preparation which is available contains an oligo-1,4-0 1,4-glucantransferase
|
|
|
- Agatha Small
- 5 years ago
- Views:
Transcription
1 THE PROPERTIES OF AN OLIGO-1,4 -- 1,4-GLUCANTRANSFERASE FROM ANIMAL. TISSUES* BY DAVID H. BROWN AND BARBARA ILLINGWORTH DEPARTMENT OF BIOLOGICAL CHEMISTRY, WASHINGTON UNIVERSITY SCHOOL OF MEDICINE, SAINT LOUIS, MISSOURI Communicated by Carl F. Cori, July 16, 1962 The limit dextrin produced by exhaustive treatment of glycogen or amylopectin with phosphorylase and inorganic phosphate was assumed by Cori and Larner to be asymmetric with respect to the number of glucose units remaining on the main and side branches.' The fact that amylo-1,6-glucosidase preparations acted without delay on such a limit dextrin to produce free glucose (see Fig. 2a of previous paper2) was taken to mean that the outer 1,6-linked branch point glucose residues had been exposed by prior phosphorylase degradation. Larner and Schliselfeld found that the rate curve for glucose liberation by glucosidase was predictable on the basis of the Km for the limit dextrin and the Ki for competitive product inhibition.3 Walker and Whelan have studied the structure of such a limit dextrin by other enzymatic means and have concluded that the arrangement of glucose residues about an outer branch point is symmetric and that four glucose units remain on each chain.4 In such a structure there are no exposed branch point glucose residues. To account for the action of amylo-1,6-glucosidase on a limit dextrin these authors have proposed that the enzyme preparation is contaminated by a transglycosylase whose action in exposing a branch point unit is required before the glucosidase can act. They found, in fact, that a partially purified preparation of the glucosidase of rabbit muscle acted on maltose and maltotriaose in such a way that it appeared to contain a transglycosylase of the type described by Giri et al.5 and by Stetten.6 This activity expressed itself as the transfer of one glucose residue in a-1,4 linkage from a donor oligosaccharide to a suitable acceptor. On the other hand, Illingworth et al. found that after amylo-1,6-glucosidase is purified more extensively by column chromatography, it has no action whatsoever on maltose or maltotriaose.7 Recently Verhue and Hers concluded that the symmetric structure of Whelan coexists with the asymmetric structure of Cori in the limit dextrin molecule, possibly because some branches are inaccessible to phosphorylase action.8 We have now been able to show that the most highly purified amylo-1,6-glucosidase preparation which is available contains an oligo-1,4-0 1,4-glucantransferase which has no action on maltose or maltotriaose, but which is able to move, preferentially, a chain of three maltosidically linked glucose residues, and, to a lesser extent, two such residues from a donor to an acceptor oligosaccharide or even to glucose itself. It has no significant action in transferring a single glucose unit. Thus, this enzyme has some of the properties found by Whelan and co-workers for the D-enzyme of potato,9 as well as some of those reported by Petrova for a transglycosylase from rabbit liver. 10 Because of its preference for the movement of either three or two glucose residues, the animal enzyme appears to have a sharper specificity than the D-enzyme of plants. The presence of this oligotransferase in amylo-1,6-glucosidase preparations does not itself prove that it is a required enzyme in glycogen degradation. This ques- 1783
2 1784 BIOCHEMISTRY: BROWN AND ILLINGWORTH PROC. N. A. S. tion can be settled only when glucosidase preparations are available without oligotransferase and vice versa. A study of the enzymatic constitution of tissues from cases of glycogen storage disease could also be decisive. Neither does the existence of the oligotransferase of itself make possible an answer to the problem of the structure of a phosphorylase limit dextrin. Despite the evidence of Hers8 that there are exposed glucose branch points in the limit dextrin, the possibility must be considered that phosphorylase preparations used to prepare limit dextrins may contain the oligotransferase whose presence would ordinarily escape detection in the absence of amylo-1,6-glucosidase which can be effectively removed from phosphorylase by recrystallization. A definitive answer to this problem requires a separate test for transferase activity. Materials and Methods.-The preparation of amylo-1,6-glucosidase has been outlined.2 The low molecular weight branched dextrins used in this work were prepared either from glycogen or from starch-u-c'4 (500,000 cpm per Amole "glucose") to which corn amylopectin was added as a diluent before a-amylase digestion.2 All reaction mixtures were deionized on Amberlite MB-3 resin columns before descending chromatography on Whatman No. 1 paper in a butanol-pyridinewater solvent (3:2:1.5). In the case of experiments involving isotopically labeled substrates, the paper chromatograms were examined for radioactive zones in a Vanguard Automatic Scanner equipped with an Automatic Data System so that the relative amount of C'4 in the different compounds could also be determined. In some cases, the radioactive substances were eluted with water so that their specific activities could be determined. Total glucose was measured microenzymatically after hydrolysis at 1000 in 1 N HCl for 3 hr. Radioactivity was measured with either a windowless gas-flow counter or a low background counter (Nuclear). Results.-The nature of the reaction catalyzed by the oligotransferase was shown in an experiment in which 4.14 Mmoles of maltotriaose-u-c14 (sp. act., 23,850 cpm/amole) was incubated in 1.0 ml of M Na citrate and 0.01 M glycylglycine and M mercaptoethanol buffer, ph 6.5, with 20 mg of human muscle glycogen and 25,ug of a rabbit muscle protein fraction (in which amylo-1,6-glucosidase had a sp. act. of 66,000 units/mg). Incubation was for 22 hr at 30 under toluene vapor. A control was incubated without added glycogen. The glycogen was isolated from the first reaction mixture by alcohol precipitation and purified by alkali-digestion and repeated reprecipitations. It was found to be unlabeled (2.36 mg had 14.1 cpm; background, 14.2 cpm). Thus, there was no transfer of any portion of the maltotriaose molecule to glycogen. Chromatography of the reaction mixture revealed that although it contained only a trace of radioactive glucose (present also in the control incubation), there was a prominent radioactive peak of maltohexaose and a lesser peak of maltopentaose. In contrast, maltotetraose was present as a barely detectable zone. Sufficient maltohexaose had been formed to allow its detection by a benzidine-trichloroacetic acid spray. The control incubation mixture contained no trace of any radioactive compound other than the maltotriaose which had been added. Scanning the experimental chromatogram showed that a total of 1,650 cpm of maltohexaose and 421 cpm of maltopentaose had been formed in the reaction mixture. The maltohexaose was eluted and found to have a sp. act. of 20,470 cpm/amole. The fact that this is 86% of that of the added maltotriaose shows that the molecule of maltohexaose consists of three labeled and three unlabeled glucose residues, the latter derived from glycogen. That the oligotransferase prefers to move three units is shown by the fact that maltopentaose was formed (by the movement of two units) in only 25%7 of the yield of the hexaose.
3 Voi.. 48, 1962 BIOCHEMISTRY: BROWN AND ILLINGWORTH 1785 That the enzyme has little or no ability to move one glucose unit is shown by the fact that maltotetraose was scarcely detectable in the reaction mixture. It should be emphasized that the oligotransferase reaction is slow and incomplete when studied in a system such as that just described. Thus, only about 4%N of the maltotriaose was converted to the two higher oligosaccharides. Maltotriaose is not the most favorable acceptor in such a system. When the singly branched compound containing five glucose residues which has been found to be acted upon directly by amylo-1,6-glucosidase2 was used instead of maltotriaose in a reaction mixture containing glycogen and the oligotransferase, 21/2 hr of incubation served to produce approximately as much of the transfer products (a branched 8-unit dextrin and a branched 7-unit dextrin) as was observed in 22 hr in the triaose experiment already described. Thus, the oligotransferase acts more readily when 90 branched dextrins are available as ac- > 80 ceptors. However, it has been found E that the branched 4-unit dextrin does m0 Fast 8 not act as an acceptor at all under con-60- ditions where the branched 5-unit com- / pound which is a glucosidase substrate v 50 is effective. Thus, the oligotransferase X appears to favor those branched oligo- a saccharides as acceptors in which at m 30 least one glucose residue is on the main 20- chain beyond the branch point, sic / Glucose If6 0 Gluctose1-4 Glucose1-4 Glucose1---- TIME OF INCUBATION (HOURS) The availability of a B7 dextrin has FIG. 1.-Rates of formation of glucose from made possible a demonstration of the Fast B1 by direct amylo-1,6-glucosidase action ability of the oligotransferase to and from move B7 by the action of the glucosidase following the action of oligotransferase. B.;, 8.1 X a maltesyl unit but not a glucosyl unit 10-i AI; B7, 8.7 X 10-4 Al. Enzyme, 29.6,4g intramolecularly as well as intermolec- mg; protein/ml; 0.01 Al Na glucosidase citrate and sp act., Al50,400 glycylglvcin units/- ularly. In Figure 1 are shown data and A1 mercaptoethanol buffer, ph 6.6; on the combined action of amylo-1, glucosidase and oligotransferase in producing glucose from an oligosaccharide designated B7 (see Fig. 1 of previous paper2) which contains a single branch point glucose residue covered by two glucose units. Inasmuch as there is no exposed branch point glucose residue in this structure an action of the glucosidase on this compound (B7) would not be expected. As shown, the rate of glucose formation is about 10 times slower from B7 than from the true glucosidase substrate, Fast B5, which lacks the maltose residue covering its branch point glucose. That the slow liberation of glucose is due to the fact that the oligotransferase must first act to expose the branch point unit was shown by the following experiments in which transfer of a maltosyl residue was detected. The enzyme preparation (13 sag of protein) was added to 0.60 ml containing 1.74 Mmoles of B7, 0.01 Ml Na citrate buffer, ph 6.7, and 4.1 4moles of either maltose-
4 1786 BIOCHEMISTRY: BROWN AND ILLINGWORTH PROC. N. A. S. UC14 (14,760 cpm/,gmole) or maltotriaose-u-c'4 (23,850 cpm//amole). Incubation was for 42 hr at 300 under toluene vapor. At the end of this time, 0.91 mole of glucose per mole of B7 had been formed in the reaction mixture containing added maltose, and 0.82 mole of glucose per mole of B7 in that containing added maltotriaose. Control incubations containing no added B7 had less than 1% of this amount of free glucose. The reaction mixtures were subjected to paper chromatography and the compounds present were detected by spraying with benzidinetrichloroacetic acid, and by scanning for radioactivity. In nearly every instance the quantity of each substance formed as well as its specific activity were determined. Table 1 gives the data from the experiment with added maltose-u-c'4 TABLE 1 SUBSTANCES FORMED FROM 1.74,UMOLES OF B7 BY OLIGOTRANSFERASE AND AMYLO-1,6-GLUCOSIDASE ACTION IN PRESENCE OF 4.1 limoles OF MALTOSE-U-C14 Detected by Amount isolated* Specific activity Substance formed spray (Mmoles) (cpm/umole) Glucose Maltotetraose ,740 Maltohexaose B about 0 Maltooctaose * Except for glucose which was determined directly, all other substances were analyzed after elution from paper chromatograms. and Table 2 the data from that with added maltotriaose-u-c.14 In both of these experiments as well as in others (not shown) in which no maltose or maltotriaose were added, maltotetraose and maltohexaose were formed from B7 in approximately equal amounts. The hexaose results from intramolecular movement of a maltosyl residue from the side chain to the main chain followed by glucosidase splitting of the TABLE 2 SUBSTANCES FORMED FROM AMOLES OF B7 BY OLIGOTRANSFERASE AND AMYLO-1,6-GLUCOSIDASE ACTION IN PRESENCE OF 4.1 1AMOLES OF MALTOTRIAOSE-U-C'4 Detected by Amount isolated* Specific activity Substance formed spray (,moles) (cpm/pmole) Glucose Maltotetraose Maltopentaose ,575 Maltohexaose Maltoheptaose ,840 B about 0 * See note to Table 1. rearranged B7 molecule. The tetraose results from intermolecular movement of a maltosyl residue from one B7 molecule to another. The products, B5 and B9, are then acted upon by glucosidase to give the tetraose and the octaose respectively. When C14-maltotriaose was added (Table 2), it acted as an acceptor of most of the maltosyl residues which escaped intramolecular transfer. Thus, maltopentaose of high specific activity was formed in an amount equal to that of the essentially unlabeled tetraose originating from B7 itself. Because of the rather high concentration of added C14-triaose, it served in this experiment as a more effective acceptor of the maltosyl residues than B7 present at lower concentration. Hence, no detectable B9 and maltooctaose were formed. Table 1 shows the formation of labeled tetraose (18% of the total), occurring because of the transfer of maltosyl resi-
5 VOL. 48, 1962 BIOCHEMISTRY: BROWN AND ILLINGWORTH 1787 dues to C'4-maltose. The amount thus formed (calculated: 0.09 Mmole) taken with the amount of octaose (0.11 jsmole) is an approximate measure of the total intermolecular transfer of maltosyl units. The sum, 0.20 jsmole, compares well with the quantity of labeled pentaose (0.29 Mmole) produced in the experiment of Table 2, whose formation measures the same kind of reaction. In other experiments which are not described, oligotransferase action on B7 has been allowed to occur in the presence of C14-glucose. Under these circumstances, C14-amylotriaose was formed demonstrating that the monosaccharide can act as an acceptor. Manners has suggested on the basis of glycogen structure that a glycosyltransferase may be missing in certain subtypes of Type III glycogen storage disease.11 The information which has been obtained on the action of the oligotransferase is being used to devise a specific assay for the enzyme in human tissue as well as to facilitate its further purification from muscle extracts. Elucidation of its role in glycogen metabolism must await the success of these experiments. Summary.-An oligo-1,4 -- 1,4-glucantransferase has been discovered in rabbit muscle as a contaminant of amylo-1,6-glucosidase preparations. The transferase acts to move preferentially maltotriosyl and, to a lesser extent, maltosyl residues from donor oligosaccharides, including glycogen, to acceptor linear and branched oligosaccharides. The enzyme has no detectable action in moving single glucose residues. With a branched 7-unit dextrin of suitable structure, it has been possible to observe intramolecular transfer from the side branch to the main chain as wvell as intermolecular transfer of a maltosyl residue. The authors wish to thank Sue Hettel for expert assistance in the chromatographic procedures involved in this work and Juanita Buster for making available the amylo-1,6-glueosidase preparations. * This work has been supported in part by a research grant (RG 4761) from the U.S. Public Health Service. 1 Cori, G. T., and J. Larner, J. Biol. Chem., 188, 17 (1951). 2 Illingworth, B., and D. H. Brown, these PROCEEDINGS, 48, 1619 (1962). 3 Larner, J., and L. H. Schliselfeld, Biochim. et Biophys. Acta, 20, 53 (1956). 4 Walker, G. J., and W. J. Whelan, Biochem. J., 76, 264 (1960). 5 Giri, K. V., A. Nagabhushanam, V. N. Nigam, and B. Belavadi, Science, 121, 898 (1955). 6 Stetten, M., J. Am. Chem. Soc., 81, 1437 (1959). 7 Illingworth, B., D. H. Brown, and C. F. Cori, Fed. Proc., 20, 86 (1961). 8Verhue, W., and H. G. Hers, Arch. Int. Physiol. et Biochim., 69, 757 (1961). 9 Walker, G. J., and W. J. Whelan, Biochem. J., 67, 548 (1957). 10 Petrova, A. N., Enzymologia, 21, 23 (1959). 1 Manners, D. J., and A. Wright, Biochem. J., 79, P (1961).
ON THE NATURE OF THE TRANSALDOLASE-DIHYDROXYACETONE
 VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
VOL. 47, 1961 BIOCHEMISTRY: HORECKER ET AL. 1949 3 Bonsignore, A., S. Pontremoli, E. Grazi, and M. Mangiarotti, Biochem. Biophys. Research Communs., 1, 79 (1959). 4 Venkataraman, R., and E. Racker, J.
Structures of Singly Branched Heptaoses Produced by Bacterial Liquefying a-amylase
 /. Biochem., 78, 889-896 (1975) Structures of Singly Branched Heptaoses Produced by Bacterial Liquefying a-amylase Kimio UMEKI and Takehiko YAMAMOTO Faculty of Science, Osaka City University, Sumiyoshi-ku,
/. Biochem., 78, 889-896 (1975) Structures of Singly Branched Heptaoses Produced by Bacterial Liquefying a-amylase Kimio UMEKI and Takehiko YAMAMOTO Faculty of Science, Osaka City University, Sumiyoshi-ku,
ACTION OF, 3-AMYLASE ON BRANCHED OLIGOSACCHARIDES*
 ACTON OF, 3-AMYLASE ON BRANCHED OLGOSACCHARDES* BY RUSS SUMMER AND DEXTER FRENCH (From the Department of Chemistry, owa State College, Ames, owa) (Received for publication, January 3, 1956) t has been
ACTON OF, 3-AMYLASE ON BRANCHED OLGOSACCHARDES* BY RUSS SUMMER AND DEXTER FRENCH (From the Department of Chemistry, owa State College, Ames, owa) (Received for publication, January 3, 1956) t has been
Mechanism of Salivary Amylase Action
 Proceedings of the Iowa Academy of Science Volume 57 Annual Issue Article 22 1950 Mechanism of Salivary Amylase Action John H. Pazur Iowa State College Dexter French Iowa State College Doris W. Knapp Iowa
Proceedings of the Iowa Academy of Science Volume 57 Annual Issue Article 22 1950 Mechanism of Salivary Amylase Action John H. Pazur Iowa State College Dexter French Iowa State College Doris W. Knapp Iowa
Experiment 1. Isolation of Glycogen from rat Liver
 Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Experiment 1 Isolation of Glycogen from rat Liver Figure 35: FIG-2, Liver, PAS, 100x. Note the presence of a few scattered glycogen granules (GG). Objective To illustrate the method for isolating glycogen.
Chapter 1. Chemistry of Life - Advanced TABLE 1.2: title
 Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
Condensation and Hydrolysis Condensation reactions are the chemical processes by which large organic compounds are synthesized from their monomeric units. Hydrolysis reactions are the reverse process.
On pages 131, 132, 136, and 137, the word conformational should be set off by quotation marks, rather than by parentheses.
 536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
536 ERRATUM: HELMREICRIAND CORI PROC. N. A. S. ERRA TUM In the article entitled "The Role of Adenylic Acid in the Activation of Phosphorylase," by Ernst Helmreich and Carl F. Cori, which appeared in the
LOCATION AND POSSIBLE ROLE OF ESTERIFIED PHOSPHORUS IN STARCH FRACTIONS 1
 LOCATION AND POSSIBLE ROLE OF ESTERIFIED PHOSPHORUS IN STARCH FRACTIONS 1 ABSTRACT Phosphorus determinations were made on various amelose and pectin fractions by a modified technique. Results were reproducible
LOCATION AND POSSIBLE ROLE OF ESTERIFIED PHOSPHORUS IN STARCH FRACTIONS 1 ABSTRACT Phosphorus determinations were made on various amelose and pectin fractions by a modified technique. Results were reproducible
Pattern of Action of Bacillus stearothermophilus Neopullulanase on Pullulan
 JOURNAL OF BACTERIOLOGY, Jan. 1989, p. 369-374 21-9193/89/1369-6$2./ Copyright 1989, American Society for Microbiology Vol. 171, No. 1 Pattern of Action of Bacillus stearothermophilus Neopullulanase on
JOURNAL OF BACTERIOLOGY, Jan. 1989, p. 369-374 21-9193/89/1369-6$2./ Copyright 1989, American Society for Microbiology Vol. 171, No. 1 Pattern of Action of Bacillus stearothermophilus Neopullulanase on
GLYCOGEN BEFORE THE LAB YOU HAVE TO READ ABOUT:
 GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
GLYCGEN BEFRE THE LAB YU HAVE T READ ABUT:. Glycogen structure. 2. Glycogen synthesis and degradation (reactions with structural formulas and enzymes). 3. The role of glycogen in liver and muscles. INTRDUCTIN
Starch Molecular Characteristics and Digestion Properties
 Starch Molecular Characteristics and Digestion Properties B.R. Hamaker, G. Zhang, Z. Ao, M. Benmoussa and S. Maghaydah Whistler Center for Carbohydrate Research Purdue University, Indiana, USA Presentation
Starch Molecular Characteristics and Digestion Properties B.R. Hamaker, G. Zhang, Z. Ao, M. Benmoussa and S. Maghaydah Whistler Center for Carbohydrate Research Purdue University, Indiana, USA Presentation
Molecular Structure and Function Polysaccharides as Energy Storage. Biochemistry
 1 1.Objectives Dr. Vijaya Khader Dr. MC Varadaraj To understand how polysaccharides act as energy source To understand the structure and energy generation process from glycogen To understand the structure
1 1.Objectives Dr. Vijaya Khader Dr. MC Varadaraj To understand how polysaccharides act as energy source To understand the structure and energy generation process from glycogen To understand the structure
Supporting Information
 Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
(I) system.1' 2, 5. been shown to occur on supplementation with vitamin K, (I) to the quinone-depleted
 ENZYMATIC FORMATION OF A PHOSPHORYLATED DERIVATIVE OF VITAMIN Ki* BY TETSUO WATANABE AND ARNOLD F. BRODIE DEPARTMENT OF MICROBIOLOGY, UNIVERSITY OF SOUTHERN CALIFORNIA SCHOOL OF MEDICINE, LOS ANGELES Communicated
ENZYMATIC FORMATION OF A PHOSPHORYLATED DERIVATIVE OF VITAMIN Ki* BY TETSUO WATANABE AND ARNOLD F. BRODIE DEPARTMENT OF MICROBIOLOGY, UNIVERSITY OF SOUTHERN CALIFORNIA SCHOOL OF MEDICINE, LOS ANGELES Communicated
Digestion of Barley Starch Granules by the Combined Action of α- and β-amylases Purified from Barley and Barley Malt
 Agricultural and Biological Chemistry ISSN: 0002-1369 (Print) (Online) Journal homepage: http://www.tandfonline.com/loi/tbbb19 Digestion of Barley Starch Granules by the Combined Action of α- and β-amylases
Agricultural and Biological Chemistry ISSN: 0002-1369 (Print) (Online) Journal homepage: http://www.tandfonline.com/loi/tbbb19 Digestion of Barley Starch Granules by the Combined Action of α- and β-amylases
Sumio KITAHATA and Shigetaka OKADA
 Agr. Biol. Chem., 39 (11), 2185 `2191, 1975 Transfer Action of Cyclodextrin Glycosyltransferase on Starch õ Sumio KITAHATA and Shigetaka OKADA Osaka Municipal Technical Research Institute, Osaka, Japan
Agr. Biol. Chem., 39 (11), 2185 `2191, 1975 Transfer Action of Cyclodextrin Glycosyltransferase on Starch õ Sumio KITAHATA and Shigetaka OKADA Osaka Municipal Technical Research Institute, Osaka, Japan
40%7 of normal. In addition, the liver glycogen isolated
 Journal of Clinical Investigation Vol. 42, No. 5, 1963 LEUKOCYTE DEBRANCHING ENZYME IN GLYCOGEN STORAGE DISEASE By HIBBARD E. WILLIAMS, ESTHER M. KENDIG, AND JAMES B. FIELD (From the Clinical Endocrinology
Journal of Clinical Investigation Vol. 42, No. 5, 1963 LEUKOCYTE DEBRANCHING ENZYME IN GLYCOGEN STORAGE DISEASE By HIBBARD E. WILLIAMS, ESTHER M. KENDIG, AND JAMES B. FIELD (From the Clinical Endocrinology
Glycogen Metabolism. BCH 340 lecture 9
 Glycogen Metabolism BC 340 lecture 9 Structure of glycogen Glycogen is homopolysaccharide formed of branched D-glucose units The primary glycosidic bond is 1-4-linkage Each branch is made of 6-12 glucose
Glycogen Metabolism BC 340 lecture 9 Structure of glycogen Glycogen is homopolysaccharide formed of branched D-glucose units The primary glycosidic bond is 1-4-linkage Each branch is made of 6-12 glucose
Energy storage in cells
 Energy storage in cells Josef Fontana EC - 58 Overview of the lecture Introduction to the storage substances of human body Overview of storage compounds in the body Glycogen metabolism Structure of glycogen
Energy storage in cells Josef Fontana EC - 58 Overview of the lecture Introduction to the storage substances of human body Overview of storage compounds in the body Glycogen metabolism Structure of glycogen
Biochemistry: A Short Course
 Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 10 Carbohydrates 2013 W. H. Freeman and Company Chapter 10 Outline Monosaccharides are aldehydes or ketones that contain two or
Tymoczko Berg Stryer Biochemistry: A Short Course Second Edition CHAPTER 10 Carbohydrates 2013 W. H. Freeman and Company Chapter 10 Outline Monosaccharides are aldehydes or ketones that contain two or
Letter to the Editor: Update on Knowledge Regarding Starch Structure and Degradation by Malt Enzymes (DP/DU and Limit Dextrinase)
 Letter to the Editor: Update on Knowledge Regarding Starch Structure and Degradation by Malt Enzymes (DP/DU and Limit Dextrinase) George N. Bathgate and Tom A. Bringhurst J. Inst. Brew. 117(1), 33 38,
Letter to the Editor: Update on Knowledge Regarding Starch Structure and Degradation by Malt Enzymes (DP/DU and Limit Dextrinase) George N. Bathgate and Tom A. Bringhurst J. Inst. Brew. 117(1), 33 38,
A BEGINNER S GUIDE TO BIOCHEMISTRY
 A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
A BEGINNER S GUIDE TO BIOCHEMISTRY Life is basically a chemical process Organic substances: contain carbon atoms bonded to other carbon atom 4 classes: carbohydrates, lipids, proteins, nucleic acids Chemical
Expression constructs
 Gene expressed in bebe3 ZmBEa Expression constructs 35S ZmBEa Pnos:Hygromycin r 35S Pnos:Hygromycin r 35S ctp YFP Pnos:Hygromycin r B -1 Chl YFP- Merge Supplemental Figure S1: Constructs Used for the Expression
Gene expressed in bebe3 ZmBEa Expression constructs 35S ZmBEa Pnos:Hygromycin r 35S Pnos:Hygromycin r 35S ctp YFP Pnos:Hygromycin r B -1 Chl YFP- Merge Supplemental Figure S1: Constructs Used for the Expression
METABOLISM OF PHENYLALANINE-CONTAINING PEPTIDE AMIDES
 METABOLISM OF PHENYLALANINE-CONTAINING PEPTIDE AMIDES IN ESCHERICHIA COLI' SOFIA SIMMONDS AND DAVID D. GRIFFITH2 Department of Biochemistry, Yale University, New Haven, Connecticut Received for publication
METABOLISM OF PHENYLALANINE-CONTAINING PEPTIDE AMIDES IN ESCHERICHIA COLI' SOFIA SIMMONDS AND DAVID D. GRIFFITH2 Department of Biochemistry, Yale University, New Haven, Connecticut Received for publication
Introduction to Carbohydrate metabolism
 Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
Introduction to Carbohydrate metabolism Some metabolic pathways of carbohydrates 1- Glycolysis 2- Krebs cycle 3- Glycogenesis 4- Glycogenolysis 5- Glyconeogenesis - Pentose Phosphate Pathway (PPP) - Curi
Topic 4 - #2 Carbohydrates Topic 2
 Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
Topic 4 - #2 Carbohydrates Topic 2 Biologically Important Monosaccharide Derivatives There are a large number of monosaccharide derivatives. A variety of chemical and enzymatic reactions produce these
Medical Biochemistry and Molecular Biology CARBOHYDRATE CHEMISTRY. By Hussein Abdelaziz
 Medical Biochemistry and Molecular Biology CARBOHYDRATE CHEMISTRY 2 By Hussein Abdelaziz Disaccharides Disaccharides consist of two sugars joined by an O-glycosidic bond. The most abundant disaccharides
Medical Biochemistry and Molecular Biology CARBOHYDRATE CHEMISTRY 2 By Hussein Abdelaziz Disaccharides Disaccharides consist of two sugars joined by an O-glycosidic bond. The most abundant disaccharides
Organic Compounds. Biology-CP Mrs. Bradbury
 Organic Compounds Biology-CP Mrs. Bradbury Carbon Chemistry The compounds that form the cells and tissues of the body are produced from similar compounds in the foods you eat. Common to most foods and
Organic Compounds Biology-CP Mrs. Bradbury Carbon Chemistry The compounds that form the cells and tissues of the body are produced from similar compounds in the foods you eat. Common to most foods and
Characterization of Starch Polysaccharides in Aqueous Systems: de facto molar masses vs supermolecular structures
 Characterization of Starch Polysaccharides in Aqueous Systems: de facto molar masses vs supermolecular structures Werner Praznik and Anton uber Department of Chemistry BKU - University of Natural Resourses
Characterization of Starch Polysaccharides in Aqueous Systems: de facto molar masses vs supermolecular structures Werner Praznik and Anton uber Department of Chemistry BKU - University of Natural Resourses
A novel α-glucosidase from the moss Scopelophila cataractae
 Vol. 54 No. 2/2007, 401 406 Regular paper on-line at: www.actabp.pl A novel α-glucosidase from the moss Scopelophila cataractae Yoshiki Yamasaki *, Susumu Nakashima and Haruyoshi Konno * Research Institute
Vol. 54 No. 2/2007, 401 406 Regular paper on-line at: www.actabp.pl A novel α-glucosidase from the moss Scopelophila cataractae Yoshiki Yamasaki *, Susumu Nakashima and Haruyoshi Konno * Research Institute
BY: RASAQ NURUDEEN OLAJIDE
 BY: RASAQ NURUDEEN LAJIDE LETURE NTENT INTRDUTIN GLYGEN BREAKDWN (Glycogenolysis) GLYGEN SYNTESIS (Glycogenesis) REGULATIN F GLYGEN METABLISM MAINTENANE F BLD GLUSE DIABETES MELLITUS AND INSULIN INTRDUTIN
BY: RASAQ NURUDEEN LAJIDE LETURE NTENT INTRDUTIN GLYGEN BREAKDWN (Glycogenolysis) GLYGEN SYNTESIS (Glycogenesis) REGULATIN F GLYGEN METABLISM MAINTENANE F BLD GLUSE DIABETES MELLITUS AND INSULIN INTRDUTIN
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/142604
ey-globulin, labeled in its protein moiety with H3-leucine, was confined to the
 THE SYNTHESIS AND SECRETION OF y-globulin BY LYMPH NODE CELLS, III. THE SLOW ACQUISITION OF THE CARBOHYDRATE MOIETY OF 7-GLOBULIN AND ITS RELATIONSHIP TO SECRETION BY ROBERT M. SWENSON* AND MILTON KERN
THE SYNTHESIS AND SECRETION OF y-globulin BY LYMPH NODE CELLS, III. THE SLOW ACQUISITION OF THE CARBOHYDRATE MOIETY OF 7-GLOBULIN AND ITS RELATIONSHIP TO SECRETION BY ROBERT M. SWENSON* AND MILTON KERN
Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol
 Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol (polyhydroxyaldehyde or polyhyroxyketone); their polymers,which
Dr. Entedhar Carbohydrates Carbohydrates are carbon compounds that have aldehyde (C-H=0) or ketone (C=O) moiety and comprises polyhyroxyl alcohol (polyhydroxyaldehyde or polyhyroxyketone); their polymers,which
TEMPORARY INHIBITION OF TRYPSIN*
 TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
TEMPORARY INHIBITION OF TRYPSIN* BY M. LASKOWSKI AND FENG CHI WU (From the Department oj Biochemistry, Marquette University School of Medicine, Milwaukee, Wisconsin) (Received for publication, April 30,
SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES
 1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
OCR (A) Biology A-level Topic 2.2: Biological molecules Notes Water Water is a very important molecule which is a major component of cells, for instance: Water is a polar molecule due to uneven distribution
Proteases in germinating finger millet (Eleusine coracana) seeds
 Biosci., Vol. 5, Number 3, September 1983, pp. 219 224. Printed in India. Proteases in germinating finger millet (Eleusine coracana) seeds Introduction U. VIDYAVATHI, B. SHIVARAJ and T. N. PATTABIRAMAN
Biosci., Vol. 5, Number 3, September 1983, pp. 219 224. Printed in India. Proteases in germinating finger millet (Eleusine coracana) seeds Introduction U. VIDYAVATHI, B. SHIVARAJ and T. N. PATTABIRAMAN
Tests for Carbohydrates
 Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
4.3 Oligosaccharides. trimethylchlorosilane, in pyridine as solvent, provides a sugar derivative with all HO-groups silylated:
 292 4 Carbohydrates trimethylchlorosilane, in pyridine as solvent, provides a sugar derivative with all HO-groups silylated: Monosaccharides form glycosides (cf. 4.2.4.5). When this occurs between the
292 4 Carbohydrates trimethylchlorosilane, in pyridine as solvent, provides a sugar derivative with all HO-groups silylated: Monosaccharides form glycosides (cf. 4.2.4.5). When this occurs between the
Enzymes - Exercise 3 - Rockville
 Enzymes - Exercise 3 - Rockville Objectives -Understand the function of an enzyme. -Know what the substrate, enzyme, and the product of the reaction for this lab. -Understand how at various environments
Enzymes - Exercise 3 - Rockville Objectives -Understand the function of an enzyme. -Know what the substrate, enzyme, and the product of the reaction for this lab. -Understand how at various environments
Chemistry of Carbon. All living things rely on one particular type of molecule: carbon
 Ach Chemistry of Carbon All living things rely on one particular type of molecule: carbon Carbon atom with an outer shell of four electrons can form covalent bonds with four atoms. In organic molecules,
Ach Chemistry of Carbon All living things rely on one particular type of molecule: carbon Carbon atom with an outer shell of four electrons can form covalent bonds with four atoms. In organic molecules,
Carbs: The Staff of Life, or The Stuff of Death? Ed Cox, M.D.
 Carbs: The Staff of Life, or The Stuff of Death? Ed Cox, M.D. Pyramid, or Paleo? Carbs defined Carbohydrates (abbrev. CHO) = saccharides Saccharide from Greek for sugar Compounds of carbon, oxygen and
Carbs: The Staff of Life, or The Stuff of Death? Ed Cox, M.D. Pyramid, or Paleo? Carbs defined Carbohydrates (abbrev. CHO) = saccharides Saccharide from Greek for sugar Compounds of carbon, oxygen and
ENZYMES QUESTIONSHEET 1
 QUESTIONSHEET 1 The apparatus illustrated below can be used to investigate the activity of the enzyme catalase, which is found in liver. The liver tissue has been ground up and mixed with a buffer solution.
QUESTIONSHEET 1 The apparatus illustrated below can be used to investigate the activity of the enzyme catalase, which is found in liver. The liver tissue has been ground up and mixed with a buffer solution.
Biochemistry Name: Practice Questions
 Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Name: Practice Questions 1. Carbohydrate molecules A and B come in contact with the cell membrane of the same cell. Molecule A passes through the membrane readily, but molecule B does not. It is most likely
Polysaccharide phosphorylase
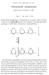 CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
CARL F. CORI and GERTY T. CORI Polysaccharide phosphorylase Nobel Lectures, December 11, 1947 Part I - by Carl F. Cori Polysaccharide phosphorylase is characterized as an enzyme which can break or make
Fundamentals of Organic Chemistry. CHAPTER 6: Carbohydrates
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 6: Carbohydrates Carbohydrates
Disaccharides. Three Important Disaccharides Maltose, Lactose, and Sucrose. The formation of these three common disaccharides are:
 DISACCHARIDES Disaccharides Three Important Disaccharides Maltose, Lactose, and Sucrose The formation of these three common disaccharides are: 2 Disaccharides Maltose (Malt Sugar) Maltose is known as malt
DISACCHARIDES Disaccharides Three Important Disaccharides Maltose, Lactose, and Sucrose The formation of these three common disaccharides are: 2 Disaccharides Maltose (Malt Sugar) Maltose is known as malt
Statement Starch Cellulose Glycogen glycosidic bonds present polymer of α-glucose unbranched chains only only found in plants
 1 The statements in the table below refer to three polysaccharide molecules. Complete the table. If the statement is correct, place a tick ( ) in the box and if the statement is incorrect place a cross
1 The statements in the table below refer to three polysaccharide molecules. Complete the table. If the statement is correct, place a tick ( ) in the box and if the statement is incorrect place a cross
A Model for the Starch Enzymatic Hydrolysis based on the Boltzmann Equation
 A Model for the Starch Enzymatic Hydrolysis based on the Boltzmann Equation A.PATSIOURA, E.REPPAS, G.MANIATIS and V.GEKAS Department of Environmental Engineering, Technical University of Crete, Polytechnioupolis,
A Model for the Starch Enzymatic Hydrolysis based on the Boltzmann Equation A.PATSIOURA, E.REPPAS, G.MANIATIS and V.GEKAS Department of Environmental Engineering, Technical University of Crete, Polytechnioupolis,
Branched Chain Amino Acid Aminotransferase of Pseudomonas
 Agric. Biol. Chem., 41 (7), 1171 `1177, 1977 Branched Chain Amino Acid Aminotransferase of Pseudomonas Yuji KOIDE, Mamoru HONMA and Tokuji SHIMOMURA Department of Agricultural Chemistry, Faculty of Agriculture,
Agric. Biol. Chem., 41 (7), 1171 `1177, 1977 Branched Chain Amino Acid Aminotransferase of Pseudomonas Yuji KOIDE, Mamoru HONMA and Tokuji SHIMOMURA Department of Agricultural Chemistry, Faculty of Agriculture,
Biological Molecules
 SIM Tuition Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t
SIM Tuition Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t
774 [Vol. 39, *) The abbreviations used are: GIcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine;
 774 [Vol. 39, 170. Separation and Identification of N-Acetylhexosamines and N Acetylneuraminic Acid by Two-dimensional Electrophoresis and Chromatography on Paper By Seiichi OHKUMA and Toshiaki SHINOHARA
774 [Vol. 39, 170. Separation and Identification of N-Acetylhexosamines and N Acetylneuraminic Acid by Two-dimensional Electrophoresis and Chromatography on Paper By Seiichi OHKUMA and Toshiaki SHINOHARA
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* Previous studies (1, 2) have shown that after the ingestion of caffeine
 A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
189,311, , ,561, ,639, ,679, Ch13; , Carbohydrates. Oligosaccharides: Determination of Sequence
 Lecture (2//7) Reading: Chs4,6,8,0,4,6,7,8; 28-29, 89,,77-80,555-557,56,62-622,69,662-66,679, 69-694 Ch; 497-50, 507-54 Problems: Ch (text); 5,6,9,0,22,24 Ch7 (study-guide: applying); 4 Ch7 (study-guide:
Lecture (2//7) Reading: Chs4,6,8,0,4,6,7,8; 28-29, 89,,77-80,555-557,56,62-622,69,662-66,679, 69-694 Ch; 497-50, 507-54 Problems: Ch (text); 5,6,9,0,22,24 Ch7 (study-guide: applying); 4 Ch7 (study-guide:
SACCHARIDES (Liquid Chromatography)
 Corn Syrup Analysis E-61-1 PRINCIPLE SCOPE A corn syrup solution is passed through a metal ion-modified cation exchange column. The individual sugars are separated by molecular exclusion and ligand exchange.
Corn Syrup Analysis E-61-1 PRINCIPLE SCOPE A corn syrup solution is passed through a metal ion-modified cation exchange column. The individual sugars are separated by molecular exclusion and ligand exchange.
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic
 Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
Learning Target: Describe characteristics and functions of carbohydrates, lipids, and proteins. Compare and contrast the classes of organic compounds. What are inorganic molecules? Molecules that CANNOT
BIOLOGY 111. CHAPTER 2: The Chemistry of Life Biological Molecules
 BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
BIOLOGY 111 CHAPTER 2: The Chemistry of Life Biological Molecules The Chemistry of Life : Learning Outcomes 2.4) Describe the significance of carbon in forming the basis of the four classes of biological
Starch Content and Amylase Activity in Avocado Fruit Pulp
 J. Amer. Soc. Hort. Sci. 103(5):673-676. 1978. Starch Content and Amylase Activity in Avocado Fruit Pulp Edna Pesis, Yoram Fuchs, and Giora Zauberman 1 Division of Fruit and Vegetable Storage, Institute
J. Amer. Soc. Hort. Sci. 103(5):673-676. 1978. Starch Content and Amylase Activity in Avocado Fruit Pulp Edna Pesis, Yoram Fuchs, and Giora Zauberman 1 Division of Fruit and Vegetable Storage, Institute
Lecture 2 Carbohydrates
 Lecture 2 Carbohydrates Sources of CHOs Wholegrains major dietary intake Vegetables, legumes ad fruit contain dietary fibre Milk products provide lactose essential for infants Glycogen is a storage carbohydrate,
Lecture 2 Carbohydrates Sources of CHOs Wholegrains major dietary intake Vegetables, legumes ad fruit contain dietary fibre Milk products provide lactose essential for infants Glycogen is a storage carbohydrate,
Separation of Saccharides Using TSKgel Amide-80, a Packing Material for High-performance Normal Phase Partition Chromatography (2) Table of Contents
 No. 079 SEPARATION REPORT Separation of Saccharides Using TSKgel Amide-80, a Packing Material for High-performance Normal Phase Partition Chromatography (2) Table of Contents 1. Introduction 1 2. Comparison
No. 079 SEPARATION REPORT Separation of Saccharides Using TSKgel Amide-80, a Packing Material for High-performance Normal Phase Partition Chromatography (2) Table of Contents 1. Introduction 1 2. Comparison
Ch18. Metabolism. Chemical processes that maintain life. From the Greek metabole change." version 1.0
 Ch18 Metabolism Chemical processes that maintain life. From the Greek metabole change." version 1.0 Nick DeMello, PhD. 2007-2015 Ch18 Metabolism Metabolism Defined Metabolic Pathways Energy stored as ATP
Ch18 Metabolism Chemical processes that maintain life. From the Greek metabole change." version 1.0 Nick DeMello, PhD. 2007-2015 Ch18 Metabolism Metabolism Defined Metabolic Pathways Energy stored as ATP
Lecture 34. Carbohydrate Metabolism 2. Glycogen. Key Concepts. Biochemistry and regulation of glycogen degradation
 Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
CARBOHYDRATE BOOKLET ANSWERS
 CARBOHYDRATE BOOKLET ANSWERS FOR: QER QUESTION APPLICATION AND EXTENSION QUESTIONS. FEEDBACK CAN BE VIEWED AT: https://thiacin.com/carbohydrateqerappextfeedback LONG ANSWER QUESTIONS WITH QER The long
CARBOHYDRATE BOOKLET ANSWERS FOR: QER QUESTION APPLICATION AND EXTENSION QUESTIONS. FEEDBACK CAN BE VIEWED AT: https://thiacin.com/carbohydrateqerappextfeedback LONG ANSWER QUESTIONS WITH QER The long
Takahiro Noda National Agricultural Research Center for Hokkaido Region (NARCH), JAPAN Workshop Japan-New Zealand (JST), 11 October 2010, Tokyo.
 National Agriculture and Food Research Organization The enzymatic digestibility and phosphate content in potato starches Takahiro Noda National Agricultural Research Center for Hokkaido Region (NARCH),
National Agriculture and Food Research Organization The enzymatic digestibility and phosphate content in potato starches Takahiro Noda National Agricultural Research Center for Hokkaido Region (NARCH),
Trypsin digestion: The lyophilized powder of the reduced S-carboxymethylated ACID) BETWEEN NORMAL (B+) AND THE COMMON NEGRO VARIANT
 A SINGLE AMINO ACID SUBSTITUTION (ASPARAGINE TO ASPARTIC ACID) BETWEEN NORMAL (B+) AND THE COMMON NEGRO VARIANT (A+) OF HUMAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE* BY AKIRA YOSHIDA DIVISION OF MEDICAL GENETICS,
A SINGLE AMINO ACID SUBSTITUTION (ASPARAGINE TO ASPARTIC ACID) BETWEEN NORMAL (B+) AND THE COMMON NEGRO VARIANT (A+) OF HUMAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE* BY AKIRA YOSHIDA DIVISION OF MEDICAL GENETICS,
Student Number: THE UNIVERSITY OF MANITOBA April 16, 2007, 9:00 AM -12:00 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination
 Name: Student Number: THE UNIVERSITY OF MANITOBA April 16, 2007, 9:00 AM -12:00 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination MBIO / CHEM.2370 Examiner: Dr. A. Scoot 1. Answer ALL
Name: Student Number: THE UNIVERSITY OF MANITOBA April 16, 2007, 9:00 AM -12:00 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination MBIO / CHEM.2370 Examiner: Dr. A. Scoot 1. Answer ALL
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI
 METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
METABOLISM OF L-RHAMNOSE BY ESCHERICHIA COLI I. L- RHAMNOSE ISOMERASE DOROTHY M. WILSON1 AND SAM AJL Department of Bacteriology, Walter Reed Army Institute of Research, Washington, D. C. The methyl pentose,
Student Number: THE UNIVERSITY OF MANITOBA April 10, 2006, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination
 Name: Student Number: April 10, 2006, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided. 2. The back
Name: Student Number: April 10, 2006, 1:30 AM - 4:30 PM Page 1 (of 4) Biochemistry II Laboratory Section Final Examination Examiner: Dr. A. Scoot 1. Answer ALL questions in the space provided. 2. The back
Biochemistry: Macromolecules
 1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
1 Biology: Macromolecules 2 Carbohydrates Carbohydrate organic compound containing carbon, hydrogen, & oxygen in a 1:2:1 ratio Meaning: hydrated carbon ratio of h:0 is 2:1 (same as in water) Source: plants
Consequently, lipoprotein fractions have been analyzed
 THE PHOSPHOLIPID COMPOSITION OF HUMAN SERUM LIPOPROTEIN FRACTIONS SEPARATED BY ULTRACENTRIFUGATION * BY GERALD B. PHILLIPS (From the Departments of Biochemistry and Medicine, College of Physicians and
THE PHOSPHOLIPID COMPOSITION OF HUMAN SERUM LIPOPROTEIN FRACTIONS SEPARATED BY ULTRACENTRIFUGATION * BY GERALD B. PHILLIPS (From the Departments of Biochemistry and Medicine, College of Physicians and
Attaching lactase to the beads is a more efficient use of lactase than adding the lactase directly to cow s milk
 Q1.Cow s milk contains the sugar lactose. Many cats are unable to digest cow s milk because they are lactose intolerant. Cow s milk can be made suitable for these cats by treating it with the enzyme lactase
Q1.Cow s milk contains the sugar lactose. Many cats are unable to digest cow s milk because they are lactose intolerant. Cow s milk can be made suitable for these cats by treating it with the enzyme lactase
HPLC Analysis of Sugars
 HPLC Analysis of Sugars Pre-Lab Exercise: 1) Read about HPLC, sugars and the experiment and its background. 2) Prepare a flowchart as appropriate for the lab exercise. 3) Note the various sugar concentrations
HPLC Analysis of Sugars Pre-Lab Exercise: 1) Read about HPLC, sugars and the experiment and its background. 2) Prepare a flowchart as appropriate for the lab exercise. 3) Note the various sugar concentrations
Higher Biology. Unit 2: Metabolism and Survival Topic 2: Respiration. Page 1 of 25
 Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
Higher Biology Unit 2: Metabolism and Survival Topic 2: Respiration Page 1 of 25 Sub Topic: Respiration I can state that: All living cells carry out respiration. ATP is the energy currency of the cell
24.1 Introduction to Carbohydrates
 24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
24.1 Introduction to Carbohydrates Carbohydrates (sugars) are abundant in nature: They are high energy biomolecules. They provide structural rigidity for organisms (plants, crustaceans, etc.). The polymer
Problem-solving Test: The Mechanism of Protein Synthesis
 Q 2009 by The International Union of Biochemistry and Molecular Biology BIOCHEMISTRY AND MOLECULAR BIOLOGY EDUCATION Vol. 37, No. 1, pp. 58 62, 2009 Problem-based Learning Problem-solving Test: The Mechanism
Q 2009 by The International Union of Biochemistry and Molecular Biology BIOCHEMISTRY AND MOLECULAR BIOLOGY EDUCATION Vol. 37, No. 1, pp. 58 62, 2009 Problem-based Learning Problem-solving Test: The Mechanism
Lecture 2: Glycogen metabolism (Chapter 15)
 Lecture 2: Glycogen metabolism (Chapter 15) First. Fig. 15.1 Review: Animals use glycogen for ENERGY STORAGE. Glycogen is a highly-branched polymer of glucose units: Basic structure is similar to that
Lecture 2: Glycogen metabolism (Chapter 15) First. Fig. 15.1 Review: Animals use glycogen for ENERGY STORAGE. Glycogen is a highly-branched polymer of glucose units: Basic structure is similar to that
SPRINGFIELD TECHNICAL COMMUNITY COLLEGE ACADEMIC AFFAIRS
 SPRINGFIELD TECHNICAL COMMUNITY COLLEGE ACADEMIC AFFAIRS Course Number: BIOL 140 Department: Biology Course Title: Biochemistry/Health Sciences Semester: Spring Year: 1997 Objectives/ Course Number: BIOL
SPRINGFIELD TECHNICAL COMMUNITY COLLEGE ACADEMIC AFFAIRS Course Number: BIOL 140 Department: Biology Course Title: Biochemistry/Health Sciences Semester: Spring Year: 1997 Objectives/ Course Number: BIOL
THE AMYLASES OF PSEUDOMONAS SACCHAROPHILA
 THE AMYLASES OF PSEUDOMONAS SACCHAROPHILA PHILIP S. THAYER' Department of Bacteriology, Univer8ity of California, Berkeley, California Received for publication June 15, 1953 In his original study of Pseudomonas
THE AMYLASES OF PSEUDOMONAS SACCHAROPHILA PHILIP S. THAYER' Department of Bacteriology, Univer8ity of California, Berkeley, California Received for publication June 15, 1953 In his original study of Pseudomonas
GENERAL TESTS FOR CARBOHYDRATE. By Sandip Kanazariya
 GENERAL TESTS FOR CARBOHYDRATE By Sandip Kanazariya Introduction Carbohydrates are of great importance to human beings. They are major part of our diet, providing 60-70% of total energy required by the
GENERAL TESTS FOR CARBOHYDRATE By Sandip Kanazariya Introduction Carbohydrates are of great importance to human beings. They are major part of our diet, providing 60-70% of total energy required by the
Communication MULTIPLE FORMS OF ACID PHOSPHATASE PRODUCED BY ASPERGJLL US OR YZAE YONEKICHI SAKURAI AND HIDEO SHIOTA
 Short Communication J. Gen. Appl. Microbiol., 16, 335-339 (1970) MULTIPLE FORMS OF ACID PHOSPHATASE PRODUCED BY ASPERGJLL US OR YZAE YONEKICHI SAKURAI AND HIDEO SHIOTA Faculty of Agriculture, Iwate University,
Short Communication J. Gen. Appl. Microbiol., 16, 335-339 (1970) MULTIPLE FORMS OF ACID PHOSPHATASE PRODUCED BY ASPERGJLL US OR YZAE YONEKICHI SAKURAI AND HIDEO SHIOTA Faculty of Agriculture, Iwate University,
The addition of sugar moiety determines the blood group
 The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
Carbohydrate Chemistry 2016 Family & Consumer Sciences Conference Karin Allen, PhD
 Carbohydrate Chemistry 2016 Family & Consumer Sciences Conference Karin Allen, PhD Overview Carbohydrate chemistry General characteristics Sugar chemistry Starch chemistry 10 minute break Iodine test for
Carbohydrate Chemistry 2016 Family & Consumer Sciences Conference Karin Allen, PhD Overview Carbohydrate chemistry General characteristics Sugar chemistry Starch chemistry 10 minute break Iodine test for
Biodegradative Threonine Dehydratase. Reduction of Ferricyanide by an Intermediate of the Enzyme-Catalyzed Reaction
 Eur. J. Biochem. Y I, 527-532 (1978) Biodegradative Threonine Dehydratase. Reduction of Ferricyanide by an Intermediate of the Enzyme-Catalyzed Reaction Prasanta DATTA and Ranjan BHADRA Department of Biological
Eur. J. Biochem. Y I, 527-532 (1978) Biodegradative Threonine Dehydratase. Reduction of Ferricyanide by an Intermediate of the Enzyme-Catalyzed Reaction Prasanta DATTA and Ranjan BHADRA Department of Biological
Highlights Pentose Phosphate Pathway
 Highlights Pentose Phosphate Pathway 1. The pentose phosphate pathway (PPP) is an interchange of metabolic pathways. 2. It is important to cells as a) an important source of NADPH, b) an important source
Highlights Pentose Phosphate Pathway 1. The pentose phosphate pathway (PPP) is an interchange of metabolic pathways. 2. It is important to cells as a) an important source of NADPH, b) an important source
ENZYME FORMATION IN LYSOZYME LYSATE OF BACILUS SUBTILIS
 The Journal of Biochemistry, Vol. 44, No. 12, 1957 ENZYME FORMATION IN LYSOZYME LYSATE OF BACILUS SUBTILIS It was already reported that the whole lysate obtained by the treat ment of Bacillus subtilis
The Journal of Biochemistry, Vol. 44, No. 12, 1957 ENZYME FORMATION IN LYSOZYME LYSATE OF BACILUS SUBTILIS It was already reported that the whole lysate obtained by the treat ment of Bacillus subtilis
Beta-amylase action on high molecular weight maltosaccharides
 Retrospective Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 1962 Beta-amylase action on high molecular weight maltosaccharides Rudolph William Youngquist Iowa State
Retrospective Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 1962 Beta-amylase action on high molecular weight maltosaccharides Rudolph William Youngquist Iowa State
Exam 3 Fall 2015 Dr. Stone 8:00. V max = k cat x E t. ΔG = -RT lnk eq K m + [S]
![Exam 3 Fall 2015 Dr. Stone 8:00. V max = k cat x E t. ΔG = -RT lnk eq K m + [S] Exam 3 Fall 2015 Dr. Stone 8:00. V max = k cat x E t. ΔG = -RT lnk eq K m + [S]](/thumbs/75/71985573.jpg) Exam 3 Fall 2015 Dr. Stone 8:00 Name There are 106 possible points (6 bonus points) on this exam. There are 8 pages. v o = V max x [S] k cat = kt e - ΔG /RT V max = k cat x E t ΔG = -RT lnk eq K m + [S]
Exam 3 Fall 2015 Dr. Stone 8:00 Name There are 106 possible points (6 bonus points) on this exam. There are 8 pages. v o = V max x [S] k cat = kt e - ΔG /RT V max = k cat x E t ΔG = -RT lnk eq K m + [S]
Qualitative analysis of carbohydrates II
 Qualitative analysis of carbohydrates II C 2 C 2 1 glycogen C 2 C 2 6 C 2 C 2 C 2 5 1 4 4 3 2 Complex carbohydrate Complex sugars consist of more than one unit of monosachride, it could be: -Disaccharides
Qualitative analysis of carbohydrates II C 2 C 2 1 glycogen C 2 C 2 6 C 2 C 2 C 2 5 1 4 4 3 2 Complex carbohydrate Complex sugars consist of more than one unit of monosachride, it could be: -Disaccharides
Characterization of a Neopullulanase and an cx-glucosidase from Bacteroides thetaiotaomicron 95-1
 JOURNAL OF BACTERIOLOGY, May 1991, p. 2962-2968 Vol. 173, No. 9 21-9193/91/92962-7$2./ Copyright 1991, American Society for Microbiology Characterization of a Neopullulanase and an cx-glucosidase from
JOURNAL OF BACTERIOLOGY, May 1991, p. 2962-2968 Vol. 173, No. 9 21-9193/91/92962-7$2./ Copyright 1991, American Society for Microbiology Characterization of a Neopullulanase and an cx-glucosidase from
Formation of Lipid-Bound Oligosaccharides Containing Mannose. Their Role
 Proc. Nat. Acad. Sci. USA Vol. 70, No. 12, Part I, pp. 3390-3394, December 1973 Formation of Lipid-Bound Oligosaccharides Containing Mannose. Their Role in Glycoprotein Synthesis (dolichol/glycolipid/gdp-man/liver
Proc. Nat. Acad. Sci. USA Vol. 70, No. 12, Part I, pp. 3390-3394, December 1973 Formation of Lipid-Bound Oligosaccharides Containing Mannose. Their Role in Glycoprotein Synthesis (dolichol/glycolipid/gdp-man/liver
III. Metabolism Glucose Catabolism Part II
 Department of Chemistry and Biochemistry University of Lethbridge III. Metabolism Glucose Catabolism Part II Slide 1 Metabolic Fates of NADH and Pyruvate Cartoon: Fate of pyruvate, the product of glycolysis.
Department of Chemistry and Biochemistry University of Lethbridge III. Metabolism Glucose Catabolism Part II Slide 1 Metabolic Fates of NADH and Pyruvate Cartoon: Fate of pyruvate, the product of glycolysis.
Comparison of Water adsorption characteristics of oligo and polysaccharides of α-glucose studied by Near Infrared Spectroscopy Alfred A.
 Comparison of Water adsorption characteristics of oligo and polysaccharides of α-glucose studied by Near Infrared Spectroscopy Alfred A. Christy, Department of Science, Faculty of Engineering and Science,
Comparison of Water adsorption characteristics of oligo and polysaccharides of α-glucose studied by Near Infrared Spectroscopy Alfred A. Christy, Department of Science, Faculty of Engineering and Science,
Citation for published version (APA): Leemhuis, R. J. (2003). What makes cyclodextrin glycosyltransferase a transglycosylase Groningen: s.n.
 University of Groningen What makes cyclodextrin glycosyltransferase a transglycosylase Leemhuis, Reinder Johannes IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
University of Groningen What makes cyclodextrin glycosyltransferase a transglycosylase Leemhuis, Reinder Johannes IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
Name. The following exam contains 44 questions, valued at 2.6 points/question. 2. Which of the following is not a principal use of proteins?
 Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Chemistry 131 Exam 3 Practice Proteins, Enzymes, and Carbohydrates Spring 2018 Name The following exam contains 44 questions, valued at 2.6 points/question 1. Which of the following is a protein? a. Amylase
Topic 3: Molecular Biology
 Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
Topic 3: Molecular Biology 3.2 Carbohydrates and Lipids Essen=al Understanding: Carbon, hydrogen and oxygen are used to supply and store energy. Carbohydrates CARBOHYDRATES CHO sugars Primarily consist
EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY
 J. Gen. App!. Microbiol,, 21, 51-59 (1975) EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY CLOSTRIDIUM ACETOBUTYLICUM BURT ENSLEY, JOHN J. McHUGH, AND LARRY L. BARTON Department
J. Gen. App!. Microbiol,, 21, 51-59 (1975) EFFECT OF CARBON SOURCES ON FORMATION OF a-amylase AND GLUCOAMYLASE BY CLOSTRIDIUM ACETOBUTYLICUM BURT ENSLEY, JOHN J. McHUGH, AND LARRY L. BARTON Department
Glycogen Storage Disease Type III, VI and IX - basics -
 Glycogen Storage Disease Type III, VI and IX - basics - Wyboston Lakes 10.10.2015 Urike Steuerwald Tórshavn / Hannover usteuerwald@web.de GSD III, VI and IX - Questions What goes wrong in glycogen storage
Glycogen Storage Disease Type III, VI and IX - basics - Wyboston Lakes 10.10.2015 Urike Steuerwald Tórshavn / Hannover usteuerwald@web.de GSD III, VI and IX - Questions What goes wrong in glycogen storage
Quiz 4 Review Guide Fall 2018
 Quiz 4 Review Guide Fall 2018 Major Topics: Enzyme Kinetics: o reaction rates and catalysis; transition state binding theory o Michaelis-Menten equation and interpretation o Inhibitors types and explanations
Quiz 4 Review Guide Fall 2018 Major Topics: Enzyme Kinetics: o reaction rates and catalysis; transition state binding theory o Michaelis-Menten equation and interpretation o Inhibitors types and explanations
College of Science Department of Biochemistry
 Dr. Abir Alghanouchi College of Science Department of Biochemistry Carbohydrates Carbohydrates are called carbohydrates because they are essentially hydrates of carbon(i.e. they are composed of carbon
Dr. Abir Alghanouchi College of Science Department of Biochemistry Carbohydrates Carbohydrates are called carbohydrates because they are essentially hydrates of carbon(i.e. they are composed of carbon
