Adverse Events After the Use of Benznidazole in Infants and Children With Chagas Disease
|
|
|
- Stewart Conley
- 5 years ago
- Views:
Transcription
1 Adverse Events After the Use of Benznidazole in Infants and Children With Chagas Disease WHAT S KNOWN ON THIS SUBJECT: Treatment of Chagas disease with benznidazole in adults leads to a high incidence of severe drug reactions. However, benznidazole seems to lead to less frequent (and less severe) ADRs in children, but there are scarce data on the subject. WHAT THIS STUDY ADDS: We describe a cohort of children with Chagas disease treated with benznidazole. A lower incidence of ADRs was observed in smaller children compared with older children and adults. All ADRs observed were mild, and treatment response was excellent. abstract BACKGROUND: Chagas disease is caused by infection with Trypanosoma cruzi. In adults, treatment with benznidazole is associated with a high incidence of adverse drug reactions (ADRs). However, in infants and children, treatment with benznidazole seems associated with a lower incidence and decreased severity of ADRs, but these effects have not been clearly characterized. OBJECTIVE: We aimed to describe ADRs observed in infants and children treated with benznidazole. PATIENTS AND METHODS: We conducted a prospective cohort study of infants and children in Argentina with Chagas disease treated with benznidazole. RESULTS: A total of 107 infants and children diagnosed with asymptomatic Chagas disease (mean age: 6.9 years) were enrolled in the study. Sixty-two events (in 44 children) were considered benznidazole related. Mean ADR duration was 8.2 days. ADRs were mild (80.6%), moderate (16%), or severe (3.2%). Most (77.3%) ADRs were in children older than 7 years. Skin was the organ with the highest incidence of ADRs (21%), followed by the central nervous system (9%) and the gastrointestinal tract (8.5%). Also, the ADR rate was lower in infants and toddlers compared with older children (18% vs 53%) (P.001). CONCLUSIONS: Treatment with benznidazole was well tolerated in children. Most ADRs were mild and did not require treatment suspension. A strong association was observed between ADR incidence and patient age, and most ADRs occurred in children older than 7 years. We believe that anxiety over potential severe ADRs in children with Chagas disease is not justified and should not be an obstacle to using benznidazole. Pediatrics 2011;127:e212 e218 AUTHORS: Jaime Altcheh, MD, a Guillermo Moscatelli, MD, a Samanta Moroni, MD, a Facundo Garcia-Bournissen, MD, b and Hector Freilij, MD a a Servicio de Parasitologia y Enfermedad de Chagas, Hospital de Niños R Gutierrez, Buenos Aires, Argentina; and b Division of Clinical Pharmacology and Toxicology, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada KEY WORDS infant, children, Chagas disease, congenital, benznidazole, adverse events, pediatric pharmacology ABBREVIATIONS ADR adverse drug reaction CI confidence interval IQR interquartile range CNS central nervous system doi: /peds Accepted for publication Sep 23, 2010 Address correspondence to Jaime Altcheh, MD, Servicio de Parasitología y Enfermedad de Chagas, Hospital de Niños R. Gutierrez, Gallo 1330, Buenos Aires 1425, Argentina. jaltcheh@gmail.com PEDIATRICS (ISSN Numbers: Print, ; Online, ). Copyright 2011 by the American Academy of Pediatrics FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose. e212
2 ARTICLES Chagas disease is a devastating disease caused by infection with the parasite Trypanosoma cruzi 1,2 and currently afflicts over 7 million people in the Americas. 1,3 5 Chagas disease has expanded to virtually all the countries of the world via immigration, with many cases reported in Europe and North America. 4 Most of the infections take place in children, by vector or congenital transmission, with other modes of infection such as blood transfusion, organ transplants, and oral route being less frequent. Most patients have mild symptoms or are asymptomatic. 1 The initial acute phase is followed by a chronic asymptomatic stage that eventually leads to irreversible heart disease many years later in up to 30% of the infected patients. 6,7 The majority of the deaths attributed to cardiac complications ( 7000 deaths per year) occur in adults infected in childhood. 5,6,8,9 Pharmacologic treatment in the acute stage can cure the disease and prevent progression. 7,10 21 However, only 2 drugs currently are available, nifurtimox and benznidazole, 21 both with similar effectiveness. 22 Unfortunately, appropriate pediatric formulations are not available, and administration of the medication to children often requires tablet fractionation. Treatment with benznidazole in adults is associated with a more than 30% incidence of adverse drug reactions (ADRs), including neuropathy and severe dermatological and gastrointestinal symptoms, and over 20% of treatments are interrupted. 23 Children seem to tolerate these treatments better, but these observations have not been clearly characterized. 1 The pharmacological basis for the differences in incidence and severity of ADRs between children and adults remain to be studied. Regardless of Chagas disease treatment guidelines 1,8 indicating that all infected children younger than 14 years should be treated, and failure to do so could be considered malpractice, many physicians and patients defer or refuse treatment because of anxiety over potential ADRs. 24 We aimed to describe ADRs observed in a prospective cohort of infants and children with Chagas disease who were treated with benznidazole. PATIENTS AND METHODS We conducted a prospective study of a cohort of infants and children with Chagas disease treated with benznidazole between September 2003 and October Treatment was open label for 60 days, with 3 years of follow-up. Infants and children diagnosed at the Parasitology and Chagas Service at the Children s Hospital Ricardo Gutierrez of Buenos Aires were evaluated. Subjects meeting all of the following criteria were considered for inclusion: were less than 20 years of age; had not received previous treatment for Chagas disease; T cruzi infection was defined as at least 2 reactive serologic tests for patients over 6 months old; or parasitemia in patients less than 6 months of age. Parasitemia was evaluated by the microhematocrit method or polymerase chain reaction for parasite DNA in blood. Serologic tests for Chagas disease were an enzyme-linked immunosorbent assay (Wiener Laboratory, Rosario, Argentina), indirect hemagglutination (Polychaco, Buenos Aires, Argentina), or particle agglutination (Fujirebio, Tokyo, Japan). Subjects who presented with any of the following criteria were excluded from the study: pregnancy; breastfeeding women; treatment with any investigational drug in the month before enrollment; cardiovascular, hepatic, neurologic, endocrine, or other major systemic diseases; and immunocompromised patients. Patients were treated with 5 to 8 mg/kg benznidazole per day, divided into 2 to 3 daily doses for 60 days (100-mg benznidazole tablets, Radanil; Roche, São Paulo, Brazil). 12 Infants doses were provided as fractioned tablets and administered with milk. Medication was provided in monthly batches, and compliance was assessed by tablet counting at each visit. Caregivers also were provided with a treatment diary to record doses administered, times of doses, symptoms, and problems associated to the treatment. A detailed clinical history, physical examination, and laboratory routine tests (hemoglobin, total white blood count, differential white blood cell count, platelets, alanine amino transferase, aspartate amino transferase, total direct and indirect bilirubin, alkaline phosphatase, total cholesterol and creatinine, and pregnancy test in adolescent girls) 1,10 were conducted at diagnosis and at 7, 30, and 60 days after the start of treatment. Eosinophilia was defined as an eosinophil level above 800 L and abnormal liver enzymes as any value of 32 IU/L for alanine amine transferase and 48 IU/L for aspartate amine transferase. Signs and symptoms suggesting ADRs were specifically inquired for and recorded during each hospital visit. After the end of the treatment, follow-up took place every 3 months for the first year and every 6 months thereafter. Additional clinical and laboratory evaluations were conducted during any unscheduled visit. Patients were advised to return on any day during the follow-up period if ADRs occurred. All concomitant medications were recorded, and caregivers were told about potential ADRs and provided a telephone number to call in case of doubt or if ADRs appeared. Clinical safety laboratory evaluations and detection of parasitemia and/or serologic tests for detection of anti- PEDIATRICS Volume 127, Number 1, January 2011 e213
3 bodies against T cruzi were performed before the start of the treatment with benznidazole and at 7, 30, and 60 days of pharmacotherapy. Cardiologic evaluation, including echocardiogram and electrocardiogram, was conducted before the start of the treatment and 1 year afterward. ADRs were defined according to World Health Organization definitions. 25,26 Causality assessment was performed using World Health Organization criteria 27,28 and the Naranjo score. 29 The study protocol was approved by the research and teaching committee and bioethics committee of the Children s Hospital Ricardo Gutierrez of Buenos Aires and the World Health Organization Secretariat Committee for Research Involving Human Subjects (Geneva, Switzerland). Written consent was required from patients legal representatives, as well as assent from the patient, as appropriate. Student s t and 2 tests were used to compare continuous and categorical variables, respectively. Continuous variables are presented as means with 95% confidence intervals (CIs) and medians and interquartile ranges (IQRs) and categorical variables as percentages. RESULTS A total of 107 of 127 eligible patients were enrolled in the study (Fig 1). Participating children mostly were from Buenos Aires. The median age was 6.9 years (range: 10 days to 19 years). Maternal origin of congenitally infected infants was Argentina in 59.8% of cases, Bolivia in 29%, Paraguay in 10.3%, and Brazil in 0.9%. Route of infection was transplacental in 67.3% of patients, vectorial in 4.7%, and undefined in 28%. All patients were asymptomatic with no cardiac involvement or other Chagas disease associated pathology at enrollment. Two patients had asthma Withdrawn n = 16 Adverse events n = 7 Lost to follow-up n = 8 Patient decision n = 1 FIGURE 1 Study flowchart. Potencial participants N = 127 Parasitemia (+) n = 96/107 PCR (+): 83/94 (88.3%) MH (+): 13 Received treatment n = 107 Completed treatment n = 91 and 1 patient had epilepsy. Five patients had preexisting laboratory abnormalities, which resolved spontaneously. Four patients had eosinophilias and 1 had mild neutropenia. The mean benznidazole dose was 6.4 mg/kg per day (range: 5 8 mg/kg per day) in 2 divided doses (76 cases) or in 3 divided doses (31 cases). Mean treatment length was 60 days (95% CI: 59 61). We observed good compliance on the basis of tablet counts and medication registry review. Fifteen subjects received concomitant medications: 5 subjects received -lactams; 4 steroids (2 budesonide, 2 methylprednisone); 1 vitamins; 1 iron; 1 acetaminophen; 1 valproic acid; 1 carbamazepine; and 2 loratadine. Only 3 patients had ADRs related to benznidazole. All patients recovered uneventfully. In 107 patients enrolled in the study, we observed 73 adverse events in 55 Not included n = 20 Declined to participate n = 1 Did not understand the study n = 5 Lived outside of the city of Buenos Aires n = 14 children. Eleven adverse events, in 11 patients, were considered unrelated to treatment after review of the time course and clinical data, using World Health Organization causality criteria. 27 The remaining 62 ADRs, in 44 patients (41.1% of all patients), were considered to be related to treatment with benznidazole. 27 The most frequent ADRs were dermatologic (22 of 107 subjects [21%]), followed by gastrointestinal (9 of 107 subjects [8.5%]), central nervous system (CNS) (10 of 107 subjects [9%]), and neuromuscular (3 of 107 subjects [2.8%]) events (Table 1). Mean duration of ADRs was 8.2 days (95% CI: ). Over two-thirds (44 of 62) of ADRs were clinical, and the remaining 18 were biochemical findings. In 5 patients, we observed both clinical and laboratory ADRs (Table 1). Approximately three-quarters of the patients (32 of 44 [73%]) who had ADRs experi- e214
4 ARTICLES TABLE 1 Breakdown of Benznidazole: Related ADRs Observed in This Cohort n % Total ADRs Clinical adverse reactions Dermatologic (n 22) 35.5 Rash Eczema Pruritus Polymorphous erythema Urticaria CNS (n 10) 16.1 Headache Blurry vision dizziness Gastrointestinal (n 9) 14.5 Vomiting Abdominal pain Anorexia Neuromuscular (n 3) 4.8 Cramps Myalgia Biochemical adverse reactions Hematologic (n 12) 19.3 Eosinophilia Leukopenia Metabolic (n 6) 9.7 Liver enzymes 5 8 Cholesterol Five patients presented clinical and biochemical adverse events. enced them in the first 10 days of treatment, with a median time to ADR of 7 days (IQR : ). Effects on the CNS predominated in the first 2 days of treatment (median: 2 days [IQR : ]), followed by gastrointestinal ADRs (median: 5 days [IQR : ]) and dermatologic ADRs (median: 9 days [IQR : ]). Differences between the time of appearance of CNS and dermatological ADRs were statistically significant (P.02; Mann- Whitney test). Mean duration of ADRs was 8.2 days (95% CI: days). Only 3 (7%) ADRs, all mild, were observed in the last 30 days of treatment. No differences were observed in the rate of ADRs between doses or among daily-dose frequencies (ie, 2 vs 3 times daily), and no differences were observed between genders. The mean age of children with ADRs was 9.9 years (95% CI: ) (n 44), which was significantly higher TABLE 2 List of Patients Who Abandoned Treatment Age, y Reason for Discontinuation Treatment Comments Day 19 Rash, moderate; patient decision 9 Good response to symptomatic treatment; 10 Eczema, moderate; parent decision 29 Good response to symptomatic treatment; 1 Erythema, polymorphous, moderate; parent decision 7 Good response to symptomatic treatment; 12 Rash, moderate 7 Good response to symptomatic treatment; 7 Eczema, severe 10 Good response to symptomatic treatment Medical decision Re-treatment with nifurtimox 18 Rash, severe; patient decision 13 Good response to symptomatic treatment; 13 Gastrointestinal, adverse events, moderate 14 Good response without treatment; lost to follow-up than the age of children without an ADR (mean: 4.8 years [95% CI: ]; n 76; P.001, t test). The incidence of ADRs was unevenly distributed and directly related to age, being more common in patients over 7 years old (70% of ADRs), which comprised 50% of the patient population (P.001, 2 test). Approximately 36% (38 of 107) of patients within our cohort were children below 2 years of age. Seven of 38 (18%) patients in this subgroup developed ADRs: 3 patients developed rashes; 2 developed eosinophilias; and 2 had increased liver function test values. All patients recovered completely with no complications. One patient, who experienced a generalized rash, was withdrawn from the study by the caregiver s decision. The rate of ADRs was significantly lower in infants and toddlers compared with older children (18% vs 53%; P.001, 2 test). No serious ADRs occurred in our cohort. Fifty (80.6%) ADRs were classified as mild, 10 (16%) as moderate, and 2 (3.2%) as severe. All patients recovered after symptomatic treatment and/or transient discontinuation of benznidazole. The 2 severe ADRs were generalized rash and led to treatment discontinuation. None of the patients had clinical criteria compatible with severe drug reactions, such as drug reaction with eosinophilia and systemic symptoms, Stevens-Johnsons syndrome, or toxic epidermal necrolysis (ie, did not satisfy criteria as outlined by Bastuji-Garin et al 30 ). No target lesions, bullae, blisters, or skin necrosis developed, and symptoms resolved quickly with symptomatic treatment and drug discontinuation. Also, no fever was present. The most common dermatological ADRs were rash and eczema, mostly on limbs and trunk, which lasted a mean of 15 days (95% CI: 7 23 days). Clinical management of dermatologic ADRs was limited mostly to antihistamine medications with good response. Two mild cases with persistent pruritus required symptomatic treatment with loratadine throughout the treatment with benznidazole, but no discontinuation was required (Table 2). Transient discontinuation of benznidazole because of ADRs was required in 12% (13 of 107) of children (mean duration: 8.9 days [95% CI: ]). Seven (7 of 13 [54%]) patients temporally discontinued treatment because of ADRs (4 because of rashes, 2 gastrointestinal discomforts, and 1 headache); 2 patients temporarily discontinued treatment because of a momentary lack of medication, 2 other patients because of patient noncompliance, and the last 2 patients because of mild viral ill- PEDIATRICS Volume 127, Number 1, January 2011 e215
5 ness. All 13 patients restarted and completed treatment without additional interruptions. Patients abandoned treatment in 6.5% (7 of 107) cases because of ADRs. Most (6 of 7) patients were over 7 years old (Table 2). One patient was successfully treated with nifurtimox (12 mg/kg per day three times per day for 60 days), and the remaining refused additional treatment. ADRs responsible for treatment interruption were dermatological in 6 cases (2 severe and 4 moderate) and gastrointestinal in 1 case. All patients completely recovered from the ADRs, as confirmed by telephone follow-up. No effects on growth or weight progression were observed in any of the patients. The most common biochemical ADRs were hematologic, mainly eosinophilia ( L) and mild leukopenia ( L) (Table 1). In 2 cases, eosinophilia was associated with mild rash. Hepatic involvement was clear in 5 cases, in which aspartate amino transferase and alanine amino transferase were increased 2 to 10 times but without signs of cholestasis (Table 2). Biochemical ADRs did not require specific measures or treatment interruption, and they resolved completely before the end of the benznidazole treatment. Treatment response was high and persistent, with over 90% of children who completed 60 days of treatment presenting a steady decrease or disappearance of specific T cruzi antibodies and a negative parasitological test (T cruzi specific polymerase chain reaction and a microhematocrit test). All patients were followed after treatment completion for up to 3 years. No long-term adverse consequences of treatment with benznidazole were observed by the treating physicians or reported by parents or patients. DISCUSSION We present here one of the largest cohorts published to date of infants and children treated with benznidazole for Chagas disease. Our results confirm clinical observations from previous pediatric cohorts, 7,13,31,32 which have suggested that benznidazoleassociated ADRs are mild in children and in most cases do not require treatment interruption. A previous randomized placebocontrolled study 31 reported a 5% incidence of noncutaneous ADRs in children older than 7 years of age who were treated with benznidazole. These ADRs mainly were anorexia, nausea, headaches, and arthralgias. Maculopapular rash and pruritus also were reported in 12% of patients and linked to treatment interruption in 2% of cases. 31 Another randomized placebo-controlled study 13 reported a 20% rate of ADRs among children 6 to 12 years old. 13 All reported ADRs were mild or moderate and disappeared with benznidazole discontinuation. Similar to our study, most common ADRs were gastrointestinal and dermatologic. A large pediatric cohort study 32 of children and adolescents showed that 50.2% of patients from Honduras and 50.8% of patients from Guatemala who were treated with benznidazole had mostly mild (3 severe) ADRs. The most frequent ADRs were gastrointestinal (26.8%), followed by dermatological (13.0%) and neurologic (10.4%). The rate of ADRs in Bolivia was lower (25.6%) in the same study, with increasing risk in patients more than 10 years old. However, 1 case of toxic epidermal necrolysis and 1 of Stevens- Johnsons syndrome were observed (both recovered well). 32 No laboratory abnormalities were reported, 32 but laboratory tests were performed less frequently than in our study. ADRs have been more commonly reported in adults, and severity seems to be much more significant. Severe ADRs reported in adults include dermatologic reactions, peripheral neuropathy, and leukopenia. 31 None of these severe reactions have been observed in children in significant numbers. Peripheral neuropathy frequently is observed in adults but is quite uncommon in children, 13,33,34 as confirmed by our study. There is limited clinical data available to suggest a correlation between benznidazole blood levels, or total benznidazole exposure, and ADRs, but peripheral neuropathy seems to be dose dependent to some extent. 34,35 We acknowledge that neuropathy is extremely difficult to rule out in small children. However, after following these children for several years after treatment, no functional deficits or any signs compatible with chronic neuropathy were noted. The incidence of ADRs in our cohort was strongly associated with the age of the patient, with most ADRs occurring in children over the age of 7 and very few ADRs in infants or toddlers. This suggests that children older than 7 years should be monitored more carefully. This finding confirms previous observations 31,32 and provides reassurance on the safety of benznidazole in younger children, which had not been fully addressed in previous studies. We also observed that neurologic ADRs seemed to occur soon after the start of the treatment, followed by gastrointestinal and then dermatologic ADRs. Evaluation of CNS ADRs, such as headaches, in infants was difficult because only older children are able to accurately express and describe its symptoms. Nevertheless, associated signs, such as unexplained irritability, food refusal, or vomiting, were not reported by caregivers. Causality evaluation of gastrointestinal ADRs also is probleme216
6 ARTICLES atic because other factors associated with drug administration, such as formulation and tablet size, could be involved in the observed intolerance. The absence of appropriate pediatric formulations complicates matters further, as tablets are not easily (or willingly) swallowed by small children, which sometimes leads to vomiting and other problems that may not be specifically related to the active drug. Cutaneous ADRs were more likely to occur in the second week of treatment, which was also observed in previous studies. 13,32 Severe reactions such as Stevens-Johnsons syndrome and toxic epidermal necrolysis rarely have been reported, with an estimated incidence of 1 of 3000 subjects, 32 which is similar to that of lamotrigine-induced Stevens-Johnsons syndrome. 36 However, an accurate evaluation of actual benznidazole-associated Stevens- Johnsons syndrome/toxic epidermal necrolysis risks requires improved pharmacovigilance efforts. We also observed a number of laboratory abnormalities, including several cases of eosinophilia and increased liver enzymes. It is interesting to note that patients who initially presented with eosinophilia did not worsen with benznidazole treatment. Asymptomatic increases in liver enzymes generally were mild and did not require treatment modifications. Progress along growth and weight curves were not affected by treatment with benznidazole. This observation is in contrast with the striking anorexia observed in patients treated with the alternative REFERENCES 1. Freilij H, Altcheh J. Congenital Chagas disease: diagnostic and clinical aspects. Clin Infect Dis. 1995;21(3): Gurtler RE, Segura EL, Cohen JE. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Infect Dis. 2003; 9(1): Bern C, Montgomery SP, Herwaldt BL, et al. anti Chagas disease medication, nifurtimox. Experimental toxicology models suggested an increased risk of lymphoma in rabbits treated with supratherapeutic benznidazole doses. 37 In our cohort, follow-up after 3 years did not show any evidence of increased risk for malignancies. It is reassuring that an increased risk for any cancers has never been observed in adult or pediatric cohorts to date. 12,23,32,38 Viotti et al 23 reviewed their experience with 1047 patients treated with benznidazole and found no increased risk for cancer (mean follow-up: 7.5 years); this also is in agreement with the experience at our institution, with more than 500 pediatric patients treated with benznidazole and prolonged follow-up. The pharmacologic basis for the marked difference in incidence and severity of ADRs reported between children and adults remains to be clarified. However, examples abound of medications metabolized at different rates in children and adults, in some cases leading to differential toxicity. Notably, children 2 to 6 years of age seem to have faster elimination rates than adults for many drugs This faster elimination rate, combined with scarce data, suggests that hepatic elimination of benznidazole provides a plausible explanation for differences in ADRs incidence among children and adults. Unfortunately, there have been no reported studies to date that evaluated the pharmacokinetics of benznidazole in children. Preliminary results from a Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA. 2007;298(18): Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102(suppl 1): Barrett MP, Burchmore RJ, Stich A, et al. The population pharmacokinetics study in our institution ( identifier NCT ) suggest that plasma concentrations are, indeed, significantly lower than those reported in adults, but these results require confirmation. 42 CONCLUSIONS Our results support previous observations 13,31,43 that benznidazoleassociated ADRs in children generally are mild. Infants and toddlers seem to be at very low risk for severe ADRs and are likely to respond well to the medication. There seem to be few reasons, at this point, to deny effective treatment to children on the basis of theoretical risks not confirmed by any studies performed to date. However, treatment for infants and children with Chagas disease still is commonly postponed to protect them from ADRs, which leads to progression of the disease, reduces the chances for cure, and potentially exposes children to a higher risk for the severe ADRs that have been observed in older patients but virtually absent in children younger than 7 years. ACKNOWLEDGMENTS This research received financial support from United Nations Children s Fund/United Nations Development Programme/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases (TDR) (ID A20772) and the Ministry of Health, Government of the City of Buenos Aires, Argentina. trypanosomiases. Lancet. 2003;362(9394): Teixeira AR, Nascimento RJ, Sturm NR. Evolution and pathology in Chagas disease: a review. Mem Inst Oswaldo Cruz. 2006; 101(5): Sosa-Estani S, Segura EL. Etiological treatment in patients infected by Trypanosoma PEDIATRICS Volume 127, Number 1, January 2011 e217
7 cruzi: experiences in Argentina. Curr Opin Infect Dis. 2006;19(6): World Health Organization Expert Committee on the Control of Chagas Disease. Control of Chagas Disease Second Report of the WHO Expert Committee. 905th ed. Geneva, Switzerland: World Health Organization; Organizacion Panamericana de la Salud. Estimación Cuantitativa de la Enfermedad de Chagas en las Américas. Washington, DC: Organizacion Panamericana de la Salud OPS/.HDM/CD/425-06; World Health Organization Expert Committee on the Control of Chagas Disease. Control of Chagas Disease Second Report of the WHO Expert Committee. Geneva, Switzerland: World Health Organization; Sosa ES, Segura EL. Treatment of Trypanosoma cruzi infection in the indeterminate phase: experience and current guidelines in Argentina [in Spanish]. Medicina (B Aires). 1999;59(suppl 2): Altcheh J, Biancardi M, Lapena A, Ballering G, Freilij H. Congenital Chagas disease: experience in the Hospital de Ninos, Ricardo Gutierrez, Buenos Aires, Argentina. Rev Soc Bras Med Trop. 2005;38(suppl 2): Sosa ES, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas disease. Am J Trop Med Hyg. 1998;59(4): Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127(1): Moya PR, Paolasso RD, Blanco S, et al. Treatment of Chagas disease with nifurtimox during the first months of life [in Spanish]. Medicina (B Aires). 1985;45(5): Russomando G, Figueredo A, Almiron M, Sakamoto M, Morita K. Polymerase chain reaction-based detection of Trypanosoma cruzi DNA in serum. J Clin Microbiol. 1992; 30(11): Fabbro DL, Streiger ML, Arias ED, Bizai ML, del Barco M, Amicone NA. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev Soc Bras Med Trop. 2007;40(1): Streiger ML, del Barco ML, Fabbro DL, Arias ED, Amicone NA. Longitudinal study and specific chemotherapy in children with chronic Chagas disease, residing in a low endemicity area of Argentina [in Portuguese]. Rev Soc Bras Med Trop. 2004;37(5): Fabbro DS, Arias E, Streiger M, et al. Evolutive behavior towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated chronic chagasic patients. Rev Inst Med Trop Sao Paulo. 2000;42(2): Streiger M, Fabbro D, del Barco M, Beltramino R, Bovero N. Congenital Chagas disease in the city of Santa Fe: diagnosis and treatment. Medicina (B Aires). 1995;55(2): Garcia-Bournissen F, Altcheh J, Giglio N, Mastrantonio G, la Vedova CO, Koren G. Pediatric clinical pharmacology studies in Chagas disease: focus on Argentina. Paediatr Drugs. 2009;11(1): Jannin J, Villa L. An overview of Chagas disease treatment. Mem Inst Oswaldo Cruz. 2007;102(suppl 1): Viotti R, Vigliano C, Lococo B, et al. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther. 2009;7(2): Cox EH, Veyrat-Follet C, Beal SL, Fuseau E, Kenkare S, Sheiner LB. A population pharmacokinetic-pharmacodynamic analysis of repeated measures time-to-event pharmacodynamic responses: the antiemetic effect of ondansetron. J Pharmacokinet Biopharm. 1999;27(6): Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237): Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf. 1994;10(2): Meyboom RH, Hekster YA, Egberts AC, Gribnau FW, Edwards IR. Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf. 1997;17(6): Meyboom RH, Egberts AC, Edwards IR, Hekster YA, de Koning FH, Gribnau FW. Principles of signal detection in pharmacovigilance. Drug Saf. 1997;16(6): Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2): Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1): de Andrade AL, Zicker F, de Oliveira RM, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348 (9039): Yun O, Lima MA, Ellman T, et al. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of Medecins Sans Frontieres. PLoS Negl Trop Dis. 2009;3(7):e Faria CR, Souza SEM, Rassi A. Peripheral polyneuropathy induced by benzonidazole in the treatment of Chagas; disease [in Portuguese]. Arq Neuropsiquiatr. 1986;44(2): Flores-Vieira CL, Barreira AA. Experimental benznidazole encephalopathy: I. Clinicalneurological alterations. J Neurol Sci. 1997; 150(1): Flores-Vieira CL, Chimelli L, Franca Fernandes RM, Barreira AA. Experimental benznidazole encephalopathy: II. Electroencephalographic and morphological alterations. J Neurol Sci. 1997;150(1): Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005;64(7): Teixeira AR, Cordoba JC, Souto M, I, Solorzano E. Chagas disease: lymphoma growth in rabbits treated with Benznidazole. Am J Trop Med Hyg. 1990;43(2): Viotti R, Vigliano C. Etiological treatment of chronic Chagas disease: neglected evidence by evidence-based medicine. Expert Rev Anti Infect Ther. 2007;5(4): Hines RN. Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol. 2007; 21(4): Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118(2): Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12): Altcheh J, Moscatelli G, Mastrantonio G, et al. Population pharmacokinetics study of benznidazole in children with Chagas disease [in Spanish] [abstract]. Presented at the 11 th Pediatric Research meeting in Catamarca, Argentina; Sosa ES, Segura EL. Treatment of Trypanosoma cruzi infection in the undetermined phase. Experience and current guidelines of treatment in Argentina. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1): e218
Safety of benznidazole use in the treatment of chronic Chagas disease
 J Antimicrob Chemother 2012; 67: 1261 1266 doi:10.1093/jac/dks027 Advance Access publication 13 February 2012 Safety of benznidazole use in the treatment of chronic Chagas disease Alejandro M. Hasslocher-Moreno,
J Antimicrob Chemother 2012; 67: 1261 1266 doi:10.1093/jac/dks027 Advance Access publication 13 February 2012 Safety of benznidazole use in the treatment of chronic Chagas disease Alejandro M. Hasslocher-Moreno,
Chagas disease. (American trypanosomiasis)
 Chagas disease (American trypanosomiasis) Epidemiology 7 to 8 million people worldwide are infected Endemic in Latin America and South America USA, Canada, Europe http://www.who.int/mediacentre/factsheets/fs340/en/index.html
Chagas disease (American trypanosomiasis) Epidemiology 7 to 8 million people worldwide are infected Endemic in Latin America and South America USA, Canada, Europe http://www.who.int/mediacentre/factsheets/fs340/en/index.html
Cycle 1 PERTuzumab (day 1) and trastuzumab (day 2) loading doses: Drug Dose BC Cancer Administration Guideline
 BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN)
 BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code: Tumour Group: Contact Physician: UBRAJTTW Breast Dr. Angela Chan ELIGIBILITY:
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer Using Weekly PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code: Tumour Group: Contact Physician: UBRAJTTW Breast Dr. Angela Chan ELIGIBILITY:
Synopsis Style Clinical Study Report SAR ACT sarilumab Version number : 1 (electronic 1.0)
 SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, placebo- and active calibrator-controlled study assessing the clinical benefit of SAR153191 subcutaneous (SC) on top of methotrexate
Impavido. (miltefosine) New Product Slideshow
 Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Chagas Disease in the Americas: Impacts and Opportunities for Control
 Chagas Disease in the Americas: Impacts and Opportunities for Control Rick Tarleton Center for Tropical and Emerging Global Diseases University of Georgia The Chagas Disease Foundation Trypanosoma cruzi
Chagas Disease in the Americas: Impacts and Opportunities for Control Rick Tarleton Center for Tropical and Emerging Global Diseases University of Georgia The Chagas Disease Foundation Trypanosoma cruzi
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications
 Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
TOXIC PROFILE OF BENZNIDAZOLE IN PATIENTS WITH CHRONIC CHAGAS DISEASE: RISK FACTORS AND COMPARISON OF THE PRODUCT FROM TWO DIFFERENT
 AAC Accepted Manuscript Posted Online 20 July 2015 Antimicrob. Agents Chemother. doi:10.1128/aac.04660-14 Copyright 2015, American Society for Microbiology. All Rights Reserved. 1 2 3 TOXIC PROFILE OF
AAC Accepted Manuscript Posted Online 20 July 2015 Antimicrob. Agents Chemother. doi:10.1128/aac.04660-14 Copyright 2015, American Society for Microbiology. All Rights Reserved. 1 2 3 TOXIC PROFILE OF
Cetirizine Proposed Core Safety Profile
 Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
SYNOPSIS Final Clinical Study Report for Study AI444031
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Name of Active Ingredient: () Individual Study Table Referring to the Dossier (For National Authority Use Only) SYNOPSIS for Study
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Name of Active Ingredient: () Individual Study Table Referring to the Dossier (For National Authority Use Only) SYNOPSIS for Study
BRAJACTT. Protocol Code. Breast. Tumour Group. Dr. Karen Gelmon. Contact Physician
 BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer using DOXOrubicin and Cyclophosphamide followed by PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code Tumour Group Contact Physician
BC Cancer Protocol Summary for Adjuvant Therapy for Breast Cancer using DOXOrubicin and Cyclophosphamide followed by PACLitaxel and Trastuzumab (HERCEPTIN) Protocol Code Tumour Group Contact Physician
Local Natalizumab Treatment Protocol
 Local Natalizumab Treatment Protocol 1. New medicine name: Natalizumab 300mg concentrate for solution for infusion (Natalizumab ) 2. Licensed indication(s): Natalizumab is indicated for single disease
Local Natalizumab Treatment Protocol 1. New medicine name: Natalizumab 300mg concentrate for solution for infusion (Natalizumab ) 2. Licensed indication(s): Natalizumab is indicated for single disease
Sponsor / Company: Sanofi Drug substance(s): Docetaxel (Taxotere )
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Chagas Initiative. Chagas disease is one of the main public health problems in Latin America, where it is more common than malaria
 Chagas Initiative Chagas Initiative The Chagas Initiative aims to increase access to effective diagnosis and treatment for patients with Chagas disease, both in endemic and non-endemic countries, and to
Chagas Initiative Chagas Initiative The Chagas Initiative aims to increase access to effective diagnosis and treatment for patients with Chagas disease, both in endemic and non-endemic countries, and to
Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List
 Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List (1) Have all important studies that you are aware of been included? No additional
Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List (1) Have all important studies that you are aware of been included? No additional
Individualized dosing for Jakafi (ruxolitinib)
 Individualized dosing for Jakafi (ruxolitinib) Indications and Usage Polycythemia vera Jakafi is indicated for treatment of patients with polycythemia vera who have had an inadequate response to or are
Individualized dosing for Jakafi (ruxolitinib) Indications and Usage Polycythemia vera Jakafi is indicated for treatment of patients with polycythemia vera who have had an inadequate response to or are
ULYRICE. Protocol Code. Lymphoma. Tumour Group. Dr. Laurie Sehn. Contact Physician
 BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
abstract BACKGROUND The role of trypanocidal therapy in patients with established Chagas cardiomyopathy
 The new england journal of medicine established in 1812 October 1, 2015 vol. 373 no. 14 Randomized Trial of for Chronic Chagas Cardiomyopathy C.A. Morillo, J.A. Marin Neto, A. Avezum, S. Sosa Estani, A.
The new england journal of medicine established in 1812 October 1, 2015 vol. 373 no. 14 Randomized Trial of for Chronic Chagas Cardiomyopathy C.A. Morillo, J.A. Marin Neto, A. Avezum, S. Sosa Estani, A.
DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD]
![DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD] DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD]](/thumbs/78/78699510.jpg) SCREENING Visit Date: DEMOGRAPHICS / / [YYYY/MM/DD] Consent Signed Date and time / / [YYYY/MM/DD] : Has written assent been obtained? If no, why not? YES NO Gender: Male Female Birthdate: Permission given
SCREENING Visit Date: DEMOGRAPHICS / / [YYYY/MM/DD] Consent Signed Date and time / / [YYYY/MM/DD] : Has written assent been obtained? If no, why not? YES NO Gender: Male Female Birthdate: Permission given
 . /////////////////// /////////////////// / Berenice? . BLOOD HEPATITIS B HEPATITIS C HIV T. pallidum T. cruzi TRANSFUSION HOST The Southern Cone Initiative, 1991 The Ministers of Health of Argentina,
. /////////////////// /////////////////// / Berenice? . BLOOD HEPATITIS B HEPATITIS C HIV T. pallidum T. cruzi TRANSFUSION HOST The Southern Cone Initiative, 1991 The Ministers of Health of Argentina,
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab
 BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
BC Cancer Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia with Fludarabine and rituximab Protocol Code Tumour Group Contact Physicians LYCLLFLUDR Lymphoma Dr.
TRANSPARENCY COMMITTEE OPINION. 19 July 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 July 2006 Keppra 250 mg, film-coated tablets Box of 60 tablets (CIP code: 356 013-6) Keppra 500 mg, film-coated
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 19 July 2006 Keppra 250 mg, film-coated tablets Box of 60 tablets (CIP code: 356 013-6) Keppra 500 mg, film-coated
The only biologic approved to treat SLE: now with multiple delivery options
 The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
Adverse effects of Immunotherapy. Asha Nayak M.D
 Adverse effects of Immunotherapy Asha Nayak M.D None Financial Disclosures Objectives Understand intensity of the AEs. Understanding unique side-effects. Develop effective monitoring and management guidelines.
Adverse effects of Immunotherapy Asha Nayak M.D None Financial Disclosures Objectives Understand intensity of the AEs. Understanding unique side-effects. Develop effective monitoring and management guidelines.
Validation of a Rapid Immunochromatographic Assay for Diagnosis of Trypanosoma cruzi Infection among Latin-American Migrants in Geneva, Switzerland
 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2010, p. 2948 2952 Vol. 48, No. 8 0095-1137/10/$12.00 doi:10.1128/jcm.00774-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Validation
JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2010, p. 2948 2952 Vol. 48, No. 8 0095-1137/10/$12.00 doi:10.1128/jcm.00774-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Validation
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
Hepatitis E FAQs for Health Professionals
 Hepatitis E FAQs for Health Professionals Index of Questions ± Overview and Statistics What is Hepatitis E? How common is Hepatitis E in the United States? Where is Hepatitis E most common? Are there different
Hepatitis E FAQs for Health Professionals Index of Questions ± Overview and Statistics What is Hepatitis E? How common is Hepatitis E in the United States? Where is Hepatitis E most common? Are there different
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Vfend /Voriconazole
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME /GENERIC DRUG NAME: Vfend /Voriconazole
Trypanosomiasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course
 Trypanosomiasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy
Trypanosomiasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy
Future of Pediatrics: Blisters, Hives and Other Tales from the Emergency Room June 14 th, 2016
 A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
BRAVTPCARB. Protocol Code: Breast. Tumour Group: Dr. Karen Gelmon. Contact Physician:
 BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab (HERCEPTIN), PACLitaxel and CARBOplatin as First-Line Treatment for Advanced Breast Cancer Protocol Code: Tumour
BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab (HERCEPTIN), PACLitaxel and CARBOplatin as First-Line Treatment for Advanced Breast Cancer Protocol Code: Tumour
Nevirapine 200mg Tablet WHOPAR part 4 May 2005 Section 7 updated: May 2016 SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Nevirapine 200mg Tablet. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nevirapine 200 mg. For excipients, see section
SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Nevirapine 200mg Tablet. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nevirapine 200 mg. For excipients, see section
MabThera. SC. The wait is over. MabThera delivered in just 5 minutes. SC= subcutaneous injection
 MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
Drafting a Coverage Authorization Request Letter
 Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
BRINCIDOFOVIR WAS USED TO SUCCESSFULLY TREAT ADENOVIRUS INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS AND OTHER IMMUNOCOMPROMISED PATIENTS
 BRINCIDOFOVIR WAS USED TO SUCCESSFULLY TREAT ADENOVIRUS INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS AND OTHER IMMUNOCOMPROMISED PATIENTS Diana F. Florescu, MD 1, Michael S. Grimley, MD 2, Genovefa
BRINCIDOFOVIR WAS USED TO SUCCESSFULLY TREAT ADENOVIRUS INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS AND OTHER IMMUNOCOMPROMISED PATIENTS Diana F. Florescu, MD 1, Michael S. Grimley, MD 2, Genovefa
Bristol-Myers Squibb
 A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
A Study of the Safety and Efficacy of plus Tenofovir in Adults with Chronic Hepatitis B Virus Infection with Previous Nucleoside/Nucleotide Treatment Failure () FINAL CLINICAL STUDY REPORT EUDRACT Number:
Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations
 122 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 104(Suppl. I): 122-135, 2009 Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations Constança Carvalho
122 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 104(Suppl. I): 122-135, 2009 Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations Constança Carvalho
PACKAGE LEAFLET: INFORMATION FOR THE USER
 PACKAGE LEAFLET: INFORMATION FOR THE USER 250 mg film-coated tablets. 500 mg film-coated tablets. 750 mg film-coated tablets. 1000 mg film-coated tablets. Levetiracetam
PACKAGE LEAFLET: INFORMATION FOR THE USER 250 mg film-coated tablets. 500 mg film-coated tablets. 750 mg film-coated tablets. 1000 mg film-coated tablets. Levetiracetam
Elements for a public summary
 VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels
VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels
Trypanosomiasis. Introduction. Epidemiology. Global Epidemiology. Trypanosomiasis Risk in UK Travellers
 Trypanosomiasis Introduction Epidemiology Risk for travellers Transmission Signs and symptoms Treatment Prevention References Reading list Links Introduction Trypanosomiasis is caused by parasitic protozoa
Trypanosomiasis Introduction Epidemiology Risk for travellers Transmission Signs and symptoms Treatment Prevention References Reading list Links Introduction Trypanosomiasis is caused by parasitic protozoa
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
Serological and parasitological response in chronic Chagas patients 3 years after nifurtimox treatment
 Jackson et al. BMC Infectious Diseases 2013, 13:85 RESEARCH ARTICLE Open Access Serological and parasitological response in chronic Chagas patients 3 years after nifurtimox treatment Yves Jackson 1*, Eric
Jackson et al. BMC Infectious Diseases 2013, 13:85 RESEARCH ARTICLE Open Access Serological and parasitological response in chronic Chagas patients 3 years after nifurtimox treatment Yves Jackson 1*, Eric
Verzenio (abemaciclib) NEW PRODUCT SLIDESHOW
 Verzenio (abemaciclib) NEW PRODUCT SLIDESHOW Introduction Brand name: Verzenio Generic name: Abemaciclib Pharmacological class: Kinase inhibitor Strength and Formulation: 50mg, 100mg, 150mg, 200mg; tabs
Verzenio (abemaciclib) NEW PRODUCT SLIDESHOW Introduction Brand name: Verzenio Generic name: Abemaciclib Pharmacological class: Kinase inhibitor Strength and Formulation: 50mg, 100mg, 150mg, 200mg; tabs
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
J O B A I D S. for MANAGING ADVERSE EVENTS FOLLOWING MASS DRUG ADMINISTRATION (AEs-f-MDA) and S E R I O U S A D V E R S E E V E N T S ( S A E s )
 J O B A I D S for MANAGING ADVERSE EVENTS FOLLOWING MASS DRUG ADMINISTRATION (AEs-f-MDA) and S E R I O U S A D V E R S E E V E N T S ( S A E s ) This Job Aid was developed by RTI International as part
J O B A I D S for MANAGING ADVERSE EVENTS FOLLOWING MASS DRUG ADMINISTRATION (AEs-f-MDA) and S E R I O U S A D V E R S E E V E N T S ( S A E s ) This Job Aid was developed by RTI International as part
SYNOPSIS. Clinical Study Report IM Double-blind Period
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Product: Talimogene Laherparepvec Clinical Study Report: Date: 31 October 2018 Page 1
 Date: 31 October 2018 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Imlygic Name of Active Ingredient: Talimogene laherparepvec Title of Study: A Phase
Date: 31 October 2018 Page 1 2. SYNOPSIS Name of Sponsor: Amgen Inc., Thousand Oaks, CA, USA Name of Finished Product: Imlygic Name of Active Ingredient: Talimogene laherparepvec Title of Study: A Phase
NCCP Chemotherapy Regimen
 INDICATIONS FOR USE: SUNitinib 50mg Therapy INDICATION ICD10 Regimen Code *Reimbursement Status Treatment of unresectable and/or metastatic malignant gastrointestinal C26 00325a CDS stromal tumour (GIST)
INDICATIONS FOR USE: SUNitinib 50mg Therapy INDICATION ICD10 Regimen Code *Reimbursement Status Treatment of unresectable and/or metastatic malignant gastrointestinal C26 00325a CDS stromal tumour (GIST)
Docetaxel + Nintedanib
 Docetaxel + Nintedanib Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Second
Docetaxel + Nintedanib Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Second
Antiemetic protocol for rare emetogenic chemotherapy - see protocol SCNAUSEA
 BCCA Protocol Summary for Treatment of Previously Untreated Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma with obinutuzumab and Chlorambucil Protocol Code Tumour Group Contact Physician
BCCA Protocol Summary for Treatment of Previously Untreated Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma with obinutuzumab and Chlorambucil Protocol Code Tumour Group Contact Physician
Sponsor / Company: Sanofi Drug substance(s): artesunate plus amodiaquine Title of the study:
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Vildagliptin belongs to a group of medicines called oral antidiabetics.
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vildagliptin belongs to a group of medicines called oral antidiabetics. Vildagliptin is used in a certain form of diabetes (type
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vildagliptin belongs to a group of medicines called oral antidiabetics. Vildagliptin is used in a certain form of diabetes (type
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Epilepsy: It is the commonest neurological condition, characterized by recurrent seizures, affecting people of all ages, race
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Epilepsy: It is the commonest neurological condition, characterized by recurrent seizures, affecting people of all ages, race
EULEXIN PRODUCT INFORMATION NAME OF MEDICINE EULEXIN DESCRIPTION
 EULEXIN PRODUCT INFORMATION NAME OF MEDICINE EULEXIN DESCRIPTION Each EULEXIN tablet contains 250 mg of flutamide, a non-steroidal, orally active antiandrogen. Each tablet also contains lactose anhydrous,
EULEXIN PRODUCT INFORMATION NAME OF MEDICINE EULEXIN DESCRIPTION Each EULEXIN tablet contains 250 mg of flutamide, a non-steroidal, orally active antiandrogen. Each tablet also contains lactose anhydrous,
Panobinostat, Bortezomib and Dexamethasone
 Panobinostat, Bortezomib and Dexamethasone Indication Treatment of relapsed/refractory multiple myeloma in patients who have received at least 2 prior regimens, including bortezomib and an immunomodulatory
Panobinostat, Bortezomib and Dexamethasone Indication Treatment of relapsed/refractory multiple myeloma in patients who have received at least 2 prior regimens, including bortezomib and an immunomodulatory
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel
 BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
Study No Title : Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Progress Toward Rubella and Congenital Rubella Syndrome Elimination in the Western Hemisphere,
 1 Introduction: Progress Toward Rubella and Congenital Rubella Syndrome Elimination in the Western Hemisphere, 2003-2008 1 Enhanced measles elimination activities in the Region of the Americas during the
1 Introduction: Progress Toward Rubella and Congenital Rubella Syndrome Elimination in the Western Hemisphere, 2003-2008 1 Enhanced measles elimination activities in the Region of the Americas during the
WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
 DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
DECLESAU (dergrafloxacin) tablets, for oral use DECLESAU (dergrafloxacin) injection, solution for intravenous use WARNING: TENDON EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS Fluoroquinolones, including
Criteria of Chagas Disease Cure
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 94, Suppl. I: 331-335, 1999 331 Criteria of Chagas Disease Cure J Romeu Cançado Cadeira de Terapêutica Clínica, Universidade Federal de Minas Gerais, Caixa Postal
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 94, Suppl. I: 331-335, 1999 331 Criteria of Chagas Disease Cure J Romeu Cançado Cadeira de Terapêutica Clínica, Universidade Federal de Minas Gerais, Caixa Postal
An overview of reports on lacosamide
 An overview of reports on lacosamide Introduction Lacosamide (Vimpat ) was registered for the European market on August 29th 2008. Since then Lareb has received several reports of possible adverse drug
An overview of reports on lacosamide Introduction Lacosamide (Vimpat ) was registered for the European market on August 29th 2008. Since then Lareb has received several reports of possible adverse drug
KELFER Capsules (Deferiprone)
 Published on: 22 Sep 2014 KELFER Capsules (Deferiprone) Composition KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg Dosage Form
Published on: 22 Sep 2014 KELFER Capsules (Deferiprone) Composition KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg Dosage Form
Antiemetic protocol for low-moderately emetogenic chemotherapy (see SCNAUSEA)
 BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Management of adverse effects of triple therapy
 Management of adverse effects of triple therapy Giovanni Battista Gaeta Cattedra di Malattie Infettive UOC Epatiti Virali Seconda Università di Napoli Disclosures Advisory board: BMS, Gilead, Janssen Speaker:
Management of adverse effects of triple therapy Giovanni Battista Gaeta Cattedra di Malattie Infettive UOC Epatiti Virali Seconda Università di Napoli Disclosures Advisory board: BMS, Gilead, Janssen Speaker:
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Pegylated Interferon Alfa-2b (Peg-Intron) Plus Ribavirin (Rebetol)in the Treatment of Chronic Hepatitis C: A Local Experience
 Pegylated Interferon Alfa-2b (Peg-Intron) Plus Ribavirin (Rebetol)in the Treatment of Chronic Hepatitis C: A Local Experience E L Seow, PH Robert Ding Island Hospital, Penang, Malaysia. Introduction Hepatitis
Pegylated Interferon Alfa-2b (Peg-Intron) Plus Ribavirin (Rebetol)in the Treatment of Chronic Hepatitis C: A Local Experience E L Seow, PH Robert Ding Island Hospital, Penang, Malaysia. Introduction Hepatitis
DOSAGE FORMS AND STRENGTHS Tablets: 25 mg, 100 mg, 150 mg, and 200 mg; scored. (3.1, 16)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMOTRIGINE TABLETS safely and effectively. See full prescribing information for LAMOTRIGINE TABLETS.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMOTRIGINE TABLETS safely and effectively. See full prescribing information for LAMOTRIGINE TABLETS.
Nevirapine 200mg Tablet WHOPAR part 3 May 2005 Updated: May 2016 PACKAGE LEAFLET
 PACKAGE LEAFLET PACKAGE LEAFLET Read this entire leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If you have further questions, please ask your
PACKAGE LEAFLET PACKAGE LEAFLET Read this entire leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If you have further questions, please ask your
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY INDICATION AUBAGIO (teriflunomide) is indicated for the treatment of patients with relapsing forms of multiple
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY INDICATION AUBAGIO (teriflunomide) is indicated for the treatment of patients with relapsing forms of multiple
List of Chapters. 5. Care of the sick child Evidence-based pediatrics (page 77 to 80)
 Illustrated Textbook of Paediatrics, 4th Edition Tom Lissauer, and Graham Clayden, 2012 List of Chapters 1. The child in society 2. History and examination 3. Normal child development, hearing and vision
Illustrated Textbook of Paediatrics, 4th Edition Tom Lissauer, and Graham Clayden, 2012 List of Chapters 1. The child in society 2. History and examination 3. Normal child development, hearing and vision
Chagas Disease in non-endemic Countries
 Chagas Disease in non-endemic Countries Tsutomu Takeuchi Institute of Tropical Medicine, Nagasaki University, Nagasaki Apparent distribution of Triatoma infestans 1983 2003 Migration flow of immigrants
Chagas Disease in non-endemic Countries Tsutomu Takeuchi Institute of Tropical Medicine, Nagasaki University, Nagasaki Apparent distribution of Triatoma infestans 1983 2003 Migration flow of immigrants
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
090177e182b31c5d\0.1\Draft\Versioned On:16-Dec :12
 MAH: / Product: IBU/PSE Protocol Number EudraCT number (if applicable) Trial report number PMID / D.O.I. (if applicable) Date of trial Is the trial Trial design Background for conducting the trial Participants
MAH: / Product: IBU/PSE Protocol Number EudraCT number (if applicable) Trial report number PMID / D.O.I. (if applicable) Date of trial Is the trial Trial design Background for conducting the trial Participants
LAMICTAL XR (lamotrigine) extended-release tablets, for oral use Initial U.S. Approval: 1994
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL XR safely and effectively. See full prescribing information for LAMICTAL XR. LAMICTAL XR
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LAMICTAL XR safely and effectively. See full prescribing information for LAMICTAL XR. LAMICTAL XR
PARASITOLOGY CASE HISTORY 5 (HISTOLOGY) (Lynne S. Garcia)
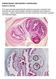 PARASITOLOGY CASE HISTORY 5 (HISTOLOGY) (Lynne S. Garcia) A 47 year-old male presented with symptoms that were consistent with possible late adult-onset epilepsy (frequent headaches and occasional seizures).
PARASITOLOGY CASE HISTORY 5 (HISTOLOGY) (Lynne S. Garcia) A 47 year-old male presented with symptoms that were consistent with possible late adult-onset epilepsy (frequent headaches and occasional seizures).
, version 1.1 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN
 Docetaxel Orion 20 mg/1 ml concentrate for solution for infusion Docetaxel Orion 80 mg/4 ml concentrate for solution for infusion Docetaxel Orion 160 mg/8 ml concentrate for solution for infusion 11.8.2014,
Docetaxel Orion 20 mg/1 ml concentrate for solution for infusion Docetaxel Orion 80 mg/4 ml concentrate for solution for infusion Docetaxel Orion 160 mg/8 ml concentrate for solution for infusion 11.8.2014,
AROMASIN 25mg (Tablets)
 APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
APPROVED PACKAGE INSERT AROMASIN SCHEDULING STATUS: S4 PROPRIETARY NAME AND DOSAGE FORM: AROMASIN 25mg (Tablets) COMPOSITION: Each sugar-coated tablet contains 25 mg exemestane. Preservative: methyl p-hydroxybenzoate
8.3 Females and Males of Reproductive Potential 2 DOSAGE AND ADMINISTRATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use BENZNIDAZOLE TABLETS safely and effectively. See full prescribing information for BENZNIDAZOLE TABLETS.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use BENZNIDAZOLE TABLETS safely and effectively. See full prescribing information for BENZNIDAZOLE TABLETS.
Technical Advisor. JICA Nicaragua Chagas Disease Control Project Nicaragua
 Kota Yoshioka is currently working as technical advisor for Chagas disease control project which isrun by Japan International Cooperation Agency (JICA) and the Ministry of Health of Nicaragua (2009-2014).
Kota Yoshioka is currently working as technical advisor for Chagas disease control project which isrun by Japan International Cooperation Agency (JICA) and the Ministry of Health of Nicaragua (2009-2014).
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS
 Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Supplementary Online Content
 Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Ninlaro. (ixazomib) New Product Slideshow
 Ninlaro (ixazomib) New Product Slideshow Introduction Brand name: Ninlaro Generic name: Ixazomib Pharmacological class: Proteasome inhibitor Strength and Formulation: 2.3mg, 3mg, 4mg; gel caps Manufacturer:
Ninlaro (ixazomib) New Product Slideshow Introduction Brand name: Ninlaro Generic name: Ixazomib Pharmacological class: Proteasome inhibitor Strength and Formulation: 2.3mg, 3mg, 4mg; gel caps Manufacturer:
EU RISK MANAGEMENT PLAN (EU RMP)
 EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
Carboplatin / Liposomal Doxorubicin CARBO/CAELYX Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
A PARTNERSHIP PLAN FOR IDIOPATHIC PULMONARY FIBROSIS
 A PARTNERSHIP PLAN FOR IDIOPATHIC PULMONARY FIBROSIS A STRATEGY FOR DEVELOPING AN IPF MANAGEMENT PLAN BASED ON REALISTIC PATIENT GOALS Indication Esbriet (pirfenidone) is indicated for the treatment of
A PARTNERSHIP PLAN FOR IDIOPATHIC PULMONARY FIBROSIS A STRATEGY FOR DEVELOPING AN IPF MANAGEMENT PLAN BASED ON REALISTIC PATIENT GOALS Indication Esbriet (pirfenidone) is indicated for the treatment of
KELFER Deferiprone. COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg
 KELFER Deferiprone COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg DOSAGE FORM Capsules PHARMACOLOGY Pharmacodynamics
KELFER Deferiprone COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg DOSAGE FORM Capsules PHARMACOLOGY Pharmacodynamics
HIV 101: Overview of the Physiologic Impact of HIV and Its Diagnosis Part 2: Immunologic Impact of HIV and its Effects on the Body
 HIV 101: Overview of the Physiologic Impact of HIV and Its Diagnosis Part 2: Immunologic Impact of HIV and its Effects on the Body Melissa Badowski, PharmD, BCPS, AAHIVP Clinical Assistant Professor University
HIV 101: Overview of the Physiologic Impact of HIV and Its Diagnosis Part 2: Immunologic Impact of HIV and its Effects on the Body Melissa Badowski, PharmD, BCPS, AAHIVP Clinical Assistant Professor University
Randomized Trial of Benznidazole for Chronic Chagas Cardiomyopathy. BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT Trial)
 Randomized Trial of Benznidazole for Chronic Chagas Cardiomyopathy BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT Trial) Carlos A. Morillo and Jose Antonio Marin-Neto Co-Principal Investigators
Randomized Trial of Benznidazole for Chronic Chagas Cardiomyopathy BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT Trial) Carlos A. Morillo and Jose Antonio Marin-Neto Co-Principal Investigators
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of
SUNitinib 37.5mg Therapy
 INDICATIONS FOR USE: SUNitinib 37.5mg Therapy Regimen Code INDICATION ICD10 Treatment of unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumours (pnet) with disease progression
INDICATIONS FOR USE: SUNitinib 37.5mg Therapy Regimen Code INDICATION ICD10 Treatment of unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumours (pnet) with disease progression
UNDERSTANDING HAIR THINNING/HAIR LOSS
 UNDERSTANDING HAIR THINNING/HAIR LOSS INDICATION AUBAGIO (teriflunomide) is indicated for the treatment of patients with relapsing forms of multiple sclerosis. IMPORTANT SAFETY INFORMATION WARNING: HEPATOTOXICITY
UNDERSTANDING HAIR THINNING/HAIR LOSS INDICATION AUBAGIO (teriflunomide) is indicated for the treatment of patients with relapsing forms of multiple sclerosis. IMPORTANT SAFETY INFORMATION WARNING: HEPATOTOXICITY
Donor-Derived Trypanosoma cruzi Infection in Solid Organ Recipients in the United States,
 American Journal of Transplantation 2013; XX: 1 8 Wiley Periodicals Inc. C Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 10.1111/ajt.12340
American Journal of Transplantation 2013; XX: 1 8 Wiley Periodicals Inc. C Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons doi: 10.1111/ajt.12340
