Subject: Temporary Prostatic Stent and Prostatic Urethral Lift
|
|
|
- Malcolm Atkinson
- 5 years ago
- Views:
Transcription
1 Original Effective Date: 03/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Temporary Prostatic Stent and Prostatic Urethral Lift THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF BENEFITS, OR A GUARANTEE OF PAYMENT, NOR DOES IT SUBSTITUTE FOR OR CONSTITUTE MEDICAL ADVICE. ALL MEDICAL DECISIONS ARE SOLELY THE RESPONSIBILITY OF THE PATIENT AND PHYSICIAN. BENEFITS ARE DETERMINED BY THE GROUP CONTRACT, MEMBER BENEFIT BOOKLET, AND/OR INDIVIDUAL SUBSCRIBER CERTIFICATE IN EFFECT AT THE TIME SERVICES WERE RENDERED. THIS MEDICAL COVERAGE GUIDELINE APPLIES TO ALL LINES OF BUSINESS UNLESS OTHERWISE NOTED IN THE PROGRAM EXCEPTIONS SECTION. Position Statement Billing/Coding Reimbursement Program Exceptions Definitions Related Guidelines Other References Updates DESCRIPTION: Benign prostatic hyperplasia (BPH) is a common disorder among older individuals that results from hyperplastic nodules in the periurethral or transitional zone of the prostate. The clinical manifestations of BPH include increased urinary frequency, nocturia, an urgency or hesitancy to urinate, and a weak stream when urinating. The urinary tract symptoms often progress with worsening hypertrophy and may lead to acute urinary retention, incontinence, renal insufficiency, and/or urinary tract infection. Temporary Prostatic Stent: Intraprostatic stenting has been investigated as a short- term treatment option permitting voluntary urination as an alternative to an indwelling catheter. The U.S. Food and Drug Administration (FDA) granted premarket approval (PMA) for The Spanner (Abbeymoore Medical Inc.) December The Spanner The device is inserted under topical anesthesia and is intended for temporary use (up to 30 days) to maintain urine flow and allow voluntary urination in patients following minimally invasive treatment for BPH and after initial post-treatment catheterization. Prostatic Urethral Lift: The prostatic urethral lift procedure involves placement of 1 or more implants in the lateral lobes of the prostate using a transurethral delivery device. The implant device is designed to retract the prostate to allow expansion of the prostatic urethra. The implants are retained in the prostate to maintain an expanded urethral lumen. One device, the NeoTract UroLift System, has been cleared for marketing by the FDA.
2 POSITION STATEMENT: Use of prostatic urethral lift in members with moderate-to-severe lower urinary tract obstruction due to benign prostatic hyperplasia meets the definition of medical necessity when ALL of the following criteria are met: 1. Member has persistent or progressive lower urinary tract symptoms despite medical therapy (α1- adrenergic antagonists maximally titrated, 5α-reductase inhibitors, or combination medication therapy maximally titrated) over a trial period of no less than 6 months, or is unable to tolerate medical therapy; 2. Prostate gland volume is 80 ml 3. Prostate anatomy demonstrates normal bladder neck without an obstructive or protruding median lobe 4. Member does not have urinary retention, urinary tract infection, or recent prostatitis (within past year) 5. Member does not have prostate-specific antigen level 3 ng/ml, or has had appropriate testing to exclude diagnosis of prostate cancer AND 6. Member does not have a contact dermatitis nickel allergy. Use of prostatic urethral lift in all other situations is considered experimental or investigational; the evidence is insufficient to determine the effects of the technology on health outcomes. Use of a temporary prostatic stent is considered experimental or investigational for all indications. There is insufficient clinical data in peer-reviewed medical literature to permit conclusions on safety, efficacy and net health outcomes. BILLING/CODING INFORMATION: CPT Coding Cystourethroscopy, with insertion of permanent adjustable transprostatic implant; single implant Cystourethroscopy, with insertion of permanent adjustable transprostatic implant; each additional permanent adjustable transprostatic implant (List separately in addition to code for primary procedure) Insertion of a temporary prostatic urethral stent, including urethral measurement (Investigational) HCPCS Coding C9739 Cystourethroscopy, with insertion of transprostatic implant; 1 to 3 implants C9740 Cystourethroscopy, with insertion of transprostatic implant; 4 or more implants
3 ICD-10 Diagnosis Codes That Support Medical Necessity: N40.1 Benign prostatic hyperplasia with lower urinary tract symptoms REIMBURSEMENT INFORMATION: Refer to section entitled POSITION STATEMENT. PROGRAM EXCEPTIONS: Federal Employee Program (FEP): Follow FEP guidelines. State Account Organization (SAO): Follow SAO guidelines. Medicare Advantage products: The following Local Coverage Determination (LCD) was reviewed on the last guideline reviewed date: Prostatic Urethral Lift (PUL) (L36775) located at fcso.com. DEFINITIONS: No guideline specific definitions apply. RELATED GUIDELINES: None applicable. OTHER: Note: The use of specific product names is illustrative only. It is not intended to be a recommendation of one product over another, and is not intended to represent a complete listing of all products available. Other names or key words used to report temporary prostatic stent: Spanner Temporary Prostatic Stent Memokath 028SW Urethral Stent Prostatic Stent, Temporary. Other names or key words used to report prostatic urethral lift: Implantable transprostatic tissue retractor system NeoTract UroLift System Transprostatic implant system. REFERENCES: 1. American Urological Association, AUA Guideline on the Management of Benign Prostatic Hyperplasia: Diagnosis and Treatment Recommendations, accessed at auanet.org. 2. Barkin J, Giddens J, et al, UroLift system for relief of prostate obstruction under local anesthesia. Can J Urol Apr;19(2):
4 3. Blue Cross Blue Shield Association Medical Policy Reference Manual, Prostatic Urethral Lift, 08/ Cantwell AL, Bogache WK, et al, Multicentre prospective crossover study of the 'prostatic urethral lift' for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. BJU Int Apr;113(4): ClinicalTrials.gov, The Effect of Prostatic Urethral Lift Versus Prostate Arterial Embolization on Quality of Life in Men With Urinary Symptoms Secondary to Benign Prostate Hyperplasia (BPH): A Prospective, Single Center, Comparative Trial, sponsored by St. Louis University, accessed 03/07/ ClinicalTrials.gov, Study of Median Lobe Prostatic UroLift Procedure, sponsored by NeoTract, Inc. Accessed 02/25/ ClinicalTrials.gov, Use of The Spanner Temporary Prostatic Stent as an Alternative to a Urinary Catheter to Achieve Bladder Drainage in Men Unfit for Other Treatments, sponsored by SRS Medical, accessed 03/07/ Dineen MK, Shore ND, Lumerman JH, et al, Use of a Temporary Prostatic Stent After Transurethral Microwave Thermotherapy Reduced Voiding Symptoms and Bother Without Exacerbating Irritative Symptoms, Urology, 2008 May; 71(5): First Coast Service Options, Inc. (FCSO), LCD for Prostatic Urethral Lift (PUL) (L36775), accessed at fcso.com. 10. Foster HE, Barry MJ, Dahm P, et al. Surgical Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA Guideline. J Urol. Jun Goh M, Kastner C, et al, First Experiences with the Spanner Temporary Prostatic Stent for Prostatic Urethral Obstruction, Urol Int. 2013;91(4): Gratzke C, Barber N, et al, Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int Nov 14. doi: /bju Grimsley SJ, Khan, MH, Lennox E, Paterson PH, Experience with the Spanner Prostatic Stent in Patients Unfit for Surgery: An Observational Study, Journal of Endourology, 09/01/07, 21(9): McKenzie P, Badlani G, Critical Appraisal of the Spanner Prostatic Stent in the Treatment of Prostatic Obstruction, Medical Devices: Evidence and Research, 2011: McNicholas TA, Benign prostatic hyperplasia and new treatment options - a critical appraisal of the UroLift system. Med Devices (Auckl) May 19;9: McNicholas TA, Woo HH, et al, Minimally invasive prostatic urethral lift: surgical technique and multinational experience. Eur Urol Aug;64(2): McVary KT, Gange SN, et al, Treatment of LUTS secondary to BPH while preserving sexual function: randomized controlled study of prostatic urethral lift. J Sex Med Jan;11(1): Ray A, Morgan H, et al, The Urolift System for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A NICE Medical Technology Guidance. Appl Health Econ Health Policy Oct;14(5): Roehrborn CG, Barkin J, et al, Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol Jun;24(3): Roehrborn, CG, Prostatic Urethral Lift: A Unique Minimally Invasive Surgical Treatment of Male Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Urol Clin North Am Aug;43(3): Roehrborn CG, et al, Three Year Results of the Prostatic Urethral L.I.F.T Study, Can J Urol Jun;22(3):
5 22. Roehrborn CG, Gange SN, et al, Durability of the prostatic urethral lift: two year results of the L.I.F.T. Study. Urology Practice 2015 (2); Roehrborn CG, Gange SN, et al, The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol Dec;190(6): Shore N, A Review of the Prostatic Urethral Lift for Lower Urinary Tract Symptoms: Symptom Relief, Flow Improvement, and Preservation of Sexual Function in Men With Benign Prostatic Hyperplasia. Curr Bladder Dysfunct Rep; published online: 27 March Shore N, Freedman S, et al, Prospective multi-center study elucidating patient experience after prostatic urethral lift. Can J Urol Feb. 26. Sønksen J, Barber NJ, et al, Prospective, Randomized, Multinational Study of Prostatic Urethral Lift Versus Transurethral Resection of the Prostate: 12-month Results from the BPH6 Study. European Urology (2015); U.S. Food and Drug Administration (FDA), accessed at fda.gov. COMMITTEE APPROVAL: This Medical Coverage Guideline (MCG) was approved by the Florida Blue Medical Policy & Coverage Committee on 09/27/18. GUIDELINE UPDATE INFORMATION: 03/15/05 New Medical Coverage Guideline. 03/15/06 Annual review: continue investigational. 02/15/07 Scheduled review. No change in investigational status. Revised when services are covered; add of temporary prostatic stent. Added temporary prostatic to Billing/Coding Information section-icd-9 diagnoses codes that support medical necessity. Updated references. 06/15/07 Reformatted guideline; references 03/15/08 Annual review: position statement maintained, description section updated, references 03/15/09 Annual review: position statement maintained and references 01/01/10 Annual HCPCS coding update: added code 53855, deleted code 0084T. 03/15/10 Annual review: position statement maintained; description section and references
6 02/15/11 Annual review: position statement maintained and references 10/15/11 Scheduled review; position statement maintained, description section and references 11/15/12 Annual review; position statement maintained and references 09/15/13 Annual review; investigational position statement maintained and references 08/15/14 Annual review; position statement maintained and references 06/15/15 Annual review; position statement, billing/coding, description, guideline title and references 04/15/16 Revision; position statements maintained and references updated 05/15/17 Revision; Investigational position statements maintained; description and references 02/15/18 Annual review; PUL coverage statement added; description, coding, & references 10/15/18 Review; Coverage criteria and references
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that can
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that can
Medical Coverage Policy Prostatic Urethral Lifts
 Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:01 01 2017 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that
FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: None Prostatic Urethral Lift Description Benign prostatic hyperplasia (BPH) is a common condition in older individuals that
Prostatic Urethral Lift Corporate Medical Policy
 Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
Medical Coverage Policy Prostatic Urethral Lifts
 Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:12 01 2018 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Medical Coverage Policy Prostatic Urethral Lifts EFFECTIVE DATE:12 01 2018 POLICY LAST UPDATED: 10 03 2017 OVERVIEW Benign prostatic hyperplasia is a common condition in older men that can lead to increased
Prostatic Urethral Lift
 Protocol Prostatic Urethral Lift (701151) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization No Review Dates: 05/17, 01/18 Preauthorization is not required. The following
Protocol Prostatic Urethral Lift (701151) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization No Review Dates: 05/17, 01/18 Preauthorization is not required. The following
Policy #: 213 Latest Review Date: September 2012
 Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Last Review Status/Date: December Summary
 Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
Section: Surgery Effective Date: January 15, 2016 Subject: Prostatic Urethral Lift Page: 1 of 9 Last Review Status/Date: December 2015 Summary Benign prostatic hyperplasia (BPH) is a common condition in
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
POLICIES AND PROCEDURE MANUAL Policy: MP048 Section: Medical Benefit Policy Subject: Surgical and Minimally Invasive Therapies for the Treatment of Benign Prostatic Hypertrophy I. Policy: Surgical and
NOTE: This policy is not effective until April 1, Transurethral Water Vapor Thermal Therapy of the Prostate
 NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
NOTE: This policy is not effective until April 1, 2019. Medical Policy Manual Surgery, Policy No. 210 Transurethral Water Vapor Thermal Therapy of the Prostate Next Review: December 2019 Last Review: December
Prostatic Urethral Lift. Summary
 Section: Surgery Effective Date: January 15, 2017 Subject: Prostatic Urethral Lift Page: 1 of 10 Last Review Status/Date: December 2016 Prostatic Urethral Lift Summary Benign prostatic hyperplasia (BPH)
Section: Surgery Effective Date: January 15, 2017 Subject: Prostatic Urethral Lift Page: 1 of 10 Last Review Status/Date: December 2016 Prostatic Urethral Lift Summary Benign prostatic hyperplasia (BPH)
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prostatic Urethral Lift Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift Page 1 of 19 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prostatic Urethral Lift Professional Institutional Original Effective Date:
Prostatic Urethral Lift
 Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
Prostatic Urethral Lift Policy Number: 7.01.151 Last Review: 10/2018 Origination: 10/2015 Next Review: 10/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Prostatic
Subject: Prostate Saturation Biopsy
 02-54000-22 Original Effective Date: 01/01/10 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Prostate Saturation Biopsy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
02-54000-22 Original Effective Date: 01/01/10 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Prostate Saturation Biopsy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
The Enlarged Prostate Symptoms, Diagnosis and Treatment
 The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
The Enlarged Prostate Symptoms, Diagnosis and Treatment MAC00031-01 Rev G Financial support for this seminar has been provided by NeoTract, Inc., the manufacturer of the UroLift System. 1 Today s Agenda
OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1
 PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
PATIENT INFORMATION BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have to be one of them! OVER 70% OF MEN IN THEIR 60s HAVE
PATIENT INFORMATION 2017 NeoTract, Inc. All rights reserved. Printed in the USA. MAC Rev A
 PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
PATIENT INFORMATION OVER 70% OF MEN IN THEIR 60s HAVE SYMPTOMS OF BPH 1 BPH affects more than 500 million men worldwide, with many men suffering from symptoms of enlarged prostate. 1 You no longer have
Related Policies None
 Medical Policy MP 7.01.151 BCBSA Ref. Policy: 7.01.151 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery End Date: 08/19/2018 Related Policies None DISCLAIMER Our medical policies are
Medical Policy MP 7.01.151 BCBSA Ref. Policy: 7.01.151 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery End Date: 08/19/2018 Related Policies None DISCLAIMER Our medical policies are
Related Policies None
 Medical Policy MP 7.01.151 Original Policy Date: August 2015 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.151 Original Policy Date: August 2015 Last Review: 12/27/2017 Effective Date: 03/01/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Subject: Percutaneous Tibial Nerve Stimulation
 02-64000-01 Original Effective Date: 05/15/08 Reviewed: 05/24/18 Revised: 06/15/18 Subject: Percutaneous Tibial Nerve Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
02-64000-01 Original Effective Date: 05/15/08 Reviewed: 05/24/18 Revised: 06/15/18 Subject: Percutaneous Tibial Nerve Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Benign Prostatic Hyperplasia: Update on Innovative Current Treatments
 Benign Prostatic Hyperplasia: Update on Innovative Current Treatments Michael Ferrandino, MD As.soc Professor Director of Minimally Invasive Urologic Surgery Division of Urologic Surgery Duke University
Benign Prostatic Hyperplasia: Update on Innovative Current Treatments Michael Ferrandino, MD As.soc Professor Director of Minimally Invasive Urologic Surgery Division of Urologic Surgery Duke University
Treating BPH: Comparing Rezum UroLift and HoLEP
 Treating BPH: Comparing Rezum UroLift and HoLEP Scott M. Cheney MD Mayo Clinic Arizona 2018 MFMER slide-1 Welcome to AZ 2018 MFMER slide-2 Outline Background on BPH, Rezum, Urolift, HoLEP AUA Guideline
Treating BPH: Comparing Rezum UroLift and HoLEP Scott M. Cheney MD Mayo Clinic Arizona 2018 MFMER slide-1 Welcome to AZ 2018 MFMER slide-2 Outline Background on BPH, Rezum, Urolift, HoLEP AUA Guideline
Original Policy Date
 MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
MP 7.01.39 Transurethral Microwave Thermotherapy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical
Subject: Chemoresistance and Chemosensitivity Assays
 05-86000-11 Original Effective Date: 12/15/02 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Chemoresistance and Chemosensitivity Assays THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
05-86000-11 Original Effective Date: 12/15/02 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Chemoresistance and Chemosensitivity Assays THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND (TRUS)
 MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
APPENDIX CLINICAL INPUT RESPONSES
 CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
CLINICAL INPUT RESPONSES AUA: American Urological Association; UCSF Med Ctr: University of California San Francisco Medical Center; PUL: prostatic urethral lift. * Indicates that information on conflicts
Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS)
 09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
REVIEW. Introduction. Accepted for publication January 2018
 REVIEW Emerging, newly-approved treatments for lower urinary tract symptoms secondary to benign prostatic hypertrophy Hannah Pham, Pranav Sharma, MD Department of Urology, Texas Tech University Health
REVIEW Emerging, newly-approved treatments for lower urinary tract symptoms secondary to benign prostatic hypertrophy Hannah Pham, Pranav Sharma, MD Department of Urology, Texas Tech University Health
HOW I DO IT. Introduction. Methods and technique
 HOW I DO IT State of the art: Advanced techniques for prostatic urethral lift for the relief of prostate obstruction under local anesthesia Lance Patrick Walsh, MD Eisenhower Medical Center, Rancho Mirage,
HOW I DO IT State of the art: Advanced techniques for prostatic urethral lift for the relief of prostate obstruction under local anesthesia Lance Patrick Walsh, MD Eisenhower Medical Center, Rancho Mirage,
rezūm system reimbursement guide
 rezūm system reimbursement guide OCTOBER 2016 Reimbursement Support 952-454-5361 rezumreimb@jdlymon.com table of contents OVERVIEW............................. 3 Benign Prostatic Hyperplasia (BPH)......................
rezūm system reimbursement guide OCTOBER 2016 Reimbursement Support 952-454-5361 rezumreimb@jdlymon.com table of contents OVERVIEW............................. 3 Benign Prostatic Hyperplasia (BPH)......................
Subject: Magnetoencephalography/Magnetic Source Imaging
 01-95805-16 Original Effective Date: 09/01/01 Reviewed: 07/26/18 Revised: 08/15/18 Subject: Magnetoencephalography/Magnetic Source Imaging THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
01-95805-16 Original Effective Date: 09/01/01 Reviewed: 07/26/18 Revised: 08/15/18 Subject: Magnetoencephalography/Magnetic Source Imaging THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Name of Policy: Transurethral Microwave Thermotherapy
 Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Name of Policy: Transurethral Microwave Thermotherapy Policy #: 449 Latest Review Date: September 2013 Category: Surgery Policy Grade: B Background/Definitions: As a general rule, benefits are payable
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of insertion of prostatic urethral lift implants to treat lower urinary tract symptoms
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of insertion of prostatic urethral lift implants to treat lower urinary tract symptoms
PATIENT SELECTION GUIDE. finding the right fit
 PATIENT SELECTION GUIDE finding the right fit IDENTIFYING PATIENTS Rezūm, a new minimally invasive treatment, is now available to offer patients as a first-line BPH therapy. BPH TREATMENT OPTIONS Behavior
PATIENT SELECTION GUIDE finding the right fit IDENTIFYING PATIENTS Rezūm, a new minimally invasive treatment, is now available to offer patients as a first-line BPH therapy. BPH TREATMENT OPTIONS Behavior
Rezūm procedure for the Prostate
 Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Subject: Gefitinib (Iressa)
 09-J2000-44 Original Effective Date: 09/15/15 Reviewed: 09/12/18 Revised: 10/15/18 Subject: Gefitinib (Iressa) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF BENEFITS,
09-J2000-44 Original Effective Date: 09/15/15 Reviewed: 09/12/18 Revised: 10/15/18 Subject: Gefitinib (Iressa) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF BENEFITS,
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technology guidance SCOPE The UroLift system for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia 1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technology guidance SCOPE The UroLift system for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia 1
50% of men. 90% of men PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS. Want more information? What are the symptoms?
 PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
PATIENT FACTSHEET: BPH CONDITION AND TREATMENTS What is Benign Prostatic Hyperplasia (enlarged prostate)? Benign prostatic hyperplasia (BPH) is a noncancerous enlargement of the prostate, the gland that
Subject: Image-Guided Radiation Therapy
 04-77260-19 Original Effective Date: 02/15/10 Reviewed: 01/25/18 Revised: 01/01/19 Subject: Image-Guided Radiation Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
04-77260-19 Original Effective Date: 02/15/10 Reviewed: 01/25/18 Revised: 01/01/19 Subject: Image-Guided Radiation Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Index. urologic.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
Index Note: Page numbers of article titles are in boldface type. A Ablative therapies, transurethral needle ablation, Adverse events, sexual side effects of BPH Aging, and incidence of BPH associated with
Cooled ThermoTherapy TM
 Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
Are BPH Medications Weighing You Down? Think Outside the Pillbox Cooled ThermoTherapy TM A non-surgical, 30 minute in-office treatment that provides long-term relief from BPH symptoms and urinary obstruction
Clinical Policy: Posterior Tibial Nerve Stimulation for Voiding Dysfunction Reference Number: CP.MP.133
 Clinical Policy: Reference Number: CP.MP.133 Effective Date: 10/16 Last Review Date: 10/16 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Clinical Policy: Reference Number: CP.MP.133 Effective Date: 10/16 Last Review Date: 10/16 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Subject: Functional Neuromuscular Stimulation
 09-E0000-54 Original Effective Date: 04/15/02 Reviewed: 08/23/18 Revised: 09/15/18 Subject: Functional Neuromuscular Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-E0000-54 Original Effective Date: 04/15/02 Reviewed: 08/23/18 Revised: 09/15/18 Subject: Functional Neuromuscular Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
MEDICAL POLICY SUBJECT: URINARY TUMOR MARKERS FOR BLADDER CANCER. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: URINARY TUMOR MARKERS FOR PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: URINARY TUMOR MARKERS FOR PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA. A Minimally Invasive Innovative Treatment
 PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
Subject: Pegvaliase-pqpz (Palynziq )
 09-J3000-07 Original Effective Date: 09/15/18 Reviewed: 08/08/18 Revised: 10/15/18 Subject: Pegvaliase-pqpz (Palynziq ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J3000-07 Original Effective Date: 09/15/18 Reviewed: 08/08/18 Revised: 10/15/18 Subject: Pegvaliase-pqpz (Palynziq ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Benign Prostatic Hyperplasia (BPH):
 Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Benign Prostatic Hyperplasia (BPH): Evidence Based Guidelines for Primary Care Providers Jeanne Martin, DNP, ANP-BC Objectives 1. Understand the pathophysiology and prevalence of BPH 2. Select the appropriate
Subject: Laboratory Tests for Heart and Kidney Transplant Rejection
 05-86000-24 Original Effective Date: 03/15/05 Reviewed: 12/06/18 Revised: 12/15/18 Subject: Laboratory Tests for Heart and Kidney Transplant Rejection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
05-86000-24 Original Effective Date: 03/15/05 Reviewed: 12/06/18 Revised: 12/15/18 Subject: Laboratory Tests for Heart and Kidney Transplant Rejection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Subject: Cannabidiol (Epidiolex )
 09-J3000-08 Original Effective Date: 09/15/18 Reviewed: 08/08/18 Revised: 00/00/00 Next Review: 04/10/19 Subject: Cannabidiol (Epidiolex ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-08 Original Effective Date: 09/15/18 Reviewed: 08/08/18 Revised: 00/00/00 Next Review: 04/10/19 Subject: Cannabidiol (Epidiolex ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Benign Prostatic Hyperplasia (BPH) Treatments
 Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0159 Benign Prostatic Hyperplasia (BPH) Treatments Table of Contents Related Coverage Resources
Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0159 Benign Prostatic Hyperplasia (BPH) Treatments Table of Contents Related Coverage Resources
Medical technologies guidance Published: 16 September 2015 nice.org.uk/guidance/mtg26
 UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia Medical technologies guidance Published: 16 September 2015 nice.org.uk/guidance/mtg26 NICE 2018. All rights reserved. Subject
UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia Medical technologies guidance Published: 16 September 2015 nice.org.uk/guidance/mtg26 NICE 2018. All rights reserved. Subject
Peter T. Chin, Damien M. Bolton, Greg Jack, Prem Rashid, Jeffrey Thavaseelan, R. James Yu, Claus G. Roehrborn, and Henry H. Woo
 Rapid Communication Prostatic Urethral Lift: Two-year Results After Treatment for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia Peter T. Chin, Damien M. Bolton, Greg Jack, Prem
Rapid Communication Prostatic Urethral Lift: Two-year Results After Treatment for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia Peter T. Chin, Damien M. Bolton, Greg Jack, Prem
Subject: Sacral Nerve Neuromodulation/Stimulation
 02-61000-23 Original Effective Date: 01/01/01 Reviewed: 06/28/18 Revised: 01/01/19 Subject: Sacral Nerve Neuromodulation/Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
02-61000-23 Original Effective Date: 01/01/01 Reviewed: 06/28/18 Revised: 01/01/19 Subject: Sacral Nerve Neuromodulation/Stimulation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Subject: Ondansetron HCl (Zofran ) Injection
 09-J0000-98 Original Effective Date: 05/15/09 Reviewed: 07/10/13 Revised: 11/01/15 Subject: Ondansetron HCl (Zofran ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-98 Original Effective Date: 05/15/09 Reviewed: 07/10/13 Revised: 11/01/15 Subject: Ondansetron HCl (Zofran ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Subject: Infrared Energy Therapy and Low Level Laser Therapy
 09-E0000-44 Original Effective Date: 08/15/03 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Infrared Energy Therapy and Low Level Laser Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-44 Original Effective Date: 08/15/03 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Infrared Energy Therapy and Low Level Laser Therapy THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Results. Conclusion. Keywords
 Functional Urology Multicentre prospective crossover study of the prostatic urethral lift for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia Anthony L. Cantwell,
Functional Urology Multicentre prospective crossover study of the prostatic urethral lift for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia Anthony L. Cantwell,
Stone Management Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Durability of the Prostatic Urethral Lift: 2-Year Results of the L.I.F.T. Study
 urologypracticejournal.com Durability of the Prostatic Urethral Lift: 2-Year Results of the L.I.F.T. Study Claus G. Roehrborn,*,y Steven N. Gange, y Neal D. Shore, Jonathan L. Giddens, Damien M. Bolton,
urologypracticejournal.com Durability of the Prostatic Urethral Lift: 2-Year Results of the L.I.F.T. Study Claus G. Roehrborn,*,y Steven N. Gange, y Neal D. Shore, Jonathan L. Giddens, Damien M. Bolton,
Corporate Medical Policy
 Corporate Medical Policy Saturation Biopsy for Diagnosis, Staging, and Management of Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_ staging_and_management_of_prostate_cancer
Corporate Medical Policy Saturation Biopsy for Diagnosis, Staging, and Management of Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_ staging_and_management_of_prostate_cancer
PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA
 St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
St. Louis Hospital PROSTATIC EMBOLIZATION FOR BENIGN HYPERPLASIA INITIAL CLINICAL RESULTS Faculty of Medical Sciences New University of Lisbon JOÃO PISCO LUÍS CAMPOS PINHEIRO TIAGO BILHIM HUGO RIO TINTO
Subject: Belinostat (Beleodaq ) Injection
 09-J2000-21 Original Effective Date: 11/15/14 Reviewed: 01/09/19 Revised: 02/15/19 Subject: Belinostat (Beleodaq ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J2000-21 Original Effective Date: 11/15/14 Reviewed: 01/09/19 Revised: 02/15/19 Subject: Belinostat (Beleodaq ) Injection THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Product Name or Headline
 Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Product Name or Headline Subhead goes here Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bronchial_thermoplasty 10/2010 3/2018 3/2019 3/2018 Description of Procedure or Service Bronchial thermoplasty
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bronchial_thermoplasty 10/2010 3/2018 3/2019 3/2018 Description of Procedure or Service Bronchial thermoplasty
Prostatic urethral lift: a novel approach for managing symptomatic BPH in the aging man Introduction: Materials and methods: Conclusion: Results:
 Prostatic urethral lift: a novel approach for managing symptomatic BPH in the aging man Daniel B. Rukstalis, MD Department of Urology, Wake Forest University School of Medicine, Winston-Salem, North Carolina,
Prostatic urethral lift: a novel approach for managing symptomatic BPH in the aging man Daniel B. Rukstalis, MD Department of Urology, Wake Forest University School of Medicine, Winston-Salem, North Carolina,
Benign Prostatic Hyperplasia. Jay Lee, MD, FRCSC Clinical Associate Professor University of Calgary
 Benign Prostatic Hyperplasia Jay Lee, MD, FRCSC Clinical Associate Professor University of Calgary Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied,
Benign Prostatic Hyperplasia Jay Lee, MD, FRCSC Clinical Associate Professor University of Calgary Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied,
Management of LUTS after TURP and MIT
 Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
Management of LUTS after TURP and MIT Hong Sup Kim Konkuk University TURP & MIT TURP : Gold standard MIT TUIP TUNA TUMT HIFU LASER Nd:YAG, ILC, HoLRP, KTP LUTS after TURP and MIT Improved : about 70% Persistent
RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, PATIENTS WITH BENIGN PROSTATIC HYPERPLASIA
 American Urological Association symptom index for BPH RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, AND DISEASE-SPECIFIC QUALITY OF LIFE QUESTION IN PATIENTS WITH
American Urological Association symptom index for BPH RELATIONSHIPS BETWEEN AMERICAN UROLOGICAL ASSOCIATION SYMPTOM INDEX, PROSTATE VOLUME, AND DISEASE-SPECIFIC QUALITY OF LIFE QUESTION IN PATIENTS WITH
What is the ICD 10 code for indwelling Foley catheter
 What is the ICD 10 code for indwelling Foley catheter 2018 ICD-10 code for Encounter for fitting and adjustment of urinary device is Z46.6. indwelling catheter - Z46.6 stent. Sep 6, 2017. Can someone to
What is the ICD 10 code for indwelling Foley catheter 2018 ICD-10 code for Encounter for fitting and adjustment of urinary device is Z46.6. indwelling catheter - Z46.6 stent. Sep 6, 2017. Can someone to
MODULE 3: BENIGN PROSTATIC HYPERTROPHY
 MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student
Experience the Innovative Therapy for Benign Prostate Enlargement
 Experience the Innovative Therapy for Benign Prostate Enlargement A Guide to Treatment of Benign Prostatic Hyperplasia 1. 2. The Prostate The prostate gland is a part of the male reproductive system. A
Experience the Innovative Therapy for Benign Prostate Enlargement A Guide to Treatment of Benign Prostatic Hyperplasia 1. 2. The Prostate The prostate gland is a part of the male reproductive system. A
cryosurgical_ablation_of_miscellaneous_solid_tumors 1/2007 5/2017 5/2018 5/2017
 Corporate Medical Policy Cryosurgical Ablation of Miscellaneous Solid Tumors Other File Name: Origination: Last CAP Review: Next CAP Review: Last Review: cryosurgical_ablation_of_miscellaneous_solid_tumors
Corporate Medical Policy Cryosurgical Ablation of Miscellaneous Solid Tumors Other File Name: Origination: Last CAP Review: Next CAP Review: Last Review: cryosurgical_ablation_of_miscellaneous_solid_tumors
Medical Technologies Evaluation Programme
 Medical Technologies Evaluation Programme MT 241 - UroLift System for the treatment of symptoms of urinary outflow obstruction secondary to benign prostatic hyperplasia Expert Adviser Questionnaire Responses
Medical Technologies Evaluation Programme MT 241 - UroLift System for the treatment of symptoms of urinary outflow obstruction secondary to benign prostatic hyperplasia Expert Adviser Questionnaire Responses
Subject: Endovascular Stent Grafts for Disorders of the Thoracic Aorta
 02-33000-29 Original Effective Date: 04/15/03 Reviewed: 07/26/18 Revised: 08/15/18 Subject: Endovascular Stent Grafts for Disorders of the Thoracic Aorta THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
02-33000-29 Original Effective Date: 04/15/03 Reviewed: 07/26/18 Revised: 08/15/18 Subject: Endovascular Stent Grafts for Disorders of the Thoracic Aorta THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH
 MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
MANAGING BENIGN PROSTATIC HYPERTROPHY IN PRIMARY CARE DR GEORGE G MATHEW CONSULTANT FAMILY PHYSICIAN FELLOW IN SEXUAL & REPRODUCTIVE HEALTH INTRODUCTION (1) Part of male sexual reproductive organ Size
The potential for NX-1207 in benign prostatic hyperplasia: an update for clinicians
 Therapeutic Advances in Chronic Disease Review The potential for NX-1207 in benign prostatic hyperplasia: an update for clinicians Neal Shore and Barrett Cowan Ther Adv Chronic Dis (2011) 2(6) 377 383
Therapeutic Advances in Chronic Disease Review The potential for NX-1207 in benign prostatic hyperplasia: an update for clinicians Neal Shore and Barrett Cowan Ther Adv Chronic Dis (2011) 2(6) 377 383
Men s Health Coding & Payment Quick Reference
 Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements. The coding options listed within this guide are commonly used codes
Corporate Medical Policy
 Corporate Medical Policy Vesicoureteral Reflux, Treatment with Periureteral Bulking Agents File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vesicoureteral_reflux_treatment_with_periureteral_bulking_agents
Corporate Medical Policy Vesicoureteral Reflux, Treatment with Periureteral Bulking Agents File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vesicoureteral_reflux_treatment_with_periureteral_bulking_agents
UroLift for treating the symptoms of benign prostatic hyperplasia
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance Assessment report overview UroLift for treating the symptoms of benign prostatic hyperplasia This assessment report overview
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance Assessment report overview UroLift for treating the symptoms of benign prostatic hyperplasia This assessment report overview
Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH
 Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH The more you know, the better you ll feel. You ve likely had a discussion with your doctor about BPH 1. What follows are some
Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH The more you know, the better you ll feel. You ve likely had a discussion with your doctor about BPH 1. What follows are some
Control & confidence. You deserve both.
 Learn more about BPH and Plasma therapy Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH Your doctor is always happy to offer all the guidance you need so that you feel completely
Learn more about BPH and Plasma therapy Control & confidence. You deserve both. YOUR GUIDE TO THE TREATMENT OF BPH Your doctor is always happy to offer all the guidance you need so that you feel completely
Subject: Neuromuscular Electrical Stimulation (NMES)
 09-E0000-25 Original Effective Date: 09/15/02 Reviewed: 08/23/18 Revised: 09/15/18 Subject: Neuromuscular Electrical Stimulation (NMES) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-E0000-25 Original Effective Date: 09/15/02 Reviewed: 08/23/18 Revised: 09/15/18 Subject: Neuromuscular Electrical Stimulation (NMES) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Subject: Vestibular Rehabilitation
 01-92502-14 Original Effective Date: 06/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Vestibular Rehabilitation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
01-92502-14 Original Effective Date: 06/15/05 Reviewed: 09/27/18 Revised: 10/15/18 Subject: Vestibular Rehabilitation THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Benign Prostatic Hyperplasia (BPH)
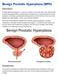 Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
Benign Prostatic Hyperplasia (BPH) Definition Prostate gland enlargement is a common condition as men get older. Also called benign prostatic hyperplasia (BPH), prostate gland enlargement can cause bothersome
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vagus_nerve_stimulation 6/1998 5/2017 5/2018 5/2017 Description of Procedure or Service Stimulation of the
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vagus_nerve_stimulation 6/1998 5/2017 5/2018 5/2017 Description of Procedure or Service Stimulation of the
Rezum: A New Non Invasive Promise for Benign Prostate Hyperplasia Treatment
 Human Journals Review Article November 2018 Vol.:13, Issue:4 All rights are reserved by Babitha. M et al. Rezum: A New Non Invasive Promise for Benign Prostate Hyperplasia Treatment Keywords: BPH, LUTS,
Human Journals Review Article November 2018 Vol.:13, Issue:4 All rights are reserved by Babitha. M et al. Rezum: A New Non Invasive Promise for Benign Prostate Hyperplasia Treatment Keywords: BPH, LUTS,
tens_(transcutaneous_electrical_nerve_stimulator) 7/ / / /2014 This policy is NOT effective until January 13, 2015
 Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Subject: Ultrasound Osteogenesis Stimulators, Non Invasive
 Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Corporate Medical Policy
 Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy TENS (Transcutaneous Electrical Nerve Stimulator) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: tens_(transcutaneous_electrical_nerve_stimulator) 7/1982
Corporate Medical Policy Automated Percutaneous and Endoscopic Discectomy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: percutaneous_discectomy 9/1991 5/2017 5/2018 5/2017 Description of Procedure or Service Surgical management
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: percutaneous_discectomy 9/1991 5/2017 5/2018 5/2017 Description of Procedure or Service Surgical management
Transurethral microwave thermotherapy
 Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Procedure 11 CLINICAL PRIVILEGE WHITE PAPER Transurethral microwave thermotherapy Background Transurethral resection of the prostate (TURP) has become the primary choice for the treatment of benign prostatic
Medical AHIMA-CCS. Medical Coding Specialist.
 Medical AHIMA-CCS Medical Coding Specialist http://killexams.com/exam-detail/ahima-ccs Question: 63 HCPCS Level II codes are updated every quarter by: A. CMS B. Medicaid C. Tricare D. Commercial payers
Medical AHIMA-CCS Medical Coding Specialist http://killexams.com/exam-detail/ahima-ccs Question: 63 HCPCS Level II codes are updated every quarter by: A. CMS B. Medicaid C. Tricare D. Commercial payers
P ROLIEVE. Thermodilatation System. The Prolieve System Patient Information is Directed to You, the Patient. A Transurethral Microwave Therapy Device
 P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
Subject: Cobimetinib (Cotellic ) Tablet
 09-J2000-53 Original Effective Date: 03/15/16 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Cobimetinib (Cotellic ) Tablet THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J2000-53 Original Effective Date: 03/15/16 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Cobimetinib (Cotellic ) Tablet THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
RATIONALE: The organs making up the urinary system consist of the kidneys, bladder, urethra, and ureters.
 Chapter 12 Section Review 12.1 1. A. Kidneys RATIONALE: The renal pelvis receives urine from the kidney, travels through the ureters on the way to the bladder, but urine is formed in the kidney. 2. C.
Chapter 12 Section Review 12.1 1. A. Kidneys RATIONALE: The renal pelvis receives urine from the kidney, travels through the ureters on the way to the bladder, but urine is formed in the kidney. 2. C.
EAU GUIDELINES POCKET EDITION 3
 EAU GUIDELINES POCKET EDITION 3 CONTENTS: BENIGN PROSTATIC HYPERPLASIA URINARY INCONTINENCE UROLITHIASIS 2 3 EAU POCKET GUIDELINES POCKET EDITION 3 This is one of a series of convenient pocket size books
EAU GUIDELINES POCKET EDITION 3 CONTENTS: BENIGN PROSTATIC HYPERPLASIA URINARY INCONTINENCE UROLITHIASIS 2 3 EAU POCKET GUIDELINES POCKET EDITION 3 This is one of a series of convenient pocket size books
Medifocus, Inc. OTCQB: MDFZF TSXV: MFS
 Medifocus, Inc. OTCQB: MDFZF TSXV: MFS www.medifocusinc.com A Leader in Focused Heat Thermotherapy for the Treatment of Benign Prostatic Hyperplasia and Breast Cancer Except for historical information,
Medifocus, Inc. OTCQB: MDFZF TSXV: MFS www.medifocusinc.com A Leader in Focused Heat Thermotherapy for the Treatment of Benign Prostatic Hyperplasia and Breast Cancer Except for historical information,
UroLift for treating lower urinary tract
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology consultation document UroLift for treating lower urinary tract The National Institute for Health and Care Excellence (NICE) is producing
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology consultation document UroLift for treating lower urinary tract The National Institute for Health and Care Excellence (NICE) is producing
Clinical Policy: Essure Removal Reference Number: CP.MP.131
 Clinical Policy: Reference Number: CP.MP.131 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.131 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
