Sequential ordering of morphed faces and facial expressions following temporal lobe damage
|
|
|
- Jane King
- 5 years ago
- Views:
Transcription
1 Neuropsychologia 44 (2006) Sequential ordering of morphed faces and facial expressions following temporal lobe damage Reiko Graham a, Orrin Devinsky b, Kevin S. LaBar a, a Center for Cognitive Neuroscience, Duke University, United States b Neurology, Psychiatry, and Neurosurgery, New York University School of Medicine, United States Received 19 April 2005; received in revised form 18 December 2005; accepted 21 December 2005 Available online 7 February 2006 Abstract A card ordering task was developed to evaluate the role of the temporal lobe in perceiving subtle featural displacements of faces that contribute to judgments of facial expression and identity. Individuals with varying degrees of temporal lobe damage and healthy controls were required to manually sort cards depicting morphs of facial expressions or facial identities so that the cards were sequentially ordered from one morph endpoint to another. Four morph progressions were used three emotion morphs (neutral-to-anger, neutral-to-fear, and fear-to-anger) and an identity morph. Five exemplars were given per morph type. Debriefing verified that participants were using feature-level cues to sort the cards. A patient with bilateral amygdala damage due to epilepsy did not differ in her sorting abilities from unilateral temporal lobectomy patients or controls. In contrast, a post-encephalitic patient with widespread left temporal lobe damage showed impairments that were most marked on the fear-to-anger and identity sorts. These results show that amygdala-damaged individuals can use information contained in facial expressions to solve tasks that rely on feature-level analysis, which recruits processing in other temporal lobe regions involved in making fine featural distinctions Elsevier Ltd. All rights reserved. Keywords: Amygdala; Emotion; Facial affect; Face morph; Brain lesions The idea that the amygdala plays a critical role in processing facial expression was advanced by Adolphs, Tranel, Damasio, and Damasio (1994), who described a patient with rare selective bilateral amygdala damage (S.M.) characterized by impairments in rating the intensity of facial expressions, especially the expression of fear. This finding was replicated in other patients with bilateral amygdala damage (e.g. Adolphs et al., 1999; Calder et al., 1996; Young, Aggleton, Hellawell, Johnson, & Broks, 1995; Young, Hellawell, Van De Wal, & Johnson, 1996) and has also been supported by neuroimaging studies showing amygdala activation to fearful faces in healthy adults (e.g. Morris et al., 1998; Whalen et al., 1998, 2001). The specificity of the amygdala s involvement in processing fearful facial expressions, however, has remained unclear. Studies of individuals with bilateral amygdala damage have provided Corresponding author at: Center for Cognitive Neuroscience, Box 90999, LSRC, Duke University, Durham, NC , United States. Tel.: ; fax: address: klabar@duke.edu (K.S. LaBar). some inconsistencies, with different patients showing varying degrees of facial emotion decoding abilities (e.g. Adolphs et al., 1999; Adolphs & Tranel, 2004; Calder et al., 1996; Hamann et al., 1996; Hamann & Adolphs, 1999; Sato et al., 2002). Neuroimaging evidence has also provided mixed results regarding amygdalar involvement in processing expressions other than fear (Adams, Gordon, Baird, Ambady, & Kleck, 2003; Blair, Morris, Frith, Perrett, & Dolan, 1999; Kesler-West et al., 2001; LaBar, Crupain, Voyvodic, & McCarthy, 2003; Whalen et al., 2001; Yang et al., 2002). A recent study by Adolphs et al. (2005) may provide an explanation for some of these inconsistencies. Their patient S.M. s deficits in processing fearful facial expression appears to stem from the fact that she fails to spontaneously attend to the eye region of faces, which is critical for identifying fear. It is possible that the apparent involvement of the amygdala in evaluating certain facial expressions may be due to its involvement in processing information from the eye region of faces. The degree to which this finding holds for other patients may provide one account for inter-individual variability in the patient findings as well as the specificity of the neuroimaging findings to some categories of emotion over others /$ see front matter 2006 Elsevier Ltd. All rights reserved. doi: /j.neuropsychologia
2 R. Graham et al. / Neuropsychologia 44 (2006) A related explanation for the variability in the patient findings is that amygdala-damaged patients may use slower, compensatory cognitive mechanisms to aid them with expression identification. This idea was supported by Graham, Devinsky, and LaBar (2006), who found that when heuristics use was discouraged by reducing exposure time to morphed facial expressions, deficits in the perception of anger and anger/fear blends, as well as fear, were evident in a patient with bilateral amygdala damage. However, with unlimited exposure durations, performance significantly improved. According to this conception, the amygdala s role in processing facial expression will vary depending on the demands of the task. The use of strategies to ameliorate deficits in facial expression processing has been found in children with autism (Teunisse & de Gelder, 2001), but the possibility that amygdala-damaged individuals could also be using strategies to differing degrees of success has not received much attention. Adolphs et al. (2005) reported that when patient S.M. was instructed to attend to the eye region of faces, her perception of fearful facial expressions appeared normal. This finding suggests that if individuals with bilateral amygdala damage are told what to look for, they can use this information strategically to help them overcome more automatic processing deficits due to amygdala damage. Amygdala activation in healthy adults is also sensitive to task demands under some circumstances, especially those that relate to intentional (explicit) versus incidental (implicit) aspects of emotion processing (e.g. Critchley et al., 2000; Whalen et al., 1998). For example, Carlsson et al. (2004) demonstrated that masked phobic stimuli elicit left amygdala activation, whereas awareness of phobic stimuli was associated with bilateral amygdala activation and additional activity in a network of cortical brain regions, including the insula, anterior cingulated, and orbitofrontal cortex. Given that the amygdala is more consistently implicated in rapid, covert processing of faces (e.g. Bishop, Duncan, & Lawrence, 2004; Vuilleumier, Armony, Driver, & Dolan, 2003; Williams, Morris, McGlone, Abbott, & Mattingley, 2004), additional task-related factors may influence its involvement on explicit emotion processing tasks. Explicit emotional judgments tend to recruit additional processing in frontotemporal cortical areas compared to non-emotional judgments (e.g. gender or age judgments) using the same facial expressions (Critchley et al., 2000; Gorno-Tempini et al., 2001; Gur et al., 2002). Therefore, task demands could affect the extent to which the amygdala versus cortical regions are preferentially recruited across different studies (see also Hariri, Bookheimer, & Mazziotta, 2000) and could also account for some of the inconsistencies observed in the current literature regarding the amygdala s role in processing facial expression. One explicit processing strategy that could aid emotion recognition judgments is feature analysis, where decisions regarding facial emotion are based on a deliberate analysis of the perceptual displacement of specific facial features. In particular, expression processing after amygdala damage could be preserved if the task emphasizes strategic, featural processing, and recruits cortical areas that are involved in making fine-grained distinctions between visual stimuli. The amygdala has been linked to the rapid processing of low frequency components of faces and facial expressions through magnocellular channels, whereas ventral and lateral temporal neocortical areas have been linked to processing high frequency components via parvocellular channels (Vuilleumier et al., 2003). This suggests that two routes exist for information regarding facial expressions of emotion: a rapid, implicit, amygdala-mediated pathway and a more voluntary, strategic, cortically-mediated pathway. Therefore, processing the high frequency information necessary to perform fine featural discriminations should not be dependent upon the amygdala, even if emotional faces are used as stimuli. Instead, more lateral and ventral temporal lobe areas may be important, consistent with their role in using featural information to discriminate objects within a category, as previously shown in monkeys (Freedman, Riesenhuber, Poggio, & Miller, 2003). In the present study, a sequential ordering task was developed using morphed faces to assess the role of the amygdala and other temporal lobe areas in perceiving the featural displacements that accompany changes in facial emotion and identity. This task involves presenting subjects with cards showing facial expression and identity morphs of varying increments, and requires the subject to order the cards in a logical progression from one morph endpoint to another. Because this task provides all the relevant perceptual cues across the morph increments simultaneously, we reasoned that the execution of this task should promote cortically-mediated strategies such as analysis of featural displacements. Accordingly, ventrolateral temporal lobe areas should be involved in performing this task, whereas medial temporal lobe structures, including the amygdala, should not be critical. The task was administered to three different patient types that varied in their degree of temporal lobe damage (1) a group of unilateral medial temporal lobectomy patients (TLBs) who underwent excision of the anteromedial temporal lobe for the surgical treatment of retractable epilepsy, (2) patient S.P., a right temporal lobectomy patient who had sustained additional damage circumscribed to her left amygdala (Phelps et al., 1998), and (3) patient C.B., a post-encephalitic patient with more widespread damage to the left temporal lobe. We hypothesized that patient S.P. and the TLB patients would show relatively intact performance on this task. We previously found that patient S.P. is impaired at two-alternative forced choice recognition of the same morphs, especially under time constraints. Therefore, a demonstration of intact performance in the present study would show a dissociation in performance as a function of task demands using identical stimuli. For patient C.B., three outcomes were considered. First, if the right ventrolateral temporal lobe areas implicated in face processing are primarily responsible for performance on this task (e.g. Haxby, Hoffman, & Gobbini, 2000; Kanwisher, McDermott, & Chun, 1997; McCarthy, 2000), then C.B. should perform normally since her right hemisphere is spared. Alternatively, if left temporal lobe areas implicated in the analysis of surface details (Peper & Irle, 1997) and face parts (Rossion et al., 2000) are critical to this task, then C.B. should have problems across all sorts presented. Finally, as a task that emphasizes both faces and feature analytic strategies, which vary in their respective dependence on the two cerebral
3 1400 R. Graham et al. / Neuropsychologia 44 (2006) hemispheres, this task may recruit right and left temporal lobe areas differentially, according to the difficulty or, perhaps, biological plausibility of the morph sort (e.g. LaBar et al., 2003). 1. Methods 1.1. Stimulus development Emotional facial expressions found to be panculturally representative of the basic emotions of fear and anger were taken from the Ekman pictures of facial affect (Ekman & Friesen, 1976). Prototypical expressions of fear and anger were morphed together, and anger and fear were also morphed with neutral expressions to create the experimental stimuli. Hence, three different emotional morph progressions were created: neutral-to-anger, neutral-to-fear, and fear-toanger. Identity was kept constant across the three emotion morphs (i.e. the same actor was used, only the expression changed). The same six actors were used to construct the three different emotion morphs. The identity morphs were created in the same manner as the expression morphs except that rather from morphing from one expression to another using the same actor, an actor with a neutral facial expression was morphed into another actor with a neutral facial expression. The faces were of actors with neutral facial expressions taken from the same Ekman picture series. Ten actors were used to create five different identity morphs. The morphs were created using the methods outlined in LaBar et al. (2003). Between each source and target emotion, eight intermediate images with 11.11% increments were created, yielding a total of 10 images in each continuum numbered from 1 to 10. For example, for the neutral-to-anger continuum, morph increment 1 was 100% neutral, increment 2 was 88.88% neutral and 11.11% angry, increment 3 was 77.77% neutral and 22.22% angry, and so on, until face 10, which depicted 100% anger. Five exemplars of each emotion sort were presented using different actors. All morph sets were printed onto 4 6 index cards that could then be shuffled and manipulated Subjects We studied S.P., a 58-year-old woman with bilateral amygdala damage associated with epilepsy, who first showed signs of neurological insult at 3 4 years of age and was later diagnosed with epilepsy. During adulthood, medically intractable complex seizures of right medial temporal lobe origin began to occur with greater frequency. At age 48, she underwent right anteromedial temporal lobe resection, including removal of the right amygdala. MR images taken prior to surgery also revealed a focal lesion in the left amygdala. S.P. completed high school and has taken some college courses. She presents a normal neuropsychological profile, including normal performance on the Wechsler Adult Intelligence Scale-Revised (verbal IQ of 104, performance IQ of 107, and full scale IQ of 106). S.P. is able to discriminate between unfamiliar faces as indexed by the Benton test of facial discrimination (Benton, Hamsher, des Varney, & Spreen, 1994). She can discriminate the age and gender of unfamiliar faces. Therefore, S.P. can successfully interpret multiple sources of non-emotional facial information. With regard to her ratings of the intensity of emotional facial expressions, S.P. has been tested on two occasions (Adolphs et al., 1999; Anderson & Phelps, 2000). On the first occasion, S.P. s ratings were impaired for fear, disgust, sadness, and anger (Adolphs et al., 1999). On the second occasion, she showed impairments in rating fear, disgust, sadness, and happiness (Anderson & Phelps, 2000). In contrast, her ability to describe her personal emotional experiences does not differ appreciably from controls (Anderson & Phelps, 2002) and her ability to judge emotions from vocalizations is intact (Anderson & Phelps, 1998). Detailed information regarding S.P. s lesions and neuropsychological status is available in Phelps et al. (1998). We also tested C.B., a 32-year-old female with an Associate s degree. At age 22, C.B. had a series of severe headaches, during which she displayed abnormal behaviors. These were followed by a series of generalized tonic clonic seizures, resulting in a diagnosis of herpes simplex encephalitis and subsequent treatment with anticonvulsant medications. MR images taken at age 32 revealed extensive damage to left temporal lobe, including the inferior and middle temporal gyri along their entire rostrocaudal extent, medial temporal occipital junction, anterior aspect of the superior temporal gyrus, and all structures contained within the anteriomedial temporal lobes (i.e. amygdala, hippocampus, entorhinal/perirhinal cortices) (Fig. 1). Additionally, there is slight damage to the left globus pallidus and inferior portion of the insula, as well as the midline basal forebrain and nucleus accumbens. Her frontal, parietal, and occipital lobes are intact in the left hemisphere, and there is no damage to the right hemisphere. Neuropsychological evaluation at age 32 indicated that C.B. performed normally on the Mini Mental State Exam (score: 28). Her IQ was low average (verbal IQ of 85, performance IQ of 84, and full scale IQ of 84). Her performance across domains of the WMS-III varied from borderline to extremely low, consistent with the extent of her left temporal lobe damage. She demonstrated some naming deficits (Short Boston Naming score: 12/15). C.B. continues to struggle with episodic memory deficits and has mild language-related impairments, such as aphasia, anomia, and paraphasia. Her face recognition performance as indexed by the Benton face recognition test falls within the normal range (long form score: 42, low average). Prior to this study, C.B. s facial emotion recognition abilities had not been assessed. Six patients with right medial temporal lobe damage (three female and three male) and seven patients (three female and four male) with left medial temporal damage comprised the TLB group. They ranged from 24 to 60 years in age (mean = 41.5 years) and had between 12 and 21 years of education (mean = 15.7 years). All TLB patients had average full scale IQs (FSIQ: , mean FSIQ = 102.3). All unilateral patients had undergone neurosurgical resection for treatment of medically refractory epilepsy 2 8 years prior to participation in this study. The exact extent of the resection varied from patient to patient according to the location of the seizure focus; however, in all patients the amygdala and adjacent medial temporal lobe structures including the hippocampus and uncus were removed unilaterally, whereas tissue from areas outside the temporal lobe was spared. The extent of temporal neocortical excision ranged from cm. Fig. 1. Structural MRI scans of patient C.B. illustrating extent of left temporal lobe damage. (a) T1-weighted scan in coronal section at the level of the amygdala and (b) FLAIR scan in axial section at the level of the middle temporal gyrus. The left hemisphere (L) is depicted on the right side of the images.
4 R. Graham et al. / Neuropsychologia 44 (2006) The task was validated with two different control groups: a group of Duke undergraduates and a group of older adults. The young control group served as the control group for patient C.B. and consisted of 8 males and 12 females ranging from 18 to 21 years of age (mean 19.4 years), who had between 12 and 15 years of education with no history of neurologic or psychiatric illness. We also tested 17 age- and education-matched controls for patient S.P., three of whom did not complete the identity sort. This control group consisted of 11 females and 6 males ranging from 50 to 66 years of age (mean 55.9 years) who had between 12 and 17 years of education and did not have a history of neurologic or psychiatric illness. Procedures for human subjects were approved by the Institutional Review Boards at Duke University and New York University. All subjects provided written informed consent to participate and were either paid or received course credit for their participation Design and procedure The morphed faces were divided into four sorting blocks, one for each morph type. Each block consisted of five sort trials. Practice trials were given for the emotion morphs. The blocks were given in the same order: neutral-to-anger, neutral-to-fear, fear-to-anger, and identity. Block order was fixed so that each participant would receive the tasks in the same order as patients S.P. and C.B. Each trial started in the same manner: the two extreme morph anchors (for example, the 100% neutral, 0% angry, and the 0% neutral, 100% angry stimuli in the neutral-to-anger morph block) were placed as anchors at the ends of a continuum. The intervening eight morph cards were shuffled and placed in random order in front of the participant, who was required to sort the morphs so that they progressed logically from one morph anchor to the other. There was a 60 s time limit for sorting and feedback was available for the practice trial. A schematic depiction of a neutral-to-anger morph sort trial is shown in Fig. 2. If the participant did not finish the sort within 1 min, the sort was terminated and the data for the trial was discarded (this occurred in a negligible number of trials, <1%). The order in which the participant placed the morphs and the sorting duration were recorded for each sort trial. After completing the experiments, subjects were asked to describe what facial information they used to complete the sort trials Data analysis Concordance was used as an estimate of sorting accuracy and was measured with Kendall s tau, a non-parametric index of the degree of the similarity between two sets of rankings, which is highly sensitive to deviations in ordering (Siegel & Castellan, 1988). As tau values approach 1.0, sorting accuracy increases. To determine if S.P. and C.B. s performance differed from that of the other groups, they were compared to TLBs, younger controls, and older controls via one sample t-tests with their respective means as the critical values. Differences between the concordance values and mean sort times across the two patients (S.P. and C.B.) could not be determined statistically because they are case reports, so they must be inferred from qualitative differences in their statistical profiles. To assess group differences between the TLB patients and controls, the mean sort times and concordance of TLBs and the two control groups were compared via repeated measures ANOVAs with group as a between subjects factor and sort block as a within subjects factor. To protect against type I error, degrees of freedom were corrected with Greenhouse-Geisser epsilon where applicable. Where significant effects were found, post hoc comparisons were conducted with Bonferroni corrections for type I error. 2. Results Debriefing confirmed that the sorting task involved featurebased analysis; all subjects reported that they focused on how one or two particular facial features changed while sorting, regardless of the morph type. For example, on the neutral-to-fear sort, most subjects (including those with temporal lobe damage) reported using increasing eye or mouth aperture to guide their sort, while on the identity sort, subjects would use features such as presence of freckles or width of the lip, nose or eyebrow. Two performance indices were examined mean sort time and concordance of each subject s ordering with the correct order. Reaction time and concordance values for the right TLB and left TLB patients were compared and found to be equal; hence, the values for the two groups were combined to form the TLB group Reaction time Mean sorting times for the different individuals and groups across the four sort types are shown in Fig. 3. Between-groups analysis of the TLB and two control groups did not find any group differences in sorting times, F < 1, but did find a difference between the sorting times for the different morph blocks, F(2, 88) = 13.21, p < Post hoc analyses showed that this effect reflects the fact that the identity morphs took longer to sort than the emotion morphs. There were no differences in sorting times for the three emotion morph types. Fig. 2. Example of the stimuli and procedure used in this experiment. One trial from a neutral-to-anger morph progression is depicted.
5 1402 R. Graham et al. / Neuropsychologia 44 (2006) Fig. 3. Mean sorting times for patient S.P., patient C.B., unilateral temporal lobectomy patients (TLB), older controls, and younger controls across the four morph types. Standard error is indicated by error bars where applicable. (*) p < 0.05 relative to S.P.; ( ) p < 0.05 relative to C.B. Comparison of S.P. s sorting times to those of the TLB group and the two control groups revealed that she performed the neutral-to-anger sorts more quickly than her age-matched controls, t(16) = 2.46, p < 0.05, and young controls, t(19) = 2.19, p < There were no differences in sorting times between S.P. and the other groups on the neutral-to-fear or the fear-to-anger morph sorts. She performed more quickly than all three groups on the identity sort (versus TLBs: t(12) = 4.49, p < 0.01; versus young controls: t(19) = 5.52, p < 0.01; versus age-matched controls: t(13) = 4.16, p < 0.01). In short, S.P. s reaction times indicated that she did not encounter difficulties with this task. Comparison of C.B. s sorting times to those of the TLB group and the two control groups revealed that she performed the neutral-to-anger sorts more slowly than the TLB group, t(12) = 4.27, p < 0.01, and young controls, t(19) = 3.20, p < She was also slower than the TLB group and young controls on the neutral-to-fear sorts (versus TLBs: t(12) = 4.66, p < 0.01; versus young controls: t(19) = 3.57, p < 0.01). C.B. s sort times were slower than those of any of the groups on the fear-to-anger sort (versus TLBs: t(12) = 6.48, p < 0.01; versus young controls: t(19) = 6.25, p < 0.01; versus older controls: t(16) = 4.48, p < 0.01) and the identity sort (versus TLBs: t(12) = 6.56, p < 0.01; versus controls: t(19) = 6.65, p < 0.01; versus older controls: t(13) = 2.40, p < 0.05). Overall, C.B. s reaction times indicate that she had more difficulty completing this task than other participants, particularly the fear-to-anger and identity blocks Concordance: Kendall s tau Concordance values for the different individuals and groups across the four sort types are shown in Fig. 4. Between-groups analysis of the TLB group and the two control groups revealed a main effect of group, F(2, 43) = 11.40, p < Post hoc tests revealed that this main effect reflected a tendency for the young controls to sort more accurately than either the TLB or the older control group, who did not differ from each other. There was also a main effect of morph type, F(3, 65) = 18.73, p < Post hoc comparisons revealed that this was due to the fact that, overall, performance on the fear-to-anger and identity sorts was lower than performance on the neutral-to-anger and fear-toanger sorts. These main effects were mediated by a significant group by morph type interaction, F(3, 65) = 3.26, p < The lower performance on the fear-to-anger and identity morphs was a pattern common to the TLB and older control groups, whereas the performance profile for the young control group showed no difference across morph types. Comparison of S.P. s concordance values to those of the TLB group and the two control groups revealed that she performed the neutral-to-anger sorts less accurately than young controls, t(19) = 2.19, p < This was also the case for the neutral-tofear, fear-to-anger, and the identity morph sorts (neutral-to-fear: t(19) = 2.63, p < 0.05; fear-to-anger: t(19) = 4.50, p < 0.01; identity: t(19) = 6.17, p < 0.01). There were no differences between the sorting performance of S.P. and the TLB group and her age-matched controls on any of the morph sorts except that she outperformed the TLB group on the identity sort, t(12) = 2.29, p < In summary, S.P. s sorting performance did not differ appreciably from any of the groups except the young control group. Importantly, there were no differences between S.P. and her age-matched controls on any of the morph sorts. Comparison of C.B. s sorting accuracy to that of the TLB group and the two control groups revealed that she performed the neutral-to-anger sorts less accurately than young Fig. 4. Mean concordance values (Kendall s tau) for patient S.P., patient C.B., unilateral temporal lobectomy patients (TLB), older controls, and younger controls across the four morph types. Standard error is indicated by error bars where applicable. (*) p < 0.05 relative to S.P.; ( ) p < 0.05 relative to C.B.
6 R. Graham et al. / Neuropsychologia 44 (2006) controls, t(19) = 3.14, p < She also sorted less accurately than young controls on the neutral-to-fear sorts, t(19) = 2.25, p < On the fear-to-anger sorts, C.B. s performance accuracy was impaired relative to the TLB patients and both control groups (versus TLBs: t(12) = 5.42, p < 0.01; versus young controls: t(19) = 12.98, p < 0.01; versus older controls: t(16) = 2.30, p < 0.05). Compared to all three groups, she also performed more poorly on the identity sort (versus TLBs: t(12) = 4.51, p < 0.01; versus young controls: t(19) = 17.67, p < 0.01; versus older controls: t(13) = 4.51, p < 0.01). In summary, analysis of C.B. s concordance values produced results that mirrored her sorting times overall, she had some difficulty on this task, especially on the fear-to-anger and identity morphs. 3. Discussion Judgments of facial expression and identity depend on multiple sources of information from the face that vary in their reliance on cortical versus subcortical routes of processing. The present study was designed to assess the role of the temporal lobe in the analysis of featural displacements, which accompany subtle changes in emotional expression and identity. Confirming our hypothesis that the card ordering task would encourage cortically-mediated strategic processing, subjects reported using specific morph features to help them perform the task. Across both facial expression and identity morph sorts, normal performance was observed in a patient with bilateral amygdala damage (S.P.) and patients with restricted unilateral anteromedial temporal lobectomy. This result is especially interesting since S.P. performs poorly on some emotion recognition judgments from the same stimulus set (Adolphs et al., 1999; Anderson & Phelps, 2000) and can use heuristic strategies to ameliorate some of her recognition deficits (Graham et al., 2006), which suggests that amygdala involvement in facial expression processing is taskdependent. In contrast to patient S.P., post-encephalitic patient C.B., who sustained more extensive left temporal lobe damage, had difficulty with the sorting task on both the fear-to-anger and identity morphs. This latter finding suggests that posterior left temporal lobe areas are recruited when difficult featural distinctions need to be made between perceptually-similar faces, on the basis of both facial expression and identity. Analysis of reaction time and accuracy suggested that performance in the patients was not associated with speed-accuracy tradeoffs. Overall, the findings support a dissociation between the roles of the amygdala and posterior temporal neocortex in featurebased processing that supports judgments of facial expression and identity. We expand on each of these findings in turn below. Debriefing confirmed our assumption that the temporal order sorting task encouraged the use of feature-analytic strategies in that subjects reported focusing on specific facial features to complete the task. An interesting observation was that these features tended to be implicitly dynamic for emotion judgments (e.g. increasing eye or mouth aperture), whereas they were static features in the identity judgments (e.g. nose or lip width, freckles). While descriptive, this tendency is congruent with the perspective that emotion judgments are dependent upon the dynamic characteristics of faces, whereas identity judgments rely more on more static facial aspects (e.g. Bruce & Young, 1986). S.P. was only outperformed on this task by the young controls, an effect that could be attributable to age. Importantly, her performance did not differ from the TLB group or her age- and education-matched control group. Therefore, bilateral amygdala damage does not appear to be associated with deficits on this task. The TLB group also did not differ from other control groups in terms of sorting time or performance. Patient S.P. actually outperformed the TLB patients on the identity sorts, but it is likely that this could be attributed to S.P. s relative familiarity with the stimulus set. S.P. s relatively quick sort times, in particular for the identity morphs, may also reflect her familiarity with the Ekman stimuli, to which she has been exposed on several occasions over a period of 10 years. The results from the TLB patients and patient S.P. confirm that the amygdala is not critically involved in face processing tasks that encourage strategic, feature-based analysis and compliment our previous finding that deficits in the perception of fear and anger in face morphs are more prominent when strategy use is discouraged, for example by limiting exposure duration (Graham et al., 2006). These results also compliment those of Adolphs et al. (2005), who found that when they instructed their patient S.M. to attend to the information in the eye region of faces, she no longer showed deficits in rating the intensity of fearful faces. Together, these findings converge to suggest that when individuals with bilateral amygdala damage are aware of the featural changes that signal different emotions (either by instruction or by having all perceptual information available at the time of test), they may be able to use this information to discriminate the feature changes that signal different emotions, and thus, potentially improve facial emotion recognition. One caveat to this implication is that in the present study, participants were not asked to label or recognize the emotions present (they were provided the emotion labels for the morph endpoints), so the extent to which featural strategies are spontaneously deployed by amygdala-lesioned patients in explicit recognition tasks requires further study (see also Adolphs et al., 2005). In combination, these results are consistent with the view that the amygdala is involved in more rapid, obligatory aspects of face processing that are less amenable to cognitive control (e.g. Critchley et al., 2000; Gläscher & Adolphs, 2003; Whalen et al., 1998; Williams et al., 2004), and that amygdala-lesioned patients can benefit from cortically-mediated cognitive strategies, including feature analysis, that rely on posterior temporal neocortex. S.P. is able to successfully pose various facial expressions of emotions, implying that she is aware of the featural changes that accompany different emotional displays (Anderson & Phelps, 2000). Therefore, she is apparently able to use this information to help her make judgments about facial expressions when those cues are simultaneously available at test, even for fearful expressions. Under certain task conditions, then, normal performance can be elicited in amygdala-lesioned patients using the same facial expression stimuli, which, under other circumstances, yield performance deficits. In contrast to S.P., patient C.B. exhibited more dramatic deficits on this task. Her performance on the neutral-to-anger
7 1404 R. Graham et al. / Neuropsychologia 44 (2006) and neutral-to-fear sorts showed evidence of subtle impairments: she sorted more slowly than the TLB and young control groups and had lower accuracy relative to the young control group. Her impairments were more marked on the fear-to-anger and identity sorts, where she had both reaction time and accuracy difficulties relative to all other groups. Between-groups analysis indicated that the control groups and the TLB group performed most poorly on the fear-to-anger and identity sorts and took the longest to sort identity morphs, indicating that these morphs were the most difficult to sort. C.B. s deficits on the more difficult morph types could be attributed to the extensive damage to ventrolateral regions of her left temporal lobe and gives us insight into the role of left temporal areas in face processing. Importantly, because her deficits were not specific to emotional expression, the results from patient C.B. implicate a more general function of left ventrolateral temporal cortex in feature-based analysis of faces. The left inferior temporal lobe has been shown to play an integral role in the discrimination of visual patterns (Kawashima et al., 1998). Single cell recordings from the human lateral temporal lobes have demonstrated that both temporal lobes are active during face processing tasks. Whereas activity in the right hemisphere appears to be face-selective, left temporal lobe activity is less specific, occurring to both faces and complex patterns (Lucas, Schoenfield-McNeill, Weber, & Ojemann, 2003). Left temporal lobe areas have been linked to processing the nonemotional surface details of faces (Peper & Irle, 1997) and face parts (Rossion et al., 2000). Because the completion of our task is dependent upon detecting subtle featural changes, this task may recruit processing in the left temporal neocortex, even when this damage is not accompanied by specific face processing deficits (e.g. prosopagnosia). One possibility is that the fine-grained part-based analysis attributed to left temporal lobe areas (Peper & Irle, 1997; Rossion et al., 2000) provide task-dependent support to the right temporal lobe areas normally recruited during face processing, and this support becomes especially important when the task involves making difficult featural discriminations. Another possibility is that while neutral-to-anger and neutral-to-fear morphs depict biologically plausible motion whose processing has been attributed to superior temporal lobe structures (e.g. Jellema & Perrett, 2003) that are right-lateralized in humans (e.g. Allison, Puce, & McCarthy, 2000; Pelphrey et al., 2003; Pelphrey, Viola, & McCarthy, 2004), the fear-to-anger and identity morphs may be less plausible and require recruitment of other temporal lobe areas whose duties are not circumscribed to face processing, including those in the left hemisphere. These possibilities warrant attention in future research. In conclusion, the present study demonstrated that individuals with amygdala damage can have intact performance on perceptual tasks employing changes in facial emotion and identity, and qualifies the conditions under which the amygdala and other temporal lobe areas are recruited for processing information in faces. Specifically, the amygdala did not play a critical role in a face task that emphasized feature-analytic strategic processing, suggesting that this structure is not important for making fine featural discriminations. Instead, more ventrolateral temporal areas appear to be important, including left temporal lobe regions that are not selectively associated with facial affect processing. Differential recruitment of these pathways across experimental paradigms may account for inconsistencies in facial affect research regarding the role of the amygdala and underscores the notion that stimuli depicting emotional states rely on multiple processing pathways, including those that do not encode emotional reactions per se. Because our data relies heavily on case reports, the task should be replicated in larger patient samples to confirm the results and to rule out alternative explanations (e.g. the larger extent of brain damage in patient C.B. or her lower IQ). Our findings complement other recent studies that emphasize the importance of task design in studying the role of temporal lobe structures in face processing and hint at the potential for cognitive remediation strategies to benefit facial evaluation in amygdala-lesioned patients. Acknowledgements This work was supported by National Institutes of Health grants R01 DA14094 and U54 MH06418, and a National Science Foundation CAREER award. The authors wish to thank Elizabeth Phelps and Martin McKeown for assistance with patient recruitment, Michael Crupain for stimulus development, and Craig Cook, Michael Zorawski and Dana Torpey for their assistance with data collection and analysis. References Adams, R. B., Gordon, H. L., Baird, A. A., Ambady, N., & Kleck, R. E. (2003). Effects of gaze on amygdala sensitivity to anger and fear faces. Science, 300, Adolphs, R., & Tranel, D. (2004). Impaired judgments of sadness but not happiness following bilateral amygdala damage. Journal of Cognitive Neuroscience, 16(3), Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372(6507), Adolphs, R., Tranel, D., Hamann, S., Young, A. W., Calder, A. J., Phelps, E. A., et al. (1999). Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia, 37(10), Adolphs, R., Gosselin, F., Buchanan, T. W., Tranel, D., Schyns, P., & Damasio, A. R. (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433, Allison, T., Puce, A., & McCarthy, G. (2000). Social perception from visual cues: role of the STS region. Trends in Cognitive Science, 4(7), Anderson, A. K., & Phelps, E. A. (1998). Intact recognition of vocal expressions of fear following bilateral lesions of the human amygdala. Neuroreport, 9(16), Anderson, A. K., & Phelps, E. A. (2000). Expression without recognition: contributions of the human amygdala to emotional communication. Psychological Science, 11(2), Anderson, A. K., & Phelps, E. A. (2002). Is the human amygdala critical for the subjective experience of emotion?: evidence of intact dispositional affect in patients with amygdala lesions. Journal of Cognitive Neuroscience, 14(5), Benton, A. L., Hamsher, K., des Varney, N. R., & Spreen, O. (1994). Contributions to neuropsychological assessment: a clinical manual. New York: Oxford University Press. Bishop, S. J., Duncan, J., & Lawrence, A. D. (2004). State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience, 24(46),
8 R. Graham et al. / Neuropsychologia 44 (2006) Blair, R. J. R., Morris, J. S., Frith, C. C., Perrett, D. I., & Dolan, R. J. (1999). Dissociable neural responses to facial expressions of sadness and anger. Brain, 122(5), Bruce, V., & Young, A. (1986). Understanding face recognition. British Journal of Psychology, 77(3), Calder, A. J., Young, A. W., Rowland, D., Perrett, D. I., Hodges, J. R., & Etcoff, N. L. (1996). Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cognitive Neuropsychology, 13(5), Carlsson, K., Petersson, K. M., Lundqvist, D., Karlsson, A., Ingvar, M., & Ohman, A. (2004). Fear and the amygdala: manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but non-feared) stimuli. Emotion, 4(4), Critchley, H., Daly, E., Phillips, M., Brammer, M., Bullmore, E., Williams, S., et al. (2000). Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping, 9, Ekman, P., & Friesen, W. (1976). Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press. Freedman, D. J., Riesenhuber, M., Poggio, T., & Miller, E. K. (2003). A comparison of primate prefrontal and inferior temporal cortices during visual categorization. Journal of Neuroscience, 23(12), Gläscher, J., & Adolphs, R. (2003). Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience, 23(32), Gorno-Tempini, M. L., Pradelli, S., Serafini, M., Pagnoni, G., Baraldi, P., Porro, C., et al. (2001). Explicit and incidental facial expression processing: an fmri study. NeuroImage, 14, Graham, R., Devinsky, O., & LaBar, K.S. (2006). Quantifying deficits in the perception of fear and anger in morphed facial expressions after bilateral amygdala damage. Neuropsychologia, submitted for publication. Gur, R. C., Schroeder, L., Turner, T., McGrath, C., Chan, R. M., Turetsky, B. I., et al. (2002). Brain activation during facial emotion processing. NeuroImage, 16, Hamann, S. B., & Adolphs, R. (1999). Normal recognition of emotional similarity between facial expressions following bilateral amygdala damage. Neuropsychologia, 37(10), Hamann, S. B., Stefanacci, L., Squire, L. R., Adolphs, R., Tranel, D., Damasio, H., et al. (1996). Recognizing facial emotion. Nature, 379(6565), 497. Hariri, A. R., Bookheimer, S. Y., & Mazziotta, J. C. (2000). Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport, 11, Haxby, J. V., Hoffman, E. A., & Gobbini, I. M. (2000). The distributed human neural system for face perception. Trends in Cognitive Science, 4, Jellema, T., & Perrett, D. I. (2003). Perceptual history influences neural responses to face and body postures. Journal of Cognitive Neuroscience, 15(7), Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17(11), Kawashima, R., Satoh, K., Goto, R., Inoue, K., Itoh, M., & Fukuda, H. (1998). The role of the left inferior temporal cortes for visual pattern discrimination: a PET study. Neuroreport, 9(7), Kesler-West, M. L., Andersen, A. H., Smith, C. D., Avison, M. J., Davis, C. E., Kryscio, R. J., et al. (2001). Neural substrates of facial emotion processing using fmri. Cognitive Brain Research, 11(2), LaBar, K. S., Crupain, M. J., Voyvodic, J. T., & McCarthy, G. (2003). Dynamic perception of facial affect and identity in the human brain. Cerebral Cortex, 13, Lucas, T. H., Schoenfield-McNeill, J., Weber, P. B., & Ojemann, G. A. (2003). A direct measure of human lateral temporal lobe neurons responsive to face matching. Cognitive Brain Research, 18, McCarthy, G. (2000). Physiological studies of face processing in humans. In M. S. Gazzaniga (Ed.), The new cognitive neurosciences (2nd ed., pp ). Cambridge, MA: The MIT Press. Morris, J. S., Friston, K. J., Buechel, C., Frith, C. D., Young, A. W., Calder, A. J., et al. (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121, Pelphrey, K. A., Mitchell, T. V., McKeown, M. J., Goldstein, J., Allison, T., & McCarthy, G. (2003). Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. Journal of Neuroscience, 23(17), Pelphrey, K. A., Viola, R. J., & McCarthy, G. (2004). When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science, 15(9), Peper, M., & Irle, E. (1997). The decoding of emotional concepts in patients with focal cerebral lesions. Brain and Cognition, 34, Phelps, E. A., LaBar, K. S., Anderson, A. K., O Connor, K. J., Fulbright, R. K., & Spencer, D. D. (1998). Specifying the contributions of the human amygdala to emotional memory: a case study. Neurocase, 4, Rossion, B., Dricot, L., Devolder, A., Bodart, J.-M., Crommelinck, M., de Gelder, B., et al. (2000). Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 12(5), Sato, W., Kubota, Y., Okada, T., Murai, T., Yoshikawa, S., & Sengoku, A. (2002). Seeing happy emotion in fearful and angry faces: qualitative analysis of facial expression recognition in a bilateral amygdala-damaged patient. Cortex, 38, Siegel, S., & Castellan, N. J., Jr. (1988). Non-parametric statistics for the behavioral sciences (2nd ed.). New York: McGraw-Hill. Teunisse, J.-P., & de Gelder, B. (2001). Impaired categorical perception of facial expressions in high-functioning adolescents with autism. Child Neuropsychology, 7(1), Vuilleumier, P., Armony, J. L., Driver, J., & Dolan, R. (2003). Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience, 6(6), Whalen, P. J., Rauch, S., Etcoff, N. L., McInerney, S. C., Lee, M. B., & Jenike, M. A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18(1), Whalen, P. J., Shin, L. M., McInerney, S. C., Fischer, H., Wright, C., & Rauch, S. L. (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion, 1(1), Williams, M. A., Morris, A. P., McGlone, F., Abbott, D. F., & Mattingley, J. B. (2004). Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. Journal of Neuroscience, 24(12), Yang, T. T., Menon, V., Eliez, S., Blasey, C., White, C. D., Reid, A. J., et al. (2002). Amygdalar activation associated with positive and negative facial expressions. Neuroreport, 13, Young, A. W., Aggleton, J. P., Hellawell, D. J., Johnson, M., & Broks, P. (1995). Face processing impairments after amygdalotomy. Brain, 118(1), Young, A. W., Hellawell, D. J., Van De Wal, C., & Johnson, M. (1996). Facial expression processing after amygdalotomy. Neuropsychologia, 34(1),
Impaired Perception of Facial Emotions Following Bilateral Damage to the Anterior Temporal Lobe
 Neuropsychology 2001, Vol. 15, No. 1, 30-38 In the public domain DOI: 10.1037//0894-4105.15.1.30 Impaired Perception of Facial Emotions Following Bilateral Damage to the Anterior Temporal Lobe Heike Schmolck
Neuropsychology 2001, Vol. 15, No. 1, 30-38 In the public domain DOI: 10.1037//0894-4105.15.1.30 Impaired Perception of Facial Emotions Following Bilateral Damage to the Anterior Temporal Lobe Heike Schmolck
Impaired Recognition of Social Emotions following Amygdala Damage
 Impaired Recognition of Social Emotions following Amygdala Damage Ralph Adolphs 1, Simon Baron-Cohen 2, and Daniel Tranel 1 Abstract & Lesion, functional imaging, and single-unit studies in human and nonhuman
Impaired Recognition of Social Emotions following Amygdala Damage Ralph Adolphs 1, Simon Baron-Cohen 2, and Daniel Tranel 1 Abstract & Lesion, functional imaging, and single-unit studies in human and nonhuman
FAILURES OF OBJECT RECOGNITION. Dr. Walter S. Marcantoni
 FAILURES OF OBJECT RECOGNITION Dr. Walter S. Marcantoni VISUAL AGNOSIA -damage to the extrastriate visual regions (occipital, parietal and temporal lobes) disrupts recognition of complex visual stimuli
FAILURES OF OBJECT RECOGNITION Dr. Walter S. Marcantoni VISUAL AGNOSIA -damage to the extrastriate visual regions (occipital, parietal and temporal lobes) disrupts recognition of complex visual stimuli
Facial Emotion Processing in Paranoid and Non-Paranoid Schizophrenia
 FACIAL EMOTION PROCESSING IN SCHIZOPHRENIA 1 Running head: FACIAL EMOTION PROCESSING IN SCHIZOPHRENIA Facial Emotion Processing in Paranoid and Non-Paranoid Schizophrenia Sophie Jacobsson Bachelor Degree
FACIAL EMOTION PROCESSING IN SCHIZOPHRENIA 1 Running head: FACIAL EMOTION PROCESSING IN SCHIZOPHRENIA Facial Emotion Processing in Paranoid and Non-Paranoid Schizophrenia Sophie Jacobsson Bachelor Degree
Psych3BN3 Topic 4 Emotion. Bilateral amygdala pathology: Case of S.M. (fig 9.1) S.M. s ratings of emotional intensity of faces (fig 9.
 Psych3BN3 Topic 4 Emotion Readings: Gazzaniga Chapter 9 Bilateral amygdala pathology: Case of S.M. (fig 9.1) SM began experiencing seizures at age 20 CT, MRI revealed amygdala atrophy, result of genetic
Psych3BN3 Topic 4 Emotion Readings: Gazzaniga Chapter 9 Bilateral amygdala pathology: Case of S.M. (fig 9.1) SM began experiencing seizures at age 20 CT, MRI revealed amygdala atrophy, result of genetic
Recognition of facial emotion in nine individuals with bilateral amygdala damage
 Neuropsychologia 37 (1999) 1111±1117 www.elsevier.com/locate/neuropsychologia Recognition of facial emotion in nine individuals with bilateral amygdala damage R. Adolphs a, *, D. Tranel a, S. Hamann b,
Neuropsychologia 37 (1999) 1111±1117 www.elsevier.com/locate/neuropsychologia Recognition of facial emotion in nine individuals with bilateral amygdala damage R. Adolphs a, *, D. Tranel a, S. Hamann b,
The previous three chapters provide a description of the interaction between explicit and
 77 5 Discussion The previous three chapters provide a description of the interaction between explicit and implicit learning systems. Chapter 2 described the effects of performing a working memory task
77 5 Discussion The previous three chapters provide a description of the interaction between explicit and implicit learning systems. Chapter 2 described the effects of performing a working memory task
Perception of Faces and Bodies
 CURRENT DIRECTIONS IN PSYCHOLOGICAL SCIENCE Perception of Faces and Bodies Similar or Different? Virginia Slaughter, 1 Valerie E. Stone, 2 and Catherine Reed 3 1 Early Cognitive Development Unit and 2
CURRENT DIRECTIONS IN PSYCHOLOGICAL SCIENCE Perception of Faces and Bodies Similar or Different? Virginia Slaughter, 1 Valerie E. Stone, 2 and Catherine Reed 3 1 Early Cognitive Development Unit and 2
Common and distinct neural responses during direct and incidental processing of multiple facial emotions
 NeuroImage 20 (2003) 84 97 www.elsevier.com/locate/ynimg Common and distinct neural responses during direct and incidental processing of multiple facial emotions J.S. Winston,* J. O Doherty, and R.J. Dolan
NeuroImage 20 (2003) 84 97 www.elsevier.com/locate/ynimg Common and distinct neural responses during direct and incidental processing of multiple facial emotions J.S. Winston,* J. O Doherty, and R.J. Dolan
Hierarchically Organized Mirroring Processes in Social Cognition: The Functional Neuroanatomy of Empathy
 Hierarchically Organized Mirroring Processes in Social Cognition: The Functional Neuroanatomy of Empathy Jaime A. Pineda, A. Roxanne Moore, Hanie Elfenbeinand, and Roy Cox Motivation Review the complex
Hierarchically Organized Mirroring Processes in Social Cognition: The Functional Neuroanatomy of Empathy Jaime A. Pineda, A. Roxanne Moore, Hanie Elfenbeinand, and Roy Cox Motivation Review the complex
Understanding emotions from standardized facial expressions in autism and normal development
 Understanding emotions from standardized facial expressions in autism and normal development autism 2005 SAGE Publications and The National Autistic Society Vol 9(4) 428 449; 056082 1362-3613(200510)9:4
Understanding emotions from standardized facial expressions in autism and normal development autism 2005 SAGE Publications and The National Autistic Society Vol 9(4) 428 449; 056082 1362-3613(200510)9:4
11, in nonhuman primates, different cell populations respond to facial identity and expression12, and
 UNDERSTANDING THE RECOGNITION OF FACIAL IDENTITY AND FACIAL EXPRESSION Andrew J. Calder* and Andrew W. Young Abstract Faces convey a wealth of social signals. A dominant view in face-perception research
UNDERSTANDING THE RECOGNITION OF FACIAL IDENTITY AND FACIAL EXPRESSION Andrew J. Calder* and Andrew W. Young Abstract Faces convey a wealth of social signals. A dominant view in face-perception research
Dynamic facial expressions of emotion induce representational momentum
 Cognitive, Affective, & Behavioral Neuroscience 2008, 8 (1), 25-31 doi: 10.3758/CABN.8.1.25 Dynamic facial expressions of emotion induce representational momentum SAKIKO YOSHIKAWA AND WATARU SATO Kyoto
Cognitive, Affective, & Behavioral Neuroscience 2008, 8 (1), 25-31 doi: 10.3758/CABN.8.1.25 Dynamic facial expressions of emotion induce representational momentum SAKIKO YOSHIKAWA AND WATARU SATO Kyoto
Henry Molaison. Biography. From Wikipedia, the free encyclopedia
 Henry Molaison From Wikipedia, the free encyclopedia Henry Gustav Molaison (February 26, 1926 December 2, 2008), known widely as H.M., was an American memory disorder patient who had a bilateral medial
Henry Molaison From Wikipedia, the free encyclopedia Henry Gustav Molaison (February 26, 1926 December 2, 2008), known widely as H.M., was an American memory disorder patient who had a bilateral medial
correlates with social context behavioral adaptation.
 REVIEW OF FRONTAL LOBE STRUCTURES Main organization of frontal cortex: 1. Motor area (precentral gyrus). 2. Premotor & supplementary motor areas (immediately anterior to motor area). Includes premotor,
REVIEW OF FRONTAL LOBE STRUCTURES Main organization of frontal cortex: 1. Motor area (precentral gyrus). 2. Premotor & supplementary motor areas (immediately anterior to motor area). Includes premotor,
Functional dissociation of amygdala-modulated arousal and cognitive appraisal, in Turner syndrome
 doi:10.1093/brain/awh562 Brain (2005), 128, 2084 2096 Functional dissociation of amygdala-modulated arousal and cognitive appraisal, in Turner syndrome D. H. Skuse, 1 J. S. Morris 1 and R. J. Dolan 2 1
doi:10.1093/brain/awh562 Brain (2005), 128, 2084 2096 Functional dissociation of amygdala-modulated arousal and cognitive appraisal, in Turner syndrome D. H. Skuse, 1 J. S. Morris 1 and R. J. Dolan 2 1
doi: /brain/awp255 Brain 2010: 133; Integration of gaze direction and facial expression in patients with unilateral amygdala damage
 doi:10.1093/brain/awp255 Brain 2010: 133; 248 261 248 BRAIN A JOURNAL OF NEUROLOGY Integration of gaze direction and facial expression in patients with unilateral amygdala damage Chiara Cristinzio, 1,2
doi:10.1093/brain/awp255 Brain 2010: 133; 248 261 248 BRAIN A JOURNAL OF NEUROLOGY Integration of gaze direction and facial expression in patients with unilateral amygdala damage Chiara Cristinzio, 1,2
Neurobehavioral Mechanisms of Fear Generalization in PTSD. Lei Zhang. Duke University, Durham Veteran Affairs Medical Center
 Running head: NEUROBEHAVIORAL MECHANISMS OF FEAR GENERALIZATION 1 Neurobehavioral Mechanisms of Fear Generalization in PTSD Lei Zhang Duke University, Durham Veteran Affairs Medical Center Running head:
Running head: NEUROBEHAVIORAL MECHANISMS OF FEAR GENERALIZATION 1 Neurobehavioral Mechanisms of Fear Generalization in PTSD Lei Zhang Duke University, Durham Veteran Affairs Medical Center Running head:
Learning Objectives.
 Emilie O Neill, class of 2016 Learning Objectives 1. Describe the types of deficits that occur with lesions in association areas including: prosopagnosia, neglect, aphasias, agnosia, apraxia 2. Discuss
Emilie O Neill, class of 2016 Learning Objectives 1. Describe the types of deficits that occur with lesions in association areas including: prosopagnosia, neglect, aphasias, agnosia, apraxia 2. Discuss
Methods to examine brain activity associated with emotional states and traits
 Methods to examine brain activity associated with emotional states and traits Brain electrical activity methods description and explanation of method state effects trait effects Positron emission tomography
Methods to examine brain activity associated with emotional states and traits Brain electrical activity methods description and explanation of method state effects trait effects Positron emission tomography
The Frontal Lobes. Anatomy of the Frontal Lobes. Anatomy of the Frontal Lobes 3/2/2011. Portrait: Losing Frontal-Lobe Functions. Readings: KW Ch.
 The Frontal Lobes Readings: KW Ch. 16 Portrait: Losing Frontal-Lobe Functions E.L. Highly organized college professor Became disorganized, showed little emotion, and began to miss deadlines Scores on intelligence
The Frontal Lobes Readings: KW Ch. 16 Portrait: Losing Frontal-Lobe Functions E.L. Highly organized college professor Became disorganized, showed little emotion, and began to miss deadlines Scores on intelligence
UNDERSTANDING THE RECOGNITION OF FACIAL IDENTITY AND FACIAL EXPRESSION
 UNDERSTANDING THE RECOGNITION OF FACIAL IDENTITY AND FACIAL EXPRESSION Andrew J. Calder* and Andrew W. Young Abstract Faces convey a wealth of social signals. A dominant view in face-perception research
UNDERSTANDING THE RECOGNITION OF FACIAL IDENTITY AND FACIAL EXPRESSION Andrew J. Calder* and Andrew W. Young Abstract Faces convey a wealth of social signals. A dominant view in face-perception research
Neural response to emotional faces with and without awareness: event-related fmri in a parietal patient with visual extinction and spatial neglect
 Neuropsychologia 40 (2002) 2156 2166 Neural response to emotional faces with and without awareness: event-related fmri in a parietal patient with visual extinction and spatial neglect P. Vuilleumier a,,
Neuropsychologia 40 (2002) 2156 2166 Neural response to emotional faces with and without awareness: event-related fmri in a parietal patient with visual extinction and spatial neglect P. Vuilleumier a,,
Neural correlates of memory for object identity and object location: effects of aging
 Neuropsychologia 40 (2002) 1428 1442 Neural correlates of memory for object identity and object location: effects of aging Alessandra Schiavetto a, Stefan Köhler a, Cheryl L. Grady a, Gordon Winocur a,c,
Neuropsychologia 40 (2002) 1428 1442 Neural correlates of memory for object identity and object location: effects of aging Alessandra Schiavetto a, Stefan Köhler a, Cheryl L. Grady a, Gordon Winocur a,c,
Dissociable Neural Pathways Are Involved in the Recognition of Emotion in Static and Dynamic Facial Expressions
 NeuroImage 18, 156 168 (2003) doi:10.1006/nimg.2002.1323 Dissociable Neural Pathways Are Involved in the Recognition of Emotion in Static and Dynamic Facial Expressions Clinton D. Kilts,*, Glenn Egan,*
NeuroImage 18, 156 168 (2003) doi:10.1006/nimg.2002.1323 Dissociable Neural Pathways Are Involved in the Recognition of Emotion in Static and Dynamic Facial Expressions Clinton D. Kilts,*, Glenn Egan,*
Differential Spatial Frequency Modulations on Facial Emotion Detection and Perception
 The Open Behavioral Science Journal, 2012, 6, 1-7 1 Open Access Differential Spatial Frequency Modulations on Facial Emotion Detection and Perception Ryan McBain 1, Daniel Norton 1 *, 1,2 and Yue Chen
The Open Behavioral Science Journal, 2012, 6, 1-7 1 Open Access Differential Spatial Frequency Modulations on Facial Emotion Detection and Perception Ryan McBain 1, Daniel Norton 1 *, 1,2 and Yue Chen
Brain and Cognition, 48(2-3), (2002) Evaluation of nonverbal emotion in face and voice: some preliminary findings on a new battery of tests
 Brain and Cognition, 48(2-3), 499-514 (2002) Evaluation of nonverbal emotion in face and voice: some preliminary findings on a new battery of tests Marc David Pell McGill University, Montréal Abstract
Brain and Cognition, 48(2-3), 499-514 (2002) Evaluation of nonverbal emotion in face and voice: some preliminary findings on a new battery of tests Marc David Pell McGill University, Montréal Abstract
Resistance to forgetting associated with hippocampus-mediated. reactivation during new learning
 Resistance to Forgetting 1 Resistance to forgetting associated with hippocampus-mediated reactivation during new learning Brice A. Kuhl, Arpeet T. Shah, Sarah DuBrow, & Anthony D. Wagner Resistance to
Resistance to Forgetting 1 Resistance to forgetting associated with hippocampus-mediated reactivation during new learning Brice A. Kuhl, Arpeet T. Shah, Sarah DuBrow, & Anthony D. Wagner Resistance to
A Role for Somatosensory Cortices in the Visual Recognition of Emotion as Revealed by Three-Dimensional Lesion Mapping
 The Journal of Neuroscience, April 1, 2000, 20(7):2683 2690 A Role for Somatosensory Cortices in the Visual Recognition of Emotion as Revealed by Three-Dimensional Lesion Mapping Ralph Adolphs, 1 Hanna
The Journal of Neuroscience, April 1, 2000, 20(7):2683 2690 A Role for Somatosensory Cortices in the Visual Recognition of Emotion as Revealed by Three-Dimensional Lesion Mapping Ralph Adolphs, 1 Hanna
Computational Explorations in Cognitive Neuroscience Chapter 7: Large-Scale Brain Area Functional Organization
 Computational Explorations in Cognitive Neuroscience Chapter 7: Large-Scale Brain Area Functional Organization 1 7.1 Overview This chapter aims to provide a framework for modeling cognitive phenomena based
Computational Explorations in Cognitive Neuroscience Chapter 7: Large-Scale Brain Area Functional Organization 1 7.1 Overview This chapter aims to provide a framework for modeling cognitive phenomena based
October 2, Memory II. 8 The Human Amnesic Syndrome. 9 Recent/Remote Distinction. 11 Frontal/Executive Contributions to Memory
 1 Memory II October 2, 2008 2 3 4 5 6 7 8 The Human Amnesic Syndrome Impaired new learning (anterograde amnesia), exacerbated by increasing retention delay Impaired recollection of events learned prior
1 Memory II October 2, 2008 2 3 4 5 6 7 8 The Human Amnesic Syndrome Impaired new learning (anterograde amnesia), exacerbated by increasing retention delay Impaired recollection of events learned prior
Fear Recognition Ability Predicts Differences in Social Cognitive and Neural Functioning in Men
 Fear Recognition Ability Predicts Differences in Social Cognitive and Neural Functioning in Men Ben Corden, Hugo D. Critchley, David Skuse, and Raymond J. Dolan Abstract & By testing the facial fear-recognition
Fear Recognition Ability Predicts Differences in Social Cognitive and Neural Functioning in Men Ben Corden, Hugo D. Critchley, David Skuse, and Raymond J. Dolan Abstract & By testing the facial fear-recognition
Frontal Contributions to Memory Encoding Before and After Unilateral Medial Temporal Lobectomy
 Frontal Contributions to Memory Encoding Before and After Unilateral Medial Temporal Lobectomy Jeff Ojemann, MD Department of Neurological Surgery University of Washington Children s Hospital & Regional
Frontal Contributions to Memory Encoding Before and After Unilateral Medial Temporal Lobectomy Jeff Ojemann, MD Department of Neurological Surgery University of Washington Children s Hospital & Regional
Top-down guidance in visual search for facial expressions
 Psychonomic Bulletin & Review 2007, 14 (1), 159-165 Top-down guidance in visual search for facial expressions SOWON HAHN AND SCOTT D. GRONLUND University of Oklahoma, Norman, Oklahoma Using a visual search
Psychonomic Bulletin & Review 2007, 14 (1), 159-165 Top-down guidance in visual search for facial expressions SOWON HAHN AND SCOTT D. GRONLUND University of Oklahoma, Norman, Oklahoma Using a visual search
Longitudinal aspects of emotion recognition in patients with traumatic brain injury
 Neuropsychologia 46 (2008) 148 159 Longitudinal aspects of emotion recognition in patients with traumatic brain injury Magdalena Ietswaart a,, Maarten Milders b, John R. Crawford b, David Currie c, Clare
Neuropsychologia 46 (2008) 148 159 Longitudinal aspects of emotion recognition in patients with traumatic brain injury Magdalena Ietswaart a,, Maarten Milders b, John R. Crawford b, David Currie c, Clare
T he role of the amygdala in processing emotional facial
 PAPER Emotional memory and perception in temporal lobectomy patients with amygdala damage B Brierley, N Medford, P Shaw, A S David... See end of article for authors affiliations... Correspondence to: Professor
PAPER Emotional memory and perception in temporal lobectomy patients with amygdala damage B Brierley, N Medford, P Shaw, A S David... See end of article for authors affiliations... Correspondence to: Professor
Taking control of reflexive social attention
 Cognition 94 (2005) B55 B65 www.elsevier.com/locate/cognit Brief article Taking control of reflexive social attention Jelena Ristic*, Alan Kingstone Department of Psychology, University of British Columbia,
Cognition 94 (2005) B55 B65 www.elsevier.com/locate/cognit Brief article Taking control of reflexive social attention Jelena Ristic*, Alan Kingstone Department of Psychology, University of British Columbia,
Autism and the development of face processing
 Clinical Neuroscience Research 6 (2006) 145 160 www.elsevier.com/locate/clires Autism and the development of face processing Golijeh Golarai a, *, Kalanit Grill-Spector a,b, Allan L. Reiss a,c a Department
Clinical Neuroscience Research 6 (2006) 145 160 www.elsevier.com/locate/clires Autism and the development of face processing Golijeh Golarai a, *, Kalanit Grill-Spector a,b, Allan L. Reiss a,c a Department
State Anxiety Modulation of the Amygdala Response to Unattended Threat-Related Stimuli
 10364 The Journal of Neuroscience, November 17, 2004 24(46):10364 10368 Behavioral/Systems/Cognitive State Anxiety Modulation of the Amygdala Response to Unattended Threat-Related Stimuli Sonia J. Bishop,
10364 The Journal of Neuroscience, November 17, 2004 24(46):10364 10368 Behavioral/Systems/Cognitive State Anxiety Modulation of the Amygdala Response to Unattended Threat-Related Stimuli Sonia J. Bishop,
The neural processing of familiar and unfamiliar faces: A review and synopsis
 726 British Journal of Psychology (2011), 102, 726 747 C 2011 The British Psychological Society The British Psychological Society www.wileyonlinelibrary.com The neural processing of familiar and unfamiliar
726 British Journal of Psychology (2011), 102, 726 747 C 2011 The British Psychological Society The British Psychological Society www.wileyonlinelibrary.com The neural processing of familiar and unfamiliar
Layered organization of cortex: Paleocortex 3 layers hippocampal formation / ventral & medial cortex closest to brainstem
 Layered organization of cortex: Paleocortex 3 layers hippocampal formation / ventral & medial cortex closest to brainstem Archicortex 3-4 layers hippocampal formation / amygdala Neocortex 6 layers more
Layered organization of cortex: Paleocortex 3 layers hippocampal formation / ventral & medial cortex closest to brainstem Archicortex 3-4 layers hippocampal formation / amygdala Neocortex 6 layers more
The Role of Working Memory in Visual Selective Attention
 Goldsmiths Research Online. The Authors. Originally published: Science vol.291 2 March 2001 1803-1806. http://www.sciencemag.org. 11 October 2000; accepted 17 January 2001 The Role of Working Memory in
Goldsmiths Research Online. The Authors. Originally published: Science vol.291 2 March 2001 1803-1806. http://www.sciencemag.org. 11 October 2000; accepted 17 January 2001 The Role of Working Memory in
Dynamic Perception of Facial Affect and Identity in the Human Brain
 Dynamic Perception of Facial Affect and Identity in the Human Brain Kevin S. LaBar, Michael J. Crupain, James T. Voyvodic 1 and Gregory McCarthy 1 Center for Cognitive Neuroscience and 1 Brain Imaging
Dynamic Perception of Facial Affect and Identity in the Human Brain Kevin S. LaBar, Michael J. Crupain, James T. Voyvodic 1 and Gregory McCarthy 1 Center for Cognitive Neuroscience and 1 Brain Imaging
Men fear other men most: Gender specific brain activations in. perceiving threat from dynamic faces and bodies. An fmri. study.
 Men fear other men most: Gender specific brain activations in perceiving threat from dynamic faces and bodies. An fmri study. Kret, ME, Pichon, S 2,4, Grèzes, J 2, & de Gelder, B,3 Cognitive and Affective
Men fear other men most: Gender specific brain activations in perceiving threat from dynamic faces and bodies. An fmri study. Kret, ME, Pichon, S 2,4, Grèzes, J 2, & de Gelder, B,3 Cognitive and Affective
York, YO10 5DD, UK. *Corresponding author: Figures: 4. Tables: 2. Abstract: 291.
 RESPONSES IN THE RIGHT POSTERIOR SUPERIOR TEMPORAL SULCUS SHOW A FEATURE- BASED RESPONSE TO FACIAL EXPRESSION Tessa R. Flack, Timothy J. Andrews, Mark Hymers, Mohammed Al-Mosaiwi, Samuel P. Marsden, James
RESPONSES IN THE RIGHT POSTERIOR SUPERIOR TEMPORAL SULCUS SHOW A FEATURE- BASED RESPONSE TO FACIAL EXPRESSION Tessa R. Flack, Timothy J. Andrews, Mark Hymers, Mohammed Al-Mosaiwi, Samuel P. Marsden, James
Title:Atypical language organization in temporal lobe epilepsy revealed by a passive semantic paradigm
 Author's response to reviews Title:Atypical language organization in temporal lobe epilepsy revealed by a passive semantic paradigm Authors: Julia Miro (juliamirollado@gmail.com) Pablo Ripollès (pablo.ripolles.vidal@gmail.com)
Author's response to reviews Title:Atypical language organization in temporal lobe epilepsy revealed by a passive semantic paradigm Authors: Julia Miro (juliamirollado@gmail.com) Pablo Ripollès (pablo.ripolles.vidal@gmail.com)
Attention modulates the processing of emotional expression triggered by foveal faces
 Neuroscience Letters xxx (2005) xxx xxx Attention modulates the processing of emotional expression triggered by foveal faces Amanda Holmes a,, Monika Kiss b, Martin Eimer b a School of Human and Life Sciences,
Neuroscience Letters xxx (2005) xxx xxx Attention modulates the processing of emotional expression triggered by foveal faces Amanda Holmes a,, Monika Kiss b, Martin Eimer b a School of Human and Life Sciences,
Visual Context Dan O Shea Prof. Fei Fei Li, COS 598B
 Visual Context Dan O Shea Prof. Fei Fei Li, COS 598B Cortical Analysis of Visual Context Moshe Bar, Elissa Aminoff. 2003. Neuron, Volume 38, Issue 2, Pages 347 358. Visual objects in context Moshe Bar.
Visual Context Dan O Shea Prof. Fei Fei Li, COS 598B Cortical Analysis of Visual Context Moshe Bar, Elissa Aminoff. 2003. Neuron, Volume 38, Issue 2, Pages 347 358. Visual objects in context Moshe Bar.
Facial Emotion Recognition after Curative Nondominant Temporal Lobectomy in Patients with Mesial Temporal Sclerosis
 Epilepsia, 47(8):1337 1342, 2006 Blackwell Publishing, Inc. C 2006 International League Against Epilepsy Facial Emotion Recognition after Curative Nondominant Temporal Lobectomy in Patients with Mesial
Epilepsia, 47(8):1337 1342, 2006 Blackwell Publishing, Inc. C 2006 International League Against Epilepsy Facial Emotion Recognition after Curative Nondominant Temporal Lobectomy in Patients with Mesial
SOCIAL JUDGMENTS ARE INFLUENCED BY BOTH FACIAL EXPRESSION AND DIRECTION OF EYE GAZE
 Social Cognition, Vol. 9, No. 4, 011, pp. 415 49 SOCIAL JUDGMENTS ARE INFLUENCED BY BOTH FACIAL EXPRESSION AND DIRECTION OF EYE GAZE Megan L. Willis Macquarie University, Sydney, Australia; University
Social Cognition, Vol. 9, No. 4, 011, pp. 415 49 SOCIAL JUDGMENTS ARE INFLUENCED BY BOTH FACIAL EXPRESSION AND DIRECTION OF EYE GAZE Megan L. Willis Macquarie University, Sydney, Australia; University
This is a repository copy of Knowing no fear. White Rose Research Online URL for this paper:
 This is a repository copy of Knowing no fear. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/1321/ Article: Sprengelmeyer, R, Young, AW orcid.org/0000-0002-1202-6297, Schroeder,
This is a repository copy of Knowing no fear. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/1321/ Article: Sprengelmeyer, R, Young, AW orcid.org/0000-0002-1202-6297, Schroeder,
Memory. Psychology 3910 Guest Lecture by Steve Smith
 Memory Psychology 3910 Guest Lecture by Steve Smith Note: Due to copyright restrictions, I had to remove the images from the Weschler Memory Scales from the slides I posted online. Wechsler Memory Scales
Memory Psychology 3910 Guest Lecture by Steve Smith Note: Due to copyright restrictions, I had to remove the images from the Weschler Memory Scales from the slides I posted online. Wechsler Memory Scales
Remembering the Past to Imagine the Future: A Cognitive Neuroscience Perspective
 MILITARY PSYCHOLOGY, 21:(Suppl. 1)S108 S112, 2009 Copyright Taylor & Francis Group, LLC ISSN: 0899-5605 print / 1532-7876 online DOI: 10.1080/08995600802554748 Remembering the Past to Imagine the Future:
MILITARY PSYCHOLOGY, 21:(Suppl. 1)S108 S112, 2009 Copyright Taylor & Francis Group, LLC ISSN: 0899-5605 print / 1532-7876 online DOI: 10.1080/08995600802554748 Remembering the Past to Imagine the Future:
Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging
 Neuropsychologia 45 (2007) 174 194 Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging Patrik Vuilleumier a,b,c,d,, Gilles Pourtois a,b,d
Neuropsychologia 45 (2007) 174 194 Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging Patrik Vuilleumier a,b,c,d,, Gilles Pourtois a,b,d
Neuroimaging and Emotion
 870 Neuroimaging and Emotion Neuroimaging and Emotion N A Harrison and H D Critchley University College London, London, UK ã 2007 Elsevier Inc. All rights reserved. Emotion Definition of Emotion Emotion
870 Neuroimaging and Emotion Neuroimaging and Emotion N A Harrison and H D Critchley University College London, London, UK ã 2007 Elsevier Inc. All rights reserved. Emotion Definition of Emotion Emotion
Chapter 2 Knowledge Production in Cognitive Neuroscience: Tests of Association, Necessity, and Sufficiency
 Chapter 2 Knowledge Production in Cognitive Neuroscience: Tests of Association, Necessity, and Sufficiency While all domains in neuroscience might be relevant for NeuroIS research to some degree, the field
Chapter 2 Knowledge Production in Cognitive Neuroscience: Tests of Association, Necessity, and Sufficiency While all domains in neuroscience might be relevant for NeuroIS research to some degree, the field
Emotion Lecture 26 1
 Emotion Lecture 26 1 The Trilogy of Mind Immanuel Kant (1791); Hilgard (1980) There are three absolutely irreducible faculties of mind: knowledge, feeling, and desire. Cognition Knowledge and Beliefs Emotion
Emotion Lecture 26 1 The Trilogy of Mind Immanuel Kant (1791); Hilgard (1980) There are three absolutely irreducible faculties of mind: knowledge, feeling, and desire. Cognition Knowledge and Beliefs Emotion
Neuroscience of Consciousness II
 1 C83MAB: Mind and Brain Neuroscience of Consciousness II Tobias Bast, School of Psychology, University of Nottingham 2 Consciousness State of consciousness - Being awake/alert/attentive/responsive Contents
1 C83MAB: Mind and Brain Neuroscience of Consciousness II Tobias Bast, School of Psychology, University of Nottingham 2 Consciousness State of consciousness - Being awake/alert/attentive/responsive Contents
Fear Selectively Modulates Visual Mental Imagery and Visual Perception
 Fear Selectively Modulates Visual Mental Imagery and Visual Perception The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Fear Selectively Modulates Visual Mental Imagery and Visual Perception The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Brain Imaging Investigation of the Impairing Effect of Emotion on Cognition
 Brain Imaging Investigation of the Impairing Effect of Emotion on Cognition Gloria Wong 1,2, Sanda Dolcos 1,3, Ekaterina Denkova 1, Rajendra A. Morey 4,5, Lihong Wang 4,5, Nicholas Coupland 1, Gregory
Brain Imaging Investigation of the Impairing Effect of Emotion on Cognition Gloria Wong 1,2, Sanda Dolcos 1,3, Ekaterina Denkova 1, Rajendra A. Morey 4,5, Lihong Wang 4,5, Nicholas Coupland 1, Gregory
Michio Nomura, a, * Hideki Ohira, a Kaoruko Haneda, a Tetsuya Iidaka, a Norihiro Sadato, b Tomohisa Okada, b and Yoshiharu Yonekura c
 Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fmri study Michio Nomura, a, * Hideki
Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fmri study Michio Nomura, a, * Hideki
This is a repository copy of Neural responses to facial and vocal expressions of fear and disgust.
 This is a repository copy of Neural responses to facial and vocal expressions of fear and disgust. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/1320/ Article: Phillips,
This is a repository copy of Neural responses to facial and vocal expressions of fear and disgust. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/1320/ Article: Phillips,
Neuropsychologia 48 (2010) Contents lists available at ScienceDirect. Neuropsychologia
 Neuropsychologia 48 (2010) 3430 3435 Contents lists available at ScienceDirect Neuropsychologia journal homepage: www.elsevier.com/locate/neuropsychologia Fear-enhanced visual search persists after amygdala
Neuropsychologia 48 (2010) 3430 3435 Contents lists available at ScienceDirect Neuropsychologia journal homepage: www.elsevier.com/locate/neuropsychologia Fear-enhanced visual search persists after amygdala
Introduction to Physiological Psychology Learning and Memory II
 Introduction to Physiological Psychology Learning and Memory II ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ksweeney/psy260.html Memory Working Memory Long-term Memory Declarative Memory Procedural Memory
Introduction to Physiological Psychology Learning and Memory II ksweeney@cogsci.ucsd.edu cogsci.ucsd.edu/~ksweeney/psy260.html Memory Working Memory Long-term Memory Declarative Memory Procedural Memory
Do women with fragile X syndrome have problems in switching attention: Preliminary findings from ERP and fmri
 Brain and Cognition 54 (2004) 235 239 www.elsevier.com/locate/b&c Do women with fragile X syndrome have problems in switching attention: Preliminary findings from ERP and fmri Kim Cornish, a,b, * Rachel
Brain and Cognition 54 (2004) 235 239 www.elsevier.com/locate/b&c Do women with fragile X syndrome have problems in switching attention: Preliminary findings from ERP and fmri Kim Cornish, a,b, * Rachel
Sex, beauty and the orbitofrontal cortex
 International Journal of Psychophysiology 63 (2007) 181 185 www.elsevier.com/locate/ijpsycho Sex, beauty and the orbitofrontal cortex Alumit Ishai Institute of Neuroradiology, University of Zurich, Winterthurerstrasse
International Journal of Psychophysiology 63 (2007) 181 185 www.elsevier.com/locate/ijpsycho Sex, beauty and the orbitofrontal cortex Alumit Ishai Institute of Neuroradiology, University of Zurich, Winterthurerstrasse
Higher Cortical Function
 Emilie O Neill, class of 2016 Higher Cortical Function Objectives Describe the association cortical areas processing sensory, motor, executive, language, and emotion/memory information (know general location
Emilie O Neill, class of 2016 Higher Cortical Function Objectives Describe the association cortical areas processing sensory, motor, executive, language, and emotion/memory information (know general location
MSc Neuroimaging for Clinical & Cognitive Neuroscience
 MSc Neuroimaging for Clinical & Cognitive Neuroscience School of Psychological Sciences Faculty of Medical & Human Sciences Module Information *Please note that this is a sample guide to modules. The exact
MSc Neuroimaging for Clinical & Cognitive Neuroscience School of Psychological Sciences Faculty of Medical & Human Sciences Module Information *Please note that this is a sample guide to modules. The exact
Differences in holistic processing do not explain cultural differences in the recognition of facial expression
 THE QUARTERLY JOURNAL OF EXPERIMENTAL PSYCHOLOGY, 2017 VOL. 70, NO. 12, 2445 2459 http://dx.doi.org/10.1080/17470218.2016.1240816 Differences in holistic processing do not explain cultural differences
THE QUARTERLY JOURNAL OF EXPERIMENTAL PSYCHOLOGY, 2017 VOL. 70, NO. 12, 2445 2459 http://dx.doi.org/10.1080/17470218.2016.1240816 Differences in holistic processing do not explain cultural differences
Motivation represents the reasons for people's actions, desires, and needs. Typically, this unit is described as a goal
 Motivation What is motivation? Motivation represents the reasons for people's actions, desires, and needs. Reasons here implies some sort of desired end state Typically, this unit is described as a goal
Motivation What is motivation? Motivation represents the reasons for people's actions, desires, and needs. Reasons here implies some sort of desired end state Typically, this unit is described as a goal
How do eye gaze and facial expression interact?
 VISUAL COGNITION, 2008, 16 (6), 708733 How do eye gaze and facial expression interact? Markus Bindemann and A. Mike Burton Department of Psychology, University of Glasgow, UK Stephen R. H. Langton Department
VISUAL COGNITION, 2008, 16 (6), 708733 How do eye gaze and facial expression interact? Markus Bindemann and A. Mike Burton Department of Psychology, University of Glasgow, UK Stephen R. H. Langton Department
Right hemispheric dominance in processing of unconscious negative emotion
 Brain and Cognition 62 (2006) 261 266 www.elsevier.com/locate/b&c Right hemispheric dominance in processing of unconscious negative emotion Wataru Sato a,, Satoshi Aoki b a Department of Psychology, Graduate
Brain and Cognition 62 (2006) 261 266 www.elsevier.com/locate/b&c Right hemispheric dominance in processing of unconscious negative emotion Wataru Sato a,, Satoshi Aoki b a Department of Psychology, Graduate
Transcranial Magnetic Stimulation Disrupts the Perception and Embodiment of Facial Expressions
 The Journal of Neuroscience, September 3, 2008 28(36):8929 8933 8929 Brief Communications Transcranial Magnetic Stimulation Disrupts the Perception and Embodiment of Facial Expressions David Pitcher, Lúcia
The Journal of Neuroscience, September 3, 2008 28(36):8929 8933 8929 Brief Communications Transcranial Magnetic Stimulation Disrupts the Perception and Embodiment of Facial Expressions David Pitcher, Lúcia
Attentional Control 1. Identifying the neural systems of top-down attentional control: A meta-analytic approach
 Attentional Control 1 Identifying the neural systems of top-down attentional control: A meta-analytic approach Barry Giesbrecht & George R. Mangun Center for Mind & Brain University of California, Davis
Attentional Control 1 Identifying the neural systems of top-down attentional control: A meta-analytic approach Barry Giesbrecht & George R. Mangun Center for Mind & Brain University of California, Davis
Brain Imaging Applied to Memory & Learning
 Brain Imaging Applied to Memory & Learning John Gabrieli Department of Brain & Cognitive Sciences Institute for Medical Engineering & Sciences McGovern Institute for Brain Sciences MIT Levels of Analysis
Brain Imaging Applied to Memory & Learning John Gabrieli Department of Brain & Cognitive Sciences Institute for Medical Engineering & Sciences McGovern Institute for Brain Sciences MIT Levels of Analysis
Auditory Processing Of Schizophrenia
 Auditory Processing Of Schizophrenia In general, sensory processing as well as selective attention impairments are common amongst people with schizophrenia. It is important to note that experts have in
Auditory Processing Of Schizophrenia In general, sensory processing as well as selective attention impairments are common amongst people with schizophrenia. It is important to note that experts have in
Michael L. Kimbarow, Ph.D. Wendy Quach, Ph.D. Marion D. Meyerson, Ph.D. San Jose State University San Jose, CA
 Michael L. Kimbarow, Ph.D. Wendy Quach, Ph.D. Marion D. Meyerson, Ph.D. San Jose State University San Jose, CA Fear Anger Sadness Disgust Happiness Expressions are universal (Ekman et al, 1987; Levensen,Eikman,Heider
Michael L. Kimbarow, Ph.D. Wendy Quach, Ph.D. Marion D. Meyerson, Ph.D. San Jose State University San Jose, CA Fear Anger Sadness Disgust Happiness Expressions are universal (Ekman et al, 1987; Levensen,Eikman,Heider
Neocortex. Hemispheres 9/22/2010. Psychology 472 Pharmacology of Psychoactive Drugs. Structures are divided into several section or lobes.
 Neocortex Psychology 472 Pharmacology of Psychoactive Drugs 1 Is the most developed in Humans Has many folds and fissures The folds of tissue are called gyri or a gyrus (single) The fissures or valleys
Neocortex Psychology 472 Pharmacology of Psychoactive Drugs 1 Is the most developed in Humans Has many folds and fissures The folds of tissue are called gyri or a gyrus (single) The fissures or valleys
Rapid fear detection relies on high spatial frequencies
 Supplemental Material for Rapid fear detection relies on high spatial frequencies Timo Stein, Kiley Seymour, Martin N. Hebart, and Philipp Sterzer Additional experimental details Participants Volunteers
Supplemental Material for Rapid fear detection relies on high spatial frequencies Timo Stein, Kiley Seymour, Martin N. Hebart, and Philipp Sterzer Additional experimental details Participants Volunteers
Dr. Mark Ashton Smith, Department of Psychology, Bilkent University
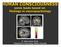 UMAN CONSCIOUSNESS some leads based on findings in neuropsychology Dr. Mark Ashton Smith, Department of Psychology, Bilkent University nattentional Blindness Simons and Levin, 1998 Not Detected Detected
UMAN CONSCIOUSNESS some leads based on findings in neuropsychology Dr. Mark Ashton Smith, Department of Psychology, Bilkent University nattentional Blindness Simons and Levin, 1998 Not Detected Detected
Frank Tong. Department of Psychology Green Hall Princeton University Princeton, NJ 08544
 Frank Tong Department of Psychology Green Hall Princeton University Princeton, NJ 08544 Office: Room 3-N-2B Telephone: 609-258-2652 Fax: 609-258-1113 Email: ftong@princeton.edu Graduate School Applicants
Frank Tong Department of Psychology Green Hall Princeton University Princeton, NJ 08544 Office: Room 3-N-2B Telephone: 609-258-2652 Fax: 609-258-1113 Email: ftong@princeton.edu Graduate School Applicants
Ch 8. Learning and Memory
 Ch 8. Learning and Memory Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by H.-S. Seok, K. Kim, and B.-T. Zhang Biointelligence
Ch 8. Learning and Memory Cognitive Neuroscience: The Biology of the Mind, 2 nd Ed., M. S. Gazzaniga, R. B. Ivry, and G. R. Mangun, Norton, 2002. Summarized by H.-S. Seok, K. Kim, and B.-T. Zhang Biointelligence
Annotation: Development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives
 Journal of Child Psychology and Psychiatry 45:7 (2004), pp 1185 1198 doi: 10.1111/j.1469-7610.2004.00316.x Annotation: Development of facial expression recognition from childhood to adolescence: behavioural
Journal of Child Psychology and Psychiatry 45:7 (2004), pp 1185 1198 doi: 10.1111/j.1469-7610.2004.00316.x Annotation: Development of facial expression recognition from childhood to adolescence: behavioural
Running head: OLFACTORY AND HIGHER CORTICAL FUNCTIONS. Association between Olfactory and Higher Cortical Functions in Alzheimer s disease
 Running head: OLFACTORY AND HIGHER CORTICAL FUNCTIONS Association between Olfactory and Higher Cortical Functions in Alzheimer s disease Alzheimer s Disease (AD) is a disease that affects many areas of
Running head: OLFACTORY AND HIGHER CORTICAL FUNCTIONS Association between Olfactory and Higher Cortical Functions in Alzheimer s disease Alzheimer s Disease (AD) is a disease that affects many areas of
Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load
 Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load Intro Examine attention response functions Compare an attention-demanding
Attention Response Functions: Characterizing Brain Areas Using fmri Activation during Parametric Variations of Attentional Load Intro Examine attention response functions Compare an attention-demanding
The Neuropsychology of
 The Neuropsychology of Stroke Tammy Kordes, Ph.D. Northshore Neurosciences Outline What is the Role of Neuropsychology Purpose of Neuropsychological Assessments Common Neuropsychological Disorders Assessment
The Neuropsychology of Stroke Tammy Kordes, Ph.D. Northshore Neurosciences Outline What is the Role of Neuropsychology Purpose of Neuropsychological Assessments Common Neuropsychological Disorders Assessment
Importance of Deficits
 Importance of Deficits In complex systems the parts are often so integrated that they cannot be detected in normal operation Need to break the system to discover the components not just physical components
Importance of Deficits In complex systems the parts are often so integrated that they cannot be detected in normal operation Need to break the system to discover the components not just physical components
Implicit Trustworthiness Decisions: Automatic Coding of Face Properties in the Human Amygdala
 Implicit Trustworthiness Decisions: Automatic Coding of Face Properties in the Human Amygdala Andrew D. Engell, James V. Haxby, and Alexander Todorov Abstract & Deciding whether an unfamiliar person is
Implicit Trustworthiness Decisions: Automatic Coding of Face Properties in the Human Amygdala Andrew D. Engell, James V. Haxby, and Alexander Todorov Abstract & Deciding whether an unfamiliar person is
25/09/2012. Capgras Syndrome. Chapter 2. Capgras Syndrome - 2. The Neural Basis of Cognition
 Chapter 2 The Neural Basis of Cognition Capgras Syndrome Alzheimer s patients & others delusion that significant others are robots or impersonators - paranoia Two brain systems for facial recognition -
Chapter 2 The Neural Basis of Cognition Capgras Syndrome Alzheimer s patients & others delusion that significant others are robots or impersonators - paranoia Two brain systems for facial recognition -
Top-Down Activation of Fusiform Cortex without Seeing Faces in Prosopagnosia
 Cerebral Cortex August 2010;20:1878--1890 doi:10.1093/cercor/bhp254 Advance Access publication November 25, 2009 Top-Down Activation of Fusiform Cortex without Seeing Faces in Prosopagnosia Ruthger Righart
Cerebral Cortex August 2010;20:1878--1890 doi:10.1093/cercor/bhp254 Advance Access publication November 25, 2009 Top-Down Activation of Fusiform Cortex without Seeing Faces in Prosopagnosia Ruthger Righart
Neural structures associated with recognition of facial expressions of basic emotions
 Neural structures associated with recognition of facial expressions of basic emotions. Sprengelmeyer 1*, M. ausch 2, U. T. Eysel 2 and H. Przuntek 1 1 Neurologische Klinik im St Josef-Hospital, uhr-universita«t
Neural structures associated with recognition of facial expressions of basic emotions. Sprengelmeyer 1*, M. ausch 2, U. T. Eysel 2 and H. Przuntek 1 1 Neurologische Klinik im St Josef-Hospital, uhr-universita«t
Cerebral Cortex 1. Sarah Heilbronner
 Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Morris water maze: standard test for spatial memory in rodents
 Vertebrate Models: The Hippocampus 34 Vertebrate Models: The Hippocampus 35 Vertebrate Models: The Hippocampus 36 Vertebrate Models: The Hippocampus 37 Animal Models of Learning (Vertebrates) Morris water
Vertebrate Models: The Hippocampus 34 Vertebrate Models: The Hippocampus 35 Vertebrate Models: The Hippocampus 36 Vertebrate Models: The Hippocampus 37 Animal Models of Learning (Vertebrates) Morris water
Human Paleoneurology and the Evolution of the Parietal Cortex
 PARIETAL LOBE The Parietal Lobes develop at about the age of 5 years. They function to give the individual perspective and to help them understand space, touch, and volume. The location of the parietal
PARIETAL LOBE The Parietal Lobes develop at about the age of 5 years. They function to give the individual perspective and to help them understand space, touch, and volume. The location of the parietal
Time perception, cognitive correlates, age and emotions
 Available online at www.sciencedirect.com ScienceDirect Procedia - Social and Behavioral Sciences 187 ( 2015 ) 695 699 PSIWORLD 2014 Time perception, cognitive correlates, age and emotions Cristian Vasile*
Available online at www.sciencedirect.com ScienceDirect Procedia - Social and Behavioral Sciences 187 ( 2015 ) 695 699 PSIWORLD 2014 Time perception, cognitive correlates, age and emotions Cristian Vasile*
Accounts for the N170 face-effect: a reply to Rossion, Curran, & Gauthier
 S. Bentin, D. Carmel / Cognition 85 (2002) 197 202 197 COGNITION Cognition 85 (2002) 197 202 www.elsevier.com/locate/cognit Discussion Accounts for the N170 face-effect: a reply to Rossion, Curran, & Gauthier
S. Bentin, D. Carmel / Cognition 85 (2002) 197 202 197 COGNITION Cognition 85 (2002) 197 202 www.elsevier.com/locate/cognit Discussion Accounts for the N170 face-effect: a reply to Rossion, Curran, & Gauthier
Amygdala Responses to Fearful and Happy Facial Expressions under Conditions of Binocular Suppression
 2898 The Journal of Neuroscience, March 24, 2004 24(12):2898 2904 Brief Communication Amygdala Responses to Fearful and Happy Facial Expressions under Conditions of Binocular Suppression Mark A. Williams,
2898 The Journal of Neuroscience, March 24, 2004 24(12):2898 2904 Brief Communication Amygdala Responses to Fearful and Happy Facial Expressions under Conditions of Binocular Suppression Mark A. Williams,
Exploration of social rule violation in patients with focal prefrontal neurosurgical lesions
 Exploration of social rule violation in patients with focal prefrontal neurosurgical lesions R G Morris 1, E Pullen 2, S Kerr 3, P R Bullock 4 and R P Selway 5 1 Neuropsychology Unit, Department of Psychology,
Exploration of social rule violation in patients with focal prefrontal neurosurgical lesions R G Morris 1, E Pullen 2, S Kerr 3, P R Bullock 4 and R P Selway 5 1 Neuropsychology Unit, Department of Psychology,
An investigation of basic facial expression recognition in autism spectrum disorders
 Cognition and Emotion ISSN: 0269-9931 (Print) 1464-0600 (Online) Journal homepage: http://www.tandfonline.com/loi/pcem20 An investigation of basic facial expression recognition in autism spectrum disorders
Cognition and Emotion ISSN: 0269-9931 (Print) 1464-0600 (Online) Journal homepage: http://www.tandfonline.com/loi/pcem20 An investigation of basic facial expression recognition in autism spectrum disorders
"False tagging mechanism False Tagging Theory All idea initially believed Doubt occur when prefrontal cortex tags it as false Provides doubt and
 Ventromedial Notes Frontal lobe Prefrontal cortex 1. dorsolateral cortex Last to myelinate Sleep deprivation Executive functions Working memory Cognitive flexibility Planning 2. Orbitofrontal cortex Controls
Ventromedial Notes Frontal lobe Prefrontal cortex 1. dorsolateral cortex Last to myelinate Sleep deprivation Executive functions Working memory Cognitive flexibility Planning 2. Orbitofrontal cortex Controls
