Nutrition and Foods Safety Agency of the Centre for Disease Prevention and Control, People s Republic of China. Testing Report
|
|
|
- Reginald Weaver
- 5 years ago
- Views:
Transcription
1 Ministry Health approved Natural Health Products Testing Agency Issued by: Public Health Inspections, Ministry Health (1996) Publication # 53 Nutrition and Foods Safety Agency the Centre for Disease Prevention and Control, People s Republic China Testing Report Sample processing number: Name Sample: Eleotin Sample received from: Botali International Enterprise Co., Ltd Tianjin Port Free Trade Zone Testing type: As per requested Publication date: January 24, 2004
2 Nutrition and Foods Safety Agency the Centre for Disease Prevention and Control, People s Republic China Testing Report Sample processing number: Page 1 10 Name Sample: Eleotin Sample received from: Botali International Enterprise Co., Ltd Tianjin Port Free Trade Zone Manufacturer: Eastwood Bio-Medical Research Inc. Sample : Expiration Date: 24 months from manufacturing date condition: store under normal room temperature Sample Description: Type: capsule Colour: contents- light brown powder Amount Sample Received: 0.3g/capsule x 30 capsules/bottle x 1 bottle g Sample Received on: May 11 th, 2004 Testing Criteria: Acute toxicity test, 3 types hereditary toxicity tests, 30-day treatment test (animal-testing) Testing Reference: Natural Health Products Testing and Evaluation Guidelines Testing Results: Acute toxicity test was performed on female and male rats orally. According to the standards set by Acute Toxicity Classification, Eleotin was determined as non-toxic. According to the results the 3 types genotoxicity assays performed (Ames test, testing for occurrences mutation the micronucleus obtained from polychromatic erythrocytes in the bone marrow mice, and testing for occurrences sperm mutation in mice), Eleotin was not determined to be a source genetic mutation.
3 No abnormality was observed with respect to the active levels and growth rats after the addition Eleotin to their normal diet at 0.75, 1.50, and 3.00g/ kg.bw. Testing biochemical indexes in blood serum: male 0.75g/ kg.bw treatment group produced cholesterol levels higher than that the control group, male 1.50 and 3.00g/kg.BW treatment groups produced ALB (Albumin) levels lower than that the control group (p<0.05), references with control group levels from past experimental records within this laboratory); except for the differences described above, all other biochemical indexes treatment groups (both male and female) are similar to those the control group. Eleotin was also observed to not have any negative effects on the following: weight, digestive efficiency & food usage, haematology indexes, mass internal organs, mass internal organs to body mass ratio, and pathological histology. (end page) Sample processing number: Page 2 10 Eleotin: Toxicology Report 1 Materials and Methods 1.1 Sample: Eleotin, provided by Botali International Enterprise Co., Ltd, Tianjin Port Free Trade Zone. Contents the capsules are light brown in powder form. The recommended dose for adults is 6 capsules a day at 0.3g/capsule (1.8g/ day), an equivalent 0.03g/kg.BW (Calculations based on an adult body weight 60kg). 1.2 Subjects: Healthy Kunming mice supplied by the Chinese Academy Medical Science, School Research Animals Breeding Centre, license #: SCXK (Beijing) B-grade SD rats provided by Beijing City, Centre for Research Animals, license #: SCXK (Beijing) Instrument and Testing Reagent: Beckman GS15R centrifuge; Hitachi 7060 c automatic biochemical analyzer; BECKMAN COULTER A T diff2 Haematology Analyzer; Nikon electron microscope. 1.4 Experimental Procedure: Acute toxicity test (: rats): maximum tolerated dose (MTD) test. Twenty healthy SD rats (10 male and 10 female) weighing g were selected for testing. Subjects were each given 10.0g/kg.BW Eleotin at 20ml/kg.BW daily for 14 days, during which, were observed for symptoms poisoning and cases death Genotoxicity assays: Ames test: Approved tester strains, TA97, TA98, TA100, and TA102 were combined with S-9 mix, Eleotin sample, a trace histidine, and molten agar in Salmonella typhimurium reverse mutation assay. The test was activated by S-9 mix obtained from PCB-induced rat liver preparations. According to the results the toxicity test, doses 0.008, 0.04, 0.02, 1.00, and 5.00 mg/dish were determined. Eleotin was dissolved in sterile water, with the largest dose being 1g Eleotin/20ml sterile
4 water; other doses were prepared by diluting doses Eleotin in sterile water, each dose with an Eleotin increase 5 times the amount the previous dose, with 100 μl prepared sample added to each dish. Unprocessed-control, solvent control, and positive control groups were also established. Tester strains were added at 0.1 ml/strain to agar dishes. Bacterial growth-promoting solution (a trace histidine) was added to the surface the agar in each dish, at 0.1 ml/dish. Prepared Eleotin dose samples were also added at 0.1ml/dish, along with 0.5ml S-9 mix/dish (when activation was necessary). Dishes were then left alone for growth in 37 C for 48 hours, after which revertant colonies were counted for each dish. When the number revertant colonies the Eleotin dose samples are at least twice more than that the solvent control group, and with dose-specific differences, the testing result are considered positive. The entire test was repeated a second time under similar conditions Test occurrences mutation the micronucleus obtained from polychromatic erythrocytes in the bone marrow mice: Eleotin solutions (or cyclophosphamide, or distilled water) were orally fed to the mice twice at t = 0h and t = 24h. Fifty mice weighing 25-30g were selected and randomly divided into 5 groups 10 (5 male and 5 female) according to their relative weights. The positive-control group was given 40 mg/kg.bw cyclophosphamide, the baseline-control group was given distilled water, Eleotin groups were given processed Eleotin (Eleotin powder diluted with distilled water) at doses 0.83, 2.50, and 7.50 g/kg.bw (high dose meaning maximum dose given at once). At t = 30h, were severed by dislocating the cervical vertebrae. Bone marrow was extracted from bones in the thoracic region (ribs), diluted with veal blood serum, smeared onto glass slides, stabilized with methanol, and finally stained with Giesma. Slides were studied under the electron microscope; the number polychromatic erythrocytes (PCE) in 200 red blood cells was counted per subject with its percentage calculated. The number mutated micronuclei in 1000 polychromatic erythrocytes was counted per subject; ( ) occurrence mutation the micronucleus was calculated. Statistical analysis was performed on all data Testing for occurrences sperm mutation in mice: Twenty-five sexually mature male mice weighing 25-30g were selected and randomly divided into 5 groups 5. The positive control group was given 40 mg/kg.bw cyclophosphamide, the baselinecontrol group was given distilled water, Eleotin groups were given processed Eleotin (Eleotin powder diluted with distilled water) at doses 0.83, 2.50, and 7.50 g/kg.bw (high dose meaning maximum dose given at once). Respective treatments were orally fed to the (Eleotin solution, cyclophosphamide, or distilled water) once a day for 5 days. Thirty days post treatment, were severed by dislocating the cervical vertebrae. Both testes were removed from, fat in the testes was removed, and the testes were cut into pieces in normal saline, centrifuged at 1000 rev./min for 7 minutes, removed the upper clear layer, smeared the remaining sample onto glass slides, stabilized with methanol, and finally stained with 1.5 % Giesma. Slides were studied under the electron microscope; 1000 sperms were counted per subject, the percentage mutated sperms was calculated. Statistical analysis was performed on all data.
5 Sample processing number: Page Thirty-Day Eleotin intake test: 80 weaned rats weighing 56-79g each, randomly divided into 4 groups, 1 control group and 3 experimental groups (at doses 0.75, 1.50, 3.00g/kg.BW, equivalent to 25, 50, and 100 times that the recommended dose for human). 10% Eleotin was added to the normal diet the treatment groups, at 0.75, 1.50, and 3.00% respectively ( total food intake). Subjects were caged and fed separately with free access to water and food. Weights were measured on a weekly basis, and amount food intake recorded. Subjects were observed for 30 days. At the end this period, blood samples were obtained after fasting, haematology indexes and blood serum biochemical indexes were tested Parameter / Index Observation: Behaviour, weight, digestive efficiency & food usage Haematology and blood serum biochemical indexes: the following were counted: leukocytes, erythrocytes, haemoglobin, AST, ALT, urea (BUN), Cre, TC, TG, blood glucose level, TP, ALB Mass internal organs to body mass ratio : At the end testing, were put down. Liver, kidneys, spleen, and testes were obtained and used in the calculation the ratio mass internal organs to body mass (unit: g) Pathological histology: Anatomical dissection and pathological histology testing. When no obvious abnormality was observed with respect to mutation/ cancer and biochemical indexes across all Eleotin dose groups, the high dose group was then more closely examined for possible pathology in the liver, kidneys, testes (or ovaries), stomach, duodenum, and spleen Statistical analysis data: mean values and standard deviations were calculated with Excel, variance analysis was performed with PEMS, appropriate log( Y i ) transformation was performed on means with non-normal distributions and/or inequality variance to stabilize the variance. 2 Results: 2.1 Acute toxicity test (rats): Table 1 shows that (male and female) that were orally fed Eleotin at 10.0g/kg.BW for 14 days did not suffer from any signs food poisoning nor death. Results showed that the maximum tolerated dose Eleotin for SD rats (male and female) was greater than 10.0g/kg.BW with a LD 50 greater than 10.0g/kg.BW as well. According to Acute Toxicity Classification, Eleotin was determined as non-toxic. Table 1. Results acute toxicity test on (rats) after taking Eleotin for 14 days Subjects Method LD 50 Rats Orally >10.0 Orally >10.0
6 Sample processing number: Page Genotoxicity assays: Ames test: Tables 2 and 3 show that the number revertant colonies the control group fall within the normal range, while the number revertant colonies for all doses Eleotin groups do not exceed those the solvent-control group by more than 2 times, hence no dose-specific differences were observed. Results the Salmonella typhimurium reverse assay (TA97, TA98, TA100, TA102, with or without S-9) determined that Eleotin does not cause genetic mutation. Table 2. Results Eleotin Ames test: 1 TA97 TA98 TA100 TA102 (mg/dish) -S9 +S9 -S9 +S9 -S9 +S9 -S9 +S9 E L ±15.3 ±11.0 ±10.8 ±8.4 ±11.5 ±22.8 ±19.1 ±4.9 E O ±9.6 ±12.0 ±9.5 ±4.6 ±21.1 ±20.6 ±1.0 ±10.0 T I ±20.0 ±6.4 ±9.5 ±9.5 ±16.0 ±18.7 ±19.0 ±30.5 N ±9.2 ±10.4 ±7.5 ±4.0 ±22.2 ±16.2 ±9.5 ± ±11.0 ±21.5 ±4.5 ±14.6 ±26.1 ±15.1 ±11.0 ±3.5 Unprocessed-control ±14.2 ±13.9 ±9.5 ±3.1 ±9.5 ±14.3 ±20.6 ±13.4 Solvent-control ± ±17.5 ±15.9 ±2.9 ±2.1 ±7.5 ±16.0 ±12.8 ±27.7 Positive control (µg/dish) NaN ± AF ±105.8 ±177.2 ± Nitro-0- phenylenediamine ± ±184.4 MMC ±94.2 1,8- Dihydroxyanthraquinone 50.0 nb. Results are mean values all 3 dishes ±23.4
7 Sample processing number: Page 5 10 Table 3. Results Eleotin Ames test: 2 TA97 TA98 TA100 TA102 (mg/dish) -S9 +S9 -S9 +S9 -S9 +S9 -S9 +S9 E L ±12.6 ±27.2 ±2.0 ±8.5 ±12.1 ±27.4 ±13.1 ±9.1 E O ±10.3 ±13.7 ±1.5 ±7.1 ±4.5 ±22.0 ±19.0 ±14.2 T I ±13.8 ±17.6 ±13.2 ±10.1 ±22.0 ±21.5 ±15.9 ±7.0 N ±2.1 ±20.8 ±10.3 ±7.9 ±21.5 ±8.7 ±17.8 ± ±8.7 ±14.2 ±7.6 ±3.5 ±9.5 ±8.1 ±16.9 ±13.3 Unprocessed-control ±6.4 ±20.8 ±8.4 ±8.3 ±8.1 ±12.3 ±18.3 ±10.0 Solvent-control ±11.4 ±3.8 ±10.5 ±1.0 ±14.7 ±7.2 ±7.8 ±12.1 Positive control (µg/dish) NaN ± AF Nitro-0- phenylenediamine ±166.0 ± ±150.2 ±235.5 ±150.4 MMC ±72.5 1,8- Dihydroxyanthraquinone 50.0 nb. Results are mean values all 3 dishes 848.7± Testing occurrence mutation the micronucleus obtained from polychromatic erythrocytes in the bone marrow mice: Table 4 shows that the % PCE for female baseline-control group and all Eleotin dose groups are between , while the % PCE for male baseline-control group and all Eleotin dose groups are between ; no cell toxicity was observed under all Eleotin doses. The occurrences mutation the micronucleus positive-control group (cyclophosphamide) for both male and female were higher than both the baseline-control group and Eleotin dose groups (Poisson Distribution, p<0.01). No statistical significance was observed with the occurrences mutation f the micronucleus between Eleotin dose groups and baselinecontrol group (p>0.05). Results showed that Eleotin dose not cause mutation the micronucleus polychromatic erythrocytes.
8 Sample processing number: Page 6 10 Table 4. Effects Eleotin on occurrences mutation the micronucleus obtained from polychromatic erythrocytes (PCE) in the bone marrow mice PCE Micronucleus Observed number Erythrocytes PCE % Observed number PCE micronucleus % Occurrence 0.00 a mg/kg.BW(CP) b a mg/kg.BW(CP) b a : Denotes comparison with each the Eleotin processed-control groups, Poisson Distribution p>0.05 b : Denotes comparison with each the Eleotin processed-control groups and baseline - control group, Poisson Distribution p< Testing for occurrences sperm mutation in mice: Table 5 shows that the occurrences sperm mutation was much higher in the positive-control group when compared with that the baseline-control group and all Eleotin dose groups (X²-test: p<0.01); while no statistical significance was observed with the differences between all Eleotin dose groups and baseline-control group (X²-test: p>0.05). Results showed that Eleotin does not cause sperm mutation in mice. Table 5. Effects Eleotin on occurrences sperm mutation in mice sperms tested mutated sperms % sperm mutation 0.00 a mg/kg.bw(cp) b a : Denotes comparison with each the Eleotin processed-control groups, X 2 -test p>0.05 b : Denotes comparison with each the Eleotin processed-control groups and baseline - control group, X 2 -test p<0.01
9 2.3 Thirty-Day Eleotin intake test: Growth and digestive efficiency & food usage: After 0.75, 1.50, and 3.00g/kg.BW Eleotin were added to the normal diet for 30 days, activities and growth all groups appeared normal, were observed with healthy and vibrant hair. Tables 6, 7, and 8 show that there was no difference in the week-4 weight, total weight, total digestive efficiency & food usage all Eleotin dose groups when compared with those the control group (p>0.05). Sample processing number: Page 7 10 Table 6. Effects Eleotin on the weight rats ( Initial Week 1 weight (g) (g) Week 2 (g) ± SD) Week 3 (g) Week 4 (g) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±25.0 Table 7. Effects Eleotin on the weekly digestive efficiency & food usage rats ( ± SD) Weekly digestive efficiency and food usage (%) Week 1 Week 2 Week 3 Week ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±10.8
10 Table 8. Effects Eleotin on the food intake and total digestive efficiency & food usage rats ( ± SD) Total digestive Total weight Total Food efficiency & gain (g) Intake (g) food usage (%) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± Haematology and biochemical indexes: Tables 9 and 10 show that after 0.75, 1.50, and 3.00g/kg.BW Eleotin were added to the normal diet for 30 days, other than the fact that the erythrocyte count the female 1.50 and 3.00g/kg.BW Eleotin groups was higher than that the control group (p<0.05, although differences did not reach biochemical significance when referenced with past experimental records within this laboratory), all remaining haematology indexes (leukocyte count, erythrocyte count, haemoglobin, etc) all other Eleotin groups (both male and female ) are similar to those the control group (p>0.05). Sample processing number: Page 8 10 Table 9. Effects Eleotin on haematology indices rats ( Leukocyte count ( 10 9 /L) Erythrocyte count ( /L) ± SD) Haemoglobin (g/l) ± ± ± ± ± ± ± ±0.20 * 146.7± ± ±0.32 * 144.3± ± ± ± ± ± ± ± ± ± ± ± ±6.0 * : Comparison with control group, p<0.05
11 Table 10. Effects Eleotin on the different types white blood cells rats ( % Lymphocytes % Neutriphils % Other types ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±1.0 ± SD) Table 11 shows that after 0.75, 1.50, and 3.00g/kg.BW Eleotin were added to the normal diet for 30 days, other than the fact that the TP level the male 0.75g/kg.BW Eleotin group was higher than that the control group, and that the ALB level the male 1.50 and 3.00g/kg.BW Eleotin groups was lower than that the control group (p<0.05, although differences did not reach any significance when referenced with past experimental records within this laboratory), all remaining biochemical indexes, AST, ALT, urea (BUN), Cre, TC, TG, blood glucose level, TP, ALB all other Eleotin groups (both male and female ) are similar to those the control group (p>0.05). Table 11. Biochemical indices (rats) after 30 days Eleotin intake ( [partial data] Alanine aminotransferase (ALT) (U/L) Aspartate aminotransferase (AST) (U/L) Urea (BUN) (mmol/l) ± SD) Cre (umol/l) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±4.9
12 Sample processing number: Page 9 10 Table 11 (continued). Biochemical indices (rats) after 30 days Eleotin intake ( ± SD) [partial data] TC (mmol/l) TG (mmol/l) Blood glucose level (mmol/l) TP (g/l) ALB (g/l) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±0.27 * 1.04± ± ± ± ± ± ± ± ±0.5 * ± ± ± ± ±0.7 * * : Comparison with control group, p< Anatomical pathology and histology: Anatomical study found one case testicular shrinkage in both testes the same from control group. No other abnormalities were observed. The differences in the ratio mass internal organs to body mass for all Eleotin dose groups when compared with that the control group did not reach statistical significance (Table 12, p>0.05). Histological studies on liver, kidneys, testes (or ovaries), stomach, duodenum, spleen: Liver: no structural changes occurred in hepatic lobules, liver cells were aligned, spot liver cell necrosis was observed in the control group (1/20 cases) and not observed in the 3.00g/kg.BW Eleotin group (0/20). No structural changes occurred in bile canaliculi and bile microcanaliculi. Kidneys: calcium and salt deposit observed in renal tubules 1/20 control group and 1/ g/kg.BW Eleotin group, renal tubule and renal cortex structures were clear, no changes, necrosis, or inflammation were observed with the epithelial cell renal tubule. Testes: Testicular shrinkage observed in 1/20 control group while no shrinkage (0/20) was observed with the 3.00g/kg.BW Eleotin group. All other groups produced healthy and some mature sperms. No structural changes were observed in the ovaries, ovarian follicles, and corpus luteum were identified. Stomach and duodenum mucosa remained the same, no signs cell necrosis, ulceration, and inflammatory cell infiltration were observed. The structures the red and white pulps the spleen were clear; the number lymphatic cells remains unchanged. The pathology observed in the liver, kidneys, and testes the 3.00g/kg.BW Eleotin group does not reach statistical significance when compared with the control group; thus it was concluded to be due to natural histological changes, which occur in animals from time to time and unrelated to Eleotin. The results showed that Eleotin does
13 not cause pathological changes in liver, kidneys, testes (or ovaries), stomach, and duodenum. Table 12. Effects Eleotin on the mass internal organs to body mass ratio rats ( ± SD) Final body mass (g) Liver Mass organ:body mass (%) Kidney Mass organ:body mass (%) Mass Mass (g) (g) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ±0.07 Sample processing number: Page Table 12 (continued). Effects Eleotin on the mass internal organs to body mass ratio rats ( ± SD) Mass (g) Spleen Mass organ:body mass (%) Mass (g) Testes Mass organ:body mass (%) ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± ± Conclusion: 3.1 Acute toxicity test (rats): (male and female) were orally fed Eleotin and results determined a LD 50 greater than 10.0g/kg.BW. According to Acute Toxicity Classification, Eleotin was determined as non-toxic. 3.2 Genotoxicity assays: According to the results the 3 types genotoxicity assays performed (Ames test, testing for occurrences mutation the micronucleus obtained
14 from polychromatic erythrocytes in the bone marrow mice, and testing for occurrences sperm mutation in mice), Eleotin was not determined to be a source genetic mutation. 3.3 Thirty-Day Eleotin intake test: After 0.75, 1.50, and 3.00g/kg.BW Eleotin were added to the normal diet for 30 days, activities and growth all groups appeared normal, were observed with healthy and vibrant hair. There was no difference in weight, food intake, total digestive efficiency & food usage all Eleotin dose groups (both male and female) when compared with those the control group (p>0.05). Haematology and biochemical indexes: After 0.75, 1.50, and 3.00g/kg.BW Eleotin were added to the normal diet for 30 days, other than the fact that the erythrocyte count the female 1.50 and 3.00g/kg.BW Eleotin groups was higher than that the control group (p<0.05, although differences did not reach biochemical significance when referenced with past experimental records within this laboratory), all remaining haematology indexes (leukocyte count, erythrocyte count, haemoglobin, etc) all other Eleotin groups (both male and female ) are similar to those the control group (p>0.05). Haematology: After 0.75, 1.50, and 3.00g/kg.BW Eleotin were added to the normal diet for 30 days, other than the fact that the TP level the male 0.75g/kg.BW Eleotin group was higher than that the control group, and that the ALB level the male 1.50 and 3.00g/kg.BW Eleotin groups was lower than that the control group ((p<0.05, although differences did not reach any significance when referenced with past experimental records within this laboratory), all remaining biochemical indexes, AST, ALT, urea (BUN), Cre, TC, TG, blood glucose level, TP, ALB all other Eleotin groups (both male and female ) are similar to those the control group (p>0.05). The differences in the ratio mass internal organs to body mass for all Eleotin dose groups when compared with that the control group did not reach statistical significance (p>0.05). Results the pathological test showed that Eleotin does not cause pathological changes.
Sample received from: Botali International Enterprise Co., Ltd Tianjin Port Free Trade Zone
 Nutrition and Foods Safety Agency of the Centre for Disease Prevention and Control, People s Republic of China Xi Yuan Hospital of China Academy of Traditional Chinese Medicine Testing Report Sample processing
Nutrition and Foods Safety Agency of the Centre for Disease Prevention and Control, People s Republic of China Xi Yuan Hospital of China Academy of Traditional Chinese Medicine Testing Report Sample processing
Evaluation of the in vitro and in vivo Genotoxicity of Almond Skins
 Biomed Environ Sci, 2011; 24(4): 415 421 415 Original Article Evaluation of the in vitro and in vivo Genotoxicity of Almond Skins ZHANG XiaoPeng, XIANG Qian, CUI WenMing, JIA XuDong, and LI Ning # Institute
Biomed Environ Sci, 2011; 24(4): 415 421 415 Original Article Evaluation of the in vitro and in vivo Genotoxicity of Almond Skins ZHANG XiaoPeng, XIANG Qian, CUI WenMing, JIA XuDong, and LI Ning # Institute
IN VIVO TRAILS ON PIGS
 IN VIVO TRAILS ON PIGS HAEMATOLOGICAL AND BIOCHEMICAL PARAMETERS OF WEANED PIGLETS FED ON FODDER MIXTURE CONTAMINATED BY ZEARALENONE WITH ADDITION OF MINAZEL PLUS Acta Veterinaria (Belgrade), Vol. 56,
IN VIVO TRAILS ON PIGS HAEMATOLOGICAL AND BIOCHEMICAL PARAMETERS OF WEANED PIGLETS FED ON FODDER MIXTURE CONTAMINATED BY ZEARALENONE WITH ADDITION OF MINAZEL PLUS Acta Veterinaria (Belgrade), Vol. 56,
Evaluation of the toxicity and safety of the antioxidant beverage effective microorganisms-x (EM-X) in animal models
 Environmental Toxicology and Pharmacology 20 (2005) 313 320 Evaluation of the toxicity and safety of the antioxidant beverage effective microorganisms-x (EM-X) in animal models Bin Ke a,, Yun-Fei Liang
Environmental Toxicology and Pharmacology 20 (2005) 313 320 Evaluation of the toxicity and safety of the antioxidant beverage effective microorganisms-x (EM-X) in animal models Bin Ke a,, Yun-Fei Liang
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/488/98-FINAL July 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS DIETHYLENE GLYCOL MONOETHYL
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/488/98-FINAL July 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS DIETHYLENE GLYCOL MONOETHYL
Understanding Blood Tests
 PATIENT EDUCATION patienteducation.osumc.edu Your heart pumps the blood in your body through a system of blood vessels. Blood delivers oxygen and nutrients to all parts of the body. It also carries away
PATIENT EDUCATION patienteducation.osumc.edu Your heart pumps the blood in your body through a system of blood vessels. Blood delivers oxygen and nutrients to all parts of the body. It also carries away
SHRIRAM INSTITUTE FOR INDUSTRIAL RESEARCH
 IN RATS SUB CHRONIC ORAL TOXICITY WITH NHH 44 Bt-COTTON SEEDS Report for: UNIVERSITY OF AGRICULTURAL SCIENCES AGRICULTURAL RESEARCH STATION DHARWAD-580007 KARNATAKA Guidelines: DBT, Guidelines for Toxicity
IN RATS SUB CHRONIC ORAL TOXICITY WITH NHH 44 Bt-COTTON SEEDS Report for: UNIVERSITY OF AGRICULTURAL SCIENCES AGRICULTURAL RESEARCH STATION DHARWAD-580007 KARNATAKA Guidelines: DBT, Guidelines for Toxicity
Pathophysiology I Liver and Biliary Disease
 Pathophysiology I Liver and Biliary Disease The Liver The liver is located in the right upper portion of the abdominal cavity just beneath the right side of the rib cage. The liver has many functions that
Pathophysiology I Liver and Biliary Disease The Liver The liver is located in the right upper portion of the abdominal cavity just beneath the right side of the rib cage. The liver has many functions that
Males- Western Diet WT KO Age (wks) Females- Western Diet WT KO Age (wks)
 Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
Relative Arv1 mrna Adrenal 33.48 +/- 6.2 Skeletal Muscle 22.4 +/- 4.93 Liver 6.41 +/- 1.48 Heart 5.1 +/- 2.3 Brain 4.98 +/- 2.11 Ovary 4.68 +/- 2.21 Kidney 3.98 +/-.39 Lung 2.15 +/-.6 Inguinal Subcutaneous
International Journal of Medicine and Health Profession Research
 Research Article ISSN: 2394 7403 International Journal of Medicine and Health Profession Research Journal home page: www.ijmhpr.com TOXICITY STUDY OF VAIVILANGAM CHOORANAM E. M. Manikgantan* 1 and R. Pattarayan
Research Article ISSN: 2394 7403 International Journal of Medicine and Health Profession Research Journal home page: www.ijmhpr.com TOXICITY STUDY OF VAIVILANGAM CHOORANAM E. M. Manikgantan* 1 and R. Pattarayan
Burak DiK 1, Emre BAHCIVAN 1,2, Hatice ESER 1,3, Kamil UNEY 1
 Burak DiK 1, Emre BAHCIVAN 1,2, Hatice ESER 1,3, Kamil UNEY 1 1 Selcuk University Faculty of Veterinary Medicine, Pharmacology and Toxicology Department, Konya, TURKEY 2 Kafkas University Faculty of Veterinary
Burak DiK 1, Emre BAHCIVAN 1,2, Hatice ESER 1,3, Kamil UNEY 1 1 Selcuk University Faculty of Veterinary Medicine, Pharmacology and Toxicology Department, Konya, TURKEY 2 Kafkas University Faculty of Veterinary
DIABETES AND LABORATORY TESTS. Author: Josephine Davis
 DIABETES AND LABORATORY TESTS Author: Josephine Davis LAB TESTS Think twice before you test. What is the reason for testing? Laboratory tests are generally requested in primary care for one of the following
DIABETES AND LABORATORY TESTS Author: Josephine Davis LAB TESTS Think twice before you test. What is the reason for testing? Laboratory tests are generally requested in primary care for one of the following
Supplementary Figure 1. Western blot of hippocampal lysates from WT and Adcy1 KO mice demonstrates the specificity of the ADCY1 antibody.
 ADCY1 13 kda β-actin 45 kda Supplementary Figure 1. Western blot of hippocampal lysates from and mice demonstrates the specificity of the ADCY1 antibody. a DHPG perk1/2 ERK1/2 Relative level min 1.6 *
ADCY1 13 kda β-actin 45 kda Supplementary Figure 1. Western blot of hippocampal lysates from and mice demonstrates the specificity of the ADCY1 antibody. a DHPG perk1/2 ERK1/2 Relative level min 1.6 *
ROTUNDA HOSPITAL DEPARTMENT OF LABORATORY MEDICINE
 This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
This active test table informs the user of Biochemistry tests available in house. s referred to other sites are recorded in the Referred Table. Issue date: 4 TH April 2016 Contact Phone Number ext.1345/2522
Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube
 Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube Background: Greiner-Bio-One, Austria has been selling plastic evacuated
Evaluation of VACUETTE CAT Serum Fast Separator Blood Collection Tube for Routine Chemistry Analytes in Comparison to VACUTAINER RST Tube Background: Greiner-Bio-One, Austria has been selling plastic evacuated
Safety of micronized palmitoylethanolamide (micropea): lack of toxicity and genotoxic potential
 ORIGINAL RESEARCH Safety of micronized palmitoylethanolamide (micropea): lack of toxicity and genotoxic potential Earle R. Nestmann Health Science Consultants Inc., Mississauga, Ontario L5N 7S2, Canada
ORIGINAL RESEARCH Safety of micronized palmitoylethanolamide (micropea): lack of toxicity and genotoxic potential Earle R. Nestmann Health Science Consultants Inc., Mississauga, Ontario L5N 7S2, Canada
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/MRL/451/98-FINAL June 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS BACITRACIN SUMMARY REPORT (1)
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/MRL/451/98-FINAL June 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS BACITRACIN SUMMARY REPORT (1)
Biochemical alterations induced by the acute exposure to combination of chlorpyrifos and lead in Wistar rats
 Biochemical alterations induced by the acute exposure to combination of chlorpyrifos and lead in Wistar rats 1 H Krishna*, 2 AV Ramachandran 1 Dhirubhai Ambani Life Sciences Centre, Reliance Life Sciences
Biochemical alterations induced by the acute exposure to combination of chlorpyrifos and lead in Wistar rats 1 H Krishna*, 2 AV Ramachandran 1 Dhirubhai Ambani Life Sciences Centre, Reliance Life Sciences
Individual Study Table Referring to Part of the Dossier. Use only) Name of Finished Product:
 SYNOPSIS Fresenius Title of the study: A double-blind, randomized study comparing the safety and torelance of SMOFlipid 20% and Intralipid 20% in long-term treatment with parenteral nutrition Coordinating
SYNOPSIS Fresenius Title of the study: A double-blind, randomized study comparing the safety and torelance of SMOFlipid 20% and Intralipid 20% in long-term treatment with parenteral nutrition Coordinating
Clinician Blood Panel Results
 Page 1 of 7 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 7 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Anatomy & Physiology Revealed Instructions. 1. From the Module dropdown menu, chose the 12. Digestive system.
 #10 - Objectives: Examine the histology of selected body organs using Anatomy & Physiology Revealed software and microscope slides. Be able to identify each organ and the specific structures indicated
#10 - Objectives: Examine the histology of selected body organs using Anatomy & Physiology Revealed software and microscope slides. Be able to identify each organ and the specific structures indicated
Fullerton Healthcare Screening Centres
 Fullerton Healthcare Screening Centres Fullerton Healthcare Screening Centre @ Ngee Ann City The Penthouse, #26-02 Ngee Ann City Tower B, 391B Orchard Road, Singapore 238874 Operating hours: Monday - Friday
Fullerton Healthcare Screening Centres Fullerton Healthcare Screening Centre @ Ngee Ann City The Penthouse, #26-02 Ngee Ann City Tower B, 391B Orchard Road, Singapore 238874 Operating hours: Monday - Friday
Food & Function Accepted Manuscript
 Food & Function Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts
Food & Function Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts
HISTOLOGY VIRTUAL LABORATORY BLOOD AND LYMPHATICS SYSTEM
 HISTOLOGY VIRTUAL LABORATORY BLOOD AND LYMPHATICS SYSTEM Login: http://histopath.westernu.edu Histology Atlas AND Virtual Histology links. I. HEMATOLOGY - PERIPHERAL BLOOD Purpose: To be able to identify
HISTOLOGY VIRTUAL LABORATORY BLOOD AND LYMPHATICS SYSTEM Login: http://histopath.westernu.edu Histology Atlas AND Virtual Histology links. I. HEMATOLOGY - PERIPHERAL BLOOD Purpose: To be able to identify
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 EMEA/MRL/578/99-FINAL-corr. 1 August 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS TIAMULIN SUMMARY REPORT (1) 1. Tiamulin is a diterpene antimicrobial with a pleuromutilin chemical structure similar
EMEA/MRL/578/99-FINAL-corr. 1 August 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS TIAMULIN SUMMARY REPORT (1) 1. Tiamulin is a diterpene antimicrobial with a pleuromutilin chemical structure similar
Evaluation of genotoxicity of gastric proton pump inhibitor omeprazole and role of vitamin-e in mice
 2016; 5(8): 66-71 ISSN: 2277-7695 TPI 2016; 5(8): 66-71 2016TPI www.thepharmajournal.com Received: 11-06-2016 Accepted: 12-07-2016 Dr. Jyothsna Guduru, Asst Professor, Dept of Pharmacology, Mallareddy
2016; 5(8): 66-71 ISSN: 2277-7695 TPI 2016; 5(8): 66-71 2016TPI www.thepharmajournal.com Received: 11-06-2016 Accepted: 12-07-2016 Dr. Jyothsna Guduru, Asst Professor, Dept of Pharmacology, Mallareddy
Cytochrome-C (rat, mouse) forward GGAGGCAAGCATAAGACTGG. mouse hexokinase 2 gene, intron 9 reverse GGGAACACAAAAGACCTCTTCTGG
 Supplementary Table 1. The sequences of oligonucleotide primers. Genes Sequence rat actin forward CGAGTACAACCTTCTTGCAG rat actin reverse GAGTCCTTCTGACCCATACC tubulin (rat, mouse) forward TAGCAGAGATCACCAATGCC
Supplementary Table 1. The sequences of oligonucleotide primers. Genes Sequence rat actin forward CGAGTACAACCTTCTTGCAG rat actin reverse GAGTCCTTCTGACCCATACC tubulin (rat, mouse) forward TAGCAGAGATCACCAATGCC
Recipes for Media and Solution Preparation SC-ura/Glucose Agar Dishes (20mL/dish, enough for 8 clones)
 Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
Protocol: 300 ml Yeast culture preparation Equipment and Reagents needed: Autoclaved toothpicks Shaker Incubator set at 30 C Incubator set at 30 C 60 mm 2 sterile petri dishes Autoclaved glass test tubes
Bani Adlina Shabrina, Juang Juansa, Nindya Budiana Putri, Rohmad Yudi Utomo, Retno Murwanti*
 Indonesian Journal of Cancer Chemoprevention, February 2016 ISSN: 2088 0197 e-issn: 2355-8989 Antigenotoxicity Activity of Papaya (Carica papaya L.) Leaf Ethanolic Extract on Swiss Mice Induced Cyclophosphamide
Indonesian Journal of Cancer Chemoprevention, February 2016 ISSN: 2088 0197 e-issn: 2355-8989 Antigenotoxicity Activity of Papaya (Carica papaya L.) Leaf Ethanolic Extract on Swiss Mice Induced Cyclophosphamide
The Endocrine System ( PART II) Individual Endocrine glands and their hormones
 The Endocrine System ( PART I) Hormone Describe the major endocrine organs, list their main locations and functions. Indicate important differences between hormonal and neural controls of body functioning.
The Endocrine System ( PART I) Hormone Describe the major endocrine organs, list their main locations and functions. Indicate important differences between hormonal and neural controls of body functioning.
SCIENTIFIC OPINION. EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) 2, 3
 EFSA Journal 2012;10(7):2825 SCIENTIFIC OPINION Scientific Opinion on the safety evaluation of the substance, 2-phenyl-3,3-bis(4-hydroxyphenyl)phthalimidine, CAS No. 6607-41-6, for use in food contact
EFSA Journal 2012;10(7):2825 SCIENTIFIC OPINION Scientific Opinion on the safety evaluation of the substance, 2-phenyl-3,3-bis(4-hydroxyphenyl)phthalimidine, CAS No. 6607-41-6, for use in food contact
GLYPHOSATE (addendum)
 GLYPHSATE (addendum) First draft prepared by Rudolf Pfeil 1 and Vicki Dellarco 2 1 Toxicology of Pesticides and Biocides, Federal Institute for Risk Assessment, Berlin, Germany 2 ffice of Pesticide Programs,
GLYPHSATE (addendum) First draft prepared by Rudolf Pfeil 1 and Vicki Dellarco 2 1 Toxicology of Pesticides and Biocides, Federal Institute for Risk Assessment, Berlin, Germany 2 ffice of Pesticide Programs,
Table 3 Insecticides 1
 Table 3 1 I07 13 rat 2.5 10 blood, liver, spleen anemia; hepatic inflamm.,siderosis of Kupffer cells, liver +spleen w. I07 13 dog 2.6 13.5 blood erythropoiesis; hematopoiesis in spleen I01 1972 52 dog
Table 3 1 I07 13 rat 2.5 10 blood, liver, spleen anemia; hepatic inflamm.,siderosis of Kupffer cells, liver +spleen w. I07 13 dog 2.6 13.5 blood erythropoiesis; hematopoiesis in spleen I01 1972 52 dog
18 Urinary system. 19 Male reproductive system. Female reproductive system. Blok 11: Genital and Urinary Tract Diseases
 Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
Blok 11: Genital and Urinary Tract Diseases 18 Urinary System 19 Male Genital System 20 Female Genital System 18 Urinary system You should be able to: 1. Describe the structures and associated functions
VICH GL23: Studies to evaluate the safety of residues of veterinary drugs in human food: genotoxicity testing
 6 November 2014 EMA/CVMP/VICH/526/2000 Committee for Medicinal Products for Veterinary Use (CVMP) VICH GL23: Studies to evaluate the safety of residues of veterinary drugs in human food: genotoxicity testing
6 November 2014 EMA/CVMP/VICH/526/2000 Committee for Medicinal Products for Veterinary Use (CVMP) VICH GL23: Studies to evaluate the safety of residues of veterinary drugs in human food: genotoxicity testing
Evaluation of new MiniCollect Z Serum (Separator) Tubes
 Evaluation of new MiniCollect Z Serum (Separator) Tubes Background: Greiner Bio-One has developed a newly designed MiniCollect tube offering an integrated collection scoop. The advantage of the new tube
Evaluation of new MiniCollect Z Serum (Separator) Tubes Background: Greiner Bio-One has developed a newly designed MiniCollect tube offering an integrated collection scoop. The advantage of the new tube
Nonalcoholic Steatohepatitis National Digestive Diseases Information Clearinghouse
 Nonalcoholic Steatohepatitis National Digestive Diseases Information Clearinghouse National Institute of Diabetes and Digestive and Kidney Diseases NATIONAL INSTITUTES OF HEALTH Nonalcoholic steatohepatitis
Nonalcoholic Steatohepatitis National Digestive Diseases Information Clearinghouse National Institute of Diabetes and Digestive and Kidney Diseases NATIONAL INSTITUTES OF HEALTH Nonalcoholic steatohepatitis
A&P 2 Endocrine Pre-Lab Guide Information from Videos and Exercises
 A&P 2 Endocrine Pre-Lab Guide Information from Videos and Exercises Read Me In this Pre-lab, we are going over some basic concepts regarding endocrine glands. These notes cover important concepts found
A&P 2 Endocrine Pre-Lab Guide Information from Videos and Exercises Read Me In this Pre-lab, we are going over some basic concepts regarding endocrine glands. These notes cover important concepts found
This is Learning Component 6 in Learning Module 1. We will show examples of features ( things ) including mineral deposits, urates, pigments, dust,
 This is Learning Component 6 in Learning Module 1. We will show examples of features ( things ) including mineral deposits, urates, pigments, dust, plant material, and amyloid. 1 Calcium salts are the
This is Learning Component 6 in Learning Module 1. We will show examples of features ( things ) including mineral deposits, urates, pigments, dust, plant material, and amyloid. 1 Calcium salts are the
Cyclophosphamide. was withdrawn by a needle and syringe, and the. samples were plated in molten agar. At the time of
 INFECTION AND IMMUNITY, Nov. 1982, P. 558-562 19-9567/82/11558-5$2./ Copyright 1982, American Society for Microbiology Vol. 38, No. 2 Increased Severity of Urinary Tract Infection and Bacteremia in Mice
INFECTION AND IMMUNITY, Nov. 1982, P. 558-562 19-9567/82/11558-5$2./ Copyright 1982, American Society for Microbiology Vol. 38, No. 2 Increased Severity of Urinary Tract Infection and Bacteremia in Mice
NEW RCPCH REFERENCE RANGES-
 s vary between populations and age groups and it is important to always check the reference Haematology: Haemoglobin Male 130 175 g/l 0 6 days 145-220 g/l Female 115 165 g/l 7 days 140-186 g/l 8 days 3
s vary between populations and age groups and it is important to always check the reference Haematology: Haemoglobin Male 130 175 g/l 0 6 days 145-220 g/l Female 115 165 g/l 7 days 140-186 g/l 8 days 3
Laboratory Protocol. November 2017 Version 3. Henrik Hasman, Yvonne Agersø and Lina M Cavaco (DTU Food)
 Laboratory Protocol Validation of selective MacConkey agar plates supplemented with 1 mg/l cefotaxime for monitoring of ESBL- and AmpCproducing E. coli in meat and caecal samples November 2017 Version
Laboratory Protocol Validation of selective MacConkey agar plates supplemented with 1 mg/l cefotaxime for monitoring of ESBL- and AmpCproducing E. coli in meat and caecal samples November 2017 Version
TRACEABILITY and UNCERTAINTY
 Cat. No. 0 79 0 90 ACP Acid phosphatase total -naphthyl phosphate NPP Acid phosphatase, non-prostatic -naphthyl phosphate (Inhib.:tartrate) ALB Albumin BCG plus ALB Albumin BCP ALP Alkaline phosphatase
Cat. No. 0 79 0 90 ACP Acid phosphatase total -naphthyl phosphate NPP Acid phosphatase, non-prostatic -naphthyl phosphate (Inhib.:tartrate) ALB Albumin BCG plus ALB Albumin BCP ALP Alkaline phosphatase
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 European Medicines Agency Veterinary Medicines and Inspections EMEA/MRL/888/03-FINAL June 2004 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS OXFENDAZOLE (Extrapolation to all ruminants) SUMMARY REPORT (4)
European Medicines Agency Veterinary Medicines and Inspections EMEA/MRL/888/03-FINAL June 2004 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS OXFENDAZOLE (Extrapolation to all ruminants) SUMMARY REPORT (4)
Clinician Blood Panel Results
 Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
HOW TO DEAL WITH THOSE ABNORMAL LIVER ENZYMES David C. Twedt DVM, DACVIM Colorado State University Fort Collins, CO
 HOW TO DEAL WITH THOSE ABNORMAL LIVER ENZYMES David C. Twedt DVM, DACVIM Colorado State University Fort Collins, CO The identification of abnormal liver enzymes usually indicates liver damage but rarely
HOW TO DEAL WITH THOSE ABNORMAL LIVER ENZYMES David C. Twedt DVM, DACVIM Colorado State University Fort Collins, CO The identification of abnormal liver enzymes usually indicates liver damage but rarely
TR-469 Toxicology and Carcinogenesis Studies of AZT (CAS No ) and AZT/-Interferon A/D B6C3F1 Mice (Gavage Studies)
 (This document has been reformatted unchanged from the original document except for the bold emphasis editing by Stuart Thomson, Director, the Gaia Research Institute. S.T.) TR-469 Toxicology and Carcinogenesis
(This document has been reformatted unchanged from the original document except for the bold emphasis editing by Stuart Thomson, Director, the Gaia Research Institute. S.T.) TR-469 Toxicology and Carcinogenesis
Plasma Red blood cells White blood cells. Leucocytes KEYWORDS Phagocytes
 Blood Plasma Red blood cells White blood cells Platelets Lymphocytes Leucocytes KEYWORDS Phagocytes Monocytes Erythocytes ABO groups Haemoglobin Blood components: Components of blood: Plasma Red blood
Blood Plasma Red blood cells White blood cells Platelets Lymphocytes Leucocytes KEYWORDS Phagocytes Monocytes Erythocytes ABO groups Haemoglobin Blood components: Components of blood: Plasma Red blood
Rapid Laboratories In House Tests
 Electrolytes CL CL (CHLORIDE) Electrolytes CO2 CO2 (BICARBONATE) Electrolytes K K (POTASSIUM) Electrolytes NA NA (SODIUM) Basic Metabolic Panel (BMP) GLU GLU (GLUCOSE) Basic Metabolic Panel (BMP) CA CA
Electrolytes CL CL (CHLORIDE) Electrolytes CO2 CO2 (BICARBONATE) Electrolytes K K (POTASSIUM) Electrolytes NA NA (SODIUM) Basic Metabolic Panel (BMP) GLU GLU (GLUCOSE) Basic Metabolic Panel (BMP) CA CA
Safety Evaluation of Modified Si Jun Zi Tang, Assessment of Controlled and Randomized, Acute and Subchronic Mouse Oral Toxicity Studies
 Safety Evaluation of Modified Si Jun Zi Tang, Assessment of Controlled and Randomized, Acute and Subchronic Mouse Oral Toxicity Studies Shuang Ma DVM, MS, Aituan Ma DVM, MS, PhD ABSTRACT In order to evaluate
Safety Evaluation of Modified Si Jun Zi Tang, Assessment of Controlled and Randomized, Acute and Subchronic Mouse Oral Toxicity Studies Shuang Ma DVM, MS, Aituan Ma DVM, MS, PhD ABSTRACT In order to evaluate
Light yellow to dark golden yellow Clear ph range Specific gravity Sediments
 #11 Objectives: Understand specific gravity and identify normal specific gravity values for urine Learn to use a urine hydrometer to measure specific gravity Define specific gravity and identify normal
#11 Objectives: Understand specific gravity and identify normal specific gravity values for urine Learn to use a urine hydrometer to measure specific gravity Define specific gravity and identify normal
SAFETY ASPECTS OF MIDAZOLAM
 Br. J. clin. Pharmac. (1983), 16, 37S-41S Biological Pharmaceutical Research Department, F. Hoffmann-La Roche & Co Ltd, CH-4002 Basle, Switzerland 1 The LD50 in the rat and the mouse is about 1600 mg/kg
Br. J. clin. Pharmac. (1983), 16, 37S-41S Biological Pharmaceutical Research Department, F. Hoffmann-La Roche & Co Ltd, CH-4002 Basle, Switzerland 1 The LD50 in the rat and the mouse is about 1600 mg/kg
Supporting information. Precise Photodynamic Therapy of Cancer via Subcellular Dynamic Tracing of Dual-loaded Upconversion Nanophotosensitizers
 Supporting information Precise Photodynamic Therapy of Cancer via Subcellular Dynamic Tracing of Dual-loaded Upconversion Nanophotosensitizers Yulei Chang 1, Xiaodan Li 1,3, Li zhang 1,3, Lu Xia 1, Xiaomin
Supporting information Precise Photodynamic Therapy of Cancer via Subcellular Dynamic Tracing of Dual-loaded Upconversion Nanophotosensitizers Yulei Chang 1, Xiaodan Li 1,3, Li zhang 1,3, Lu Xia 1, Xiaomin
Teratogenic and Anti-mutagenic Potentials of Aqueous Leaf Extract of Momordica charantia Linn.
 International Journal of Sciences: Basic and Applied Research (IJSBAR) ISSN 2307-4531 (Print & Online) http://gssrr.org/index.php?journal=journalofbasicandapplied ---------------------------------------------------------------------------------------------------------------------------
International Journal of Sciences: Basic and Applied Research (IJSBAR) ISSN 2307-4531 (Print & Online) http://gssrr.org/index.php?journal=journalofbasicandapplied ---------------------------------------------------------------------------------------------------------------------------
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/339/98-FINAL May 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS VALNEMULIN SUMMARY REPORT 1.
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/339/98-FINAL May 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS VALNEMULIN SUMMARY REPORT 1.
Practical approaches for endocrine toxicity in preclinical safety assessment
 Practical approaches for endocrine toxicity in preclinical safety assessment Akira Inomata, D.V.M., Ph.D., D.J.S.T.P. Tsukuba Drug Safety, Global Drug Safety, Biopharmaceutical Assessments Core Function
Practical approaches for endocrine toxicity in preclinical safety assessment Akira Inomata, D.V.M., Ph.D., D.J.S.T.P. Tsukuba Drug Safety, Global Drug Safety, Biopharmaceutical Assessments Core Function
What is the composition of blood, including blood cells? What organs and structures control the flow of blood throughout the body?
 3 Chapter 10: Circulatory System and Lymphatic System In this chapter, you will learn about the structure and function of the circulatory system and lymphatic system. What is the composition of blood,
3 Chapter 10: Circulatory System and Lymphatic System In this chapter, you will learn about the structure and function of the circulatory system and lymphatic system. What is the composition of blood,
HYPOGLYCAEMIC ACTION OF THE FLAVONOID FRACTION OF ARTOCARPUS HETEROPHYLLUS LEAF
 42 Research Paper ISSN 0189-6016 2006 Afr. J. Traditional, Complementary and Alternative Medicines www.africanethnomedicines.net HYPOGLYCAEMIC ACTION OF THE FLAVONOID FRACTION OF ARTOCARPUS HETEROPHYLLUS
42 Research Paper ISSN 0189-6016 2006 Afr. J. Traditional, Complementary and Alternative Medicines www.africanethnomedicines.net HYPOGLYCAEMIC ACTION OF THE FLAVONOID FRACTION OF ARTOCARPUS HETEROPHYLLUS
PATHOLOGY Intracellular Degeneration LAB 1
 PATHOLOGY Intracellular Degeneration LAB 1 Cellular swelling Liver Organ :- Liver Lesion :- 1. Narrowing of hepatic sinusoids due to the swelling of hepatocyte. 2. The cytoplasm of affected hepatocyte
PATHOLOGY Intracellular Degeneration LAB 1 Cellular swelling Liver Organ :- Liver Lesion :- 1. Narrowing of hepatic sinusoids due to the swelling of hepatocyte. 2. The cytoplasm of affected hepatocyte
Supporting Information (SI) for: Renal Clearable Peptide Functionalized NaGdF 4 Nanodots for. High-Efficiency Tracking Orthotopic Colorectal Tumor in
 Supporting Information (SI) for: Renal Clearable Peptide Functionalized NaGdF 4 Nanodots for High-Efficiency Tracking Orthotopic Colorectal Tumor in Mouse Hongda Chen, a,c Xiaodong Li, b Fuyao Liu, a Huimao
Supporting Information (SI) for: Renal Clearable Peptide Functionalized NaGdF 4 Nanodots for High-Efficiency Tracking Orthotopic Colorectal Tumor in Mouse Hongda Chen, a,c Xiaodong Li, b Fuyao Liu, a Huimao
The Endocrine System PART B
 9 The Endocrine System PART B PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Thyroid Gland Found
9 The Endocrine System PART B PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Thyroid Gland Found
Unit Seven Blood and Immunity
 Unit Seven Blood and Immunity I. Introduction A. Definition Blood is a sticky fluid that is heavier and thicker than water. Blood is a type of, whose cells and suspended in a liquid intercellular material.
Unit Seven Blood and Immunity I. Introduction A. Definition Blood is a sticky fluid that is heavier and thicker than water. Blood is a type of, whose cells and suspended in a liquid intercellular material.
INTERNATIONAL JOURNAL OF PHYTOTHERAPY RESEARCH ISSN Research article
 Research article EVALUATION OF SAFETY OF VETTUMARAN GUTIKA THROUGH SUB ACUTE TOXICITY STUDY IN WISTAR RATS Sanjaya Kumar Y.R*, Sushant S Parekar, Thamizh Selvam N, Sanal Gopi C.G and Sudarsanan Nair PK
Research article EVALUATION OF SAFETY OF VETTUMARAN GUTIKA THROUGH SUB ACUTE TOXICITY STUDY IN WISTAR RATS Sanjaya Kumar Y.R*, Sushant S Parekar, Thamizh Selvam N, Sanal Gopi C.G and Sudarsanan Nair PK
The Effects of Feeding MIN-AD and Sodium Bicarbonate on Early Lactation Performance of Dairy Cattle
 D-3.0-06/04 The Effects of Feeding MIN-AD and Sodium Bicarbonate on Early Lactation Performance of Dairy Cattle Abstract To determine the effects of MIN-AD on early lactation performance, 56 pregnant primi-
D-3.0-06/04 The Effects of Feeding MIN-AD and Sodium Bicarbonate on Early Lactation Performance of Dairy Cattle Abstract To determine the effects of MIN-AD on early lactation performance, 56 pregnant primi-
Organochloride pesticides impaired mitochondrial function in hepatocytes and. aggravated disorders of fatty acids metabolism
 Supplementary Materials Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acids metabolism Qian Liu 1,2,3*, Qihan Wang 4*, Cheng Xu 2,3, Wentao
Supplementary Materials Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acids metabolism Qian Liu 1,2,3*, Qihan Wang 4*, Cheng Xu 2,3, Wentao
Aspartate Transaminase (AST) Color Endpoint Assay Kit Manual Catalog #:
 Aspartate Transaminase (AST) Color Endpoint Assay Kit Manual Catalog #: 5605-01 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf
Aspartate Transaminase (AST) Color Endpoint Assay Kit Manual Catalog #: 5605-01 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf
The Development of Chronic Hepatitis in Rabbits Experimentally Infected. with HEV Isolate from Rabbit
 The Development of Chronic Hepatitis in Rabbits Experimentally Infected with HEV Isolate from Rabbit Peking University, Beijing, China Ling Wang October 7 2014 Hepatitis E Virus (HEV) Characteristics Faecal-oral
The Development of Chronic Hepatitis in Rabbits Experimentally Infected with HEV Isolate from Rabbit Peking University, Beijing, China Ling Wang October 7 2014 Hepatitis E Virus (HEV) Characteristics Faecal-oral
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017
 M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
Dr/ Sherein Saeid AbdElgayed, ph.d
 هللامسب Dr/ Sherein Saeid AbdElgayed, ph.d Professor of Veterinary Pathology, Cairo University, Giza, Egypt. Chairman of the Editorial Board of Arab Journal of Science & Research Publishing (AJSRP) http://www.ajsrp.com
هللامسب Dr/ Sherein Saeid AbdElgayed, ph.d Professor of Veterinary Pathology, Cairo University, Giza, Egypt. Chairman of the Editorial Board of Arab Journal of Science & Research Publishing (AJSRP) http://www.ajsrp.com
DICHLOROACETONITRILE. 1. Exposure Data
 DICHLOROACETONITRILE Data were last evaluated in IARC (1991). 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature Chem. Abstr. Serv. Reg. No.: 3018-12-0 Chem. Abstr. Name: Dichloroacetonitrile
DICHLOROACETONITRILE Data were last evaluated in IARC (1991). 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature Chem. Abstr. Serv. Reg. No.: 3018-12-0 Chem. Abstr. Name: Dichloroacetonitrile
TABLE OF CONTENTS GENERAL INFORMATION... 1
 BIOO RESEARCH PRODUCTS Glucose Assay Kit Manual Catalog #: 5611-01 BIOO Scientific Corp. 2011 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview... 1 Required Materials
BIOO RESEARCH PRODUCTS Glucose Assay Kit Manual Catalog #: 5611-01 BIOO Scientific Corp. 2011 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview... 1 Required Materials
Genotoxicity of 2-Hydroxy-1,4-naphthoquinone (Lawsone, CAS )
 Genotoxicity of 2-Hydroxy-1,4-naphthoquinone (Lawsone, CAS 83-72-7) Bewertung des BgVV vom 21. Juni 2002 Die Substanz Lawson (2-Hydroxy-1,4-naphthochinon) ist ein Bestandteil von Haarfärbemitteln wie z.b.
Genotoxicity of 2-Hydroxy-1,4-naphthoquinone (Lawsone, CAS 83-72-7) Bewertung des BgVV vom 21. Juni 2002 Die Substanz Lawson (2-Hydroxy-1,4-naphthochinon) ist ein Bestandteil von Haarfärbemitteln wie z.b.
Function Alimentary Canal
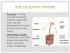 THE DIGESTIVE SYSTEM Function: to help convert food into simpler molecules that can be absorbed and used by the cells of the body. Alimentary Canala one way tube that passes through the body. (found in
THE DIGESTIVE SYSTEM Function: to help convert food into simpler molecules that can be absorbed and used by the cells of the body. Alimentary Canala one way tube that passes through the body. (found in
i. Where is the participant seen?
 PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
Complete Medical History
 Lab Results for Ben Greenfield Last Test Date: Your medical history is not complete. Complete Medical History Complete Medical History What's Next Blood Draw Blood draw scheduled Complete your medical
Lab Results for Ben Greenfield Last Test Date: Your medical history is not complete. Complete Medical History Complete Medical History What's Next Blood Draw Blood draw scheduled Complete your medical
TRACP & ALP double-stain Kit
 Table of Content I. Description... 2 II. Introduction... 2 III. Principles... 2 IV. Kit components... 3 V. Storage... 3 VI. Preparation of reagents... 3 VII. Methods... 4-7 Cell fixation... 4 Activity
Table of Content I. Description... 2 II. Introduction... 2 III. Principles... 2 IV. Kit components... 3 V. Storage... 3 VI. Preparation of reagents... 3 VII. Methods... 4-7 Cell fixation... 4 Activity
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes
 Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
Comparison of VACUETTE Heparin Gel Tubes for Common Chemistry Analytes Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood collection since 9. The
COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE
 European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/77290/05-FINAL March 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE FLUAZURON SUMMARY REPORT 1. Fluazuron is an insect
European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/77290/05-FINAL March 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE FLUAZURON SUMMARY REPORT 1. Fluazuron is an insect
Excretion and Water Balance
 Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions
 Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
Stability of VACUETTE Lithium Heparin Separator tubes with modified centrifugation conditions Background: Greiner-Bio-One, Austria has been selling plastic evacuated tubes (VACUETTE ) for venous blood
Chapter 19(1) An Introduction to the Circulatory System and Blood
 Chapter 19(1) An Introduction to the Circulatory System and Blood Circulatory System circulatory system = heart, blood vessels and blood cardiovascular system = heart and blood vessels hematology = the
Chapter 19(1) An Introduction to the Circulatory System and Blood Circulatory System circulatory system = heart, blood vessels and blood cardiovascular system = heart and blood vessels hematology = the
HEALTH SCREEN CARE. VIGNE Healthcare provides a comprehensive Health Screening Package of EXCLUSIVE HEALTH SCREENING EXPERIENCE
 HEALTH SCREEN CARE VIGNE Healthcare provides a comprehensive Health Screening Package of Silver Gold Platinum Cancer EXCLUSIVE HEALTH SCREENING EXPERIENCE Meet & Greet Medical Review with Doctor Appointment
HEALTH SCREEN CARE VIGNE Healthcare provides a comprehensive Health Screening Package of Silver Gold Platinum Cancer EXCLUSIVE HEALTH SCREENING EXPERIENCE Meet & Greet Medical Review with Doctor Appointment
Adrenal Glands. Adrenal Glands. Adrenal Glands. Adrenal Glands. Adrenal Glands 4/12/2016. Controlled by both nerves and hormones.
 Glands http://www.hawaiilife.com/articles/2012/03/good-news-vacation-rental-owners/ 70 Figure 10.14a gland Glands cortex Mineralocorticoids Gonadocorticoids Glucocorticoids medulla Epinephrine Norepinephrine
Glands http://www.hawaiilife.com/articles/2012/03/good-news-vacation-rental-owners/ 70 Figure 10.14a gland Glands cortex Mineralocorticoids Gonadocorticoids Glucocorticoids medulla Epinephrine Norepinephrine
Name: Period: Review for Animal Systems Test II - KEY
 Name: Period: Review for Animal Systems Test II - KEY Questions 1-9: Write the main functions of the following body s in the spaces below. Then, write the levels of organization for each body. Give specific
Name: Period: Review for Animal Systems Test II - KEY Questions 1-9: Write the main functions of the following body s in the spaces below. Then, write the levels of organization for each body. Give specific
Effects of Dietary Zinc and Endotoxin Challenge on Immune Function in Weanling Pigs
 2000 Animal Science Research Report Pages 154-159 Effects of Dietary Zinc and Endotoxin Challenge on Immune Function in Weanling Pigs S. Mandali, M.Rincker, S.D. Carter, A.B. Arquitt, E. Droke, B.J.Stoecker
2000 Animal Science Research Report Pages 154-159 Effects of Dietary Zinc and Endotoxin Challenge on Immune Function in Weanling Pigs S. Mandali, M.Rincker, S.D. Carter, A.B. Arquitt, E. Droke, B.J.Stoecker
H. M. Carleton, Lecturer in Histology, University of Oxford. (From the Department of Physiology.) INTRODUCTORY.
 Note on the Comparative Effects on Tissues of Isotonic Saline and Distilled Water when used as Solvents for Mercuric Chloride and Formol in Histological Fixation. By H. M. Carleton, Lecturer in Histology,
Note on the Comparative Effects on Tissues of Isotonic Saline and Distilled Water when used as Solvents for Mercuric Chloride and Formol in Histological Fixation. By H. M. Carleton, Lecturer in Histology,
PRODUCT INFORMATION WARTEC SOLUTION
 PRODUCT INFORMATION WARTEC SOLUTION NAME OF THE MEDICINE WARTEC Solution contains podophyllotoxin as the active ingredient. CAS number: 518-28-5 DESCRIPTION WARTEC Solution is a blue solution for topical
PRODUCT INFORMATION WARTEC SOLUTION NAME OF THE MEDICINE WARTEC Solution contains podophyllotoxin as the active ingredient. CAS number: 518-28-5 DESCRIPTION WARTEC Solution is a blue solution for topical
BIOO RESEARCH PRODUCTS. Aspartate Transaminase (AST) Color Endpoint Assay Kit Manual Catalog #:
 BIOO RESEARCH PRODUCTS Aspartate Transaminase (AST) Color Endpoint Assay Kit Manual Catalog #: 5605-01 BIOO Scientific 2010 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure
BIOO RESEARCH PRODUCTS Aspartate Transaminase (AST) Color Endpoint Assay Kit Manual Catalog #: 5605-01 BIOO Scientific 2010 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure
Chapter 16 Lymphatic System and Immunity. Lymphatic Pathways. Lymphatic Capillaries. network of vessels that assist in circulating fluids
 Chapter 16 Lymphatic System and Immunity network of vessels that assist in circulating fluids closely associated with the cardiovascular system transports excess fluid away from interstitial spaces transports
Chapter 16 Lymphatic System and Immunity network of vessels that assist in circulating fluids closely associated with the cardiovascular system transports excess fluid away from interstitial spaces transports
HUMAN BIOLOGY (SPECIFICATION A) Unit 7 The Human Life-span
 Surname Other Names Leave blank Centre Number Candidate Number Candidate Signature General Certificate of Education June 2003 Advanced Level Examination HUMAN BIOLOGY (SPECIFICATION A) Unit 7 The Human
Surname Other Names Leave blank Centre Number Candidate Number Candidate Signature General Certificate of Education June 2003 Advanced Level Examination HUMAN BIOLOGY (SPECIFICATION A) Unit 7 The Human
The Lymphoid System Pearson Education, Inc.
 23 The Lymphoid System Introduction The lymphoid system consists of: Lymph Lymphatic vessels Lymphoid organs An Overview of the Lymphoid System Lymph consists of: Interstitial fluid Lymphocytes Macrophages
23 The Lymphoid System Introduction The lymphoid system consists of: Lymph Lymphatic vessels Lymphoid organs An Overview of the Lymphoid System Lymph consists of: Interstitial fluid Lymphocytes Macrophages
Antimicrobial AlphaSan Test Report Summary Table 08/21/01
 AlphaSan RC 5000 Physical Chemical Properties Acute Oral Toxicity, Rat EPA FIFRA 81-1 Acute Dermal Toxicity, Rat EPA FIFRA 81-2 Primary Dermal Irritation Rabbit EPA FIFRA 81-5 Primary Eye Irritation Rabbit
AlphaSan RC 5000 Physical Chemical Properties Acute Oral Toxicity, Rat EPA FIFRA 81-1 Acute Dermal Toxicity, Rat EPA FIFRA 81-2 Primary Dermal Irritation Rabbit EPA FIFRA 81-5 Primary Eye Irritation Rabbit
IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS PDE FOR CUMENE
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE DRAFT CONSENSUS GUIDELINE IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS Released for
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE DRAFT CONSENSUS GUIDELINE IMPURITIES: GUIDELINE FOR RESIDUAL SOLVENTS Released for
Sinusoids and venous sinuses
 LYMPHOID SYSTEM General aspects Consists of organs that are made of lymphoid tissue; Immune defense Breakdown of red blood cells. 1 Sinusoids In place of capillaries Endothelium; often fenestrated More
LYMPHOID SYSTEM General aspects Consists of organs that are made of lymphoid tissue; Immune defense Breakdown of red blood cells. 1 Sinusoids In place of capillaries Endothelium; often fenestrated More
Experiment 6. Determination of the enzyme ALT or SGPT activity in serum by enzymatic method using Biophotometer
 Experiment 6 Determination of the enzyme ALT or SGPT activity in serum by enzymatic method using Biophotometer Background: Alanine aminotransferase (glutamate pyruvate transaminase) belongs to the group
Experiment 6 Determination of the enzyme ALT or SGPT activity in serum by enzymatic method using Biophotometer Background: Alanine aminotransferase (glutamate pyruvate transaminase) belongs to the group
Etiology Bacteria Sand particles Particles of ingesta / intestinal contents Desquamated epithelium
 10 CONCRETIONS Concretions Calculi o Urinary Calculi o Biliary Calculi o Salivary Calculi o Pancreatic Calculi o Enteric Calculi Piliconcretions Phytoconcretions Polyconcretions Model Questions CONCRETIONS
10 CONCRETIONS Concretions Calculi o Urinary Calculi o Biliary Calculi o Salivary Calculi o Pancreatic Calculi o Enteric Calculi Piliconcretions Phytoconcretions Polyconcretions Model Questions CONCRETIONS
Effect of PHYTASE 5000 (Granular) on the Performance and Nutrient Digestibility of Growing Finishing Pigs
 Effect of PHYTASE 5000 (Granular) on the Performance and Nutrient Digestibility of Growing Finishing Pigs 1. Introduction Vegetal materials contain plenty of phytate phosphorus, the utilization rate of
Effect of PHYTASE 5000 (Granular) on the Performance and Nutrient Digestibility of Growing Finishing Pigs 1. Introduction Vegetal materials contain plenty of phytate phosphorus, the utilization rate of
How to interpret your urine sample results
 How to interpret your urine sample results Chronic UTI Info Factsheet Series Once you have submitted your urine sample for analysis, it will be sent off to the local laboratory or hospital laboratory if
How to interpret your urine sample results Chronic UTI Info Factsheet Series Once you have submitted your urine sample for analysis, it will be sent off to the local laboratory or hospital laboratory if
complemented with SipA ( SipA/pSipA) or SL1344 WT for 48 hours, after which the
 P-gp expression (% of control) 12 1 8 6 4 2 * * Untreated SipA SipA/pSipA WT Supplementary Figure 1. SipA modulates the expression of P-gp in healthy murine intestinal epithelium in vivo. Salmonella Typhimurium
P-gp expression (% of control) 12 1 8 6 4 2 * * Untreated SipA SipA/pSipA WT Supplementary Figure 1. SipA modulates the expression of P-gp in healthy murine intestinal epithelium in vivo. Salmonella Typhimurium
