Warranties for Hip and Knee Implants Consumers Union Safe Patient Project September 9, 2013
|
|
|
- Brent Washington
- 6 years ago
- Views:
Transcription
1 Warranties for Hip and Knee Implants Consumers Union Safe Patient Project September 9, 2013 Millions of Americans now have hip and knee implants, and thanks to aggressive marketing to younger consumers and aging Baby Boomers, demand for these surgeries will only grow. In 2011, doctors performed more than 711,000 knee replacement/reconstructions along with 464,000 hip replacements (total and partial). 1 Demand for hip and knee replacement surgery could reach 4 million a year by 2030, and more than 50% of patients will be under age Why does that matter? Because younger people will need their implants to last a long time, but the vast majority of knee and hip implants do not come with a manufacturers warranty. 3 For other kinds of products, including other types of implants, the warranty specifies the length of time the manufacturer guarantees the item will last and gives consumers a process to address defects when they occur. 4 According to recall data from the Food and Drug Administration (FDA), over the last ten years every major manufacturer of knee and hip implants has voluntarily recalled a product, or line of products, due to defects. Problems range from having the wrong size etched on the device to post-operative fracturing to early wear and delamination. 5 Some designs proved to be fundamentally unsafe: entire lines of metal on metal hips have been recalled after thousands of patients had to have them removed. 6 Most hip and knee implants are exempted from safety testing before they are put on the U.S. market. Only1% of medical devices are approved through the more rigorous Premarket Approval Process which requires safety testing. 7 Unlike Australia, there is no national U.S. registry that tracks which implants fail or a system to notify the patients who have them. As consumers line up for the promise to feel better, play longer on their new joints, they are buying into a loosely regulated marketplace that can leave them holding the bag for costly and painful revision surgery when these implants fail or break. When implants go bad, the cost of additional surgery to remove and replace the implant is now largely borne by patients (and their insurance companies or Medicare). Revision surgery costs more, is surgically demanding, results in longer hospital stays and often leads to additional revision surgeries. 8 When patients outlive their implants, revision surgery is also required and they should have solid information about the expected lifespan of the device when making their initial decision to get a knee or hip replacement. Since manufacturers are not required to report what percent of their products fail or even how often it happens, it s impossible to calculate the overall cost to Medicare, insurance companies or patients when implants turn out to be defective. It is estimated that nearly 18% of hip replacement and 8% of knee replacement surgeries performed in the U.S. are for revisions. 9 The costs for revision procedures is likely in the hundreds of millions of dollars per year based on the huge volume of revisions performed. 10
2 Manufacturers should bear their fair share of this cost, and assist consumers when their products fail. Warranties would do that. Consumers Union calls on the manufacturers of knee and hip implants sold in the United States to back their products with a 20-year warranty. A warranty will communicate to patients how long devices can be expected to last [ this is officially how long we will stand by our product ] and provide a straightforward process to replace a defective product with as little hassle and cost as possible. Transparency and clear lines of accountability will ultimately move companies to reduce defects and improve quality because it will be in their own financial best interest to do so. Loopholes prevent FDA from adequately protecting consumers against defective implants Unlike prescription drugs, which must be tested to demonstrate they are safe and effective before they are put on the market, most joint replacement products are not required to undergo rigorous pre-market testing. Medical devices like hip and knee implants do not have to be reviewed for safety by the FDA if they are similar to a product already on the market. Since most design changes are incremental, nearly every hip and knee currently being installed in patients is similar to something that came before it, and therefore device makers gain approval of their new implants through what is known as the FDA s 510(k) clearance process (based on its substantial equivalence to a device already on the market). Under this section of federal law, implant makers do not need to provide evidence from clinical trials to demonstrate a product s safety and effectiveness, as required by the FDA s more rigorous premarket approval or PMA process 11 FDA s premarket approval process, while rarely used, does appear to improve implant safety. The GAO surveyed device recalls from Among all classes of devices that were eventually recalled, 87% had been approved through the 510(k) process, while only about 8% of the recalled devices went to market after the more rigorous Premarket Approval process. 12 Instead of pre-market safety testing, the FDA primarily relies on post-market reporting to Medwatch, a system for physicians and patients to report problems with implants. When enough problems or signals are reported, FDA may send out notice to the companies and the companies can choose to voluntarily recall their products. This means virtually every hip and knee implant patient is an unwitting participant in a giant, post market test to see which products are actually safe and which ones turn out to harmful or ineffective. While FDA cannot require clinical safety testing for any product that is substantially equivalent to another implant, a 2012 amendment to federal law created a more streamlined process for the FDA to require clinical testing. 13 In January 2013, the FDA proposed a reclassification of metal-on-metal hips, which, when finalized, would require more rigorous testing and reporting before the design could be marketed again. 14 Defects happen We know that hip and knee products can be defective - some have failed spectacularly, while defects limited to certain lots or components can also have serious implications for patients. Imagine you just opened the box of a complicated piece of build-it-yourself furniture. You carefully lay out all the parts, you pull out the custom tools helpfully provided, and you read all the instructions until you feel you understand the steps. You start building it only to find, when 2
3 you are well into the project, that some of the screws are the wrong size. Now imagine this is your surgeon, and the item under construction is your knee, and you are in the operating room under anesthetic. Literally hundreds of knee components have been recalled since 2003, 15 frequently because they ship with the wrong part, a wrong size part, a missing part, or a part built for the left side but etched as a right (or vice versa). Since most parts must be opened immediately before surgery to keep them sterile, these kinds of errors have serious implications for patients. Reasons for product recalls straight from the FDA s own database include the following packaging issues (quoted verbatim): Labeling Discrepancy -- Mislabeled and mis-etched as to size. Inserts were labeled and packaged as size Standard; 12.5mm Inserts but were actually a 10mm Inserts. Risks include but not limited to: a delay in the procedure while the proper component is located; and tight joint with constricted movement as a result of implantation of the wrong thickness of insert. The product contained screws with the incorrect length (incorrectly contained 15 mm length screws instead of the correct 5 mm length screws). Mislabeled--The product is not collared; although the label states that it is collared. A non-collared component will provide a reduced contact with bone interface and/or a delay in the procedure may be incurred while clarifying package contents. The firm is initiating a removal of one lot of the Bigliani/Flatow Humeral Provisional Stem ( ; lot ) as the stems manufactured under the lot are 14mm x 170mm devices incorrectly etched and packaged as 12mm x 170mm devices. A box labeled as a Scorpio NRG Femoral Size 7 Right; may actually contain a Scorpio PS Femoral Size 5 Right. Zimmer had determined that two lots of these tibial implants have incorrectly positioned or missing flange plugs that were not seated in the device upon receipt to the customer. A missing flange plug could lead to a delay of surgery or possibility of wear leading to more surgery. And those specialized tools included in the product to facilitate installation are sometimes a problem as well. The instrument may have been assembled improperly; which may result in reversed resection cuts on the femur and affect implant performance; resulting in the need for revision surgery. Triathlon Offset Adaptors may seize during surgery and the OR staff may be unable to disassemble the instrument using the removal tool. The standard Triathlon Femoral Stylus does not fit into the Specialty Sizers and the "R" and "L" markings on the Right Sizer are reversed. The instrument's sizing line is in the wrong place. Of course, some knee components have been recalled because they simply weren t manufactured to spec or started to fail once installed in people. Fracture of SPIRALOK Anchors post op; requiring patient revision 3
4 The firm has received complaints of loosening of the implanted device requiring revision surgery. There have been 114 MDRs filed all reported that the device loosened and the patient required additional surgery to replace the device. The device was cut to an incorrect angle; which may result in an incorrect bone cut. Raw material supplied by a third party used to manufacture various medical devices does not comply with metallurgical requirements outlines in ASTM standard for titanium surgical implants. An impurity in the metal may affect the strength of the screw; resulting in breakage and/or surgical delays. Hip implant devices, tools and components have been recalled for similar reasons, but the story of the metal-on-metal hip is an abject lesson in the failings of our current oversight systems. Metal on metal hip implants, where the ball and socket of the hip joint are both made of metal, were a popular innovation because they promised to last longer than other devices made with ceramic and plastic. Because the basic design was similar to previous hip replacement designs, the FDA allowed the new product to be fast tracked to the market without safety testing. 16 It was popular: an estimated 755,000 Americans got these hips over the past decade. 17 Some patients with the new hips started to report swelling at the site, along with unexpected symptoms like rash, cardiomyopathy, neurological problems, cognitive impairments, and more. It turned out that when metal rubbed against metal, chromium and cobalt ions were released from the surface, first into the hip itself and later into the bloodstream, causing these and other health problems. The metal being released into the nearby tissue sometimes caused damage to surrounding bone, tissue, muscles and nerves, making it difficult to replace the metal hip. 18 Starting in 2004 and 2005, Johnson & Johnson introduced DePuy ASR and ASR XL metal on metal hips. As early as 2007, the Australian Joint Registry recorded unusually high revision rates for the DePuy ASR. DePuy stopped selling the ASR in Australia in The National Joint Registry of England and Wales identified similarly high revision rates. 19 Even as scientific evidence mounted, patients in the U.S. continued to get all-metal hips implanted. In August of 2010, DePuy recalled all 93,000 ASR XL hips worldwide after it became clear that the device was failing far more often than average and producing serious injuries. While it s unclear how many people actually had to have their artificial hip removed, an article in the British Medical Journal called it one of the biggest disasters in orthopaedic history. 20 Australian regulators spotted trouble with these hips early, in part because the country has a national device registry. The U.S. does not. Because the U.S. lacks a national device registry, U.S. doctors and patients don t learn about a recall in a systematic way and patients may not know to revisit their doctor to have their implant checked. The Australian Joint Replacement Registry allows officials to easily notify doctors about a recalled device, who can then notify their patients. 21 The societal implications of failing knees and hips 4
5 Years ago, when implant patients were most often elders, the longevity of a new knee or hip may have mattered less than the immediate relief it provided from pain and diminished mobility. Today, people under the age of 65 routinely undergo hip or knee replacement, and they will likely remain active for 20 or 30 more years after their surgery. And, many people over 65 tend to be more active than in the past and are living longer. Implant advertising is geared toward younger and athletic older audiences, as aging sports stars like former tennis player Billy Jean King promote knee replacements as a way to extend an active life. Researchers estimate that 50 percent of hip replacement patients are already under the age of 65, and soon the same will be true for patients with knee replacements. 22 Younger customers mean bigger business and the hip and knee industry is big already. In 2012 worldwide revenue for hips reached $5.8 billion and for knees another $6.9 billion. Yearly, 1.4 million hip replacements and 1.1 million knee replacements are performed worldwide. 23 In the near future, Medicare hip and knee replacements could make up a significant percentage of all Medicare spending. Hospitals and surgeons are currently exploring cost control methods such as bundling all costs associated with a hip or knee replacement. The cost of a hip or knee implant can be as much as 50 percent of Medicare payment. 24 Younger patients are more likely to need revision surgery in their lifetimes simply because they are living longer with an implant. In addition to the increased cost, revision surgery is often more complicated than the original procedure. A patient cannot endure multiple revisions as more tissue and bone are destroyed with each surgery. According to one study, revision surgery resulted in more infections, longer hospital stays, and higher costs for the surgery. 25 A warranty would help make implants safer and spread costs more fairly A strong warranty that gives consumers reasonable compensation when a joint fails will improve consumers experience and encourage companies to make their products safer and more durable. Manufacturers should have some skin in the game by guaranteeing that their products will last for at least 20 years, and not expect patients, insurers or taxpayer-supported health programs to pick up the cost for failed devices. As these products are marketed to younger and younger consumers, potential patients need information about the lifespan of their implant and a guarantee to back up company promises. For most products on the market today, a manufacturer s warranty sets out how long the buyer can expect the product to work. If the product doesn t work or breaks during that time period, the consumer can request repair or replacement under the terms of the warranty. For hip and knee replacements, consumers expect the product to last 20 years, or even a lifetime. Of the people who responded to our detailed questions about their joint replacement, about two-thirds thought a 20 year or lifetime warranty was appropriate. 26 Hip and knee manufacturers claim this kind of system can t work for them, because problems people experience may not be due to product failure. Perhaps the patient gained weight, fell on the implant, or otherwise damaged it due to inappropriate activity; perhaps the failure was due to a surgical infection. 27 These debates are not new to any product on the market -- installers can make mistakes, consumers can drop appliances or use their vehicle inappropriately. Any good 5
6 warranty creates a procedure for resolving such disputes and getting consumers a replacement product with as little hassle and cost as possible. Currently, there is only one implant manufacturer that provides any kind of warranty at all. The Biomet Oxford Partial Knee comes with a lifetime warranty. Biomet pays the cost of a replacement implant one time (parts) but does not include costs associated with replacement surgery (labor). Consumers Union believes this warranty is inadequate because it doesn t cover the associated costs to replace the implant. When a customer triggers an appliance warranty, or takes a car back to the dealership for a warranty repair, the warranty covers parts and labor because the labor associated with replacement is part of the cost of failure. A good knee or hip warranty should do the same. Consumers Union believes a standard warranty should come with every knee and hip device. The warranty should be given to patients as part of the implant procedure and should meet the following basic requirements: Covers the implant for at least 20 years Covers the full costs of replacing the flawed device, including the device, surgeon and hospital costs as well as the related patient out of pocket costs. Does not require the failed device be replaced with the same product or a product from the original device maker if the product has been recalled by FDA or the company, is the subject of FDA warnings, is under investigation by the FDA or if the product is no longer being sold by the company. Establishes a clear system for patients to use, including a toll-free number and a registration number to track the claims process, with physicians charging the device company, not the patient. Does not require the patient pay out-of-pocket expenses; for example, the patient should not have to pay the device maker or surgeon first and get reimbursed later. Provides the patient with full information concerning a warranty claim denial and provides a process to allow the patient to appeal the decision. Does not limit or eliminate a patient s right to sue if they use the warranty. Does not disqualify patients across the board because they have specific diseases or illnesses that are not related to the failure of a device. Does not disqualify patients for normal activities, including falls. Does not disqualify patients due to information that is not routinely available to them, such as information that is on the device packaging or placed into their medical records and not routinely provided directly to the patients in the course of getting the implant. Patients do not waive the right to sue if they use the warranty. For more information contact: Suzanne Henry 506 West 14 th. Street, Ste.A Austin, TX
7 1 Agency for Healthcare Quality Research, Healthcare cost and Utilization Project (HCUP): Outcomes for CCS principle category Arthroplasty of knee and hip replacements, total and partial, 2011 weighted national estimates from the HCUP Nationwide Inpatient Sample (NIS); 2 Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ., Future Young Patient Demand for Primary and Revision Joint Replacement. Clinical Orthopedic Related Research, 2009 Oct; 467(10): doi: /s Epub 2009 Apr 10. Also, Kurtz SM, Ong K, Lau E, Mowat F, Halpern M, Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030, Journal of Bone and Joint Surgery Am., April 2007; 89(4): Consumers Union talked to representatives of the top six sellers of hip and knee implants and found only one offered a warranty on a hip or knee product: Biomet s Oxford Partial Knee Implant; 4 Examples of warranties for St. Jude s implantable products ( Biotronik ( ) and Boston Scientific Cardiac Pulse Generators ( and Medtronic diabetes products ( 5 Consumers Union review of 10 years of hip and knee recalls from the FDA recall database, 2003 to 2013, accessed on 7/23/13 and 7/24/13 respectively. ( 6 Cohen, D., Out of Joint: The story of the ASR; British Medical Journal;342:d2905, May US Government Accountability Office, FDA Should Take steps to ensure that high risk device types are approved through the most stringent premarket review process, GAO : January Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT, Risk of subsequent revision after primary and revision total joint arthroplasty, Clinical Orthopedic Related Research,, Nov 2010; 468(11): Also,Vincent KR, Vincent HK, Lee LW, Weng J, Alfano AP, Outcomes after inpatient rehabilitation of primary and revision total hip arthroplasty. Arch Phys Med Rehabil. August 2006; 87: Also, Lavernia C, Lee D, Hernandez V, The increasing financial burden of knee revision surgery in the United States, Clinical Orthopedic and Related Research, May 2006: 446, pp Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002, J Bone Joint Surg Am. July 2005; 87(7): Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Mobility/Orthopedic Procedures 2011 to HCUP Projections Report # ONLINE September 20, U.S. Agency for Healthcare Research and Quality, Available: Estimated US hip revision incidence for all adults: 12,000 at the end of 2012, p. 47. Average hospital cost for all adults for hip replacement revision surgery estimated at $24,500 at the end of 2012, p. 51. Estimated total cost of hip replacement revisions: $294 million at the end of Estimated US knee revision incidence for all adults, 15,500 at the end of 2012, p. 75. Average hospital cost for all adults for knee replacement revision surgery estimated at $25,000 at the end of 2012, p. 79. Estimated total cost of knee replacement revisions, $387 million CFR 807 Subpart E ; Premarket Notification 510(k); 12 Government Accountability Office (GAO); June 2011; FDA Should Enhance its Oversight of Recalls; 13 Food and Drug Administration Safety and Innovation Act, Public Law , Sec. 608, pp , 112th Congress, July 9, 2012; 14 Proposed Order for the reclassification of metal-on-metal hip joint to require filing of a premarket approval application (PMA); Jan. 18, FDA recall database on knee replacements, 2003 to accessed on [FIND DATE AND CONFIRM RANGE OF DATES FOR THIS AND FOOTNOTE 5 ABOVE] 16 Ardaugh B.; Graves S.; Redberg R; The 510(k) Ancestry of a Metal-on-Metal Hip Implant; New England Journal of Medicine, Jan. 10, 2013, 368;2, pp Consumer Reports, FDA cracks down on all-metal hip replacements; Jan. 18, Food and Drug Administration, Concerns about Metal-on Metal Hip Implants; Jan. 17, 2013; 19 Australian Government, Department of Health and Ageing, Therapeutic Goods Administration, Recall of DePuy Orthopaedics ASR hip, May 16, 2011, Also, National Joint Registry for England and Wales, 7th Annual Report, 2010, p Cohen, D., Out of Joint: The story of the ASR; British Medical Journal;342:d2905, May Also, Consumer Reports, Dangerous Devices; May, Australian Orthopaedic Association National Joint Replacement Registry, Patient Information; 22 Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ, Future Young Patient Demand for Primary and Revision Joint Replacement, Clin Orthop Relat Res Oct; 467(10): doi: /s Epub 2009 Apr Orthoknow, Orthopedic Industry Annual Report 2012; June Full report available at 24 Rikkers, Laing, A Surgeon's Look at Costs In Total Joint Arthroplasty, Orthopedic and Dental Industry News, March 31, 2006; 25 Ong KL, Lau E, Suggs J, Kurtz SM, Manley MT, Risk of subsequent revision after primary and revision total joint arthroplasty, Clinical Orthopedic Related Research,, Nov 2010; 468(11): Of the more than 700 people who have experienced a hip or knee replacement and responded to Consumers Union s detailed questionnaire about warranties with us, 232 supported the idea of a 20 year warranty and 197 supported a lifetime warranty. This was not a scientific survey. 27 Weyman, Andy, MD; Chief Medical Officer; Smith & Nephew; letter to Consumers Union in response to request to warranty their hip and knee implant products; July,
8 8
A Summary of Knee Recalls Consumers Union Safe Patient Project September 9, 2013
 A Summary of Knee Recalls Consumers Union Safe Patient Project September 9, 213 An estimated 4.4 million Americans are living with knee implants. In 211, over 711, 1 knee replacements were performed primarily
A Summary of Knee Recalls Consumers Union Safe Patient Project September 9, 213 An estimated 4.4 million Americans are living with knee implants. In 211, over 711, 1 knee replacements were performed primarily
Comments of the Patient, Consumer, and Public Health Coalition. Strengthening the Center for Devices and Radiological Health s 510(k) Review Process
 March 19, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Comments of the Patient, Consumer, and Public Health Coalition on Strengthening
March 19, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Comments of the Patient, Consumer, and Public Health Coalition on Strengthening
FDA Safety Communication: Metal-on- Metal Hip Implants
 FDA Safety Communication: Metal-on- Metal Hip Implants Date Issued: Jan. 17, 2013 Audience: Orthopaedic surgeons Health care providers responsible for the ongoing care of patients with metal-onmetal hip
FDA Safety Communication: Metal-on- Metal Hip Implants Date Issued: Jan. 17, 2013 Audience: Orthopaedic surgeons Health care providers responsible for the ongoing care of patients with metal-onmetal hip
No two knees are alike
 No two knees are alike That s why we custom-fit your surgery just for you Custom-Fit Surgery: The right fit for nearly every knee It s not surprising. Research proves that differences in bone shape influence
No two knees are alike That s why we custom-fit your surgery just for you Custom-Fit Surgery: The right fit for nearly every knee It s not surprising. Research proves that differences in bone shape influence
Table of Contents: JULY TH EDITION
 Table of Contents: Table of Contents of the worldwide Orthopaedic Contract manufacturing Market 2016-2021 & Top 100 player profiles 275+ pages 450+ exhibits July 2017 5 th edition I- Worldwide orthopaedic
Table of Contents: Table of Contents of the worldwide Orthopaedic Contract manufacturing Market 2016-2021 & Top 100 player profiles 275+ pages 450+ exhibits July 2017 5 th edition I- Worldwide orthopaedic
Preparing a US FDA Medical Device 510(K) Submission
 Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
What Are Hip and Knee Replacement Implants Made Of?
 What Are Hip and Knee Replacement Implants Made Of? Hip and knee replacement surgery involve replacing the worn-out bone and cartilage lining your hip or knee joint with new implants that are composed
What Are Hip and Knee Replacement Implants Made Of? Hip and knee replacement surgery involve replacing the worn-out bone and cartilage lining your hip or knee joint with new implants that are composed
Total Hip Replacement. Find out why Total Hip Replacement may be right for you.
 Total Hip Replacement Find out why Total Hip Replacement may be right for you. UNDERSTANDING TOTAL HIP REPLACEMENT This brochure offers a brief overview of hip anatomy, arthritis and total hip arthroplasty.
Total Hip Replacement Find out why Total Hip Replacement may be right for you. UNDERSTANDING TOTAL HIP REPLACEMENT This brochure offers a brief overview of hip anatomy, arthritis and total hip arthroplasty.
WHAT IS THE BEST BONE FIXATION TYPE? 2/11/2011
 WHAT IS THE BEST BONE FIXATION TYPE? 2/11/2011 A Comparison of cement vs. bone ingrowth. Thomas P. Gross, M.D. At 2 years of follow-up cemented and uncemented femoral resurfacing is equivalent. Femoral
WHAT IS THE BEST BONE FIXATION TYPE? 2/11/2011 A Comparison of cement vs. bone ingrowth. Thomas P. Gross, M.D. At 2 years of follow-up cemented and uncemented femoral resurfacing is equivalent. Femoral
GENESIS II/ LEGION Total Knee System
 GENESIS II/ LEGION Total Knee System The GENESIS II/LEGION knee with the VERILAST technology The knee tested to simulate 30 years of wear performance DISCLAIMER: Always speak to your doctor about the options
GENESIS II/ LEGION Total Knee System The GENESIS II/LEGION knee with the VERILAST technology The knee tested to simulate 30 years of wear performance DISCLAIMER: Always speak to your doctor about the options
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
YOUR TOTAL HIP REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE
 YOUR TOTAL HIP REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE IMPORTANT. PLEASE NOTE. This brochure offers a brief overview of hip anatomy, arthritis and hip replacement surgery. The
YOUR TOTAL HIP REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE IMPORTANT. PLEASE NOTE. This brochure offers a brief overview of hip anatomy, arthritis and hip replacement surgery. The
Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide,
 Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Robots: Executive Summary The study is designed
Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Robots: Executive Summary The study is designed
YOUR TOTAL HIP REPLACEMENT SURGERY
 YOUR TOTAL HIP REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE Exactech_030H Rev A_Total Hip Replacement Surgery_PRINT.indd 1 IMPORTANT. PLEASE NOTE. This brochure offers a brief overview
YOUR TOTAL HIP REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE Exactech_030H Rev A_Total Hip Replacement Surgery_PRINT.indd 1 IMPORTANT. PLEASE NOTE. This brochure offers a brief overview
Bad Bearings: THE DEVOLUTION OF HIP REPLACEMENT IN AMERICA
 Bad Bearings: THE DEVOLUTION OF HIP REPLACEMENT IN AMERICA 1970-2014 Stephen S. Tower, M.D. Orthopedic Surgeon Affiliated Professor University of Alaska President of the Alaska Arthroplasty Initiative
Bad Bearings: THE DEVOLUTION OF HIP REPLACEMENT IN AMERICA 1970-2014 Stephen S. Tower, M.D. Orthopedic Surgeon Affiliated Professor University of Alaska President of the Alaska Arthroplasty Initiative
RECLAIM REVISION HIP SYSTEM
 RECLAIM REVISION HIP SYSTEM Where Strength and Modularity Connect DESIGN RATIONALE System Overview RECLAIM Modular Revision Hip System WHERE STRENGTH & MODULARITY CONNECT 2 Offset Options Proximal Body
RECLAIM REVISION HIP SYSTEM Where Strength and Modularity Connect DESIGN RATIONALE System Overview RECLAIM Modular Revision Hip System WHERE STRENGTH & MODULARITY CONNECT 2 Offset Options Proximal Body
InPulse. GLFHC staff donate in record numbers during 2018 Employee Appeal. Get Social with GLFHC. Volume 3, Issue 7 January continued on page 3
 Get Social with GLFHC The mission of Greater Lawrence Family Health Center is to improve and maintain the health of individuals and families in the Merrimack Valley by providing a network of high quality,
Get Social with GLFHC The mission of Greater Lawrence Family Health Center is to improve and maintain the health of individuals and families in the Merrimack Valley by providing a network of high quality,
Hip and Knee Orthopedic Surgical Implants Market Growth, Research & Trend to Radiant Insights, Inc
 Hip and Knee Orthopedic Surgical Implants Market Growth, Research & Trend to 2016-2022 Radiant Insights, Inc Orthopedic surgical implants help replace deteriorated joints. This deterioration could be due
Hip and Knee Orthopedic Surgical Implants Market Growth, Research & Trend to 2016-2022 Radiant Insights, Inc Orthopedic surgical implants help replace deteriorated joints. This deterioration could be due
Enabling Greater Market Awareness for Hernia Practitioners. Applying the Centers of Excellence Model to Hernia Health Care
 Enabling Greater Market Awareness for Hernia Practitioners Applying the Centers of Excellence Model to Hernia Health Care A Hernia Centers of Excellence (HCoE) White Paper Executive Summary Situation:
Enabling Greater Market Awareness for Hernia Practitioners Applying the Centers of Excellence Model to Hernia Health Care A Hernia Centers of Excellence (HCoE) White Paper Executive Summary Situation:
The S.T.A.R. Scandinavian Total Ankle Replacement. Patient Information
 The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
Knee Replacement Complications
 Knee Replacement Complications Knee replacements have become a routine surgery in the United States. Nearly 700,000 people each year receive this life-improving surgery and are able to enjoy richer, more
Knee Replacement Complications Knee replacements have become a routine surgery in the United States. Nearly 700,000 people each year receive this life-improving surgery and are able to enjoy richer, more
WHAT YOU IS BACK WITHIN ARM S REACH
 YOUR TOTAL SHOULDER REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE WHAT YOU IS BACK WITHIN ARM S REACH Nathan Richardson, MD Orthopedics, Shoulder & Elbow Surgeon Board Certified in
YOUR TOTAL SHOULDER REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE WHAT YOU IS BACK WITHIN ARM S REACH Nathan Richardson, MD Orthopedics, Shoulder & Elbow Surgeon Board Certified in
SHOULDER REPLACEMENT PATIENT S GUIDE
 SHOULDER REPLACEMENT PATIENT S GUIDE HIGHLIGHTS FOR PATIENTS Remember to get up and MOVE! DISCLAIMER HIGHLIGHTS FOR PATIENTS The following information is provided about shoulder replacement in general.
SHOULDER REPLACEMENT PATIENT S GUIDE HIGHLIGHTS FOR PATIENTS Remember to get up and MOVE! DISCLAIMER HIGHLIGHTS FOR PATIENTS The following information is provided about shoulder replacement in general.
Medical Devices and the Public s Health: The FDA 510(k) Clearance Process at 35 Years. Written Statement of
 Medical Devices and the Public s Health: The FDA 510(k) Clearance Process at 35 Years Written Statement of Dr. David R. Challoner Vice President for Health Affairs, Emeritus University of Florida and Chair,
Medical Devices and the Public s Health: The FDA 510(k) Clearance Process at 35 Years Written Statement of Dr. David R. Challoner Vice President for Health Affairs, Emeritus University of Florida and Chair,
Using Recall Data to Assess the 510(k) Process
 Using Recall Data to Assess the 510(k) Process Ralph F. Hall Distinguished Professor and Practitioner University of Minnesota Law School July 28, 2010 Acknowledgments 2 Disclosures 3 Agenda I. Research
Using Recall Data to Assess the 510(k) Process Ralph F. Hall Distinguished Professor and Practitioner University of Minnesota Law School July 28, 2010 Acknowledgments 2 Disclosures 3 Agenda I. Research
HP KNEE EXTRACTION Instrumentation. Product Overview
 HP KNEE EXTRACTION Instrumentation Product Overview HP Extraction Knee Instruments DePuy Synthes Joint Reconstruction is working with Symmetry Medical to distribute Advanced Knee Extraction instruments.
HP KNEE EXTRACTION Instrumentation Product Overview HP Extraction Knee Instruments DePuy Synthes Joint Reconstruction is working with Symmetry Medical to distribute Advanced Knee Extraction instruments.
Evidence-based Practice And Standards For Medical Devices. Rita F. Redberg, MD, MSc, FACC, FAHA University of California, San Francisco
 Evidence-based Practice And Standards For Medical Devices Rita F. Redberg, MD, MSc, FACC, FAHA University of California, San Francisco 2 3 Regulatory Principles Data Transparency and Accessibility High
Evidence-based Practice And Standards For Medical Devices Rita F. Redberg, MD, MSc, FACC, FAHA University of California, San Francisco 2 3 Regulatory Principles Data Transparency and Accessibility High
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN
 MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
MAASH HIP REPLACEMENT: FAST TRACK, NO COMPLICATIONS
 MAASH HIP REPLACEMENT: FAST TRACK, NO COMPLICATIONS SUMMARY New European hip replacement technique, minimally invasive, that preserves ligaments, capsule and nerves of the hip. Flash recovery: walking
MAASH HIP REPLACEMENT: FAST TRACK, NO COMPLICATIONS SUMMARY New European hip replacement technique, minimally invasive, that preserves ligaments, capsule and nerves of the hip. Flash recovery: walking
Integra. Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION
 Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN
 2:17-cv-11244-LVP-EAS Doc # 1 Filed 04/20/17 Pg 1 of 22 Pg ID 1 ROSETTA VENTIMIGLIA, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Defendant. / John A.
2:17-cv-11244-LVP-EAS Doc # 1 Filed 04/20/17 Pg 1 of 22 Pg ID 1 ROSETTA VENTIMIGLIA, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Defendant. / John A.
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN
 MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
KIP A. PETROFF Board Certified Personal Injury and Civil Trial Lawyer Texas Board of Legal Specialization
 KIP A. PETROFF Board Certified Personal Injury and Civil Trial Lawyer Texas Board of Legal Specialization 10440 N. Central Expressway Telephone: (972) 294-7530 Suite 1540 web: KipPetroff.com Dallas, TX
KIP A. PETROFF Board Certified Personal Injury and Civil Trial Lawyer Texas Board of Legal Specialization 10440 N. Central Expressway Telephone: (972) 294-7530 Suite 1540 web: KipPetroff.com Dallas, TX
2/17/2018. Prof. Steven S. Saliterman Department of Biomedical Engineering, University of Minnesota
 Department of Biomedical Engineering, University of Minnesota http://saliterman.umn.edu/ FDA Authority Medical Device Act of 1976 FDA Modernization Act of 1997 Federal Food, Drug and Cosmetic Act (FFDCA)
Department of Biomedical Engineering, University of Minnesota http://saliterman.umn.edu/ FDA Authority Medical Device Act of 1976 FDA Modernization Act of 1997 Federal Food, Drug and Cosmetic Act (FFDCA)
Prof. Steven S. Saliterman. Department of Biomedical Engineering, University of Minnesota
 Department of Biomedical Engineering, University of Minnesota http://saliterman.umn.edu/ FDA Authority Medical Device Act of 1976 FDA Modernization Act of 1997 Federal Food, Drug and Cosmetic Act (FFDCA)
Department of Biomedical Engineering, University of Minnesota http://saliterman.umn.edu/ FDA Authority Medical Device Act of 1976 FDA Modernization Act of 1997 Federal Food, Drug and Cosmetic Act (FFDCA)
Hip and Knee Orthopedic Surgical Implants: Market Shares, Strategies, and Forecasts, Worldwide,
 Hip and Knee Orthopedic Surgical Implants: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Implants: Executive Summary The study is designed
Hip and Knee Orthopedic Surgical Implants: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Implants: Executive Summary The study is designed
Certain Antibiotics Spur Widening Reports of
 Page 1 of 6 Certain Antibiotics Spur Widening Reports of Severe Side Effects June 16, 2011 at 12:00 AM EDT A class of antibiotics called quinolones is raising concerns after some patients report becoming
Page 1 of 6 Certain Antibiotics Spur Widening Reports of Severe Side Effects June 16, 2011 at 12:00 AM EDT A class of antibiotics called quinolones is raising concerns after some patients report becoming
The proposed rule is significant, and the requirements and exceptions are complex. Key provisions of the proposal are described below.
 ADVISORY Food & Drug FDA ISSUES PROPOSED RULE TO ESTABLISH A UNIQUE DEVICE IDENTIFICATION SYSTEM FOR MEDICAL DEVICES July 16, 2012 On July 11, 2012, the Food and Drug Administration (FDA) published in
ADVISORY Food & Drug FDA ISSUES PROPOSED RULE TO ESTABLISH A UNIQUE DEVICE IDENTIFICATION SYSTEM FOR MEDICAL DEVICES July 16, 2012 On July 11, 2012, the Food and Drug Administration (FDA) published in
About this consent form
 Protocol Title: Development of the smoking cessation app Smiling instead of Smoking Principal Investigator: Bettina B. Hoeppner, Ph.D. Site Principal Investigator: n/a Description of Subject Population:
Protocol Title: Development of the smoking cessation app Smiling instead of Smoking Principal Investigator: Bettina B. Hoeppner, Ph.D. Site Principal Investigator: n/a Description of Subject Population:
Free Yourself Tips for living without back pain
 spring 2010 Free Yourself Tips for living without back pain - Log on to live healthier - Diabetes do s and don ts - How to appeal a claim best friends enjoying Idaho's city of rocks one TO one newsletter
spring 2010 Free Yourself Tips for living without back pain - Log on to live healthier - Diabetes do s and don ts - How to appeal a claim best friends enjoying Idaho's city of rocks one TO one newsletter
Insurance Guide For Dental Healthcare Professionals
 Insurance Guide For Dental Healthcare Professionals Dental Benefits Basics What is dental insurance? Unlike traditional insurance, dental benefits are not meant to cover all oral healthcare needs. The
Insurance Guide For Dental Healthcare Professionals Dental Benefits Basics What is dental insurance? Unlike traditional insurance, dental benefits are not meant to cover all oral healthcare needs. The
DMA will take your dental practice to the next level
 DMA will take your dental practice to the next level A membership payment plan created by dentists for dentists and patients Traditionally dentists have only been able to grow their practices by a mix
DMA will take your dental practice to the next level A membership payment plan created by dentists for dentists and patients Traditionally dentists have only been able to grow their practices by a mix
2:12-cv VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN
 2:12-cv-13397-VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 CLAUDIA D. ORR, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Case No. Hon. Defendant. /
2:12-cv-13397-VAR-LJM Doc # 1 Filed 08/02/12 Pg 1 of 12 Pg ID 1 CLAUDIA D. ORR, UNITED STATES DISTRICT COURT EASTERN DISTRICT OF MICHIGAN vs. Plaintiff, SMITH & NEPHEW, INC., Case No. Hon. Defendant. /
STATE OF ARIZONA Department of Revenue
 STATE OF ARIZONA Department of Revenue TAXPAYER INFORMATION RULING LR 18-005 Douglas A. Ducey Governor David Briant Director Thank you for your letter dated February 12, 2018, requesting a taxpayer information
STATE OF ARIZONA Department of Revenue TAXPAYER INFORMATION RULING LR 18-005 Douglas A. Ducey Governor David Briant Director Thank you for your letter dated February 12, 2018, requesting a taxpayer information
Primer: Medical Device User Fee Amendments Han Zhong l September 2011
 Primer: Medical Device User Fee Amendments Han Zhong l September 2011 Introduction The Medical Device User Fee and Modernization Act (MDUFMA or MDUFA) is a set of agreements between the Food and Drug Administration
Primer: Medical Device User Fee Amendments Han Zhong l September 2011 Introduction The Medical Device User Fee and Modernization Act (MDUFMA or MDUFA) is a set of agreements between the Food and Drug Administration
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a
It s your knee. Help keep it that way PERSONALIZED TOTAL KNEE IMPLANTS
 It s your knee Help keep it that way PERSONALIZED TOTAL KNEE IMPLANTS Osteoarthritis the disease Osteoarthritis (OA) is the most common form of arthritis, affecting tens of millions of people worldwide.
It s your knee Help keep it that way PERSONALIZED TOTAL KNEE IMPLANTS Osteoarthritis the disease Osteoarthritis (OA) is the most common form of arthritis, affecting tens of millions of people worldwide.
Spinal Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide,
 Spinal Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Spinal Surgical Robot: Executive Summary The study is designed to give a comprehensive overview
Spinal Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Spinal Surgical Robot: Executive Summary The study is designed to give a comprehensive overview
Wear Properties of UHMWPE in CHARITE Artificial Inter-vertebral Disc
 Mechanics of Contact and Lubrication, MTM G230 Department of Mechanical & Industrial Enineering Northeastern University Spring 2006 Wear Properties of UHMWPE in CHARITE Artificial Inter-vertebral Disc
Mechanics of Contact and Lubrication, MTM G230 Department of Mechanical & Industrial Enineering Northeastern University Spring 2006 Wear Properties of UHMWPE in CHARITE Artificial Inter-vertebral Disc
New York Attorney General Targets Supplements at Major Retailers
 New York Attorney General Targets Supplements at Major Retailers By ANAHAD O'CONNOR NY Times FEBRUARY 3, 2015 12:00 AM A Target in East Harlem. It and three other retailers GNC, Walgreens and Walmart were
New York Attorney General Targets Supplements at Major Retailers By ANAHAD O'CONNOR NY Times FEBRUARY 3, 2015 12:00 AM A Target in East Harlem. It and three other retailers GNC, Walgreens and Walmart were
Improve your quality of life after medially stabilized knee arthroplasty
 G K SPHERE MEDIALLY STABILIZED KNEE Improve your quality of life after medially stabilized knee arthroplasty IMPROVE YOUR QUALI 2 All trademarks and registered trademarks are the property of their respective
G K SPHERE MEDIALLY STABILIZED KNEE Improve your quality of life after medially stabilized knee arthroplasty IMPROVE YOUR QUALI 2 All trademarks and registered trademarks are the property of their respective
Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis?
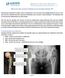
![Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis?](/thumbs/78/78456133.jpg) Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Diagnosis: Ceramic head fracture In the 1970 s, Boutin implemented ceramic in modern total hip arthroplasty (THA).
Case report: Pain L THR [ post THR 2 years; with history of trivial fall] Your Diagnosis? Diagnosis: Ceramic head fracture In the 1970 s, Boutin implemented ceramic in modern total hip arthroplasty (THA).
National Joint Replacement Registry. Lay Summary 2015 Annual Report Hip and Knee Replacement
 National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
Nautilus & Athletic Journal Articles
 Nautilus & Athletic Journal Articles ArthurJonesExercise.com Featuring the Lower Back Machine While the title of this article may lend itself to argument, it is certainly true that the lower back is, at
Nautilus & Athletic Journal Articles ArthurJonesExercise.com Featuring the Lower Back Machine While the title of this article may lend itself to argument, it is certainly true that the lower back is, at
What is the Best Bearing Type? 1/11/2011
 What is the Best Bearing Type? 1/11/2011 A comparison of modern bearing types. Thomas P. Gross, M.D. As a patient there are four reasons you should consider a metal-on-metal bearing total hip replacement
What is the Best Bearing Type? 1/11/2011 A comparison of modern bearing types. Thomas P. Gross, M.D. As a patient there are four reasons you should consider a metal-on-metal bearing total hip replacement
Carry this card with you at all times. Show this card to any medical professional treating you. Patient Implant Card. Option ELITE Vena Cava Filter.
 Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
Produced on: Licenced for use until: Corail Stem (Standard Offset Collared)
 Implant Bespoke Report for: DePuy Comprising PRIMARY hips implanted up to: 09 October 2017 NJR Database extract: 08 December 2017 Produced on: Licenced for use until: 29 December 2017 29 April 2018 Contents
Implant Bespoke Report for: DePuy Comprising PRIMARY hips implanted up to: 09 October 2017 NJR Database extract: 08 December 2017 Produced on: Licenced for use until: 29 December 2017 29 April 2018 Contents
Straight Spine Safe Spine Newsletter May Is National Correct Posture Month, but Every Day Should Be Perfect Posture Day
 Straight Spine Safe Spine Newsletter May Is National Correct Posture Month, but Every Day Should Be Perfect Posture Day May is Correct Posture Month, but every day should be Perfect Posture Day. This may
Straight Spine Safe Spine Newsletter May Is National Correct Posture Month, but Every Day Should Be Perfect Posture Day May is Correct Posture Month, but every day should be Perfect Posture Day. This may
Online Orthopaedics Online Orthopaedics
 Our patients who are in need of a total knee replacement have traditionally left the decision entirely in our hands as orthopaedic joint replacement surgeons. A patient rarely comes to the office asking
Our patients who are in need of a total knee replacement have traditionally left the decision entirely in our hands as orthopaedic joint replacement surgeons. A patient rarely comes to the office asking
Produced on: Licenced for use until: Corail Stem (Standard Offset Non-Collared)
 Implant Bespoke Report for: DePuy Comprising PRIMARY hips implanted up to: 09 October 2017 NJR Database extract: 08 December 2017 Produced on: Licenced for use until: 29 December 2017 29 December 2018
Implant Bespoke Report for: DePuy Comprising PRIMARY hips implanted up to: 09 October 2017 NJR Database extract: 08 December 2017 Produced on: Licenced for use until: 29 December 2017 29 December 2018
Total Hip Replacement
 PATIENT EDUCATION Total Hip Replacement A guide to understanding total hip replacement surgery, featuring The George Archer Story. BACK TO MOBILITY George Archer s Personal Adventure On April16, 1996,
PATIENT EDUCATION Total Hip Replacement A guide to understanding total hip replacement surgery, featuring The George Archer Story. BACK TO MOBILITY George Archer s Personal Adventure On April16, 1996,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
The Reprocessing of Single- Use Medical Devices; Regulations Coming to Europe? CleanMed Europe Malmo, Sweden 26 September 2012
 1 The Reprocessing of Single- Use Medical Devices; Regulations Coming to Europe? CleanMed Europe Malmo, Sweden 26 September 2012 2 Topics to be Covered Introduction to AMDR Introduction to single-use medical
1 The Reprocessing of Single- Use Medical Devices; Regulations Coming to Europe? CleanMed Europe Malmo, Sweden 26 September 2012 2 Topics to be Covered Introduction to AMDR Introduction to single-use medical
GENERAL Why did Harvard Pilgrim implement an MSK program and why is it expanding to include hip, knee, shoulder and spine surgeries?
 National Imaging Associates, Inc. (NIA) Musculoskeletal Care Management (MSK) Program Hip, Knee, Shoulder & Spine Surgeries Frequently Asked Questions (FAQ s) Harvard Pilgrim Health Care Ordering Physicians
National Imaging Associates, Inc. (NIA) Musculoskeletal Care Management (MSK) Program Hip, Knee, Shoulder & Spine Surgeries Frequently Asked Questions (FAQ s) Harvard Pilgrim Health Care Ordering Physicians
Shoulder Joint Replacement
 Shoulder Joint Replacement Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was first performed
Shoulder Joint Replacement Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was first performed
Are You Living with. Hip Pain? MAKOplasty may be the right treatment option for you.
 Are You Living with Hip Pain? MAKOplasty may be the right treatment option for you. Hip pain shouldn t keep you from doing the things you love. Understanding Common Causes of Hip Pain If you are one of
Are You Living with Hip Pain? MAKOplasty may be the right treatment option for you. Hip pain shouldn t keep you from doing the things you love. Understanding Common Causes of Hip Pain If you are one of
Special Education Fact Sheet. Special Education Impartial Hearings in New York City
 New York Lawyers For The Public Interest, Inc. 151 West 30 th Street, 11 th Floor New York, NY 10001-4017 Tel 212-244-4664 Fax 212-244-4570 TTD 212-244-3692 www.nylpi.org Special Education Fact Sheet Special
New York Lawyers For The Public Interest, Inc. 151 West 30 th Street, 11 th Floor New York, NY 10001-4017 Tel 212-244-4664 Fax 212-244-4570 TTD 212-244-3692 www.nylpi.org Special Education Fact Sheet Special
Gender Solutions Patello-Femoral Joint System
 Zimmer Biomet is the leading company in partial knee arthroplasty (PKA) 1 with over 40 years experience, offering a comprehensive range of anatomic and innovative solutions. Research shows that surgeons
Zimmer Biomet is the leading company in partial knee arthroplasty (PKA) 1 with over 40 years experience, offering a comprehensive range of anatomic and innovative solutions. Research shows that surgeons
2. STRAUMANN PRODUCTS COVERED BY THE STRAUMANN GUARANTEE. Abutment attached to an implant* Replacement with equivalent ceramic abutment**
 Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Straumann Guarantee Straumann Guarantee* 1. GUARANTEE BENEFICIARY AND SCOPE This guarantee (the Straumann Guarantee as defined below) from the Institut Straumann AG, Basel, Switzerland ( Straumann ) applies
Air Toxics. Questions and Answers
 Air Toxics Questions and Answers What is going on? Possibly unsafe levels of arsenic and cadmium have been found in the air around the Bullseye Glass Company in Southeast Portland. Cadmium has been found
Air Toxics Questions and Answers What is going on? Possibly unsafe levels of arsenic and cadmium have been found in the air around the Bullseye Glass Company in Southeast Portland. Cadmium has been found
March 19, Dear Ms. Strohkirch:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 19, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Treace
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 19, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Treace
SUPERIOR COURT OF WASHINGTON FOR KING COUNTY
 1 1 1 1 1 1 1 1 0 1 SUPERIOR COURT OF WASHINGTON FOR KING COUNTY ALAN ROSSI, MD; JAMES MILLARD; and ) ELLEN PARDEE; ) ) Plaintiffs, ) v. ) No.: ) BIOMET, INC.; BIOMET ORTHOPEDICS, LLC; ) COMPLAINT FOR
1 1 1 1 1 1 1 1 0 1 SUPERIOR COURT OF WASHINGTON FOR KING COUNTY ALAN ROSSI, MD; JAMES MILLARD; and ) ELLEN PARDEE; ) ) Plaintiffs, ) v. ) No.: ) BIOMET, INC.; BIOMET ORTHOPEDICS, LLC; ) COMPLAINT FOR
April 2016 Can We Get Stronger as We Age? The answer to that question is
 Can We Get Stronger as We Age? The answer to that question is absolutely! After age 40 or so, we all begin to lose muscle strength and bone density, and our hormone production slows. While these factors
Can We Get Stronger as We Age? The answer to that question is absolutely! After age 40 or so, we all begin to lose muscle strength and bone density, and our hormone production slows. While these factors
DEPUY SYNTHES JOINT RECONSTRUCTION PATIENT EDUCATION SEMINAR
 DEPUY SYNTHES JOINT RECONSTRUCTION PATIENT EDUCATION SEMINAR Welcome! Thank you for joining us! We hope today s information will help you on a path to reducing your pain and gaining mobility. This informational
DEPUY SYNTHES JOINT RECONSTRUCTION PATIENT EDUCATION SEMINAR Welcome! Thank you for joining us! We hope today s information will help you on a path to reducing your pain and gaining mobility. This informational
Musculoskeletal Management (MSK) Program Frequently Asked Questions (FAQ s) For Physicians
 Musculoskeletal Management (MSK) Program Frequently Asked Questions (FAQ s) For Physicians Question GENERAL Why does Harvard Pilgrim have an MSK Program? Answer The MSK program is designed to improve quality
Musculoskeletal Management (MSK) Program Frequently Asked Questions (FAQ s) For Physicians Question GENERAL Why does Harvard Pilgrim have an MSK Program? Answer The MSK program is designed to improve quality
PROVIDER CONTRACT ISSUES
 211 East Chicago Avenue T 312.440.2500 Chicago, Illinois 60611 F 312.440.7494 www.ada.org TOP 10 CLAIM CONCERNS: ADA, NADP SHARE VIEWS ON DENTISTS CONCERNS The ADA Council on Dental Benefit Programs continually
211 East Chicago Avenue T 312.440.2500 Chicago, Illinois 60611 F 312.440.7494 www.ada.org TOP 10 CLAIM CONCERNS: ADA, NADP SHARE VIEWS ON DENTISTS CONCERNS The ADA Council on Dental Benefit Programs continually
introduction jointunderstanding benefits of knee-replacement surgery
 introduction When knee pain becomes so severe that drugs do not provide relief or when knee problems make daily activities painful, difficult, or even impossible, surgeons can sometimes replace the damaged
introduction When knee pain becomes so severe that drugs do not provide relief or when knee problems make daily activities painful, difficult, or even impossible, surgeons can sometimes replace the damaged
CPCS. Training Manual. Collarless Polished Cemented Stem
 SMITH & NEPHEW ORTHOPAEDICS ~ TRUST. INNOVATION. PERFORMANCE. CPCS Collarless Polished Cemented Stem Training Manual This document is for internal use by Smith & Nephew and sales force only and is not
SMITH & NEPHEW ORTHOPAEDICS ~ TRUST. INNOVATION. PERFORMANCE. CPCS Collarless Polished Cemented Stem Training Manual This document is for internal use by Smith & Nephew and sales force only and is not
your guide to Total Knee replacement surgery
 your guide to Total Knee replacement surgery Arthritis and Knee understanding replacement Joint deterioration can affect every aspect of a person s life. In its early stages, it is common for people to
your guide to Total Knee replacement surgery Arthritis and Knee understanding replacement Joint deterioration can affect every aspect of a person s life. In its early stages, it is common for people to
 1 816.759.0123 www.burlesonorthodontics.com Introductory Letter from Dr. Burleson Dear Friend, If you are researching orthodontists and different types of braces for yourself, your children, or a loved
1 816.759.0123 www.burlesonorthodontics.com Introductory Letter from Dr. Burleson Dear Friend, If you are researching orthodontists and different types of braces for yourself, your children, or a loved
Vasu Pai D orth, MS, National Board [orth],mch, FRACS, FICMR Total Hip Arthroplasty
![Vasu Pai D orth, MS, National Board [orth],mch, FRACS, FICMR Total Hip Arthroplasty Vasu Pai D orth, MS, National Board [orth],mch, FRACS, FICMR Total Hip Arthroplasty](/thumbs/90/102636395.jpg) Vasu Pai D orth, MS, National Board [orth],mch, FRACS, FICMR Total Hip Arthroplasty Introduction Hip arthritis is a common problem, most often due to osteoarthritis. In hip arthritis affects a patient,
Vasu Pai D orth, MS, National Board [orth],mch, FRACS, FICMR Total Hip Arthroplasty Introduction Hip arthritis is a common problem, most often due to osteoarthritis. In hip arthritis affects a patient,
COMMUNITY HOSPICE & PALLIATIVE CARE NOTICE OF PRIVACY PRACTICES
 COMMUNITY HOSPICE & PALLIATIVE CARE NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE
COMMUNITY HOSPICE & PALLIATIVE CARE NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE
MAR (k) SUMMARY. Stanmore Implants Worldwide Ltd JTS Extendible Implant Preparation Date: March 17, A ppl ica nt/spo0nsor:
 510(k) SUMMARY MAR 2 2 201 Stanmore Implants Worldwide Ltd JTS Extendible Implant Preparation Date: March 17, 2011 A ppl ica nt/spo0nsor: Contact Person: Proprietary name: Common or Usual Name: Classification
510(k) SUMMARY MAR 2 2 201 Stanmore Implants Worldwide Ltd JTS Extendible Implant Preparation Date: March 17, 2011 A ppl ica nt/spo0nsor: Contact Person: Proprietary name: Common or Usual Name: Classification
Smith & Nephew. Polarstem Cementless
 Implant Smith & Nephew Comprising PRIMARY hips implanted up to: 09 September 2018 NJR Database extract: 08 November 2018 Produced on: Licenced for use until: 20 November 2018 20 March 2019 Contents Recorded
Implant Smith & Nephew Comprising PRIMARY hips implanted up to: 09 September 2018 NJR Database extract: 08 November 2018 Produced on: Licenced for use until: 20 November 2018 20 March 2019 Contents Recorded
Clinical Practice Guidelines: Can I Learn to Live With Them? What s in the News These Days 2/11/2016. Excessive Costs of Medical Care
 Orthopaedic Trauma Fracture Care: Pushing the Envelope San Diego Clinical Practice Guidelines: Can I Learn to Live With Them? Andrew H. Schmidt, MD What s in the News These Days Excessive Costs of Medical
Orthopaedic Trauma Fracture Care: Pushing the Envelope San Diego Clinical Practice Guidelines: Can I Learn to Live With Them? Andrew H. Schmidt, MD What s in the News These Days Excessive Costs of Medical
Summary HTA. Arthroplasty register for Germany. HTA-Report Summary. Gorenoi V, Schönermark MP, Hagen A
 Summary HTA HTA-Report Summary Arthroplasty register for Germany Gorenoi V, Schönermark MP, Hagen A Health political and scientific background Joint prostheses are man-made replacement joints. The hip
Summary HTA HTA-Report Summary Arthroplasty register for Germany Gorenoi V, Schönermark MP, Hagen A Health political and scientific background Joint prostheses are man-made replacement joints. The hip
Table 1. Existing products in the market Product Benefits Costs. Breg FreeSport Knee Brace [10] Does not monitor
![Table 1. Existing products in the market Product Benefits Costs. Breg FreeSport Knee Brace [10] Does not monitor Table 1. Existing products in the market Product Benefits Costs. Breg FreeSport Knee Brace [10] Does not monitor](/thumbs/81/83168072.jpg) IntelliSleeve - Novel Monitoring of Knee Biomechanics for Rehabilitation Sriram Boppana, Daniel Haugan, Rohith Jaykumar, and Jacques Stites - Purdue University, Weldon School of Biomedical Engineering,
IntelliSleeve - Novel Monitoring of Knee Biomechanics for Rehabilitation Sriram Boppana, Daniel Haugan, Rohith Jaykumar, and Jacques Stites - Purdue University, Weldon School of Biomedical Engineering,
Contents. Helpline No. UK/ North Ireland Rep. Ireland Web Support Model Number: 12005
 Contents 1 02 03 04 06 07 11 12 13 15 17 18 19 19 Contents Welcome Safety Instructions Box Contents Notes on Measurements Getting Started Measuring Weight Saving Personal Data Measuring Body Fat, Water
Contents 1 02 03 04 06 07 11 12 13 15 17 18 19 19 Contents Welcome Safety Instructions Box Contents Notes on Measurements Getting Started Measuring Weight Saving Personal Data Measuring Body Fat, Water
U. AUG skeletal dynamics. 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System
 U. AUG 23 2011 00 skeletal dynamics 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System Submitter: Skeletal Dynamics, LLC 6905 SW 67 Avenue Suite 201 Miami,
U. AUG 23 2011 00 skeletal dynamics 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System Submitter: Skeletal Dynamics, LLC 6905 SW 67 Avenue Suite 201 Miami,
DIABETES TEST STRIPS REIMBURSEMENT REDUCTIONS TO INDEPENDENT PHARMACIES WILL NEGATIVELY IMPACT MEDICARE PATIENTS
 DIABETES TEST STRIPS REIMBURSEMENT REDUCTIONS TO INDEPENDENT PHARMACIES WILL NEGATIVELY IMPACT MEDICARE PATIENTS NCPA Member Survey Results September 2012 CMS is considering cutting payments to pharmacies
DIABETES TEST STRIPS REIMBURSEMENT REDUCTIONS TO INDEPENDENT PHARMACIES WILL NEGATIVELY IMPACT MEDICARE PATIENTS NCPA Member Survey Results September 2012 CMS is considering cutting payments to pharmacies
How to Regulate E-Cigarettes? Are we asking the right questions?
 How to Regulate E-Cigarettes? Are we asking the right questions? Eric N. Lindblom Director, Tobacco Control and Food & Drug Law O Neill Institute for National & Global Health Law Georgetown University
How to Regulate E-Cigarettes? Are we asking the right questions? Eric N. Lindblom Director, Tobacco Control and Food & Drug Law O Neill Institute for National & Global Health Law Georgetown University
A Patient s Guide to Artificial Joint Replacement of the Ankle
 A Patient s Guide to Artificial Joint Replacement of the Ankle Introduction Surgery to replace the ankle joint with an artificial joint (called ankle arthroplasty) is becoming more common. This surgery
A Patient s Guide to Artificial Joint Replacement of the Ankle Introduction Surgery to replace the ankle joint with an artificial joint (called ankle arthroplasty) is becoming more common. This surgery
July 10, X-spine Systems, Incorporated David Kirschman, MD Chief Medical Officer 452 Alexandersville Road Miamisburg, Ohio 45342
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
How Clinical Trials Work
 A Guide to: How Clinical Trials Work Clinical trials are rigorously controlled tests designed to examine the safety and / or effectiveness of medicines, devices, treatments, or preventive measures in humans.
A Guide to: How Clinical Trials Work Clinical trials are rigorously controlled tests designed to examine the safety and / or effectiveness of medicines, devices, treatments, or preventive measures in humans.
COSMETIC DENTAL CARE. Guide to DenTal Implants. cosmeticdentalcare.com.au
 COSMETIC DENTAL CARE Guide to DenTal Implants cosmeticdentalcare.com.au Matching Mother Nature We ve often been told that there s simply no way to fill a gap in your teeth so that it feels like your real
COSMETIC DENTAL CARE Guide to DenTal Implants cosmeticdentalcare.com.au Matching Mother Nature We ve often been told that there s simply no way to fill a gap in your teeth so that it feels like your real
What is the Economic Impact of Autism Spectrum Disorder?
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/autism-spectrum/what-is-economic-impact-autism-spectrumdisorder/10263/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/autism-spectrum/what-is-economic-impact-autism-spectrumdisorder/10263/
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels
 SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
SFIREG Issue Paper: Pesticide Use on Cannabis State Established Pesticide Residue Action Levels Introduction to Issue: In recent years, with the approval of medical and recreational use of marijuana in
2016 Report to the Public About. Hip and Knee Replacements
 2016 Report to the Public About Hip and Knee Replacements Using Data to Improve Patient Care Joint replacement surgery has helped relieve pain and restore function for millions of people. Most people who
2016 Report to the Public About Hip and Knee Replacements Using Data to Improve Patient Care Joint replacement surgery has helped relieve pain and restore function for millions of people. Most people who
LEGION Total Knee System. Exceptional Durability
 LEGION Total Knee System Exceptional Durability How many knee systems have been lab-tested to 30 years of simulated wear? Just one: LEGION Primary Knee System with VERILAST Technology The LEGION Primary
LEGION Total Knee System Exceptional Durability How many knee systems have been lab-tested to 30 years of simulated wear? Just one: LEGION Primary Knee System with VERILAST Technology The LEGION Primary
