ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
|
|
|
- Helena Brooks
- 5 years ago
- Views:
Transcription
1 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access to the peripheral vascular system. The insertion device consists of an ergonomically designed handle with an integral echogenic needle that contains a passively-activated needle protection mechanism, guidewire with slider advancer, catheter release tab, and single-lumen catheter (refer to Figure 1). The insertion device is designed to allow the user to intuitively advance the guidewire and release the catheter with minimal insertion steps. The catheter is advanced over the needle and threaded over a guidewire into a peripheral vessel. Throughout catheter insertion, blood is contained within the device to aid in prevention of blood exposure. The catheter system consists of a translucent radiopaque polyurethane catheter, a needle with openings to enhance flashback visibility, a seal in the catheter hub designed to reduce blood exposure, a stabilization platform with a strain relief nose designed to reduce kinking at the hub of the catheter, extension tubing with Luer hub, a vent plug, and a clamp. The catheter is intended for short-term use to permit delivery of infusion therapies, infusion of blood and blood products, pressure monitoring, high pressure injection at a maximum of 325 psi, and withdrawal of blood. The catheter system may be used for any patient population with consideration given to adequacy of vascular anatomy and appropriateness of the procedure. Indications: The ARROW Endurance catheter system permits access to the patient s peripheral vascular system for short-term use to sample blood, monitor blood pressure, or administer fluids. The catheter may be used for high pressure injection. The safety feature is intended to minimize the risk of sharps injuries. Contraindications: None known. Catheter Release Tab Guidewire Slider Handle Guard Catheter Juncture Hub Extension Line Clamp Needle Support Juncture Hub Advancer Vent Plug Extension Line Luer Hub Front Grip Location Front Grip Location Rear Grip Location Figure 1 1 EV B1.indd 1
2 General Warnings and Cautions 1. Sterile, Single use: Do not reuse, reprocess or resterilize. Reuse of device creates a potential risk of serious injury and/ or infection which may lead to death. 2. Read all package insert warnings, precautions and instructions prior to use. Failure to do so may result in severe patient injury or death. 3. To minimize the risk of air embolism and blood loss associated with disconnects use only securely tightened Luer-Lock connections. Cautions: 1. Do not alter the catheter, guidewire, or any other kit/set component during insertion, use, or removal. 2. Procedure must be performed by trained personnel well versed in anatomical landmarks, safe technique, and potential complications. 3. Some disinfectants used at catheter insertion site contain solvents which can weaken the catheter material. Alcohol, acetone, and polyethylene glycol can weaken the structure of polyurethane materials. These agents may also weaken the adhesive bond between catheter stabilization device and skin. Do not use acetone on catheter surface. Do not use alcohol to soak catheter surface or allow alcohol to dwell in a catheter lumen to restore catheter patency or as an infection prevention measure. Do not use polyethylene glycol containing ointments at insertion site. Take care when infusing drugs with a high concentration of alcohol. Allow insertion site to dry completely prior to applying dressing. 4. Indwelling catheter should be routinely inspected for desired patency, security of dressing, and possible migration. 5. Do not use syringes smaller than 3 ml to flush or infuse to reduce risk of catheter damage. Venous Use Warnings 1. Practitioners must be aware of complications associated with peripheral venous catheterization including but not limited to: phlebitis, thrombophlebitis, venous thrombosis, occlusion, infiltration, catheter embolus, septicemia, inadvertent arterial puncture, nerve injury, hematoma, air embolism, site infection, and cellulitis. 2. Therapies not appropriate for administration via a peripheral vein include vesicants, known irritants, parenteral nutrition solutions, drug/solutions with ph <5 and >9 or >600 mosm/l. Arterial Use Warnings 1. Practitioners must be aware of complications associated with arterial catheterization including but not limited to: septicemia, vessel wall perforation, intravascular thrombosis and embolization, hematoma, arterial spasm, tissue necrosis, hemorrhage, peripheral ischemia and infarction, peripheral nerve injury, air embolism, site infection, and cellulitis. 2. Practitioners must ascertain that definite evidence of adequate collateral ulnar flow exists prior to radial artery catheterization. 3. In brachial artery catheterization, collateral flow cannot be guaranteed. Therefore, intravascular thrombosis can compromise circulation and result in patient injury. 4. Accidental infusions of drugs or therapeutics or pressure injection into an arterial system may result in severe patient injury or death. Pressure Injection Warnings and Cautions 1. Assess each patient for appropriateness of a pressure injection procedure. 2. Pressure injection procedures must be performed by trained personnel well versed in safe technique and potential complications. 3. Do not pressure inject if catheter is placed into an artery. Pressure injection into an artery may result in severe patient injury or death. 4. Ensure catheter patency prior to pressure injection to reduce risk of catheter failure and/or patient complications. Inability to obtain brisk blood return or to flush easily indicates a possible problem with the catheter and catheter should not be used. 5. Avoid kinking or obstructing the catheter system during pressure injection to reduce risk of catheter failure. 6. Discontinue pressure injection at first sign of infiltration/ extravasation. Cautions: 1. Do not exceed maximum pressure limit of 325 psi on high pressure injector equipment or catheter s maximum recommended flow rate for corresponding injectate viscosity to reduce risk of catheter failure. 2. Pressure limit settings on high pressure injector equipment may not prevent over-pressurizing an occluded or partially occluded catheter, which may cause catheter failure. 3. Follow the contrast media manufacturer s specified instructions for use, contraindications, warnings, and precautions. 4. The single flow rate on venous placement label is the maximum recommended flow rate for high pressure injection using injectate of 11.8 centipoise (cp) viscosity. For higher viscosity media or a high pressure injector setting lower than 325 psi, this flow rate may not be achievable. 5. Due to variations in add-on devices, tubing, injectate temperature and pressure limit settings, the maximum pressure injection flow rates may not be achievable. Kits/Sets may not contain all accessory components detailed in these instructions for use. Become familiar with instructions for individual component(s) before beginning the procedure. 2 EV B1.indd 2
3 Insertion Instructions A Suggested Procedure: Use Sterile Technique. Prepare for Insertion: 1. Select most appropriate insertion site. Use of ultrasound has been shown to increase success with catheter placement. For upper arm insertion ultrasound is recommended. Selecting a vessel with an appropriate subcutaneous depth optimizes the success of peripheral catheter placement. 2. Prep and drape anticipated insertion site per institutional policies and procedures. 3. Administer local anesthetic per institutional policies and procedures. A Protected Needle/Safety Needle should be used in accordance with manufacturer s instructions for use. 4. Check that extension line clamp is not engaged and vent plug is secure (refer to Figure 1). 5. Remove needle guard (refer to Figure 2). Caution: Prior to insertion, distal tip of catheter must be fully retracted past needle bevel or catheter tip damage may occur (refer to Figure 4). Caution: Prior to insertion, guidewire must be fully retracted into needle or blood flashback may be inhibited. NOTE: Priming catheter prior to insertion may reduce or impede blood flashback. Insert Catheter System: 7. Access chosen vessel using a continuous, controlled, slow, forward motion. Observe blood flashback through the catheter. When appropriate, utilizing lower insertion angles during vessel access optimizes the success of peripheral catheter placement. Optionally, front grip locations (refer to Figure 1) may be utilized. If utilized, switch to rear grip location prior to catheter advancement. NOTE: Rate of blood flashback may slow at catheter hub before flowing to the extension line. Blood flashback indicates part of the needle bevel is in the vessel. 8. Carefully advance an additional 1 mm to ensure bevel is fully seated within vessel (refer to Figure 5). Figure 2 6. Prepare catheter by loosening catheter tip and seal adhesion. This is achieved by: Retract the guidewire slider to the 2 cm mark (refer to Figure 3). Advance the catheter forward approximately 3 mm (refer to Figure 3). Figure 5 9. Stabilize position of needle/handle and carefully advance guidewire into vessel by pulling guidewire slider back into handle (refer to Figure 6). The guidewire slider has centimeter markings to indicate how far the guidewire is advanced past bevel of needle. When guidewire slider has the 0 mark covered by the device, tip of guidewire is positioned at needle tip. Figure 3 Return catheter to its original position making sure juncture hub and catheter release tab fit tightly together and the distal tip of catheter is retracted past needle bevel (refer to Figure 4). Advance the guidewire slider back to its original position making sure the 0 mark on the slider is visible. This will withdraw the guidewire into the device and return the needle support to its original position (refer to Figure 4). Figure 4 Figure 6 Caution: Do not advance guidewire unless the needle is confirmed to be in the vessel. Caution: Do not force guidewire if resistance is encountered during advancement. Caution: Blood flashback may slow or stop upon guidewire advancement. 10. Fully advance guidewire. Guidewire should advance smoothly without resistance. Warning: Do not retract guidewire through needle while in vessel to reduce risk of guidewire damage and embolization. If it is necessary to withdraw guidewire once deployed, needle and guidewire should be removed as a unit. 11. While firmly holding device needle/handle stationary, advance catheter over guidewire into vessel with the opposite hand (refer to Figure 8). Advance catheter using a continuous, controlled, slow, forward motion. Catheter should advance smoothly without resistance. Optionally, the catheter release tab may be utilized to initiate catheter advancement. Push forward on catheter release tab to advance catheter over needle bevel (refer to Figure 7). The catheter release tab has a stop at 1 cm; at this point the needle bevel will be covered by the catheter. 3 EV B1.indd 3
4 Figure 7 Caution: Do not attempt to force catheter release tab past the stopping point. Optionally maintain needle/handle stability by anchoring hand while advancing catheter with opposite hand. Centimeter markings on catheter body indicate length of catheter inserted. Complete Insertion: 14. Upon removal of needle/handle, ensure guidewire is intact. Slowly retract guidewire into needle for disposal. When guidewire slider has the 0 mark covered by the device, tip of guidewire should be positioned at needle tip. 15. Remove juncture hub advancer from catheter by gently lifting up on the catheter while holding the juncture hub advancer (refer to Figure 10). Once disengaged, remove juncture hub advancer and discard. Caution: Do not raise the catheter beyond 90 relative to the skin to avoid catheter kinking and damage. Figure 8 Caution: Always keep needle/handle stationary while threading catheter. Do not retract needle/handle while threading catheter. Failure to keep needle/ handle stationary may prevent catheter from threading into vessel. Caution: Do not force catheter if resistance is encountered during advancement. 12. Fully advance catheter until the juncture hub advancer nose is against the insertion site. Juncture hub advancer remains with catheter during advancement and will be removed prior to securement/dressing. The final mark on the catheter should be against the insertion site. This allows 5 mm between insertion site and nose of catheter juncture hub which facilitates dressing. 13. Hold catheter stationary by grasping catheter juncture hub with one hand. With the other hand, withdraw needle/guidewire out of catheter. The needle protection assembly will remain attached to proximal end of catheter hub until needle protection is activated. Visually confirm needle tip is enclosed in the needle protection (refer to Figure 9). Figure Engage extension line clamp and remove the vent plug from catheter extension line Luer hub, attach a 10 ml syringe filled with normal saline for injection, unclamp and check for brisk free-flowing blood return, flush, and reengage extension line clamp. Inability to obtain blood return and/or flush indicates catheter is not in place. 17. Remove syringe and attach needleless connector or stopcock and flush per institutional policies and procedures. Secure Catheter: 18. Secure catheter and dress according to institutional policies and procedures. Ensure all external portions of the catheter except for extension line are completely under the dressing. Attach supplied venous or arterial placement label to extension line to indicate whether the catheter is in a vein or artery. Warning: After insertion, use supplied labels to distinguish if the catheter was placed into a vein or an artery. Failure to do so creates a potential risk of catheter misuse which may result in severe patient injury or death. Caution: Do not apply tape, staples, or sutures directly to the catheter body to reduce risk of damaging catheter, impeding catheter flow, or adversely affecting monitoring capabilities. Secure only at indicated stabilization locations. Caution: Avoid placement or securement in an area of flexion. Caution: Avoid kinking when securing catheter to patient. Caution: Minimize catheter manipulation throughout procedure to maintain proper catheter tip position. Catheter Stabilization Device (where provided): A catheter stabilization device should be used in accordance with manufacturer s instructions for use. 19. Document insertion procedure. Caution: Never attempt to insert a needle or a blunt cannula into the seal in back of catheter hub (refer to Figure 11). Figure 9 Caution: Do not attempt to advance needle back into catheter after catheter is partially threaded off needle to reduce risk of catheter damage. Warning: If resistance is met, do not apply excessive force in removing the needle/guidewire. Remove system as a unit. Caution: Do not attempt to remove needle protection assembly from needle. Figure 11 4 EV B1.indd 4
5 Care and Maintenance: Dressing: Dress according to institutional policies, procedures, and practice guidelines. Change immediately if the integrity becomes compromised (i.e., dressing becomes damp, soiled, loosened or no longer occlusive). Catheter Patency: Maintain catheter patency according to institutional policies, procedures and practice guidelines. All personnel who care for patients with peripheral intravascular devices must be knowledgeable about effective management to prolong catheter s dwell time and prevent injury. Pressure Injection Instructions Pressure injection information on product labeling provides assistance in making catheter-related decisions when pressure injecting with ARROW Endurance Extended Dwell Peripheral Catheter. The ARROW Endurance catheter s indication for high pressure injection implies the catheter s ability to withstand the procedure, but does not imply appropriateness of the procedure for a particular patient. Use sterile technique. 1. Inspect catheter site and catheter to ensure there are no signs and/or symptoms of phlebitis, erythema or tenderness at site. Ensure that there are no kinks or bends in the catheter body and that the dressing is clean, dry and intact. 2. Remove needleless connector from extension line Luer hub if required. It may be acceptable to pressure inject through specifically labeled needleless connectors - refer to the needleless connector manufacturer s instructions for use. 3. Check for patency: Attach a 10 ml syringe filled with normal saline for injection. Flush catheter gently. Check for brisk free-flowing blood return. Vigorously flush catheter. Warning: Ensure catheter patency prior to pressure injection to reduce risk of catheter failure and/or patient complications. Inability to obtain brisk blood return or to flush easily indicates a possible problem with the catheter and catheter should not be used. 4. Detach syringe. 5. Attach pressure injection administration set tubing to extension line Luer hub according to manufacturer s recommendations. Caution: Do not exceed maximum pressure limit of 325 psi on high pressure injector equipment or catheter s maximum recommended flow rate for corresponding injectate viscosity to reduce risk of catheter failure. 6. Inject contrast media in accordance with institutional policies and procedures. Caution: Follow the contrast media manufacturer s specified instructions for use, contraindications, warnings, and precautions. 7. Disconnect catheter from high pressure injector equipment. 8. Flush catheter using a 10 ml syringe filled with normal saline for injection. 9. Disconnect syringe and replace sterile needleless connector, as required, on catheter extension line Luer hub and flush per institutional policies and procedures. Catheter Removal Instructions: Use aseptic technique per institutional policies and procedures. 1. Remove dressing. Warning: Do not use scissors to remove dressing to reduce the risk of cutting the catheter. 2. Remove catheter securement device or sutures being careful not to cut catheter. 3. Remove catheter slowly. Warning: Do not use excessive force in removing catheter. If resistance is met on removal, stop and follow institutional policies and procedures for difficult to remove catheters. 4. Apply pressure using a gauze pad at site after catheter is removed per institutional policies and procedures. 5. Cover site with a sterile occlusive dressing. 6. Document catheter removal procedure including confirmation that entire catheter length has been removed per institutional policies and procedures. Catheter Specification Testing Details Refer to product labeling for catheter specifications. The following section explains how some catheter specifications were generated. Priming volumes are approximate and are done via direct connection to catheter hub (no needleless connector). Gravity flow rates were determined using room temperature water at 100 cm head height and represent approximate flow capabilities. Pump flow rates are determined at pump pressure of 10 psig and represent approximate flow capabilities. Maximum pressure injection flow rate is the maximum indicated flow rate for high pressure injection. Pressure injection flow rates are determined at injector pressure limit setting of 325 psi maximum, with 152 cm administration set tubing, and using injectate viscosities as identified on labeling. For reference information concerning patient assessment, clinical education, potential complications, and specific techniques for this procedure, consult standard textbooks, medical literature, and Arrow International, Inc. website: 5 EV B1.indd 5
6 Symbol Glossary Caution Consult instructions for use Do not reuse Do not resterilize Sterilized by ethylene oxide Keep away from sunlight Keep dry Do not use if package is damaged Not made with natural rubber latex Catalogue number Lot number Use by Manufacturer Teleflex, the Teleflex logo, Arrow, the Arrow logo and ARROW Endurance are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries Teleflex Incorporated. All rights reserved. Arrow International, Inc. Subsidiary of Teleflex Incorporated 2400 Bernville Road Reading, PA USA EV B, Rev. 1 (2/17) 6 EV B1.indd 6
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Groshong* PICC and Catheters
 Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer
 Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Xcela Hybrid PICC with PASV Valve Technology
 Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
2. Need for serial arterial blood gas determinations. 2. Anticipation of the initiation of thrombolytic therapy
 I. Subject: Arterial Cannulation II. Policy: Arterial cannulation will be performed upon a physician's order by Cardiopulmonary and Respiratory Therapy personnel certified in the arterial catheterization
I. Subject: Arterial Cannulation II. Policy: Arterial cannulation will be performed upon a physician's order by Cardiopulmonary and Respiratory Therapy personnel certified in the arterial catheterization
Successful IV Starts Revised February 2014
 Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
BARD. Instructions For Use
 BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
The Power of Purple* Polyurethane PICC. Patient Guide. Access Systems
 The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
Pressure Injectable PICC Product. Venous Access Critical Care
 Pressure Injectable PICC Product Venous Access Critical Care Symbol Legend: Symbols and definitions of symbols are provided for reference. Some symbols may not apply to this product. Refer to product labeling
Pressure Injectable PICC Product Venous Access Critical Care Symbol Legend: Symbols and definitions of symbols are provided for reference. Some symbols may not apply to this product. Refer to product labeling
Radiology Polyurethane Catheter
 Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
BioFlo PICC. with ENDEXO Technology. Directions For Use A
 BioFlo PICC with ENDEXO Technology Directions For Use... 3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, BioFlo PICCwith ENDEXO Technology, 14600123-01A 14600123-01
BioFlo PICC with ENDEXO Technology Directions For Use... 3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, BioFlo PICCwith ENDEXO Technology, 14600123-01A 14600123-01
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Polyurethane Radiology PICC
 Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
The High-Flow Port Designed & Indicated for Apheresis
 The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES
 EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
EL DORADO COUNTY EMS AGENCY FIELD PROCEDURES Effective: July 1, 2017 Reviewed: November 9, 2016 Revised: November 9, 2016 EMS Agency Medical Director INTRAOSSEOUS INFUSION PURPOSE: To establish immediate
Quattro Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC4593/
 Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
Caution: Federal law restricts this device to sale by or on the order of a physician. includes: Quantity English 1 Quattro Catheter 3-Luer 9.3 French x 45cm Triple Infusion Luer Extension Line Clamps Radiopaque
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter
 Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Instructions for Use Reprocessed LASSO Circular Mapping Diagnostic Electrophysiology (EP) Catheter Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. DEVICE DESCRIPTION
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
ICY Intravascular Heat Exchange Catheter (Custom Luer) Instructions for Use Model IC-3893A/
 Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Caution: Federal law restricts this device to sale by or on the order of a physician. ICY Intravascular Heat Exchange Catheter (ICY Catheter) Kit includes: Quantity 1 English ICY Catheter 3-Luer 9.3 French
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Instructions for Use 1
 Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
SARASOTA MEMORIAL HOSPITAL. NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) 12/18 12/18 1 of 7 RESPONSIBILITY:
 SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
SARASOTA MEMORIAL HOSPITAL TITLE: ISSUED FOR: NURSING PROCEDURE INTRAOSSEOUS NEEDLE: INSERTION, CARE, AND REMOVAL (inv08) Nursing DATE: REVIEWED: PAGES: 12/18 12/18 1 of 7 RESPONSIBILITY: PS1094 Insertion-
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE
 LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
ASEPT Pleural Drainage System
 ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
ORDERING INFORMATION ASEPT Drainage PRODUCTS (Provided separately, see package label for contents) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT Pleural drainage catheter and insertion
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE
 Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use
 Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
Ponsky * PEG Safety System - "Pull" Bard * PEG Safety System - "Guidewire" Information for Use Rx only Single patient use DEHP-Free This product and package do not contain natural rubber latex STERILE
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Designed to Reduce the Risk of Occlusion (1)
 Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Peripherally Inserted Central Catheter (PICC) Booklet
 Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices
 Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
Curraheen, Co. Cork. Guidelines on the Management and Care of Central Venous Access Devices Date re-approved: 27 th Jan 2015. Version No: 2 Revision Due: 2018 Index code: CLIN028 Disclaimer: The information
Hospital of the University of Pennsylvania NURSING. Insertion of Peripherally Inserted Central Catheter (PICC) and Midline Catheter (MLC)
 Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
Page 1 of 11 KEYWORDS: Central Catheter Sterility REFER TO: 4B-02-09 Nursing Management of the Patient with a Central Venous Access Device HUP 1-12-24 Consent to Health Care Services SCOPE Registered Nurses
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Figure 1: Revolution TM Peripheral Atherectomy System Diagram. Table 1: Revolution TM Peripheral Atherectomy System Specifications Minimum Burr
 Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Instructions For Use All instructions should be read before use CAUTION Investigational Device. Limited by Federal (or United States) law to investigational use. DEVICE DESCRIPTION: The Revolution TM Peripheral
Central Line Care and Management
 Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement
 Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
Title/Description: Peripherally Inserted Central Catheter & Midline Placement with ECG Confirmation of Tip Placement Department: Patient Care Services Personnel: Nursing Services Effective Date: April
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation.
 Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
Intraosseous Infusion System Directions for Use 01/2006, Vidacare Corporation, all rights reserved. Vidacare, EZ-IO Product System and EZ-Connect are trademarks of the Vidacare Corporation. EC REP Emerge
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5575 Entity: Fairview Pharmacy Services
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS
 DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
DIGESTIVE HEALTH ENTERAL FEEDING PRODUCTS HALYARD* MIC* AND MIC-KEY* INTRODUCER KITS HALYARD* MIC* and MIC-KEY* Introducer Kits provide physicians with an innovative solution to facilitate the primary
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
CATHETERIZATION TRAINER ON A STAND
 CATHETERIZATION TRAINER ON A STAND Parts identification: Clamp Prostate velcro strap (male genitalia only) Genitalia stand male female Urethra channel Bladder tubing and luer locks Female genitalia Male
CATHETERIZATION TRAINER ON A STAND Parts identification: Clamp Prostate velcro strap (male genitalia only) Genitalia stand male female Urethra channel Bladder tubing and luer locks Female genitalia Male
Single Pass MCA Revascularization with Trevo
 Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
Single Pass MCA Revascularization with Trevo By Concentric Medical ProVueTM Retriever Case Reported By Sascha Prothmann, MD Klinikum rechts der Isar, Munich, Germany Images contained herein courtesy of
ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Do not Do not YOUR KIT CONTAINS:
 ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Read the Instructions for Use before you start using ALPROLIX and each time you get a refill. There may be new information.
ALPROLIX Coagulation Factor IX (Recombinant), Fc Fusion Protein INSTRUCTIONS FOR USE Read the Instructions for Use before you start using ALPROLIX and each time you get a refill. There may be new information.
PATIENT CARE PLAN FOR CARE OF PERIPHERAL MIDLINE. Manufacturers specific recommendations should be noted and adhered to by individual practitioners.
 PATIENT CARE PLAN FOR CARE OF PERIPHERAL MIDLINE The care plan is designed to be used in conjunction with CINS Guidelines for vascular devices. Manufacturers specific recommendations should be noted and
PATIENT CARE PLAN FOR CARE OF PERIPHERAL MIDLINE The care plan is designed to be used in conjunction with CINS Guidelines for vascular devices. Manufacturers specific recommendations should be noted and
Department Policy. Code: D:PC Entity: Fairview Pharmacy Services. Department: Fairview Home Infusion. Manual: Policy and Procedure Manual
 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Midline (Extended Dwell Peripheral) Catheter Care and Management
Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Midline (Extended Dwell Peripheral) Catheter Care and Management
AXIOS Stent and Electrocautery Enhanced Delivery System. Quick Reference Guide
 AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
AXIOS Stent and Electrocautery Enhanced Delivery System Quick Reference Guide AXIOS Stent (front and side views) UPN Codes Flange Diameter (mm) Lumen Diamter (mm) Saddle Length (mm) Catheter OD (Fr) Catheter
Arterial Line Insertion Pre Reading
 PROCEDURE ACCREDITATION THE CANBERRA HOSPITAL EMERGENCY DEPARTMENT Arterial Line Insertion Pre Reading Indications Requirement for continuous blood pressure monitoring (all patients on pressors, inotropes,
PROCEDURE ACCREDITATION THE CANBERRA HOSPITAL EMERGENCY DEPARTMENT Arterial Line Insertion Pre Reading Indications Requirement for continuous blood pressure monitoring (all patients on pressors, inotropes,
Exceptional Performance and Ease of Placement
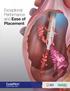 Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
Emergency clamp should always be readily available in case of accidental catheter fracture
 Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
ASAHI Corsair INSTRUCTIONS FOR USE
 ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
ASAHI Corsair INSTRUCTIONS FOR USE SYMBOLS Legal manufacturer Do not use if package is damaged GC Minimum GC I.D. REF Catalogue number Caution, consult accompanying documents Maximum injection pressure
Parts identification:
 TRANSPARENT CATHETERIZATION SIMULATOR Parts identification: Clamp Urethra channel Prostate velcro strap (male genitalia only) Transparent Simulator Bladder tubing and luer locks Female genitalia Male genitalia
TRANSPARENT CATHETERIZATION SIMULATOR Parts identification: Clamp Urethra channel Prostate velcro strap (male genitalia only) Transparent Simulator Bladder tubing and luer locks Female genitalia Male genitalia
Intravenous Catheter Complications
 Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
Central Venous Access Devices. Stephanie Cunningham Amy Waters
 Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
A step-by-step preparation guide
 A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use
 Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use DEVICE DESCRIPTION The Detachable Azur CX 35 Peripheral Coil System (Azur system) consists of a coil implant attached to a delivery system.
Azur CX 35 Peripheral Coil System (Detachable) Instructions for Use DEVICE DESCRIPTION The Detachable Azur CX 35 Peripheral Coil System (Azur system) consists of a coil implant attached to a delivery system.
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Sterile Technique & IJ/Femoral Return Demonstration
 Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Sterile Technique & IJ/Femoral Return Demonstration Sterile Technique Description: This is a return demonstration checklist used to evaluate participants in the simulated hands on skills portions for certification
Percutaneous Sheath Introducer Product with Arrow Raulerson Syringe
 K09907100A.f Page 1 Monday, October 25, 1999 1:08 PM Safety and Efficacy Considerations: The product is designed for single use only. Do not resterilize or reuse. Do not alter the sheath or any other kit/set
K09907100A.f Page 1 Monday, October 25, 1999 1:08 PM Safety and Efficacy Considerations: The product is designed for single use only. Do not resterilize or reuse. Do not alter the sheath or any other kit/set
Quick Start Guide. Venipuncture and Intramuscular Injection Training Arm
 Venipuncture and Intramuscular Injection Training Arm Quick Start Guide Realistic training arm for demonstrating and practicing proper venipuncture procedure and deltoid intramuscular injections. The venous
Venipuncture and Intramuscular Injection Training Arm Quick Start Guide Realistic training arm for demonstrating and practicing proper venipuncture procedure and deltoid intramuscular injections. The venous
Vascular access device selection & placement. Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University
 Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
Education for Self Administration of Intravenous Therapy HOME IV THERAPY PICC. Portacath
 HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
Mary Lou Garey MSN EMT-P MedFlight of Ohio
 Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
IO considerations. Daniel Dunham
 IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
IO considerations Daniel Dunham If patient is conscious Advise of EMERGENT NEED for this procedure and obtain informed consent Rule out contraindications Fracture. Excessive tissue and/or absence of adequate
BP and Heart Rate by Telemetry
 BP and Heart Rate by Telemetry Version: 1 Modified from: Butz et al. Physiol Genomics. 2001 Mar 8;5(2):89-97. Edited by: Dr. Lynette Bower, UC Davis Summary Reagents and Materials Protocol Reagent Preparation
BP and Heart Rate by Telemetry Version: 1 Modified from: Butz et al. Physiol Genomics. 2001 Mar 8;5(2):89-97. Edited by: Dr. Lynette Bower, UC Davis Summary Reagents and Materials Protocol Reagent Preparation
Chapter Goal. Learning Objectives 9/11/2012. Chapter 6. Venous Access
 Chapter 6 Venous Access Chapter Goal Understand basic principles of venous access & IV therapy, as well as relate importance of employing appropriate BSI precautions when employing these precautions Learning
Chapter 6 Venous Access Chapter Goal Understand basic principles of venous access & IV therapy, as well as relate importance of employing appropriate BSI precautions when employing these precautions Learning
Infusion Skills Competency Checklist To be used at annual skills fair or at any other time for IV Competency
 Employee Profile Infusion Skills Checklist Last Name First Name Middle Initial Employee Number Employee Discipline Check one: RN LPN Per state specific LPN Practice Acts Direct Supervisor s Name: Date
Employee Profile Infusion Skills Checklist Last Name First Name Middle Initial Employee Number Employee Discipline Check one: RN LPN Per state specific LPN Practice Acts Direct Supervisor s Name: Date
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use. Directions for Use. Contents
 NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
