Commissioning Policy TBC 2018
|
|
|
- June Young
- 5 years ago
- Views:
Transcription
1 Commissioning Policy Exogen Ultrasound Bone Healing System for Bone Fractures with Non-Union TBC 2018 This commissioning policy has been endorsed by and applies to patients within: NHS North Staffordshire Clinical Commissioning Group (CCG) NHS Stoke on Trent Clinical Commissioning Group (CCG) Version: Ratified by (name and date of Committee): V1 Planning and Commissioning Committee Date issued: Expiry date: (Document is not valid after this date) Any revisions to the policy will be based on local and national evidence of effectiveness and cost effectiveness together with recommendations and guidelines from local, national and international clinical professional bodies and withdrawal of the outcome guarantee by the company Review date: March 2019 Lead Executive/Director: Cheryl Hardisty Director of Commissioning Name of originator/author: Target audience: Distribution: Equality & Diversity Impact Assessment Kathryn Whitfield Commissioning Manager NHS and Private Providers who undertake NHS funded treatment GPs/Providers/GP Practices/CCG Websites N/A 1
2 Circulated to the following individuals/groups for comments Dr John Harvey Name Designation Consultant in Public Health Review and Amendment Log Version Type of change Date Description of change Number V0.1 Initial policy Policy development 2
3 1 Policy statement: Following a review of the evidence and consideration of the local circumstances for use, North Staffordshire and Stoke on Trent Clinical Commissioning Groups will separately fund (in accordance with this policy): 1.1 Exogen ultrasound bone healing system for the treatment of bone fractures with nonunion, in accordance with defined clinical and process criteria 2 Scope of policy: 2.1 This policy should be considered in line with all other North Staffordshire and Stoke on Trent Commissioning Policies. Copies of these Commissioning Policies are available on the CCGs websites 2.2 This policy relates to the use of Exogen ultrasound bone healing system when used for management of bone fractures which are subject to non-union, in an out-patient setting 3 Background 3.1 NHS principles have been applied in the agreement of this policy. 3.2 Following immediate management of a presenting fracture through casting, traction or surgical intervention, patients receive regular follow-up to determine progression of healing. In children and adolescents healing rates in the order of 99% are usual whilst in adults this is around 80% depending on the bone involved 3.3 Healing of fractures varies according to the nature of the fracture and affected bone, host factors including age, co-morbidities and lifestyle factors and other issues such as surgical aspects and infection. The definition of non-union therefore can vary according to these parameters. It is usual practice to consider non-union from around 6 months following fracture or 3 months for fracture neck of femur in some cases, at this stage re-intervention is considered. However nearly all fractures heal within 3-4 months 1. The difference between delayed healing and non-union is arbitrary and radiological evidence of a persistent fracture line is designated as delayed healing or non-union. However there are differing clinical observations in the different stages of delayed healing which can be seen in the table below 2 Table 1: Frequent observations in patients with different stages of impaired fracture healing Delayed Union Non-union Pseud arthrosis Symptoms Painful Painful No pain Radiograph Hypertrophic Hypertrophic or atrophic Hypertrophic or atrophic Healing Spontaneous healing No spontaneous healing Only surgical treatment Source: reference 2 below 3.4 Exogen has been available since 1997 and since this time has been assessed in the form of observational studies. In January 2013, the National Institute for Health and Clinical Excellence 1 Frolke, JPM and Patka, P (2007) Definition and classification of fracture non-unions, Injury, Internal Journal Care Injured. 2 Frolke, JPM and Patka, P (2007) Definition and classification of fracture non-unions, Injury, Internal Journal Care Injured. 3
4 (NICE 2013) completed a review of the technology and the associated evidence for use. The outcomes of this review were published as Medical Technology Guidance NICE medical technologies guidance helps the NHS to adopt medical technologies more rapidly and consistently by advising on efficacy and cost effectiveness. NICE has not issued a mandatory requirement to fund this intervention The Exogen ultrasound bone healing system delivers low-intensity pulsed ultrasound waves with the aim of stimulating bone healing. It is thought that healing is promoted by stimulating the production of growth factors and proteins that increase the removal of old bone, increase the production of new bone and increase the rate at which fibrous matrix at a fracture site is converted to mineralised bone 3.6 Long bone fractures are suitable for treatment if the fracture is stable and well aligned. EXOGEN is not indicated for use in fractures of the skull or vertebrae or in children or adolescents because of their skeletal immaturity 3.7 The EXOGEN system is available as 2 disposable devices, which differ only in the number of treatments they deliver: The EXOGEN is intended for use in patients with non-union fractures (fractures that have failed to heal after 9 months). The device delivers a minimum of 191x20 minute treatments (more than 6 months' treatment). The EXOGEN Express is intended for use in patients with delayed healing fractures (fractures that have no radiological evidence of healing after 3 months). The device delivers a maximum of 150x20 minute treatments (less than 5 months' treatment). (NB: EXOGEN will only be funded under this policy) 3.8 The EXOGEN device consists of a main operating unit with a permanently connected transducer and a separate fixture strap. The strap is placed around the fractured bone, coupling gel is applied to the transducer head (to aid conduction of ultrasound) and the transducer is secured directly over the fracture site by a fixture on the strap. The ultrasound signal emitted by the device is derived from a combination of defined electrical signal parameters and the proprietary transducer design, which generate an acoustic wave pattern specific to EXOGEN. If the patient's limb is immobilised in a cast then a hole is cut in the cast to allow access of the transducer to the skin. The device is programmed to deliver ultrasound in 20-minute sessions and these are self- administered by the patient each day. It is intended to be used in the patient's home 4 Relevant National Guidance and Research 4.1 NICE published Medical Technology Guidance (MTG12) for Exogen in January 2013.This demonstrates that this ultrasound technique is cost-saving over traditional surgery when used for treatment of long bone fractures with non-union. The NICE recommendations are: The case for adopting the EXOGEN ultrasound bone healing system to treat long bone fractures with non-union (failure to heal after 9 months) is supported by the clinical evidence, which shows high rates of fracture healing 4
5 The EXOGEN ultrasound bone healing system to treat long bone fractures with non-union is associated with an estimated provider cost saving of 1164 per patient compared with current management, through avoiding theatre time and bed days saved for NHS Trusts. (Note: this level of cost-saving has not been established locally) There is some radiological evidence of improved healing when the EXOGEN ultrasound bone healing system is used for long bone fractures with delayed healing (no radiological evidence of healing after approximately 3 months). There are substantial uncertainties about the rate at which bone healing progresses without adjunctive treatment between 3 and 9 months after fracture, and about whether or not surgery would be necessary. These uncertainties result in a range of cost consequences, some cost-saving and others that are more costly than current management 4.2 NICE estimates assume the following: 21.4% of fractures are non-union after 9 months Around 50% of non-unions are not suitable for Exogen therapy 5 Evidence for Use 5.1 It should be noted that all the evidence associated with Exogen when used for long-bone fracture with non-union is from observational studies with limited outcomes but with good clinical results, with healing rates ranging from 75% to 100% (depending on the long bone involved and duration of non-healing) over a period of 4.6 to 7.3 months and hence the reason for support from NICE. A small study suggests that where Exogen is used in patients with non-union > 12 months, much lower healing rates are observed e.g. 65% Although limited, the evidence also suggests different healing rates associated with differing long- bones, with the tibia appearing to have the best outcomes (some reports of 100%); this is also the bone which most commonly fractures and for which non-union most commonly occurs. This evidence was supported by the views of clinical experts involved in the NICE MTG Comparative evidence with surgery is limited. Healing rates from surgical intervention as identified in case series/cohort studies range from 62 to 100% over a period of 9 weeks to 24 weeks Different trials used different definitions for non-union including: failure of fracture to unite at a minimum of 6 months from fracture, no progression towards radiographic healing or healing had stopped for a minimum period of 3 months before Exogen. The largest trial (256 patients) used the definition of 9 months from fracture 5
6 5.2 The evidence has not been assessed for other indications associated with use of Exogen ultrasound bone healing system and these would be outside the scope of this policy 5.4 Adverse events associated with use of Exogen appear to be minimal, with 3 cases of skin irritation (from the coupling gel) and 1 report of chest pain (associated with a cardiac pacemaker) during a 1 year period of use reported on a database operated by the FDA and MAUDE (Manufacturer and User facility Device Experience). The manufacturer suggested that 55,000 devices were used during this time period None of the clinical studies reported device-related events and no safety concerns were identified by the external assessment centre in relation to Exogen. Reports on surgical treatment of non-union and delayed healing fractures documented adverse events including postoperative wound infection, osteomyelitis and pain 6
7 6 Commissioning Policy NHS North Staffordshire Clinical Commissioning Group and NHS Stoke on Trent Clinical Commissioning Group,(termed the Commissioners ) consider all lives of all patients whom it serves to be of equal value and, in making decisions about funding treatment for patients, will seek not to discriminate on the grounds of sex, age, sexual orientation, ethnicity, educational level, employment, marital status, religion or disability except where a difference in the treatment options made available to patients is directly related to the patient s clinical condition or is related to the anticipated benefits to be derived from a proposed form of treatment. 6.1 Use of Exogen for bone fractures with non-union Exogen will be funded where all the following criteria are met: Clinical Patient age > 18 years Non-union of any bone fracture > 9 months and < 12 months Not to be used in cases of unstable surgical fixation, not well aligned or where interfragment gap is > 10mm Not to be used in cases with infection Not to be used in pregnancy, patients with pacemakers or vertebral/skull fractures Note: patients with lifestyle factors which are known to delay fracture healing rates e.g. smoking and excess alcohol intake, will be appropriately counselled and required to eliminate these risks before determining non-union status and ultimately eligibility for Exogen. Where appropriate, referrals to specific support services should be arranged e.g. smoking cessation service Process Patient ability to comply with usage protocol and criteria Patients to be screened and referred by Consultant Orthopaedic Surgeon following review on at least two occasions at least 4 weeks apart to allow examination of serial x- rays Further assessment in non-union clinic by surgeon with expertise of dealing with nonunion of bones Regular audit of outcomes to be undertaken and participation in regional network where available These criteria will be reviewed and updated on publication of new evidence in the form of relevant trial data, updated national guidance or national or local audit outcomes. 6.2 Reporting requirements and funding arrangements are detailed in Appendix 1 Use of Exogen forany bone fracture with delayed healing. Exogen will not be funded for use in this indication Other indications for use of Exogen No other indications for use of Exogen ultrasound bone healing system without these indications will be funded Any identified new indications for use require submission of a new technology request form for consideration to the Clinical Priorities Advisory Group (CPAG) 7
8 7 Clinically Exceptional Circumstances 7.1 If there is demonstrable evidence of a patient s clinical exceptional circumstances, the referring practitioner should refer to the Commissioner s Operational Policy for Individual Funding Requests(IFR) document for further guidance on the process for consideration. 8
9 8 Appendix 1: Reporting Requirements and Funding Arrangements 1. Funding Arrangements Commissioner funded Exogen Ultrasound Bone Healing System for Any Bone Fractures with Non-union 1.1 Exogen will be funded for patients meeting the clinical criteria listed in section 6.2. Via the BlueTeq prior approval route during 2018/19 so that the commissioners can have an audit trail and determine activity levels and costs for 2019/ For treatment failures where there is continued non-union of the anybone fracture after six months of Exogen therapy providers will need to recover costs via the manufacturers money back guarantee arrangement (see appendix 2). This will then need to be credited back to the commissioners Additional points to note: Local intelligence suggests that activity levels for each Trust will <10 per annum, however this will be monitored on a quarterly basis CCG financial risk Audit of use during 2018/19 will inform the funding arrangements for 2019/20 1. Reporting Requirements All Approved Indications The information detailed in the table below should be provided to commissioners every 6 months. The preferred route for submission of data is via Blue Teq via the CSU in the absence of the Blue Teq system within the Trust. Table 1: Information requirements for the use of Exogen therapy in long bone non-union of fractures Purchaser Hospital Pseudonymised Stability Code Site Patient Number Y/N Date initiated Duration Nonhealing (weeks) Type or location of fracture Alternative treatment procedure code Treatment Success/Failur e S/F Date of final assessm ent Time to heal/fail (weeks) Refund for failure Y/N Cost Exogen claimed 9
10 9 Appendix 2: Exogen Performance Guarantee PERFORMANCE GUARANTEE The EXOGEN Performance Guarantee is a Bioventus program that refunds to buyers participating in the program, the payment for EXOGEN if progression of healing (progression to bony union) is not shown per criteria below. It is also designed to help reinforce patients adherence for the prescribed treatment. Criteria Buyers of EXOGEN participating in the program are eligible when the device has been prescribed by a qualified physician to treat a stable, established non-union fracture with a fracture gap less than 10 millimetres (excluding vertebra and skull fractures). Patients must treat their non-union fracture with EXOGEN per product instructions, for a minimum of 120 days and achieve a 90% minimum adherence. Evaluation Absence of healing (progression to bony union e.g. callus formation) is determined by the prescribing physician comparing the patient s X-Rays taken prior to using EXOGEN to one taken at 120 days or beyond. The EXOGEN device contains an internal patient usage monitor that records the date, time and duration of each treatment. This monitor will be used by Bioventus to confirm that the 90% treatment adherence is met. SMK Exclusions Fracture types: - Fresh fractures - Unstable - Greater than10 millimetres fracture gap - Vertebra and skull - Pathological Treatment of multiple fractures (the guarantee is only valid to treat a defined fracture) Modified and/or altered devices Guarantee is void if alternative interventions occur during the 120 day treatment period EXOGEN must be purchased and received directly from Bioventus Any other costs associated with the purchase (only the cost of the EXOGEN device will be refunded) Valid only in UK and Ireland Claims Customers may contact a Bioventus Customer Care Representative at (UK) or (Ireland) for assistance. All claims must be accompanied by the following: 1. Registration form to the Performance Guarantee Program sent to Bioventus within the first 30 days of the initial treatment. 2. Prescribing physician s written assessment, using the EXOGEN Claim Form 3. Prescribed EXOGEN device returned to Bioventus Claims must be received by Bioventus within one year of first EXOGEN treatment date. Summary of Indications for Use EXOGEN is indicated for the non-invasive treatment of osseous defects (excluding vertebra and skull) that includes the treatment of delayed unions, non-unions, stress fractures and joint fusion. EXOGEN is also indicated for the acceleration of fresh fracture heal time, repair following osteotomy, repair in bone transport procedures and repair in distraction osteogenesis procedures. A non-union is considered to be established when the fracture site shows no visibly progressive signs of healing. There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel. Full prescribing information can be found in product labelling, at Bioventus Coöperatief U.A. Taurusavenue LS Hoofddorp The Netherlands EXOGEN and the Bioventus logo are registered trademarks of Bioventus LLC Bioventus LLC Customer Care T (UK): (toll free) T (IR): (toll free) E: customercare-international@bioventusglobal.com 10
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
Commissioning Policy. Exogen Ultrasound Bone Healing System for Long Bone Fractures with Non-Union or Delayed Healing.
 Commissioning Policy Exogen Ultrasound Bone Healing System for Long Bone Fractures with Non-Union or Delayed Healing September 2014 This commissioning policy has been endorsed by and applies to patients
Commissioning Policy Exogen Ultrasound Bone Healing System for Long Bone Fractures with Non-Union or Delayed Healing September 2014 This commissioning policy has been endorsed by and applies to patients
Commissioning Policy. Treatment of Snoring. April 2010
 Commissioning Policy Treatment of Snoring April 2010 This commissioning policy applies to patients within: South Worcestershire Clinical Commissioning Group (CCG) Redditch & Bromsgrove Clinical Commissioning
Commissioning Policy Treatment of Snoring April 2010 This commissioning policy applies to patients within: South Worcestershire Clinical Commissioning Group (CCG) Redditch & Bromsgrove Clinical Commissioning
Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016
 Commissioning Policy Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible:
Commissioning Policy Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible:
Mechanism of Action. Activating bone healing at every stage
 Mechanism of Action Activating bone healing at every stage Ultrasound Bone Healing System Mechanism of Action The Bone Healing System is a unique fracture healing device that uses low intensity pulsed
Mechanism of Action Activating bone healing at every stage Ultrasound Bone Healing System Mechanism of Action The Bone Healing System is a unique fracture healing device that uses low intensity pulsed
Corporate Medical Policy
 Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Corporate Medical Policy Ultrasound Accelerated Fracture Healing Device File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultrasound_accelerated_fracture_healing_device 12/1994 2/2017
Commissioning Policy. The use of Acupuncture in the Management of Musculoskeletal Pain. July 2013
 Commissioning Policy The use of Acupuncture in the Management of Musculoskeletal Pain July 2013 This commissioning policy applies to patients within: South Worcestershire Clinical Commissioning Group (CCG)
Commissioning Policy The use of Acupuncture in the Management of Musculoskeletal Pain July 2013 This commissioning policy applies to patients within: South Worcestershire Clinical Commissioning Group (CCG)
Mechanism of Action. Activating bone healing at every stage
 Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Bone Healing System is a unique fracture healing device that uses low intensity pulsed ultrasound
Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Bone Healing System is a unique fracture healing device that uses low intensity pulsed ultrasound
Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181)
 Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Subject: Ultrasound Osteogenesis Stimulators, Non Invasive
 Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Date Printed: March 7, 2016: 10:37 AM Private Property of Blue Cross and Blue Shield of Florida. This medical policy (medical coverage guideline) is Copyright 2016, Blue Cross and Blue Shield of Florida
Mechanism of Action. Activating bone healing at every stage
 Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Ultrasound Bone Healing System is an FDA-approved bone healing device that uses low intensity
Mechanism of Action Activating bone healing at every stage EXOGEN Ultrasound Bone Healing System The EXOGEN Ultrasound Bone Healing System is an FDA-approved bone healing device that uses low intensity
Specialised Services Commissioning Policy. CP29: Bariatric Surgery
 Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
Specialised Services Commissioning Policy CP29: Bariatric Surgery Document Author: Specialist Planner, Cardiothoracic Executive Lead: Director of Planning Approved by: Management Group Issue Date: 12 June
Putting NICE guidance into practice. Resource impact report: Hearing loss in adults: assessment and management (NG98)
 Putting NICE guidance into practice Resource impact report: Hearing loss in adults: assessment and management (NG98) Published: June 2018 Summary This report focuses on the recommendation from NICE s guideline
Putting NICE guidance into practice Resource impact report: Hearing loss in adults: assessment and management (NG98) Published: June 2018 Summary This report focuses on the recommendation from NICE s guideline
MT 154 EXOGEN Ultrasound Bone Healing System for long bone fractures with non-union or delayed healing. Consultation Comments table
 National Institute for Health and Clinical Excellence Medical Technologies Evaluation Programme MTAC date: 19 October 2012 MT 154 EXOGEN Ultrasound Bone Healing System for long bone fractures with non-union
National Institute for Health and Clinical Excellence Medical Technologies Evaluation Programme MTAC date: 19 October 2012 MT 154 EXOGEN Ultrasound Bone Healing System for long bone fractures with non-union
Reversal of Sterilisation (Men and Women) January 2018
 Commissioning Policy Reversal of Sterilisation (Men and Women) January 2018 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible: NHS South Worcestershire
Commissioning Policy Reversal of Sterilisation (Men and Women) January 2018 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible: NHS South Worcestershire
Specialised Services Policy:
 Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
CATARACT REFERRAL FOR ASSESSMENT OF SURGICAL TREATMENT CRITERIA BASED ACCESS POLICY
 CATARACT REFERRAL FOR ASSESSMENT OF SURGICAL TREATMENT CRITERIA BASED ACCESS POLICY Version: Recommendation by: 1516.V1a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: MAY 2015
CATARACT REFERRAL FOR ASSESSMENT OF SURGICAL TREATMENT CRITERIA BASED ACCESS POLICY Version: Recommendation by: 1516.V1a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: MAY 2015
Case scenarios: Patient Group Directions
 Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
GROMMET INSERTION RECURRENT ACUTE OTITIS MEDIA (WITHOUT EFFUSION) SECONDARY CARE PRIOR APPROVAL POLICY
 Version: 1718.v1 Ratified by: SCCG COG Date Ratified: 05 April 2017 Name of Originator/Author: Name of Responsible Committee/Individual: IFR SCCG CCPF/ IFR Date issued: 18 April 2017 Review date: Target
Version: 1718.v1 Ratified by: SCCG COG Date Ratified: 05 April 2017 Name of Originator/Author: Name of Responsible Committee/Individual: IFR SCCG CCPF/ IFR Date issued: 18 April 2017 Review date: Target
EXTRACORPOREAL SHOCKWAVE THERAPY (ESWT) INDIVIDUAL FUNDING REQUESTS POLICY
 EXTRACORPOREAL SHOCKWAVE THERAPY (ESWT) INDIVIDUAL FUNDING REQUESTS POLICY Version: 1718.v1 Recommendation by: Date Ratified: 01 November 2017 Name of Originator/Author: Approved by Responsible Committee/Individual:
EXTRACORPOREAL SHOCKWAVE THERAPY (ESWT) INDIVIDUAL FUNDING REQUESTS POLICY Version: 1718.v1 Recommendation by: Date Ratified: 01 November 2017 Name of Originator/Author: Approved by Responsible Committee/Individual:
Smoking cessation interventions and services
 National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS)
 09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
09-E0000-22 Original Effective Date: 06/15/00 Reviewed: 04/28/16 Revised: 05/15/16 Subject: Non-Invasive Electrical Bone Growth Stimulators (EBGS) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION,
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/15 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/15 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Enhanced Service Specification. Childhood seasonal influenza vaccination programme 2017/18
 Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
REVERSAL OF STERILISATION/ VASECTOMY INDIVIDUAL FUNDING REQUEST POLICY
 REVERSAL OF STERILISATION/ VASECTOMY INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1516.v1.1a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 23 September 2015 Name
REVERSAL OF STERILISATION/ VASECTOMY INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1516.v1.1a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 23 September 2015 Name
GOVERNING BOARD. Assisted Conception (IVF): Review of access criteria. Date of Meeting 21 January 2015 Agenda Item No 13. Title
 GOVERNING BOARD Date of Meeting 21 January 2015 Agenda Item No 13 Title Assisted Conception (IVF): Review of access criteria Purpose of Paper The SHIP (Southampton, Hampshire, Isle of Wight and Portsmouth)
GOVERNING BOARD Date of Meeting 21 January 2015 Agenda Item No 13 Title Assisted Conception (IVF): Review of access criteria Purpose of Paper The SHIP (Southampton, Hampshire, Isle of Wight and Portsmouth)
West Hampshire Clinical Commissioning Group Board
 West Hampshire Clinical Commissioning Group Board Date of meeting 25 July 2013 Agenda Item 9 Paper No WHCCG13/089 Priorities Committee Statement Assisted Conception/IVF Key issues An Interim Policy Statement
West Hampshire Clinical Commissioning Group Board Date of meeting 25 July 2013 Agenda Item 9 Paper No WHCCG13/089 Priorities Committee Statement Assisted Conception/IVF Key issues An Interim Policy Statement
The Newcastle upon Tyne Hospitals NHS Foundation Trust. Pre-filled Patient Controlled Analgesia (PCA) syringes
 The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
ASSISTED CONCEPTION NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA & POLICY GUIDANCE
 ASSISTED CONCEPTION NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA & POLICY GUIDANCE Version 1.0 Page 1 of 11 MARCH 2014 POLICY DOCUMENT VERSION CONTROL CERTIFICATE TITLE Title: Assisted Conception
ASSISTED CONCEPTION NHS FUNDED TREATMENT FOR SUBFERTILITY ELIGIBILITY CRITERIA & POLICY GUIDANCE Version 1.0 Page 1 of 11 MARCH 2014 POLICY DOCUMENT VERSION CONTROL CERTIFICATE TITLE Title: Assisted Conception
making a referral for breast imaging Standard Operating Procedure
 Document Control Title Reporting Radiographer Author Directorate Surgery Date Version Issued 0.1 May 2016 Status Draft Author s job title Reporting Radiographer Department Breast Imaging Comment / Changes
Document Control Title Reporting Radiographer Author Directorate Surgery Date Version Issued 0.1 May 2016 Status Draft Author s job title Reporting Radiographer Department Breast Imaging Comment / Changes
Blackpool CCG. Policies for the Commissioning of Healthcare. Assisted Conception
 1 Introduction Blackpool CCG Policies for the Commissioning of Healthcare Assisted Conception 1.1 This policy describes circumstances in which NHS Blackpool Clinical Commissioning Group (CCG) will fund
1 Introduction Blackpool CCG Policies for the Commissioning of Healthcare Assisted Conception 1.1 This policy describes circumstances in which NHS Blackpool Clinical Commissioning Group (CCG) will fund
Draft Falls Prevention Strategy
 Cheshire West & Chester Council Draft Falls Prevention Strategy 2017-2020 Visit: cheshirewestandchester.gov.uk Visit: cheshirewestandchester.gov.uk 02 Cheshire West and Chester Council Draft Falls Prevention
Cheshire West & Chester Council Draft Falls Prevention Strategy 2017-2020 Visit: cheshirewestandchester.gov.uk Visit: cheshirewestandchester.gov.uk 02 Cheshire West and Chester Council Draft Falls Prevention
Enhanced Service Specification. Childhood seasonal influenza vaccination programme 2018/19
 Enhanced Service Specification Childhood seasonal influenza vaccination programme 2018/19 Contents Childhood seasonal influenza vaccination programme... 1 Contents... 4 1 Introduction... 5 2 Background...
Enhanced Service Specification Childhood seasonal influenza vaccination programme 2018/19 Contents Childhood seasonal influenza vaccination programme... 1 Contents... 4 1 Introduction... 5 2 Background...
Osteoporosis: fragility fracture risk. Costing report. Implementing NICE guidance
 Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
28 th September Author Jeremy Gilbert Bariatric Nurse Specialist
 POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
Fertility Policy. December Introduction
 Fertility Policy December 2015 Introduction Camden Clinical Commissioning Group (CCG) is responsible for commissioning a range of health services including hospital, mental health and community services
Fertility Policy December 2015 Introduction Camden Clinical Commissioning Group (CCG) is responsible for commissioning a range of health services including hospital, mental health and community services
TRIGGER FINGER CRITERIA BASED ACCESS POLICY
 TRIGGER FINGER CRITERIA BASED ACCESS POLICY Version: Discussion and Recommendation by the Somerset CCG Clinical Commissioning Policy Forum 1617.v1b Date: 16 June 2016 Name of Originator/Author: Name of
TRIGGER FINGER CRITERIA BASED ACCESS POLICY Version: Discussion and Recommendation by the Somerset CCG Clinical Commissioning Policy Forum 1617.v1b Date: 16 June 2016 Name of Originator/Author: Name of
SCHEDULE 2 THE SERVICES. A. Service Specifications
 SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No. 04/MSKT/0013 Service PAN DORSET FRACTURE LIAISON SERVICE Commissioner Lead CCP for Musculoskeletal & Trauma Provider Lead Deputy
SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No. 04/MSKT/0013 Service PAN DORSET FRACTURE LIAISON SERVICE Commissioner Lead CCP for Musculoskeletal & Trauma Provider Lead Deputy
Protocol. Ultrasound Accelerated Fracture Healing Device
 Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/17 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
Protocol Ultrasound Accelerated Fracture Healing Device (10105) Medical Benefit Effective Date: 04/01/14 Next Review Date: 01/17 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10, 05/11, 01/12,
NHS Grampian Tobacco Policy 2016
 NHS Grampian Tobacco Policy 2016 Co-ordinator: Tobacco Policy Review Group Reviewer: GAPF Policies Subgroup Approver: Grampian Area Partnership Forum (GAPF) Revised 2016 Review date: 2017 Uncontrolled
NHS Grampian Tobacco Policy 2016 Co-ordinator: Tobacco Policy Review Group Reviewer: GAPF Policies Subgroup Approver: Grampian Area Partnership Forum (GAPF) Revised 2016 Review date: 2017 Uncontrolled
St Helens CCG NHS Funded Treatment for Subfertility Policy 2015/16
 St Helens CCG NHS Funded Treatment for Subfertility Policy 2015/16 1 Standard Operating Procedure St Helens CCG NHS Funded Treatment for Sub Fertility Policy Version 1 Implementation Date May 2015 Review
St Helens CCG NHS Funded Treatment for Subfertility Policy 2015/16 1 Standard Operating Procedure St Helens CCG NHS Funded Treatment for Sub Fertility Policy Version 1 Implementation Date May 2015 Review
COMMISSIONING POLICY FOR IN VITRO FERTILISATION (IVF)/ INTRACYTOPLASMIC SPERM INJECTION (ICSI) WITHIN TERTIARY INFERTILITY SERVICES V2.
 COMMISSIONING POLICY FOR IN VITRO FERTILISATION (IVF)/ INTRACYTOPLASMIC SPERM INJECTION (ICSI) WITHIN TERTIARY INFERTILITY SERVICES V2.3 2017 Agreed at Cannock Chase CCG Signature: Designation: Chair of
COMMISSIONING POLICY FOR IN VITRO FERTILISATION (IVF)/ INTRACYTOPLASMIC SPERM INJECTION (ICSI) WITHIN TERTIARY INFERTILITY SERVICES V2.3 2017 Agreed at Cannock Chase CCG Signature: Designation: Chair of
HALTON CLINICAL COMMISSIONING GROUP NHS FUNDED TREATMENT FOR SUBFERTILITY. CONTENTS Page
 HALTON CLINICAL COMMISSIONING GROUP NHS FUNDED TREATMENT FOR SUBFERTILITY CONTENTS Page 1. INTRODUCTION 2 2. GENERAL PRINCIPLES 2 3. DEFINITION OF SUBFERTILITY AND TIMING OF ACCESS TO TREATMENT 3 4. DEFINITION
HALTON CLINICAL COMMISSIONING GROUP NHS FUNDED TREATMENT FOR SUBFERTILITY CONTENTS Page 1. INTRODUCTION 2 2. GENERAL PRINCIPLES 2 3. DEFINITION OF SUBFERTILITY AND TIMING OF ACCESS TO TREATMENT 3 4. DEFINITION
Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital
 Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
Please Note: This policy is currently under review and is still fit for purpose. Venous Thromboembolism (VTE) Prevention and Treatment of VTE in Patients Admitted to Hospital This procedural document supersedes
LIPOSUCTION (COSMETIC) INDIVIDUAL FUNDING REQUEST POLICY
 LIPOSUCTION (COSMETIC) INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1617.v2a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 13 July 2016 Name of Originator/Author:
LIPOSUCTION (COSMETIC) INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1617.v2a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 13 July 2016 Name of Originator/Author:
Corporate Medical Policy
 Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2017 6/2018 6/2017 Description
Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2017 6/2018 6/2017 Description
Patient Group Directions Policy
 Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Medical technologies guidance Published: 9 January 2013 nice.org.uk/guidance/mtg12
 EXOGEN ultrasound bone healing system for long bone fractures with non- union or delayed ed healing Medical technologies guidance Published: 9 January 2013 nice.org.uk/guidance/mtg12 NICE 2017. All rights
EXOGEN ultrasound bone healing system for long bone fractures with non- union or delayed ed healing Medical technologies guidance Published: 9 January 2013 nice.org.uk/guidance/mtg12 NICE 2017. All rights
Specialised Services Policy Position PP151
 Specialised Services Policy Position PP151 Complex Devices: Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for arrhythmias and heart failure January 2019 Version 1.0 Document
Specialised Services Policy Position PP151 Complex Devices: Implantable Cardioverter Defibrillators and Cardiac Resynchronisation Therapy for arrhythmias and heart failure January 2019 Version 1.0 Document
Health and Safety Policy Arrangements: Radiation Protection Guidelines
 Health and Safety Policy Arrangements: Radiation Protection Guidelines Author: Dr N. Sarrami Date of Approval: 12//2010 Due Review Date: 12//2012 1 Radiation Protection Guidelines CONTENTS Section Section
Health and Safety Policy Arrangements: Radiation Protection Guidelines Author: Dr N. Sarrami Date of Approval: 12//2010 Due Review Date: 12//2012 1 Radiation Protection Guidelines CONTENTS Section Section
National Institute for Health and Care Excellence IP1714 Low intensity pulsed ultrasound to promote healing of delayed-union and non-union fractures
 National Institute for Health and Care Excellence IP1714 Low intensity pulsed ultrasound to promote healing of delayed-union and non-union fractures IPAC 10/05/18: Com. no. Consultee name and organisation
National Institute for Health and Care Excellence IP1714 Low intensity pulsed ultrasound to promote healing of delayed-union and non-union fractures IPAC 10/05/18: Com. no. Consultee name and organisation
Surgical Intervention for Simple Snoring Individual Funding Requests Policy
 Surgical Intervention for Simple Snoring Individual Funding Requests Policy Version: Recommendation by: 1516.v1.1a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 23 September 2015
Surgical Intervention for Simple Snoring Individual Funding Requests Policy Version: Recommendation by: 1516.v1.1a Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 23 September 2015
Insulin Pumps and Glucose Monitors in Adults Policy
 Insulin Pumps and Glucose Monitors in Adults Policy Version: 2016-19 Ratified by: NHS Leeds West CCG Assurance Committee on; 16 November 2016 NHS Leeds North CCG Governance on Performance and Risk Committee
Insulin Pumps and Glucose Monitors in Adults Policy Version: 2016-19 Ratified by: NHS Leeds West CCG Assurance Committee on; 16 November 2016 NHS Leeds North CCG Governance on Performance and Risk Committee
Directed Enhanced Service (DES) for H1N1 Vaccination Programme JCVI priority groups
 Directed Enhanced Service (DES) for H1N1 Vaccination Programme JCVI priority groups October 2009 Introduction NHS Employers and the General Practitioners Committee (GPC) of the BMA have agreed arrangements
Directed Enhanced Service (DES) for H1N1 Vaccination Programme JCVI priority groups October 2009 Introduction NHS Employers and the General Practitioners Committee (GPC) of the BMA have agreed arrangements
A patient s guide to. Radial Extracorporeal Shockwave Therapy (ESWT)
 A patient s guide to Radial Extracorporeal Shockwave Therapy (ESWT) The Foot & Ankle Unit at the Royal National Orthopaedic Hospital is made up of a multi-disciplinary team. The team consists of four specialist
A patient s guide to Radial Extracorporeal Shockwave Therapy (ESWT) The Foot & Ankle Unit at the Royal National Orthopaedic Hospital is made up of a multi-disciplinary team. The team consists of four specialist
Commissioning policy for: Hallux Valgus (bunions)
 Commissioning policy for: Hallux Valgus (bunions) 01 April 2016 VERSION CONTROL Version: 3.0 Ratified by: NHS Warwickshire rth CCG Governing Body Date ratified: 24 March 2016 Name of originator/author:
Commissioning policy for: Hallux Valgus (bunions) 01 April 2016 VERSION CONTROL Version: 3.0 Ratified by: NHS Warwickshire rth CCG Governing Body Date ratified: 24 March 2016 Name of originator/author:
Specialised Services Policy: CP23 Vagal Nerve Stimulation
 Specialised Services Policy: CP23 Vagal Nerve Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning and Performance
Specialised Services Policy: CP23 Vagal Nerve Stimulation Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of Planning and Performance
CETUXIMAB FOR THE FIRST LINE TREATMENT OF METASTATIC COLORECTAL CANCER (MCRC)
 CETUXIMAB FOR THE FIRST LINE TREATMENT OF METASTATIC COLORECTAL CANCER (MCRC) 1. BACKGROUND submitted evidence to NICE on the 2 nd cetuximab in the treatment of mcrc. May 2008 for the appraisal of The
CETUXIMAB FOR THE FIRST LINE TREATMENT OF METASTATIC COLORECTAL CANCER (MCRC) 1. BACKGROUND submitted evidence to NICE on the 2 nd cetuximab in the treatment of mcrc. May 2008 for the appraisal of The
Putting NICE guidance into practice. Resource impact report: ifuse for treating chronic sacroiliac joint pain (MTG39)
 Putting NICE guidance into practice Resource impact report: ifuse for treating chronic sacroiliac joint pain (MTG39) Published: October 2018 Summary NICE has recommended ifuse for treating chronic sacroiliac
Putting NICE guidance into practice Resource impact report: ifuse for treating chronic sacroiliac joint pain (MTG39) Published: October 2018 Summary NICE has recommended ifuse for treating chronic sacroiliac
HC 963 SesSIon november Department of Health. Young people s sexual health: the National Chlamydia Screening Programme
 Report by the Comptroller and Auditor General HC 963 SesSIon 2008 2009 12 november 2009 Department of Health Young people s sexual health: the National Chlamydia Screening Programme 4 Summary Young people
Report by the Comptroller and Auditor General HC 963 SesSIon 2008 2009 12 november 2009 Department of Health Young people s sexual health: the National Chlamydia Screening Programme 4 Summary Young people
Hip Replacement Surgery Including referral for Surgical Assessment of Osteoarthritis Criteria Based Access Policy
 Hip Replacement Surgery Including referral for Surgical Assessment of Osteoarthritis Criteria Based Access Policy Version: 1617.v6 Recommendation by: Somerset CCG Clinical Commissioning Policy Forum (CCPF)
Hip Replacement Surgery Including referral for Surgical Assessment of Osteoarthritis Criteria Based Access Policy Version: 1617.v6 Recommendation by: Somerset CCG Clinical Commissioning Policy Forum (CCPF)
Management of AIDS/HIV Infected Healthcare Workers Policy
 Management of AIDS/HIV Infected Healthcare Workers Policy DOCUMENT CONTROL: Version: 4 Ratified by: Corporate Policy Panel Date ratified: 20 July 2017 Name of originator/author: HR Manager Name of responsible
Management of AIDS/HIV Infected Healthcare Workers Policy DOCUMENT CONTROL: Version: 4 Ratified by: Corporate Policy Panel Date ratified: 20 July 2017 Name of originator/author: HR Manager Name of responsible
BREAST IMPLANT SURGERY INDIVIDUAL FUNDING REQUEST POLICY
 BREAST IMPLANT SURGERY INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1617.V2b Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 13 July 2016 Name of Originator/Author:
BREAST IMPLANT SURGERY INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1617.V2b Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 13 July 2016 Name of Originator/Author:
ifuse implant system for treating chronic sacroiliac joint pain
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance SCOPE ifuse implant system for treating chronic sacroiliac joint pain 1 Technology 1.1 Description of the technology The ifuse
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance SCOPE ifuse implant system for treating chronic sacroiliac joint pain 1 Technology 1.1 Description of the technology The ifuse
GOVERNING BODY MEETING in Public 22 February 2017 Agenda Item 3.4
 GOVERNING BODY MEETING in Public 22 February 2017 Paper Title Purpose of paper Redesign of Services for Frail Older People in Eastern Cheshire To seek approval from Governing Body for the redesign of services
GOVERNING BODY MEETING in Public 22 February 2017 Paper Title Purpose of paper Redesign of Services for Frail Older People in Eastern Cheshire To seek approval from Governing Body for the redesign of services
Director of Commissioning, Telford and Wrekin CCG and Shropshire CCG. Version No. Approval Date August 2015 Review Date August 2017
 Commissioning Policy for In Vitro Fertilisation (IVF)/ Intracytoplasmic Sperm Injection (ICSI) within tertiary Infertility Services, in Shropshire and Telford and Wrekin Owner(s) Version No. Director of
Commissioning Policy for In Vitro Fertilisation (IVF)/ Intracytoplasmic Sperm Injection (ICSI) within tertiary Infertility Services, in Shropshire and Telford and Wrekin Owner(s) Version No. Director of
PATIENT GROUP DIRECTION PROCEDURE
 PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
PATIENT GROUP DIRECTION PROCEDURE Date approved 2 October 2015 Version 3 Approved by Yvette Oade, Chief Medical Officer Procedure Lead Clinical Governance Lead - Medicines Management Procedure Author Karen
Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641
 Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641 Introduction 1. All GMS practices are expected to provide essential and those additional
Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641 Introduction 1. All GMS practices are expected to provide essential and those additional
Policy updated: November 2018 (approved by Haringey and Islington s Executive Management Team on 5 December 2018)
 Islington CCG Fertility Policy First approved: 29 January 2015 Policy updated: November 2018 (approved by Haringey and Islington s Executive Management Team on 5 December 2018) Introduction Islington CCG
Islington CCG Fertility Policy First approved: 29 January 2015 Policy updated: November 2018 (approved by Haringey and Islington s Executive Management Team on 5 December 2018) Introduction Islington CCG
Commissioning Policy Individual Funding Request
 Commissioning Policy Individual Funding Request Female Sterilisation Prior Approval Policy Date Adopted: 6 th February 2017 Version: 1617.1 Individual Funding Request Team - A partnership between Bristol,
Commissioning Policy Individual Funding Request Female Sterilisation Prior Approval Policy Date Adopted: 6 th February 2017 Version: 1617.1 Individual Funding Request Team - A partnership between Bristol,
UTILIZATION MANAGEMENT POLICY
 TITLE: BONE GROWTH STIMULATORS EFFECTIVE DATE: July 1, 2015 UTILIZATION MANAGEMENT POLICY DOCUMENT HISTORY Original Endorsement Date: May, 1991 Subsequent Endorsement Date(s): 02/1992, 03/1994, 03/1995,
TITLE: BONE GROWTH STIMULATORS EFFECTIVE DATE: July 1, 2015 UTILIZATION MANAGEMENT POLICY DOCUMENT HISTORY Original Endorsement Date: May, 1991 Subsequent Endorsement Date(s): 02/1992, 03/1994, 03/1995,
Extra-corporeal Shock Wave Therapy (ESWT) for non-insertional Achilles tendinopathy Inflamed Achilles tendon
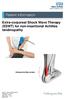 Extra-corporeal Shock Wave Therapy (ESWT) for non-insertional Achilles tendinopathy Inflamed Achilles tendon Source: Trauma & Orthopaedics Reference No: 6459-1 Issue date: 1/2/19 Review date: 1/2/22 Page
Extra-corporeal Shock Wave Therapy (ESWT) for non-insertional Achilles tendinopathy Inflamed Achilles tendon Source: Trauma & Orthopaedics Reference No: 6459-1 Issue date: 1/2/19 Review date: 1/2/22 Page
Local Enhanced Service Specification. MenACWY vaccination programme for 15 and 16 year olds (school year 11) in Cornwall and the Isles of Scilly
 Local Enhanced Service Specification MenACWY vaccination programme for 15 and 16 year olds (school year 11) in Cornwall and the Isles of Scilly 1 st January 2016 to 31 st August 2016 1 Local Enhanced Service
Local Enhanced Service Specification MenACWY vaccination programme for 15 and 16 year olds (school year 11) in Cornwall and the Isles of Scilly 1 st January 2016 to 31 st August 2016 1 Local Enhanced Service
Bone Growth Stimulation
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
No Smoking Policy. No Smoking Policy
 No Smoking Policy Document Status Version: V4.0 Approved DOCUMENT CHANGE HISTORY Initiated by Date Author HR Version Date Comments (i.e. viewed, or reviewed, amended approved by person or committee) V1.1
No Smoking Policy Document Status Version: V4.0 Approved DOCUMENT CHANGE HISTORY Initiated by Date Author HR Version Date Comments (i.e. viewed, or reviewed, amended approved by person or committee) V1.1
Haringey CCG Fertility Policy April 2014
 Haringey CCG Fertility Policy April 2014 1 SUMMARY This policy describes the clinical pathways and entry criteria for Haringey patients wishing to access NHS funded fertility treatment. 2 RESPONSIBLE PERSON:
Haringey CCG Fertility Policy April 2014 1 SUMMARY This policy describes the clinical pathways and entry criteria for Haringey patients wishing to access NHS funded fertility treatment. 2 RESPONSIBLE PERSON:
IT and Information Acceptable Use Policy
 BMI IMpol04 Information Management IT and Information Acceptable Use Policy This is a controlled document and whilst this document may be printed, the electronic version posted on the intranet/shared drive
BMI IMpol04 Information Management IT and Information Acceptable Use Policy This is a controlled document and whilst this document may be printed, the electronic version posted on the intranet/shared drive
SHIP8 Clinical Commissioning Groups Priorities Committee (Southampton, Hampshire, Isle of Wight and Portsmouth CCGs)
 SHIP8 Clinical Commissioning Groups Priorities Committee (Southampton, Hampshire, Isle of Wight and Portsmouth CCGs) Policy Recommendation 002: Assisted Conception Services Date of Issue: September 2014
SHIP8 Clinical Commissioning Groups Priorities Committee (Southampton, Hampshire, Isle of Wight and Portsmouth CCGs) Policy Recommendation 002: Assisted Conception Services Date of Issue: September 2014
Commissioning Policy Individual Funding Request
 Commissioning Policy Individual Funding Request Extracorporeal Shockwave Therapy (ESWT) Individual Funding Requests Policy Date Adopted: 6 th February 2017 Version: 1617.1 Individual Funding Request Team
Commissioning Policy Individual Funding Request Extracorporeal Shockwave Therapy (ESWT) Individual Funding Requests Policy Date Adopted: 6 th February 2017 Version: 1617.1 Individual Funding Request Team
DRAFT. Greater Manchester EUR Policy Statement
 DRAFT Greater Manchester EUR Policy Statement Title/Topic: Ultrasound and pulsed electromagnetic systems (PES) for bone healing Reference: GM063 Date: November 2015 VERSION CONTROL Version Date Details
DRAFT Greater Manchester EUR Policy Statement Title/Topic: Ultrasound and pulsed electromagnetic systems (PES) for bone healing Reference: GM063 Date: November 2015 VERSION CONTROL Version Date Details
Prescription only medicines (POMs)
 Prescription only medicines (POMs) 2017 Learning objectives Explain the legal framework with which registered health care professionals can administer prescription only medicines Define the role and limitations
Prescription only medicines (POMs) 2017 Learning objectives Explain the legal framework with which registered health care professionals can administer prescription only medicines Define the role and limitations
Approved January Waltham Forest CCG Fertility policy
 Approved January 2015 Waltham Forest CCG Fertility policy Contents 1 Introduction 1 2 Individual Funding Requests 1 2.1 Eligibility criteria 1 2.2 Number of cycles funded 2 2.3 Treatment Pathway 3 Page
Approved January 2015 Waltham Forest CCG Fertility policy Contents 1 Introduction 1 2 Individual Funding Requests 1 2.1 Eligibility criteria 1 2.2 Number of cycles funded 2 2.3 Treatment Pathway 3 Page
Guidelines for the appointment of. General Practitioners with Special Interests in the Delivery of Clinical Services. Epilepsy
 Guidelines for the appointment of General Practitioners with Special Interests in the Delivery of Clinical Services Epilepsy April 2003 Epilepsy This general practitioner with special interest (GPwSI)
Guidelines for the appointment of General Practitioners with Special Interests in the Delivery of Clinical Services Epilepsy April 2003 Epilepsy This general practitioner with special interest (GPwSI)
Warrington Centre for Sexual Health WCSH Local Enhanced Service LES
 Warrington Centre for Sexual Health WCSH Local Enhanced Service LES 2015-19 NOTE: This LES is only for asymptomatic persons and does not cover testing and treatment of symptomatic persons Introduction
Warrington Centre for Sexual Health WCSH Local Enhanced Service LES 2015-19 NOTE: This LES is only for asymptomatic persons and does not cover testing and treatment of symptomatic persons Introduction
COMMISSIONING POLICY FOR IN VITRO FERTILISATION (IVF)/ INTRACYTOPLASMIC SPERM INJECTION (ICSI) WITHIN TERTIARY INFERTILITY SERVICES
 COMMISSIONING POLICY FOR IN VITRO FERTILISATION (IVF)/ INTRACYTOPLASMIC SPERM INJECTION (ICSI) WITHIN TERTIARY INFERTILITY SERVICES Version number V2.3 Responsible individual Author(s) Barry Weaver Trish
COMMISSIONING POLICY FOR IN VITRO FERTILISATION (IVF)/ INTRACYTOPLASMIC SPERM INJECTION (ICSI) WITHIN TERTIARY INFERTILITY SERVICES Version number V2.3 Responsible individual Author(s) Barry Weaver Trish
pat hways Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166
 pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
North Staffordshire Clinical Commissioning Group. Infertility and Assisted Reproduction Commissioning Policy and Eligibility Criteria
 North Staffordshire Clinical Commissioning Group Infertility and Assisted Reproduction Commissioning Policy and Eligibility Criteria Policy Infertility and Assisted Reproduction Commissioning Policy and
North Staffordshire Clinical Commissioning Group Infertility and Assisted Reproduction Commissioning Policy and Eligibility Criteria Policy Infertility and Assisted Reproduction Commissioning Policy and
BUNION (AND OTHER PAINFUL TOE CONDITION) SURGICAL TREATMENT POLICY PRIOR APPROVAL
 BUNION (AND OTHER PAINFUL TOE CONDITION) SURGICAL TREATMENT POLICY PRIOR APPROVAL Version: 1718.v3 Recommendation by: Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 12 July 2017
BUNION (AND OTHER PAINFUL TOE CONDITION) SURGICAL TREATMENT POLICY PRIOR APPROVAL Version: 1718.v3 Recommendation by: Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 12 July 2017
FERTILITY SERVICE POLICY
 FERTILITY SERVICE POLICY Page 1 of 8 FERTILITY SERVICE POLICY Please note that all Clinical Commissioning policies are currently under review and elements within the individual policies may have been replaced
FERTILITY SERVICE POLICY Page 1 of 8 FERTILITY SERVICE POLICY Please note that all Clinical Commissioning policies are currently under review and elements within the individual policies may have been replaced
Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical Commissioning Group
 BEFORE USING THIS POLICY ALWAYS ENSURE YOU ARE USING THE MOST UP TO DATE VERSION Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical
BEFORE USING THIS POLICY ALWAYS ENSURE YOU ARE USING THE MOST UP TO DATE VERSION Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical
SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD
 AGENDA ITEM 8 GOVERNING BODY MEETING IN PUBLIC ON 25 TH SEPTEMBER 2014 SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD Date of the meeting 25 th September 2014 Author Sponsoring Board
AGENDA ITEM 8 GOVERNING BODY MEETING IN PUBLIC ON 25 TH SEPTEMBER 2014 SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD Date of the meeting 25 th September 2014 Author Sponsoring Board
ABDOMINOPLASTY/APRONECTOMY INDIVIDUAL FUNDING REQUEST POLICY
 ABDOMINOPLASTY/APRONECTOMY INDIVIDUAL FUNDING REQUEST POLICY Version: Ratified by: 1516.v1.2 Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 23 September 2015 Name of Originator/Author:
ABDOMINOPLASTY/APRONECTOMY INDIVIDUAL FUNDING REQUEST POLICY Version: Ratified by: 1516.v1.2 Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 23 September 2015 Name of Originator/Author:
SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT
 SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT PROTOCOL This document sets out the details of the administrative protocol for the direct referral by Optometrists/OMPs of cataract patients.
SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT PROTOCOL This document sets out the details of the administrative protocol for the direct referral by Optometrists/OMPs of cataract patients.
Governing Body Meeting
 Agenda Item No: 13 Date of Meeting: 26 th November 2015 Governing Body Meeting Paper Title: East and North Hertfordshire CCG (ENHCCG) Policy on Fertility treatment and referral criteria for specialist
Agenda Item No: 13 Date of Meeting: 26 th November 2015 Governing Body Meeting Paper Title: East and North Hertfordshire CCG (ENHCCG) Policy on Fertility treatment and referral criteria for specialist
Policy statement. Commissioning of Fertility treatments
 Policy statement Commissioning of Fertility treatments NB: The policy relating to commissioning of fertility treatments is unchanged from the version approved by the CCG in March 2017. The clinical thresholds
Policy statement Commissioning of Fertility treatments NB: The policy relating to commissioning of fertility treatments is unchanged from the version approved by the CCG in March 2017. The clinical thresholds
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation of the Spine as an Adjunct
FEP Medical Policy Manual Effective Date: October 15, 2017 Related Policies: 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton 7.01.85 Electrical Stimulation of the Spine as an Adjunct
Richard Watson, Chief Transformation Officer. Dr P Holloway, GP Clinical Lead for Cancer Lisa Parrish, Senior Transformation Lead
 GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
GOVERNING BODY Agenda Item No. 08 Reference No. IESCCG 18-02 Date. 23 January 2018 Title Lead Chief Officer Author(s) Purpose Cancer Services Update Richard Watson, Chief Transformation Officer Dr P Holloway,
Primary Health Networks
 Primary Health Networks Drug and Alcohol Treatment Activity Work Plan 2016-17 to 2018-19 Hunter New England & Central Coast Please note: This Activity Work Plan was developed in response to the HNECC PHN
Primary Health Networks Drug and Alcohol Treatment Activity Work Plan 2016-17 to 2018-19 Hunter New England & Central Coast Please note: This Activity Work Plan was developed in response to the HNECC PHN
Psychosis & Schizophrenia: The Updated NICE Quality Standard. Dr Tony Gill Mental Health Practitioner University of Leeds 7 th June 2015
 Psychosis & Schizophrenia: The Updated NICE Quality Standard Dr Tony Gill Mental Health Practitioner University of Leeds 7 th June 2015 NICE.what is it? The National Institute for Health & Care Excellence
Psychosis & Schizophrenia: The Updated NICE Quality Standard Dr Tony Gill Mental Health Practitioner University of Leeds 7 th June 2015 NICE.what is it? The National Institute for Health & Care Excellence
