Student number. University of Guelph Department of Chemistry and Biochemistry Structure and Function In Biochemistry
|
|
|
- Bonnie Leonard
- 6 years ago
- Views:
Transcription
1 University of Guelph Department of Chemistry and Biochemistry Structure and Function In Biochemistry Midterm Test, March 3, Time allowed, 90 min. Answer questions 120 on the computer scoring sheet provided. Only one option is correct for each of these questions. Use pencil to fill in the circles representing your chosen answer; and erase cleanly if you make an error. Do not use ink or whiteout on the computer scoring sheet. You may mark your answer on the question paper for your own records, however in case of a discrepancy between question paper and the computer scoring sheet, the choice shown on the computer sheet will be taken as final. Answer questions 2124 directly on the question paper. Questions 120 have a weight of each Questions 2124 have marks as indicated 20 marks 20 marks ======= 40 marks total Q.2122 Q.23 Q. 24 subtotal
2 Answer questions 120 on the computer scoring sheet. Value each 1. Thalassemia with two b globin genes defective is not always lethal because: A) α hemoglobin functions adequately on its own. B) The other β globin genes make adequate supplies of hemoglobin. C) Fetal γglobins may continue to be expressed in adult life. D) The body makes do with myoglobin provided no strenuous activity is undertaken. E) This condition is never observed. 1) A B C D E 2. The Bohr effect is the reduced affinity of hemoglobin for O 2 A) Due to binding of 2,3bisphosphoglycerate B) Due to high altitude. C) Due to lower ph D) In fetal hemoglobin. E) Resulting from the hemoglobin S mutation. 2) A B C D E 3. ATP acts on aspartate transcarbamoylase: A) By providing energy for the reaction. B) By phosphorylating the substrate. C) As a homotropic effector D) As a feedback inhibitor. E) By binding and stabilizing the R state of the enzyme. 3) A B C D E 4. Vitamin K is required for normal blood clotting because: A) Vitamin K binds to thrombin making it more active. B) Vitamins make you more healthy. C) Vitamin K is required for formation of γcarboxyglutamate in the active site of thrombin. D) Vitamin K is required for formation of γcarboxyglutamate in prothrombin. E) Vitamin K binds Ca 2+ to neutralize the negative charge or fibrinogen. 4) A B C D E 5. Which of the following does not increase activity in the cyclic AMPprotein kinase cascade? A) Adenylate cyclase B) Protein kinase A C) Glycogen Synthase D) Phosphorylase kinase E) Phosphorylase 5) A B C D E 6. Caffeine acts as a stimulant by: A) Increasing synthesis of cyclic AMP B) Stimulating Gprotein to bind GTP C) Preventing breakdown of cyclic AMP D) Binding to protein kinase A. E) Binding to a hormone receptor on the cell surface. 6) A B C D E 7. The initial activation of trypsinogen requires: A) Enteropeptidase B) Pepsinogen C) Chymotrypsinogen D) Chymotrypsin E) Pancreatic trypsin inhibitor. 7) A B C D E
3 8. Which statement about 2,3 bisphosphoglycerate (BPG) is untrue? A) People who live at high altitude have higher BPG levels to enhance O 2 release in tissues. B) BPG binds to a positively charged region between the βglobin subunits. C) BPG binds more weakly to fetal hemoglobin, so O 2 binding is stronger. D) BPG binding favours the R state of hemoglobin. E) BPG is derived from intermediates of the glycolysis pathway. 8) A B C D E 9. In the MonodWymanChangeux model of allosteric cooperative binding, e.g. of O 2 by hemoglobin, which one of the following is assumed? A) That all subunits change from T to R together. B) Each subunit changes from T to R as it binds O 2. C) All subunits bind O 2 simultaneously. D) high affinity sites are occupied first. E) O 2 can t bind until hemoglobin is in the R state. 9) A B C D E 10. Which statement about Histidine F8 in the globin sequence is incorrect: A) changes position in the R state to T state transition B) is the 5th ligand for Fe 2+. C) forces the 6th ligand into a bent configuration. D) pulls Fe 2+ out of the heme plane. E) is found in myoglobin and all hemoglobins. 10) A B C D E 11. Plots of O 2 binding by myoglobin (Mb) are made in terms of fractional occupancy θ. Which formula correctly represents Y? [Mb] A) θ = [Mb]+[MbO B) θ = C) θ = 2 ] [Mb] [Mb]+ D) θ = [MbO E) θ = 11) A B C D E 2 ] [Mb][O 2 ] 12. Which of the following correctly completes the statement Binding of aspartate by ATCase is a sigmoidal function of [aspartate] because A) Aspartate molecules bind to all catalytic subunits simultaneously. B) As each aspartate binds, it changes the csubunit it s bound to into the Rstate. C) Aspartate is a positive heterotropic effector for ATCase. D) Each aspartate bound increases the probability that the whole c 6 r 6 will convert to the R state. E) Binding negatively charged aspartate favours the Tstate. 12) A B C D E 13. Which of the following is not a serine protease? A) Factor XIIa B) Fibrin C) Plasmin D) Thrombin E) Tissue Plasminogen Activator 13) A B C D E
4 14. A blood clotting factor which is activated by both intrinsic and extrinsic pathways is: A) Factor VIII B) Factor IX C) Factor VII D) Factor XI E) Factor X. 14) A B C D E 15. Cleavage of the bond between Arg 15 and Ile 16 activates chymotrypsinogen because: A) this releases a peptide that blocked the active site. B) overall, the protein becomes less negative. C) substrate can now bind to Ile 16. D) New Nterminal Ile 16 is positive and can attract Asp 194 into the correct position for active enzyme. E) Ile 16 attracts Asp 102, setting up the catalytic triad. 15) A B C D E 16. Classical hemophilia can be treated by replacement of missing A) Vitamin K B) Factor VIII C) Factor XIII D) Factor XI E) Factor VII. 16) A B C D E 17. Carbon dioxide helps O 2 release from hemoglobin by: A) Binding to heme Fe and displacing O 2. B) Binding to Histidine E7 and displacing O 2 C) Binding to the Nterminal amino groups and changing their charge from positive to negative. D) Reducing the partial pressure of O 2. E) increasing the ph in peripheral tissues. 17) A B C D E 18. The c 6 r 6 organization of ATCase is dissociated into smaller subunits by covalent binding of phydroxymercuribenzoate. Which of the following is not true: A) the c 3 component is catalytically active. B) the r 2 component can bind ATP or CTP. C) the c 3 component is under allosteric control.d) the r 2 component does not bind substrate. E) the r 2 and c 3 components can recombine into fully functional allosterically regulated ATCase. 18) A B C D E 19. The sedimentation rate of ATCase is easily measured in the ultracentrifuge. When ATP is added to a sample, which of the following is true? A) Sedimentation rate increases because ATP favours the more open R state. B) Sedimentation rate decreases because ATP favours the more open R state. C) Sedimentation rate increases because ATP favours the more compact R state. D) Sedimentation rate increases because of added mass of ATP. E) Sedimentation rate decreases because the ATCase breaks up into c 3 and r 2 components. 19) A B C D E 20. Which of the following amino acids is the most hydrophobic? A) Asp B) Ser C) His D) Val E) Lys 20) A B C D E
5 21. Factor XIIIa plays a unique role in the blood clotting system. i) Which serine protease activates factor XIII to give to XIIIa? ii) Once factor XIIIa is active, what reaction does it catalyze, and what effect does this have on the blood clot? 3 marks 22. Prothrombin and the zymogen forms of several serine protease blood clotting factors, e.g. factor X, have an unusually large propeptide which is important in the activation process. What modified amino acid is found in this propeptide? How does this modified amino acid participate in the activation mechanism? Explain why blood clots in contact with glass, which has a negatively charged surface. What natural negatively charged surface triggers blood clotting in the body? 4 marks
6 23. The diagram on the right shows a plot of f R, the fraction of ATCase molecules in the R allosteric state, as a function of [succinate]. Also plotted is Y, the fraction of substrate sites occupied by succinate f R Y What instrumental method was used to measure f R? What property of ATCase changes on adopting R state to allow this measurement? Why was the experiment done with succinate instead of aspartate, the natural substrate? CH 2 CH 2 CH 2 NH 2 CH succinate aspartate What is the significance of the fact that the curve for f R lies to the left of the curve for occupancy, Y? What conclusion might be drawn if the two curves were superimposed? 3 marks [succinate] Aspartate is one substrate for aspartate transcarbamoylase (ATCase); name the other:
7 24. The arrangement of subunits in hemoglobin is best described in terms of two globin pairs, α 1 β 1 and α 2 β 2 (subscripts identify different subunits rather than showing stoichiometry). What structural changes are noted in the change from T to R state of hemoglobin? How is the orientation of α 1 β 1 to α 2 β 2 changed? Which combination is least affected by change from T to R? Which combination alters its hydrogen bonding pattern on change T to R? Which combination moves further apart on change T to R? Which combination moves closert together on change T to R? Which combination contains the 2,3bisphosphoglycerate binding site? Which combination contains salt bridges that help restore the molecule to the T state? 3 marks
Student number. University of Guelph Department of Chemistry and Biochemistry Structure and Function In Biochemistry
 University of Guelph Department of Chemistry and Biochemistry 19356 Structure and Function In Biochemistry Midterm Test, March 3, 1998. Time allowed, 90 min. Answer questions 120 on the computer scoring
University of Guelph Department of Chemistry and Biochemistry 19356 Structure and Function In Biochemistry Midterm Test, March 3, 1998. Time allowed, 90 min. Answer questions 120 on the computer scoring
University of Guelph Department of Chemistry and Biochemistry Structure and Function In Biochemistry
 University of Guelph Department of Chemistry and Biochemistry 19-356 Structure and Function In Biochemistry Final Exam, April 21, 1997. Time allowed, 120 min. Answer questions 1-30 on the computer scoring
University of Guelph Department of Chemistry and Biochemistry 19-356 Structure and Function In Biochemistry Final Exam, April 21, 1997. Time allowed, 120 min. Answer questions 1-30 on the computer scoring
The R-subunit would not the able to release the catalytic subunit, so this mutant of protein kinase A would be incapable of being activated.
 1. Explain how one molecule of cyclic AMP can result in activation of thousands of molecules of glycogen phosphorylase. Technically it takes four molecules of cyclic AMP to fully activate one molecule
1. Explain how one molecule of cyclic AMP can result in activation of thousands of molecules of glycogen phosphorylase. Technically it takes four molecules of cyclic AMP to fully activate one molecule
Chapter 10. Regulatory Strategy
 Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Enzyme Regulation I. Dr. Kevin Ahern
 Enzyme Regulation I Dr. Kevin Ahern Enzyme Regulation Mechanisms Enzyme Regulation Mechanisms 1. Allosterism Enzyme Regulation Mechanisms 1. Allosterism 2. Covalent Modification Enzyme Regulation Mechanisms
Enzyme Regulation I Dr. Kevin Ahern Enzyme Regulation Mechanisms Enzyme Regulation Mechanisms 1. Allosterism Enzyme Regulation Mechanisms 1. Allosterism 2. Covalent Modification Enzyme Regulation Mechanisms
Tala Saleh. Ahmad Attari. Mamoun Ahram
 23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
Lecture 19: Review of regulation
 Chem*3560 Lecture 19: Review of regulation What is meant by cooperative allosteric regulation? Positive cooperativity - characteristic is the sigmoidal binding/activity curve T-state has weaker affinity,
Chem*3560 Lecture 19: Review of regulation What is meant by cooperative allosteric regulation? Positive cooperativity - characteristic is the sigmoidal binding/activity curve T-state has weaker affinity,
CHAPTER 10: REGULATORY STRATEGIES. Traffic signals control the flow of traffic
 CHAPTER 10: REGULATORY STRATEGIES Traffic signals control the flow of traffic INTRODUCTION CHAPTER 10 The activity of enzymes must often be regulated so that they function at the proper time and place.
CHAPTER 10: REGULATORY STRATEGIES Traffic signals control the flow of traffic INTRODUCTION CHAPTER 10 The activity of enzymes must often be regulated so that they function at the proper time and place.
Biological Sciences 4087 Exam I 9/20/11
 Name: Biological Sciences 4087 Exam I 9/20/11 Total: 100 points Be sure to include units where appropriate. Show all calculations. There are 5 pages and 11 questions. 1.(20pts)A. If ph = 4.6, [H + ] =
Name: Biological Sciences 4087 Exam I 9/20/11 Total: 100 points Be sure to include units where appropriate. Show all calculations. There are 5 pages and 11 questions. 1.(20pts)A. If ph = 4.6, [H + ] =
REGULATION OF ENZYME ACTIVITY. Medical Biochemistry, Lecture 25
 REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
Lecture 6: Allosteric regulation of enzymes
 Chem*3560 Lecture 6: Allosteric regulation of enzymes Metabolic pathways do not run on a continuous basis, but are regulated according to need Catabolic pathways run if there is demand for ATP; for example
Chem*3560 Lecture 6: Allosteric regulation of enzymes Metabolic pathways do not run on a continuous basis, but are regulated according to need Catabolic pathways run if there is demand for ATP; for example
University of Guelph Department of Chemistry and Biochemistry Structure and Function In Biochemistry
 University of Guelph Department of Chemistry and Biochemistry 19-356 Structure and Function In Biochemistry Final Exam, April 22, 1998. Time allowed, 120 min. Answer questions 1-30 on the computer scoring
University of Guelph Department of Chemistry and Biochemistry 19-356 Structure and Function In Biochemistry Final Exam, April 22, 1998. Time allowed, 120 min. Answer questions 1-30 on the computer scoring
Margaret A. Daugherty Fall 2003
 Enzymes & Kinetics IV Regulation and Allostery ENZYME-SUBSTRATE INTERACTIONS THE LOCK & KEY MODEL Margaret A. Daugherty Fall 2003 A perfect match between enzyme and substrate can explain enzyme specificity
Enzymes & Kinetics IV Regulation and Allostery ENZYME-SUBSTRATE INTERACTIONS THE LOCK & KEY MODEL Margaret A. Daugherty Fall 2003 A perfect match between enzyme and substrate can explain enzyme specificity
University of Guelph Department of Chemistry and Biochemistry Structure and Function In Biochemistry
 University of Guelph Department of Chemistry and Biochemistry 19-356 Structure and Function In Biochemistry Final Exam, April 21, 1997. Time allowed, 120 min. Answer questions 1-30 on the computer scoring
University of Guelph Department of Chemistry and Biochemistry 19-356 Structure and Function In Biochemistry Final Exam, April 21, 1997. Time allowed, 120 min. Answer questions 1-30 on the computer scoring
Chapter 7. Heme proteins Cooperativity Bohr effect
 Chapter 7 Heme proteins Cooperativity Bohr effect Hemoglobin is a red blood cell protein that transports oxygen from the lungs to the tissues. Hemoglobin is an allosteric protein that displays cooperativity
Chapter 7 Heme proteins Cooperativity Bohr effect Hemoglobin is a red blood cell protein that transports oxygen from the lungs to the tissues. Hemoglobin is an allosteric protein that displays cooperativity
Lecture 5. Dr. Sameh Sarray Hlaoui
 Lecture 5 Myoglobin & Hemoglobin Dr. Sameh Sarray Hlaoui Myoglobin and Hemoglobin Myoglobin - Myoglobin and Hemoglobin are (metalloprotein containing a heme prosthetic group). hemeproteins - Function as
Lecture 5 Myoglobin & Hemoglobin Dr. Sameh Sarray Hlaoui Myoglobin and Hemoglobin Myoglobin - Myoglobin and Hemoglobin are (metalloprotein containing a heme prosthetic group). hemeproteins - Function as
Globular proteins Proteins globular fibrous
 Globular proteins Globular proteins Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form in a biologically functional way. Globular
Globular proteins Globular proteins Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form in a biologically functional way. Globular
Bio 100 Serine Proteases 9/26/11
 Assigned Reading: 4th ed. 6.4.1 The Chymotrypsin Mechanism Involves Acylation And Deacylation Of A Ser Residue p. 213 BOX 20-1 Penicillin and β-lactamase p. 779 6.5.7 Some Enzymes Are Regulated By Proteolytic
Assigned Reading: 4th ed. 6.4.1 The Chymotrypsin Mechanism Involves Acylation And Deacylation Of A Ser Residue p. 213 BOX 20-1 Penicillin and β-lactamase p. 779 6.5.7 Some Enzymes Are Regulated By Proteolytic
Chapter 15. Enzyme Regulation. Activity? Part 1 Factors that influence enzymatic activity
 Chapter 15 Enzyme Regulation http://lms.ls.ntou.edu.tw/course/106ls tw/course/106 hanjia@mail.ntou.edu.tw Reginald H. Garrett Charles M. Grisham Essential Questions Before this class, ask your self the
Chapter 15 Enzyme Regulation http://lms.ls.ntou.edu.tw/course/106ls tw/course/106 hanjia@mail.ntou.edu.tw Reginald H. Garrett Charles M. Grisham Essential Questions Before this class, ask your self the
GENERAL THOUGHTS ON REGULATION. Lecture 16: Enzymes & Kinetics IV Regulation and Allostery REGULATION IS KEY TO VIABILITY
 GENERAL THOUGHTS ON REGULATION Lecture 16: Enzymes & Kinetics IV Regulation and Allostery Margaret A. Daugherty Fall 2004 1). Enzymes slow down as product accumulates 2). Availability of substrates determines
GENERAL THOUGHTS ON REGULATION Lecture 16: Enzymes & Kinetics IV Regulation and Allostery Margaret A. Daugherty Fall 2004 1). Enzymes slow down as product accumulates 2). Availability of substrates determines
Chapter 11: Enzyme Catalysis
 Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Biol220 Cell Signalling Cyclic AMP the classical secondary messenger
 Biol220 Cell Signalling Cyclic AMP the classical secondary messenger The classical secondary messenger model of intracellular signalling A cell surface receptor binds the signal molecule (the primary
Biol220 Cell Signalling Cyclic AMP the classical secondary messenger The classical secondary messenger model of intracellular signalling A cell surface receptor binds the signal molecule (the primary
Anas Kishawi. Zaid Emad. Nafez abu tarboush
 24 Anas Kishawi Zaid Emad Nafez abu tarboush Hello everybody, this sheet is done according to Dr. Nafith s lecture so try to use his slides for the best understanding, and good luck. WAYS OF CHANGING THE
24 Anas Kishawi Zaid Emad Nafez abu tarboush Hello everybody, this sheet is done according to Dr. Nafith s lecture so try to use his slides for the best understanding, and good luck. WAYS OF CHANGING THE
Biochemistry 15 Doctor /7/2012
 Heme The Heme is a chemical structure that diffracts by light to give a red color. This chemical structure is introduced to more than one protein. So, a protein containing this heme will appear red in
Heme The Heme is a chemical structure that diffracts by light to give a red color. This chemical structure is introduced to more than one protein. So, a protein containing this heme will appear red in
Q1: Circle the best correct answer: (15 marks)
 Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Q1: Circle the best correct answer: (15 marks) 1. Which one of the following incorrectly pairs an amino acid with a valid chemical characteristic a. Glycine, is chiral b. Tyrosine and tryptophan; at neutral
Exam 2 Review Problems DO NOT BRING TO EXAM
 This packet contains problems from old exams, your book, supplemental materials, and even stuff from a TA from many years past. Use this as practice only. This is not, by any means, a definitive indication
This packet contains problems from old exams, your book, supplemental materials, and even stuff from a TA from many years past. Use this as practice only. This is not, by any means, a definitive indication
2018 Biochemistry 110 California Institute of Technology Lecture 11: Enzyme Regulatory Strategies
 2018 Biochemistry 110 California Institute of Technology Lecture 11: Enzyme Regulatory Strategies 1. Aspartate Transcarbamoylase (ATCase) 2. Zymogen and Digestive Enzyme Regulation 3. Blood Clotting and
2018 Biochemistry 110 California Institute of Technology Lecture 11: Enzyme Regulatory Strategies 1. Aspartate Transcarbamoylase (ATCase) 2. Zymogen and Digestive Enzyme Regulation 3. Blood Clotting and
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Key Concepts. Learning Objectives
 Lectures 8 and 9: Protein Function, Ligand Binding -- Oxygen Binding and Allosteric Regulation in Hemoglobin [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 7, pp. 183-199 problems in textbook: chapter
Lectures 8 and 9: Protein Function, Ligand Binding -- Oxygen Binding and Allosteric Regulation in Hemoglobin [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 7, pp. 183-199 problems in textbook: chapter
PROTEOMICS August 27 31, 2007 Peter D'Eustachio - MSB
 PROTEOMICS August 27 31, 2007 Peter D'Eustachio - MSB 328 e-mail: deustp01@med.nyu.edu GOALS OF THIS SEGMENT OF THE COURSE Recognize the structures of the 20 amino acids; understand their properties as
PROTEOMICS August 27 31, 2007 Peter D'Eustachio - MSB 328 e-mail: deustp01@med.nyu.edu GOALS OF THIS SEGMENT OF THE COURSE Recognize the structures of the 20 amino acids; understand their properties as
Vets 111/Biov 111 Cell Signalling-2. Secondary messengers the cyclic AMP intracellular signalling system
 Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
BIOCHEMISTRY I HOMEWORK III DUE 10/15/03 66 points total + 2 bonus points = 68 points possible Swiss-PDB Viewer Exercise Attached
 BIOCHEMISTRY I HOMEWORK III DUE 10/15/03 66 points total + 2 bonus points = 68 points possible Swiss-PDB Viewer Exercise Attached 1). 20 points total T or F (2 points each; if false, briefly state why
BIOCHEMISTRY I HOMEWORK III DUE 10/15/03 66 points total + 2 bonus points = 68 points possible Swiss-PDB Viewer Exercise Attached 1). 20 points total T or F (2 points each; if false, briefly state why
Lecture 18 (10/27/17) Lecture 18 (10/27/17)
 Reading: Ch6; 225-232 Lecture 18 (10/27/17) Problems: Ch5 (text); 2 Ch6 (study guide-facts); 5, 6, 7, 14 NEXT Reading: Ch5; 164, 166-169 Problems: none Remember Monday at 6:30 in PHO-206 is the first MB
Reading: Ch6; 225-232 Lecture 18 (10/27/17) Problems: Ch5 (text); 2 Ch6 (study guide-facts); 5, 6, 7, 14 NEXT Reading: Ch5; 164, 166-169 Problems: none Remember Monday at 6:30 in PHO-206 is the first MB
The hemoglobin (Hb) can bind a maximum of 220 ml O2 per liter.
 Hemoglobin Hemoglobin The most important function of the red blood cells is totransport (O2) from the lungs into the tissues, and carbon dioxide (CO2) from the tissues back into the lungs. O2 is poorly
Hemoglobin Hemoglobin The most important function of the red blood cells is totransport (O2) from the lungs into the tissues, and carbon dioxide (CO2) from the tissues back into the lungs. O2 is poorly
2. Which of the following amino acids is most likely to be found on the outer surface of a properly folded protein?
 Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
Name: WHITE Student Number: Answer the following questions on the computer scoring sheet. 1 mark each 1. Which of the following amino acids would have the highest relative mobility R f in normal thin layer
PAPER No. : 16, Bioorganic and biophysical chemistry MODULE No. : 22, Mechanism of enzyme catalyst reaction (I) Chymotrypsin
 Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
Subject Paper No and Title 16 Bio-organic and Biophysical Module No and Title 22 Mechanism of Enzyme Catalyzed reactions I Module Tag CHE_P16_M22 Chymotrypsin TABLE OF CONTENTS 1. Learning outcomes 2.
االمتحان النهائي لعام 1122
 االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
االمتحان النهائي لعام 1122 Amino Acids : 1- which of the following amino acid is unlikely to be found in an alpha-helix due to its cyclic structure : -phenylalanine -tryptophan -proline -lysine 2- : assuming
PBL SEMINAR. HEMOGLOBIN, O 2 -TRANSPORT and CYANOSIS An Overview
 1 University of Papua New Guinea School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PBL SEMINAR HEMOGLOBIN, O 2 -TRANSPORT and CYANOSIS
1 University of Papua New Guinea School of Medicine and Health Sciences Division of Basic Medical Sciences Discipline of Biochemistry and Molecular Biology PBL SEMINAR HEMOGLOBIN, O 2 -TRANSPORT and CYANOSIS
Past Years Questions Chpater 6
 Past Years Questions Chpater 6 **************************************** 1) Which of the following about enzymes is Incorrect? A) Most enzymes are proteins. B) Enzymes are biological catalysts. C) Enzymes
Past Years Questions Chpater 6 **************************************** 1) Which of the following about enzymes is Incorrect? A) Most enzymes are proteins. B) Enzymes are biological catalysts. C) Enzymes
1. Hemoglobin and the Movement of Oxygen. Respirator system/biochemistry
 1. Hemoglobin and the Movement of Oxygen Respirator system/biochemistry YOU MUST BE ABLE TO: Hemoglobin and the Movement of Oxygen specific aims 1. Compare structure of myoglobin and hemoglobin 2. Understand
1. Hemoglobin and the Movement of Oxygen Respirator system/biochemistry YOU MUST BE ABLE TO: Hemoglobin and the Movement of Oxygen specific aims 1. Compare structure of myoglobin and hemoglobin 2. Understand
PHRM 836 September 1, 2015
 PRM 836 September 1, 2015 Protein structure- function relationship: Catalysis example of serine proteases Devlin, section 9.3 Physiological processes requiring serine proteases Control of enzymatic activity
PRM 836 September 1, 2015 Protein structure- function relationship: Catalysis example of serine proteases Devlin, section 9.3 Physiological processes requiring serine proteases Control of enzymatic activity
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
بسم هللا الرحمن الرحيم Q1: the overall folding of a single protein subunit is called : -tertiary structure -primary structure -secondary structure -quaternary structure -all of the above Q2 : disulfide
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
270,000,000 hemoglobin units are. hemoglobin has 4 heme units; 2 α and 2 β units. Active site of a heme unit has an Iron ion
 RBC strange shape a biconcave disc that is round and flat RBC has no nucleus. The nucleus is extruded from the cell as it matures. An RBC can change shape to an amazing extent, without breaking, as it
RBC strange shape a biconcave disc that is round and flat RBC has no nucleus. The nucleus is extruded from the cell as it matures. An RBC can change shape to an amazing extent, without breaking, as it
SC/BIOL Biochemistry
 SC/BIOL 2020.03 Biochemistry Midterm #1 Name: Student ID: Feb 7 th, 2013 Time: 1 hr and 15 min This test has multiple choice: 24 Marks. Fill in the blanks: 10 Marks Peptide structure: 6 Marks There are
SC/BIOL 2020.03 Biochemistry Midterm #1 Name: Student ID: Feb 7 th, 2013 Time: 1 hr and 15 min This test has multiple choice: 24 Marks. Fill in the blanks: 10 Marks Peptide structure: 6 Marks There are
Notes 11/2. Heather Graehl
 Lecture Page 1 Notes 11/2 Friday, November 02, 2007 9:56 AM Heather Graehl Notes 1112 Audio recording started: 10:02 AM Friday, November 02, 2007 Slide 1: Enzymes: Serine Proteases (Nov 7th Midterm will
Lecture Page 1 Notes 11/2 Friday, November 02, 2007 9:56 AM Heather Graehl Notes 1112 Audio recording started: 10:02 AM Friday, November 02, 2007 Slide 1: Enzymes: Serine Proteases (Nov 7th Midterm will
Catalysis & specificity: Proteins at work
 Catalysis & specificity: Proteins at work Introduction Having spent some time looking at the elements of structure of proteins and DNA, as well as their ability to form intermolecular interactions, it
Catalysis & specificity: Proteins at work Introduction Having spent some time looking at the elements of structure of proteins and DNA, as well as their ability to form intermolecular interactions, it
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/ MCQs. Location : 102, 105, 106, 301, 302
 FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
ENZYMOLOGY. Regulation of enzyme activity. P.C. Misra Professor Department of Biochemistry Lucknow University Lucknow
 ENZYMOLOGY Regulation of enzyme activity P.C. Misra Professor Department of Biochemistry Lucknow University Lucknow-226 007 5-May-2006 (Revised 17-Aug-2006) CONTENTS Introduction Regulation of activity
ENZYMOLOGY Regulation of enzyme activity P.C. Misra Professor Department of Biochemistry Lucknow University Lucknow-226 007 5-May-2006 (Revised 17-Aug-2006) CONTENTS Introduction Regulation of activity
Enzymes: The Catalysts of Life
 Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
Chapter 6 Enzymes: The Catalysts of Life Lectures by Kathleen Fitzpatrick Simon Fraser University Activation Energy and the Metastable State Many thermodynamically feasible reactions in a cell that could
The MOLECULES of LIFE
 The MOLECULES of LIFE Physical and Chemical Principles Solutions Manual Prepared by James Fraser and Samuel Leachman Chapter 16 Principles of Enzyme Catalysis Problems True/False and Multiple Choice 1.
The MOLECULES of LIFE Physical and Chemical Principles Solutions Manual Prepared by James Fraser and Samuel Leachman Chapter 16 Principles of Enzyme Catalysis Problems True/False and Multiple Choice 1.
We will usually use the common name for an enzyme, such as carboxypeptidase, or chymotrypsin.
 Chapter 11 - Enzymatic Catalysis Introduction: In the late 1800's the Buchner brothers discovered that yeast extracts were capable of alcholic fermentation, thus refuting Pasteur s contention that intact
Chapter 11 - Enzymatic Catalysis Introduction: In the late 1800's the Buchner brothers discovered that yeast extracts were capable of alcholic fermentation, thus refuting Pasteur s contention that intact
Chemistry and Biochemistry 153A Spring Exam 2
 hemistry and Biochemistry 153A Spring 2011 Exam 2 Instructions: You will have 1 hour 45 minutes to complete the exam. You may use a pencil (recommended) or blue or black ink pen to write your answers.
hemistry and Biochemistry 153A Spring 2011 Exam 2 Instructions: You will have 1 hour 45 minutes to complete the exam. You may use a pencil (recommended) or blue or black ink pen to write your answers.
Amino acids. Side chain. -Carbon atom. Carboxyl group. Amino group
 PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
PROTEINS Amino acids Side chain -Carbon atom Amino group Carboxyl group Amino acids Primary structure Amino acid monomers Peptide bond Peptide bond Amino group Carboxyl group Peptide bond N-terminal (
Pharmacist. Drugs. body physiology. ( molecular constituents)
 Why? Pharmacist Drugs body physiology ( molecular constituents) Mechanistic levels of response: Altered patient response physiologic systems Vascular system blood, muscle, liver tissues / organs cellular
Why? Pharmacist Drugs body physiology ( molecular constituents) Mechanistic levels of response: Altered patient response physiologic systems Vascular system blood, muscle, liver tissues / organs cellular
1. Measurement of the rate constants for simple enzymatic reaction obeying Michaelis- Menten kinetics gave the following results: =3x10-5 = 30μM
 1. Measurement of the rate constants for simple enzymatic reaction obeying Michaelis- Menten kinetics gave the following results: k 1 = 2 x 10 8 M -1 s -1, k 2 = 1 x 10 3 s -1, k 3 = 5 x 10 3 s -1 a) What
1. Measurement of the rate constants for simple enzymatic reaction obeying Michaelis- Menten kinetics gave the following results: k 1 = 2 x 10 8 M -1 s -1, k 2 = 1 x 10 3 s -1, k 3 = 5 x 10 3 s -1 a) What
PPP_glycogen_metabolism Part 2 الفريق الطبي األكاديمي. Done By: - Shady Soghayr
 PPP_glycogen_metabolism Part 2 الفريق الطبي األكاديمي Done By: - Shady Soghayr لكية الطب البرشي البلقاء التطبيقية / املركز 6166 6102/ **How we get glucose-1-phosphate from glucose (source of glucose-1-
PPP_glycogen_metabolism Part 2 الفريق الطبي األكاديمي Done By: - Shady Soghayr لكية الطب البرشي البلقاء التطبيقية / املركز 6166 6102/ **How we get glucose-1-phosphate from glucose (source of glucose-1-
BCH 4053 THIRD EXAM November 5, 1999
 BCH 4053 THIRD EXAM November 5, 1999 I remind you that you are bound by the Academic Honor Code. This means (1) you will not give or receive information during this exam, nor will you consult with unauthorized
BCH 4053 THIRD EXAM November 5, 1999 I remind you that you are bound by the Academic Honor Code. This means (1) you will not give or receive information during this exam, nor will you consult with unauthorized
Biochem sheet (5) done by: razan krishan corrected by: Shatha Khtoum DATE :4/10/2016
 Biochem sheet (5) done by: razan krishan corrected by: Shatha Khtoum DATE :4/10/2016 Note about the last lecture: you must know the classification of enzyme Sequentially. * We know that a substrate binds
Biochem sheet (5) done by: razan krishan corrected by: Shatha Khtoum DATE :4/10/2016 Note about the last lecture: you must know the classification of enzyme Sequentially. * We know that a substrate binds
Enzymes: Regulation 2-3
 Enzymes: Regulation 2-3 Reversible covalent modification Association with regulatory proteins Irreversible covalent modification/proteolytic cleavage Reading: Berg, Tymoczko & Stryer, 6th ed., Chapter
Enzymes: Regulation 2-3 Reversible covalent modification Association with regulatory proteins Irreversible covalent modification/proteolytic cleavage Reading: Berg, Tymoczko & Stryer, 6th ed., Chapter
number Done by Corrected by Doctor Nayef Karadsheh
 number 15 Done by BaraaAyed Corrected by Mamoon Alqtamin Doctor Nayef Karadsheh 1 P a g e Regulation of glycogen synthesis and degradation Regulation of glycogen synthesis and degradation involves two
number 15 Done by BaraaAyed Corrected by Mamoon Alqtamin Doctor Nayef Karadsheh 1 P a g e Regulation of glycogen synthesis and degradation Regulation of glycogen synthesis and degradation involves two
Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 30, 2014
 Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 30, 2014 PHRM 836 Exam I - 1 Name: Instructions 1. Check your exam to make certain that it has 10 pages including this cover
Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 30, 2014 PHRM 836 Exam I - 1 Name: Instructions 1. Check your exam to make certain that it has 10 pages including this cover
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
UNIVERSITY OF GUELPH CHEM 4540 ENZYMOLOGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS
 UNIVERSITY F GUELPH CHEM 4540 ENZYMLGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS Instructions: Time allowed = 80 minutes. Total marks = 30. This quiz represents
UNIVERSITY F GUELPH CHEM 4540 ENZYMLGY Winter 2005 Quiz #2: March 24, 2005, 11:30 12:50 Instructor: Prof R. Merrill ANSWERS Instructions: Time allowed = 80 minutes. Total marks = 30. This quiz represents
Regulation Through Conformational Changes
 Regulation Through Conformational Changes These regulatory mechanisms (conformational changes) include: 1. Allosteric activation and inhibition. 2. Phoshorylation or other covalent modification. 3. Protein-protein
Regulation Through Conformational Changes These regulatory mechanisms (conformational changes) include: 1. Allosteric activation and inhibition. 2. Phoshorylation or other covalent modification. 3. Protein-protein
BIOCHEMISTRY 460 FIRST HOUR EXAMINATION FORM A (yellow) ANSWER KEY February 11, 2008
 WRITE YOUR AND I.D. NUMBER LEGIBLY ON EVERY PAGE PAGES WILL BE SEPARATED FOR GRADING! CHECK TO BE SURE YOU HAVE 6 PAGES, (print): ANSWERS INCLUDING COVER PAGE. I swear/affirm that I have neither given
WRITE YOUR AND I.D. NUMBER LEGIBLY ON EVERY PAGE PAGES WILL BE SEPARATED FOR GRADING! CHECK TO BE SURE YOU HAVE 6 PAGES, (print): ANSWERS INCLUDING COVER PAGE. I swear/affirm that I have neither given
FUNDAMENTALS OF BIOCHEMISTRY, CELL BIOLOGY AND BIOPHYSICS Vol. I - Biochemistry of Vitamins, Hormones and Other Messenger Molecules - Chris Whiteley
 BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
BIOCHEMISTRY OF VITAMINS, HORMONES AND OTHER MESSENGER MOLECULES Chris Whiteley Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa Keywords: phosphorylation, phosphorylase,
Previous Class. Today. Term test I discussions. Detection of enzymatic intermediates: chymotrypsin mechanism
 Term test I discussions Previous Class Today Detection of enzymatic intermediates: chymotrypsin mechanism Mechanistic Understanding of Enzymemediated Reactions Ultimate goals: Identification of the intermediates,
Term test I discussions Previous Class Today Detection of enzymatic intermediates: chymotrypsin mechanism Mechanistic Understanding of Enzymemediated Reactions Ultimate goals: Identification of the intermediates,
Ch. 45 Blood Plasma proteins, Coagulation and Fibrinolysis Student Learning Outcomes: Describe basic components of plasma
 Chapt. 45 Ch. 45 Blood Plasma proteins, Coagulation and Fibrinolysis Student Learning Outcomes: Describe basic components of plasma Inheritance of X-linked gene for Factor VIII hemophilia A Explain the
Chapt. 45 Ch. 45 Blood Plasma proteins, Coagulation and Fibrinolysis Student Learning Outcomes: Describe basic components of plasma Inheritance of X-linked gene for Factor VIII hemophilia A Explain the
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2011
 Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2011 I. (25 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2011 I. (25 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
Enzymes. Gibbs Free Energy of Reaction. Parameters affecting Enzyme Catalysis. Enzyme Commission Number
 SCBC203 Enzymes Jirundon Yuvaniyama, Ph.D. Department of Biochemistry Faculty of Science Mahidol University Gibbs Free Energy of Reaction Free Energy A B + H 2 O A OH + B H Activation Energy Amount of
SCBC203 Enzymes Jirundon Yuvaniyama, Ph.D. Department of Biochemistry Faculty of Science Mahidol University Gibbs Free Energy of Reaction Free Energy A B + H 2 O A OH + B H Activation Energy Amount of
Amino acids & Protein Structure Chemwiki: Chapter , with most emphasis on 16.3, 16.4 and 16.6
 Amino acids & Protein Structure Chemwiki: Chapter 16. 16.1, 16.3-16.9 with most emphasis on 16.3, 16.4 and 16.6 1 1. Most jobs (except information storage) in cells are performed by proteins. 2. Proteins
Amino acids & Protein Structure Chemwiki: Chapter 16. 16.1, 16.3-16.9 with most emphasis on 16.3, 16.4 and 16.6 1 1. Most jobs (except information storage) in cells are performed by proteins. 2. Proteins
Chem Exam 2 (A) Name
 Chem 4511 Exam 2 (A) Name No credit will be given for answers (or work) that are on the backsides of the pages. You may use the backsides as scratch paper, but put all of your answers on the front sides.
Chem 4511 Exam 2 (A) Name No credit will be given for answers (or work) that are on the backsides of the pages. You may use the backsides as scratch paper, but put all of your answers on the front sides.
Lecture 34. Carbohydrate Metabolism 2. Glycogen. Key Concepts. Biochemistry and regulation of glycogen degradation
 Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
Lecture 34 Carbohydrate Metabolism 2 Glycogen Key Concepts Overview of Glycogen Metabolism Biochemistry and regulation of glycogen degradation Biochemistry and regulation of glycogen synthesis What mechanisms
Review of Biochemistry
 Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Review of Biochemistry Chemical bond Functional Groups Amino Acid Protein Structure and Function Proteins are polymers of amino acids. Each amino acids in a protein contains a amino group, - NH 2,
Tuesday, Sept. 14, Is an enzyme a rigid system?
 Tuesday, Sept. 14, Is an enzyme a rigid system? Early researchers thought of enzymes as rigid entities, recognizing their substrates the way a lock would recognize a key. Today's researchers, however,
Tuesday, Sept. 14, Is an enzyme a rigid system? Early researchers thought of enzymes as rigid entities, recognizing their substrates the way a lock would recognize a key. Today's researchers, however,
Gas Exchange in the Tissues
 Gas Exchange in the Tissues As the systemic arterial blood enters capillaries throughout the body, it is separated from the interstitial fluid by only the thin capillary wall, which is highly permeable
Gas Exchange in the Tissues As the systemic arterial blood enters capillaries throughout the body, it is separated from the interstitial fluid by only the thin capillary wall, which is highly permeable
Regulation of Enzymatic Activity. Lesson 4
 Regulation of Enzymatic Activity Lesson 4 Regulation of Enzymatic Activity no real regulation: - regulation of enzyme expression and turnover - control of enzyme trafficking - supply of cofactors real
Regulation of Enzymatic Activity Lesson 4 Regulation of Enzymatic Activity no real regulation: - regulation of enzyme expression and turnover - control of enzyme trafficking - supply of cofactors real
Blood clotting. Subsequent covalent cross-linking of fibrin by a transglutaminase (factor XIII) further stabilizes the thrombus.
 Blood clotting It is the conversion, catalyzed by thrombin, of the soluble plasma protein fibrinogen (factor I) into polymeric fibrin, which is deposited as a fibrous network in the primary thrombus. Thrombin
Blood clotting It is the conversion, catalyzed by thrombin, of the soluble plasma protein fibrinogen (factor I) into polymeric fibrin, which is deposited as a fibrous network in the primary thrombus. Thrombin
Blood coagulation and fibrinolysis. Blood clotting (HAP unit 5 th )
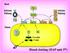 Blood coagulation and fibrinolysis Blood clotting (HAP unit 5 th ) Vessel injury Antithrombogenic (Favors fluid blood) Thrombogenic (Favors clotting) 3 Major systems involved Vessel wall Endothelium ECM
Blood coagulation and fibrinolysis Blood clotting (HAP unit 5 th ) Vessel injury Antithrombogenic (Favors fluid blood) Thrombogenic (Favors clotting) 3 Major systems involved Vessel wall Endothelium ECM
CH395 G Exam 2 Multiple Choice - Fall 2004
 C395 G Exam 2 Multiple Choice - Fall 2004 1. Which of the following fatty acids has the lowest melting point? a. Fatty acids with sites of unsaturation with cis double bonds b. Fatty acids with sites of
C395 G Exam 2 Multiple Choice - Fall 2004 1. Which of the following fatty acids has the lowest melting point? a. Fatty acids with sites of unsaturation with cis double bonds b. Fatty acids with sites of
Chemical Mechanism of Enzymes
 Chemical Mechanism of Enzymes Enzyme Engineering 5.2 Definition of the mechanism 1. The sequence from substrate(s) to product(s) : Reaction steps 2. The rates at which the complex are interconverted 3.
Chemical Mechanism of Enzymes Enzyme Engineering 5.2 Definition of the mechanism 1. The sequence from substrate(s) to product(s) : Reaction steps 2. The rates at which the complex are interconverted 3.
Chymotrypsin Lecture. Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin
 Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Chymotrypsin Lecture Aims: to understand (1) the catalytic strategies used by enzymes and (2) the mechanism of chymotrypsin What s so great about enzymes? They accomplish large rate accelerations (10 10-10
Scantron Instructions
 BIOLOGY 1A MIDTERM # 1 February 17 th, 2012 NAME SECTION # DISCUSSION GSI 1. Sit every other seat and sit by section number. Place all books and paper on the floor. Turn off all phones, pagers, etc. and
BIOLOGY 1A MIDTERM # 1 February 17 th, 2012 NAME SECTION # DISCUSSION GSI 1. Sit every other seat and sit by section number. Place all books and paper on the floor. Turn off all phones, pagers, etc. and
Biology 638 Biochemistry II Exam-2
 Biology 638 Biochemistry II Exam-2 Biol 638, Exam-2 (Code-1) 1. Assume that 16 glucose molecules enter into a liver cell and are attached to a liner glycogen one by one. Later, this glycogen is broken-down
Biology 638 Biochemistry II Exam-2 Biol 638, Exam-2 (Code-1) 1. Assume that 16 glucose molecules enter into a liver cell and are attached to a liner glycogen one by one. Later, this glycogen is broken-down
Theme 1 CONTROL OF ENZYME ACTIVITY
 Theme 1 CONTROL OF ENZYME ACTIVITY Included in this section: Enzyme Activity: Control Notes in study material as well as slides used in class. Please use the study material provide as well as Matthews
Theme 1 CONTROL OF ENZYME ACTIVITY Included in this section: Enzyme Activity: Control Notes in study material as well as slides used in class. Please use the study material provide as well as Matthews
ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIDS. Enzyme Kinetics and Control Reactions
 Handout Enzyme Kinetics and Control Reactions ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIDS Enzyme Kinetics and Control Reactions I. Kinetics A. Reaction rates 1. First order (reaction rate is
Handout Enzyme Kinetics and Control Reactions ANSC 689 PHYSIOLOGICAL CHEMISTRY OF LIVESTOCK SPECIDS Enzyme Kinetics and Control Reactions I. Kinetics A. Reaction rates 1. First order (reaction rate is
Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 30, 2014
 Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 30, 2014 PHRM 836 Exam I - 1 Name: Instructions 1. Check your exam to make certain that it has 10 pages including this cover
Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 30, 2014 PHRM 836 Exam I - 1 Name: Instructions 1. Check your exam to make certain that it has 10 pages including this cover
Part IV Antithrombotics, Anticoagulants and Fibrinolytics
 Part IV Antithrombotics, Anticoagulants and Fibrinolytics "The meaning of good and bad, of better and worse, is simply helping or hurting" Emerson Chapter 16: Blood Coagulation and Fibrinolytic System
Part IV Antithrombotics, Anticoagulants and Fibrinolytics "The meaning of good and bad, of better and worse, is simply helping or hurting" Emerson Chapter 16: Blood Coagulation and Fibrinolytic System
Biology 638 Biochemistry II Exam-1
 Biology 638 Biochemistry II Exam-1 Using the following values, answer questions 1-3. ATP + H 2 O ADP + P i ΔG = -30 kj/mol Creatine-phosphate + H 2 O Creatine + P i ΔG = -12 kj/mol ½O 2 + 2H + + 2e - H
Biology 638 Biochemistry II Exam-1 Using the following values, answer questions 1-3. ATP + H 2 O ADP + P i ΔG = -30 kj/mol Creatine-phosphate + H 2 O Creatine + P i ΔG = -12 kj/mol ½O 2 + 2H + + 2e - H
2008MSC'STRUCTURAL'BIOCHEMISTRY'
 LearningObjectives: 2008MSC'STRUCTURAL'BIOCHEMISTRY' Module'1' 'Amino'Acids'and'Proteins:'Structure'and'Function' 1 Understandthestructureandstereochemistryofalpha1aminoacids 1 Beabletodescribetheclassesofaminoacidsandtheirproperties
LearningObjectives: 2008MSC'STRUCTURAL'BIOCHEMISTRY' Module'1' 'Amino'Acids'and'Proteins:'Structure'and'Function' 1 Understandthestructureandstereochemistryofalpha1aminoacids 1 Beabletodescribetheclassesofaminoacidsandtheirproperties
v o = V max [S] rate = kt[s] e V max = k cat E t ΔG = -RT lnk eq K m + [S]
![v o = V max [S] rate = kt[s] e V max = k cat E t ΔG = -RT lnk eq K m + [S] v o = V max [S] rate = kt[s] e V max = k cat E t ΔG = -RT lnk eq K m + [S]](/thumbs/81/84404059.jpg) Exam 3 Spring 2017 Dr. Stone 8:00 Name There are 100 possible points on this exam. -ΔG / RT v o = V max [S] rate = kt[s] e V max = k cat E t ΔG = -RT lnk eq K m + [S] h rate forward = k forward [reactants]
Exam 3 Spring 2017 Dr. Stone 8:00 Name There are 100 possible points on this exam. -ΔG / RT v o = V max [S] rate = kt[s] e V max = k cat E t ΔG = -RT lnk eq K m + [S] h rate forward = k forward [reactants]
Figure 1 Original Advantages of biological reactions being catalyzed by enzymes:
 Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Enzyme basic concepts, Enzyme Regulation I III Carmen Sato Bigbee, Ph.D. Objectives: 1) To understand the bases of enzyme catalysis and the mechanisms of enzyme regulation. 2) To understand the role of
Properties of Allosteric Enzymes
 Properties of Allosteric Enzymes (1) An allosteric enzyme possesses at least spatially distinct binding sites on the protein molecules the active or the catalytic site and the regulator or the allosteric
Properties of Allosteric Enzymes (1) An allosteric enzyme possesses at least spatially distinct binding sites on the protein molecules the active or the catalytic site and the regulator or the allosteric
Lecture 15. Signal Transduction Pathways - Introduction
 Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Examination I Key PHRM 836 Biochemistry for Pharmaceutical Sciences II September 26, 2012
 Examination I Key PHRM 836 Biochemistry for Pharmaceutical Sciences II September 26, 2012 PHRM 836 Exam I - 1 Correct answers in multiple choice questions are indicated in RED and underlined. Correct answers
Examination I Key PHRM 836 Biochemistry for Pharmaceutical Sciences II September 26, 2012 PHRM 836 Exam I - 1 Correct answers in multiple choice questions are indicated in RED and underlined. Correct answers
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site. Still having trouble understanding the material? Check
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site. Still having trouble understanding the material? Check
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2005
 Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2005 I. (20 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
Metabolic Biochemistry / BIBC 102 Midterm Exam / Spring 2005 I. (20 points) Fill in all of the enzyme catalyzed reactions which convert glycogen to lactate. Draw the correct structure for each intermediate
Review II: The Molecules of Life
 Review II: The Molecules of Life Judy Wieber BBSI @ Pitt 2007 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2007 Outline Introduction Proteins Carbohydrates Lipids
Review II: The Molecules of Life Judy Wieber BBSI @ Pitt 2007 Department of Computational Biology University of Pittsburgh School of Medicine May 24, 2007 Outline Introduction Proteins Carbohydrates Lipids
