pat hways Medtech innovation briefing Published: 1 October 2018 nice.org.uk/guidance/mib161
|
|
|
- Julianna Gallagher
- 5 years ago
- Views:
Transcription
1 pat hways myairvo2 for the treatment of chronic obstructive pulmonary disease Medtech innovation briefing Published: 1 October 2018 nice.org.uk/guidance/mib161 Summary The technology described in this briefing is myairvo2. It is designed to warm and humidify respiratory gases used for nasal high-flow therapy. This briefing focuses on its potential use in a community setting. The innovative aspects are that myairvo2 is designed to allow the delivery of nasal high-flow therapy in a community setting. It is also designed to allow clinicians to titrate flow and oxygen independently from one another and does not need a sealed interface. The intended place in therapy would be as well as standard management in the home or longterm care environment for people with chronic obstructive pulmonary disease (COPD) having nasal high-flow oxygen therapy. The main points from the evidence summarised in this briefing are from 5 studies, 1 randomised controlled trial and 4 randomised crossover trials including a total of 232 adults. They show that myairvo2 is at least as effective as non-humidified and unwarmed gases in patients with COPD and has the potential to reduce hospital admissions. Key uncertainties around the evidence relate to the lack of follow-up to show the long-term positive effects of myairvo2 and the generalisability to UK NHS practice. There are also uncertainties around which patient group nasal high-flow therapy would most benefit in a community setting and if it should be used with or instead of current treatments. Page 1 of
2 The cost of myairvo2 is 2,475 per unit (excluding VAT). The overall resource impact is uncertain because there is no published evidence assessing the resource consequences of myairvo2 in a community setting. More information on this would be helpful. The technology The myairvo2 system delivers warmed and humidified respiratory gases, including at high-flow rates. It includes a humidifier with an integrated flow generator that is designed for use in patients who are breathing without help and has interfaces to suit both upper and bypassed airways (tracheostomy patients). Other features of myairvo2 are that it allows clinicians to titrate flow (from 10 to 60 litres/min on adult mode) and oxygen independently from one another and does not need a sealed interface. The product is marketed as AIRVO2 and myairvo2. myairvo2 is a device that is being used in the home or other domiciliary care environment. This briefing focuses on myairvo2 delivering nasal high-flow therapy for treating chronic obstructive pulmonary disease (COPD), including in a community setting. Innovations myairvo2 differs from standard respiratory gas devices in being designed to allow the delivery of nasal high-flow therapy in a community setting. It also uses room air, removing the need for piped medical air, and humidifies the delivered air to reduce the risk of dryness and trauma to the upper airway mucosa. Current care pathway The NICE guideline on chronic obstructive pulmonary disease recommends treatment options including smoking cessation, inhaled therapy, oral therapy, oxygen therapy, non-invasive ventilation, pulmonary rehabilitation and surgery. The guideline makes detailed recommendations for patient selection and use of long-term oxygen therapy and non-invasive ventilation. The guideline does not include recommendations on nasal high-flow therapy as an alternative or adjunct to these interventions. The British Thoracic Society guideline on oxygen use in adults in healthcare and emergency settings highlights that, as part of good clinical practice, high-flow nasal oxygen should be considered as an alternative to reservoir mask treatment in patients with acute respiratory failure without hypercapnia. Page 2 of
3 Population, setting and intended user The place in treatment of myairvo2 in a community setting is uncertain because patient selection for nasal high-flow oxygen (or oxygen-enriched gases) isn't well defined. It could be used in place of, or as well as, long-term oxygen therapy or non-invasive ventilation. myairvo2 would be prescribed by a healthcare professional and administered by a carer or community nursing staff or the patient themselves. Costs myairvo2 costs 2,475 (including 3 filters) and has a lifespan of 5 years with no additional servicing costs or needs. The consumable components cost 136 per patient per 2-month period and 9 per filter, which needs replacing every 3 months or 1,000 hours of use. Based on these costings, the total device costs of myairvo2 are 1,320 per patient in the first year and 1,347 in the remaining 4 years. Standard of care Nasal high-flow therapy is not routinely delivered in a community setting in the NHS so direct comparative costs are not available. Interventions that myairvo2 might replace or be used alongside include a domiciliary non-invasive ventilation service ( 2,373 in the first year, excluding device costs, and 1,536 in subsequent years [Dretzke et al. 2015]). Resource consequences myairvo2 would represent an additional cost to standard care, which could potentially be offset if it resulted in a reduction in exacerbations, admissions to hospital, or antibiotic use. Other cost savings may be possible if myairvo2 helps support early discharge from hospital. myairvo2 is for use in the patient's home and in community care facilities. It is anticipated that introducing this technology for community-based patients would need some pathway redesign with changes in resourcing because of this. Regulatory information myairvo2 is a CE marked class IIa medical device. The following Medical Device Alert for this technology has been identified: Page 3 of
4 Risk of undetected auditory alarm (2016) updated instructions for use to check the speaker before each use because undetected auditory alarms could result in the patient becoming hypoxic. All actions outlined for this alert have been completed and the alert has been archived. Equality considerations NICE is committed to promoting equality, eliminating unlawful discrimination and fostering good relations between people with particular protected characteristics and others. In producing guidance and advice, NICE aims to comply fully with all legal obligations to: promote race and disability equality and equality of opportunity between men and women, eliminate unlawful discrimination on grounds of race, disability, age, sex, gender reassignment, marriage and civil partnership, pregnancy and maternity (including women post-delivery), sexual orientation, and religion or belief (these are protected characteristics under the Equality Act 2010). Chronic obstructive pulmonary disease (COPD) is a chronic condition, which may mean someone is disabled if this has a substantial and long-term effect on their ability to do daily activities. Disability is a protected characteristic under the Equality Act. Clinical and technical evidence A literature search was carried out for this briefing in accordance with the interim process and methods statement. This briefing includes the most relevant or best available published evidence relating to the clinical effectiveness of the technology. Further information about how the evidence for this briefing was selected is available on request by contacting mibs@nice.org.uk. Published evidence The evidence base for myairvo2 includes over 20 studies identified as being potentially relevant, including more than 10 randomised trials. Seven studies were published in abstract form only. Five studies (1 randomised controlled trial and 4 randomised crossover trials) are summarised in this briefing, including 232 patients. Table 1 summarises the clinical evidence as well as its strengths and limitations. Overall assessment of the evidence The evidence suggests that myairvo2 shows efficacy in treating patients with chronic obstructive pulmonary disease compared with non-humidified unheated respiratory support. One study reported a reduction in hospital admissions. However, there is little evidence of the long-term Page 4 of
5 effects of myairvo2. As well as the lack of long-term studies, it is uncertain how generalisable the studies are to UK NHS practice because none of the studies were done in the UK. Only 1 study assessed myairvo2 in a community setting. Table 1 Summary of selected studies Storgaard et al. (2018) Study size, design and location Intervention and comparator(s) Key outcomes Prospective RCT of 200 patients diagnosed with COPD with chronic hypoxaemic respiratory failure. Study done at 4 outpatient clinics in Denmark. LTOT with myairvo2 (n=100) at home. LTOT (n=100) at home. Rate of AECOPD myairvo vs 4.95 (control) per patient/year (p<0.001). Hospital admissions 0.79 vs 1.39/patient/year for 12- vs 1-month use of HFNC respectively (p<0.001). Dyspnoea (mmrc score) At 3 months, myairvo2 group had improved mmrc scores (p<0.05), and from 3 months onward, they had lower mmrc scores vs controls (p<0.001). QoL myairvo group had better SGRQ at both 6 (p=0.002) and 12 months (p=0.033) vs controls. PaCO 2 Over 12 months, PaCO 2 decreased in myairvo2 group and increased for controls (p=0.005). All-cause mortality myairvo2 15% vs control 12% (p=0.636) Exercise performance significant difference at 12 months (p=0.005) in favour of myairvo2 group (excluding 'non-walkers'). Strengths and limitations Treatment allocation was fully described. The baseline dyspnoea score was significantly different between groups (p=0.008). Neither the participants nor the assessors could be blinded because it was obvious if they were being treated using the myairvo2 device. McKinstry et al. (2018) Page 5 of
6 Study size, design and location Intervention and comparator(s) Key outcomes Single-centre randomised crossover trial in New Zealand, including 48 patients with stable COPD. Participants had all 4 interventions in a randomised order; NHF using myairvo2 at 15 litres/min, 30 litres/min, 45 litres/min and room air in a hospital clinical setting. Mean (95% CI) change in PtCO2 at 20 minutes compared with room air: 15 litres/min: 0.6 mmhg ( 1.1 to 0.0), p= litres/min: 1.3 mmhg ( 1.9 to 0.8), p< litres/min: 2.4 mmhg ( 2.9 to 1.8), p< Mean (95% CI) change in respiratory rate at 20 minutes compared with room air: 15 litres/min: 1.5 ( 2.7 to 0.3), p= litres/min: 4.1 ( 5.3 to 2.9), p< litres/min: 4.3 ( 5.5 to 3.1), p< Strengths and limitations Treatment allocation was fully described. Crossover design, therefore participants served as their own control, reducing the influence of selection bias. Patients said to be blinded, however it is feasible they could have differentiated between the 4 treatments. Pisani et al. (2017) Study size, design and location Intervention and comparator(s) Single-centre randomised crossover trial in Italy, including 14 COPD patients with stable CHRF. Participants completed in 5 randomised order, 30-minute trials (HFOT was delivered at 2 flow rates, 20 and 30 litres/min with the participants' mouth open and closed, and the fifth trial was NIV) in a hospital clinic setting. Page 6 of
7 Key outcomes The data were analysed by assessing differences between baseline and the 5 trials. TI,p (seconds) no difference between trials. TE,p (seconds) breathing frequency (breaths/min) was significantly prolonged compared with baseline for all the settings. Tidal volume (ml) significantly higher compared with baseline for all settings. Pdi swing (cmh 2 O) reduced compared with baseline in all trials. PTPdi/min (cmh 2 Oxs/min) reduced compared with baseline in all trials. PEEPi,dyn (cmh 2 O) significantly reduced compared with baseline in all trials. PaCO 2 (mm Hg) decreased but not significantly. HFOT at 30 litres/min and NIV compared with standard oxygen. PaO 2 (mm Hg) no difference in HFOT 20 closed group, lower in the HFOT 30 group and significantly higher in NIV group compared baseline. Comfort score did not vary among the different trials. Strengths and limitations Blinding and dropout rates were not reported. Crossover design, therefore participants served as their own control, reducing the influence of selection bias. Small numbers of participants and only short-term effects reported. Cirio et al. (2016) Study size, design and location Intervention and comparator(s) Key outcomes Single-centre pilot randomised crossover trial in Italy, including 12 patients with severe COPD. Participants completed in a randomised order 2 constant-load, symptomlimited exercise tests at 75% of maximum workload achieved with the incremental test, with (HFNC-test) and without (control-test) HFNC using myairvo2 in a hospital clinic setting. Exercise duration mean difference 109±104, p<0.015, resulting in a mean increase of 41±36%. Dyspnoea p=0.002 Leg fatigue p=0.002 SaO 2 p< Page 7 of
8 Strengths and limitations Treatment allocation was not described fully. Crossover design, therefore participants served as their own control, reducing the influence of selection bias. The washout period was 24 hours. Unclear if this exercise test would be generalisable to rehabilitation. Fraser et al. (2016) Study size, design and location Intervention and comparator(s) Single-centre pilot randomised crossover trial in New Zealand, including 30 patients with COPD. Intervention: NHF using air supplemented with the equivalent FiO 2 to a total flow of 30 litres/min from an AIRVO2 through an Optiflow nasal interface in a hospital clinic setting. Comparator: current LTOT in a hospital clinic setting. Key outcomes RR p=0.001 SaO 2 p=0.06 Dyspnoea p<0.001 Tidal volume p=0.003 End-expiratory lung impedance p< Strengths and limitations Treatment allocation fully described in supplementary document. Crossover design, therefore participants served as their own control, reducing the influence of selection bias. Male-only participants, therefore difficult to generalise results to female participants. This is a short-term study, therefore it is unclear if these benefits can be realised long term. Abbreviations: AECOPD, acute exacerbation of COPD; CHRF, chronic hypercapnic respiratory failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FiO 2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; HFOT, high-flow oxygen therapy; LTOT, long-term oxygen therapy; mmrc, modified Medical Research Council; NHF, nasal high-flow; NIV, noninvasive ventilation; PaCO 2, partial pressure carbon dioxide; PaO 2, partial pressure oxygen; Pdi swing, transdiaphragmatic pressure; PEEPi,dyn, dynamic intrinsic positive end-expiratory pressure; PTPdi, diaphragm pressure time product; QoL, quality of life; RCT, randomised controlled trial; RR, respiratory rate; SaO 2, oxygen saturation; SGRQ, St George's Respiratory Questionnaire; TE,p, patient's own expiratory time; TI,p, patient's own inspiratory time; vs, versus. Page 8 of
9 Recent and ongoing studies Feasibility of using daily home HNHF-O2 during sleep and/or daytime in hypercapnic COPD patients following recent hospitalization for AECOPD for 90 days. ClinicalTrials.gov identifier: NCT Estimated completion date: September Indication: COPD. Devices: myairvo2. USA. Specialist commentator comments Comments on this technology were invited from clinical specialists working in the field and relevant patient organisations. The comments received are individual opinions and do not represent NICE's view. All 5 of the specialist commentators were familiar with or had used this technology before. Level of innovation One commentator felt this technology was a variation to current therapy. Three experts think myairvo2 is a novel and developing concept. One commentator added that nasal high-flow therapy is innovative but not new; however, the application of nasal high-flow therapy using this technology to support early discharge from hospital or as an alternative to long-term oxygen therapy or domiciliary continuous positive airway pressure (CPAP)/non-invasive ventilation in the community setting is novel. One commentator stated that the myairvo2 is not a new technology. Potential patient impact One specialist commentator stated the myairvo2 can improve stability of the disease. Two specialist commentators stated humidified air benefits patients by helping mucus clearance and helping to wash out CO 2 from the airway dead space. One commentator concluded the main benefit of the myairvo2 is that humidified therapy with a high percentage of oxygen can be offered through nasal interfaces. This improves a patient's quality of life because they are able to communicate, eat and drink easily, which are limited with a face mask or with non-invasive ventilation. The high-flow aspect makes the device suitable for those patients who are extremely breathless. One specialist noted possible patient benefits such as the potential to avoid long-term supplemental oxygen therapy, domiciliary CPAP or non-invasive ventilation, the ability to relieve breathlessness at rest or during physical activity, and a reduction in complications related to noninvasive ventilation interfaces. The myairvo2 provides greater flexibility to titrate respiratory therapy in comparison to long-term oxygen therapy and has the potential to reduce exacerbations Page 9 of
10 of chronic obstructive pulmonary disease (COPD) and prevent admission to hospital. Three commentators feel that the myairvo2 would be of benefit for patients having end-of-life care. Potential system impact Two commentators feel there is potential for myairvo2 to reduce the number of COPD exacerbations and hospital admissions and the associated costs. One specialist highlighted a recent publication (Storgaard et al. 2018), citing improved admission-free survival in COPD patients treated with home nasal high-flow therapy. One commentator stated that this technology provides another treatment option. One specialist commentator stated that there is potential for myairvo2 to support early discharge from hospital after exacerbation of COPD, reducing the amount of time in hospital. The same commentator also highlighted the potential to reduce costs compared with alternative domiciliary respiratory support therapies such as long-term oxygen therapy and non-invasive ventilation. One specialist outlined that it is expected that the community staff and service needs will be similar to home oxygen or non-invasive ventilation. There is also potential that there would be a little more pressure on the outpatient services that would support the equipment such as home ventilation services. General comments Specialist commentators identified a diverse range of patient groups for whom myairvo2 could be suitable, some of which are outside the scope of this briefing. In the community, this device may be considered for some patients with bronchiectasis or cystic fibrosis and in patients with a tracheostomy with thick tenacious secretions needing humidification. One expert concluded that myairvo2 and nasal high-flow therapy is a developing field, which appears to be having a real impact on patient care. One expert advised that to address some of the uncertainty in this promising technology, UK-based research exploring clinical outcomes (for example, length of stay, hospital admissions, rates of exacerbations, antibiotic and steroid use, arterial blood gas measurements, patient-reported outcomes) as well as an economic evaluation should be done. This could include a more detailed cost comparison for a patient cohort. Studies should also address the following: Directly comparing the use of AIRVO2 in comparison to each of long-term oxygen therapy and non-invasive ventilation across a range of specific clinical states. Exploring the role of AIRVO2 specifically for early supported discharge following exacerbation of COPD. Page 10 of
11 Exploring the role of AIRVO2 specifically when long-term oxygen therapy is not tolerated/ adhered to or non-invasive ventilation is not tolerated/adhered to. Specialist commentators The following clinicians contributed to this briefing: Mr Iain Wheatley, Nurse Consultant acute and respiratory care, Association of Respiratory Nurse Specialists (ARNS), did not declare any interests. Mr Matthew Quint, Respiratory Clinical Specialist, Member of the Chartered Society of Physiotherapy, received financial support for travel and accommodation at 2 UK conferences by the company producing the technology. Dr Christopher Carlin, Consultant Respiratory Physician and Honorary Senior Lecturer, received financial support for travel, accommodation and catering for attendance at 2 UK conferences by the company producing the technology. Ms Ema Swingwood, Respiratory Pathway Lead/Physiotherapist, Member of Association for Chartered Physiotherapists in Respiratory Care (ACPRC), consultancy fee received from the company producing the technology. Mr Gareth Cornell, Clinical Specialist Physiotherapist Critical Care, member of Association of Chartered Physiotherapists in Respiratory Care, did not declare any interests. Development elopment of this briefing This briefing was developed by NICE. The interim process and methods statement sets out the process NICE uses to select topics, and how the briefings are developed, quality-assured and approved for publication. ISBN: Page of
pat hways Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87
 pat hways CytoSorb therapy for sepsis Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87 Summary The technology described in this briefing is CytoSorb therapy. It is an
pat hways CytoSorb therapy for sepsis Medtech innovation briefing Published: 29 November 2016 nice.org.uk/guidance/mib87 Summary The technology described in this briefing is CytoSorb therapy. It is an
Key uncertainties around the evidence or technology are that the test has only been validated in biochemical studies.
 pat hways PredictSure-IBD for inflammatory bowel disease prognosis Medtech innovation briefing Published: 25 March 2019 nice.org.uk/guidance/mib178 Summary The technology described in this briefing is
pat hways PredictSure-IBD for inflammatory bowel disease prognosis Medtech innovation briefing Published: 25 March 2019 nice.org.uk/guidance/mib178 Summary The technology described in this briefing is
The innovative aspects are that ORA G3 is currently the only device capable of measuring corneal hysteresis.
 pat hways ORA G3 to measure corneal hysteresis Medtech innovation briefing Published: 18 June 2018 nice.org.uk/guidance/mib150 Summary The technology described in this briefing is Ocular Response Analyzer
pat hways ORA G3 to measure corneal hysteresis Medtech innovation briefing Published: 18 June 2018 nice.org.uk/guidance/mib150 Summary The technology described in this briefing is Ocular Response Analyzer
The innovative aspects are that the test automatically generates a report on ALK status that any clinician can interpret.
 pat hways HTG EdgeSeq ALKPlus Assay EU for ALK status testing in non-small-cell lung cancer Medtech innovation briefing Published: 7 November 2017 nice.org.uk/guidance/mib128 Summary The technology described
pat hways HTG EdgeSeq ALKPlus Assay EU for ALK status testing in non-small-cell lung cancer Medtech innovation briefing Published: 7 November 2017 nice.org.uk/guidance/mib128 Summary The technology described
GDm-Health is free to download and use. Its use may result in efficiency savings from reducing face-to-face clinic appointments.
 pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
pat hways Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157
 pat hways AlignRT in breast cancer radiotherapy Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157 Summary The technology described in this briefing is the AlignRT patient
pat hways AlignRT in breast cancer radiotherapy Medtech innovation briefing Published: 24 August 2018 nice.org.uk/guidance/mib157 Summary The technology described in this briefing is the AlignRT patient
The innovative aspect is that it detects bladder cancer based on the novel biomarker, minichromosome maintenance complex component 5 (MCM5).
 pat hways ADXBLADDER for detecting bladder cancer Medtech innovation briefing Published: 12 April 2019 nice.org.uk/guidance/mib180 Summary The technology described in this briefing is ADXBLADDER. It is
pat hways ADXBLADDER for detecting bladder cancer Medtech innovation briefing Published: 12 April 2019 nice.org.uk/guidance/mib180 Summary The technology described in this briefing is ADXBLADDER. It is
Commissioning for Better Outcomes in COPD
 Commissioning for Better Outcomes in COPD Dr Matt Kearney Primary Care & Public Health Advisor Respiratory Programme, Department of Health General Practitioner, Runcorn November 2011 What are the Commissioning
Commissioning for Better Outcomes in COPD Dr Matt Kearney Primary Care & Public Health Advisor Respiratory Programme, Department of Health General Practitioner, Runcorn November 2011 What are the Commissioning
Nasal High Flow Humidification with or without Oxygen for COPD Management. Shereen Bailey, RCP, RRT, NPS
 Nasal High Flow Humidification with or without Oxygen for COPD Management Shereen Bailey, RCP, RRT, NPS Objectives How it works COPD Management today The role of NHFC Evidence Research/Case Studies Types
Nasal High Flow Humidification with or without Oxygen for COPD Management Shereen Bailey, RCP, RRT, NPS Objectives How it works COPD Management today The role of NHFC Evidence Research/Case Studies Types
Endobronchial valve insertion to reduce lung volume in emphysema
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
pat hways Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76
 pat hways Axxent brachytherapy system for early stage breast cancer Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76 Summary The technology described in this briefing is
pat hways Axxent brachytherapy system for early stage breast cancer Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib76 Summary The technology described in this briefing is
pat hways Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77
 pat hways OSNA for colon cancer staging Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77 Summary The technology described in this briefing is the OSNA in vitro diagnostic
pat hways OSNA for colon cancer staging Medtech innovation briefing Published: 24 August 2016 nice.org.uk/guidance/mib77 Summary The technology described in this briefing is the OSNA in vitro diagnostic
The technology described in this briefing is Permacol, a collagen paste that is injected into anal fistulae.
 pat hways Permacol for treating anal fistulae Medtech innovation briefing Published: 23 May 2017 nice.org.uk/guidance/mib105 Summary The technology described in this briefing is Permacol, a collagen paste
pat hways Permacol for treating anal fistulae Medtech innovation briefing Published: 23 May 2017 nice.org.uk/guidance/mib105 Summary The technology described in this briefing is Permacol, a collagen paste
BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012
 BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012 Evidence base for Home Oxygen therapy in COPD, non-copd respiratory disease and nonrespiratory
BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012 Evidence base for Home Oxygen therapy in COPD, non-copd respiratory disease and nonrespiratory
Improving Care & Outcomes
 Improving Care & Outcomes Macquarie Technology Day, 20 October 2011 1 Improving Care & Outcomes The Care Continuum - Matthew Payton F&P Optiflow - Matthew Payton F&P Info Technologies - Lewis Gradon ICON
Improving Care & Outcomes Macquarie Technology Day, 20 October 2011 1 Improving Care & Outcomes The Care Continuum - Matthew Payton F&P Optiflow - Matthew Payton F&P Info Technologies - Lewis Gradon ICON
The technology described in this briefing is Promonitor. It is used to monitor response to biologic therapies.
 pat hways Promonitor for monitoring response to biologics in rheumatoid arthritis Medtech innovation briefing Published: 27 October 2017 nice.org.uk/guidance/mib126 Summary The technology described in
pat hways Promonitor for monitoring response to biologics in rheumatoid arthritis Medtech innovation briefing Published: 27 October 2017 nice.org.uk/guidance/mib126 Summary The technology described in
QOF indicator area: Chronic Obstructive Pulmonary disease (COPD)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Cost impact statement: Chronic Obstructive Pulmonary Disease QOF indicator area:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE QUALITY AND OUTCOMES FRAMEWORK (QOF) INDICATOR DEVELOPMENT PROGRAMME Cost impact statement: Chronic Obstructive Pulmonary Disease QOF indicator area:
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE
 Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist. This program has been approved for 1 hour of continuing education credit.
 Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist This program has been approved for 1 hour of continuing education credit. Course Objectives Identify at least four goals of home NIV Identify candidates
Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist This program has been approved for 1 hour of continuing education credit. Course Objectives Identify at least four goals of home NIV Identify candidates
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600
 Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
pat hways Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120
 pat hways Caris Molecular Intelligence for guiding cancer treatment Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120 Summary The technology described in this briefing
pat hways Caris Molecular Intelligence for guiding cancer treatment Medtech innovation briefing Published: 20 September 2017 nice.org.uk/guidance/mib120 Summary The technology described in this briefing
What is the next best step?
 Noninvasive Ventilation William Janssen, M.D. Assistant Professor of Medicine National Jewish Health University of Colorado Denver Health Sciences Center What is the next best step? 65 year old female
Noninvasive Ventilation William Janssen, M.D. Assistant Professor of Medicine National Jewish Health University of Colorado Denver Health Sciences Center What is the next best step? 65 year old female
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Motor neurone disease: the use of non-invasive ventilation in the management of motor neurone disease 1.1 Short title Motor
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Motor neurone disease: the use of non-invasive ventilation in the management of motor neurone disease 1.1 Short title Motor
pat hways Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147
 pat hways AlignRT for intracranial anial stereotactic radiosurgery Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147 Summary The technology and indication described in this
pat hways AlignRT for intracranial anial stereotactic radiosurgery Medtech innovation briefing Published: 16 May 2018 nice.org.uk/guidance/mib147 Summary The technology and indication described in this
The technology described in this briefing is minimally invasive percutaneous nephrolitholapaxy medium (MIP-M). It is used to remove kidney stones.
 pat hways Minimally invasive percutaneous nephrolitholapaxy medium (MIP-M) for removing kidney stones Medtech innovation briefing Published: 26 January 2018 nice.org.uk/guidance/mib8 Summary The technology
pat hways Minimally invasive percutaneous nephrolitholapaxy medium (MIP-M) for removing kidney stones Medtech innovation briefing Published: 26 January 2018 nice.org.uk/guidance/mib8 Summary The technology
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
2014 Full Year Results Presentation. Year ended 31 March 2014
 2014 Full Year Results Presentation Year ended 31 March 2014 26-30 May 2014 1 Full year result highlights 12 months to 31 March 2014 NZ$M PCP CC 1 Record net profit after tax 97.1 +26% +46% Record operating
2014 Full Year Results Presentation Year ended 31 March 2014 26-30 May 2014 1 Full year result highlights 12 months to 31 March 2014 NZ$M PCP CC 1 Record net profit after tax 97.1 +26% +46% Record operating
pat hways Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166
 pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
OXYGEN USE IN PHYSICAL THERAPY PRACTICE. Rebecca H. Crouch, PT,DPT,MS,CCS,FAACVPR
 OXYGEN USE IN PHYSICAL THERAPY PRACTICE Rebecca H. Crouch, PT,DPT,MS,CCS,FAACVPR Supplemental Oxygen Advantages British Medical Research Council Clinical Trial Improved survival using oxygen 15 hrs/day
OXYGEN USE IN PHYSICAL THERAPY PRACTICE Rebecca H. Crouch, PT,DPT,MS,CCS,FAACVPR Supplemental Oxygen Advantages British Medical Research Council Clinical Trial Improved survival using oxygen 15 hrs/day
Oxygen & High flow nasal Oxygen therapy. Learning points. Why? 18/07/
 Oxygen & High flow nasal Oxygen therapy 13.07.2017 Learning points Update on BTS guidance May 2017 Help you understand the mechanism of action of high flow nasal oxygen therapy Help you think about the
Oxygen & High flow nasal Oxygen therapy 13.07.2017 Learning points Update on BTS guidance May 2017 Help you understand the mechanism of action of high flow nasal oxygen therapy Help you think about the
Chronic Obstructive Pulmonary Disease (COPD).
 Chronic Obstructive Pulmonary Disease (COPD). Linde: Living healthcare 02 03 Chronic Obstructive Pulmonary Disease (COPD). A pocket guide for healthcare professionals. COPD the facts Moderate to severe
Chronic Obstructive Pulmonary Disease (COPD). Linde: Living healthcare 02 03 Chronic Obstructive Pulmonary Disease (COPD). A pocket guide for healthcare professionals. COPD the facts Moderate to severe
Chronic obstructive pulmonary disease in over 16s: diagnosis and management
 National Institute for Health and Care Excellence Final Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B] Oxygen therapy in people with stable COPD NICE guideline NG115 Evidence
National Institute for Health and Care Excellence Final Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B] Oxygen therapy in people with stable COPD NICE guideline NG115 Evidence
The product website contains useful information and videos that cover both the patient and the clinician interfaces for mycopd
 mycopd universal guidance The product website contains useful information and videos that cover both the patient and the clinician interfaces for mycopd https://mymhealth.com/mymhealth/mycopd The clinician
mycopd universal guidance The product website contains useful information and videos that cover both the patient and the clinician interfaces for mycopd https://mymhealth.com/mymhealth/mycopd The clinician
For personal use only
 FY2015 Half Year Results Presentation 6 months ended 30 September 2014 24 28 November 2014 1 First half result highlights H1 FY15 (6 months to 30 September 2014) PCP CC 1 Operating profit +8% +64% RAC
FY2015 Half Year Results Presentation 6 months ended 30 September 2014 24 28 November 2014 1 First half result highlights H1 FY15 (6 months to 30 September 2014) PCP CC 1 Operating profit +8% +64% RAC
Bi-Level Therapy: Boosting Comfort & Compliance in Apnea Patients
 Bi-Level Therapy: Boosting Comfort & Compliance in Apnea Patients Objectives Describe nocturnal ventilation characteristics that may indicate underlying conditions and benefits of bilevel therapy for specific
Bi-Level Therapy: Boosting Comfort & Compliance in Apnea Patients Objectives Describe nocturnal ventilation characteristics that may indicate underlying conditions and benefits of bilevel therapy for specific
National Horizon Scanning Centre. Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis. April 2008
 Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room
 IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room 1) Decreasing Telemetry Alarms Janice Marlett, BSN, RN, Nursing Staff Educator To decrease tele alarms: Properly prep the skin Shave
IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room 1) Decreasing Telemetry Alarms Janice Marlett, BSN, RN, Nursing Staff Educator To decrease tele alarms: Properly prep the skin Shave
Trial protocol - NIVAS Study
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Trial protocol - NIVAS Study METHODS Study oversight The Non-Invasive Ventilation after Abdominal Surgery
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Trial protocol - NIVAS Study METHODS Study oversight The Non-Invasive Ventilation after Abdominal Surgery
The cost of the Axonics sacral neuromodulation system is 475 for the trial phase, 9,210 for a
 pat hways Axonics sacral al neuromodulation system for overactive bladder and faecal incontinence Medtech innovation briefing Published: 10 December 2018 nice.org.uk/guidance/mib164 Summary The technology
pat hways Axonics sacral al neuromodulation system for overactive bladder and faecal incontinence Medtech innovation briefing Published: 10 December 2018 nice.org.uk/guidance/mib164 Summary The technology
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
Web Appendix 1: Literature search strategy. BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up. Sources to be searched for the guidelines;
 Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Average volume-assured pressure support
 Focused review Average volume-assured pressure support Abdurahim Aloud MD Abstract Average volume-assured pressure support (AVAPS) is a relatively new mode of noninvasive positive pressure ventilation
Focused review Average volume-assured pressure support Abdurahim Aloud MD Abstract Average volume-assured pressure support (AVAPS) is a relatively new mode of noninvasive positive pressure ventilation
Fisher & Paykel Healthcare
 Fisher & Paykel Healthcare Deutsche Bank / Craigs New Zealand Corporate Day 1 Sydney March 2014 Investment Highlights A leader in respiratory and OSA treatment devices Consistent growth strategy Estimated
Fisher & Paykel Healthcare Deutsche Bank / Craigs New Zealand Corporate Day 1 Sydney March 2014 Investment Highlights A leader in respiratory and OSA treatment devices Consistent growth strategy Estimated
NI 60. Non-invasive ventilation without compromise. Homecare Pneumology Neonatology Anaesthesia. Sleep Diagnostics Service Patient Support
 NI 60 Non-invasive ventilation without compromise Homecare Pneumology Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support NI 60 Non-invasive ventilation without
NI 60 Non-invasive ventilation without compromise Homecare Pneumology Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support NI 60 Non-invasive ventilation without
Surveillance report Published: 6 April 2016 nice.org.uk. NICE All rights reserved.
 Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP)
 Introduction NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP) Noninvasive ventilation (NIV) is a method of delivering oxygen by positive pressure mask that allows for the prevention or postponement of invasive
Introduction NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP) Noninvasive ventilation (NIV) is a method of delivering oxygen by positive pressure mask that allows for the prevention or postponement of invasive
Cardiorespiratory Physiotherapy Tutoring Services 2017
 VENTILATOR HYPERINFLATION ***This document is intended to be used as an information resource only it is not intended to be used as a policy document/practice guideline. Before incorporating the use of
VENTILATOR HYPERINFLATION ***This document is intended to be used as an information resource only it is not intended to be used as a policy document/practice guideline. Before incorporating the use of
Disclosure. Learning Objectives. Bernadette Zelaya, RRT. Area Clinical Manager
 High Velocity Nasal Insufflation An Important Therapeutic Approach for Use in the Emergency Department Presented by Vapotherm Accredited for 1 CEU by the American Association for Respiratory Care Provider
High Velocity Nasal Insufflation An Important Therapeutic Approach for Use in the Emergency Department Presented by Vapotherm Accredited for 1 CEU by the American Association for Respiratory Care Provider
1.1.2 CPAP therapy is used for patients who are suffering from an acute type 1 respiratory failure (Pa02 <8kPa with a normal or low Pac02).
 Guidelines for initiating and managing CPAP (Continuous Positive Airway Pressure) on a general ward. B25/2006 1.Introduction and Who Guideline applies to 1.1.1 This document provides guidance for Healthcare
Guidelines for initiating and managing CPAP (Continuous Positive Airway Pressure) on a general ward. B25/2006 1.Introduction and Who Guideline applies to 1.1.1 This document provides guidance for Healthcare
Systems differ in their ability to deliver optimal humidification
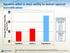 Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Keywords: Non-invasive mechanical ventilation, Respiratory Failure, Respiratory muscles, Hypercapnia, Breathing pattern.
 Monaldi Arch Chest Dis 2004; 61: 2, 81-85 ORIGINAL ARTICLE Inspiratory muscle workload due to dynamic intrinsic PEEP in stable COPD patients: effects of two different settings of non-invasive pressure-support
Monaldi Arch Chest Dis 2004; 61: 2, 81-85 ORIGINAL ARTICLE Inspiratory muscle workload due to dynamic intrinsic PEEP in stable COPD patients: effects of two different settings of non-invasive pressure-support
High Flow Oxygen Therapy in Acute Respiratory Failure. Laurent Brochard Toronto
 High Flow Oxygen Therapy in Acute Respiratory Failure Laurent Brochard Toronto Conflicts of interest Our clinical research laboratory has received research grants for clinical research projects from the
High Flow Oxygen Therapy in Acute Respiratory Failure Laurent Brochard Toronto Conflicts of interest Our clinical research laboratory has received research grants for clinical research projects from the
Chronic obstructive pulmonary disease
 0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
(To be filled by the treating physician)
 CERTIFICATE OF MEDICAL NECESSITY TO BE ISSUED TO CGHS BENEFICIAREIS BEING PRESCRIBED BILEVEL CONTINUOUS POSITIVE AIRWAY PRESSURE (BI-LEVEL CPAP) / BI-LEVEL VENTILATORY SUPPORT SYSTEM Certification Type
CERTIFICATE OF MEDICAL NECESSITY TO BE ISSUED TO CGHS BENEFICIAREIS BEING PRESCRIBED BILEVEL CONTINUOUS POSITIVE AIRWAY PRESSURE (BI-LEVEL CPAP) / BI-LEVEL VENTILATORY SUPPORT SYSTEM Certification Type
What s New in Acute COPD? Dr Nick Scriven Consultant AIM President SAM
 What s New in Acute COPD? Dr Nick Scriven Consultant AIM President SAM Covering: Basic Definition New assessment criteria Some newer treatments BiPAP Not Covering: Definitions: Chronic Obstructive Pulmonary
What s New in Acute COPD? Dr Nick Scriven Consultant AIM President SAM Covering: Basic Definition New assessment criteria Some newer treatments BiPAP Not Covering: Definitions: Chronic Obstructive Pulmonary
High flow nasal Oxygen therapy. Learning points. Part 1: Oxygen 21/06/ Oxford Advanced Course: Newcastle
 High flow nasal Oxygen therapy 15.06.2017 Oxford Advanced Course: Newcastle Learning points Update on BTS guidance May 2017 Help you understand the mechanism of action of high flow nasal oxygen therapy
High flow nasal Oxygen therapy 15.06.2017 Oxford Advanced Course: Newcastle Learning points Update on BTS guidance May 2017 Help you understand the mechanism of action of high flow nasal oxygen therapy
What you need to know about: High flow nasal oxygen therapy
 What you need to know about: High flow nasal oxygen therapy Main introduction Adequate oxygenation is essential in many disorders, and this article will discuss the physiology, practicalities and indications
What you need to know about: High flow nasal oxygen therapy Main introduction Adequate oxygenation is essential in many disorders, and this article will discuss the physiology, practicalities and indications
Up to 50% of continuous flow oxygen therapy patients experience clinically significant nocturnal desaturation. 1
 Up to 50% of continuous flow oxygen therapy patients experience clinically significant nocturnal desaturation. 1 Continuous Flow Oxygen Delivery & Sleep A number of theories and studies are published surrounding
Up to 50% of continuous flow oxygen therapy patients experience clinically significant nocturnal desaturation. 1 Continuous Flow Oxygen Delivery & Sleep A number of theories and studies are published surrounding
Oxygen Therapy: When, What and Why
 Oxygen Therapy: When, What and Why SOUTH EAST LONDON OXYGEN STUDY DAY 26/5/2016 Dr Irem Patel, Integrated Respiratory Physician, King s Health Partners Oxygen: A medicine to treat hypoxia What I will cover
Oxygen Therapy: When, What and Why SOUTH EAST LONDON OXYGEN STUDY DAY 26/5/2016 Dr Irem Patel, Integrated Respiratory Physician, King s Health Partners Oxygen: A medicine to treat hypoxia What I will cover
Adult Nasal High Flow: Clinical Paper Summaries
 Adult Nasal High Flow: Clinical Paper Summaries Table of Contents KEY REFERENCES THERAPY OVERVIEW Research in high flow therapy (Dysart).. S1 SPECIFIC PATIENT POPULATION High-flow nasal cannulae in post-cardiac
Adult Nasal High Flow: Clinical Paper Summaries Table of Contents KEY REFERENCES THERAPY OVERVIEW Research in high flow therapy (Dysart).. S1 SPECIFIC PATIENT POPULATION High-flow nasal cannulae in post-cardiac
The Use of Active Cycle of Breathing Technique (ACBT) In Pulmonary Physiotherapy: A Critical Review of the Literature Lauro G. Villegas Jr.
 The Use of Active Cycle of Breathing Technique (ACBT) In Pulmonary Physiotherapy: A Critical Review of the Literature Lauro G. Villegas Jr., PTRP Keywords: Active Cycle of Breathing Technique (ACBT), Pulmonary
The Use of Active Cycle of Breathing Technique (ACBT) In Pulmonary Physiotherapy: A Critical Review of the Literature Lauro G. Villegas Jr., PTRP Keywords: Active Cycle of Breathing Technique (ACBT), Pulmonary
Oxygen: Is there a problem? Tom Heaps Acute Physician
 Oxygen: Is there a problem? Tom Heaps Acute Physician Case 1 79-year-old female, diabetic, morbidly obese Admitted with LVF Overnight Reduced GCS?cause 15l NRB in situ ABG showed ph 6.9, pco 2 15.9kPa
Oxygen: Is there a problem? Tom Heaps Acute Physician Case 1 79-year-old female, diabetic, morbidly obese Admitted with LVF Overnight Reduced GCS?cause 15l NRB in situ ABG showed ph 6.9, pco 2 15.9kPa
Supplementary Online Content
 Supplementary Online Content Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD
Supplementary Online Content Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD
Analgesia for chest trauma - RVI
 Analgesia for chest trauma - RVI Northern Network Initial Management Patients with blunt chest trauma will be managed in a standard fashion within the context of the well established trauma systems at
Analgesia for chest trauma - RVI Northern Network Initial Management Patients with blunt chest trauma will be managed in a standard fashion within the context of the well established trauma systems at
Chronic Obstructive Pulmonary Disease (COPD) Measures Document
 Chronic Obstructive Pulmonary Disease (COPD) Measures Document COPD Version: 3 - covering patients discharged between 01/10/2017 and present. Programme Lead: Jo Higgins Clinical Lead: Dr Paul Albert Number
Chronic Obstructive Pulmonary Disease (COPD) Measures Document COPD Version: 3 - covering patients discharged between 01/10/2017 and present. Programme Lead: Jo Higgins Clinical Lead: Dr Paul Albert Number
Home Mechanical Ventilation
 The International Convention Centre (ICC), Birmingham 11 12 September 2017 Home Mechanical Ventilation Martin Latham Nurse Specialist in Sleep Disordered Breathing St James s University Hospital Leeds
The International Convention Centre (ICC), Birmingham 11 12 September 2017 Home Mechanical Ventilation Martin Latham Nurse Specialist in Sleep Disordered Breathing St James s University Hospital Leeds
Bronchodilator Delivery and Nebuliser Trials in Adults
 Bronchodilator Delivery and Nebuliser Trials in Adults Acute Management Favour the use of MDI (+/- Spacer) If considering nebuliser Short term treatment Approx. < 3 weeks See optimisation of inhaled bronchodilators
Bronchodilator Delivery and Nebuliser Trials in Adults Acute Management Favour the use of MDI (+/- Spacer) If considering nebuliser Short term treatment Approx. < 3 weeks See optimisation of inhaled bronchodilators
Exacerbations of COPD. Dr J Cullen
 Exacerbations of COPD Dr J Cullen Definition An AECOPD is a sustained worsening of the patient s clinical condition from their stable state that is beyond their usual day-to-day variation is acute in onset
Exacerbations of COPD Dr J Cullen Definition An AECOPD is a sustained worsening of the patient s clinical condition from their stable state that is beyond their usual day-to-day variation is acute in onset
Evaluation of Effect of Breathe Ventilation System on Work of Breathing in COPD patients. Matthew Cohn, M.D.
 Evaluation of Effect of Breathe Ventilation System on Work of Breathing in COPD patients Matthew Cohn, M.D. 1 11/4/2013 Disclosure Slide- Matthew Cohn, M.D. Personal financial relationships with commercial
Evaluation of Effect of Breathe Ventilation System on Work of Breathing in COPD patients Matthew Cohn, M.D. 1 11/4/2013 Disclosure Slide- Matthew Cohn, M.D. Personal financial relationships with commercial
BiPAPS/TVAPSCPAPASV???? Lori Davis, B.Sc., R.C.P.T.(P), RPSGT
 BiPAPS/TVAPSCPAPASV???? Lori Davis, B.Sc., R.C.P.T.(P), RPSGT Modes Continuous Positive Airway Pressure (CPAP): One set pressure which is the same on inspiration and expiration Auto-PAP (APAP) - Provides
BiPAPS/TVAPSCPAPASV???? Lori Davis, B.Sc., R.C.P.T.(P), RPSGT Modes Continuous Positive Airway Pressure (CPAP): One set pressure which is the same on inspiration and expiration Auto-PAP (APAP) - Provides
Clinical Commissioning Policy Proposition: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages)
 Clinical Commissioning Policy Proposition: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England E03X05/01 Information Reader Box (IRB) to be inserted on inside
Clinical Commissioning Policy Proposition: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England E03X05/01 Information Reader Box (IRB) to be inserted on inside
Position Description
 Position Description Position Title Service Group Team Reports to Direct Reports Authority Level Sleep Physiologist Medical Services Clinical Physiology Team Leader Clinical Physiology None None Issue
Position Description Position Title Service Group Team Reports to Direct Reports Authority Level Sleep Physiologist Medical Services Clinical Physiology Team Leader Clinical Physiology None None Issue
Non-invasive ventilation (also called bi-level or BIPAP)
 Patient information service Bristol Royal Infirmary Non-invasive ventilation (also called bi-level or BIPAP) Respecting everyone Embracing change Recognising success Working together Our hospitals. 2 Why
Patient information service Bristol Royal Infirmary Non-invasive ventilation (also called bi-level or BIPAP) Respecting everyone Embracing change Recognising success Working together Our hospitals. 2 Why
Putting NICE guidance into practice. Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80)
 Putting NICE guidance into practice Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80) Published: November 2017 Summary This report focuses on the recommendations
Putting NICE guidance into practice Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80) Published: November 2017 Summary This report focuses on the recommendations
CARE OF THE ADULT COPD PATIENT
 CARE OF THE ADULT COPD PATIENT Target Audience: The target audience for this clinical guideline is all MultiCare providers and staff including those associated with our Clinically Integrated Network. The
CARE OF THE ADULT COPD PATIENT Target Audience: The target audience for this clinical guideline is all MultiCare providers and staff including those associated with our Clinically Integrated Network. The
Saline (0.9%) Nebuliser Guideline
 Saline (0.9%) Nebuliser Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Version: 3 Ratified by: Scope (Target audience, state if Trust wide): Review date (when
Saline (0.9%) Nebuliser Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Version: 3 Ratified by: Scope (Target audience, state if Trust wide): Review date (when
Charisma High-flow CPAP solution
 Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
BTS/ICS Guidelines for the ventilatory management of acute hypercapnic respiratory failure in adults
 BTS/ICS Guidelines for the ventilatory management of acute hypercapnic respiratory failure in adults British Thoracic Society Intensive Care Society Introduction Acute Hypercapnic Respiratory Failure (AHRF)
BTS/ICS Guidelines for the ventilatory management of acute hypercapnic respiratory failure in adults British Thoracic Society Intensive Care Society Introduction Acute Hypercapnic Respiratory Failure (AHRF)
COPD Challenge CASE PRESENTATION
 Chronic obstructive pulmonary disease (COPD) exacerbations may make up more than 10% of acute medical admissions [1], and they are increasingly recognised as a cause of significant morbidity and mortality
Chronic obstructive pulmonary disease (COPD) exacerbations may make up more than 10% of acute medical admissions [1], and they are increasingly recognised as a cause of significant morbidity and mortality
Airway Clearance Devices
 Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
NON-INVASIVE VENTILATION. Lijun Ding 23 Jan 2018
 NON-INVASIVE VENTILATION Lijun Ding 23 Jan 2018 Learning objectives What is NIV The difference between CPAP and BiPAP The indication of the use of NIV Complication of NIV application Patient monitoring
NON-INVASIVE VENTILATION Lijun Ding 23 Jan 2018 Learning objectives What is NIV The difference between CPAP and BiPAP The indication of the use of NIV Complication of NIV application Patient monitoring
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION
 SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
OHTAC Recommendation: Chronic Obstructive Pulmonary Disease (COPD) Ontario Health Technology Advisory Committee
 OHTAC Recommendation: Chronic Obstructive Pulmonary Disease (COPD) Ontario Health Technology Advisory Committee March 2012 Background The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made
OHTAC Recommendation: Chronic Obstructive Pulmonary Disease (COPD) Ontario Health Technology Advisory Committee March 2012 Background The Chronic Obstructive Pulmonary Disease Mega-Analysis series is made
Supplementary Online Content 2
 Supplementary Online Content 2 van Meenen DMP, van der Hoeven SM, Binnekade JM, et al. Effect of on demand vs routine nebulization of acetylcysteine with salbutamol on ventilator-free days in intensive
Supplementary Online Content 2 van Meenen DMP, van der Hoeven SM, Binnekade JM, et al. Effect of on demand vs routine nebulization of acetylcysteine with salbutamol on ventilator-free days in intensive
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline
 umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Smoking cessation interventions and services
 National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
Chronic Obstructive Pulmonary Disease (COPD) Copyright 2014 by Mosby, an imprint of Elsevier Inc.
 Chronic Obstructive Pulmonary Disease () 8.18.18 Copyright 2014 by Mosby, an imprint of Elsevier Inc. Description Airflow limitation not fully reversible progressive Abnormal inflammatory response of lungs
Chronic Obstructive Pulmonary Disease () 8.18.18 Copyright 2014 by Mosby, an imprint of Elsevier Inc. Description Airflow limitation not fully reversible progressive Abnormal inflammatory response of lungs
POLICY. Number: Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE. Authorization
 POLICY Number: 7311-60-024 Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE Authorization [ ] President and CEO [ x ] Vice President, Finance and Corporate Services Source:
POLICY Number: 7311-60-024 Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE Authorization [ ] President and CEO [ x ] Vice President, Finance and Corporate Services Source:
Non-invasive Ventilation protocol For COPD
 NHS LANARKSHIRE MONKLANDS HOSPITAL Non-invasive Ventilation protocol For COPD April 2017 S Baird Review Date: Oct 2019 Approved by Medical Directorate Indications for Non-Invasive Ventilation (NIV) NIV
NHS LANARKSHIRE MONKLANDS HOSPITAL Non-invasive Ventilation protocol For COPD April 2017 S Baird Review Date: Oct 2019 Approved by Medical Directorate Indications for Non-Invasive Ventilation (NIV) NIV
Tissue is the Issue. PEEP CPAP FiO2 HFNC PSV HFNC. DO 2 = CO [(Hb x 1.34) SaO PaO 2 ] perfusione
![Tissue is the Issue. PEEP CPAP FiO2 HFNC PSV HFNC. DO 2 = CO [(Hb x 1.34) SaO PaO 2 ] perfusione Tissue is the Issue. PEEP CPAP FiO2 HFNC PSV HFNC. DO 2 = CO [(Hb x 1.34) SaO PaO 2 ] perfusione](/thumbs/79/78902905.jpg) Tissue is the Issue perfusione PEEP CPAP FiO2 HFNC PSV HFNC DO 2 = CO [(Hb x 1.34) SaO 2 + 0.003 PaO 2 ] O2 HFNC PEEP CPAP PSV ARF ACPE HIGH FLOW NASAL CANNULA High and Exact FiO2, High Flow heating and
Tissue is the Issue perfusione PEEP CPAP FiO2 HFNC PSV HFNC DO 2 = CO [(Hb x 1.34) SaO 2 + 0.003 PaO 2 ] O2 HFNC PEEP CPAP PSV ARF ACPE HIGH FLOW NASAL CANNULA High and Exact FiO2, High Flow heating and
LEARNING OBJECTIVES FOR COPD EDUCATORS
 LEARNING OBJECTIVES FOR COPD EDUCATORS For further Information contact: INTERNATIONAL NETWORK FOR RESPIRATORY CARE 16851 Mount Wolfe Road Caledon, ON Canada L7E 3P6 Phone: 905 880-1092 Fax: 905 880-9733
LEARNING OBJECTIVES FOR COPD EDUCATORS For further Information contact: INTERNATIONAL NETWORK FOR RESPIRATORY CARE 16851 Mount Wolfe Road Caledon, ON Canada L7E 3P6 Phone: 905 880-1092 Fax: 905 880-9733
Non-Invasive Ventilation
 Khusrav Bajan Head Emergency Medicine, Consultant Intensivist & Physician, P.D. Hinduja National Hospital & M.R.C. 112 And the Lord God formed man of the dust of the ground and breathed into his nostrils
Khusrav Bajan Head Emergency Medicine, Consultant Intensivist & Physician, P.D. Hinduja National Hospital & M.R.C. 112 And the Lord God formed man of the dust of the ground and breathed into his nostrils
COPD is a syndrome of chronic limitation in expiratory airflow encompassing emphysema or chronic bronchitis.
 1 Definition of COPD: COPD is a syndrome of chronic limitation in expiratory airflow encompassing emphysema or chronic bronchitis. Airflow obstruction may be accompanied by airway hyper-responsiveness
1 Definition of COPD: COPD is a syndrome of chronic limitation in expiratory airflow encompassing emphysema or chronic bronchitis. Airflow obstruction may be accompanied by airway hyper-responsiveness
호흡재활치료 울산의대서울아산병원 호흡기내과 이상도
 호흡재활치료 울산의대서울아산병원 호흡기내과 이상도 Systemic (Extrapulmonary) effects in COPD Skeletal muscle dysfunction Osteoporosis Weight loss Sexual dysfunction Cardiovascular diseases (Gross et al., Curr Opin Pulm Med 2001;7:84)
호흡재활치료 울산의대서울아산병원 호흡기내과 이상도 Systemic (Extrapulmonary) effects in COPD Skeletal muscle dysfunction Osteoporosis Weight loss Sexual dysfunction Cardiovascular diseases (Gross et al., Curr Opin Pulm Med 2001;7:84)
High Flow Nasal Cannula in Children During Sleep. Brian McGinley M.D. Associate Professor of Pediatrics University of Utah
 High Flow Nasal Cannula in Children During Sleep Brian McGinley M.D. Associate Professor of Pediatrics University of Utah Disclosures Conflicts of Interest: None Will discuss a product that is commercially
High Flow Nasal Cannula in Children During Sleep Brian McGinley M.D. Associate Professor of Pediatrics University of Utah Disclosures Conflicts of Interest: None Will discuss a product that is commercially
