MEDICAL POLICY No R2 OSTEOARTHRITIS OF THE KNEE
|
|
|
- Dwain Bradford
- 6 years ago
- Views:
Transcription
1 OSTEOARTHRITIS OF THE KNEE Effective Date: May 10, 2017 Review Dates: 2/10, 2/11, 2/12, 2/13, 2/14, 2/15, 2/16, 2/17 Date Of Origin: February 10, 2010 Status: Current Summary of Changes Clarifications: Deletions: Additions: Pg. 1, Section I, 3, criteria updated to reflect MAKOplasty knee resurfacing is considered experimental, investigational, or unproven, and therefore is not covered. I. POLICY/CRITERIA The following treatments for osteoarthritis of the knee are considered experimental, investigational, or unproven, and therefore, are not covered: 1. The use of patellofemoral replacement (PFR) for isolated patellofemoral osteoarthritis 2. Mesenchymal stem cell injections of the knee 3. MAKOplasty knee resurfacing. The MAKOplasty device may also be used for computer assisted navigation; as with other similar devices, this is not separately payable. II. MEDICAL NECESSITY REVIEW Required Not Required Not Applicable III. APPLICATION TO PRODUCTS Coverage is subject to member s specific benefits. Group specific policy will supersede this policy when applicable. HMO/EPO: This policy applies to insured HMO/EPO plans. POS: This policy applies to insured POS plans. PPO: This policy applies to insured PPO plans. Consult individual plan documents as state mandated benefits may apply. If there is a conflict between this policy and a plan document, the provisions of the plan document will govern. Page 1 of 7
2 ASO: For self-funded plans, consult individual plan documents. If there is a conflict between this policy and a self-funded plan document, the provisions of the plan document will govern. INDIVIDUAL: For individual policies, consult the individual insurance policy. If there is a conflict between this medical policy and the individual insurance policy document, the provisions of the individual insurance policy will govern. MEDICARE: Coverage is determined by the Centers for Medicare and Medicaid Services (CMS); if a coverage determination has not been adopted by CMS, this policy applies. MEDICAID/HEALTHY MICHIGAN PLAN: For Medicaid/Healthy Michigan Plan members, this policy will apply. Coverage is based on medical necessity criteria being met and the appropriate code(s) from the coding section of this policy being included on the Michigan Medicaid Fee Schedule located at: If there is a discrepancy between this policy and the Michigan Medicaid Provider Manual located at: the Michigan Medicaid Provider Manual will govern. For Medical Supplies/DME/Prosthetics and Orthotics, please refer to the Michigan Medicaid Fee Schedule to verify coverage. IV. DESCRIPTION Patellofemoral replacement (PFR) is the implantation of prostheses that replace the surfaces of the patella and femur at the knee joint. Potential candidates for PFR replacement have severe pain from isolated patellofemoral osteoarthritis (PFOA) that does not respond to nonsurgical or surgical therapies, and in whom there is no malalignment of the kneecap. Isolated patellofemoral osteoarthritis, involving only the junction between the patella and the femur, affects 5% to 10% of patients who present with knee pain. The disease is diagnosed through a history of patellofemoral pain and stiffness, and demonstration of damage to the articular surface in the presence of normal tibiofemoral articulation on plain radiographs, computed tomography, magnetic resonance imaging, or arthroscopy. Review of available evidence on the efficacy and safety of PFR for isolated patellofemoral osteoarthritis show benefits in some patients, however all of the available studies are limited by small populations and the lack of controls, and in some studies, inadequate follow-up times and an unacceptable loss of patients to follow-up can bias the results. In addition, there are no randomized controlled studies comparing PFR with total knee replacement or with other therapies for patellofemoral osteoarthritis including other surgeries. Additional information from well-designed studies is needed to determine the effects of factors such as age, gender, preoperative diagnosis, device/implant design (e.g., off-the-shelf and customized implants), and anatomical variations, etc., on the outcomes of PFR. Mesenchymal stem cells (MSCs) are self-renewing and multipotent cells capable of differentiating into multiple cell types. They were originally isolated from the bone marrow stroma but have recently been identified in other tissues. Bone marrow aspirate is considered to be the most accessible source and the most Page 2 of 7
3 common place to isolate MSCs for treatment of musculoskeletal disease. Bone marrow aspirate concentrate (BMAC) can be extracted and derived from different bones in the body. For orthopedic indications, bone marrow is generally extracted from the iliac crest, though other sites may be utilized. BMAC is under investigation as an alternative to autologous bone grafting from the iliac crest, Centrifugation of bone marrow aspirate (e.g. Harvest SmartPrep centrifuge) to concentrate MSCs is being utilized to increase the concentration of osteoprogenitor cells. Some research has suggested that stem cell concentration may relate to overall effectiveness, hence the use of centrifugation to create BMAC. MAKOplasty : Although there was a moderate amount of literature addressing robotic-assisted and computer-navigated orthopedic procedures for the knee, only 8 abstracts were found that specifically mentioned Mako surgery, Makoplasty, or RIO System components for knee procedures. The retrospective clinical studies evaluated the risk for overcorrection and the impact of residual osteoarthritis (patellofemoral and lateral compartment) on medial unicompartmental knee replacement. The case report described clinical experience in a patient with combined medial compartment osteoarthritis and a subchondral defect of the medial femoral condyle. Conclusions: There is insufficient published evidence to assess the safety and/or impact on health outcomes or patient management of Makoplasty for osteoarthritis of the knee. (Hayes February 2016) American Academy of Orthopaedic Surgeons: MAKO Surgical s RIO Robotic Arm Interactive Orthopedic System (A) is now available for use in MAKOplasty total hip arthroplasty (THA) procedures. The total hip replacement application is designed to support the surgeon s ability to more accurately align and position the implants relative to the needs of a patient. MAKOplasty THA provides the surgeon with a preoperative 3-dimensional (3-D) reconstruction of the patient s hip, which is used to develop the patient-specific surgical plan. The robotic arm then assists the surgeon during the procedure to accurately prepare the joint and optimally place hip implants. No AAOS statement found for use in knees (Accessed December 15, 2016) V. CODING INFORMATION ICD-10 Codes that may apply: M17.0 Bilateral primary osteoarthritis of knee M17.11 Unilateral primary osteoarthritis, right knee M17.12 Unilateral primary osteoarthritis, left knee M17.2 Bilateral post-traumatic osteoarthritis of knee M17.31 Unilateral post-traumatic osteoarthritis, right knee Page 3 of 7
4 M17.32 Unilateral post-traumatic osteoarthritis, left knee M17.4 Other bilateral secondary osteoarthritis of knee M17.5 Other unilateral secondary osteoarthritis of knee M17.9 of knee, unspecified CPT/HCPCS Codes: Unlisted procedure, femur or knee Arthroplasty, patella; with prosthesis Arthroplasty, femoral condyles or tibial plateau(s), knee; Unlisted procedure, musculoskeletal system, general VI. REFERENCES 1. Hayes Technology Brief Patellofemoral replace for October 13, American Academy of Orthopaedic Surgeons (AAOS). Treatment of osteoarthritis of the knee (non-arthroplasty). Rosemont (IL): American Academy of Orthopaedic Surgeons; Available at: 3. Lonner JH. Patellofemoral arthroplasty: the impact of design on outcomes. Orthop Clin North Am. 2008;39(3): Arthritis Foundation [website]. One In Two Adults At Risk For Painful Knee Arthritis [press release]. September 3, Available at: 08.pdf. 5. Delanois RE, McGrath MS, Ulrich SD, et al. Results of total knee replacement for isolated patellofemoral arthritis: when not to perform a patellofemoral arthroplasty. Orthop Clin N Am. 2008;39: Sisto DJ, Sarin VK. Patellofemoral arthroplasty with a customized trochlear prosthesis. Orthop Clin North Am. 2008;39(3): , vi-vii. 7. Sisto DJ, Sarin VK. Custom patellofemoral arthroplasty of the knee. J Bone Joint Surg Am. 2006;88(7): van Wagenberg JM, Speigner B, Gosens T, de Waal Malefijt J. Midterm clinical results of the Autocentric II patellofemoral prosthesis. Int Orthop Feb 18. [Epub ahead of print]. Available at: 9. Ackroyd CE, Newman JH, Evans R, et al. The Avon patellofemoral arthroplasty: five-year survivorship and functional results. J Bone Joint Surg Br. 2007;89(3): Leadbetter WB, Kolisek FR, Levitt RL, et al. Patellofemoral arthroplasty: a multi-centre study with minimum 2-year follow-up. Int Orthop Dec 5. [Epub ahead of print]. Available at: Page 4 of 7
5 11. American Association of Hip and Knee Surgeons (AAHKS). Total Knee Replacement. Updated April American Academy of Orthopaedic Surgeons [website]. Available at: Leadbetter WB, Ragland PS, Mont MA. The appropriate use of patellofemoral arthroplasty: An analysis of reported indications, contraindications, and failures. Clin Orthop Relat Res. 2005;436: Hogan K, Del Schutte H Jr. Patellofemoral arthritis. Updated September 12, emedicine [website]. Available at: Ackroyd CE, Chir B. Development and early results of a new patellofemoral arthroplasty. Clin Orthop Relat Res. 2005;(436): Argenson JN, Flecher X, Parratte S, Aubaniac JM. Patellofemoral arthroplasty: an update. Clin Orthop Relat Res. 2005;440: Merchant AC. A modular prosthesis for patellofemoral arthroplasty: design and initial results. Clin Orthop Relat Res. 2005;(436): Nicol SG, Loveridge JM, Weale AE, et al. Arthritis progression after patellofemoral joint replacement. Knee. 2006;13(4): Hendrix MR, Ackroyd CE, Lonner JH. Revision patellofemoral arthroplasty: three- to seven-year follow-up. J Arthroplasty. 2008;23(7): Butler JE, Shannon R. Patellofemoral arthroplasty with a custom-fit femoral prosthesis. Orthopedics. 2009;32(2): Van Jonbergen HPW, Werkman DM, van Kampen A. Conversion of patellofemoral arthroplasty to total knee arthroplasty: A matched case-control study of 13 patients. Acta Orthop. 2009;80(1): Available at: Paxton EW, Fithian DC. Outcome instruments for patellofemoral arthroplasty. Clin Orthop Relat Res. 2005;(436): Credentialing Resource Center (CRC). Orthopedic surgery. Clin Privil White Pap. 2008;(149): Orthopaedic Nurses Certification Board (ONCB) [website]. Certifying Excellence in Orthopaedic Nursing. Updated May Available at: Accessed October 12, Food and Drug Administration (FDA) [website]. Center for Devices and Radiological Health (CDRH). Devices@FDA [search: ]. Knee joint patellofemoral polymer/metal semi-constrained cemented prosthesis. Updated October 6, Available at: Cross MJ. Complications of total knee arthroplasty. Updated June 24, emedicine [website]. Available at: Lonner JH. Patellofemoral arthroplasty. J Am Acad Orthop Surg. 2007;15(8): Centers for Medicare & Medicaid Services (CMS) [website]. Medicare Coverage Database. National Coverage Documents [search: patellofemoral]. Page 5 of 7
6 Updated October 12, Available at: Aetna [website]. Clinical Policy Bulletins Available at: Anthem Blue Cross [website]. Medical Policies and Clinical UM Guidelines Available at: Blue Cross/Blue Shield Association [website]. Technology Evaluation Center (TEC). TEC Assessments Available at: CIGNA HealthCare [website]. Knee Arthroplasty/Replacement. Medical Coverage Policy No Reviewed May 15, Available at: ons/medical/ mm_0347_coveragepositioncriteria_minimally_invasive_knee_replacement.p df. 32. Humana [website]. Medical Coverage Policies Available at: Regence Group [website]. Medical Policy Available at: United Healthcare (UHC) [website]. Policies, Protocols and Administrative Guides Available at: Accessed October 5, Wellmark Blue Cross Blue Shield (BC/BS) [website]. Medical Policies & Authorizations Available at: iesandauthorizationshome.aspx. 36. ClinicalTrials.gov [website]. Search for Clinical Trials [search: patellofemoral replacement] Available at: Hayes, Inc. Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma for Orthopedic Use, June , Selected Treatments, Aetna Clinical Policy ( Retrieved January 11, 2016) 39. Bone and Tendon Graft Substitutes and Adjuncts, Aetna Clinical Policy (Retrieved January 11, 2016) 40. Hayes, Inc. MAKOplasty (MAKO Surgical Corporation) for Knee Arthroplasty, February 4, American Academy/Association or Orthopaedic (Accessed December 15, 2016) 42. Joint Resurfacing, Aetna Clinical Policy (Retrieved December 15, 2016) 43. Computer-Assisted Musculoskeletal Surgical Navigational Orthopedic Procedures of the Appendicular System, Anthem Blue Page 6 of 7
7 (Retrieved December 15, 2016) AMA CPT Copyright Statement: All Current Procedure Terminology (CPT) codes, descriptions, and other data are copyrighted by the American Medical Association. This document is for informational purposes only. It is not an authorization, certification, explanation of benefits, or contract. Receipt of benefits is subject to satisfaction of all terms and conditions of coverage. Eligibility and benefit coverage are determined in accordance with the terms of the member s plan in effect as of the date services are rendered. Priority Health s medical policies are developed with the assistance of medical professionals and are based upon a review of published and unpublished information including, but not limited to, current medical literature, guidelines published by public health and health research agencies, and community medical practices in the treatment and diagnosis of disease. Because medical practice, information, and technology are constantly changing, Priority Health reserves the right to review and update its medical policies at its discretion. Priority Health s medical policies are intended to serve as a resource to the plan. They are not intended to limit the plan s ability to interpret plan language as deemed appropriate. Physicians and other providers are solely responsible for all aspects of medical care and treatment, including the type, quality, and levels of care and treatment they choose to provide. The name Priority Health and the term plan mean Priority Health, Priority Health Managed Benefits, Inc., Priority Health Insurance Company and Priority Health Government Programs, Inc. Page 7 of 7
MEDICAL POLICY No R1 MEDICAL MANAGEMENT OF OBESITY
 MEDICAL MANAGEMENT OF OBESITY Effective Date: May 10, 2017 Review Dates: 8/11, 12/11, 2/12, 2/13, 2/14, 2/15, 2/16, 2/17 Date Of Origin: August 10, 2011 Status: Current Note: This medical policy does not
MEDICAL MANAGEMENT OF OBESITY Effective Date: May 10, 2017 Review Dates: 8/11, 12/11, 2/12, 2/13, 2/14, 2/15, 2/16, 2/17 Date Of Origin: August 10, 2011 Status: Current Note: This medical policy does not
ORTHOGNATHIC SURGERY
 ORTHOGNATHIC SURGERY MEDICAL POLICY Effective Date: February 1, 2017 Review Dates: 1/93, 7/95, 10/97, 4/99, 10/00, 8/01, 12/01, 4/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11,
ORTHOGNATHIC SURGERY MEDICAL POLICY Effective Date: February 1, 2017 Review Dates: 1/93, 7/95, 10/97, 4/99, 10/00, 8/01, 12/01, 4/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11,
MEDICAL POLICY No R9 DETOXIFICATION I. POLICY/CRITERIA
 DETOXIFICATION MEDICAL POLICY Effective Date: January 1, 2018 Review Dates: 1/93, 2/97, 4/99, 2/01, 12/01, 2/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11, 12/12, 12/13, 11/14,
DETOXIFICATION MEDICAL POLICY Effective Date: January 1, 2018 Review Dates: 1/93, 2/97, 4/99, 2/01, 12/01, 2/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11, 12/12, 12/13, 11/14,
MEDICAL POLICY No R4 NEUROPSYCHOLOGICAL AND PSYCHOLOGICAL TESTING
 NEUROPSYCHOLOGICAL AND PSYCHOLOGICAL TESTING Effective Date: October 1, 2015 Review Dates: 7/07, 6/08, 6/09, 8/09, 8/10, 8/11, 8/12, 8/13, 8/14, 8/15, 8/16, 8/17 Date Of Origin: July 2007 Status: Current
NEUROPSYCHOLOGICAL AND PSYCHOLOGICAL TESTING Effective Date: October 1, 2015 Review Dates: 7/07, 6/08, 6/09, 8/09, 8/10, 8/11, 8/12, 8/13, 8/14, 8/15, 8/16, 8/17 Date Of Origin: July 2007 Status: Current
For Commercial products, please refer to the following policy: Preauthorization via Web-Based Tool for Procedures
 Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
Medical Coverage Policy Total Joint Arthroplasty Hip and Knee EFFECTIVE DATE: 08/01/2017 POLICY LAST UPDATED: 06/06/2017 OVERVIEW Joint replacement surgery, also known as arthroplasty, has proved to be
MEDICAL POLICY No R2 DRUG TESTING
 Summary of Changes MEDICAL POLICY DRUG TESTING Effective Date: April 10, 2017 Review Dates: 5/15, 5/16, 11/16, 2/17 Date Of Origin: May 13, 2015 Status: Current Clarifications: Deletions: Additions: Pg.
Summary of Changes MEDICAL POLICY DRUG TESTING Effective Date: April 10, 2017 Review Dates: 5/15, 5/16, 11/16, 2/17 Date Of Origin: May 13, 2015 Status: Current Clarifications: Deletions: Additions: Pg.
MEDICAL POLICY No R10 INFUSION SERVICES & EQUIPMENT
 INFUSION SERVICES & EQUIPMENT Effective Date: August 1, 2017 Review Dates: 10/95, 12/99, 12/01, 11/02, 11/03, 11/04, 10/05, 10/06, 10/07, 10/08, 10/09, 4/10, 4/11, 4/12, 4/13, 5/14, 5/15, 2/16, 2/17, 5/17
INFUSION SERVICES & EQUIPMENT Effective Date: August 1, 2017 Review Dates: 10/95, 12/99, 12/01, 11/02, 11/03, 11/04, 10/05, 10/06, 10/07, 10/08, 10/09, 4/10, 4/11, 4/12, 4/13, 5/14, 5/15, 2/16, 2/17, 5/17
OSTEOCHONDRAL ALLOGRAFTS AND AUTOGRAFTS IN THE TREATMENT OF FOCAL ARTICULAR CARTILAGE LESIONS
 Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-115 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-115 Effective Date: 10/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
MEDICAL POLICY No R3 REFRACTIVE KERATOPLASTY / LASIK
 REFRACTIVE KERATOPLASTY / LASIK Effective Date: November 10, 2017 Review Dates: 7/07, 6/08, 6/09, 6/10, 8/10, 8/11, 8/12, 8/13, 8/14, 8/15, 8/16, 8/17 Date Of Origin: July 2007 Status: Current Summary
REFRACTIVE KERATOPLASTY / LASIK Effective Date: November 10, 2017 Review Dates: 7/07, 6/08, 6/09, 6/10, 8/10, 8/11, 8/12, 8/13, 8/14, 8/15, 8/16, 8/17 Date Of Origin: July 2007 Status: Current Summary
MEDICAL POLICY No R8 EATING DISORDERS POLICY/CRITERIA
 EATING DISORDERS MEDICAL POLICY Effective Date: June 27, 2016 Review Dates: 1/93, 8/96, 4/99, 12/01, 12/02, 11/03, 11/04, 10/05, 10/06, 10/07, 8/08, 8/09, 8/10, 8/11, 8/12, 8/13, 5/14, 5/15, 5/16 Date
EATING DISORDERS MEDICAL POLICY Effective Date: June 27, 2016 Review Dates: 1/93, 8/96, 4/99, 12/01, 12/02, 11/03, 11/04, 10/05, 10/06, 10/07, 8/08, 8/09, 8/10, 8/11, 8/12, 8/13, 5/14, 5/15, 5/16 Date
Corporate Medical Policy
 Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY No R1 GENDER REASSIGNMENT SURGERY
 GENDER REASSIGNMENT SURGERY Effective Date: January 1, 2017* Review Dates: 8/15, 8/16, 11/16 Date Of Origin: August 12, 2015 Status: Current *Note: For fully funded commercial (individual or group), this
GENDER REASSIGNMENT SURGERY Effective Date: January 1, 2017* Review Dates: 8/15, 8/16, 11/16 Date Of Origin: August 12, 2015 Status: Current *Note: For fully funded commercial (individual or group), this
MEDICAL POLICY No R0
 TRANSCUTANEOUS ELECTRICAL ACUSTIMULATION (TEAS) FOR HYPEREMESIS GRAVIDARUM Effective Date: November 19, 2010 Review Dates: 10/10, 10/11, 10/12, 10/13, 11/14, 11/15, 11/16 Date Of Origin: October 13, 2010
TRANSCUTANEOUS ELECTRICAL ACUSTIMULATION (TEAS) FOR HYPEREMESIS GRAVIDARUM Effective Date: November 19, 2010 Review Dates: 10/10, 10/11, 10/12, 10/13, 11/14, 11/15, 11/16 Date Of Origin: October 13, 2010
Original Date: December 2015 Page 1 of 8 FOR CMS (MEDICARE) MEMBERS ONLY
 National Imaging Associates, Inc. Clinical guidelines TOTAL JOINT ARTHROPLASTY -Total Hip Arthroplasty -Total Knee Arthroplasty -Replacement/Revision Hip or Knee Arthroplasty CPT4 Codes: Please refer to
National Imaging Associates, Inc. Clinical guidelines TOTAL JOINT ARTHROPLASTY -Total Hip Arthroplasty -Total Knee Arthroplasty -Replacement/Revision Hip or Knee Arthroplasty CPT4 Codes: Please refer to
MEDICAL POLICY No R1 COMPUTERIZED TOMOGRAPHIC ANGIOGRAPHY CORONARY ARTERIES (CCTA)
 COMPUTERIZED TOMOGRAPHIC ANGIOGRAPHY CONARY ARTERIES (CCTA) Effective Date: June 19, 2017 Review Dates: 11/15, 11/16 Date Of Origin: November 11, 2015 Status: Current Summary of Changes Clarifications:
COMPUTERIZED TOMOGRAPHIC ANGIOGRAPHY CONARY ARTERIES (CCTA) Effective Date: June 19, 2017 Review Dates: 11/15, 11/16 Date Of Origin: November 11, 2015 Status: Current Summary of Changes Clarifications:
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED NAVIGATION FOR KNEE AND HIP ARTHROPLASTY
 MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: COMPUTER ASSISTED PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
Cigna Medical Coverage Policies Musculoskeletal Knee Arthroplasty Total and Partial
 Cigna Medical Coverage Policies Musculoskeletal Knee Arthroplasty Total and Partial Effective January 25, 2017 Instructions for use The following coverage policy applies to health benefit plans administered
Cigna Medical Coverage Policies Musculoskeletal Knee Arthroplasty Total and Partial Effective January 25, 2017 Instructions for use The following coverage policy applies to health benefit plans administered
Corporate Medical Policy
 Corporate Medical Policy Computer Assisted Surgical Navigational Orthopedic Procedures File Name: Origination: Last CAP Review: Next CAP Review: Last Review: computer_assisted_surgical_navigational_orthopedic_procedures
Corporate Medical Policy Computer Assisted Surgical Navigational Orthopedic Procedures File Name: Origination: Last CAP Review: Next CAP Review: Last Review: computer_assisted_surgical_navigational_orthopedic_procedures
AUTOLOGOUS CHONDROCYTE IMPLANTATION FOR FOCAL ARTICULAR CARTILAGE LESIONS
 CARTILAGE LESIONS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
CARTILAGE LESIONS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
B i co m pa r t m e n ta l Knee Ar t h r o p l a s t y. Surgical Technique Overview
 B i co m pa r t m e n ta l Knee Ar t h r o p l a s t y Surgical Technique Overview Femoral array Medial parapatellar incision Tibial array 1. Perform a medial incision and parapatellar arthrotomy to expose
B i co m pa r t m e n ta l Knee Ar t h r o p l a s t y Surgical Technique Overview Femoral array Medial parapatellar incision Tibial array 1. Perform a medial incision and parapatellar arthrotomy to expose
Custom Patellofemoral Arthroplasty of the Knee. Surgical Technique
 This is an enhanced PDF from The Journal of Bone and Joint Surgery The PDF of the article you requested follows this cover page. Custom Patellofemoral Arthroplasty of the Knee. Surgical Technique Domenick
This is an enhanced PDF from The Journal of Bone and Joint Surgery The PDF of the article you requested follows this cover page. Custom Patellofemoral Arthroplasty of the Knee. Surgical Technique Domenick
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
7.01.140 Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery Section 7.0 Surgery Subsection Description Effective Date November 26, 2014
MEDICAL POLICY No R2 COMPUTERIZED TOMOGRAPHIC ANGIOGRAPHY CORONARY ARTERIES (CCTA)
 COMPUTERIZED TOMOGRAPHIC ANGIOGRAPHY CONARY ARTERIES (CCTA) Effective Date: January 1, 2018 Review Dates: 11/15, 11/16, 11/17 Date Of Origin: November 11, 2015 Status: Current Summary of Changes Clarifications:
COMPUTERIZED TOMOGRAPHIC ANGIOGRAPHY CONARY ARTERIES (CCTA) Effective Date: January 1, 2018 Review Dates: 11/15, 11/16, 11/17 Date Of Origin: November 11, 2015 Status: Current Summary of Changes Clarifications:
Medical Policy Original Effective Date: Revised Date: 07/26/17 Page 1 of 9
 Page 1 of 9 Disclaimer Description Coverage Determination/ Clinical Indications Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on
Page 1 of 9 Disclaimer Description Coverage Determination/ Clinical Indications Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on
Cigna Medical Coverage Policies Musculoskeletal Knee Arthroplasty Total and Partial
 Cigna Medical Coverage Policies Musculoskeletal Knee Arthroplasty Total and Partial Effective February 1, 2016 Instructions for use The following coverage policy applies to health benefit plans administered
Cigna Medical Coverage Policies Musculoskeletal Knee Arthroplasty Total and Partial Effective February 1, 2016 Instructions for use The following coverage policy applies to health benefit plans administered
Are You Living with. Knee Pain? MAKOplasty may be the right treatment option for you.
 Are You Living with Knee Pain? MAKOplasty may be the right treatment option for you. Understanding Osteoarthritis Osteoarthritis shouldn t keep you from doing the things you love. Osteoarthritis (OA) is
Are You Living with Knee Pain? MAKOplasty may be the right treatment option for you. Understanding Osteoarthritis Osteoarthritis shouldn t keep you from doing the things you love. Osteoarthritis (OA) is
P a rt i a l Knee Ar t h r o p l a s t y (PKA) Surgical Technique Overview
 P a rt i a l Knee Ar t h r o p l a s t y (PKA) Surgical Technique Overview Femoral array Medial parapatellar incision Tibial array 1. Perform a medial incision and parapatellar arthrotomy to expose the
P a rt i a l Knee Ar t h r o p l a s t y (PKA) Surgical Technique Overview Femoral array Medial parapatellar incision Tibial array 1. Perform a medial incision and parapatellar arthrotomy to expose the
Why precision is powerful
 Why precision is powerful A new answer for isolated patellofemoral OA First generation PFJ implants had sharp, constraining trochlear grooves and were prone to complications such as maltracking and catching
Why precision is powerful A new answer for isolated patellofemoral OA First generation PFJ implants had sharp, constraining trochlear grooves and were prone to complications such as maltracking and catching
why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS
 why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS TKR is not always the answer Today, many patients with medial or lateral disease and patellofemoral involvement receive a Total
why bicompartmental? A REVOLUTIONARY ALTERNATIVE TO TOTAL KNEE REPLACEMENTS TKR is not always the answer Today, many patients with medial or lateral disease and patellofemoral involvement receive a Total
Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain
 Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain Policy #: 015 Latest Review Date: February 2010 Category: Therapy Policy Grade:
Name of Policy: Sympathetic Therapy and Bioelectrical Nerve Block or Electroanalgesic Nerve Block for the Treatment of Pain Policy #: 015 Latest Review Date: February 2010 Category: Therapy Policy Grade:
JOHNS HOPKINS HEALTHCARE
 Page 1 of 5 ACTION: New Policy Effective Date: 03/15/2012 Revising Policy Number Review Dates: 10/22/07, 09/08/08, 05/24/11, Superseding Policy Number 05/29/12, 09/05/14, 09/01/17 Archiving Retiring Policy
Page 1 of 5 ACTION: New Policy Effective Date: 03/15/2012 Revising Policy Number Review Dates: 10/22/07, 09/08/08, 05/24/11, Superseding Policy Number 05/29/12, 09/05/14, 09/01/17 Archiving Retiring Policy
Unicompartmental Knee Replacement
 Unicompartmental Knee Replacement Results and Techniques Alexander P. Sah, MD California Orthopaedic Association Meeting Laguna Niguel, CA May 20th, 2011 Overview Why partial knee replacement? - versus
Unicompartmental Knee Replacement Results and Techniques Alexander P. Sah, MD California Orthopaedic Association Meeting Laguna Niguel, CA May 20th, 2011 Overview Why partial knee replacement? - versus
Partial Knee Replacement
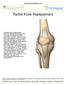 Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Patellofemoral Replacement
 Patellofemoral Replacement During knee replacement surgery, damaged bone and cartilage is resurfaced with metal and plastic components. Patellofemoral replacement is a type of "partial" knee replacement
Patellofemoral Replacement During knee replacement surgery, damaged bone and cartilage is resurfaced with metal and plastic components. Patellofemoral replacement is a type of "partial" knee replacement
MAKOplasty may be the right treatment option for you.
 Are You Living with Knee Pain? MAKOplasty may be the right treatment option for you. Osteoarthritis shouldn t keep you from doing the things you love. Understanding Osteoarthritis Osteoarthritis (OA) is
Are You Living with Knee Pain? MAKOplasty may be the right treatment option for you. Osteoarthritis shouldn t keep you from doing the things you love. Understanding Osteoarthritis Osteoarthritis (OA) is
SEVERE VARUS AND VALGUS DEFORMITIES TREATED BY TOTAL KNEE ARTHROPLASTY
 SEVERE VARUS AND VALGUS DEFORMITIES TREATED BY TOTAL KNEE ARTHROPLASTY Th. KARACHALIOS, P. P. SARANGI, J. H. NEWMAN From Winford Orthopaedic Hospital, Bristol, England We report a prospective case-controlled
SEVERE VARUS AND VALGUS DEFORMITIES TREATED BY TOTAL KNEE ARTHROPLASTY Th. KARACHALIOS, P. P. SARANGI, J. H. NEWMAN From Winford Orthopaedic Hospital, Bristol, England We report a prospective case-controlled
Original Policy Date
 MP 8.01.30 Orthopedic Applications of Stem Cell Therapy Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return
MP 8.01.30 Orthopedic Applications of Stem Cell Therapy Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return
Fresh Osteochondral Allograft Reimbursement Reference Document
 Fresh Osteochondral Allograft Reimbursement Reference Document This information is provided as a coding guide based on the experiences of clinicians with whom we have worked with over the years, and is
Fresh Osteochondral Allograft Reimbursement Reference Document This information is provided as a coding guide based on the experiences of clinicians with whom we have worked with over the years, and is
MEDICAL POLICY No R8 VENTRICULAR ASSIST DEVICES & ARTIFICIAL HEARTS
 VENTRICULAR ASSIST DEVICES & ARTIFICIAL HEARTS Effective Date: January 29, 2018 Review Dates: 8/05, 6/06, 6/07, 6/08, 10/08, 10/09, 10/10, 10/11, 10/12, 10/13, 11/14, 11/15, 11/16, 11/17, 11/18 Date Of
VENTRICULAR ASSIST DEVICES & ARTIFICIAL HEARTS Effective Date: January 29, 2018 Review Dates: 8/05, 6/06, 6/07, 6/08, 10/08, 10/09, 10/10, 10/11, 10/12, 10/13, 11/14, 11/15, 11/16, 11/17, 11/18 Date Of
Clinical Policy: Radial Head Implant Reference Number: CP.MP.148
 Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.148 Effective Date: 08/17 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR
 CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
National Joint Replacement Registry. Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY
 National Joint Replacement Registry Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY Report 2017 AOAnjrr 2016 supplementary report AOAnjrr 2016 supplementary report Contents SUMMARY...
National Joint Replacement Registry Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY Report 2017 AOAnjrr 2016 supplementary report AOAnjrr 2016 supplementary report Contents SUMMARY...
Outcome of patellofemoral arthroplasty, determinants for success
 Acta Orthop. Belg., 2015, 81, 759-767 ORIGINAL STUDY Outcome of patellofemoral arthroplasty, determinants for success Philippe Willekens, Jan Victor, Dimitri Verbruggen, Michiel Vande Kerckhove, Catherine
Acta Orthop. Belg., 2015, 81, 759-767 ORIGINAL STUDY Outcome of patellofemoral arthroplasty, determinants for success Philippe Willekens, Jan Victor, Dimitri Verbruggen, Michiel Vande Kerckhove, Catherine
Clinical Policy: Articular Cartilage Defect Repairs Reference Number: CP.MP.26
 Clinical Policy: Reference Number: CP.MP.26 Effective Date: 10/08 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.26 Effective Date: 10/08 Last Review Date: 05/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
New Directions in Osteoarthritis Research
 New Directions in Osteoarthritis Research Kananaskis October 22, 2015 Nick Mohtadi MD MSc FRCSC No conflicts of interest related to this presentation 1 Osteoarthritis: Disease? Fact of Life? Strong family
New Directions in Osteoarthritis Research Kananaskis October 22, 2015 Nick Mohtadi MD MSc FRCSC No conflicts of interest related to this presentation 1 Osteoarthritis: Disease? Fact of Life? Strong family
Case report. Open Access. Abstract
 Open Access Case report Dissociation of mobile-bearing patellar component in low contact stress patellofemoral arthroplasty, its mechanism and management: two case reports Hans-Peter W van Jonbergen*,
Open Access Case report Dissociation of mobile-bearing patellar component in low contact stress patellofemoral arthroplasty, its mechanism and management: two case reports Hans-Peter W van Jonbergen*,
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14 THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: LIMB PNEUMATIC COMPRESSION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
2016 CELEBRATING 15 YEARS OF DATA REPORT NATIONAL JOINT REPLACEMENT REGISTRY. Outcomes of Classes No Longer Used Hip and Knee Arthroplasty
 NATIONAL JOINT REPLACEMENT REGISTRY Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY REPORT 2016 CELEBRATING 15 YEARS OF DATA Contents INTRODUCTION...3 HIP REPLACEMENT...4 Partial
NATIONAL JOINT REPLACEMENT REGISTRY Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY REPORT 2016 CELEBRATING 15 YEARS OF DATA Contents INTRODUCTION...3 HIP REPLACEMENT...4 Partial
Clinical Policy: Digital Breast Tomosynthesis Reference Number: CP.MP.90
 Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Patello-Femoral Replacement (PFR) Do you have severe pain under or around your kneecap?
 KineMatch Patient-Specific PFR Patello-Femoral Replacement (PFR) Do you have severe pain under or around your kneecap? Have you been told by your doctor that you may need knee replacement surgery? Are
KineMatch Patient-Specific PFR Patello-Femoral Replacement (PFR) Do you have severe pain under or around your kneecap? Have you been told by your doctor that you may need knee replacement surgery? Are
Name of Policy: Magnetic Resonance Neurography
 Name of Policy: Magnetic Resonance Neurography Policy #: 177 Latest Review Date: June 2011 Category: Radiology Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue
Name of Policy: Magnetic Resonance Neurography Policy #: 177 Latest Review Date: June 2011 Category: Radiology Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue
Osteochondral allografting for all other joints is not covered as the evidence is insufficient to determine the
 Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
MEDICAL POLICY No R8 VENTRICULAR ASSIST DEVICES & ARTIFICIAL HEARTS
 VENTRICULAR ASSIST DEVICES & ARTIFICIAL HEARTS Effective Date: January 29, 2018 Review Dates: 8/05, 6/06, 6/07, 6/08, 10/08, 10/09, 10/10, 10/11, 10/12, 10/13, 11/14, 11/15, 11/16, 11/17 Date Of Origin:
VENTRICULAR ASSIST DEVICES & ARTIFICIAL HEARTS Effective Date: January 29, 2018 Review Dates: 8/05, 6/06, 6/07, 6/08, 10/08, 10/09, 10/10, 10/11, 10/12, 10/13, 11/14, 11/15, 11/16, 11/17 Date Of Origin:
Patellofemoral Arthroplasty: A Systematic Review of the Literature
 340 The Open Orthopaedics Journal, 2012, 6, (Suppl 2: M12) 340-347 Patellofemoral Arthroplasty: A Systematic Review of the Literature Payam Tarassoli 1, Shahid Punwar *,2, Wasim Khan 3 and David Johnstone
340 The Open Orthopaedics Journal, 2012, 6, (Suppl 2: M12) 340-347 Patellofemoral Arthroplasty: A Systematic Review of the Literature Payam Tarassoli 1, Shahid Punwar *,2, Wasim Khan 3 and David Johnstone
PARTIAL KNEE REPLACEMENT
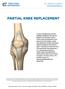 PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
The future of knee surgery.
 The future of knee surgery. 1 Adelaide Orthopaedic and Trauma Specialists - OrthoRobotics Adelaide Orthopaedic and Trauma Specialists (AOTS) are excited to introduce MAKOplasty, an innovation for those
The future of knee surgery. 1 Adelaide Orthopaedic and Trauma Specialists - OrthoRobotics Adelaide Orthopaedic and Trauma Specialists (AOTS) are excited to introduce MAKOplasty, an innovation for those
Policy #: 291 Latest Review Date: February 2013
 Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Clinical Policy: Essure Removal Reference Number: CP.MP.131
 Clinical Policy: Reference Number: CP.MP.131 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.131 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis
 Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis Effective January 1, 2016 Instructions for use The following coverage policy applies to health benefit
Cigna Medical Coverage Policies Musculoskeletal Shoulder Arthroplasty (Total, Hemi, Reverse)/Arthrodesis Effective January 1, 2016 Instructions for use The following coverage policy applies to health benefit
MEDICAL POLICY No R5 SURGICAL TREATMENT OF OBESITY
 SURGICAL TREATMENT OF OBESITY Effective Date: May 10, 2017 Review Dates: 8/11, 12/11, 2/12, 2/13, 2/14, 11/14, 2/15, 2/16, 2/17 Date Of Origin: August 10, 2011 Status: Current Note: This medical policy
SURGICAL TREATMENT OF OBESITY Effective Date: May 10, 2017 Review Dates: 8/11, 12/11, 2/12, 2/13, 2/14, 11/14, 2/15, 2/16, 2/17 Date Of Origin: August 10, 2011 Status: Current Note: This medical policy
Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide,
 Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Robots: Executive Summary The study is designed
Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Robots: Executive Summary The study is designed
Why do I perform PFP? Fernando Fonseca, MD PhD Head of Department Orthopaedics
 Why do I perform PFP? Fernando Fonseca, MD PhD Head of Department Orthopaedics Acknowledges to António Completo Paulo Flores Susana Meireles André Castro Disclosures: None ... Knee is one of the most complex
Why do I perform PFP? Fernando Fonseca, MD PhD Head of Department Orthopaedics Acknowledges to António Completo Paulo Flores Susana Meireles André Castro Disclosures: None ... Knee is one of the most complex
Gender Solutions Patello-Femoral Joint System
 Zimmer Biomet is the leading company in partial knee arthroplasty (PKA) 1 with over 40 years experience, offering a comprehensive range of anatomic and innovative solutions. Research shows that surgeons
Zimmer Biomet is the leading company in partial knee arthroplasty (PKA) 1 with over 40 years experience, offering a comprehensive range of anatomic and innovative solutions. Research shows that surgeons
Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS)
 09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
The UniSpacer : correcting varus malalignment in medial gonarthrosis
 DOI 10.1007/s00264-009-0908-9 ORIGINAL PAPER The UniSpacer : correcting varus malalignment in medial gonarthrosis Michael Clarius & Justus F. Becker & Holger Schmitt & Joern B. Seeger Received: 20 October
DOI 10.1007/s00264-009-0908-9 ORIGINAL PAPER The UniSpacer : correcting varus malalignment in medial gonarthrosis Michael Clarius & Justus F. Becker & Holger Schmitt & Joern B. Seeger Received: 20 October
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: subtalar_arthroereisis 6/2009 2/2018 2/2019 2/2018 Description of Procedure or Service Arthroereisis (also
Are You Living with. Hip Pain? MAKOplasty may be the right treatment option for you.
 Are You Living with Hip Pain? MAKOplasty may be the right treatment option for you. Hip pain shouldn t keep you from doing the things you love. Understanding Common Causes of Hip Pain If you are one of
Are You Living with Hip Pain? MAKOplasty may be the right treatment option for you. Hip pain shouldn t keep you from doing the things you love. Understanding Common Causes of Hip Pain If you are one of
Clinical Policy: Total Artificial Heart Reference Number: CP.MP.127
 Clinical Policy: Reference Number: CP.MP.127 Effective Date: 12/16 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.127 Effective Date: 12/16 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
INTRA-ARTICULAR HYALURONAN INJECTIONS
 INTRA-ARTICULAR HYALURONAN INJECTIONS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
INTRA-ARTICULAR HYALURONAN INJECTIONS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: facet_joint_denervation 6/2009 4/2017 4/2018 4/2017 Description of Procedure or Service Facet joint denervation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: facet_joint_denervation 6/2009 4/2017 4/2018 4/2017 Description of Procedure or Service Facet joint denervation
Integra. Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION
 Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
Corporate Medical Policy Investigational (Experimental) Services
 Corporate Medical Policy Investigational (Experimental) Services File Name: Origination: investigational_(experimental)_services 1/1996 Description of Procedure or Service BCBSNC defines the terms "investigational"
Corporate Medical Policy Investigational (Experimental) Services File Name: Origination: investigational_(experimental)_services 1/1996 Description of Procedure or Service BCBSNC defines the terms "investigational"
MEDICAL POLICY MEDICAL POLICY DETAILS POLICY STATEMENT. Page: 1 of 7
 Page: 1 of 7 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title METAL-ON-METAL TOTAL HIP RESURFACING Policy Number 7.01.74 Category Technology Assessment Effective Date 06/15/06 Revised Date 07/19/07,
Page: 1 of 7 MEDICAL POLICY MEDICAL POLICY DETAILS Medical Policy Title METAL-ON-METAL TOTAL HIP RESURFACING Policy Number 7.01.74 Category Technology Assessment Effective Date 06/15/06 Revised Date 07/19/07,
CLINICAL PAPER / ORTHOPEDIC
 HIP LEG LENGTH AND OFFSET Kelley T.C. and Swank M.L. (2009) Using CAS leads to more accurate positioning within the safe zone (inclination between 30 and 50, anteversion between 5 and 25 ) CAS improves
HIP LEG LENGTH AND OFFSET Kelley T.C. and Swank M.L. (2009) Using CAS leads to more accurate positioning within the safe zone (inclination between 30 and 50, anteversion between 5 and 25 ) CAS improves
OIG Work Plan for Orthotics
 OIG Work Plan for Orthotics February 1, 2018 We recently heard that the government will be focusing audits on off the shelf orthotics. We have tried to find information but have not been successful. Are
OIG Work Plan for Orthotics February 1, 2018 We recently heard that the government will be focusing audits on off the shelf orthotics. We have tried to find information but have not been successful. Are
Ideal Candidate for Cartilage Restoration. Large or Complex Lesions
 Complex Biological Knee Reconstruction: Bipolar, Multifocal Lesions and Osteoarthritis William Bugbee, MD Attending Physician, Scripps Clinic 18 th International Sports Medicine Fellow s Conference Ideal
Complex Biological Knee Reconstruction: Bipolar, Multifocal Lesions and Osteoarthritis William Bugbee, MD Attending Physician, Scripps Clinic 18 th International Sports Medicine Fellow s Conference Ideal
PRE & POST OPERATIVE RADIOLOGICAL ASSESSMENT IN TOTAL KNEE REPLACEMENT. Dr. Divya Rani K 2 nd Year Resident Dept. of Radiology
 PRE & POST OPERATIVE RADIOLOGICAL ASSESSMENT IN TOTAL KNEE REPLACEMENT Dr. Divya Rani K 2 nd Year Resident Dept. of Radiology PRE OPERATIVE ASSESSMENT RADIOGRAPHS Radiographs are used for assessment and
PRE & POST OPERATIVE RADIOLOGICAL ASSESSMENT IN TOTAL KNEE REPLACEMENT Dr. Divya Rani K 2 nd Year Resident Dept. of Radiology PRE OPERATIVE ASSESSMENT RADIOGRAPHS Radiographs are used for assessment and
Protocol. Hip Resurfacing
 Protocol Hip Resurfacing (70180) Medical Benefit Effective Date: 10/01/14 Next Review Date: 11/18 Preauthorization No Review Dates: 07/12, 07/13, 07/14, 07/15, 11/15, 11/16, 11/17 Preauthorization is not
Protocol Hip Resurfacing (70180) Medical Benefit Effective Date: 10/01/14 Next Review Date: 11/18 Preauthorization No Review Dates: 07/12, 07/13, 07/14, 07/15, 11/15, 11/16, 11/17 Preauthorization is not
Subject: Ultrasound Osteogenesis Stimulators, Non Invasive
 Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers
 ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
GENERAL Why did Tufts Health Plan implement a Spinal Conditions Management Program and why is it expanding to include joint surgeries?
 National Imaging Associates, Inc. (NIA) Spinal Conditions Management Program and Joint Surgery Program Frequently Asked Questions (FAQ s) For Tufts Health Plan Ordering Physicians Question GENERAL Why
National Imaging Associates, Inc. (NIA) Spinal Conditions Management Program and Joint Surgery Program Frequently Asked Questions (FAQ s) For Tufts Health Plan Ordering Physicians Question GENERAL Why
Clinical Policy: Cochlear Implant Replacements Reference Number: CP.MP.14
 Clinical Policy: Reference Number: CP.MP.14 Effective Date: 02/09 Last Review Date: 09/17 Revision Log Coding Implications See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.14 Effective Date: 02/09 Last Review Date: 09/17 Revision Log Coding Implications See Important Reminder at the end of this policy for important regulatory and
Corporate Medical Policy
 Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Kinematic vs. mechanical alignment: What is the difference?
 Kinematic vs. mechanical alignment: What is the difference? In this 4 Questions interview, Stephen M. Howell, MD, explains the potential benefits of 3D alignment during total knee replacement. Introduction
Kinematic vs. mechanical alignment: What is the difference? In this 4 Questions interview, Stephen M. Howell, MD, explains the potential benefits of 3D alignment during total knee replacement. Introduction
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vagus_nerve_stimulation 6/1998 5/2017 5/2018 5/2017 Description of Procedure or Service Stimulation of the
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vagus_nerve_stimulation 6/1998 5/2017 5/2018 5/2017 Description of Procedure or Service Stimulation of the
Surgical Technique. Poly UHMWPE. Bio Hyaluronic Acid. Advancing Materials. Advancing Outcomes.
 Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Pre-Operative Planning Prior to implantation of the BioPoly RS-Trochlea Implant, pre-operative planning will
Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Pre-Operative Planning Prior to implantation of the BioPoly RS-Trochlea Implant, pre-operative planning will
SUBJECT: LIMB PNEUMATIC COMPRESSION EFFECTIVE DATE: 06/27/13 DEVICES FOR VENOUS REVISED DATE: 06/26/14, 09/15/15,09/21/17. THROMBOEMBOLISM PROPHYLAXIS
 MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY REVISED DATE: 06/26/14, 09/15/15,09/21/17. PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
The Avon patellofemoral arthroplasty
 The Avon patellofemoral arthroplasty FIVE-YEAR SURVIVORSHIP AND FUNCTIONAL RESULTS C. E. Ackroyd, J. H. Newman, R. Evans, J. D. J. Eldridge, C. C. Joslin From Southmead Hospital, Bristol, England We report
The Avon patellofemoral arthroplasty FIVE-YEAR SURVIVORSHIP AND FUNCTIONAL RESULTS C. E. Ackroyd, J. H. Newman, R. Evans, J. D. J. Eldridge, C. C. Joslin From Southmead Hospital, Bristol, England We report
Robotic-Arm Assisted Total Knee Arthroplasty Demonstrated Greater Accuracy to Plan Compared to Manual Technique
 EPiC Series in Health Sciences Volume 1, 2017, Pages 283 287 CAOS 2017. 17th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery Health Sciences Robotic-Arm Assisted Total
EPiC Series in Health Sciences Volume 1, 2017, Pages 283 287 CAOS 2017. 17th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery Health Sciences Robotic-Arm Assisted Total
Related Policies None
 Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.153 BCBSA Ref. Policy: 7.01.153 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Viscosupplementation VISCOSUPPLEMENTATION AND CANALOSTOMY HS-270. Policy Number: HS-270. Original Effective Date: 1/8/2015. Revised Date(s): 1/7/2016
 Easy Choice Health Plan, Inc. Exactus Pharmacy Solutions, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Incorporated WellCare Health Insurance of Arizona, Inc., operating in Hawai i as Ohana
Easy Choice Health Plan, Inc. Exactus Pharmacy Solutions, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Incorporated WellCare Health Insurance of Arizona, Inc., operating in Hawai i as Ohana
Related Policies None
 Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Section of total knee replacement. Total Knee Replacement System. Knieendoprothesen System. Système de prothèse totale de genou
 Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Lumify. Lumify reimbursement guide {D DOCX / 1
 Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Lumify Lumify reimbursement guide {D0672917.DOCX / 1 {D0672917.DOCX / 1 } Contents Overview 4 How claims are paid 4 Documentation requirements 5 Billing codes for ultrasound: Non-hospital setting 6 Billing
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207
 Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: Robotic Surgery Reference Number: CP.MP. 207 Effective Date: 03/05 Last Review Date: 10/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Name of Policy: Computerized Pulse Waveform Analysis
 Name of Policy: Computerized Pulse Waveform Analysis Policy #: 020 Latest Review Date: September 2012 Category: Medical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Name of Policy: Computerized Pulse Waveform Analysis Policy #: 020 Latest Review Date: September 2012 Category: Medical Policy Grade: Active Policy but no longer scheduled for regular literature reviews
Corporate Medical Policy
 Corporate Medical Policy Orthotics File Name: Origination: Last CAP Review: Next CAP Review: Last Review: orthotics 6/1990 2/2017 2/2018 2/2017 Description of Procedure or Service An orthotic (orthosis)
Corporate Medical Policy Orthotics File Name: Origination: Last CAP Review: Next CAP Review: Last Review: orthotics 6/1990 2/2017 2/2018 2/2017 Description of Procedure or Service An orthotic (orthosis)
Medical Coverage Policy Computer-Assisted. Musculoskeletal Surgical Navigational Orthopedic Procedure
 Medical Coverage Policy Computer-Assisted Musculoskeletal Surgical Navigational Orthopedic Procedure EFFECTIVE DATE: 06 06 2017 POLICY LAST UPDATED: 04 03 2018 OVERVIEW Computer-assisted navigation (CAN)
Medical Coverage Policy Computer-Assisted Musculoskeletal Surgical Navigational Orthopedic Procedure EFFECTIVE DATE: 06 06 2017 POLICY LAST UPDATED: 04 03 2018 OVERVIEW Computer-assisted navigation (CAN)
