, Chief Medical Officer HCR ManorCare Heart Failure Fastest growing clinical cardiac disease in the United
ASTELLAS AND MEDIVATION INITIATE PHASE III TRIAL OF ENZALUTAMIDE IN PATIENTS WITH TRIPLE-NEGATIVE BREAST CANCER
 Astellas Contact: Medivation Contacts: For Media For Media Tyler Marciniak Samina Bari Director, Communications Vice President, Corporate (847) 736-7145 Communications tyler.marciniak@astellas.com (415)
Astellas Contact: Medivation Contacts: For Media For Media Tyler Marciniak Samina Bari Director, Communications Vice President, Corporate (847) 736-7145 Communications tyler.marciniak@astellas.com (415)
The Role of Information Technology in Disease Management: A Case for Heart Failure
 The Role of Information Technology in Disease Management: A Case for Heart Failure Teresa De Peralta, MSN, APN-C Heart Failure Product Workflow Consultant Medtronic Population Management Level 3: As patient
The Role of Information Technology in Disease Management: A Case for Heart Failure Teresa De Peralta, MSN, APN-C Heart Failure Product Workflow Consultant Medtronic Population Management Level 3: As patient
Neprilysin Inhibitor (Entresto ) Prior Authorization and Quantity Limit Program Summary
 Neprilysin Inhibitor (Entresto ) Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Indication Entresto Reduce the risk of cardiovascular (sacubitril/valsartan) death
Neprilysin Inhibitor (Entresto ) Prior Authorization and Quantity Limit Program Summary FDA APPROVED INDICATIONS DOSAGE 1 Indication Entresto Reduce the risk of cardiovascular (sacubitril/valsartan) death
(direct) (609) (mobile)
 MEDIA Sanofi-aventis Bristol-Myers Squibb Ingrid Görg-Armbrecht Laura Hortas + 33 153-774-625 (direct) (609) 252-4587 +33 686-056-688 (mobile) laura.hortas@bms.com ingrid.goerg-armbrecht@sanofi-aventis.com
MEDIA Sanofi-aventis Bristol-Myers Squibb Ingrid Görg-Armbrecht Laura Hortas + 33 153-774-625 (direct) (609) 252-4587 +33 686-056-688 (mobile) laura.hortas@bms.com ingrid.goerg-armbrecht@sanofi-aventis.com
Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference. February 7, 2007
 Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference February 7, 2007 Information related to forward-looking statements This presentation includes forward-looking statements
Merrill Lynch's Global Pharmaceutical, Biotechnology, and Medical Device Conference February 7, 2007 Information related to forward-looking statements This presentation includes forward-looking statements
PULMONARY HYPERTENSION & THALASSAEMIA
 3rd Pan-American Thalassaemia Conference Buenos Aires 2010 Dr Malcolm Walker Cardiologist University College & the Heart Hospital LONDON Clinical Director Hatter Cardiovascular Institute - UCLH PULMONARY
3rd Pan-American Thalassaemia Conference Buenos Aires 2010 Dr Malcolm Walker Cardiologist University College & the Heart Hospital LONDON Clinical Director Hatter Cardiovascular Institute - UCLH PULMONARY
PRESS RELEASE. New NICE guidance will improve diagnosis and treatment of chronic heart failure
 Tel: 0845 003 7782 www.nice.org.uk Ref: 2010/118 ISSUED: WEDNESDAY, 25 AUGUST 2010 PRESS RELEASE New NICE guidance will improve diagnosis and treatment of chronic heart failure The National Institute for
Tel: 0845 003 7782 www.nice.org.uk Ref: 2010/118 ISSUED: WEDNESDAY, 25 AUGUST 2010 PRESS RELEASE New NICE guidance will improve diagnosis and treatment of chronic heart failure The National Institute for
Intravenous Inotropic Support an Overview
 Intravenous Inotropic Support an Overview Shaul Atar, MD Western Galilee Medical Center, Nahariya Affiliated with the Faculty of Medicine of the Galilee, Safed, Israel INOTROPES in Acute HF (not vasopressors)
Intravenous Inotropic Support an Overview Shaul Atar, MD Western Galilee Medical Center, Nahariya Affiliated with the Faculty of Medicine of the Galilee, Safed, Israel INOTROPES in Acute HF (not vasopressors)
Aurinia Pharmaceuticals Announces Voclosporin Meets Primary Endpoint in Phase IIB AURA-LV Study in Lupus Nephritis
 August 15, 2016 Aurinia Pharmaceuticals Announces Voclosporin Meets Primary Endpoint in Phase IIB AURA-LV Study in Lupus Nephritis Conference call and webcast at 8am ET First therapeutic agent to meet
August 15, 2016 Aurinia Pharmaceuticals Announces Voclosporin Meets Primary Endpoint in Phase IIB AURA-LV Study in Lupus Nephritis Conference call and webcast at 8am ET First therapeutic agent to meet
Antialdosterone treatment in heart failure
 Update on the Treatment of Chronic Heart Failure 2012 Antialdosterone treatment in heart failure 전남의대윤현주 Chronic Heart Failure Prognosis of Heart failure Cecil, Text book of Internal Medicine, 22 th edition
Update on the Treatment of Chronic Heart Failure 2012 Antialdosterone treatment in heart failure 전남의대윤현주 Chronic Heart Failure Prognosis of Heart failure Cecil, Text book of Internal Medicine, 22 th edition
Pathophysiology: Heart Failure
 Pathophysiology: Heart Failure Mat Maurer, MD Irving Assistant Professor of Medicine Outline Definitions and Classifications Epidemiology Muscle and Chamber Function Pathophysiology Heart Failure: Definitions
Pathophysiology: Heart Failure Mat Maurer, MD Irving Assistant Professor of Medicine Outline Definitions and Classifications Epidemiology Muscle and Chamber Function Pathophysiology Heart Failure: Definitions
Committed to Transforming the Treatment Paradigm for Migraine Prevention
 June 14, 2018 Committed to Transforming the Treatment Paradigm for Migraine Prevention September 6, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking
June 14, 2018 Committed to Transforming the Treatment Paradigm for Migraine Prevention September 6, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking
GIAPREZA (angiotensin II) Update
 Corporate Presentation GIAPREZA (angiotensin II) Update NASDAQ: LJPC December 2017 0 Forward Looking Statement These slides contain forward-looking statements as that term is defined in the Private Securities
Corporate Presentation GIAPREZA (angiotensin II) Update NASDAQ: LJPC December 2017 0 Forward Looking Statement These slides contain forward-looking statements as that term is defined in the Private Securities
Congestive Heart Failure
 Sheri Saluga Anatomy and Physiology II March 4, 2010 Congestive Heart Failure Scenario George is in congestive heart failure. Because of his condition, his ankles and feet appear to be swollen and he has
Sheri Saluga Anatomy and Physiology II March 4, 2010 Congestive Heart Failure Scenario George is in congestive heart failure. Because of his condition, his ankles and feet appear to be swollen and he has
