pat hways Medtech innovation briefing Published: 10 November 2015 nice.org.uk/guidance/mib45
|
|
|
- Camilla Davidson
- 6 years ago
- Views:
Transcription
1 pat hways PneuX for preventing enting ventilator-associated pneumonia in intensive e care Medtech innovation briefing Published: 10 November 2015 nice.org.uk/guidance/mib45 Summary The PneuX tube system is intended for airway management in critically ill patients who are having mechanical ventilation. It is designed to prevent ventilator-associated pneumonia by minimising the risk of pulmonary aspiration and micro-aspiration in patients having ventilation for 24 hours or more. One randomised controlled trial in high-risk cardiac patients found that the PneuX system was associated with a statistically significant reduction in the incidence of ventilator-associated pneumonia compared with a standard endotracheal tube. A PneuX endotracheal tube costs 150 and a PneuX tracheostomy tube costs 175 (both excluding VAT). Page 1 of
2 Product summary and likely place in therapy PneuX is a endotracheal/ tracheostomy tube system for airway management designed to prevent ventilator-associated pneumonia (VAP) in patients having ventilation for 24 hours or more. The system could be used to replace standard endotracheal and tracheostomy tubes that have no subglottic drainage access, subglottic drainage access but with a high pressure cuff, or no continuous cuff-pressure monitor. Effectiveness eness and safety One UK-based randomised controlled trial in 240 high-risk patients having cardiac surgery found that PneuX was associated with a significant reduction in VAP incidence compared with a standard endotracheal tube (10.8% compared with 21%, p=0.03). There was no statistically significant difference in any other outcome measures. One UK-based retrospective cohort study in a medical and surgical ICU reported VAP incidence of 6% in 48 patients with the PneuX system. Unplanned extubation was reported in 17% of patients; 10% of patients self-extubated. One UK-based retrospective cohort study in 53 critically ill patients with the PneuX system found no incidence of VAP while the PneuX system was in place. One UK-based retrospective cohort study analysing records of 185 intubations using the PneuX system found that the incidence of unplanned extubations was 0.1% over 982 intubation days. Page 2 of
3 Technical and patient factors The PneuX system consists of an endotracheal or tracheostomy tube, the PneuX tracheal seal monitor and an extension tube. The manufacturer recommends that the tube and monitor should only be used together and not with other devices. The PneuX endotracheal/tracheostomy tube has multiple access ports for subglottic drainage and a low-volume, low pressure cuff made from soft silicone. The PneuX tracheal seal monitor has a default setting to maintain a constant low pressure on the tracheal wall (30 cm H 2 O, which requires an intra-cuff pressure of 80 cm H 2 O). Cost and resource use A single-use PneuX endotracheal tube costs 150 (excluding VAT). A single-use PneuX tracheostomy tube costs 175 (excluding VAT). The PneuX tracheal seal monitor is provided to the hospital on a loan basis. The extension tube is provided at no charge to the hospital. The PneuX endotracheal/tracheostomy tube is available in 3 sizes, with an inner diameter of 7.0, 8.0 or 9.0 mm. A smaller PneuX endotracheal tube with an inner diameter of 6.0 mm is also available. Both the PneuX endotracheal/tracheostomy tube and the extension tube are supplied sterile and for single use. Introduction Ventilator-associated pneumonia (VAP) is a hospital-acquired infection. Although there is no consensus definition, it is often defined as pneumonia that occurs in patients who have had intubation with an endotracheal or tracheostomy tube to help or control respiratory function continuously for at least 48 hours before the onset of the pneumonia (American Thoracic Society and Infectious Diseases Society of America, 2005). The presence of a tracheal tube interferes with the normal protective reflexes of the upper airway, such as coughing. This can result in impaired clearance of micro-organisms and rapid colonisation of the oropharyngeal secretions with aerobic Gram-negative bacteria. These contaminated secretions gather above the cuff of the tracheal tube Page 3 of
4 and slowly leak down into the airway, leading to the unintentional entry of very small amounts of contaminated material into the respiratory tract (micro-aspiration). This is the main cause of VAP (Hunter 2012). There are no standard criteria to diagnose VAP; a diagnosis is generally based on clinical signs and symptoms, chest X-rays and microbiological confirmation (American Thoracic Society and Infectious Diseases Society of America, 2005). Around 83,500 intubations were carried out in England in (Health and Social Care Information Centre, 2015). Because of a lack of standardised diagnostic criteria, it is difficult to quantify the exact incidence of VAP. However, VAP is a common complication of mechanical ventilation and the most common infection in ICU (Bouza et al. 2006, Zuschneid et al. 2007, Hunter 2012). Between 10% and 20% of patients who have mechanical ventilation for longer than 48 hours will develop VAP. Critically ill patients who develop VAP appear to be twice as likely to die compared with similar patients without VAP (Safdar et al. 2005). Risk factors for the development of VAP include prolonged mechanical ventilation, older age, lying face-up and comorbidities (Bauer et al. 2000). The risk for patients is highest during early ICU stay when it is estimated to be 3% per day during days 1 5 of ventilation, 2% per day during days 5 10 of ventilation and 1% per day thereafter (Masterton, 2008). Various strategies have been developed to reduce the risk of ICU patients developing VAP, including specialised endotracheal tubes. These tubes include features for continuous subglottic drainage and adequate pressure of the endotracheal-tube cuff to prevent leakage of colonised subglottic secretions into the lower airway (Kollef, 1999; Dodek et al. 2004). Technology overview This briefing describes the regulated use of the technology for the indication specified, in the setting described, and with any other specific equipment referred to. It is the responsibility of healthcare professionals to check the regulatory status of any intended use of the technology in other indications and settings. About the technology CE marking The PneuX system consists of a class III device (endotracheal/tracheal tube) and a class IIb device (PneuX tracheal seal monitor, previously known as Venner tracheal seal monitor). The manufacturer, Venner Medical (Singapore) Pte, received a CE mark for the PneuX endotracheal and Page 4 of
5 tracheostomy tubes on 20 March 2007 and for the tracheal seal monitor and extension tube on 22 October Description The PneuX system was formerly known as the Venner PneuX P.Y. - VAP Prevention System and the Lo-Trach system. It has also been referred to as the PneuX VAP prevention system, the PneuX P.Y. system and the Venner-PneuX system. The system is designed to prevent VAP by minimising the risk of pulmonary aspiration and micro-aspiration during long-term mechanical ventilation, which is expected to be more than 24 hours but no more than 30 days. The PneuX system consists of 3 component parts: PneuX endotracheal/tracheostomy tube a flexible silicone tube with a cuff, a flange, a drain tube, an inflation tube, a reservoir and a 15 mm standard connector. The tubes are compatible with magnetic resonance imaging (MRI) and are available in 3 sizes: 7.0, 8.0 or 9.0 mm inner diameter. A smaller PneuX endotracheal tube with an inner diameter of 6.0 mm is also available. The PneuX tracheal seal monitor an electronic automatic pressure controller for the inflation volume and pressure within the tube cuff during use. Extension tube a 2-metre extension tube for the PneuX tracheal seal monitor. It connects the air outlet on the PneuX tracheal seal monitor and the pilot valve of the PneuX endotracheal/ tracheostomy tube. The system has the following main features: Low-volume, low-pressure cuff and PneuX tracheal seal monitor the low volume cuff is made from a soft silicone material. The PneuX tracheal seal monitor is an electronic automatic pressure controller which controls and maintains the safe inflation volume and pressure within the cuff during use. Its default setting is to maintain a constant low pressure on the tracheal wall (30 cm H 2 O), which is recognised to reduce the risk of tracheal mucosa injury compared with higher pressures. An intra-cuff pressure of approximately 80 cm H 2 O is needed to produce a pressure of 30 cm H 2 O on the tracheal wall. By continuously adjusting and maintaining the pressure of the cuff against the trachea wall, the monitor is designed to allow the cuff to produce a tracheal seal without creases, which the manufacturer claims is optimal for reducing the risk of aspiration. The cuff and monitor also allow for intermittent increases in Page 5 of
6 cuff pressure, allowing for safe airway irrigation. This is designed to reduce the risk of upper airway colonisation and to make it easier to remove detritus from the oropharynx. Wire-reinforced tube the PneuX endotracheal/tracheostomy tube is a soft silicone wire-reinforced tube, which conforms to the anatomy and requires a bougie for intubation. Non-stick coating of the inner lumen the non-stick lining reduces the risk of biofilm formation. Boat tip the tip of the PneuX endotracheal/tracheostomy tube is bevelled with a Murphy eye (an additional hole at the tip) to aid the tube's passage into the trachea. This is intended to allow atraumatic intubation and optimum adaptation to the airway. Three suction ports there are 3 subglottic secretion drainage and irrigation ports above the proximal end of the cuff to allow the tube to function properly even if a port is blocked. The small size of the subglottic ports is intended to prevent damage to the tracheal mucosa. The manufacturer recommends that the PneuX endotracheal/tracheostomy tube and the PneuX tracheal seal monitor are used together, and so neither should be used with other devices. Setting and intended use The PneuX system can be used in intensive or critical care patients having mechanical ventilation, particularly in cases where the duration of intubation is expected to be more than 24 hours (but not more than 30 days). The PneuX system is also compatible with tracheal intubation during routine anaesthesia. It can be placed by anaesthetists and maintained by critical care nurses. Current NHS options VAP prevention strategies vary considerably in current practice. In 2008, the Working Party on Hospital-Acquired Pneumonia of the British Society for Antimicrobial Chemotherapy produced evidence-based guidance (Masterton et al. 2008). The scope of the guidance excluded oral antiseptic treatments, severely immunocompromised patients, children under 16 years old and people with cystic fibrosis. The guidance states that measures should be taken to prevent VAP by reducing aspiration via subglottic secretion drainage, correct positioning of the tube and sufficient cuff pressure to avoid aspiration and tracheal damage. The Patient Safety First How to Guide for critical care (2008) provides advice on mechanical ventilation and the NHS ventilator care bundle. Page 6 of
7 The NHS ventilator care bundle has 4 key components: elevation of the head of the bed to between 30 and 45 degrees daily sedative interruption and daily assessment of readiness to extubate peptic ulcer disease prophylaxis and venous thromboembolism prophylaxis appropriate humidification of inspired gas and appropriate tubing management. The guide recommends that centres should monitor care bundle compliance and patient outcomes in order to assess the efficacy of this approach. These measurements include: ventilator care bundle compliance, by determining the percentage of patients having all 4 components of the care bundle cumulative count of the number of days that have passed without VAP being reported average length of stay on mechanical ventilation average length of stay in ICU VAP rate per 1000 ventilator days. Subglottic secretion drainage/suctioning is not included in the care bundle, because the bundle is intended to offer solutions that are rapidly and readily available to hospitals. However, the guide states that subglottic suctioning is an effective therapy for reducing VAP rates and that if an ICU has experience of doing the procedure it should continue to offer it. The guide also states that although the bundle has omitted some interventions, the selection of a few well-established measures serves to minimise risks to the patient. It also states that proper use of the ventilator bundle leads to near zero rates of VAP over prolonged periods of time. The Scottish Intensive Care Society/Health Protection Scotland VAP prevention bundle Guidance for implementation recommends 5 key elements to be addressed together to minimise the risk of VAP: sedation, weaning and extubation, patient positioning, oral antiseptics and subglottic secretion drainage. Of these the PneuX system would specifically address the last point, subglottic secretion drainage. In 2011, the National Resource for Infection Control published High impact intervention No.5: Care bundle to reduce ventilation-associated pneumonia. It outlined 6 key ways to prevent VAP. Page 7 of
8 These relate to patient positioning, sedation level, oral hygiene, subglottic aspiration, tracheal tube cuff pressure and stress ulcer prophylaxis. It recommends secretion drainage through the subglottic secretion port every 1 2 hours and cuff pressure measurements every 4 hours to maintain a pressure of cm H2O. The PneuX system with the wall seal pressure maintained at approximately 30 cm H2O would specifically address the recommendation of subglottic aspiration drainage. Overall, the various guidelines recommend the use of prophylactic antimicrobials, care bundles and patient positioning. Measures that are both specific to intubation tube selection and design and common to the various guidelines are secretion drainage through subglottic ports and sufficient cuff pressure to avoid aspiration and tracheal damage. The PneuX system includes both those features. NICE is aware of other CE-marked devices that appear to fulfil a similar function to the PneuX system. Examples include: Microcuff endotracheal tube (Kimberly-Clark) Mallinckrodt Evac oral tracheal tube Seal Guard, Murphy Eye (Covidien) Mallinckrodt Seal Guard (Covidien) TaperGuard Evac oral tracheal tube (Covidien) UnoFlex reinforced endotracheal tube (ConvaTec). Costs and use of the technology The PneuX system has the following costs (unit price, exclusive of VAT and carriage): Endotracheal tube (size of 7.0, 8.0 or 9.0 mm inner diameter; MRI-compatible): 150 Tracheostomy tube (size of 7.0, 8.0, or 9.0 mm inner diameter; MRI-compatible): 175 Tracheal seal monitor: provided on loan basis to the hospital Extension tube: provided at no charge to the hospital. The PneuX endotracheal/tracheostomy tube and the extension tube are sterile and for single patient use. The shelf life for the PneuX endotracheal/tracheostomy tube is 2 years. The tubes are packed as single units and supplied as a box of 10 units of a single size. The extension tube is Page 8 of
9 available separately. The manufacturer provides a comprehensive training programme at no additional cost to the hospital/trust. There are no maintenance or calibration requirements for the system and the PneuX tracheal seal monitor is serviced at 2-year intervals by the manufacturer at no additional cost. In the NHS, the standard intubation tube (and their cost) varies from department to department. A number of example standard tubes and their costs is shown below (price of each tube exclusive of VAT; supplied in a box of 10 tubes): With subglottic suction: Smiths Medical Portex oral endotracheal tube cuffed with Murphy eye and SACETT suction above cuff (inner diameter 7.0 mm, outer diameter 10.4 mm): 7.80 Covidien TaperGuard EVAC Tracheal standard endotracheal tube, cuffed with Murphy eye (inner diameter 7.0 mm, outer diameter 9.5 mm): PROACT PRO-Breathe standard endotracheal Tube, cuffed with Murphy eye evacuation lumen (inner diameter 7.0 mm): Without subglottic suction: Smiths Medical Portex standard endotracheal tube cuffed with Murphy eye, clear low-pressure cuff (inner diameter 7.0 mm): 1.35 PROACT PRO-Breathe standard endotracheal tube, cuffed with Murphy eye oral/nasal PVC low-profile cuff (inner diameter 7.0 mm): Likely place in therapy The PneuX system would be used in place of standard endotracheal or tracheostomy tubes which have no subglottic access, subglottic access but with a high-pressure cuff, or no continuous cuff-pressure monitor, during long-term intubation (which is expected to be more than 24 hours but no more than 30 days). Specialist commentator comments One specialist commentator considered that maintaining a constant cuff pressure is a very beneficial feature of the PneuX system, and that continuous cuff-pressure monitoring should further reduce the risk of micro-aspiration. At present, in their ICUs, cuff pressure is measured Page 9 of
10 once per nursing shift (that is, once every 8 hours) and there is a tendency for cuff pressure to fall during this time. One specialist commentator was concerned that there may be some difficulties in predicting which patients will be intubated for more than hours, which could lead to an increased risk of having to change the endotracheal tube. Reintubation may cause a problem for some patients, particularly those with severe burns and swollen airways in whom it would not be advisable to change a tube. A specialist commentator noted that the smaller PneuX endotracheal/tracheostomy tubes have a significantly larger external diameter than comparable tubes. For example, the PneuX endotracheal/tracheostomy tube with an inner diameter of 6.0 mm has an outer diameter of 9.9 mm, compared with 9.0 mm for other endotracheal tubes. This may lead to difficulties during tube changes if the anaesthetist is not aware of the difference. Another specialist commentator thought that the PneuX system may assist critical care nurses and doctors in reducing incidences of VAP, but that more robust research needs to be done. Equality considerations NICE is committed to promoting equality, eliminating unlawful discrimination and fostering good relations between people with particular protected characteristics and others. In producing guidance and advice, NICE aims to comply fully with all legal obligations to: promote race and disability equality and equality of opportunity between men and women eliminate unlawful discrimination on grounds of race, disability, age, sex, gender reassignment, pregnancy and maternity (including women post-delivery), sexual orientation, and religion or belief (these are protected characteristics under the Equality Act 2010). Risk factors for VAP include age (incidence increases with advancing age) and chronic illnesses (underlying chronic lung disease), which may significantly affect activities of daily living to the point where a person can be considered to be disabled. Age and disability are protected characteristics under the Equality Act (2010). Page 10 of
11 Evidence review Clinical and technical evidence Regulatory bodies A search of the Medicines and Healthcare Products Regulatory Agency website for manufacturer Field Safety Notices or Medical Device Alerts for this device revealed one advisory notice issued on 12 October The notice reported that, in an isolated incident, a LoTrach (that is, PneuX) tracheostomy tube migrated through its locking mechanism, which resulted in the loss of the patient's airway. It was stated that LoTrach tracheostomy tubes and endotracheal tubes of all sizes were potentially affected. The patient was re-intubated and suffered no long-term harm. No component failures were identified. No reports of adverse events were identified from a search of the US Food and Drug Administration (FDA) database: Manufacturer and User Device Facility Experience (MAUDE). Clinical evidence A literature search identified 3 published studies relevant to this briefing; 1 was a randomised controlled trial (Gopal et al. 2015) and 2 were retrospective cohort studies (Smith et al. 2014; Doyle et al. 2011). They are summarised in tables 1 to 6 of this briefing. One study published as an abstract only was also identified to be relevant (Hodd et al. 2009). The study is summarised in table 7. Four conference abstracts were also identified, 1 (Gopal et al. 2013) reporting the published Gopal et al. (2015) study, 1 (Smith et al. 2011) reporting the published Smith et al. (2014) study and 2 (Carter et al. 2009; Fletcher et al. 2009) reporting data from the published study by Doyle et al. (2011). None of the abstracts provided any additional information to the published papers, and so they were not summarised in the briefing. One study, published as an abstract only (Crommett et al. 2013), used the PneuX system in 39 critically ill patients. The only outcome measured was the amount of secretions on the inner lumen of the PneuX endotracheal/tracheostomy tube. This study was not included in this briefing because it did not report patient outcomes. The trial by Gopal et al. (2015) was a UK-based, single-centre randomised controlled trial comparing the PneuX system with a standard endotracheal tube in high-risk adults having cardiac surgery. Each intubation group comprised 120 patients and the follow-up period was 48 hours after Page 11 of
12 extubation. The primary outcome measure was the incidence of VAP, confirmed using the Hospitals in Europe Link for Infection Control through Surveillance definition. VAP incidence was significantly lower in the PneuX group than in the standard group (10.8% compared with 21%, p=0.03). The between-group difference with 95% confidence intervals (CI) was not reported in the paper. These data were used to calculate an absolute risk difference of 10% (95% CI 1% to 19%), which equates to a number needed to treat of 10 (95% CI 5.3 to 100.0). There was no significant difference between the 2 groups in terms of length of ICU stay (p=0.2) and in-hospital mortality (p=0.2). There were also no statistically significant differences between the 2 groups in terms of re-exploration rates, intubation duration and cardiopulmonary bypass duration. Smith et al. (2014) reported on a retrospective cohort study in an ICU in an NHS hospital trust, which included 48 patients who were intubated with the PneuX system during The follow-up period was not specified but the primary outcome was incidence of VAP. The diagnosis of VAP was based on the recommendations of the American Thoracic Society and the Infectious Diseases Society of America Three of the 48 patients in the study developed VAP (6.25%, 95% CI 1.3% to 17%). In these 3 patients, VAP was first identified on days 3, 7 and 9 after intubation. There was a 17% incidence of unplanned extubation, of which 5 incidents (10%) were classed as self-extubation. The authors concluded that, compared with other published studies, there appeared to be a higher rate of unplanned extubations, mostly self-extubations, raising concerns about patient safety. They suggested that further trials would be needed to determine the value of the system in clinical practice. The study by Doyle et al. (2011) was a retrospective cohort study in a general ICU at a NHS hospital trust, which analysed 53 critically ill patients who sequentially were intubated with the PneuX system over 14 months. The primary outcome was incidence of VAP but the follow-up period was not specified. VAP was diagnosed based on clinical suspicion, international consensus criteria or clinical pulmonary infection score. There were no VAP episodes while the PneuX endotracheal tube was in place. One patient needed re-intubation following elective extubation, and developed VAP 2 days later while the conventional tube was in place. This gave a VAP rate in the 53 patients of 1.8%. The abstract by Hodd et al. (2009) reported a retrospective cohort study in an ICU at a NHS hospital trust. The electronic medical records of all ICU patients intubated with the PneuX system over (185 intubations) were analysed. The primary outcome was the incidence of Page 12 of
13 unplanned extubations. There was 1 unplanned extubation (self-extubation) during that period, resulting in an incidence of 0.1% (1.02 unplanned extubations per 1000 intubation days). Recent and ongoing studies No ongoing or in-development trials on the PneuX system for patients in ICU at risk of VAP were identified. Costs and resource consequences No published evidence was identified on the direct costs and resource consequences related to using the PneuX system in preventing VAP. If the PneuX system reduced the incidence of VAP, then resources related to the treatment of VAP and VAP-related emergency department, ICU and hospital stay could be saved. According to the manufacturer, 3 NHS hospitals are currently using the PneuX system. One abstract (Carter et al. 2009) reported the same data as the published paper by Doyle et al. (2011). No cases of VAP were seen in the 58 patients with the PneuX system in place. On an intention-to-treat basis the VAP rate was 1.8%, because 1 patient who needed reintubation with a conventional endotracheal tube developed VAP while the tube was in place. With a 10 20% expected incidence of VAP, the authors extrapolated that 5 10 episodes of VAP were expected in this study. The authors stated that the price of the PneuX system was $250 (approximately 160) per patient, making a total cost per year of $11,400 (approximately 7480) in their institution; for comparison, other researchers have estimated that treating a single case of VAP costs around $10,019 (approximately 6490). Gopal et al. (2015) cited a review paper that puts the cost of VAP treatment in the USA at around $40,000 (approximately 25,900) per patient. The authors then stated that in their study, there were 12 fewer patients with VAP in the PneuX group than in the standard endotracheal tube group. This was equal to a potential cost saving of $480,000 (approximately 310,750). The authors estimated that, assuming using PneuX costs $100 (approximately 65) per patient, about 4800 patients could have been intubated using the PneuX system with at least a halving of the VAP rate. The cost of the PneuX system assumed by the authors is considerably lower than the current list price, so these results should be interpreted with caution. Page 13 of
14 Strengths and limitations of the evidence The evidence is based on 3 published studies and 1 study available as an abstract. The VAP diagnosis criteria used varied across the studies where the incidence of VAP was assessed. The follow-up period was reported in only 1 study (Gopal et al. 2015). The Gopal et al. (2015) study was a single-centre randomised controlled trial. The trial may have been subject to a number of known sources of potential bias. The treatment assignment method using a computer-generated randomisation software program was appropriate, but it was unclear whether the random allocation of the treatments was concealed. A sample size calculation was done and the recruited number of patients in each group was sufficient. However, it was unclear whether a blinding method was applied to the treatments, for example to blind the outcome assessors, or those who analysed the data. Performance bias was minimised because other than the treatments being compared, patients in both groups received the same care, with both groups having routine respiratory care in the ICU as per the NHS ventilator care bundle and the same routine gastric stress ulcer prophylaxis. The patients included in this study were high-risk patients (over 70 years old or with impaired left ventricular ejection fraction of less than 50%) having elective and urgent cardiac surgery. This patient group may not be fully representative, being a specific subgroup of the patient population for whom the use of the PneuX system is intended. Because this was a single and relatively small study, the estimates of the effectiveness of the PneuX system are imprecise. In the study by Smith et al. (2014) the patients were recruited using consecutive sampling, because it included all patients who met the inclusion criteria during the study period. In the study by Doyle et al. (2011) inclusions were sequential patients who had the PneuX system for mechanical ventilation during the study period. However, because both studies had a non-comparative single-cohort, the observed VAP rate with the PneuX system was not compared to that with an alternative tube. As such, conclusions regarding the comparative effectiveness of the PneuX system cannot be drawn from these studies. One study published as an abstract only (Hodd et al. 2009) provided limited information in terms of study methods, characteristics and results, making it hard to judge the quality of the evidence in the abstracts. All 4 studies were UK-based. Their results are likely to be generalisable to the UK healthcare settings. Page 14 of
15 Three authors of the Gopal et al. (2015) study received a grant from the distributor of the PneuX system to present the data following the completion of the study. The PneuX system used in the Smith et al. (2014) study was provided by the manufacturer. In the Doyle et al. (2011) study, 1 author is the inventor of the PneuX system and is a minor shareholder in the intellectual property ownership of the PneuX system and tracheal seal monitor. In the past this author received funding and consultancy fees from the manufacturer. Relevance to NICE guidance programmes The use of the PneuX system is not currently planned into any NICE guidance programme. References American Thoracic Society; Infectious Diseases Society of America (2005) Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilatorassociated, and healthcare-associated pneumonia. American Journal of Respiratory and Critical Care Medicine 171: Bauer TT, Ferrer R, Angrill J et al. (2000) Ventilator-associated pneumonia: incidence, risk factors, and microbiology. Seminars in Respiratory Infections 15: 2 79 Bouza E, Hortal J, Munoz P et al. (2006) Post-operative infections after major heart surgery and prevention of ventilator-associated pneumonia: a one day European prevalence study (ESGNI-008). Journal of Hospital Infection 64: Carter J, Hodd J, Young P (2009) The PneuX P.Y. tracheal tube: cost effectiveness in the general intensive care unit. Critical Care Medicine 37: A466 Crommett J, Dinh A, Chang E et al. (2012) Mass of luminal secretion: comparison between PneuX and PVC tracheal tubes. Critical Care Medicine 40: U285 Dodek P, Keenan S, Cook D et al. (2004) Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Annals of Internal Medicine 141: Doyle A, Fletcher A, Carter J et al. (2011) The incidence of ventilator-associated pneumonia using the PneuX System with or without elective endotracheal tube exchange: a pilot study. BMC Research Notes 4: 92 Page 15 of
16 Fletcher A, Carter J, Blunt M et al. (2009) Incidence of ventilator-associated pneumonia in patients undergoing elective tube exchange to LoTrach endotracheal tubes. Critical Care 13: S122 Gopal S, Luckraz H, Giri R et al. (2015) Significant reduction in ventilator-associated pneumonia with the Venner-PneuX System in high-risk patients undergoing cardiac surgery: the Low Ventilator-Associated-Pneumonia study. European Journal of Cardio-Thoracic Surgery 47: e92 6 Gopal S, Luckraz H, Giri Ret al. (2013) Benefits of the Venner PneuX endotracheal tube in high-risk patients undergoing cardiac surgery. Critical Care Medicine 41: A4 Health and Social Care Information Centre (2015) Adult Critical Care Data in England April 2013 to March [online; accessed 01 October 2015] Hodd J, Doyle A, Irvine M et al. (2009) Preventing unplanned extubation with the Pneux P.Y. System (LoTrach) An engineered solution. Critical Care Medicine 37: A192 Hunter JD (2012) Ventilator associated pneumonia. British Medical Journal 344: e3325 Kollef MH (1999) The prevention of ventilator-associated pneumonia. The New England Journal of Medicine 340: 6 34 Masterton RG, Galloway A, French G et al. (2008) Guidelines for the management of hospitalacquired pneumonia in the UK: report of the working party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. Journal of Antimicrobial Chemotherapy 62: 5 34 Safdar N, Dezfulian C, Collard HR et al. (2005) Clinical and economic consequences of ventilatorassociated pneumonia: a systematic review. Critical Care Medicine 33: Smith N, Khan F, Gratrix A (2014) A retrospective review of patients managed with the Pneux PY VAP prevention system. Journal of the Intensive Care Society 15(2): Smith N, Khan F, Gratrix A (2011) The PneuX pneumonia prevention system and the incidence of ventilator associated pneumonia. Intensive Care Medicine 37: S6 Zuschneid I, Schwab F, Geffers C et al. (2007) Trends in ventilator-associated pneumonia rates within the German nosocomial infection surveillance system (KISS). Infection Control and Hospital Epidemiology 28: Page 16 of
17 Search strategy and evidence selection Search strategy 1. The following databases were searched from inception to July 2015 using the keyword "Pneux", "LoTrach", and "Lo Trach": Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R); Embase (via OVID); Cochrane Library; CAB Abstracts; Web of Science Science Citation Index. These citations were sifted through to identify any relevant material, using the inclusion criteria in the 'Evidence selection' section below. 2. The internet was searched using the above keywords. 3. ClinicalTrials.gov, WHO ICTRP, and Current Controlled Trials were also searched for ongoing trials. 4. Information provided by the manufacturer in supporting this briefing was checked to identify any further information. 5. The manufacturer's website was thoroughly investigated. 6. Information provided by the manufacturer was thoroughly checked for relevant studies. Evidence selection The inclusion criteria were as follows: Population: adult cardiac patients requiring ventilation in a critical care setting who are at high risk of developing ventilator-associated pneumonia (VAP). Intervention: ention: the PneuX system, used for the prevention of ventilator-associated pneumonia. It was formerly known as the Venner PneuX P.Y. - VAP Prevention System and the Lo-Trach system. It has also been referred as the PneuX VAP prevention system, the PneuX P.Y. system, and the Venner-PneuX system. Comparator: ator: any other equivalent or similar technology aimed at VAP prevention, or conventional endotracheal or tracheal intubation tube. Page 17 of
18 Outcomes: any clinical efficacy and safety outcomes, including but not limited to: incidence of VAP length of ICU/ITU stay length of hospital stay incidence of secondary infection incidence of aspiration duration of mechanical ventilation antibiotic usage mortality adverse events cost savings. Study type: published randomised or non-randomised controlled trials; published cohort studies. For safety aspect of the device published case report studies were included. Proof-of-concept, bench-top or basic science studies were excluded. Non-English language studies were excluded. Appendix Contents Data tables Table 1: Overview of the Gopal et al. (2015) study Table 2: Summary of results of the Gopal et al. (2015) study Table 3: Overview of the Smith et al. (2014) study Table 4: Summary of results of the Smith et al. (2014) study Page 18 of
19 Table 5: Overview of the Doyle et al. (2011) study Table 6: Summary of results of the Doyle et al. (2011) study Table 7: Summary of Hodd et al. (2009) abstract Table 1 Overview of the Gopal et al. (2015) study Study component Objectives/ hypotheses Study design Setting Inclusion/ exclusion criteria Primary outcomes Statistical methods Patients included Results Description To assess whether the PneuX system is associated with a reduction in VAP when compared with the standard ET tube in high-risk patients undergoing cardiac surgery. Single centre, randomised controlled trial comparing the PneuX tube with the standard tube. Follow-up period: 48 hours after extubation. Intermittent subglottic suctioning was performed at 6-hourly intervals. Department of Cardiothoracic Surgery, Heart and Lung Centre, Wolverhampton, UK. Patients were transferred to the ICU post-operatively. Inclusion criteria: patients over the age of 70 years or with impaired LVEF (<50%) undergoing elective and urgent cardiac surgery. Patients who were recruited to other studies were excluded. Incidence of VAP. A diagnosis of VAP was confirmed via the Hospitals in Europe Link for Infection Control through Surveillance (HELICS) definition. Power calculations suggested 107 patients per group (power of 0.9 and an alpha of 0.01). High-risk patients undergoing cardiac surgery; n=240 (120 in each group). VAP incidence was significantly lower in the PneuX tube group than in the standard ET group (10.8% compared with 21%, p=0.03). a There was no significant difference between the two groups in terms of intensive care unit stay (p=0.2) and in-hospital mortality (p=0.2). Page 19 of
20 Conclusions The PneuX system is associated with a significant reduction in VAP. This can potentially lead to significant cost reductions and should be implemented as part of the VAP reduction bundle. Abbreviations: CI, confidence interval; ET, endotracheal; ICU, intensive care unit; LVEF, left ventricular ejection fraction; n, number of patients; VAP, ventilator-associated pneumonia. a The between-group difference with 95% CI was not reported in the paper. The External Assessment Centre (principal authors of the briefing) used the presented data to calculate a risk difference of 10% (95% CI 1 to19%) which equates to a number needed to treat of 10 (95% CI 5.3 to 100). Table 2 Summary of results from the Gopal et al. (2015) study PneuX tube Standard tube Analysis Randomised n=120 n=120 Efficacy n=120 n=120 Primary outcome: 13 (11%) 25 (21%) p=0.03 VAP incidence (n;%) a Selected secondary outcomes: VAP incidence <0.01 density b Survival 98% 99% p=0.2 Median ICU stay (days) Re-exploration (n;%) Median intubation time (hours) p= (14%) 10 (8%) p= p=0.5 Page 20 of
21 Mean CPB time (minutes±sd) 110±58 105±62 p=0.3 Mortality (n; 2 (1.6%) 1 (0.8%) p=0.2 %) c Safety The anaesthetists reported no difficulties in intubating patients with the PneuX tube. There was no accidental tube displacement or dislodgement. There was 1 case of a patient experiencing oxygen desaturation following suctioning and irrigation, which resulted in a transient increase in oxygen requirement. The patient was subsequently extubated uneventfully 12 hours later. An independent clinician from an external organisation was asked to comment on whether the patient had potentially aspirated the sterile water during the reported episode. The conclusion was that it was impossible to state with any certainty that the patient had aspirated the sterile water during the period of suction and irrigation. There were no reports of failure of the continuous cuff-pressure monitoring device. Abbreviations: CPB, cardiopulmonary bypass; ET, endotracheal tube; EuroSCORE, European system for cardiac operative risk evaluation; ICU, intensive care unit; LVEF, left ventricular ejection fraction; n, number of patients; NR, not reported; SD, standard deviation; VAP, ventilator-associated pneumonia. a The between-group difference with 95% CI was not reported in the paper. The External Assessment Centre (principal authors of the briefing) used the presented data to calculate a risk difference of 10% (95%CI 1 to19%) which equates to a number needed to treat of 10 (95%CI 5.3 to 100). b Number of VAP episodes per 1000 ventilator days. c None of the 3 patients who died had VAP. Table 3 Overview of the Smith et al. (2014) study Study component Objectives/ hypotheses Study design Description To review the impact of the PneuX system on the incidence of VAP and its effects on local practice. Retrospective cohort study. Follow-up period: not reported. Page 21 of
22 Setting Inclusion/ exclusion criteria Primary outcomes Statistical methods Results A mixed medical and surgical ICU, Hull and East Yorkshire Hospitals NHS Trust, in All patients were intubated with the PneuX system and received its associated care package in the intensive care department during Identified using both paper and electronic hospital records. Incidence of VAP. Diagnosis of VAP was based on the recommendations of the American Thoracic Society and the Infectious Diseases Society of America. a Clinical diagnosis of VAP was made if new radiographic infiltrates were present on a chest X-ray at 48 hours after initial intubation, in combination with at least 2 of the following: fever, leukocytosis, or purulent trachea-bronchial secretions. Not specified. Median (range) was used for the duration of the PneuX system being in place. 95% CI was reported for VAP incidence. The incidence of VAPs/ 1000 bed days was calculated based on the cumulative total of PneuX system use and the occurrence of VAP. Three of the 48 patients developed VAP (6.25%, 95% CI %). Two more cases of VAP were excluded due to pre-existing VAP. Conclusions The PneuX system facilitated lower VAP rates than those documented elsewhere and highlighted the incidence of unplanned extubations in local practice. Further evaluation of the implementation of the PneuX system in intensive care areas, in tandem with large-scale evaluation of its effectiveness, are still required. Abbreviations: CI, confidence internal; ICU, intensive care unit; VAP, ventilator-associated pneumonia. a VAP as diagnosed by the ATS/IDSA guidelines (American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American Journal of Respiratory and Critical Care Medicine (2005) 171: Page 22 of
23 Table 4 Summary of results from the Smith et al. (2014) study Patients included Forty eight adult patients who were expected to be intubated for at least 24 hours, including 14 who had primary intubation with the PneuX system and 34 who underwent tracheal tube exchange to the PneuX system. Mean age 57.5 years. 56% male. Type of patient: 32 medical, 7 surgical and 9 neurosurgical. Primary outcomes Three of the 48 patients developed VAP (6.25%, 95% CI %); 2 more cases of VAP were excluded due to pre-existing pneumonia. Of the 3 patients who were diagnosed with VAP, the onset of VAP was identified on days 3, 7 and 9 after initial intubation. Safety The majority of extubations were planned in advance but 17% (n=8) were classified as unplanned, with a majority (62%, n=5) of these being unplanned self-extubations. Patient self-extubation occurred in 13% (n=6) of the 48 patients. a Physiotherapists commented that the tracheal tube felt 'too long,' which prevented suction catheters entering the trachea sufficiently to provide adequate suction. Some staff felt that while the bite block and lock nut secured the tube in the right position, it also acted as a grip for patients to achieve unplanned self-extubation. Abbreviations: CI, confidence internal; ICU, intensive care unit; VAP, ventilator-associated pneumonia; n, number of patients. a The paper stated that "the majority (66%) of extubations were planned. However, of the remaining unplanned extubations, two were deemed to be accidental, where the patient was not responsible for tracheal tube removal, and 5 were classed as self-extubation. In a single case the tracheal tube was removed for clinical reasons". There are some discrepancies regarding the reported extubation rates. Table 5 Overview of the Doyle et al. (2011) study Study component Objectives/ hypotheses Description To determine the incidence of VAP in patients who were intubated with the PneuX system and to establish whether intermittent subglottic secretion drainage could be performed reliably and safely using the PneuX system. Page 23 of
24 Study design Setting Inclusion/ exclusion criteria Primary outcomes Statistical methods Retrospective cohort study. Follow-up period: not reported. At a Queen Elizabeth Hospital, NHS Trust. Follow-up period unclear. Sequential critically ill patients aged more than 18 years who received the PneuX tracheal tube and cuff pressure controller for mechanical ventilation during a 14-month period in that hospital. The use of the PneuX system was restricted to those patients who were anticipated to require more than 24 hours of intubation. Incidence of VAP. VAP was defined as any pneumonia that occurred after more than 48 hours of intubation and mechanical ventilation with the PneuX system. It was diagnosed by (i) clinical suspicion (including the use of any antibiotics for the treatment of colonisation or infection within the tracheobronchial tree or lungs); and/or (ii) international consensus criteria: the presence of new, persistent pulmonary infiltrates not otherwise explained, appearing on chest radiographs and at least two of the following criteria: temperature of greater than 38 degrees Centigrade; leukocytosis of greater than 10,000 cells/mm 3 and purulent respiratory secretions; or (iii) clinical pulmonary infection score (a fall in the PaO 2 /FiO 2 ratio of greater than 25% and a clinical pulmonary infection score of greater than 5 in the presence of bacteria in a qualitative endotracheal aspirate). Not specified. Conclusions The study demonstrates that a low incidence of VAP is possible using the PneuX system. It also demonstrates that elective exchange and intermittent subglottic secretion drainage can be performed reliably and safely using the PneuX system. Abbreviations: ICU, intensive care unit; VAP, ventilator-associated pneumonia. Table 6 Summary of results from the Doyle et al. (2011) study Patients included Fifty-three critically ill patients aged older than 18 years received the PneuX system. All patients included in the study were intubated with the PneuX system and received mechanical ventilation for more than 48 hours. No patients intubated with the PneuX system were excluded from the analysis. Page 24 of
25 Primary outcomes There were a total of 306 days of intubation with a mean of 5.3 days (range 1 18 days). Forty-four (83%) patients underwent a tube exchange and 17% were primary intubations with the PneuX system on the ICU. There were no episodes of VAP while the PneuX system was in situ. On an ITT basis there was a 1.8% VAP rate as 1 patient, who required reintubation following elective extubation, received a conventional tube and developed a VAP 2 days later with the conventional tube in situ. No antimicrobial therapy was initiated for chest infections in patients intubated with the PneuX system. Safety There were no complications from, or failure of, subglottic secretion drainage during the study. Abbreviations: ICU, intensive care unit; ITT, intention to treat; VAP, ventilator-associated pneumonia. Table 7 Summary of data from the Hodd et al. (2009) abstractact Objectives/ hypotheses Study design To determine the incidence of unplanned extubations using the PneuX system and to compare this with previously reported data. Hypothesis: the incidence of unplanned extubation is decreased when using the PneuX system compared to historical data. Retrospective cohort study analysis of the electronic medical records of ICU patients intubated with the PneuX system. Setting At a Queen Elizabeth Hospital, NHS Trust, during the period Follow-up period unclear. Inclusion/ exclusion criteria Primary outcomes Statistical methods The electronic medical records of all ICU patients intubated with the PneuX system from in the hospital. Incidence of unplanned extubation. Not specified. Page 25 of
26 Patients included Results A total of 185 intubations with the PneuX system. No further information on the population. During the 3-year period there were a total of 185 intubations with the PneuX system and 982 intubation days (mean 5.31, median 4). There was 1 unplanned extubation (self-extubation) during that period. This resulted in an incidence for unplanned extubations of 0.1% (1.02 per 1000 intubation days). Conclusions The results suggest that the PneuX system compares favourably with figures reported in largest published database (7.6% incidence of unplanned extubation; 9.4 per 1000 intubation days). Engineered efforts to minimise unplanned extubation should be encouraged. Abbreviations: ICU, intensive care unit; VAP, ventilator-associated pneumonia. About this briefing Medtech innovation briefings summarise the published evidence and information available for individual medical technologies. The briefings provide information to aid local decision-making by clinicians, managers and procurement professionals. Medtech innovation briefings aim to present information and critically review the strengths and weaknesses of the relevant evidence, but contain no recommendations and are not formal NICE guidance. Development of this briefing This briefing was developed for NICE by The Birmingham and Brunel Consortium. The interim process & methods statement sets out the process NICE uses to select topics, and how the briefings are developed, quality-assured and approved for publication. Project team Birmingham and Brunel Consortium Medical Technologies Evaluation Programme, NICE Page 26 of
pat hways Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87
 pat hways CytoSorb therapy for sepsis Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87 Summary The technology described in this briefing is CytoSorb therapy. It is an
pat hways CytoSorb therapy for sepsis Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87 Summary The technology described in this briefing is CytoSorb therapy. It is an
pat hways Medtech innovation briefing Published: 2 March 2015 nice.org.uk/guidance/mib22
 pat hways Shiley Endotracheal Tube with TaperGuard Cuff for intensive e care patients at risk of ventilator-associated pneumonia Medtech innovation briefing Published: 2 March 2015 nice.org.uk/guidance/mib22
pat hways Shiley Endotracheal Tube with TaperGuard Cuff for intensive e care patients at risk of ventilator-associated pneumonia Medtech innovation briefing Published: 2 March 2015 nice.org.uk/guidance/mib22
VAP Prevention bundles
 VAP Prevention bundles Dr. Shafiq A.Alimad MD Head of medical department at USTH YICID workshop, 15-12-2014 Care Bundles What are they & why use them? What are Care Bundles? Types of Care Bundles available
VAP Prevention bundles Dr. Shafiq A.Alimad MD Head of medical department at USTH YICID workshop, 15-12-2014 Care Bundles What are they & why use them? What are Care Bundles? Types of Care Bundles available
Targeted literature review:
 Targeted literature review: What are the key infection prevention and control recommendations to inform a minimising ventilator associated pneumonia (VAP) quality improvement tool? Part of HAI Delivery
Targeted literature review: What are the key infection prevention and control recommendations to inform a minimising ventilator associated pneumonia (VAP) quality improvement tool? Part of HAI Delivery
Key uncertainties around the evidence or technology are that the test has only been validated in biochemical studies.
 pat hways PredictSure-IBD for inflammatory bowel disease prognosis Medtech innovation briefing Published: 25 March 2019 nice.org.uk/guidance/mib178 Summary The technology described in this briefing is
pat hways PredictSure-IBD for inflammatory bowel disease prognosis Medtech innovation briefing Published: 25 March 2019 nice.org.uk/guidance/mib178 Summary The technology described in this briefing is
KIMBERLY-CLARK* MICROCUFF* Endotracheal Tube.. Revolutionary cuff material designed to reduce micro-aspiration
 KIMBERLY-CLARK* MICROCUFF* Endotracheal Tube.. Revolutionary cuff material designed to reduce micro-aspiration Ventilator-Associated Pneumonia VAP is a major clinical concern...... associated with high
KIMBERLY-CLARK* MICROCUFF* Endotracheal Tube.. Revolutionary cuff material designed to reduce micro-aspiration Ventilator-Associated Pneumonia VAP is a major clinical concern...... associated with high
GDm-Health is free to download and use. Its use may result in efficiency savings from reducing face-to-face clinic appointments.
 pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
The innovative aspects are that the test automatically generates a report on ALK status that any clinician can interpret.
 pat hways HTG EdgeSeq ALKPlus Assay EU for ALK status testing in non-small-cell lung cancer Medtech innovation briefing Published: 7 November 2017 nice.org.uk/guidance/mib128 Summary The technology described
pat hways HTG EdgeSeq ALKPlus Assay EU for ALK status testing in non-small-cell lung cancer Medtech innovation briefing Published: 7 November 2017 nice.org.uk/guidance/mib128 Summary The technology described
Moving from VAP to VAC
 Moving from VAP to VAC Cindy Munro, PhD, RN, ANP-BC, FAANP, FAAN Associate Dean of Research and Innovation Professor College of Nursing Conflict of interest: No relationships with pharmaceutical companies,
Moving from VAP to VAC Cindy Munro, PhD, RN, ANP-BC, FAANP, FAAN Associate Dean of Research and Innovation Professor College of Nursing Conflict of interest: No relationships with pharmaceutical companies,
The innovative aspects are that ORA G3 is currently the only device capable of measuring corneal hysteresis.
 pat hways ORA G3 to measure corneal hysteresis Medtech innovation briefing Published: 18 June 2018 nice.org.uk/guidance/mib150 Summary The technology described in this briefing is Ocular Response Analyzer
pat hways ORA G3 to measure corneal hysteresis Medtech innovation briefing Published: 18 June 2018 nice.org.uk/guidance/mib150 Summary The technology described in this briefing is Ocular Response Analyzer
ANWICU knowledge
 ANWICU knowledge www.anwicu.org.uk This presenta=on is provided by ANWICU We are a collabora=ve associa=on of ICUs in the North West of England. Permission to provide this presenta=on has been granted
ANWICU knowledge www.anwicu.org.uk This presenta=on is provided by ANWICU We are a collabora=ve associa=on of ICUs in the North West of England. Permission to provide this presenta=on has been granted
pat hways Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76
 pat hways Axxent brachytherapy system for early stage breast cancer Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76 Summary The technology described in this briefing is
pat hways Axxent brachytherapy system for early stage breast cancer Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76 Summary The technology described in this briefing is
IS YOUR CUFF DOING THE JOB?
 MICROCUFF * Endotracheal Tubes IS YOUR CUFF DOING THE JOB? Polyurethane cuff and subglottic secretion drainage help prevent early- and late-onset VAP 1 LEADING AUTHORITIES: SUBGLOTTIC SUCTIONING IS A BEST
MICROCUFF * Endotracheal Tubes IS YOUR CUFF DOING THE JOB? Polyurethane cuff and subglottic secretion drainage help prevent early- and late-onset VAP 1 LEADING AUTHORITIES: SUBGLOTTIC SUCTIONING IS A BEST
Collaborative Regional Benchmarking Group (North of England, North Yorkshire & Humber and West Yorkshire)
 Collaborative Regional Benchmarking Group (North of England, North Yorkshire & Humber and West Yorkshire) Best Practice Guidance Endo Tracheal Tube Care These recommendations are based on the current evidence
Collaborative Regional Benchmarking Group (North of England, North Yorkshire & Humber and West Yorkshire) Best Practice Guidance Endo Tracheal Tube Care These recommendations are based on the current evidence
The innovative aspect is that it detects bladder cancer based on the novel biomarker, minichromosome maintenance complex component 5 (MCM5).
 pat hways ADXBLADDER for detecting bladder cancer Medtech innovation briefing Published: 12 April 2019 nice.org.uk/guidance/mib180 Summary The technology described in this briefing is ADXBLADDER. It is
pat hways ADXBLADDER for detecting bladder cancer Medtech innovation briefing Published: 12 April 2019 nice.org.uk/guidance/mib180 Summary The technology described in this briefing is ADXBLADDER. It is
American College of Surgeons Critical Care Review Course 2012: Infection Control
 American College of Surgeons Critical Care Review Course 2012: Infection Control Overview: I. Central line associated blood stream infection (CLABSI) II. Ventilator associated pneumonia (VAP) I. Central
American College of Surgeons Critical Care Review Course 2012: Infection Control Overview: I. Central line associated blood stream infection (CLABSI) II. Ventilator associated pneumonia (VAP) I. Central
BIP Endotracheal Tube
 Bactiguard Infection Protection BIP Endotracheal Tube For prevention of healthcare associated infections Ventilator associated pneumonia Infections of the respiratory tract are serious and common healthcare
Bactiguard Infection Protection BIP Endotracheal Tube For prevention of healthcare associated infections Ventilator associated pneumonia Infections of the respiratory tract are serious and common healthcare
SUBGLOTTIC SECRETION REMOVAL:
 ARROW REGIONAL TELEFLEX ANAESTHESIA ISIS Nullan utpat, Better vulputpatie access. ero Best dion practice. ulputat SUBGLOTTIC SECRETION REMOVAL: A VAP REDUCTION STRATEGY Ventilator-Associated Pneumonia
ARROW REGIONAL TELEFLEX ANAESTHESIA ISIS Nullan utpat, Better vulputpatie access. ero Best dion practice. ulputat SUBGLOTTIC SECRETION REMOVAL: A VAP REDUCTION STRATEGY Ventilator-Associated Pneumonia
Endobronchial valve insertion to reduce lung volume in emphysema
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
INDEPENDENT LUNG VENTILATION
 INDEPENDENT LUNG VENTILATION Giuseppe A. Marraro, MD Director Anaesthesia and Intensive Care Department Paediatric Intensive Care Unit Fatebenefratelli and Ophthalmiatric Hospital Milan, Italy gmarraro@picu.it
INDEPENDENT LUNG VENTILATION Giuseppe A. Marraro, MD Director Anaesthesia and Intensive Care Department Paediatric Intensive Care Unit Fatebenefratelli and Ophthalmiatric Hospital Milan, Italy gmarraro@picu.it
VAP Definitions. CDC New Approach to VAP Surveillance. Conflict of Interest Disclosure Robert M Kacmarek. Artificial Airways, Cuffs, Bioflim and VAP
 Conflict of Interest Disclosure Robert M Kacmarek Artificial Airways, Cuffs, Bioflim and VAP Bob Kacmarek PhD, RRT Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts 9-14-18
Conflict of Interest Disclosure Robert M Kacmarek Artificial Airways, Cuffs, Bioflim and VAP Bob Kacmarek PhD, RRT Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts 9-14-18
AIRWAY MANAGEMENT PRODUCTS
 QUICK REFERENCE GUIDE AIRWAY MANAGEMENT PRODUCTS Shiley Endotracheal Tubes SHILEY ENDOTRACHEAL TUBE CATALOG NUMBER CHARTS DESCRIPTION QTY PER BOX SIZE (MM) 1.0 1.5 2.0 2.5 3.0 3.5 4.0 VENTILATOR-ASSOCIATED
QUICK REFERENCE GUIDE AIRWAY MANAGEMENT PRODUCTS Shiley Endotracheal Tubes SHILEY ENDOTRACHEAL TUBE CATALOG NUMBER CHARTS DESCRIPTION QTY PER BOX SIZE (MM) 1.0 1.5 2.0 2.5 3.0 3.5 4.0 VENTILATOR-ASSOCIATED
Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600
 Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Respiratory Guard System: New Technology
 Respiratory Guard System: New Technology Zvi Peled, Avishai Zisser, Michal Fertouk, Victor Kerzman, Keren Bitton-Worms and Gil Bolotin. Department of Cardiac Surgery, Rambam Health Care Campus, Technion
Respiratory Guard System: New Technology Zvi Peled, Avishai Zisser, Michal Fertouk, Victor Kerzman, Keren Bitton-Worms and Gil Bolotin. Department of Cardiac Surgery, Rambam Health Care Campus, Technion
A NEW direction for subglottic secretion management
 A NEW direction for subglottic secretion management The SIMEX Subglottic Aspiration System, cuff M and cuff S are the most advanced solution for the aspiration of subglottic secretion, featuring all new
A NEW direction for subglottic secretion management The SIMEX Subglottic Aspiration System, cuff M and cuff S are the most advanced solution for the aspiration of subglottic secretion, featuring all new
Pneumonia (PNEU) and Ventilator-Associated Pneumonia (VAP) Prevention. Basics of Infection Prevention 2-Day Mini-Course 2016
 Pneumonia (PNEU) and Ventilator-Associated Pneumonia (VAP) Prevention Basics of Infection Prevention 2-Day Mini-Course 2016 Objectives Differentiate long term care categories of respiratory infections
Pneumonia (PNEU) and Ventilator-Associated Pneumonia (VAP) Prevention Basics of Infection Prevention 2-Day Mini-Course 2016 Objectives Differentiate long term care categories of respiratory infections
pat hways Medtech innovation briefing Published: 1 October 2018 nice.org.uk/guidance/mib161
 pat hways myairvo2 for the treatment of chronic obstructive pulmonary disease Medtech innovation briefing Published: 1 October 2018 nice.org.uk/guidance/mib161 Summary The technology described in this
pat hways myairvo2 for the treatment of chronic obstructive pulmonary disease Medtech innovation briefing Published: 1 October 2018 nice.org.uk/guidance/mib161 Summary The technology described in this
Cuffed Tracheal Tubes in Children - Myths and Facts. PD Dr. Markus Weiss Department of Anaesthesia University Children s Hospital Zurich Switzerland
 Cuffed Tracheal Tubes in Children - Myths and Department of Anaesthesia University Children s Hospital Zurich Switzerland PRO Reduced gas leak, low fresh gas flow Decreased atmospheric pollution Constant
Cuffed Tracheal Tubes in Children - Myths and Department of Anaesthesia University Children s Hospital Zurich Switzerland PRO Reduced gas leak, low fresh gas flow Decreased atmospheric pollution Constant
Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Care Unit (FELLOW)
 Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Data Analysis Plan: Apneic Oxygenation vs. No Apneic Oxygenation Background Critically ill patients
Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Data Analysis Plan: Apneic Oxygenation vs. No Apneic Oxygenation Background Critically ill patients
PERFORMANCE UNDER PRESSURE.
 PERFORMANCE UNDER PRESSURE. IntelliCuff Cuff Pressure Controller and SubG Endotracheal Tube Smart technology to help reduce risk of VAP and tracheal injuries Replace Complexity with Efficiency. Managing
PERFORMANCE UNDER PRESSURE. IntelliCuff Cuff Pressure Controller and SubG Endotracheal Tube Smart technology to help reduce risk of VAP and tracheal injuries Replace Complexity with Efficiency. Managing
[No conflicts of interest]
![[No conflicts of interest] [No conflicts of interest]](/thumbs/86/94144068.jpg) [No conflicts of interest] Patients and staff at: Available evidence pre-calories Three meta-analyses: Gramlich L et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes
[No conflicts of interest] Patients and staff at: Available evidence pre-calories Three meta-analyses: Gramlich L et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device
 I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
Liberation from Mechanical Ventilation in Critically Ill Adults
 Liberation from Mechanical Ventilation in Critically Ill Adults 2017 ACCP/ATS Clinical Practice Guidelines Timothy D. Girard, MD, MSCI Clinical Research, Investigation, and Systems Modeling of Acute Illness
Liberation from Mechanical Ventilation in Critically Ill Adults 2017 ACCP/ATS Clinical Practice Guidelines Timothy D. Girard, MD, MSCI Clinical Research, Investigation, and Systems Modeling of Acute Illness
Alcohol interventions in secondary and further education
 National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
Translaryngeal tracheostomy
 Translaryngeal tracheostomy Issued: August 2013 NICE interventional procedure guidance 462 guidance.nice.org.uk/ipg462 NICE has accredited the process used by the NICE Interventional Procedures Programme
Translaryngeal tracheostomy Issued: August 2013 NICE interventional procedure guidance 462 guidance.nice.org.uk/ipg462 NICE has accredited the process used by the NICE Interventional Procedures Programme
TELEFLEX ISIS TM. Better access. Best practice.
 TELEFLEX ISIS TM HVT Better access. Best practice. SUBGLOTTIC SECRETION REMOVAL: A VAP REDUCTION STRATEGY. Ventilator-Associated Pneumonia (VAP) is a nosocomial pneumonia that develops more than 48 hours
TELEFLEX ISIS TM HVT Better access. Best practice. SUBGLOTTIC SECRETION REMOVAL: A VAP REDUCTION STRATEGY. Ventilator-Associated Pneumonia (VAP) is a nosocomial pneumonia that develops more than 48 hours
pat hways Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157
 pat hways AlignRT in breast cancer radiotherapy Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157 Summary The technology described in this briefing is the AlignRT patient
pat hways AlignRT in breast cancer radiotherapy Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157 Summary The technology described in this briefing is the AlignRT patient
Rota-Trach Double Lumen Tracheostomy Tube VITALTEC
 Rota-Trach Double Lumen Tracheostomy Tube VITALTEC Contents INTRODUCTION HISTORY REVIEW ANATOMY & PHYSIOLOGY OPERATIVE PROCEDURE THE RANGE OF TRACHEOSTOMY TUBES ROTA-TRACH TRACHEOSTOMY TUBE INTRODUCTION
Rota-Trach Double Lumen Tracheostomy Tube VITALTEC Contents INTRODUCTION HISTORY REVIEW ANATOMY & PHYSIOLOGY OPERATIVE PROCEDURE THE RANGE OF TRACHEOSTOMY TUBES ROTA-TRACH TRACHEOSTOMY TUBE INTRODUCTION
All bedside percutaneously placed tracheostomies
 Page 1 of 5 Scope: All bedside percutaneously placed tracheostomies Population: All ICU personnel Outcomes: To standardize and outline the steps necessary to safely perform a percutaneous tracheostomy
Page 1 of 5 Scope: All bedside percutaneously placed tracheostomies Population: All ICU personnel Outcomes: To standardize and outline the steps necessary to safely perform a percutaneous tracheostomy
Diagnosis of Ventilator- Associated Pneumonia: Where are we now?
 Diagnosis of Ventilator- Associated Pneumonia: Where are we now? Gary French Guy s & St. Thomas Hospital & King s College, London BSAC Guideline 2008 Masterton R, Galloway A, French G, Street M, Armstrong
Diagnosis of Ventilator- Associated Pneumonia: Where are we now? Gary French Guy s & St. Thomas Hospital & King s College, London BSAC Guideline 2008 Masterton R, Galloway A, French G, Street M, Armstrong
The first convertible endotracheal tube
 TELEFLEX ISIS The first convertible endotracheal tube teleflex isis the right product, every time The unique convertible nature of the Teleflex ISIS frees clinicians from the uncertain burden of choosing
TELEFLEX ISIS The first convertible endotracheal tube teleflex isis the right product, every time The unique convertible nature of the Teleflex ISIS frees clinicians from the uncertain burden of choosing
pat hways Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77
 pat hways OSNA for colon cancer staging Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77 Summary The technology described in this briefing is the OSNA in vitro diagnostic
pat hways OSNA for colon cancer staging Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77 Summary The technology described in this briefing is the OSNA in vitro diagnostic
Web Appendix 1: Literature search strategy. BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up. Sources to be searched for the guidelines;
 Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Trial protocol - NIVAS Study
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Trial protocol - NIVAS Study METHODS Study oversight The Non-Invasive Ventilation after Abdominal Surgery
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Trial protocol - NIVAS Study METHODS Study oversight The Non-Invasive Ventilation after Abdominal Surgery
Cuffed or uncuffed ETT in pediatric anesthesia? Dr. Renata Haghedooren Dr. Sophie Chullikal Dr. Julie Lauweryns
 Cuffed or uncuffed ETT in pediatric anesthesia? Dr. Renata Haghedooren Dr. Sophie Chullikal Dr. Julie Lauweryns Overview History Survey Tradition Pro-Con Debate Conclusions History of intubation 1878:
Cuffed or uncuffed ETT in pediatric anesthesia? Dr. Renata Haghedooren Dr. Sophie Chullikal Dr. Julie Lauweryns Overview History Survey Tradition Pro-Con Debate Conclusions History of intubation 1878:
Tracheostomy and laryngectomy airway emergencies: an overview for medical and nursing staff
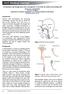 2013 Medical Journal Tracheostomy and laryngectomy airway emergencies: an overview for medical and nursing staff Steven Lobaz 1 and Paul Bush 2 1 ST6 and 2 Consultant Department of Anaesthesia and Intensive
2013 Medical Journal Tracheostomy and laryngectomy airway emergencies: an overview for medical and nursing staff Steven Lobaz 1 and Paul Bush 2 1 ST6 and 2 Consultant Department of Anaesthesia and Intensive
Tracheostomy: Procedures, Timing and Tubes
 TRACHEOSTOMY: PROCEDURES, TIMING AND TUBES Gail M. Sudderth RRT Clinical Specialist Passy-Muir Inc. gsudderth@passy-muir.com (949) 833-8255 Objectives Explain how the timing of the tracheotomy and tube
TRACHEOSTOMY: PROCEDURES, TIMING AND TUBES Gail M. Sudderth RRT Clinical Specialist Passy-Muir Inc. gsudderth@passy-muir.com (949) 833-8255 Objectives Explain how the timing of the tracheotomy and tube
Oral care & swallowing
 Oral care & swallowing Oral care is important as it has a role to play in preventing healthcare associated infections. Dental plaque and the oropharynx can become colonized by bacteria and a biofilm can
Oral care & swallowing Oral care is important as it has a role to play in preventing healthcare associated infections. Dental plaque and the oropharynx can become colonized by bacteria and a biofilm can
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance FINAL SCOPE ENDURALIFE-powered CRT-D devices for the treatment of heart failure 1 Technology 1.1 Description of the technology
Exclusion Criteria 1. Operator or supervisor feels specific intra- procedural laryngoscopy device will be required.
 FELLOW Study Data Analysis Plan Direct Laryngoscopy vs Video Laryngoscopy Background Respiratory failure requiring endotracheal intubation occurs in as many as 40% of critically ill patients. Procedural
FELLOW Study Data Analysis Plan Direct Laryngoscopy vs Video Laryngoscopy Background Respiratory failure requiring endotracheal intubation occurs in as many as 40% of critically ill patients. Procedural
1.40 Prevention of Nosocomial Pneumonia
 1.40 Prevention of Nosocomial Pneumonia Purpose Audience Policy Statement: The guideline is designed to reduce the incidence of pneumonia and other acute lower respiratory tract infections. All UTMB healthcare
1.40 Prevention of Nosocomial Pneumonia Purpose Audience Policy Statement: The guideline is designed to reduce the incidence of pneumonia and other acute lower respiratory tract infections. All UTMB healthcare
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H
 Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
Deep vein thrombosis and its prevention in critically ill adults Attia J, Ray J G, Cook D J, Douketis J, Ginsberg J S, Geerts W H Authors' objectives To systematically review the incidence of deep vein
Foundations of Critical Care Nursing Course. Tracheostomy Workbook
 Foundations of Critical Care Nursing Course Tracheostomy Workbook 1 Key Reference: Dawson D (2014) Essential principles:trachepstomy care in the adult patient, Nursing in Critical Care, Vol 19, 2 p.63-72.
Foundations of Critical Care Nursing Course Tracheostomy Workbook 1 Key Reference: Dawson D (2014) Essential principles:trachepstomy care in the adult patient, Nursing in Critical Care, Vol 19, 2 p.63-72.
The essential principles of tracheostomy care
 The essential principles of tracheostomy care Deborah Dawson Consultant Nurse Critical Care Excellence in specialist and community healthcare Key publications https://www.stgeorges.nhs.uk/gps-andclinicians/clinical-resources/tracheostomyguidelines/
The essential principles of tracheostomy care Deborah Dawson Consultant Nurse Critical Care Excellence in specialist and community healthcare Key publications https://www.stgeorges.nhs.uk/gps-andclinicians/clinical-resources/tracheostomyguidelines/
DRAFT FOR CONSULTATION. Clinical Commissioning Policy Proposition: Robotic assisted trans-oral surgery for throat and voice box cancers
 Clinical Commissioning Policy Proposition: Robotic assisted trans-oral surgery for throat and voice box cancers Information Reader Box (IRB) to be inserted on inside front cover for documents of 6 pages
Clinical Commissioning Policy Proposition: Robotic assisted trans-oral surgery for throat and voice box cancers Information Reader Box (IRB) to be inserted on inside front cover for documents of 6 pages
Saudi Journal of Medical and Pharmaceutical Sciences. DOI: /sjmps. Review Article. Available Online:
 DOI: 10.21276/sjmps Saudi Journal of Medical and Pharmaceutical Sciences Scholars Middle East Publishers Dubai, United Arab Emirates Website: http://scholarsmepub.com/ ISSN 2413-4929 (Print) ISSN 2413-4910
DOI: 10.21276/sjmps Saudi Journal of Medical and Pharmaceutical Sciences Scholars Middle East Publishers Dubai, United Arab Emirates Website: http://scholarsmepub.com/ ISSN 2413-4929 (Print) ISSN 2413-4910
TRACHEOSTOMY EMERGENCIES
 TRACHEOSTOMY EMERGENCIES MODULE: AIRWAY TARGET: ALL ANAESTHETISTS, INTENSIVISTS, ED & ACUTE PHYSICIANS, FOUNDATION DOCTORS BACKGROUND: Around 16% of ICU patients may have a tracheostomy. Life- threatening
TRACHEOSTOMY EMERGENCIES MODULE: AIRWAY TARGET: ALL ANAESTHETISTS, INTENSIVISTS, ED & ACUTE PHYSICIANS, FOUNDATION DOCTORS BACKGROUND: Around 16% of ICU patients may have a tracheostomy. Life- threatening
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement
 CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
CHEST VOLUME 117 / NUMBER 4 / APRIL, 2000 Supplement Evidence-Based Assessment of Diagnostic Tests for Ventilator- Associated Pneumonia* Executive Summary Ronald F. Grossman, MD, FCCP; and Alan Fein, MD,
TRACHEOSTOMY CARE. Tracheostomy- Surgically created hole that extends from the neck skin into the windpipe or trachea.
 1 TRACHEOSTOMY CARE Definitions: Trachea-Windpipe Tracheostomy- Surgically created hole that extends from the neck skin into the windpipe or trachea. Outer Cannula- The outer part of a trach tube. Usually
1 TRACHEOSTOMY CARE Definitions: Trachea-Windpipe Tracheostomy- Surgically created hole that extends from the neck skin into the windpipe or trachea. Outer Cannula- The outer part of a trach tube. Usually
PRODUCTS FOR THE DIFFICULT AIRWAY. Courtesy of Cook Critical Care
 PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
PRODUCTS FOR THE DIFFICULT AIRWAY Courtesy of Cook Critical Care EMERGENCY CRICOTHYROTOMY Thyroid Cartilage Access Site Cricoid Cartilage Identify the cricothyroid membrane between the cricoid and thyroid
The technology described in this briefing is Promonitor. It is used to monitor response to biologic therapies.
 pat hways Promonitor for monitoring response to biologics in rheumatoid arthritis Medtech innovation briefing Published: 27 October 2017 nice.org.uk/guidance/mib126 Summary The technology described in
pat hways Promonitor for monitoring response to biologics in rheumatoid arthritis Medtech innovation briefing Published: 27 October 2017 nice.org.uk/guidance/mib126 Summary The technology described in
*gurgle* *snore* *slaver* Tracheostomy Emergencies with Trachy Tracey Helen Lyall ACCP LUHT 03/06/2016
 *gurgle* *snore* *slaver* Tracheostomy Emergencies with Trachy Tracey Helen Lyall ACCP LUHT 03/06/2016 Learning objectives Describe the difference in anatomy between a tracheotomy and a laryngectomy Understand
*gurgle* *snore* *slaver* Tracheostomy Emergencies with Trachy Tracey Helen Lyall ACCP LUHT 03/06/2016 Learning objectives Describe the difference in anatomy between a tracheotomy and a laryngectomy Understand
Health technology description. What is an evidence note. Key points. Literature search
 Health technology description In response to an enquiry from the Scottish Dental Clinical Effectiveness Programme Number 66 April 2017 After dental instruments have been sterilised unwrapped and are dry,
Health technology description In response to an enquiry from the Scottish Dental Clinical Effectiveness Programme Number 66 April 2017 After dental instruments have been sterilised unwrapped and are dry,
Adult Patients Going Home with a Tracheostomy
 Physiotherapy Department Adult Patients Going Home with a Tracheostomy Darent Valley Hospital Darenth Wood Road Dartford Kent DA2 8DA www.dvh.nhs.uk In case of emergencies you will need to dial 999 If
Physiotherapy Department Adult Patients Going Home with a Tracheostomy Darent Valley Hospital Darenth Wood Road Dartford Kent DA2 8DA www.dvh.nhs.uk In case of emergencies you will need to dial 999 If
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
COBIS Management of airway burns and inhalation injury PAEDIATRIC
 COBIS Management of airway burns and inhalation injury PAEDIATRIC 1 A multidisciplinary team should provide the management of the child with inhalation injury. Childhood inhalation injury mandates transfer
COBIS Management of airway burns and inhalation injury PAEDIATRIC 1 A multidisciplinary team should provide the management of the child with inhalation injury. Childhood inhalation injury mandates transfer
The technology described in this briefing is minimally invasive percutaneous nephrolitholapaxy medium (MIP-M). It is used to remove kidney stones.
 pat hways Minimally invasive percutaneous nephrolitholapaxy medium (MIP-M) for removing kidney stones Medtech innovation briefing Published: 26 January 2018 nice.org.uk/guidance/mib8 Summary The technology
pat hways Minimally invasive percutaneous nephrolitholapaxy medium (MIP-M) for removing kidney stones Medtech innovation briefing Published: 26 January 2018 nice.org.uk/guidance/mib8 Summary The technology
Emergency)tracheostomy)management)/)Patent)upper)airway)
 Emergency)tracheostomy)management)/)Patent)upper)airway) Call,for,airway,expert,help,,Look,,listen,&,feel,at,the,mouth,and,tracheostomy) A)Mapleson)C)system)(e.g.) Waters)circuit ))may)help)assessment)if)available)
Emergency)tracheostomy)management)/)Patent)upper)airway) Call,for,airway,expert,help,,Look,,listen,&,feel,at,the,mouth,and,tracheostomy) A)Mapleson)C)system)(e.g.) Waters)circuit ))may)help)assessment)if)available)
Other methods for maintaining the airway (not definitive airway as still unprotected):
 Page 56 Where anaesthetic skills and drugs are available, endotracheal intubation is the preferred method of securing a definitive airway. This technique comprises: rapid sequence induction of anaesthesia
Page 56 Where anaesthetic skills and drugs are available, endotracheal intubation is the preferred method of securing a definitive airway. This technique comprises: rapid sequence induction of anaesthesia
Ventilator Associated
 Ventilator Associated Pneumonia: Key and Controversial Issues Christopher P. Michetti, MD, FACS Inova Fairfax Hospital, Falls Church, VA Forrest Dell Moore, MD, FACS Banner Healthcare System, Phoenix,
Ventilator Associated Pneumonia: Key and Controversial Issues Christopher P. Michetti, MD, FACS Inova Fairfax Hospital, Falls Church, VA Forrest Dell Moore, MD, FACS Banner Healthcare System, Phoenix,
Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41
 Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technologies guidance SCOPE CardioQ-ODM oesophageal Doppler monitor for patients undergoing major or high-risk surgery and patients in critical
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technologies guidance SCOPE CardioQ-ODM oesophageal Doppler monitor for patients undergoing major or high-risk surgery and patients in critical
Clinical Practice Management Guideline for Ventilator-Associated Pneumonia: Diagnosis, Treatment & Prevention
 Clinical for Ventilator-Associated Pneumonia: Diagnosis, Treatment & Prevention Background Ventilator-associated pneumonia (VAP), a pneumonia that develops 48hrs after initiation of mechanical ventilation,
Clinical for Ventilator-Associated Pneumonia: Diagnosis, Treatment & Prevention Background Ventilator-associated pneumonia (VAP), a pneumonia that develops 48hrs after initiation of mechanical ventilation,
HAP/VAP care bundle interventions - a UK approach. Dr R G Masterton NHS Ayrshire & Arran
 HAP/VAP care bundle interventions - a UK approach Dr R G Masterton NHS Ayrshire & Arran How Hazardous Is Health Care? (Leape and Amalberti) Total lives lost per year 100,000 10,000 1,000 100 10 1 HAZARDOUS
HAP/VAP care bundle interventions - a UK approach Dr R G Masterton NHS Ayrshire & Arran How Hazardous Is Health Care? (Leape and Amalberti) Total lives lost per year 100,000 10,000 1,000 100 10 1 HAZARDOUS
pat hways Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166
 pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
National Horizon Scanning Centre. Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis. April 2008
 Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
REHABILITATION OF PATIENTS MANAGED IN ICU
 REHABILITATION OF PATIENTS MANAGED IN ICU RECOMMENDATIONS Safety to mobilize / exercise: on the website Recommendation 1 All critically ill patients nursed in ICU should be screened closely before active
REHABILITATION OF PATIENTS MANAGED IN ICU RECOMMENDATIONS Safety to mobilize / exercise: on the website Recommendation 1 All critically ill patients nursed in ICU should be screened closely before active
pat hways Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120
 pat hways Caris Molecular Intelligence for guiding cancer treatment Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120 Summary The technology described in this briefing
pat hways Caris Molecular Intelligence for guiding cancer treatment Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120 Summary The technology described in this briefing
28 th September Author Jeremy Gilbert Bariatric Nurse Specialist
 POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
POLICY FOR SELF ADMINISTRATION OF CONTINUOUS POSITIVE AIRWAY PRESSURE BY COMPETENT PATIENTS COMING IN FOR METABOLIC AND OBESITY SURGERY (BARIATRIC SURGERY) TO PENDENNIS WARD 28 th September 2014 Author
Ventilator Associated Pneumonia. ICU Fellowship Training Radboudumc
 Ventilator Associated Pneumonia ICU Fellowship Training Radboudumc Attributable mortality VAP Meta-analysis of individual patient data from randomized prevention studies Attributable mortality mainly results
Ventilator Associated Pneumonia ICU Fellowship Training Radboudumc Attributable mortality VAP Meta-analysis of individual patient data from randomized prevention studies Attributable mortality mainly results
Ventilator associated events, conditions and prevention of VAP. Dr.Pratap Upadhya
 Ventilator associated events, conditions and prevention of VAP Dr.Pratap Upadhya Introduction Pathogenesis of vap Diagnosis of vap Ventilator-Associated Events: New Terminology and Its Relationship to
Ventilator associated events, conditions and prevention of VAP Dr.Pratap Upadhya Introduction Pathogenesis of vap Diagnosis of vap Ventilator-Associated Events: New Terminology and Its Relationship to
Weaning from Mechanical Ventilation. Dr Azmin Huda Abdul Rahim
 Weaning from Mechanical Ventilation Dr Azmin Huda Abdul Rahim Content Definition Classification Weaning criteria Weaning methods Criteria for extubation Introduction Weaning comprises 40% of the duration
Weaning from Mechanical Ventilation Dr Azmin Huda Abdul Rahim Content Definition Classification Weaning criteria Weaning methods Criteria for extubation Introduction Weaning comprises 40% of the duration
pat hways Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147
 pat hways AlignRT for intracranial anial stereotactic radiosurgery Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147 Summary The technology and indication described in this
pat hways AlignRT for intracranial anial stereotactic radiosurgery Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147 Summary The technology and indication described in this
The technology described in this briefing is Permacol, a collagen paste that is injected into anal fistulae.
 pat hways Permacol for treating anal fistulae Medtech innovation briefing Published: 23 May 2017 nice.org.uk/guidance/mib105 Summary The technology described in this briefing is Permacol, a collagen paste
pat hways Permacol for treating anal fistulae Medtech innovation briefing Published: 23 May 2017 nice.org.uk/guidance/mib105 Summary The technology described in this briefing is Permacol, a collagen paste
Ventilator Associated Pneumonia. ICU Fellowship Training Radboudumc
 Ventilator Associated Pneumonia ICU Fellowship Training Radboudumc Attributable mortality VAP Meta-analysis of individual patient data from randomized prevention studies Attributable mortality mainly results
Ventilator Associated Pneumonia ICU Fellowship Training Radboudumc Attributable mortality VAP Meta-analysis of individual patient data from randomized prevention studies Attributable mortality mainly results
Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516
 Cabozantinib for treating medullary thyroid cancer Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Cabozantinib for treating medullary thyroid cancer Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
Tracheostomy practice in adults with acute respiratory failure
 本檔僅供內部教學使用檔案內所使用之照片之版權仍屬於原期刊公開使用時, 須獲得原期刊之同意授權 Tracheostomy practice in adults with acute respiratory failure Bradley D. Freeman, MD, FACS; Peter E. Morris, MD, FCCP Crit Care Med 2012 Vol. 40, No. 10
本檔僅供內部教學使用檔案內所使用之照片之版權仍屬於原期刊公開使用時, 須獲得原期刊之同意授權 Tracheostomy practice in adults with acute respiratory failure Bradley D. Freeman, MD, FACS; Peter E. Morris, MD, FCCP Crit Care Med 2012 Vol. 40, No. 10
The Impact of a Unique Airway Clearance System on Airway Mechanics in Ventilated Patients
 The Impact of a Unique Airway Clearance System on Airway Mechanics in Ventilated Patients Schofield, L. 1, Shorr, A.F. 2, Washington, J. 1, Carlson, M. 1, Wagner, W. 1.1 McLaren Northern Michigan Hospital,
The Impact of a Unique Airway Clearance System on Airway Mechanics in Ventilated Patients Schofield, L. 1, Shorr, A.F. 2, Washington, J. 1, Carlson, M. 1, Wagner, W. 1.1 McLaren Northern Michigan Hospital,
Clinical Commissioning Policy Statement: Patent Foramen Ovale (PFO) Closure. April Reference: NHSCB/A09/PS/a
 Clinical Commissioning Policy Statement: Patent Foramen Ovale (PFO) Closure April 2013 Reference: NHSCB/A09/PS/a NHS Commissioning Board Clinical Commissioning Policy Statement: Patent Foramen Ovale (PFO)
Clinical Commissioning Policy Statement: Patent Foramen Ovale (PFO) Closure April 2013 Reference: NHSCB/A09/PS/a NHS Commissioning Board Clinical Commissioning Policy Statement: Patent Foramen Ovale (PFO)
GASTRO-INTESTINAL (ENTERAL)
 GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 127 B A C D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. Bard 12 French, 5 cc balloon,
GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 127 B A C D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. Bard 12 French, 5 cc balloon,
Urinary Catheters. Prevalence of Infections
 Urinary Catheterisation Urinary catheterisation is defined as an intervention to enable the emptying of the bladder by insertion of a catheter. Catheters can be short term less than 28 days or long term
Urinary Catheterisation Urinary catheterisation is defined as an intervention to enable the emptying of the bladder by insertion of a catheter. Catheters can be short term less than 28 days or long term
RSPT Tracheal Aspiration. Tracheal Aspiration. RSPT 1410 Tracheal Aspiration
 1 RSPT 1410 2 is the use of to facilitate the removal of secretions from the respiratory tract. Under normal circumstances, patients with normal coughing do not have difficulty in removing secretions.
1 RSPT 1410 2 is the use of to facilitate the removal of secretions from the respiratory tract. Under normal circumstances, patients with normal coughing do not have difficulty in removing secretions.
What is the next best step?
 Noninvasive Ventilation William Janssen, M.D. Assistant Professor of Medicine National Jewish Health University of Colorado Denver Health Sciences Center What is the next best step? 65 year old female
Noninvasive Ventilation William Janssen, M.D. Assistant Professor of Medicine National Jewish Health University of Colorado Denver Health Sciences Center What is the next best step? 65 year old female
Tracheostomy. Hope Building Neurosurgery
 Tracheostomy Hope Building Neurosurgery 0161 206 5055 All Rights Reserved 2017. Document for issue as handout. Unique Identifier: CS36(17). Review date: November 2019 What is a tracheostomy? A tracheostomy
Tracheostomy Hope Building Neurosurgery 0161 206 5055 All Rights Reserved 2017. Document for issue as handout. Unique Identifier: CS36(17). Review date: November 2019 What is a tracheostomy? A tracheostomy
Charisma High-flow CPAP solution
 Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504
 Transcatheter valve-in-valve e implantation for aortic bioprosthetic valve dysfunction Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504 Your responsibility This
Transcatheter valve-in-valve e implantation for aortic bioprosthetic valve dysfunction Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504 Your responsibility This
Neonatal Airway Disorders, Treatments, and Outcomes. Steven Goudy, MD Pediatric Otolaryngology Emory University Medical Center
 Neonatal Airway Disorders, Treatments, and Outcomes Steven Goudy, MD Pediatric Otolaryngology Emory University Medical Center Disclosure I have nothing to disclose Neonatal and Pediatric Tracheostomy Tracheostomy
Neonatal Airway Disorders, Treatments, and Outcomes Steven Goudy, MD Pediatric Otolaryngology Emory University Medical Center Disclosure I have nothing to disclose Neonatal and Pediatric Tracheostomy Tracheostomy
