BIOLOX delta Option Ceramic Femoral Head System
|
|
|
- Cory Cole
- 6 years ago
- Views:
Transcription
1 BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Knees Hips Extremities Cement and Accessories PMI Technology
2 Product Features BIOLOX delta ceramic heads are composed of 75% aluminum oxide and approximately 25% zirconia. The combination of these two materials provides low wear and higher strength as compared to alumina ceramics. 1 In addition, BIOLOX delta ceramic heads combined with Antioxidant Infused E1 bearings show wear rates similar to that of metal-on-metal articulations. 2 BIOLOX delta Option Ceramic Femoral Head Component The BIOLOX delta Option Ceramic Femoral Head is a two-piece component consisting of a titanium neck sleeve and a BIOLOX delta ceramic head. Unlike the traditional BIOLOX delta one piece ceramic head, which is indicated for primary arthroplasty only, the BIOLOX delta Option component is designed to allow surgeons to utilize a ceramic head in both primary and revision arthroplasty. 2.0 Volumetric Wear Rates of 38mm Acetabular Liners 2 5 Million Cycles 38mm Head Size Head Diameters Neck Sleeve Taper Neck Length mm 44mm Type I -6, -3, Std., +3, +6 12/14-3, Std., +4, +7 Volumetric Wear Rate (mm 3 /MC) BIOLOX delta/ E1 Liner Metal-on-Metal (Run-in Wear) Type 1-6mm -3mm Std +3mm +6mm 1
3 Instructions for Use BIOLOX delta Option ceramic femoral heads and titanium neck sleeves are compatible with Biomet Type 1 and 12/14 stem tapers and can be utilized in both primary and revision total hip arthroplasty with Biomet polyethylene and antioxidant infused liners. Head Removal In the case of a revision surgery, extraction of the existing femoral head should be done with suitable extraction instruments to avoid unnecessary damage to the trunnion. Figure 1: Taper with scratch/defect at a height of 0.25mm Stem Trunnion Inspection The BIOLOX delta Option Ceramic Head component can be used on both a new stem, or a previously implanted stem. BIOLOX delta Option Ceramic Head components should not be used on trunnions with scratches or defects greater than 0.25mm in height. Inspect the taper for damage prior to placement of the modular head components by measuring scratches or defects, verifying that the height is less than 0.25mm (Figure 1). Figure 2: Taper with broad truncation The conditions shown in Figures 2, 3 and 4 are also considered unsuitable for the use of the Biomet BIOLOX delta Option Ceramic Head and can be expected to cause failure. Figure 3: Slanted taper Biomet does not practice medicine. Each surgeon is responsible for determining the appropriate device and surgical technique to utilize on each individual patient. Figure 4: Crushed taper 2
4 Trialing Verify the taper type on the existing stem or the stem to be inserted, taking care to select the correct trial and final implant that matches the stem taper. Determine the appropriate neck length and verify joint stability. Assembly Instructions Verify the correct selection of BIOLOX delta Option Ceramic Head and neck sleeve as predetermined during the trialing process. Any neck sleeve can be used with any BIOLOX delta Option Ceramic Head. Note: Heads and neck sleeves are packaged separately. Assemble the modular head components prior to positioning them onto the stem by aligning the head onto the neck sleeve axially and apply pressure. A slight resistance will be felt once the taper is engaged (Figure 5 & 6). Figure 5 Head/Stem Positioning Assure that all tapers, neck sleeve to ceramic head and ceramic head component to stem trunnion, are clean and dry before assembly. Impact the modular head components onto the stem with a light tap that firmly and definitively seats the head using a plastic head impactor only. Note: The use of metal impactors or any other metallic objects may scratch or crack the modular head bearing surface, compromising the integrity of the component. If the modular ceramic head becomes scratched or cracked, the head and neck sleeve must be replaced. Figure 6 To verify fixation of the head, attempt to remove the head by hand. 3
5 BIOLOX delta Option Ceramic Femoral Head Implants Part Number Description Size Biolox delta Option Ceramic Head Biolox delta Option Ceramic Head Biolox delta Option 12/14 Insert Biolox delta Option 12/14 Insert Biolox delta Option 12/14 Insert Biolox delta Option 12/14 Insert 40mm 44mm -6mm -3mm Std +3mm +6mm -3mm Std +4mm +7mm Instruments Part Number Description Size M 2 a-magnum Head Trial M 2 a-magnum Head Trial 12/14 Neck Trial 12/14 Neck Trial 12/14 Neck Trial 12/14 Neck Trial 40mm 44mm -6mm -3mm Std +3mm +6mm -3mm Std +4mm +7mm Note: The head trial and Type 1 neck trials are currently available in the M 2 a-magnum instrument sets. 4
6 Biomet Orthopedics East Bell Drive Date: 11/09 P.O. Box 587 Warsaw, Indiana USA Date: 11/09 lta Option Modular Head Hip Join Biomet Biolox delta Option Modular Head Hip Joint Prostheses ATTENTION OPERATING SURGEON DESCRIPTION The Biomet Biolox delta Option Ceramic modular head components are a Transition- Toughened-Platelet Alumina Composite ceramic (TTPA) material with highly polished surfaces in a variety of head sizes. The highly polished surface is designed to reduce friction and minimizes wear. The titanium sleeves adapt the ceramic heads to either a Biomet Type I or a Biomet 12/14 taper and allows them to be used in both primary and revision total hip arthroplasty. Taper with scratch/defect at a Height of 0.25mm Figure A Taper with Broad Truncation Figure B MATERIALS Head - TTPA Ceramic Sleeve - Titanium Alloy (Ti-6Al-4V) INDICATIONS 1. Noninflammatory degenerative joint disease including osteoarthritis and avascular necrosis. 2. Rheumatoid arthritis. 3. Correction of functional deformity. 4. Treatment of non-union, femoral neck fracture and trochanteric fractures of the proximal femur with head involvement, unmanageable by other techniques. 5. Revision procedures where other devices or treatments have failed. CONTRAINDICATIONS Absolute contraindications include: infection, sepsis, and osteomyelitis. Relative contraindications include: 1) uncooperative patient or patient with neurologic disorders who are incapable of following directions, 2) osteoporosis, 3) metabolic disorders which may impair bone formation, 4) osteomalacia, 5) distant foci of infections which may spread to the implant site, 6) rapid joint destruction, marked bone loss or bone resorption apparent on roentgenogram, 7) vascular insufficiency, muscular atrophy, or neuromuscular disease. WARNINGS Improper selection, placement, positioning, alignment and fixation of the implant components may result in unusual stress conditions which may lead to subsequent reduction in the service life of the prosthetic components. Malalignment of the components or inaccurate implantation can lead to excessive wear and/or failure of the implant or procedure. Inadequate preclosure cleaning (removal of surgical debris) can lead to excessive wear. Improper preoperative or intraoperative implant handling or damage (scratches, dents, etc.) can lead to crevice corrosion, fretting, fatigue fracture and/or excessive wear. Use clean gloves when handling implants. Laboratory testing indicates that implants subjected to body fluids, surgical debris or fatty tissue, have lower adhesion strength to cement than implants handled with clean gloves. Do not modify implants. The surgeon is to be thoroughly familiar with the implants and instruments, prior to performing surgery. 1. Use Biomet Biolox delta Option ceramic modular head with Biomet metallic femoral components. Do not use Biomet Biolox delta Option ceramic modular heads with femoral stems or acetabular components offered by other manufacturers. Mismatching of components or taper sizes can be expected to cause intraoperative or postoperative fracture of ceramic heads. 2. Sleeves labeled Type I Taper are to be used with femoral stem components labeled Type I Taper. 3. Sleeves labeled 12/14 are to be used with femoral stem components labeled 12/14 taper. 4. Use only with Ultra-High Molecular Weight Polyethylene (UHMWPE) or metal-backed UHMWPE acetabular components. 5. Do not use ceramic heads that have been dropped, rubbed, scratched, or disfigured. Blemishes can be expected to cause failure. 6. Do not use a metallic hammer when seating the ceramic head. Use a nylon or polyethylene seating instrument. Do not use excessive force. The TTPA ceramic head can fracture with excessive force. 7. The femoral stem trunion, sleeves and the bore of the ceramic head should be dry and free of contamination prior to assembly. 8. During a revision surgery, extraction of the femoral head should be done with suitable extraction instruments to avoid unnecessary damage to the trunion. 9. Biomet Biolox delta Option modular head components should not be used on trunnions with scratches or defects greater than 0.25mm in height. The surgeon should inspect the taper for damage prior to placement of the modular head components by measuring, with a measuring device, any scratches or defects, and verifying that the height is less than 0.25mm (see Figure A). The conditions shown in Figures B, C and D are also considered unsuitable for the use of the Biomet Biolox delta Option Ceramic Head and can be expected to cause failure: 10. Patient smoking may result in delayed healing, non-healing and/or compromised stability in or around the placement site. Slanted Taper - Figure C Crushed Taper - Figure D Biomet joint replacement prostheses provide the surgeon with a means of reducing pain and restoring function for many patients. While these devices are generally successful in attaining these goals, they cannot be expected to withstand the activity levels and loads of normal healthy bone and joint tissue. Accepted practices in postoperative care are important. Failure of the patient to follow postoperative care instructions involving rehabilitation can compromise the success of the procedure. The patient is to be advised of the limitation of the reconstruction and the need for protection of the implants from full load bearing until adequate fixation and healing have occurred. Excessive, unusual and/or awkward movement and/or activity, trauma, excessive weight, and obesity have been implicated with premature failure of certain implants by loosening, fracture, dislocation, subluxation and/or wear. Loosening of the implants can result in increased production of wear particles, as well as accelerate damage to bone making successful revision surgery more difficult. The patient is to be made aware and warned of general surgical risks, possible adverse effects as listed, and to follow the instructions of the treating physician including follow-up visits. PRECAUTIONS Specialized instruments are designed for Biomet joint replacement systems to aid in the accurate implantation of the prosthetic components. The use of instruments or implant components from other systems can result in inaccurate fit, sizing, and/or excessive wear and device failure. Intraoperative fracture or breaking of instruments has been reported. Surgical instruments are subject to wear with normal usage. Instruments, which have experienced extensive use or excessive force, are susceptible to fracture. Surgical instruments should only be used for their intended purpose. Biomet recommends that all instruments be regularly inspected for wear and disfigurement. Do not reuse implants. While an implant may appear undamaged, previous stress may have created imperfections that would reduce the service life of the implant. Do not treat patients with implants that have been, even momentarily, placed in a different patient. Patient selection factors to be considered include: 1) need to obtain pain relief and improve function, 2) ability and willingness of the patient to follow instructions, including control of weight and activity levels, 3) a good nutritional state of the patient, and 4) the patient must have reached full skeletal maturity. POSSIBLE ADVERSE EFFECTS 1. Material sensitivity reactions. Implantation of foreign material in tissues can result in histological reactions involving various sizes of macrophages and fibroblasts. The clinical significance of this effect is uncertain, as similar changes may occur as a precursor to or during the healing process. Particulate wear debris and discoloration from metallic and polyethylene components of joint implants may be present in adjacent tissue or fluid. It has been reported that wear debris may initiate a cellular response resulting in osteolysis or osteolysis may be a result of loosening of the implant. 2. Early or late postoperative infection and allergic reaction. 3. Intraoperative bone perforation or fracture may occur, particularly in the presence of poor bone stock caused by osteoporosis, bone defects from previous surgery, bone resorption, or while inserting the device. 4. Loosening, migration, or fracture of the implants can occur due to loss of fixation, trauma, malalignment, malposition, non-union, bone resorption, and or excessive, unusual and/or awkward movement and/or activity. The trunnion must also be free of scratches or defects greater than 0.25mm in height, free of slants, free of broad truncations, and free of crushed ends (see warning #9 for clarification). 5. Periarticular calcification or ossification, with or without impediment of joint mobility. 6. Inadequate range of motion due to improper selection or positioning of components. 7. Undesirable shortening of limb. 8. Dislocation and subluxation due to inadequate fixation, malalignment, malposition, excessive, unusual and/or awkward movement and/or activity, trauma, weight gain, or obesity. Muscle and fibrous tissue laxity can also contribute to these conditions. 9. Fretting and crevice corrosion can occur at interfaces between components. 10. Wear and/or deformation of articulating surfaces. 11. Trochanteric avulsion or non-union as a result of excess muscular tension, early weight bearing, or inadequate reattachment. The information contained in this package insert was current on the date this brochure was printed. However, the package insert may have been revised after that date. To obtain a current package insert, please contact Biomet at the contact information provided herein. 5
7 12. Problems of the knee or ankle of the affected limb or contralateral limb aggravated by leg length discrepancy, too much femoral medialization or muscle deficiencies. 13. The TTPA ceramic modular head is composed of ceramic material with limited clinical history. Although mechanical testing demonstrates that, when used with polyethylene acetabular components, ceramic balls produce a relatively low amount of particles, the total amount of particulate produced remains undetermined. Because of the limited clinical and preclinical experience, the long-term biological effects of these particulates are unknown. 14. Intraoperative and postoperative bone fracture and/or postoperative pain. 15. Ceramic head fractures have been reported. Manufacturer Symbol Legend ASSEMBLY INSTRUCTIONS 1. The modular head components must be assembled together before positioning them onto the stem. 2. Verify that the appropriate head size and matching tapers are being utilized before assembly. 3. The modular head components are assembled by placing the head onto the sleeve as shown in Figure E and Figure F. Assure that the tapers are clean and dry and that they are aligned axially before applying pressure. The tapers are engaged once resistance is felt. Date of Manufacture Do Not Reuse Caution Figure E Figure F Sterilized using Ethylene Oxide Sterilized using Irradiation Sterile Sterilized using Aseptic Processing Techniques Sterilized using Steam or Dry Heat Use By 4. Impact the modular head components onto the stem with a light tap that firmly and definitively seats the head using a plastic head impactor only. Metal impactors or any other metallic objects may scratch or crack the modular head bearing surface and, therefore, should not be used. 5. If the modular ceramic head becomes scratched or cracked, the head and sleeve must be replaced. CAUTION: In cases in which the BIOLOX OPTION system can only be put on the shaft taper using more pressure, because there are scratches or warping of more than 0.25mm, the BIOLOX OPTION system shall not be used. STERILITY Prosthetic components are sterilized by exposure to a minimum dose of 25 kgy of gamma radiation. Single Use Only. Do not resterilize. Do not use any component from an opened or damaged package. Do not use implants after expiration date. WEEE Device Catalogue Number Batch Code Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Comments regarding this device can be directed to Attn: Regulatory Dept., Biomet Inc., P.O. Box 587, Warsaw, IN USA, FAX: Flammable All trademarks herein are the property of Biomet, Inc. or its subsidiaries unless otherwise indicated. Biolox is a trademark of CERASIV GMBH The information contained in this package insert was current on the date this brochure was printed. However, the package insert may have been revised after that date. To obtain a current package insert, please contact Biomet at the contact information provided herein. 6
8 References Data on file at Biomet. Bench test results not necessarily indicative of clinical performance. BIOLOX delta is a trademark of CeramTec AG. All trademarks herein are the property of Biomet, Inc. or its subsidiaries unless otherwise indicated. This material is intended for the sole use and benefit of the Biomet sales force and physicians. It is not to be redistributed, duplicated or disclosed without the express written consent of Biomet. For product information, including indications, contraindications, warnings, precautions and potential adverse effects, see the package insert herein and Biomet s website. P.O. Box 587, Warsaw, IN x Biomet Orthopedics biomet.com Form No. BIV REV061510
BIOLOX delta Option Ceramic Femoral Head System. Product Features and Instructions for Use
 BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Product Features BIOLOX delta ceramic heads are composed of 75% aluminum oxide and approximately 25% zirconia.
BIOLOX delta Option Ceramic Femoral Head System Product Features and Instructions for Use Product Features BIOLOX delta ceramic heads are composed of 75% aluminum oxide and approximately 25% zirconia.
Positioning Sleeve Surgical Technique
 Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Active Articulation. Surgical Technique. Dual Mobility Hip System. Knees Hips Extremities Cement and Accessories PMI Trauma Technology
 Active Articulation Dual Mobility Hip System Surgical Technique Knees Hips Extremities Cement and Accessories PMI Trauma Technology Active Articulation Dual Mobility Hip System Contents Preoperative Planning,
Active Articulation Dual Mobility Hip System Surgical Technique Knees Hips Extremities Cement and Accessories PMI Trauma Technology Active Articulation Dual Mobility Hip System Contents Preoperative Planning,
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician.
 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH Mpact 3D Metal Implants and Augments 3D Metal INSTRUCTION FOR USE Important notice: the device(s) can
- October 2013 English
 TOTAL HIP JOINT REPLACEMENT FOR CEMENTED APPLICATIONS FOR THE ATTENTION OF THE OPERATING SURGEON Total hip replacement provides the surgeon with a means of restoring mobility and reducing pain with the
TOTAL HIP JOINT REPLACEMENT FOR CEMENTED APPLICATIONS FOR THE ATTENTION OF THE OPERATING SURGEON Total hip replacement provides the surgeon with a means of restoring mobility and reducing pain with the
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Description Materials Indications Patient selection factors to be considered include: Contraindications Absolute contraindications include:
 Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Taperloc Complete Hip System. Surgical Technique
 Taperloc Complete Hip System Surgical Technique One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized care to one patient. The science and art of medical
Taperloc Complete Hip System Surgical Technique One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized care to one patient. The science and art of medical
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Technique Guide Hip Resurfacing System
 Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Regenerex Primary Tibial Tray
 Cruciate Retaining and Posterior Stabilized Surgical Technique Addendum to the Vanguard Complete Knee System Knees Hips Extremities Cement and Accessories PMI Technology Contents Microplasty Knee Instrumentation
Cruciate Retaining and Posterior Stabilized Surgical Technique Addendum to the Vanguard Complete Knee System Knees Hips Extremities Cement and Accessories PMI Technology Contents Microplasty Knee Instrumentation
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Ceramic Femoral Heads and Acetabular Cup Liners
 Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
Important Information: Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution: Federal (U.S.A) law restricts this device to sale by or on the
REPIPHYSIS LIMB SALVAGE SYSTEM
 REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
REPIPHYSIS LIMB SALVAGE SYSTEM 150800-0 For additional information please contact the manufacturer or local distributor. M MicroPort Orthopedics Inc. 5677 Airline Rd Arlington, TN 38002 U.S.A. i Y October
Important notice: the device(s) can be prescribed and implanted only by a doctor legally authorized to perform this type of surgery.
 rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
rev.10 CAUTION Federal law (USA) restricts this device to sale, by or on the order of a physician. ENGLISH - EVOLIS/GMK KNEE PROSTHESIS - INSTRUCTIONS FOR USE Important notice: the device(s) can be prescribed
Important Notice to Users in the United States: General Safety Instructions
 SLR-PLUS Revision Stem, SLR-PLUS Revision Stem Lateral Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com non-sterile cemented non-
SLR-PLUS Revision Stem, SLR-PLUS Revision Stem Lateral Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com non-sterile cemented non-
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
Greater Component Accuracy...
 Greater Component Accuracy... StageOne cement spacer molds are designed to provide the surgeon with choices never before offered in two-stage revision knee surgery for an infected total joint. StageOne
Greater Component Accuracy... StageOne cement spacer molds are designed to provide the surgeon with choices never before offered in two-stage revision knee surgery for an infected total joint. StageOne
StelKast Acetabular. Surgical Protocol and Product Specifications. Including ProForm, Provident, Provident Apical Threaded Hole & Systems
 StelKast Acetabular Surgical Protocol and Product Specifications Including ProForm, Provident, Provident Apical Threaded Hole & Systems StelKast Acetabular Surgical Protocol Introduction StelKast offers
StelKast Acetabular Surgical Protocol and Product Specifications Including ProForm, Provident, Provident Apical Threaded Hole & Systems StelKast Acetabular Surgical Protocol Introduction StelKast offers
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
TriboFit Hip System. Issue 3 - August 2013 English
 Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
Important Information: Please read prior to use in a clinical setting. The surgeon should be familiar with the operative technique and all the information in this insert. Description: The TriboFit Hip
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ArComXL Polyethylene: A New Way to Process Highly Crosslinked Polyethylene
 TM ArComXL Polyethylene: A New Way to Process Highly Crosslinked Polyethylene Several years ago, our competitors introduced highly crosslinked polyethylene (HXLP). Biomet chose not to follow that path.
TM ArComXL Polyethylene: A New Way to Process Highly Crosslinked Polyethylene Several years ago, our competitors introduced highly crosslinked polyethylene (HXLP). Biomet chose not to follow that path.
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM
 CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
Important Notice to Users in the United States: General Safety Instructions
 POLARSTEM Femoral Stems with Ti/HA (Standard, Lateral, Valgus and Collar) Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com 02/2017
POLARSTEM Femoral Stems with Ti/HA (Standard, Lateral, Valgus and Collar) Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340 Baar Phone + 41 41 766 22 22 swiss.implants@smith-nephew.com 02/2017
Echo Bi-Metric. " Hips " Extremities " Cement and Accessories " PMI " Technology
 Echo Bi-Metric " Hips " Extremities " Cement and Accessories " PMI " Technology Echo Bi-Metric Figure Figure Biomets Echo Hip System Offers Three Stem Variations Forged cobalt chromium alloy Interlok gritblasted
Echo Bi-Metric " Hips " Extremities " Cement and Accessories " PMI " Technology Echo Bi-Metric Figure Figure Biomets Echo Hip System Offers Three Stem Variations Forged cobalt chromium alloy Interlok gritblasted
BIOFOAM ANKLE SPACER BLOCK
 BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
BIOFOAM ANKLE SPACER BLOCK 145782-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
Cementless Tapered Femoral Stem Surgical technique
 Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
Instructions for use for ADLER Cemented Bipolar hemi-arthroplasty Prosthesis
 Page 1 of 8 Instructions use Important Inmation on For use by an Accredited Orthopaedic Surgeon only 1. Device Description: ADLER Cemented Bipolar hemi-arthroplasty implants are used during a surgical
Page 1 of 8 Instructions use Important Inmation on For use by an Accredited Orthopaedic Surgeon only 1. Device Description: ADLER Cemented Bipolar hemi-arthroplasty implants are used during a surgical
MICROPORT KNEE SYSTEMS The following languages are included in this packet:
 EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
EN English (en) MICROPORT KNEE SYSTEMS 151251-0 The following languages are included in this packet: For additional languages, visit our website ortho.microport.com Then click on the Prescribing Information
POLARCUP Dual Mobility System. General Safety Instructions. Instructions for Use. Duty to inform the patient. Implant passport
 Important Notice to Users in the United States: This package insert is for product distributed in US only. U.S. Federal law restricts this device to sale by or on the order of a physician. General Safety
Important Notice to Users in the United States: This package insert is for product distributed in US only. U.S. Federal law restricts this device to sale by or on the order of a physician. General Safety
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
Cementless Tapered Femoral Stem Surgical technique
 Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
Cementless Tapered Femoral Stem Surgical technique Contents Operative summary 4 Pre-operative planning 5 Femoral neck osteotomy 5 Femoral canal preparation 5 Intra-medullary (IM) reamer 6 Sequential rasping
SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA
 SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340
SL-PLUS MIA Stem, SL-PLUS MIA Stem Lateral, SL-PLUS/ INTEGRATION-PLUS MIA Stem with Ti/HA, SL-PLUS/INTEGRATION-PLUS MIA Stem Lateral with Ti/HA Smith & Nephew Orthopaedics AG Oberneuhofstrasse 10d CH-6340
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
Arcos Modular Femoral Revision System
 Arcos Modular Femoral Revision System Arcos System Simplify the Complex The Arcos Modular Femoral Revision System meets the demands of complex hip revision surgery by offering surgeons and OR staff the
Arcos Modular Femoral Revision System Arcos System Simplify the Complex The Arcos Modular Femoral Revision System meets the demands of complex hip revision surgery by offering surgeons and OR staff the
UTF Stem. reduced. Surgical Protocol
 UTF Stem TM reduced Surgical Protocol Table of Contents Surgical Protocol Device... Preoperative Planning and Templating... Femoral Osteotomy... Femoral Canal Accessing... Canal Reaming... Canal Broaching...
UTF Stem TM reduced Surgical Protocol Table of Contents Surgical Protocol Device... Preoperative Planning and Templating... Femoral Osteotomy... Femoral Canal Accessing... Canal Reaming... Canal Broaching...
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
CMA TM Cemented Modular Augmentable. Baseplate. 2 Knee
 CMA TM Cemented Modular Augmentable Baseplate 2 Knee More can be addressed The U2 Cemented Modular Augmentable Baseplate is an optimal solution for tibial bone defect in primary total knee replacement.
CMA TM Cemented Modular Augmentable Baseplate 2 Knee More can be addressed The U2 Cemented Modular Augmentable Baseplate is an optimal solution for tibial bone defect in primary total knee replacement.
Enhanced Stability Constrained Liners. Design Rationale Surgical Technique
 Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Echo. Femoral Hip System. Hip Fracture Surgical Technique. Endo II Uni-polar Acetabular Component RingLoc Bi-polar Acetabular Component
 Echo Femoral Hip System Hip Fracture Surgical Technique Endo II Uni-polar Acetabular Component RingLoc Bi-polar Acetabular Component One Surgeon. One Patient. Over 1 million times per year, Biomet helps
Echo Femoral Hip System Hip Fracture Surgical Technique Endo II Uni-polar Acetabular Component RingLoc Bi-polar Acetabular Component One Surgeon. One Patient. Over 1 million times per year, Biomet helps
Modular Calcar Revision System
 Modular Calcar Revision System Engineered for long term stability, pain reduction, and optimal fixation for revision cases The Mallory/Head modular revision system offers stability at the time of surgery,
Modular Calcar Revision System Engineered for long term stability, pain reduction, and optimal fixation for revision cases The Mallory/Head modular revision system offers stability at the time of surgery,
For use by an Accredited Orthopaedic Surgeon only
 Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
Instructions use Page 1 of 6 For use by an Accredited Orthopaedic Surgeon only 1. DEVICE DESCRIPTION: The advancement of partial and total hip replacement has provided surgeons with the means of restoring
INVISION TOTAL ANKLE REVISION SYSTEM The following languages are included in this packet:
 INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
INVISION TOTAL ANKLE REVISION SYSTEM 151678-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 -
Furlong H-A.C. THR System
 Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
INBONE II. Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION
 INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT TIBIAL REAMING SPECIFICATION INBONE II Total Ankle System SURGICAL TECHNIQUE SUPPLEMENT Tibial Reaming Specification Contents Chapter 1 4 Product
Surgical Protocol written by Keith Lawhorn, M.D.
 Surgical Protocol written by Keith Lawhorn, M.D. The MaxFire Meniscal Repair System is an all-inside, all-suture meniscal repair system utilizing ZipLoop Technology. Anchors are preloaded on needle inserters,
Surgical Protocol written by Keith Lawhorn, M.D. The MaxFire Meniscal Repair System is an all-inside, all-suture meniscal repair system utilizing ZipLoop Technology. Anchors are preloaded on needle inserters,
CAUTION: Ceramic liners are not approved for use in the United States.
 Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS
 SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
TOTAL ELBOW SYSTEM The following languages are included in this packet:
 TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
TOTAL ELBOW SYSTEM 115298-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
CONSENSUS ORTHOPEDICS INC.
 CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. CONSENSUS HIP SYSTEMS Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
Instructions for use for Adler Cemented Total Hip Replacement Prosthesis
 0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
0434 Page 1 of 5 Instructions use Important Inmation on Adler Cemented Femoral Hip Stems and Modular Heads For use by an Accredited Orthopaedic Surgeon only DEVICE DESCRIPTION General Inmation The advancement
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Optimizing function Maximizing survivorship Accelerating recovery
 Surgical Technique Optimizing Function Maximizing Survivorship Accelerating Recovery The company believes in an approach to patient treatment that places equal importance on: Optimizing function Maximizing
Surgical Technique Optimizing Function Maximizing Survivorship Accelerating Recovery The company believes in an approach to patient treatment that places equal importance on: Optimizing function Maximizing
Revision. Hip Stem. Surgical Protocol
 U2 TM Revision Hip Stem Surgical Protocol U2 Revision Hip Stem Table of Contents Introduction... 1 Preoperative Planning... 2 Femoral Preparation... 3 Trial Reduction... 5 Implant Insertion... 6 Ordering
U2 TM Revision Hip Stem Surgical Protocol U2 Revision Hip Stem Table of Contents Introduction... 1 Preoperative Planning... 2 Femoral Preparation... 3 Trial Reduction... 5 Implant Insertion... 6 Ordering
TaperFill. Surgical Technique
 TaperFill Surgical Technique Table of Contents Indications and Contraindications 3 TaperFill Hip Size Charts 4-5 DJO Surgical 9800 Metric Boulevard Austin, TX (800) 456-8696 www.djosurgical.com Preoperative
TaperFill Surgical Technique Table of Contents Indications and Contraindications 3 TaperFill Hip Size Charts 4-5 DJO Surgical 9800 Metric Boulevard Austin, TX (800) 456-8696 www.djosurgical.com Preoperative
Dual Mobility System Evaluation surgical technique
 Trinity Dual Mobility System Evaluation surgical technique Contents Operative summary 4 Overview 5 Operative technique 6 1. Acetabular reaming 6 Reamer guide 6 2. Acetabular shell trial 6 3. Acetabular
Trinity Dual Mobility System Evaluation surgical technique Contents Operative summary 4 Overview 5 Operative technique 6 1. Acetabular reaming 6 Reamer guide 6 2. Acetabular shell trial 6 3. Acetabular
TM UCP STEM Surgical Protocol
 UCP TM STEM Surgical Protocol Table of Contents Surgical Protocol Device... Preoperative Planning and Templating... Femoral Osteotomy... Femoral Canal Accessing... Canal Broaching... Trial Reduction...
UCP TM STEM Surgical Protocol Table of Contents Surgical Protocol Device... Preoperative Planning and Templating... Femoral Osteotomy... Femoral Canal Accessing... Canal Broaching... Trial Reduction...
THE NATURAL FIT. Surgical Technique. Hip Knee Spine Navigation
 THE NATURAL FIT Surgical Technique Hip Knee Spine Navigation MiniMAX Surgical Technique Hip Knee Spine Navigation INTRODUCTION The MiniMAX TM is a cementless anatomic stem available in 9 right sizes and
THE NATURAL FIT Surgical Technique Hip Knee Spine Navigation MiniMAX Surgical Technique Hip Knee Spine Navigation INTRODUCTION The MiniMAX TM is a cementless anatomic stem available in 9 right sizes and
Comprehensive Reverse Shoulder System
 Comprehensive Reverse Shoulder System Comprehensive Reverse Shoulder System Simple. Versatile. The Comprehensive Reverse Shoulder System is the next generation reverse shoulder prosthesis, offering unmatched
Comprehensive Reverse Shoulder System Comprehensive Reverse Shoulder System Simple. Versatile. The Comprehensive Reverse Shoulder System is the next generation reverse shoulder prosthesis, offering unmatched
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Dual Mobility System Surgical technique
 Trinity Dual Mobility System Surgical technique 2 Contents Operative summary 4 Overview 5 Operative technique 6 1. Acetabular reaming 6 Reamer guide 6 2. Acetabular shell trial 7 3. Acetabular shell implantation
Trinity Dual Mobility System Surgical technique 2 Contents Operative summary 4 Overview 5 Operative technique 6 1. Acetabular reaming 6 Reamer guide 6 2. Acetabular shell trial 7 3. Acetabular shell implantation
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
The short stem hip system Surgical Technique
 The short stem hip system Surgical Technique The short stem hip system cementless The Aida hip system was developed in co-operation with the Aida inventors group, founded by surgeons of 10 surgical department
The short stem hip system Surgical Technique The short stem hip system cementless The Aida hip system was developed in co-operation with the Aida inventors group, founded by surgeons of 10 surgical department
comprehensive Design Rationale shoulder system Knees Hips Extremities Cement and Accessories PMI TEchnology
 comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Peanut Growth Control Plating System
 Surgical Technique Peanut Growth Control Plating System An Innovative Approach To Hemi-Epiphysiodesis Provides controlled growth of healthy epiphysis, restoring proper anatomic alignment Advanced instrumentation
Surgical Technique Peanut Growth Control Plating System An Innovative Approach To Hemi-Epiphysiodesis Provides controlled growth of healthy epiphysis, restoring proper anatomic alignment Advanced instrumentation
CC TRIO VERSAFITCUP. Surgical Technique. each to their own. Hip Knee Spine Navigation
 VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
VERSAFITCUP CC TRIO each to their own Surgical Technique Hip Knee Spine Navigation Versafitcup CC TRIO Surgical Technique Hip Knee Spine Navigation EACH TO THEIR OWN The Versafitcup CC Trio is a range
CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM
 CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR WITH THE SURGICAL TECHNIQUE.
CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR WITH THE SURGICAL TECHNIQUE.
S H O U L D E R Solutions by Tornier. Aequalistm. Pyrocarbon Humeral Head. surgical technique
 S H O U L D E R Solutions by Tornier Aequalis TM Pyrocarbon humeral head SURGICAL TECHNIQUE Aequalistm Pyrocarbon Humeral Head surgical technique Aequalis TM Pyrocarbon humeral head surgical TECHNIQUE
S H O U L D E R Solutions by Tornier Aequalis TM Pyrocarbon humeral head SURGICAL TECHNIQUE Aequalistm Pyrocarbon Humeral Head surgical technique Aequalis TM Pyrocarbon humeral head surgical TECHNIQUE
HELIOS h i p s y s t e m
 HELIOS h i p s y s t e m Design The Helios stem is a highly polished, High Nitrogen Stainless Steel (ISO5832-9) dual tapered cemented stem. The design of the stem is based on the clinically lly successful
HELIOS h i p s y s t e m Design The Helios stem is a highly polished, High Nitrogen Stainless Steel (ISO5832-9) dual tapered cemented stem. The design of the stem is based on the clinically lly successful
Total Wrist System. Surgical TEchniquE Maestro. Surgical Technique. Optional additional Descriptor Optional additional Descriptor
 Surgical TEchniquE Maestro Total Wrist System Surgical Technique Optional additional Descriptor Optional additional Descriptor Knees Hips Extremities Cement and Accessories PMI Technology Maestro Total
Surgical TEchniquE Maestro Total Wrist System Surgical Technique Optional additional Descriptor Optional additional Descriptor Knees Hips Extremities Cement and Accessories PMI Technology Maestro Total
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation. Surgical Technique
 JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
JuggerLoc Bone-to-Bone System for Ankle Syndesmosis Fixation Surgical Technique Table of Contents Position and Preparation... 2 Incision... 2 Fracture Reduction... 2 Drill Fibula and Tibia... 3 JuggerLoc
EVOLVING OUR HERITAGE, MEETING YOUR NEEDS. Surgical Technique
 EVOLVING OUR HERITAGE, MEETING YOUR NEEDS Surgical Technique Joint Spine Sports Med Mpact DM Surgical Technique Joint Spine Sports Med INTRODUCTION The Mpact DM is part of the Mpact Acetabular System and
EVOLVING OUR HERITAGE, MEETING YOUR NEEDS Surgical Technique Joint Spine Sports Med Mpact DM Surgical Technique Joint Spine Sports Med INTRODUCTION The Mpact DM is part of the Mpact Acetabular System and
BiMobile Dual Mobility System Cementless & Cemented. Surgical Technique
 BiMobile Dual Mobility System Cementless & Cemented Surgical Technique Presented by: Waldemar Link GmbH & Co. KG Barkhausenweg 10 22339 Hamburg Germany Phone +49 40 53995-0 info@linkhh.de www.linkorthopaedics.com
BiMobile Dual Mobility System Cementless & Cemented Surgical Technique Presented by: Waldemar Link GmbH & Co. KG Barkhausenweg 10 22339 Hamburg Germany Phone +49 40 53995-0 info@linkhh.de www.linkorthopaedics.com
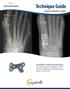
Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis?
![Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis?](/thumbs/78/78456133.jpg) Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Diagnosis: Ceramic head fracture In the 1970 s, Boutin implemented ceramic in modern total hip arthroplasty (THA).
Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Diagnosis: Ceramic head fracture In the 1970 s, Boutin implemented ceramic in modern total hip arthroplasty (THA).
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
Instructions for use Prodisc -C Vivo Cervical disc prosthesis
 Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
Instructions for use Prodisc -C Vivo Cervical disc prosthesis Instructions for use Prodisc-C Vivo Cervical disc prosthesis Safety precautions Please read these instructions for use, the Synthes brochure
