CORE DECOMPRESSION FOR AVASCULAR NECROSIS
|
|
|
- Ariel Dickerson
- 5 years ago
- Views:
Transcription
1 UnitedHealthcare Commercial Medical Policy CORE DECOMPRESSION FOR AVASCULAR NECROSIS Policy Number: PAI006 Effective Date: February 1, 2019 Table of Contents Page COVERAGE RATIONALE... 1 APPLICABLE CODES... 1 DESCRIPTION OF SERVICES... 1 CLINICAL EVIDENCE... 2 U.S. FOOD AND DRUG ADMINISTRATION (FDA)... 5 CENTERS FOR MEDICARE AND MEDICAID SERVICES (CMS)... 6 REFERENCES... 6 POLICY HISTORY/REVISION INFORMATION... 6 INSTRUCTIONS FOR USE... 7 COVERAGE RATIONALE Core decompression is proven and medically necessary for treating early (pre-collapse stage I and II) avascular necrosis of the femoral head. Core decompression is unproven and not medically necessary for treating late avascular necrosis of the femoral head or for avascular necrosis elsewhere, including the humeral head, the distal femur, the talus, or the mandibular condyle due to insufficient evidence of efficacy. APPLICABLE CODES The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. Listing of a code in this policy does not imply that the service described by the code is a covered or noncovered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Coverage Determination Guidelines may apply. CPT Code Description Unlisted craniofacial and maxillofacial procedure Unlisted procedure, shoulder Unlisted procedure, pelvis or hip joint Unlisted procedure, femur or knee Unlisted procedure, leg or ankle CPT is a registered trademark of the American Medical Association HCPCS Code S2325 DESCRIPTION OF SERVICES Hip core decompression Description Avascular necrosis (AVN), also known as osteonecrosis, aseptic necrosis and ischemic bone necrosis, is a relatively common disease characterized by death of cellular elements of bone or marrow. Avascular necrosis (AVN) occurs when the blood flow to the bone has been interrupted leading to the death of bone. As the bone tissue dies, the bone structure collapses which results in pain and loss of joint function. This condition occurs most often in the femoral head but can affect other bones and joints. There are many risk factors for the disease including hemoglobinopathies, dislocation of the hip, alcoholism, fracture of the femoral neck, use of corticosteroids, as well as collagen vascular disease. AVN is a progressive disorder that often results in the eventual collapse of the bone and the need for joint replacement or other arthroplasty. Core decompression of the hip is usually employed before collapse and fracture of the femoral head and/or neck to delay or avoid reconstructive surgery of the affected joint. It is generally carried out to preserve the function and the Core Decompression for Avascular Necrosis Page 1 of 7
2 structure of the hip as well as to relieve pain associated with AVN. Core decompression consists of drilling one or more small channels into the dead bone (necrotic lesion). The procedure is designed to decrease pressure within the bone by restoring blood flow to the bone. Bone grafting may or may not be used with core decompression. Severity of avascular necrosis is determined by the staging system based on the consensus of the Subcommittee of Nomenclature of the International Association on Bone Circulation and Bone Necrosis (Tofferi, 2008). Staging is as follows: Stage Stage 0 Stage I Stage II Stage III Stage IV Stage V CLINICAL EVIDENCE Clinical Findings Patient is asymptomatic Radiography findings are normal Histology findings demonstrate osteonecrosis Patient may or may not be symptomatic Radiography and CT scan findings are unremarkable AVN is considered likely based on MRI and bone scan results [may be subclassified by extent of involvement (see below)] Histology findings are abnormal Patient is symptomatic Plain radiography findings are abnormal and include osteopenia, osteosclerosis, or cysts Subchondral radiolucency is absent MRI findings are diagnostic Patient is symptomatic Radiographic findings include subchondral lucency (crescent sign) and subchondral collapse Shape of the femoral head is generally preserved on radiographs and CT scans Subclassification depends on the extent of crescent, as follows: o Stage IIIa: Crescent is less than 15% of the articular surface o Stage IIIb: Crescent is 15-30% of the articular surface o Stage IIIc: Crescent is more than 30% of the articular surface Joint space may be irregular CT scanning is more sensitive than radiography Subclassification depends on the extent of collapsed surface, as follows: o Stage IVa: Less than 15% of surface is collapsed o Stage IVb: Approximately 15-30% of surface is collapsed o Stage IVc: More than 30% of surface is collapsed Radiography findings include narrowing of the joint space, osteoarthritis with sclerosis of acetabulum, and marginal osteophytes Stage VI Findings include extensive destruction of the femoral head and joint Core Decompression of the Femoral Head (Hip) A network meta-analysis was conducted by Yoon et al. (2018) to assess the efficacy of various core decompression (CD) modalities and non-operative treatment. Nine randomized controlled trials with a minimum two year follow-up were included. A total of 453 hips were included in the meta-analysis; 151 hips in CD, 70 hips in CD combining bone grafting (BG), 116 hips in CD combining bone marrow mononuclear cells (BMMC), 25 hips in CD combining BG and BMMC, and 91 hips in non-operative treatment. The rate of conversion to total hip arthroplasty (THA) and the radiologic progression were compared among the five treatments. The pooled risk ratio compared with non-operative treatment for THA conversion was 0.92 in traditional CD; 4.10 in CD combining BG; 0.30 in CD combining BMMC; and 1.78 in CD combining BG and BMMC. No significant differences were found in terms of the radiologic progression and the rates of THA conversion across all CD modalities and non-operative treatment. The authors concluded that these results question the assumption that CD changes the natural course of osteonecrosis of the femoral head. If the size of necrotic portion is the major determinant of future collapse of the necrotic femoral head and the collapse does not occur in small lesions even without any treatment, a large-scale randomized controlled trial is necessary to confirm the effectiveness of CD. Roth et al. (2016) conducted a systematic review based on the published literature from January 1, 1970 to April 31, 2013 for the treatment of adult non-traumatic avascular necrosis of the femoral head (AVN; N-ANFH). Inclusion criteria were systematic reviews, meta-analyses and relevant peer review publications. Systematic literature search was done using the databases of the US National Library of Medicine National Institutes of Health and the Cochrane Library and a total of 159 articles were included for detailed evaluation. The authors concluded that core Core Decompression for Avascular Necrosis Page 2 of 7
3 decompression is indicated and should be offered in early and potential reversible stages of N-ANFH, Association Research Circulation Osseous (ARCO) stage 1, but also stage 2 if the area of necrosis is medial or central and <30%. According to the authors, in ARCO stage 3 and 4, core decompression is not indicated and total hip replacement (THR) should be discussed. Sadile et al. (2016) conducted a meta-analysis to assess the efficacy of core decompression (CD) compared with all other joint preserving treatments (JPT) in delaying the natural osteonecrosis evolution to hip osteoarthritis (HOA). Medline and Scopus databases were searched for 15- to 70-year-olds with osteonecrosis of the femoral head (ONFH) with a minimum follow-up of 24 months. The outcomes evaluated were patient clinical status, radiographic progression and total hip arthroplasty or further surgery need. A total of 12 studies which included randomized controlled trials, controlled clinical trials and prospective studies (776 patients) met the inclusion criteria. Clinical outcome, radiographic progression and the need for total hip arthroplasty/further surgery suggested a slight superiority of other JPT compared with CD. The authors identified that high heterogeneity of the primary investigations was the main limitation of the study. They concluded that the efficacy and effectiveness of core decompression for ONFH are, at best, no better than other joint preserving strategies. The more recent scientific evidence seems to suggest that such procedure is less successful than other joint preserving strategies. According to the authors further studies are needed to identify the best therapeutic approach to the ONFH. A systematic review regarding the use of core decompression for the treatment of osteonecrosis of the hip noted that core decompression has been the surgical option since the 1960s (Rajagopal et al., 2012). The study authors also noted that that their systematic review was performed to evaluate core decompression with regard to pain relief, need for total hip arthroplasty (THA), lesion size, and Ficat stage. Only 4 articles of level IV evidence (139 total cases) met their inclusion criteria. Three studies reported improvement in outcomes. Overall, outcomes were "good" in one study and either "fair" or "poor" in the others. One-fourth (25.8%) of patients required THA. Patients with necrotic lesion size <50% had the best outcomes with core decompression. Although core decompression may become a standard treatment option to prevent THA in early stages of osteonecrosis, there are currently no rigorous studies that provide data regarding long-term health outcomes. Marti-Carvajal and colleagues (2014, updated 2016) conducted a systematic review to compare the effect of surgical treatments with non-surgical treatment of avascular necrosis (AVN) in individuals with sickle cell disease (SCD). Only 1 trial was identified and included 46 participants. Eight patients withdrew after randomization as they declined to participate in the trial. The remaining 38 patients were randomized to receive either hip core decompression and physical therapy or physical therapy alone. After a mean follow-up of 3 years, the surgical group (hip core decompression and physical therapy) showed no clinical improvement compared with the non-surgical group. There were also no significant differences between the study groups in terms of major complications (hip pain; vasocclusions; and acute chest syndrome). This study did not report patient-relevant outcomes, such as mortality or quality of life (QOL).The authors concluded that the addition of core decompression to physical therapy did not improve outcomes for patients with SCD and AVN. Additional studies, preferably RCTs, are necessary to evaluate the role of hip-core depression in patients with SCD. Shah et al. (2015) undertook a study (n=20) to analyze the clinical, functional and radiological outcome of core decompression and bone grafting in patients with osteonecrosis of the femoral head (ONFH) up to stage IIB. The procedure adopted in 26 hips was core decompression and cancellous bone grafting. Two hips were treated with core decompression and fibular grafting. Functional outcome was assessed by Harris hip score, wherein 19 hips had good or excellent outcome; 1 hip had fair outcome and 8 hips showed poor result. For stage I, 12/13 hips improved, whereas for Stage IIA, 6/11 hips showed improvement and for stage IIB, only 2/4 hips showed improvement. Core decompression procedures showed improvement in patients who had abduction more than 30 degrees and adduction more than 108 degrees. It also showed more improvement for internal rotation in comparison with external rotation. Out of the 28 hips, a majority had pain relief immediately following operation but as time passed deterioration was seen. At 3 months, 22 hips had pain relief but only 19 hips had pain relief at final follow-up. Less than 25% of the hips required a replacement or salvage procedure. The success rate was 92.3% for grade I, 100% for grade IIA and 50% for grade IIB. The authors concluded that core decompression and bone grafting provide satisfactory outcome when patients are carefully selected in early stages of the disease, before the stage of collapse. Wei et al. (2011) conducted a study on the effect of core decompression combined with an allogeneic, antigenextracted, autolysed fibular allograft and autologous impacted bone grafting for the treatment of osteonecrosis of the femoral head. The study included 162 patients (223 hips; 61 females, 101 males; mean age 33.5 years, range years) with stage II-III avascular necrosis of the femoral head. The outcome was determined by changes in the Harris hip score, by progression in radiographic stages, and by the need for hip replacement. The mean follow-up was 24 months. Excellent and good results were obtained in 93.3% of cases in stage II, and 87% in stages III with a survivorship of 81% in all cases. According to the authors, core decompression combined with an allogeneic, antigenextracted, autolysed fibular allograft and autologous impacted bone grafting may be the treatment of choice, particularly in the pre-collapse stage. Core Decompression for Avascular Necrosis Page 3 of 7
4 Yu et al. (2015) conducted a study to review the outcomes of using synthetic bone graft substitute (calcium sulfate and calcium phosphate) for the treatment of late-stage osteonecrosis of the femoral head. The study consisted of 19 hips in 18 patients with osteonecrosis of the femoral head [6 hips in Association Research Circulation Osseous (ARCO) stage IIC and 13 hips in stage IIIA] who were treated with core decompression combined with PRO-DENSE (Injectable Regenerative Graft). The clinical failure was defined as conversion to total hip arthroplasty or progression in head collapse. At the conclusion of the study, 3 in the 6 stage IIC hips and 8 in the 13 stage IIIA hips were converted to total hip arthroplasty postoperatively. Advanced collapse of the femoral head waiting for total hip arthroplasty was observed in the other six hips. Of the 19 hips, only 2 hips survived without further collapse in the 5- year follow-up. This resulted in 89.5% failure rate. The authors concluded that core decompression combined with an injectable (PRO-DENSE) were associated with high failure rates in the early postoperative period. It is not recommended for the treatment of ARCO stage IIC and IIIA osteonecrosis of the femoral head. Von Stechow and Drees (2007) stated that untreated osteonecrosis will eventually destroy the affected femoral head. Depending on the location and the extent of the osteonecrosis, several surgical options are available. For early small and medium-sized pre-collapse lesions, core decompression is the treatment of choice. Core Decompression in the Shoulder, Knee, and Ankle While available evidence indicates that core decompression is effective in treating early stages of AVN of the hip, there is currently insufficient evidence that this procedure is effective in treating AVN of the shoulder, knee or ankle. The majority of studies involved a small number of patients and lacked appropriate control groups. Furthermore, several of the studies were published by the same group of investigators. Prospective, well-designed, randomized, controlled trials are needed to ascertain the clinical value of core decompression for joints other than the hip. Humeral Head (Shoulder) Franceschi et al. (2016) performed a systematic review to identify published studies and analyze clinical evidence available related to the surgical management of osteonecrosis of the humeral head. A search was performed using the PubMed (MEDLINE), EMBASE and Cochrane Library databases. Twelve studies were included: five prospective case series and seven retrospective case series. A total of 309 patients, comprising 382 shoulders, were included. Three main surgical procedures were evaluated: core decompression, hemi-arthroplasty and total shoulder arthroplasty. The authors concluded that based on the current available data, core decompression is a safe and effective option for treating low-grade osteonecrosis of the humeral head, while hemi-arthroplasty and total shoulder arthroplasty should be considered for high-grade osteonecrosis. According to the authors more studies and better-designed trials are needed and the level of evidence had poor reference standard; analyses with no sensitivity analyses. Kennon and colleagues (2016) evaluated radiographic and functional outcomes after procedures for humeral head atraumatic avascular necrosis (HAAVN), decompression efficacy in chronic steroid-induced (CSI) and sickle cell disease (SCD) populations. Twenty-five shoulders were treated surgically for HAAVN. Stage I/II disease received core decompression and ultrasound bone stimulation. Stage III received surface replacement or hemiarthroplasty, and arthroplasty was performed for stage IV/V. Radiographs and clinical scores were recorded preoperatively and postoperatively. Eleven shoulders (stage I/II disease) underwent core decompression. Seven of 8 shoulders (88%) progressed to stage III/IV after decompression. All SCD patients progressed to collapse. The procedure in 19 shoulders was surface replacement, hemiarthroplasty, or total shoulder arthroplasty (TSA). Constant, American Shoulder and Elbow Surgeons, Simple Shoulder Test-12, and University of California Los Angeles Shoulder scores were significantly higher at 1- and 2-year follow-up with arthroplasty; 13 of 16 arthroplasty patients (81%) had satisfactory to excellent results. One surface replacement was revised to reverse TSA. The authors concluded that the results suggest core decompression for AVN in SCD patients does not alter osteonecrosis progression and humeral head collapse. They suggest that resurfacing and hemiarthroplasty are viable treatment options for stage III, whereas shoulder replacement for stage IV/V disease appears to offer better functional results. Harreld et al. (2009) conducted a small study to evaluate humeral head core decompression involving percutaneous perforations. During this study, shoulder arthroplasty was avoided in all 15 patients (26 shoulders) for a mean followup of 32 months. Of the 26 shoulders, 25 had successful clinical and functional outcomes, and 1 showed radiographic progression of the disease but has not needed further operative treatment. Decompression results were compared with those of a nonoperative historical control group, identified through a literature search. There was a 48% (143/299) rate of progression to arthroplasty in the control group at a follow-up ranging from 2 to 4.5 years. According to the authors, percutaneous decompression appears to be a low-morbidity method for relieving symptoms and deferring shoulder arthroplasty in patients with symptomatic osteonecrosis of the humeral head. This study is limited by lack of randomization, and small sample size. Femoral Condyle or Distal Femur (Knee) The knee is the second most common location for osteonecrosis with about a 10% incidence of the disease in the hip. Core Decompression for Avascular Necrosis Page 4 of 7
5 One retrospective, uncontrolled study (n=248 knees), provided weak but positive evidence of the long-term effectiveness of core decompression in delaying secondary surgery in the early stages of AVN of the femoral condyle. A second core decompression procedure was performed in 16% of patients; the criteria for repeat core decompression were not reported. Only 7 knees were at stage III at the time of diagnosis. The overall survival rate for knees included in the 2000 report (stages I through III) was 79%, based on a mean of 7 years of follow-up (minimum of 2 years) (Mont et al., 2000). Comparability of these results (Mont et al., 2000) with those of future studies may be limited. First, patients were selected for core decompression only after 3 months of conservative treatment failed to relieve symptoms. This is a reasonable selection process but not one reported by other authors. Results from core decompression might have been more favorable in patients whose symptoms had not already been shown to be unresponsive to conservative treatment. Secondly, 16% of patients had two, rather than one, core decompression procedures for AVN in the knee, which may have inflated results. Talus (Ankle) A systematic review which included forty-one studies was conducted by Dhillon et al. (2018) to identify and summarize the available evidence in the literature for the treatment of talar avascular necrosis (AVN). They concluded that the literature was inconclusive regarding the ideal modality of treatment of the talar AVN, and the factors that should guide such treatment have not been well explained. They summarized that early talar AVN seems best treated with protected weight bearing and possibly in combination with extracorporeal shock wave therapy. If that fails, core decompression can be considered. Arthrodesis should be saved as a salvage procedure in late cases with arthritis and collapse, and a tibiotalocalcaneal fusion with bone grafting may be needed in cases of significant bone loss. Future prospective, randomized studies are necessary to guide the conservative and surgical management of talar AVN. Gross and colleagues (2014) conducted a systematic review evaluating various interventions for talar avascular necrosis, including hind foot fusion, conservation treatment approaches, bone grafts, core decompression, and talar replacement. A total of 19 studies (321 ankles) were included for detailed review. All individual studies were considered poor quality, and the overall body of evidence was considered very low quality due to study limitations, such as imprecise and sparse data and possible reporting bias. Study author concluded that additional randomized studies are needed to inform and guide specific treatments for avascular necrosis of the talus. Marulanda et al (2010) conducted a non-randomized study to examine the results of percutaneous drilling to treat osteonecrosis of the ankle in 31 patients (44 ankles). At a mean follow-up duration of 45 +/- 12 months, 40 (91%) ankles had achieved a successful clinical outcome. There were no perioperative complications, although 3 ankles subsequently collapsed and required arthrodesis. According to the authors, the percutaneous drilling technique appears to be a useful method for the relief of symptomatic ankle osteonecrosis. This study is limited by lack of randomization, control and small sample size. Beck et al. (2016) conducted a prospective case series to assess the effectiveness of endoscopic core decompression (ECD) in the treatment of osteochondral lesions of the talus (OLT). All patients suffered from chronic ankle pain due to an OLT of the medial talar dome and had undergone non-operative treatment for at least 3 months without improvement of their symptoms. The diagnosis was confirmed by plain radiographs and an MRI of the ankle joint. Seven patients underwent core decompression of the lesion. Outcome and patient satisfaction were evaluated according to the American Orthopedic Foot and Ankle Society Score (AOFAS) and the Foot and Ankle Disability Index (FADI) preoperatively and after a mean follow-up of 24.1 months. Remodeling of the OLT and bone ingrowth into the graft substitute were evaluated by means of radiographs of the ankle joint, as well as an MRI 1year after treatment. The authors found that the AOFAS significantly improved from 71.0±2.4 to 90.3±5.9, and the FADI improved from 71.8±11.1 to 91.7±4.8. The radiographic controls showed good bone remodelling with invisibility of the bone graft substitute in x-ray within 8 12 weeks. The ECD led to a good restoration of the medial talar dome contour with an almost complete resection of the OLT. MRI imaging showed an alteration of the bony signal in the treated OLT and drill meatus for more than 12 months. This is an uncontrolled study with a small sample size. Mandibular Condyle Osteonecrosis of the mandibular condyle has only recently been reported, and there is limited information on the efficacy of core decompression. In 8 of 9 patients (16 joints) with histologically confirmed osteonecrosis of the mandible, core decompression resulted in substantial pain reduction over a mean follow-up period of 34 months. (Chuong et al., 1995) In a second group of 8 patients (15 joints) with more severe lesions, core decompression with bone grafting resulted in significant clinical improvement in 11 joints during the follow-up period (mean 28 months). U.S. FOOD AND DRUG ADMINISTRATION (FDA) Core decompression is a surgical procedure and is not regulated by FDA. The procedure is performed with ordinary surgical instruments. The FDA has not approved any devices specifically for core decompression. Approval has been Core Decompression for Avascular Necrosis Page 5 of 7
6 granted to numerous bone graft substitutes (product code LYC), some of which may be used in conjunction with core decompression. Available at: (Accessed June 10, 2018) CENTERS FOR MEDICARE AND MEDICAID SERVICES (CMS) Medicare does not have National Coverage Determinations (NCDs) for core decompression for the treatment of avascular necrosis of the femoral head, humeral head, distal femur, mandibular condyle or the talus. Local Coverage Determinations (LCDs) do not exist at this time. (Accessed June 29, 2018) REFERENCES Beck S, Claben T, Haversath M, et al. Operative technique and clinical outcome in endoscopic core decompression of osteochondral lesions of the talus: A pilot study. Med Sci Monit. 2016; 22: Chuong R, Piper MA, Boland TJ. Osteonecrosis of the mandibular condyle. Pathophysiology and core decompression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod May;79(5): Dhillon MS, Rana B, Panda I, et al. Management options in avascular necrosis of talus. Indian J Orthop May-Jun; 52(3): Franceschi F, Franceschetti E, Paciotti M, et al. Surgical management of osteonecrosis of the humeral head: a systematic review. Knee Surg Sports Traumatol Arthrosc May 19. Gross CE, Haughom B, Chahal J, Holmes GB. Treatments for avascular necrosis of the talus: a systematic review. Foot Ankle Spec. 2014; 7(5): Harreld KL, Marulanda GA, Ulrich SD, et al. Small-diameter percutaneous decompression for osteonecrosis of the shoulder Am J Orthop (Belle Mead NJ) Jul; 38(7): Kennon JC, Smith JP, Crosby LA. Core decompression and arthroplasty outcomes for atraumatic osteonecrosis of the humeral head. J Shoulder Elbow Surg Apr 13. Martí-Carvajal AJ, Solà I, Agreda-Pérez LH. Treatment for avascular necrosis of bone in people with sickle cell disease. Cochrane Database Syst Rev. 2014; 10;7:CD Updated Marulanda GA, McGrath MS, Ulrich SD, et al. Percutaneous drilling for the treatment of atraumatic osteonecrosis of the ankle. J Foot Ankle Surg Jan-Feb; 49(1): Mont MA, Baumgarten KM, Rifai A, et al. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82(9): Rajagopal M, Balch Samora J, Ellis TJ, et al. Efficacy of core decompression as treatment for osteonecrosis of the hip: a systematic review. Hip Int Sep-Oct;22(5): Roth A, Beckmann J, Bohndorf K, et al. S3-Guideline non-traumatic adult femoral head necrosis. Arch Orthop Trauma Surg (2016) 136: Sadile F, Bernasconi A, Russo S, et al. Core decompression versus other joint preserving treatments for osteonecrosis of the femoral head: a meta-analysis. Br. Med. Bull. Published June 1, Volume 118, Issue 1; Pages Shah S, Kapoor C, Jhaveri M, et al. Analysis of outcome of avascular necrosis of femoral head treated by core decompression and bone grafting. Journal of Clinical Orthopaedics and Trauma 6 (2015) Tofferi JK, Gilliland W. Avascular Necrosis: Differential diagnoses & workup. emedicine. October 24, Updated December Von Stechow D, Drees P. Surgical treatment concepts for femoral head necrosis. Orthopade. 2007;36(5): Wei BF and Ge XH. Treatment of osteonecrosis of the femoral head with core decompression and bone grafting. Hip Int. 2011Apr 6; 21(2): Yoon BH, Lee YK, Kim KC, et al. No differences in the efficacy among various core decompression modalities and nonoperative treatment: a network meta-analysis. Int Orthop May 31. Yu PA, Peng KT, Huang TW, et al. Injectable synthetic bone graft substitute combined with core decompression in the treatment of advanced osteonecrosis of the femoral head: A 5-year follow-up. Biomed J May-Jun;38(3): POLICY HISTORY/REVISION INFORMATION Date Action/Description 01/09/2019 Corporate Medical Affairs Committee Core Decompression for Avascular Necrosis Page 6 of 7
7 Date 10/25/ /27/ /28/ /27/ /24/ /20/ /21/ /24/ /26/ /26/ /23/ /20/2009 Action/Description INSTRUCTIONS FOR USE This Medical Policy provides assistance in interpreting UnitedHealthcare standard benefit plans. When deciding coverage, the member specific benefit plan document must be referenced as the terms of the member specific benefit plan may differ from the standard plan. In the event of a conflict, the member specific benefit plan document governs. Before using this policy, please check the member specific benefit plan document and any applicable federal or state mandates. UnitedHealthcare reserves the right to modify its Policies and Guidelines as necessary. This Medical Policy is provided for informational purposes. It does not constitute medical advice. UnitedHealthcare may also use tools developed by third parties, such as the MCG Care Guidelines, to assist us in administering health benefits. UnitedHealthcare Medical Policies are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice. Core Decompression for Avascular Necrosis Page 7 of 7
CORE DECOMPRESSION FOR AVASCULAR NECROSIS
 UnitedHealthcare Community Plan Medical Policy CORE DECOMPRESSION FOR AVASCULAR NECROSIS Policy Number: CS025.E Effective Date: September 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT
UnitedHealthcare Community Plan Medical Policy CORE DECOMPRESSION FOR AVASCULAR NECROSIS Policy Number: CS025.E Effective Date: September 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT
CORE DECOMPRESSION FOR AVASCULAR NECROSIS
 UnitedHealthcare Oxford Clinical Policy CORE DECOMPRESSION FOR AVASCULAR NECROSIS Policy Number: SURGERY 063.13 T2 Effective Date: October 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS
UnitedHealthcare Oxford Clinical Policy CORE DECOMPRESSION FOR AVASCULAR NECROSIS Policy Number: SURGERY 063.13 T2 Effective Date: October 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS
For Commercial products, please refer to the following policy: Preauthorization via Web-Based Tool for Procedures
 Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
Clinical Policy Bulletin: Core Decompression for Avascular
 Page 1 of21 Aetna Better Health 2000 Market Street, Suite 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Clinical Policy Bulletin: Core Decompression for Avascular Necrosis Revised April 2014 Number: 0753
Page 1 of21 Aetna Better Health 2000 Market Street, Suite 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Clinical Policy Bulletin: Core Decompression for Avascular Necrosis Revised April 2014 Number: 0753
Original Date: December 2015 Page 1 of 8 FOR CMS (MEDICARE) MEMBERS ONLY
 National Imaging Associates, Inc. Clinical guidelines TOTAL JOINT ARTHROPLASTY -Total Hip Arthroplasty -Total Knee Arthroplasty -Replacement/Revision Hip or Knee Arthroplasty CPT4 Codes: Please refer to
National Imaging Associates, Inc. Clinical guidelines TOTAL JOINT ARTHROPLASTY -Total Hip Arthroplasty -Total Knee Arthroplasty -Replacement/Revision Hip or Knee Arthroplasty CPT4 Codes: Please refer to
OSTEOCHONDRAL ALLOGRAFTS AND AUTOGRAFTS IN THE TREATMENT OF FOCAL ARTICULAR CARTILAGE LESIONS
 Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-115 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-115 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis
 Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis Effective January 1, 2016 Instructions for use The following coverage policy applies to health benefit
Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis Effective January 1, 2016 Instructions for use The following coverage policy applies to health benefit
MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE
 UnitedHealthcare Commercial Utilization Review Guideline MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE Guideline Number: URG-13.01 Effective Date: February 1, 2019 Table
UnitedHealthcare Commercial Utilization Review Guideline MAGNETIC RESONANCE IMAGING (MRI) AND COMPUTED TOMOGRAPHY (CT) SCAN SITE OF CARE Guideline Number: URG-13.01 Effective Date: February 1, 2019 Table
SHOULDER ARTHROPLASTY (TOTAL, HEMI, REVERSE)/ARTHRODESIS
 evicore healthcare. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Imaging requests for patients with atypical symptoms or clinical presentations
evicore healthcare. Clinical Decision Support Tool Diagnostic Strategies This tool addresses common symptoms and symptom complexes. Imaging requests for patients with atypical symptoms or clinical presentations
Speaker s Disclosure Statement. Starvation, Death and Destruction: The Battlefield of AVN. Objectives. Risk Factors
 Starvation, Death and Destruction: The Battlefield of AVN Speaker s Disclosure Statement I have no industry relationships to disclose I will discuss off-label use of medications Dana-Farber/Boston Children
Starvation, Death and Destruction: The Battlefield of AVN Speaker s Disclosure Statement I have no industry relationships to disclose I will discuss off-label use of medications Dana-Farber/Boston Children
MEDICAL POLICY MEDICAL POLICY DETAILS POLICY STATEMENT. Page: 1 of 6
 Page: 1 of 6 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title SHOULDER ARTHROPLASTY (TOTAL, PARTIAL AND REVERSE) Policy Number 7.01.95 Category Technology Assessment Effective Date 6/21/18 Revised
Page: 1 of 6 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title SHOULDER ARTHROPLASTY (TOTAL, PARTIAL AND REVERSE) Policy Number 7.01.95 Category Technology Assessment Effective Date 6/21/18 Revised
Post-traumatic osteonecrosis of distal tibia
 Injury Extra (2007) 38, 262 266 www.elsevier.com/locate/inext CASE REPORT Post-traumatic osteonecrosis of distal tibia D. Chakravarty a, *, A. Khanna a,1, A. Kumar b,2 a Department of Orthopaedics, Peterborough
Injury Extra (2007) 38, 262 266 www.elsevier.com/locate/inext CASE REPORT Post-traumatic osteonecrosis of distal tibia D. Chakravarty a, *, A. Khanna a,1, A. Kumar b,2 a Department of Orthopaedics, Peterborough
Sympathetic Electrical Stimulation Therapy for Chronic Pain
 Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Sympathetic Electrical Stimulation Therapy for Chronic Pain Policy Number: 015M0076A Effective Date: April 01, 015 RETIRED 5/11/017 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE
Isolated Subtalar Arthrodesis for Avascular Necrosis of the Talus
 Isolated Subtalar Arthrodesis for Avascular Necrosis of the Talus Travis J. Dekker, MD Manuel J. Pelligrini, MD Adam P. Schiff, MD James K. DeOrio, MD Mark E. Easley, MD James A. Nunley, MD Samuel B. Adams,
Isolated Subtalar Arthrodesis for Avascular Necrosis of the Talus Travis J. Dekker, MD Manuel J. Pelligrini, MD Adam P. Schiff, MD James K. DeOrio, MD Mark E. Easley, MD James A. Nunley, MD Samuel B. Adams,
Screening for and Assessment of Osteonecrosis in Oncology Patients. Sue C. Kaste, DO SPR Postgraduate Course 2015
 Screening for and Assessment of Osteonecrosis in Oncology Patients Sue C. Kaste, DO SPR Postgraduate Course 2015 The author declares no potential conflicts of interest or financial disclosures Osteonecrosis
Screening for and Assessment of Osteonecrosis in Oncology Patients Sue C. Kaste, DO SPR Postgraduate Course 2015 The author declares no potential conflicts of interest or financial disclosures Osteonecrosis
Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow.
 Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow. Prepared for The Canadian Orthopaedic Association Contents Executive
Preliminary Report Choosing Wisely Identifying Musculoskeletal Interventions with Limited Levels of Efficacy in the Shoulder & Elbow. Prepared for The Canadian Orthopaedic Association Contents Executive
INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement
 016625 REVISION R INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement CASE STUDY Patient History The patient was a 65-year-old
016625 REVISION R INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement CASE STUDY Patient History The patient was a 65-year-old
Evangelia E. Vassalou MD,PhD Radiologist Department of Medical Imaging, Heraklion University Hospital Department of Medical Imaging, Sitia General
 Evangelia E. Vassalou MD,PhD Radiologist Department of Medical Imaging, Heraklion University Hospital Department of Medical Imaging, Sitia General Hospital Osteonecrosis pathophysiology epidemiology imaging
Evangelia E. Vassalou MD,PhD Radiologist Department of Medical Imaging, Heraklion University Hospital Department of Medical Imaging, Sitia General Hospital Osteonecrosis pathophysiology epidemiology imaging
A Patient s Guide to Avascular Necrosis of the Hip
 A Patient s Guide to Avascular Necrosis of the Hip 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled from a variety
A Patient s Guide to Avascular Necrosis of the Hip 2350 Royal Boulevard Suite 200 Elgin, IL 60123 Phone: 847.931.5300 Fax: 847.931.9072 DISCLAIMER: The information in this booklet is compiled from a variety
HYSTERECTOMY FOR BENIGN CONDITIONS
 UnitedHealthcare Commercial Medical Policy HYSTERECTOMY FOR BENIGN CONDITIONS Policy Number: 2018T0572G Effective Date: September 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
UnitedHealthcare Commercial Medical Policy HYSTERECTOMY FOR BENIGN CONDITIONS Policy Number: 2018T0572G Effective Date: September 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Idiopathic osteonecrosis of the medial tibial plateau
 Idiopathic osteonecrosis of the medial tibial plateau J. R. Valenti J. A. Illescas A. Barriga R. Dölz ABSTRACT Osteonecrosis of the medial tibial plateau is characterized by acute pain on the medial aspect
Idiopathic osteonecrosis of the medial tibial plateau J. R. Valenti J. A. Illescas A. Barriga R. Dölz ABSTRACT Osteonecrosis of the medial tibial plateau is characterized by acute pain on the medial aspect
Case Study: David. Conditions Treated Femoral Neck Fracture with Avascular Necrosis of the Hip. Age Range During Treatment 16 Years
 Case Study: David Conditions Treated Femoral Neck Fracture with Avascular Necrosis of the Hip Age Range During Treatment 16 Years David S. Feldman, MD Chief of Pediatric Orthopedic Surgery Professor of
Case Study: David Conditions Treated Femoral Neck Fracture with Avascular Necrosis of the Hip Age Range During Treatment 16 Years David S. Feldman, MD Chief of Pediatric Orthopedic Surgery Professor of
Clinical Policy: Radial Head Implant Reference Number: CP.MP.148
 Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
CLINICAL GUIDELINES. CMM-318: Shoulder Arthroplasty/ Replacement/Resurfacing/ Revision/Arthrodesis. Version Effective October 22, 2018
 CLINICAL GUIDELINES CMM-318: / Replacement/Resurfacing/ Revision/Arthrodesis Version 20.0.2018 Effective October 22, 2018 Clinical guidelines for medical necessity review of speech therapy services. CMM-318:
CLINICAL GUIDELINES CMM-318: / Replacement/Resurfacing/ Revision/Arthrodesis Version 20.0.2018 Effective October 22, 2018 Clinical guidelines for medical necessity review of speech therapy services. CMM-318:
POSITION STATEMENT The Use of Osteochondral Transplantation for the Treatment of Osteochondral Lesions of the Talus
 Position Statement POSITION STATEMENT The Use of Osteochondral Transplantation for the Treatment of Osteochondral Lesions of the Talus The American Orthopaedic Foot & Ankle Society (AOFAS) endorses the
Position Statement POSITION STATEMENT The Use of Osteochondral Transplantation for the Treatment of Osteochondral Lesions of the Talus The American Orthopaedic Foot & Ankle Society (AOFAS) endorses the
Ankle Replacement Surgery
 Ankle Replacement Surgery Ankle replacement surgery is performed to replace the damaged articular surfaces of the three bones of the ankle joint with artificial implants. This procedure is now being preferred
Ankle Replacement Surgery Ankle replacement surgery is performed to replace the damaged articular surfaces of the three bones of the ankle joint with artificial implants. This procedure is now being preferred
MEDICAL POLICY MEDICAL POLICY DETAILS POLICY STATEMENT. Page: 1 of 5
 Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title HIP ARTHROPLASTY Policy Number 7.01.96 Category Technology Assessment Effective Date 06/21/18 Revised Date 12/20/18 Product Disclaimer
Page: 1 of 5 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title HIP ARTHROPLASTY Policy Number 7.01.96 Category Technology Assessment Effective Date 06/21/18 Revised Date 12/20/18 Product Disclaimer
Cpt code for bone density of hips only
 Cpt code for bone density of hips only Enter a location: Find a13 This policy may apply to the following codes. Inclusion of a code in this section does not guarantee that it will be reimbursed. For further
Cpt code for bone density of hips only Enter a location: Find a13 This policy may apply to the following codes. Inclusion of a code in this section does not guarantee that it will be reimbursed. For further
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma >
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
Management of arthritis of the shoulder. Omar Haddo Consultant Orthopaedic Surgeon
 Management of arthritis of the shoulder Omar Haddo Consultant Orthopaedic Surgeon Diagnosis Pain - with activity initially. As disease progresses night pain is common and sleep difficult Stiffness trouble
Management of arthritis of the shoulder Omar Haddo Consultant Orthopaedic Surgeon Diagnosis Pain - with activity initially. As disease progresses night pain is common and sleep difficult Stiffness trouble
Fresh Osteochondral Allograft Reimbursement Reference Document
 Fresh Osteochondral Allograft Reimbursement Reference Document This information is provided as a coding guide based on the experiences of clinicians with whom we have worked with over the years, and is
Fresh Osteochondral Allograft Reimbursement Reference Document This information is provided as a coding guide based on the experiences of clinicians with whom we have worked with over the years, and is
MEDICAL POLICY MEDICAL POLICY DETAILS POLICY STATEMENT. Page: 1 of 7
 Page: 1 of 7 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title METAL-ON-METAL TOTAL HIP RESURFACING Policy Number 7.01.74 Category Technology Assessment Effective Date 06/15/06 Revised Date 07/19/07,
Page: 1 of 7 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title METAL-ON-METAL TOTAL HIP RESURFACING Policy Number 7.01.74 Category Technology Assessment Effective Date 06/15/06 Revised Date 07/19/07,
Shoulder Joint Replacement
 Shoulder Joint Replacement Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was first performed
Shoulder Joint Replacement Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was first performed
Clinical Policy: Articular Cartilage Defect Repairs Reference Number: CP.MP.26
 Clinical Policy: Reference Number: CP.MP.26 Effective Date: 10/08 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.26 Effective Date: 10/08 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
CLINICAL PAPER / ORTHOPEDIC
 HIP LEG LENGTH AND OFFSET Kelley T.C. and Swank M.L. (2009) Using CAS leads to more accurate positioning within the safe zone (inclination between 30 and 50, anteversion between 5 and 25 ) CAS improves
HIP LEG LENGTH AND OFFSET Kelley T.C. and Swank M.L. (2009) Using CAS leads to more accurate positioning within the safe zone (inclination between 30 and 50, anteversion between 5 and 25 ) CAS improves
Osteochondral allografting for all other joints is not covered as the evidence is insufficient to determine the
 Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18
 Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Partial hip resurfacing is considered investigational/not medically necessary for all other indications not listed above.
 Subject: Policy #: Current Effective Date: 11/13/2006 Status: Revised Last Review Date: 09/14/2006 Description/Scope Hip resurfacing can be categorized in two ways: as a partial (hemi) hip resurfacing
Subject: Policy #: Current Effective Date: 11/13/2006 Status: Revised Last Review Date: 09/14/2006 Description/Scope Hip resurfacing can be categorized in two ways: as a partial (hemi) hip resurfacing
Synthetic Cartilage Implants for Joint Pain
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Synthetic Cartilage Implants for Joint Pain
 Synthetic Cartilage Implants for Joint Pain Policy Number: 7.01.160 Last Review: 3/2018 Origination: 2/2018 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Synthetic Cartilage Implants for Joint Pain Policy Number: 7.01.160 Last Review: 3/2018 Origination: 2/2018 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Clinical Policy: Trigger Point Injections for Pain Management
 Clinical Policy: for Pain Management Reference Number: CP.MP.169 Last Review Date: 08/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Clinical Policy: for Pain Management Reference Number: CP.MP.169 Last Review Date: 08/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Operations included in the National Joint Registry (NJR)
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
Medical Policy Original Effective Date: Revised Date: 07/26/17 Page 1 of 9
 Page 1 of 9 Disclaimer Description Coverage Determination/ Clinical Indications Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on
Page 1 of 9 Disclaimer Description Coverage Determination/ Clinical Indications Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on
Avascular Necrosis of the Foot. Dr. Hema Choudur MD, FRCPC Associate Professor. Dept. of Radiology. McMaster University, Hamilton, Canada.
 Avascular Necrosis of the Foot Dr. Hema Choudur MD, FRCPC Associate Professor. Dept. of Radiology. McMaster University, Hamilton, Canada. Avascular Necrosis: Pathophysiology Ischemia to the bone from oxygen
Avascular Necrosis of the Foot Dr. Hema Choudur MD, FRCPC Associate Professor. Dept. of Radiology. McMaster University, Hamilton, Canada. Avascular Necrosis: Pathophysiology Ischemia to the bone from oxygen
Pei An Yu, Kuo Ti Peng, Tsan Weng Huang, Robert Wen Wei Hsu, Wei Hsiu Hsu, Mel S. Lee
 Original Article 257 Injectable Synthetic Bone Graft Substitute Combined with Core Decompression in the Treatment of Advanced Osteonecrosis of the Femoral Head: A 5 Year Follow Up Pei An Yu, Kuo Ti Peng,
Original Article 257 Injectable Synthetic Bone Graft Substitute Combined with Core Decompression in the Treatment of Advanced Osteonecrosis of the Femoral Head: A 5 Year Follow Up Pei An Yu, Kuo Ti Peng,
MEDICAL POLICY SUBJECT: OSTEOCHONDRAL GRAFTING
 MEDICAL POLICY PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Arthritis of the Shoulder
 Arthritis of the Shoulder In 2011, more than 50 million people in the United States reported that they had been diagnosed with some form of arthritis, according to the National Health Interview Survey.
Arthritis of the Shoulder In 2011, more than 50 million people in the United States reported that they had been diagnosed with some form of arthritis, according to the National Health Interview Survey.
Ideal Candidate for Cartilage Restoration. Large or Complex Lesions
 Complex Biological Knee Reconstruction: Bipolar, Multifocal Lesions and Osteoarthritis William Bugbee, MD Attending Physician, Scripps Clinic 18 th International Sports Medicine Fellow s Conference Ideal
Complex Biological Knee Reconstruction: Bipolar, Multifocal Lesions and Osteoarthritis William Bugbee, MD Attending Physician, Scripps Clinic 18 th International Sports Medicine Fellow s Conference Ideal
Medial circumflex artery Lateral circumflex artery
 Femoral Head Fractures: A Critical But Frequently Missed Injury Susanna C. Spence MD Manickam Kumaravel MBBS University of Texas Health Science Center at Houston Background Femoral head fractures: A complication
Femoral Head Fractures: A Critical But Frequently Missed Injury Susanna C. Spence MD Manickam Kumaravel MBBS University of Texas Health Science Center at Houston Background Femoral head fractures: A complication
HYSTERECTOMY FOR BENIGN CONDITIONS
 HYSTERECTOMY FOR BENIGN CONDITIONS UnitedHealthcare Oxford Clinical Policy Policy Number: SURGERY 104.7 T2 Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
HYSTERECTOMY FOR BENIGN CONDITIONS UnitedHealthcare Oxford Clinical Policy Policy Number: SURGERY 104.7 T2 Effective Date: April 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
Integra. Titan Modular Shoulder System, 2.5
 Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
Treatment of malunited fractures of the ankle
 Treatment of malunited fractures of the ankle A LONG-TERM FOLLOW-UP OF RECONSTRUCTIVE SURGERY I. I. Reidsma, P. A. Nolte, R. K. Marti, E. L. F. B. Raaymakers From Academic Medical Center, Amsterdam, Netherlands
Treatment of malunited fractures of the ankle A LONG-TERM FOLLOW-UP OF RECONSTRUCTIVE SURGERY I. I. Reidsma, P. A. Nolte, R. K. Marti, E. L. F. B. Raaymakers From Academic Medical Center, Amsterdam, Netherlands
Bisphosphonate combination therapy for non-femoral avascular necrosis
 Agarwala and Vijayvargiya Journal of Orthopaedic Surgery and Research (2019) 14:112 https://doi.org/10.1186/s13018-019-1152-7 RESEARCH ARTICLE Open Access Bisphosphonate combination therapy for non-femoral
Agarwala and Vijayvargiya Journal of Orthopaedic Surgery and Research (2019) 14:112 https://doi.org/10.1186/s13018-019-1152-7 RESEARCH ARTICLE Open Access Bisphosphonate combination therapy for non-femoral
End-Stage Hemophilic Arthropathy: The Journey to Joint Replacement
 Slide no 1 End-Stage Hemophilic Arthropathy: The Journey to Joint Replacement Husam Darwish, MD, FRCSC Asst. Professor and Consultant Department of Orthopaedic Surgery King Abdulaziz University Jeddah,
Slide no 1 End-Stage Hemophilic Arthropathy: The Journey to Joint Replacement Husam Darwish, MD, FRCSC Asst. Professor and Consultant Department of Orthopaedic Surgery King Abdulaziz University Jeddah,
MOTORIZED SPINAL TRACTION
 MOTORIZED SPINAL TRACTION UnitedHealthcare Commercial Medical Policy Policy Number: PAI010 Effective Date: January 1, 2019 Table of Contents Page COVERAGE RATIONALE... 1 APPLICABLE CODES... 1 DESCRIPTION
MOTORIZED SPINAL TRACTION UnitedHealthcare Commercial Medical Policy Policy Number: PAI010 Effective Date: January 1, 2019 Table of Contents Page COVERAGE RATIONALE... 1 APPLICABLE CODES... 1 DESCRIPTION
Mid-term Outcomes of Arthroscopic-Assisted Core Decompression of Pre-collapse Osteonecrosis of Femoral Head Minimum of 5 Year Follow-up
 Arthroscopic-Assisted Core Decompression of Pre-collapse Osteonecrosis of Femoral Head Minimum of 5 Year Follow-up Mark R. Nazal*, Ali Parsa, Scott D. Martin Sports Medicine, Department of Orthopaedic
Arthroscopic-Assisted Core Decompression of Pre-collapse Osteonecrosis of Femoral Head Minimum of 5 Year Follow-up Mark R. Nazal*, Ali Parsa, Scott D. Martin Sports Medicine, Department of Orthopaedic
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
AUTOLOGOUS CHONDROCYTE IMPLANTATION FOR FOCAL ARTICULAR CARTILAGE LESIONS
 CARTILAGE LESIONS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
CARTILAGE LESIONS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A AAOS. See American Academy of Orthopaedic Surgeons (AAOS) ACA. See Affordable Care Act (ACA) ACL injuries. See Anterior cruciate ligament (ACL)
Note: Page numbers of article titles are in boldface type. A AAOS. See American Academy of Orthopaedic Surgeons (AAOS) ACA. See Affordable Care Act (ACA) ACL injuries. See Anterior cruciate ligament (ACL)
A Patient s Guide to Osteonecrosis of the Humeral Head
 A Patient s Guide to Osteonecrosis of the Humeral Head 20295 NE 29th Place, Ste 300 Aventura, FL 33180 Phone: (786) 629-0910 Fax: (786) 629-0920 admin@instituteofsports.com DISCLAIMER: The information
A Patient s Guide to Osteonecrosis of the Humeral Head 20295 NE 29th Place, Ste 300 Aventura, FL 33180 Phone: (786) 629-0910 Fax: (786) 629-0920 admin@instituteofsports.com DISCLAIMER: The information
Osteonecrosis of the knee Treatment with ESWT. Dr Shrenik Shah Shrey hospital Ahmedabad
 Osteonecrosis of the knee Treatment with ESWT Dr Shrenik Shah Shrey hospital Ahmedabad Osteonecrosis(ON) of the knee SPONK- Spontaneous ON of the knee Secondary ON of the knee Postarthrosopic ON of the
Osteonecrosis of the knee Treatment with ESWT Dr Shrenik Shah Shrey hospital Ahmedabad Osteonecrosis(ON) of the knee SPONK- Spontaneous ON of the knee Secondary ON of the knee Postarthrosopic ON of the
Treatment of Femoral Head Necrosis with Chinese Medicine
 Treatment of Femoral Head Necrosis with Chinese Medicine DaxinCao From the First Affiliated hospital of Nanjing Medical University and Jiangsu Province Hospital www.dxc-online.com Tel:00862584438382, 00862584274745
Treatment of Femoral Head Necrosis with Chinese Medicine DaxinCao From the First Affiliated hospital of Nanjing Medical University and Jiangsu Province Hospital www.dxc-online.com Tel:00862584438382, 00862584274745
Clinical Policy Title: Genicular nerve block
 Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Priorities Forum Statement GUIDANCE
 Priorities Forum Statement Number 21 Subject Knee Arthroscopy including arthroscopic knee washouts Date of decision November 2016 Date refreshed March 2017 Date of review November 2018 Osteoarthritis of
Priorities Forum Statement Number 21 Subject Knee Arthroscopy including arthroscopic knee washouts Date of decision November 2016 Date refreshed March 2017 Date of review November 2018 Osteoarthritis of
Medical Affairs Policy
 Service: Acupuncture Therapy PUM 250-0002-1803 Medical Affairs Policy Medical Policy Committee Approval 03/16/18 Effective Date 07/01/18 Prior Authorization Needed Yes-if not an exclusion of the health
Service: Acupuncture Therapy PUM 250-0002-1803 Medical Affairs Policy Medical Policy Committee Approval 03/16/18 Effective Date 07/01/18 Prior Authorization Needed Yes-if not an exclusion of the health
Medical Policy Partial or Total Replacement of First Metatarsophalangeal Joint
 Medical Policy Partial or Total Replacement of First Metatarsophalangeal Joint Subject: Partial or Total Replacement of First Metatarsophalangeal (MTP) Joint Background: Underlying causes of disease or
Medical Policy Partial or Total Replacement of First Metatarsophalangeal Joint Subject: Partial or Total Replacement of First Metatarsophalangeal (MTP) Joint Background: Underlying causes of disease or
O20 FEMORAL HEAD NECROSIS AND HYPERBARIC OXYGEN A MRI STUDY STUDY PROTOCOL ADOPTED FROM THE COST B14 WORKING GROUP
 O20 FEMORAL HEAD NECROSIS AND HYPERBARIC OXYGEN A MRI STUDY STUDY PROTOCOL ADOPTED FROM THE COST B14 WORKING GROUP "2" L. Ditri, F. Grigoletto, Y. Melamed, D. Reis, L. Cucci, P. Germonprè, T. Mesimeris,
O20 FEMORAL HEAD NECROSIS AND HYPERBARIC OXYGEN A MRI STUDY STUDY PROTOCOL ADOPTED FROM THE COST B14 WORKING GROUP "2" L. Ditri, F. Grigoletto, Y. Melamed, D. Reis, L. Cucci, P. Germonprè, T. Mesimeris,
Osteoarthritis. Dr Anthony Feher. With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides
 Osteoarthritis Dr Anthony Feher With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides No Financial Disclosures Number one chronic disability in the United States
Osteoarthritis Dr Anthony Feher With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides No Financial Disclosures Number one chronic disability in the United States
Osteonecrosis - Spectrum of imaging findings
 Osteonecrosis - Spectrum of imaging findings Poster No.: C-1861 Congress: ECR 2016 Type: Educational Exhibit Authors: P. Ninitas, A. L. Amado Costa, A. Duarte, I. Távora ; Lisbon/ 1 1 2 1 1 2 PT, Costa
Osteonecrosis - Spectrum of imaging findings Poster No.: C-1861 Congress: ECR 2016 Type: Educational Exhibit Authors: P. Ninitas, A. L. Amado Costa, A. Duarte, I. Távora ; Lisbon/ 1 1 2 1 1 2 PT, Costa
Clinical Policy Title: Platelet rich plasma
 Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Augmented Glenoid Component for Bone Deficiency in Shoulder Arthroplasty
 Clin Orthop Relat Res (2008) 466:579 583 DOI 10.1007/s11999-007-0104-4 SYMPOSIUM: NEW APPROACHES TO SHOULDER SURGERY Augmented Glenoid Component for Bone Deficiency in Shoulder Arthroplasty Robert S. Rice
Clin Orthop Relat Res (2008) 466:579 583 DOI 10.1007/s11999-007-0104-4 SYMPOSIUM: NEW APPROACHES TO SHOULDER SURGERY Augmented Glenoid Component for Bone Deficiency in Shoulder Arthroplasty Robert S. Rice
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS
 why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS TKR is not always the answer Today, many patients with medial or lateral disease and patellofemoral involvement receive a Total
why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS TKR is not always the answer Today, many patients with medial or lateral disease and patellofemoral involvement receive a Total
THE DIAGNOSIS AND MANAGEMENT OF SPONTANEOUS AND POST-ARTHROSCOPY OSTEONECROSIS OF THE KNEE
 THE DIAGNOSIS AND MANAGEMENT OF SPONTANEOUS AND POST-ARTHROSCOPY OSTEONECROSIS OF THE KNEE Abstract Spontaneous osteonecrosis of the knee (SPONK) and osteonecrosis in the postoperative knee (ONPK) are
THE DIAGNOSIS AND MANAGEMENT OF SPONTANEOUS AND POST-ARTHROSCOPY OSTEONECROSIS OF THE KNEE Abstract Spontaneous osteonecrosis of the knee (SPONK) and osteonecrosis in the postoperative knee (ONPK) are
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14 THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
Tibiotalocalcaneal fusion over retrograde SIGN Nail
 Tibiotalocalcaneal fusion over retrograde SIGN Nail PROF (DR.) SYED ANWARUZZAMAN Professor and Head Department of Orthopedic &trauma Comilla Medical college & Hospital Comilla, Bangladesh Introduction
Tibiotalocalcaneal fusion over retrograde SIGN Nail PROF (DR.) SYED ANWARUZZAMAN Professor and Head Department of Orthopedic &trauma Comilla Medical college & Hospital Comilla, Bangladesh Introduction
Integra Cadence Total Ankle System PATIENT INFORMATION
 Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
Non-Arthroplasty Hip Surgery. Javad Parvizi MD FRCS Professor of Orthopaedic Surgery
 Non-Arthroplasty Hip Surgery Javad Parvizi MD FRCS Professor of Orthopaedic Surgery Subcapital reduction osteotomy Relative lengthening of femoral neck (Perthes) AVN surgery Femoral osteotomy Trap door
Non-Arthroplasty Hip Surgery Javad Parvizi MD FRCS Professor of Orthopaedic Surgery Subcapital reduction osteotomy Relative lengthening of femoral neck (Perthes) AVN surgery Femoral osteotomy Trap door
Corporate Medical Policy
 Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
October 1999, Supplement 1 Volume 15 Number 7
 October 1999, Supplement 1 Volume 15 Number 7
October 1999, Supplement 1 Volume 15 Number 7
Case 27 Clinical Presentation
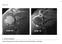 53 Case 27 Clinical Presentation 40-year-old man presents with acute shoulder pain and normal findings on radiographs. 54 RadCases Musculoskeletal Radiology Imaging Findings (,) Coronal images of the shoulder
53 Case 27 Clinical Presentation 40-year-old man presents with acute shoulder pain and normal findings on radiographs. 54 RadCases Musculoskeletal Radiology Imaging Findings (,) Coronal images of the shoulder
Late Results of Total Shoulder Replacement in Patients With Rheumatoid Arthritis
 CLINICAL ORTHOPAEDICS AND RELATED RESEARCH Number 366, pp. 39-45 0 1999 Lippincott Williams & Wilkins, Inc. Late Results of Total Shoulder Replacement in Patients With Rheumatoid Arthritis Jens 0. S@jbjerg,
CLINICAL ORTHOPAEDICS AND RELATED RESEARCH Number 366, pp. 39-45 0 1999 Lippincott Williams & Wilkins, Inc. Late Results of Total Shoulder Replacement in Patients With Rheumatoid Arthritis Jens 0. S@jbjerg,
Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis?
![Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis?](/thumbs/78/78456133.jpg) Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Diagnosis: Ceramic head fracture In the 1970 s, Boutin implemented ceramic in modern total hip arthroplasty (THA).
Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Diagnosis: Ceramic head fracture In the 1970 s, Boutin implemented ceramic in modern total hip arthroplasty (THA).
DISEASES AND DISORDERS
 DISEASES AND DISORDERS 9. 53 10. Rheumatoid arthritis 59 11. Spondyloarthropathies 69 12. Connective tissue diseases 77 13. Osteoporosis and metabolic bone disease 95 14. Crystal arthropathies 103 15.
DISEASES AND DISORDERS 9. 53 10. Rheumatoid arthritis 59 11. Spondyloarthropathies 69 12. Connective tissue diseases 77 13. Osteoporosis and metabolic bone disease 95 14. Crystal arthropathies 103 15.
Total Hip Arthroplasty Performed Using Conventional and Computer-Assisted, Tissue- Preserving Techniques 6
 Total Hip Arthroplasty Performed Using Conventional and Computer-Assisted, Tissue- Preserving Techniques 6 Stephen B. Murphy, MD, Timo M. Ecker, MD and Moritz Tannast, MD Introduction Less invasive techniques
Total Hip Arthroplasty Performed Using Conventional and Computer-Assisted, Tissue- Preserving Techniques 6 Stephen B. Murphy, MD, Timo M. Ecker, MD and Moritz Tannast, MD Introduction Less invasive techniques
FREIBERG S INFRACTION TREATMENT WITH METATARSAL NECK DORSAL CLOSING WEDGE OSTEOTOMY: REPORT OF TWO CASES
 FREIBERG S INFRACTION TREATMENT WITH METATARSAL NECK DORSAL CLOSING WEDGE OSTEOTOMY: REPORT OF TWO CASES Sung-Yen Lin, 1 Yuh-Min Cheng, 1,2 and Peng-Ju Huang 1,2 1 Department of Orthopedics, Kaohsiung
FREIBERG S INFRACTION TREATMENT WITH METATARSAL NECK DORSAL CLOSING WEDGE OSTEOTOMY: REPORT OF TWO CASES Sung-Yen Lin, 1 Yuh-Min Cheng, 1,2 and Peng-Ju Huang 1,2 1 Department of Orthopedics, Kaohsiung
BRONCHIAL THERMOPLASTY
 BRONCHIAL THERMOPLASTY UnitedHealthcare Community Plan Medical Policy Policy Number: CS014.E Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
BRONCHIAL THERMOPLASTY UnitedHealthcare Community Plan Medical Policy Policy Number: CS014.E Effective Date: July 1, 2017 Table of Contents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS... 1 COVERAGE
OCD: Beyond Microfracture. Disclosures. OCD Talus: My Approach 2/23/2018
 OCD: Beyond Microfracture Gregory C Berlet MD, FRCS(C), FAOA Orthopedic Foot and Ankle Center Columbus Ohio Disclosures Consultant/Speaker Bureau/Royalties/ Stock: Wright Medical, Stryker, ZimmerBiomet,
OCD: Beyond Microfracture Gregory C Berlet MD, FRCS(C), FAOA Orthopedic Foot and Ankle Center Columbus Ohio Disclosures Consultant/Speaker Bureau/Royalties/ Stock: Wright Medical, Stryker, ZimmerBiomet,
Knee Cartilage Transplants
 Knee Cartilage Transplants Date of Origin: 3/2005 Last Review Date: 8/23/2017 Effective Date: 8/23/2017 Dates Reviewed: Developed By: Medical Necessity Criteria Committee I. Description Allograft transplants
Knee Cartilage Transplants Date of Origin: 3/2005 Last Review Date: 8/23/2017 Effective Date: 8/23/2017 Dates Reviewed: Developed By: Medical Necessity Criteria Committee I. Description Allograft transplants
Clinical Policy Title: Platelet rich plasma
 Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review
Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review
Medical Affairs Policy
 Medical Affairs Policy Service: Back Pain: Sacroiliac and Coccydynia Treatments PUM 250-0024-1706 Medical Policy Committee Approval 06/15/18 Effective Date 10/01/18 Prior Authorization Needed Yes Disclaimer:
Medical Affairs Policy Service: Back Pain: Sacroiliac and Coccydynia Treatments PUM 250-0024-1706 Medical Policy Committee Approval 06/15/18 Effective Date 10/01/18 Prior Authorization Needed Yes Disclaimer:
CC TRIO VERSAFITCUP. Surgical Technique. each to their own. Hip Knee Spine Navigation
 VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
Bone Bangalore
 Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) HOD & Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital,
Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) HOD & Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital,
Arthritis of the Shoulder
 Page 1 of 7 Arthritis of the Shoulder This article is also available in Spanish: Artritis del hombro (Arthritis of the Shoulder) (topic.cfm?topic=a00723). In 2011, more than 50 million people in the United
Page 1 of 7 Arthritis of the Shoulder This article is also available in Spanish: Artritis del hombro (Arthritis of the Shoulder) (topic.cfm?topic=a00723). In 2011, more than 50 million people in the United
Care of the Foot and Ankle
 Care of the Foot and Ankle DaVinci Christopher W. DiGiovanni, MD Chief, Division of Foot and Ankle Professor & Program Director, Dept. Orthopaedic Surgery The Warren Alpert School of Medicine at Brown
Care of the Foot and Ankle DaVinci Christopher W. DiGiovanni, MD Chief, Division of Foot and Ankle Professor & Program Director, Dept. Orthopaedic Surgery The Warren Alpert School of Medicine at Brown
