MEDICAL POLICY SUBJECT: BULKING AGENTS FOR TREATMENT OF URINARY OR FECAL INCONTINENCE. POLICY NUMBER: CATEGORY: Technology Assessment
|
|
|
- Constance Stevens
- 6 years ago
- Views:
Transcription
1 MEDICAL POLICY SUBJECT: BULKING AGENTS FOR (ARCHIVED DATE: 05/28/09-, EDITED DATE: 05/27/10, 05/19/11, 05/24/12, 05/23/13) PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied. Medical policies apply to commercial and Medicaid products only when a contract benefit for the specific service exists. Medical policies only apply to Medicare products when a contract benefit exists and where there are no National or Local Medicare coverage decisions for the specific service. POLICY STATEMENT: I. Based upon our criteria and review of the peer-reviewed literature, the use of urethral bulking agents has been medically proven to be effective. Types of urethral bulking agents that have received FDA approval include: A. Collagen implants have been medically proven to be effective and therefore, can be considered a medically appropriate treatment option in the management of stress incontinence due to urethral sphincter dysfunction that is unresponsive to conservative therapy, for the following: 1. Male or female patients with congenital sphincter weakness secondary to conditions such as myelomeningocele or epispadias; 2. Male or female patients with acquired sphincter weakness secondary to spinal cord lesion; 3. Male patients following trauma, including prostatectomy and/or radiation; and 4. Female patients without urethral hypermobility and with abdominal leak point pressure of 100 cm H 2 O or less. B. The following bulking agents have been medically proven to be effective, and therefore, can be considered a medically appropriate treatment option in the management of only women with stress incontinence due to ISD that is unresponsive to conservative therapy: 1. Carbon-coated beads (Durasphere) 2. Spherical particles of calcium hydroxylapatite (Coaptite ); and 3. Polydimethylsiloxane (silicone elastomer) particles (Macroplastique ). II. Based upon our criteria and assessment of peer-reviewed literature, the following urethral bulking agents have not been medically proven to be effective and therefore, are considered investigational for the treatment of urinary stress incontinence: A. Polytetrafluoroethylene (Teflon ), B. Autologous fat, and C. Microballoons (UroVive ). III. Based upon our criteria and assessment of peer-reviewed literature, urethral bulking agents when used in the treatment of other types of urinary incontinence (e.g., urinary retention with overflow incontinence, ectopic ureter, neurogenic bladder dysfunction, urinary fistulas and psychogenic incontinence), as yet, have not demonstrated a benefit to patient outcomes and are considered not medically necessary. IV. Based upon our criteria and assessment of peer-reviewed literature, the use of perianal bulking agents to treat fecal incontinence is considered investigational. Refer to Corporate Medical Policy # regarding Endoscopic Injection of Bulking Agents for the Treatment of Vesicoureteral Reflux. A nonprofit independent licensee of the BlueCross BlueShield Association
2 (ARCHIVED DATE: 05/28/09-, PAGE: 2 OF: 5 POLICY GUIDELINES: I. Use of Durasphere is restricted to prescription use by doctors trained in cystoscope use who have completed the appropriate implant training program. II. Patients whose incontinence does not improve with 5 injection procedures (e.g., 5 separate treatment sessions) are considered treatment failures and further treatment of urinary incontinence by Durasphere implant is considered not medically necessary. III. The Federal Employee Health Benefit Program (FEHBP/FEP) requires that procedures, devices or laboratory tests approved by the U.S. Food and Drug Administration (FDA) may not be considered investigational and thus these procedures, devices or laboratory tests may be assessed only on the basis of their medical necessity. DESCRIPTION: One physiologic cause of urinary incontinence is intrinsic sphincter deficiency (ISD). ISD describes a condition in which the urethral sphincter has been damaged by surgery, neurological disorders, or trauma. The result is sphincter incompetence and urinary leakage. There are two types of ISD: I. Total ISD is characterized by constant involuntary dripping of urine from the urethra day and night without bladder distention, as determined by physical exam of the abdomen and measurement of residual urine. II. Partial sphincter deficiency, also known as stress incontinence, affects patients who may experience involuntary loss of urine when coughing, bending, lifting or performing any maneuver that increases transabdominal pressure. Urethral bulking agents are substances that have been developed for use in the treatment of stress incontinence due to ISD. These substances are injected at the area of the bladder neck and proximal urethra. The increased bulk caused by these substances enhances urethral resistance to the outflow of urine, thus reducing urine leakage. The goal of this procedure is to create enhanced urethral coaptation by insertion of a bulking agent that is nonimmunogenic, and will not migrate from where it is injected. Urethral bulking agents may be injected over a course of several treatments until the desired effect is achieved. The procedure for injecting urethral-bulking agents is either performed in the doctor s office or the outpatient department of a hospital. Pyrolytic carbon-coated beads (Durasphere ) are indicated for use in the treatment of adult women with stress urinary incontinence due to ISD. Carbon beads do not require skin testing, as there is no associated antigenicity. Durasphere is injected sub-mucosally at the bladder neck in females. The final bulking result is derived from the combination of the carbon-coated beads and the body s own collagen. Coaptite is an injectable implant composed of spherical particles of calcium hydroxylapatite. It is indicated for the treatment of stress urinary incontinence due to (ISD) in adult females. Macroplastique is an injectable soft-tissue urethral bulking agent for treating stress urinary incontinence primarily due to intrinsic sphincter deficiency. Macroplastique is made up of a water-soluble gel (polyvinylpyrrolidone) that is absorbed and removed from the body in urine, and the man-made, rubber-like, silicone elastomer implant material (cross-linked polydimethylsiloxane) that is permanent and not absorbed by the body. The silicone elastomer causes the bulking effect around the urethra after implantation. Following the success of periurethral bulking agents for treating SUI, bulking agents injected into the anal canal have been proposed for treating fecal incontinence associated with internal anal sphincter (IAS) dysfunction. The bulking agent is injected into the submucosa of the anal canal to increase tissue bulk in the area, which narrows the opening of the anus. Current treatment options for fecal incontinence include conservative measures e.g., dietary changes, pharmacotherapy and pelvic floor muscle exercises, sacral nerve stimulation, and surgical interventions to correct an underlying problem.
3 (ARCHIVED DATE: 05/28/09-, PAGE: 3 OF: 5 Several agents identical to or similar to those used for urinary incontinence (e.g., Durasphere, silicone biomaterial) have been studied for the treatment of fecal incontinence. However Solesta, (Q-Med), a formulation of non-animal stabilized hyaluronic acid/dextranomer in stabilized hyaluronic acid (NASHA Dx) has FDA approval for treating fecal incontinence. RATIONALE: Urinary Incontinence Cross-linked collagen, carbon-coated beads (Durasphere ), Coaptite, and Macroplastique have been approved by the FDA, but only for the treatment of urinary stress incontinence due to ISD. Durasphere, Coaptite, and Macroplastique are approved only for use in women. Other synthetic urethral bulking agents do not have FDA approval. Though autologous fat injections do not require FDA approval, results of clinical studies have shown poor health outcomes associated with reabsorption and fibrotic ingrowth. Clinical trials investigating the safety and efficacy of Durasphere have shown that they have improved the net health outcomes of patients in the short term by achieving dryness or significant improvements in the symptoms of incontinence and provided benefits comparable to alternative treatments such as surgery. Long-term effectiveness of these urethral-bulking agents has yet to be established. Fecal Incontinence Maeda et al., in a Cochrane Review (2013) determined the effectiveness of perianal injection of bulking agents for the treatment of fecal incontinence in adults. Five eligible randomised trials with a total of 382 patients were identified. Four of the trials were at an uncertain or high risk of bias. Most trials reported a short term benefit from injections regardless of the material used, including placebo saline injection. One study demonstrated dextranomer in stabilized hyaluronic acid (NASHA Dx) to be more effective than sham injection but with more adverse effects. Dextranomer in stabilized hyaluronic acid (NASHA Dx) was better than sham injections at six months (65/136, 48% versus 48/70, 69%) participants not improved, defined as less than 50% reduction in incontinence episodes, (RR 0.70, 95% CI 0.55 to 0.88); with more incontinence free days (3.1 days compared with 1.7 in the sham treatment group, median 1.40 days, 95% CI 0.33 to 2.47). Another study comparing silicone material (PTQ ) to saline injections was too small to demonstrate a clinical benefit compared to the control injection of normal saline. A silicone biomaterial (PTQ ) was shown to provide some advantages and was safer in treating fecal incontinence than carboncoated beads (Durasphere ) in the short term. Similarly, there were short term benefits from injections delivered under ultrasound guidance compared with digital guidance. No long term evidence on outcomes was available and further conclusions were not warranted from the available data. None of the studies reported patient evaluation of outcomes and thus it is difficult to gauge whether the improvement in incontinence scores matched practical symptom improvements that mattered to the patients. The authors concluded one large randomised controlled trial has shown that this form of treatment using dextranomer in stabilized hyaluronic acid (NASHA Dx) improves continence for a little over half of patients in the short term. However, the number of identified trials was limited and most had methodological weaknesses. CODES: Number Description Eligibility for reimbursement is based upon the benefits set forth in the member s subscriber contract. CODES MAY NOT BE COVERED UNDER ALL CIRCUMSTANCES. PLEASE READ THE POLICY AND GUIDELINES STATEMENTS CAREFULLY. Codes may not be all inclusive as the AMA and CMS code updates may occur more frequently than policy updates. CPT: Endoscopic injection of implant material into the submucosal tissues of the urethra and/or bladder neck Copyright 2014 American Medical Association, Chicago, IL HCPCS: C9735 Anoscopy; with directed submucosal injection(s), any substance (used for the Solesta Procedure)
4 (ARCHIVED DATE: 05/28/09-, PAGE: 4 OF: 5 L8603 L8604 L8605 L8606 Injectable bulking agent, collagen implant, urinary tract, per 2.5 ml syringe, includes shipping and necessary supplies Injectable bulking agent, dextranomer/hyaluronic acid copolymer implant, urinary tract, 1 ml syringe, includes shipping and necessary supplies Injectable bulking agent, dextranomer/hyaluronic acid copolymer implant, anal canal, 1 ml, includes shipping and necessary supplies Injectable bulking agent, synthetic implant, urinary tract, 1 ml syringe, includes shipping and necessary supplies ICD9: Intrinsic (urethral) sphincter deficiency (ISD) Stress incontinence, female Urinary incontinence, unspecified Urge incontinence Stress incontinence, male Mixed incontinence (female) (male) Incontinence without sensory awareness Nocturnal enuresis Continuous leakage Other urinary incontinence ICD10: N36.42-N36.43 Urethral functional and muscular disorders (code range) N39.3 Stress incontinence (female) (male) N39.41-N39.46, N39.44-N39.46 Other specified urinary incontinence (code range) N Other specified urinary incontinence R32 REFERENCES: Unspecified urinary incontinence BlueCross BlueShield Association. Periurethral bulking agents for the treatment of urinary and fecal incontinence. Medical Policy Reference Manual Policy # Mar 14. Davila GW. Nonsurgical outpatient therapies for the management of female stress urinary incontinence: long-term effectiveness and durability. Adv Urol 2011;2011: Keegan PE et al. Periurethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2009;1:CD Lovatsis D, et al; Society of Obstetricians and Gynaecologists of Canada Urogynaecology Committee. Guidelines for the evaluation and treatment of recurrent urinary incontinence following pelvic floor surgery. J Obstet Gynaecol Can 2010; 32(9): Maeda Y, et al. Perianal injectable bulking agents as a treatment for faecal incontinence in adults. Cochrane Database Syst Rev Feb 28;2:CD
5 (ARCHIVED DATE: 05/28/09-, PAGE: 5 OF: 5 Maslekar S, et al. Injectable collagen for the treatment of fecal incontinence: long-term results. Dis Colon Rectum 2013 Mar;56(3): U.S. Food and Drug Administration. Summary of safety and effectiveness data for Coaptite. Available at [ accessed 2/3/14. U.S. Food and Drug Administration. Durasphere injectable bulking agent. Available at [ accessed 2/3/14. U.S. Food and Drug Administration. Summary of safety and effectiveness data for Macroplastique. Available at [ ApprovedDevices/ucm htm] accessed 1/31/14. KEY WORDS: Autologous fat, periurethral, Bovine collagen, Carbon coated beads, Coaptite, Collagen, GAX, Macroplastique, Microballoons, Teflon, Uryx, Tegress, Solesta. CMS COVERAGE FOR MEDICARE PRODUCT MEMBERS There is currently a National Coverage Determination (NCD) for Incontinence Control Devices. Please refer to the following NCD website for Medicare Members:
Subject: Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 09-A9000-03 Original Effective Date: 02/15/12 Reviewed: 12/06/18 Revised: 12/15/18 Subject: Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence THIS MEDICAL COVERAGE GUIDELINE
09-A9000-03 Original Effective Date: 02/15/12 Reviewed: 12/06/18 Revised: 12/15/18 Subject: Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence THIS MEDICAL COVERAGE GUIDELINE
Injectable Bulking Agents for the Treatment of Fecal Incontinence. Policy Specific Section: September 27, 2013 January 1, 2015
 Medical Policy Injectable Bulking Agents for the Treatment of Fecal Incontinence Type: Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: September 27,
Medical Policy Injectable Bulking Agents for the Treatment of Fecal Incontinence Type: Investigational / Experimental Policy Specific Section: Surgery Original Policy Date: Effective Date: September 27,
INJECTABLE BULKING AGENTS FOR THE TREATMENT OF URINARY AND FECAL INCONTINENCE
 FECAL INCONTINENCE Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FECAL INCONTINENCE Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Corporate Medical Policy
 Corporate Medical Policy Injectable Bulking Agents for the Treatment of Urinary and Fecal File Name: Origination: Last CAP Review: Next CAP Review: Last Review: injectable_bulking_agents_for_the_treatment_of_urinary_and_fecal_incontinence
Corporate Medical Policy Injectable Bulking Agents for the Treatment of Urinary and Fecal File Name: Origination: Last CAP Review: Next CAP Review: Last Review: injectable_bulking_agents_for_the_treatment_of_urinary_and_fecal_incontinence
Periurethral Bulking Agents for the Treatment of Urinary Incontinence. Original Policy Date
 MP 7.01.14 Periurethral Bulking Agents for the Treatment of Urinary Incontinence Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature
MP 7.01.14 Periurethral Bulking Agents for the Treatment of Urinary Incontinence Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature
Medical Coverage Policy Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Medical Coverage Policy Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence EFFECTIVE DATE:02 5 2013 POLICY LAST UPDATED: 12 18 2018 OVERVIEW Bulking agents are injectable substances
Medical Coverage Policy Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence EFFECTIVE DATE:02 5 2013 POLICY LAST UPDATED: 12 18 2018 OVERVIEW Bulking agents are injectable substances
Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Policy Number: 7.01.19 Last Review: 6/2014 Origination: 2/1994 Next Review: 6/2015 Policy Blue Cross and Blue Shield of Kansas
Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Policy Number: 7.01.19 Last Review: 6/2014 Origination: 2/1994 Next Review: 6/2015 Policy Blue Cross and Blue Shield of Kansas
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Injectable Bulking Agents for the Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Injectable Bulking Agents for the Treatment of Urinary and
Injectable Bulking Agents for the Page 1 of 18 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Injectable Bulking Agents for the Treatment of Urinary and
Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 7.01.19 Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Section 7.0 Surgery Subsection Effective Date February 27, 2015 Original Policy Date February 27, 2015 Next Review
7.01.19 Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Section 7.0 Surgery Subsection Effective Date February 27, 2015 Original Policy Date February 27, 2015 Next Review
Protocol. Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Injectable Bulking Agents for the Treatment of Urinary and Fecal (70119) Medical Benefit Effective Date: 07/01/15 Next Review Date: 05/18 Preauthorization No Review Dates: 01/08, 11/08, 09/09, 09/10, 09/11,
Injectable Bulking Agents for the Treatment of Urinary and Fecal (70119) Medical Benefit Effective Date: 07/01/15 Next Review Date: 05/18 Preauthorization No Review Dates: 01/08, 11/08, 09/09, 09/10, 09/11,
Medical Policy Title: Periurethral Bulking ARBenefits Approval: <Date>
 Medical Policy Title: Periurethral Bulking ARBenefits Approval: Agents for the Treatment of Incontinence Effective Date: 01/01/2012 Document: ARB0279 Revision Date: Code(s): 51715 Endoscopic injection
Medical Policy Title: Periurethral Bulking ARBenefits Approval: Agents for the Treatment of Incontinence Effective Date: 01/01/2012 Document: ARB0279 Revision Date: Code(s): 51715 Endoscopic injection
MP Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Medical Policy MP 7.01.19 BCBSA Ref. Policy: 7.01.19 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: Surgery Related Policies 1.01.17 Pelvic Floor Stimulation as a Treatment of Urinary and
Medical Policy MP 7.01.19 BCBSA Ref. Policy: 7.01.19 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: Surgery Related Policies 1.01.17 Pelvic Floor Stimulation as a Treatment of Urinary and
Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Last Review Status/Date: September 2015 Page: 1 of 16 Urinary and Fecal Incontinence Description Bulking agents are injectable substances that used to increase tissue bulk. They can be injected periurethrally
Last Review Status/Date: September 2015 Page: 1 of 16 Urinary and Fecal Incontinence Description Bulking agents are injectable substances that used to increase tissue bulk. They can be injected periurethrally
Name of Policy: Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Name of Policy: Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Policy #: 455 Latest Review Date: July 2014 Category: Surgery Policy Grade: B Background/Definitions: As a
Name of Policy: Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Policy #: 455 Latest Review Date: July 2014 Category: Surgery Policy Grade: B Background/Definitions: As a
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND (TRUS)
 MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Policy Number: 7.01.19 Last Review: 6/2017 Origination: 2/1994 Next Review: 6/2018 Policy Blue Cross and Blue Shield of Kansas
Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence Policy Number: 7.01.19 Last Review: 6/2017 Origination: 2/1994 Next Review: 6/2018 Policy Blue Cross and Blue Shield of Kansas
Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Last Review Status/Date: March 2017 Page: 1 of 16 Urinary and Fecal Incontinence Description Bulking agents are injectable substances used to increase tissue bulk. They can be injected periurethrally to
Last Review Status/Date: March 2017 Page: 1 of 16 Urinary and Fecal Incontinence Description Bulking agents are injectable substances used to increase tissue bulk. They can be injected periurethrally to
MP Injectable Bulking Agents for the Treatment of Urinary and Fecal Incontinence
 Medical Policy MP 7.01.19 BCBSA Ref. Policy: 7.01.19 Last Review: 08/30/2017 Effective Date: 08/30/2017 Section: Surgery End Date: 08/19/2018 Related Policies 1.01.17 Pelvic Floor Stimulation as a Treatment
Medical Policy MP 7.01.19 BCBSA Ref. Policy: 7.01.19 Last Review: 08/30/2017 Effective Date: 08/30/2017 Section: Surgery End Date: 08/19/2018 Related Policies 1.01.17 Pelvic Floor Stimulation as a Treatment
Clinical Policy: Urinary Incontinence Devices and Treatments
 Clinical Policy: Reference Number: CP.MP.142 Last Review Date: 03/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications Revision Log Description
Clinical Policy: Reference Number: CP.MP.142 Last Review Date: 03/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications Revision Log Description
Fecal Incontinence. Sphincter Augmentation. Alaiyan Bilal MD Hadassah mt scopus
 Fecal Incontinence Sphincter Augmentation Alaiyan Bilal MD Hadassah mt scopus AUGMENTATION METHODS Injectables( Bulking agents) Radiofrequency INJECTABLES Biomaterials Polytef, Autologous fat, GAX (glutaraldehyde
Fecal Incontinence Sphincter Augmentation Alaiyan Bilal MD Hadassah mt scopus AUGMENTATION METHODS Injectables( Bulking agents) Radiofrequency INJECTABLES Biomaterials Polytef, Autologous fat, GAX (glutaraldehyde
Urinary Incontinence. Biopolymer GmbH & Co.KG - Germany
 Urinary Incontinence Treatment Options: 1. Physical Therapy: a conservative early stage treatment for UI, including pelvic floor muscles training known as Kegel exercise. 2. Medications: by means of pharmaceutical
Urinary Incontinence Treatment Options: 1. Physical Therapy: a conservative early stage treatment for UI, including pelvic floor muscles training known as Kegel exercise. 2. Medications: by means of pharmaceutical
Clinical Policy Title: Injectable bulking agents fecal incontinence
 Clinical Policy Title: Injectable bulking agents fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June 5, 2018
Clinical Policy Title: Injectable bulking agents fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June 5, 2018
Clinical Policy Title: Injectable bulking agents for fecal incontinence
 Clinical Policy Title: Injectable bulking agents for fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June
Clinical Policy Title: Injectable bulking agents for fecal incontinence Clinical policy number: 08.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June
A Case of Fecal Incontinence: Medical and Interventional Treatment Options
 A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
6 Page Male Incontinence Booklet 10/09/ :44 Page 1. The Natural Non-Surgical Option for Male Urinary Incontinence
 6 Page Male Incontinence Booklet /9/ :44 Page The Natural Non-Surgical Option for Male Urinary Incontinence 6 Page Male Incontinence Booklet /9/ :44 Page Stress Urinary Incontinence - A Stressful Prospect
6 Page Male Incontinence Booklet /9/ :44 Page The Natural Non-Surgical Option for Male Urinary Incontinence 6 Page Male Incontinence Booklet /9/ :44 Page Stress Urinary Incontinence - A Stressful Prospect
DOWNLOAD OR READ : URINARY FECAL INCONTINENCE CURRENT MANAGEMENT CONCEPTS URINARY AND FECAL INCONTINENCE PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : URINARY FECAL INCONTINENCE CURRENT MANAGEMENT CONCEPTS URINARY AND FECAL INCONTINENCE PDF EBOOK EPUB MOBI Page 1 Page 2 urinary fecal incontinence current pdf Discusses urinary incontinence
DOWNLOAD OR READ : URINARY FECAL INCONTINENCE CURRENT MANAGEMENT CONCEPTS URINARY AND FECAL INCONTINENCE PDF EBOOK EPUB MOBI Page 1 Page 2 urinary fecal incontinence current pdf Discusses urinary incontinence
Medical Review Criteria Invasive Treatment for Urinary Incontinence
 Medical Review Criteria Invasive Treatment for Urinary Incontinence Effective Date: December 21, 2016 Subject: Invasive Treatment for Urinary Incontinence Background: Urinary incontinence (the involuntary
Medical Review Criteria Invasive Treatment for Urinary Incontinence Effective Date: December 21, 2016 Subject: Invasive Treatment for Urinary Incontinence Background: Urinary incontinence (the involuntary
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Injectable Bulking Agents for Urinary Conditions and Fecal Incontinence Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information...
Cigna Medical Coverage Policy Subject Injectable Bulking Agents for Urinary Conditions and Fecal Incontinence Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information...
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14 THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE
 493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
The Management of Female Urinary Incontinence. Part 1: Aetiology and Investigations
 The Management of Female Urinary Incontinence Part 1: Aetiology and Investigations Dr Oseka Onuma Gynaecologist and Pelvic Reconstructive Surgeon 4 Robe Terrace Medindie SA 5081 Urinary incontinence has
The Management of Female Urinary Incontinence Part 1: Aetiology and Investigations Dr Oseka Onuma Gynaecologist and Pelvic Reconstructive Surgeon 4 Robe Terrace Medindie SA 5081 Urinary incontinence has
Duc M. Vo, MD, FACS Northwest Surgical Specialists
 Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
MEDICAL POLICY SUBJECT: PERCUTANEOUS POSTERIOR TIBIAL NERVE STIMULATION (PPTNS)
 MEDICAL POLICY 03/19/15, 05/17/16 PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria
MEDICAL POLICY 03/19/15, 05/17/16 PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria
MEDICAL POLICY SUBJECT: BIOFEEDBACK
 MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Corporate Medical Policy
 Corporate Medical Policy Vesicoureteral Reflux, Treatment with Periureteral Bulking Agents File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vesicoureteral_reflux_treatment_with_periureteral_bulking_agents
Corporate Medical Policy Vesicoureteral Reflux, Treatment with Periureteral Bulking Agents File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vesicoureteral_reflux_treatment_with_periureteral_bulking_agents
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER AUGMENTATION FOR THE TREATMENT OF GASTROESOPHAGEAL REFLUX DISEASE (GERD)
 MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
MEDICAL POLICY SUBJECT: MAGNETIC ESOPHAGEAL RING/ MAGNETIC SPHINCTER PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence
 A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
Transvaginal and Transurethral Radiofrequency Tissue Remodeling for Urinary Stress Incontinence
 Transvaginal and Transurethral Radiofrequency Tissue Remodeling for Urinary Stress Incontinence Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Transvaginal and Transurethral Radiofrequency Tissue Remodeling for Urinary Stress Incontinence Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137
 Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137 Effective Date: 01/18 Last Review Date: 12/16 Coding Implications Revision Log Description Fecal incontinence defined as the
Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137 Effective Date: 01/18 Last Review Date: 12/16 Coding Implications Revision Log Description Fecal incontinence defined as the
Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury. Original Policy Date
 MP 7.01.58 Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury Medical Policy Section Issue 12:2013 Original Policy Date 12:2013 Last Review
MP 7.01.58 Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury Medical Policy Section Issue 12:2013 Original Policy Date 12:2013 Last Review
MEDICAL POLICY EFFECTIVE DATE: 04/28/11 REVISED DATE: 04/26/12, 04/25/13, 04/24/14, 06/25/15, 06/22/16, 06/22/17
 MEDICAL POLICY SUBJECT: STANDARD DIALECTICAL BEHAVIOR A nonprofit independent licensee of the BlueCross BlueShield Association PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered,
MEDICAL POLICY SUBJECT: STANDARD DIALECTICAL BEHAVIOR A nonprofit independent licensee of the BlueCross BlueShield Association PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered,
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION. POLICY NUMBER: CATEGORY: Therapy/Rehabilitation
 MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
Loss of Bladder Control
 BLADDER HEALTH: Surgery for Urinary Incontinence Loss of Bladder Control Surgery for Urinary Incontinence Don t Let Urinary Incontinence Keep You from Enjoying Life. What is Urinary Incontinence? What
BLADDER HEALTH: Surgery for Urinary Incontinence Loss of Bladder Control Surgery for Urinary Incontinence Don t Let Urinary Incontinence Keep You from Enjoying Life. What is Urinary Incontinence? What
072 Posted: 10/17/06 Page 1 of 25
 072 Posted: 10/17/06 Page 1 of 25 Incontinence Therapy Selected Treatments and Devices Medical Policy When services are covered We cover the following treatments for urinary incontinence: 1 Behavioral
072 Posted: 10/17/06 Page 1 of 25 Incontinence Therapy Selected Treatments and Devices Medical Policy When services are covered We cover the following treatments for urinary incontinence: 1 Behavioral
MEDICAL POLICY. SUBJECT: BRACHYTHERAPY OR RADIOACTIVE SEED IMPLANTATION FOR PROSTATE CANCER POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: BRACHYTHERAPY OR PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: BRACHYTHERAPY OR PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
Pelvic Floor Disorders. Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon
 Pelvic Floor Disorders Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon What is Pelvic Floor Disorder Surgical perspective symptoms of RED, FI or prolapse on the background
Pelvic Floor Disorders Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon What is Pelvic Floor Disorder Surgical perspective symptoms of RED, FI or prolapse on the background
MEDICAL POLICY SUBJECT: URINARY TUMOR MARKERS FOR BLADDER CANCER. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: URINARY TUMOR MARKERS FOR PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: URINARY TUMOR MARKERS FOR PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011
 Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011 Treatment, Urinary Stress Incontinence, Transurethral Effective Date: 01/01/2012 Document: ARB0359 Revision Date: Code(s): 53860 Transurethral
Medical Policy Title: Radiofrequency ARBenefits Approval: 10/19/2011 Treatment, Urinary Stress Incontinence, Transurethral Effective Date: 01/01/2012 Document: ARB0359 Revision Date: Code(s): 53860 Transurethral
Neurogenic bladder. Neurogenic bladder is a type of dysfunction of the bladder due to neurological disorder.
 Definition: Neurogenic bladder Neurogenic bladder is a type of dysfunction of the bladder due to neurological disorder. Types: Nervous system diseases: Congenital: like myelodysplasia like meningocele.
Definition: Neurogenic bladder Neurogenic bladder is a type of dysfunction of the bladder due to neurological disorder. Types: Nervous system diseases: Congenital: like myelodysplasia like meningocele.
URINARY INCONTINENCE
 Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
Pelvic Health Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Posterior Tibial Nerve Stimulation for Voiding Dysfunction
 Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
Close. Number: Policy. Last Review 07/14/2016 Effective: 04/30/2002 Next Review: 07/13/2017. Review History
 1 of 55 Close Number: 0611 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Last Review 07/14/2016 Effective: 04/30/2002 Next Review: 07/13/2017 I. Aetna considers the following
1 of 55 Close Number: 0611 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Last Review 07/14/2016 Effective: 04/30/2002 Next Review: 07/13/2017 I. Aetna considers the following
URINARY INCONTINENCE. Urology Division, Surgery Department Medical Faculty, University of Sumatera Utara
 URINARY INCONTINENCE Urology Division, Surgery Department Medical Faculty, University of Sumatera Utara Definition The involuntary loss of urine May denote a symptom, a sign or a condition Symptom the
URINARY INCONTINENCE Urology Division, Surgery Department Medical Faculty, University of Sumatera Utara Definition The involuntary loss of urine May denote a symptom, a sign or a condition Symptom the
Title: Zuidex versus Contigen for Stress Urinary Incontinence. Date: May 30, 2007
 Title: Zuidex versus Contigen for Stress Urinary Incontinence Date: May 30, 2007 Context and policy issues: Stress urinary incontinence (SUI) is the involuntary leakage of urine during exercise or movements
Title: Zuidex versus Contigen for Stress Urinary Incontinence Date: May 30, 2007 Context and policy issues: Stress urinary incontinence (SUI) is the involuntary leakage of urine during exercise or movements
Product Name or Headline
 Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Incontinence; Lets talk about it. Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery
 Incontinence; Lets talk about it Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery Select the most appropriate subtitle for this talk A: Bladders gone wild! B: There s no such thing
Incontinence; Lets talk about it Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery Select the most appropriate subtitle for this talk A: Bladders gone wild! B: There s no such thing
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
Men s Health Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
A70.4 Insertion of neurostimulator electrodes into peripheral nerve Z12.2 Posterior tibial nerve R15.X Faecal incontinence
 The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- AIDED DETECTION (CAD) POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Post- prostatectomy Incontinence - PPI
 Post- prostatectomy Incontinence - PPI Dr. Kolombo Ivan, MD, FEBU Assoc.Prof. Popken Gralf MD, PhD, Assoc.Prof. Otčenášek Michal MD, PhD, Dr. Klézl Petr MD, MBA, Dr. Kolombová Jitka MD, MBA, Assoc.Prof.
Post- prostatectomy Incontinence - PPI Dr. Kolombo Ivan, MD, FEBU Assoc.Prof. Popken Gralf MD, PhD, Assoc.Prof. Otčenášek Michal MD, PhD, Dr. Klézl Petr MD, MBA, Dr. Kolombová Jitka MD, MBA, Assoc.Prof.
W17: Intrinsic Sphincteric Deficiency, Diagnosis and Management Workshop Chair: Sherif Mourad, Egypt 20 October :00-18:00
 W7: Intrinsic Sphincteric Deficiency, Diagnosis and Management Workshop Chair: Sherif Mourad, Egypt 20 October 204 4:00-8:00 Start End Topic Speakers 4:00 4:05 Introduction Sherif Mourad 4:05 4:20 Pathophysiology
W7: Intrinsic Sphincteric Deficiency, Diagnosis and Management Workshop Chair: Sherif Mourad, Egypt 20 October 204 4:00-8:00 Start End Topic Speakers 4:00 4:05 Introduction Sherif Mourad 4:05 4:20 Pathophysiology
Urinary Incontinence Treatment URINARY INCONTINENCE TREATMENT HS-080. Policy Number: HS-080. Original Effective Date: 2/2/2009
 Easy Choice Health Plan, Inc. Exactus Pharmacy Solutions, Inc. Harmony Health Plan, Inc. Missouri Care, Incorporated WellCare Health Insurance of Arizona, Inc., operating in Hawai i as Ohana Health Plan,
Easy Choice Health Plan, Inc. Exactus Pharmacy Solutions, Inc. Harmony Health Plan, Inc. Missouri Care, Incorporated WellCare Health Insurance of Arizona, Inc., operating in Hawai i as Ohana Health Plan,
MEDICAL POLICY SUBJECT: SACRAL NERVE STIMULATION
 MEDICAL POLICY 01/16/14, 01/22/15, 03/15/16 PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY 01/16/14, 01/22/15, 03/15/16 PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
2017 Hospital Coding and Payment Guide
 Reimbursement Men s Health 2017 Hospital Coding and Payment Guide This coding reference guide is intended to illustrate the common coding and payment groups for male prosthetic urology procedures and related
Reimbursement Men s Health 2017 Hospital Coding and Payment Guide This coding reference guide is intended to illustrate the common coding and payment groups for male prosthetic urology procedures and related
Novel Options for the Management of Fecal Incontinence
 Novel Options for the Management of Fecal Incontinence Arnold Wald, MD, MACG University of Wisconsin School of Medicine and Public Health, Madison WI ANORECTAL CONTINENCE MECHANISMS Reservoir Elements
Novel Options for the Management of Fecal Incontinence Arnold Wald, MD, MACG University of Wisconsin School of Medicine and Public Health, Madison WI ANORECTAL CONTINENCE MECHANISMS Reservoir Elements
Urogynecology ICD-9 to ICD-10 Crosswalks
 1100 Wayne Ave, Suite 825 Silver Spring, MD 20910 301.273.0570 Fax 301.273.0778 info@augs.org www.augs.org Urogynecology ICD-9 to ICD-10 Crosswalks ICD 9 ICD 9 Description ICD 10 Code ICD 10 Description
1100 Wayne Ave, Suite 825 Silver Spring, MD 20910 301.273.0570 Fax 301.273.0778 info@augs.org www.augs.org Urogynecology ICD-9 to ICD-10 Crosswalks ICD 9 ICD 9 Description ICD 10 Code ICD 10 Description
Pelvic Floor. Reimbursement & Coding Guide
 Pelvic Floor Reimbursement & Coding Guide Pelvic Floor Reimbursement and Coding Guide ACell Pelvic Floor Matrix products are biologically-derived devices comprised of porcine Urinary Bladder Matrix (UBM),
Pelvic Floor Reimbursement & Coding Guide Pelvic Floor Reimbursement and Coding Guide ACell Pelvic Floor Matrix products are biologically-derived devices comprised of porcine Urinary Bladder Matrix (UBM),
OHTAC Recommendation
 OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
Glossary of terms Urinary Incontinence
 Patient Information English Glossary of terms Urinary Incontinence Anaesthesia (general, spinal, or local) Before a procedure you will get medication to make sure that you don t feel pain. Under general
Patient Information English Glossary of terms Urinary Incontinence Anaesthesia (general, spinal, or local) Before a procedure you will get medication to make sure that you don t feel pain. Under general
Loss of Bladder Control
 BLADDER HEALTH Loss of Bladder Control SURGERY TO TREAT URINARY INCONTINENCE AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is Urinary Incontinence? Urinary incontinence
BLADDER HEALTH Loss of Bladder Control SURGERY TO TREAT URINARY INCONTINENCE AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is Urinary Incontinence? Urinary incontinence
Urogynecology in EDS. Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018
 Urogynecology in EDS Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018 One in three like me Voiding Issues Frequency/Urgency Urinary Incontinence neurogenic bladder Neurologic supply
Urogynecology in EDS Joan L. Blomquist, MD Greater Baltimore Medical Center August 2018 One in three like me Voiding Issues Frequency/Urgency Urinary Incontinence neurogenic bladder Neurologic supply
Clinical Policy: Fecal Incontinence Treatments Reference Number: CP.MP.137 Last Review Date: 12/17
 Clinical Policy: Reference Number: CP.MP.137 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Fecal
Clinical Policy: Reference Number: CP.MP.137 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Fecal
Urogynaecology. Colm McAlinden
 Urogynaecology Colm McAlinden Definitions Urinary incontinence compliant of any involuntary leakage of urine with many different causes Two main types: Stress Urge Definitions Nocturia: More than a single
Urogynaecology Colm McAlinden Definitions Urinary incontinence compliant of any involuntary leakage of urine with many different causes Two main types: Stress Urge Definitions Nocturia: More than a single
Incontinence Supplies
 Incontinence Supplies Policy Number: Original Effective Date: MM.12.020 07/01/2015 Lines of Business: Current Effective Date: QUEST Integration 09/28/2018 Section: Other/Miscellaneous Place(s) of Service:
Incontinence Supplies Policy Number: Original Effective Date: MM.12.020 07/01/2015 Lines of Business: Current Effective Date: QUEST Integration 09/28/2018 Section: Other/Miscellaneous Place(s) of Service:
Original Policy Date
 MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
Medical Policy. Description/Scope. Position Statement
 Subject: Document #: Current Effective Date: 03/29/2017 Status: Revised Last Review Date: 02/02/2017 Description/Scope This document addresses the following treatments for urinary incontinence: Vaginal
Subject: Document #: Current Effective Date: 03/29/2017 Status: Revised Last Review Date: 02/02/2017 Description/Scope This document addresses the following treatments for urinary incontinence: Vaginal
Various Types. Ralph Boling, DO, FACOG
 Various Types Ralph Boling, DO, FACOG The goal of this lecture is to increase assessment and treatment abilities for physicians managing urinary incontinence (UI) patients. 1. Effectively communicate with
Various Types Ralph Boling, DO, FACOG The goal of this lecture is to increase assessment and treatment abilities for physicians managing urinary incontinence (UI) patients. 1. Effectively communicate with
MEDICAL POLICY SUBJECT: FEMALE STERILIZATION. POLICY NUMBER: CATEGORY: Contract Clarification
 MEDICAL POLICY SUBJECT: FEMALE STERILIZATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: FEMALE STERILIZATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Incontinence. Anatomy The human body has two kidneys. The kidneys continuously filter the blood and make urine.
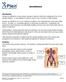 Incontinence Introduction Urinary incontinence occurs when a person cannot control the emptying of his or her urinary bladder. It can happen to anyone, but is very common in older people. Urinary incontinence
Incontinence Introduction Urinary incontinence occurs when a person cannot control the emptying of his or her urinary bladder. It can happen to anyone, but is very common in older people. Urinary incontinence
Periurethral bulking injections for stress urinary incontinence
 n The Leeds Teaching Hospitals NHS Trust Periurethral bulking injections for stress urinary incontinence Information for patients Leeds Centre for Women s Health Urinary incontinence is the involuntary
n The Leeds Teaching Hospitals NHS Trust Periurethral bulking injections for stress urinary incontinence Information for patients Leeds Centre for Women s Health Urinary incontinence is the involuntary
Subject: Percutaneous Tibial Nerve Stimulation
 02-64000-01 Original Effective Date: 05/15/08 Reviewed: 05/24/18 Revised: 06/15/18 Subject: Percutaneous Tibial Nerve Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
02-64000-01 Original Effective Date: 05/15/08 Reviewed: 05/24/18 Revised: 06/15/18 Subject: Percutaneous Tibial Nerve Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Bard: Continence Therapy. Stress Urinary Incontinence. Regaining Control. Restoring Your Lifestyle.
 Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
THE EFFECT OF ENDOSCOPIC INJECTIONS OF DEXTRANOMER BASED IMPLANTS ON CONTINENCE AND BLADDER CAPACITY: A PROSPECTIVE STUDY OF 31 PATIENTS
 0022-5347/02/1684-1863/0 Vol. 168, 1863 1867, October 2002 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2002 by AMERICAN UROLOGICAL ASSOCIATION, INC. DOI: 10.1097/01.ju.0000029638.43807.5b THE EFFECT
0022-5347/02/1684-1863/0 Vol. 168, 1863 1867, October 2002 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2002 by AMERICAN UROLOGICAL ASSOCIATION, INC. DOI: 10.1097/01.ju.0000029638.43807.5b THE EFFECT
Management of Female Stress Incontinence
 Management of Female Stress Incontinence Dr. Arvind Goyal Associate Professor (Urology& Renal Transplant) Dayanand Medical College & Hospital, Ludhiana, Punjab, India Stress Incontinence Involuntary loss
Management of Female Stress Incontinence Dr. Arvind Goyal Associate Professor (Urology& Renal Transplant) Dayanand Medical College & Hospital, Ludhiana, Punjab, India Stress Incontinence Involuntary loss
Comparison of Autologous, Non-autologous, and Synthetic Periurethral Bulking Agents
 Comparison of Autologous, Non-autologous, and Synthetic Periurethral Bulking Agents Abstract Andrew J. Steward Department of Aerospace and Mechanical Engineering University of Notre Dame, Notre Dame, IN
Comparison of Autologous, Non-autologous, and Synthetic Periurethral Bulking Agents Abstract Andrew J. Steward Department of Aerospace and Mechanical Engineering University of Notre Dame, Notre Dame, IN
Operative Approach to Stress Incontinence. Goals of presentation. Preoperative evaluation: Urodynamic Testing? Michelle Y. Morrill, M.D.
 Operative Approach to Stress Incontinence Goals of presentation Michelle Y. Morrill, M.D. Director of Urogynecology The Permanente Medical Group Kaiser, San Francisco Review preoperative care & evaluation
Operative Approach to Stress Incontinence Goals of presentation Michelle Y. Morrill, M.D. Director of Urogynecology The Permanente Medical Group Kaiser, San Francisco Review preoperative care & evaluation
Urinary incontinence. Urology Department. Patient Information Leaflet
 Urinary incontinence Urology Department Patient Information Leaflet Introduction This leaflet is for people who have been diagnosed with urinary incontinence. It contains information about the bladder,
Urinary incontinence Urology Department Patient Information Leaflet Introduction This leaflet is for people who have been diagnosed with urinary incontinence. It contains information about the bladder,
Ben Herbert Alex Wojtowicz
 Ben Herbert Alex Wojtowicz 54 year old female presenting with: Dragging sensation Urinary incontinence Some faecal incontinence HPC Since May 14 had noticed a mass protruding from the vagina when going
Ben Herbert Alex Wojtowicz 54 year old female presenting with: Dragging sensation Urinary incontinence Some faecal incontinence HPC Since May 14 had noticed a mass protruding from the vagina when going
Objectives. Prevalence of Urinary Incontinence URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS
 URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS Lisa S Pair, MSN, CRNP Division of Urogynecology and Pelvic Reconstructive Surgery Department of Obstetrics and Gynecology University of Alabama
URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS Lisa S Pair, MSN, CRNP Division of Urogynecology and Pelvic Reconstructive Surgery Department of Obstetrics and Gynecology University of Alabama
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
Sympathetic Electrical Stimulation Therapy for Chronic Pain
 Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
REVISED DATE: 07/19/12, 06/20/13, 05/22/14, 04/16/15, 03/17/16, 03/16/17, 03/15/18 POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: PLUGS FOR FISTULA REPAIR PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: PLUGS FOR FISTULA REPAIR PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
This information is intended as an overview only
 This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL REVASCULARIZATION
 MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
Posterior Tibial Nerve Stimulation
 Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566
 Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Lumify. Lumify reimbursement guide {D DOCX / 1
 Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Medical Policy Title: Periureteral Bulking ARBenefits Approval: 10/26/201
 Medical Policy Title: Periureteral Bulking ARBenefits Approval: 10/26/201 Agents as a Treatment of Vesicoureteral Reflux (VUR) Effective Date: 01/01/2012 Document: ARB0278 Revision Date: Code(s): 52327
Medical Policy Title: Periureteral Bulking ARBenefits Approval: 10/26/201 Agents as a Treatment of Vesicoureteral Reflux (VUR) Effective Date: 01/01/2012 Document: ARB0278 Revision Date: Code(s): 52327
