ORIGINAL ARTICLE. DD Woodhead, DK Lambert, JM Clark and RD Christensen. Intermountain Healthcare, McKay-Dee Hospital, Ogden, UT, USA
|
|
|
- Poppy Briggs
- 6 years ago
- Views:
Transcription
1 ORIGINAL ARTICLE Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial DD Woodhead, DK Lambert, JM Clark and RD Christensen Intermountain Healthcare, McKay-Dee Hospital, Ogden, UT, USA (2006) 26, r 2006 Nature Publishing Group All rights reserved /06 $30 Objective: We compared two methods of delivering high-flow gas therapy by nasal cannula, applied immediately after planned endotracheal extubations of NICU patients. Study design and methods: Thirty NICU patients who were about to be extubated from mechanical ventilation were randomized into two groups; Group 1 received Vapotherm s for the first 24 h after extubation, then standard high-flow nasal cannula for the next 24 h, and received standard high-flow therapy for the first 24 h, then Vapotherm s for the next 24 h. At 24 h after extubation and again 48 h after extubation, a neonatologist who was not aware which modality the patient had been receiving examined the nasal mucosa and applied a scoring system. A research nurse who was unaware of the modality abstracted respiratory rates and respiratory effort scores from a specific study-bedside record. The experimental design was such that a patient could fail extubation either by reintubation for mechanical ventilation, or by rescue to the opposite modality before completing the 24-h test period. Results: Fifteen patients were randomized to Group 1 and 15 to. No differences were apparent between the groups in birth weight, gestational age, age at study entry, gender or underlying pulmonary disorder. Respiratory rates were similar while on Vapotherm s (52±13 breaths/min, mean±s.d.) and high-flow (54±14/min). At 24 h after starting the modality, those on Vapotherm s had more normal examinations of the nasal mucosa (2.7±1.2 vs 7.8±1.7, P<0.0005) and lower respiratory effort scores (1.2±0.6 vs 2.0±0.9, P<0.05) than did those on high-flow. No patients failed while on Vapotherm s, but seven failed while on high-flow (two reintubations and five rescue switches to Vapotherm s, P<0.005). Conclusions: Among NICU patients immediately following extubation, Vapotherm s performed better than a standard high-flow nasal cannula in maintaining a normal appearing nasal mucosa, a lower respiratory effort score, and averting reintubation. Correspondence: Dr RD Christensen, Intermountain Healthcare, McKay-Dee Hospital, 4403 Harrison Blvd, Ogden, UT 84403, USA. rdchris4@ihc.com Received 6 February 2006; revised 3 April 2006; accepted 5 April 2006; published online 25 May 2006 (2006) 26, doi: /sj.jp ; published online 25 May 2006 Keywords: high-flow nasal cannula; endotracheal extubation; reintubation; Vapotherm s Introduction One potential use of a high-flow nasal cannula in the NICU is to reduce the need for reintubation following extubation from mechanical ventilation. 1,2 The high-flow nasal cannula system has been reported to be less invasive than nasal CPAP. 1,2 However, the high-flow cannula can dry the nasal mucosa, particularly when gas flows exceed 1 or 2 l/min. 2,3 Mucosal dryness and thick nasal secretions sometimes occur when neonates are treated with highflow nasal cannula gas delivery. Vapotherm s was devised as a method, in the opinion of the company, for humidifying and warming inhaled gas to prevent dryness of the nasal mucosa. 4 6 However, to date no prospective, randomized studies have been published directly comparing Vapotherm s with a standard highflow nasal cannula on recently extubated neonates. To obtain this comparison, we devised a randomized, prospective, masked, crossover trial. Methods Patients were deemed eligible to participate in the study if they were in the NICU with an endotracheal tube in place for mechanical ventilation, and had a physician s order to extubate to a highflow (X1 l/min) nasal cannula. They were only eligible if the attending neonatologist predicted that they were likely to need a high-flow nasal cannula for at least the next 48 h, and if the parent or responsible guardian signed the informed consent document. Patients were ineligible for study participation if they had what the attending neonatologist judged to be a lethal congenital abnormality or if they were judged by the attending neonatologist as likely to be transferred to another hospital before the study (48 h) had ended.
2 482 Vapotherm s vs high-flow nasal cannula after extubation Eligible patients were randomized before extubation, using a list constructed by a random number table, to either Group 1 or Group 2. Group 1 patients received Vapotherm s for the first 24 h after extubation and were then crossed over to a standard high-flow nasal cannula for the next 24 h. patients received standard high-flow nasal cannula treatment for the first 24 h after extubation and then were crossed over to Vapotherm s for the next 24 h. To avoid maldistribution, by chance, with a preponderance of smaller subjects in one study group, the randomization was blocked on birth weight, using three categories; <1000, and >1500 g. Thirty patients, 15 per group, were planned for this pilot study, as a convenience sample, based on the projection that about one patient per week could be studied and the desire to conduct the study within a 6-month period. Guidelines of the American Association of Respiratory Care were used to assess when a patient should be extubated from mechanical ventilation. 7,8 Specific factors considered included; acceptable blood gasses on weaning ventilator settings, adequate breath sounds, an improving chest X-ray, and anticipated stamina sufficient to not need mechanical ventilation any longer. Immediately following extubation, a nasal cannula was put in place. Depending on the size of the patient either the premature (model MA1100A), the neonate (model MN1100B), or the infant (model MI1300) cannula was used (manufactured by Vapotherm Inc., Stevensville, MD, USA). The cannula tubing was then attached to either Vapotherm s or a standard high-flow system. In each individual, the same cannula was used for the entire 48 h of study. Adjustments in the FiO 2 and gas flow were made by the respiratory therapist and/or nurse bedside in consultation with the neonatologist and in accordance with manufacture s recommendations order to provide what they interpreted as optimal individual support to that patient. At 24 h after extubation the nasal cannula was briefly and temporarily discontinued while a neonatologist who was unaware of whether the patient had been receiving high-flow or Vapotherm s performed an examination of the nasal mucosa using a speculum and otoscope. The scoring system is shown in Table 1. Before each examination of the nasal mucosa, the nasal prongs were slid out of the nares so that the nasal mucosa could be seen. When the examining neonatologist left the bedside, the respiratory therapist set up the opposite modality for delivering nasal gas flow for the next 24-h period. A second nasal examination was performed, using the same procedure, after a 24-h period on the second modality. To help keep the examining neonatologist unaware of which modality was being used, a Vapotherm s unit was always set up at the bedside of study patients (whether they were actually receiving Vapotherm s or high-flow). In this way, it was not obvious to the examiner whether the patient had been receiving Vapotherm s or high-flow. A research nurse (DKL) who was unaware of the randomization, recorded each respiratory rate and respiratory effort score from a study-specific bedside chart, on which the bedside nurse or Table 1 Scoring the nasal examinations Right nare Left nare 1 1 Completely normal 2 2 Erythematous mucosa 3 3 Erythematous and edematous mucosa 4 4 Erythema or edema and thick mucous 5 5 Occluded with thick mucous and edema and/or hemorrhagic A neonatologist that was unaware of whether the patient had been on Vapotherm s or standard high-flow for the past 24 h examined the nasal mucosa. Two such examinations were performed on each patient, one after each 24-h study period. The findings of each examination were recorded on a score sheet by circling the appropriate numbers (below). The scores were the sum of the numbers recorded for each nare, therefore, the values ranged from 2 (minimum) to 10 (maximum). respiratory therapist periodically recorded respiratory rates and a description of retractions during the two 24 h study periods. The research nurse recorded the data in an office, where she could not see which treatment devise the patient was receiving. The respiratory effort score was listed as; 0 (no retractions), 1 (mild retractions), 2 (moderate retractions) or 3 (severe retractions). Five categories of retractions were listed: supraclavicular, suprasternal, intercostal, substernal and subcostal. In that way respiratory effort scores could range from a low of 0 (no retractions in any of the five categories) to a high of 15 (three in each of the five categories). Reintubation of a study patient, for return to mechanical ventilation, occurred under the direction of the clinical care team (neonatologist, neonatal nurse practitioner, respiratory therapist and bedside nurse). The team members were aware of whether the patient was receiving Vapotherm s or standard high-flow nasal cannula. No set criteria were used for determining when a patient required reintubation for mechanical ventilation. However, considerations included a consistently rising FiO 2, deteriorating blood gases, increased respiratory effort, and increased apnea and bradycardia requiring repeated bag/mask breaths. In a similar fashion, a patient receiving one modality could be switched to the opposite modality if the clinical care team judged the patient was failing that modality, but not to the point where reintubation was needed immediately. If patients were reintubated a tracheal aspirate was obtained for Gram stain and culture. Descriptive statistics were calculated using SPSS (v 13.0) for Windows. Between group means were tested using independent samples t-tests when parametric assumptions were met, with Wilcoxon Rank-Sum tests used for non-parametric comparisons. Proportions were compared between groups using w 2 tests or, when expected counts were small, Fisher s exact test. For all tests, 2-tailed tests were used, and a was set at The study protocol was approved by the Intermountain Healthcare Institutional Review
3 483 Board, and parents of the study subjects signed informed consent documents. Results During the months of July 2005 through November 2005, the families of 35 NICU patients who were receiving mechanical ventilation were contacted to inquire whether they were interested in learning about the extubation study. Thirty-four of the 35 families gave written consent for their neonate to participate in the study, and of these, 30 were enrolled in the study. Three of the consented patients were not enrolled because at the time of endotracheal extubation the attending neonatologist decided that the patient would not be extubated to a high-flow nasal cannula, but rather to a low-flow (<0.5 l/min) nasal cannula. One of the consented patients was not enrolled because he was transferred to another hospital before endotracheal extubation. No patients were excluded from the study on the basis that they were deemed to have a lethal congenital abnormality or on the basis that they were likely to be transferred to another hospital before the study had ended. Table 2 Characteristics of the study subjects Group 1 (received Vapotherm s first) n ¼ 15 (received highflow first) n ¼ 15 Birth weight (grams, mean±s.d.) 1630± ±880 Gestational age at delivery (weeks days; 31.0± ±3.1 mean±s.d.) Age at study entry (days; median, range) 3 (1 19) 5 (1 16) Gender (% male) Initial primary lung disorder: HMD treated with surfactant 60% 73% HMD not treated with surfactant 33% 27% Pneumonia/sepsis 7% F Meconium aspiration F F TTN F F Other F F Race/ethnicity White 80% 87% Hispanic 20% 13% Black F F American Indian F F Pacific Islander F F Other F F F ¼ zero percent. Thirty patients were enrolled, randomized, and begun on one modality or the other for delivering high-flow nasal gas therapy. The characteristics of the 30 are given in Table 2. No significant differences were observed in the characteristics of the 15 randomized to Group 1 and the 15 randomized to. Immediately following extubation the subjects were treated with either Vapotherm s (Group 1) or high-flow (), but during the first 24 h of study, seven were weaned to a low-flow (<0.5 l/ min) nasal cannula. Three of these were in Group 1 and four were in (Table 3). These seven continued to wean from supplemental O 2, none required reintubation and none were discharged home on supplemental O 2. Of the remaining 23 patients, 14 finished the 48-h study, but seven failed. None failed while receiving Vapotherm s ; all seven failures were while receiving high-flow (Table 3). Two failed during the first 24 h after extubation due to reintubation because of increasing PCO 2 and FiO 2 and increasing atelectasis on chest X-ray, all of which were unresponsive to increasing cannula gas flow (from 1 to 2 l/min). Tracheal aspirates of these two following reintubation grew no organisms. Five failed during the second 24 h because they were switched from high-flow back to Vapotherm s before the 24-h study period on high-flow ended. These five were switched after 9.5 h (median; range, 1.5 to 20 h) on high-flow. Two of the five were switched on the basis of increasing episodes of apnea and bradycardia that subsided when switched back to Vapotherm s ; two others were switched because of increasing PCO 2 and respiratory rate, which improved when moved back to Vapotherm s, and the fifth was switched because of increasing FiO 2 with diminished breath sounds, which improved when moved back to Vapotherm s. Results of the Vapotherm s and standard high-flow nasal cannula treatment on respiratory rate, examinations of the nasal mucosa, and respiratory effort scores, are shown in Table 4. All 30 patients had a test period on high-flow, but only 28 had a test period on Vapotherm s. This is because two (both in ) failed high-flow within the first 24 h and were reintubated without ever receiving a trial of Vapotherm s. No differences were observed in respiratory rates during the period on Vapotherm s vs the period Table 3 Short-term outcomes of patients randomized to Group 1 and Group 1 (n ¼ 15) (n ¼ 15) Weaned to low-flow in first 24 h Failed in first 24 h Crossed to opposite modality at 24 h Failed in second 24 h Group 1 Vapotherm for first 24 h, then high-flow for second 24 h. High-flow for first 24 h, then Vapotherm for second 24 h.
4 484 Table 4 Respiratory rates, nasal mucosa examination scores, and respiratory effort scores while on Vapotherm s vs while on the standard high-flow cannula Respiratory rate a during the test period Nasal exam score b at the end of the test period Respiratory effort score c at the end of the test period Vapotherm s 52±13 (24) 2.7±1.2 (20) 1.2±0.6 (21) High-flow 54±14 (27) 7.8±1.7 (16) 2.0±0.9 (16) P-value NS < <0.05 a All respiratory rates (breaths per minute) charted during the test period. b Nasal examination scores could range from a low of 2 (normal) to a high of 10 (bilaterally occluded or hemorrhagic nares see Table 1). c Respiratory effort scores tabulated retractions, and could range from a low of 0 (no retractions) to a high of 15 (severe supraclavicular, suprasternal, intercostal, substernal, and subcostal retractions). (n) ¼ number of patients available to be included in this score. (Patients not available to be included had either no nasal exam score or no respiratory effort score at the end of the study period, because they had failed and were either intubated or rescued to Vapotherm. One nasal exam was inadvertently missed on one Vapotherm patient). NS ¼ not significant (P>0.05). on high-flow. One nasal examination was inadvertently missed in one subject after the period on Vapotherm s. At the conclusion of 24 h on Vapotherm s, nares were significantly more normal appearing (score of 2.7±1.2), than at the conclusion of 24 h on high-flow cannula treatment (score of 7.8±1.7, P<0.0005). At the conclusion of 24 h on Vapotherm s, respiratory effort scores were slightly lower (less retractions) than were those on high-flow (1.2±0.6 vs 2.0±0.9, P<0.05). When respiratory effort scores were compared before vs at the end of the 24-h period, no subjects had an increase in score during the period on Vapotherm s.in contrast, six subjects had an increase in respiratory effort score during the 24-h period on high-flow (P<0.05). The nasal gas flow used during the period on Vapotherm s was 3.1±0.6 l/min, compared with 1.8±0.4 l/min during the period on standard high-flow (P<0.01). The FiO 2 used during the period on Vapotherm s was 0.31±0.04, compared with 0.32±0.04 during the period on standard high-flow. Caffeine was administered to all of the eight extubated subjects <1000 g birth weight, to five of the 12 who were g birth weight (two in Group 1 and three in ), and to none of the 10 who were >1500 g birth weight. The subjects on caffeine treatment all received doses while on Vapotherm and while on high-flow. Three of the 30 patients had a documented infection during the hospitalization. None were in proximity to the study. One was early-onset Escherichia coli sepsis (12 days before the study). One developed clinical sepsis with Enterobacter cloacae grown from a tracheal aspirate through a newly placed endotracheal tube (but not grown from blood or spinal fluid). This occurred 10 days after completing the study. For the 8 days preceding the onset of symptoms that patient was on a low-flow (0.3 l/min) nasal cannula (not Vapotherm s ). The third infection was a coagulase negative Staphylococcus grown from one of two blood cultures eight days following completion of the study, when the patient was receiving low-flow (0.3 l/min) nasal cannula (not Vapotherm s ). Three of the 30 patients had a documented occurrence of extraventilatory air during the hospitalization. None were in proximity to the study. Two had a pneumothorax and one had a pneumomediastinum. These all occurred two to three days before extubation and thus before study-onset. No cases of pneumothorax or pulmonary interstitital emphysemia occurred after study-onset. Discussion Vapotherm s was developed, in the opinion of the company, to deliver molecular water vapor through a nasal cannula with nearly 100% relative humidity at body temperature. 4 6 The device was designed to deliver gasses in the range of 5 to 40 l/min, but for use in neonates a low-flow vapor transfer cartridge was developed that allows flows, according to manufacture instructions, of 1 to 8 l/ min. 4,5 Vapotherm s has been used recently in many NICU s for a variety of indications, including as a substitute for nasal CPAP among neonates recently extubated. 9 Despite its apparent popularity, we could find no published, peer-reviewed, randomized trials testing Vapotherm s in a NICU population. 9 For the present study, we focused on the problems following extubation from mechanical ventilation, and we postulated that Vapotherm s would perform better than a standard high-flow nasal cannula, among recently extubated neonates. The specific performance measures we selected were; (1) appearance of the nasal mucosa, respiratory rate, respiratory effort score, and failure of extubation. We used a crossover design so each study patient would be treated with both modalities. We randomized patients regarding which of the two test treatments would be administered first, in case the first treatment biased or conditioned the patient regarding the response to the second treatment. We observed that following extubation, patients placed on standard high-flow cannula treatment were more likely to experience an increase in retractions than were those on Vapotherm s. Furthermore, while on Vapotherm s, the patients were more likely to have a normal appearance of their nasal mucosa and fewer extubation failures. We speculate that the better respiratory effort scores were, at least in part, due to the higher gas flow during the Vapotherm s period. However, despite the higher flows, the nasal mucosa during Vapotherm s appeared more normal. We speculate that this was the result of the higher humidity and body temperature of the gas. During the course of our study, the Centers for Disease Control and Prevention began an investigation of Ralstonia in association with Vapotherm s use. 10 This issue had not been widely recognized as a concern during the time our study was being designed, and
5 485 consequently no cultures of the nasal mucosa, equipment, or trachea were included in the study protocol. However, we obtained tracheal aspirates in the two patients who were reintubated, and both were sterile. These two had been on high-flow and not Vapotherm s at the time of the reintubation. In no case was Ralstonia 10 or Pseudomonas recovered from any culture of any of the 30 study patients at any time during their hospitalization. Another concern regarding Vapotherm s is the unmeasured end-expiratory distending pressures generated, and the attendant fear that, under some circumstances, very high continuous positive end-expiratory pressures (CPAP) can inadvertently be administered. 9,11,12 As commented on recently by Finer, 12 efforts are needed to quantify and monitor unregulated CPAP, when using high-flow rates through a nasal cannula. We observed no cases of pneumothorax or pulmonary interstitial emphysemia on any study patient between study entry and discharge home, although this was not specifically sought as part of the study design. We recognize various shortcomings of our study. First, although the neonatologist conducting the nasal examinations and the research nurse abstracting the charts were effectively blinded to the study arm, we had no way of similarly blinding the bedside nurse, bedside respiratory therapist, and attending neonatologist. We realize that this could introduce a source of bias. Second, we knew that the convenience sample of only 30 subjects would be too small to evaluate relatively rare adverse events due to one modality or the other. Indeed, the lack of infections and pneumothoracies among our 30 subjects during the study does not exclude these as real risks of Vapotherm s. Third, the method by which retractions are scored (absent, mild, moderate or severe) on our bedside charts is subjective, thus minimizing the importance of the observed better respiratory effort scores while on Vapotherm s. In conclusion, among 30 NICU patients in a cross-over study immediately following endotracheal extubation, it appears that Vapotherm s performed better than a standard high-flow nasal cannula in maintaining normal appearing nasal mucosa, a lower respiratory effort, and averting reintubation, with no recognized complications. Acknowledgments The authors thank the nurses and respiratory therapists at the McKay-Dee Hospital NICU for their valuable assistance with the study. We also thank Gorgi Rigby, RRT, LDS Hospital NICU, Salt Lake City, UT for helpful discussions about the research project. References 1 Sreenan C, Lemke RP, Hudson-Mason A, Osiovich H. High-flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001; 107: Myers TR. AARC clinical practice guideline. Selection of an oxygen delivery devise for neonatal and pediatric patients. Respir Care 2002; 47: Kopelman A, Holbert D. Use of oxygen cannulas in extremely low birth weight infants is associated with mucosal trauma and bleeding, and possibly with coagulase-negative staphylococcal sepsis. J Perinatol 2003; 23: In-house literature. Vapotherm Inc. 198 Log Canoe Circle: Stevensville, MD. 6 Waugh JB, Granger WM. An evaluation of 2 new devices for nasal high-flow gas therapy. Respir Care 2004; 49: A Collective Task Force Facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine. Evidence-based guidelines for weaning and discontinuing ventilatory support. Respir Care 2002; 47: Durbin Jr CG, Campbell RS, Bransone RD. AARC clinical practice guideline. Removal of the endotracheal tube. Respir Care 1999; 44: deklerk A. Humidified high flow nasal cannulae. Hot Topics Neonatol 2005; 4 6: Centers for Disease Control and Prevention. Ralstonia associated with Vapotherm oxygen delivery device United States, Morb Mortal Wkly Rep 2005; 54: Locke RG, Wolfson MR, Shaffer TH, Rubinstein SD, Greenspan JS. Inadvertent administration of positive-end-distending pressure during nasal cannula flow. Pediatrics 1993; 91: Finer NN. Nasal cannula use in the preterm infant: oxygen or pressure? Pediatrics 2005; 116:
Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D.
 Heated, Humidified High-Flow Nasal Cannula (HHHFNC) vs. Nasal Intermittent Positive Pressure Ventilation (NIPPV) for the Primary Treatment of RDS, A Randomized, Controlled, Prospective, Pilot Study Kugelman
Heated, Humidified High-Flow Nasal Cannula (HHHFNC) vs. Nasal Intermittent Positive Pressure Ventilation (NIPPV) for the Primary Treatment of RDS, A Randomized, Controlled, Prospective, Pilot Study Kugelman
Work of breathing using high-flow nasal cannula in preterm infants
 (), 7 8 r Nature Publishing Group All rights reserved. 73-83/ $3 www.nature.com/jp ORIGINAL ARTICLE Work of breathing using high-flow nasal cannula in preterm infants JG Saslow 1,, ZH Aghai 1,, TA Nakhla
(), 7 8 r Nature Publishing Group All rights reserved. 73-83/ $3 www.nature.com/jp ORIGINAL ARTICLE Work of breathing using high-flow nasal cannula in preterm infants JG Saslow 1,, ZH Aghai 1,, TA Nakhla
Provide guidelines for the management of mechanical ventilation in infants <34 weeks gestation.
 Page 1 of 5 PURPOSE: Provide guidelines for the management of mechanical ventilation in infants
Page 1 of 5 PURPOSE: Provide guidelines for the management of mechanical ventilation in infants
OmniOx (HFT 500) Jan Prepared by MEKICS. OmniOx-HFT500 Jan ver1.1 1
 OmniOx (HFT 500) Jan. 2013. Prepared by MEKICS OmniOx-HFT500 Jan. 2013 ver1.1 1 0. Index 1. Introduce of OmniOx (HTF500) 2. Specification (HFT, CPAP+) 3. The World Best Features 2 OmniOx-HFT500 Jan. 2013
OmniOx (HFT 500) Jan. 2013. Prepared by MEKICS OmniOx-HFT500 Jan. 2013 ver1.1 1 0. Index 1. Introduce of OmniOx (HTF500) 2. Specification (HFT, CPAP+) 3. The World Best Features 2 OmniOx-HFT500 Jan. 2013
Volume Guarantee Initiation and ongoing clinical management of an infant supported by Volume Guarantee A Case Study
 D-32084-2011 Volume Guarantee Initiation and ongoing clinical management of an infant supported by Volume Guarantee A Case Study Robert DiBlasi RRT-NPS, FAARC Respiratory Care Manager of Research & Quality
D-32084-2011 Volume Guarantee Initiation and ongoing clinical management of an infant supported by Volume Guarantee A Case Study Robert DiBlasi RRT-NPS, FAARC Respiratory Care Manager of Research & Quality
Guidelines and Best Practices for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) NICU POCKET GUIDE
 Guidelines and Best Practices for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) TM NICU POCKET GUIDE Patient Selection Diagnoses Patient presents with one or more of the following symptoms: These
Guidelines and Best Practices for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) TM NICU POCKET GUIDE Patient Selection Diagnoses Patient presents with one or more of the following symptoms: These
5/3/2012. Goals and Objectives HFNC. High-Flow Oxygen Therapy: Real Benefit or Just a Fad?
 High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
High-Flow Oxygen Therapy: Real Benefit or Just a Fad? Timothy R. Myers MBA, RRT-NPS Director, Women s & Children s Respiratory Care & Procedural Services and Pediatric Heart Center Rainbow Babies & Children
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION
 CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
Simulation 3: Post-term Baby in Labor and Delivery
 Simulation 3: Post-term Baby in Labor and Delivery Opening Scenario (Links to Section 1) You are an evening-shift respiratory therapist in a large hospital with a level III neonatal unit. You are paged
Simulation 3: Post-term Baby in Labor and Delivery Opening Scenario (Links to Section 1) You are an evening-shift respiratory therapist in a large hospital with a level III neonatal unit. You are paged
Name and title of the investigators responsible for conducting the research: Dr Anna Lavizzari, Dr Mariarosa Colnaghi
 Protocol title: Heated, Humidified High-Flow Nasal Cannula vs Nasal CPAP for Respiratory Distress Syndrome of Prematurity. Protocol identifying number: Clinical Trials.gov NCT02570217 Name and title of
Protocol title: Heated, Humidified High-Flow Nasal Cannula vs Nasal CPAP for Respiratory Distress Syndrome of Prematurity. Protocol identifying number: Clinical Trials.gov NCT02570217 Name and title of
ROLE OF PRESSURE IN HIGH FLOW THERAPY
 ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
ROLE OF PRESSURE IN HIGH FLOW THERAPY Thomas L. Miller, PhD, MEd Director, Clinical Research and Education Vapotherm, Inc. Research Assistant Professor of Pediatrics Jefferson Medical College This information
AEROSURF Phase 2 Program Update Investor Conference Call
 AEROSURF Phase 2 Program Update Investor Conference Call November 12, 2015 Forward Looking Statement To the extent that statements in this presentation are not strictly historical, including statements
AEROSURF Phase 2 Program Update Investor Conference Call November 12, 2015 Forward Looking Statement To the extent that statements in this presentation are not strictly historical, including statements
Surfactant Administration
 Approved by: Surfactant Administration Gail Cameron Senior Director Operations, Maternal, Neonatal & Child Health Programs Dr. Paul Byrne Medical Director, Neonatology Neonatal Policy & Procedures Manual
Approved by: Surfactant Administration Gail Cameron Senior Director Operations, Maternal, Neonatal & Child Health Programs Dr. Paul Byrne Medical Director, Neonatology Neonatal Policy & Procedures Manual
Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline
 EOE Neonatal ODN Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline Authors: Dr Eliana Panayiotou and Dr Bharat Vakharia For use in: EoE Neonatal Units Guidance specific to
EOE Neonatal ODN Clinical Guideline: Heated Humidified High Flow Nasal Cannula (HHHFNC) Guideline Authors: Dr Eliana Panayiotou and Dr Bharat Vakharia For use in: EoE Neonatal Units Guidance specific to
NON INVASIVE LIFE SAVERS. Non Invasive Ventilation (NIV)
 Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
Guidelines and Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide
 Guidelines Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide Patient Selection Diagnoses Patient presents with one or more of the following signs or symptoms of respiratory distress:
Guidelines Best Practices for High Flow Nasal Cannula (HFNC) Pediatric Pocket Guide Patient Selection Diagnoses Patient presents with one or more of the following signs or symptoms of respiratory distress:
Hyaline membrane disease. By : Dr. Ch Sarishma Peadiatric Pg
 Hyaline membrane disease By : Dr. Ch Sarishma Peadiatric Pg Also called Respiratory distress syndrome. It occurs primarily in premature infants; its incidence is inversely related to gestational age and
Hyaline membrane disease By : Dr. Ch Sarishma Peadiatric Pg Also called Respiratory distress syndrome. It occurs primarily in premature infants; its incidence is inversely related to gestational age and
Systems differ in their ability to deliver optimal humidification
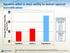 Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Practical Application of CPAP
 CHAPTER 3 Practical Application of CPAP Dr. Srinivas Murki Neonatologist Fernadez Hospital, Hyderabad. A.P. Practical Application of CPAP Continuous positive airway pressure (CPAP) applied to premature
CHAPTER 3 Practical Application of CPAP Dr. Srinivas Murki Neonatologist Fernadez Hospital, Hyderabad. A.P. Practical Application of CPAP Continuous positive airway pressure (CPAP) applied to premature
Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet
 Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1. Name of Guideline / Policy/ Procedure 2. Purpose of Procedure/ Guidelines/ Protocol Guideline for
Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1. Name of Guideline / Policy/ Procedure 2. Purpose of Procedure/ Guidelines/ Protocol Guideline for
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device
 I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
GE Healthcare. Non Invasive Ventilation (NIV) For the Engström Ventilator. Relief, Relax, Recovery
 GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
Advantages and disadvantages of different nasal CPAP systems in newborns
 Intensive Care Med (2004) 30:926 930 DOI 10.1007/s00134-004-2267-8 N E O N A T A L A N D P A E D I A T R I C I N T E N S I V E C A R E V. Buettiker M. I. Hug O. Baenziger C. Meyer B. Frey Advantages and
Intensive Care Med (2004) 30:926 930 DOI 10.1007/s00134-004-2267-8 N E O N A T A L A N D P A E D I A T R I C I N T E N S I V E C A R E V. Buettiker M. I. Hug O. Baenziger C. Meyer B. Frey Advantages and
CLINICAL CONSIDERATIONS FOR THE BUNNELL LIFE PULSE HIGH-FREQUENCY JET VENTILATOR
 CLINICAL CONSIDERATIONS FOR THE BUNNELL LIFE PULSE HIGH-FREQUENCY JET VENTILATOR 801-467-0800 Phone 800-800-HFJV (4358) Hotline TABLE OF CONTENTS Respiratory Care Considerations..3 Physician Considerations
CLINICAL CONSIDERATIONS FOR THE BUNNELL LIFE PULSE HIGH-FREQUENCY JET VENTILATOR 801-467-0800 Phone 800-800-HFJV (4358) Hotline TABLE OF CONTENTS Respiratory Care Considerations..3 Physician Considerations
CPAP failure in preterm infants: incidence, predictors and consequences
 CPAP failure in preterm infants: incidence, predictors and consequences SUPPLEMENTAL TEXT METHODS Study setting The Royal Hobart Hospital has an 11-bed combined Neonatal and Paediatric Intensive Care Unit
CPAP failure in preterm infants: incidence, predictors and consequences SUPPLEMENTAL TEXT METHODS Study setting The Royal Hobart Hospital has an 11-bed combined Neonatal and Paediatric Intensive Care Unit
Neonatal Respiratory Physiotherapy. Nicky Hawkes Advanced Respiratory Physiotherapist Oct 2011
 Neonatal Respiratory Physiotherapy Nicky Hawkes Advanced Respiratory Physiotherapist Oct 2011 Respiratory Physiotherapy?..Not just percussion! Assessment of baby s respiratory status and deciding what
Neonatal Respiratory Physiotherapy Nicky Hawkes Advanced Respiratory Physiotherapist Oct 2011 Respiratory Physiotherapy?..Not just percussion! Assessment of baby s respiratory status and deciding what
CHEST PHYSIOTHERAPY IN NICU PURPOSE POLICY STATEMENTS SITE APPLICABILITY PRACTICE LEVEL/COMPETENCIES. The role of chest physiotherapy in the NICU
 PURPOSE The role of chest physiotherapy in the NICU POLICY STATEMENTS In principle chest physiotherapy should be limited to those infants considered most likely to benefit with significant respiratory
PURPOSE The role of chest physiotherapy in the NICU POLICY STATEMENTS In principle chest physiotherapy should be limited to those infants considered most likely to benefit with significant respiratory
SARASOTA MEMORIAL HOSPITAL DEPARTMENT POLICY
 PS1006 SARASOTA MEMORIAL HOSPITAL DEPARTMENT POLICY TITLE: NON-INVASIVE VENTILATION FOR THE Job Title of Reviewer: EFFECTIVE DATE: REVISED DATE: Director, Respiratory Care Services 126.685 (neo) 3/26/15
PS1006 SARASOTA MEMORIAL HOSPITAL DEPARTMENT POLICY TITLE: NON-INVASIVE VENTILATION FOR THE Job Title of Reviewer: EFFECTIVE DATE: REVISED DATE: Director, Respiratory Care Services 126.685 (neo) 3/26/15
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE
 Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
 NEONATAL LIFE SUPPORT PROVIDER (NLSP) CERTIFICATION EXAMINATION 1. To determine if an infant requires resuscitation, you must rapidly assess gestation period, presence of meconium in amniotic fluid, breaths
NEONATAL LIFE SUPPORT PROVIDER (NLSP) CERTIFICATION EXAMINATION 1. To determine if an infant requires resuscitation, you must rapidly assess gestation period, presence of meconium in amniotic fluid, breaths
NEONATAL NEWS Here s Some More Good Poop
 NEONATALNEWS Here ssomemoregoodpoop WINTEREDITION2010 THISNEWSLETTERISPUBLISHEDPERIODICALLYBYTHENEONATOLOGISTSOF ASSOCIATESINNEWBORNMEDICINETOCONVEYNEWANDUPDATEDPOLICIES ANDGUIDELINESANDPROVIDEGENERALEDUCATIONTONICUCARETAKERSAT
NEONATALNEWS Here ssomemoregoodpoop WINTEREDITION2010 THISNEWSLETTERISPUBLISHEDPERIODICALLYBYTHENEONATOLOGISTSOF ASSOCIATESINNEWBORNMEDICINETOCONVEYNEWANDUPDATEDPOLICIES ANDGUIDELINESANDPROVIDEGENERALEDUCATIONTONICUCARETAKERSAT
Neonatal Life Support Provider (NLSP) Certification Preparatory Materials
 Neonatal Life Support Provider (NLSP) Certification Preparatory Materials NEONATAL LIFE SUPPORT PROVIDER (NRP) CERTIFICATION TABLE OF CONTENTS NEONATAL FLOW ALGORITHM.2 INTRODUCTION 3 ANTICIPATION OF RESUSCITATION
Neonatal Life Support Provider (NLSP) Certification Preparatory Materials NEONATAL LIFE SUPPORT PROVIDER (NRP) CERTIFICATION TABLE OF CONTENTS NEONATAL FLOW ALGORITHM.2 INTRODUCTION 3 ANTICIPATION OF RESUSCITATION
Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Care Unit (FELLOW)
 Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Data Analysis Plan: Apneic Oxygenation vs. No Apneic Oxygenation Background Critically ill patients
Facilitating EndotracheaL Intubation by Laryngoscopy technique and Apneic Oxygenation Within the Intensive Data Analysis Plan: Apneic Oxygenation vs. No Apneic Oxygenation Background Critically ill patients
Title Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline. Department Paediatrics / Neonates Date Issued
 Document Control Title Author Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline Author s job title Directorate Medical Version Department Paediatrics / Neonates Date Issued Status
Document Control Title Author Neonatal and Paediatric High-Flow Nasal Cannula Oxygen Therapy Guideline Author s job title Directorate Medical Version Department Paediatrics / Neonates Date Issued Status
Neonatal Resuscitation Using a Nasal Cannula: A Single-Center Experience
 Original Article Neonatal Resuscitation Using a Nasal Cannula: A Single-Center Experience Pedro Paz, MD, MPH 1 Rangasamy Ramanathan, MD 1,2 Richard Hernandez, RCP 2 Manoj Biniwale, MD 1 1 Division of Neonatal
Original Article Neonatal Resuscitation Using a Nasal Cannula: A Single-Center Experience Pedro Paz, MD, MPH 1 Rangasamy Ramanathan, MD 1,2 Richard Hernandez, RCP 2 Manoj Biniwale, MD 1 1 Division of Neonatal
MASTER SYLLABUS
 MASTER SYLLABUS 2018-2019 A. Academic Division: Health Science B. Discipline: Respiratory Care C. Course Number and Title: RESP 2490 Practicum IV D. Course Coordinator: Tricia Winters, BBA, RRT, RCP Assistant
MASTER SYLLABUS 2018-2019 A. Academic Division: Health Science B. Discipline: Respiratory Care C. Course Number and Title: RESP 2490 Practicum IV D. Course Coordinator: Tricia Winters, BBA, RRT, RCP Assistant
** SURFACTANT THERAPY**
 ** SURFACTANT THERAPY** Full Title of Guideline: Surfactant Therapy Author (include email and role): Stephen Wardle (V4) Reviewed by Dushyant Batra Consultant Neonatologist Division & Speciality: Division:
** SURFACTANT THERAPY** Full Title of Guideline: Surfactant Therapy Author (include email and role): Stephen Wardle (V4) Reviewed by Dushyant Batra Consultant Neonatologist Division & Speciality: Division:
Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome
 European Review for Medical and Pharmacological Sciences 2015; 19: 573-577 Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome B.
European Review for Medical and Pharmacological Sciences 2015; 19: 573-577 Usefulness of DuoPAP in the treatment of very low birth weight preterm infants with neonatal respiratory distress syndrome B.
COMPLICATED STAPHYLOCOCCAL INFECTION IN A NEONATE. A. Ansary*, D. Varghese, L. Jackson ROYAL HOSPITAL FOR CHILDREN, GLASGOW,UK
 COMPLICATED STAPHYLOCOCCAL INFECTION IN A NEONATE A. Ansary*, D. Varghese, L. Jackson ROYAL HOSPITAL FOR CHILDREN, GLASGOW,UK Complicated staphylococcal infection in a neonate Overview Case Radiology/biochemistry/microbiology
COMPLICATED STAPHYLOCOCCAL INFECTION IN A NEONATE A. Ansary*, D. Varghese, L. Jackson ROYAL HOSPITAL FOR CHILDREN, GLASGOW,UK Complicated staphylococcal infection in a neonate Overview Case Radiology/biochemistry/microbiology
Von Reuss and CPAP, Disclosures CPAP. Noninvasive respiratory therapieswhy bother? Noninvasive respiratory therapies- types
 Noninvasive respiratory therapiesby a nose? NEO- The Conference for Neonatology February 21, 2014 Disclosures I have no relevant financial relationships to disclose or conflicts of interest to release.
Noninvasive respiratory therapiesby a nose? NEO- The Conference for Neonatology February 21, 2014 Disclosures I have no relevant financial relationships to disclose or conflicts of interest to release.
ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME
 INDIAN PEDIATRICS VOLUME 35-FEBRUAKY 1998 ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME Kanya Mukhopadhyay, Praveen Kumar and Anil Narang From the Division of Neonatology, Department
INDIAN PEDIATRICS VOLUME 35-FEBRUAKY 1998 ROLE OF EARLY POSTNATAL DEXAMETHASONE IN RESPIRATORY DISTRESS SYNDROME Kanya Mukhopadhyay, Praveen Kumar and Anil Narang From the Division of Neonatology, Department
Review of Neonatal Respiratory Problems
 Review of Neonatal Respiratory Problems Respiratory Distress Occurs in about 7% of infants Clinical presentation includes: Apnea Cyanosis Grunting Inspiratory stridor Nasal flaring Poor feeding Tachypnea
Review of Neonatal Respiratory Problems Respiratory Distress Occurs in about 7% of infants Clinical presentation includes: Apnea Cyanosis Grunting Inspiratory stridor Nasal flaring Poor feeding Tachypnea
9/15/2017. Disclosures. Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Objectives. Aerogen Pharma
 Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Rob DiBlasi RRT-NPS, FAARC Program Manager Research/QI, Respiratory Therapy Principle Investigator, Seattle Children s Research Institute
Heated High Flow Nasal Cannula: Hot Air or Optimal Noninvasive Support? Rob DiBlasi RRT-NPS, FAARC Program Manager Research/QI, Respiratory Therapy Principle Investigator, Seattle Children s Research Institute
Cover Page. The handle holds various files of this Leiden University dissertation
 Cover Page The handle http://hdl.handle.net/1887/46692 holds various files of this Leiden University dissertation Author: Zanten, Henriëtte van Title: Oxygen titration and compliance with targeting oxygen
Cover Page The handle http://hdl.handle.net/1887/46692 holds various files of this Leiden University dissertation Author: Zanten, Henriëtte van Title: Oxygen titration and compliance with targeting oxygen
Simulation 08: Cyanotic Preterm Infant in Respiratory Distress
 Flow Chart Simulation 08: Cyanotic Preterm Infant in Respiratory Distress Opening Scenario Section 1 Type: DM As staff therapist assigned to a Level 2 NICU in a 250 bed rural medical center you are called
Flow Chart Simulation 08: Cyanotic Preterm Infant in Respiratory Distress Opening Scenario Section 1 Type: DM As staff therapist assigned to a Level 2 NICU in a 250 bed rural medical center you are called
Exclusion Criteria 1. Operator or supervisor feels specific intra- procedural laryngoscopy device will be required.
 FELLOW Study Data Analysis Plan Direct Laryngoscopy vs Video Laryngoscopy Background Respiratory failure requiring endotracheal intubation occurs in as many as 40% of critically ill patients. Procedural
FELLOW Study Data Analysis Plan Direct Laryngoscopy vs Video Laryngoscopy Background Respiratory failure requiring endotracheal intubation occurs in as many as 40% of critically ill patients. Procedural
Introducing Infant Flow Advance SIPAP. By Joanne Cookson March 2008
 Introducing Infant Flow Advance SIPAP By Joanne Cookson March 2008 Aim To introduce clinical practioners to the new SiPAP machine Objectives To define what is SiPAP To look at different modes able to be
Introducing Infant Flow Advance SIPAP By Joanne Cookson March 2008 Aim To introduce clinical practioners to the new SiPAP machine Objectives To define what is SiPAP To look at different modes able to be
This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review.
 This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review. The version of record [Journal of Neonatal Nursing (February 2013)
This is a pre-copyedited, author-produced PDF of an article accepted for publication in Journal of Neonatal Nursing following peer review. The version of record [Journal of Neonatal Nursing (February 2013)
Research in high flow therapy: Mechanisms of action. Kevin Dysart, Thomas L. Miller, Marla R. Wolfson, Thomas H. Shaffer
 Research in high flow therapy: Mechanisms of action Kevin Dysart, Thomas L. Miller, Marla R. Wolfson, Thomas H. Shaffer 64666_03_Mech0fAction.indd 1 Respiratory Medicine (2009) xx, 1e6 ARTICLE IN PRESS
Research in high flow therapy: Mechanisms of action Kevin Dysart, Thomas L. Miller, Marla R. Wolfson, Thomas H. Shaffer 64666_03_Mech0fAction.indd 1 Respiratory Medicine (2009) xx, 1e6 ARTICLE IN PRESS
Bubble CPAP for Respiratory Distress Syndrome in Preterm Infants
 R E S E A R C H P A P E R Bubble CPAP for Respiratory Distress Syndrome in Preterm Infants JAGDISH KOTI*, SRINIVAS MURKI, PRAMOD GADDAM, ANUPAMA REDDY AND M DASARADHA RAMI REDDY From Fernandez Hospital
R E S E A R C H P A P E R Bubble CPAP for Respiratory Distress Syndrome in Preterm Infants JAGDISH KOTI*, SRINIVAS MURKI, PRAMOD GADDAM, ANUPAMA REDDY AND M DASARADHA RAMI REDDY From Fernandez Hospital
Charisma High-flow CPAP solution
 Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
High Flow Nasal Cannula Oxygen HFNC. Dr I S Kalla Department of Pulmonology University of the Witwatersrand
 786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
786 High Flow Nasal Cannula Oxygen HFNC Dr I S Kalla Department of Pulmonology University of the Witwatersrand Disclaimer I was a scep@c un@l I used it Now I am a firm believer HFNC The Fisher and Paykel
Neonatal Resuscitation in What is new? How did we get here? Steven Ringer MD PhD Harvard Medical School May 25, 2011
 Neonatal Resuscitation in 2011- What is new? How did we get here? Steven Ringer MD PhD Harvard Medical School May 25, 2011 Conflicts I have no actual or potential conflict of interest in relation to this
Neonatal Resuscitation in 2011- What is new? How did we get here? Steven Ringer MD PhD Harvard Medical School May 25, 2011 Conflicts I have no actual or potential conflict of interest in relation to this
High-Flow Nasal Cannulae in Very Preterm Infants after Extubation
 original article High-Flow Nasal Cannulae in Very Preterm Infants after Extubation Brett J. Manley, M.B., B.S., Louise S. Owen, M.D., Lex W. Doyle, M.D., Chad C. Andersen, M.B., B.S., David W. Cartwright,
original article High-Flow Nasal Cannulae in Very Preterm Infants after Extubation Brett J. Manley, M.B., B.S., Louise S. Owen, M.D., Lex W. Doyle, M.D., Chad C. Andersen, M.B., B.S., David W. Cartwright,
Trial protocol - NIVAS Study
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Trial protocol - NIVAS Study METHODS Study oversight The Non-Invasive Ventilation after Abdominal Surgery
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Trial protocol - NIVAS Study METHODS Study oversight The Non-Invasive Ventilation after Abdominal Surgery
Disclosure. Learning Objectives. Bernadette Zelaya, RRT. Area Clinical Manager
 High Velocity Nasal Insufflation An Important Therapeutic Approach for Use in the Emergency Department Presented by Vapotherm Accredited for 1 CEU by the American Association for Respiratory Care Provider
High Velocity Nasal Insufflation An Important Therapeutic Approach for Use in the Emergency Department Presented by Vapotherm Accredited for 1 CEU by the American Association for Respiratory Care Provider
Test Bank Pilbeam's Mechanical Ventilation Physiological and Clinical Applications 6th Edition Cairo
 Instant dowload and all chapters Test Bank Pilbeam's Mechanical Ventilation Physiological and Clinical Applications 6th Edition Cairo https://testbanklab.com/download/test-bank-pilbeams-mechanical-ventilation-physiologicalclinical-applications-6th-edition-cairo/
Instant dowload and all chapters Test Bank Pilbeam's Mechanical Ventilation Physiological and Clinical Applications 6th Edition Cairo https://testbanklab.com/download/test-bank-pilbeams-mechanical-ventilation-physiologicalclinical-applications-6th-edition-cairo/
10/17/2016 OXYGEN DELIVERY: INDICATIONS AND USE OF EQUIPMENT COURSE OBJECTIVES COMMON CAUSES OF RESPIRATORY FAILURE
 OXYGEN DELIVERY: INDICATIONS AND USE OF EQUIPMENT J U L I E Z I M M E R M A N, R N, M S N C L I N I C A L N U R S E S P E C I A L I S T E L O I S A C U T L E R, R R T, B S R C C L I N I C A L / E D U C
OXYGEN DELIVERY: INDICATIONS AND USE OF EQUIPMENT J U L I E Z I M M E R M A N, R N, M S N C L I N I C A L N U R S E S P E C I A L I S T E L O I S A C U T L E R, R R T, B S R C C L I N I C A L / E D U C
ENDOTRACHEAL INTUBATION POLICY
 POLICY Indications: Ineffective ventilation with mask and t-piece, or mask and bag technique Inability to maintain a patent airway Need or anticipation of need for prolonged ventilation Need for endotracheal
POLICY Indications: Ineffective ventilation with mask and t-piece, or mask and bag technique Inability to maintain a patent airway Need or anticipation of need for prolonged ventilation Need for endotracheal
Non Invasive Ventilation In Preterm Infants. Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid
 Non Invasive Ventilation In Preterm Infants Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid Summary Noninvasive ventilation begings in the delivery room
Non Invasive Ventilation In Preterm Infants Manuel Sanchez Luna Hospital General Universitario Gregorio Marañón Complutense University Madrid Summary Noninvasive ventilation begings in the delivery room
Nottingham Children s Hospital
 High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
High Flow Nasal Cannula Therapy Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Guide line for the use of HFNCT (High Flow Nasal Cannula Therapy) Contact Name
Objectives. Apnea Definition and Pitfalls. Pathophysiology of Apnea. Apnea of Prematurity and hypoxemia episodes 5/18/2015
 Apnea of Prematurity and hypoxemia episodes Deepak Jain MD Care of Sick Newborn Conference May 2015 Objectives Differentiating between apnea and hypoxemia episodes. Pathophysiology Diagnosis of apnea and
Apnea of Prematurity and hypoxemia episodes Deepak Jain MD Care of Sick Newborn Conference May 2015 Objectives Differentiating between apnea and hypoxemia episodes. Pathophysiology Diagnosis of apnea and
Equipment: NRP algorithm, MRSOPA table, medication chart, SpO 2 table Warm
 NRP Skills Stations Performance Skills Station OR Integrated Skills Station STATION: Assisting with and insertion of endotracheal tube (ETT) Equipment: NRP algorithm, MRSOPA table, medication chart, SpO
NRP Skills Stations Performance Skills Station OR Integrated Skills Station STATION: Assisting with and insertion of endotracheal tube (ETT) Equipment: NRP algorithm, MRSOPA table, medication chart, SpO
Sample Case Study. The patient was a 77-year-old female who arrived to the emergency room on
 Sample Case Study The patient was a 77-year-old female who arrived to the emergency room on February 25 th with a chief complaint of shortness of breath and a deteriorating pulmonary status along with
Sample Case Study The patient was a 77-year-old female who arrived to the emergency room on February 25 th with a chief complaint of shortness of breath and a deteriorating pulmonary status along with
Presented By : Kamlah Olaimat
 Presented By : Kamlah Olaimat 18\7\2010 Transient Tachpnea of the Definition:- newborn (TTN) TTN is a benign disease of near term or term infant who display respiratory distress shortly after delivery.
Presented By : Kamlah Olaimat 18\7\2010 Transient Tachpnea of the Definition:- newborn (TTN) TTN is a benign disease of near term or term infant who display respiratory distress shortly after delivery.
Protocol Update 2019
 Protocol Update 2019 There have been several questions revolving around protocol updates and how they are to be conducted. As many of you are aware there is a protocol submission process in the appendix
Protocol Update 2019 There have been several questions revolving around protocol updates and how they are to be conducted. As many of you are aware there is a protocol submission process in the appendix
I. Subject: Pressure Support Ventilation (PSV) with BiPAP Device/Nasal CPAP
 I. Subject: Pressure Support Ventilation (PSV) with BiPAP Device/Nasal CPAP II. Policy: PSV with BiPAP device/nasal CPAP will be initiated upon a physician's order by Respiratory Therapy personnel trained
I. Subject: Pressure Support Ventilation (PSV) with BiPAP Device/Nasal CPAP II. Policy: PSV with BiPAP device/nasal CPAP will be initiated upon a physician's order by Respiratory Therapy personnel trained
IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room
 IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room 1) Decreasing Telemetry Alarms Janice Marlett, BSN, RN, Nursing Staff Educator To decrease tele alarms: Properly prep the skin Shave
IICU Staff Meeting Minutes May 15 and 16, 2013 IICU Conference Room 1) Decreasing Telemetry Alarms Janice Marlett, BSN, RN, Nursing Staff Educator To decrease tele alarms: Properly prep the skin Shave
Acute Paediatric Respiratory Pathway
 Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
Guideline for the use of high Flow Nasal Cannula Oxygen Therapy (Optiflow or Airvo) in Children with Bronchiolitis or an acute respiratory illness Introduction: High flow nasal cannula (HFNC) oxygen enables
Clinicoetiological profile and risk assessment of newborn with respiratory distress in a tertiary care centre in South India
 International Journal of Contemporary Pediatrics Sahoo MR et al. Int J Contemp Pediatr. 2015 Nov;2(4):433-439 http://www.ijpediatrics.com pissn 2349-3283 eissn 2349-3291 Research Article DOI: http://dx.doi.org/10.18203/2349-3291.ijcp20150990
International Journal of Contemporary Pediatrics Sahoo MR et al. Int J Contemp Pediatr. 2015 Nov;2(4):433-439 http://www.ijpediatrics.com pissn 2349-3283 eissn 2349-3291 Research Article DOI: http://dx.doi.org/10.18203/2349-3291.ijcp20150990
High-flow nasal cannula use in a paediatric intensive care unit over 3 years
 High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
High-flow nasal cannula use in a paediatric intensive care unit over 3 years Tracey I Wraight and Subodh S Ganu Respiratory illness and/or distress is the commonest reason for non-elective paediatric intensive
INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2
 2 Effects of CPAP INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2 ). The effect on CO 2 is only secondary to the primary process of improvement in lung volume and
2 Effects of CPAP INTRODUCTION The effect of CPAP works on lung mechanics to improve oxygenation (PaO 2 ). The effect on CO 2 is only secondary to the primary process of improvement in lung volume and
Nasal versus oral intubation for mechanical ventilation of newborn infants (Review)
 Nasal versus oral intubation for mechanical ventilation of newborn infants (Review) Spence K, Barr P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
Nasal versus oral intubation for mechanical ventilation of newborn infants (Review) Spence K, Barr P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
Interfacility Protocol Protocol Title:
 Interfacility Protocol Protocol Title: Mechanical Ventilator Monitoring & Management Original Adoption Date: 05/2009 Past Protocol Updates 05/2009, 12/2013 Date of Most Recent Update: March 23, 2015 Medical
Interfacility Protocol Protocol Title: Mechanical Ventilator Monitoring & Management Original Adoption Date: 05/2009 Past Protocol Updates 05/2009, 12/2013 Date of Most Recent Update: March 23, 2015 Medical
Airway Clearance Devices
 Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
The Turkish Journal of Pediatrics 2014; 56:
 The Turkish Journal of Pediatrics 2014; 56: 232-237 Original INSURE method (INtubation-SURfactant-Extubation) in early and late premature neonates with respiratory distress: factors affecting the outcome
The Turkish Journal of Pediatrics 2014; 56: 232-237 Original INSURE method (INtubation-SURfactant-Extubation) in early and late premature neonates with respiratory distress: factors affecting the outcome
SWISS SOCIETY OF NEONATOLOGY. Potentially fatal complications of peripherally inserted central venous catheters (PICCs)
 SWISS SOCIETY OF NEONATOLOGY Potentially fatal complications of peripherally inserted central venous catheters (PICCs) October 2001 2 Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital
SWISS SOCIETY OF NEONATOLOGY Potentially fatal complications of peripherally inserted central venous catheters (PICCs) October 2001 2 Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital
PUMANI bcpap GUIDELINES FOR CLINICIANS. An Overview of the Pumani bcpap, Indications for bcpap, and Instructions for Use
 An Overview of the Pumani bcpap, Indications for bcpap, and Instructions for Use What is bcpap? bcpap stands for bubble Continuous Positive Airway Pressure. Sometimes called Continuous Distending Pressure,
An Overview of the Pumani bcpap, Indications for bcpap, and Instructions for Use What is bcpap? bcpap stands for bubble Continuous Positive Airway Pressure. Sometimes called Continuous Distending Pressure,
Steven Ringer MD PhD April 5, 2011
 Steven Ringer MD PhD April 5, 2011 Disclaimer Mead Johnson sponsors programs such as this to give healthcare professionals access to scientific and educational information provided by experts. The presenter
Steven Ringer MD PhD April 5, 2011 Disclaimer Mead Johnson sponsors programs such as this to give healthcare professionals access to scientific and educational information provided by experts. The presenter
Pedi-Cap CO 2 detector
 Pedi-Cap CO 2 detector Presentation redeveloped for this program by Rosemarie Boland from an original presentation by Johnston, Adams & Stewart, (2006) Background Clinical methods of endotracheal tube
Pedi-Cap CO 2 detector Presentation redeveloped for this program by Rosemarie Boland from an original presentation by Johnston, Adams & Stewart, (2006) Background Clinical methods of endotracheal tube
SARASOTA MEMORIAL HOSPITAL NURSING PROCEDURE
 SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE Management, Monitoring & Documentation of a Clinically Significant Cardiopulmonary Event (CSCPE) (NUR47) DATE: REVIEWED: PAGES: 9/09 9/17 1 of 6 PS1094
SARASOTA MEMORIAL HOSPITAL TITLE: NURSING PROCEDURE Management, Monitoring & Documentation of a Clinically Significant Cardiopulmonary Event (CSCPE) (NUR47) DATE: REVIEWED: PAGES: 9/09 9/17 1 of 6 PS1094
Clearing the air.. How to assist and rescue neck breathing patients. Presented by: Don Hall MCD, CCC/SLP Sarah Markel RRT, MHA
 Clearing the air.. How to assist and rescue neck breathing patients Presented by: Don Hall MCD, CCC/SLP Sarah Markel RRT, MHA Learning Objectives Define common terms identified with total (laryngectomy)
Clearing the air.. How to assist and rescue neck breathing patients Presented by: Don Hall MCD, CCC/SLP Sarah Markel RRT, MHA Learning Objectives Define common terms identified with total (laryngectomy)
Addendum to the NRP Provider Textbook 6 th Edition Recommendations for specific modifications in the Canadian context
 Addendum to the NRP Provider Textbook 6 th Edition Recommendations for specific modifications in the Canadian context A subcommittee of the Canadian Neonatal Resuscitation Program (NRP) Steering Committee
Addendum to the NRP Provider Textbook 6 th Edition Recommendations for specific modifications in the Canadian context A subcommittee of the Canadian Neonatal Resuscitation Program (NRP) Steering Committee
Admission/Discharge Form for Infants Born in Please DO NOT mail or fax this form to the CPQCC Data Center. This form is for internal use ONLY.
 Selection Criteria Admission/Discharge Form for Infants Born in 2016 To be eligible, you MUST answer YES to at least one of the possible criteria (A-C) A. 401 1500 grams o Yes B. GA range 22 0/7 31 6/7
Selection Criteria Admission/Discharge Form for Infants Born in 2016 To be eligible, you MUST answer YES to at least one of the possible criteria (A-C) A. 401 1500 grams o Yes B. GA range 22 0/7 31 6/7
Table 1: The major changes in AHA / AAP neonatal resuscitation guidelines2010 compared to previous recommendations in 2005
 Table 1: The major changes in AHA / AAP neonatal guidelines2010 compared to previous recommendations in 2005 Resuscitation step Recommendations (2005) Recommendations (2010) Comments/LOE 1) Assessment
Table 1: The major changes in AHA / AAP neonatal guidelines2010 compared to previous recommendations in 2005 Resuscitation step Recommendations (2005) Recommendations (2010) Comments/LOE 1) Assessment
Evaluating the Effect of Flow and Interface Type on Pressures Delivered With Bubble CPAP in a Simulated Model
 Evaluating the Effect of Flow and Interface Type on Pressures Delivered With Bubble CPAP in a Simulated Model Stephanie A Bailes RRT-NPS NREMT-B, Kimberly S Firestone MSc RRT, Diane K Dunn RRT-NPS, Neil
Evaluating the Effect of Flow and Interface Type on Pressures Delivered With Bubble CPAP in a Simulated Model Stephanie A Bailes RRT-NPS NREMT-B, Kimberly S Firestone MSc RRT, Diane K Dunn RRT-NPS, Neil
5 Million neonatal deaths each year worldwide. 20% caused by neonatal asphyxia. Improvement of the outcome of 1 million newborns every year
 1 5 Million neonatal deaths each year worldwide 20% caused by neonatal asphyxia Improvement of the outcome of 1 million newborns every year International Liaison Committee on Resuscitation (ILCOR) American
1 5 Million neonatal deaths each year worldwide 20% caused by neonatal asphyxia Improvement of the outcome of 1 million newborns every year International Liaison Committee on Resuscitation (ILCOR) American
Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018
 Postnatal Steroids Use for Bronchopulmonary Dysplasia in 2018 + = Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018 AAP Policy Statement - 2002 This statement is intended for
Postnatal Steroids Use for Bronchopulmonary Dysplasia in 2018 + = Noah Hillman M.D. IPOKRaTES Conference Guadalajaira, Mexico August 23, 2018 AAP Policy Statement - 2002 This statement is intended for
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES
 DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
Mechanical Ventilation Principles and Practices
 Mechanical Ventilation Principles and Practices Dr LAU Chun Wing Arthur Department of Intensive Care Pamela Youde Nethersole Eastern Hospital 6 October 2009 In this lecture, you will learn Major concepts
Mechanical Ventilation Principles and Practices Dr LAU Chun Wing Arthur Department of Intensive Care Pamela Youde Nethersole Eastern Hospital 6 October 2009 In this lecture, you will learn Major concepts
Small Volume Nebulizer Treatment (Hand-Held)
 Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
WILAflow Elite Neonatal Ventilator. Non-invasive treatment for the most delicate patients.
 EN WILAflow Elite Neonatal Ventilator Non-invasive treatment for the most delicate patients. 0197 Infant Ventilation redefined A new generation in Infant Ventilation WILAflow Elite is a microprocessor
EN WILAflow Elite Neonatal Ventilator Non-invasive treatment for the most delicate patients. 0197 Infant Ventilation redefined A new generation in Infant Ventilation WILAflow Elite is a microprocessor
NRP Raising the Bar for Providers and Instructors
 NRP 2011 Raising the Bar for Providers and Instructors What is the same? 1. Minimum course requirement is Lessons 1 through 4 and Lesson 9. The NRP Provider Card requires renewal every 2 years. Your facility
NRP 2011 Raising the Bar for Providers and Instructors What is the same? 1. Minimum course requirement is Lessons 1 through 4 and Lesson 9. The NRP Provider Card requires renewal every 2 years. Your facility
Aim: Reduction in the rate of CLD in ELBW infants (<1000 grams) by 30% from its baseline of 72 % by January 2016.
 LIVE (Less Invasive Ventilation of ELBW infants) HEALTHY WVU Children s Hospital, Morgantown, WV, USA Rebecca Tilley, RN; Jamie Karr, RT; Tiffany Blosser, RN; Christy Dixon, RT; Melinda Connolly, ANP;
LIVE (Less Invasive Ventilation of ELBW infants) HEALTHY WVU Children s Hospital, Morgantown, WV, USA Rebecca Tilley, RN; Jamie Karr, RT; Tiffany Blosser, RN; Christy Dixon, RT; Melinda Connolly, ANP;
PROFESSOR DR. NUMAN NAFIE HAMEED الاستاذ الدكتور نعمان نافع الحمداني
 Lecture 6 PROFESSOR DR. NUMAN NAFIE HAMEED الاستاذ الدكتور نعمان نافع الحمداني Neonatal Resuscitation Program (NRP) 2010 MCQ? In neonatal resuscitation program, the preterm neonates need special preparations
Lecture 6 PROFESSOR DR. NUMAN NAFIE HAMEED الاستاذ الدكتور نعمان نافع الحمداني Neonatal Resuscitation Program (NRP) 2010 MCQ? In neonatal resuscitation program, the preterm neonates need special preparations
Respiratory Care Equipment
 Respiratory Care Equipment 1 / 7 2 / 7 3 / 7 Respiratory Care Equipment Respiratory. For patients that require medication delivery or disease state management, we also offer medication nebulizers, peak
Respiratory Care Equipment 1 / 7 2 / 7 3 / 7 Respiratory Care Equipment Respiratory. For patients that require medication delivery or disease state management, we also offer medication nebulizers, peak
Non-invasive ventilatory strategies, such as
 R E S E A R C H P A P E R Heated Humidified High Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure as Primary Mode of Respiratory Support for Respiratory Distress in Preterm Infants DEEPARAJ
R E S E A R C H P A P E R Heated Humidified High Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure as Primary Mode of Respiratory Support for Respiratory Distress in Preterm Infants DEEPARAJ
