NAVINTA LLC Fax:
|
|
|
- Frederick Francis
- 5 years ago
- Views:
Transcription
1 AVITA LLC Fax: ! 2011 FEB - 8 A 10: 2q February 7, 2011 VIA OVERIGHT DELIVERY Division of Documents Management Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane Room 1061 Rockville, MD CITIZE PETITIO The undersigned submits this petition under Section 505 of the Federal Food, Drug, and Cosmetic Act (FDC Act), 21 U.S.C. 355, to request the Commissioner of Food and Drugs to refrain from approving any pending or new applications [submitted under 5050) or 505(b)(2)] of Iron Sucrose Injection drug prodilct therapeutically equivalent to the Reference Listed Drug product (RLD) Venofer (Iron sucrose injection). A. Action Requested This petition is submitted by avinta LLC (avinta) and requests that the Food and Drug Administration (FDA) refrain from granting final approval of any pending or new applications [submitted under 505(j) or 505(b)(2)] of Iron Sucrose Injection drug product, therapeutically equivalent to Venofer@ and do not exhibit pharmaceutical equivalence to Venofer with respect to the Sodium COlltent as described within the scope of this petition. -;:::OIJ c}d11 p. cx>? 1499 Lower Ferry Rd., Ewing, J Page 1 of6 d
2 Dr. Paokaj Dave AVITA LLC Fax: B. Statement of Grounds Iron sucrose Injection (DA ) was approved by the agency on ov 06, 2000 and currently it is marketed in the US as a Reference Listed Drug (RLD) under the trade name "Venofer ". Venofer (Iron Sucrose Injection, USP) is a brown, sterile, aqueous, complex of polynuclear iron (III)-hydroxide in sucrose for intravenous use. Iron sucrose injection has a molecular weight of approximately 34,000-60,000 daltons and a proposed structural formula: here, n is the degree of iron polymerization and m is the number of sucrose molecules associated with the iron (III)-hydroxide. Each ml of injection contains 20 mg elemental iron as iron sucrose in water for injection. Venofer is available in 5 ml single dose vials (100 mg elemental iron per 5 ml) and 10 ml single dose vials (200 mg elemental iron per 10 ml). The drug product contains approximately 30% sucrose w/v (300 mglml) and has a ph of The product contains no preservatives. The Osmolarity ofthe injection is 1250 mosmolll as provided in physician's inert labeling for Venofer (Exhibit A) Lower Ferry Rd., Ewing, J Page 2 of6
3 AVITA LLC Viee President Phone: Fax: Additionally, United States phannacopoeia (USP) has a monograph for Iron sucrose injection (Exhibit B), which describes that Iron sucrose injection is a sterile, colloidal solution of ferric hydroxide in complex with sucrose in water for injection. It contains not less than 95.0 percent and not more than percent ofthe labeled amount of iron. Sodium hydroxide may be added to adjust the ph. It contains no antimicrobial agent, chelating agent, dextran, gluconate, or other added substances. Elemental Components in Venofer : As described in the molecular formula of Iron sucrose complex printed in the Venofer Label, one can infer that Iron sucrose molecule contains Ferric Iron as polynuclear Iron(III) hydroxide, Sodium and Sucrose. USP monograph has defined specific tests for the control on content of Iron and content of Sucrose in the drug product, whereas a specific control on the content of sodium was not provided, even though sodium has been described as part of the Iron sucrose molecule (Ref: Venofer Label). As stated in the USP monograph, sodium hydroxide may be added to adjust the ph. This process of adding sodium hydroxide would introduce additional sodium level into the drug product. Alkalinity Test: USP monograph for Iron sucrose injection has a test for alkalinity, specifying that each ml of Iron sucrose injection should consume not less than 0.5 ml and not more than 0.8 ml of 0.1 Hydrochloric acid to bring down the ph to 7.4. Assuming the USP monograph test "Alkalinity" is 1499 Lower Ferry Rd., Ewing, J Page 3 of6
4 AVITA LLC Fax: intended to control the amount of sodium present in the Iron sucrose injection, the amount of sodium equivalent to 0.5 ml and 0.8 ml of 0.1 Hydrochloric acid would be correspondingly 1.15 mg/ml and 1.84 mg/ml of injection. Sodium Content: In order to verify the sodium content present in Venofer, three lots were tested for sodium content by ICPMS system using a validated analytical method. The sodium results obtained with three lots ofvenofer are provided in the Table 1. Table 1: Observed Sodium Content in Venofer (Iron Sucrose Injection) Venofer Lot o. Exp. Date Content of Sodium, mglml I 9500 July August August As evident from the sodium content data reported in Table 1, the values for sodium content are significantly higher than the sodium equivalent to the USP alkalinity test specification. This proves that significant amount of sodium is present in the drug product which did not contribute to the test for alkalinity, meaning this could be the sodium bound to the molecule and hence not neutralized during the titration against 0.1 hydrochloric acid as given in the test for alkalinity described in USP monograph Lower Ferry Rd., Ewing, J Page 4 of6
5 AVITA LLC Fax: Therefore, it is essential to have a control on the total sodium content in the Iron sucrose injection product so as to ensure phannaceutical equivalence of composition of a 505(j) and/or 505(b)(2) application drug product to that ofvenofer. Based on the sodium content data (about 5.7 mg/ml) observed in Venofer, the agency shall refrain from approving any pending or new applications referencing to Venofer if the sodium content is not within a proposed range ofbetween 5.1 mg/ml and 6.6 mg/ml ofdrug product. For the scientific reasons stated within the scope of this petition, all pending or new applications [submitted under 505(j) or 505(b)(2)] of Iron Sucrose Injection drug product], before being eligible to receive tentative or final approvals, besides meeting the USP monograph specification for Iron sucrose injection, shall be required to submit evidence to show the amount of sodium present in the respective drug product is similar to that found in Venofer at a proposed specification of between 5.1 mg/ml and 6.6 mg/ml of injection. c. Environmental Impact The relief requested by this petition is categorically exempt from the requirement for an environmental assessment under 21 C.F.R and D. Economic Impact Information regarding economic impact will be submitted upon request Lower Ferry Rd., Ewing, J Page 50f6
6 AVITA LLC Fax: E. Certification I certify that, to my best knowledge and belief: (a) this petition includes all information and views upon which the petition relies; (b) this petition includes representative data and/or information known to the petitioner which are unfavorable to the petition; and (c) I have taken reasonable steps to ensure that any representative data and/or information which are unfavorable to the petition were disclosed to me. If I received or expect to receive payments, including. cash and other forms of consideration, to file this information or its contents, I received or expect to receive those payments from the following persons or organizations: one, other than my usual salary and benefits as an employee ofavinta. I verify under penalty ofperjury that the foregoing is true and correct as ofthe date ofthe submission ofthis petition. Respectfully submitted, aj Dave, PhD. V President, a inta LLC 1499 Lower Ferry Road Ewing, J (609) Lower Ferry Rd., Ewing, J Page 60f6
7 ",-- l '.-,III _- DR.PAKAI DAVE AVITA, LLC. 0.5 LBS LTR 1 OF LO!R. Pl!RRY RD. EIG J SHIP TO: DMSIO OF DOCUMETS MAAGEMET FOOD AD DRUG ADMIISTRATIO DMSIO OF DOCUMETS MAAGEMET DEPT. OF HEALTH & HUMA SERVICES 5630 FISHERS LAE, ROOM# 1061 ROCKVILLE MD MD S: F '0.l> V. '0 II " UPS EXT DAY AIR I TRACKIG #: 1Z E BIUJG: PIP lim '" V"IS 12.OA * L i '".[ (:, I - CD.-:!.. 00 r.. '0.I>
NAVINTA LLC FEB loa II: 2 2. February 9, 2011
 February 9, 2011 NAVINTA LLC 2011 FEB loa II: 2 2 Fax: 609883 1137 Ms. Michelle Bigesby, Division ofdocuments Management, Food and Drug Administration, Department ofhealth and Human Services; 5630 Fishers
February 9, 2011 NAVINTA LLC 2011 FEB loa II: 2 2 Fax: 609883 1137 Ms. Michelle Bigesby, Division ofdocuments Management, Food and Drug Administration, Department ofhealth and Human Services; 5630 Fishers
FEB 28 A 9 :09
 InvaGen Pharmaceutic als. Inc. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4 2 3 7 11 FEB 28 A 9 :09 Division of Dockets Management Food and Drug Administration Department of Health
InvaGen Pharmaceutic als. Inc. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4 2 3 7 11 FEB 28 A 9 :09 Division of Dockets Management Food and Drug Administration Department of Health
InvaGen. FDP..90 fit P(Do47. Citizen petitian
 InvaGen Pharmaceuticals, I nt. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4:91. 6 11. Division of Dockets Management Food and Drug Administration Department of Health and Human Services
InvaGen Pharmaceuticals, I nt. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4:91. 6 11. Division of Dockets Management Food and Drug Administration Department of Health and Human Services
LACHMAN CONSULTANT SERVICES, INC STEWART AVENUE, WESTBURY, NY (516) " FAX (516) $,7,1 OVERNIGHT COURIER 11/16/09
 LACHMAN CONSULTANT SERVICES, INC. CONSULTANTS TO THE PHARMACEUTICAL AND ALLIED INDUSTRIES 1600 STEWART AVENUE, WESTBURY, NY 11590 (516) 222-6222 " FAX (516) 683-18$,7,1 November 16, 2009 OVERNIGHT COURIER
LACHMAN CONSULTANT SERVICES, INC. CONSULTANTS TO THE PHARMACEUTICAL AND ALLIED INDUSTRIES 1600 STEWART AVENUE, WESTBURY, NY 11590 (516) 222-6222 " FAX (516) 683-18$,7,1 November 16, 2009 OVERNIGHT COURIER
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
 UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Citizen Petition to: Margaret A. Hamburg, M.D, Commissioner of Food and Drugs Docket No. For Review of Standard of Identity
UNITED STATES DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION Citizen Petition to: Margaret A. Hamburg, M.D, Commissioner of Food and Drugs Docket No. For Review of Standard of Identity
ANDA Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY December 3, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
INRANGE SYSTEMS. Citizen Petition
 INRANGE SYSTEMS I~ I~ ;111Q I~ I tim@ II lifi11 Ill~ 1!1 II: I@ ;I@~ lij December 26, 2014 ZOI5 JA. -5 P 1: 15 Division of Dockets Management Food and Drug Administration 5630 Fishers Lane, Room 1061 (HFA-305)
INRANGE SYSTEMS I~ I~ ;111Q I~ I tim@ II lifi11 Ill~ 1!1 II: I@ ;I@~ lij December 26, 2014 ZOI5 JA. -5 P 1: 15 Division of Dockets Management Food and Drug Administration 5630 Fishers Lane, Room 1061 (HFA-305)
Listing of Color Additives Exempt from Certification; Synthetic Iron Oxide
 This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
BUG 8C BEA.$DSLEY, LLP. 919 EIGHTEENTH STREET, N.W. SUITE C300 WASHINGTON, D.C. 2000C3-55ro. October 22, 2009
 BUG 8C BEA.$DSLEY, LLP 919 EIGHTEENTH STREET, N.W. SUITE C300 WASHINGTON, D.C. 2000C3-55ro OCT 22?3 '07 W$rrEa's TmmEPjKoxE 202-736-3615 TE-1-Rp$oxE 202-736-3800 FAcsnKn.R 202-736-3608 Dockets Management
BUG 8C BEA.$DSLEY, LLP 919 EIGHTEENTH STREET, N.W. SUITE C300 WASHINGTON, D.C. 2000C3-55ro OCT 22?3 '07 W$rrEa's TmmEPjKoxE 202-736-3615 TE-1-Rp$oxE 202-736-3800 FAcsnKn.R 202-736-3608 Dockets Management
SUMMARY: The Food and Drug Administration (FDA or we) is amending the color additive
 This document is scheduled to be published in the Federal Register on 08/21/2015 and available online at http://federalregister.gov/a/2015-20676, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 08/21/2015 and available online at http://federalregister.gov/a/2015-20676, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Food Additives Permitted for Direct Addition to Food for Human Consumption; Vitamin D 2
 This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
Withdrawal of Proposed Rule on Supplemental Applications Proposing Labeling Changes for
 This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Janssen T. October 31, 2016
 Docket No. FDA-2011-P-0086-0001/CP Janssen Research & Development, LLC. 1125 Trenton-Harbourton Road, P.O. Box 200 Titusville, NJ 08560 Janssen T October 31, 2016 Division of Dockets Management (HF A 305)
Docket No. FDA-2011-P-0086-0001/CP Janssen Research & Development, LLC. 1125 Trenton-Harbourton Road, P.O. Box 200 Titusville, NJ 08560 Janssen T October 31, 2016 Division of Dockets Management (HF A 305)
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of
 This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION
 rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION HAR 1 0 2017 Anthony Maffia Vice President, Regulatory Affairs Sandoz Inc. 1 00 College Road West Princeton, NJ 08540 Dear Mr. Maffia: Re: Docket No. FDA-2016-P-3356
rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION HAR 1 0 2017 Anthony Maffia Vice President, Regulatory Affairs Sandoz Inc. 1 00 College Road West Princeton, NJ 08540 Dear Mr. Maffia: Re: Docket No. FDA-2016-P-3356
Clinical Policy: Iron Sucrose (Venofer) Reference Number: CP.PHAR.167
 Clinical Policy: (Venofer) Reference Number: CP.PHAR.167 Effective Date: 03/16 Last Review Date: 03/17 Revision Log Coding Implications See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Venofer) Reference Number: CP.PHAR.167 Effective Date: 03/16 Last Review Date: 03/17 Revision Log Coding Implications See Important Reminder at the end of this policy for important regulatory
the May 2010 Draft Guidance on Azelaic Acid, and you request that the FDA take the following actions relating to those comments:
 (.t ~stltvic's. ~"~Iy"~ DEPARTMENT OF HEALTH &. HUMAN SERVICES JUN 22 2012 Food and Drug Administration Rockvile MD 20857. David L. Rosen Foley & Lardner LLP 3000 K Street, N.W., Suite 500 Washington,
(.t ~stltvic's. ~"~Iy"~ DEPARTMENT OF HEALTH &. HUMAN SERVICES JUN 22 2012 Food and Drug Administration Rockvile MD 20857. David L. Rosen Foley & Lardner LLP 3000 K Street, N.W., Suite 500 Washington,
Indirect Food Additives: Adhesives and Components of Coatings and Paper and Paperboard Components
 4 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Parts 175 and 176 [Docket No. 99F-09253 Indirect Food Additives: Adhesives and Components of Coatings and Paper and Paperboard
4 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Parts 175 and 176 [Docket No. 99F-09253 Indirect Food Additives: Adhesives and Components of Coatings and Paper and Paperboard
Agency Information Collection Activities; Submission for Office of Management and Budget
 This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
ACTION: Notification; declaratory order; extension of compliance date.
 This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
National Drug Code Directory
 The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
SUMMARY: The Food and Drug Administration (FDA) is requesting public input on updated
 This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
JAN MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY. Docket No.
 DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
THE WEINBERG GROUP VIA FEDEX. October l, 2009
 .. 0 THE WEINBERG GROUP VIA FEDEX October l, 2009 Division of Dockets Management Food and Drug Administration (HFA-305) Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD
.. 0 THE WEINBERG GROUP VIA FEDEX October l, 2009 Division of Dockets Management Food and Drug Administration (HFA-305) Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD
DATES: This rule is effective December 8, See section X for further information on the
 This document is scheduled to be published in the Federal Register on 11/07/2017 and available online at https://federalregister.gov/d/2017-24194, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/07/2017 and available online at https://federalregister.gov/d/2017-24194, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
National Organic Program (NOP); Sunset 2017 Amendments to the National List
 This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
 In re Patent Application of: Neil P. DESAI et al. Docket No.: 638772000109 (PATENT) IN THE UNITED STATES PATENT AND TRADEMARK OFFICE Application No.: 11/520,479 Filed: September 12, 2006 For: NOVEL FORMULATIONS
In re Patent Application of: Neil P. DESAI et al. Docket No.: 638772000109 (PATENT) IN THE UNITED STATES PATENT AND TRADEMARK OFFICE Application No.: 11/520,479 Filed: September 12, 2006 For: NOVEL FORMULATIONS
Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen
 Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
D1)-(c790//./0, o 93/ op
 ZUCKERMAN SPAEDER 1900 M STREET, NW SUITE 1000 WASHINGTON, DC 20036-5802 202.778.1800 202.822.8106 fax www.zuckerman.com Kate C. Beardsley Partner (202) 778-1866 kbeardsley@zuckerman.com Division of Dockets
ZUCKERMAN SPAEDER 1900 M STREET, NW SUITE 1000 WASHINGTON, DC 20036-5802 202.778.1800 202.822.8106 fax www.zuckerman.com Kate C. Beardsley Partner (202) 778-1866 kbeardsley@zuckerman.com Division of Dockets
FDA 510(k) 101 The Basics
 FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
Martin Shimer Deputy Director, Division of Legal and Regulatory Support Office of Generic Drug Policy
 M E M O R A N D U M DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR DRUG EVALUATION AND RESEARCH DATE: November 9, 2017 FROM: Martin Shimer Deputy
M E M O R A N D U M DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR DRUG EVALUATION AND RESEARCH DATE: November 9, 2017 FROM: Martin Shimer Deputy
SUMMARY: The Food and Drug Administration (FDA, the Agency, or we) is proposing to
 This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25850, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25850, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
SUMMARY: The Food and Drug Administration (FDA, the Agency, or we) is amending its
 This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25851, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25851, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
June 9, U.S. Food and Drug Administration Division of Dockets Management, HFA Fishers Lane, Room 1061 Rockville, MD 20852
 June 9, 2014 U.S. Food and Drug Administration Division of Dockets Management, HFA-305 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Implementation of the Food and Drug Administration Food Safety
June 9, 2014 U.S. Food and Drug Administration Division of Dockets Management, HFA-305 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Implementation of the Food and Drug Administration Food Safety
URGENT: Important Safety Information
 July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
Three-Month Extension of Certain Tobacco Product Compliance Deadlines Related to the
 This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Venofer) Reference Number: CP.PHAR.167 Effective Date: 07.01.18 Last Review Date: 02.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Venofer) Reference Number: CP.PHAR.167 Effective Date: 07.01.18 Last Review Date: 02.18 Line of Business: Oregon Health Plan Coding Implications Revision Log See Important Reminder at
Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry
 Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry Draft Guidance This guidance is being distributed for comment purposes only. Although you can comment on any guidance at
Fruit Juice and Vegetable Juice as Color Additives in Food: Guidance for Industry Draft Guidance This guidance is being distributed for comment purposes only. Although you can comment on any guidance at
Town and Country Compounding and Consultation Services, LLC 10/17/17
 Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Highly Concentrated Caffeine in Dietary Supplements; Guidance for Industry; Availability
 This document is scheduled to be published in the Federal Register on 04/16/2018 and available online at https://federalregister.gov/d/2018-07836, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 04/16/2018 and available online at https://federalregister.gov/d/2018-07836, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
[Version 8, 10/2012] ANNEX I SUMMARY OF PROPOSED PRODUCT CHARACTERISTICS
![[Version 8, 10/2012] ANNEX I SUMMARY OF PROPOSED PRODUCT CHARACTERISTICS [Version 8, 10/2012] ANNEX I SUMMARY OF PROPOSED PRODUCT CHARACTERISTICS](/thumbs/77/75608970.jpg) [Version 8, 10/2012] ANNEX I SUMMARY OF PROPOSED PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Uniferon 200 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
[Version 8, 10/2012] ANNEX I SUMMARY OF PROPOSED PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Uniferon 200 mg/ml solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA Telephone: 510/
 Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
Department of Health and Human Services Public Health Service Food and Drug Administration San Francisco District 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Telephone: 510/337-6700 WARNING LETTER December
Soma)con PHARMACEUTICALS
 Soma)con PHARMACEUTICALS 5357 11 OCT 20 A10 AO Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852
Soma)con PHARMACEUTICALS 5357 11 OCT 20 A10 AO Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Products
 Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Before the OFFICE OF MANAGEMENT AND BUDGET Washington, D.C.
 Before the OFFICE OF MANAGEMENT AND BUDGET Washington, D.C. In the Matter of ) ) ) Notice of Information Collection Being ) OMB Control No. 3060-0761 Submitted to the Office of Management and ) Budget
Before the OFFICE OF MANAGEMENT AND BUDGET Washington, D.C. In the Matter of ) ) ) Notice of Information Collection Being ) OMB Control No. 3060-0761 Submitted to the Office of Management and ) Budget
Overview of Dietary Supplement GMP Inspection Trends Quality Session 6
 Overview of Dietary Supplement GMP Inspection Trends Quality Session 6 Presented by: Dean R. Cirotta, MBA President & Chief Operating Officer EAS Consulting Group, LLC 1700 Diagonal Road, Suite 750 Alexandria,
Overview of Dietary Supplement GMP Inspection Trends Quality Session 6 Presented by: Dean R. Cirotta, MBA President & Chief Operating Officer EAS Consulting Group, LLC 1700 Diagonal Road, Suite 750 Alexandria,
Food Additives Program
 U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
Talon Compounding Pharmacy 10/3/17
 Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
I. BACKGROUND. Docket No. FDA-2009-P Dear Dr. Aikman:
 DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 Mark S. Aikman, Pharm.D. Vice President, Regulatory Affairs and Quality Assurance Osmotica Pharmaceutical Corp. 1205
DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 Mark S. Aikman, Pharm.D. Vice President, Regulatory Affairs and Quality Assurance Osmotica Pharmaceutical Corp. 1205
Compare Results. 153 Replacements 26 Insertions 344 Deletions. Total Changes. Styling and. Content. 0 Annotations. Old File: New File:
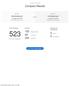 3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 03/04/2019 and available online at https://federalregister.gov/d/2019-03824, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 03/04/2019 and available online at https://federalregister.gov/d/2019-03824, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Inspections, Compliance, Enforcement, and Criminal Investigations
 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Dimitri Sirakoff 9/12/13 Department
Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Dimitri Sirakoff 9/12/13 Department
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Re: National Bioengineered Food Disclosure Standard; Proposed Rule; Request for Comments, 83 Fed. Reg (May 4, 2018), Docket No.
 VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
Clinical Policy: Pralatrexate (Folotyn) Reference Number: CP.PHAR.313 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Folotyn) Reference Number: CP.PHAR.313 Effective Date: 02.01.17 Last Review Date: 11.17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Clinical Policy: (Folotyn) Reference Number: CP.PHAR.313 Effective Date: 02.01.17 Last Review Date: 11.17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Ingredient Listing Qty. Unit NDC # Supplier. q.s. to ml
 12/20/2018; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier, USP 10 MU Sucrose, NF 23.00 g Propylene Glycol, USP 7.0 ml Medisca Oral Mix (Flavored Suspending Vehicle) 50.0 ml Medisca
12/20/2018; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier, USP 10 MU Sucrose, NF 23.00 g Propylene Glycol, USP 7.0 ml Medisca Oral Mix (Flavored Suspending Vehicle) 50.0 ml Medisca
Clinical Policy: Ferric Carboxymaltose (Injectafer) Reference Number: CP.PHAR.234
 Clinical Policy: (Injectafer) Reference Number: CP.PHAR.234 Effective Date: 06/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Injectafer) Reference Number: CP.PHAR.234 Effective Date: 06/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Raritan Pharmaceuticals, Inc. 6/20/17
 Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Flavored Milk; Petition to Amend the Standard of Identity for Milk and 17 Additional Dairy
 This document is scheduled to be published in the Federal Register on 02/20/2013 and available online at http://federalregister.gov/a/2013-03835, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 02/20/2013 and available online at http://federalregister.gov/a/2013-03835, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
NEW PROVIDER ENROLLMENT FOR ADULT SITE
 New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email completed New Provider Enrollment
New Jersey Department of Health Vaccines for Children (NJVFC) Program P.O. Box 369 Trenton, NJ 08625-0369 Phone: (609) 826-4862 Fax: (609) 826-4868 INSTRUCTIONS: Email completed New Provider Enrollment
ferric carboxymaltose 50mg iron/ml solution for injection/infusion (Ferinject ) SMC No. (463/08) Vifor Pharma UK Ltd
 Resubmission ferric carboxymaltose 50mg iron/ml solution for injection/infusion (Ferinject ) SMC No. (463/08) Vifor Pharma UK Ltd 06 May 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
Resubmission ferric carboxymaltose 50mg iron/ml solution for injection/infusion (Ferinject ) SMC No. (463/08) Vifor Pharma UK Ltd 06 May 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
Q&A for submission of applications for prequalification of Zinc Sulfate tablets and Zinc Sulfate oral liquid (solution)
 Q&A for submission of applications for prequalification of Zinc Sulfate tablets and Zinc Sulfate oral liquid (solution) This document should not be treated as a comprehensive guideline; it serves as a
Q&A for submission of applications for prequalification of Zinc Sulfate tablets and Zinc Sulfate oral liquid (solution) This document should not be treated as a comprehensive guideline; it serves as a
MYCAMINE National Drug Code Directory
 0469-3250-10 MYCAMINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
0469-3250-10 MYCAMINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
Procedure for Registration / Renewal of Third Party Hazmat/Industrial Tank Cleaning Contractors
 Procedure for Registration / Renewal of Third Party Hazmat/Industrial Tank Cleaning Contractors 1. PURPOSE: The competency and integrity of the 3 rd party Hazmat/ Industrial Tank Cleaning contractors are
Procedure for Registration / Renewal of Third Party Hazmat/Industrial Tank Cleaning Contractors 1. PURPOSE: The competency and integrity of the 3 rd party Hazmat/ Industrial Tank Cleaning contractors are
Donald W. Guthner Orgenix, LLC Ill Hill Road. Douglassville, PA (888) ORGENIX ( ) (484) (FAX) Intramedullary Nails, Nails
 K070741 ~, SANATMETAL Manufacturer of Orthopaedic and Traumatologic Products H-3301, Eger Faiskola ut 5. Phone: +(36)36-512-900 Fax: +(36)36-512-932 e-mail: metalasanatmetal.hu 510(k) Statement of Summary
K070741 ~, SANATMETAL Manufacturer of Orthopaedic and Traumatologic Products H-3301, Eger Faiskola ut 5. Phone: +(36)36-512-900 Fax: +(36)36-512-932 e-mail: metalasanatmetal.hu 510(k) Statement of Summary
Listing of Color Additives Exempt From Certification; Spirulina Extract
 This document is scheduled to be published in the Federal Register on 08/13/2013 and available online at http://federalregister.gov/a/2013-19550, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 08/13/2013 and available online at http://federalregister.gov/a/2013-19550, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
SODIUM BICARBONATE National Drug Code Directory
 0409-6625-02 SODIUM BICARBONATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of
0409-6625-02 SODIUM BICARBONATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of
Hogan Lovells. December 11, 2013 BY HAND DELIVERY
 Hogan Lovells 13 777 BY HAND DELIVERY Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, Maryland 20852 Re: CITIZEN PETITION SUPPLEMENT On behalf of AbbVie Inc. ("AbbVie") 1, the undersigned
Hogan Lovells 13 777 BY HAND DELIVERY Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, Maryland 20852 Re: CITIZEN PETITION SUPPLEMENT On behalf of AbbVie Inc. ("AbbVie") 1, the undersigned
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Center for Drug Evaluation and Research
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Center for Drug Evaluation and Research DATE: February 16, 2017 TO: FROM: Ganciclovir injection 500mg/250ml (NDA
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Center for Drug Evaluation and Research DATE: February 16, 2017 TO: FROM: Ganciclovir injection 500mg/250ml (NDA
Food Additives Permitted for Direct Addition to Food for Human Consumption; Acacia (Gum
 This document is scheduled to be published in the Federal Register on 12/06/2013 and available online at http://federalregister.gov/a/2013-29073, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 12/06/2013 and available online at http://federalregister.gov/a/2013-29073, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
DEXAMETHASONE SODIUM PHOSPHATE National Drug Code Directory
 0641-6146-25 DEXAMETHASONE SODIUM PHOSPHATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current
0641-6146-25 DEXAMETHASONE SODIUM PHOSPHATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current
Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference
 Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference Donald Kautter US Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food
Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference Donald Kautter US Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food
ANDA Labeling Question Based Review September 11, 2013 GPhA/FDA ANDA Labeling Workshop/USP User Forum
 ANDA Labeling Question Based Review September 11, 2013 GPhA/FDA ANDA Labeling Workshop/USP User Forum Sarah Park, PharmD Jeanne Skanchy, RPh. Labeling Reviewers Office of Generic Drugs/Division of Labeling
ANDA Labeling Question Based Review September 11, 2013 GPhA/FDA ANDA Labeling Workshop/USP User Forum Sarah Park, PharmD Jeanne Skanchy, RPh. Labeling Reviewers Office of Generic Drugs/Division of Labeling
CLIENT PROCEDURE FOR ANNUAL APPROVAL OF SHIP REPAIR COMPANIES
 CLIENT PROCEDURE FOR ANNUAL APPROVAL OF SHIP REPAIR COMPANIES 1.0 PURPOSE Safe work environment is essential for performing all kinds of ship repair operations. In this regard and to enable safe and smooth
CLIENT PROCEDURE FOR ANNUAL APPROVAL OF SHIP REPAIR COMPANIES 1.0 PURPOSE Safe work environment is essential for performing all kinds of ship repair operations. In this regard and to enable safe and smooth
BEFORE THE FEDERAL COMMUNICATIONS COMMISSION WASHINGTON, DC 20554
 BEFORE THE FEDERAL COMMUNICATIONS COMMISSION WASHINGTON, DC 20554 In the Matter of ) ) Telecommunications Relay Services and ) CG Docket No. 03-123 Speech-to-Speech Services for Individuals ) With Hearing
BEFORE THE FEDERAL COMMUNICATIONS COMMISSION WASHINGTON, DC 20554 In the Matter of ) ) Telecommunications Relay Services and ) CG Docket No. 03-123 Speech-to-Speech Services for Individuals ) With Hearing
Intent to Consider the Appropriate Classification of Hyaluronic Acid Intra-articular Products
 This document is scheduled to be published in the Federal Register on 12/18/2018 and available online at https://federalregister.gov/d/2018-27351, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/18/2018 and available online at https://federalregister.gov/d/2018-27351, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
OCT 1 7 A10 51
 '. ' October 11, 2013 Division of Dockets Management Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD 20852 4 6 6 4 13 OCT 1 7 A10 51 CITIZEN
'. ' October 11, 2013 Division of Dockets Management Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD 20852 4 6 6 4 13 OCT 1 7 A10 51 CITIZEN
The undersigned submit this petition under 42 U.S.C. 263b of the Mammogram Quality Standards Advisory Act of 1992 as amended and renewed in the
 The undersigned submit this petition under 42 U.S.C. 263b of the Mammogram Quality Standards Advisory Act of 1992 as amended and renewed in the Mammogram Quality Standards Reauthorization Act of 1998 and
The undersigned submit this petition under 42 U.S.C. 263b of the Mammogram Quality Standards Advisory Act of 1992 as amended and renewed in the Mammogram Quality Standards Reauthorization Act of 1998 and
Please see accompanying Full Prescribing Information.
 To Help Restore Joint Function Adequan i.m. is recommended for the intramuscular treatment of non-infectious degenerative and/or traumatic joint dysfunction and associated lameness of the carpal and hock
To Help Restore Joint Function Adequan i.m. is recommended for the intramuscular treatment of non-infectious degenerative and/or traumatic joint dysfunction and associated lameness of the carpal and hock
in the ICH Regions Table of Content Annexes to Guideline and 3. Why is Q4B necessary? Q4B Annexes? for Human Use
 Frequently Asked Questions Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions The Q4B Expert Working Group developed a set of frequently asked questions to help users
Frequently Asked Questions Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions The Q4B Expert Working Group developed a set of frequently asked questions to help users
Hieber's Pharmacy 12/5/17
 Hieber's Pharmacy 12/5/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973) 331-4969 CERTIFIED MAIL RETURN RECEIPT REQUESTED
Hieber's Pharmacy 12/5/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973) 331-4969 CERTIFIED MAIL RETURN RECEIPT REQUESTED
Food Labeling: Health Claims; Dietary Saturated Fat and Cholesterol and Risk of Coronary
 This document is scheduled to be published in the Federal Register on 12/19/2016 and available online at https://federalregister.gov/d/2016-29997, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 12/19/2016 and available online at https://federalregister.gov/d/2016-29997, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
6/7/2017 CHANGES IN PHARMA REGULATIONS HOW IT AFFECTS THE OR NURSE DISCLOSURE OBJECTIVES
 CHANGES IN PHARMA REGULATIONS HOW IT AFFECTS THE OR NURSE John Karwoski, RPh, MBA DISCLOSURE I, John Karwoski, RPH, MBA have business relationships with the companies MOBIUS THERAPEUTICS, LLC, Cubex, and
CHANGES IN PHARMA REGULATIONS HOW IT AFFECTS THE OR NURSE John Karwoski, RPh, MBA DISCLOSURE I, John Karwoski, RPH, MBA have business relationships with the companies MOBIUS THERAPEUTICS, LLC, Cubex, and
GLP VIRUCIDAL TESTING OF ZEROMOLD PLUS FOR HIV-1
 Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
Southwest Research Institute Page 1 of 8 Microencapsulation and Nanomaterials Department Amended Final Report: GLP Virucidal Testing of ZeroMold Plus for HIV-1 Protocol No.: GLP-SP-239 FINAL REPORT STUDY
STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS. Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails
 STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails NOTICE OF GRANT OPPORTUNITY (Updated) Announcement Date: September
STATE OF NEW JERSEY DEPARTMENT OF CORRECTIONS Medication Assisted Treatment For Substance Use Disorder In the New Jersey County Jails NOTICE OF GRANT OPPORTUNITY (Updated) Announcement Date: September
ENQUIRY NO. OGC/RFQ/17/ ENQUIRY FOR INTEGRATED MANAGEMENT SYSTEMS (IMS) CERTIFICATION
 ENQUIRY NO. OGC/RFQ/17/7000003450 ENQUIRY FOR INTEGRATED MANAGEMENT SYSTEMS (IMS) CERTIFICATION P.O Box: 799, Postal Code: 133, Al-Khuwair, Sultanate of Oman Enquiry No: OGC/RFQ/17/7000003450 Page 1 of
ENQUIRY NO. OGC/RFQ/17/7000003450 ENQUIRY FOR INTEGRATED MANAGEMENT SYSTEMS (IMS) CERTIFICATION P.O Box: 799, Postal Code: 133, Al-Khuwair, Sultanate of Oman Enquiry No: OGC/RFQ/17/7000003450 Page 1 of
Residual Solvents: FDA/ Regulatory Perspective
 Rosa Motta Compliance Officer Residual Solvents: FDA/ Regulatory Perspective PDA/USP Residual Solvents Conference January 18-19, 2007 1 Outline Laws and regulations governing the compliance requirements
Rosa Motta Compliance Officer Residual Solvents: FDA/ Regulatory Perspective PDA/USP Residual Solvents Conference January 18-19, 2007 1 Outline Laws and regulations governing the compliance requirements
Qualification of Drivers; Exemption Applications; Epilepsy and Seizure Disorders; AGENCY: Federal Motor Carrier Safety Administration (FMCSA), DOT.
 This document is scheduled to be published in the Federal Register on 02/29/2012 and available online at http://federalregister.gov/a/2012-04869, and on FDsys.gov DEPARTMENT OF TRANSPORTATION Federal Motor
This document is scheduled to be published in the Federal Register on 02/29/2012 and available online at http://federalregister.gov/a/2012-04869, and on FDsys.gov DEPARTMENT OF TRANSPORTATION Federal Motor
IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION
 Case 1:13-cv-03675-WBH Document 14 Filed 01/07/14 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION UNITED STATES OF AMERICA, Plaintiff, v. CIVIL ACTION
Case 1:13-cv-03675-WBH Document 14 Filed 01/07/14 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF GEORGIA ATLANTA DIVISION UNITED STATES OF AMERICA, Plaintiff, v. CIVIL ACTION
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
ASSEMBLY COMMITTEE ON HUMAN SERVICES Blanca Rubio, Chair AB 2702 (McCarty) As Amended April 2, 2018
 Page 1 Date of Hearing: April 24, 2018 SUBJECT: Trustline registry. ASSEMBLY COMMITTEE ON HUMAN SERVICES Blanca Rubio, Chair AB 2702 (McCarty) As Amended April 2, 2018 SUMMARY: Requires the Department
Page 1 Date of Hearing: April 24, 2018 SUBJECT: Trustline registry. ASSEMBLY COMMITTEE ON HUMAN SERVICES Blanca Rubio, Chair AB 2702 (McCarty) As Amended April 2, 2018 SUMMARY: Requires the Department
Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public Hearing;
 4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
STATE OF FLORIDA BOARD OF PHYSICAL THERAPY PRACTICE
 STATE OF FLORIDA BOARD OF PHYSICAL THERAPY PRACTICE DEPARTMENT OF HEALTH, Petitioner, VI CASE NO. 2016-00119 ANTHONY PAUL PRIBILA, P.T., Respondent. ADMINISTRATIVE COMPLAINT COMES NOW the Petitioner, Department
STATE OF FLORIDA BOARD OF PHYSICAL THERAPY PRACTICE DEPARTMENT OF HEALTH, Petitioner, VI CASE NO. 2016-00119 ANTHONY PAUL PRIBILA, P.T., Respondent. ADMINISTRATIVE COMPLAINT COMES NOW the Petitioner, Department
