2014 Physician Quality Reporting System Data Collection Form: Cataracts (for patients aged 18 and older)
|
|
|
- Kristian McDowell
- 6 years ago
- Views:
Transcription
1 2014 Physician Quality Reporting System Data Collection Form: Cataracts (for patients aged 18 and older) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy Gender: M F Patient Insured - Traditional Medicare*: Medicare Advantage: Other: *Note: A minimum of 11 patients must be Traditional Medicare Part B **Note: Currently, ICD-10 codes are not required. You may choose to use either the ICD-9 codes listed in each data collection form, or the ICD-10 codes in the document: PQRS2014 Applicable Measure Group Codes. This document also contains a list of ICD-9, encounter, and procedure codes. Not all measures groups require all 3 code types. Medical Record Number: Appointment Date: / / (1/1/14 12/31/14) mm dd yyyy ICD-9 (or ICD-10) Diagnosis Code**: Encounter Code: N/A Procedure Code: Patient sample criteria for the Cataracts Measures Group are: patients aged 18 years and older with specific procedure for cataract surgery performed: One of the following procedure codes indicating cataract surgery: 66840, 66850, 66852, 66920, 66930, 66940, 66983, WITHOUT Modifier 56 (pre-operative management only) IMPORTANT NOTE: Eligible professionals wanting to report this measures group must engage a third party to administer, receive results, and review the surveys required for measures #303 and #304. Eligible professionals who wish to report these measures must advise Covisint who will be administering, receiving and reviewing the surveys. Do not report measures #303 or #304 when the cataract surgery includes modifier 55 (post-operative management only). Include only procedures performed January 1 through September 30, 2014 of the reporting period. This will allow the post-operative period to occur within the reporting year. PLEASE REFER TO THE CATARACTS MEASURES GROUP IN THE CMS 2014 PQRS MEASURES GROUPS SPECIFICATIONS MANUAL FOR FURTHER INFORMATION.
2 Patient Name: Page 2 of 5 NOTE: Measures #191 and #192 need only be reported when the patient also has a diagnosis of uncomplicated cataract. Refer to the 2014 CMS Measure Group specifications manual for Cataracts for specific codes indicating a significant ocular (co-morbid) condition that excludes a patient from reporting for each of these two measures. Physician Quality Reporting Measure # 191 : Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery Percentage of patients aged 18 years and older with a diagnosis of uncomplicated cataract who had cataract surgery and no significant ocular conditions impacting the visual outcome of surgery and had bestcorrected visual acuity of 20/40 or better (distance or near) achieved within 90 days following the cataract surgery group specifications for exclusion criteria related to this measure Best-corrected visual acuity of 20/40 or better (distance or near) achieved within 90 days following cataract surgery Not Eligible patient has Complicated Cataract OR Other Significant Ocular Condition(s) Best-corrected visual acuity of 20/40 or better (distance or near) not achieved within 90 days following cataract surgery, reason not otherwise specified
3 Patient Name: Page 3 of 5 Physician Quality Reporting Measure # 192 : Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures Percentage of patients aged 18 years and older with a diagnosis of uncomplicated cataract who had cataract surgery and had any of a specified list of surgical procedures in the 30 days following cataract surgery which would indicate the occurrence of any of the following major complications: retained nuclear fragments, endophthalmitis, dislocated or wrong power IOL, retinal detachment, or wound dehiscence group specifications for exclusion criteria related to this measure Must take into account codes for major complications (e.g., retained nuclear fragments, endophthalmitis, dislocated or wrong power IOL, retinal detachment, or wound dehiscence): 65235, 65800, 65810, 65815, 65860, 65880, 65900, 65920, 65930, 66030, 66250, 66820, 66825, 66830, 66852, 66986, 67005, 67010, 67015, 67025, 67028, 67030, 67031, 67036, 67039, 67041, 67042, 67043, 67101, 67105, 67107, 67108, 67110, 67112, 67141, 67145, 67250, Measure Note: For performance, a lower rate indicates better performance. Surgical procedure performed within 30 days following cataract surgery for major complications Not Eligible patient has Complicated Cataract OR Other Significant Ocular Condition(s) Surgical procedure not performed within 30 days following cataract surgery for major complications
4 Patient Name: Page 4 of 5 Physician Quality Reporting Measure # 303 : Cataracts: Improvement in Patient s Visual Function within 90 Days Following Cataract Surgery Measure Note: Surveys must be administered by third party and sent to Covisint for review Percentage of patients aged 18 years and older in sample who had cataract surgery and had improvement in visual function achieved within 90 days following the cataract surgery, based on completing a pre-operative and post-operative visual function survey group specifications for important definitions related to this measure Improvement in visual function achieved within 90 days following cataract surgery Patient care survey was not completed by patient Improvement in visual function not achieved within 90 days following cataract surgery Not Eligible patient care limited to postoperative management ONLY Physician Quality Reporting Measure # 304 : Cataracts: Patient Satisfaction within 90 Days Following Cataract Surgery Measure Note: Surveys must be administered by third party and sent to Covisint for review Percentage of patients aged 18 years and older in sample who had cataract surgery and were satisfied with their care within 90 days following the cataract surgery, based on completion of the Consumer Assessment of Healthcare Providers and Systems Surgical Care Survey group specifications for important definitions related to this measure Satisfaction with care achieved within 90 days following cataract surgery Patient care survey was not completed by patient Satisfaction with care not achieved within 90 days following cataract surgery Not Eligible patient care limited to postoperative management ONLY
5 Patient Name: Page 5 of 5
2016 Physician Quality Reporting System Data Collection Form: Parkinson s Disease (for patients aged 18 and older)
 2016 Physician Quality Reporting System Data Collection Form: Parkinson s Disease (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered
2016 Physician Quality Reporting System Data Collection Form: Parkinson s Disease (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered
2016 Physician Quality Reporting System Data Collection Form: Chronic Obstructive Pulmonary Disease (COPD) (for patients aged 18 and older)
 2016 Physician Quality Reporting System Data Collection Form: Chronic Obstructive Pulmonary Disease (COPD) (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse
2016 Physician Quality Reporting System Data Collection Form: Chronic Obstructive Pulmonary Disease (COPD) (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse
2016 Physician Quality Reporting System Data Collection Form: Total Knee Replacement
 2016 Physician Quality Reporting System Data Collection Form: Total Knee Replacement IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered satisfactory reporting.
2016 Physician Quality Reporting System Data Collection Form: Total Knee Replacement IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered satisfactory reporting.
2016 Physician Quality Reporting System Data Collection Form: Coronary Artery Bypass Graft (CABG) (for patients aged 18 years and older)
 2016 Physician Quality Reporting System Data Collection Form: Coronary Artery Bypass Graft (CABG) (for patients aged 18 years and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse
2016 Physician Quality Reporting System Data Collection Form: Coronary Artery Bypass Graft (CABG) (for patients aged 18 years and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse
2014 Physician Quality Reporting System Data Collection Form: Asthma (for patients aged 5-64)
 2014 Physician Quality Reporting System Data Collection Form: Asthma (for patients aged 5-64) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy Gender: M F Patient Insured - Traditional
2014 Physician Quality Reporting System Data Collection Form: Asthma (for patients aged 5-64) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy Gender: M F Patient Insured - Traditional
CATARACTS MEASURES GROUP OVERVIEW
 2016 PQRS OPTIONS FOR MEASURES GROUPS: CATARACTS MEASURES GROUP OVERVIEW 2016 PQRS MEASURES IN CATARACTS MEASURES GROUP: #130 Documentation of Current Medications in the Medical Record #191 Cataracts:
2016 PQRS OPTIONS FOR MEASURES GROUPS: CATARACTS MEASURES GROUP OVERVIEW 2016 PQRS MEASURES IN CATARACTS MEASURES GROUP: #130 Documentation of Current Medications in the Medical Record #191 Cataracts:
2016 Physician Quality Reporting System Data Collection Form: Sleep Apnea (for patients aged 18 and older)
 2016 Physician Quality Reporting System Data Collection Form: Sleep Apnea (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered
2016 Physician Quality Reporting System Data Collection Form: Sleep Apnea (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered
2016 Physician Quality Reporting System Data Collection Form: Coronary Artery Disease (CAD) (for patients aged 18 and older)
 2016 Physician Quality Reporting System Data Collection Form: Coronary Artery Disease (CAD) (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures)
2016 Physician Quality Reporting System Data Collection Form: Coronary Artery Disease (CAD) (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures)
Measure #192: Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures
 Measure #192: Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #192: Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
 Measure #192 (NQF 0564): Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures National Quality Strategy Domain: Patient Safety 2016 PQRS OPTIONS FOR
Measure #192 (NQF 0564): Cataracts: Complications within 30 Days Following Cataract Surgery Requiring Additional Surgical Procedures National Quality Strategy Domain: Patient Safety 2016 PQRS OPTIONS FOR
2015 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients aged 18 and older)
 2015 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures)
2015 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures)
2015 Physician Quality Reporting System Data Collection Form: Oncology (for patients aged 18 and older)
 2015 Physician Quality Reporting System Data Collection Form: Oncology (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered satisfactory
2015 Physician Quality Reporting System Data Collection Form: Oncology (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered satisfactory
2014 Physician Quality Reporting System Data Collection Form: Oncology (for patients aged 18 and older)
 2014 Physician Quality Reporting System Data Collection Form: Oncology (for patients aged 18 and older) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy Gender: M F Medical Record
2014 Physician Quality Reporting System Data Collection Form: Oncology (for patients aged 18 and older) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy Gender: M F Medical Record
Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery
 Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
The CSAC will review recommendations from the Eye Care and Ear, Nose and Throat (EENT) Conditions project at its October 13 conference call.
 TO: FR: Consensus Standards Approval Committee (CSAC) EENT Project Team RE: Eye Care and Ear, Nose and Throat Conditions Member Voting Results DA: October 13, 2015 The CSAC will review recommendations
TO: FR: Consensus Standards Approval Committee (CSAC) EENT Project Team RE: Eye Care and Ear, Nose and Throat Conditions Member Voting Results DA: October 13, 2015 The CSAC will review recommendations
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Outcome High Priority
 Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management
Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Management
Patient sample criteria for the Preventive Care Measure Group are patients aged 50 years and older with a specific patient encounter:
 2016 Physician Quality Reporting System Data Collection Form: Preventive Care (for patients aged 50 and older) NOTE: Individual measures may have more restrictive age and gender requirements. IMPORTANT:
2016 Physician Quality Reporting System Data Collection Form: Preventive Care (for patients aged 50 and older) NOTE: Individual measures may have more restrictive age and gender requirements. IMPORTANT:
2014 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients aged 18 and older)
 2014 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients aged 18 and older) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy
2014 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients aged 18 and older) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES:
Quality ID #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES:
2016 Physician Quality Reporting System Data Collection Form: Multiple Chronic Conditions (for patients aged 66 and older)
 2016 Physician Quality Reporting System Data Collection Form: Multiple Chronic Conditions (for patients aged 66 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures)
2016 Physician Quality Reporting System Data Collection Form: Multiple Chronic Conditions (for patients aged 66 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures)
2016 Physician Quality Reporting System Data Collection Form: Sinusitis (for patients aged 18 and older)
 2016 Physician Quality Reporting System Data Collection Form: Sinusitis (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered
2016 Physician Quality Reporting System Data Collection Form: Sinusitis (for patients aged 18 and older) IMPORTANT: Any measure with a 0% performance rate (100% for inverse measures) is not considered
Eye Care and Ear, Nose, and Throat Conditions,
 Eye Care and Ear, Nose, and Throat Conditions, 2014-2016 FINAL TECHNICAL REPORT February 29, 2016 This report is funded by the Department of Health and Human Services under contract HHSM-500-2012-00009I
Eye Care and Ear, Nose, and Throat Conditions, 2014-2016 FINAL TECHNICAL REPORT February 29, 2016 This report is funded by the Department of Health and Human Services under contract HHSM-500-2012-00009I
2016 PQRS Dementia Measures Group
 Measures #47: Care Plan #134: Preventive Care and Screening: Screening for Clinical Depression and Follow-Up Plan #280: : Staging of #281: : Cognitive Assessment #282 : Functional Status Assessment #283
Measures #47: Care Plan #134: Preventive Care and Screening: Screening for Clinical Depression and Follow-Up Plan #280: : Staging of #281: : Cognitive Assessment #282 : Functional Status Assessment #283
Eye Care and Ear, Nose and Throat Conditions
 Eye Care and Ear, Nose and Throat Conditions DRAFT REPORT FOR COMMENT July 10, 2015 This report is funded by the Department of Health and Human Services under contract HHSM-500-2012-00009I Task Order HHSM-500-T0008
Eye Care and Ear, Nose and Throat Conditions DRAFT REPORT FOR COMMENT July 10, 2015 This report is funded by the Department of Health and Human Services under contract HHSM-500-2012-00009I Task Order HHSM-500-T0008
Tobacco Use: Screening & Cessation Intervention
 Tobacco Use: Screening and Cessation Intervention MSSP ACO Measure Tobacco Use: Screening & Cessation Intervention Domain: Preventive Care and Screening ACO 17 PREV- 10 PQRS - 226 NQF 0028 Measure Steward:
Tobacco Use: Screening and Cessation Intervention MSSP ACO Measure Tobacco Use: Screening & Cessation Intervention Domain: Preventive Care and Screening ACO 17 PREV- 10 PQRS - 226 NQF 0028 Measure Steward:
2017 Data Collection Form: Orthopedics Advanced
 2017 Data Collection Form: Orthopedics Advanced Physician Name: The following Quality measures are included in this ADVANCED specialty set: o 21 Perioperative - Selection of Prophylactic Antibiotic o 23
2017 Data Collection Form: Orthopedics Advanced Physician Name: The following Quality measures are included in this ADVANCED specialty set: o 21 Perioperative - Selection of Prophylactic Antibiotic o 23
Note: This is an outcome measure and can be calculated solely using registry data.
 Measure #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery -- National Quality Strategy Domain: Effective Clinical Care DESCRIPTION: Percentage of patients
Measure #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery -- National Quality Strategy Domain: Effective Clinical Care DESCRIPTION: Percentage of patients
revolutionehr.com 2019 Clinical Quality Measure Scoring Guide
 revolutionehr.com 2019 Clinical Quality Measure Scoring Guide Clinical quality measures, or CQMs, are statistics that seek to quantify the quality of services performed by health care providers. These
revolutionehr.com 2019 Clinical Quality Measure Scoring Guide Clinical quality measures, or CQMs, are statistics that seek to quantify the quality of services performed by health care providers. These
Optometric Cataract Refined Referral
 Optometric Cataract Refined Referral Guidance Notes for Optometrists Version Control: v1: April 2013 v2: August 2015 REFINED CATARACT REFERRAL PATHWAY GUIDANCE FOR OPTOMETRISTS Background Approximately
Optometric Cataract Refined Referral Guidance Notes for Optometrists Version Control: v1: April 2013 v2: August 2015 REFINED CATARACT REFERRAL PATHWAY GUIDANCE FOR OPTOMETRISTS Background Approximately
2017 Clinical Quality Measures
 2017 Clinical Quality Measures Clinical quality measures, or CQMs, are statistics that seek to quantify the quality of services performed by health care providers. These statistics involve data related
2017 Clinical Quality Measures Clinical quality measures, or CQMs, are statistics that seek to quantify the quality of services performed by health care providers. These statistics involve data related
Note: This is an outcome measure and will be calculated solely using registry data.
 Measure #303 (NQF 1536): Cataracts: Improvement in Patient s Visual Function within 90 Days Following Cataract Surgery National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes
Measure #303 (NQF 1536): Cataracts: Improvement in Patient s Visual Function within 90 Days Following Cataract Surgery National Quality Strategy Domain: Person and Caregiver-Centered Experience and Outcomes
2016 PQRS Diabetes Measures Group
 Measures #1 : Hemoglobin A1c Poor Control #110 Preventive Care and Screening: Influenza Immunization #117 : Eye Exam #119 : Medical Attention for Nephropathy #126 Mellitus: Diabetic Foot and Ankle Care,
Measures #1 : Hemoglobin A1c Poor Control #110 Preventive Care and Screening: Influenza Immunization #117 : Eye Exam #119 : Medical Attention for Nephropathy #126 Mellitus: Diabetic Foot and Ankle Care,
Informed Consent For Cataract Surgery. And/Or Implantation of an Intraocular Lens INTRODUCTION
 Informed Consent For Cataract Surgery And/Or Implantation of an Intraocular Lens INTRODUCTION This information is given to you so that you can make an informed decision about having eye surgery. Take as
Informed Consent For Cataract Surgery And/Or Implantation of an Intraocular Lens INTRODUCTION This information is given to you so that you can make an informed decision about having eye surgery. Take as
2013 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients 18 and older)
 2013 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients 18 and older) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy Gender:
2013 Physician Quality Reporting System Data Collection Form: Inflammatory Bowel Disease (IBD) (for patients 18 and older) Physician Name: Patient Name: Last First MI Date of Birth: / / mm dd yyyy Gender:
Incremental cost-effectiveness of initial cataract surgery Busbee B G, Brown M M, Brown G C, Sharma S
 Incremental cost-effectiveness of initial cataract surgery Busbee B G, Brown M M, Brown G C, Sharma S Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion
Incremental cost-effectiveness of initial cataract surgery Busbee B G, Brown M M, Brown G C, Sharma S Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion
Consent for Cataract Surgery
 Consent for Cataract Surgery Introduction This information is given to you so that you can make an informed decision about having an eye operation, before you sign the INFORMED CONSENT DOCUMENT. You have
Consent for Cataract Surgery Introduction This information is given to you so that you can make an informed decision about having an eye operation, before you sign the INFORMED CONSENT DOCUMENT. You have
Cataract and Refractive Surgery Co-Management Policy and Procedure Manual
 Cataract and Refractive Surgery Co-Management Policy and Procedure Manual Michael R. George, M.D. Chief Surgeon and Medical Director Tylock-George Eye Care Index of Cataract and Refractive Surgery Manual
Cataract and Refractive Surgery Co-Management Policy and Procedure Manual Michael R. George, M.D. Chief Surgeon and Medical Director Tylock-George Eye Care Index of Cataract and Refractive Surgery Manual
VISIONCARE S IMPLANTABLE MINIATURE TELESCOPE (by Dr. Isaac Lipshitz)
 PATIENT INFORMATION BOOKLET PAGE 1 OF 32 VISIONCARE S IMPLANTABLE MINIATURE TELESCOPE (by Dr. Isaac Lipshitz) AN INTRAOCULAR TELESCOPE FOR TREATING SEVERE TO PROFOUND VISION IMPAIRMENT DUE TO BILATERAL
PATIENT INFORMATION BOOKLET PAGE 1 OF 32 VISIONCARE S IMPLANTABLE MINIATURE TELESCOPE (by Dr. Isaac Lipshitz) AN INTRAOCULAR TELESCOPE FOR TREATING SEVERE TO PROFOUND VISION IMPAIRMENT DUE TO BILATERAL
Progressive Symptomatic Retinal Detachment Complicating Retinoschisis. Initial Reporting Questionnaire
 Progressive Symptomatic Retinal Detachment Complicating Retinoschisis In association with the British Ophthalmological Surveillance Unit Ethics ref: 13/NW/0037 Initial Reporting Questionnaire Case Definition:
Progressive Symptomatic Retinal Detachment Complicating Retinoschisis In association with the British Ophthalmological Surveillance Unit Ethics ref: 13/NW/0037 Initial Reporting Questionnaire Case Definition:
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
2014 Oncology Measures Group Overview
 2014 Oncology Measures Group Overview The Oncology Measures Group is a reporting option that significantly reduces the burden of participation in the Physician Quality Reporting System (PQRS). Source:
2014 Oncology Measures Group Overview The Oncology Measures Group is a reporting option that significantly reduces the burden of participation in the Physician Quality Reporting System (PQRS). Source:
SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT
 SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT PROTOCOL This document sets out the details of the administrative protocol for the direct referral by Optometrists/OMPs of cataract patients.
SWINDON PCT CATARACT DIRECT REFERRAL SCHEME SERVICE LEVEL AGREEMENT PROTOCOL This document sets out the details of the administrative protocol for the direct referral by Optometrists/OMPs of cataract patients.
2016 PQRS. Rheumatiod Arthritis Me asures Group. Measures. Reporting Instructions
 Measures #108 Rheumatoid Arthritis (RA): Disease Modifying Anti-Rheumatic Drug (DMARD) Therapy #128 Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow-Up Plan #131 Pain Assessment
Measures #108 Rheumatoid Arthritis (RA): Disease Modifying Anti-Rheumatic Drug (DMARD) Therapy #128 Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow-Up Plan #131 Pain Assessment
84 Year Old with Rosacea
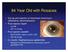 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
DIRECT REFERRAL OF CATARACT PATIENTS COMMUNITY OPTOMETRIST PROTOCOL AND GUIDELINES
 DIRECT REFERRAL OF CATARACT PATIENTS COMMUNITY OPTOMETRIST PROTOCOL AND GUIDELINES October 2013 Preoperative assessment History Symptoms/social history: is the patient visually affected and do they want
DIRECT REFERRAL OF CATARACT PATIENTS COMMUNITY OPTOMETRIST PROTOCOL AND GUIDELINES October 2013 Preoperative assessment History Symptoms/social history: is the patient visually affected and do they want
2016 PQRS Recommended Measures for: Ophthalmology
 Measures Groups Choose 1 Measures Group Report on a minimum of 20 eligible patients (at least 11 must be Medicare Part B FFS patients) #130: Documentation of Current Medications in the Medical Record #226:
Measures Groups Choose 1 Measures Group Report on a minimum of 20 eligible patients (at least 11 must be Medicare Part B FFS patients) #130: Documentation of Current Medications in the Medical Record #226:
PREAMBLE TO MSC PAYMENT SCHEDULE: OPTOMETRY SERVICES
 PREAMBLE TO MSC PAYMENT SCHEDULE: OPTOMETRY SERVICES A. GENERAL PROVISIONS 1. Eye Examination Benefits Optometric benefits are services defined in Section 23 of the Medical and Health Care Services Regulations,
PREAMBLE TO MSC PAYMENT SCHEDULE: OPTOMETRY SERVICES A. GENERAL PROVISIONS 1. Eye Examination Benefits Optometric benefits are services defined in Section 23 of the Medical and Health Care Services Regulations,
Cost-utility analysis of cataract surgery in the second eye Busbee B G, Brown M M, Brown G C, Sharma S
 Cost-utility analysis of cataract surgery in the second eye Busbee B G, Brown M M, Brown G C, Sharma S Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion
Cost-utility analysis of cataract surgery in the second eye Busbee B G, Brown M M, Brown G C, Sharma S Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion
The risks and benefits of cataract surgery
 The risks and benefits of cataract surgery Other formats If you need this information in another format such as audio tape or computer disk, Braille, high contrast, British Sign Language or translated
The risks and benefits of cataract surgery Other formats If you need this information in another format such as audio tape or computer disk, Braille, high contrast, British Sign Language or translated
Welcome to Saratoga Ophthalmology!
 Amjad M. Hammad, MD, MBA Salman J. Yousuf, DO The Center for Vitreo-Retinal Surgery Charles H. Rheeman, MD Gregory B. Krohel, MD The Center for Oculoplastics & Neuro-Ophthalmology Kamran I. Chaudhri, MD
Amjad M. Hammad, MD, MBA Salman J. Yousuf, DO The Center for Vitreo-Retinal Surgery Charles H. Rheeman, MD Gregory B. Krohel, MD The Center for Oculoplastics & Neuro-Ophthalmology Kamran I. Chaudhri, MD
TRANSITION OF CARE APPLICATION
 Dear Member: Thank you for enrolling in MedStar Medicare Choice. You may currently be receiving services from healthcare providers that are not part of the MedStar Medicare Choice Provider Network. An
Dear Member: Thank you for enrolling in MedStar Medicare Choice. You may currently be receiving services from healthcare providers that are not part of the MedStar Medicare Choice Provider Network. An
ASCRS completes fourth annual Clinical Survey
 ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
TECHNICAL SPECIFICATIONS FOR KEY PERFORMANCE INDICATORS (KPI) CLINICAL SERVICES MEDICAL PROGRAMME 2017 OPHTHALMOLOGY
 OPHTHALMOLOGY TYPE NO SUBSPECIALTY INDICATOR DIMENSION STANDARD D 1 - D 2 - D 3 - I 4 General I 5 General I 6 General/ Public Health I 7 Surgical Retina I 8 Medical Retina I 9 Cornea I 10 Glaucoma I 11
OPHTHALMOLOGY TYPE NO SUBSPECIALTY INDICATOR DIMENSION STANDARD D 1 - D 2 - D 3 - I 4 General I 5 General I 6 General/ Public Health I 7 Surgical Retina I 8 Medical Retina I 9 Cornea I 10 Glaucoma I 11
2018 MIPS Quality Category Measures and Benchmarks for Ophthalmology
 2018 MIPS Quality Category Measures and Benchmarks for Ophthalmology Physicians must report on 60% of all patients, if reporting via registry or EHR, and 60% of all Medicare Part B patients if reporting
2018 MIPS Quality Category Measures and Benchmarks for Ophthalmology Physicians must report on 60% of all patients, if reporting via registry or EHR, and 60% of all Medicare Part B patients if reporting
Child s Name Birth Date / / Age. Mother's Name. Father's Name. Phone: Home Cell. Address. Address Number & Street City State Zip
 Welcome! Thank you for choosing our practice for your health needs. Your first visit to our center is an opportunity for us to learn all about you. If you have any questions or concerns, do not hesitate
Welcome! Thank you for choosing our practice for your health needs. Your first visit to our center is an opportunity for us to learn all about you. If you have any questions or concerns, do not hesitate
2017 MIPS Quality Category Measures and Benchmarks for Ophthalmology
 2017 MIPS Quality Category Measures and Benchmarks for Ophthalmology Physicians must report on 50% of all patients, if reporting via registry or EHR, and 50% of all Medicare Part B patients if reporting
2017 MIPS Quality Category Measures and Benchmarks for Ophthalmology Physicians must report on 50% of all patients, if reporting via registry or EHR, and 50% of all Medicare Part B patients if reporting
Clinical Evaluation of the BunnyLens IOL
 Clinical Evaluation of the BunnyLens IOL Introduction: BunnyLens is a foldable Hydrophlic Acrylic IOL with four ear shaped haptic design. The lens design offers many advantages in terms of: 1. Centration
Clinical Evaluation of the BunnyLens IOL Introduction: BunnyLens is a foldable Hydrophlic Acrylic IOL with four ear shaped haptic design. The lens design offers many advantages in terms of: 1. Centration
NQF. National Voluntary Consensus Standards for Ambulatory Care Additional Eye Care and Melanoma Performance Measures EYE CARE AND MELANOMA
 NQF NATIONAL QUALITY FORUM EYE CARE AND MELANOMA National Voluntary Consensus Standards for Ambulatory Care Additional Eye Care and Melanoma Performance Measures A CONSENSUS REPORT National Voluntary
NQF NATIONAL QUALITY FORUM EYE CARE AND MELANOMA National Voluntary Consensus Standards for Ambulatory Care Additional Eye Care and Melanoma Performance Measures A CONSENSUS REPORT National Voluntary
FIRST NAME MI LAST NAME BIRTH DATE (MM/DD/YYYY) GENDER. Name of person previously tested and relationship:
 REQUEST FOR GERMLINE BAP1TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
REQUEST FOR GERMLINE BAP1TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
American College of Physicians Genesis Registry
 Powered by Premier American College of Physicians Genesis Registry This registry has been approved by CMS as a Qualified Clinical Data Registry (QCDR) for Eligible Clinicians and group practices for the
Powered by Premier American College of Physicians Genesis Registry This registry has been approved by CMS as a Qualified Clinical Data Registry (QCDR) for Eligible Clinicians and group practices for the
Cataract Enhanced Scheme (CES):
 Cataract Enhanced Scheme (CES): Direct access cataract referral service for Optometrists Re-launch Event: Wed 4 Oct 17 Akil Kanani BSc (Hons) MCOptom Prof Cert Glauc Lead Optometrist for CES Introduction
Cataract Enhanced Scheme (CES): Direct access cataract referral service for Optometrists Re-launch Event: Wed 4 Oct 17 Akil Kanani BSc (Hons) MCOptom Prof Cert Glauc Lead Optometrist for CES Introduction
Posterior Capsular Rupture A Practical Guide To Prevention And Management
 Posterior Capsular Rupture A Practical Guide To Prevention And Management We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing
Posterior Capsular Rupture A Practical Guide To Prevention And Management We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing
ADDITIONAL INFORMATION REGARDING EP CLINICAL QUALITY MEASURES FOR 2014 EHR INCENTIVE PROGRAMS
 ADDITIONAL INFORMATION REGARDING EP CLINICAL QUALITY MEASURES FOR 2014 EHR INCENTIVE PROGRAMS The table below entitled Clinical s for 2014 CMS EHR Incentive Programs for Eligible Professionals contains
ADDITIONAL INFORMATION REGARDING EP CLINICAL QUALITY MEASURES FOR 2014 EHR INCENTIVE PROGRAMS The table below entitled Clinical s for 2014 CMS EHR Incentive Programs for Eligible Professionals contains
Information for Patients. Vitrectomy
 Manchester Royal Eye Hospital Vitreoretinal Services Information for Patients Vitrectomy Your eye doctor has advised you that you require vitrectomy surgery. This leaflet gives you information that will
Manchester Royal Eye Hospital Vitreoretinal Services Information for Patients Vitrectomy Your eye doctor has advised you that you require vitrectomy surgery. This leaflet gives you information that will
2016 PQRS Inflammatory Bowel Disease (IBD) Measures Group
 Measures #110 Preventive Care and Screening: Influenza Immunization #111 Pneumonia Vaccination Status for Older Adults #226 Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention
Measures #110 Preventive Care and Screening: Influenza Immunization #111 Pneumonia Vaccination Status for Older Adults #226 Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #14 (NQF 0087): Age-Related Macular Degeneration (AMD): Dilated Macular Examination National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Quality ID #14 (NQF 0087): Age-Related Macular Degeneration (AMD): Dilated Macular Examination National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Current Quality Measurement Priorities and Challenges in the Medical Environment
 Convened by the American Medical Association The Physician Consortium for Performance Improvement Current Quality Measurement Priorities and Challenges in the Medical Environment Mark S. Antman, DDS, MBA
Convened by the American Medical Association The Physician Consortium for Performance Improvement Current Quality Measurement Priorities and Challenges in the Medical Environment Mark S. Antman, DDS, MBA
04/11/2014. Retina Coding and Reimbursement 101. Financial Disclosure. Chief Complaint
 Retina Coding and Reimbursement 101 William T. Koch, COA, COE, CPC Administrative Director Director of Billing Operations The Retina Institute St. Louis, Missouri Advisory Boards Allergan Genentech Regeneron
Retina Coding and Reimbursement 101 William T. Koch, COA, COE, CPC Administrative Director Director of Billing Operations The Retina Institute St. Louis, Missouri Advisory Boards Allergan Genentech Regeneron
MARCH Vision Care. Kansas Specific Information. Table of Contents
 Kansas Specific Information This document contains information specific to the State of Kansas. Please refer to the Provider Reference Guide for general information regarding plan administration. Table
Kansas Specific Information This document contains information specific to the State of Kansas. Please refer to the Provider Reference Guide for general information regarding plan administration. Table
American College of Physicians Genesis Registry
 Powered by Premier American College of Physicians Genesis Registry This registry has been approved by CMS as a Qualified Clinical Data Registry (QCDR) for Eligible Clinicians and group practices for the
Powered by Premier American College of Physicians Genesis Registry This registry has been approved by CMS as a Qualified Clinical Data Registry (QCDR) for Eligible Clinicians and group practices for the
Pediatric cataract. Nikos Kozeis MD, PhD, FICO, FEBO, MRCOphth. Surgical challenges and postoperative complications
 Pediatric cataract Surgical challenges and postoperative complications Nikos Kozeis MD, PhD, FICO, FEBO, MRCOphth Consultant Paediatric Ophthalmologist Thessaloniki, Greece Pediatric Cataract 2.4 / 10000
Pediatric cataract Surgical challenges and postoperative complications Nikos Kozeis MD, PhD, FICO, FEBO, MRCOphth Consultant Paediatric Ophthalmologist Thessaloniki, Greece Pediatric Cataract 2.4 / 10000
Cataract. A cataract is a clouding of the lens in your eye. It
 Cataract A cataract is a clouding of the lens in your eye. It affects your vision. Cataracts are very common in older people. By age 80, more than half of all Americans either have a cataract or have had
Cataract A cataract is a clouding of the lens in your eye. It affects your vision. Cataracts are very common in older people. By age 80, more than half of all Americans either have a cataract or have had
INFORMED CONSENT FOR CATARACT SURGERY
 DESERT OPHTHALMOLOGY 1180 N Indian Canyon Drive W100 Palm Springs CA 92262 35900 Bob Hope Drive Suite 205 Rancho Mirage CA 92270 Phone (760) 320-8497 Fax (760) 320-5444 INFORMED CONSENT FOR CATARACT SURGERY
DESERT OPHTHALMOLOGY 1180 N Indian Canyon Drive W100 Palm Springs CA 92262 35900 Bob Hope Drive Suite 205 Rancho Mirage CA 92270 Phone (760) 320-8497 Fax (760) 320-5444 INFORMED CONSENT FOR CATARACT SURGERY
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
CORONARY ARTERY DISEASE (CAD) MEASURES GROUP OVERVIEW
 CONARY ARTERY DISEASE (CAD) MEASURES GROUP OVERVIEW 2014 PQRS OPTIONS F MEASURES GROUPS: 2014 PQRS MEASURES IN CONARY ARTERY DISEASE (CAD) MEASURES GROUP: #6. Coronary Artery Disease (CAD): Antiplatelet
CONARY ARTERY DISEASE (CAD) MEASURES GROUP OVERVIEW 2014 PQRS OPTIONS F MEASURES GROUPS: 2014 PQRS MEASURES IN CONARY ARTERY DISEASE (CAD) MEASURES GROUP: #6. Coronary Artery Disease (CAD): Antiplatelet
Eylea (aflibercept) Document Number: IC-0026
 Eylea (aflibercept) Document Number: IC-0026 Last Review Date: 3/1/2018 Date of Origin: 02/07/2013 Dates Reviewed: 03/07/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015,
Eylea (aflibercept) Document Number: IC-0026 Last Review Date: 3/1/2018 Date of Origin: 02/07/2013 Dates Reviewed: 03/07/2013, 06/2013, 09/2013, 12/2013, 03/2014, 06/2014, 09/2014, 12/2014, 03/2015, 04/2015,
Technicians & Nurses Program
 ASCRS ASOA Symposium & Congress Technicians & Nurses Program May 6-10, 2016 New Orleans ICD-10 Implementation: Tips and Pearls for Technicians Financial Disclosure The instructor acknowledges a financial
ASCRS ASOA Symposium & Congress Technicians & Nurses Program May 6-10, 2016 New Orleans ICD-10 Implementation: Tips and Pearls for Technicians Financial Disclosure The instructor acknowledges a financial
Public Health and Eye Care
 Public Health and Eye Care Rohit Varma, MD, MPH Professor and Chair USC Department of Ophthalmology Director, USC Eye Institute Associate Dean, Keck School of Medicine of USC Los Angeles, CA 1 Prevalence
Public Health and Eye Care Rohit Varma, MD, MPH Professor and Chair USC Department of Ophthalmology Director, USC Eye Institute Associate Dean, Keck School of Medicine of USC Los Angeles, CA 1 Prevalence
04/06/2015. Documentation Do s and Don ts In The Retina Practice. Financial Disclosure. Documentation Dos and Don ts
 Documentation Do s and Don ts In The Retina Practice William T. Koch, COA, COE, CPC Administrative Director Director of Billing Operations The Retina Institute St. Louis, Missouri Advisory Boards Allergan
Documentation Do s and Don ts In The Retina Practice William T. Koch, COA, COE, CPC Administrative Director Director of Billing Operations The Retina Institute St. Louis, Missouri Advisory Boards Allergan
Practical Care of the Cataract Patient with Retinal Disease
 Practical Care of the Cataract Patient with Retinal Disease Brooks R. Alldredge, OD, FAAO Kelly L. Cyr, OD, FAAO The Retina Center Eye Associates of New Mexico 4411 The 25 Way NE, Suite 325 Albuquerque,
Practical Care of the Cataract Patient with Retinal Disease Brooks R. Alldredge, OD, FAAO Kelly L. Cyr, OD, FAAO The Retina Center Eye Associates of New Mexico 4411 The 25 Way NE, Suite 325 Albuquerque,
George M. Salib, M.D., Inc.
 George M. Salib, M.D., Inc. Patient Acknowledgement Regarding Precautions Following Dilation It may be necessary to dilate your eyes during your eye examination or treatment. Dilation results in light
George M. Salib, M.D., Inc. Patient Acknowledgement Regarding Precautions Following Dilation It may be necessary to dilate your eyes during your eye examination or treatment. Dilation results in light
LOCSU Community Services
 LOCSU Community Services Pre- and Post-Operative Cataract Community Service Pathway Issued by Local Optical Committee Support Unit December 2008 [Revised 14 March 2016, Version 3.2] Contents Page Outline
LOCSU Community Services Pre- and Post-Operative Cataract Community Service Pathway Issued by Local Optical Committee Support Unit December 2008 [Revised 14 March 2016, Version 3.2] Contents Page Outline
Clinical Policy: Implantable Miniature Telescope for Age Related Macular Degeneration Reference Number: CP.MP.517
 Clinical Policy: Implantable Miniature Telescope for Age Related Macular Reference Number: CP.MP.517 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Clinical Policy: Implantable Miniature Telescope for Age Related Macular Reference Number: CP.MP.517 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Note: This is an outcome measure and will be calculated solely using registry data.
 Quality ID #303 (NQF 1536): Cataracts: Improvement in Patient s Visual Function within 90 Days Following Cataract Surgery National Quality Strategy Domain: Person and Caregiver-Centered Experience and
Quality ID #303 (NQF 1536): Cataracts: Improvement in Patient s Visual Function within 90 Days Following Cataract Surgery National Quality Strategy Domain: Person and Caregiver-Centered Experience and
Advanced Eyecare of Orange County/ Kim T. Doan, M.D.
 Patient Information Sheet: Cataract Surgery And/Or Implantation of an Intraocular Lens This information is given to you so that you can prepare for the discussion with your eye surgeon. This document will
Patient Information Sheet: Cataract Surgery And/Or Implantation of an Intraocular Lens This information is given to you so that you can prepare for the discussion with your eye surgeon. This document will
Humber. Cataract Surgery Commissioning Policy
 Intervention Elective Eye Surgery for the treatment of Cataracts in adults OPCS codes C62 Incision of iris C621 Iridosclerotomy C622 Surgical iridotomy C623 Laser iridotomy C624 Correction iridodialysis
Intervention Elective Eye Surgery for the treatment of Cataracts in adults OPCS codes C62 Incision of iris C621 Iridosclerotomy C622 Surgical iridotomy C623 Laser iridotomy C624 Correction iridodialysis
NOA 3rd Party Newsletter PQRS EDITION - Page 1 CONTENTS. Traffic Sheet P.3. Flowsheet & Detailed Directions P.11.
 NOA 3rd Party Newsletter - 2016 PQRS EDITION - Page 1 CONTENTS EYE MEASURES Measure #12 :Primary Open-Angle Glaucoma: Optic Nerve Evaluation Traffic Sheet P.2. Flowsheet & Detailed Directions P.8. Measure
NOA 3rd Party Newsletter - 2016 PQRS EDITION - Page 1 CONTENTS EYE MEASURES Measure #12 :Primary Open-Angle Glaucoma: Optic Nerve Evaluation Traffic Sheet P.2. Flowsheet & Detailed Directions P.8. Measure
The Visian ICL Advantages
 The Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features found with the Visian ICL. These include:
The Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features found with the Visian ICL. These include:
Information about cases being considered by the Case Examiners
 Information about cases being considered by the Case Examiners 13 October 2016 1 Contents Purpose... 3 What should I do next?... 3 Background... 4 Criteria that Case Examiners will consider... 5 Closing
Information about cases being considered by the Case Examiners 13 October 2016 1 Contents Purpose... 3 What should I do next?... 3 Background... 4 Criteria that Case Examiners will consider... 5 Closing
ASCRS launches new Annual Clinical Survey
 ASCRS launches new Annual Clinical Survey Survey of more than 1,000 members measures current clinical opinions and practice patterns Survey overview The American Society of Cataract & Refractive Surgery
ASCRS launches new Annual Clinical Survey Survey of more than 1,000 members measures current clinical opinions and practice patterns Survey overview The American Society of Cataract & Refractive Surgery
Cataracts in adults: management
 Cataracts in adults: management NICE guideline: short version Draft for consultation, May 0 This guideline covers managing cataracts in adults aged and over. It aims to improve care before, during and
Cataracts in adults: management NICE guideline: short version Draft for consultation, May 0 This guideline covers managing cataracts in adults aged and over. It aims to improve care before, during and
CATARACT & LENS SURGERY CATARACT SURGERY
 GENERAL INFORMATION CATARACT & LENS SURGERY CATARACT SURGERY WHAT IS A CATARACT? A cataract is not a growth, but rather a clouding of the normally transparent and flexible lens of the eye. This condition
GENERAL INFORMATION CATARACT & LENS SURGERY CATARACT SURGERY WHAT IS A CATARACT? A cataract is not a growth, but rather a clouding of the normally transparent and flexible lens of the eye. This condition
NICE guideline Published: 26 October 2017 nice.org.uk/guidance/ng77
 Cataracts acts in adults: management NICE guideline Published: 26 October 2017 nice.org.uk/guidance/ng77 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Cataracts acts in adults: management NICE guideline Published: 26 October 2017 nice.org.uk/guidance/ng77 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Sample page. Ophthalmology A comprehensive illustrated guide to coding and reimbursement CODING COMPANION
 CODING COMPANION 2018 Ophthalmology A comprehensive illustrated guide to coding and reimbursement POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com.
CODING COMPANION 2018 Ophthalmology A comprehensive illustrated guide to coding and reimbursement POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com.
Cataract Surgery: What You Must Know Before Having It Done
 DAVID D. RICHARDSON, MD, INC. DAVID RICHARDSON, M.D. SAN MARINO EYE 2020 Huntington Drive San Marino, CA 91108 Telephone: (626) 289-7856 Fax: (626) 284-6532 Cataract Surgery: What You Must Know Before
DAVID D. RICHARDSON, MD, INC. DAVID RICHARDSON, M.D. SAN MARINO EYE 2020 Huntington Drive San Marino, CA 91108 Telephone: (626) 289-7856 Fax: (626) 284-6532 Cataract Surgery: What You Must Know Before
Coding Guidance for HIV Clinical Practices: Diagnosis Coding for HIV Patients
 Coding Guidance for HIV Clinical Practices: Diagnosis Coding for HIV Patients This module discusses diagnosis coding used by medical practices when treating patients with HIV. Diagnosis coding establishes
Coding Guidance for HIV Clinical Practices: Diagnosis Coding for HIV Patients This module discusses diagnosis coding used by medical practices when treating patients with HIV. Diagnosis coding establishes
ASCRS completes third annual Clinical Survey
 ASCRS completes third annual Clinical Survey More than 2,000 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education Survey Overview The third annual
ASCRS completes third annual Clinical Survey More than 2,000 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education Survey Overview The third annual
Electronic poster presentations
 Electronic poster presentations Cataract Surgery E-00002 Blue-light exposure in an animal model of uveal melanoma B.F. Fernandes, S. Di Cesare, S. Maloney, J.-C. Marshall, W. Dawson, M.N. Burnier, Jr.
Electronic poster presentations Cataract Surgery E-00002 Blue-light exposure in an animal model of uveal melanoma B.F. Fernandes, S. Di Cesare, S. Maloney, J.-C. Marshall, W. Dawson, M.N. Burnier, Jr.
