Bowel Disease Research Foundation
|
|
|
- Steven Hancock
- 5 years ago
- Views:
Transcription
1 Bowel Disease Research Foundation of The Association of Coloproctology of Great Britain and Ireland Advancing the cure and treatment of bowel disease Royal College of Surgeons of England Lincoln's Inn Fields London WC2A 3PE Tel: Web: Research Project Final Report Principal investigator Miss Kathryn Gill Institution Sandwell and West Birmingham Hospitals NHS Trust Project Title UK Multi-centre trial of transcutaneous posterior tibial nerve stimulation in patients with faecal incontinence- using a novel device. Start date 01/08/2012 Finish date Lay Summary (max 500 words) Please use the following format: a) Problem addressed, background and strategic significance b) Method(s) used c) Hoped for results d) Actual results and implications for treatment/understanding 10/01/2014 Problem addressed: Faecal incontinence (FI), defined as uncontrolled loss of solids, liquids or gas from the anus, is a common and distressing condition. Whilst milder symptoms may be managed by several drug and behavioural strategies many patients still resort to surgery, often with poor results. Recent treatments that stimulate the pelvic floor nerves and muscles have proved successful but are currently delivered by invasive techniques. This project uses a new technology called Geko to stimulate a nerve at the ankle (tibial nerve) to treat FI. Geko is a discrete, self-contained device that sticks to the skin and can thus be worn at home without impeding normal activities. Methods used: 8 UK centres recruited a combined total of 43 patients with symptoms of FI. Bowel diaries and questionnaires are being used to determine the severity of symptoms for 2 weeks prior to starting treatment. Each patient is trained how to use the device at a visit to the trial centre before taking the devices home for the duration of the trial. The device is used for either one or 4 hours, 2 days per week for 6 weeks in total. Whether it is used for one or four hours is decided by a randomisation procedure at the trial centre at the initial visit. The bowel diaries and questionnaires are then repeated to determine if there is any improvement in symptoms in one or both groups after the treatment period has finished. Hoped for results: The study was hoping to determine whether the Geko device was effective in treating FI symptoms in patients, paving the way for a large-scale definitive trial. Also the study was aiming to identify whether there were any side effects or adverse effects the treatment may have on patients (ideally none). Actual results: 43 patients (38 females) entered into the study from eight
2 centres. 22 were placed randomly in to the 1-hour treatment group and 21 in to the 4-hour treatment group. Improvement in the number of incontinent episodes per week was observed, with larger improvements seen in the 4-hour treatment group. The amount of time patients could delay opening their bowels for was again improved in both groups but larger improvements were seen in the 4-hour treatment group. There was also an improvement in quality of life and symptom severity scores which patients undertook before and after the study. These improvements were seen in both groups. There were no side effects from the treatment and patients were happy with the treatment. Implications: These are encouraging results, but a bigger trial is needed to confirm these results. If the results are proven in a larger trial, then the Geko device could increase the number of treatment options available for FI to improve patient care, increase compliance with treatment, reduce the number of hospital visits and requirement for invasive treatments as well as NHS costs. It may increase choices for patients who are marginalized from current treatments e.g. elderly with other medical problems and improve their quality of life. Background (purpose for project) Faecal incontinence is a socially disabling condition defined as uncontrolled loss of solids, liquids or gas from the anus. Current epidemiological information shows that between 1 and 15% of adults outside of nursing institutions are affected, depending upon the definition and frequency of FI used. It is likely that 1-2% of adults experience regular faecal incontinence which impacts on quality of life with associated high healthcare utilisation and job absenteeism and thus is a major public health issue. The combined healthcare costs for urinary and faecal incontinence is estimated to account for 2% of the total healthcare budget, which is reflective of the unmet clinical need in this area. Until recently, in those who fail conservative management, the treatment of faecal incontinence has been limited to either major irreversible anal sphincter surgical interventions or stoma formation. Such procedures may be costly, are often unsuccessful and carry a high risk of associated morbidity. Recent attention, has therefore focused on non-invasive or minimally surgically invasive therapies to optimise the residual continence mechanisms by altering the nerve impulses to this area (termed neuromodulation), such modalities include percutaneous tibial nerve stimulation (PTNS) and sacral nerve stimulation (SNS). On the basis of the success of PTNS and SNS, recent attempts have been made to employ transcutaneous electrical nerve stimulation (TENS) to provide similar neuromodulation by stimulating the posterior tibial nerve at the ankle. The method applies stick-on electrocardiograph type electrodes over the posterior tibial nerve. Several studies attest to the success of such techniques in urinary incontinence; three short follow up (3-6 months) observational studies have been published reporting functional success ranging from 26-60% in patients with faecal incontinence. The advantage of a cheap and completely non-invasive technique is obvious, however TENS necessarily involves hard wiring to a stimulation unit that must be carried by the patient with limited if any ambulation. There is therefore a limitation on stimulation duration and an impetus to improve upon the acceptability of such devices. One such mode of delivery may be the The Geko device. It is a wearable, discrete, self-contained device that is designed to adhere to the skin. Geko is currently employed in hospitals in the form of a CE marked, disposable device for prevention of deep vein thrombosis with no reported morbidity. Introduction Faecal incontinence (FI) is a socially disabling condition defined as uncontrolled loss of solids, liquids or gas from the anus. Current epidemiological information
3 Bowel Disease Research Foundation of The Association of Coloproctology of Great Britain and Ireland Advancing the cure and treatment of bowel disease Royal College of Surgeons of England Lincoln's Inn Fields London WC2A 3PE Tel: Web: shows that between 1 and 15% of adults outside of nursing institutions are affected, depending upon the definition and frequency of FI used. (1,2) It is likely that 1-2% of adults experience regular faecal incontinence which impacts on quality of life (2) with associated high healthcare utilisation and job absenteeism and thus is a major public health issue. The combined healthcare costs for urinary and faecal incontinence is estimated to account for 2% of the total healthcare budget (3), which is reflective of the unmet clinical need in this area. Diagnosis includes a combination of detailed clinical history and examination with prospective stool diaries. A step-wise approach is taken in the management of FI with the first line management consisting of conservative measures including dietary advice, drugs, nurse-led bowel retraining and biofeedback, however a significant proportion do not respond to conservative treatment. (4) Until recently, in those who fail conservative management, the treatment of faecal incontinence has been limited to either major irreversible anal sphincter surgical interventions or stoma formation. Such procedures may be costly, are often unsuccessful and carry a high risk of associated morbidity (4-6). Recent attention has therefore focused on non-invasive or minimally surgically invasive therapies to optimise the residual continence mechanisms by altering the nerve impulses to this area (termed neuromodulation). One such modality is sacral nerve stimulation (SNS)(7). SNS is a surgical technique to apply low voltage electrical stimulation to the sacral nerve roots via an electrode through the sacral foramen in the spine. This is a less invasive therapeutic option for most patients failing conservative therapies regardless of FI aetiology e.g. sphincter injury, neurological impairment (9). Review data suggests complete continence with SNS in 41-75% and > 50 % improvement in symptoms in % of patients up to 3 years (10). However SNS has limitations namely: 1. Two operations with potential complications (11) 2. Cost implications - a recent HITEC review concluded that SNS compared with conservative management resulted in an additional cost of 9,142 per patient over a 10-year time horizon with a cost per QALY gained of 22,784 (11). Estimated current costs of implementing NICE guidelines on FI are million of which million is for SNS (4). An evolving alternative to SNS is percutaneous tibial nerve stimulation (PTNS). PTNS is a technique where low amplitude electrical stimulation is applied via a narrow calibre (akin to acupuncture) needle to the posterior tibial nerve at the ankle. It is thought that stimulation of the tibial nerve thence modulates bowel motility and/or sphincter function by transmitting impulses to the sacral nerves. The posterior tibial nerve is easily accessible at the medial aspect of the ankle and contains fibres arising from S2-S4 sacral nerve roots. These are the same spinal segments that innervate the bladder, rectum and pelvic floor. Published studies have shown statistically significant improvement for bladder control and faecal incontinence from PTNS (60-85%). Further, unlike SNS, PTNS can be administered within an outpatient setting and without the need for surgery,
4 confirming percutaneous stimulation of the tibial nerve is a low cost, low- risk technique with almost no associated morbidity (12). Transcutaneous nerve stimulation On the basis of the success of PTNS, recent attempts have been made to employ transcutaneous electrical nerve stimulation (TENS) to provide similar neuromodulation of the lumbosacral outflow by stimulating afferent input at the ankle. The method applies stick-on electrocardiograph type electrodes over the posterior tibial nerve. Several studies attest to the success of such techniques in urinary incontinence (13,14); three short follow up (3-6 months) observational studies have been published reporting functional success ranging from 26-60% in patients with FI (15-17). The advantage of a cheap and completely noninvasive technique is obvious however TENS necessarily involves hard wiring to a stimulation unit that must be carried by the patient with limited if any ambulation. There is therefore a limitation on stimulation duration and an impetus to improve upon the acceptability of such devices. One such mode of delivery may be the The Geko device. It is a wearable, discrete, self-contained device that is designed to adhere to the skin. Geko is currently employed in hospitals in the form of a CE marked, disposable device for prevention of deep vein thrombosis with no reported morbidity. The Geko TM Device In its original application, the Geko triggers small electrical impulses that gently activate the motor nerves within the popliteal fossa, behind the knee, in turn activating the venous muscle pumps of the calf and foot. Substantive increases in lower limb blood flow have been demonstrated to improve venous return and thereby reduce stasis. The OnPulse technology may be adapted for application in other anatomical regions and has already been successfully proven to improve post-training fatigue and injury recovery in elite athletes. Thus, proof of concept is embodied by the ability of the technology to provide effective stimulation levels to large human muscles with subsequent beneficial trophic effects albeit not yet at the pelvic floor. Given success of ankle based neuromodulation (above), the proposed study will use this technology to evoke stimulation of the posterior tibial nerve at the site of this nerve behind the medial malleolus. If the treatment proves successful it would have the following advantages over sacral and needle posterior tibial methods of neuromodulation: Non-invasive with subsequent reduction in operative intervention with associated morbidity and mortality Reduction in the cost of mild to moderate faecal incontinence. Estimated cost of 12 week course of GEKO is approximately 300 compared to 1,800 or 16,000 NHS tariffs for PTNS and SNS respectively. The treatment is patient administered therefore reducing the inconvenience of hospital appointments and an opportunity to increase community delivered care in line with Governmant policy. First fully ambulatory treatment modality so patients can continue their normal activities such as work and child care. Has the potential to expand choices for patients marginalised from interventional therapy (e.g. the elderly with poor performance status) and improve quality of life. Given all the information above, this treatment potentially will change the face of FI treatment if it is successful.
5 Bowel Disease Research Foundation of The Association of Coloproctology of Great Britain and Ireland Advancing the cure and treatment of bowel disease Royal College of Surgeons of England Lincoln's Inn Fields London WC2A 3PE Tel: Web: Methods SPECIFIC AIMS 1. To determine the effect of a 6 week course of Geko treatment on frequency and severity of FI; 2. To estimate dose response and acceptability of 2 treatment duration paradigms 3. To record any side effects. DESIGN The study is an early phase study of clinical efficacy (phase II exploratory pilot). A two arm randomised design has been chosen to mitigate against the limitations of a simple single arm before and after study. The trials pragmatic design, which includes a large and small dose of neurostimulation, has the advantage of giving basic information on dosing and acceptability as well as providing a surrogate sham i.e. if the basic hypothesis is true and the treatment has efficacy then one would expect to see a difference in effect size between the larger and smaller dose. A diagram has been included to show the basic trial design (Figure 1). In Suitable brief, patients Eligibility with FI have Eligibility been identified confirmed from within participating centres by patients responsible determined clinical staff (medical and nursing). Patients undergo eligibility Outcome data assessment identified to define whether Demonstration they are suitable of use for the study, recruitment and consent, in clinic baseline Consent assessment period (weeks 0 to 2), allocation by randomisation, nurse led training, 6 week treatment protocol and post treatment assessment (weeks 8-10). Figure 1: Research Plan Standard Care bowel diary Baseline assessment -bowel diary -QOL 6 weeks intervention hours 2 times a week i.e.12 treatments Outcome assessment -bowel diary -QoL TIME (weeks)
6 Visit 1: Potential Eligibility: Once identified the local lead investigator is informed and case notes are reviewed for general eligibility. Potentially eligible subjects are provided with adequate explanation of aims, methods, anticipated benefits and hazards of the study and will take away a patient information sheet containing this information. These patients are provided with a standard care bowel diary for completion over a two-week period. Visit 2: Consent- baseline assessment: A GCP trained local investigator determines eligibility by interview on the basis of defined inclusion and exclusion criteria including the standard care bowel diary information. If the patient is still eligible, a member of the local team will answer any outstanding questions and informed consent will be obtained. Patient demographics will be collected and participants will be given the research bowel diary to complete. An appointment time for visit 2 will be made for 2 weeks later to allow patients time to record baseline bowel diaries. Inclusion criteria: Eligibility will be a failure of nurse-led conservative therapies in patients with symptoms sufficiently severe to warrant intervention (FI defined as greater than 2 incontinence episodes per month or ability to defer for less than 5 minutes); All patients male and female aged 18-80; Although not mandatory it is expected that most patients will have undergone NICE recommended appropriate specialist investigation including structural and functional anorectal assessment. Anal sphincter injury is not a contra-indication. Exclusion criteria: Inability to fill in the detailed bowel diaries required for outcome assessment (this will exclude patients who do not speak/read English) Neurological disorders such as diabetic neuropathy, multiple sclerosis, and Parkinson s disease (any patient with painful peripheral neuropathy) Anatomical limitations that would prevent successful placement of surface electrodes Congenital anorectal anomalies or absence of native rectum due to surgery Present evidence of a full thickness rectal prolapse Previous rectal surgery (resection/rectopexy) performed less than 12 months previously (24 months for cancer) Stoma in situ Chronic bowel disease such as inflammatory bowel disease leading to chronic uncontrolled diarrhoea Pregnancy or intention to become pregnant Previous experience of SNS or PTNS Cardiac pacemaker Psychiatric disorders Visit 3. - Allocation- training. A GCP trained local investigator will determine final eligibility. Eligible participants are asked to complete FIQoL, EQ-5D quality of life questionnaires. Participants are randomised to treatment group by sequential opening of sealed envelopes. Full training in device application is given and first treatment commenced under direct observation. Contact details are given for the CNS in case of any questions pertaining to the use of the device.
7 Bowel Disease Research Foundation of The Association of Coloproctology of Great Britain and Ireland Advancing the cure and treatment of bowel disease Royal College of Surgeons of England Lincoln's Inn Fields London WC2A 3PE Tel: Web: Interventions: All patients will apply the Geko device once daily for 2 days of each of the 6-week trial (weeks 2 to 8). The duration of application will depend on randomisation (1 or 4 hours). With the device attached, the patient can resume normal daily activities with no restriction to ambulation. Application of Geko: Following simple skin preparation the device is applied via its self-adhesive gel to the area of the posterior tibial nerve 1cm behind the medial malleolus in a vertical position with the head of the device (control unit) uppermost and the indicator strip aligned with the centre of the medial malleolus. Either ankle can be employed. Visit 4: Outcome assessments: The patients will attend to record all outcome data at end 10 weeks with a preceding 2 week bowel diary completed from end of treatment (end week 8). These outcomes will be recorded blind to allocation. Study Milestones and Progress Since being awarded the grant from the BDRF, prompt progress has been made with the trial including gaining ethical approval, MHRA approval, NIHR portfolio status and visiting/opening all centres (including recruiting one new centre outside of the original planned trial centres). Below are details of the milestones, which show the progress of the study. Ethical Approval: Obtained: 28/02/2012. MHRA Approval: 27/06/2012 NIHR CRN Portfolio Status Obtained: 23/05/2012 Centres Opening Dates and Recruitment Centre Opening Date Number of Patients Recruited Sandwell and West Birmingham NHS Hospitals Trust University Hospital Southampton NHS Foundation Trust Oxford Radcliffe Hospitals NHS Trust 01/07/ /07/ /07/2012 5
8 Barts and the London NHS Trust University Hospitals Bristol NHS Foundation Trust Hull and East Yorkshire Hospitals NHS Trust Dorset County Hospitals NHS Foundation Trust 14/12/ /07/ /12/ /12/ Musgrove Hospital Park 01/07/ Results and discussion RESULTS Of 69 consecutive screened patients, 43 patients (38 female) met the eligibility criteria and agreed to participate. 21 patients were randomised to 4-hour treatment group and 22 patients to the 1-hour treatment group. All patients completed the study. Baseline demographic, medical and physiological characteristics were similar in both treatment groups, aside from the previous episiotomy characteristic (table 1). All patients were asked to complete a case reporting form detailing the duration of treatment and the timing of treatment i.e. which days the device was used on and this was reviewed to ensure compliance and record variance from protocol. Patients were instructed on how to use the device at their first treatment. They were given contact details of a local investigator, should there be any problems with using the devices. No patients made contact in relation to difficulties placing or using the device. All patients were asked at the end of the study whether they had encountered any technical difficulties with the devices for assessment of patient acceptability. Acceptability and adverse events All patients found the device easy to use. There were no unexpected serious adverse events relating to the Geko TM device. Two patients had mild irritation at the site of the device application. Both of these patients symptoms resolved spontaneously within 48 hours. The patients used the contralateral limb for the remaining study period with no further irritation apparent. Clinical outcome data Both the 1 and 4-hour treatment groups led to some improvements in the main clinical outcomes (table 2). In accord with the principles of pilot studies 25, we did not compare the two groups directly, but within group effects were analysed (table 3). Significant improvements were identified in the 4-hour treatment group with regards to a number of bowel diary variables but less so for the 1-hour treatment regimen. There were modest but significant reductions in CCIS in both groups, however a significant within group effect was noted for the modified ICIQ-B (PROM) score only for the 4-hour group. Significant improvements were observed in some domains for SF-36 generic quality of life outcome measures but not for EQ-5D although the EQ-5D VAS was improved in the 4-hour group. Patients felt that both regimens of therapy were successful with significant (approx. 5 points;
9 Bowel Disease Research Foundation of The Association of Coloproctology of Great Britain and Ireland Advancing the cure and treatment of bowel disease Royal College of Surgeons of England Lincoln's Inn Fields London WC2A 3PE Tel: Web: P<0.001) changes in Likert global scale. DISCUSSION This study has shown that transcutaneous posterior tibial nerve stimulation using the Geko device is safe and has some effect in both the 1 and 4-hour treatment groups. Both treatment groups showed an improvement in FI symptoms and QOL/symptom severity outcome measures. The 4-hour group (group 1) had a significant effect on the change in total FIE (faecal incontinence episodes) per week, passive FIE per week and deferment time. The 1-hour group (group 2) also had a significant effect on deferment time. Both groups showed a significant improvement in the Cleveland clinic incontinence score. In the 4-hour treatment group (group 1), however, the effect on FI symptoms (i.e. deferment time, FI episodes per week) appeared to be greater when compared to the 1-hour group (group 2). Interestingly, there are differences in both groups when analysing the generic QOL outcome measures, which may represent a lack of specificity of these outcome measures as reported previously 27. Both groups had a significant effect on three domains of the SF-36 QOL measure, namely, change in physical functioning, physical role and health transition. Only the 4-hour group (group 1) had a significant effect on the modified ICIQ-B. Overall the differences seen in the 4-hour group (group 1) between the pre and post-treatment results imply a dose effect. There were no serious adverse events and the patients, as recorded on the post-treatment Likert scale, were satisfied with the treatment. The limitations of the current study are mainly focused around the pilot design and the small number of patients. The aim of this pilot was to provide effect estimates for two differing regimens of therapy. The latter objective aimed to provide dosing information to guide subsequent more definitive studies of effectiveness but also to provide some reassurance that the therapy was not entirely dependent on placebo response i.e. to test the hypothesis that a higher dose had more effect than a lower one. This design did not allow for direct statistical comparison between group effects and was not statistically powered to detect within group effects. Thus, while within group effect size estimates will be useful for future trial design, conclusions regarding efficacy for either dose of therapy should be tempered on the basis of current data. Further limitations include the lack of control arm in the study; since both dose regimens used conscious levels of stimulation, a realistic sham will be difficult to develop. Finally, the short-term follow-up means that any conclusions regarding clinical effectiveness must be avoided without further study. Future trials would need to address these limitations. Conclusion This study provides evidence that transcutaneous posterior tibial nerve
10 stimulation via the Geko device does improve symptoms for patients with FI at that this is dose-dependent. The modest effects observed in this study need to be balanced with the potential benefits of transcutaneous stimulation with the Geko device. When compared with other modalities of delivery of transcutaneous and percutaneous posterior tibial nerve stimulation, as well as sacral nerve stimulation a major benefit will be that the Geko is a fully ambulatory therapy. Alongside this is the potential reduction in the cost of treatment when compared to other neuromodulatory treatments: Geko approx. 300 vs. PTNS 2,356 vs. SNS 12,748. The treatment is patient-administered which reduces the requirement for multiple hospital appointments whilst increasing communitydelivered care in line with Department of Health policy. The fact that the device is non-invasive may help to expand treatment choices for patients marginalised from interventional therapy (e.g. elderly patients with poor performance status). The potential reduction in operative intervention will also mean less associated morbidity and mortality. Recommendations for future work Whilst the effect size seems small when compared to more established neurostimulation techniques i.e. PTNS/SNS, the non-invasive, ambulatory nature alongside the decreased cost of the Geko device suggests that a larger study is warranted to identify whether this therapy may have a future clinical role. References (author, title, date of publication) 1. Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002; 50: Nelson RL. Epidemiology of fecal incontinence. Gastroenterology 2004; 126 (suppl 1): S3-S7. 3. Finne-Soveri H, Sorbye LW, Jonsson PV, Carpenter GI, Bernabei R. Increased work-load associated with faecal incontinence among home care patients in 11 European countries. Eur J Public Health 2008; 18: National Institute for Health and Clinical Excellence (NICE). Faecal incontinence: The management of faecal incontinence in adults. NICE guideline CG no.49, June Tan JJY. Evolving therapy for fecal incontinence. DCR 2007; 50: Mowatt G, Glazener C, Jarrett M. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev Jul 18;(3): CD Rev Malouf AJ, Norton CS, Engel AF, Nicholls RJ, Kamm MA. Long-term results of overlapping anterior anal-sphincter repair for obstetric trauma. Lancet. 2000; 355(9200): Tillin T, Gannon K, Feldman RA, Williams NS. Third-party prospective evaluation of patient outcomes after dynamic graciloplasty. Br J Surg 2006; 93: Tjandra JJ, Chan MK, Yeh CH, Murray-Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum 2008; 51: Jarrett ME, Mowatt G, Glazener CM, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg 2004; 91: Jarret ME Br J Surg Sprange K Evidence Review: SNS for FI Healthcare Innovation and Technology Health Assessment (HITEC) Crown Allison M. Percutaneous tibial nerve stimulation: a new treatment for faecal incontinence. Gastrointestinal Nursing 2009; 7: Van Balken M Posterior tibial nerve stimulation in the treatment of voiding dysfunction: urodynamic data. J Urol 172:
11 Bowel Disease Research Foundation of The Association of Coloproctology of Great Britain and Ireland Advancing the cure and treatment of bowel disease Royal College of Surgeons of England Lincoln's Inn Fields London WC2A 3PE Tel: Web: Chang PL Urodynamic studies in acupuncture for women with frequency, urgency and dysuria. J Urol 140: Eleouet M Chronic posterior tibial nerve transcutaneous electrical nerve stimulation (TENS) to treat faecal incontinence Int J of Colorectal Disease ; Vitton V. Transcutaneous electrical posterior tibial nerve stimulation for faecal incontinence: effects on symptoms and quality of life Int J of Colorectal Disease; 25(8); , 17. Queralto M. Preliminary results of peripheral transcutaneous neuromodulation in the treatment of idiopathic faecal incontinence. Int J of Colorectal Disease 2006:21; Appendix Table 1: Numbers and percentages or means and standard deviations of baseline characteristics by treatment type Group 1 (N=21) Group 2 (N=22) Demographic Patients recruited per centre (n = 7) 3 (range 2-4) 3 (range 2-5) Female 18 (86%) 20 (91%) Age 59 years (SD 11) 62 years (SD 12) History Parity 2 (range 0-7) 2 (range 0-5)
12 History of instrumental delivery 2 (11%) 3 (14%) Previous episiotomy 3 (14%) 8 (36%)* Previous tear 6 (29%) 6 (27%) Previous Caesarean section 1 (5%) 2 (10%) Previous anal surgery (STARR) 1 (5%) 1 (5%) Investigation Sphincter defect on EAUS 11 (52%) 9 (41%) Previous / on-going treatments Nurse led conservative management 21 (100%) 22 (100%) Biofeedback 9 (43%) 7 (32%) Incontinence pad usage 21 (100%) 20 (91%) Anal plug 2 (10%) 1 (5%) Anti-diarrhoeal medications 16 (76%) 18 (82%) Suppository use 1 (5%) 2 (9%) Enema use 2 (10%) 2 (9%) Irrigation 1 (5%) 0 (0%) Main Symptoms Urge Incontinence 6 (29%) 8 (36%) Passive Incontinence 8 (38%) 3 (14%) Mixed Incontinence 7 (33%) 10 (46%) Deferment time (median: mins) 0.5 (range 0.5-5) 2 (range 0.5-5) Key: SD = standard deviation; * P = (no significant differences in all other baseline comparisons); STARR = stapled transanal resection of rectum. EAUS = endoanal ultrasound
13 Bowel Disease Research Foundation of The Association of Coloproctology of Great Britain and Ireland Advancing the cure and treatment of bowel disease Royal College of Surgeons of England Lincoln's Inn Fields London WC2A 3PE Tel: Web: Table 2: Main clinical outcomes by group Group 1 (n = 21) Group 2 (n = 22) Variable Before After Before After Daily bowel frequency 2 (1-4) 1 (1-5) 1.5 (1-4) 1.5 (1-4) Urge FIE per week 4 (0-16) 1 (0-8) 2 (0-16) 0.5 (0-12) Passive FIE per week 5 (0-19) 2 (0-15) 1.5 (0-14.5) 0.5 (0-11) Deferment time (min) 0.5 (0.5-5) 5 (0.5-15) 2 (0.5-5) 2 (0.5 10) Social limitation 11 / 21 (54%) 4 / 21 (19%) 11 / 22 (50%) 8 / 22 (36%) CCIS 14 (6-20) 12 (2-18) 15 (5-18) 11 (5-18) Key: All variables shown as medians and ranges. CCIS = Cleveland clinic incontinence score. Table 3: Within group effect estimates (mean) and 95% confidence intervals Group 1 (n = 21) Group 2 (n = 22) Variable Mean 95% CI Mean 95% CI Change total FIE per week to 0.5* to
14 Change passive FIE per week to 0.7* to Change deferment time (min) to to + 2.4* Change in CCIS to to 0.1* Change EQ-5D-3L to to Change EQ-5D VAS to to Change modified ICIQ-B to 0.2* to Change physical functioning to * to * Change physical role to * to * Change bodily pain to to Change general health to to Change vitality to to Change social functioning to to Change emotional role to to * Change mental health to to Change health transition to to Likert scale of success to to Key: CCIS = Cleveland clinic incontinence score. Significant difference in mean from zero on onesample t-test at: * <0.05 level; < 0.01 level; < level
PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS. A08/S/d Colorectal: Faecal Incontinence (Adult)
 A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
GI Physiology - Investigating and treating patients with pelvic floor dysfunction. Lynne Smith Department of GI Physiology NGH Sheffield
 GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
GI Physiology - Investigating and treating patients with pelvic floor dysfunction Lynne Smith Department of GI Physiology NGH Sheffield Aims o o o To give an overview of lower GI investigations To demonstrate
SACRAL NERVE STIMULATION FOR EXPERIENCE IN CHILDREN
 SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
SACRAL NERVE STIMULATION FOR COLORECTAL DISEASES: EXPERIENCE IN CHILDREN C. LOUIS-BORRIONE - JM. GUYS TIMONE-ENFANTS MARSEILLE SACRAL NEUROMODULATION IN CHILDREN 26 : Humphreys et al - 23 children with
A70.4 Insertion of neurostimulator electrodes into peripheral nerve Z12.2 Posterior tibial nerve R15.X Faecal incontinence
 The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
Randomised Mixed Methods Pilot Trial of Sacral and Percutaneous Tibial Nerve Stimulation for Faecal Incontinence
 Research for Patient Benefit Randomised Mixed Methods Pilot Trial of Sacral and Percutaneous Tibial Nerve Stimulation for Faecal Incontinence Thin NN 1, Taylor SJC 2, Bremner SA 2, Hounsome N 2, Alam A
Research for Patient Benefit Randomised Mixed Methods Pilot Trial of Sacral and Percutaneous Tibial Nerve Stimulation for Faecal Incontinence Thin NN 1, Taylor SJC 2, Bremner SA 2, Hounsome N 2, Alam A
Tertiary, regional and local pelvic floor service providers: the future. model? Andrew Williams
 Tertiary, regional and local pelvic floor service providers: the future Andrew Williams model? Pelvic Floor Unit Guy s and St Thomas NHS Foundation Trust Background 23% women suffer at least one pelvic
Tertiary, regional and local pelvic floor service providers: the future Andrew Williams model? Pelvic Floor Unit Guy s and St Thomas NHS Foundation Trust Background 23% women suffer at least one pelvic
Conservative Management of Functional Bowel & Pelvic Floor Disorders
 Conservative Management of Functional Bowel & Pelvic Floor Disorders Kathy Davis PhD BSc(Hons)SRN Specialist Nurse Consultant Parkside Hospital & Minerva Medical Clinic Overview Burden of disease Aims
Conservative Management of Functional Bowel & Pelvic Floor Disorders Kathy Davis PhD BSc(Hons)SRN Specialist Nurse Consultant Parkside Hospital & Minerva Medical Clinic Overview Burden of disease Aims
Lets talk about Faecal incontinence (FI) in Scleroderma
 Lets talk about Faecal incontinence (FI) in Scleroderma Dr. Shamaila Butt Gastroenterology Research Registrar GI Physiology unit University College Hospital London GI manifestations in Scleroderma Oesophagus
Lets talk about Faecal incontinence (FI) in Scleroderma Dr. Shamaila Butt Gastroenterology Research Registrar GI Physiology unit University College Hospital London GI manifestations in Scleroderma Oesophagus
Biofeedback Therapy A nurse led management service for functional bowel disorders
 Biofeedback Therapy A nurse led management service for functional bowel disorders Brigitte Collins Lead Nurse BSc, MSc GI Nursing, Dip/Hypnotherapy St Marks Hospital Is biofeedback necessary? Conservative
Biofeedback Therapy A nurse led management service for functional bowel disorders Brigitte Collins Lead Nurse BSc, MSc GI Nursing, Dip/Hypnotherapy St Marks Hospital Is biofeedback necessary? Conservative
Posterior Tibial Nerve Stimulation
 Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
Sacral Nerve Stimulation for Faecal Incontinence
 Sacral Nerve Stimulation for Faecal Incontinence Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital
Sacral Nerve Stimulation for Faecal Incontinence Questions & Answers GLASGOW COLORECTAL CENTRE Ross Hall Hospital 221 Crookston Road Glasgow G52 3NQ e-mail: info@colorectalcentre.co.uk Ph: Main hospital
Pelvic Floor Disorders. Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon
 Pelvic Floor Disorders Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon What is Pelvic Floor Disorder Surgical perspective symptoms of RED, FI or prolapse on the background
Pelvic Floor Disorders Amir Darakhshan MD FRCS (Gen Surg) Consultant Colorectal and General Surgeon What is Pelvic Floor Disorder Surgical perspective symptoms of RED, FI or prolapse on the background
2/5/2016. Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach
 Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach Holly E Richter, PhD, MD, FACOG, FACS J Marion Sims Professor Obstetrics and Gynecology Professor
Evolving Surgical Treatment Approaches for Fecal Incontinence in Women: An Evidence and Cased-Based Approach Holly E Richter, PhD, MD, FACOG, FACS J Marion Sims Professor Obstetrics and Gynecology Professor
Duc M. Vo, MD, FACS Northwest Surgical Specialists
 Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
Duc M. Vo, MD, FACS Northwest Surgical Specialists Disclosures none Outline Definition Etiologies Exam findings Additional testing Medical management Surgical options What is fecal incontinence? Recurrent
University College Hospital at Westmoreland Street. Percutaneous Tibial Nerve Stimulation (PTNS)
 University College Hospital at Westmoreland Street Percutaneous Tibial Nerve Stimulation (PTNS) Urology Directorate If you need a large print, audio or translated copy of this document, please contact
University College Hospital at Westmoreland Street Percutaneous Tibial Nerve Stimulation (PTNS) Urology Directorate If you need a large print, audio or translated copy of this document, please contact
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE The management of faecal incontinence in adults 1.1 Short title Faecal incontinence 2 Background (a) (b) (c) The National Institute
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE The management of faecal incontinence in adults 1.1 Short title Faecal incontinence 2 Background (a) (b) (c) The National Institute
Posterior Tibial Nerve Stimulation for Voiding Dysfunction
 Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
Posterior Tibial Nerve Stimulation for Voiding Dysfunction Corporate Medical Policy File name: Posterior Tibial Nerve Stimulation for Voiding Dysfunction File code: UM.NS.05 Origination: 8/2011 Last Review:
OHTAC Recommendation
 OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
OHTAC Recommendation Sacral Nerve Stimulation for the Management of Urge Incontinence, Urgency-Frequency, Urinary Retention and Fecal Incontinence March 2, 2005 1 The Ontario Health Technology Advisory
A Case of Fecal Incontinence: Medical and Interventional Treatment Options
 A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
A Case of Fecal Incontinence: Medical and Interventional Treatment Options HPI JP is a 69 year-old F with a 12-month history of FI. Her symptoms began after a colonoscopy She has been experiencing passive
PERCUTANEOUS TIBIAL NERVE STIMULATION
 PERCUTANEOUS TIBIAL NERVE STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical
PERCUTANEOUS TIBIAL NERVE STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical
MEDICAL POLICY SUBJECT: PERCUTANEOUS POSTERIOR TIBIAL NERVE STIMULATION (PPTNS) POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: PERCUTANEOUS POSTERIOR Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally
MEDICAL POLICY SUBJECT: PERCUTANEOUS POSTERIOR Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally
2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)
 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service
2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service
MEDICAL POLICY SUBJECT: PERCUTANEOUS POSTERIOR TIBIAL NERVE STIMULATION (PPTNS)
 MEDICAL POLICY 03/19/15, 05/17/16 PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria
MEDICAL POLICY 03/19/15, 05/17/16 PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria
Clinical Commissioning Policy Statement: Sacral Nerve Stimulation (SNS) for Faecal Incontinence in Adults April Reference: NHSCB/A08/PS/b
 Clinical Commissioning Policy Statement: Sacral Nerve Stimulation (SNS) for Faecal Incontinence in Adults April 2013 Reference: NHSCB/A08/PS/b NHS Commissioning Board Clinical Commissioning Policy Statement:
Clinical Commissioning Policy Statement: Sacral Nerve Stimulation (SNS) for Faecal Incontinence in Adults April 2013 Reference: NHSCB/A08/PS/b NHS Commissioning Board Clinical Commissioning Policy Statement:
ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION. Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital
 ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital Accidental Bowel Leakage What Gets the Woman into Your Office 67%
ACCIDENTAL BOWEL LEAKAGE: A PRACTICAL APPROACH TO EVALUATION Tristi W. Muir, MD Chair, Department of OB/GYN Houston Methodist Hospital Accidental Bowel Leakage What Gets the Woman into Your Office 67%
Faecal Incontinence: Assessment and Management
 Mrs PK; 56 yrs; Married; 2 children Faecal Incontinence: Assessment and Management Professor Marc A Gladman MBBS DFFP PhD MRCOG FRCS (UK) FRACS Professor of Colorectal Surgery >10 years of incontinence
Mrs PK; 56 yrs; Married; 2 children Faecal Incontinence: Assessment and Management Professor Marc A Gladman MBBS DFFP PhD MRCOG FRCS (UK) FRACS Professor of Colorectal Surgery >10 years of incontinence
Fecal Incontinence. What is fecal incontinence?
 Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Faecal Incontinence Information Leaflet THE DIGESTIVE SYSTEM
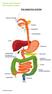 THE DIGESTIVE SYSTEM This factsheet is about faecal incontinence Faecal (or anal) incontinence is the loss of stool, liquid or gas from the bowel at an undesirable time. Males and females of any age may
THE DIGESTIVE SYSTEM This factsheet is about faecal incontinence Faecal (or anal) incontinence is the loss of stool, liquid or gas from the bowel at an undesirable time. Males and females of any age may
PERCUTANEOUS TIBIAL NERVE STIMULATION
 PERCUTANEOUS TIBIAL NERVE STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical
PERCUTANEOUS TIBIAL NERVE STIMULATION Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical
The cost of the Axonics sacral neuromodulation system is 475 for the trial phase, 9,210 for a
 pat hways Axonics sacral al neuromodulation system for overactive bladder and faecal incontinence Medtech innovation briefing Published: 10 December 2018 nice.org.uk/guidance/mib164 Summary The technology
pat hways Axonics sacral al neuromodulation system for overactive bladder and faecal incontinence Medtech innovation briefing Published: 10 December 2018 nice.org.uk/guidance/mib164 Summary The technology
Incidence of Colorectal Cancers- Australia. Anterior Resection 5/23/2018. What spurs us to investigate?
 Incidence of Colorectal Cancers- Australia 17,000 Colorectal cancers in 2018 20% of Colorectal cancers are in the Rectum 12.3% of all new cancers Anterior Resection Syndrome (ARS) Lisa Wilson. Colorectal
Incidence of Colorectal Cancers- Australia 17,000 Colorectal cancers in 2018 20% of Colorectal cancers are in the Rectum 12.3% of all new cancers Anterior Resection Syndrome (ARS) Lisa Wilson. Colorectal
Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137
 Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137 Effective Date: 01/18 Last Review Date: 12/16 Coding Implications Revision Log Description Fecal incontinence defined as the
Clinical Policy: Fecal Incontinence Treatments Reference Number: PA.CP.MP.137 Effective Date: 01/18 Last Review Date: 12/16 Coding Implications Revision Log Description Fecal incontinence defined as the
Commissioning guide:
 2017 Commissioning guide: Sponsoring Organisation: Association of Coloproctology of Great Britain and Ireland Date of evidence search: 2016 Date of publication: January 2017, revised from November 2014
2017 Commissioning guide: Sponsoring Organisation: Association of Coloproctology of Great Britain and Ireland Date of evidence search: 2016 Date of publication: January 2017, revised from November 2014
Percutaneous Tibial Nerve Stimulation for Overactive Bladder Symptoms. Patient Information Leaflet
 Percutaneous Tibial Nerve Stimulation for Overactive Bladder Symptoms Patient Information Leaflet About this leaflet The information provided in this leaflet should be used as a guide. There may be some
Percutaneous Tibial Nerve Stimulation for Overactive Bladder Symptoms Patient Information Leaflet About this leaflet The information provided in this leaflet should be used as a guide. There may be some
Stapled transanal rectal resection for obstructed defaecation syndrome
 Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
A prospective multicentre study to investigate percutaneous tibial nerve stimulation for the treatment of faecal incontinence
 Original article doi:1.1111/j.1463-1318.29.22.x A prospective multicentre study to investigate percutaneous tibial nerve stimulation for the treatment of faecal incontinence B. Govaert, D. Pares, S. Delgado-Aros,
Original article doi:1.1111/j.1463-1318.29.22.x A prospective multicentre study to investigate percutaneous tibial nerve stimulation for the treatment of faecal incontinence B. Govaert, D. Pares, S. Delgado-Aros,
URINARY INCONTINENCE
 Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
Management of Neurogenic Bowel Dysfunction. Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders
 Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Management of Neurogenic Bowel Dysfunction Fiona Paul, DNP, RN, CPNP Center for Motility and Functional Gastrointestinal Disorders DEFECATION Delivery of colon contents to the rectum Rectal compliance
Prolapse & Urogynaecology. Hester Mannion and Fabi Sica
 Prolapse & Urogynaecology Hester Mannion and Fabi Sica Take home messages Prolapse and associated incontinence is very common It has a devastating effect on the QoL of the patient and their partner Strategies
Prolapse & Urogynaecology Hester Mannion and Fabi Sica Take home messages Prolapse and associated incontinence is very common It has a devastating effect on the QoL of the patient and their partner Strategies
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews The effect of probiotics on functional constipation: a systematic review of randomised controlled trials EIRINI DIMIDI, STEPHANOS CHRISTODOULIDES,
PROSPERO International prospective register of systematic reviews The effect of probiotics on functional constipation: a systematic review of randomised controlled trials EIRINI DIMIDI, STEPHANOS CHRISTODOULIDES,
Optimising the outcome of neuromodulation for faecal incontinence and constipation. Mr Gregory Paul Thomas. Imperial College. London, United Kingdom
 Optimising the outcome of neuromodulation for faecal incontinence and constipation Mr Gregory Paul Thomas Imperial College London, United Kingdom Department of Surgery and Cancer MD (Res) 1 Declaration
Optimising the outcome of neuromodulation for faecal incontinence and constipation Mr Gregory Paul Thomas Imperial College London, United Kingdom Department of Surgery and Cancer MD (Res) 1 Declaration
03/13/18. A. Symptoms lasting for greater than or equal to 12 months that have resulted to significant impairment in activities of daily living; and
 Reference #: MC/I008 Page: 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
Reference #: MC/I008 Page: 1 of 5 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services, Inc. (PAS) Non-ERISA PreferredOne Community Health Plan
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE SCOPE. Urinary incontinence: the management of urinary incontinence in women
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence: the management of urinary incontinence in women 1.1 Short title Urinary incontinence 2 Background a) The National
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence: the management of urinary incontinence in women 1.1 Short title Urinary incontinence 2 Background a) The National
NEUROMODULATION FOR UROGYNAECOLOGISTS
 NEUROMODULATION FOR UROGYNAECOLOGISTS Introduction The pelvic floor is highly complex structure made up of skeletal and striated muscle, support and suspensory ligaments, fascial coverings and an intricate
NEUROMODULATION FOR UROGYNAECOLOGISTS Introduction The pelvic floor is highly complex structure made up of skeletal and striated muscle, support and suspensory ligaments, fascial coverings and an intricate
LONG TERM OUTCOME OF ELECTIVE SURGERY
 LONG TERM OUTCOME OF ELECTIVE SURGERY Roberto Persiani Associate Professor Mini-invasive Oncological Surgery Unit Institute of Surgical Pathology (Dir. prof. D. D Ugo) Dis Colon Rectum, March 2000 Dis
LONG TERM OUTCOME OF ELECTIVE SURGERY Roberto Persiani Associate Professor Mini-invasive Oncological Surgery Unit Institute of Surgical Pathology (Dir. prof. D. D Ugo) Dis Colon Rectum, March 2000 Dis
Sacral Nerve Neuromodulation/Stimulation
 Protocol Sacral Nerve Neuromodulation/Stimulation (70169) Medical Benefit Effective Date: 01/01/14 Next Review Date: 09/14 Preauthorization No Review Dates: 01/08, 11/08, 09/09, 09/10, 09/11, 09/12, 09/13
Protocol Sacral Nerve Neuromodulation/Stimulation (70169) Medical Benefit Effective Date: 01/01/14 Next Review Date: 09/14 Preauthorization No Review Dates: 01/08, 11/08, 09/09, 09/10, 09/11, 09/12, 09/13
Corporate Medical Policy
 Corporate Medical Policy Percutaneous Tibial Nerve Stimulation for Voiding Dysfunction File Name: Origination: Last CAP Review: Next CAP Review: Last Review: percutaneous_tibial_nerve_stimulation_for_voiding_dysfunction
Corporate Medical Policy Percutaneous Tibial Nerve Stimulation for Voiding Dysfunction File Name: Origination: Last CAP Review: Next CAP Review: Last Review: percutaneous_tibial_nerve_stimulation_for_voiding_dysfunction
Overactive Bladder Syndrome
 Overactive Bladder Syndrome behavioural modifications to pharmacological and surgical treatments Dr Jos Jayarajan Urologist Austin Health, Eastern Health Warringal Private, Northpark Private, Epworth Overactive
Overactive Bladder Syndrome behavioural modifications to pharmacological and surgical treatments Dr Jos Jayarajan Urologist Austin Health, Eastern Health Warringal Private, Northpark Private, Epworth Overactive
Feasibility of using a novel non-invasive ambulatory tibial nerve stimulation device for the home-based treatment of overactive bladder symptoms
 Original Article Feasibility of using a novel non-invasive ambulatory tibial nerve stimulation device for the home-based treatment of overactive bladder symptoms Jai H. Seth 1, Gwen Gonzales 1, Collette
Original Article Feasibility of using a novel non-invasive ambulatory tibial nerve stimulation device for the home-based treatment of overactive bladder symptoms Jai H. Seth 1, Gwen Gonzales 1, Collette
Sacral nerve neuromodulation for the treatment of lower bowel motility disorders
 The Royal College of Surgeons of England HUNTERIAN LECTURE doi 10.1308/003588406X149174 Sacral nerve neuromodulation for the treatment of lower bowel motility disorders NICHOLAS J KENEFICK St Mark s Hospital,
The Royal College of Surgeons of England HUNTERIAN LECTURE doi 10.1308/003588406X149174 Sacral nerve neuromodulation for the treatment of lower bowel motility disorders NICHOLAS J KENEFICK St Mark s Hospital,
Incontinence; Lets talk about it. Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery
 Incontinence; Lets talk about it Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery Select the most appropriate subtitle for this talk A: Bladders gone wild! B: There s no such thing
Incontinence; Lets talk about it Karanvir Virk M.D. Minimally Invasive and Pelvic Reconstructive Surgery Select the most appropriate subtitle for this talk A: Bladders gone wild! B: There s no such thing
OBSTETRICALLY-CAUSED ANAL SPHINCTER INJURY PREDICTION, MANAGEMENT, PREVENTION
 OBSTETRICALLY-CAUSED ANAL SPHINCTER INJURY PREDICTION, MANAGEMENT, PREVENTION COLM O HERLIHY, MD Professor and Chair University College Dublin Department of Obstetrics and Gynaecology National Maternity
OBSTETRICALLY-CAUSED ANAL SPHINCTER INJURY PREDICTION, MANAGEMENT, PREVENTION COLM O HERLIHY, MD Professor and Chair University College Dublin Department of Obstetrics and Gynaecology National Maternity
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal mesh background of, 84 85 Age as factor in PFDs, 8 Anal plugs in FI management in women, 107 Anterior compartment native tissue
Index Note: Page numbers of article titles are in boldface type. A Abdominal mesh background of, 84 85 Age as factor in PFDs, 8 Anal plugs in FI management in women, 107 Anterior compartment native tissue
Sacral Neuromodulation Beyond Pelvic Pain!!!
 Sacral Neuromodulation Beyond Pelvic Pain!!! Dr. Hirachand S Mutagi. Senior Consultant Pain Physician. Head -Sakra World Hospital. Director ReLeaf Pain Services. Rapid advances in neurostimulation therapy
Sacral Neuromodulation Beyond Pelvic Pain!!! Dr. Hirachand S Mutagi. Senior Consultant Pain Physician. Head -Sakra World Hospital. Director ReLeaf Pain Services. Rapid advances in neurostimulation therapy
2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)
 E10d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No.
E10d 2012/13 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No.
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transabdominal artificial bowel sphincter implantation for faecal incontinence
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transabdominal artificial bowel sphincter implantation for faecal incontinence
Sacral Nerve Neuromodulation/Stimulation for Pelvic Floor Dysfunction
 Medical Policy Manual Surgery, Policy No. 134 Sacral Nerve Neuromodulation/Stimulation for Pelvic Floor Dysfunction Next Review: December 2018 Last Review: June 2018 Effective: July 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Surgery, Policy No. 134 Sacral Nerve Neuromodulation/Stimulation for Pelvic Floor Dysfunction Next Review: December 2018 Last Review: June 2018 Effective: July 1, 2018 IMPORTANT REMINDER
Percutaneous Tibial Nerve Stimulation
 Percutaneous Tibial Nerve Stimulation Policy Number: 7.01.106 Last Review: 11/2018 Origination: 5/2008 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) may provide coverage
Percutaneous Tibial Nerve Stimulation Policy Number: 7.01.106 Last Review: 11/2018 Origination: 5/2008 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) may provide coverage
Treatments for Fecal Incontinence A Review of the Research for Adults
 Treatments for Fecal Incontinence A Review of the Research for Adults e Is This Information Right for Me? This information is right for you if: Your health care professional* said you or your loved one
Treatments for Fecal Incontinence A Review of the Research for Adults e Is This Information Right for Me? This information is right for you if: Your health care professional* said you or your loved one
Medical Policy. MP Percutaneous Tibial Nerve Stimulation
 Medical Policy MP 7.01.106 BCBSA Ref. Policy: 7.01.106 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: Surgery Related Policies 1.01.17 Pelvic Floor Stimulation as a Treatment of Urinary and
Medical Policy MP 7.01.106 BCBSA Ref. Policy: 7.01.106 Last Review: 08/20/2018 Effective Date: 08/20/2018 Section: Surgery Related Policies 1.01.17 Pelvic Floor Stimulation as a Treatment of Urinary and
Advanced Care for Female Overactive Bladder & Urinary Incontinence. Department of Urology Kaiser Permanente Santa Rosa
 Advanced Care for Female Overactive Bladder & Urinary Incontinence Department of Urology Kaiser Permanente Santa Rosa Goals Participants will: Review normal urinary tract anatomy and function Understand
Advanced Care for Female Overactive Bladder & Urinary Incontinence Department of Urology Kaiser Permanente Santa Rosa Goals Participants will: Review normal urinary tract anatomy and function Understand
Coding for Sacral Neuromodulation
 301.273.0570 Fax 301.273.0778 Coding for Sacral Neuromodulation Sacral Neuromodulation (SNS) is a widely used technique in Female Pelvic Medicine and Reconstructive Surgery (FPMRS), with several FDA-approved
301.273.0570 Fax 301.273.0778 Coding for Sacral Neuromodulation Sacral Neuromodulation (SNS) is a widely used technique in Female Pelvic Medicine and Reconstructive Surgery (FPMRS), with several FDA-approved
Sacral Nerve Neuromodulation / Stimulation
 Sacral Nerve Neuromodulation / Stimulation Policy Number: 7.01.69 Last Review: 2/2018 Origination: 2/2001 Next Review: 2/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Sacral Nerve Neuromodulation / Stimulation Policy Number: 7.01.69 Last Review: 2/2018 Origination: 2/2001 Next Review: 2/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Description. Section: Medicine Effective Date: April 15, 2016 Subsection: Medicine Original Policy Date: June 7, Page: 1 of 6.
 Section: Medicine Effective Date: April 15, 2016 Page: 1 of 6 Last Review Status/Date: March 2016 Description Radiofrequency (RF) energy has been investigated as a minimally invasive treatment of fecal
Section: Medicine Effective Date: April 15, 2016 Page: 1 of 6 Last Review Status/Date: March 2016 Description Radiofrequency (RF) energy has been investigated as a minimally invasive treatment of fecal
BEST PRACTICE ADVOCACY CENTRE NEW ZEALAND SCOPE. Urinary incontinence in women: the management of urinary incontinence in women
 BEST PRACTICE ADVOCACY CENTRE NEW ZEALAND SCOPE 1 Guideline title Urinary incontinence in women: the management of urinary incontinence in women 2 Guideline Contextualisation This is a contextualisation
BEST PRACTICE ADVOCACY CENTRE NEW ZEALAND SCOPE 1 Guideline title Urinary incontinence in women: the management of urinary incontinence in women 2 Guideline Contextualisation This is a contextualisation
PREPARING FOR ANORECTOAL MANOMETRY. ManoScan Anorectal Manometry System
 PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
PREPARING FOR ANORECTOAL MANOMETRY ManoScan Anorectal Manometry System WHAT IS ANORECTAL MANOMETRY? Anorectal manometry is a test used to evaluate the function and coordination of the sphincter and pelvic
Sphincter exercises for people with bowel control problems. Information for patients. Physiotherapy Department
 Sphincter exercises for people with bowel control problems Information for patients Physiotherapy Department 01625 661481 @EastCheshireNHS Leaflet Ref: 11229 Published: June 18 Review: 31/0/5/2021 Page
Sphincter exercises for people with bowel control problems Information for patients Physiotherapy Department 01625 661481 @EastCheshireNHS Leaflet Ref: 11229 Published: June 18 Review: 31/0/5/2021 Page
Comparison of blood pressure measured at the arm, ankle and calf
 doi:10.1111/j.1365-2044.2008.05633.x Comparison of blood pressure measured at the arm, ankle and calf C. Moore, 1 A. Dobson, 2 M. Kinagi 2 and B. Dillon 3 1 SpR Anaesthetics, 2 Consultant Anaesthetist,
doi:10.1111/j.1365-2044.2008.05633.x Comparison of blood pressure measured at the arm, ankle and calf C. Moore, 1 A. Dobson, 2 M. Kinagi 2 and B. Dillon 3 1 SpR Anaesthetics, 2 Consultant Anaesthetist,
International Neuromodulation Society 14th World Congress May 25-30, 2019, Preliminary Scientific Program*
 International Neuromodulation Society 14th World Congress May 25-30, 2019, Preliminary Scientific Program* *Subject to change Pre-Conference Days May 25-26 May 25 - Pre-Conference: Noninvasive Brain Stimulation
International Neuromodulation Society 14th World Congress May 25-30, 2019, Preliminary Scientific Program* *Subject to change Pre-Conference Days May 25-26 May 25 - Pre-Conference: Noninvasive Brain Stimulation
Postpartum Complications
 ACOG Postpartum Toolkit Postpartum Complications Introduction The effects of pregnancy on many organ systems begin to resolve spontaneously after birth of the infant and delivery of the placenta. The timeline
ACOG Postpartum Toolkit Postpartum Complications Introduction The effects of pregnancy on many organ systems begin to resolve spontaneously after birth of the infant and delivery of the placenta. The timeline
Objectives. Prevalence of Urinary Incontinence URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS
 URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS Lisa S Pair, MSN, CRNP Division of Urogynecology and Pelvic Reconstructive Surgery Department of Obstetrics and Gynecology University of Alabama
URINARY INCONTINENCE: EVALUATION AND CURRENT TREATMENT OPTIONS Lisa S Pair, MSN, CRNP Division of Urogynecology and Pelvic Reconstructive Surgery Department of Obstetrics and Gynecology University of Alabama
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Posterior Tibial Nerve Stimulation Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Posterior Tibial Nerve Stimulation Professional Institutional Original
Posterior Tibial Nerve Stimulation Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Posterior Tibial Nerve Stimulation Professional Institutional Original
VTE Prophylaxis. Serving an unmet need in high-risk acute stroke patients.
 VTE Prophylaxis Serving an unmet need in high-risk acute stroke patients www.gekodevices.com Providing venous thromboembolism (VTE) prophylaxis to at-risk stroke patients The risk of venous thromboembolism
VTE Prophylaxis Serving an unmet need in high-risk acute stroke patients www.gekodevices.com Providing venous thromboembolism (VTE) prophylaxis to at-risk stroke patients The risk of venous thromboembolism
Medical Review Criteria Implantable Neurostimulators
 Medical Review Criteria Implantable Neurostimulators Subject: Implantable Neurostimulators Effective Date: April 14, 2017 Authorization: Prior authorization is required for covered implantable stimulators
Medical Review Criteria Implantable Neurostimulators Subject: Implantable Neurostimulators Effective Date: April 14, 2017 Authorization: Prior authorization is required for covered implantable stimulators
CONTINENCE MODULE 1 MIMIMUM STANDARDS FOR THE SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF FEMALE LOWER URINARY TRACT SYMPTOMS
 CONTINENCE MODULE 1 MIMIMUM STANDARDS FOR THE SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF FEMALE LOWER URINARY TRACT SYMPTOMS The minimum standards required to initiate specialised conservative
CONTINENCE MODULE 1 MIMIMUM STANDARDS FOR THE SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF FEMALE LOWER URINARY TRACT SYMPTOMS The minimum standards required to initiate specialised conservative
Description. Section: Medicine Effective Date: April 15, 2017 Subsection: Medicine Original Policy Date: June 7, Page: 1 of 6.
 Page: 1 of 6 Last Review Status/Date: March 2017 Description Radiofrequency energy has been investigated as a minimally invasive treatment of fecal incontinence, in a procedure referred to as the Secca
Page: 1 of 6 Last Review Status/Date: March 2017 Description Radiofrequency energy has been investigated as a minimally invasive treatment of fecal incontinence, in a procedure referred to as the Secca
A non-invasive, safe and effective treatment that improves pelvic floor muscle contraction and diminishes urine loss in pelvic floor disorders 1
 A non-invasive, safe and effective treatment that improves pelvic floor muscle contraction and diminishes urine loss in pelvic floor disorders 1 INNOVO INNOVO has changed my life for the better. I no longer
A non-invasive, safe and effective treatment that improves pelvic floor muscle contraction and diminishes urine loss in pelvic floor disorders 1 INNOVO INNOVO has changed my life for the better. I no longer
restore the pelvic floor
 restore the pelvic floor A non-invasive, safe, and effective treatment that improves pelvic floor muscle contraction and diminishes urine loss in pelvic floor disorders 1 has changed my life for the better.
restore the pelvic floor A non-invasive, safe, and effective treatment that improves pelvic floor muscle contraction and diminishes urine loss in pelvic floor disorders 1 has changed my life for the better.
Saint Mary s Hospital. The Warrell Unit. Perineal Clinic
 Saint Mary s Hospital The Warrell Unit Perineal Clinic 2 What is the Perineal Clinic? The Perineal Clinical is a clinic for women who have had a tear of the anal sphincter during the delivery of their
Saint Mary s Hospital The Warrell Unit Perineal Clinic 2 What is the Perineal Clinic? The Perineal Clinical is a clinic for women who have had a tear of the anal sphincter during the delivery of their
Integrated Continence Service Policy. January SafeCare Council January Carol Giffin, Continence Advisor
 Policy No: OP51 Version: 1.0 Name of Policy: Integrated Continence Service Policy Effective From: January 2008 Approved by: SafeCare Council January 2008 Next Review Date: January 2010 Reviewed by: Carol
Policy No: OP51 Version: 1.0 Name of Policy: Integrated Continence Service Policy Effective From: January 2008 Approved by: SafeCare Council January 2008 Next Review Date: January 2010 Reviewed by: Carol
2/5/2016. ABS Complications. Anal Slings-investigational
 ABS Complications Anal Slings-investigational Similar to transvaginal tape or transobturator tape for UI Dacron, mersilene, polyester, and teflon mesh, fascia lata Wound infections, sinus tract, t ulcer
ABS Complications Anal Slings-investigational Similar to transvaginal tape or transobturator tape for UI Dacron, mersilene, polyester, and teflon mesh, fascia lata Wound infections, sinus tract, t ulcer
Subject: Sacral Nerve Neuromodulation/Stimulation
 02-61000-23 Original Effective Date: 01/01/01 Reviewed: 06/28/18 Revised: 01/01/19 Subject: Sacral Nerve Neuromodulation/Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
02-61000-23 Original Effective Date: 01/01/01 Reviewed: 06/28/18 Revised: 01/01/19 Subject: Sacral Nerve Neuromodulation/Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
WHAT IS LARS? LOW ANTERIOR RESECTIONSYNDROME. Sophie Pilkington. Colorectal Surgeon University Hospital Southampton
 WHAT IS LARS? LOW ANTERIOR RESECTIONSYNDROME Sophie Pilkington Colorectal Surgeon University Hospital Southampton INTRODUCTION UK Bowel cancer 2013 41,100 new cases INTRODUCTION UK Bowel cancer 2013 41,100
WHAT IS LARS? LOW ANTERIOR RESECTIONSYNDROME Sophie Pilkington Colorectal Surgeon University Hospital Southampton INTRODUCTION UK Bowel cancer 2013 41,100 new cases INTRODUCTION UK Bowel cancer 2013 41,100
MEDICAL POLICY SUBJECT: SACRAL NERVE STIMULATION
 MEDICAL POLICY 01/16/14, 01/22/15, 03/15/16 PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY 01/16/14, 01/22/15, 03/15/16 PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
Please complete this voiding diary and questionnaire. Bring both of them with you to your next appointment with your provider.
 Please complete this voiding diary and questionnaire. Bring both of them with you to your next appointment with your provider. To begin the diary, please choose two days when you will be at home. The two
Please complete this voiding diary and questionnaire. Bring both of them with you to your next appointment with your provider. To begin the diary, please choose two days when you will be at home. The two
Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211
 Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
Prucalopride for the treatment of chronic constipation in women Technology appraisal guidance Published: 15 December 2010 nice.org.uk/guidance/ta211 NICE 2018. All rights reserved. Subject to Notice of
Motility Disorders. Pelvic Floor. Colorectal Center for Functional Bowel Disorders (N = 701) January 2010 November 2011
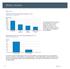 Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Motility Disorders Pelvic Floor Colorectal Center for Functional Bowel Disorders (N = 71) January 21 November 211 New Patients 35 3 25 2 15 1 5 Constipation Fecal Incontinence Rectal Prolapse Digestive-Genital
Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders
 Original article doi:10.1111/j.1463-1318.2011.02797.x Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders D. S. Y. Chan*, A. Saklani, P. R. Shah, M. Lewis and P. N. Haray
Original article doi:10.1111/j.1463-1318.2011.02797.x Rectal irrigation: a useful tool in the armamentarium for functional bowel disorders D. S. Y. Chan*, A. Saklani, P. R. Shah, M. Lewis and P. N. Haray
Stress Incontinence. Susannah Elvy Urogynaecology CNS
 Stress Incontinence Susannah Elvy Urogynaecology CNS Definitions Prevalence Assessment Investigation Treatment Surgery Men International Continence Society define as the complaint of any involuntary leakage
Stress Incontinence Susannah Elvy Urogynaecology CNS Definitions Prevalence Assessment Investigation Treatment Surgery Men International Continence Society define as the complaint of any involuntary leakage
A. Service Specifications
 A. Service Specifications SCHEDULE 2 THE SERVICES Service Specification No: Service Commissioner Lead Specialised Complex Surgery for Urinary Incontinence and Vaginal and Uterine Prolapse For local completion
A. Service Specifications SCHEDULE 2 THE SERVICES Service Specification No: Service Commissioner Lead Specialised Complex Surgery for Urinary Incontinence and Vaginal and Uterine Prolapse For local completion
CONTINENCE MODULE 3 MIMIMUM STANDARDS FOR SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF CONSTIPATION AND FAECAL INCONTINENCE
 CONTINENCE MODULE 3 MIMIMUM STANDARDS FOR SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF CONSTIPATION AND FAECAL INCONTINENCE The minimum standards required to initiate specialised conservative treatment
CONTINENCE MODULE 3 MIMIMUM STANDARDS FOR SPECIALIST ASSESSMENT & CONSERVATIVE MANAGEMENT OF CONSTIPATION AND FAECAL INCONTINENCE The minimum standards required to initiate specialised conservative treatment
Your guidebook for getting results with Medtronic Bladder Control Therapy Delivered by the NURO System TAKE BACK CONTROL.
 Your guidebook for getting results with Medtronic Bladder Control Therapy Delivered by the NURO System TAKE BACK CONTROL. RESET. RESTORE. * REGAIN CONTROL. Congratulations! You ve decided to try a therapy
Your guidebook for getting results with Medtronic Bladder Control Therapy Delivered by the NURO System TAKE BACK CONTROL. RESET. RESTORE. * REGAIN CONTROL. Congratulations! You ve decided to try a therapy
FECAL INCONTINENCE. John H. Winston, III, M.D., M.B.A.
 FECAL INCONTINENCE John H. Winston, III, M.D., M.B.A. Diplomate, American Board of Colon & Rectal Surgery Diplomate, American Board of Surgery www.colorectalsurgeryservices.com Fecal Incontinence (FI)
FECAL INCONTINENCE John H. Winston, III, M.D., M.B.A. Diplomate, American Board of Colon & Rectal Surgery Diplomate, American Board of Surgery www.colorectalsurgeryservices.com Fecal Incontinence (FI)
Onpulse TM technology and the geko TM device: The development of a novel system for the prevention of venous thromboembolism
 Onpulse TM technology and the geko TM device: The development of a novel system for the prevention of venous thromboembolism Tucker AT, Bain DS Jawad H, Bain DS, Dawson H, Adams K, Elnahhas T, Johnston
Onpulse TM technology and the geko TM device: The development of a novel system for the prevention of venous thromboembolism Tucker AT, Bain DS Jawad H, Bain DS, Dawson H, Adams K, Elnahhas T, Johnston
Guidelines for the Manual Evacuation of Faeces
 Rationale Guidelines for the Manual Evacuation of Faeces These guidelines are to provide the required information for designated registered nurses, health care assistants and bank support workers to perform
Rationale Guidelines for the Manual Evacuation of Faeces These guidelines are to provide the required information for designated registered nurses, health care assistants and bank support workers to perform
Sacral Nerve Neuromodulation/Stimulation. Description
 Subject: Sacral Nerve Neuromodulation/Stimulation Page: 1 of 17 Last Review Status/Date: September 2015 Sacral Nerve Neuromodulation/Stimulation Description Sacral nerve neuromodulation (SNM), also referred
Subject: Sacral Nerve Neuromodulation/Stimulation Page: 1 of 17 Last Review Status/Date: September 2015 Sacral Nerve Neuromodulation/Stimulation Description Sacral nerve neuromodulation (SNM), also referred
McAlees et al. Trials (2018) 19:336 https://doi.org/ /s (Continued on next page)
 McAlees et al. Trials (2018) 19:336 https://doi.org/10.1186/s13063-018-2689-1 STUDY PROTOCOL Efficacy and mechanism of sub-sensory sacral (optimised) neuromodulation in adults with faecal incontinence:
McAlees et al. Trials (2018) 19:336 https://doi.org/10.1186/s13063-018-2689-1 STUDY PROTOCOL Efficacy and mechanism of sub-sensory sacral (optimised) neuromodulation in adults with faecal incontinence:
