Comments on CCNFSDU CODEX ALIMENTARIUS
|
|
|
- Franklin Gibbs
- 5 years ago
- Views:
Transcription
1 Comments on CCNFSDU CODEX ALIMENTARIUS Dear Dr. Yetley: Written by Scott C. Tips Category: Codex Published: September 2002 BY & FAX TO (202) Dr. Elizabeth Yetley # U.S. Codex Office Room 4861, South Building Washington, D.C Re: Comments on CCNFSDU CODEX ALIMENTARIUS On behalf of the National Health Federation, the nation's oldest nonprofit organization dedicated to ensuring consumers' rights to freedom of choice in food, dietary-supplement and medical matters, we respectfully submit the following comments on the draft guidelines of the Codex Committee on Nutrition and Foods for Special Dietary Uses, due August 23, 2002:/ 1. "Positive Lists" for Vitamins & Minerals: While superficially attractive, "positive lists" of "approved" vitamins and minerals, are counterproductive, obsolete before they can even be implemented, and illegal under United States law. Therefore, you have no choice but to strenuously oppose any and all implementation of "positive lists" within the Codex Committee system. a. Counterproductive. The so-called "positive lists" are counterproductive because they will mislead consumers and governmental bodies into thinking that only those vitamins and minerals appearing on the list are safe and acceptable./ Eventually, if not more immediately, such a list will become the basis of law that only those vitamins and minerals appearing on this list will be allowed to be lawfully sold. Many vitamins and minerals, or other associated nutrients and co-factors, especially those yet to be fully investigated or even discovered, will not appear on this list because the committee process (particularly the international committee process) will be slow, arduous, and subject to arbitrary dispute by those countries with, frankly, political agendas and/or insufficient sophistication in food matters to support their inclusion. Vitamins and minerals that might otherwise help people nutritionally will be omitted from the list, either forever or for sufficiently long periods of time so as to negatively impact consumers' health. b. Obsolete Before Publication. Because of the slow process mentioned above in implementing and then publicizing such a list, the current accelerating pace of advances
2 in knowledge of clinical nutrition will make such a list obsolete before it is even fixed and published. Therefore, such a list will be not only counterproductive but backward. It will be the same spirit as mandating gas-lighting standards during the time that electrical lighting was being introduced. Knowledge is not static and what we know today about clinical nutrition is far beyond the knowledge we possessed even in 1985, slightly more than fifteen years ago. And in 15 years' time, today's knowledge on the subject will appear equally quaint. For this reason, as both a practical matter and a philosophical approach, the free market, not agency edict, is the best mechanism here for maximizing the health of the public. c. Illegal. The Dietary Supplement Health and Education Act of 1994 ("DSHEA") as well as the anti-harmonization provisions of the FDA Modernization Act of 1997 prohibit positive lists of approved vitamins and minerals. Vitamins and minerals are not "approved" as are drugs; rather, they exist and, except for newly discovered vitamins, they can be freely sold within the United States as dietary supplements provided that they are appropriately labeled and make no disease claims. The publication and use of a positive list of vitamins and minerals would be inconsistent with American law in this regard by its creation of a two-tier system of "approved" vitamins and minerals and "non-approved" vitamins and minerals. Furthermore, the anti-harmonization provisions of the FDA Modernization Act of 1997 prohibit the Food and Drug Administration from engaging in any action that would subvert DSHEA and/or other existing American law. Agreeing and committing the United States government to such a list would accordingly violate U.S. law. 2. "Negative Lists" for Vitamins & Minerals: Not even superficially attractive, the socalled "negative lists" for prohibited vitamins and minerals have all of the problems mentioned for positive lists. They are counterproductive, obsolete before they can even be implemented, and illegal under United States law. Moreover, negative lists would especially invite abuse, since they would proscribe certain vitamins and minerals, perhaps at certain levels, based upon data that is in dispute. Indeed, even the Food and Drug Administration has yet to define for the Pearson v. Shalala Court the term "substantial scientific agreement." 3. Upper & Lower Potency Limits for Vitamins & Minerals: Subsumed within the positive and negative lists are presumed upper and lower limits for vitamins and minerals. Such limits would suffer from all of the above-mentioned problems and illegalities. It would be exactly the same as bureaucratically prescribing the techniques for manufacturing early airplanes from the 1910s; knowledge advances but the rules governing such prior knowledge, being less elastic, retard the progress of knowledge and, hence, society in general. Most importantly, United States law flatly prohibits the Secretary from imposing maximum limits on the potency of safe vitamins and minerals. (See the "Proxmire" Amendment of 1976, Pub. L. No , 501, 90 Stat. 410.) Read in juxtaposition with
3 the FDA Modernization Act of 1997, this Amendment completely prevents you from agreeing to any maximum limits on vitamin-and-mineral potency, no matter how well meaning or based your intentions might be. You have no choice but to reject upper limits. Moreover, the practical problem with upper limits is self-evident. First of all, if they are based on common European misconceptions, then they will be far to low to be efficacious in any genuine respect. We rather suspect that that is the true intent. Assuming the best, however, that is, that the motives are sincere, then the concept of upper limits on vitamins and minerals is still greatly misguided because they will be based upon RDIs that were created to avoid deficiencies in those particular vitamins and minerals in general populations, not with the goal of maximizing health in individuals. Those are two very different goals. Lower limits for vitamins "sound" as if they might be a valid concept, but when you consider the effect, you will also realize that, however well-intentioned, the effect will be equally counterproductive. Consider multivitamin capsules or tablets that, of course, only have a finite amount of capacity available for filling. If a lower limit has been set, but inadequate space remains in which one may fill that space with a particular vitamin or mineral, then the manufacturer must omit that ingredient and substitute a useless filler or excipient instead. The result: the consumer will have lost out on receiving at least some of an important nutrient. Under the philosophy that something is better than nothing, the argument is made here that the consumer will have suffered a loss. It would be indefensible for you to say that you are "protecting" the consumers' health by causing manufacturers' to omit healthful ingredients from their products. Rather, if a genuine concern exists about consumers being misled by their intake amount of a particular vitamin or mineral, then the level can be clearly and adequately disclosed on the product label. That is a situation that already exists and is already addressed with current label laws and regulations. 4. National Authorities Determination of Whether Vitamins & Minerals May be Treated as "Foods" of "Drugs" (Agenda Item 5): The proposed draft guidelines make it clear that most of the Europeans would like vitamin and mineral supplements to be tightly regulated and not to be sold in a free and open market. Therefore, right out of the chute, the draft guidelines are heavily biased to the restrictive European viewpoint: if a country's laws treat vitamin and mineral supplements as drugs, then the Codex guidelines would not apply to those supplements since the Codex guidelines are intended only for food. Therefore, the precious European national laws making drugs out of natural vitamins and minerals would not be touched. The only touchable laws would be those food and dietary supplement laws (such as in the U.S.) that treat vitamins and minerals with actual concern for consumer freedom of choice. The playing field has thus been ipso facto unfairly defined. 5. Substances Must Prove Their Nutritive Value for Humans Before They Can Be Acceptable (Agenda Item 5): The National Health Federation absolutely opposes any provision that would revise the Composition section of the Proposed Draft Guidelines for Vitamin and Mineral Supplements to indicate that substances would only be acceptable
4 if scientific data had proven their nutritive value for human beings and if criteria such as safety and bioavailability were considered in their selection. Such a provision would be absolutely insane! The National Health Federation cannot even believe that anyone would be so ignorant as to propose such a provision. If someone must first prove the "value" of a substance to humans before it can even be used, then the currently feeble knowledge of humans and incomplete understanding of dietary substances will prevent many useful and important nutritional substances from being available to nourish us until human knowledge catches up with reality. And that may never occur. Moreover, once again we must decide upon what constitutes "value" and how that term is defined. This whole area is a veritable minefield of disasters. You not only should, but you must, fight against any such limiting provision. To do otherwise is to betray your duty to Americans to protect their health. 6. General Comments About Nutrient-Content Claims (Agenda Item 10): The National Health Federation's position is that all dietary supplements, including vitamins and minerals, should be permitted to have labels and labeling that advise consumers of truthful and non misleading information about the product. We know that the official U.S. position has been to push for limits and lists based upon "science-based risk assessment" methods. The question, though, is upon whose "science" will this science-based risk assessment be based? One of the risks in adopting such science-based risk assessment standards is that they will not be fair and objective, but will instead be used to create artificial barriers that will only restrict freedom of choice. And compliance with those standards could be equally difficult if lengthy, expensive, drug-like tests, trials, and clinical studies must first be conducted before the standards are established and implemented. Either way, United States law will be broken if dietary supplements are required to comply with standards different than those already set forth in DSHEA. While there is merit to the claim that the Europeans would be better off with vitamin-and-mineral potencies based upon a science-based risk assessment standard rather than their current, completely arbitrary standard, the Food and Drug Administration's first priority is not to convert foreign agencies to American practices but rather to safeguard American health based upon American law. 7. Conclusion: These comments are relatively general in nature and intended as an overview of the Federation's positions on the subject. Nevertheless, the U.S. position absolutely must be one that stresses the importance of consumer choice and access to vitamin and mineral supplements. Furthermore, you are bound by United States law to reject any lists or limits on vitamins and minerals or other dietary supplements. You cannot commit the United States to being a party to any agreement or protocol that would foist such dietary restrictions upon the United States. I have been disappointed that during the last Codex meeting in Berlin in November 2001, none of the comments or suggestions that I made to you concerning the above were considered or implemented. Rather, the United States
5 delegate's approach was to compromise away our rights and, in doing so, to violate American law. The United States' delegate must re-think its Codex position and follow American law. Sincerely yours, 2002 Scott C. Tips SCT:mm cc: Ms. Maureen Kennedy Salaman, NHF President
American Holistic Health Association Position Paper November 2004 Codex Meeting
 American Holistic Health Association Position Paper November 2004 Codex Meeting Written by American Holistic Health Association Category: Codex Published: 9 September 2004 The following are the AHHA comments
American Holistic Health Association Position Paper November 2004 Codex Meeting Written by American Holistic Health Association Category: Codex Published: 9 September 2004 The following are the AHHA comments
Codex Alimentarius and the US Dietary Supplement Industry. Mark A. Le Doux, Chairman and CEO Natural Alternatives International, Inc.
 Codex Alimentarius and the US Dietary Supplement Industry Mark A. Le Doux, Chairman and CEO Natural Alternatives International, Inc. UNDERSTANDING THE CODEX ALIMENTARIUS Since the first steps were taken
Codex Alimentarius and the US Dietary Supplement Industry Mark A. Le Doux, Chairman and CEO Natural Alternatives International, Inc. UNDERSTANDING THE CODEX ALIMENTARIUS Since the first steps were taken
RE: Permits under the Arizona Pharmacy Act should not be required for dietary supplements
 Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
Codex Alimentarius and dietary supplements, April 2005
 Many companies that sell herbal products in the U.S. and internationally have contacted AHPA 1 in the past several weeks to ask questions about the Codex Alimentarius Commission. Numerous consumers of
Many companies that sell herbal products in the U.S. and internationally have contacted AHPA 1 in the past several weeks to ask questions about the Codex Alimentarius Commission. Numerous consumers of
Nutrition Labeling Laws for Food and Supplements Timeline
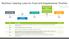 Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
 UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Citizen Petition to: Margaret A. Hamburg, M.D, Commissioner of Food and Drugs Docket No. For Review of Standard of Identity
UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Citizen Petition to: Margaret A. Hamburg, M.D, Commissioner of Food and Drugs Docket No. For Review of Standard of Identity
Working Document prepared by the Commission services - does not prejudice the Commission's final decision 3/2/2014 COMMISSION STAFF WORKING DOCUMENT
 COMMISSION STAFF WORKING DOCUMENT on certain requirements for FSMPs [Supporting Document for the Expert Group meeting of 7 February 2014] Introduction Following the discussions in the Expert Group meeting
COMMISSION STAFF WORKING DOCUMENT on certain requirements for FSMPs [Supporting Document for the Expert Group meeting of 7 February 2014] Introduction Following the discussions in the Expert Group meeting
FDA s Nutrition Facts Panel and the Labeling of Added Sugars September 8, 2016 Bruce Silverglade Principal, OFW Law
 FDA s Nutrition Facts Panel and the Labeling of Added Sugars September 8, 2016 Bruce Silverglade Principal, OFW Law November 7, 2017 Sweetener Systems Conference bsilverglade@ofwlaw.com (1) 202 518 6316
FDA s Nutrition Facts Panel and the Labeling of Added Sugars September 8, 2016 Bruce Silverglade Principal, OFW Law November 7, 2017 Sweetener Systems Conference bsilverglade@ofwlaw.com (1) 202 518 6316
The Nutrition (Amendment) (EU Exit) Regulations 2018
 The Nutrition (Amendment) (EU Exit) Regulations 2018 A public consultation Contents Introduction... 3 Why we are consulting... 4 Nutrition and Health Claims... 6 Proposals... 6 Vitamins, minerals, and
The Nutrition (Amendment) (EU Exit) Regulations 2018 A public consultation Contents Introduction... 3 Why we are consulting... 4 Nutrition and Health Claims... 6 Proposals... 6 Vitamins, minerals, and
E-FORUM ON THE ROLE OF CODEX IN THE IMPLEMENTATION OF THE GLOBAL STRATEGY ON DIET, PHYSICAL ACTIVITY AND HEALTH
 E-FORUM ON THE ROLE OF CODEX IN THE IMPLEMENTATION OF THE GLOBAL STRATEGY ON DIET, PHYSICAL ACTIVITY AND HEALTH COMMENTS FROM THE U.S. DELEGATE TO THE CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL
E-FORUM ON THE ROLE OF CODEX IN THE IMPLEMENTATION OF THE GLOBAL STRATEGY ON DIET, PHYSICAL ACTIVITY AND HEALTH COMMENTS FROM THE U.S. DELEGATE TO THE CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL
FDA Regulation of Claims on Dietary Supplement and Food Products
 FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
Dagmar Roth-Behrendt Vice-President of the European Parliament
 1 Dagmar Roth-Behrendt Vice-President of the European Parliament Herbal medicinal products: new Committee & European dimension Meeting on 23 September 2004 at the European Medicines Agency (EMEA) in London
1 Dagmar Roth-Behrendt Vice-President of the European Parliament Herbal medicinal products: new Committee & European dimension Meeting on 23 September 2004 at the European Medicines Agency (EMEA) in London
perpetuate -- and perhaps even intensify -- that controversy. 1 On July 18th, the Fifth Circuit affirmed FDA s longstanding position that
 Food & Drug July 29, 2008 Fifth Circuit Rules that FDA May Regulate Compounded Drugs as New Drugs Update on Medical Center Pharmacy v. Mukasey For decades, the pharmacy compounding industry has disputed
Food & Drug July 29, 2008 Fifth Circuit Rules that FDA May Regulate Compounded Drugs as New Drugs Update on Medical Center Pharmacy v. Mukasey For decades, the pharmacy compounding industry has disputed
May 7, Dear Mr. Landa:
 Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Dietary Supplement Health and Education Act of 1994 Public Law rd Congress
 Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Respondent brews beer. In 1987, respondent applied to the Bureau of Alcohol, Tobacco
 ORubin v. Coors Brewing Company 514 U.S. 476 (1995) Justice Thomas: Respondent brews beer. In 1987, respondent applied to the Bureau of Alcohol, Tobacco and Firearms (BATF), an agency of the Department
ORubin v. Coors Brewing Company 514 U.S. 476 (1995) Justice Thomas: Respondent brews beer. In 1987, respondent applied to the Bureau of Alcohol, Tobacco and Firearms (BATF), an agency of the Department
Maia Jack, Ph.D. CPGglobal, LLC 2013 Food Additives USDA FAS EMP: A Global Perspective on Safety Evaluation and Use
 Codex Global Harmonization and Note 161 Maia Jack, Ph.D. CPGglobal, LLC 2013 Food Additives USDA FAS EMP: A Global Perspective on Safety Evaluation and Use Codex Alimentarius Overview of Codex Committee
Codex Global Harmonization and Note 161 Maia Jack, Ph.D. CPGglobal, LLC 2013 Food Additives USDA FAS EMP: A Global Perspective on Safety Evaluation and Use Codex Alimentarius Overview of Codex Committee
AGENDA ITEM NO. 7 CX/FL 08/36/10 E
 AGENDA ITEM NO. 7 E JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON FOOD LABELLING THIRTY-SIXTH SESSION OTTAWA, CANADA, APRIL 28 - MAY 2, 2008 DRAFT DEFINITION OF ADVERTISING IN RELATION TO NUTRITION
AGENDA ITEM NO. 7 E JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON FOOD LABELLING THIRTY-SIXTH SESSION OTTAWA, CANADA, APRIL 28 - MAY 2, 2008 DRAFT DEFINITION OF ADVERTISING IN RELATION TO NUTRITION
Discussion Paper on NUTRITION CLAIMS AND FUNCTIONAL CLAIMS
 SANCO/1341/2001 Discussion Paper on NUTRITION CLAIMS AND FUNCTIONAL CLAIMS Prepared by Directorate General Health and Consumer Protection (SANCO D4) European Commission http://europa.eu.int/comm/dgs/health_consumer/index_en.htm
SANCO/1341/2001 Discussion Paper on NUTRITION CLAIMS AND FUNCTIONAL CLAIMS Prepared by Directorate General Health and Consumer Protection (SANCO D4) European Commission http://europa.eu.int/comm/dgs/health_consumer/index_en.htm
The full opinion and all the legal papers are available at:
 Alliance for Open Society International v. USAID Questions and Answers About the August 8, 2008 Ruling Granting InterAction and Global Health Council a Preliminary Injunction A sweeping speech restriction
Alliance for Open Society International v. USAID Questions and Answers About the August 8, 2008 Ruling Granting InterAction and Global Health Council a Preliminary Injunction A sweeping speech restriction
EUROPEAN COMMISSION SUMMARY REPORT OF THE STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH HELD IN BRUSSELS ON 13 JUNE 2014
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Brussels, SANCO G ARES(2014)2253750 SUMMARY REPORT OF THE STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH HELD IN BRUSSELS ON 13 JUNE
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Brussels, SANCO G ARES(2014)2253750 SUMMARY REPORT OF THE STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH HELD IN BRUSSELS ON 13 JUNE
October 31, Draft Guidance for Industry, Frequently Asked Questions About Medical Foods; Second Edition
 October 31, 2013 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Rockville, MD 20852 Re: Docket No. FDA-2013-D-0880 Draft Guidance for
October 31, 2013 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Rockville, MD 20852 Re: Docket No. FDA-2013-D-0880 Draft Guidance for
Iowa District Court Polk County, Iowa. CARL OLSEN, ) ) Petitioner, ) ) vs. ) ) Docket No. CV IOWA BOARD OF PHARMACY ) ) Respondent.
 Iowa District Court Polk County, Iowa CARL OLSEN, ) ) Petitioner, ) ) vs. ) ) Docket No. CV 51068 IOWA BOARD OF PHARMACY ) ) Respondent. ) MEMORANDUM IN SUPPORT OF PETITION FOR JUDICIAL REVIEW Federal
Iowa District Court Polk County, Iowa CARL OLSEN, ) ) Petitioner, ) ) vs. ) ) Docket No. CV 51068 IOWA BOARD OF PHARMACY ) ) Respondent. ) MEMORANDUM IN SUPPORT OF PETITION FOR JUDICIAL REVIEW Federal
Module 34: Legal aspects, ADI and GRAS status of food additives
 Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
Revised Version Text of final Codex Vitamin and Mineral Guideline. Marked for Amendment. Deletions struck through; additions underlined.
 Revised Version Text of final Codex Vitamin and Mineral Guideline. Marked for Amendment Deletions struck through; additions underlined. Finalized by the Codex Alimentarius Commission July 4, 2005 Guidelines
Revised Version Text of final Codex Vitamin and Mineral Guideline. Marked for Amendment Deletions struck through; additions underlined. Finalized by the Codex Alimentarius Commission July 4, 2005 Guidelines
NDI: LOOKING BACK & AHEAD
 NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
NDI: LOOKING BACK & AHEAD Chi Hee Kim D i r e c t o r, W o r l d w i d e R e g u l a t o r y, G o v e r n m e n t a n d I n d u s t r y A f f a i r s H e r b a l i f e I n t e r n a t i o n a l o f A m
DEPARTMENT OF VETERANS AFFAIRS SUMMARY: The Department of Veterans Affairs (VA) is amending its medical
 This document is scheduled to be published in the Federal Register on 03/23/2017 and available online at https://federalregister.gov/d/2017-05799, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
This document is scheduled to be published in the Federal Register on 03/23/2017 and available online at https://federalregister.gov/d/2017-05799, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
December 4, 2017 VIA ELECTRONIC SUBMISSION
 VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
Re: Docket No. FDA D Presenting Risk Information in Prescription Drug and Medical Device Promotion
 1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
Office of University Counsel and Secretary of the Board of Regents
 To: From: University of Colorado Research Faculty President Bruce D. Benson University Counsel Patrick T. O Rourke Date: March 11, 2014 Re: Legality of Marijuana Research Colorado is one of twenty states
To: From: University of Colorado Research Faculty President Bruce D. Benson University Counsel Patrick T. O Rourke Date: March 11, 2014 Re: Legality of Marijuana Research Colorado is one of twenty states
GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS
 1 CAC/GL 23-1997 GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS CAC/GL 23-1997 Nutrition claims should be consistent with national nutrition policy and support that policy. Only nutrition claims that
1 CAC/GL 23-1997 GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS CAC/GL 23-1997 Nutrition claims should be consistent with national nutrition policy and support that policy. Only nutrition claims that
The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016
 INFORMATION NOTE INTENDED FOR KNOWLEDGE HUB The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016 This note is to alert you to the fact that the English Statutory
INFORMATION NOTE INTENDED FOR KNOWLEDGE HUB The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016 This note is to alert you to the fact that the English Statutory
COMMUNITY HOSPICE & PALLIATIVE CARE NOTICE OF PRIVACY PRACTICES
 COMMUNITY HOSPICE & PALLIATIVE CARE NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE
COMMUNITY HOSPICE & PALLIATIVE CARE NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE
COMMISSION DELEGATED REGULATION (EU).../... of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/10355/2015 (POOL/E4/2015/10355/10355-EN. doc) [...1(2015) XXX draft COMMISSION DELEGATED REGULATION (EU).../... of XXX supplementmg Regulation (EU) No 609/2013 of
EUROPEAN COMMISSION Brussels, XXX SANTE/10355/2015 (POOL/E4/2015/10355/10355-EN. doc) [...1(2015) XXX draft COMMISSION DELEGATED REGULATION (EU).../... of XXX supplementmg Regulation (EU) No 609/2013 of
Attn: Alicia Richmond Scott, Pain Management Task Force Designated Federal Officer
 March 18, 2019 Office of the Assistant Secretary of Health U.S. Department of Health and Human Services 200 Independence Avenue SW, Room 736E Washington, DC 20201 Attn: Alicia Richmond Scott, Pain Management
March 18, 2019 Office of the Assistant Secretary of Health U.S. Department of Health and Human Services 200 Independence Avenue SW, Room 736E Washington, DC 20201 Attn: Alicia Richmond Scott, Pain Management
Model Intervention for Students with Substance Abuse Problems Act
 Model Intervention for Students with Substance Abuse Problems Act MODEL INTERVENTION FOR STUDENTS WITH SUBSTANCE ABUSE PROBLEMS ACT Table of Contents G-103 Policy Statement G-105 Highlights Section One
Model Intervention for Students with Substance Abuse Problems Act MODEL INTERVENTION FOR STUDENTS WITH SUBSTANCE ABUSE PROBLEMS ACT Table of Contents G-103 Policy Statement G-105 Highlights Section One
Theoriginalhcgdrops.com 11/28/11
 Theoriginalhcgdrops.com 11/28/11 UNITED STATES OF AMERICA FEDERAL TRADE COMMISSION BUREAU OF CONSUMER PROTECTION WASHINGTON, D.C. 20580 DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
Theoriginalhcgdrops.com 11/28/11 UNITED STATES OF AMERICA FEDERAL TRADE COMMISSION BUREAU OF CONSUMER PROTECTION WASHINGTON, D.C. 20580 DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
WTO THEMATIC SESSION ON REGULATORY COOPERATION BETWEEN MEMBERS 9 NOVEMBER 2016 Codex Food Labelling Standards an Overview.
 WTO THEMATIC SESSION ON REGULATORY COOPERATION BETWEEN MEMBERS 9 NOVEMBER 2016 Codex Food Labelling Standards an Overview Codex Secretariat Background Codex texts are recommendations for governments to
WTO THEMATIC SESSION ON REGULATORY COOPERATION BETWEEN MEMBERS 9 NOVEMBER 2016 Codex Food Labelling Standards an Overview Codex Secretariat Background Codex texts are recommendations for governments to
A. Concentrated Omega-3 Dietary Supplements Provide Recognized Health Benefits
 DEANNA TANNER OKUN Tel: (202) 407-8615 okun@adduci.com VIA ELECTRONIC FILING AND OVERNIGHT MAIL The Honorable Lisa R. Barton Secretary to the Commission United States International Trade Commission 500
DEANNA TANNER OKUN Tel: (202) 407-8615 okun@adduci.com VIA ELECTRONIC FILING AND OVERNIGHT MAIL The Honorable Lisa R. Barton Secretary to the Commission United States International Trade Commission 500
Food Standards should be Reviewed and Extended
 Term Paper Food Standards should be Reviewed and Extended By SS 07 ANR 490 / 811 Food Regulation in the United States Michigan State University April 2, 2007 I. Introduction The U.S. Congress passed the
Term Paper Food Standards should be Reviewed and Extended By SS 07 ANR 490 / 811 Food Regulation in the United States Michigan State University April 2, 2007 I. Introduction The U.S. Congress passed the
FAQ FOR THE NATA AND APTA JOINT STATEMENT ON COOPERATION: AN INTERPRETATION OF THE STATEMENT September 24, 2009
 FAQ FOR THE NATA AND APTA JOINT STATEMENT ON COOPERATION: AN INTERPRETATION OF THE STATEMENT September 24, 2009 Introduction The NATA considers the Joint Statement on Cooperation a very positive resolution
FAQ FOR THE NATA AND APTA JOINT STATEMENT ON COOPERATION: AN INTERPRETATION OF THE STATEMENT September 24, 2009 Introduction The NATA considers the Joint Statement on Cooperation a very positive resolution
June 9, U.S. Food and Drug Administration Division of Dockets Management, HFA Fishers Lane, Room 1061 Rockville, MD 20852
 June 9, 2014 U.S. Food and Drug Administration Division of Dockets Management, HFA-305 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Implementation of the Food and Drug Administration Food Safety
June 9, 2014 U.S. Food and Drug Administration Division of Dockets Management, HFA-305 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Implementation of the Food and Drug Administration Food Safety
CHE8000 Major Research Project A. The Complementary Medicines Reforms and Its Impact to the Complementary Medicine Industry and the Consumer
 CHE8000 Major Research Project A The Complementary Medicines Reforms and Its Impact to the Complementary Medicine Industry and the Consumer Completed by: Lina Karlina (ID: 2067226) Lina Karlina (2067226)
CHE8000 Major Research Project A The Complementary Medicines Reforms and Its Impact to the Complementary Medicine Industry and the Consumer Completed by: Lina Karlina (ID: 2067226) Lina Karlina (2067226)
SOUTH DAKOTA BOARD OF REGENTS. Policy Manual
 SUBJECT: Drug Free Environment NUMBER: 4:27 SOUTH DAKOTA BOARD OF REGENTS Policy Manual Drug Free Workplace Policy The South Dakota Board of Regents is committed to providing a drug free workplace. Additional
SUBJECT: Drug Free Environment NUMBER: 4:27 SOUTH DAKOTA BOARD OF REGENTS Policy Manual Drug Free Workplace Policy The South Dakota Board of Regents is committed to providing a drug free workplace. Additional
Policy on Alcohol at - CSU Channel IslandsCI
 Page 1 of 5 Policy on Alcohol at - CSU Channel IslandsCI PURPOSE: The University Alcohol Policy relates to all members of the campus community with the exception of tenants leasing space in the academic
Page 1 of 5 Policy on Alcohol at - CSU Channel IslandsCI PURPOSE: The University Alcohol Policy relates to all members of the campus community with the exception of tenants leasing space in the academic
Animal Products Notice
 Animal Products Notice Labelling Requirements for Exports of Dairy Based Infant Formula Products and Formulated Supplementary Food for Young Children 18 December 2014 An animal products notice issued under
Animal Products Notice Labelling Requirements for Exports of Dairy Based Infant Formula Products and Formulated Supplementary Food for Young Children 18 December 2014 An animal products notice issued under
Bill C-51 and Natural Health Products - The Facts
 Bill C-51 and Natural Health Products - The Facts 1. How will Bill C-51 change the way natural health products are regulated? Bill C-51 will not affect the way that natural health products are regulated
Bill C-51 and Natural Health Products - The Facts 1. How will Bill C-51 change the way natural health products are regulated? Bill C-51 will not affect the way that natural health products are regulated
STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH SECTION ON GENERAL FOOD LAW Summary Record of Meeting of 25 June 2007
 STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH SECTION ON GENERAL FOOD LAW Summary Record of Meeting of 25 June 2007 Chairman: Mr Basil Mathioudakis (except points 1 and 7) 1. Consultation paper
STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH SECTION ON GENERAL FOOD LAW Summary Record of Meeting of 25 June 2007 Chairman: Mr Basil Mathioudakis (except points 1 and 7) 1. Consultation paper
Statement regarding: Key ideas of a draft legal proposal on information to patients by the European Commission (DG Enterprise and Industry)
 STATEMENT 7 April 2008 European Commission Statement regarding: Key ideas of a draft legal proposal on information to patients by the European Commission (DG Enterprise and Industry) 1. Background history,
STATEMENT 7 April 2008 European Commission Statement regarding: Key ideas of a draft legal proposal on information to patients by the European Commission (DG Enterprise and Industry) 1. Background history,
January 29, Re: FNS Dear Sir or Madam:
 January 29, 2018 School Programs Branch, Policy and Program Development Division Food and Nutrition Service U.S. Department of Agriculture 3101 Park Center Drive, 12 th Floor Alexandria, Virginia 22302
January 29, 2018 School Programs Branch, Policy and Program Development Division Food and Nutrition Service U.S. Department of Agriculture 3101 Park Center Drive, 12 th Floor Alexandria, Virginia 22302
ALCOHOL POLICY FOR GRADUATE STUDENT EVENTS
 ALCOHOL POLICY FOR GRADUATE STUDENT EVENTS POLICY STATEMENT Yeshiva University is committed to creating and maintaining an environment that is free of alcohol abuse. The University expects that the consumption
ALCOHOL POLICY FOR GRADUATE STUDENT EVENTS POLICY STATEMENT Yeshiva University is committed to creating and maintaining an environment that is free of alcohol abuse. The University expects that the consumption
Research on the Administrative Rules of APIs, Pharmaceutical Excipients and Auxiliary Materials Master File. Translation version
 Research on the Administrative Rules of APIs, Pharmaceutical Excipients and Auxiliary Materials Master File Division of Pharmaceuticals Department of Drug Registration Hou Renping Translation version Main
Research on the Administrative Rules of APIs, Pharmaceutical Excipients and Auxiliary Materials Master File Division of Pharmaceuticals Department of Drug Registration Hou Renping Translation version Main
Preparing for an Oral Hearing: Taxi, Limousine or other PDV Applications
 Reference Sheet 12 Preparing for an Oral Hearing: Taxi, Limousine or other PDV Applications This Reference Sheet will help you prepare for an oral hearing before the Passenger Transportation Board. You
Reference Sheet 12 Preparing for an Oral Hearing: Taxi, Limousine or other PDV Applications This Reference Sheet will help you prepare for an oral hearing before the Passenger Transportation Board. You
SUMMARY REPORT OF THE STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH HELD IN BRUSSELS ON 10 DECEMBER 2012 (Section General Food Law)
 EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Brussels, SANCO E 1718316 SUMMARY REPORT OF THE STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH HELD IN BRUSSELS ON 10 DECEMBER 2012 (Section
EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Brussels, SANCO E 1718316 SUMMARY REPORT OF THE STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH HELD IN BRUSSELS ON 10 DECEMBER 2012 (Section
Ornsurang Teerawat
 ASEAN TRADITIONAL MEDICINES AND HEALTH SUPPLEMENTS REGULATORY AND HARMONIZATION UPDATE Ornsurang Teerawat 21.10.2014 \ http://trainstudenytp.wordpress.com ACCSQ (ASEAN Consultative Committee for Standard
ASEAN TRADITIONAL MEDICINES AND HEALTH SUPPLEMENTS REGULATORY AND HARMONIZATION UPDATE Ornsurang Teerawat 21.10.2014 \ http://trainstudenytp.wordpress.com ACCSQ (ASEAN Consultative Committee for Standard
In a world that respects individual
 by Jonathan W. Emord Codex Standards, the EU Directive and the Future of Health Freedom In a world that respects individual liberty, ingestible substances that cause no harm at recommended dose levels
by Jonathan W. Emord Codex Standards, the EU Directive and the Future of Health Freedom In a world that respects individual liberty, ingestible substances that cause no harm at recommended dose levels
Working Through The Haze: What Legal Marijuana Means For Nevada Employers
 Working Through The Haze: What Legal Marijuana Means For Nevada Employers Publication 12/14/2016 Dora Lane Partner 775.327.3045 Reno, Las Vegas dlane@hollandhart.com Anthony Hall Partner 775.327.3000 Reno,
Working Through The Haze: What Legal Marijuana Means For Nevada Employers Publication 12/14/2016 Dora Lane Partner 775.327.3045 Reno, Las Vegas dlane@hollandhart.com Anthony Hall Partner 775.327.3000 Reno,
Agenda Item 6 CX/NFSDU 08/30/6 September 2008 JOINT FAO/WHO FOOD STANDARDS PROGRAMME
 CX/NFSDU 08/30/6 1 Agenda Item 6 CX/NFSDU 08/30/6 September 2008 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL DIETARY USES Thirthieth Session Cape Town, South
CX/NFSDU 08/30/6 1 Agenda Item 6 CX/NFSDU 08/30/6 September 2008 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL DIETARY USES Thirthieth Session Cape Town, South
Draft Guidance for Industry: ) Evidence-Based Review System for the ) Docket No. 2007D-0125 Scientific Evaluation of Health Claims )
 Food and Drug Administration Division of Dockets Management (HFM-305) Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, Maryland 20852 Draft Guidance for Industry: ) Evidence-Based
Food and Drug Administration Division of Dockets Management (HFM-305) Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, Maryland 20852 Draft Guidance for Industry: ) Evidence-Based
METROLINX ADMINISTRATIVE FEE DISPUTE RESOLUTION PROCESS RULES OF PRACTICE
 METROLINX ADMINISTRATIVE FEE DISPUTE RESOLUTION PROCESS RULES OF PRACTICE Overview The Metrolinx Act, 2006, gives Metrolinx ( Metrolinx ) the authority to establish a system of administrative fees to ensure
METROLINX ADMINISTRATIVE FEE DISPUTE RESOLUTION PROCESS RULES OF PRACTICE Overview The Metrolinx Act, 2006, gives Metrolinx ( Metrolinx ) the authority to establish a system of administrative fees to ensure
IMPORTANT DISCLAIMER. Note
 yn EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL June 2012 DRAFT GUIDANCE DOCUMENT FOR COMPETENT AUTHORITIES FOR THE CONTROL OF COMPLIANCE WITH EU LEGISLATION ON: Regulation (EU) No 1169/2011
yn EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL June 2012 DRAFT GUIDANCE DOCUMENT FOR COMPETENT AUTHORITIES FOR THE CONTROL OF COMPLIANCE WITH EU LEGISLATION ON: Regulation (EU) No 1169/2011
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels
 SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
DEPARTMENT OF VETERANS AFFAIRS SUMMARY: The Department of Veterans Affairs (VA) proposes to amend its medical
 This document is scheduled to be published in the Federal Register on 08/05/2016 and available online at http://federalregister.gov/a/2016-18660, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
This document is scheduled to be published in the Federal Register on 08/05/2016 and available online at http://federalregister.gov/a/2016-18660, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
Canada would provide a proposed draft definition for consideration by the next session based on these comments.
 E Agenda Item 7 CX/FL 11/39/14-Rev1 JOINT FAO/WHO FOOD STANDARDS PROGRAM CODEX COMMITTEE ON FOOD LABELLING Thirty-ninth Session Québec City, Canada 9 13 May 2011 Proposed Draft Definition of Nutrient Reference
E Agenda Item 7 CX/FL 11/39/14-Rev1 JOINT FAO/WHO FOOD STANDARDS PROGRAM CODEX COMMITTEE ON FOOD LABELLING Thirty-ninth Session Québec City, Canada 9 13 May 2011 Proposed Draft Definition of Nutrient Reference
State of Connecticut Department of Education Division of Teaching and Learning Programs and Services Bureau of Special Education
 State of Connecticut Department of Education Division of Teaching and Learning Programs and Services Bureau of Special Education Introduction Steps to Protect a Child s Right to Special Education: Procedural
State of Connecticut Department of Education Division of Teaching and Learning Programs and Services Bureau of Special Education Introduction Steps to Protect a Child s Right to Special Education: Procedural
Angelman s Inc.
 STATE OF CALIFORNIA DEPARTMENT OF CALIFORNIA HIGHWAY PATROL CONTROLLED SUBSTANCES & ALCOHOL TESTING COMPLIANCE CHECKLIST DRIVER RECORDS - CONTROLLED SUBSTANCES AND ALCOHOL TESTING Do you ensure that all
STATE OF CALIFORNIA DEPARTMENT OF CALIFORNIA HIGHWAY PATROL CONTROLLED SUBSTANCES & ALCOHOL TESTING COMPLIANCE CHECKLIST DRIVER RECORDS - CONTROLLED SUBSTANCES AND ALCOHOL TESTING Do you ensure that all
December 29, Via Electronic Mail
 December 29, 2015 Via Electronic Mail Mr. Ted Elkin Deputy Director for Regulatory Affairs Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway Room 4B-006,
December 29, 2015 Via Electronic Mail Mr. Ted Elkin Deputy Director for Regulatory Affairs Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway Room 4B-006,
Introduction on the Regulations on Imported Nutrition Supplements of the Republic of China
 Introduction on the Regulations on Imported Nutrition Supplements of the Republic of China Preface With the aging of population in Taiwanese society, the people of Taiwan have become more health-conscious
Introduction on the Regulations on Imported Nutrition Supplements of the Republic of China Preface With the aging of population in Taiwanese society, the people of Taiwan have become more health-conscious
Discussion points on Bill S-5
 Disclaimer: The following document was developed to provide a brief set of discussion points for individuals wishing to discuss Bill S-5 with policy makers, politicians, senators or their advisers. It
Disclaimer: The following document was developed to provide a brief set of discussion points for individuals wishing to discuss Bill S-5 with policy makers, politicians, senators or their advisers. It
C. No employee shall report to work or remain on duty while having a detectable blood alcohol concentration.
 1 Series 3000 Personnel Section 3100 General Provisions Policy 3101 Drug Free Work Place File: 3101 3101.1 STATEMENT OF PURPOSE: The WV Board of Education and the Harrison County Board of Education recognize
1 Series 3000 Personnel Section 3100 General Provisions Policy 3101 Drug Free Work Place File: 3101 3101.1 STATEMENT OF PURPOSE: The WV Board of Education and the Harrison County Board of Education recognize
Reishi D. International, Inc. 2/6/18
 Reishi D. International, Inc. 2/6/18 San Francisco District Office 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Via UPS Overnight February 7, 2018 Mr. Zheng Xiong Li, CEO Reishi D. International, Inc.
Reishi D. International, Inc. 2/6/18 San Francisco District Office 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Via UPS Overnight February 7, 2018 Mr. Zheng Xiong Li, CEO Reishi D. International, Inc.
Joint FAO/WHO Food Standards Programme Food and Agriculture Organization of the United Nations Lusaka, Zambia, 3-5 July 2002
 FAO/WHO CODEX ALIMENTARIUS COMMISSION (CODEX) Joint FAO/WHO Food Standards Programme Food and Agriculture Organization of the United Nations Lusaka, Zambia, 3-5 July 2002 1. Introducing Codex Alimentarius
FAO/WHO CODEX ALIMENTARIUS COMMISSION (CODEX) Joint FAO/WHO Food Standards Programme Food and Agriculture Organization of the United Nations Lusaka, Zambia, 3-5 July 2002 1. Introducing Codex Alimentarius
Alberta - US Comparator: Standard-Making and Enforcement Functions
 Alberta - US Comparator: Standard-Making and Enforcement Functions Current Reliability Standards Below is a link to current reliability standards in Alberta: http://www.aeso.ca/rulesprocedures/17006.html
Alberta - US Comparator: Standard-Making and Enforcement Functions Current Reliability Standards Below is a link to current reliability standards in Alberta: http://www.aeso.ca/rulesprocedures/17006.html
GUIDELINES FOR MAKING PRODUCT CLAIMS
 GUIDELINES FOR MAKING PRODUCT CLAIMS This practical guide to making claims provides valuable information for Independent Business Owners (IBOS) to use in their everyday operations. It describes various
GUIDELINES FOR MAKING PRODUCT CLAIMS This practical guide to making claims provides valuable information for Independent Business Owners (IBOS) to use in their everyday operations. It describes various
Nutrition is Dangerous?
 Codex Alimentarius (Latin for food code ) is the proposed set of international guidelines for nutritional supplements, food handling, production, and trade which is now gradually being ratified in countries
Codex Alimentarius (Latin for food code ) is the proposed set of international guidelines for nutritional supplements, food handling, production, and trade which is now gradually being ratified in countries
COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE
 COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE June 13 2013 Version 3.0 i FOREWORD Guidance documents are meant to provide assistance to industry and health care practitioners on how to comply
COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE June 13 2013 Version 3.0 i FOREWORD Guidance documents are meant to provide assistance to industry and health care practitioners on how to comply
AIDS LAW PROJECT/TREATMENT ACTION CAMPAIGN: SUBMISSION ON THE REGULATIONS RELATING TO THE LABELLING AND ADVERTISING OF FOODSTUFFS 1
 AIDS LAW PROJECT/TREATMENT ACTION CAMPAIGN: SUBMISSION ON THE REGULATIONS RELATING TO THE LABELLING AND ADVERTISING OF FOODSTUFFS 1 The AIDS Law Project (ALP) and the Treatment Action Campaign (TAC) focus
AIDS LAW PROJECT/TREATMENT ACTION CAMPAIGN: SUBMISSION ON THE REGULATIONS RELATING TO THE LABELLING AND ADVERTISING OF FOODSTUFFS 1 The AIDS Law Project (ALP) and the Treatment Action Campaign (TAC) focus
COUNCIL OF THE EUROPEAN UNION. Brussels, 7 September 2009 (OR. en) 11261/09 Interinstitutional File: 2008/0002 (COD) DENLEG 51 CODEC 893
 COUNCIL OF THE EUROPEAN UNION Brussels, 7 September 2009 (OR. en) 11261/09 Interinstitutional File: 2008/0002 (COD) DLEG 51 CODEC 893 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: Common Position with
COUNCIL OF THE EUROPEAN UNION Brussels, 7 September 2009 (OR. en) 11261/09 Interinstitutional File: 2008/0002 (COD) DLEG 51 CODEC 893 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: Common Position with
Substance Abuse Policy. Substance Abuse Policy for Employees and Students
 College Rules and Regulations 2.2008.1 Substance Abuse Policy Substance Abuse Policy for Employees and Students I. Substance Abuse Policy for Employees and Students A. Purpose The County College of Morris
College Rules and Regulations 2.2008.1 Substance Abuse Policy Substance Abuse Policy for Employees and Students I. Substance Abuse Policy for Employees and Students A. Purpose The County College of Morris
on the approximation of the laws of the Member States concerning food additives authorized for use in foodstuffs intended for human consumption
 11. 2. 89 Official Journal of the European Communities No L 40/ 27 COUNCIL DIRECTIVE of 21 December 1988 on the approximation of the laws of the Member States concerning food additives authorized for use
11. 2. 89 Official Journal of the European Communities No L 40/ 27 COUNCIL DIRECTIVE of 21 December 1988 on the approximation of the laws of the Member States concerning food additives authorized for use
EUROPEAN COMMISSION SUMMARY REPORT OF THE STANDING COMMITTEE ON PLANTS, ANIMALS, FOOD AND FEED HELD IN BRUSSELS ON 10 FEBRUARY 2015
 EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Brussels sante.ddg2.g.dir(2015)934853 SUMMARY REPORT OF THE STANDING COMMITTEE ON PLANTS, ANIMALS, FOOD AND FEED HELD IN BRUSSELS ON 10 FEBRUARY
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Brussels sante.ddg2.g.dir(2015)934853 SUMMARY REPORT OF THE STANDING COMMITTEE ON PLANTS, ANIMALS, FOOD AND FEED HELD IN BRUSSELS ON 10 FEBRUARY
Policy / Drug and Alcohol-Free Workshops
 Policy 4118.235/4218.235 Drug and Alcohol-Free Workshops DATE: February 13, 2017 PREVIOUS ITEM: None ENCLOSURES: CABE s Suggested Policy 4118.235/4218.235 CABE s July 15, 2016 Policy Update REASON: To
Policy 4118.235/4218.235 Drug and Alcohol-Free Workshops DATE: February 13, 2017 PREVIOUS ITEM: None ENCLOSURES: CABE s Suggested Policy 4118.235/4218.235 CABE s July 15, 2016 Policy Update REASON: To
May 6, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 May 6, 2016 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted electronically via www.regulations.gov Re: Docket No. FDA-2014-N-1207
May 6, 2016 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted electronically via www.regulations.gov Re: Docket No. FDA-2014-N-1207
Code of Ethics, International Federation of Sports Physical Therapists, adopted GM, Cape Town September 2013
 Code of Ethics, The following Code of Ethics of International Federations of Sports Physical Therapists (IFSPT) is based on The International Federation of Sports Medcine (FIMS) Code of Ethics (http://www.fims.org/en/general/code-of-ethics/)
Code of Ethics, The following Code of Ethics of International Federations of Sports Physical Therapists (IFSPT) is based on The International Federation of Sports Medcine (FIMS) Code of Ethics (http://www.fims.org/en/general/code-of-ethics/)
Consumers Association s response to the European Commission s Discussion paper on nutrition claims and functional claims
 Introduction Consumers Association s response to the European Commission s Discussion paper on nutrition claims and functional claims Consumers Association (CA), publisher of Which?, Health Which? and
Introduction Consumers Association s response to the European Commission s Discussion paper on nutrition claims and functional claims Consumers Association (CA), publisher of Which?, Health Which? and
Roadmap to review the Nutrition and Health Claims legislation expression of interest to contribute to the upcoming external study
 European Commission DG SANTE Directorate-General for Health and Food Safety www.eucope.org Telephone: Telefax: E-Mail: 25 April 2016 Roadmap to review the Nutrition and Health Claims legislation expression
European Commission DG SANTE Directorate-General for Health and Food Safety www.eucope.org Telephone: Telefax: E-Mail: 25 April 2016 Roadmap to review the Nutrition and Health Claims legislation expression
House Committee on Energy and Commerce House Committee on Energy and Commerce. Washington, DC Washington, DC 20515
 February 28, 2018 The Honorable Michael Burgess, M.D. The Honorable Gene Green Chairman Ranking Member Subcommittee on Health Subcommittee on Health House Committee on Energy and Commerce House Committee
February 28, 2018 The Honorable Michael Burgess, M.D. The Honorable Gene Green Chairman Ranking Member Subcommittee on Health Subcommittee on Health House Committee on Energy and Commerce House Committee
CIAA COMMENTS ON DG SANCO DISCUSSION PAPER ON NUTRITIONAL AND FUNCTIONAL CLAIMS
 CIAA COMMENTS ON DG SANCO DISCUSSION PAPER ON NUTRITIONAL AND FUNCTIONAL CLAIMS SANCO/1341/2001 MIN/144/01E Final CIAA 2 CIAA KEY PRINCIPLES ON CLAIMS The Food and Drink industry has responded to government
CIAA COMMENTS ON DG SANCO DISCUSSION PAPER ON NUTRITIONAL AND FUNCTIONAL CLAIMS SANCO/1341/2001 MIN/144/01E Final CIAA 2 CIAA KEY PRINCIPLES ON CLAIMS The Food and Drink industry has responded to government
COMMISSION OF THE EUROPEAN COMMUNITIES REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT
 EN EN EN COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 5.12.2008 COM(2008) 824 final REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT on the use of substances other than vitamins
EN EN EN COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 5.12.2008 COM(2008) 824 final REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT on the use of substances other than vitamins
JICPA comments on the IESBA Exposure Draft, Proposed Revisions to the Code Pertaining to the Offering and Accepting of Inducements
 The Japanese Institute of Certified Public Accountants 4-4-1 Kudan-Minami, Chiyoda-ku, Tokyo 102-8264, Japan Phone: 81-3-3515-1130 Fax: 81-3-5226-3355 Email: international@sec.jicpa.or.jp December 8, 2017
The Japanese Institute of Certified Public Accountants 4-4-1 Kudan-Minami, Chiyoda-ku, Tokyo 102-8264, Japan Phone: 81-3-3515-1130 Fax: 81-3-5226-3355 Email: international@sec.jicpa.or.jp December 8, 2017
Sexual Assault. Attachment 1. Approval Date: Policy No.: The University of British Columbia Board of Governors
 Attachment 1 Policy No.: Approval Date: The University of British Columbia Board of Governors 131 Title: Background & Purposes: Sexual Assault Responsible Executive: Vice-President, Students Vice-President,
Attachment 1 Policy No.: Approval Date: The University of British Columbia Board of Governors 131 Title: Background & Purposes: Sexual Assault Responsible Executive: Vice-President, Students Vice-President,
DELTA SIGMA THETA SORORITY, INC. A Service Sorority
 DELTA SIGMA THETA SORORITY, INC. A Service Sorority ALCOHOL AND ILLEGAL DRUG USAGE POLICY EXECUTIVE SUMMARY Delta Sigma Theta Sorority, Incorporated ( Delta ) is revising its alcohol and drug policy to
DELTA SIGMA THETA SORORITY, INC. A Service Sorority ALCOHOL AND ILLEGAL DRUG USAGE POLICY EXECUTIVE SUMMARY Delta Sigma Theta Sorority, Incorporated ( Delta ) is revising its alcohol and drug policy to
JOINT FAO/WHO FOOD STANDARDS PROGRAMME
 Agenda Item 8 CX/NFSDU 10/32/8 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL DIETARY USES Thirty second Session Crowne Plaza Hotel, Santiago, Chile 1 5 November
Agenda Item 8 CX/NFSDU 10/32/8 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL DIETARY USES Thirty second Session Crowne Plaza Hotel, Santiago, Chile 1 5 November
THE INSTITUTE OF INTERNAL AUDITORS INSTRUCTOR CODE OF CONDUCT & ETHICS
 THE INSTITUTE OF INTERNAL AUDITORS INSTRUCTOR CODE OF CONDUCT & ETHICS 1. PURPOSE/SCOPE OF CODE OF CONDUCT & ETHICS The IIA is committed to the highest ethical standards to merit and maintain the confidence
THE INSTITUTE OF INTERNAL AUDITORS INSTRUCTOR CODE OF CONDUCT & ETHICS 1. PURPOSE/SCOPE OF CODE OF CONDUCT & ETHICS The IIA is committed to the highest ethical standards to merit and maintain the confidence
NOTICE OF INITIATION OF DISQUALIFICATION PROCEEDINGS AND OPPORTUNITY TO EXPLAIN
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 1401 Rockville Pike Rockville, MD 20852-1448 September 26, 2011 By Overnight Delivery Michael Dean Berger, M.D.
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 1401 Rockville Pike Rockville, MD 20852-1448 September 26, 2011 By Overnight Delivery Michael Dean Berger, M.D.
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011
 Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
The science behind labeling issues and health claims a European perspective
 2005 CSL/JIFSAN Joint Symposium on Food Safety and Nutrition: Bioactive Food Components The science behind labeling issues and health claims a European perspective Dr Michele Kellerhals Scientific and
2005 CSL/JIFSAN Joint Symposium on Food Safety and Nutrition: Bioactive Food Components The science behind labeling issues and health claims a European perspective Dr Michele Kellerhals Scientific and
Chapter 2 Evaluating Nutrition Information
 1 Chapter 2 Evaluating Nutrition Information Chapter 2 Learning Outcomes 2.1 The Importance of Nutrition 2.1.1 Explain how Joseph Goldberger developed a hypothesis for the cause of pellagra. 2.1.2 Explain
1 Chapter 2 Evaluating Nutrition Information Chapter 2 Learning Outcomes 2.1 The Importance of Nutrition 2.1.1 Explain how Joseph Goldberger developed a hypothesis for the cause of pellagra. 2.1.2 Explain
II. The Federal Government s Current Approach to Compensation. The issue of compensation for vaccine related injuries has been brought to
 13 II. The Federal Government s Current Approach to Compensation The issue of compensation for vaccine related injuries has been brought to congressional and wider public attention most dramatically in
13 II. The Federal Government s Current Approach to Compensation The issue of compensation for vaccine related injuries has been brought to congressional and wider public attention most dramatically in
JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL DIETARY USES
 Agenda Item 4 CX/NFSDU 01/4 September 2001 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL DIETARY USES Twenty-third Session Berlin, Germany, 26-30 November 2001
Agenda Item 4 CX/NFSDU 01/4 September 2001 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX COMMITTEE ON NUTRITION AND FOODS FOR SPECIAL DIETARY USES Twenty-third Session Berlin, Germany, 26-30 November 2001
