BASE 24-HOUR URINE COLLECTION LITHOLINK CORE LAB
|
|
|
- Georgina Goodwin
- 6 years ago
- Views:
Transcription
1 CHAPTER 34. BASE 24-HOUR URINE COLLECTION LITHOLINK CORE LAB 34.1 Background Participants who are enrolled in the CKD BASE Study will collect urine over a 24-hour period at B0, W12 and W28. A 50 ml tube of urine will be shipped overnight to Litholink Corporation in Chicago, Illinois. Two additional tubes containing 10 ml of urine will be saved as back up and frozen at each local site. For general collection and processing questions, call For additional supplies needed and shipment notification, contact Susan Donohue at donahus@labcorp.com and Ignacio Granja at granjai@labcorp.com. Contact information can be found in the CKD BASE Address Directory Schedule, Type and Volume of Urine Required Tube Size 50 ml provided by Litholink BASE 24-Hour Urine Samples Collected for Litholink Collection Periods, Quantity Required, Storage and Shipping 1 x 50 ml urine Storage: room temperature B W12 W28 1 x 50 ml urine Storage: room temperature 1 x 50 ml urine Storage: room temperature 15 ml provided by local sites 2 10 ml urine Storage: In -20 o or colder freezer locally 2 10 ml urine Storage: In -20 o or colder freezer locally 2 10 ml urine Storage: In -20 o or colder freezer locally 34.3 Training Requirements for Personnel Sending Samples Each person shipping diagnostic specimens should be trained according to DOT and IATA regulations. Someone at each BASE site will be responsible for training the appropriate personnel Logistics for Litholink Overview: The Litholink Corporation will provide 24-hour urine collection kits see section 34.5 for specific contents. Participants will collect their urine over the course of 24-hours and bring in the urine collection container(s) for processing by the study coordinator or other designated person. The labels to be used locally for the backup tubes will be supplied to the sites by the DCC with detailed completion instructions provided. The tube labels include space to write in the BASE participant s 6 digit-identification number. The labels will also include two spaces in which the study staff should mark the participant s alphanumeric code, which will be used as an alternative ID. Tubes should be labeled immediately prior to when urine from the participant is poured into the tubes. CKD Manual of Operations BASE Chapter 34 Page 1 of 12
2 34.5 Kits and Shipping Materials Litholink Kit Contents include: 1 Orange urine collection container 1 Sealed tube of liquid urine preservative (contains Gentamicin 20 mg, Germall II 1.25 g, Potassium Fluoride 306 mg) 1 Green-topped 50 ml tube 1-Safeguard Specimen (biohazard) Bag with absorbent material 1-Pre-addressed shipping box (printed with appropriate DOT labels) 1-Preprinted Fedex Priority Overnight label (See address directory for name of individual at Litholink who will receive the specimen box) 1-Customer service card with toll-free phone number Note: Litholink will send female collection aids ( hats ) in one bulk shipment. Local Site provides: Extra 15 ml tubes (for storing and freezing two additional tubes containing 10 ml/tube of backup urine) Tube labels (provided to each site by DCC) Freezer rack/storage box to hold frozen urine tubes backup -20 o or colder freezer 34.6 Instructing the Participant on Collecting Urine Provide the participant with items needed for urine collection including: orange urine collection container, sealed tube of liquid urine preservative, how to instructions, and if female, urine collection aid hat. (It is suggested that the urine jug be marked with some identifying information to separate multiple samples in the event that more than one participant comes in for a study visit on the same day.). For the initial collection, it is recommended that the participant be given two jugs since each 4 liter jug should not be filled more than 4,000 mls of urine. In the event that the participant s output exceeds the 4,000 mls, the second jug could then be used to complete the collection. Note, any collections of over 4,500 mls in a jug will need to be repeated. If the participant does not use the second jug for the collection, the participant should return the unused jug to the site and the site could use it for another participant. However, if the participant does use the second jug or the urine volume is close to 4,000 mls, a second jug should be given at subsequent visits where a 24-hour collection is needed. Thoroughly review the instructions with the participant (and caregiver, if he or she is accompanying the study participant). Urine collection should start the date prior to the in-person study visit* and should be written on participant s instruction sheet. Be sure to provide the participant with study personnel contact information and an emergency contact, if needed. Remind the participant that all urine is collected even if there is nighttime urination. Collections should be as close to 24-hours as possible prior to the in-person study visit. Urine jug can be stored at room temperature. Collection time should be between 20 and 28 hours. If during Baseline, the collection is <20 or >28 hours, an additional collection is required for randomization. CKD Manual of Operations BASE Chapter 34 Page 2 of 12
3 *For in-person visits requiring 24-hour urine collection (B1, W12 and W28), it is helpful to schedule in-person visits on a Monday so urine can be collected over the weekend and brought in to the visit. Participant Instructions sheets on collecting their urines are provided in this chapter s appendices. Appendix A includes two IRB-reviewed participant instructions for females and for males prepared by University of Utah. Appendix B provides a generic template that can be modified accordingly. Any participant urine collection instructions must be submitted to your local IRB. For female participants, instruct the participant to try to avoid collecting the sample during their menstrual cycle. If they happen to do the collection during this time, the sample should still be sent to Litholink with a note stating that the sample was collected during the participant s menstrual cycle. If the participant comes in for the W28 visit, does not bring in a 24-hour urine collection, and study medications are stopped, the 24-hour urine collection for W28 will be missing and should not be recollected. If the 24-hour urine was collected after the participant stopped study medications at W28, the urine results would not provide the pharmacodynamics data that is needed since the pharmacodynamic effect is expected to dissipate within a day or so of stopping BASE study medications Litholink Urine Sample Processing Information Participant will return urine collection jug and instruction sheet where actual dates and times of start/stop urine collection are written in by the participant. Record this information on Form 326 (start and end date of urine collection and start and end time of urine collection). Process any urine regardless of under or over collection. If during baseline, if the collection is < 20 hours or > 28 hours, the collection will need to be repeated in order for the participant to be randomized. Also, filling a 4-liter jug with more than 4,000 mls of urine may compromise results from Litholink. Any collection that exceeds 4,500 mls in one jug will need to be repeated. 24-Hour Urine Collection processing (for 1 or 2 jugs) 1. Before measuring urine volume, it is important to tighten the lid. 2. Place the collection container on a flat surface turning the jug upright so the handle is vertical. 3. Use the measuring tool along the side of the container (jug lines) to read how much fluid is inside the container. Estimate to the nearest 100 mls and record on Form 326 item 14a (Estimated volume of this 24-hour urine collection to the nearest 100 mls). If a second jug was used, estimate the second jug to the nearest 100 mls and record on Form 326 item 14b (Estimated volume of additional urine (second jug) collected to the nearest 100 mls). 4. Weigh the urine collection (includes the urine, jug with lid and the preservative). Enter value on Form 326 item 15a (Weight of the 24-hr urine collection jug). If a second jug was used, weigh the second jug urine collection (includes the urine, jug with lid and the CKD Manual of Operations BASE Chapter 34 Page 3 of 12
4 preservative). Enter the value on Form 326 item 15b (Weight of the second 24-hr urine collection jug). 5. Thoroughly mix or shake the urine collection container(s). 6. Fill the green-topped tube marked Collection 1 about ¾ full with the urine sample. If a second jug was used, fill a second green-topped tube marked Collection 2 about ¾ full with the urine sample. 7. Twist green top(s) tightly to seal. Make sure that the top(s) is on secure and evenly so this does not leak when shipping to Litholink. Be sure the 50 ml tube(s) used for shipping is labeled with the BASE study participant identification number and alphacode, start date/time of urine collection, and visit type. Do not spin the tube(s). Tube(s) can sit at room temperature until ready to ship. 8. Proceed to processing the backup tubes in Section Complete a paper copy of Litholink 24-Hr Urine Mailing form (Form 326) and make sure a copy is made of the completed document so it can be included in the shipment to Litholink. The information on the form must also be entered in the database. All efforts should be made to ship the 50 ml tube of urine to Litholink the day the collection ends. The urine collection will no longer be good if the urine collection is received at Litholink > 96 hours after the time the urine collection ended as documented on Form 326 (Litholink 24-hr Urine Mailing Form). Specimens older than four days (96 hours) yield suboptimal results that could impact the quality of study data thus the participant should be instructed to re-collect the sample. In Baseline, the following criteria must be met in order for the 24-urine to meet randomization eligibility criteria: Form 326 shows preservative observed in urine container (Q9=1)... Yes Form 326 shows preservative added (Q10=1 or 2)... Yes Form 326 shows that collection was complete (Q11=1)... Yes Form 326 shows tube sent to Litholink (Q16=1 or 2)... Yes Collection was between 20 and 28 hours?... Yes At least one Litholink urine results Form 355 with ammonium results... YES 34.8 Processing Backup Tubes for Freezing 1. Complete and attach the participant ID labels provided by the DCC to the urine tubes prior to filling. Place the label lengthwise on the tubes do not overlap. DO NOT write the participant s name or any other personal identification information (e.g., SS#, DOB) on the tubes. Sample label: BASE Study-Litholink ID: - Visit: Baseline W12 W28 Start Date: / / CKD Manual of Operations BASE Chapter 34 Page 4 of 12
5 The tube labels include 8 spaces on which the study team member will write in the CKD BASE Participants' 6 digit ID and alpha code with a fine-tipped permanent marker (like a Sharpie). Circle the visit: Baseline, W12 or W28. Write the start date of the urine collect. 2. Pour 10 ml of urine into each tube and make sure seal is closed tightly. Place tubes in storage container (rack/box) in the designated -20 o or colder freezer. Every effort should be made to freeze the back-up samples within 48 hours from the time the urine collection ended as documented on Form 326 (Litholink 24-hr Urine Mailing Form). Note, if two urine collection jugs were used by the participant, than two back up samples are needed from each jug for a total of four back-up samples. 3. Dispose of any remaining urine and urine jug according to your site s local disposal policy. 4. Do not ship tubes to Litholink unless told to do so. Await word from either Litholink or the DCC indicating the status of the original 50 ml urine tube. 5. After receiving successful receipt and status information on the 50 ml urine tube from either Litholink or the DCC, complete Form 15 - Back-up Litholink Sample Discard Form. This form documents the disposition of the two frozen tubes for the urine collection. a. If the 50 ml sample was analyzed and results reported, dispose of the frozen tubes according to your site s local policy and by completing Form 15. b. If the original sample could not be analyzed sufficiently, Litholink will contact your site to ship the tubes that were frozen previously. Complete Form 327-Litholink Backup Mailing Form indicating that frozen tubes are being shipped. 6. If shipment to Litholink is required, remove frozen tubes from freezer. Verify ID and alphacode and other information that appears on the tubes labels. Do not thaw out the urine. Place frozen tubes into the biohazard bag with the absorbent material included. Ship at room temperature. (Do not ship on a cold pack.) 7. Complete Form 327 Litholink Back-up Mailing Form and make a copy for your files. Place the completed F327 inside the shipping box and send by FedEx to Litholink. Note: If frozen urine is requested on a Friday, keep the tubes in the freezer and ship on Monday. CKD Manual of Operations BASE Chapter 34 Page 5 of 12
6 34.9 Shipping Instructions CKD Manual of Operations BASE Chapter 34 Page 6 of 12
7 Appendix A: Consent Form Univ. of Utah BASE Study Instructions for Collecting the Urine Sample - Female Collecting a 24-hour sample of urine is a very important part of this study. This sample will be used to do tests that will help us determine if sodium bicarbonate might be helpful for patients like you. If you have any questions about how to collect the sample, please contact one of the following people: Before starting: Check to be sure you have: 1 large orange collection jug 1 collection aid (aka hat ) 1 tube of liquid urine preservative Pen or pencil Instructions: Katt Mackin at (801) Megan Varner at (801) Jennifer Zitterkoph at (801) Dr. Kalani Raphael at (801) ext o The urine collection will start the day before your study visit. My urine collection will begin on o When you wake up in the morning, flush your first urine in the toilet. DO NOT collect this urine. Please write down the exact time: o Open the tube of urine preservative and empty it into the collection container. CKD Manual of Operations BASE Chapter 34 Page 7 of 12
8 o Drop the urine preservative tube and lid into the collection container. This ensures that every drop of the preservative gets into the container. Important: When you bring your urine collection to the study visit, the study coordinator will check to make sure the preservative tube and lid are inside the collection container. If they are not, you will be asked to repeat the urine collection. o Collect all of your urine into the container over the next 24 hours, including the very first urine the following morning and any urine produced during the night. To perform the collection, place the collection aid ( hat ) over the toilet and then carefully pour the urine into the orange collection container. The first urine collected the following morning is your stop time. Please write down this exact time: o Bring the orange jug with the lid firmly attached to your study visit. Appointment Information: Date: Time: Location: Center for Clinical and Translational Science (CCTS) on the 5 th floor of the hospital. CKD Manual of Operations BASE Chapter 34 Page 8 of 12
9 BASE Study Instructions for Collecting the Urine Sample - Male Collecting a 24-hour sample of urine is a very important part of this study. This sample will be used to do tests that will help us determine if sodium bicarbonate might be helpful for patients like you. If you have any questions about how to collect the sample, please contact one of the following people: Katt Mackin at (801) Megan Varner at (801) Jennifer Zitterkoph at (801) Dr. Kalani Raphael at (801) ext Before starting: Check to be sure you have: 1 large orange collection jug 1 tube of liquid urine preservative Pen or pencil Instructions: o The urine collection will start the day before your study visit. My urine collection will begin on o When you wake up in the morning, flush your first urine in the toilet. DO NOT collect this urine. Please write down the exact time: o Open the tube of urine preservative and empty it into the collection container. CKD Manual of Operations BASE Chapter 34 Page 9 of 12
10 o Drop the urine preservative tube and lid into the collection container. This ensures that every drop of the preservative gets into the container. Important: When you bring your urine collection to the study visit, the study coordinator will check to make sure the preservative tube and lid are inside the collection container. If they are not, you will be asked to repeat the urine collection. o Collect all of your urine into the container over the next 24 hours, including the very first urine the following morning and any urine produced during the night. The first urine collected the following morning is your stop time. Please write down this exact time: o Bring the orange jug with the lid firmly attached to your study visit. Appointment Information: Date: Time: Location: Center for Clinical and Translational Science (CCTS) on the 5 th floor of the hospital. CKD Manual of Operations BASE Chapter 34 Page 10 of 12
11 Appendix B: Template Consent Form BASE Study - Instructions for Collecting the Urine Sample Collecting a 24-hour sample of urine is a very important part of this study. This sample will be used to do tests that will help us determine if sodium bicarbonate might be helpful for persons with kidney conditions like you. If you have any questions about how to collect the sample, please call one of the following people: Before starting: Check to be sure you have: 1 large orange collection jug 1 tube of liquid urine preservative 1 collection aid, if needed ( hat for females, urinal for males, if requested) Pen or pencil This instruction sheet Instructions: BASE Study Team Contacts: Coordinator: xxx-xxx-xxxx Coordinator: xxx-xxx-xxxx Study Nurse: xxx-xxx-xxxx Study PI: xxx-xxx-xxxx o The urine collection will start the day before your study visit. My study coordinator says to start on this day (write in day, date): My actual urine collection began on (write in day, date): o When you wake up in the morning, flush your first urine in the toilet. DO NOT save this urine. This is the time I first went to the bathroom after waking up: Circle one: am pm o Open the tube of urine preservative and empty it into the collection container. CKD Manual of Operations BASE Chapter 34 Page 11 of 12
12 o Drop the urine preservative tube and lid into the orange jug. This ensures that every drop of the preservative gets into the container. Important: When you bring your urine collection to the study visit, the study coordinator will check to make sure the preservative tube and lid are inside the collection container. If these two items are not in the jug, you will be asked to repeat the urine collection. o Collect all of your urine into the container over the next 24 hours. Keep the jug at room temperature (no need to refrigerate it). To perform the collection, place the collection aid ( hat ) over the toilet and then carefully pour the urine into the orange collection container. o Collect all urine produced during the night. The last time you collect your urine in the jug is the very first urine the following morning. The first urine collected the following morning is your stop time. This is the time I first went to the bathroom after waking up the next day: o Circle one: am pm o Bring the orange jug with the lid firmly attached to your study visit instruction sheet. Appointment Information: Date: Time: Location: CKD Manual of Operations BASE Chapter 34 Page 12 of 12
Biospecimen Adult Urine Procedures. Event: Pre-Preg, PV1, PV2, Birth, 6M, 12M, 36M, and 60M
 Biospecimen Adult Urine Procedures Event: Pre-Preg, PV1, PV2, Birth, 6M, 12M, 36M, and 60M Domain: Type of Document: Biospecimen Standard Operating Procedures This page intentionally left blank. BIO Adult
Biospecimen Adult Urine Procedures Event: Pre-Preg, PV1, PV2, Birth, 6M, 12M, 36M, and 60M Domain: Type of Document: Biospecimen Standard Operating Procedures This page intentionally left blank. BIO Adult
Specimen Collection. Urine Vacutainers. Laboratory Services September 2018
 1 Specimen Collection Urine Vacutainers Laboratory Services September 2018 Agenda Before you begin Collection Labeling Packaging & Transport Resources Click on the links above to be taken directly to that
1 Specimen Collection Urine Vacutainers Laboratory Services September 2018 Agenda Before you begin Collection Labeling Packaging & Transport Resources Click on the links above to be taken directly to that
Monitoring the Health of Transplanted Organs DONOR GENOTYPING MANUAL
 Monitoring the Health of Transplanted Organs DONOR GENOTYPING MANUAL 10101 Innovation Drive. Suite 600. Wauwatosa, WI 53226 1-888-214-3151 MKT-008 v3.0 MKT-008 v3.0 TABLE OF CONTENTS I Intended Use...
Monitoring the Health of Transplanted Organs DONOR GENOTYPING MANUAL 10101 Innovation Drive. Suite 600. Wauwatosa, WI 53226 1-888-214-3151 MKT-008 v3.0 MKT-008 v3.0 TABLE OF CONTENTS I Intended Use...
Baseline Patient Forms
 Revision of 06/14/2016 Page 1 of 7 CKD PILOT STUDIES Forms Completion Schedule - COMBINE Non-Patient Forms In order to make a clinical site ready to enroll F09 Clinical Center F10 Study Personnel Review
Revision of 06/14/2016 Page 1 of 7 CKD PILOT STUDIES Forms Completion Schedule - COMBINE Non-Patient Forms In order to make a clinical site ready to enroll F09 Clinical Center F10 Study Personnel Review
Providence Medford Medical Center Pathology Department
 Providence Medford Medical Center Pathology Department Anatomic pathology services including histology, cytology and autopsies are offered through Providence Medford Medical Center Pathology Department.
Providence Medford Medical Center Pathology Department Anatomic pathology services including histology, cytology and autopsies are offered through Providence Medford Medical Center Pathology Department.
ANTIBODY SCREENING by Uni-Gold Recombigen HIV
 ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
Sample Submission Instructions. STEP 1: Complete the Test Requisition Form and Informed Consent
 STEP : Complete the Test Requisition Form and Informed Consent Please fill out all sections of the Test Requisition Form Patient Information Required fields are highlighted in yellow Patient Name (Last
STEP : Complete the Test Requisition Form and Informed Consent Please fill out all sections of the Test Requisition Form Patient Information Required fields are highlighted in yellow Patient Name (Last
Stool Collection Guidelines
 Stool Collection Guidelines Your child s stool (bowel movement) must be tested so we can plan the treatment for your child. You will need to collect the stool specimen at home. Bring it to a Laboratory
Stool Collection Guidelines Your child s stool (bowel movement) must be tested so we can plan the treatment for your child. You will need to collect the stool specimen at home. Bring it to a Laboratory
ARCADIA Study Coordinator Training Investigator Meeting
 ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
Sputum DNA Collection, Preservation and Isolation Kit 50 Individual Devices
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Sputum DNA Collection, Preservation and Isolation Kit 50 Individual
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Sputum DNA Collection, Preservation and Isolation Kit 50 Individual
How to use your gonal-f pre-filled pen
 How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
24 hour urine collection for 5HIAA
 24 hour urine collection for 5HIAA Your doctor has asked that you collect a 24 hour sample of your urine to see if your body is making too much of a substance called 5-HIAA (which stands for 5- hydroxyindole
24 hour urine collection for 5HIAA Your doctor has asked that you collect a 24 hour sample of your urine to see if your body is making too much of a substance called 5-HIAA (which stands for 5- hydroxyindole
APHL/CDC HIV Demonstration Project Demonstration Project for HIV Nucleic Acid Testing (NAT) Referral Updated: January 2018
 APHL/CDC HIV Demonstration Project Demonstration Project for HIV Nucleic Acid Testing (NAT) Referral Updated: January 2018 Shipping and Submission Instructions Thank you for participating as a submitting
APHL/CDC HIV Demonstration Project Demonstration Project for HIV Nucleic Acid Testing (NAT) Referral Updated: January 2018 Shipping and Submission Instructions Thank you for participating as a submitting
CRC Screening Materials
 CRC Screening Materials Item Page A. Screening Invitation Letter 2 B. CRC Screening Brochure 3 C. Navigation Script 5 D. Screening Plan Template 13 E. Colonoscopy Reminder 14 F. SBT Reminder 15 G. Screening
CRC Screening Materials Item Page A. Screening Invitation Letter 2 B. CRC Screening Brochure 3 C. Navigation Script 5 D. Screening Plan Template 13 E. Colonoscopy Reminder 14 F. SBT Reminder 15 G. Screening
PROCEDURES FOR COLLECTING SHIPPING SPECIMENS FOR MEASUREMENT OF NICOTINE, COTININE, CAFFEINE AND CATECHOLAMINES 2017
 PROCEDURES FOR COLLECTING AND SHIPPING SPECIMENS FOR MEASUREMENT OF NICOTINE, COTININE AND OTHER NICOTINE METABOLITES, TOBACCO ALKALOIDS, AND CARCINOGEN BIOMARKERS Clinical Pharmacology Laboratory San
PROCEDURES FOR COLLECTING AND SHIPPING SPECIMENS FOR MEASUREMENT OF NICOTINE, COTININE AND OTHER NICOTINE METABOLITES, TOBACCO ALKALOIDS, AND CARCINOGEN BIOMARKERS Clinical Pharmacology Laboratory San
How to use your Pergoveris pre-filled pen
 How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
Monitoring the Health of Transplanted Organs INSTRUCTION MANUAL
 Monitoring the Health of Transplanted Organs INSTRUCTION MANUAL 10101 Innovation Drive. Suite 600. Wauwatosa, WI 53226 1-888-214-3151 MKT-005 v2.0 TABLE OF CONTENTS SECTION I: INTENDED USE I Intended Use...
Monitoring the Health of Transplanted Organs INSTRUCTION MANUAL 10101 Innovation Drive. Suite 600. Wauwatosa, WI 53226 1-888-214-3151 MKT-005 v2.0 TABLE OF CONTENTS SECTION I: INTENDED USE I Intended Use...
AVIAN INFLUENZA COMMODITIES TRAINING GUIDE MODULE 3 - SAMPLING, RAPID TESTING AND PACKAGING PARTICIPANT HANDOUTS
 AVIAN INFLUENZA COMMODITIES TRAINING GUIDE MODULE 3 - SAMPLING, RAPID TESTING AND PACKAGING PARTICIPANT HANDOUTS MARCH 2007 MODULE 3 PARTICIPANT HANDOUTS Participant Handouts Participant Handout # 1 Flu
AVIAN INFLUENZA COMMODITIES TRAINING GUIDE MODULE 3 - SAMPLING, RAPID TESTING AND PACKAGING PARTICIPANT HANDOUTS MARCH 2007 MODULE 3 PARTICIPANT HANDOUTS Participant Handouts Participant Handout # 1 Flu
Oregon Immunization Program
 Oregon Immunization Program Standard Operating Procedures for Vaccine Management It is the direct responsibility of the staff person designated below to safeguard and ensure the maintenance of vaccines
Oregon Immunization Program Standard Operating Procedures for Vaccine Management It is the direct responsibility of the staff person designated below to safeguard and ensure the maintenance of vaccines
TB LAM Ag Lateral Flow Assay Standard Operating Procedure
 TB LAM Ag Lateral Flow Assay Standard Operating Procedure 1.0. Purpose The purpose of this standard operating procedure (SOP) is to detail the steps for correctly performing, interpreting, and documenting
TB LAM Ag Lateral Flow Assay Standard Operating Procedure 1.0. Purpose The purpose of this standard operating procedure (SOP) is to detail the steps for correctly performing, interpreting, and documenting
Consent and Authorization Document
 Kalani Raphael, MD Page 1 of 14 Consent and Authorization Document BACKGROUND You are being asked to participate in this research study because you have chronic kidney disease, also called CKD, a condition
Kalani Raphael, MD Page 1 of 14 Consent and Authorization Document BACKGROUND You are being asked to participate in this research study because you have chronic kidney disease, also called CKD, a condition
CORTISOL AWAKENING RESPONSE
 CORTISOL AWAKENING RESPONSE TEST INSTRUCTIONS Read through this booklet completely before testing. Watch our instructional video at dutchtest.com/car-instructions. CREATORS OF THE DUTCH TEST BEFORE YOU
CORTISOL AWAKENING RESPONSE TEST INSTRUCTIONS Read through this booklet completely before testing. Watch our instructional video at dutchtest.com/car-instructions. CREATORS OF THE DUTCH TEST BEFORE YOU
EMORY NEUROMUSCULAR LABORATORY
 EMORY NEUROMUSCULAR LABORATORY The lab processes all nerve and muscle biopsies sent to Emory University. The lab is located within The School of Medicine, Department of Neurology. We are a full service
EMORY NEUROMUSCULAR LABORATORY The lab processes all nerve and muscle biopsies sent to Emory University. The lab is located within The School of Medicine, Department of Neurology. We are a full service
Lab Director: Russell P. Tracy, Ph.D. Coordinator: Elaine Cornell Lab/Project Manager: Rebekah Boyle, M.S. Repository Manager: Sarah Nightingale
 Presented by: Jessica Rooney Colchester Research Facility Laboratory for Clinical Biochemistry Research (LCBR) University of Vermont Department of Pathology and Laboratory Medicine Lab Director: Russell
Presented by: Jessica Rooney Colchester Research Facility Laboratory for Clinical Biochemistry Research (LCBR) University of Vermont Department of Pathology and Laboratory Medicine Lab Director: Russell
Controlled Substances Program. For Academic Units
 Brigham Young University Page 1 Provo, Utah Controlled Substances Program For Academic Units Last Revised: November 30, 2009 Brigham Young University Page 2 TABLE OF CONTENTS Section Title Page 1.0 Overview
Brigham Young University Page 1 Provo, Utah Controlled Substances Program For Academic Units Last Revised: November 30, 2009 Brigham Young University Page 2 TABLE OF CONTENTS Section Title Page 1.0 Overview
Standard Operating Procedures Clinical and Translational Research Center
 Standard Operating Procedures Clinical and Translational Research Center Title: Approved By: Number of Pages: Frequently Sampled Intravenous Glucose Tolerance Test (FSIVGTT) Effective March 19, 2012 Date:
Standard Operating Procedures Clinical and Translational Research Center Title: Approved By: Number of Pages: Frequently Sampled Intravenous Glucose Tolerance Test (FSIVGTT) Effective March 19, 2012 Date:
The REDOXS Study. REducing Deaths due to OXidative Stress. A randomized trial of glutamine and antioxidant supplementation in critically ill patients
 The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
Clostridium difficile Specimen Collection
 Clostridium difficile Specimen Collection Collect stool specimens into a clean, airtight container with no preservative. All stool specimens should be tested as soon as possible. Ideally, stool specimens
Clostridium difficile Specimen Collection Collect stool specimens into a clean, airtight container with no preservative. All stool specimens should be tested as soon as possible. Ideally, stool specimens
The REDOXS Study. REducing Deaths due to OXidative Stress. A randomized trial of glutamine and antioxidant supplementation in critically ill patients
 The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide)
 PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide) Section 1. WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN Read this section completely before you begin. Then, move
PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide) Section 1. WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN Read this section completely before you begin. Then, move
Lilly. Disposable Insulin Delivery Device User Manual
 1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
PVDOMICS: ECG Training Module
 PVDOMICS: ECG Training Module Cardiovascular Physiology Core Cleveland Clinic, Cleveland OH November 7, 2016 NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood
PVDOMICS: ECG Training Module Cardiovascular Physiology Core Cleveland Clinic, Cleveland OH November 7, 2016 NHLBI Pulmonary Vascular Disease Phenomics Program Funded by the National Heart, Lung, and Blood
Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN
 Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN Site: VFC#: Date of this Management Plan: / _/ (please update annually) This document identifies the key elements of vaccine storage and handling
Maryland Vaccines for Children Program VACCINE MANAGEMENT PLAN Site: VFC#: Date of this Management Plan: / _/ (please update annually) This document identifies the key elements of vaccine storage and handling
NexScreen Cup. Urine Drug Screen * READ POLICY PRIOR TO STARTING TUTORIAL
 NexScreen Cup Urine Drug Screen * READ POLICY PRIOR TO STARTING TUTORIAL Before Testing a Patient Orient yourself to your working area: Locate the NexScreen Cup. Locate testing supplies: Positive and Negative
NexScreen Cup Urine Drug Screen * READ POLICY PRIOR TO STARTING TUTORIAL Before Testing a Patient Orient yourself to your working area: Locate the NexScreen Cup. Locate testing supplies: Positive and Negative
The RE-ENERGIZE Study. A RandomizEd trial of ENtERal Glutamine to minimize thermal injury. Lab Study Manual
 The RE-ENERGIZE Study A RandomizEd trial of ENtERal Glutamine to minimize thermal injury Lab Study Manual Human Research Protection Office (HRPO) Log Number A-15774.0 HRPO Proposal Number 09155001 Clinical
The RE-ENERGIZE Study A RandomizEd trial of ENtERal Glutamine to minimize thermal injury Lab Study Manual Human Research Protection Office (HRPO) Log Number A-15774.0 HRPO Proposal Number 09155001 Clinical
APPENDIX E COLLECTION AND SUBMISSION OF SPECIMENS FOR TB TESTING
 APPENDIX E COLLECTION AND SUBMISSION OF SPECIMENS FOR TB TESTING For additional information about the collection, transport or submission of specimens for AFB examination, contact BC Public Health & Microbiology
APPENDIX E COLLECTION AND SUBMISSION OF SPECIMENS FOR TB TESTING For additional information about the collection, transport or submission of specimens for AFB examination, contact BC Public Health & Microbiology
Autologous plasma eye drops. Patient information
 Autologous plasma eye drops Patient information Autologous plasma eye drops are made from the patient s own blood and are used to help treat their corneal surface defects and diseases. This leaflet gives
Autologous plasma eye drops Patient information Autologous plasma eye drops are made from the patient s own blood and are used to help treat their corneal surface defects and diseases. This leaflet gives
Line Listing and Specimen Collection. Module 5
 Line Listing and Specimen Collection Module 5 Learning Outcomes By the end of this module you will be able to: Explain the role of the line list in outbreak management. Identify when cases should be added.
Line Listing and Specimen Collection Module 5 Learning Outcomes By the end of this module you will be able to: Explain the role of the line list in outbreak management. Identify when cases should be added.
Health Services. Procedure Manual
 Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Collecting Stool Specimens for Culture Collecting Stool Specimens For Parasitology Collection of 72 Hour Stool Fat
 Stool Collection Instructions: Click on the title of the Patient Information sheet(s) you would like to read Table of Contents Collecting Stool Specimens for Culture 814902 Collecting Stool Specimens For
Stool Collection Instructions: Click on the title of the Patient Information sheet(s) you would like to read Table of Contents Collecting Stool Specimens for Culture 814902 Collecting Stool Specimens For
To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status.
 Module 9 Performing HIV Rapid Tests Purpose To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status. Pre-requisite Modules Module 3: Overview
Module 9 Performing HIV Rapid Tests Purpose To provide you with necessary knowledge and skills to accurately perform 3 HIV rapid tests and to determine HIV status. Pre-requisite Modules Module 3: Overview
Instructions for Use. Welcome!
 Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
Center for Family Health Policy
 Subject: Vaccine Management and Administration (Immunization) BOARD APPROVED: 7/30/96 REVISION APPROVED: 05/03/05, 04/08/08, 04/07/09, 04/12/11 Purpose: To ensure safe and appropriate storage, handling
Subject: Vaccine Management and Administration (Immunization) BOARD APPROVED: 7/30/96 REVISION APPROVED: 05/03/05, 04/08/08, 04/07/09, 04/12/11 Purpose: To ensure safe and appropriate storage, handling
If you notice discrepancies between the package insert and the WSLH instructions, follow the WSLH instructions!
 HIV Oral Fluid Testing Wisconsin State Laboratory of Hygiene Introduction This training is based on the Wisconsin State Laboratory of Hygiene s (WSLH) experience with HIV oral fluid testing using the OraSure
HIV Oral Fluid Testing Wisconsin State Laboratory of Hygiene Introduction This training is based on the Wisconsin State Laboratory of Hygiene s (WSLH) experience with HIV oral fluid testing using the OraSure
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM
 THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT TO PERFORM THERAPEUTIC DONOR INSEMINATION WITH ANONYMOUS DONOR SPERM Partner #1 Last Name (Surname): Partner #1 First Name: Partner
COMPONENT SECTION MANUAL THE PROCEDURE FOR THE PREPARATION OF WHOLE BLOOD FOR NEONATAL EXCHANGE TRANSFUSION
 SOP from a Massachusetts BLOOD BANK DONOR/TRANSFUSION SERVICE COMPONENT SECTION MANUAL THE PROCEDURE FOR THE PREPARATION OF WHOLE BLOOD FOR I. PURPOSE: To describe the steps necessary to prepare reconstituted
SOP from a Massachusetts BLOOD BANK DONOR/TRANSFUSION SERVICE COMPONENT SECTION MANUAL THE PROCEDURE FOR THE PREPARATION OF WHOLE BLOOD FOR I. PURPOSE: To describe the steps necessary to prepare reconstituted
For use only with INSTRUCTIONS FOR USE
 TM For use only with INSTRUCTIONS FOR USE IMPORTANT NOTICE: Please read this safety information first. 1. Follistim Pen is a precision device. It is very important that you read and follow all directions
TM For use only with INSTRUCTIONS FOR USE IMPORTANT NOTICE: Please read this safety information first. 1. Follistim Pen is a precision device. It is very important that you read and follow all directions
Brown-Lupton Health Service Texas Christian University Campus P.O. Box Fort Worth, TX Dear Student,
 Brown-Lupton Health Service Texas Christian University Campus P.O. Box 297400 Fort Worth, TX 76129 817-257-7940 Dear Student, Enclosed you will find our policies, procedures and student consent form for
Brown-Lupton Health Service Texas Christian University Campus P.O. Box 297400 Fort Worth, TX 76129 817-257-7940 Dear Student, Enclosed you will find our policies, procedures and student consent form for
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial
 Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Introduction to Immunisations & Cold Chain Management in General Practice. April 2016
 Introduction to Immunisations & Cold Chain Management in General Practice April 2016 Introduction to Immunisations & Cold Chain Management This session will cover: The role of immunisations in General
Introduction to Immunisations & Cold Chain Management in General Practice April 2016 Introduction to Immunisations & Cold Chain Management This session will cover: The role of immunisations in General
Temperature monitors for vaccines and the cold chain
 WHO/V&B/99.15 ORIGINAL: ENGLISH DISTR.: GENERAL Temperature monitors for vaccines and the cold chain Cold-chain monitor Vaccine vial monitor Freeze Watch TM Stop!Watch TM DT and TT shipping indicator DEPARTMENT
WHO/V&B/99.15 ORIGINAL: ENGLISH DISTR.: GENERAL Temperature monitors for vaccines and the cold chain Cold-chain monitor Vaccine vial monitor Freeze Watch TM Stop!Watch TM DT and TT shipping indicator DEPARTMENT
BCG Bladder Therapy. Information for patients. Where to find us: TGH Cystoscopy Clinic 2NU (Room 291) Toronto General Hospital Phone:
 Form: D-5660 BCG Bladder Therapy Information for patients Where to find us: TGH Cystoscopy Clinic 2NU (Room 291) Toronto General Hospital Phone: 416 340 3882 Your doctor has decided that BCG bladder therapy
Form: D-5660 BCG Bladder Therapy Information for patients Where to find us: TGH Cystoscopy Clinic 2NU (Room 291) Toronto General Hospital Phone: 416 340 3882 Your doctor has decided that BCG bladder therapy
INSTRUCTIONS FOR USE. EMGALITY TM (em-gal-it-ē) (galcanezumab-gnlm)
 1 INSTRUCTIONS FOR USE EMGALITY TM (em-gal-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use Prefilled Pen For subcutaneous injection only. Before you use the EMGALITY prefilled pen (Pen), read
1 INSTRUCTIONS FOR USE EMGALITY TM (em-gal-it-ē) (galcanezumab-gnlm) injection, for subcutaneous use Prefilled Pen For subcutaneous injection only. Before you use the EMGALITY prefilled pen (Pen), read
Patient/Carer instructions for the administration of Subcutaneous Cytarabine
 Patient/Carer instructions for the administration of Subcutaneous Cytarabine This document covers the following information: What cytarabine is What subcutaneous means What happens if you decide to inject
Patient/Carer instructions for the administration of Subcutaneous Cytarabine This document covers the following information: What cytarabine is What subcutaneous means What happens if you decide to inject
MYALEPT (MAI-uh-lept) (metreleptin) for injection for subcutaneous use
 .3 mg per vial _ A healthcare provider should show you how to inject MYALEPT before you use it for the first time. A healthcare provider should also watch you inject your MYALEPT dose the first time you
.3 mg per vial _ A healthcare provider should show you how to inject MYALEPT before you use it for the first time. A healthcare provider should also watch you inject your MYALEPT dose the first time you
Pathology Specimen Handling Requirements
 CONWAY REGIONAL HEALTH SYSTEM CLINICAL LABORATORY Pathology Specimen Handling Requirements POLICY: Tissue or body fluids, etc. removed or collected during any procedure for purposes of Pathologist examination
CONWAY REGIONAL HEALTH SYSTEM CLINICAL LABORATORY Pathology Specimen Handling Requirements POLICY: Tissue or body fluids, etc. removed or collected during any procedure for purposes of Pathologist examination
Instructions For Use. SOLUTION FOR INJECTION IN PRE-FILLED PEN 250 micrograms
 Instructions For Use SOLUTION FOR INJECTION IN PRE-FILLED PEN 250 micrograms Contents 1. How to use your Ovitrelle pre-filled pen 2. Before you start using your Ovitrelle pre-filled pen 3. Getting your
Instructions For Use SOLUTION FOR INJECTION IN PRE-FILLED PEN 250 micrograms Contents 1. How to use your Ovitrelle pre-filled pen 2. Before you start using your Ovitrelle pre-filled pen 3. Getting your
Hazardous Drug Safety
 Annual Compliance Education This course contains annual compliance education necessary to meet compliance and regulatory requirements. Instructions: To receive credit for completion: 1. Read the content
Annual Compliance Education This course contains annual compliance education necessary to meet compliance and regulatory requirements. Instructions: To receive credit for completion: 1. Read the content
CATHETER PASSPORT. Looking after your Urinary Catheter. The Catheter Passport should be given to all patients with a urinary catheter.
 CATHETER PASSPORT Looking after your Urinary Catheter The Catheter Passport should be given to all patients with a urinary catheter. Health care staff should ensure that page 10 is completed. This document
CATHETER PASSPORT Looking after your Urinary Catheter The Catheter Passport should be given to all patients with a urinary catheter. Health care staff should ensure that page 10 is completed. This document
Gastrostomy Tube Feeding
 Gastrostomy Tube Feeding A gastrostomy tube (g-tube) is a tube that enters through your abdomen and rests in your stomach. This tube is used for tube feeding formula, water, and medicine (instead of taking
Gastrostomy Tube Feeding A gastrostomy tube (g-tube) is a tube that enters through your abdomen and rests in your stomach. This tube is used for tube feeding formula, water, and medicine (instead of taking
Investigator Initiated Study Proposal Form
 Please submit completed form to IISReview@KCI1.com Date Submitted Name & Title Institution Address Phone Number Email Address Principal Investigator / Institution YES NO Multi Center Study Acelity Product(s)
Please submit completed form to IISReview@KCI1.com Date Submitted Name & Title Institution Address Phone Number Email Address Principal Investigator / Institution YES NO Multi Center Study Acelity Product(s)
QUICK REFERENCE INSTRUCTIONS For use with Sofia only.
 QUICK REFERENCE INSTRUCTIONS For use with Sofia only. CLIA Complexity: Waived Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package
QUICK REFERENCE INSTRUCTIONS For use with Sofia only. CLIA Complexity: Waived Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete Package
Education for self administration of intravenous therapy HOME IV THERAPY. 30 minute - Baxter Pump Tobramycin
 HOME IV THERAPY Tobramycin Tobramycin Check the order on the drug chart This can change when the results from your blood test come through. Your doctor will change the order, if required. A copy of the
HOME IV THERAPY Tobramycin Tobramycin Check the order on the drug chart This can change when the results from your blood test come through. Your doctor will change the order, if required. A copy of the
24-Hour Urine Collection: Split Daytime and Nighttime
 2019 24-Hour Urine Collection: Split Daytime and Nighttime www.nshealth.ca 24-Hour Urine Collection: Split Daytime and Nighttime Why do I need to do a 24-hour split urine collection? A 24-hour split urine
2019 24-Hour Urine Collection: Split Daytime and Nighttime www.nshealth.ca 24-Hour Urine Collection: Split Daytime and Nighttime Why do I need to do a 24-hour split urine collection? A 24-hour split urine
Giving Yourself an Insulin Injection With the Lantus SoloStar Pen
 PATIENT & CAREGIVER EDUCATION Giving Yourself an Insulin Injection With the Lantus SoloStar Pen This information describes how to prepare and give yourself an insulin injection (shot) with the Lantus SoloStar
PATIENT & CAREGIVER EDUCATION Giving Yourself an Insulin Injection With the Lantus SoloStar Pen This information describes how to prepare and give yourself an insulin injection (shot) with the Lantus SoloStar
Inspection Window. Syringe Body
 Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
1.1 Study Objectives Scientific Background Selection Criteria Contacting Pathology Departments/Laboratories...
 PATHOLOGY TISSUE PROCESS MANUAL TABLE OF CONTENTS 1. INTRODUCTION...2 1.1 Study Objectives...2 1.2 Scientific Background...2 2. SAMPLE SELECTION...3 2.1 Selection Criteria...3 2.2 Contacting Pathology
PATHOLOGY TISSUE PROCESS MANUAL TABLE OF CONTENTS 1. INTRODUCTION...2 1.1 Study Objectives...2 1.2 Scientific Background...2 2. SAMPLE SELECTION...3 2.1 Selection Criteria...3 2.2 Contacting Pathology
How to Use ENBREL : Vial Adapter Method
 How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
Human TSH ELISA Kit. User Manual
 Human TSH ELISA Kit User Manual Catalog number: GTX15585 GeneTex Table of Contents A. Product Description... 2 B. Kit Components... 3 C. Additional Required Materials (not included)... 3 D. Reagent Preparation...
Human TSH ELISA Kit User Manual Catalog number: GTX15585 GeneTex Table of Contents A. Product Description... 2 B. Kit Components... 3 C. Additional Required Materials (not included)... 3 D. Reagent Preparation...
Self Catheterisation for Men
 Intermittent Self Catheterisation for Men www.fittleworth.com Opening Hours: 8 am to 8 pm Monday to Friday 9 am to 1 pm Saturday National: 0800 378 846 Scotland: 0800 783 7148 Intermittent Self Catheterisation
Intermittent Self Catheterisation for Men www.fittleworth.com Opening Hours: 8 am to 8 pm Monday to Friday 9 am to 1 pm Saturday National: 0800 378 846 Scotland: 0800 783 7148 Intermittent Self Catheterisation
Therapeutic Sperm Banking
 Therapeutic Sperm Banking An Option for Preserving Male Fertility Andrology Laboratory and Reproductive Tissue Bank Information For Patients Men undergoing cancer treatment, including certain types of
Therapeutic Sperm Banking An Option for Preserving Male Fertility Andrology Laboratory and Reproductive Tissue Bank Information For Patients Men undergoing cancer treatment, including certain types of
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
GYNAECOLOGICAL CYTOLOGY
 The laboratory runs a tour of the department for Practice Nurses on request, as part of their training, explaining the procedures and requirements for Gynae LBC. RECOMMENDED PROTOCOL FOR SMEAR TAKERS Only
The laboratory runs a tour of the department for Practice Nurses on request, as part of their training, explaining the procedures and requirements for Gynae LBC. RECOMMENDED PROTOCOL FOR SMEAR TAKERS Only
Best Practice Guidance 12_Version 1.2. Title: Collection of Biological Samples
 This document is for guidance on the collection of biological samples that have been granted ethical approval under CUREC procedures. Certain research involving the taking of samples that consist of, or
This document is for guidance on the collection of biological samples that have been granted ethical approval under CUREC procedures. Certain research involving the taking of samples that consist of, or
HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE
 1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
PLEASE READ THIS USER MANUAL BEFORE USE
 USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
Determining the Molecular Mass of an Unknown Acid by Titration
 Determining the Molecular Mass of an Unknown Acid by Titration Objectives: To perform an analytical titration. To standardize a basic solution. To determine the equivalent mass of an unknown acid. Background:
Determining the Molecular Mass of an Unknown Acid by Titration Objectives: To perform an analytical titration. To standardize a basic solution. To determine the equivalent mass of an unknown acid. Background:
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations
 Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
Calgary Diabetes Centre Insulin Pump Therapy: Preparation and Expectations This is a long and important document. It lists the steps for starting insulin pump therapy at the Calgary Diabetes Centre. It
Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions.
 1 INSTRUCTIONS FOR USE TALTZ (tȯl(t)s) (ixekizumab) injection, for subcutaneous use Autoinjector Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions. Important
1 INSTRUCTIONS FOR USE TALTZ (tȯl(t)s) (ixekizumab) injection, for subcutaneous use Autoinjector Before you use the TALTZ autoinjector, read and carefully follow all the step-by-step instructions. Important
Treatment. Zinbryta (daclizumab)
 Information for people living with multiple sclerosis Treatment What is Zinbryta and how does it work? How is Zinbryta administered? Before you take Zinbryta While you are taking Zinbryta What are the
Information for people living with multiple sclerosis Treatment What is Zinbryta and how does it work? How is Zinbryta administered? Before you take Zinbryta While you are taking Zinbryta What are the
Cap Clip Rubber Seal Plunger Pen Body Dose Window
 Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
What is a catheter? What do I need to learn about catheter care?
 Catheter care What is a catheter? A catheter is a tube that drains urine from your child s body. The catheter is connected to tubing and a bag to collect the urine. Catheters come in different sizes and
Catheter care What is a catheter? A catheter is a tube that drains urine from your child s body. The catheter is connected to tubing and a bag to collect the urine. Catheters come in different sizes and
Adult Metabolic Diseases Clinic 4 th Floor 2775 Laurel Street Vancouver, BC V5Z 1M9 Tel: Fax:
 Adult Metabolic Diseases Clinic 4 th Floor 2775 Laurel Street Vancouver, BC V5Z 1M9 Tel: 604-875-5965 Fax: 604-875-5967 PROTOCOL FOR CSF COLLECTION FOR NEUROTRANSMITTERS WITH OR WITHOUT AMINO ACIDS Indications:
Adult Metabolic Diseases Clinic 4 th Floor 2775 Laurel Street Vancouver, BC V5Z 1M9 Tel: 604-875-5965 Fax: 604-875-5967 PROTOCOL FOR CSF COLLECTION FOR NEUROTRANSMITTERS WITH OR WITHOUT AMINO ACIDS Indications:
Caring for Your Urinary (Foley ) Catheter
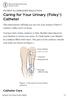 PATIENT & CAREGIVER EDUCATION Caring for Your Urinary (Foley ) Catheter This information will help you care for your urinary (Foley ) catheter while you re at home. You have had a Foley catheter (a thin,
PATIENT & CAREGIVER EDUCATION Caring for Your Urinary (Foley ) Catheter This information will help you care for your urinary (Foley ) catheter while you re at home. You have had a Foley catheter (a thin,
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only
 QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
QUICK REFERENCE INSTRUCTIONS For use with Sofia and Sofia 2. Rx only Test Procedure Study the Package Insert and User Manual thoroughly before using Quick Reference Instructions. This is not a complete
Rhino Clear Sprint Atomizer
 Visit us online to view our wide range of sinus therapy medications for use with the Rhino Clear Sprint atomizer. www.woodlandhillspharmacy.com 20631 Ventura Blvd, Ste 305 Woodland Hills, CA 91364 Phone:
Visit us online to view our wide range of sinus therapy medications for use with the Rhino Clear Sprint atomizer. www.woodlandhillspharmacy.com 20631 Ventura Blvd, Ste 305 Woodland Hills, CA 91364 Phone:
Urinary Catheter Passport
 Urinary Catheter Passport Catheter Change Record and Looking After Your Urinary Catheter Please take this booklet with you if you are admitted to hospital or have an appointment Patient details Name Address
Urinary Catheter Passport Catheter Change Record and Looking After Your Urinary Catheter Please take this booklet with you if you are admitted to hospital or have an appointment Patient details Name Address
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe
 Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
Materials: Activity: Explanation:
 Children will model how fat can be digested by bile. Materials: Whole milk Water Shallow dish or pie pan Food coloring Liquid dish or hand soap Cotton swabs Activity: 1) Pour about ½ inch of milk into
Children will model how fat can be digested by bile. Materials: Whole milk Water Shallow dish or pie pan Food coloring Liquid dish or hand soap Cotton swabs Activity: 1) Pour about ½ inch of milk into
Rapid-VIDITEST FOB Blister
 Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector
 SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
Please note: Forms with PXXXX designations can be found at All other forms can be found at
 ADULT SAFETY NET (ASN) PROGRAM 2018 COMPLIANCE SITE VISIT FOLLOW-UP PLAN This document is used to track required follow-up actions at the completion of an ASN compliance site visit. When all follow-up
ADULT SAFETY NET (ASN) PROGRAM 2018 COMPLIANCE SITE VISIT FOLLOW-UP PLAN This document is used to track required follow-up actions at the completion of an ASN compliance site visit. When all follow-up
Testing for Fabry Disease. What You Need to Know
 Testing for Fabry Disease What You Need to Know Identification of One Family Member Can Enable Earlier Screening for Others A woman with Fabry disease may have inherited it from either her mother or her
Testing for Fabry Disease What You Need to Know Identification of One Family Member Can Enable Earlier Screening for Others A woman with Fabry disease may have inherited it from either her mother or her
Study Design and Materials
 Study Coordinator Training November 13, 2008 Agenda Study Design and Materials Screening and Randomization Study Medication Height and Waist Measurements Data Collection Procedures Site Monitoring Data
Study Coordinator Training November 13, 2008 Agenda Study Design and Materials Screening and Randomization Study Medication Height and Waist Measurements Data Collection Procedures Site Monitoring Data
For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges.
 MS9662 MS9663 HumaPen LUXURA INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. Your HumaPen LUXURA
MS9662 MS9663 HumaPen LUXURA INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. Your HumaPen LUXURA
User Bulletin 7500 Fast Precision Plate Holder for MicroAmp Tube Strips
 User Bulletin 7500 Fast Precision Plate Holder for MicroAmp Tube Strips December 19, 2007 SUBJECT: Using MicroAmp Fast 8-Tube Strips (0.1-mL) on the 7500 Fast Precision Plate Holder for MicroAmp Tube Strips
User Bulletin 7500 Fast Precision Plate Holder for MicroAmp Tube Strips December 19, 2007 SUBJECT: Using MicroAmp Fast 8-Tube Strips (0.1-mL) on the 7500 Fast Precision Plate Holder for MicroAmp Tube Strips
WITHDRAWN. Treatment. Zinbryta (daclizumab)
 Information for people living with multiple sclerosis What is Zinbryta and how does it work? How is Zinbryta administered? Before you take Zinbryta While you are taking Zinbryta What are the potential
Information for people living with multiple sclerosis What is Zinbryta and how does it work? How is Zinbryta administered? Before you take Zinbryta While you are taking Zinbryta What are the potential
For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges.
 MS9673 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The colour of your HumaPen
MS9673 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The colour of your HumaPen
ORMOM 2015 PATIENT FLOW-2015
 Onsite Lead: All Departments ORMOM 2015 PATIENT FLOW-2015 Patient Line/Wristbands Security will be onsite with the patient line overnight. Volunteers will be monitoring the patient line starting at 3:30
Onsite Lead: All Departments ORMOM 2015 PATIENT FLOW-2015 Patient Line/Wristbands Security will be onsite with the patient line overnight. Volunteers will be monitoring the patient line starting at 3:30
