Biology 13A Lab #13: Nutrition and Digestion
|
|
|
- Clare Robbins
- 6 years ago
- Views:
Transcription
1 Biology 13A Lab #13: Nutrition and Digestion Lab #13 Table of Contents: Expected Learning Outcomes Introduction Activity 1: Food Chemistry & Nutrition Activity 2: Parts of the Digestive System Activity 3: Chemical Digestion Expected Learning Outcomes At the end of this lab, you will be able to list the essential nutrients found in food; describe the basic chemical composition of carbohydrates, proteins, fats, and vitamins; identify nutrient content in foods and test for nutrients in unknown samples; learn the parts of the digestive system; demonstrate how nutrients are broken down in the presence of digestive enzymes; and explain functions of major nutrients in the body. Figure 13.1 Digestive System Lab #13 Nutrition and Digestion 102
2 Introduction Food, glorious food! Movement, processing information and responding to the environment, and maintenance of homeostasis all require energy. Ultimately, energy is derived from food. In addition, food provides building material for cells and tissues. The job of the digestive system is to break down food and absorb nutrients: carbohydrates, proteins, and lipids and smaller quantities of vitamins and minerals. Most of the water we need also comes from food. Few foods combine all six nutrients. As primates, we are omnivores, adapted to eat a wide variety of foods to obtain a full complement of necessary nutrients. Our digestive system anatomy and physiology reflects the eclectic diet for which we are adapted. Releasing nutrients from food requires mechanical digestion where large pieces are crushed and ground primarily by teeth, with the aid of tongue and saliva. This increases the surface area for chemical digestion in which digestive enzymes break down complex large molecules such as proteins and carbohydrates to their basic components (e.g. amino acids and simple sugars). Chemical digestion is performed by many organs: for example, salivary glands produce amylase, an enzyme that breaks down starches (polysaccharides) to disaccharides; the pancreas and small intestine produce numerous enzymes that complete the breakdown of proteins, lipids, and carbohydrates to forms usable by cells. The nutrients are absorbed through the lining of the small intestine and are then transported throughout the body. Today we will examine nutrients in food, review the structures of the digestive system, and investigate enzymes responsible for chemical digestion. Testing Your Comprehension: Answer the following questions based on your reading of the introduction. 1. List the five nutrients found in food. 2. What is an omnivore? How does the fact that humans are omnivores influence our anatomy and behavior? 3. Where and how does mechanical digestion occur? 4. What molecules are necessary for chemical digestion? Give examples of organs that perform chemical digestion. Lab #13 Nutrition and Digestion 103
3 Activity 1: Food Chemistry & Nutrition Carbohydrates, proteins, lipids, and vitamins and minerals are the nutrients in food. Carbohydrates are either simple sugars (monosaccharides) consisting of a single sugar molecule such as glucose, or disaccharides, two monosaccharides joined together (e.g. sucrose, or table sugar), or polysaccharides, large chains of monosaccharides. Starch and glycogen are polysaccharides. They are major sources of energy for cellular work. Common sources of carbohydrates in the diet are breads, cereals, fruits and vegetables. Proteins have numerous functions. They are the basis for tissue and organ structure; some are capable of movement (socalled motor proteins ) while others act as enzymes. All proteins are chains of amino acids. Twenty amino acids combine to form thousands of different proteins. Twelve amino acids can be assembled in the body but eight must be obtained directly from the diet. Dietary sources of proteins include fish, soybeans, meat, and dairy products. Lipids are hydrophobic (insoluble in water). They include fats, oils, waxes, phospholipids, and steroids. Concentrated sources of energy, each gram of lipid has more calories than a gram of protein or carbohydrate. In addition to energy storage, lipids form the basic structure of plasma membranes and provide support for joints, tendons, and internal organs. Dietary sources of lipids include nuts, meat, butter and cheese, and vegetable oils. Although only minute quantities of vitamins and minerals are required, a deficiency can have devastating effects. Vitamins help control chemical reactions, often facilitating the actions of enzymes. They are necessary for normal growth and metabolism. Thirteen vitamins are essential for health four of those are fat soluble and are stored for months at a time in adipose tissue; nine are water soluble and must be regularly replaced. Minerals such as calcium and phosphorus are also derived from the diet and perform vital functions. Vitamins are obtained from a wide variety of foods. For example, vitamin C is obtained from citrus fruits and tomatoes whereas vitamin B is found in nuts, whole grains, and beans. Vitamin pills may supplement the diet. Each vitamin has specific functions in the body, leading to particular symptoms if there is a lack. The first symptom of vitamin C deficiency is fatigue, followed by anemia, back and joint pain, bleeding of the gums, and poor wound healing. With time, death ensues, as was the fate of numerous sailors who succumbed to scurvy. Lab #13 Nutrition and Digestion 104
4 Testing Your Comprehension: Answer the following questions based on your reading. 1. Define monosachharide, disaccharide, and polysaccharide, and give examples of each. 2. What is the basic building block of proteins? What are dietary sources of proteins? 3. List several functions of lipids. 4. What causes scurvy and what are some of its symptoms? Simple chemical tests using indicators can be used to determine the presence of nutrients in food. A color change of an indicator is usually a positive test for the presence of a certain nutrient. In this experiment, you will use several indicators to test for the presence of nutrients in solutions. This purpose of this lab is to demonstrate how different foods can contain one, some, or all four of these organic compounds that are important to cells. Summary of Activities 1. Make a hypothesis about the content of food samples you and your lab partners have brought from home. 2. Test each food, along with the appropriate positive and negative controls, for protein, monosaccharide, and complex carbohydrate. We will be using three different tests to identify protein, monosaccharide (glucose and/or fructose), and complex carbohydrates (starch) in various foods. Monosaccharides and starch are both carbohydrates. Monosaccharides are the small organic molecules that are the subunits used to build large starch macromolecules. Bring one food or drink to lab that you think contains one of these compounds as an experimental sample. Some examples of what to bring: juice or milk fruit or vegetables flour, or tofu, or bread anything that can mix with water NO: oily foods uncooked beans or pasta, or other dry unhydrated food dark-colored foods or drinks acidic, or sour foods like orange juice These items will not react well with the test chemicals. Foods will contain at least one type of organic compound. Before beginning, make a prediction about which type of compound or compounds you think your experimental sample contains. Record your prediction on your RESULTS page. You will perform three different tests on the same four experimental samples (share with your lab partners). Each test will use a different indicator reagent that will change color in the presence of the particular organic compound that is being tested for. As part of the experimental method, you must include control samples to insure the validity of your results. A control is a test sample with a known result. If your control samples do not give you the expected result, then your experimental results are not valid and you must reevaluate your experimental set-up (maybe your test chemicals are no good). A negative control will result in no change in color. It will either contain no sample at all or it will contain a nonreactive sample like water. For example, if you are testing for the presence of monosaccharides, the test chemical, called Benedict's solution, will remain the original color blue when mixed with water. A positive control will result in a color change indicating the presence of the compound you are testing for. For example, a 5% glucose solution will react with Benedict's solution and change it from blue to rust (brown-red). You can compare your experimental results to your control results to determine if you obtained a positive reaction. To Prepare Your Samples: 1. If your experimental sample is solid, chop or break it up into the smallest pieces possible using a razor blade or glass rod, whichever is Lab #13 Nutrition and Digestion 104
5 appropriate. Use a pinch of sample small enough so that the material can be easily suspended in 1.5 ml of water and does not fill the bottom of the tube. Lab #13 Nutrition and Digestion 105
6 Add 1.5 ml distilled water to your sample and mix it well. 2. If your sample is a liquid, add 1.5 ml of the liquid to the appropriate tube. 3. Each tube should have the same volume of fluid, regardless of the type of sample. I. Identification of Protein Materials: test tube rack test tubes tape for labeling your tubes 0.5% CuSO4 10% NaOH albumin protein solution (egg white) distilled water four different experimental samples (share with the people at your table) The test chemicals used in this experiment react with the covalent bonds that link amino acids together in protein chains. In the presence of protein, the chemicals will turn varying shades of purple. NOTE: NaOH (sodium hydroxide) is very caustic and will burn your skin and damage your clothes. Handle it with caution. If you do come into contact with it, notify the instructor and flush the area thoroughly with running water. NaOH is the ingredient in hair removal products like Nair. It works by dissolving protein, which is what hair is made of. Procedure: 1. Predict which organic compounds your experimental samples might contain. 2. Label your tubes 1 through Prepare your sample following the instructions from above. 4. CAUTIOUSLY add 20 drops of 10% NaOH solution to each tube. Agitate the tube gently. See instructor for demonstration. 5. Add 4 drops of 0.5% CuSO4 to each tube. Agitate the tubes again. 6. Let the tubes sit at room temperature a few minutes, until you see a color change in your positive control. 7. Record the results. II. Identification of Monosaccharides Materials: hot plate 400 ml beaker half filled with boiling water test tube clamp for picking hot test tubes Benedict's solution 5% glucose solution 5% fructose solution 5% sucrose solution distilled water 4 experimental samples Benedict's solution is a blue solution that will change color in the presence of monosaccharides, such as glucose or fructose. Benedict's changes from blue --> green --> orange --> brown, depending on the amount of monosaccharide present. Any change in color indicates the presence of monosaccharides. Procedure: 1. Set up a boiling water bath using a 400 ml beaker filled with approximately 150 ml of tap water on a hot plate. Add 2 or 3 boiling chips to the water in the beaker. These will prevent boiling water from slashing up when you add your test tubes. Set the hot plate on high to get the water boiling, then reduce the temperature to a setting of "2" to keep the water simmering. The water should be boiling BEFORE you place your tubes in it. 2. Label your tubes 1 through 8. If you are using tape, make sure the tape is high enough on the tube so that it does not get wet in the water bath. Otherwise, the tape will fall off and you will not be able to identify your samples. 3. Prepare your samples as before. 4. Add 1.5 ml of Benedict's solution to each test tube, for a total of 3 ml. 5. Agitate your tubes so that the sample and Benedict's is well mixed. See instructor for demonstration. Lab #13 Nutrition and Digestion 106
7 6. Place the all the test tubes at the same time in the boiling water bath for 5 minutes. 7. Remove the tubes using the test tube clamp and record the resulting color. Do not mix the tubes again once they have been boiled. If your sample was a solid, note the color change in the immediate vicinity of your sample. The rest of the Benedict's may stay blue since a solid cannot mix well. III. Identification of Starch (Complex Carbohydrate) Materials: Iodine solution (IKI) Starch suspension distilled water 4 experimental samples Iodine will react with starch and turn from a yellow/brown color to a purple/black color. Only the purple/black color is an indication of starch. Procedure: 1. Number your tubes. 2. Prepare your samples as before. Remember, each tube should have 1.5 ml of liquid. 3. Add 2-3 drops IKI to each tube. Agitate your tubes. 4. Record your results. 5 Lab #13 Nutrition and Digestion 105
8 Data Sheets: Turn in with lab report Before you begin, predict which organic compounds may be found in each of your samples. Experimental Samples #1: #2: #3: #4: Color of Sample Predicted Organic Compound(s) Part 1: Testing Samples Where Nutrient Content is Known In the first part of the experiment, you will determine the presence of nutrients in prepared samples where the contents are known, at least to the teacher! You won t know what s in them until you run the tests, then you can check to determine whether your results are accurate. We will then proceed to testing nutrient contents of a variety of foods. Procedure 1. Obtain 4 small plastic medicine cups. Use a wax pencil to label them from #1 through #4, for the four samples. 2. Pour approximately 5 ml of each of the samples into the corresponding labeled cups. Use the ml marks on the cup to measure the amount. 3. Each group should obtain 1 white spot plate. Each plate has 4 columns with 3 rows (figure 13.2). Use the wax pencil to number the wells 1 through 12 from left to right (e.g. 1, 5, and 9 will be in Col. 1). Use a plastic pipette to transfer 1 ml of Nutrient Sample #1 to each of the four wells across Row 1 of the spot plate. Rinse the pipette. Add 1 ml of Sample #2 to the four wells in Row #2 then rinse. Add 1 ml of Sample #3 to Row 3. Figure 13.2 Spot Plate Lab #13 Nutrition and Digestion 106
9 4. Use a clean pipette to add 1-2 drops of indophenol solution to Wells #1, 5, and 9 in column 1. Indophenol is an indicator for ascorbic acid (vitamin C). If ascorbic acid is present, it will bleach the indophenol and change the color from blue to colorless. Record observations in Table If the color changed, put a + in the appropriate column. 5. Use a clean pipette and add 1-2 drops of iodine solution to Wells #2, 6, and 10 in Column 2. Iodine is an indicator for starch. If starch is present, the mixture will change color from yellow-brown to blue-black. Record observations in Table Use a clean pipette to add 1-2 drops of Biuret reagent to Wells # 3, 7, and 11 in Column 3 of the spot plate. In the presence of protein, the mixture will change color from light blue to pinkish or purplish violet. Record observations in Table Use a clean pipette to add 1-2 drops of Sudan III to Wells #4, 8, and 12 in Column 4. Sudan III is an indicator for lipids and if they are present, the mixture will stain red. Record observations in Table Table 13.1 Results for Nutrient Sample Tests Sample1 Sample2 Sample3 Sample4 Color (+/-) Color (+/-) Color (+/-) Color (+/-) Indophenol Iodine Solution Biuret Reagent Sudan III Lab #13 Nutrition and Digestion 107
10 Part 2: Testing Samples Where Nutrient Content is Not Known Now that you know how to test for carbohydrates, proteins, Vitamin C, and lipids, we can apply the tests to some common foods to determine what nutrients they contain. 1. Thoroughly rinse the spot plate and dry it. Use a clean pipette to transfer 1 ml of Nutrient Sample #4 to each of the four wells across Row 1 of the spot plate. 2. There are food samples prepared for you to test. Each group will be assigned 1 to 2 samples. Predict what nutrients you will find in: Potato Chips Orange Juice Strawberries Rice Test the food samples as you did the unknown nutrient samples in steps #3-7 on pages Record results in Table Clean Up!!! Lab #13 Nutrition and Digestion 108
11 Indophenol Table 13.2 Results for Known Food Items Potato Chip Orange Juice Strawberries Color (+/-) Color (+/-) Color (+/-) Rice Color (+/-) Iodine Solution Biuret Reagent Sudan III Testing Your Comprehension: Answer the following questions based on the experiment. 1. Which nutrients were identified in the unknown nutrient samples? What were the samples? Record answers in the following table. Nutrient(s) Sample 1 Sample 2 Sample 3 Sample 4 Sample I.D. 2. Which indicators are used to detect lipids, starch, vitamin C, and proteins? 3. Which of the following do you think might test positive for the presence of all the nutrients: carbohydrates, proteins, fats, minerals, and vitamins? Circle your choice. Corn, potatoes, olive oil, red meat, dairy products Lab #13 Nutrition and Digestion 109
12 4. What nutrients did you find when you tested potato chips, orange juice, strawberries, and rice? Did the results match your predictions? 5. Why is it important to eat a variety of foods? In your answer, refer to the results from tests of different food items. Activity 2: Parts of the Digestive System The previous experiment explored some of the nutrients in food. How are nutrients extracted from the food we eat? In this activity, we will follow a bite of food through the digestive system and identify the structures that it passes through. 1. Get a hand-out with the diagram of the digestive system from the instructor. 2. Use the torso model to examine the parts of the system. Beginning at the mouth, follow an imaginary bite of food through the system. 3. On the diagram, place an arrow to the place where mechanical digestion occurs. What structures are involved in mechanical digestion? 4. On the diagram, place an arrow and label the place where absorption of nutrients occurs. What structures are involved? 5. On the diagram, place an arrow and label the location where most water is reabsorbed. Why is reabsorption of water important? Activity 3: Chemical Digestion Chemical digestion is due to enzymes that break down large food molecules to their basic components. Several organs produce enzymes that break down specific food molecules. The mouth contains amylase, an enzyme from saliva that breaks starches (polysaccharides) into disaccharides. Cells in the lining of the stomach produce pepsin, which in the presence of hydrochloric acid serves to break proteins into peptide chains. Chemical digestion is completed in the first part of the small intestine. Enzymes such as lactase, maltase, and sucrase break disaccharides into simple sugars. Polypeptide chains are further broken down to single amino acids by trypsin and peptidase. Lipids are broken into fatty acids and glycerol by lipases. The smaller molecules can then be absorbed by the villi of the small intestine. Procedure Lab #13 Nutrition and Digestion 110
13 Each group needs the following: 1 spot plate ph test strips toothpicks 3 ml Albumin 2% solution 3 ml Biuret solution plastic pipettes Starch solution Amylase solution Corn oil Lipase solution 10% Liquid soap Protein Digestion 1. Label the wells of a spot plate 1 through 12 with #1 to 4 going from left to right across the top row. 2. Place 5 drops of the albumin solution in wells 1 to 4, across the top. 3. To wells #2, #4 and #5, add 5 drops of hydrochloric acid. To wells #3 and #4 add 5 drops of pepsin. 4. Stir the mixture in each well using a clean toothpick. Allow the mixture in each well to remain undisturbed for 5 10 minutes. 5. Determine the ph in each of the wells. Dip a separate ph strip in each well and compare the color change to the ph chart provided on the package. Record results in Table Test for the presence of protein by adding 2 drops of Biuret reagent to each well. A positive test for protein is indicated by a pinkish color. In the observations column, write down whether the sample is a control, whether the protein is completely digested, etc. Lab #13 Nutrition and Digestion 111
14 Table 13.3 Protein Digestion Test Results Well # Contents PH Protein Test (+/-) Observations 1 Albumin 2 Albumin + HCl 3 Albumin + Pepsin 4 Albumin + Pepsin + Hydrochloric Acid 5 Hydrochloric Acid Questions: 1. How could you tell that protein digestion took place? 2. Explain what may have happened in well #2 after the addition of hydrochloric acid. 3. Explained what happened after addition of pepsin in #3. 4. Explain what happened in well #4 after adding pepsin and HCl. 5. Which well showed the greatest degree of protein digestion and why? 6. Does the effect of pepsin depend on ph? Explain your answer with reference to human physiology. Lab #13 Nutrition and Digestion 110
15 Carbohydrate Digestion 1. Use a clean spot plate. Label wells 1 and 2. Place 15 drops of starch solution in the wells of the spot plate that are numbered 1 and To well #2, add 5 drops of amylase solution. Stir thoroughly with a toothpick and leave the mixture undisturbed for 5 to 10 minutes. 3. Test for the presence of starch (a complex carbohydrate) in the two wells. Using a clean pipette, add 1 drop IKI (potassium iodine) solution to each of the two wells. In the presence of starch, the mixture will change color from yellow-brown to blue-black. 4. Observe any color changes and record results in Table Table 13.4 Carbohydrate Digestion Test Results Well # Contents Starch Test Observations 1 Starch 2 Starch & Amylase Questions: 1. How do you know that carbohydrate digestion has taken place? Explain the process. 2. Explain what happened in well #2 after the addition of amylase. 3. What is the purpose of well #1? Fat Digestion 1. Clean and dry the spot plate thoroughly. Use a wax pencil to number the first three wells in the top row # Place 10 drops of water and 3 drops of corn oil in the wells of the spot plate numbered Stir each well thoroughly using a toothpick. Lab #13 Nutrition and Digestion 111
16 4. Test the ph of each of the wells by dipping a ph strip in the well and comparing the color change to the ph chart provided on the package. Record the ph of each well in data table Add 5 drops of lipase solution to well #2, then add 5 drops of lipase solution and 2 drops of soap solution to well #3. 6. Stir the mixture in each well thoroughly with a toothpick and allow the spot plate to remain undisturbed for about 20 minutes. 7. After 20 minutes, test the ph in each well and record results and observations in table Table 13.5 Fat Digestion Test Results Well # Contents ph Observations 1 Oil and water 2 Oil, water, and lipase 3 Oil, water, lipase, and liquid soap 1. What are the end products of digestion of fats? How does this relate to ph? 2. In this experiment, what data were collected? How were the data used to show that fat digestion occurred? 3. What is the role of the soap in the experiment? What substance in the body performs a similar function? 4. Which well showed the greatest amount of fat digestion? Explain why. Lab #13 Nutrition and Digestion 112
Figure 2. Figure 1. Name: Bio AP Lab Organic Molecules
 Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Name: Bio AP Lab Organic Molecules BACKGROUND: A cell is a living chemistry laboratory in which most functions take the form of interactions between organic molecules. Most organic molecules found in living
Lab 3 MACROMOLECULES INTRODUCTION I. IDENTIFICATION OF MACROMOLECULES. A. Carbohydrates
 Lab 3 MACROMOLECULES OBJECTIVES Define macromolecule, vitamin, mineral, carbohydrate, monosaccharide, disaccharide, polysaccharide, lipid, protein, amino acid, calorie; Describe the basic structures of
Lab 3 MACROMOLECULES OBJECTIVES Define macromolecule, vitamin, mineral, carbohydrate, monosaccharide, disaccharide, polysaccharide, lipid, protein, amino acid, calorie; Describe the basic structures of
Organic Chemistry Worksheet
 Organic Chemistry Worksheet Name Section A: Intro to Organic Compounds 1. Organic molecules exist in all living cells. In terms of biochemistry, what does the term organic mean? 2. Identify the monomer
Organic Chemistry Worksheet Name Section A: Intro to Organic Compounds 1. Organic molecules exist in all living cells. In terms of biochemistry, what does the term organic mean? 2. Identify the monomer
Chemical Tests For Biologically Important Molecules Do not write on this document
 Chemical Tests For Biologically Important Molecules Do not write on this document Introduction The most common and important organic molecules found in living things fall into four classes: carbohydrates,
Chemical Tests For Biologically Important Molecules Do not write on this document Introduction The most common and important organic molecules found in living things fall into four classes: carbohydrates,
EXERCISE 3 Carbon Compounds
 LEARNING OBJECTIVES EXERCISE 3 Carbon Compounds Perform diagnostic tests to detect the presence of reducing sugars (Benedict s), starch (Lugol s), protein (Biuret), lipid (SudanIV) and sodium chloride
LEARNING OBJECTIVES EXERCISE 3 Carbon Compounds Perform diagnostic tests to detect the presence of reducing sugars (Benedict s), starch (Lugol s), protein (Biuret), lipid (SudanIV) and sodium chloride
Lab 2. The Chemistry of Life
 Lab 2 Learning Objectives Compare and contrast organic and inorganic molecules Relate hydrogen bonding to macromolecules found in living things Compare and contrast the four major organic macromolecules:
Lab 2 Learning Objectives Compare and contrast organic and inorganic molecules Relate hydrogen bonding to macromolecules found in living things Compare and contrast the four major organic macromolecules:
LAB 3: Biomolecules and Digestion
 Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Page 3.1 LAB 3: Biomolecules and Digestion Food taken into our bodies must first be broken down by mechanical and chemical digestion before it can be absorbed and used as an energy source. The chemical
Biomolecule: Carbohydrate
 Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Name: Period: Date: Testing for Biological Macromolecules Lab
 Testing for Biological Macromolecules Lab Introduction: All living organisms are composed of various types of organic molecules, such as carbohydrates, starches, proteins, lipids and nucleic acids. These
Testing for Biological Macromolecules Lab Introduction: All living organisms are composed of various types of organic molecules, such as carbohydrates, starches, proteins, lipids and nucleic acids. These
Six Nutrients. Nutrients: substances in food that your body needs to stay healthy. Carbohydrates Protein Fat Minerals Vitamins Water
 Nutrients Six Nutrients Nutrients: substances in food that your body needs to stay healthy Carbohydrates Protein Fat Minerals Vitamins Water Water Function: most essential nutrient Helps digest and absorb
Nutrients Six Nutrients Nutrients: substances in food that your body needs to stay healthy Carbohydrates Protein Fat Minerals Vitamins Water Water Function: most essential nutrient Helps digest and absorb
Chew on This. Investigating the Function of the Digestive System
 Chew on This Investigating the Function of the Digestive System OBJECTIVE Students will identify the structure and function of the digestive system. The student will investigate the role of enzymes in
Chew on This Investigating the Function of the Digestive System OBJECTIVE Students will identify the structure and function of the digestive system. The student will investigate the role of enzymes in
You Are What You Eat
 An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.5 Lab Protocol..6 Post-Lab Questions and Analysis 9 Last updated: September 26 th, 2017 1 Introduction When deciding
An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.5 Lab Protocol..6 Post-Lab Questions and Analysis 9 Last updated: September 26 th, 2017 1 Introduction When deciding
Introduction: Lab Safety: Student Name: Spring 2012 SC135. Laboratory Exercise #4: Biologically Important Molecules
 FMCC Student Name: Spring 2012 SC135 Introduction: Laboratory Exercise #4: Biologically Important Molecules The major groups of biologically important molecules are: Carbohydrates, Lipids, Proteins and
FMCC Student Name: Spring 2012 SC135 Introduction: Laboratory Exercise #4: Biologically Important Molecules The major groups of biologically important molecules are: Carbohydrates, Lipids, Proteins and
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction
 Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Lab #4: Nutrition & Assays for Detecting Biological Molecules - Introduction Most biological molecules fall into one of four varieties: proteins, carbohydrates, lipids and nucleic acids. These are sometimes
Chapter 15 Food and Digestion
 Chapter 15 Food and Digestion 15.1A Food and Energy Functions of Nutrients 1. 2. 3. 4. Calories = amt. of energy in food RDA depends on age, gender, size and activity level Types of Nutrients (includes
Chapter 15 Food and Digestion 15.1A Food and Energy Functions of Nutrients 1. 2. 3. 4. Calories = amt. of energy in food RDA depends on age, gender, size and activity level Types of Nutrients (includes
Macromolecules Materials
 Macromolecules Materials Item per bench per class Test tubes 19 a bunch Benedict s reagent 1 bottle 6 Iodine bottle 1 bottle 6 Sudan IV bottle 1 bottle 6 Biuret s Bottle 1 bottle 6 250 ml beaker 1 6 heat
Macromolecules Materials Item per bench per class Test tubes 19 a bunch Benedict s reagent 1 bottle 6 Iodine bottle 1 bottle 6 Sudan IV bottle 1 bottle 6 Biuret s Bottle 1 bottle 6 250 ml beaker 1 6 heat
Digestive Enzyme Lab
 Digestive Enzyme Lab Objectives 1. To describe the function of enzymes 2. To define: reactants, products, activation energy 3. To describe the enzymatic digestion of carbohydrates by salivary amylase 4.
Digestive Enzyme Lab Objectives 1. To describe the function of enzymes 2. To define: reactants, products, activation energy 3. To describe the enzymatic digestion of carbohydrates by salivary amylase 4.
The Digestive System. 1- Carbohydrates 2- Proteins 3- Lipids 4- Water 5- Vitamins 6- Minerals 7- Fibers
 I. Type of food: The Digestive System 1- Carbohydrates 2- Proteins 3- Lipids 4- Water 5- Vitamins 6- Minerals 7- Fibers 1- Carbohydrates: are energy foods (sugars). They are made of C,H, and O atoms. They
I. Type of food: The Digestive System 1- Carbohydrates 2- Proteins 3- Lipids 4- Water 5- Vitamins 6- Minerals 7- Fibers 1- Carbohydrates: are energy foods (sugars). They are made of C,H, and O atoms. They
of Life Chemical Aspects OBJ ECTIVESshould be able to: ENCOUNTERS WITH LIFE H" ~ ~O N-C-C H R OH After completing this exercise, the student
 ENCOUNTERS WT LFE Chemical Aspects of Life C 20 C--O. /1 '\. O \/ '\./ C C / \. O / -, O \.1 C--C 1 O GLYCEROL After completing this exercise, the student OBJ ECTVESshould be able to: Define organic and
ENCOUNTERS WT LFE Chemical Aspects of Life C 20 C--O. /1 '\. O \/ '\./ C C / \. O / -, O \.1 C--C 1 O GLYCEROL After completing this exercise, the student OBJ ECTVESshould be able to: Define organic and
HW #1 Molecules of Life Packet
 Name Hour Due: HW #1 Molecules of Life Packet Lab Molecule ID Chemistry Fats, carbs WS HW Page 1 Page 2 Your Points Total Points Possible 5 pts Macromolecules in Foods Lab Introduction: The food we eat
Name Hour Due: HW #1 Molecules of Life Packet Lab Molecule ID Chemistry Fats, carbs WS HW Page 1 Page 2 Your Points Total Points Possible 5 pts Macromolecules in Foods Lab Introduction: The food we eat
Testing for the Presence of Macromolecules
 5 McMush Lab Testing for the Presence of Macromolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These macromolecules are large carbon-based
5 McMush Lab Testing for the Presence of Macromolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These macromolecules are large carbon-based
For example, monosaccharides such as glucose are polar and soluble in water, whereas lipids are nonpolar and insoluble in water.
 Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Lab: Organic Compounds
 Lab: Organic Compounds Name(s) Date Period Benchmark: SC.912.L.18.1: Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. Background:
Lab: Organic Compounds Name(s) Date Period Benchmark: SC.912.L.18.1: Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. Background:
WEAR GOGGLES, GLOVES AND A LAB APRON!!!!
 Organic Food Lab =) Problem: What test are used to discover if certain organic molecules are present in food? Could these tests be used to identify an unknown food? Background: We will be studying various
Organic Food Lab =) Problem: What test are used to discover if certain organic molecules are present in food? Could these tests be used to identify an unknown food? Background: We will be studying various
Identification of Organic Compounds Lab
 Identification of Organic Compounds Lab Introduction All organic compounds contain the element carbon (C). Organic compounds usually also contain oxygen (O) or hydrogen (H) or both. They may also contain
Identification of Organic Compounds Lab Introduction All organic compounds contain the element carbon (C). Organic compounds usually also contain oxygen (O) or hydrogen (H) or both. They may also contain
4. 10/09/14 Ch. 5: Populations /22/14 Ch. 2: Chemistry of Life 55
 Table of Contents # Date Title Page # 1. 1 2. 09/02/14 Ch. 1: The Science of Biology 09/16/14 Ch. 4: Ecosystems and Communities 17 3. 09/23/14 Ch. 3: The Biosphere 26 4. 10/09/14 Ch. 5: Populations 45
Table of Contents # Date Title Page # 1. 1 2. 09/02/14 Ch. 1: The Science of Biology 09/16/14 Ch. 4: Ecosystems and Communities 17 3. 09/23/14 Ch. 3: The Biosphere 26 4. 10/09/14 Ch. 5: Populations 45
(LM pages 91 98) Time Estimate for Entire Lab: 2.5 to 3.0 hours. Special Requirements
 Laboratory 7 Chemical Aspects of Digestion (LM pages 91 98) Time Estimate for Entire Lab: 2.5 to 3.0 hours Special Requirements Incubation. Students should start these sections at the beginning of the
Laboratory 7 Chemical Aspects of Digestion (LM pages 91 98) Time Estimate for Entire Lab: 2.5 to 3.0 hours Special Requirements Incubation. Students should start these sections at the beginning of the
McMush Lab Testing for the Presence of Macromolecules
 5 Testing for the Presence of Macromolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These macromolecules are large carbon based structures.
5 Testing for the Presence of Macromolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These macromolecules are large carbon based structures.
Carbohydrates Chemical Composition and Identification
 Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
Carbohydrates Chemical Composition and Identification Introduction: Today, scientists use a combination of biology and chemistry for their understanding of life and life processes. Thus, an understanding
Who took Kaleb s ipod? -- An organic compound mystery
 Who took Kaleb s ipod? -- An organic compound mystery Dr. Jennifer Doherty, Dr. Ingrid Waldron and Dr. Lori Spindler, Department of Biology, University of Pennsylvania, copyright 2009 Adapted from Identity
Who took Kaleb s ipod? -- An organic compound mystery Dr. Jennifer Doherty, Dr. Ingrid Waldron and Dr. Lori Spindler, Department of Biology, University of Pennsylvania, copyright 2009 Adapted from Identity
Chapter 15 Food and Digestion
 Chapter 15 Food and Digestion Activity: Use Qualitative Observations (5 senses) to describe: What happens when you see candy? How does it smell? How do you chomp it into smaller pieces or swallow candy
Chapter 15 Food and Digestion Activity: Use Qualitative Observations (5 senses) to describe: What happens when you see candy? How does it smell? How do you chomp it into smaller pieces or swallow candy
Name Date Period. Macromolecule Virtual Lab. Name: Go to the website:
 Macromolecule Virtual Lab Name: Go to the website: http://faculty.kirkwood.edu/apeterk/learningobjects/biologylabs.htm The most common organic compounds found in living organisms are lipids, carbohydrates,
Macromolecule Virtual Lab Name: Go to the website: http://faculty.kirkwood.edu/apeterk/learningobjects/biologylabs.htm The most common organic compounds found in living organisms are lipids, carbohydrates,
1.3.1 Function of Food. Why do we need food?
 1.3.1 Function of Food Why do we need food? Need to know The Function of Food Three reasons for requiring food 2 Food is needed for: 1.Energy 2.Growth of new cells and Repair of existing cells, tissues,
1.3.1 Function of Food Why do we need food? Need to know The Function of Food Three reasons for requiring food 2 Food is needed for: 1.Energy 2.Growth of new cells and Repair of existing cells, tissues,
Laboratory 3 Organic Molecules
 Laboratory 3 Organic Molecules MATERIALS Distilled water, vegetable oil, and solutions of glucose, starch, and gelatin Dairy products (half and half, heavy cream and whole milk) Non-dairy soy and almond
Laboratory 3 Organic Molecules MATERIALS Distilled water, vegetable oil, and solutions of glucose, starch, and gelatin Dairy products (half and half, heavy cream and whole milk) Non-dairy soy and almond
Digestive and Excretory Systems
 Chapter 38 Digestive and Excretory Systems Section 38 1 Food and Nutrition (pages 971 977) This section identifies the nutrients your body needs and explains why water is such an important nutrient Food
Chapter 38 Digestive and Excretory Systems Section 38 1 Food and Nutrition (pages 971 977) This section identifies the nutrients your body needs and explains why water is such an important nutrient Food
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System
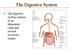 The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
The digestive system consists of an alimentary canal and several accessory organs. The Digestive System The Digestive System The digestive system mechanically and chemically breaks down food. Mechanical
McMush Lab Testing for the Presence of Biomolecules
 Biology McMush Lab Testing for the Presence of Biomolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These biomolecules are large carbon-based
Biology McMush Lab Testing for the Presence of Biomolecules Carbohydrates, lipids, proteins, and nucleic acids are organic molecules found in every living organism. These biomolecules are large carbon-based
CONCEPTS: OBJECTIVES: MATERIALS:
 CONCEPTS: Adolescence is considered to be the period of maximum growth both in terms of height and weight. Nutrition plays an important role in providing fuel and nutrients to support this rapid growth.
CONCEPTS: Adolescence is considered to be the period of maximum growth both in terms of height and weight. Nutrition plays an important role in providing fuel and nutrients to support this rapid growth.
LAB 4 Macromolecules
 LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
LAB 4 Macromolecules Overview In addition to water and minerals, living things contain a variety of organic molecules. Most of the organic molecules in living organisms are of 4 basic types: carbohydrate,
B. Element - each different kind of atom is a different element 1. Examples: C = carbon H = hydrogen
 I. Chemistry study of what substances are made of and how they change and combine Structural Formula A. Atom fundamental unit of matter 1. Subatomic particles: n o = neutron p + = proton e - = electron
I. Chemistry study of what substances are made of and how they change and combine Structural Formula A. Atom fundamental unit of matter 1. Subatomic particles: n o = neutron p + = proton e - = electron
Biology 20 Laboratory Life s Macromolecules OBJECTIVE INTRODUCTION
 Biology 20 Laboratory Life s Macromolecules OBJECTIVE To observe and record reactions between three classes of macromolecules in the presence of simple chemical indictors. To be able to distinguish positive
Biology 20 Laboratory Life s Macromolecules OBJECTIVE To observe and record reactions between three classes of macromolecules in the presence of simple chemical indictors. To be able to distinguish positive
Section 38-1 Food and Nutrition (pages )
 Name Class Date Section 38-1 Food and Nutrition (pages 971-977) Key Concepts What are the nutrients your body needs? Why is water such an important nutrient? Food and Energy (page 971) 1. Cells convert
Name Class Date Section 38-1 Food and Nutrition (pages 971-977) Key Concepts What are the nutrients your body needs? Why is water such an important nutrient? Food and Energy (page 971) 1. Cells convert
Tiny structures that carry out cellular functions (cell parts) Ex: nucleus, mitochondria, ribosomes
 ALL living things are Building from smallest to LARGEST: Organelles- Cells- Tiny structures that carry out cellular functions (cell parts) Ex: nucleus, mitochondria, ribosomes The basic unit of structure
ALL living things are Building from smallest to LARGEST: Organelles- Cells- Tiny structures that carry out cellular functions (cell parts) Ex: nucleus, mitochondria, ribosomes The basic unit of structure
This section identifies the nutrients your body needs and explains why water is such an important nutrient.
 Chapter 38 Digestive and Excretory Systems Section 38-1 Food and Nutrition (pages 971-977) 44P TEKS FOCUS: 9A Structure and function of biomolecules; 11 C Importance of nutrition on health This section
Chapter 38 Digestive and Excretory Systems Section 38-1 Food and Nutrition (pages 971-977) 44P TEKS FOCUS: 9A Structure and function of biomolecules; 11 C Importance of nutrition on health This section
#9 - Digestion. Objectives: Prelab Activity. I Digestive System
 #9 - Objectives: Observe and understand the process of emulsification Understand the digestion of fats by pancreatic lipase and the purpose of bile Understand the digestion of protein by pepsin and the
#9 - Objectives: Observe and understand the process of emulsification Understand the digestion of fats by pancreatic lipase and the purpose of bile Understand the digestion of protein by pepsin and the
2-2 Properties of Water
 2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
FOOD. Why do we need food? What's in our food? There are 3 trace elements, Iron (Fe), Copper (Cu) and Zinc (Zn).
 Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
CHEMISTRY OF LIFE 30 JANUARY 2013
 CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules
 Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules Introduction: There are four broad classes of macromolecules that can be found in living systems. Each type of
Name: Per. Date: / 71 points MACROMOLECULE LAB: Testing for the Presence of Macromolecules Introduction: There are four broad classes of macromolecules that can be found in living systems. Each type of
You Are What You Eat
 You Are What You Eat An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.6 Lab Protocol..7 Post-Lab Questions and Analysis 11 Last updated: 10/15/18 1 You Are What
You Are What You Eat An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.6 Lab Protocol..7 Post-Lab Questions and Analysis 11 Last updated: 10/15/18 1 You Are What
Chapter 9: Digestion Review Assignment
 _ Date: Mark: /45 Chapter 9: Digestion Review Assignment 45 Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following roles do
_ Date: Mark: /45 Chapter 9: Digestion Review Assignment 45 Multiple Choice = 45 Marks Identify the choice that best completes the statement or answers the question. 1. Which of the following roles do
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014
 REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
REVISION: CHEMISTRY OF LIFE 19 MARCH 2014 Lesson Description In this lesson we revise: The Chemistry of Life Food tests Summary Inorganic Nutrients Water Solvent Medium in which chemical reactions occur
Organic Molecule Composition of Milk: Lab Investigation
 Name: Organic Molecule Composition of Milk: Lab Investigation Introduction & Background Milk & milk products have been a major food source from earliest recorded history. Milk is a natural, nutritionally
Name: Organic Molecule Composition of Milk: Lab Investigation Introduction & Background Milk & milk products have been a major food source from earliest recorded history. Milk is a natural, nutritionally
1.1.1 Protein. 1 Quiz: Protein. 1. The main reason why the body needs protein is for growth, repair and maintenance.
 1 Quiz: Protein 1.1.1 Protein 1. The main reason why the body needs protein is for growth, repair and maintenance. 2. An example of a protein alternative is mycoprotein, e.g. Quorn. 3. An example of a
1 Quiz: Protein 1.1.1 Protein 1. The main reason why the body needs protein is for growth, repair and maintenance. 2. An example of a protein alternative is mycoprotein, e.g. Quorn. 3. An example of a
ARE YOU WHAT YOU EAT? TEACHER HANDBOOK
 ARE YOU WHAT YOU EAT? TEACHER HANDBOOK Alabama Course of Study: Science Biology: 1. Select appropriate laboratory glassware, balances, time measuring equipment, and optical instruments to conduct an experiment.
ARE YOU WHAT YOU EAT? TEACHER HANDBOOK Alabama Course of Study: Science Biology: 1. Select appropriate laboratory glassware, balances, time measuring equipment, and optical instruments to conduct an experiment.
Lesson Two Nutrients and the Body
 Lesson Two Nutrients and the Body Objectives After participating in this lesson, students will Be able to identify key nutrients the body needs and describe their function and importance. Understand that
Lesson Two Nutrients and the Body Objectives After participating in this lesson, students will Be able to identify key nutrients the body needs and describe their function and importance. Understand that
Nutrients and Digestion
 Nutrients and Digestion Nutrition what is needed to be taken in to keep the body healthy Essential Nutrients Carbohydrates Fats Proteins Minerals Vitamins Water Carbohydrates Types of sugars combined in
Nutrients and Digestion Nutrition what is needed to be taken in to keep the body healthy Essential Nutrients Carbohydrates Fats Proteins Minerals Vitamins Water Carbohydrates Types of sugars combined in
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is food made of?
 What is food made of? Food: Nutrients and Food Any substance that is ingested (eaten) and sustains life Meat, fish, nuts, fruits, vegetables, grain products, etc. Nutrients: Food is broken down into substances
What is food made of? Food: Nutrients and Food Any substance that is ingested (eaten) and sustains life Meat, fish, nuts, fruits, vegetables, grain products, etc. Nutrients: Food is broken down into substances
Reading Comprehension of the digestive tract
 Reading Comprehension of the digestive tract Digestion is a process that break-down food into small molecule called nutrient. These small molecule called nutrients pass through the cell membrane or absorb
Reading Comprehension of the digestive tract Digestion is a process that break-down food into small molecule called nutrient. These small molecule called nutrients pass through the cell membrane or absorb
AP Biology Macromolecules
 AP Biology Macromolecules Introduction: There are four broad classes macromolecules that can be found in living systems. Each type macromolecule has a characteristic structure and function in living organisms.
AP Biology Macromolecules Introduction: There are four broad classes macromolecules that can be found in living systems. Each type macromolecule has a characteristic structure and function in living organisms.
Organic Compounds in the Foods
 Organic Compounds in the Foods Purpose: This lab activity will help you understand the chemical composition (i.e., carbohydrates, proteins, and fats) of the foods that you eat. Materials we will be using:
Organic Compounds in the Foods Purpose: This lab activity will help you understand the chemical composition (i.e., carbohydrates, proteins, and fats) of the foods that you eat. Materials we will be using:
Name Unit # Period Score 159 points possible Dietary Guidelines, Food Pyramid and Nutrients Test
 Name Unit # Period Score 159 points possible Dietary Guidelines, Food Pyramid and Nutrients Test 1. List the ten dietary guidelines recommended for Americans. (10) a. b. c. d. e. f. g. h. i. j. Multiple
Name Unit # Period Score 159 points possible Dietary Guidelines, Food Pyramid and Nutrients Test 1. List the ten dietary guidelines recommended for Americans. (10) a. b. c. d. e. f. g. h. i. j. Multiple
Macromolecule Virtual Lab
 Part A Macromolecule Virtual Lab Go to the website: http://faculty.kirkwood.edu/apeterk/learningobjects/biologylabs.htm CARBOHYDRATES Scroll down to the bottom and click on Carbohydrate 1. What do carbohydrates
Part A Macromolecule Virtual Lab Go to the website: http://faculty.kirkwood.edu/apeterk/learningobjects/biologylabs.htm CARBOHYDRATES Scroll down to the bottom and click on Carbohydrate 1. What do carbohydrates
Section 38 1 Food and Nutrition (pages )
 Chapter 38 Digestive and Excretory Systems Section 38 1 Food and Nutrition (pages 971 977) Key Concepts What are the nutrients your body needs? Why is water such an important nutrient? Food and Energy
Chapter 38 Digestive and Excretory Systems Section 38 1 Food and Nutrition (pages 971 977) Key Concepts What are the nutrients your body needs? Why is water such an important nutrient? Food and Energy
Nutrition for Health. Nutrients. Before You Read
 CHAPTER 10 LESSON 2 Nutrition for Health Nutrients BIG Idea Each nutrient in your diet plays a unique and essential role in keeping you healthy. Before You Read Sometimes figuring out what to eat can be
CHAPTER 10 LESSON 2 Nutrition for Health Nutrients BIG Idea Each nutrient in your diet plays a unique and essential role in keeping you healthy. Before You Read Sometimes figuring out what to eat can be
CIE Biology GCSE 7: Human nutrition
 CIE Biology GCSE 7: Human nutrition Notes Humans need many different nutrients to survive. To receive these nutrients in the correct quantities, a balanced diet must be eaten. A balanced diet includes
CIE Biology GCSE 7: Human nutrition Notes Humans need many different nutrients to survive. To receive these nutrients in the correct quantities, a balanced diet must be eaten. A balanced diet includes
Lab 6: Cellular Respiration
 Lab 6: Cellular Respiration Metabolism is the sum of all chemical reactions in a living organism. These reactions can be catabolic or anabolic. Anabolic reactions use up energy to actually build complex
Lab 6: Cellular Respiration Metabolism is the sum of all chemical reactions in a living organism. These reactions can be catabolic or anabolic. Anabolic reactions use up energy to actually build complex
Biochemistry. Chapter 6
 Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Biochemistry Chapter 6 Game Plan for Today. - Collect your papers - Hand back quests - Go over Amoeba Sister Chart - Biochem Notes - Video Carbohydrate Lab Food Label Lab! Testing For Carbohydrates Benedict's
Tests for Carbohydrates
 Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
Goals bserve physical and chemical properties of some common carbohydrates. Use physical and chemical tests to distinguish between monosaccharides, disaccharides, and polysaccharides. Identify an unknown
Lab Activity 30. Digestive Enzymes. Portland Community College BI 233
 Lab Activity 30 Digestive Enzymes Portland Community College BI 233 Cellular Reactions All molecular bonds have energy barriers that prevent spontaneous breakdown Enzymes lowering these activation energy
Lab Activity 30 Digestive Enzymes Portland Community College BI 233 Cellular Reactions All molecular bonds have energy barriers that prevent spontaneous breakdown Enzymes lowering these activation energy
C H O N P S. Name : Color the Elements on the Periodic Table as listed below
 Name : Unit 9: and Changes in Digestion (if found, please return to Room 714) 7.6A Organic Compounds Identify that organic compounds contain CHONPS. Organic Compounds contain what? 6 1 8 7 15 16 C H O
Name : Unit 9: and Changes in Digestion (if found, please return to Room 714) 7.6A Organic Compounds Identify that organic compounds contain CHONPS. Organic Compounds contain what? 6 1 8 7 15 16 C H O
3/9/2011. I. Main nutritional requirements. WARM-UP (GRAB A SHEET ON YOUR WAY IN) TERMS STUDENT LEARNING OBJECTIVES OBJECTIVE 1
 (GRAB A SHEET ON YOUR WAY IN) What 7 things make up your favorite salad? (if you don t like salad pick 7 things anyway) What food group do each of them fall under? (the food groups are Grains, Vegetables,
(GRAB A SHEET ON YOUR WAY IN) What 7 things make up your favorite salad? (if you don t like salad pick 7 things anyway) What food group do each of them fall under? (the food groups are Grains, Vegetables,
LAB 5 - Enzymes BACKGROUND INFORMATION
 LAB 5 - Enzymes BACKGROUND INFORMATION Chemical Reactions The cells of organisms, from bacteria to plants to animals, carry out hundreds to thousands of chemical reactions that must be properly coordinated
LAB 5 - Enzymes BACKGROUND INFORMATION Chemical Reactions The cells of organisms, from bacteria to plants to animals, carry out hundreds to thousands of chemical reactions that must be properly coordinated
THE DIGESTIVE SYSTEM. Video Quiz
 1 Video Quiz At the conclusion of the videotape, there will be a short quiz with these questions. Write your answers in the space provided. Use the back of this sheet if necessary. Question 1: What is
1 Video Quiz At the conclusion of the videotape, there will be a short quiz with these questions. Write your answers in the space provided. Use the back of this sheet if necessary. Question 1: What is
Nutrition, part 2. Because 1 part isn t enough!
 Nutrition, part 2 Because 1 part isn t enough! 4. Calories and Caloric Intake Calories per gram of our Macro and Micro nutrients Macro Carbohydrates: 4 cal/g Fats: 9 cal/g Proteins: 4 cal/g Micro Vitamins:
Nutrition, part 2 Because 1 part isn t enough! 4. Calories and Caloric Intake Calories per gram of our Macro and Micro nutrients Macro Carbohydrates: 4 cal/g Fats: 9 cal/g Proteins: 4 cal/g Micro Vitamins:
Name Group Members. Table 1 Observation (include details of what you observe)
 Name Group Members Macromolecules, Part 1 - PROTEINS There are four classes of macromolecules that are important to the function of all living things. These include carbohydrates, lipids, proteins and
Name Group Members Macromolecules, Part 1 - PROTEINS There are four classes of macromolecules that are important to the function of all living things. These include carbohydrates, lipids, proteins and
The Structure and Function of Macromolecules: Carbohydrates, Lipids, Proteins & Nucleic Acids.
 The Structure and Function of Macromolecules: Carbohydrates, Lipids, Proteins & Nucleic Acids. Biological Compounds Carbohydrates Lipids Proteins Nucleic Acids Introduction Cells join smaller organic molecules
The Structure and Function of Macromolecules: Carbohydrates, Lipids, Proteins & Nucleic Acids. Biological Compounds Carbohydrates Lipids Proteins Nucleic Acids Introduction Cells join smaller organic molecules
Ch 2 Molecules of life
 Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Ch 2 Molecules of life Think about (Ch 2, p.2) 1. Water is essential to life. If there is water on a planet, it is possible that life may exist on the planet. 2. Water makes up the largest percentage by
Digestive System Practice Test
 Name: Class Period: Section 1: Digestive System Practice Test Directions: Match the items in Column B to the definitions or explanations offered in Column A. Write the matching letter, on the line provided
Name: Class Period: Section 1: Digestive System Practice Test Directions: Match the items in Column B to the definitions or explanations offered in Column A. Write the matching letter, on the line provided
Who took Jerell s ipod? An organic compound mystery 1
 Who took Jerell s ipod? An organic compound mystery 1 Jerell is a 10 th grade student who works at McDonald s on the weekends. While on break, Jerell was studying for his biology test and listening to
Who took Jerell s ipod? An organic compound mystery 1 Jerell is a 10 th grade student who works at McDonald s on the weekends. While on break, Jerell was studying for his biology test and listening to
Lesson 1 Carbohydrates, Fats & Proteins pages
 Lesson 1 Carbohydrates, Fats & Proteins pages 190-201 What are the 3 classes of nutrients that supply your body with energy and how does the body obtain the energy from foods? Describe the roles that carbohydrates,
Lesson 1 Carbohydrates, Fats & Proteins pages 190-201 What are the 3 classes of nutrients that supply your body with energy and how does the body obtain the energy from foods? Describe the roles that carbohydrates,
1 Small molecules are used as the basic units in the synthesis of large food molecules. Which statement is correct? A
 1 Small molecules are used as the basic units in the synthesis of large food molecules. Which statement is correct? mino acids are basic units of carbohydrates. Fatty acids are basic units of glycogen.
1 Small molecules are used as the basic units in the synthesis of large food molecules. Which statement is correct? mino acids are basic units of carbohydrates. Fatty acids are basic units of glycogen.
The Digestive System
 Digestive System 1 Name The Digestive System Purpose: To describe how food moves through the digestive system. To identify the parts of the digestive system. Background Information: Food provides us with
Digestive System 1 Name The Digestive System Purpose: To describe how food moves through the digestive system. To identify the parts of the digestive system. Background Information: Food provides us with
The Chemistry of Life
 The Chemistry of Life Biomolecules Warm-up List the percentages of each: Total Fats Saturated Fats 25% Carbohydrates 10% Protein 7% 20% What Biomolecule would cholesterol be classified as? Lipids (fats)
The Chemistry of Life Biomolecules Warm-up List the percentages of each: Total Fats Saturated Fats 25% Carbohydrates 10% Protein 7% 20% What Biomolecule would cholesterol be classified as? Lipids (fats)
When people don t eat enough complex carbohydrates they don t have enough energy and feel tired and less alert. They also may not get enough fiber.
 Carbohydrates Carbohydrates are compounds that come from plants and contain carbon, hydrogen, and oxygen. These nutrients supply energy, which all living things need. Carbohydrates are the body s most
Carbohydrates Carbohydrates are compounds that come from plants and contain carbon, hydrogen, and oxygen. These nutrients supply energy, which all living things need. Carbohydrates are the body s most
3. Beans are rich in which may be deficient in the diet of a vegetarian. A. proteins B. carbohydrates C. vitamins D. lipids
 Topic 5: Food and humans 1. The sugar found in human blood is: A. Fructose B. Starch C. Glucose D. Glycogen 2. Fat is formed from the condensation of: A. Amino acids B. Amino acids and glycerol C. Fatty
Topic 5: Food and humans 1. The sugar found in human blood is: A. Fructose B. Starch C. Glucose D. Glycogen 2. Fat is formed from the condensation of: A. Amino acids B. Amino acids and glycerol C. Fatty
9. Determine the mass of the fat you removed from the milk and record in the table. Calculation:
 Chemistry 100 Instructor s Initials Name: Experiment 14: Biochemistry Analysis of milk for the lipids, carbohydrates, and proteins. A. Determining the % Fat in Whole Milk 1. Weigh a clean, dry, empty 50
Chemistry 100 Instructor s Initials Name: Experiment 14: Biochemistry Analysis of milk for the lipids, carbohydrates, and proteins. A. Determining the % Fat in Whole Milk 1. Weigh a clean, dry, empty 50
Name... Class... Date...
 Required practical 4: Food tests Aiming for 8 Specification references: Required practical 4: Use qualitative reagents to test for a range of carbohydrates, lipids, and proteins. To include: Benedict s
Required practical 4: Food tests Aiming for 8 Specification references: Required practical 4: Use qualitative reagents to test for a range of carbohydrates, lipids, and proteins. To include: Benedict s
In this lab, you will determine, through observation, which protease is secreted into the stomach, and which is secreted into the small intestine.
 Lab 2: Protein and Fat Digestion LABORATORY OBJECTIVES To investigate the effect of ph and digestive enzymes on the digestion of proteins To investigate the action of lipase on the breakdown of fats INTRODUCTION:
Lab 2: Protein and Fat Digestion LABORATORY OBJECTIVES To investigate the effect of ph and digestive enzymes on the digestion of proteins To investigate the action of lipase on the breakdown of fats INTRODUCTION:
The function of the digestive system is to break down
 Curriculum Set #3 The function of the digestive system is to break down food into its components for use by the body. This is accomplished both mechanically and chemically. Mechanical digestion changes
Curriculum Set #3 The function of the digestive system is to break down food into its components for use by the body. This is accomplished both mechanically and chemically. Mechanical digestion changes
Student Book. Grains: 5 10 ounces a day (at least half whole grains) Self-Check
 ETR Associates Middle School I read and followed directions. My work is neat and complete. This is my best work. HealthSmart Actions Lesson at a Glance Student Book The HealthSmart Actions student book
ETR Associates Middle School I read and followed directions. My work is neat and complete. This is my best work. HealthSmart Actions Lesson at a Glance Student Book The HealthSmart Actions student book
All organisms must obtain and process essential nutrients (food) *** Exception: Venus Fly Traps undergo photosynthesis but needs source of nitrogen
 All organisms must obtain and process essential nutrients (food) AUTOTROPHS self feeder makes their own food eg. Plants do not require a digestive tract *** Exception: Venus Fly Traps undergo photosynthesis
All organisms must obtain and process essential nutrients (food) AUTOTROPHS self feeder makes their own food eg. Plants do not require a digestive tract *** Exception: Venus Fly Traps undergo photosynthesis
6 The chemistry of living organisms
 Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Nutrition Notes website.notebook October 19, Nutrition
 Nutrition Nutrition Notes website.notebook October 19, 2016 Food is any substance that is ingested ("eaten") and helps sustain life. Food categories: Meats and Alternative Dairy Products Fruits and Vegetables
Nutrition Nutrition Notes website.notebook October 19, 2016 Food is any substance that is ingested ("eaten") and helps sustain life. Food categories: Meats and Alternative Dairy Products Fruits and Vegetables
Nutrients. The food you eat is a source of nutrients. Nutrients are defined as the substances found in food that keep your body functioning.
 Nutrients The food you eat is a source of nutrients. Nutrients are defined as the substances found in food that keep your body functioning. Your body needs nutrients to Provide energy. Build and repair
Nutrients The food you eat is a source of nutrients. Nutrients are defined as the substances found in food that keep your body functioning. Your body needs nutrients to Provide energy. Build and repair
Molecules. Background
 Background A molecule is a group of two or more atoms. Compounds are also two or more atoms. Compounds are made from different types of atoms. can be made of different types of atoms or can also be made
Background A molecule is a group of two or more atoms. Compounds are also two or more atoms. Compounds are made from different types of atoms. can be made of different types of atoms or can also be made
There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids
 There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids Before you can understand the topics in this unit there are some key vocabulary terms you need to
There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids Before you can understand the topics in this unit there are some key vocabulary terms you need to
Chewing the fat about fat!
 Chewing the fat about fat! When we talk about fat, most people think of fatty foods, like fries and fatty meats. But fat is an essential nutrient. It plays an important role in the many functions that
Chewing the fat about fat! When we talk about fat, most people think of fatty foods, like fries and fatty meats. But fat is an essential nutrient. It plays an important role in the many functions that
