PARATEST STABILITY STUDY, SYSTEM USED FOR DIAGNOSIS OF INTESTINAL PARASITES
|
|
|
- Aldous Hunter
- 6 years ago
- Views:
Transcription
1 PARATEST STABILITY STUDY, SYSTEM USED FOR DIAGNOSIS OF INTESTINAL PARASITES Rev.00 January 10, Introduction Intestinal parasitic diseases represent a serious public health issue in underdeveloped and developing countries, affecting adults and children. The latter may suffer losses in physical and intellectual development resulting from the action of the parasites on the host, such as nutritional and blood deprivation, inflammation, tissue injuries, gastrointestinal and digestive disorders among others. Although mortality is not too high in human illnesses by intestinal parasites, there are still reports of death by hyperparasitism in the country, demonstrating the neglect that these health disorders have been submitted to. Accurate diagnosis of parasitic diseases, with subsequent referral to the appropriate therapy, represents an important action in individual and collective health that leads to improved quality of life. With this objective, the system PARATEST is evaluated in this study as an alternative approach to parasitological diagnosis that offers less biohazard and more practicality to carry out the examinations, while maintaining the quality standard of results. 2. Objectives This study aims to evaluate the stability of the system for diagnosis PARATEST in relation to: a) Kit storage temperature in the original conditions of production, i.e. before addition and dilution of sample (feces) in the diluent / preservative liquid.
2 b) Kit storage temperature, after addition and dilution of feces in the diluent / preservative liquid. c) Kit storage time, especially at room temperature (22 ºC - 25 ºC) in the production original condition, i.e., before addition and dilution of biological sample (feces) in the diluent / preservative liquid. d) Kit storage time, especially at room temperature (22 ºC - 25 ºC), after dilution of feces in the diluent / preservative liquid. 3. Method 3.1. System (kit) PARATEST The System (Kit) PARATEST produced by the company Diagnostek consists of a plastic bottle whose cap is equipped with a filter membrane with pores of about 266 µm which retain much of the debris and residues from fecal bolus and allow parasitic forms that can be identified by microscope to pass through. The bottle from the Kit PARATEST contains a volume of 7 ml diluent / preservative liquid of 5% buffered formalin with sodium phosphate buffer at ph 7.0. For stool examinations about two grams of feces newly issued by the patient are diluted in this solution, and from the suspension thus obtained, a small fraction is dropped on a microscope slide and examined microscopically by a specialist in parasitology. The diagnosis is made by morphological identification of the parasitic forms observed on microscopic examination. All bottles in the Kit have lot identification, with information on the week, month and year of manufacture, which contributes to the accuracy of stability analyses Selection of PARATEST Kit batches for stability studies For stability analysis in relation to temperature, the variable related to the lot was fixed, using bottles from the same batch at different temperatures.
3 For stability analysis in relation to storage time, before the addition of fecal samples, bottles from different lots produced on different dates were used. For the stability analysis in relation to time after the addition of fecal sample, bottles from the same batch, with same date of manufacture were used Concept of stability for PARATEST Kit and the analysis criterion With regard to time or temperature, with and / or without diluted biological sample, the stability of the lots of PARATEST Kit is defined as the capacity of preservation (fixation) and sustenance of the integrity of parasitic forms in the different conditions tested. This stability was measured based on parasite morphology analyzed after being kept at different temperatures and different times. Therefore, the criterion for samples analyses to verify the stability of the kit was based on the morphology of the parasitic forms as described by microscopists according to the following convention: a) Intact and well parasitic forms: when the analyst noted parasitic forms with identical or very close morphology to the original morphology described in the literature and observed in fresh stool specimens and not (control sample), only diluted in the preservative liquid of PARATEST (morphological gold standard) at the time of analysis. b) Changed and not parasitic forms: when the analyst noted parasitic forms with different and distorted morphology in relation to the original morphology described in the literature and compared to that observed in fresh stool specimens not (control sample), only diluted in the preservative liquid of PARATEST (morphological gold standard) at the time of analysis Control samples
4 The controls of the tests at different times and different temperatures were made by comparison to intact / well parasitic forms (the morphological gold standard) examined simultaneously in real time to test samples. This control was obtained from fresh fecal samples known to be positive which were diluted in the preservative solution of PARATEST at the exact moment (time zero) of microscopic analysis, at room temperature, and monitored microscopically over time Description of tests Evaluation of the diluent / preservative liquid stability from PARATEST Kit in its original condition of production, in relation to storage temperature For this test, three bottles from the PARATEST Kit were used, and from the same production batch, with production date less than a week, which were kept at different temperatures before the addition of stool sample (Bottle X - room temperature, between 22ºC and 25ºC; Bottle Y - refrigerated at 4 º C and Bottle Z greenhouse at 37 º C) for 7 days before the beginning of the study. The purpose was to determine whether the change in temperature would affect the stability of the diluent / preservative liquid. During testing, two grams of the same fresh fecal sample, poly-infected, and known positive for protozoan cysts and helminth eggs and larvae, were added to these three bottles. Immediately after the feces homogenization, an aliquot of the suspension from each bottle was dripped on a microscope slide and examined under an optical microscope by a specialist in parasitology, aiming at the description of morphology. Morphological analysis was performed in comparison with the gold standard of the pre-set control in this study and the morphology was described as agreement morphology, which is also defined in this study, according to item After the first examination, immediately after dilution of the stool (time zero), the bottles were incubated at the initial temperatures and their contents were re-examined after 24
5 hours (time 1) and 48 hours (time 2). The results of these analyses are described in Section 4.1, Table 1. The option to use bottles belonging to lots manufactured less than a week was only a safety margin to eliminate possible variable related to storage time Evaluation of the diluent/preservative liquid stability in the PARATEST Kit, in relation to temperature variation after addition of stool For this test, three bottles from the Kit PARATEST were used, from the same production batch, with production date with less than a week, which were kept at room temperature until the time of the test (bottle M, bottle N and bottle O). Two grams of the same fresh fecal sample, poly-infected, and known positive for protozoan cysts and helminth eggs and larvae were added to each bottle. Immediately after homogenization in the diluent / preservative liquid from the PARATEST Kit, an aliquot of the suspension from each of the three bottles was dripped on a slide and examined under an optical microscope, settling upon these initial analyses (zero time at room temperature) the gold standard of morphological forms of the parasite forms observed. The qualitative results of these immediate analyses represented controls for the subsequent analysis of the contents of these bottles subjected to different temperatures. Next, one of these bottles were incubated at room temperature (bottle M), the second was incubated under refrigeration at 4 ºC (bottle N), and the third was incubated at 37ºC (bottle O). Over seven days, the tests were performed after 24 hours, 72 hours, 120 hours and 168 hours, when a new aliquot from each of the three bottles was dripped on a microscope slide for analysis. These subsequent microscopic examinations were performed by the same analyst, aiming at the description of morphology. This was compared to the gold standard, pre-defined in this study and in line with the convention of the morphological pattern description, also previously defined in this study (as per item 3.1.2). The results of these tests are described in section 4.2, Table 2.
6 The option to use bottles belonging to lots manufactured less than a week was only a safety margin to eliminate possible variable related to storage time Evaluation of the diluent/preservative liquid stability in the PARATEST Kit, compared to the storage time at room temperature (22 ºC - 25 ºC) before adding the biological sample fecal For this test, seven bottles of PARATEST Kit have been used, from different batches, and produced in different dates over a period of three years, namely: Bottle k, one week of production; Bottle P, one month of production; Bottle Q, three months of production; Bottle R, six months of production; Bottle S, one year of production; Bottle T, two years of production, and Bottle U, 3 years of production. room temperature until the time of the test (bottle M, bottle N and bottle O). Two grams of the same fresh fecal sample, poly-infected, and known positive for protozoan cysts and helminth eggs and larvae were added to each bottle. Immediately after homogenization in the diluent / preservative liquid of PARATEST Kit (time zero), an aliquot of the suspension from each of the three bottles was dripped on a slide and examined under an optical microscope. Morphological analysis of the parasite forms was confronted with the gold standard of the control sample, prepared in real time, examined at time zero and after one week. The test was conducted at room temperature. As a control, two tests were made for each bottle: time zero and after one week (time 1). The results of these tests are presented in section 4.3, Table Evaluation of the diluent / preservative liquid stability in PARATEST Kit, compared to the time of storage at room temperature (22ºC - 25ºC), after addition of the biological sample fecal For this test three bottles (bottle G, bottle H, and bottle I) PARATEST Kit have been used, belonging to the same lot, manufactured with less than a week before the test. This time of manufacture of the lot was random, aiming to eliminate only the variable time of the
7 test. Room temperature was adopted to perform the test during the whole period of analysis, standardized to a month. Two grams of the same fresh fecal sample, poly-infected, and known positive for protozoan cysts and helminth eggs and larvae were added to each bottle. Immediately after homogenization in the diluent/ preservative liquid of PARATEST Kit, an aliquot of the suspension from each of the three bottles was dripped on a slide and examined under an optical microscope (time zero). Morphological analysis of the parasitic forms, at time zero, represented the morphological gold standard, which was confronted with the parasitic forms in the bottles throughout the month. The results of these tests are presented in section 4.4, Table Results 4.1. Stability of diluent / preservative liquid of PARATEST Kit in its original condition of production, in relation to temperature variation As shown in the description of parasitic forms in Table 1, it was found that stability was not affected at the three tested PARATEST Kit storage temperatures, ensuring the sample conservation and morphology preservation of the parasitic forms, in optimized conditions, compared to the gold standard of control. After the analysis at time zero, the bottles were kept for over 48 hours at the original storage temperatures, without the morphology of the parasitic forms being affected. It was noted, however, that the evaporation of preservative / diluent liquid of PARATEST Kit is accelerated at the temperature of 37ºC.
8 Table 1: Influence of PARATEST Kit storage temperature on the morphology of the parasitic forms. Initial temperature of the diluent / preservative liquid Description of parasitic forms over time Time Zero (Immediate examination) Time 1 (After 24 hours) Time 2 (After 48 hours) Note Room T (22 o C-25 o C) (Bottle X) Similar to the gold standard 4 o C T (Bottle Y) Similar to the gold standard 37 o C T (Bottle Z) Similar to the gold standard Control* Represents the gold standard * Gold Standard 4.2. Result of the diluent / preservative liquid of PARATEST Kit stability evaluation in relation to temperature variation after the addition of stool As shown in Table 2 below, the variation in incubation temperature of PARATEST after the addition of fecal samples did not exert any deleterious effect on the conservation of parasitic forms for up to seven days (168 hours) after the date of dilution. However, it was observed an increased evaporation of the preservative liquid at the temperature of 37ºC.
9 Table 2 - Influence of temperature variation followed by addition of the biological sample on the parasitic forms. Description of parasitic forms over time Bottle / (temperature) Time Zero (Immediate examination) Time 1 24 hours Time 2 72 hours Time hours Time hours Bottle M Room T (22 o C-25 o C) Bottle N ( 4 o C T) Bottle O (37 o C T) Control* (gold standard) 4.3. Result of the diluent / preservative liquid in PARATEST Kit stability evaluation, compared to the time of storage at room temperature (22 ºC - 25 ºC) before adding the biological fecal sample As shown in Table 3 below, the test performed as described in Section showed that the kit is stable before addition of the biological sample for a period of up to three years when stored at room temperature. It also revealed that after dilution of stool, parasitic forms are kept intact and for a period of up to four weeks.
10 Table 3. Stability evaluation of the Kit in relation to storage time prior to sample dilution. Description of parasitic forms over time Bottle / (production time) Time Zero (Immediate examination) Time 1 (1 week) Time 2 (2 weeks) Time 3 (3 weeks) Time 4 (4 weeks) Bottle K (1 week) Bottle P (1 month) Bottle Q (3 months) Bottle R (6 months) Bottle S (1 year) Bottle T (2 years) Bottle U Ongoing Ongoing Ongoing (3 years) study study study Control* * Gold Standard
11 4.4. Result of stability evaluation of the diluent / preservative liquid of PARATEST Kit, compared to the storage time at room temperature (22 ºC - 25 ºC), after adding the biological sample fecal As shown in Table 4 below, the parasitic forms are kept intact and for up to four weeks after dilution in the diluent / preservative liquid of PARATEST Kit. These results confirm those presented in section 4.3. Table 4. Influence of fecal sample permanence time in the diluent/preservative liquid on the morphology of the parasitic forms. Description of parasitic forms over time Bottle Room T (22 o C-25 o C) Time Zero (Immediate examination) Time 1 (1 week) Time 2 (2 weeks) Time 3 (3 weeks) Time 4 (4 weeks) Bottle G * Bottle H Bottle I Control* * Gold Standard 5. Conclusion
12 Based on stability studies described above it was concluded that: Regarding the variable Temperature : - The system PARATEST without the addition of biological samples (feces) maintains stability when stored at room temperature or under refrigeration or at 37 ºC for a period of three years ensuring the integrity and preservation of forms of the parasite when added to diluent /preservative liquid. Diagnostek recommends storage at room temperature (23 o C 25 o C). - PARATEST system after the addition of the biological sample (feces) maintains intact and well the parasitic forms at room temperature or refrigerated, as at the temperature up to 37ºC, for a period of one week. Diagnostek recommends storage, after biological sample dilution, at room temperature and stresses that temperatures around 37ºC could accelerate the evaporation of the diluent/ preservative liquid. Regarding the variable Time - The system PARATEST without the addition of biological samples (feces) is stable for a period of three years at room temperature, keeping parasitic forms intact and well, when the fecal sample is added to the liquid diluent/ preservative. - The system PARATEST, after the addition of biological samples (feces) maintains the parasitic forms intact and well at room temperature for at least thirty days (four weeks). Diagnostek recommends that examination be made within 10 days after the addition of stool in the diluent / preservative liquid.
Parasitology. Helminthology (Helminths)
 Parasitology Protozoology (Protozoa) Helminthology (Helminths) Entomology (Arthropodes) Platyhelminthes (flat worms) Nematheminthes (round worms) Trematodes Nematodes Cestodes Collection of the specimens
Parasitology Protozoology (Protozoa) Helminthology (Helminths) Entomology (Arthropodes) Platyhelminthes (flat worms) Nematheminthes (round worms) Trematodes Nematodes Cestodes Collection of the specimens
Cryptosporidium Veterinary
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Prof. Mahmoud Rushdi. Assiut University Egypt.
 By Prof. Mahmoud Rushdi Faculty of Veterinary Mdii Medicine Assiut University Egypt Precautions to be taken during collection and preservation of fecal sample 2 1 Precautions during collection of fecal
By Prof. Mahmoud Rushdi Faculty of Veterinary Mdii Medicine Assiut University Egypt Precautions to be taken during collection and preservation of fecal sample 2 1 Precautions during collection of fecal
Rapid-VIDITEST Calprotectin
 Rapid-VIDITEST Calprotectin One Step Calprotectin Card Test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz INTENDED
Rapid-VIDITEST Calprotectin One Step Calprotectin Card Test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz INTENDED
Catalog # OKDA00123 SUMMARY AND EXPLANATION
 Aviva Systems Biology 5754 Pacific Center Blvd., Suite 201, San Diego, CA 92121, USA Tel: 858-552-6979 Fax: 858-552-6975 www.avivasysbio.com Email: info@avivasysbio.com Giardia lamblia ELISA Kit Catalog
Aviva Systems Biology 5754 Pacific Center Blvd., Suite 201, San Diego, CA 92121, USA Tel: 858-552-6979 Fax: 858-552-6975 www.avivasysbio.com Email: info@avivasysbio.com Giardia lamblia ELISA Kit Catalog
E. Histolytica IgG (Amebiasis)
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Campylobacter Antigen ELISA Kit
 Campylobacter Antigen ELISA Kit Catalog Number KA3204 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Campylobacter Antigen ELISA Kit Catalog Number KA3204 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX
 Q3 2018 Parasitology American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX 77005 800-234-5315 281-436-5357 Specimen 1 Referees Extent 1 Extent 2 Total Few to 534 Giardia lamblia Many
Q3 2018 Parasitology American Association of Bioanalysts 5615 Kirby Drive, Suite 870 Houston, TX 77005 800-234-5315 281-436-5357 Specimen 1 Referees Extent 1 Extent 2 Total Few to 534 Giardia lamblia Many
Toxocara. Cat # Toxocara ELISA. Enzyme Linked Immunosorbent Assay. ELISA-Indirect; Antigen Coated Plate
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Rapid-VIDITEST Swine Flu
 Rapid-VIDITEST Swine Flu One Step Influenza type A Antigen Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST Swine Flu One Step Influenza type A Antigen Card test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST FOB Blister
 Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST FOB Blister One Step Fecal Occult Blood Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565,
IVD. DRG Toxocara canis ELISA (EIA-3518) Revised 12 Feb rm (Vers. 6.1)
 Please use only the valid version of the package insert provided with the kit. Intended Use For the qualitative screening of serum IgG antibodies to Toxocara using an Enzyme-Linked Immunosorbent Assay
Please use only the valid version of the package insert provided with the kit. Intended Use For the qualitative screening of serum IgG antibodies to Toxocara using an Enzyme-Linked Immunosorbent Assay
Rapid-VIDITEST. Helicobacter pylori. One step Helicobacter pylori Blister test. Instruction manual
 Rapid-VIDITEST Helicobacter pylori One step Helicobacter pylori Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST Helicobacter pylori One step Helicobacter pylori Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565,
IBD EZ VUE I. PRINCIPLE
 IBD EZ VUE INTENDED USE The IBD EZ VUE test is an immunochromatographic test for the qualitative detection of elevated levels of lactoferrin, a marker for fecal leukocytes and an indicator of intestinal
IBD EZ VUE INTENDED USE The IBD EZ VUE test is an immunochromatographic test for the qualitative detection of elevated levels of lactoferrin, a marker for fecal leukocytes and an indicator of intestinal
Human Influenza A (Swine Flu) Rapid test
 Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Trident Membrane Protein Extraction Kit
 Cat. No. Size Shelf life GTX16373 5/ 20 tests 12 months at the appropriate storage temperatures (see below) Contents Component Storage Amount for 5 tests Amount for 20 tests Buffer A -20 o C 2.5 ml 10
Cat. No. Size Shelf life GTX16373 5/ 20 tests 12 months at the appropriate storage temperatures (see below) Contents Component Storage Amount for 5 tests Amount for 20 tests Buffer A -20 o C 2.5 ml 10
Rapid-VIDITEST Enterovirus
 Rapid-VIDITEST Enterovirus A rapid one step Enterovirus Card test for the qualitative detection of Enterovirus antigens in human feces. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365,
Rapid-VIDITEST Enterovirus A rapid one step Enterovirus Card test for the qualitative detection of Enterovirus antigens in human feces. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365,
Crypto / Giardia Ag Combo (Fecal) ELISA
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
REFERENCES HemaPrompt FG Product Information. June, Aerscher Diagnostics, Chestertown, MD.
 Applies To: SRMC Hospital and Clinics Responsible Department: Rapid Response Laboratory Revised: 3/2014 Procedure Patient Age Group: ( ) N/A (X ) All Ages ( ) Newborns ( ) Pediatric ( ) Adult DESCRIPTION/OVERVIEW
Applies To: SRMC Hospital and Clinics Responsible Department: Rapid Response Laboratory Revised: 3/2014 Procedure Patient Age Group: ( ) N/A (X ) All Ages ( ) Newborns ( ) Pediatric ( ) Adult DESCRIPTION/OVERVIEW
HAV IgG/IgM Rapid Test
 HAV IgG/IgM Rapid Test Cat. No.:DTS649 Pkg.Size: Intended use CD HAV IgG/IgM Rapid Test is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM
HAV IgG/IgM Rapid Test Cat. No.:DTS649 Pkg.Size: Intended use CD HAV IgG/IgM Rapid Test is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM
Herpes Simplex Virus 2 IgG HSV 2 IgG
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
See external label 2 C-8 C = C-REACTIVE PROTEIN (CRP) LATEX SLIDE TEST
 CORTEZ DIAGNOSTICS, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
CORTEZ DIAGNOSTICS, INC. 21250 Califa Street, Suite 102 and 116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Rotavirus Test Kit. Instructions For Use. Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA
 Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
Rotavirus Test Kit Instructions For Use Format: Cassette Specimen: Fecal Extract Catalog Number: VEL-001-ROTA * Please read the instructions carefully before use INTENDED USE Velotest Rotavirus Test is
Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister
 Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister One
Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Rapid-VIDITEST C. difficile Ag (GDH) Card/Blister One
Ralstonia solanacearum PathoScreen Kit DAS ELISA, peroxidase label Catalog number: PSP 33900
 List of contents Ralstonia solanacearum PathoScreen Kit Lot number Item 96 wells 288 wells 480 wells Antibody coated 96-well microtiter plates 1 3 5 Peroxidase enzyme conjugate, ready to use 11 ml 33 ml
List of contents Ralstonia solanacearum PathoScreen Kit Lot number Item 96 wells 288 wells 480 wells Antibody coated 96-well microtiter plates 1 3 5 Peroxidase enzyme conjugate, ready to use 11 ml 33 ml
Fecal H. pylori Antigen Rapid Test (Strip)
 Fecal H. pylori Antigen Rapid Test (Strip) Cat. No.:DTS526 Pkg.Size: Intended use This H. pylori Antigen Rapid Test is intended for the direct qualitative detection of the presence of H. pylori antigen
Fecal H. pylori Antigen Rapid Test (Strip) Cat. No.:DTS526 Pkg.Size: Intended use This H. pylori Antigen Rapid Test is intended for the direct qualitative detection of the presence of H. pylori antigen
Brief Survey of Common Intestinal Parasites in the Tokyo Metropolitan Area. Tsukasa NOZAKI1), Kouichi NAGAKURA2)*, Hisae FUSEGAWA3)
 Brief Survey of Common Intestinal Parasites in the Tokyo Metropolitan Area Tsukasa NOZAKI1), Kouichi NAGAKURA2)*, Hisae FUSEGAWA3) and Yasuhiko AND01),3) 1) Central Clinical Laboratoly, Tokai University
Brief Survey of Common Intestinal Parasites in the Tokyo Metropolitan Area Tsukasa NOZAKI1), Kouichi NAGAKURA2)*, Hisae FUSEGAWA3) and Yasuhiko AND01),3) 1) Central Clinical Laboratoly, Tokai University
Porcine/Canine Insulin ELISA
 Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
Origination Date: 25 Mar 2004 Total Pages: Effective Date: 18 Sep 2012 SOP Number LTC-SOP-17 v2.0. Network Name, Title Signature Date
 Title: Stool Processing and Cryptosporidium/Giardia Origination Date: 25 Mar 2004 Total Pages: Effective Date: 18 Sep 2012 SOP Number LTC-SOP-17 v2.0 Prepared By: Bill Kabat and Nancy Isham of the Supersedes
Title: Stool Processing and Cryptosporidium/Giardia Origination Date: 25 Mar 2004 Total Pages: Effective Date: 18 Sep 2012 SOP Number LTC-SOP-17 v2.0 Prepared By: Bill Kabat and Nancy Isham of the Supersedes
DEVELOPMENTAL VALIDATION OF SPERM HY-LITER EXPRESS Jennifer Old, Chris Martersteck, Anna Kalinina, Independent Forensics, Lombard IL
 DEVELOPMENTAL VALIDATION OF SPERM HY-LITER EXPRESS Jennifer Old, Chris Martersteck, Anna Kalinina, Independent Forensics, Lombard IL Background and Introduction SPERM HY-LITER is the first immunofluorescent
DEVELOPMENTAL VALIDATION OF SPERM HY-LITER EXPRESS Jennifer Old, Chris Martersteck, Anna Kalinina, Independent Forensics, Lombard IL Background and Introduction SPERM HY-LITER is the first immunofluorescent
In vitro cultivation of Plasmodium falciparum
 Chapter 2 In vitro cultivation of Plasmodium falciparum In vitro cultivation of Plasmodium falciparum 2.1 INTRODUCTION Malaria represents the world s greatest public health problem in terms of number of
Chapter 2 In vitro cultivation of Plasmodium falciparum In vitro cultivation of Plasmodium falciparum 2.1 INTRODUCTION Malaria represents the world s greatest public health problem in terms of number of
Taenia solium IgG ELISA Kit
 Taenia solium IgG ELISA Kit Catalog Number KA3191 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Taenia solium IgG ELISA Kit Catalog Number KA3191 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Rapid-VIDITEST. Calprotectin-Lactoferrin
 Rapid-VIDITEST Calprotectin-Lactoferrin One step calprotectin and lactoferrin Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.:
Rapid-VIDITEST Calprotectin-Lactoferrin One step calprotectin and lactoferrin Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.:
District NTD Training module 9 Learners Guide
 District NTD Training module 9 Learners Guide Session 3: Diagnostic and laboratory tools for filarial worms, Soil- Transmitted Helminths and Schistosomiasis Key message addressed to communities: Community
District NTD Training module 9 Learners Guide Session 3: Diagnostic and laboratory tools for filarial worms, Soil- Transmitted Helminths and Schistosomiasis Key message addressed to communities: Community
Sure-Vue Signature Cryptosporidium/Giardia Test
 SAMPLE PROCEDURE This procedure is provided to Fisher Healthcare customers to assist with the development of laboratory procedures. This document was derived from, and was current with, the instructions
SAMPLE PROCEDURE This procedure is provided to Fisher Healthcare customers to assist with the development of laboratory procedures. This document was derived from, and was current with, the instructions
IV2-113E Use by. Invitron Glargine ELISA Kit REF LOT IVD. Definitions. English. For in-vitro diagnostic use. Instructions for use.
 Definitions Instructions for use REF Catalogue number IV2-113E Use by English Invitron Glargine ELISA Kit For in-vitro diagnostic use Σ 96 LOT IVD Lot/Batch Code Storage temperature limitations In vitro
Definitions Instructions for use REF Catalogue number IV2-113E Use by English Invitron Glargine ELISA Kit For in-vitro diagnostic use Σ 96 LOT IVD Lot/Batch Code Storage temperature limitations In vitro
Veterinary Parasitology and Parasitic Diseases Department of Pathology and Animal Health Faculty of Veterinary Medicine, University of Naples
 Veterinary Parasitology and Parasitic Diseases Department of Pathology and Animal Health Faculty of Veterinary Medicine, University of Naples Federico II Via della Veterinaria, 1-80137 Naples, Italy www.flotac.unina.it
Veterinary Parasitology and Parasitic Diseases Department of Pathology and Animal Health Faculty of Veterinary Medicine, University of Naples Federico II Via della Veterinaria, 1-80137 Naples, Italy www.flotac.unina.it
AFLATOXIN M1 CAT. NO. 961AFLMO1M
 h AFLATOXIN M1 CAT. NO. 961AFLMO1M Competitive ELISA Immunoassay for the quantitative detection of Aflatoxin M1 in milk, milk powder and cheese. General Aflatoxins are toxic metabolites produced by a variety
h AFLATOXIN M1 CAT. NO. 961AFLMO1M Competitive ELISA Immunoassay for the quantitative detection of Aflatoxin M1 in milk, milk powder and cheese. General Aflatoxins are toxic metabolites produced by a variety
H.pylori IgA Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Optimal algorithm for HER2 testing
 Optimal algorithm for HER2 testing The revised definition of IHC 2+ (equivocal) is invasive breast cancer with Weak to moderate complete membrane staining observed in >10% of tumor cells. (see Figure 1
Optimal algorithm for HER2 testing The revised definition of IHC 2+ (equivocal) is invasive breast cancer with Weak to moderate complete membrane staining observed in >10% of tumor cells. (see Figure 1
MALARIA P. FALCIPARUM / P. VIVAX
 MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
Rapid-VIDITEST. FOB+Tf. One step Fecal Occult Blood Card Test. Instruction manual
 Rapid-VIDITEST FOB+Tf One step Fecal Occult Blood Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Rapid-VIDITEST FOB+Tf One step Fecal Occult Blood Card Test Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice, Czech Republic, Tel.: +420 261 090 565, www.vidia.cz
Veterinary Parasitology and Parasitic Diseases Department of Pathology and Animal Health Faculty of Veterinary Medicine, University of Naples
 Veterinary Parasitology and Parasitic Diseases Department of Pathology and Animal Health Faculty of Veterinary Medicine, University of Naples Federico II Via della Veterinaria, 1-80137 Naples, Italy www.flotac.unina.it
Veterinary Parasitology and Parasitic Diseases Department of Pathology and Animal Health Faculty of Veterinary Medicine, University of Naples Federico II Via della Veterinaria, 1-80137 Naples, Italy www.flotac.unina.it
Triglyceride Assay Kit-Bulk 5 Plate Kit Cat# TG-5-RB
 Triglyceride Assay Kit-Bulk 5 Plate Kit Cat# TG-5-RB INSTRUCTION MANUAL (ZBM-14) STORAGE CONDITIONS Reagents A & B, Buffers: Store at 2-8 C. Glycerol Standard -20 C For Research Use Only Not For Use In
Triglyceride Assay Kit-Bulk 5 Plate Kit Cat# TG-5-RB INSTRUCTION MANUAL (ZBM-14) STORAGE CONDITIONS Reagents A & B, Buffers: Store at 2-8 C. Glycerol Standard -20 C For Research Use Only Not For Use In
Giardia lamblia ELISA Kit
 Giardia lamblia ELISA Kit Catalog Number KA2093 96 assay Version: 01 Intend for research use only www.abnova.com Introduction and Background A. Intended Use Giardia lamblia ELISA Kit is an Enzyme-Linked
Giardia lamblia ELISA Kit Catalog Number KA2093 96 assay Version: 01 Intend for research use only www.abnova.com Introduction and Background A. Intended Use Giardia lamblia ELISA Kit is an Enzyme-Linked
Cryptosporidium Proficiency Test Program for Laboratories using US EPA Method 1623: Cryptosporidium and Giardia
 Cryptosporidium Proficiency Test Program for Laboratories using US EPA Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA, December 2005 version Preferable Specifications to Establish
Cryptosporidium Proficiency Test Program for Laboratories using US EPA Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA, December 2005 version Preferable Specifications to Establish
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
PARA-TECT Cryptosporidium/Giardia Direct Fluorescent Assay Directions For Use For In Vitro Diagnostic Use
 Medical Chemical Corp., 19430 Van Ness Avenue, Torrance, California 90501 (310)787-6800, Fax (310)787-4464 Catalog # MCC-C/G - DFA, 75 Test PARA-TECT Cryptosporidium/Giardia Direct Fluorescent Assay Directions
Medical Chemical Corp., 19430 Van Ness Avenue, Torrance, California 90501 (310)787-6800, Fax (310)787-4464 Catalog # MCC-C/G - DFA, 75 Test PARA-TECT Cryptosporidium/Giardia Direct Fluorescent Assay Directions
H.Pylori IgG
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Nature Protocols: doi: /nprot Supplementary Figure 1. Fluorescent titration of probe CPDSA.
 Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
Supplementary Figure 1 Fluorescent titration of probe CPDSA. Fluorescent titration of probe CPDSA (10 um) upon addition of GSH in HEPES (10 mm, ph = 7.4) containing 10% DMSO. Each spectrum was recorded
H.Pylori IgG Cat # 1503Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Insulin ELISA. For the quantitative determination of insulin in serum and plasma
 Insulin ELISA For the quantitative determination of insulin in serum and plasma For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin ELISA For the quantitative determination of insulin in serum and plasma For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin (Porcine/Canine) ELISA
 Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
HumaTex CRP. Design Verification. Contents
 Design Verification HumaTex CRP Contents 1 Function... 2 2 Reproducibility... 2 3 Sensitivity and dynamic range... 2 Preparation of serum control panel... 2 Sensitivity test results... 2 Prozone check...
Design Verification HumaTex CRP Contents 1 Function... 2 2 Reproducibility... 2 3 Sensitivity and dynamic range... 2 Preparation of serum control panel... 2 Sensitivity test results... 2 Prozone check...
Comparison of Polyvinyl Alcohol Fixative with Three Less Hazardous Fixatives for Detection and Identification of Intestinal Parasites
 JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2000, p. 1592 1598 Vol. 38, No. 4 0095-1137/00/$04.00 0 Comparison of Polyvinyl Alcohol Fixative with Three Less Hazardous Fixatives for Detection and Identification
JOURNAL OF CLINICAL MICROBIOLOGY, Apr. 2000, p. 1592 1598 Vol. 38, No. 4 0095-1137/00/$04.00 0 Comparison of Polyvinyl Alcohol Fixative with Three Less Hazardous Fixatives for Detection and Identification
Reagent Set DAS ELISA, Alkaline phosphatase label SRA 22001, SRA 23203, SRA 27703, SRA & SRA ToRSV, ArMV, GFLV, AnFBV and PDV
 List of contents Lot number Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
List of contents Lot number Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
E. Histolytica IgG ELISA Kit
 E. Histolytica IgG ELISA Kit Catalog Number KA3193 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
E. Histolytica IgG ELISA Kit Catalog Number KA3193 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Bovine Insulin ELISA
 Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
Trichinella Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Annexin V-PE Apoptosis Detection Kit
 Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
Annexin V-PE Apoptosis Detection Kit Catalog Number KA0716 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
See external label 96 tests HSV 2 IgA. Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
 MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
MEDICAL ELECTRONIC SYSTEMS 5757 W. Century Blvd. Suite 805 Los Angeles, CA. 90045 Remember, it ALL Started with a Sperm! www.mes global.com SQA-V Gold Semiannual Validation/Proficiency/QC Recommendations
Cryptosporidium spp. ELISA Kit
 Cryptosporidium spp. ELISA Kit Catalog Number KA2094 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Cryptosporidium spp. ELISA Kit Catalog Number KA2094 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Please use only the valid version of the package insert provided with the kit.
 Please use only the valid version of the package insert provided with the kit. Intended Use For the qualitative screening of serum IgG antibodies to Trichinella using an Enzyme Linked Immunoabsorbant Assay
Please use only the valid version of the package insert provided with the kit. Intended Use For the qualitative screening of serum IgG antibodies to Trichinella using an Enzyme Linked Immunoabsorbant Assay
Data sheet. TBARS Assay kit. (Colorimetric/Fluorometric) Kit Contents. MDA-TBA Adduct. 2-Thiobarbituric Acid. Cat. No: CA995.
 Data sheet Cat. No: CA995 TBARS Assay kit (Colorimetric/Fluorometric) Introduction Oxidative stress in the cellular environment results in the formation of highly reactive and unstable lipid hydroperoxides.
Data sheet Cat. No: CA995 TBARS Assay kit (Colorimetric/Fluorometric) Introduction Oxidative stress in the cellular environment results in the formation of highly reactive and unstable lipid hydroperoxides.
INFRAFRONTIER-I3 - Cryopreservation training course. Hosted by the Frozen Embryo and Sperm Archive, MRC - Harwell
 Hosted by the Frozen Embryo and Sperm Archive, MRC - Harwell IVF recovery procedure incorporting methyl-β-cyclodextrin and reduced glutathione This protocol is based on the work published by Takeo et al.,
Hosted by the Frozen Embryo and Sperm Archive, MRC - Harwell IVF recovery procedure incorporting methyl-β-cyclodextrin and reduced glutathione This protocol is based on the work published by Takeo et al.,
CHEMILUMINESCENCE ENZYME IMMUNOASSAY (CLIA) Toxoplasma IgG. Cat # (20-25 C Room temp.) Volume
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
IVD information *Droppers for the sensitized and control cells. Not for use other than dispensing the sensitized and control cells.
 In Vitro Diagnostic Reagent Instruction Manual of Diagnostic Reagent for Determination of anti-hbs Thoroughly read this instruction manual before use of this kit Background of the development and features
In Vitro Diagnostic Reagent Instruction Manual of Diagnostic Reagent for Determination of anti-hbs Thoroughly read this instruction manual before use of this kit Background of the development and features
Mouse Ultrasensitive Insulin ELISA
 Mouse Ultrasensitive Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use
Mouse Ultrasensitive Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use
Aac Reagent Set ELISA for the detection of Acidovorax avenae subsp. citrulli Catalog number: SRA 14800
 List of contents Lot number Aac Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
List of contents Lot number Aac Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525
Toxoplasma gondii IgM ELISA Kit
 Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Blueberry scorch virus Reagent Set DAS ELISA, Alkaline phosphatase label for BlScV Catalog number: SRA 19100
 List of contents Lot number Blueberry scorch virus Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150
List of contents Lot number Blueberry scorch virus Reagent Set Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150
SIV p27 Antigen ELISA Catalog Number:
 INTENDED USE The RETRO-TEK SIV p27 Antigen ELISA is for research use only and is not intended for in vitro diagnostic use. The RETRO-TEK SIV p27 Antigen ELISA is an enzyme linked immunoassay used to detect
INTENDED USE The RETRO-TEK SIV p27 Antigen ELISA is for research use only and is not intended for in vitro diagnostic use. The RETRO-TEK SIV p27 Antigen ELISA is an enzyme linked immunoassay used to detect
TRACP & ALP double-stain Kit
 Table of Content I. Description... 2 II. Introduction... 2 III. Principles... 2 IV. Kit components... 3 V. Storage... 3 VI. Preparation of reagents... 3 VII. Methods... 4-7 Cell fixation... 4 Activity
Table of Content I. Description... 2 II. Introduction... 2 III. Principles... 2 IV. Kit components... 3 V. Storage... 3 VI. Preparation of reagents... 3 VII. Methods... 4-7 Cell fixation... 4 Activity
IgG Antibodies To Toxoplasma Gondii ELISA Kit Protocol
 IgG Antibodies To Toxoplasma Gondii ELISA Kit Protocol (Cat. No.:EK-310-85) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED
IgG Antibodies To Toxoplasma Gondii ELISA Kit Protocol (Cat. No.:EK-310-85) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED
Stool Collection Guidelines
 Stool Collection Guidelines Your child s stool (bowel movement) must be tested so we can plan the treatment for your child. You will need to collect the stool specimen at home. Bring it to a Laboratory
Stool Collection Guidelines Your child s stool (bowel movement) must be tested so we can plan the treatment for your child. You will need to collect the stool specimen at home. Bring it to a Laboratory
See external label 2 C-8 C Σ=96 tests Cat # 1505Z. MICROWELL ELISA H.Pylori IgA Cat # 1505Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
See external label 2 C-8 C 96 tests CHEMILUMINESCENCE. CMV IgG. Cat # Step (20-25 C Room temp.) Volume
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Galactose and Lactose Assay Kit
 Galactose and Lactose Assay Kit Catalog Number KA0842 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Galactose and Lactose Assay Kit Catalog Number KA0842 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Item Catalog Number Manufacturer 1,4-Dithioerythritol (1 g) D9680 Sigma-Aldrich
 SOP: Nuclei isolation from fresh mouse tissues and DNaseI treatment Date modified: 01/12/2011 Modified by: E. Giste/ T. Canfield (UW) The following protocol describes the isolation of nuclei and subsequent
SOP: Nuclei isolation from fresh mouse tissues and DNaseI treatment Date modified: 01/12/2011 Modified by: E. Giste/ T. Canfield (UW) The following protocol describes the isolation of nuclei and subsequent
Giardia lamblia (flagellates)
 Giardia lamblia (flagellates) Dr. Hala Al Daghistani Giardia lamblia (Giardia duodenalis or Giardia intestinalis) is the causative agent of giardiasis and is the only common pathogenic protozoan found
Giardia lamblia (flagellates) Dr. Hala Al Daghistani Giardia lamblia (Giardia duodenalis or Giardia intestinalis) is the causative agent of giardiasis and is the only common pathogenic protozoan found
Mouse C-peptide ELISA
 Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Preparation
Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Preparation
H. pylori IgM CLIA kit
 H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT. Product: Bioelisa HIV 1+2 Ag/Ab Number: PQDx
 WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: Bioelisa HIV 1+2 Ag/Ab Number: PQDx 0183-060-00 Abstract Bioelisa HIV 1+2 Ag/Ab with product codes 3000-1172 and 300-1173,
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: Bioelisa HIV 1+2 Ag/Ab Number: PQDx 0183-060-00 Abstract Bioelisa HIV 1+2 Ag/Ab with product codes 3000-1172 and 300-1173,
BÜHLMANN fcal turbo. Calprotectin turbidimetric assay for professional use. Reagent Kit B-KCAL-RSET. Revision date:
 BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
BÜHLMANN fcal turbo Calprotectin turbidimetric assay for professional use Reagent Kit B-KCAL-RSET Revision date: 2017-02-24 BÜHLMANN LABORATORIES AG Baselstrasse 55 4124 Schönenbuch, Switzerland Tel.:
(For In Vitro Diagnostic Use)
 Read this insert carefully before performing the assay and keep for future reference. The reliability of assay procedure other than those described in this package insert cannot be guaranteed. 207727 (For
Read this insert carefully before performing the assay and keep for future reference. The reliability of assay procedure other than those described in this package insert cannot be guaranteed. 207727 (For
Malaria Pf/pan antigen Rapid Test
 Malaria Pf/pan antigen Rapid Test Cat. No.:IVDTS003 Pkg.Size:10T/50T Intended use The Malaria Pf/pan antigen Rapid Test is a self-performing, qualitative, sandwich immunoassay, utilizing whole blood for
Malaria Pf/pan antigen Rapid Test Cat. No.:IVDTS003 Pkg.Size:10T/50T Intended use The Malaria Pf/pan antigen Rapid Test is a self-performing, qualitative, sandwich immunoassay, utilizing whole blood for
H. pylori IgM. Cat # H. pylori IgM ELISA. ELISA: Enzyme Linked Immunosorbent Assay. ELISA - Indirect; Antigen Coated Plate
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
Mouse C-peptide ELISA
 Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calculation
Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calculation
Helicobacter pylori Antigen Test
 Helicobacter pylori Antigen Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-HP(s) Specimen: Fecal Specimen * Please read the instructions carefully before use INTENDED USE Helicobacter
Helicobacter pylori Antigen Test Instructions For Use Format: Cassette For: Catalog Number: VEL-001-HP(s) Specimen: Fecal Specimen * Please read the instructions carefully before use INTENDED USE Helicobacter
Rapid-VIDITEST. Astrovirus Card
 Rapid-VIDITEST Astrovirus Card One step Astrovirus Card Test. Instruction manual INTENDED USE: The Rapid-VIDITEST Astrovirus Card is a one step coloured chromatographic immunoassay for the qualitative
Rapid-VIDITEST Astrovirus Card One step Astrovirus Card Test. Instruction manual INTENDED USE: The Rapid-VIDITEST Astrovirus Card is a one step coloured chromatographic immunoassay for the qualitative
Reagent Set DAS ELISA, Alkaline phosphatase label
 List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525 ml 2.525 ml
List of contents Lot number Item 96 wells 500 wells 1000 wells 5000 wells Capture antibody 0.150 ml 0.275 ml 0.525 ml 2.525 ml Alkaline phosphatase enzyme conjugate 0.150 ml 0.275 ml 0.525 ml 2.525 ml
Egg Protein Residue ELISA Kit
 Egg Protein Residue ELISA Kit Catalog Number KA3208 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Egg Protein Residue ELISA Kit Catalog Number KA3208 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
Cereal yellow dwarf virus - RPV ELISA Kit
 Cereal yellow dwarf virus - RPV ELISA Kit Cat. No.:DEIA9232 Pkg.Size:96T Intended use The Cereal yellow dwarf virus - RPV ELISA Kit is a qualitative serological assay for the detection of CYDV-RPV in plant
Cereal yellow dwarf virus - RPV ELISA Kit Cat. No.:DEIA9232 Pkg.Size:96T Intended use The Cereal yellow dwarf virus - RPV ELISA Kit is a qualitative serological assay for the detection of CYDV-RPV in plant
Toxoplasma gondii IgM ELISA Kit
 Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Toxoplasma gondii IgM ELISA Kit Catalog Number KA0226 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: DPP HIV 1/2 Assay WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: DPP HIV 1/2 Assay WHO reference number: PQDx 0053-006-00 DPP HIV 1/2 Assay with product code 65-9506-0, manufactured by Chembio Diagnostic
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: DPP HIV 1/2 Assay WHO reference number: PQDx 0053-006-00 DPP HIV 1/2 Assay with product code 65-9506-0, manufactured by Chembio Diagnostic
PARASITOLOGY CASE HISTORY 15 (HISTOLOGY) (Lynne S. Garcia)
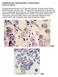 PARASITOLOGY CASE HISTORY 15 (HISTOLOGY) (Lynne S. Garcia) A biopsy was performed on a 27-year-old man with no known travel history, presenting with a perianal ulcer. The specimen was preserved in formalin
PARASITOLOGY CASE HISTORY 15 (HISTOLOGY) (Lynne S. Garcia) A biopsy was performed on a 27-year-old man with no known travel history, presenting with a perianal ulcer. The specimen was preserved in formalin
