Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
|
|
|
- Osborn Jackson
- 6 years ago
- Views:
Transcription
1 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE
2 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized Do not use if the product sterilization barrier or its packaging is compromised. CAUTION: Federal (USA) law restricts this device for sale by or on the order of a physician Catalog Number Part Number Synovis Internal Code Manufacturer Authorized Representative Inner Diameter Use by Length Lot PRODUCT SIZES Catalog Number Nerve Gap Size of Neurotube* GEM0240NT _> 8mm _< 30mm 2.3 mm dia. X 40 mm length GEM0420NT _> 8mm _< 10mm 4.0 mm dia. X 20 mm length GEM0820NT _> 8mm _< 10mm 8.0 mm dia. X 20 mm length * Nominal 2
3 INDICATIONS: The Neurotube of 2.3mm diameter is intended for use in patients with an injury to a digital nerve with 8-30mm nerve gap. The Neurotubes of 4mm and 8mm diameters are intended for use in patients with an injury to a sensory nerve with 8-10mm nerve gap. IMPORTANT NOTE: This Instructions for Use manual is designed to provide instructions for proper use of the Neurotube product. It is not intended as a reference to surgical technique. DESCRIPTION: The Neurotube is an absorbable woven polyglycolic acid mesh tube, which is designed for primary or secondary peripheral nerve repair or reconstruction. The tube replaces the classic nerve graft technique for the repair of nerve gaps. The walls are corrugated for strength and flexibility. The corrugations prevent the tube from collapsing under normal physiological soft tissue pressures. The Neurotube is resorbed through the process of hydrolysis. ENGLISH INSTRUCTIONS FOR USE: 1. Aseptically transfer the inner pouch into the sterile field. 2. Visually inspect the pouch for any holes or tears; do not use if damaged. 3. Open the pouch and inspect the tube; do not use if tube is kinked, brittle, or degraded. 4. The nerve is surgically exposed at the appropriate incision site with the extremity under tourniquet control. 5. The injured segment of the nerve must be resected distally and proximally until a nerve stump is identified with no residual intrafascicular scarring. 6. Place the nerve on a wooden support (tongue depressor) and serially section with a #11 blade. Micro-scissors and larger scissors can cause the extrusion of intrafascicular components of the nerve. 7. The tourniquet is released and meticulous hemostasis is obtained, so the resected end of the nerve will not fill the Neurotube with blood. The resulting blood clot would create a barrier to neural regeneration. 8. Measure the nerve gap (distance between the nerve ends). 9. Measure the nerve diameter and carefully select the appropriate nerve tube diameter so as not to compress the repaired nerve. 10. If using the 2.3 mm diameter Neurotube, trim the Neurotube with scissors to a length 10 mm longer than the measured nerve gap, so the nerve ends may be inserted 5 mm into each end of the tube. 3
4 ENGLISH 11. A horizontal mattress stitch is used to draw the nerve end 5 mm into the Neurotube. An 8-0 suture with a 140-micron, 135 curve needle is recommended. The stitch is passed through the Neurotube from the outside to inside, transversely through the epineurium of the nerve end, and back through the tube, from the inside to the outside. Irrigation with heparinized saline facilitates drawing the nerve end 5 mm into the Neurotube and a knot is tied. It may be necessary to use a second stitch (anchor stitch) placing the suture superficially through the epineurium of the nerve and through the end of the tube. 12. The corrugated external surface of the Neurotube prevents kinking as the Neurotube goes about a curve or overlies a joint surface. After one end of the nerve is secured within the Neurotube, the tube is filled with heparinized saline (10 units per cc). The second nerve end is drawn into the opposite end of the Neurotube with a horizontal mattress stitch, and if necessary, an anchor stitch may be placed. 13. The tube is refilled with heparinized saline. 14. An attempt should be made to position the Neurotube in a soft tissue bed, which will facilitate mobilization of subcutaneous fat between the tube and the overlying skin. 15. Close the site. STORAGE: Store at room temperature, approximately 80 F (27 C) or less; avoid exposure to high humidity and prolonged extreme temperatures. WARNINGS: Complete hemostasis should be obtained in the surgical field before the Neurotube is positioned to bridge the gap between the nerve ends. Blood clot(s) in the lumen of the tube will impede neural regenerations. For hand surgeries, the patient s hand should be immobilized for three weeks following nerve reconstruction with the Neurotube. A plaster or fiberglass cast may be used for the first week and extension- block protective splint may be used for the second and third weeks. Cautiously supervised movement of the hand may be initiated before three weeks if a tendon repair is associated with the nerve reconstruction. Aggressive movement may cause the device to migrate to the surface of the skin. Should the Neurotube become exposed by movement before neural regeneration has been completed through the tube, it is suggested that the tube be removed and replaced with an autologous nerve graft. The nerve ends should never be inserted into the Neurotube under tension. If the nerve gap is greater than 30 mm when applying the 2.3 mm diameter Neurotube, an autologous nerve graft should be used. If the nerve gap is greater than 10 mm when applying the 4 mm or 8 mm diameter Neurotube, an autologous nerve graft should be used. Do not resterilize. Discard open, unused Neurotubes. 4
5 DISCLAIMER OF WARRANTIES: Synovis Micro Companies Alliance, Inc. (SMCA), a subsidiary of Synovis Life Technologies, Inc. warrants that reasonable care has been used in the manufacture of this device. This warranty is exclusive and in lieu of all other warranties whether expressed, implied, written or oral, including, but not limited to, any implied warranties of merchantability or fitness. Since SMCA has no control over the conditions under which the device is used, diagnosis of the patient, methods of administration, or its handling after it leaves its possession, SMCA does not warrant either a good effect or against an ill effect following its use. The manufacturer shall not be liable for any incidental or consequential loss, damage or expense arising directly or indirectly from the use of this device. SMCA will replace any device which is defective at the time of shipment. No representative of SMCA may change any of the foregoing or assume any additional liability or responsibility in connection with this device. 5
6 Synovis GEM0240NT _> 8mm _< 30mm 2.3 mm X 40 mm GEM0420NT _> 8mm _< 10mm 4.0 mm X 20 mm GEM0820NT _> 8mm _< 10mm 8.0 mm X 20 mm * * 6
7 mm 10 mm 5 mm 7
8 11. 5 mm mm 80 F (27 C) 2.3 mm 30 mm 4 mm 8 mm 10 mm 8
9 Synovis Life Technologies, Inc. Synovis Micro Companies Alliance, Inc. (SMCA) SMCA SMCA SMCA SMCA 9
10 10
11 11
12 A Subsidiary of Synovis Life Technologies, Inc. 439 Industrial Lane Birmingham, AL USA (USA) (fax) synovismicro.com Neurotube is a registered trademark in the U.S.A. and Canada only U.S. Patents 4,870,966 and 5,147,399 MS-13150A 07/12
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Cardiva Catalyst III INSTRUCTIONS FOR USE
 Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
Cardiva Catalyst III INSTRUCTIONS FOR USE IFU 2469 Rev. G CAUTION Federal (USA) law restricts this device to sale by or on the order of a physician. For single use only GRAPHICAL SYMBOLS ON THE CARDIVA
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
HeliMEND Advanced. Absorbable Collagen Membrane. Instructions for Use
 HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
HeliMEND Advanced Absorbable Collagen Membrane Instructions for Use 2 Indications HeliMEND Advanced absorbable collagen membrane is an absorbable, implantable material that is indicated for guided tissue
AXS Catalyst Distal Access Catheter
 AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
AXS Catalyst Distal Access Catheter Directions for Use 2 AXS Catalyst Distal Access Catheter ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning Contents
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Device Preparation (all steps to be performed per standard interventional technique)
 sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
sertera Biopsy Device Instructions for Use Please read all information carefully. Failure to properly follow the instructions may lead to unintended consequences. Important: This package insert is designed
SYNTHECEL Dura Repair. Assurance and Versatility for Dura Reconstruction.
 SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
SYNTHECEL Dura Repair Assurance and Versatility for Dura Reconstruction. SYNTHECEL Dura Repair is an assured and versatile solution for your dura reconstruction needs. Clinically proven One product choice
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp
 Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Radux StandTall Instructions for Use Sheath Extender and Securement Clasp USA Caution Federal (USA) law restricts this device to sale by or on the order of a health care professional. Caution The Radux
Integra. TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE
 Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
Integra TenoGlide Tendon Protector Sheet SURGICAL TECHNIQUE Table of Contents Description...2 Indications...2 Contraindications...2 Surgical Technique...4 Step 1 Surgical Approach...4 Step 2 Remove TenoGlide
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
INSTRUCTIONS FOR USE FOR: en English INSTRUCTIONS FOR USE GORE ENFORM PREPERITONEAL BIOMATERIAL Carefully read all instructions prior to use. Observe all instructions, warnings, and precautions noted throughout.
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15. Instructions for Use
 Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
Advisor FL Circular Mapping Catheter, Sensor Enabled D-AVSE-D10-F20, D-AVSE-DF10-F20, D-AVSE-D10-F15, D-AVSE-DF10-F15 Instructions for Use For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
P e r i - G u a r d I N S T R U C C I O N E S D E U S O M O D E D E M P L O I... 6 I S T R U Z I O N I P E R L U S O
 MS-12788D Peri-Guard CE IFU:12788b.qxd 12/12/2011 2:49 PM Page 1 P e r i - G u a r d I N S T R U C T I O N S F O R U S E......... 2 M O D E D E M P L O I.............. 6 G E B R A U C H S A N L E I T U
MS-12788D Peri-Guard CE IFU:12788b.qxd 12/12/2011 2:49 PM Page 1 P e r i - G u a r d I N S T R U C T I O N S F O R U S E......... 2 M O D E D E M P L O I.............. 6 G E B R A U C H S A N L E I T U
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
NeuroMend. Collagen Wrap Conduits. Operative Technique
 NeuroMend Collagen Wrap Conduits Operative Technique Stryker NeuroMend Collagen Wrap Conduits Wrap a protective environment around your patients injured peripheral nerves Common Indications The NeuroMend
NeuroMend Collagen Wrap Conduits Operative Technique Stryker NeuroMend Collagen Wrap Conduits Wrap a protective environment around your patients injured peripheral nerves Common Indications The NeuroMend
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Page 1 of 5 Reprocessed by Instructions for Use Reprocessed Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE
 CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
CAREFULLY READ ALL INSTRUCTIONS PRIOR TO USE INDICATIONS FOR USE The LATERA Absorbable Nasal Implant is indicated for supporting upper and lower lateral nasal cartilage. CAUTION: Federal law restricts
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use. Polyurethane Dual Lumen Occlusion Catheter
 Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
Polyurethane Dual Lumen Occlusion Catheter English Instructions for Use Polyurethane Dual Lumen Occlusion Catheter Polyurethane Dual Lumen Occlusion Catheter (Model Numbers 4F-2L-40, 4F-2L-80, 5F-2L-40,
eptfe Vascular Graft Instructions for Use
 TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
TM eptfe Vascular Graft Instructions for Use R Instructions For Use Caution: Federal (U.S.A) law restricts this device to sale by or on the order of a physician. Device Description, Indications, Contraindications,
JuggerKnot Soft Anchor 1.0 mm Mini
 JuggerKnot Soft Anchor 1.0 mm Mini Ulnar Collateral Ligament (UCL) Repair of the Thumb Surgical Technique Surgical Protocols by Mark Rekant, M.D. A. Lee Osterman, M.D. One Surgeon. One Patient. Over 1
JuggerKnot Soft Anchor 1.0 mm Mini Ulnar Collateral Ligament (UCL) Repair of the Thumb Surgical Technique Surgical Protocols by Mark Rekant, M.D. A. Lee Osterman, M.D. One Surgeon. One Patient. Over 1
QuadsTape System TM. For Quadriceps Tendon Reconstruction. Surgical Technique Manual
 QuadsTape System TM For Quadriceps Tendon Reconstruction Surgical Technique Manual 0086 Introduction QuadsTape System TM The QuadsTape System comprises a wide open weave Poly-Tape prosthesis with associated
QuadsTape System TM For Quadriceps Tendon Reconstruction Surgical Technique Manual 0086 Introduction QuadsTape System TM The QuadsTape System comprises a wide open weave Poly-Tape prosthesis with associated
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh
 Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
Ventralight ST Mesh with Echo PS Positioning System Catalog Number Shape Mesh Size 5955450 Circle 4.5" (11.4 cm) 5955460 Ellipse 4" x 6" (10.2 cm x 15.2 cm) 5955600 Circle 6" (15.2 cm) 5955680 Ellipse
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology
 Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Reprocessed by Instructions for Use Reprocessed Ethicon ENDOPATH XCEL TM Bladeless Trocar with OPTIVIEW Technology Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Balloon in Balloon (BIB )
 Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
Balloon in Balloon (BIB ) Stent Placement Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device. Instructions for Use
 The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
The SplitWire Percutaneous Transluminal Angioplasty Scoring Device Instructions for Use Contents Contains one (1) SplitWire device. Sterile. Sterilized with ethylene oxide gas. Radiopaque. For single use
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Pruitt Irrigation Occlusion Catheter. English Instructions for Use. Pruitt Irrigation Occlusion Catheter
 English Instructions for Use Pruitt Irrigation Occlusion Catheter (Model Numbers 2102-09, e2102-09) Instructions for Use - English Concept Balloon tipped catheters have been used to occlude vessels for
English Instructions for Use Pruitt Irrigation Occlusion Catheter (Model Numbers 2102-09, e2102-09) Instructions for Use - English Concept Balloon tipped catheters have been used to occlude vessels for
RECOMMENDED INSTRUCTIONS FOR USE
 Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Rapid Exchange PTCA Dilatation Catheter RECOMMENDED INSTRUCTIONS FOR USE Available in diameters 1.25mm to 4.5mm and in lengths 09mm to 40mm Caution: This device should be used only by physicians trained
Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas. Reprocessed Device for Single Use.
 Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed ENDOPATH XCEL Bladeless Endoscopic Trocars and Cannulas Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
NEPHROCHECK Liquid Control Kit Package Insert
 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
V a s c u - G u a r d
 MS 12786C IFU, Vascu Guard CE:12786b 12/14/2011 12:01 PM Page 1 V a s c u - G u a r d I N S T R U C T I O N S F O R U S E........... 2 M O D E D E M P L O I................. 6 G E B R A U C H S A N L E
MS 12786C IFU, Vascu Guard CE:12786b 12/14/2011 12:01 PM Page 1 V a s c u - G u a r d I N S T R U C T I O N S F O R U S E........... 2 M O D E D E M P L O I................. 6 G E B R A U C H S A N L E
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER
 Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
Page 1 of 5 NON-COMPLIANT PTCA RAPID EXCHANGE DILATATION CATHETER STERILE. SINGLE USE ONLY. Sterilized with ethylene oxide gas. Non pyrogenic. Do not resterilize. Do not use opened or damaged packages.
JuggerKnot Soft Anchor 1.0 mm Mini
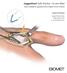 JuggerKnot Soft Anchor 1.0 mm Mini Ulnar Collateral Ligament (UCL) Repair of the Thumb Surgical Technique Surgical Protocols by Mark Rekant, M.D. A. Lee Osterman, M.D. One Surgeon. One Patient. Over 1
JuggerKnot Soft Anchor 1.0 mm Mini Ulnar Collateral Ligament (UCL) Repair of the Thumb Surgical Technique Surgical Protocols by Mark Rekant, M.D. A. Lee Osterman, M.D. One Surgeon. One Patient. Over 1
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM
 EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
EndoRelease ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM SURGICAL TECHNIQUE Up p e r Ex t r e m i t y So l u t i o n s ENDOSCOPIC CUBITAL TUNNEL RELEASE SYSTEM Description: The EndoRelease Endoscopic Cubital
Low Profile Neuro Plating System. Surgical Technique
 Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
Low Profile Neuro Plating System Surgical Technique TABLE OF CONTENTS INTRODUCTION Low Profile Neuro Plating System 2 SURGICAL TECHNIQUE Technique 5 PRODUCT INFORMATION Low Profile Neuro Plates 10 Low
The following languages are included in this packet:
 OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
OSTEOSET XR BONE VOID FILLER 150841-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE
 CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
CELLPLEX TCP SYNTHETIC CANCELLOUS BONE 129257-9 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -
Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters
 Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
Reprocessed by Instructions for Use Reprocessed 3D Diagnostic Ultrasound Catheters Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a
WallFlex Biliary RX Partially Covered Stent System Prescriptive Information
 Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Caution/Rx Only: Federal Law (USA) restricts this device to sale by or on the order of a physician. Intended Use/Indications for Use The WallFlex Biliary RX is indicated for use in the palliative treatment
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
The Ceterix NovoStitch Disposable Suture Passer. Do Not Reuse
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedics 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241-1748 IK Sterile D Do Not Reuse i See Instructions for Use THE CETERIX
Peritoneal Drainage System
 ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
ASEPT Drainage Parts and Accessories (Provided separately, see package label for contents.) ASEPT Pleural Drainage System 622289 (1 each) (includes ASEPT drainage catheter and insertion kit) ASEPT Peritoneal
OptiFix Absorbable Fixation System
 OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
OptiFix Absorbable Fixation System Absorbable Fixation Redefined Advancing the Fixation Experience. SOFT TISSUE REPAIR Right Procedure. Right Product. Right Outcome. Hernia Repair Fixation Challenges and
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES
 Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
Look For These Other Products by PARÉ Surgical, Inc. INNOVATIVE MEDICAL DEVICES Quik-Stitch-Endoscopic Suturing Device Disposable & Reusable The giflx- Disposable Rat tooth Jaw grasping Forceps with Ratcheting
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
LeMaitre Embolectomy Catheter
 Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Instructions for Use - English Lemaitre Embolectomy Catheter (Model Numbers 1601-24, e1601-24, 1601-26, e1601-26, 1601-28, e1601-28, 1601-34, e1601-34, 1601-38, e1601-38, 1601-44, e1601-44, 1601-48, e1601-48,
Fascial Turn-Down Flap Repair of Chronic Achilles Tendon Rupture
 19 Fascial Turn-Down Flap Repair of Chronic Achilles Tendon Rupture S. Ghosh, P. Laing, and Nicola Maffulli Introduction Fascial turn-down flaps can be used for an anatomic repair of chronic Achilles tendon
19 Fascial Turn-Down Flap Repair of Chronic Achilles Tendon Rupture S. Ghosh, P. Laing, and Nicola Maffulli Introduction Fascial turn-down flaps can be used for an anatomic repair of chronic Achilles tendon
JuggerKnot Soft Anchor 1.0 mm Mini. Scapholunate Ligament Repair/Reconstruction. Brochure and Surgical Technique
 JuggerKnot Soft Anchor 1.0 mm Mini Scapholunate Ligament Repair/Reconstruction Brochure and Surgical Technique One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide
JuggerKnot Soft Anchor 1.0 mm Mini Scapholunate Ligament Repair/Reconstruction Brochure and Surgical Technique One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide
Balloon in Balloon (BIB )
 in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
in (BIB ) Dilatation Catheter INSTRUCTIONS FOR USE CAUTION: FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Read all instructions prior to use. Distributed by: B. Braun
ENDOBUTTON Fixation Device
 ENDOBUTTON Fixation Device Distal Biceps Repair A Shoulder Series Technique Guide As described by: Felix Buddy Savoie, MD PRELIMINARY - NOT FOR DISTRIBUTION As described by: Felix Buddy Savoie, MD Chief
ENDOBUTTON Fixation Device Distal Biceps Repair A Shoulder Series Technique Guide As described by: Felix Buddy Savoie, MD PRELIMINARY - NOT FOR DISTRIBUTION As described by: Felix Buddy Savoie, MD Chief
Model 380B DNA Synthesizer Version 1.1
 Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
Model 380B DNA Synthesizer Version 1.1 User s Manual Copyright 2001, Applied Biosystems. All rights reserved. For Research Use Only. Not for use in diagnostic procedures. APPLIED BIOSYSTEMS LIMITED WARRANTY
Surgical Technique. Achilles Tendon Repair Using Conexa Reconstructive Tissue Matrix. conexatm. Surgical Technique Described by Tom Chang, DPM
 Surgical Technique Achilles Tendon Repair Using Conexa Reconstructive Tissue Matrix Surgical Technique Described by Tom Chang, DPM conexatm r e c o n s t r u c t i v e t i s s u e m a t r i x Achilles
Surgical Technique Achilles Tendon Repair Using Conexa Reconstructive Tissue Matrix Surgical Technique Described by Tom Chang, DPM conexatm r e c o n s t r u c t i v e t i s s u e m a t r i x Achilles
Positioning System. Laparoscopic ventral hernia repair KEY BENEFITS SOFT TISSUE REPAIR
 Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
Echo PS Positioning System with Ventralight ST Mesh or Composix L/P Mesh Laparoscopic ventral hernia repair Echo PS Positioning System with Ventralight ST Mesh Echo PS Positioning System with Composix
INSTRUCTIONS FOR USE
 PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
PERICARDIUM COVERED STENT (PCS) INSTRUCTIONS FOR USE STERILE - Ethylene Oxide Sterilized. For single use only. Do not re-sterilize. THE CLINICAL TECHNIQUES AND PROCEDURES DESCRIBED HERE DO NOT REPRESENT
Mini External Fixator
 Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Stabilize the Phalanges and Metacarpals Mini External Fixator Surgical Technique Table of Contents Introduction Mini External Fixator 2 Indications 4 Surgical Technique Technique Overview 5 Product Information
Integra. Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE
 Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
Integra Endoscopic Gastrocnemius Release System SURGICAL TECHNIQUE Table of Contents Indications... 2 Contraindications... 2 System Description... 2 Features and Benefits... 2 Surgical Site Preparation...3
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Integra. DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE
 Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
ER REBOA Catheter. Instructions for Use
 ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
ER REBOA Catheter Instructions for Use Prytime Medical Devices, Inc. 229 N. Main Street Boerne, TX 78006, USA feedback@prytimemedical.com www.prytimemedical.com US 1 210 340 0116 U.S. and Foreign Patents
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
TruGuard Custom Tongue and Jaw Positioner Instructions for Use
 TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
TruGuard Custom Tongue and Jaw Positioner Instructions for Use Caution: Federal Law restricts this device to sale by or on the order of a licensed Physician or Radiation Therapist. DEVICE DESCRIPTION Bionix
Visit Linvatec.com today to learn more. Surgical Technique: Sequential Meniscal Running Stitch
 ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
ORDERING INFORMATION Sequent Meniscal Repair System MR004S MR007S MR004C MR007C SC047D Straight Needle, 4 Implants Straight Needle, 7 Implants Curved Needle, 4 Implants Curved Needle, 7 implants Sequent
Surgical Protocol written by Keith Lawhorn, M.D.
 Surgical Protocol written by Keith Lawhorn, M.D. The MaxFire Meniscal Repair System is an all-inside, all-suture meniscal repair system utilizing ZipLoop Technology. Anchors are preloaded on needle inserters,
Surgical Protocol written by Keith Lawhorn, M.D. The MaxFire Meniscal Repair System is an all-inside, all-suture meniscal repair system utilizing ZipLoop Technology. Anchors are preloaded on needle inserters,
BIOKNOTLESSRC ROTATOR CUFF REPAIR SUTURE ANCHOR SURGICAL TECHNIQUE. Surgical Technique for Arthroscopic Rotator Cuff Repair. Raymond Thal, M.D.
 SURGICAL TECHNIQUE ROTATOR CUFF REPAIR BIOKNOTLESSRC SUTURE ANCHOR Surgical Technique for Arthroscopic Rotator Cuff Repair Raymond Thal, M.D. Town Center Orthopaedic Associates Reston, Virginia Surgical
SURGICAL TECHNIQUE ROTATOR CUFF REPAIR BIOKNOTLESSRC SUTURE ANCHOR Surgical Technique for Arthroscopic Rotator Cuff Repair Raymond Thal, M.D. Town Center Orthopaedic Associates Reston, Virginia Surgical
3.5 mm Clavicle Hook Plates
 A Single Solution for Lateral Clavicle Fractures and Acromioclavicular Joint Dislocations 3.5 mm Clavicle Hook Plates Surgical Technique Discontinued December 2017 DSUS/TRM/1016/1126(1) Table of Contents
A Single Solution for Lateral Clavicle Fractures and Acromioclavicular Joint Dislocations 3.5 mm Clavicle Hook Plates Surgical Technique Discontinued December 2017 DSUS/TRM/1016/1126(1) Table of Contents
SYNPOR POROUS POLYETHYLENE IMPLANTS. For craniofacial and orbital augmentation and reconstruction
 SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
SYNPOR POROUS POLYETHYLENE IMPLANTS For craniofacial and orbital augmentation and reconstruction SURGICAL TECHNIQUE TABLE OF CONTENTS INTRODUCTION SYNPOR Porous Polyethylene Implants 2 Indications and
Tension Band Pin System 2. Surgical Technique
 Tension Band Pin System 2 Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
Tension Band Pin System 2 Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches that improve
Doc no: IFU/CPL Issue date: Rev no: 00 Rev date: 1
 FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
FUTURA SURGICARE PVT LTD INSTRUCTION FOR USE Brand name: PROGUT Material: Catgut Plain. Absorbable surgical suture U.S.P Description: Progut surgical catgut suture is a sterile absorbable suture composed
Titanium Wire With Barb and Needle
 For Canthal Tendon Procedures Titanium Wire With Barb and Needle Surgical Technique Table of Contents Introduction Titanium Wire With Barb and Needle 2 Indications 2 Surgical Technique Preoperative Planning
For Canthal Tendon Procedures Titanium Wire With Barb and Needle Surgical Technique Table of Contents Introduction Titanium Wire With Barb and Needle 2 Indications 2 Surgical Technique Preoperative Planning
Extensor Mechanism. Quadriceps Tendon Reconstruction Patellar Tendon Reconstruction Post Patellectomy Reconstruction. Surgical Technique Manual
 Extensor Mechanism Quadriceps Tendon Reconstruction Patellar Tendon Reconstruction Post Patellectomy Reconstruction Surgical Technique Manual 0086 Introduction QuadsTape and PatellarTape Systems The QuadsTape
Extensor Mechanism Quadriceps Tendon Reconstruction Patellar Tendon Reconstruction Post Patellectomy Reconstruction Surgical Technique Manual 0086 Introduction QuadsTape and PatellarTape Systems The QuadsTape
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
Integra. Silicone PIP Implant SURGICAL TECHNIQUE
 Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
Integra Silicone PIP Implant SURGICAL TECHNIQUE Table of Contents Indications For Use...2 Contraindications...2 Warnings and Precautions...2 Surgical Technique Preoperative Assessment... 3 Step 1: Initial
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
North Medical Endoscopic Biliary Stent (BilS)
 North Medical Endoscopic Biliary Stent (BilS) Instructions for Use Manufactured by North Medical DEVICE NAME North Medical Endoscopic Biliary Stent (BilS) DEVICE DESCRIPTION North Medical's BilS stent
North Medical Endoscopic Biliary Stent (BilS) Instructions for Use Manufactured by North Medical DEVICE NAME North Medical Endoscopic Biliary Stent (BilS) DEVICE DESCRIPTION North Medical's BilS stent
Passer. Sterile. D i. Do Not PASSER DESCRIPTION. The Ceterix. Page 1
 The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedic cs 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241 1748 IK D i Sterile Do Not Reusee See Instructions for Use THE
The Ceterix NovoStitch Disposable Suture Passer Ceterix Orthopaedic cs 959 Hamilton Avenue Menlo Park, CA 94025 Customer Service: (650) 241 1748 IK D i Sterile Do Not Reusee See Instructions for Use THE
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation. Surgical Technique
 JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
AchilloCord PLUS System
 AchilloCord PLUS System For Acute Achilles Tendon Reconstruction Surgical Technique Manual 0086 Introduction AchilloCord PLUS System The AchilloCord PLUS System provides a non-harvesting, quick, easy and
AchilloCord PLUS System For Acute Achilles Tendon Reconstruction Surgical Technique Manual 0086 Introduction AchilloCord PLUS System The AchilloCord PLUS System provides a non-harvesting, quick, easy and
Allium Trans-hepatic Biliary Stent (BIS)
 Allium Trans-hepatic Biliary (BIS) Instructions for Use Manufactured by Allium Ltd. DEVICE NAME Allium Trans-hepatic Biliary (BIS) DEVICE DESCRIPTION The Trans-hepatic BIS is a flexible, self-expanding
Allium Trans-hepatic Biliary (BIS) Instructions for Use Manufactured by Allium Ltd. DEVICE NAME Allium Trans-hepatic Biliary (BIS) DEVICE DESCRIPTION The Trans-hepatic BIS is a flexible, self-expanding
Integra BraIn MaPPIng
 Integra BraIn MaPPIng Product catalogue Innovations. For Life. brain mapping Ordering and Warranty Information Pricing: Prices stated in specific written quotations are firm for thirty days from the date
Integra BraIn MaPPIng Product catalogue Innovations. For Life. brain mapping Ordering and Warranty Information Pricing: Prices stated in specific written quotations are firm for thirty days from the date
FIXED PERFORMANCE. Soft Tissue ACL Reconstruction
 ADJUSTABLE CONVENIENCE, FIXED PERFORMANCE Soft Tissue ACL Reconstruction Surgical Technique The RIGIDLOOP Adjustable Cortical System The RIGIDLOOP Adjustable Cortical System is an innovative technology
ADJUSTABLE CONVENIENCE, FIXED PERFORMANCE Soft Tissue ACL Reconstruction Surgical Technique The RIGIDLOOP Adjustable Cortical System The RIGIDLOOP Adjustable Cortical System is an innovative technology
SureLock All-Suture Anchor System
 SureLock All-Suture Anchor System Guide for Bankart Repair Surgical Technique 2 SureLock All-Suture System Bankart Repair Surgical Technique Figure 1 *Curved and straight drill guides are available for
SureLock All-Suture Anchor System Guide for Bankart Repair Surgical Technique 2 SureLock All-Suture System Bankart Repair Surgical Technique Figure 1 *Curved and straight drill guides are available for
OSSIX PLUS The Resorbable Collagen Membrane Instructions for Use for OSSIX PLUS
 OSSIX PLUS The Resorbable Collagen Membrane Instructions for Use for OSSIX PLUS DESCRIPTION OSSIX PLUS is a biodegradable and biocompatible collagen membrane intended for use during the process of guided
OSSIX PLUS The Resorbable Collagen Membrane Instructions for Use for OSSIX PLUS DESCRIPTION OSSIX PLUS is a biodegradable and biocompatible collagen membrane intended for use during the process of guided
