REVIEW ARTICLE. C. V. Rider,* J. R. Furr,* V. S. Wilson* and L. E. Gray Jr* Summary
|
|
|
- Jodie Fisher
- 6 years ago
- Views:
Transcription
1 international journal of andrology ISSN REVIEW ARTICLE Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity C. V. Rider,* J. R. Furr,* V. S. Wilson* and L. E. Gray Jr* *MD-72, Reproductive Toxicology Branch, T A Division, NHEERL, ORD, US Environmental Protection Agency, RTP, NC, and Duke University, Durham, NC, USA Summary Keywords: antiandrogens and 2,3,7,8 TCDD, male reproduction, reproductive system Correspondence: Leon Earl Gray Jr, MD-72, Reproductive Toxicology Branch, T A Division, NHEERL, ORD, US Environmental Protection Agency, RTP, NC 27711, USA. gray.earl@epa.gov Received 5 October 29; revised 3 December 29; accepted 15 December 29 doi:1.1111/j x Although risk assessments are typically conducted on a chemical-by-chemical basis, the 1996 Food Quality Protection Act required the US Environmental Protection Agency to consider cumulative risk of chemicals that act via a common mechanism of toxicity. To this end, we are conducting studies with mixtures of chemicals to elucidate mechanisms of joint action at the systemic level with the goal of providing a framework for assessing the cumulative effects of reproductive toxicants. Previous mixture studies conducted with antiandrogenic chemicals are reviewed briefly and two new studies are described. In all binary mixture studies, rats were dosed during pregnancy with chemicals, singly or in pairs, at dosage levels equivalent to approximately one-half of the ED5 for hypospadias or epididymal agenesis. The binary mixtures included androgen receptor (AR) antagonists (vinclozolin plus procymidone), phthalate esters [di(n-butyl) phthalate (DBP) plus benzyl n-butyl phthalate (BBP) and diethyl hexyl phthalate (DEHP) plus DBP], a phthalate ester plus an AR antagonist (DBP plus procymidone), a mixed mechanism androgen signalling disruptor (linuron) plus BBP, and two chemicals which disrupt epididymal differentiation through entirely different toxicity pathways: DBP (AR pathway) plus 2,3,7,8 TCDD (AhR pathway). We also conducted multi-component mixture studies combining several antiandrogens. In the first study, seven chemicals (four pesticides and three phthalates) that elicit antiandrogenic effects at two different sites in the androgen signalling pathway (i.e. AR antagonist or inhibition of androgen synthesis) were combined. In the second study, three additional phthalates were added to make a 1 chemical mixture. In both the binary mixture studies and the multi-component mixture studies, chemicals that targeted male reproductive tract development displayed cumulative effects that exceeded predictions based on a response-addition model and most often were in accordance with predictions based on dose-addition models. In summary, our results indicate that compounds that act by disparate mechanisms of toxicity to disrupt the dynamic interactions among the interconnected signalling pathways in differentiating tissues produce cumulative dose-additive effects, regardless of the mechanism or mode of action of the individual mixture component. Introduction Although risk assessments have been historically conducted on a chemical-by-chemical basis, regulatory agencies are beginning to consider cumulative risk of chemicals. However, a consistent framework for cumulative risk assessment is yet to be developed. It is well known that humans (Eskenazi et al., 1999; Landrigan et al., 1999; Silva, 24; Wolff et al., 27, 28; Calafat et al., 28), fish (Jobling et al., 1998, 26; Jobling & Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
2 Cumulative effect of antiandrogens in utero C. V. Rider et al. Tyler, 26; Ankley et al., 27) and wildlife (Hall & Thomas, 27) are continuously exposed to multiple contaminants. The chemicals found in some aquatic systems include pesticides (Hela et al., 25; Jaspers et al., 26) and industrial chemicals (Hall & Thomas, 27), as well as pharmaceuticals and hormones (Kolpin et al., 22; Durhan et al., 26). The effects of mixtures of chemicals like the ubiquitous phthalates are of concern as humans are exposed to multiple phthalates at one time (Silva, 24; Wolff et al., 27). To address this issue, in 26, the U.S. Environmental Protection Agency (EPA) requested that the National Academy of Sciences (NAS) establish a panel to provide the EPA with recommendations on whether to perform a cumulative risk assessment of the phthalates. This review will present the major conclusions of the NAS panel (Box from p 116 of the NAS report) and discuss the data from our laboratory on the reproductive effects of mixtures (NAS, 28). Our working hypothesis is that chemicals that disrupt a common system or tissue during development contribute to cumulative toxicity and should therefore be included in cumulative risk assessments. The results of our studies support this hypothesis and are in agreement with the conclusions of the NAS Report (28) (Table 1). This represents a shift from the current cumulative risk assessment process, which only includes chemicals that share a narrowly defined mechanism of action. As a result of growing concerns about mixtures, the field of mixtures toxicology is emerging as an area of increasing scientific and regulatory focus. For example, in 1996, the EPA began considering the cumulative risk of chemicals that act via a common mechanism of toxicity as mandated in the Food Quality Protection Act (FQPA). The EPA s Offices of Water and Research and Development and the EPA Superfund, Solid Waste and Air Programs also have ongoing programmes in the area of mixtures toxicology. In this regard, the research from our laboratory, described herein, is intended to contribute to the development of a guidance framework for assessing cumulative risk to reproduction and development from exposures during pregnancy. Our research has included mixtures of pesticides, phthalates and 2,3,7,8-Tetrachlorodibenzo-p-dioxin (2,3,7,8 TCDD). These chemicals disrupt sexual differentiation by acting as androgen receptor (AR) antagonists, inhibitors of foetal testosterone synthesis or as an aryl hydrocarbon receptor (AhR) agonist (Figs 1 & 2). We initially conducted a series of binary mixture studies exposing pregnant dams during foetal sexual differentiation with pairs of chemicals (Gray et al., 21; Hotchkiss et al., 24a; Howdeshell et al., 27, 28b; Rider et al., 29). In the binary studies, rats were dosed with chemicals singly or in pairs at dosage levels equivalent to about one-half of the ED5 for hypospadias or epididymal agenesis (2 2 factorial designs). We predicted that by itself each chemical in a pair would cause a minimal rate of malformations, whereas when two chemicals were mixed, they would produce predictable, doseadditive effects on common target tissues. We found that the binary combinations produced cumulative, dose-additive effects on the androgen-dependent tissues that were a common component of each chemical s phenotype. Several of the studies have been reviewed in detail previously (Gray et al., 21; Gray, 22; Howdeshell et al., 28b; Rider et al., 28, 29) and will only be briefly reviewed, whereas the phthalate plus dioxin binary study is new and will be presented in more detail. In addition to the binary studies, we have conducted three mixture studies with larger numbers of chemicals. One study combined five phthalates in the mixture to determine if they produced dose-additive effects on foetal testosterone production (Howdeshell et al., 28b), foetal testis gene expression and post-natal development of the male and female offspring. These phthalates all shared a common mechanism of toxicity. The remaining two multi-component mixture studies combined chemicals that elicit antiandrogenic effects at two different sites in the androgen signalling pathway (i.e. AR antagonist or inhibition of androgen synthesis). One study combined seven chemicals and the second combined 1 chemicals in the mixture. The text that follows in this article will (i) briefly describe the mechanisms and modes of toxicity in vitro Table 1 Main points from National Academy of Science report: Phthalates and Cumulative Risk Assessment: The Task Ahead Mixtures of phthalates produce effects that are well-predicted using the dose-addition model. Other antiandrogen mixtures (including mixtures of phthalates with other antiandrogens) also conform to the dose-addition model. Dose-addition is an appropriate model for mixtures of antiandrogenic chemicals including phthalates. The response-addition model is not an appropriate model for antiandrogenic chemicals. The criteria for including chemicals in cumulative risk assessments are too narrow and should be expanded to include chemicals with similar effects. The current cumulative risk assessment process is not protective because it does not include all chemicals, which elicit dose additive toxicity. 444 Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
3 C. V. Rider et al. Cumulative effect of antiandrogens in utero Expanding the framework for cumulative risk assessment from mechanisms of toxicity, to toxicity pathways and then to systems biology Common mechanisms of toxicity Toxic mechanism A:A R antagonist Toxic mechanism Toxic mechanism B: Enzyme inhibition C: Unknown Toxic mechanism D: AhR agonist Figure 1 A diagram showing how different mechanisms of toxicity and disruption of diverse toxicity pathways (Androgen-vs. Ah receptor signalling pathways) converge as an integrated network of pathways to disrupt the development of the reproductive tract during a critical developmental period. Common toxicity pathways AR signaling AhR signaling Common systems biology AR bound to antagonist Fewer free AR to bind androgens Reduced steroid enzyme activity Less androgen to bind AR Reduced AR-hormone dimers and androgen -dependent gene activation Abnormal reproductive tract differentiation Abnormal leydig cell development Androgensignaling pathway Increased AhR activation Reduced AhR-dependent gene activation AhRsignaling pathway and in vivo of the individual chemicals we selected to study in our research programme as these have been reviewed in detail previously (Gray et al., 21; Howdeshell et al., 28b; Rider et al., 28, 29), (ii) describe the mathematical modelling procedures we used to characterize the effects of the individual chemicals and derive predictions for how the mixtures would behave, (iii) present a summary of the results of our mixture studies on chemicals that act via common mechanisms of toxicity (Section A), chemicals that act via disparate mechanisms of toxicity, but disrupt the same signalling pathway (Section B), and chemicals that disrupt common developing tissues via alteration of diverse signalling pathways (Section C) and (iv) describe a proposed framework for cumulative risk assessment based on disruption of a common developing system. The review will include new data from two studies, one a multicomponent mixture study of 1 chemicals that disrupt androgen signalling by diverse mechanisms of toxicity and another study combining chemicals that disrupt different signalling pathways, the AhR agonist 2,3,7,8 TCDD and dibutyl phthalate that reduces foetal testosterone levels. Mechanisms and modes of action of the individual chemicals used in mixture studies Over the last several decades, our laboratory has studied the post-natal effects of in utero exposure to a variety of environmental chemicals with diverse endocrine and nonendocrine mechanisms of reproductive toxicity. We are now using the dose response information from these studies to design mixture studies to determine how chemicals from different classes with similar and diverse mechanisms and modes of action interact in the mixture. The objective of our research is to define a framework for conducting cumulative risk assessments of reproductive toxicants. The chemicals selected for our current mixture studies are shown in Fig. 2. In this figure, the red indicates stronger effects, whereas the black areas indicate the absence of effects. Reviewing the columns associated with each chemical describes (i) the known mechanisms of toxicity as determined from in vitro and short-term in vivo screening studies and (ii) the overall phenotype in the male offspring after exposure during foetal life. Comparing the different chemicals by rows (the endpoints) allows one to compare the relative potencies displayed by the toxicants to one another. For each endpoint, we predict that all the chemicals with shading (dark red to light red) will interact jointly when combined in a mixture, but those with black squares will have no effect when included in the mixture. For example, of the chemicals in this chart, only the phthalates reduce insulin-like peptide hormone-3 (insl3) and cause gubernacular agenesis. Therefore, combining a phthalate with any of the other chemicals in the chart will not enhance the phthalateinduced reduction in insl3 mrna levels in the foetal testis or increase the incidence of gubernacular agenesis. Dicarboximide fungicides: vinclozolin and procymidone Of the dicarboximide fungicides, vinclozolin (Kelce et al., 1994), iprodione (Blystone et al., 29) and procymidone (Hosokawa et al., 1993; Ostby et al., 1999; Vinggaard et al., 1999; Nellemann et al., 23) act as AR antagonists in vitro and or in vivo. These pesticides or their Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
4 Cumulative effect of antiandrogens in utero C. V. Rider et al. Heat map of the reproductive toxicants used in our mixture studies Vinclozolin Procymidone Prochloraz Linuron Six phthalates 2, 3, 7, 8 TCDD Upstream mechanism of toxicity Androgen receptor antagonism Reduced T production: in vitro Reduced T production: in the fetus Reduced STAR, CYP11, CYP 17 Reduced Insl3 mrna levels Ah receptor agonist Downsteam effects: In utero in F1 males Reduced AGD in F1 male rats Retained nipples as infants Gubernacular agenesis Undescended testes with gubernaculum Hypospadias Epididymal agenesis or hypoplasia Reduced sperm counts Delayed puberty in males w/o Malfs Downsteam effects: In utero in F1 females Delayed puberty Vaginal threads Uterine and vaginal agenesis Avg Max Figure 2 Heat map displaying the intensity of effects of each chemical in vitro, in short-term in vivo screens and on F1 male and female offspring after exposure in utero during sexual differentiation. The red shading indicates stronger effects, whereas the black areas indicate the absence of effects. Reviewing the columns associated with each chemical describes (i) the known mechanisms of toxicity as determined from in vitro and short-term in vivo screening studies and (ii) the overall phenotype in the male offspring after exposure during foetal life. Comparing the different chemicals by rows (the endpoints) allows one to compare the relative potencies displayed by the toxicants to one another. For each endpoint, we predict that all the chemicals with shading (light red to dark red) will interact jointly when combined in a mixture, but those with black squares will have no effect when included in the mixture. metabolites competitively inhibit the binding of androgens to AR, which leads to an inhibition of androgendependent gene expression in vitro and in vivo (Kelce & Wilson, 1997). Linuron (herbicide) While some toxicants disrupt sexual differentiation predominantly via one mechanism of toxicity (i.e. AR antagonists or inhibitors of testosterone synthesis), some pesticides including linuron and prochloraz act via dual mechanisms of toxicity. These pesticides display AR antagonist activity and inhibit testosterone synthesis with varying potencies. The herbicide linuron is an AR antagonist in vitro (Lambright et al., 2; McIntyre et al., 2, 22a,b; Turner et al., 23). In contrast to some AR antagonists, neither short-term (Lambright et al., 2; O Connor et al., 22) nor long-term (Gray et al., 1999) administration with linuron induces elevated serum LH levels. Prochloraz (fungicide) Prochloraz is a fungicide that also disrupts reproductive development and function by several modes of action (Noriega et al., 25; Vinggaard et al., 25, 26). Prochloraz inhibits the steroidogenic enzymes 17, 2 lyase and aromatase and it also is an AR antagonist (Blystone et al., 27). In a study in which rat dams were dosed from GD 14 18, Wilson et al. (24) found that prochloraz reduced foetal testis testosterone 446 Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
5 C. V. Rider et al. Cumulative effect of antiandrogens in utero levels and increased progesterone production 1-fold on GD 18 without affecting Leydig cell insl3 mrna levels. Phthalates In utero, some phthalates alter male and female reproductive tract differentiation via unknown mechanisms of action. The mode of action in the male involves altered Leydig cell migration and differentiation and abnormal gonocyte development (Parks et al., 2; Mahood et al., 25, 26; Hallmark et al., 27). The Leydig cell alterations result in reductions in foetal testis testosterone production and mrna levels for key proteins in the steroidogenic pathway including StAR and CYP11, as well as insl-3, which is critical for gubernacular development and testis descent (Kumagai et al., 22; Hughes & Acerini, 28). 2,3,7,8 Tetrachlorodibenzo dioxin (TCDD) 2,3,7,8 Tetrachlorodibenzo dioxin binds with high affinity to the cytosolic AhR. This basic-helix-loop-helix receptor acts as a transcription factor in a manner similar to the steroid hormone receptors. Although the normal role of this transcription factor in development remains unknown, gestational TCDD treatment induces malformations of the external genitalia and subfertility in female rat (Gray & Ostby, 1995; Flaws et al., 1997; Gray et al., 1997a) and hamster (Wolf et al., 1999) offspring and alters reproductive development of the male rat (Gray et al., 1995, 1997b; Mably et al., 1992; Simanainen et al., 24) and hamster (Gray et al., 1995). Several studies have shown that TCDD administration does not reduce adult (Gray et al., 1995; Theobald et al., 2) or foetal androgen levels in the male rat or mouse (Ko et al., 24; Haavisto et al., 21, 26), even though several of the effects in the male offspring include suppressed development of some androgen-dependent tissues. In mice, it is known that TCDD directly inhibits the androgen-dependent processes by which the urogenital sinus of foetal mice forms prostatic epithelial buds (Lin et al., 24) without affecting androgen levels. Additional mechanistic studies proposed that TCDD acted directly on AhR, ARNT and AhR-induced transcripts in the periprostatic mesenchyme (Vezina et al., 28, 29). This tissue intimately contacts urogenital sinus epithelium where buds are specified and they proposed that activation of AhR signalling disrupted dorsoventral patterning of the urogenital sinus, reprogramming the areas where prostatic buds are specified and prostate lobes are formed. Administration of TCDD on the 15th day of gestation (GD 15) at doses lower than or equal to 1 lg kg both demasculinizes and feminizes male rat reproductive morphology and behaviour. In our first series of studies, Long Evans Hooded and Holtzman rats were dosed by gavage with 1 lg TCDD kg on GD 8 (a period of major organogenesis), or Syrian hamsters, a species relatively insensitive to the lethal effects of TCDD, were dosed on GD 11 (a period equivalent to GD 15 in the rat), with TCDD at 2 lg kg. In Long Evans and Holtzman F1 male rats exposed on GD 15 or hamsters exposed on GD 11, puberty (preputial separation) was delayed by about 3 days, ejaculated sperm counts were reduced by at least 58 and epididymal sperm storage was reduced by 3. Testicular sperm production was less affected. The sex accessory glands were also reduced in size in Long Evans offspring treated on GD 15 in spite of the fact that serum testosterone levels, testosterone production by the testis in vitro and AR levels were not reduced. Some reproductive measures, like anogenital distance and male sex behaviour, were altered by TCDD-treatment in rat, but not hamster offspring (Gray et al., 1995). In the F1 female offspring, in utero TCDD exposure (1 lg kg on GD 15) induces cleft phallus, vaginal thread formation and reduces ovarian weight (Gray & Ostby, 1995; Flaws et al., 1997; Gray et al., 1997b). TCDD treatment on GD 15 at,.5,.2 or.8 lg kg, delays vaginal opening and induces malformations of the external genitalia (cleft phallus and persistent vaginal thread formation) in the female progeny (Flaws et al., 1997; Gray & Ostby, 1995; Gray et al., 1997b). Mixture modelling Over the past several decades, research in several laboratories including our own has described the effects of individual chemicals on the reproductive development of male rats. Individual chemical data can be used in mathematical models to make predictions about the potential effects of mixtures on male reproductive tract development. Predicted mixture responses can then be compared with the observed effects of the mixtures to determine the type of joint action (dose-addition, response-addition, synergy, antagonism) exhibited by the mixture. For our mixture studies, most of the individual data were compiled from studies conducted in our laboratory over the past 2 years (see individual chemical data in Rider et al., 28). Occasionally, data from the literature were used to increase the robustness of the individual chemical dose response analyses (i.e. add data points to a partial dose response curve). While these historical data included studies conducted by different researchers with several rat strains and slightly different dosing schedules, all studies included exposure to the chemical during the critical in utero window of male rat reproductive tract differentiation. The use of historical data as input for Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
6 Cumulative effect of antiandrogens in utero C. V. Rider et al. mixture modelling introduces a certain degree of variability, but is necessary given that developmental studies with an in utero exposure and a postnatal evaluation of the offspring are resource-intensive precluding the inclusion of all the individual dose response groups within the mixture study. In addition, we found surprisingly good agreement between overlapping studies within our lab and between labs. We attribute this agreement to the relatively standardized procedures for measuring organ weights and identifying reproductive tract malformations among labs from which data were used. The greatest limitation in the modelling of mixtures of chemicals in utero is that complete dose response curves cannot be generated for each individual chemical for many endpoints because the systemic toxicity of some of the chemicals restrains using high-dosage levels. Assumptions are a necessary part of risk assessment as data sets are often incomplete. Therefore, the utility of a given mixture toxicity model depends in part on whether it can consistently produce accurate predictions even when input data contain logical assumptions. We present our mixture work here as a body of evidence supporting the presumption that the dose-addition model consistently provides a relatively good fit to observed data despite the use of imperfect input data. Using graphpad prism 5. software (GraphPad Software Inc., La Jolla, CA, USA), we transformed the data to fit a 1 scale. For continuous endpoints (AGD and organ weights), we converted the data to percentage change from the control value and input the mean, standard error and sample size for each group. For malformation data, we presented the data as percentage incidence. We then graphed the data on a log-linear scale and fitted the data with a sigmoidal (variable slope) equation in graphpad prism (see eqn 1): Y ¼ Bottom þðtop BottomÞ= ð1 þ 1^ððLogED5 XÞ HillSlopeÞÞ ð1þ where Y is the response, X is the chemical dose, Top and Bottom refer to the minimum and maximum effect calculated from the data and ED5 is the exposure dose eliciting a 5 response. The parameters (Hill slope and ED5) generated from the logistic fit to the individual chemical data were used in models to make predictions of the mixture responses. Predicted responses from models vs. observed responses We modelled the observed mixture responses (means, standard errors and sample sizes) using prism software and generated predictions of the mixture effects with three types of models (described in more detail in Rider et al., 28): l Dose-addition l Response-addition, and l Integrated-addition The dose-addition model is commonly applied to mixtures of chemicals that have the same mechanism of action (Altenburger et al., 2; Silva et al., 22), whereas the response-addition model has been proposed as an alternative for use with mixtures containing chemicals with different mechanisms of action (Backhaus et al., 2). The integrated-addition model represents a melding of the dose- and response-addition models in an attempt to deal with mixtures containing chemicals with similar and dissimilar mechanisms of toxicity (Altenburger et al., 25; Rider & LeBlanc, 25; Rider et al., 28). Application of the dose-addition (DA) model requires putting chemicals in equivalent terms by accounting for the individual chemical potencies. This is done by dividing the concentration of the individual chemical in the mixture by the ED5 of that chemical (see eqn 2). Once this is accomplished, these adjusted doses can be added to arrive at a total mixture dose. The predicted response is then calculated by determining the response level that corresponds with the total mixture dose. In summary, the slope and ED5 derived from individual chemical dose response analyses are input into the dose-addition equation to calculate the overall response of the mixture associated with any given mixture dose. The dose-addition equation we used to calculate predicted responses of mixtures is: 1 R ¼ 1 1 þ q P n D i ED5 i i¼1 ð2þ where R is the response to the mixture, D i is the concentration of chemical i in the mixture, ED5 i is the concentration of chemical i that causes a 5 response, and q is the average Hill slope (i.e. slope associated with a logistic fit of the individual chemical dose response curve) associated with the chemicals. The response-addition (RA) model or independent joint action model has been suggested as the more appropriate model for mixtures of chemicals with different mechanisms of action (Greco et al., 1992). The equation for response-addition is based on probability theory and is expressed as: R ¼ 1 Yn i¼1 ð1 R i Þ ð3þ 448 Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
7 C. V. Rider et al. Cumulative effect of antiandrogens in utero As this equation is specific to situations in which response level increases with increasing dose, endpoints were converted to fit this criterion (e.g. AGD, which decreases in males with increasing antiandrogen dose, was expressed as change from control). The integrated-addition (IA) model provides an intermediate model that incorporates both dose-addition and response-addition (Teuschler et al., 24; Altenburger et al., 25; Rider & LeBlanc, 25). Chemicals with the same mechanism of action are grouped and the total dose associated with each group is calculated using doseaddition. The groups are then combined using responseaddition. The integrated-addition model is expressed mathematically as: 8 9 R ¼ 1 YN I¼1 >< 1 >= þ q P n D >: i >; ED5 i i¼1 ð4þ Identifying a framework for cumulative risk assessment The following discussion will present a summary of the data that support the conclusions of the NAS report and our hypothesis that the framework for cumulative risk assessments should be broadly based on disruption of common systems or target tissues rather than narrowly based on specific mechanisms of toxicity. Mixtures Section A. Chemicals that act via common mechanisms of toxicity l Androgen receptor antagonists (vinclozolin plus procymidone). l Phthalate esters [Di(n-butyl) phthalate (DBP) plus benzyl n-butyl phthalate (BBP)]. l Phthalate esters [diethyl hexyl phthalate (DEHP) plus DBP]. Mixtures Section B. Chemicals that disrupt a common pathway via disparate mechanisms of toxicity (i.e. disruption of the androgen signalling pathway) l A phthalate ester plus an AR antagonist (DBP plus procymidone). l A pesticide plus a phthalate (linuron plus BBP). l A multi-component mixture of seven chemicals (vinclozolin, procymidone, prochloraz, linuron, DBP, BBP, and DEHP). l A multi-component mixture study of 1 chemicals [diisoheptyl phthalate (DiHP), di-n-pentyl (DPP) and di-iso-butyl phthalate (DiBP)] and the chemicals used in the seven chemical study (above). Mixtures Section C. Chemicals that disrupt a common tissue via different signalling pathways and diverse mechanisms of toxicity (i.e. disruption of the androgen and AhR signalling pathways in the foetal male rat reproductive tract) l 2,3,7,8 tetrachlorodibenzo dioxin plus a phthalate (TCDD plus DBP). Several different frameworks have been proposed for including chemicals in a cumulative risk assessment ranging from one limited to chemicals that clearly display a common cellular and molecular mechanism of action, to a much broader framework based on disruption of a common target tissue (Figs 1 & 2). The broader framework was recommended by a recent NAS panel report (Table 1) (NAS, 28). The frameworks presented here include grouping chemicals in a cumulative risk assessment based on (i) common mechanisms of toxicity, (ii) disruption of common signalling pathways (a toxicity pathway approach) or (iii) a broader approach, based on disruption of common reproductive target tissues in the foetus (a systems approach including multiple signalling pathways) (Fig. 1). If cumulative risk assessments were conducted on the 11 chemicals (six phthalates, four pesticides and TCDD) discussed herein using the framework based on common mechanisms of toxicity, then they would be clustered into at least four different assessments (i.e. 1. AR antagonists, 2. inhibitors of foetal testosterone production, 3. dual mechanism antiandrogens and 4. AhR-mediated toxicant). The assumption would be that chemicals with different mechanisms of toxicity interact independently and that the mixture outcome could be predicted by responseaddition modelling, whereas dose-addition models would over-predict the effects. In reviewing the data, one finds that dose-addition models consistently yield more accurate predictions than those from either integrated-addition or response-addition models. Grouping the chemicals for cumulative risk assessments based on disruption of a common signalling pathway or toxicity pathway rather than common mechanisms of toxicity assumes that chemicals that disrupt androgen signalling will act jointly and the effects can be predicted by dose-addition modelling, but these chemicals would act independently of those that disrupt the same tissue via the AhR pathway. This would result in the 11 chemicals in the current study being grouped into two independent risk assessments (i.e. Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
8 Cumulative effect of antiandrogens in utero C. V. Rider et al. 1. androgen signalling disruptors and 2. AhR signalling disruptor). Grouping the chemicals for cumulative risk assessments based on disruption of a common system or target tissue via multiple pathways and mechanisms of toxicity assumes that chemicals that disrupt androgen signalling and AhR signalling will act jointly and the effects of all 11 chemicals can be predicted by a single-dose addition model. This is the approach recommended in the NAS report (Table 1) and the approach supported by our studies. Under this framework, all 11 chemicals in our studies would be included in a single cumulative risk assessment (i.e. chemicals that target the developing male reproductive tract). Mixtures Section A. Disruption of reproductive differentiation by common mechanisms of toxicity Mixtures of AR antagonists, vinclozolin and procymidone In the late 199s and in 25, the EPA began an examination of whether some or all members of the dicarboximide class of fungicides, which includes vinclozolin, iprodione and procymidone, shared a common mechanism of toxicity. At that time, the scientific information on the mechanisms of toxicity of the imide group of dicarboximide fungicides was incomplete and the EPA did not therefore recommend a cumulative risk assessment of vinclozolin, procymidone and iprodione ( epa.gov/opp1/reregistration/vinclozolin/) ( epa.gov/oppsrrd1/reds/procymidone_tred.pdf). Since then, several studies from our laboratory and other laboratories have been completed that address these uncertainties. These studies demonstrate that vinclozolin and procymidone share a common mechanism of toxicity and interact in a cumulative manner (Gray et al., 21; Nellemann et al., 23). In our AR antagonist binary study (Gray et al., 21), in utero exposure to vinclozolin alone resulted in 1 incidence of hypospadias and incidence of vaginal pouch development in male rats, while procymidone exposure resulted in incidence of either malformation. The combination exposure, however, resulted in 96 incidence of hypospadias and 54 incidence of vaginal pouch in treated animals. Alteration in foetal Leydig cell differentiation and hormone production by phthalates The effects of mixtures of phthalates are a concern as humans are exposed to multiple phthalates at one time (Silva, 24; Wolff et al., 27). To address this issue, in 26, the EPA requested that the NAS establish a panel to provide the EPA with recommendations on how to address the cumulative effects of the phthalates. The data from several of the phthalate mixture studies presented herein were given to the NAS panel for their reanalysis and evaluation (NAS, 28). We conducted three studies with mixtures of phthalate esters. The first study combined two phthalate esters with a common active metabolite (DBP and BBP). The second study combined two phthalate esters with different active metabolites (DEHP and DBP) (Howdeshell et al., 27). In the third phthalate mixture study, we combined five phthalates and examined the effects of the mixture on foetal testosterone synthesis and gene expression levels and post-natal effects in the male and female offspring (Howdeshell et al., 28c; Howdeshell et al. in prep). The results from all three of these studies were consistent with predictions based on a dose-addition model for inducing abnormal development in utero in male rats. Exposure to the individual chemicals resulted in no malformations or low incidence of malformations, whereas the combination exposures typically resulted in 5 or greater incidence of malformations (Howdeshell et al., 27). In the third mixture study, we assessed the cumulative effects on foetal testosterone production, gene expression levels and post-natal development of the male and female offspring following in utero exposure to a mixture of five phthalates: DBP, di-iso-butyl phthalate (DiBP), BBP, diethyl hexyl phthalate (DEHP) and di-n-pentyl phthalate (DPP) (Howdeshell et al., 28c). First, we characterized the dose response effects of the six individual phthalates [BBP, DBP, DEHP, diethyl phthalate (DEP), DiBP, and DPP] on gestation day (GD) 18 testicular testosterone production following exposure of Sprague Dawley rats on GD8-18. BBP, DBP, DEHP, and DiBP were equipotent (ED5 of 44 ± 16 mg kg day), DPP was about threefold more potent (ED5 = 13 mg kg day) and DEP had no effect on foetal testosterone production. We hypothesized that co-administration of the five antiandrogenic phthalates would reduce testosterone production and insulin-like-3, StAR and Cyp11a mrna levels and induce male reproductive tract malformations in a doseadditive fashion as they act via a common mode of toxicity. In the five phthalate mixture study, dams were dosed at 1, 8, 6, 4, 2, 1, 5 or of the mixture. The top dose contained 13 mg of total phthalates kg day including BBP, DBP, DEHP, DiBP (3 mg kg day per chemical) and DPP (1 mg DPP kg day). This mixture ratio was selected such that each phthalate would contribute equally to the reduction in testosterone. As hypothesized, foetal testosterone production (Howdeshell et al., 28c) and gene expression levels were reduced and male reproductive tract malformations were increased (Howdeshell et al. in prep) in a dose-additive manner. From these mixture studies, we conclude that the above pairs of chemicals target the androgen signalling pathway via the same mechanism of action and produced dose- 45 Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
9 C. V. Rider et al. Cumulative effect of antiandrogens in utero additive effects when presented as mixtures. These results indicate that these chemicals would be good candidates for cumulative risk assessment and supports the use of the dose-addition model for determining the effects of these mixtures. Mixtures Section B Disruption of the androgen signalling pathway by disparate mechanisms of toxicity: AR antagonism and inhibition of foetal testosterone synthesis Environmental chemicals can disrupt the androgen signalling pathway in the male rat foetus during sexual differentiation and alter this process via several diverse endocrine mechanisms of toxicity (Fig. 2). EDCs known to interfere with the androgen signalling pathway include dicarboximide fungicides (e.g. vinclozolin (Kelce et al., 1994)), organochlorine-based insecticides (e.g. p,p DDT and p,p DDT (Kelce et al., 1995)), conazole fungicides (e.g. prochloraz (Noriega et al., 25; Vinggaard et al., 1999)), plasticizers (e.g. phthalates), polybrominated diphenyl ethers (PBDEs; Gray et al., 24; Stoker et al., 25) and urea-based herbicides (e.g. linuron) (Lambright et al., 2; McIntyre et al., 2). Mixture studies with pesticides and phthalates with diverse modes of toxicity We conducted two binary mixture studies to determine how procymidone and linuron interacted in mixtures with phthalates. In the first study, we combined BBP (reduces foetal testosterone) with linuron, an antiandrogen with multiple mechanisms of action (AR antagonist and reduces foetal testosterone levels) (Hotchkiss et al., 24b, Wilson et al., 24). The second study combined DBP with the AR antagonist procymidone. In these studies, pregnant rats were dosed on GD14-18 with either the individual compounds or the binary mixture at a dose level equivalent to approximately one-half of the ED5 value for malformations. Following this, we conducted a study with a mixture of seven chemicals that included vinclozolin, procymidone, linuron, prochloraz, and three phthalates DBP, DEHP and BBP (Rider et al., 28) and we will present new data herein on a multi-component mixture study with a 1 chemical mixture (the seven chemicals listed above and three additional phthalates). Benzyl butyl phthalate plus linuron In the BBP and linuron study, in utero exposure to BBP alone elicited a incidence of hypospadias and vaginal pouch formation and 12 incidence of epididymal agenesis in male rats. In utero exposure to linuron alone resulted in incidence of hypospadias and vaginal pouch development and 63 incidence of epididymal agenesis. However, exposure to the combination resulted in cumulative effects with males displaying 56, 4, and 97 incidence of hypospadias, vaginal pouch and epididymal agenesis, respectively (Hotchkiss et al. 24b). Di-n-butyl phthalate plus procymidone In the procymidone plus DBP study, procymidone or DBP alone induced low incidence rates of hypospadias (1.5 and, respectively) and vaginal pouch (), whereas the males treated with the combination of procymidone and DBP displayed 49 and 27 incidence rates of hypospadias and vaginal pouch, respectively indicating that the interaction was at least dose-additive. Seven chemical mixture study, including vinclozolin, procymidone, prochloraz, linuron and three phthalates or six phthalates To test our hypothesis further, we designed two multicomponent mixture studies, one with seven antiandrogenic chemicals and the other with 1 chemicals (see below). The chemicals in these multi-component mixtures alter the androgen signalling pathway via diverse mechanisms of action including AR antagonists (vinclozolin and procymidone), mixed mechanism chemicals that bind to the AR and decrease testosterone production (linuron and prochloraz) and testosterone synthesis inhibiting phthalates (BBP, DBP, and DEHP in the seven chemical study (Rider et al., 28) and BBP, DBP, DiBP, DEHP, DPP and DiHP in the new 1 chemical study (see below). According to the current mixtures paradigm, these mixtures should conform to a model of integrated-addition. However, we found that models of integrated-addition or response-addition frequently underestimated the effects of these mixtures. Ten chemical mixture study (original data) The main objective of this study was to expand upon our previous seven chemical mixture study by including a greater number of chemicals to define more clearly the differences in predictions generated from the dose-addition model vs. the integrated- and response-addition models. Increasing the number of chemicals allowed for the use of lower concentrations of individual chemicals in the mixture further testing our working hypothesis that chemicals present below their no observed adverse effect levels can contribute to mixture toxicity as long as they have a common target. The dosage levels of each chemical at each dilution of the mixture are shown in Table 2. The ED5 values of the individual chemicals used in the models to predict the effects of the mixture are shown in Table 3. When compared with the previous seven chemical study, this study included three more phthalates (DiBP, DiHP and DPP), slightly larger numbers of litters per dose-group Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
10 Cumulative effect of antiandrogens in utero C. V. Rider et al. Table 2 Chemical doses in the 1 chemical mixture study Mixture groups ( of top dose) Chemical mg kg day mg kg day mg kg day mg kg day mg kg day mg kg day mg kg day DBP DIBP BBP DEHP DIHP DPP Vinclozolin Procymidione Linuron Prochloraz Total dose (mg kg day) Necropsy 2 (6) 2 (8) 4 (22) 3 (17) 3 (14) 4 (26) 5 (26) Litter # (pup #) AGD Litter # (pup #) 3 (21) 2 (8) 4 (3) 3 (17) 3 (15) 4 (28) 6 (35) and a larger number of dilutions near the top dose to provide greater distinction among the model predictions in the higher dosage ranges where the dose-, integratedand response-addition models separate. Materials and Methods Animals Thirty timed pregnant Sprague Dawley rats were purchased from Charles River Breeding Laboratory (Raleigh, NC, USA) on gestation day (GD) 2. Animals were housed individually in clear polycarbonate cages ( cm) with a bedding of heat-treated laboratory-grade pine shavings (Northeastern Products, Warrensburg, NY, USA). Animals were fed Purina Rat Chow 58 (pregnant and lactating females) or Purina Rat Chow 51 (weanling and adult rats) (Ralston Purina Co., St. Louis, MO, USA) and provided with access to filtered municipal drinking water (Durham, NC, USA) ad libitum. The current study was conducted under protocols approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee. Table 3 Dose response parameters (ED5 and Hill slope) for the 1 chemicals included in the new multi-component mixture study. Parameters were derived from a logistic fit to individual chemical dose response data Vinclozolin Linuron Procymidone Phthalates DBP, DiBP, BBP, DEHP, DiHP a Prochloraz More Potent Phthalate (DPP) ED5 (mg kg) Hill slope ED5 (mg kg) Hill slope ED5 (mg kg) Hill slope ED5 (mg kg) Hill slope ED5 (mg kg) Hill slope ED5 (mg kg) Hill slope AGD PND 13 nips Hypospadias Ectopic testes Epididymal agenesis Epididymal weight Seminal vesicle weight Ventral prostate weight Levator Ani Bulbocavernosus Muscles weight a Phthalate (DBP, DiBP, BBP, DEHP, and DiHP) parameters were based on individual chemical dose response data for DBP and DEHP (for which the most complete dose response data were available) because previous studies with incomplete dose response data from the other phthalates in this group supported the assumption that these phthalates were approximately equipotent. 452 Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
11 C. V. Rider et al. Cumulative effect of antiandrogens in utero Treatment administration Our goal in dose selection of the seven chemical study (four pesticides and three phthalates) was to use doses of each chemical that would contribute to the induction of malformations approximately equally. The mixture contained each chemical at 1 7th of its respective ED1 for inducing reproductive tract malformations. In the current study with 1 chemicals, we kept the mixture ratio for the four pesticides and total phthalate dose constant as in the seven chemical study. As this study has six phthalates (see below), rather than three, the contribution of each of the six phthalates in the mixture was half of the contribution of the three phthalates in the seven chemical study. Thirty pregnant rats [n = 4 (mixture groups) to 6 dams (control group); Table 2] were dosed with the vehicle (corn oil at 2.5 ml kg; Sigma, St Louis, MO, USA) or a dilution of the high-dose of the mixture by oral gavage on GD It is noteworthy that the goal of these studies is to fit the full dose response curve for as many endpoints as possible to determine which model best predicts the observed effects of the mixture. The goal of these studies is not to establish no-observed-adverse-effect levels, which would require using larger numbers of litters in the low-dose range and a longer intrauterine exposure period. Chemical information (reported purity) l Benzyl n-butyl phthalate (BBP) 98, Aldrich CAS ; lot # 8523JQ; cat # 3,85-1. l Di (n-butyl) phthalate (DBP) 98, Sigma CAS ; lot # 19F386; cat # D-227. l Diethylhexyl phthalate or Phthalic Acid bis(2-ethylhexyl Ester) (DEHP) Aldrich CAS , cat # D21154, lot # 9428JC, purity = 99. l Diisobutyl Phthalate (DiBP) Aldrich CAS , cat # , lot # 1314LC, purity = 99. l Diisoheptyl Phthalate (DiHP) Aldrich CAS , cat # , lot # 695EC, purity = technical grade. l Dipentyl Phthalate (DPP) Fluka CAS , ca t#8154, lot # , purity = 99. l 3-(3, 4-dichlorophenyl)-1-methoxy-1-methylurea (Linuron) 99, Crescent Chemical Company CAS ; cat # W-1464; lot # l N-propyl-N-(2-(2,4,6-trichlorophenoxy)ethyl)- 1H-imidazole-1-carboxamide (Prochloraz) 99.5, Riedel-de-Haen CAS ; cat # 45631; lot # 96X. l N-(3, 5-dichlorophenyl)-1, 2-imethylcyclopropane-1, 2-dicarboxamide (Procymidone) 99, Chem Services CAS ; cat # PS-2126; lot # 231-1A. l N-3, 5-dichlorophenyl-5-methyl-5-vinyl-1, 3-oxazolidine-2, 4-dione (Vinclozolin) 99.4, Riedel-de-Haen CAS ; cat # 35625; lot # 9126X. l Vehicle Corn Oil Sigma CAS ; cat # C-8267; lot # 126K117. Male offspring assessment On postnatal day (PND) 3, all pups were sexed, weighed and anogenital distances (AGD) were measured using a dissecting microscope with an ocular micrometer (Hotchkiss et al. 24b) (Fig. 3). At PND 14, each male rat was examined in a blinded manner for areolae or nipples. At about 2 days of age, all male rats were anaesthetized with CO 2, decapitated and examined for reproductive tract alterations. The ventral surface of each male rat was shaved and examined for the presence of nipples. Rats were then assessed for abnormalities including cleft phallus, hypospadias, epididymal agenesis, testicular atrophy, undescended testes, fluid filled testes, ventral prostate or seminal vesicle agenesis and malformations of the gubernacular cords (elongated or absent). Weights were taken on body, glans penis, ventral prostate, seminal vesicles, testes, epididymis and levator ani bulbocavernosus muscle (LABC) (Fig. 4a). AGD, retained nipple and organ weight data were analysed using litter mean values on sas 9.1 (SAS, Cary, NC, USA) with proc glm (p <.5 was the critical value for statistical significance). The incidence of reproductive tract malformations was similarly analysed using the litter mean incidence of each malformation. graphpad prism output from the observed mixture responses included the ED5 [with standard errors and 95 confidence limits (CL)], and the goodness of fit (R Square) of the observed data to the best-fit model. For statistical purposes, we compared the ED5s of the observed responses with the dose, response and integrated addition predictions of the ED5s. Predicted ED5s differed significantly from the observed ED5 if the predicted value was outside the 95 CL of the ED5 of the observed response. Results In the 1 chemical study, no maternal toxicity (maternal weight loss or weight gain) or treatment-related pup mortality was observed (data not shown). The body weight of the male offspring was significantly reduced (by Dunnett s post-hoc test) in the 1 dose group. A significant incidence of female-like retained nipples was observed in the 2 1 dose groups, whereas most other tissues were significantly affected at 4 of the top dose and above. The gubernaculum was the least sensitive tissue, being affected only at 6 of the top dose and above. Journal compilation ª 21 European Academy of Andrology International Journal of Andrology 33 (21),
L Earl Gray Jr. Research Biologist
 EFFECTS OF MIXTURES OF PHTHALATES, PESTICIDES AND TCDD ON SEXUAL DIFFERENTIATON IN RATS: A RISK FRAMEWORK BASED UPON DISRUPTION OF COMMON DEVELOPING SYSTEMS. USEPA scientist grapples with difficult environmental
EFFECTS OF MIXTURES OF PHTHALATES, PESTICIDES AND TCDD ON SEXUAL DIFFERENTIATON IN RATS: A RISK FRAMEWORK BASED UPON DISRUPTION OF COMMON DEVELOPING SYSTEMS. USEPA scientist grapples with difficult environmental
Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals 1
 international journal of andrology ISSN 0105-6263 Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals 1 L. Earl Gray Jr,* Vickie S. Wilson,* Tammy Stoker,*
international journal of andrology ISSN 0105-6263 Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals 1 L. Earl Gray Jr,* Vickie S. Wilson,* Tammy Stoker,*
Effects of in Utero Exposure to Finasteride on Androgen-Dependent Reproductive Development in the Male Rat
 TOXICOLOGICAL SCIENCES 74, 393 406 (2003) DOI: 10.1093/toxsci/kfg128 Effects of in Utero Exposure to Finasteride on Androgen-Dependent Reproductive Development in the Male Rat Christopher J. Bowman, 1
TOXICOLOGICAL SCIENCES 74, 393 406 (2003) DOI: 10.1093/toxsci/kfg128 Effects of in Utero Exposure to Finasteride on Androgen-Dependent Reproductive Development in the Male Rat Christopher J. Bowman, 1
Dioxin toxicology. David R Bell
 Dioxin toxicology David R Bell Dioxins, furans, PCBs, Cl Cl Cl Cl Cl Cl Cl x O O O O Cl Cl Cl Cl Cl Cl OCDD Cl x Highly toxic, carcinogenic, teratogenic, Ubiquitous at low level Family of congeners which
Dioxin toxicology David R Bell Dioxins, furans, PCBs, Cl Cl Cl Cl Cl Cl Cl x O O O O Cl Cl Cl Cl Cl Cl OCDD Cl x Highly toxic, carcinogenic, teratogenic, Ubiquitous at low level Family of congeners which
Setting the scene: Is mixtures risk assessment necessary and feasible?
 3 rd SETAC Special Science Symposium Setting the scene: Is mixtures risk assessment necessary and feasible? Andreas Kortenkamp The School of Pharmacy, University of London 2-3 February 2011, Brussels The
3 rd SETAC Special Science Symposium Setting the scene: Is mixtures risk assessment necessary and feasible? Andreas Kortenkamp The School of Pharmacy, University of London 2-3 February 2011, Brussels The
A Testing Strategy for the Identification of mammalian Endocrine Disruptors with particular focus on steroids
 A Testing Strategy for the Identification of mammalian Endocrine Disruptors with particular focus on steroids Tzutzuy Ramirez, Robert Landsiedel, Susanne Kolle, Hennicke Kamp, Bennard van Ravenzwaay EUSAAT
A Testing Strategy for the Identification of mammalian Endocrine Disruptors with particular focus on steroids Tzutzuy Ramirez, Robert Landsiedel, Susanne Kolle, Hennicke Kamp, Bennard van Ravenzwaay EUSAAT
Classification of developmentally toxic pesticides, low dose effects, mixtures perspective of the industry
 Classification of developmentally toxic pesticides, low dose effects, mixtures perspective of the industry Steffen Schneider 9th Berlin Workshop on Developmental Toxicology Berlin September 13, 2018 What
Classification of developmentally toxic pesticides, low dose effects, mixtures perspective of the industry Steffen Schneider 9th Berlin Workshop on Developmental Toxicology Berlin September 13, 2018 What
pesticides in the EU regulatory framework Prof Andreas Kortenkamp
 Addressing combined effects of pesticides in the EU regulatory framework Prof Andreas Kortenkamp Brunel University London Info session on cumulative risk assessment for o sess o o cu u at e s assess e
Addressing combined effects of pesticides in the EU regulatory framework Prof Andreas Kortenkamp Brunel University London Info session on cumulative risk assessment for o sess o o cu u at e s assess e
Elkton Road, Newark, DE 19714, USA; 2 Pfizer, Inc., Drug Safety Evaluation, MS , Eastern Point Road, Groton, CT , USA
 Pure Appl. Chem., Vol. 75, Nos. 11 12, pp. 2099 2123, 2003. 2003 IUPAC Topic 3.10 Critical evaluation of observed adverse effects of endocrine active substances on reproduction and development, the immune
Pure Appl. Chem., Vol. 75, Nos. 11 12, pp. 2099 2123, 2003. 2003 IUPAC Topic 3.10 Critical evaluation of observed adverse effects of endocrine active substances on reproduction and development, the immune
COLLECTIVE EXPERT APPRAISAL: SUMMARY AND CONCLUSIONS
 COLLECTIVE EXPERT APPRAISAL: SUMMARY AND CONCLUSIONS Related to theestablishment of a Toxicity Reference Value based on the reprotoxic effects of linuron (CAS No. 330-55-2) AFSSET Solicited Request No.
COLLECTIVE EXPERT APPRAISAL: SUMMARY AND CONCLUSIONS Related to theestablishment of a Toxicity Reference Value based on the reprotoxic effects of linuron (CAS No. 330-55-2) AFSSET Solicited Request No.
Combined exposure to endocrine disrupting pesticides impairs parturition, causes pup mortality and affects sexual differentiation in rats
 international journal of andrology ISSN 15-6263 ORIGINAL ARTICLE Combined exposure to endocrine disrupting pesticides impairs parturition, causes pup mortality and affects sexual differentiation in rats
international journal of andrology ISSN 15-6263 ORIGINAL ARTICLE Combined exposure to endocrine disrupting pesticides impairs parturition, causes pup mortality and affects sexual differentiation in rats
Assessment and regulation of endocrine disrupters under European chemical legislations Susy Brescia Chemicals Regulation Directorate (CRD) HSE
 Health and and Safety Executive Assessment and regulation of endocrine disrupters under European chemical legislations Susy Brescia Chemicals Regulation Directorate (CRD) HSE EDs and the current EU legislative
Health and and Safety Executive Assessment and regulation of endocrine disrupters under European chemical legislations Susy Brescia Chemicals Regulation Directorate (CRD) HSE EDs and the current EU legislative
COMMENTS AND RESPONSE TO COMMENTS ON CLH: PROPOSAL AND JUSTIFICATION
 COMMENTS AND RESPONSE TO COMMENTS ON CLH: PROPOSAL AND JUSTIFICATION Comments provided during public consultation are made available in this table as submitted by the webform. Please note that the comments
COMMENTS AND RESPONSE TO COMMENTS ON CLH: PROPOSAL AND JUSTIFICATION Comments provided during public consultation are made available in this table as submitted by the webform. Please note that the comments
Effects of environmental antiandrogens on reproductive development in experimental animals
 Human Reproduction Update, Vol.7, No.3 pp. 248±264, 2001 Effects of environmental antiandrogens on reproductive development in experimental animals L.E.Gray, Jr 1,5, J.Ostby 1, J.Furr 1, C.J.Wolf 1, C.Lambright
Human Reproduction Update, Vol.7, No.3 pp. 248±264, 2001 Effects of environmental antiandrogens on reproductive development in experimental animals L.E.Gray, Jr 1,5, J.Ostby 1, J.Furr 1, C.J.Wolf 1, C.Lambright
COMMISSION IMPLEMENTING DECISION. of
 EUROPEAN COMMISSION Brussels, 4.7.2017 C(2017) 4462 final COMMISSION IMPLEMENTING DECISION of 4.7.2017 on the identification of bis(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), benzyl butyl
EUROPEAN COMMISSION Brussels, 4.7.2017 C(2017) 4462 final COMMISSION IMPLEMENTING DECISION of 4.7.2017 on the identification of bis(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), benzyl butyl
Exposures and Heritable Health Effects
 Exposures and Heritable Health Effects Thaddeus T. Schug Population Health Branch National Institute of Environmental Health Sciences November 30, 2017 Outline Developmental exposures and health effects
Exposures and Heritable Health Effects Thaddeus T. Schug Population Health Branch National Institute of Environmental Health Sciences November 30, 2017 Outline Developmental exposures and health effects
Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism
 Research article Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism Michelle Welsh, Philippa T.K. Saunders,
Research article Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism Michelle Welsh, Philippa T.K. Saunders,
and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in three differentially TCDD-sensitive rat lines.
 ToxSci Advance Access published April 14, 2004 Pattern of male reproductive system effects after in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in three differentially TCDD-sensitive
ToxSci Advance Access published April 14, 2004 Pattern of male reproductive system effects after in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in three differentially TCDD-sensitive
Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice
 Biology of Reproduction, 217, (), 1 16 doi:1.193/biolre/iox154 Research Article Advance Access Publication Date: 2 November 217 Research Article Prenatal exposure to di(2-ethylhexyl) phthalate disrupts
Biology of Reproduction, 217, (), 1 16 doi:1.193/biolre/iox154 Research Article Advance Access Publication Date: 2 November 217 Research Article Prenatal exposure to di(2-ethylhexyl) phthalate disrupts
Substance Name: Diisobutyl phthalate (DIBP) EC Number: CAS Number: SUPPORT DOCUMENT TO THE OPINION OF THE MEMBER STATE COMMITTEE
 Substance Name: Diisobutyl phthalate (DIBP) EC Number: 201-553-2 CAS Number: 84-69-5 SUPPORT DOCUMENT TO THE OPINION OF THE MEMBER STATE COMMITTEE FOR IDENTIFICATION OF DIISOBUTYL PHTHALATE (DIBP) AS A
Substance Name: Diisobutyl phthalate (DIBP) EC Number: 201-553-2 CAS Number: 84-69-5 SUPPORT DOCUMENT TO THE OPINION OF THE MEMBER STATE COMMITTEE FOR IDENTIFICATION OF DIISOBUTYL PHTHALATE (DIBP) AS A
DANIEL R. DOERGE U.S. Food and Drug Administration National Center for Toxicological Research Jefferson, AR
 Research support by Interagency Agreement between NTP/NIEHS and NCTR/FDA The opinions presented are not necessarily those of the U.S. FDA or NTP NCTR/FDA Research on BPA: Integrating pharmacokinetics in
Research support by Interagency Agreement between NTP/NIEHS and NCTR/FDA The opinions presented are not necessarily those of the U.S. FDA or NTP NCTR/FDA Research on BPA: Integrating pharmacokinetics in
Endocrine Disrupting Chemicals: New considerations in the toxics space TURI Annual Meeting
 Endocrine Disrupting Chemicals: New considerations in the toxics space TURI Annual Meeting Laura N. Vandenberg, PhD UMass Amherst School of Public Health April 25, 2018 Disclosure statement I am funded
Endocrine Disrupting Chemicals: New considerations in the toxics space TURI Annual Meeting Laura N. Vandenberg, PhD UMass Amherst School of Public Health April 25, 2018 Disclosure statement I am funded
Diethylstilbestrol (DES) General Information
 Use of the DES Paradigm to Inform Risk Assessment for Weakly Estrogenic Chemicals Robert Golden PhD ToxLogic Potomac, MD Diethylstilbestrol (DES) General Information DES ranges from slightly less to several
Use of the DES Paradigm to Inform Risk Assessment for Weakly Estrogenic Chemicals Robert Golden PhD ToxLogic Potomac, MD Diethylstilbestrol (DES) General Information DES ranges from slightly less to several
Decreased Insulin Receptor Kinase Activity in Gestational Diabetes Mellitus
 ENDOCRINOLOGY Decreased Insulin Receptor Kinase Activity in Gestational Diabetes Mellitus during pregnancy decrease in insulin sensitivity helps provide adequate glucose for the developing fetus 14% of
ENDOCRINOLOGY Decreased Insulin Receptor Kinase Activity in Gestational Diabetes Mellitus during pregnancy decrease in insulin sensitivity helps provide adequate glucose for the developing fetus 14% of
11. SEXUAL DIFFERENTIATION. Germinal cells, gonocytes. Indifferent stage INDIFFERENT STAGE
 11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
EOH3101 PRINCIPLES OF ENVIRONMENTAL HEALTH ENVIRONMENTAL HEALTH ISSUES: ENVIROMENTAL HORMONES
 EOH3101 PRINCIPLES OF ENVIRONMENTAL HEALTH ENVIRONMENTAL HEALTH ISSUES: ENVIROMENTAL HORMONES INTRODUCTION Endocrine disruptors are chemicals that may interfere with the body s endocrine system and produce
EOH3101 PRINCIPLES OF ENVIRONMENTAL HEALTH ENVIRONMENTAL HEALTH ISSUES: ENVIROMENTAL HORMONES INTRODUCTION Endocrine disruptors are chemicals that may interfere with the body s endocrine system and produce
ISRTP Workshop. Cumulative Risk for Endocrine Disrupters: The Case FOR a dose addition approach. Andreas Kortenkamp
 ISRTP Workshop Cumulative Risk for Endocrine Disrupters: The Case FOR a dose addition approach Andreas Kortenkamp The School of Pharmacy, University of London 19-20 February 2008 Endocrine disruption:
ISRTP Workshop Cumulative Risk for Endocrine Disrupters: The Case FOR a dose addition approach Andreas Kortenkamp The School of Pharmacy, University of London 19-20 February 2008 Endocrine disruption:
Title: Report on Public Health Concerns - Phthalates and Bisphenol A
 Title: Report on Public Health Concerns - Phthalates and Bisphenol A To: Public Health Subcommittee, Health and Government Operations Committee of the Maryland General Assembly Plain Language Summary Prepared
Title: Report on Public Health Concerns - Phthalates and Bisphenol A To: Public Health Subcommittee, Health and Government Operations Committee of the Maryland General Assembly Plain Language Summary Prepared
Effects of Prenatal Testosterone Propionate on the Sexual Development of Male and Female Rats: A Dose-Response Study
 TOXICOLOGICAL SCIENCES 65, 71 86 (2002) Effects of Prenatal Testosterone Propionate on the Sexual Development of Male and Female Rats: A Dose-Response Study Cynthia J. Wolf,*, Andrew Hotchkiss, Joseph
TOXICOLOGICAL SCIENCES 65, 71 86 (2002) Effects of Prenatal Testosterone Propionate on the Sexual Development of Male and Female Rats: A Dose-Response Study Cynthia J. Wolf,*, Andrew Hotchkiss, Joseph
Mode of action (MoA) in toxicology: general concept
 Toxicogenomics toxicology at a molecular level (mrna, mirna, proteins, metabolites ) Mode of action (MoA) in toxicology: general concept Chemical substance Key event 1 (Molecular initiating event) Key
Toxicogenomics toxicology at a molecular level (mrna, mirna, proteins, metabolites ) Mode of action (MoA) in toxicology: general concept Chemical substance Key event 1 (Molecular initiating event) Key
PHTHALATES STAKEHOLDER WORKSHOP SUMMARY REPORT
 PHTHALATES STAKEHOLDER WORKSHOP SUMMARY REPORT March 26, 2014 Ottawa, Canada Contents Acronyms... 3 INTRODUCTION... 4 Overview Phthalate Substances Grouping and the Chemicals Management Plan... 5 The Proposed
PHTHALATES STAKEHOLDER WORKSHOP SUMMARY REPORT March 26, 2014 Ottawa, Canada Contents Acronyms... 3 INTRODUCTION... 4 Overview Phthalate Substances Grouping and the Chemicals Management Plan... 5 The Proposed
Maldescended testis in Adults. Dr. BG GAUDJI Urologist STEVE BIKO ACADEMIC HOSPITAL
 Maldescended testis in Adults Dr. BG GAUDJI Urologist STEVE BIKO ACADEMIC HOSPITAL Definitions Cryptorchid: testis neither resides nor can be manipulated into the scrotum Ectopic: aberrant course Retractile:
Maldescended testis in Adults Dr. BG GAUDJI Urologist STEVE BIKO ACADEMIC HOSPITAL Definitions Cryptorchid: testis neither resides nor can be manipulated into the scrotum Ectopic: aberrant course Retractile:
Endocrine disrupting chemicals & the grain industry
 Endocrine disrupting chemicals & the grain industry 21 August 2018 Riana Bornman, SHSPH, UP riana.bornman@up.ac.za Conflict of interest statement Opinions expressed and conclusions arrived at are those
Endocrine disrupting chemicals & the grain industry 21 August 2018 Riana Bornman, SHSPH, UP riana.bornman@up.ac.za Conflict of interest statement Opinions expressed and conclusions arrived at are those
Consideration of Reproductive/Developmental Mode of Action Data from Laboratory Animals in the Risk Assessment of Environmental Chemicals
 Consideration of Reproductive/Developmental Mode of Action Data from Laboratory Animals in the Risk Assessment of Environmental Chemicals Susan Makris U.S. EPA, National Center for Environmental Assessment
Consideration of Reproductive/Developmental Mode of Action Data from Laboratory Animals in the Risk Assessment of Environmental Chemicals Susan Makris U.S. EPA, National Center for Environmental Assessment
Endocrine Disrupting Activity Associated With Oil and Natural Gas Extraction
 Endocrine Disrupting Activity Associated With Oil and Natural Gas Extraction Susan C Nagel, PhD Associate Professor Obstetrics, Gynecology, and Women s Health University of Missouri NIH R21ES026395 and
Endocrine Disrupting Activity Associated With Oil and Natural Gas Extraction Susan C Nagel, PhD Associate Professor Obstetrics, Gynecology, and Women s Health University of Missouri NIH R21ES026395 and
IN SUMMARY HST 071 NORMAL & ABNORMAL SEXUAL DIFFERENTIATION Fetal Sex Differentiation Postnatal Diagnosis and Management of Intersex Abnormalities
 Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 Title: Fetal Sex Differentiation Postnatal Diagnosis
Harvard-MIT Division of Health Sciences and Technology HST.071: Human Reproductive Biology Course Director: Professor Henry Klapholz IN SUMMARY HST 071 Title: Fetal Sex Differentiation Postnatal Diagnosis
DRAFT REPORT OF THE OECD VALIDATION OF THE RAT HERSHBERGER BIOASSAY: PHASE 3: CODED TESTING OF ANDROGEN AGONISTS, ANDROGEN ANTAGONISTS AND NEGATIVE
 DRAFT REPORT OF THE OECD VALIDATION OF THE RAT HERSHBERGER BIOASSAY: PHASE 3: CODED TESTING OF ANDROGEN AGONISTS, ANDROGEN ANTAGONISTS AND NEGATIVE REFERENCE CHEMICALS BY MULTIPLE LABORATORIES. SURGICAL
DRAFT REPORT OF THE OECD VALIDATION OF THE RAT HERSHBERGER BIOASSAY: PHASE 3: CODED TESTING OF ANDROGEN AGONISTS, ANDROGEN ANTAGONISTS AND NEGATIVE REFERENCE CHEMICALS BY MULTIPLE LABORATORIES. SURGICAL
Hormonally active contaminants- Interactions and effects on food safety. Helen Håkansson
 Hormonally active contaminants- Interactions and effects on food safety Contents EDC definition and effects Effects of dioxin-like compounds TEQ concept and TEF values for dioxin-like compound Need of
Hormonally active contaminants- Interactions and effects on food safety Contents EDC definition and effects Effects of dioxin-like compounds TEQ concept and TEF values for dioxin-like compound Need of
Development of the Genital System
 Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Development of the Genital System Professor Alfred Cuschieri Department of Anatomy University of Malta The mesonephros develops primitive nephrotomes draining into a mesonephric duct nephrotome mesonephric
Annex XV report PROPOSAL FOR IDENTIFICATION OF A SUBSTANCE OF VERY HIGH CONCERN ON THE BASIS OF THE CRITERIA SET OUT IN REACH ARTICLE 57
 Annex XV report PROPOSAL FOR IDENTIFICATION OF A SUBSTANCE OF VERY HIGH CONCERN ON THE BASIS OF THE CRITERIA SET OUT IN REACH ARTICLE 57 Substance Name(s): Bis(2-ethylhexyl) phthalate (DEHP) EC Number(s):
Annex XV report PROPOSAL FOR IDENTIFICATION OF A SUBSTANCE OF VERY HIGH CONCERN ON THE BASIS OF THE CRITERIA SET OUT IN REACH ARTICLE 57 Substance Name(s): Bis(2-ethylhexyl) phthalate (DEHP) EC Number(s):
Bioassay in Rats: A Short-term Screening Assay for (Anti)Androgenic Properties
 27-04-2009 DRAFT GUIDANCE DOCUMENT on the Weanling Hershberger Bioassay in Rats: A Short-term Screening Assay for (Anti)Androgenic Properties INTRODUCTION 1. The OECD initiated a high-priority activity
27-04-2009 DRAFT GUIDANCE DOCUMENT on the Weanling Hershberger Bioassay in Rats: A Short-term Screening Assay for (Anti)Androgenic Properties INTRODUCTION 1. The OECD initiated a high-priority activity
REVIEW Fifteen Years after Wingspread Environmental Endocrine Disrupters and Human and Wildlife Health: Where We are Today and Where We Need to Go
 TOXICOLOGICAL SCIENCES 105(2), 235 259 (2008) doi:10.1093/toxsci/kfn030 Advance Access publication February 16, 2008 REVIEW Fifteen Years after Wingspread Environmental Endocrine Disrupters and Human and
TOXICOLOGICAL SCIENCES 105(2), 235 259 (2008) doi:10.1093/toxsci/kfn030 Advance Access publication February 16, 2008 REVIEW Fifteen Years after Wingspread Environmental Endocrine Disrupters and Human and
APPLICATION FOR AUTHORISATION: ESTABLISHING REFERENCE DNELS FOR BBP
 Helsinki, 2 September 20 RAC/26/20/07 Rev. (Agreed at RAC-26) APPLICATION FOR AUTHORISATION: ESTABLISHING REFERENCE DNELS FOR BBP Background At the 22 nd meeting of the Committee for Risk Assessment (RAC)
Helsinki, 2 September 20 RAC/26/20/07 Rev. (Agreed at RAC-26) APPLICATION FOR AUTHORISATION: ESTABLISHING REFERENCE DNELS FOR BBP Background At the 22 nd meeting of the Committee for Risk Assessment (RAC)
Endocrine Disrupting Chemicals. - according to REACH regulation
 Endocrine Disrupting Chemicals - according to REACH regulation Lone Mikkelsen Policy officer Chemicals Copenhagen Chemicals Summit 29/9 2016 Agenda Absence of adequate regulation for endocrine disrupting
Endocrine Disrupting Chemicals - according to REACH regulation Lone Mikkelsen Policy officer Chemicals Copenhagen Chemicals Summit 29/9 2016 Agenda Absence of adequate regulation for endocrine disrupting
CONTRIBUTED ARTICLES Weight of the Evidence Evaluation of Low-Dose Reproductive and Developmental Effects of Bisphenol A
 Human and Ecological Risk Assessment, 10: 875 921, 2004 Copyright C ASP ISSN: 1080-7039 print / 1549-7680 online DOI: 10.1080/10807030490513883 CONTRIBUTED ARTICLES Weight of the Evidence Evaluation of
Human and Ecological Risk Assessment, 10: 875 921, 2004 Copyright C ASP ISSN: 1080-7039 print / 1549-7680 online DOI: 10.1080/10807030490513883 CONTRIBUTED ARTICLES Weight of the Evidence Evaluation of
Fifteen Years after Wingspread Environmental Endocrine Disrupters and Human and Wildlife Health: Where We are Today and Where We Need to Go
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Environmental Protection Agency Papers U.S. Environmental Protection Agency 2008 Fifteen Years after Wingspread Environmental
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Environmental Protection Agency Papers U.S. Environmental Protection Agency 2008 Fifteen Years after Wingspread Environmental
Angeliki Lyssimachou, PhD Environmental Scientist/Toxicologist
 Angeliki Lyssimachou, PhD Environmental Scientist/Toxicologist Pesticides a health concern Designed to be toxic to living organisms they pass through biological membranes May interact with hormones, their
Angeliki Lyssimachou, PhD Environmental Scientist/Toxicologist Pesticides a health concern Designed to be toxic to living organisms they pass through biological membranes May interact with hormones, their
I have no financial disclosures
 The Environment and Reproductive Health: Why What We Eat, Breath and Touch Matters Susan.Davidson@SSMHealth.com Susan Davidson, MD Dean Medical Center I have no financial disclosures 1 Many Complex Factors
The Environment and Reproductive Health: Why What We Eat, Breath and Touch Matters Susan.Davidson@SSMHealth.com Susan Davidson, MD Dean Medical Center I have no financial disclosures 1 Many Complex Factors
Hershberger Assays for Di-2-ethylhexyl Phthalate and Its Substitute Candidates
 Dev. Reprod. Vol. 22, No. 1, 19~27, March, 2018 https://doi.org/10.12717/dr.2018.22.1.019 ISSN 2465-9525 (Print) ISSN 2465-9541 (Online) Hershberger Assays for Di-2-ethylhexyl
Dev. Reprod. Vol. 22, No. 1, 19~27, March, 2018 https://doi.org/10.12717/dr.2018.22.1.019 ISSN 2465-9525 (Print) ISSN 2465-9541 (Online) Hershberger Assays for Di-2-ethylhexyl
Endocrine Disrupting Chemicals (EDCs): Its Impact On Health
 Endocrine Disrupting Chemicals (EDCs): Its Impact On Health Mustafa AM a a Faculty of Medicine, University of Malaya, Kuala Lumpur ABSTRACT: Endocrine disruptors or EDCs are compounds that interfere with
Endocrine Disrupting Chemicals (EDCs): Its Impact On Health Mustafa AM a a Faculty of Medicine, University of Malaya, Kuala Lumpur ABSTRACT: Endocrine disruptors or EDCs are compounds that interfere with
COLLECTIVE EXPERT APPRAISAL: SUMMARY AND CONCLUSIONS
 COLLECTIVE EXPERT APPRAISAL: SUMMARY AND CONCLUSIONS Related to the establishment of a Toxicity Reference Value based on the reprotoxic effects of nonylphenols AFSSET Only the French language version of
COLLECTIVE EXPERT APPRAISAL: SUMMARY AND CONCLUSIONS Related to the establishment of a Toxicity Reference Value based on the reprotoxic effects of nonylphenols AFSSET Only the French language version of
FSANZ Response to Studies Cited as Evidence that BPA may cause Adverse Effects in Humans
 Annex 1 FSANZ Response to Studies Cited as Evidence that BPA may cause Adverse Effects in Humans STUDY KEY FINDINGS/CLAIMS FSANZ RESPONSE Relative binding affinity-serum modified access (RBA-SMA) assay
Annex 1 FSANZ Response to Studies Cited as Evidence that BPA may cause Adverse Effects in Humans STUDY KEY FINDINGS/CLAIMS FSANZ RESPONSE Relative binding affinity-serum modified access (RBA-SMA) assay
Topic 3.13 Use of NOAEL, benchmark dose, and other models for human risk assessment of hormonally active substances*,
 Pure Appl. Chem., Vol. 75, Nos. 11 12, pp. 2151 2158, 2003. 2003 IUPAC Topic 3.13 Use of NOAEL, benchmark dose, and other models for human risk assessment of hormonally active substances*, R. Woodrow Setzer,
Pure Appl. Chem., Vol. 75, Nos. 11 12, pp. 2151 2158, 2003. 2003 IUPAC Topic 3.13 Use of NOAEL, benchmark dose, and other models for human risk assessment of hormonally active substances*, R. Woodrow Setzer,
Inter-Laboratory Control Data for Reproductive Endpoints Required in the OPPTS / OECD 416 Reproduction and Fertility Test
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Environmental Protection Agency Papers U.S. Environmental Protection Agency 2009 Inter-Laboratory Control Data for
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Environmental Protection Agency Papers U.S. Environmental Protection Agency 2009 Inter-Laboratory Control Data for
DANIEL R. DOERGE U.S. Food and Drug Administration National Center for Toxicological Research Jefferson, AR
 NCTR Research Plan for BPA: Integrating pharmacokinetics in rodent and primate species, rat toxicology studies, human biomonitoring, and PBPK modeling to assess potential human risks from dietary intake
NCTR Research Plan for BPA: Integrating pharmacokinetics in rodent and primate species, rat toxicology studies, human biomonitoring, and PBPK modeling to assess potential human risks from dietary intake
To: National Toxicology Program (NTP); Center for the Evaluation of Risks to Human Reproduction (CERHR)
 February 2, 2007 To: National Toxicology Program (NTP); Center for the Evaluation of Risks to Human Reproduction (CERHR) From: Natural Resources Defense Council These comments are submitted by Natural
February 2, 2007 To: National Toxicology Program (NTP); Center for the Evaluation of Risks to Human Reproduction (CERHR) From: Natural Resources Defense Council These comments are submitted by Natural
Hormone Disrupting Chemicals 1993: Sharpe and Skakkebæk publicerer østrogen hypotesen
 Hormone Disrupting Chemicals 1993: Sharpe and Skakkebæk publicerer østrogen hypotesen We argue that the increasing incidence of reproductive abnormalities in the human male may be related to increased
Hormone Disrupting Chemicals 1993: Sharpe and Skakkebæk publicerer østrogen hypotesen We argue that the increasing incidence of reproductive abnormalities in the human male may be related to increased
ENDOSULFAN AND ENDOCRINE DISRUPTION: HUMAN RISK CHARACTERIZATION. Prepared by
 ENDOSULFAN AND ENDOCRINE DISRUPTION: HUMAN RISK CHARACTERIZATION Prepared by Laura M. Plunkett, Ph.D., DABT Integrative Biostrategies, LLC Houston, Texas Prepared for Makhteshim-Agan of North America,
ENDOSULFAN AND ENDOCRINE DISRUPTION: HUMAN RISK CHARACTERIZATION Prepared by Laura M. Plunkett, Ph.D., DABT Integrative Biostrategies, LLC Houston, Texas Prepared for Makhteshim-Agan of North America,
Chapter 7: Causal Criteria for Assessing Endocrine Disruptors A Proposed Framework
 Chapter 7: Causal Criteria for Assessing Endocrine Disruptors A Proposed Framework 7.1 Introduction To create an objective and unbiased assessment of the hypothesis that chemicals with endocrine activity
Chapter 7: Causal Criteria for Assessing Endocrine Disruptors A Proposed Framework 7.1 Introduction To create an objective and unbiased assessment of the hypothesis that chemicals with endocrine activity
Draft OECD Guideline for the Testing of Chemicals. The Hershberger Bioassay in rats: a short term test for (anti)androgenic properties
 Draft OECD Guideline for the Testing of Chemicals The Hershberger Bioassay in rats: a short term test for (anti)androgenic properties Revised 20 November 2008 DRAFT OECD GUIDELINE FOR THE TESTING OF CHEMICALS
Draft OECD Guideline for the Testing of Chemicals The Hershberger Bioassay in rats: a short term test for (anti)androgenic properties Revised 20 November 2008 DRAFT OECD GUIDELINE FOR THE TESTING OF CHEMICALS
Decoding UCMR3: Clear Communication about Drinking Water Contaminants
 Decoding UCMR3: Clear Communication about Drinking Water Contaminants Christopher P Weis, Ph.D., D.A.B.T. Office of the Director National Institute of Environmental Health Science and National Toxicology
Decoding UCMR3: Clear Communication about Drinking Water Contaminants Christopher P Weis, Ph.D., D.A.B.T. Office of the Director National Institute of Environmental Health Science and National Toxicology
The Big Picture of Trace chemicals
 Impacts of Trace Chemicals on the Environment David O. Norris Integrative Physiology University of Colorado at Boulder david.norris@colorado.edu The Big Picture of Trace chemicals And how we all fit into
Impacts of Trace Chemicals on the Environment David O. Norris Integrative Physiology University of Colorado at Boulder david.norris@colorado.edu The Big Picture of Trace chemicals And how we all fit into
Towards 2020: Linda S. Birnbaum, Ph.D., D.A.B.T., A.T.S. Director National Institute of Environmental Health Sciences National Toxicology Program
 Towards 2020: What are the Critical Environmental Health Challenges? Linda S. Birnbaum, Ph.D., D.A.B.T., A.T.S. Director National Institute of Environmental Health Sciences National Toxicology Program
Towards 2020: What are the Critical Environmental Health Challenges? Linda S. Birnbaum, Ph.D., D.A.B.T., A.T.S. Director National Institute of Environmental Health Sciences National Toxicology Program
Endocrine disruptors and effects on reproduction DEMETER: a support tool for occupational risks assessment
 Endocrine disruptors and effects on reproduction DEMETER: a support tool for occupational risks assessment ISSA Symposium 1 June 2016 Stéphane Malard Medical Studies and Assistance Division, INRS, Paris
Endocrine disruptors and effects on reproduction DEMETER: a support tool for occupational risks assessment ISSA Symposium 1 June 2016 Stéphane Malard Medical Studies and Assistance Division, INRS, Paris
Chapter 15 Toxicological Implications of PCBs, PCDDs and PCDFs in Mammals
 Chapter 15 Toxicological Implications of PCBs, PCDDs and PCDFs in Mammals Matthew Zwiernik 1 Frouke Vermeulen 2 Steven Bursian 1 1 Michigan State University, College of Veterinary Medicine, Department
Chapter 15 Toxicological Implications of PCBs, PCDDs and PCDFs in Mammals Matthew Zwiernik 1 Frouke Vermeulen 2 Steven Bursian 1 1 Michigan State University, College of Veterinary Medicine, Department
Age at Puberty and First Litter Size in Early and Late Paired Rats 2
 BIOLOGY OF REPRODUCTION 34, 322-3 26 (1986) Age at Puberty and First Litter Size in Early and Late Paired Rats 2 ALIDA M. EVANS Cancer Prevention Program 1300 University Avenue-7C University of Wisconsin
BIOLOGY OF REPRODUCTION 34, 322-3 26 (1986) Age at Puberty and First Litter Size in Early and Late Paired Rats 2 ALIDA M. EVANS Cancer Prevention Program 1300 University Avenue-7C University of Wisconsin
CEE 697z Organic Compounds in Water and Wastewater
 Print version CEE 697z Organic Compounds in Water and Wastewater PPCPs: Introduction Lecture #16 For Background see: http://www.ecs.umass.edu/eve/background/chemicals/ppcps/ppcp%20intro.html EDCs & PPCPs
Print version CEE 697z Organic Compounds in Water and Wastewater PPCPs: Introduction Lecture #16 For Background see: http://www.ecs.umass.edu/eve/background/chemicals/ppcps/ppcp%20intro.html EDCs & PPCPs
Efferent Ducts and Epididymis
 increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
increase) the secretion of each of the androgen regulated proteins. Regulation of spermatogenesis is therefore an extremely complex cascade of cell-cell interactions with the Leydig cells supporting germ
Prenatal Exposure of 2,2,4,4-Tetrabromodiphenyl Ether (PBDE 47) Inducing Sperm Superoxide Anion Generation and Hyperactivation in Male Offspring Mice
 Prenatal Exposure of 2,2,4,4-Tetrabromodiphenyl Ether (PBDE 47) Inducing Sperm Superoxide Anion Generation and Hyperactivation in Male Offspring Mice Hsu PC 1, Li CH 1, Chen LW 1, Tseng LH 2, Guo YL 3,
Prenatal Exposure of 2,2,4,4-Tetrabromodiphenyl Ether (PBDE 47) Inducing Sperm Superoxide Anion Generation and Hyperactivation in Male Offspring Mice Hsu PC 1, Li CH 1, Chen LW 1, Tseng LH 2, Guo YL 3,
Sex Determination and Development of Reproductive Organs
 Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
Sex Determination and Development of Reproductive Organs Sex determination The SRY + gene is necessary and probably sufficient for testis development The earliest sexual difference appears in the gonad
AGREEMENT OF THE MEMBER STATE COMMITTEE ON THE IDENTIFICATION OF 4,4'-ISOPROPYLIDENEDIPHENOL (BISPHENOL A) AS A SUBSTANCE OF VERY HIGH CONCERN
 AGREEMENT OF THE MEMBER STATE COMMITTEE ON THE IDENTIFICATION OF 4,4'-ISOPROPYLIDENEDIPHENOL (BISPHENOL A) AS A SUBSTANCE OF VERY HIGH CONCERN According to Articles 57 and 59 of Regulation (EC) 1907/2006
AGREEMENT OF THE MEMBER STATE COMMITTEE ON THE IDENTIFICATION OF 4,4'-ISOPROPYLIDENEDIPHENOL (BISPHENOL A) AS A SUBSTANCE OF VERY HIGH CONCERN According to Articles 57 and 59 of Regulation (EC) 1907/2006
Endocrine Disruptors, Crustacean Molting and Bivalve Populations. Peter L. defur Virginia Commonwealth University Richmond VA
 Endocrine Disruptors, Crustacean Molting and Bivalve Populations Peter L. defur Virginia Commonwealth University Richmond VA Not so long ago, and not so far away We are certain of the following: a large
Endocrine Disruptors, Crustacean Molting and Bivalve Populations Peter L. defur Virginia Commonwealth University Richmond VA Not so long ago, and not so far away We are certain of the following: a large
The Biology of Sex: How We Become Male or Female.
 The Biology of Sex: How We Become Male or Female. Dr. Tamatha Barbeau, Dept. of Biology Guest Lecture for Gender 200 March 2017 Objectives: 1. Sex vs. Gender defined. 2. Biological sex based on inheritance
The Biology of Sex: How We Become Male or Female. Dr. Tamatha Barbeau, Dept. of Biology Guest Lecture for Gender 200 March 2017 Objectives: 1. Sex vs. Gender defined. 2. Biological sex based on inheritance
Strategies for health interpretation: development of HBM healthbased guidance values for individual phthalates and BPA
 Strategies for health interpretation: development of HBM healthbased guidance values for individual phthalates and BPA Workshop on policy uptake of HBM-results Brussels - Nov 2018 Eva Ougier (ANSES) &
Strategies for health interpretation: development of HBM healthbased guidance values for individual phthalates and BPA Workshop on policy uptake of HBM-results Brussels - Nov 2018 Eva Ougier (ANSES) &
A Japanese View on Assessment of Endocrine Disrupting Chemicals
 A Japanese View on Assessment of Endocrine Disrupting Chemicals Hiroaki Aoyama, Ph.D. Executive Director, Toxicology Division Institute of Environmental Toxicology Food Safety Committee of Japan (FSCJ)
A Japanese View on Assessment of Endocrine Disrupting Chemicals Hiroaki Aoyama, Ph.D. Executive Director, Toxicology Division Institute of Environmental Toxicology Food Safety Committee of Japan (FSCJ)
Supplemental Information. Characterization of Adipogenic Activity of House Dust Extracts and Semi-Volatile Indoor. Contaminants in 3T3-L1 Cells
 Supplemental Information Characterization of Adipogenic Activity of House Dust Extracts and Semi-Volatile Indoor Contaminants in 3T3-L1 Cells Christopher D. Kassotis, Kate Hoffman, and Heather M. Stapleton*
Supplemental Information Characterization of Adipogenic Activity of House Dust Extracts and Semi-Volatile Indoor Contaminants in 3T3-L1 Cells Christopher D. Kassotis, Kate Hoffman, and Heather M. Stapleton*
DOHaD: Role of environmental chemical exposures
 DOHaD: Role of environmental chemical exposures Linda S. Birnbaum, Ph.D., D.A.B.T., A.T.S. Director, National Institute of Environmental Health Sciences and National Toxicology Program PPTox-IV Boston,
DOHaD: Role of environmental chemical exposures Linda S. Birnbaum, Ph.D., D.A.B.T., A.T.S. Director, National Institute of Environmental Health Sciences and National Toxicology Program PPTox-IV Boston,
 TOXICITY DATA (continued): KETOCONAZOLE (continued): TDLo (Oral-Rat) 1760 mg/kg: female 1-21 day(s) after conception: Reproductive: Maternal Effects: other effects; Fertility: post-implantation mortality
TOXICITY DATA (continued): KETOCONAZOLE (continued): TDLo (Oral-Rat) 1760 mg/kg: female 1-21 day(s) after conception: Reproductive: Maternal Effects: other effects; Fertility: post-implantation mortality
Environmental and Developmental Origins of Ovarian Reserve. Nick Macklon Professor of Obstetrics and Gynaecology, University of Southampton, UK.
 Environmental and Developmental Origins of Ovarian Reserve Nick Macklon Professor of Obstetrics and Gynaecology, University of Southampton, UK. Why worry? Women delaying childbirth Diminished ovarian
Environmental and Developmental Origins of Ovarian Reserve Nick Macklon Professor of Obstetrics and Gynaecology, University of Southampton, UK. Why worry? Women delaying childbirth Diminished ovarian
Environmental contaminants and food safety
 Environmental contaminants and food safety Agneta Oskarsson Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences, Uppsala Sweden 26 th NKVet Symposium
Environmental contaminants and food safety Agneta Oskarsson Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences, Uppsala Sweden 26 th NKVet Symposium
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
WWF's RESPONSE TO THE COMMUNITY STRATEGY FOR ENDOCRINE DISRUPTORS
 WWF's RESPONSE TO THE COMMUNITY STRATEGY FOR ENDOCRINE DISRUPTORS WWF WWF is the world s largest and most experienced independent conservation organisation. It has 4.7 million regular supporters and a
WWF's RESPONSE TO THE COMMUNITY STRATEGY FOR ENDOCRINE DISRUPTORS WWF WWF is the world s largest and most experienced independent conservation organisation. It has 4.7 million regular supporters and a
ENDOCRINE-DISRUPTING ACTIVITIES OF PCBs AND OTHER FOOD CONTAMINANTS
 ENDOCRINE-DISRUPTING ACTIVITIES OF PCBs AND OTHER FOOD CONTAMINANTS Miroslav Machala 1, Jan Vondráček 2 1 ; 2 Institute of Biophysics, ASCR, Brno, Czech Republic MECHANISTIC STUDIES REVEALED MULTIPLE MODES
ENDOCRINE-DISRUPTING ACTIVITIES OF PCBs AND OTHER FOOD CONTAMINANTS Miroslav Machala 1, Jan Vondráček 2 1 ; 2 Institute of Biophysics, ASCR, Brno, Czech Republic MECHANISTIC STUDIES REVEALED MULTIPLE MODES
State of the Science Evaluation:
 State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures Photo image area measures 2 H x 6.93 W and
State of the Science Evaluation: Nonmonotonic Dose Responses as They Apply to Estrogen, Androgen, and Thyroid Pathways and EPA Testing and Assessment Procedures Photo image area measures 2 H x 6.93 W and
Development of the urogenital system
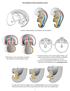 Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
Development of the urogenital system Location of the pronephros, mesonephros and metanephros Differentiation of the intermedierm mesoderm into nephrotome and mesonephric tubules Connection between aorta
Cumulative Risk: The Argument Against Estrogen Equivalents. Conducting and Assessing the Results of Endocrine Screening
 Cumulative Risk: The Argument Against Estrogen Equivalents Conducting and Assessing the Results of Endocrine Screening ISRTP Workshop, Lister Hill Auditorium Bethesda, MD. February 19-20, 2008 Christopher
Cumulative Risk: The Argument Against Estrogen Equivalents Conducting and Assessing the Results of Endocrine Screening ISRTP Workshop, Lister Hill Auditorium Bethesda, MD. February 19-20, 2008 Christopher
Basic Reproduction & Genetics. Steve Pritchard UNL Extension Educator Boone-Nance Counties
 Basic Reproduction & Genetics Steve Pritchard UNL Extension Educator Boone-Nance Counties Hormonal Regulation of the Estrous Cycle Several hormones regulate the estrous cycle Changes in the concentrations
Basic Reproduction & Genetics Steve Pritchard UNL Extension Educator Boone-Nance Counties Hormonal Regulation of the Estrous Cycle Several hormones regulate the estrous cycle Changes in the concentrations
Endocrine Disrupters as Obesogens
 Endocrine Disrupters as Obesogens Is the environment making us fat? Bruce Blumberg, Ph.D. Department of Developmental and Cell Biology Department of Pharmaceutical Sciences Developmental Biology Center
Endocrine Disrupters as Obesogens Is the environment making us fat? Bruce Blumberg, Ph.D. Department of Developmental and Cell Biology Department of Pharmaceutical Sciences Developmental Biology Center
Esposizione a livelli ambientali di xenoestrogeni: modelli di studio nel ratto in vivo e rilevanza degli effetti
 Esposizione a livelli ambientali di xenoestrogeni: modelli di studio nel ratto in vivo e rilevanza degli effetti Francesca Farabollini (a), Leonida Fusani (c), Daniele Della Seta (a), Francesco Dessì-Fulgheri
Esposizione a livelli ambientali di xenoestrogeni: modelli di studio nel ratto in vivo e rilevanza degli effetti Francesca Farabollini (a), Leonida Fusani (c), Daniele Della Seta (a), Francesco Dessì-Fulgheri
ENDOCRINE DISRUPTORS: Endometriosis and Infertility
 ENDOCRINE DISRUPTORS: Roma 8 Aprile 2010 iintroduction P.G.Signorile Rachel Carson 1962 book, "Silent Spring Endocrine Disruptors 1980s, was researching the health of vertebrates living in the Great Lakes.
ENDOCRINE DISRUPTORS: Roma 8 Aprile 2010 iintroduction P.G.Signorile Rachel Carson 1962 book, "Silent Spring Endocrine Disruptors 1980s, was researching the health of vertebrates living in the Great Lakes.
Chapter 16: Steroid Hormones (Lecture 17)
 Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
Chapter 16: Steroid Hormones (Lecture 17) A) 21 or fewer carbon atoms B) Precursor: 27 carbon cholesterol C) major classes of steroid hormones 1) progestagens a) progesterone- prepares lining of uterus
Pesticides and Health. Lynn R. Goldman, MD, MPH Johns Hopkins Blooomberg School of Public Health
 Pesticides and Health Lynn R. Goldman, MD, MPH Johns Hopkins Blooomberg School of Public Health lgoldman@jhsph.edu What are pesticides? insecticides herbicides rodenticides fungicides other living thing
Pesticides and Health Lynn R. Goldman, MD, MPH Johns Hopkins Blooomberg School of Public Health lgoldman@jhsph.edu What are pesticides? insecticides herbicides rodenticides fungicides other living thing
Environmental contaminants and cancers of the reproductive tract
 Chapter 14 Environmental contaminants and cancers of the reproductive tract Gail S. Prins and Esther L. Calderon Introduction Many environmental contaminants have been shown to directly or indirectly contribute
Chapter 14 Environmental contaminants and cancers of the reproductive tract Gail S. Prins and Esther L. Calderon Introduction Many environmental contaminants have been shown to directly or indirectly contribute
Endocrine Disrupter Chemicals: Universal Access to Healthcare
 Endocrine Disrupter Chemicals: the right to know Riana Bornman, School of Health Systems and Public Health, University of Pretoria SAMA Conference 2016 Universal Access to Healthcare Concern started SOS-EDCs
Endocrine Disrupter Chemicals: the right to know Riana Bornman, School of Health Systems and Public Health, University of Pretoria SAMA Conference 2016 Universal Access to Healthcare Concern started SOS-EDCs
Determining the critical window of influence of PCB perinatally on behavioral and hormonal development in Sprague-Dawley rat pups
 Bowling Green State University ScholarWorks@BGSU Honors Projects Honors College Fall 12-15-2014 Determining the critical window of influence of PCB perinatally on behavioral and hormonal development in
Bowling Green State University ScholarWorks@BGSU Honors Projects Honors College Fall 12-15-2014 Determining the critical window of influence of PCB perinatally on behavioral and hormonal development in
Justification for the selection of a candidate CoRAP substance
 Justification for the selection of a candidate CoRAP substance Substance Name (Public Name): Diethyl phthalate Chemical Group: EC Number: 201-550-6 CAS Number: 84-66-2 Submitted by: Germany/Portugal Published:
Justification for the selection of a candidate CoRAP substance Substance Name (Public Name): Diethyl phthalate Chemical Group: EC Number: 201-550-6 CAS Number: 84-66-2 Submitted by: Germany/Portugal Published:
Fertility and early embryonic development. Chris Willoughby, Huntingdon Life Sciences 11 October 2012
 Fertility and early embryonic development Chris Willoughby, Huntingdon Life Sciences 11 October 2012 Outline 2 What sort of compounds may affect fertility? What areas of reproduction are we assessing in
Fertility and early embryonic development Chris Willoughby, Huntingdon Life Sciences 11 October 2012 Outline 2 What sort of compounds may affect fertility? What areas of reproduction are we assessing in
REPRODUCCIÓN. La idea fija. Copyright 2004 Pearson Education, Inc., publishing as Benjamin Cummings
 REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
REPRODUCCIÓN La idea fija How male and female reproductive systems differentiate The reproductive organs and how they work How gametes are produced and fertilized Pregnancy, stages of development, birth
Endocrine disrupters a role in human health?
 9 In: Endocrine Disrupters T. Grotmol, A. Bernhoft, G.S. Eriksen and T.P. Flaten, eds. Oslo: The Norwegian Academy of Science and Letters, 2006. Endocrine disrupters a role in human health? Erik Dybing
9 In: Endocrine Disrupters T. Grotmol, A. Bernhoft, G.S. Eriksen and T.P. Flaten, eds. Oslo: The Norwegian Academy of Science and Letters, 2006. Endocrine disrupters a role in human health? Erik Dybing
Biology of Reproduction-Biol 326
 Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
Biology of Reproduction-Biol 326 READ ALL INSTRUCTIONS CAREFULLY. ANSWER ALL THE QUESTIONS ON THE ANSWER SHEET. THE ANSWER ON THE ANSWER SHEET IS YOUR OFFICIAL ANSWER REGARDLESS OF WHAT YOU MARK ON THE
