Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
|
|
|
- Jasper Lane
- 6 years ago
- Views:
Transcription
1 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis
2 Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated specifically for therapeutic apheresis. * Seal Keeps system closed when accessed Valve Keeps system closed when deaccessed With an ever-growing number of apheresis procedures and a rising chronic apheresis patient population, there s a need for long-term vascular access options. Bard is the global leader in ports and we ve listened to the challenges and needs of the apheresis community. Users asked for a durable device that can provide easy, reliable access to the vascular system and deliver ideal flow rates that apheresis procedures demand. The POWERFLOW Implantable Apheresis IV Port is engineered to meet these needs, designed for long device life, maximum flow, and patient comfort. Robust Titanium Funnel guides IV catheter for reliable and quick access Needle Stop Prevents needle from piercing the valve Pre-cut Silicone Valve accepts 16 and 14 gauge IV catheters * As of April After 1000 IV catheter insertions, bench top leak testing was performed both with the device accessed (both 14G and 16G IV catheters tested separately) and with no IV catheter present. Bench testing may not be indicative of actual clinical performance. Different test methods may yield different results. Data on file, Bard Peripheral Vascular, Inc., Tempe, Arizona.
3 Engineered for Maximum Flow and Patient Comfort 9.6F CHRONOFLEX Large inner diameter polyurethane catheter delivers max flows Silicone Body Soft and lightweight to help address patient comfort 1.5 mm 2.6 mm 2.0 mm 3.1 mm 1.5 mm 3.1 mm 8F CHRONOFLEX Polyurethane Catheter 9.6F CHRONOFLEX Polyurethane Catheter 9.6F Silicone Catheter IV Access Replaces needle with IV catheter Bard s Largest Inner Diameter Polyurethane Port Catheter Totally Implantable To help address patient lifestyle Comparison of other Bard catheters; catheter depiction for reference only, not drawn to scale.
4 Bench Tested for High Flow Performance Designed for Easy Access The s unique design and access delivers flow rates up to 150 ml/min * at low pressure. access is performed at a shallow angle by percutaneous insertion of an overthe-needle soft peripheral intravenous catheter. Flow Rates at Normal Operating Pressure (-100 mmhg) with 9.6F CHRONOFLEX Catheter, 14 Ga IV Catheter ml/min * with 9.6F CHRONOFLEX Catheter, 16 Ga IV Catheter ml/min * Designed for Patient Comfort and Mobility accessed with 16G IV Catheter * Mean flow rates, 25 cm catheter, when tested in a benchtop model using a blood simulant with viscosity of 3.5cP, N=27. Simulated testing may not be indicative of actual clinical performance. Changes in blood viscosity, catheter length, and IV type will affect achievable flow rates.
5 The is part of the BARD PORTREADY Program, an initiative designed to provide resources for patient education and clinician training. Learn more at POWERFLOW Apheresis IV Port Description French Size Tray Type Product Code POWERFLOW Apheresis IV Port Intermediate Tray Components 9.6F Intermediate A POWERFLOW Apheresis IV Port CHRONOFLEX Radiopaque Polyurethane Single-Lumen Catheter, 9.6F, 45 cm Length 2 BD Insyte Autoguard Shielded IV Catheters, 16 G (1.7 mm OD) x 45 mm AIRGUARD Valved Introducer, 10F, Peel-Apart Sheath Syringe, 12 ml Introducer Needle, 18 G x 70 mm Tunneler 2 Catheter Locks STRUXURE Guidewire, 3 mm radius, J tip with Straightener Flushing Connector, 17 G Product and Packaging Do Not Contain Natural Rubber Latex REPRESENTATIVE S NAME CONTACT PHONE NUMBER PHYSICIAN S SIGNATURE POWERFLOW Implantable Apheresis IV Port Indications For Use: The Bard POWERFLOW Implantable Apheresis IV Port is indicated for patient therapies requiring repeated access to the vascular system. The port system can be used for long-term therapeutic apheresis, withdrawal of blood, and infusion of medications, IV fluids, parenteral nutrition solutions, blood and blood products. The Bard POWERFLOW Implantable Apheresis IV Port is indicated for power injection of contrast media. For power injection of contrast media, the maximum recommended infusion rate is 5 ml/s. Contraindications: 1) Catheter insertion in the subclavian vein medial to the border of the first rib, an area which is associated with higher rates of pinch-off. Port catheter may be placed in lateral subclavian vein based on evaluation by a qualified practitioner. 2) When the presence of infection, bacteremia, or septicemia is known or suspected at the prospective port placement site. 3) When the patient s vasculature and/or body size is insufficient for the size of the implanted device. 4) Placement in the patient arm, due to device size and potential heating effect in an MR system. 5) When the patient is known or is suspected to be allergic to materials contained in the device or placement components. The implanted device is primarily composed of titanium, silicone, polycarbonate and/or polyurethane. 6) If severe chronic obstructive pulmonary disease exists. 7) If the prospective insertion site has been previously irradiated. 8) If the prospective placement site has previously suffered episodes of venous thrombosis or vascular surgical procedures. 9) If local tissue factors will prevent proper device stabilization and/or access. 10) Hemodialysis, as the safety and effectiveness has not been established for this therapy. Warnings: 1) Needle access should be accomplished using Seldinger technique. Passage of the guidewire, sheath, introducer, and catheter should also be performed with Seldinger technique with careful attention to the signs and symptoms of vessel perforation. Should there be concern for vessel perforation, appropriate diagnostic testing should be performed. 2) Do not power inject through a port system that exhibits signs of clavicle-first rib compression or pinch-off as it may result in port system failure. 3) Failure to confirm IV catheter placement may result in infiltration/ extravasation. 4) If high dose heparin (1,000 5,000 units/ml) is used, aspirate the solution out of the device before use to prevent systemic heparinization of the patient. 5) DO NOT USE A SYRINGE SMALLER THAN 10 ml TO FLUSH AND CONFIRM PATENCY. Patency should be assessed with a 10 ml syringe or larger with sterile normal saline. Upon confirmation of patency, administration of medication should be given in a syringe appropriately sized for the dose. Do not infuse against resistance. 6) s are only power injectable when accessed with the BD Insyte Autoguard Shielded IV Catheter. 7) Failure to warm contrast media to body temperature prior to power injection may result in port system failure. 8) Failure to ensure patency of the catheter prior to power injection studies may result in port system failure. 9) Power injector machine pressure limiting feature may not prevent over pressurization of an occluded catheter. 10) Exceeding the maximum power injection flow rate may result in port system failure. 11) system indication for power injection of contrast media implies the port s ability to withstand the procedure, but it does not imply appropriateness of the procedure for a particular patient nor for a particular IV catheter. A suitably trained clinician is responsible for evaluating the health status of a patient as it pertains to a power injection procedure and for evaluating the suitability of any IV catheter used to access the port. 12) Do not exceed a 300 psi pressure limit setting on the power injection machine, or the maximum recommended flow rate on the IV catheter, if power injecting through the (max infusion rate is 5mL/s). 13) If local pain, swelling or signs of extravasation are noted during power injection, the injection should be stopped immediately. Precautions: 1) Check patient s records, and ask patient, whether they have any known allergies to chemicals or materials that will be used during the placement procedure. 2) Fill (prime) the device with sterile normal saline solution to help avoid air embolism. 3) Bard recommends the use of components provided in the kit. If additional items are to be used, check for proper fit prior to utilization. 4) Carefully follow the connection technique given in the IFU to ensure proper catheter connection and to avoid catheter damage. 5) Remember that some patients may be hyper-sensitive to heparin or suffer from heparin induced thrombocytopenia (HIT). These patients must not have their port locked with heparinized saline. 6) Encourage patient to keep patient ID card and present it to clinicians accessing their port. 7) Care should be taken to avoid excessive force when accessing an implanted port. 8) POWERFLOW IV Port is not accessed with Huber non-coring needles. To access the PowerFlow IV Port, use BD Insyte Autoguard Shielded IV Catheters, 16G or 14G, 1.75 inches (44mm) or longer. The exact IV catheter length should be determined by the clinical situation. 9) Do not bend the needle while using the product. 10) Do not reuse or reinsert the needle into the IV catheter. Reinsertion of the needle may cause damage to the IV catheter, which may lead to extravasation. 11) The IV catheter hub should not be left open to air while it is in the port. 12) During MRI, RF heating behavior for the Bard POWERFLOW Apheresis IV Port does not scale with static field strengths and has not been evaluated for static field strengths above 3T. Possible Complications: Air embolism Allergic Reaction Catheter or port erosion through the skin Device rotation or extrusion Extravasation Fibrin sheath formation Infection, including, but no limited to, pocket, catheter tunnel, and/or blood stream. Please consult package insert for more detailed safety information and instructions for use. For technical inquiries, contact the Bard Clinical Information Hotline at Bard, Advancing Lives and the Delivery of Health Care, AirGuard, PortReady, PowerFlow, and StruXure are trademarks and/or registered trademarks of C. R. Bard, Inc. or an affiliate. ChronoFlex is a trademarks and/or registered trademark of AdvanSource Biomaterials Corp. All other trademarks are property of their respective owners. Copyright 2017, C. R. Bard, Inc. All Rights Reserved. Illustrations by Mike Austin. Copyright All Rights Reserved. Bard Peripheral Vascular, Inc W. 3rd Street Tempe, AZ BPV/PRT3/1016/0002(1)
The High-Flow Port Designed & Indicated for Apheresis
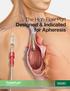 The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
40 35
 NURSING GUIDE 40 35 TABLE OF CONTENTS POWERFLOW Implantable Apheresis IV Port Summary Safety Information Description... 2 Indications for Use... 3 Contraindications... 4 Warnings... 4 Precautions... 5
NURSING GUIDE 40 35 TABLE OF CONTENTS POWERFLOW Implantable Apheresis IV Port Summary Safety Information Description... 2 Indications for Use... 3 Contraindications... 4 Warnings... 4 Precautions... 5
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Exceptional Performance and Ease of Placement
 Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
Exceptional Performance and Ease of Placement S IMU LATED FLO W PERFO RMANC E Higher Flow Rates Higher is Better GLIDEPATH catheters demonstrated on average in forward Precision catheters Lower Pressures
PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS
 PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS As a single-source provider, Bard Access Systems sets the standard for implanted ports and vascular access devices. We make it possible for
PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS As a single-source provider, Bard Access Systems sets the standard for implanted ports and vascular access devices. We make it possible for
Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE
 DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Vaxcel Implantable Ports Valved and Non-Valved. A Patient s Guide
 Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
The Power of Purple* Polyurethane PICC. Patient Guide. Access Systems
 The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
The Power of Purple* Polyurethane PICC Patient Guide Access Systems Preamble Your doctor is giving you a PowerPICC* catheter so that you can easily get the intravenous (IV) medicines you need. This catheter
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT
 PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
PLASTIC POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to
Designed to Reduce the Risk of Occlusion (1)
 Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Designed to Reduce the Risk of Occlusion (1) more efficient flushing better outcomes The Vortex* ports feature the Vortex Technology for a more efficient flushing. The Vortex Technology sets up efficient
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE
 Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Vascu-PICC WITH CUFF PERIPHERALLY INSERTED CENTRAL VEIN ACCESS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Vascu-PICC with cuff Peripherally Inserted Central Vein Catheters are designed for
Instructions For Use. Implantable Ports. with Open-Ended and Groshong Catheters
 Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS
 A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
A CATHETER FAMILY FOR ALL YOUR PATIENT NEEDS MAHURKAR * Elite acute dialysis catheter family EFFICIENCY OF DIALYSIS IN REVERSE FLOW Less than one percent recirculation Symmetrical tip design minimizes
POWER INJECTABLE IMPLANTABLE INFUSION PORT
 POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to the vascular
POWER INJECTABLE IMPLANTABLE INFUSION PORT INSTRUCTIONS FOR USE DESCRIPTION: The Power Injectable Implantable Infusion Port is an implantable access device designed to provide repeated access to the vascular
FLEXIC ATH LTD. Peripherally Inserted. Instructions n For Use.
 FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
FLEXIC ATH LTD * M/29M Peripherally Inserted Catheter Instructions n For Use This leaflet contains instructions for both stan- dard needle-introducer er and protection con- tained M/29 models, i.e., with
BARD. Instructions For Use
 BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
BARD Instructions For Use 1 Product Description PowerMidline Catheters are a family of peripherally placed catheters made from radiopaque body-softening polyurethane materials. Each PowerMidline Catheter
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
PICC and Midline Catheters
 The Argon Family of PICC and Midline Catheters Providing Optimal Outcomes for Long-Term & Intermediate Therapies Argon Medical Devices Argon knows the importance to both clinician and patient of thoughtfully
The Argon Family of PICC and Midline Catheters Providing Optimal Outcomes for Long-Term & Intermediate Therapies Argon Medical Devices Argon knows the importance to both clinician and patient of thoughtfully
Polyurethane Radiology PICC
 Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
Polyurethane Radiology PICC with Microintroducer Revised date: February 2016 Bard, PowerPICC, StatLock, and The Power of Purple are trademarks and/or registered trademarks of C. R. Bard, Inc. All other
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Mary Lou Garey MSN EMT-P MedFlight of Ohio
 Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
ARROW ENDURANCE. Extended Dwell Peripheral Catheter System. Rx only.
 ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
ARROW ENDURANCE Extended Dwell Peripheral Catheter System Rx only. Product Description: The ARROW Endurance catheter system is a sterile, single use peripheral intravascular device designed to permit access
Groshong* PICC and Catheters
 Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
Bard Access Systems Groshong* PICC and Catheters Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description, Indications & Contraindications 3 Contraindications,
THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS)
 Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Polyurethane Catheter
 References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
References 1. Aitken, D.R. and Minton, J.P. The Pinch-Off Sign: A Subclavian Catheters, American Journal of Surgery, Vol. 148, Nov. 1984, pp. 633-636. 2. Rubenstein, R.B., Alberty, R.E., et al. Hickman
CLARIVEIN INFUSION CATHETER
 CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
CLARIVEIN INFUSION CATHETER General Product Description Overview The ClariVein Infusion Catheter (ClariVein -IC) is an infusion catheter system designed to introduce physician-specified medicaments into
Finally, the Control You Need to Deliver Accurate Treatment
 Finally, the Control You Need to Deliver Accurate Treatment LIFESTREAM Covered Stent. has joined BD When you reach for a balloon expandable stent, you require accuracy. The LIFESTREAM Balloon Expandable
Finally, the Control You Need to Deliver Accurate Treatment LIFESTREAM Covered Stent. has joined BD When you reach for a balloon expandable stent, you require accuracy. The LIFESTREAM Balloon Expandable
Radiology Polyurethane Catheter
 Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Radiology Polyurethane Catheter with Microintroducer Revised date: January 2018 Bard, PowerPICC, Provena and StatLock are trademarks and/or registered trademarks of C. R. Bard, Inc. All other trademarks
Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer
 Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
Bard Access Systems Groshong Catheters with Introcan Safety Peripheral IV Catheter Introducer Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description 2 Product Description,
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family
 Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family MICRO-INTRODUCERS Micro-Introducer Kits Galt s Micro-Introducer Kits with smooth transition,
Galt Medical Corp. A leader in vascular and interventional medical devices. part of the GaltNeedleTech TM Family MICRO-INTRODUCERS Micro-Introducer Kits Galt s Micro-Introducer Kits with smooth transition,
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Successful IV Starts Revised February 2014
 Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
Successful IV Starts Revised February 2014 Why Intravenous Therapy? Used for access to the body s circulation Indications: Administer fluids, blood, medications, and nutrition Obtain laboratory specimens
BD PosiFlush Pre-Filled Syringes * Average reflux as measured in 4Fr PICC; data on file at BD.
 Positively Unique... BD PosiFlush Pre-Filled Syringes * Average reflux as measured in 4Fr PICC; data on file at BD. Designed to Eliminate Syringe-Induced Reflux * What is syringe-induced blood reflux?
Positively Unique... BD PosiFlush Pre-Filled Syringes * Average reflux as measured in 4Fr PICC; data on file at BD. Designed to Eliminate Syringe-Induced Reflux * What is syringe-induced blood reflux?
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
Extended Dwell Catheter Product Offerings. Providing optimal outcomes for long-term and intermediate therapies
 Extended Dwell Catheter Product Offerings Providing optimal outcomes for long-term and intermediate therapies Argon Medical Devices Extended Dwell Catheter Product Offerings The Argon family of PICC and
Extended Dwell Catheter Product Offerings Providing optimal outcomes for long-term and intermediate therapies Argon Medical Devices Extended Dwell Catheter Product Offerings The Argon family of PICC and
ompanionport Speciality Medical Devices For The Veterinary Community Surgical Suggestions
 Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Speciality Medical Devices For The Veterinary Community suture holes Place the CompanionPort in the subcutaneous port pocket off to one side so that the septum of the port will not lie directly beneath
Protecting Patients. Preserving Access.
 MAHURKAR * Family of Acute Care Catheters Protecting Patients. Preserving Access. A full spectrum of acute care catheters for use in hemodialysis, apheresis, infusion and renal replacement therapy The
MAHURKAR * Family of Acute Care Catheters Protecting Patients. Preserving Access. A full spectrum of acute care catheters for use in hemodialysis, apheresis, infusion and renal replacement therapy The
Instructions for Use 1
 Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Instructions for Use 1 Instructions for Use IMPORTANT! Please read before use. Description The Sniper is a dual lumen catheter comprised of an inner guidewire/infusion lumen and an outer, annular lumen
Proven Performance Through Innovative Design *
 Proven Performance Through Innovative Design * Deliver Our Next Generation AV Covered Stent Results The COVERA Vascular Covered Stent builds upon proven technologies from the category leader in AV Access.
Proven Performance Through Innovative Design * Deliver Our Next Generation AV Covered Stent Results The COVERA Vascular Covered Stent builds upon proven technologies from the category leader in AV Access.
Totally indwelling venous access devices
 Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE
 LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE LONG-TERM HEMODIALYSIS CATHETER INSTRUCTIONS FOR USE INDICATIONS FOR USE: The Centros and CentrosFLO long-term hemodialysis catheter are indicated
IV Drug Delivery Systems used in Cancer Care
 IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports
 Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
Xcela Hybrid PICC with PASV Valve Technology
 Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Xcela Hybrid PICC with PASV Valve Technology Directions For Use...3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, XCELA HYBRID PICC w/ PASV VALVE TECHNOLOGY, 14600125-01A
Quick Reference Guide
 Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Quick Reference Guide Indications for Use The AFX Endovascular AAA System is indicated for endovascular treatment in patients with AAA. The devices are indicated for patients with suitable aneurysm morphology
Victoria Chapman BS, RN, HP (ASCP)
 Victoria Chapman BS, RN, HP (ASCP) Considerations: Age Sex Body Composition Hydration Status Chemotherapy Use Access History Considerations: Immunosuppression Use Chemotherapy Frequency of plasma exchanges
Victoria Chapman BS, RN, HP (ASCP) Considerations: Age Sex Body Composition Hydration Status Chemotherapy Use Access History Considerations: Immunosuppression Use Chemotherapy Frequency of plasma exchanges
Central Line Care and Management
 Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
LINEAR 7.5Fr. IAB Catheter
 LINEAR 7.5Fr. IAB Catheter The LINEAR 7.5Fr. Catheter Easier to insert* Proprietary IAB Membrane provides better performance* Abrasion resistance improved 43%* Improved fatigue resistance* Immediate inflation
LINEAR 7.5Fr. IAB Catheter The LINEAR 7.5Fr. Catheter Easier to insert* Proprietary IAB Membrane provides better performance* Abrasion resistance improved 43%* Improved fatigue resistance* Immediate inflation
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
1/22/2016. Disclaimer. Disclaimer
 Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
Disclaimer Omnicare, Inc., as a provider of Infusion Pharmacy Services, is committed to the establishment and maintenance of the highest quality of care in infusion therapy services. This Infusion Therapy
Groshong Central Venous Catheters
 Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
Bard Access Systems Groshong Central Venous Catheters Long Term Instructions For Use Table of Contents Contents Page Introduction... 1 Description Placement Schematics Groshong Valve Function Indications
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE REF LOT STERILE EO. Manufactured for: 824 Twelfth Avenue Bethlehem, PA
 ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
ASEPT Pleural Drainage System LS-00116-01-AB 017-11 INSTRUCTIONS FOR USE Do not reuse STERILIZE Do not resterilize 30 C Rx only 15 C REF Store at controlled room temperature 15-30 C (59-86 F) Keep away
Denver ascites shunts. For patients with refractory ascites
 Denver ascites shunts For patients with refractory ascites Silique surface treatment For patients with refractory ascites, consider peritoneovenous shunting (PVS) with the Denver shunt. Denver shunts now
Denver ascites shunts For patients with refractory ascites Silique surface treatment For patients with refractory ascites, consider peritoneovenous shunting (PVS) with the Denver shunt. Denver shunts now
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use. Directions for Use. Contents
 NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
NexSite HD, Hemodialysis Symmetric Tip Catheter for long term use Directions for Use Contents Note to Physicians: Please consult the on-line training video on www.marvaomedical.com for information on the
Intravenous Catheter Complications
 Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Short-Term Dialysis Catheters
 Short-Term Dialysis Catheters Instructions For Use Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT 84116 USA 1-801-522-5000 Clinical Information Hotline: 1-800-443-3385 Ordering Information:
Short-Term Dialysis Catheters Instructions For Use Bard Access Systems, Inc. 605 North 5600 West Salt Lake City, UT 84116 USA 1-801-522-5000 Clinical Information Hotline: 1-800-443-3385 Ordering Information:
DISCOVER NEW HORIZONS IN FLUID DRAINAGE. Bringing Safety and Convenience to Fluid Drainage Management
 DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
DISCOVER NEW HORIZONS IN FLUID DRAINAGE Bringing Safety and Convenience to Fluid Drainage Management DRAIN ASEPT Pleural and Peritoneal Drainage Catheter System 600mL or 1,000mL Evacuated Drainage Bottle
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
Directions For Use. All directions should be read before use. Page 1 of 8
 Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
Directions For Use All directions should be read before use Page 1 of 8 WARNING: For single use only. Do not reuse, reprocess or re-sterilize. Reuse, reprocessing or re-sterilization may compromise the
ASEPT. Pleural Drainage System INSTRUCTIONS FOR USE. Rx only REF LOT. STERILE EO Sterilized using ethylene oxide
 ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
ASEPT Pleural Drainage System INSTRUCTIONS FOR USE L1171/D REV 2014-02 2 Do not reuse 2 STERILIZE Do not resterilize Store at room temperature STERILE EO Sterilized using ethylene oxide Rx only Do not
BioFlo PICC. with ENDEXO Technology. Directions For Use A
 BioFlo PICC with ENDEXO Technology Directions For Use... 3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, BioFlo PICCwith ENDEXO Technology, 14600123-01A 14600123-01
BioFlo PICC with ENDEXO Technology Directions For Use... 3 Navilyst Medical, Master DFU Template 8in x 8in Global, 146T0808 Rev/Ver. A, DFU, BioFlo PICCwith ENDEXO Technology, 14600123-01A 14600123-01
LINEAR. 7.5Fr. IAB Catheter. This document is intended to provide information to an international audience outside of the US.
 LINEAR 7.5Fr. IAB Catheter This document is intended to provide information to an international audience outside of the US. Maquet offers a range of convenient and innovative clinical solutions for counterpulsation
LINEAR 7.5Fr. IAB Catheter This document is intended to provide information to an international audience outside of the US. Maquet offers a range of convenient and innovative clinical solutions for counterpulsation
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template
 ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ARROW EZ-IO Intraosseous Vascular Access System Procedure Template PURPOSE To provide procedural guidance for establishment of intraosseous vascular access using the ARROW EZ-IO Intraosseous Vascular Access
ROTABLATORTM. Peripheral. Rotational Atherectomy System. Quick Reference Cards
 Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
Peripheral ROTABLATORTM Rotational Atherectomy System Quick Reference Cards Technical Assistance: 1.800.949.6708 Customer Service: 1.888.272.1001 Complaints: 1.800.811.3211 Setup Video: www.bostonscientific.com/rotablatorsetup
External Ref: Andres, D.A., et al. Catheter Pinch-Off Syndrome: Recognition and Management.
 Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Complications With Intravenous
Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Complications With Intravenous
Port Design. Page 1. Port Placement, Removal, and Management. Selecting a Vascular Access Device. Thomas M. Vesely, MD
 Non-Dialysis Procedures Port Placement, Removal, and Management Thomas M. Vesely, MD Saint Louis, Missouri Selecting a Vascular Access Device Duration of use Number of lumens Frequency used Blood flow
Non-Dialysis Procedures Port Placement, Removal, and Management Thomas M. Vesely, MD Saint Louis, Missouri Selecting a Vascular Access Device Duration of use Number of lumens Frequency used Blood flow
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE
 Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
Split-Stream LONG-TERM HEMODIALYSIS INSTRUCTIONS FOR USE INDICATIONS FOR USE: INSTRUCTIONS FOR USE The Medcomp Split-Stream is indicated for use in attaining Long-Term vascular access for Hemodialysis
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
Protecting Patients. Preserving Access.
 MAHURKAR * Family of Acute Care Catheters Protecting Patients. Preserving Access. The Gold Standard Mahurkar * Acute Care Catheter Family Ease of insertion. Optimal flow rates. Patient comfort. A variety
MAHURKAR * Family of Acute Care Catheters Protecting Patients. Preserving Access. The Gold Standard Mahurkar * Acute Care Catheter Family Ease of insertion. Optimal flow rates. Patient comfort. A variety
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Advanced Innovation for Exceptional Performance
 Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
Advanced Innovation for Exceptional Performance longest BALLOON LENGTHS ON THE MARKET Bard Peripheral Vascular Ultraverse 014 300 mm Boston Scientific Coyote 220 mm Cordis SLEEK RX 220 mm Aviator Plus
Trust Cook s line of ports to meet your vascular access needs.
 Trust Cook s line of ports to meet your vascular access needs. Vascular access sets The convenience of power injection with the comfort of Vital-Port. Features: Maximum flow rate: 5 ml/sec Maximum pressure
Trust Cook s line of ports to meet your vascular access needs. Vascular access sets The convenience of power injection with the comfort of Vital-Port. Features: Maximum flow rate: 5 ml/sec Maximum pressure
The next step in dialysis catheter technology
 arrow nextstep retrograde The next step in dialysis catheter technology NEXTSTEP NOW STEP-TIP EASE MEETS SPLIT-TIP FLOW The Arrow NextStep retrograde is the first of its kind, a chronic hemodialysis catheter
arrow nextstep retrograde The next step in dialysis catheter technology NEXTSTEP NOW STEP-TIP EASE MEETS SPLIT-TIP FLOW The Arrow NextStep retrograde is the first of its kind, a chronic hemodialysis catheter
Appendix E: Overview of Vascular
 Appendix E: Overview of Vascular 56 Peripheral Short Catheter, less than 3 inches (7.5 cm) in length; over-the-needle catheter is most common. Inserted by percutaneous venipuncture, generally into a hand
Appendix E: Overview of Vascular 56 Peripheral Short Catheter, less than 3 inches (7.5 cm) in length; over-the-needle catheter is most common. Inserted by percutaneous venipuncture, generally into a hand
Emergency clamp should always be readily available in case of accidental catheter fracture
 Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
Note: Please see individual policies for further information. Flushing best practice: Always use a 10 diameter syringe or larger when first accessing and when flushing vascular access device (VAD) Use
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services
Central Venous Access Devices. Stephanie Cunningham Amy Waters
 Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System
 100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
100000017144.3 100000017144.3 Instructions for Use Cordis PRECISE PRO Rx Nitinol Stent System Figure 1.065" (1.65 mm) OR.078" (1.98 mm) 16 4 13 10 11 15 8 3 14.062" (1.57 mm) OR.065" (1.65 mm).038" (0.97
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Lifecath Twin & Dualyse/Trilyse Permanent and Temporary Renal Catheters
 Lifecath Twin & Dualyse/Trilyse Permanent and Temporary Renal Catheters Accurate locking dose Excellent flow rates Safe connections vygon@vygon.co.uk www.vygon.co.uk Product Information Product Information
Lifecath Twin & Dualyse/Trilyse Permanent and Temporary Renal Catheters Accurate locking dose Excellent flow rates Safe connections vygon@vygon.co.uk www.vygon.co.uk Product Information Product Information
IV Fluids Nursing B23 Objectives Serum Osmolality 275 to 295 Isotonic
 1 IV Fluids Nursing B23 2 Objectives 3 Serum Osmolality Serum osmolality solute concentration of a solution Higher osmolality means greater pulling power for water Normal serum osmolality is 275 to 295
1 IV Fluids Nursing B23 2 Objectives 3 Serum Osmolality Serum osmolality solute concentration of a solution Higher osmolality means greater pulling power for water Normal serum osmolality is 275 to 295
Complications Associated With IV Therapy
 Occlusion is the partial or complete obstruction of a catheter, which obstructs the infusion of solutions or medications. Occlusions can result from the coagulation of blood (thrombotic) or from obstruction
Occlusion is the partial or complete obstruction of a catheter, which obstructs the infusion of solutions or medications. Occlusions can result from the coagulation of blood (thrombotic) or from obstruction
Fundamentals of Flushing and Locking
 Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
Fundamentals of Flushing and Locking Vascular Access Devices Fundamentals of Flushing and Locking Purpose, Product, and Process 2016 BD. BD and the BD Logo are trademarks of Becton, Dickinson and Company.
High Flow Family. Short Term Dialysis Catheters. Dialysis
 High Flow Family Short Term Dialysis Catheters Dialysis High Flow Triple Lumen 13.5 Fr Unique tip design, superior to the classic sidehole design: Clotting risk is considerably reduced Adhesion risk to
High Flow Family Short Term Dialysis Catheters Dialysis High Flow Triple Lumen 13.5 Fr Unique tip design, superior to the classic sidehole design: Clotting risk is considerably reduced Adhesion risk to
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections M A R C H 15, 2017
 The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections D A R C Y DOELLMAN M S N, RN, CRNI, VA - BC M A R C H 15, 2017 LOUISVILLE, KENTUCKY Cincinnati Children s Hospital 642
The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections D A R C Y DOELLMAN M S N, RN, CRNI, VA - BC M A R C H 15, 2017 LOUISVILLE, KENTUCKY Cincinnati Children s Hospital 642
PTA Balloon Dilatation Catheter
 PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
PTA Balloon Dilatation Catheter March 2016 1/6(3214-0) Catalogue number Serial number Lot number Use by Do not reuse Do not resterilize Do not use if package is damaged Consult instructions for use Keep
Advocate Christ Medical Center CVC Placement Certification Course
 Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
IV Therapy January, 08 Tip of the Month
 Every Hub Every Time IV Therapy January, 08 Tip of the Month Every Hub Every Time No matter what the occasion, SCRUB the catheter ports every single time before access. Evidence Supports SCRUBBING using
Every Hub Every Time IV Therapy January, 08 Tip of the Month Every Hub Every Time No matter what the occasion, SCRUB the catheter ports every single time before access. Evidence Supports SCRUBBING using
DURABLE. CONSISTENT. SAFE. IN.PACT Admiral Drug-Coated Balloon
 DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
DURABLE. CONSISTENT. SAFE. Drug-Coated Balloon THE SCIENCE BEHIND THE OUTCOMES DCB has proven, long-term durable outcomes across multiple clinical trials, as well as across complex patient and lesion types.
