NeuRx Diaphragm Pacing System Patient/Caregiver Information and Instruction Manual For Amyotrophic Lateral Sclerosis
|
|
|
- Aubrey Sylvia Murphy
- 5 years ago
- Views:
Transcription
1 NeuRx Diaphragm Pacing System Patient/Caregiver Information and Instruction Manual For Amyotrophic Lateral Sclerosis
2 TABLE OF CONTENTS GLOSSARY OF TERMS... 3 DEVICE DESCRIPTION... 5 CONTRAINDICATIONS... 8 INDICATIONS FOR USE... 8 INFORMATION ON CONDITION OR DISEASE... 8 WARNINGS AND PRECAUTIONS... 9 RISKS AND PROBABLE BENEFITS ALTERNATIVE PROCEDURES AND TREATMENTS BEFORE SURGERY: WHAT TO EXPECT SURGERY: WHAT TO EXPECT AFTER SURGERY: WHAT TO EXPECT FUNCTIONAL FEATURES CONDITIONING SESSION CONDITIONING WARNINGS BATTERY INSTALLATION ALARMS CARE OF PATIENT CABLE CARE OF THE EXITING ELECTRODE WIRES CARE OF EXIT SITES AND CONNECTOR CARE OF EXIT SITES HOW TO SHOWER OR BATHE CLEANING OF COMPONENTS TROUBLESHOOTING NeuRx EPG BACKUP INDIFFERENT ELECTRODE INTERCONNECT CLINICAL STUDIES WARRANTY STATEMENT TRAVELING WITH THE NeuRx DPS SERVICE REPLACEMENT PARTS STORAGE AND DISPOSAL OF PARTS SPECIFICATIONS (DETAILS OF DEVICE OPERATION) LABEL SYMBOLS USER ASSISTANCE
3 GLOSSARY OF TERMS Alcohol Wipe individually packaged pad saturated with 70% isopropyl alcohol. Used to cleanse the skin s surface. For single use only. ALS (amyotrophic lateral sclerosis) See the section of this manual called Information on Condition or Disease. Arrhythmia when your heart is beating either very slow, very fast or it feels like it skips a beat. It is also called an irregular heartbeat. Arterial Blood Gas (ABG) a blood test that measures the amounts of gases in the blood. The gases measured are oxygen and carbon dioxide. Backup Indifferent Electrode Interconnect can be used instead of the indifferent electrode if that part fails. ( Indifferent Electrode is explained further down in this glossary.) The difference is that it goes on the skin instead of under the skin. Capnothorax a complication caused by air going through the diaphragm and into your chest during the surgical procedure. Chronic Hypoventilation state in which too little air enters the lungs. This causes low levels of oxygen and high levels of carbon dioxide in the blood. This can be due to breathing that is not deep enough or too slow. Conditioning use of the NeuRx DPS to cause the breathing muscle (diaphragm) to contract. Connector Holder plastic shell which holds the electrode connector against the skin. Sticks to the skin with an adhesive like a bandage. Covering Bandage a bandage that covers the electrode wires where they come out through the skin. Diaphragm the main muscle that is used for breathing. Diathermy Treatment deep tissue heat treatment using energy from an electrical device. DPS See NeuRx DPS. ECG (electrocardiogram) test to check the electrical activity of the heart. The test helps to see if there has been a heart attack, an irregular heartbeat, or other heart problems. Electrode Connector a part of the NeuRx DPS which connects the electrode wires to the patient cable. Electrode Wire See Percutaneous Electrode Wire. EPG (NeuRx DPS External Pulse Generator) (stimulator) the part of the NeuRx DPS System that puts out the electrical signal which stimulates the diaphragm. It 3
4 runs on batteries. It is outside the body. It is connected (through the electrode connector) to the electrode wires which go into the diaphragm. Exit Site (electrode wire exit site) place where an electrode wire passes through your skin. Forced Vital Capacity (FVC) the volume of air that one can exhale from the lungs with force. Indifferent Electrode the one wire which is placed under the skin in the chest area. Laparoscope a small surgeon s tool with a camera on the end. The surgeon puts it into the abdomen through a half inch long incision. It lets the surgeon look inside the abdomen without the need for a large incision. Maximum Inspiratory Pressure (MIP or PImax) a measure of how well one can breathe in. NeuRx DPS (Diaphragm Pacing System) device which sends a small amount of electricity to the diaphragm muscle. This makes the diaphragm contract. Parts include the electrode wires, the external pulse generator ( EPG ) box, and the patient cable. Non-Invasive Ventilation (NIV) breathing help from a small machine that uses a mask that fits over the nose or nose and mouth. Patient Cable cable which connects the NeuRx EPG (external pulse generator box) to the electrode wires. It carries the electrical signal from the NeuRx EPG to the wires. Percutaneous Electrode Wire thin wire which is used to send a small amount of electricity to the diaphragm muscle. One end of the wire is implanted in the diaphragm. The other end is inserted in an electrode connector outside the body over the chest area. Percutaneous Endoscopic Gastrostomy (PEG) a tube through which nutrition is given. It is inserted by a surgeon through the skin and into the stomach. Phrenic Nerves the nerves which carry the signals that tell your diaphragm to either inhale or exhale. Pulmonary Function Test (PFT) breathing tests that show how well your lungs are working (exhaling and inhaling). One such test checks the maximum amount of air you can force out of your lungs (forced vital capacity or FVC). Another test checks how strong your breathing muscles are when you breathe in (maximum inspiratory pressure or MIP). Stimulator See EPG (External Pulse Generator). 4
5 DEVICE DESCRIPTION The name of the device is the NeuRx Diaphragm Pacing System (DPS). The NeuRx DPS has implantable parts and external parts. The implantable parts consist of five electrode wires which go through the skin. The tips of four electrode wires are placed into the diaphragm by your surgeon. The diaphragm is the breathing muscle. The tip of the fifth electrode wire is placed under the skin in the chest area to complete the wiring. The other ends of the five electrode wires come out through the skin in the chest area. The outside ends of the electrode wires will be placed in an electrode connector. The electrode connector is attached to the skin with the connector holder. The connector holder has an adhesive patch like a bandage. The electrode connector has a socket to plug in one end of the patient cable. The other end of the patient cable is connected to the NeuRx EPG. The NeuRx External Pulse Generator (EPG) is a stimulator box. The patient kit contains the external parts. These parts include the NeuRx EPG, patient cables, batteries and connector holders. The patient cable goes between the EPG and the electrode connector. The EPG is programmed by your healthcare provider. The EPG sends electrical signals to the diaphragm. The signal causes the muscle to contract in order to condition the muscle. See Figure 1 on page 6 for a picture of the NeuRx TM EPG, patient cable, connector holder, electrode connector, and electrode wires. See Figure 2 on page 7 for a drawing showing where the electrode wires are implanted in the body. This drawing also shows the position of the electrode wires, the patient cable, and the NeuRx EPG. 5
6 Electrode Wires Electrode Connector Connector Holder Patient Cable NeuRx External Pulse Generator (EPG) Figure 1. NeuRx TM External Pulse Generator (EPG), Patient Cable, Connector Holder, Electrode Connector, and Electrode Wires. 6
7 B A C Figure 2. Location in the body where the four NeuRx TM DPS electrode wires (A) are implanted in the diaphragm. A yellow arrow points to each of the four wire implant locations. The patient cable (B) carries the electrical signal from the EPG (C) to the electrode wires (A) which stimulate the diaphragm to contract. 7
8 CONTRAINDICATIONS Are there Any Reasons Why You Should Not Use the NeuRx Diaphragm Pacing System (Contraindications)? None. INDICATIONS FOR USE HUMANITARIAN USE DEVICE Humanitarian Device. Authorized by Federal law for use in the treatment of chronic hypoventilation in ALS patients. The effectiveness of this device for this use has not been demonstrated. CAUTION: Federal law (USA) restricts this device to sale, distribution and use by or on the order of a physician. The NeuRx Diaphragm Pacing System (DPS) is a device. It is meant for patients who have both ALS and diagnosed breathing problems. For the NeuRx DPS to work, your breathing muscle (diaphragm) must be able to respond to stimulation. This can be shown before surgery by a diaphragm movement test or by a breathing nerve test. You must be able to exhale at least 45% of the air that a typical healthy person could (in an FVC test). You must also be 21 years of age or older. The breathing nerves (phrenic nerves) provide a path from the spinal cord to the diaphragm. It is over this path that your brain sends messages to inhale. Both the right and left phrenic nerves must be at least partially working for the NeuRx DPS to work. Your phrenic nerves can be tested by your neurologist. See BEFORE SURGERY: WHAT TO EXPECT for information about these tests. INFORMATION ON CONDITION OR DISEASE Amyotrophic lateral sclerosis (ALS) is a disease that affects nerve cells in the brain and the spinal cord. Certain nerve cells called motor neurons reach from the brain to the spinal cord and from the spinal cord to the muscles throughout the body. When the motor neurons die because of ALS, the ability of the brain to start and control muscle movement is lost. Patients in the later stages of the disease may become totally paralyzed. Often ALS affects the motor neurons which reach to the muscles used in breathing. In this way, ALS can cause the breathing problems described in the Indications for Use section above. For more about ALS see the website of the ALS Association: 8
9 WARNINGS AND PRECAUTIONS WARNING: If you use the device full time, then you must always have a back-up means of breathing help (ventilation) available. If you do not feel that you are receiving enough breathing help from the NeuRx DPS, then you should use your back-up means right away. If you think there is any problem with your NeuRx DPS, then you should use your back-up means right away. When you switch to your backup means of breathing help, you should turn off the NeuRx DPS. WARNING: If this device is not used properly, it may cause injury or electrical hazard. Follow your healthcare provider s directions in the use of the NeuRx DPS. WARNING: Do NOT use the NeuRx DPS if you are pregnant. It is unknown if it is safe to use the NeuRx DPS if you are pregnant. The clinical trial did not involve pregnant women. WARNING: Avoid eating or drinking while conditioning with the NeuRx DPS. There is a risk of food or liquid entering your lungs. Do NOT have any food or liquid in your mouth when you start conditioning. If you use the NeuRx DPS full time, talk to your healthcare provider who is helping you with your NeuRx DPS about the ways to reduce the risk of food or liquid entering your lungs. WARNING: NeuRx DPS could interfere with some medical equipment. Some medical equipment could interfere with the NeuRx DPS. Call your healthcare provider who is helping you with your NeuRx DPS before having any of the following: Implanted cardiac pacemaker or defibrillator. The NeuRx DPS may interfere with these devices. There is not enough information to know for sure whether your NeuRx DPS can be used safely with these devices. Your healthcare provider should get an opinion from a specialist. Your healthcare provider should also test whether your NeuRx DPS interferes with devices like these if you need them. Surgery. Use of high-frequency surgical equipment may cause burns where the electrode wires pass through the skin. It might also damage the NeuRx EPG if connected. Magnetic Resonance Imaging (MRI) test. The NeuRx DPS has not been tested with MRI. MRI could cause the electrode wires to move. MRI could also cause unwanted tissue heating through the electrode wires. Diathermy treatment. Diathermy treatment is deep tissue heat treatment. It should not be done around the implanted electrode wires. Unwanted tissue heating through the electrode wires could occur. 9
10 External electrical stimulation such as transcutaneous electrical nerve stimulation (TENS). Such stimulation should not be done in the chest area near the electrode wires. Unwanted diaphragm contraction could occur. Shortwave or microwave therapy. Operating the NeuRx DPS close to (about 3 feet from) such equipment may interfere with your NeuRx DPS. WARNING: Do NOT use this device if the skin around the electrode wires gets swollen, infected, or inflamed. Call your healthcare provider who is helping you with your NeuRx DPS. WARNING FLAMMABILITY: Do NOT use the NeuRx EPG in rooms or spaces where oxygen is being used. This could cause a fire. CAUTION: Federal law (USA) restricts this device to sale, distribution and use by or on the order of a physician. CAUTION: If you think the device is not providing enough stimulation, then call your healthcare provider who is helping you with your NeuRx DPS. This could mean that the NeuRx DPS may not cause your diaphragm to contract. CAUTION: Some patients may feel skin irritation or sensitivity because of the NeuRx DPS. This may be due to the stimulation. It may also be due to the adhesive on the skin bandage. It could also be due to the transparent dressing used over the gauze that covers the electrode wires. (Tegaderm and Op-Site are examples of transparent dressings.) Call your healthcare provider who is helping you with your NeuRx DPS if this occurs. Irritation can usually be reduced by changing the settings of the EPG or removing the adhesive. CAUTION: Avoid touching the patient cable or electrode wires to other metal objects. Metal objects can conduct electricity. You may accidently get shocked from another metal surface. CAUTION: To avoid damage to the device, keep this device out of the reach of children. CAUTION: Do NOT attempt to open the NeuRx External Pulse Generator (EPG). Doing so can damage the device. CAUTION: To protect the NeuRx EPG against damage due to mechanical shock: Do NOT drop the NeuRx EPG. The EPG may break and not be available for use when needed. Use the protective black carrying case provided for transporting the NeuRx EPG when not in use. 10
11 CAUTION: To protect the NeuRx EPG against damage due to moisture, do NOT allow the NeuRx EPG to get wet. The EPG may quit working and not be available for use when needed. CAUTION: Do NOT get wet while using the NeuRx EPG. This includes bathing, showering, swimming, or any other activity in which you could get wet. While the NeuRx EPG is designed to keep moisture out, the EPG is not waterproof. If the location where the electrode wires pass through your skin (the exit sites) gets wet, it may affect treatment. You may receive less stimulation. You may also feel the sensation of the stimulation. Take steps to keep the exit sites from getting wet. Protect the exit sites with a gauze pad and transparent dressing (such as Tegaderm or Op-Site ). If the exit site gets wet, clean it with an alcohol wipe. Use an individually packaged pad saturated with 70% isopropyl alcohol. Allow the alcohol to air dry before use. CAUTION: Do NOT have the EPG connected during any type of electrical diagnostic test such as an electromyogram (EMG) or electrocardiogram (ECG). The use of the EPG during such tests could interfere with those tests. CAUTION ELECTROMAGNETIC INTERFERENCE: Some electrically powered equipment gives off electromagnetic waves which could interfere with your NeuRx EPG. When using your NeuRx EPG around electrical equipment, check the NeuRx EPG screen to make sure the EPG is working. To reduce the possibility of interference from other electrical equipment, do NOT use cables or accessories with your NeuRx EPG other than those specified. 11
12 RISKS AND PROBABLE BENEFITS INTRODUCTION The NeuRx DPS System was evaluated in a clinical study. The study involved 106 patients at 9 hospitals (8 in the U.S. and 1 in France). From the 106 patients in the study, 86 would have met the indications for the NeuRx DPS. The results discussed here are from those 86 patients. The results show that the benefits of using the NeuRx DPS System outweigh the health risks. Risks and probable benefits of the NeuRx DPS are described below. The results of the clinical study are also described. SURGICAL PROCEDURE RISKS There are risks of the surgery to implant the electrode wires. These serious adverse effects were seen in the study: Breathing failure occurred in one of the 86 patients in the study after surgery. This patient also had a feeding tube inserted during the DPS surgery. The feeding tube moved out of place and caused problems. Those problems led to the patient needing breathing support, through a tube in their throat, from a breathing machine. Problems caused by the abdominal air getting through the diaphragm and going into the chest cavity during the surgery (capnothorax). This happened in 15 out of 86 patients in the study (in about 1 out of every 6 patients). Two of these patients (in about 1 out of every 43 patients) needed special treatment for this problem. In one case, a small tube was placed in the chest for a short time. In one case, the patient had to stay in the hospital longer than normal. In the other 13 patients (in about 1 out of every 7 patients), the problem went away on its own. Reaction to anesthesia. One patient had a serious reaction to anesthesia. After starting anesthesia in the operating room the patient had a low heart rate and low blood pressure. The surgery was then cancelled before any incision was made. 12
13 Table 1. Serious adverse events from the study which related to the device or the surgery. Serious Adverse Event Reported Occurrence (Likelihood) of Serious Adverse Event Adverse Effect Reported Occurrence (Likelihood) of Adverse Effect capnothorax 2 out of 86 patients (1 out of every 43 patients) (2.3%) catheter placement extended hospitalization 1 out of 2 patients who had serious capnothorax 1 out of 2 patients who had serious capnothorax respiratory failure 1 out of 86 patients (1.2%) tracheostomy, mechanical ventilation 1 out of 1 patient who had respiratory failure related to the surgery anesthesia reaction 1 out of 87* patients (1.1%) low heart rate (bradycardia), low blood pressure (hypotension), and cancellation of surgery 1 out of 1 patient who had serious anesthesia reaction *87 patients = the 86 patients who were implanted and the 1 patient who was not implanted because of anesthesia reaction There are other surgical risks which were not seen in the clinical study. These include: Nerve, tissue or organ damage as a result of the procedure. Irregular heartbeat (cardiac arrhythmia) when the electrode wires are implanted in the chest. 13
14 DEVICE RISKS Once the surgery is over and you have recovered, there are risks associated with having the NeuRx DPS System. The following adverse effects were seen in the study but none were considered serious: Table 2. Adverse events related to the device (other than due to surgery). All were non-serious. Device Adverse Event infection where electrode wire passes through skin (exit site) discomfort from stimulation wire leading to diaphragm break outside the body wire under skin in chest area (indifferent electrode) break outside the body Reported Occurrence (Likelihood) of Device Adverse Event 8 out of 86 patients (1 out of every 11 patients) 22 out of 86 patients (1 out of every 4 patients) 28 out of 86 patients (about 1 out of every 3 patients) 18 out of 86 patients (about 1 out of every 5 patients) Notes (About Adverse Effects, Resolution, etc.) 7 resolved with antibiotics. 1 resolved after the wires were adjusted in the healthcare provider s office. None required removal of the wires. Resolved in 20 patients. Unresolved in 2 patients but tolerable (the patients continued using the NeuRx DPS ). Affected wire does not deliver therapy until repaired. Requires healthcare provider appointment to repair. No patients had to return to surgery to fix. The NeuRx DPS system does not deliver therapy until repair. Requires healthcare provider appointment to repair. No patients had to return to surgery to fix. 14
15 Device Adverse Event wire under skin in chest area (indifferent electrode) came out of the body broken NeuRx EPG (stimulator) broken patient cables Reported Occurrence (Likelihood) of Device Adverse Event 6 out of 86 patients (in about 1 out of every 14 patients) 6 out of 86 patients (in about 1 out of every 14 patients) 4 out of 86 patients (in about 1 out of every 22 patients) Notes (About Adverse Effects, Resolution, etc.) No patients had to return to surgery to fix. Fix involves reinserting the wire under the skin in the healthcare provider s office under topical anesthetic. Patient may temporarily use an alternate part (the backup indifferent electrode interconnect) until an appointment can be made for the healthcare provider to reinsert the wire under the skin. No NeuRx DPS therapy can occur without a working NeuRx EPG. A replacement NeuRx EPG can be shipped to the patient. No NeuRx DPS therapy can occur without a working patient cable. Two cables are provided with each kit. The patient cables are meant to be disposable. More replacement cables can be shipped to patient. pain where electrode wire passes through skin (exit site) skin irritation or inflammation 3 out of 86 patients (about 1 out of every 29 patients) 5 out of 86 patients (about 1 out of every 17 patients) All 3 cases were mild pain. One case resolved in 12 days. One case resolved in 5 days. One case did not resolve. The healthcare provider s report described said that pain was really mild and occurs rarely. All 5 cases were mild in severity. All cases resolved within a month and a half (except one case in which the time to resolve was unknown). The average time to resolve was 18 days. The longest known time to resolve was 43 days. 15
16 There are some device risks which were not seen in the study but are considered possible. These device risks include: Food or liquid entering your lungs while using the device. Bleeding where the electrode wire tip is attached to the diaphragm muscle. Electrode wire breaks inside the body. This could lead to a loss of therapy or less therapy. It could require another surgical procedure to correct. Allergic reaction to the materials which are used in the electrode wires. Use of the NeuRx DPS causing problems with devices such as heart pacemakers and defibrillators. (See the warning about this under Warnings and Precautions in this manual.) Problem interactions with some medical equipment: o High-frequency surgical equipment may cause burns where the electrode wires pass through the skin. It might also damage the NeuRx EPG if connected. o MRI could cause the electrode wires to move. MRI could also cause unwanted tissue heating through the electrode wires. o Diathermy treatment equipment used for deep tissue heat treatment. Unwanted tissue heating through the electrode wires could occur. o External electrical stimulation such as transcutaneous electrical nerve stimulation (TENS). If this is performed in the chest area near the electrode wires, unwanted diaphragm contraction could occur. o Shortwave or microwave therapy. Operating the NeuRx DPS close to (about 3 feet from) such equipment may interfere with your NeuRx DPS. o Electrical diagnostic test equipment such as an electromyogram (EMG) or electrocardiogram (ECG). The use of the EPG during such tests could interfere with those tests. Impeding treatment if the location where the electrode wires pass through your skin (the exit sites) gets wet. Electromagnetic interference: Some electrically powered equipment gives off electromagnetic waves which could interfere with your NeuRx EPG. Accidently getting shocked from another metal surface if the patient cable or electrode wires touch other metal objects. 16
17 PROBABLE BENEFITS You may get help from the NeuRx DPS because in a study: The patients who used the NeuRx DPS plus non-invasive ventilation (NIV) survived16 months longer (on average) than patients who just used NIV. This 16 month time frame is from the time of diagnosis. Survival time was measured until death or the need for a full time ventilator and a tube in the throat (tracheostomy). The patients who just used NIV were similar patients in a different study. The patients who used the NeuRx DPS plus NIV survived 9 months longer (on average) than patients who just used NIV. This is from the time they started using NIV. Some patients had a feeding tube placed with the NeuRx DPS. All of these patients survived past 30 days. Normally, 2 to 25 patients out of 100 (up to a fourth of all patients) would not survive this long. Some patients could not get used to or were unable to use NIV but used the NeuRx DPS. They survived an average of 16 months after getting the NeuRx DPS. Some patients had their sleep tested after using the NeuRx DPS for 4 months. They tended to have better sleep than before they received NeuRx DPS. ALTERNATIVE PROCEDURES AND TREATMENTS Other breathing treatments exist for patients with ALS. These include non-invasive ventilation through a mask or invasive ventilation with a tube through the throat. Non-invasive ventilation (NIV). The common device used for NIV is a bilevel or continuous positive airway pressure machine. These supply air to the mouth or nose. The choice of mask depends on fit, air leaks, looks, and claustrophobic concerns. A number of current papers show NIV may help patients with ALS. Current treatment advice is that all patients with ALS and respiratory symptoms, or an FVC less than 50%, should be offered NIV. In general, NIV may be used with the NeuRx DPS. Mechanical ventilation. At some point, ALS reduces the ability to breathe so much that invasive ventilation becomes the only choice for survival. Invasive ventilation uses a tube placed through the throat connected to a ventilator. This can prolong life for several years. 17
18 BEFORE SURGERY: WHAT TO EXPECT Your healthcare provider will need to do breathing tests to see if your breathing problems can be treated using the NeuRx DPS. These tests include checking how well your lungs are working (breathing out and breathing in). One test checks the maximum amount of air you can force out of your lungs (forced vital capacity or FVC). Another test checks how strong your breathing muscles are when you breathe in. A blood test may be done to see how much oxygen and carbon dioxide are in your blood. In another test, a small monitor is placed over your finger during sleep. The monitor measures the level of oxygen in your blood. The results of any one of these tests can show if you could be a candidate for the NeuRx DPS. SURGERY: WHAT TO EXPECT The surgeon will make a short cut (incision) in the skin of your abdomen. This incision will give the surgeon access to your diaphragm. The incision will be about half an inch long. A tube will be placed into the incision. Carbon dioxide gas will be pumped through the tube to fill your abdomen. A small tool with a camera on the end (laparoscope) will be inserted into the tube in your abdomen. After the surgeon looks into your abdomen, 3 more incisions will be made. These incisions will also be about half an inch long. A tube will be placed in each incision for the surgeon to work through. The surgeon will then insert a tool called a probe through one of the tubes. The probe will help the surgeon find the best location to place the electrode wire tips in the diaphragm. The surgeon will use the probe to test several locations on the diaphragm. Once the surgeon has found the 4 best locations, the surgeon will remove the probe. The surgeon will then insert the tips of 4 electrode wires in these locations. The surgeon will put the other ends of the 4 electrode wires on the outside of your body. These 4 wires will come out through your skin in the same area. A fifth electrode wire is then placed just beneath the skin in the same area to complete the wiring. After surgery, you will be able to see about 1 to 2 inches of each electrode wire outside your body. After surgery you may have an x-ray. This is done to check for abdominal air that may have gone to your chest (capnothorax). If needed, a tube may be placed in your chest to remove the carbon dioxide. After the surgery, your surgeon will decide how long you will have to stay in the hospital. 18
19 AFTER SURGERY: WHAT TO EXPECT Your NeuRx DPS may be implanted as a same-day surgery. Depending on your condition before and after surgery, you may need to spend the night. If you have a second procedure at the same time as your DPS implant you may need a longer stay in the hospital. You should discuss this with your surgeon prior to surgery. While you are recovering from surgery, a healthcare provider will adjust the settings on your NeuRx EPG. The purpose is to give the right amount of stimulation to your diaphragm. If the stimulation makes you uncomfortable, tell your healthcare provider. He or she can change the settings to reduce or eliminate the discomfort. Before you leave the hospital, the staff will give you detailed instructions on how to use the NeuRx DPS. You will also be given this manual. You will use the NeuRx DPS to condition your diaphragm. In conditioning, the NeuRx EPG sends a small amount of electricity through the wires to your diaphragm. This causes your diaphragm to contract. When your diaphragm is conditioned, it will do a better job of helping your breathing. See the sections on Conditioning Sessions and Conditioning Warnings for information about conditioning your diaphragm. You will need to keep dry while using the NeuRx EPG. The NeuRx EPG is not waterproof. Refer to the section HOW TO SHOWER OR BATHE on page 34 for instructions on bathing, showering, swimming, or any activity during which you could get wet. If your NeuRx EPG is properly cared for, the device should keep working for years. However, you will need to change the battery in your NeuRx EPG about every 500 hours of use. The following sections will walk you through the use, care and troubleshooting of the NeuRx EPG. 19
20 FUNCTIONAL FEATURES To turn the NeuRx EPG ON: Press and release the two buttons on the front of the NeuRx EPG at the same time. To turn the NeuRx EPG OFF: Press and release the two buttons on the front of the NeuRx EPG at the same time. The buttons must be pressed at the same time. This is a safety feature to guard against accidentally turning the EPG on or off (Figure 3). Figure 3. On/Off Buttons. The NeuRx EPG has a screen that shows what the NeuRx EPG is currently doing and if it is working properly. The NeuRx EPG screen shows the breaths-per-minute (BPM) and when the individual electrode wires are active. When the EPG sends electricity to the diaphragm to trigger inhaling, a letter A, B or C is shown below each of the electrode wire numbers (1, 2, 3, and 4). This shows that the NeuRx EPG is working properly. The example in Figure 4 below shows the NeuRx EPG with AAAA on its screen. This means that it is working properly during inhaling. When any letter A, B or C appears below the electrode wire numbers 1, 2, 3, and 4, it means that the NeuRx EPG is working. Other examples could be AABB, BABA, BACC, BBBB, BBCC, CCCC, etc., just as long A, B or C appears below each of the four electrode wire numbers. Figure 4. A, B or C showing that the NeuRx EPG is working properly during inhaling for each electrode wire (number 1, 2, 3, or 4). 20
21 During exhaling, a - character is shown below each electrode number (1, 2, 3, and 4) on the screen (Figure 5). This means that the NeuRx EPG is not active. It is not sending electrical stimulation. Figure 5. - shows that the NeuRx EPG is not active for each wire (number 1, 2, 3, and 4) during exhaling. Your NeuRx EPG has been programmed with settings that your healthcare provider determines are right for you. A? appearing below an electrode number (1, 2, 3, or 4) on the screen can occur normally when the EPG s electric signal is at a low level (Figure 6). Later the? should switch to a letter (A, B, or C) when the EPG s electric signal level gets high enough. Figure 6.? below an associated electrode wire (number 1, 2, 3, or 4) can occur normally when the EPG s electric signal is at a low level. If X appears below an electrode wire number (Figure 7), it could mean one of these things: 21
22 The wire is broken. The wire is loose in the electrode connector. The patient cable is broken The patient cable is not connected properly. The NeuRx EPG is broken. Please follow the troubleshooting guide provided in this manual. If you see that an electrode wire is loose or broken, call your healthcare provider who is helping you with your NeuRx DPS to discuss how to get it repaired. Figure 7. "X" could mean loose or broken electrode wires or patient cable or a broken EPG. CONDITIONING SESSION You will use the NeuRx DPS to condition your diaphragm. In conditioning, the NeuRx EPG sends a small amount of electricity through the wires to your diaphragm. This causes your diaphragm to contract. When your diaphragm is conditioned, it will do a better job of helping your breathing. Your healthcare provider will tell you how to use the NeuRx EPG. You should condition your diaphragm with the NeuRx EPG at least 3 times per day. Each session should last at least 30 minutes. You may find it helpful to use the NeuRx EPG for longer periods to help with breathing. The NeuRx EPG may be used at the same time as non-invasive ventilation. You may also sleep with the NeuRx EPG to assist with breathing difficulties at night. At visits to your ALS healthcare provider, the settings of your NeuRx EPG may be adjusted as needed. The number of breaths or stimulations you receive per minute may be changed. The power or charge of each stimulation may also be changed. 22
23 The following describes the process for a single conditioning session: 1. Wash and dry your hands before starting the conditioning session. 2. Clear secretions from your mouth before you condition. Do this again throughout the conditioning session. 3. Connect the patient cable to the electrode connector. To do this, hold the electrode connector between two fingers. Slide the patient cable into the connector holder. Secure the patient cable to the electrode connector (Figure 8). Figure 8. Connecting the patient cable to the electrode connector. 4. Insert the other end of the patient cable into the top of the NeuRx EPG. To do this, line up the arrows on the patient cable with the top of the EPG. Push the cable into the connection until it is secure (Figure 9). Figure 9. Connecting the patient cable to the NeuRx EPG. 23
24 5. Press the two buttons at the same time to turn the NeuRx EPG on. 6. When the conditioning session is over, press the two buttons at the same time to turn the NeuRx EPG off. 7. To disconnect the patient cable from the NeuRx EPG, firmly hold the EPG in one hand. Grasp the cable with two fingers as shown and pull (Figure 10). Figure 10. Disconnecting the patient cable. 8. Disconnect the patient cable from the electrode connector. To do this, hold the electrode connector between two fingers. Gently pull the patient cable out of the connector holder (Figure 11). Figure 11. Disconnecting the patient cable from the electrode connector. 9. Store all items in the patient kit provided. 24
25 CONDITIONING WARNINGS STOP the conditioning session if: You notice any change in heart rate or a feeling of chest discomfort. You have shortness of breath or if any discomfort persists or worsens. You want to eat or drink. You cannot manage secretions. WARNING: Avoid eating or drinking while conditioning with the NeuRx DPS. There is a risk of food or liquid entering your lungs. Do NOT have any food or liquid in your mouth when you start conditioning. If you use the NeuRx DPS full time, talk to your healthcare provider who is helping you with your NeuRx DPS about the ways to reduce the risk of food or liquid entering your lungs. CAUTION: Do NOT bathe, shower, swim or participate in any other aquatic activities while using the NeuRx EPG. The NeuRx EPG is not waterproof. If the exit site gets wet, clean it with an alcohol wipe. Use an individually packaged pad saturated with 70% isopropyl alcohol. Allow the alcohol to air dry before use. (See additional information under the Warnings and Precautions section.) BATTERY INSTALLATION Replace the battery every 500 hours of NeuRx DPS use. This is about every 20 days if you are using NeuRx DPS full time. The NeuRx EPG screen will initially show REPLACE BATT. It will alarm for 10 seconds every hour when your battery needs to be replaced. Replace the battery immediately if the NeuRx EPG screen shows LOW BATTERY and if the NeuRx EPG alarms for 20 seconds every minute. WARNING: The NeuRx EPG has a lithium battery. Take care to prevent fire or explosion. Do NOT short-circuit, recharge, puncture, burn, or crush the battery. Do NOT immerse the battery in water. Do NOT expose the battery to temperatures above 302ºF (150 C). To change the battery, follow these instructions: 1. Make sure that the NeuRx EPG is turned OFF prior to replacing the battery. 2. Use the provided flat blade screwdriver to loosen the screws on the back bottom of the NeuRx EPG. Remove the battery cover located on the back bottom of the NeuRx EPG (Figure 12). 3. Remove the old battery. Replace it with a new battery (Figure 13). 25
26 IMPORTANT: Use only the kind of battery specified in this manual. Do NOT use a standard alkaline battery in the NeuRx EPG. IMPORTANT: Put the battery in the correct position (see Figure 13 on page 27). 4. Replace the battery cover and secure with mounting screws. 5. Follow the local regulations when you dispose of old batteries. Figure 12. Loosen these screws on the battery cover to replace battery. 26
27 Figure 13. Replacing the disposable battery. 27
28 ALARMS The NeuRx EPG sounds an alarm if it detects any of the following problems: A beep lasting the duration of each inhaled breath will sound: o if the patient cable gets disconnected from the NeuRx EPG or if the connection is loose. o if the patient cable gets disconnected from the electrode connector or if the connection is loose. o if an exiting electrode wire is broken at the electrode connector. The alarm will repeat until the problem is resolved. A beep lasting 10 seconds may sound and REPLACE BATT appears on the screen. The 10 second alarm repeats once every hour. This means the NeuRx EPG has switched to the internal backup battery. It means that it is time to replace the main battery. A beep lasting 20 seconds may sound and LOW BATTERY appears on the screen. The 20 second alarm repeats once every minute. This means the internal backup battery is low. It means that it is time to replace the main battery immediately. CARE OF PATIENT CABLE The patient cable is shown in Figure 14. It runs between the electrode connector and the NeuRx EPG (as shown in Figure 1 near the front of this manual). Do NOT cut, kink or pull the patient cable. Do NOT manipulate the metal pins in the end pieces of the patient cable. Do NOT immerse the patient cable in water. Keep extra patient cables in a dry secure location. When in use, the one end of the patient cable should fit securely into the electrode connector. The other end should fit securely into the NeuRx EPG. Arrange the patient cable so that it is comfortable. It should allow you to move around without pulling on the exit site connector. Call your healthcare provider if you need replacement cables. 28
29 Figure 14. Patient Cable. CARE OF THE EXITING ELECTRODE WIRES Do NOT pull on the electrode wires coming through the skin. Do NOT cut the electrode wires. Use extreme caution when shaving the skin around the electrode wire exit site. CARE OF EXIT SITES AND CONNECTOR Keep the skin at the exit sites clean and dry. Do NOT scratch skin at exit sites. Clean the exit sites with an alcohol wipe. Use an individually packaged pad saturated with 70% isopropyl alcohol. Allow the alcohol to air dry before use. Then place a gauze dressing over the exit site. Be sure to cover all the wire with the gauze. Place a transparent dressing over the gauze. (Tegaderm and Op-Site are examples of transparent dressings.) Change the dressings every 3 days or more often if the dressing becomes wet or soiled. If the area becomes red, swollen, painful, or drainage appears, call your healthcare provider. Avoid touching the metal pins in the electrode connector. The connector holder should lie flat against the surface of the skin. Call your healthcare provider if any of the exiting electrode wires are frayed or broken. This electrode connector will snap into the connector holder (provided in the patient kit). Call your healthcare provider if the electrode connector appears cracked or broken. You should change the connector holder weekly or if it becomes soiled. Follow the steps beginning on the next page under Care of Exit Sites. 29
30 CARE OF EXIT SITES The steps described below will show you how to take care of the exit sites. The exit sites are the locations where the electrode wires pass through your skin. These steps will show you how to: remove the electrode connector from the old connector holder, clean the exit sites, place the electrode connector into a new connector holder, apply a new connector holder onto the skin, and cover the exiting electrode wires with gauze and transparent dressing. You should perform these steps every 3 days or more often if the dressing becomes wet or otherwise soiled. IMPORTANT REMINDER: Do NOT pull the electrode wires. Doing so may pull more electrode wire from under the skin. (See step 6 below.) 1. Wash and dry your hands before caring for the exit sites. 2. Using two fingers, grasp the electrode connector and tilt it down as shown in Figure 15. Figure 15 30
31 3. Remove the electrode connector from the connector holder. Now remove the connector holder (Figure 16). Figure Clean the exit sites with an alcohol wipe (with 70% isopropyl alcohol). Always wipe toward the exit site. Allow the alcohol to air dry before use (Figure 17). Figure 17 31
32 5. Once dry, place the electrode connector into a new connector holder as shown in Figure 18. Snap the electrode connector down into the connector holder. The gold pins should be facing out for the cable to plug into them. Figure Carefully remove the paper backing from a new connector holder. Make sure you do NOT pull on the exiting electrode wires (Figure 19). Figure 19 32
33 7. Carefully press the new connector holder onto the skin (Figure 20). Figure Place a 2 X 2 gauze pad over the exiting electrode wires (Figure 21). Figure Place a transparent dressing over the gauze. (Tegaderm and Op-Site are examples of transparent dressings.) Do NOT cover the gold pins with the transparent dressing (Figure 22). Figure 22 33
34 HOW TO SHOWER OR BATHE CAUTION: Do NOT get wet while using the NeuRx EPG. This includes bathing, showering, or swimming. The NeuRx EPG is not waterproof. If the exit site gets wet, clean it with an alcohol wipe. Use an individually packaged pad saturated with 70% isopropyl alcohol. Allow it to air dry before use. (See more information under the Warnings and Precautions section.) The following instructions are written for bathing. However, these instructions should be followed for any activity in which you would get wet. 1. Before bathing, disconnect the patient cable from the electrode connector (Figure 23). Figure Before bathing, cover the wires and the connector holder with a waterproof dressing (Figure 24). Figure 24 34
35 3. When you are done bathing, carefully uncover the wires and the connector holder. 4. If you notice moisture on the electrode connector, wipe it with an alcohol wipe. Use a wipe with 70% isopropyl alcohol. Allow the alcohol to air dry before use. CLEANING OF COMPONENTS CAUTION: Do NOT soak the NeuRx EPG or the patient cable in liquid. Clean the NeuRx EPG and the patient cable as needed. Wipe the outside of the NeuRx EPG and the patient cable using a clean, damp cloth with an anti-bacterial cleaning solution. Be sure to wipe away any leftover cleaner. TROUBLESHOOTING NeuRx EPG If you think that the NeuRx EPG is not working properly or multiple X s appear on the screen, please follow the steps below: 1. Check the connection between the patient cable and the NeuRx EPG. Check the connection between the patient cable and the electrode connector. Make sure these connections are secure. 2. Replace the patient cable with the spare cable in the patient kit. 3. Disconnect the patient cable from the NeuRx EPG. 4. Insert the test plug. a. When the test plug is inserted into the NeuRx EPG, the screen should show AAAA when activated. b. If AAAA does not appear on the screen or if there is still a problem, call Synapse Biomedical. For help with your NeuRx DPS device, first call your healthcare provider who is helping you with your NeuRx DPS. As a back-up you may also call: Synapse Biomedical Customer Service U.S. Toll Free: From outside the U.S.:
36 Use the following troubleshooting guide to help solve problems with your NeuRx TM EPG: Problem Action Pacing of the diaphragm stops. Discomfort while pacing. Bleeding, bruising, or infection where the electrode wires pass through the skin. Pain at the electrode wire exit site. Skin irritation or hypersensitivity to stimulation. Multiple X s appear on the NeuRx EPG screen. The NeuRx EPG is exposed to substantial amount of water or fluid. Alarm sounds during each stimulation. The NeuRx EPG beeps for 10 seconds every hour. (This means the NeuRx EPG is running on its backup battery.) Check the connection of the patient cable to the electrode connector. Check the connection of the patient cable to the NeuRx EPG. Try a different patient cable. Inspect the connections of the electrode wires to the electrode connector for breakage. STOP use of the NeuRx EPG and call your healthcare provider who is helping you with your NeuRx DPS. The EPG settings may need adjustment. Call your healthcare provider who is helping you with your NeuRx DPS. STOP use of the NeuRx EPG and call your healthcare provider who is helping you with your NeuRx DPS. Call your healthcare provider who is helping you with your NeuRx DPS. Try a different patient cable. If the problem continues, disconnect the patient cable from the NeuRx EPG and insert the test plug (found in the patient kit). If the EPG is working properly, use the Backup Indifferent Electrode Interconnect. If the problem continues, call your healthcare provider who is helping you with your NeuRx DPS. STOP use of the NeuRx EPG. Call your healthcare provider who is helping you with your NeuRx DPS. Check the connection of the patient cable to the electrode connector. Check the connection of the patient cable to the NeuRx EPG. Try a different patient cable. Inspect the connections of the electrode wires to the electrode connector for breakage. Replace the main battery as described in this manual. See the Battery Installation section of this manual for the steps. 36
37 Problem The NeuRx EPG beeps for 20 seconds every minute. (This means the NeuRx EPG is running on its backup battery and the backup battery is getting low.) Action Replace the main battery right away. See the Battery Installation section of this manual for the steps. 37
38 BACKUP INDIFFERENT ELECTRODE INTERCONNECT The troubleshooting section of this manual may tell you to use the backup indifferent electrode interconnect. This is called the backup connector for short. It is part number of the patient kit. The backup connector may be useful when the indifferent electrode under the skin has failed. This is when the NeuRx EPG displays XXXX (see Figure 25 below) but the patient cable and EPG still work correctly. Figure 25. EPG displaying XXXX. Follow these steps to use the backup connector: 1. Disconnect the patient cable from the electrode connector (Figure 26). Figure 26 38
39 2. Remove the backup connector (Figure 27) and the surface anode (Figure 28) from the kit. Figure 27. Backup Indifferent Electrode Interconnect Figure 28. Surface Anode Packet 3. Remove one surface anode (Figure 29) from the packet. Connect the brown cable to the white cable (Figure 30). Figure 29 Figure 30 39
40 4. Plug the backup connector into the electrode connector that is secured in the connector holder (Figure 31 and Figure 32). Figure 31 Figure Remove the plastic backing from the surface anode. Place the surface anode on the patient near the electrode connector holder. Plug the patient cable into the backup connector (Figure 33). Figure 33 40
41 If steps 1 through 5 were successful, then during conditioning the NeuRx EPG screen will show four letters in a row. An example ( AAAA ) is shown Figure 34. Other examples of correct operation are described under FUNCTIONAL FEATURES on page 20. If the system does not seem to be working correctly, refer to TROUBLESHOOTING NeuRx EPG on page 35. Figure 34. A, B or C showing that the NeuRx EPG is working properly during inhaling for each electrode wire (number 1, 2, 3, or 4). CLINICAL STUDIES The NeuRx DPS System was evaluated in a clinical study. The study was done at 9 clinical centers (8 in the U.S. and 1 in France). There were 106 patients with ALS who received the device. Eighty-six patients had breathing problems like those described in the NeuRx DPS Indications for Use. The risks and benefits of the NeuRx DPS are based on the results of these 86 patients. See the Risks and Probable Benefits section of this manual for the study results. WARRANTY STATEMENT The NeuRx EPG has a one year unconditional warranty from the date of installation. The patient cable has a 90-day unconditional warranty from the date of installation. 41
42 TRAVELING WITH THE NeuRx DPS The government puts out information for travelers with disabilities and medical conditions. You should check the Transportation Security Administration website for the latest advice. The address is: SERVICE You should not try to fix the NeuRx EPG on your own. Units needing service should be returned to Synapse Biomedical, Inc. REPLACEMENT PARTS The following replacement parts may be ordered from an authorized supplier. ITEM PART NUMBER ORDER QUANTITY Patient Cable Lithium Batteries (3 per pack) Connector Holder (30 per pack) Surface Electrodes (4 per pack) Airline Travel Batteries (3 per pack) Each 1 pack 1 pack 1 pack 1 pack STORAGE AND DISPOSAL OF PARTS Store all parts at a temperature between +20ºF and +140ºF. Secure parts in the patient kit provided when not in use. Dispose of all used connector holders and broken patient cables in the trash. Follow the local regulations when you dispose of old batteries. Take care to prevent fire or explosion. Do NOT burn the batteries. 42
43 SPECIFICATIONS (DETAILS OF DEVICE OPERATION) This section is included to comply with safety standards. Power Source 3.6-volt lithium battery Battery Life 500 hours Operating Temperature +41 to +100ºF Storage Temperature +20 to +140ºF Relative Humidity 10% to 85% Pulse Waveform type Pulse Amplitude Pulse Width Pulse Period Inspiration Interval Inspiration Rate regulated-current biphasic 5mA to 25mA 10usec to 200usec 20msec to 250msec 0.8sec to 1.5sec 8 to 18 breaths per minute 43
44 LABEL SYMBOLS Below is an explanation of the symbols used on this product and its packaging. SYMBOL EXPLANATION This symbol appears on the back of your EPG. It is just a reminder to look into this manual first when you have questions. This symbol appears on the back of your EPG. It means that the EPG has been tested to protect you from electrical shock. This is because the EPG is battery powered. IEC , Type BF Equipment. This symbol appears on the back of your EPG. It means that the EPG has met standards in Europe. The device meets Medical Device Directive 93/42/EEC. This has been certified by notified body number IPX4 This symbol appears on the back of your EPG. It means that it is protected against splashing water. This symbol appears on the top of your EPG near the output connector. It warns that output voltages may approach 50 volt D.C. while you are using the EPG. This symbol appears on the back of your EPG. It shows the serial number of your specific device. This is the company address outside the U.S.: Moustapha Diop 156 Place des Aubépines Montlignon France 44
45 USER ASSISTANCE For assistance with your NeuRx DPS device including troubleshooting, first call your healthcare provider who is helping you with your NeuRx DPS. Below are spaces to write down your healthcare provider s name and phone number: Healthcare Provider Name: Healthcare Provider Phone Number: If you cannot reach your healthcare provider, as a back-up you may also call: Synapse Biomedical Customer Service U.S. Toll Free: From outside the U.S.:
46 46
47 47
48 300 Artino Street Oberlin, Ohio U.S.A. Tel: Fax: France +33 (0) Other European Countries +33 (0) Synapse Biomedical Inc All Rights Reserved Rev A
NeuRx Diaphragm Pacing System Patient/Caregiver Instruction Manual
 NeuRx Diaphragm Pacing System Patient/Caregiver Instruction Manual Key Contact Information Doctor Nurse Contact Name Center Name Telephone Fax # Address Table of Contents LABEL SYMBOLS... 3 CONTROL SWITCH
NeuRx Diaphragm Pacing System Patient/Caregiver Instruction Manual Key Contact Information Doctor Nurse Contact Name Center Name Telephone Fax # Address Table of Contents LABEL SYMBOLS... 3 CONTROL SWITCH
Transcutaneous Electrical Nerve Stimulator (TENS)
 Transcutaneous Electrical Nerve Stimulator (TENS) How does TENS work? A TENS unit sends electrical pulses through the skin to start your body s own pain killers. The electrical pulses can release endorphins
Transcutaneous Electrical Nerve Stimulator (TENS) How does TENS work? A TENS unit sends electrical pulses through the skin to start your body s own pain killers. The electrical pulses can release endorphins
SIX PACK ABS Item No INSTRUCTION MANUAL. Read entire manual before operating this product. Use only as directed.
 SIX PACK ABS Item No. 206098 INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. WARNINGS If you are in the care of a physician, consult your physician before using
SIX PACK ABS Item No. 206098 INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. WARNINGS If you are in the care of a physician, consult your physician before using
Compatible with the following products TENS. Replacement Pads. User Manual. Last revised: V Last revised: V
 Compatible with the following products TENS Replacement Pads User Manual Last revised: V11-160923 Last revised: V4-170619 Contents Introduction 2-3 Parts 3 General Warnings and Safety 4-10 Using your TENS
Compatible with the following products TENS Replacement Pads User Manual Last revised: V11-160923 Last revised: V4-170619 Contents Introduction 2-3 Parts 3 General Warnings and Safety 4-10 Using your TENS
AWAY Model # RTLAGF-900 REV
 AWAY Model # RTLAGF-900 REV2.4.9.15 Contents User manual English 2 General Description 2 Intended Use 3 Included in this Package 4 Instructions for Use 7 Application Duration 7 Electrode Placement 8 Tips
AWAY Model # RTLAGF-900 REV2.4.9.15 Contents User manual English 2 General Description 2 Intended Use 3 Included in this Package 4 Instructions for Use 7 Application Duration 7 Electrode Placement 8 Tips
FlexAbs EMS ABDOMINAL STIMULATOR. FlexBody. EMS Body Stimulator INSTRUCTION MANUAL
 FlexAbs TM EMS ABDOMINAL STIMULATOR FlexBody TM EMS Body Stimulator INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. INTRODUCTION The FlexAbs TM and FlexBody TM
FlexAbs TM EMS ABDOMINAL STIMULATOR FlexBody TM EMS Body Stimulator INSTRUCTION MANUAL Read entire manual before operating this product. Use only as directed. INTRODUCTION The FlexAbs TM and FlexBody TM
Transcutaneous electrical nerve stimulation (TENS) in children
 Transcutaneous electrical nerve stimulation (TENS) in children Information for families Great Ormond Street Hospital for Children NHS Foundation Trust This information sheet explains about the use of transcutaneous
Transcutaneous electrical nerve stimulation (TENS) in children Information for families Great Ormond Street Hospital for Children NHS Foundation Trust This information sheet explains about the use of transcutaneous
Electrodes (for TransAeris System)
 Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
Surgical Procedures for Implantation of TransLoc Electrodes (for TransAeris System) 1 R L 1 TransAeris System TransLoc Electrode Kit 3 3 TransAeris Patient Kit (FrictionLoc) TransAeris Patient Kit (TransAeris
User Manual.
 User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
Patient & Family Guide. Using TENS for Pain.
 Patient & Family Guide 2017 Using TENS for Pain www.nshealth.ca Using TENS for Pain TENS (Transcutaneous Electrical Nerve Stimulation) provides stimulation that your body responds to by making natural
Patient & Family Guide 2017 Using TENS for Pain www.nshealth.ca Using TENS for Pain TENS (Transcutaneous Electrical Nerve Stimulation) provides stimulation that your body responds to by making natural
There are different types of ICDs:
 Guidelines for Patients with Implantable Devices o Implantable Cardioverter Defibrillator (ICD) Your ICD is approximately the size of a pager and includes the following parts: The ICD: A battery powered
Guidelines for Patients with Implantable Devices o Implantable Cardioverter Defibrillator (ICD) Your ICD is approximately the size of a pager and includes the following parts: The ICD: A battery powered
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T
 Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Transcutaneous Electrical Nerve Stimulation Device GF-3 / GF-3T Operation Manual Read Before Using GF-3-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
X-Plain Pacemaker Reference Summary
 X-Plain Pacemaker Reference Summary Introduction A pacemaker is a device that regulates the heart beat. More than half a million Americans use a pacemaker. If your doctor recommends that you have a pacemaker,
X-Plain Pacemaker Reference Summary Introduction A pacemaker is a device that regulates the heart beat. More than half a million Americans use a pacemaker. If your doctor recommends that you have a pacemaker,
THE REBUILDER SYSTEM. ReBuilder Model 300
 All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
User Manual Table of Contents. Instructions for Using the LaserTouchOne Charging the Unit... 5 Holding the Device... 5 Treatment Steps...
 User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
User Manual User Manual Table of Contents Introduction The LaserTouchOne... 3 Device Features and Controls (Photo of device)... 4 Product Contents... 4 Laser Safety... 4 Electrical Safety... 5 Instructions
THE REBUILDER SYSTEM. ReBuilder Model 300. ReBuilder Medical Inc. 636 Treeline Drive, Suite A Charles Town, WV
 THE REBUILDER SYSTEM ReBuilder Model 300 ReBuilder Medical Inc. 636 Treeline Drive, Suite A Charles Town, WV 25414 Phone: 304-725-2202 Fax: 304-725-4915 GET TO KNOW YOUR REBUILDER Confirm all the following
THE REBUILDER SYSTEM ReBuilder Model 300 ReBuilder Medical Inc. 636 Treeline Drive, Suite A Charles Town, WV 25414 Phone: 304-725-2202 Fax: 304-725-4915 GET TO KNOW YOUR REBUILDER Confirm all the following
Drainage Frequency: PATIENT GUIDE. Dressing Frequency: Every Drainage Weekly Drainage. Physician Contact Information. Dr. Phone:
 Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
Drainage Frequency: PATIENT GUIDE Dressing Frequency: Every Drainage Weekly Drainage Physician Contact Information Dr. Phone: CHEST DRAINAGE Pleural Space Insertion Site Cuff Exit Site Catheter Valve Connector
The Biomet EBI Bone Healing System. Patient Manual
 The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
Mini Pulse Electronic Stimulator
 Mini Pulse Electronic Stimulator Model: PM-180 Operating Manual IMPORTANT: Please read all instructions before using this product. Retain this manual for future reference. www.santamedical.com IMPORTANT
Mini Pulse Electronic Stimulator Model: PM-180 Operating Manual IMPORTANT: Please read all instructions before using this product. Retain this manual for future reference. www.santamedical.com IMPORTANT
THE REBUILDER SYSTEM. ReBuilder Model 2407
 All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
All warranty claims are to be processed by the manufacturer directly and must be accompanied by a receipt for purchase or copy of a dated order to dispense by a certified physician s office. DO NOT RETURN
Going Home with Your Peripheral Nerve Catheter and Pain Relief Pump
 Northwestern Memorial Hospital Patient Education CARE AND TREATMENT If you have any questions about the pain relief pump, please call the Anesthesia Pain Service. Going Home with Your Peripheral Nerve
Northwestern Memorial Hospital Patient Education CARE AND TREATMENT If you have any questions about the pain relief pump, please call the Anesthesia Pain Service. Going Home with Your Peripheral Nerve
NovoTTF -100A System. Patient Information and Operation Manual
 NovoTTF -100A System Patient Information and Operation Manual Document number QSD-QR-331 Revision 10 Release date: January 22, 2014 Table of contents 2 Glossary of Medical Terms 5 3 What is NovoTTF-100A
NovoTTF -100A System Patient Information and Operation Manual Document number QSD-QR-331 Revision 10 Release date: January 22, 2014 Table of contents 2 Glossary of Medical Terms 5 3 What is NovoTTF-100A
Biomet SpinalPak Non-Invasive Spine Fusion Stimulator System
 Biomet SpinalPak Non-Invasive Spine Fusion Stimulator System A Patient s Guide 100 Interpace Parkway Parsippany, NJ 07054 800-526-2579 www.biomet.com BNS231003 2009 EBI, LLC. All trademarks are the property
Biomet SpinalPak Non-Invasive Spine Fusion Stimulator System A Patient s Guide 100 Interpace Parkway Parsippany, NJ 07054 800-526-2579 www.biomet.com BNS231003 2009 EBI, LLC. All trademarks are the property
BLINDED MODE USERS GUIDE SUPPLEMENT
 BLINDED MODE USERS GUIDE SUPPLEMENT BLINDED MODE user s guide SUPPLEMENT WARNING: The SEVEN PLUS when set to Blinded Mode using the Data Manager Software will not provide real-time continuous glucose readings,
BLINDED MODE USERS GUIDE SUPPLEMENT BLINDED MODE user s guide SUPPLEMENT WARNING: The SEVEN PLUS when set to Blinded Mode using the Data Manager Software will not provide real-time continuous glucose readings,
Central venous access devices for children with lysosomal storage disorders
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
The ALS patient s guide to the NeuRx Diaphragm Pacing System
 The ALS patient s guide to the NeuRx Diaphragm Pacing System What is ALS? pacing the diaphragm: The NeuRx DPS for people with Amyotrophic Lateral Sclerosis Amyotrophic lateral sclerosis (ALS) is a disease
The ALS patient s guide to the NeuRx Diaphragm Pacing System What is ALS? pacing the diaphragm: The NeuRx DPS for people with Amyotrophic Lateral Sclerosis Amyotrophic lateral sclerosis (ALS) is a disease
Pain Relief Patch. Operation Manual Read Before Using MODEL # ET
 Pain Relief Patch Operation Manual Read Before Using Intended Use The ireliev Pain Relief Patch is intended for temporary relief of pain associated with sore and aching muscles in the upper and lower extremities
Pain Relief Patch Operation Manual Read Before Using Intended Use The ireliev Pain Relief Patch is intended for temporary relief of pain associated with sore and aching muscles in the upper and lower extremities
Information for patients, parents and guardians. Your child s doctor has recommended that your child has a procedure called an ablation.
 Having an ablation Information for patients, parents and guardians Your child s doctor has recommended that your child has a procedure called an ablation. An ablation is a treatment for an abnormal heartbeat.
Having an ablation Information for patients, parents and guardians Your child s doctor has recommended that your child has a procedure called an ablation. An ablation is a treatment for an abnormal heartbeat.
ICD Implantation Patient Information
 Melbourne Heart Rhythm ICD Implantation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers (the right atrium
Melbourne Heart Rhythm ICD Implantation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers (the right atrium
INSTRUCTIONS FOR USE TYMLOS (tim lows ) (abaloparatide) injection, for subcutaneous use
 INSTRUCTIONS FOR USE TYMLOS (tim lows ) (abaloparatide) injection, for subcutaneous use Instructions for Use Read and follow this Instructions for Use so that you inject TYMLOS pen the right way. Call
INSTRUCTIONS FOR USE TYMLOS (tim lows ) (abaloparatide) injection, for subcutaneous use Instructions for Use Read and follow this Instructions for Use so that you inject TYMLOS pen the right way. Call
E09 PEG. Expires end of March 2018 VITALITY.CO.UK
 VITALITY.CO.UK E09 PEG Expires end of March 2018 You can get more information and share your experiences at www.aboutmyhealth.org Tell us how useful you found this document at www.patientfeedback.org eidohealthcare.com
VITALITY.CO.UK E09 PEG Expires end of March 2018 You can get more information and share your experiences at www.aboutmyhealth.org Tell us how useful you found this document at www.patientfeedback.org eidohealthcare.com
PREPARING FOR REFLUX TESTING. Bravo Reflux Testing System. A simple way to evaluate your gastroesophageal reflux symptoms
 PREPARING FOR REFLUX TESTING Bravo Reflux Testing System A simple way to evaluate your gastroesophageal reflux symptoms HOW IT WORKS The test involves a miniature ph capsule, which is approximately the
PREPARING FOR REFLUX TESTING Bravo Reflux Testing System A simple way to evaluate your gastroesophageal reflux symptoms HOW IT WORKS The test involves a miniature ph capsule, which is approximately the
Part I: How to Assemble the Pumani CPAP Part II: How to Prepare the Baby for CPAP Part III: How to Attach the Baby to the Pumani CPAP
 Pumani CPAP User Manual Table of Contents Part I: How to Assemble the Pumani CPAP 3 Pumani CPAP Components 4 Assembly Instructions 5-15 Part II: How to Prepare the Baby for CPAP 16-18 Part III: How to
Pumani CPAP User Manual Table of Contents Part I: How to Assemble the Pumani CPAP 3 Pumani CPAP Components 4 Assembly Instructions 5-15 Part II: How to Prepare the Baby for CPAP 16-18 Part III: How to
Instructions for Use. Welcome!
 Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
User s instructions m-series (Micro) IN-m. Behind-the-ear
 User s instructions m-series (Micro) IN-m Behind-the-ear 2 The hearing aid and accessories shown in these instructions may not look the same as the ones you have. We furthermore reserve the right to make
User s instructions m-series (Micro) IN-m Behind-the-ear 2 The hearing aid and accessories shown in these instructions may not look the same as the ones you have. We furthermore reserve the right to make
Portable Equine Nebuliser System. User Manual
 Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
SLEEP IMPROVING WRISTBAND. Item No Owner s Guide
 SLEEP IMPROVING WRISTBAND Item No. 205350 Owner s Guide Thank you for purchasing the Sharper Image Sleep Improving Wristband. Based on ancient Chinese acupuncture principles, this biofeedback device uses
SLEEP IMPROVING WRISTBAND Item No. 205350 Owner s Guide Thank you for purchasing the Sharper Image Sleep Improving Wristband. Based on ancient Chinese acupuncture principles, this biofeedback device uses
InsuPad User Manual. Charger Base The charger base can be connected by the attached USB cable to the power adapter supplied for charging.
 InsuPad User Manual System overview The InsuPad is designed to improve the delivery of injected insulin into the blood by controlled warming of the area which surrounds the point of injection. The device
InsuPad User Manual System overview The InsuPad is designed to improve the delivery of injected insulin into the blood by controlled warming of the area which surrounds the point of injection. The device
FREQUENTLY ASKED QUESTIONS. Frequently Asked Questions
 Frequently Asked Questions THE DEVICE Do I need a prescription to use the device? Yes. You need a prescription from your eye care professional to use the TrueTear device. How and where can I purchase the
Frequently Asked Questions THE DEVICE Do I need a prescription to use the device? Yes. You need a prescription from your eye care professional to use the TrueTear device. How and where can I purchase the
Muscle Stimulation. Page 1
 Muscle Stimulation Page 1 Page 2 Page 3 Table of Contents: Get Started with the E-Wave Pg 4 Controls and Features Pg 4 Factory Settings Pg 6 Electrodes and Skin Care Pg 7 Batteries Pg 7 Indications Pg
Muscle Stimulation Page 1 Page 2 Page 3 Table of Contents: Get Started with the E-Wave Pg 4 Controls and Features Pg 4 Factory Settings Pg 6 Electrodes and Skin Care Pg 7 Batteries Pg 7 Indications Pg
Pacemaker and AV Node Ablation Patient Information
 Melbourne Heart Rhythm Pacemaker and AV Node Ablation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers
Melbourne Heart Rhythm Pacemaker and AV Node Ablation Patient Information The Heart The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two upper chambers
Compact Massager Instructions
 Instructions 2 Please read all instructions carefully to familiarise yourself with your new massager before using. Save these instructions for further reference. For any further assistance or information
Instructions 2 Please read all instructions carefully to familiarise yourself with your new massager before using. Save these instructions for further reference. For any further assistance or information
Complex Care Hub Manual: Continuous Positive Airway Pressure (CPAP) Ventilation
 Complex Care Hub Manual: Continuous Positive Airway Pressure (CPAP) Ventilation Table of Contents 1. What is Continuous Positive Airway Pressure (CPAP)?... 2 2. Important information for support worker...
Complex Care Hub Manual: Continuous Positive Airway Pressure (CPAP) Ventilation Table of Contents 1. What is Continuous Positive Airway Pressure (CPAP)?... 2 2. Important information for support worker...
INSTRUCTIONS FOR USE HUMIRA 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE
 INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
Algovita. MRI Procedure Guidelines. Spinal Cord Stimulation System. 201x
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 201x Algovita
Lesson 1: Types of ECG s
 Electrocardiography Lesson 1: Types of ECG s Objectives: Distinguish between a single-channel ECG machine and a multi-channel machine. Distinguish between a manual ECG machine and an automatic ECG machine.
Electrocardiography Lesson 1: Types of ECG s Objectives: Distinguish between a single-channel ECG machine and a multi-channel machine. Distinguish between a manual ECG machine and an automatic ECG machine.
STATE OF OKLAHOMA 2014 EMERGENCY MEDICAL SERVICES PROTOCOLS
 5M VENTRICULAR ASSIST DEVICE (VAD) MANAGEMENT ADULT EMERGENCY MEDICAL DISPATCHER EMERGENCY MEDICAL RESPONDER EMT EMT-INTERMEDIATE 85 ADVANCED EMT PARAMEDIC A Ventricular Assist Device, or VAD, is a mechanical
5M VENTRICULAR ASSIST DEVICE (VAD) MANAGEMENT ADULT EMERGENCY MEDICAL DISPATCHER EMERGENCY MEDICAL RESPONDER EMT EMT-INTERMEDIATE 85 ADVANCED EMT PARAMEDIC A Ventricular Assist Device, or VAD, is a mechanical
User s instructions The Flash Series. FL-CIC Completely-in-canal
 User s instructions The Flash Series FL-CIC Completely-in-canal The hearing aid and accessories shown in these user s instructions may not look the same as the ones you have. We furthermore reserve the
User s instructions The Flash Series FL-CIC Completely-in-canal The hearing aid and accessories shown in these user s instructions may not look the same as the ones you have. We furthermore reserve the
Procedures/Risks:central venous catheter
 Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
CAUTION...2 INSTRUCTION...5 IF-4000 INTERFERENTIAL STIMUATOR CONTROLS AND FUCTIONS...7 HOW TO USE YOUR IF-4000 STIMULATOR...14 ELECTRODES CARE...
 CONTENTS CAUTION...2 INSTRUCTION...5 IF-4000 INTERFERENTIAL STIMUATOR CONTROLS AND FUCTIONS...7 HOW TO USE YOUR IF-4000 STIMULATOR...14 SKIN CARE...17 ELECTRODES CARE...18 BATTERY REPLACEMENT...20 TECHNICAL
CONTENTS CAUTION...2 INSTRUCTION...5 IF-4000 INTERFERENTIAL STIMUATOR CONTROLS AND FUCTIONS...7 HOW TO USE YOUR IF-4000 STIMULATOR...14 SKIN CARE...17 ELECTRODES CARE...18 BATTERY REPLACEMENT...20 TECHNICAL
Pacemaker and ICD Interrogation
 Pacemaker and ICD Interrogation To receive the maximum benefit from your pacemaker, you will need to have regular follow-up appointments to ensure that it is working properly. This follow up can be arranged
Pacemaker and ICD Interrogation To receive the maximum benefit from your pacemaker, you will need to have regular follow-up appointments to ensure that it is working properly. This follow up can be arranged
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT. Instruction Manual
 HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
Your Nerve Block & Home Pump For Hip Surgery
 Your Nerve Block & Home Pump For Hip Surgery Department of Anesthesiology and Perioperative Medicine www.happypatient.org Updated 3/10 Why? To Help Control Postoperative Pain at Home A pump connected to
Your Nerve Block & Home Pump For Hip Surgery Department of Anesthesiology and Perioperative Medicine www.happypatient.org Updated 3/10 Why? To Help Control Postoperative Pain at Home A pump connected to
Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System.
 Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Algovita Spinal Cord Stimulation System MRI Procedure Guidelines Read this manual before performing an MRI scan on a patient implanted with an Algovita Spinal Cord Stimulation System. ONLY 0300-000175-001
Cough Assist. Information for patients, families and carers Therapy Services
 Cough Assist Information for patients, families and carers Therapy Services PROUD TO MAKE A DIFFERENCE SHEFFIELD TEACHING HOSPITALS NHS FOUNDATION TRUST page 2 of 16 Table of contents Why do I need a Cough
Cough Assist Information for patients, families and carers Therapy Services PROUD TO MAKE A DIFFERENCE SHEFFIELD TEACHING HOSPITALS NHS FOUNDATION TRUST page 2 of 16 Table of contents Why do I need a Cough
Compressor Nebulizer. Guidebook
 NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe. Plunger rod Used plunger rod
 Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe Guide to parts Before use After use Plunger rod Used plunger rod Finger grip Label
Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe Guide to parts Before use After use Plunger rod Used plunger rod Finger grip Label
Positive Pressure Therapy
 Positive Pressure Therapy Positive Pressure Therapy...2 What is Sleep Apnea?....2 Positive Pressure Machines..................................................... 4 Types..................................................................................
Positive Pressure Therapy Positive Pressure Therapy...2 What is Sleep Apnea?....2 Positive Pressure Machines..................................................... 4 Types..................................................................................
You and your pacemaker
 You and your pacemaker You and your pacemaker Why do I need a pacemaker? A pacemaker will help improve your heart s rate. It is used to help your heart beat at a normal rate. What is a pacemaker? A pacemaker
You and your pacemaker You and your pacemaker Why do I need a pacemaker? A pacemaker will help improve your heart s rate. It is used to help your heart beat at a normal rate. What is a pacemaker? A pacemaker
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
FDA CLEARED for OVER THE COUNTER SALES (OTC) NO PRESCRIPTION REQUIRED. HiDow Wireless Tens/EMS Systems WIRELESS DEVICE INSTRUCTION MANUAL
 FDA CLEARED for OVER THE COUNTER SALES (OTC) NO PRESCRIPTION REQUIRED HiDow Wireless Tens/EMS Systems WIRELESS DEVICE INSTRUCTION MANUAL 1 INTRODUCTION TO TENS EXPLANATION OF PAIN Pain is a warning system
FDA CLEARED for OVER THE COUNTER SALES (OTC) NO PRESCRIPTION REQUIRED HiDow Wireless Tens/EMS Systems WIRELESS DEVICE INSTRUCTION MANUAL 1 INTRODUCTION TO TENS EXPLANATION OF PAIN Pain is a warning system
TWIST STEPPER NO. 045
 TWIST STEPPER NO. 045 IMPORTANT: Read all instructions carefully before using this product. Retain owner s manual for future reference. For customer service, please contact: support@sunnyhealthfitness.com
TWIST STEPPER NO. 045 IMPORTANT: Read all instructions carefully before using this product. Retain owner s manual for future reference. For customer service, please contact: support@sunnyhealthfitness.com
Children's (Pediatric) PICC Line Placement
 Scan for mobile link. Children's (Pediatric) PICC Line Placement A peripherally inserted central catheter (PICC line) is most often used to deliver medication over a long period. The doctor or nurse inserts
Scan for mobile link. Children's (Pediatric) PICC Line Placement A peripherally inserted central catheter (PICC line) is most often used to deliver medication over a long period. The doctor or nurse inserts
IC031 Magnetic Belt Drive Performance Indoor Cycle Bike
 IC031 Magnetic Belt Drive Performance Indoor Cycle Bike USER MANUAL IMPORTANT: Read all instructions carefully before using this product. Retain owner s manual for future reference. For customer service,
IC031 Magnetic Belt Drive Performance Indoor Cycle Bike USER MANUAL IMPORTANT: Read all instructions carefully before using this product. Retain owner s manual for future reference. For customer service,
Thank you very much for agreeing to participate in the Ontario Sleep and Brain Health Study
 Thank you very much for agreeing to participate in the Ontario Sleep and Brain Health Study By participating in this study, you will play a key role in helping us better understand the links between sleep
Thank you very much for agreeing to participate in the Ontario Sleep and Brain Health Study By participating in this study, you will play a key role in helping us better understand the links between sleep
Your Nerve Block & Home Pump For Shoulder Surgery
 Your Nerve Block & Home Pump For Shoulder Surgery Department of Anesthesiology and Perioperative Medicine www.happypatient.org Updated 10/09 Why? To Help Control Postoperative Pain at Home A pump connected
Your Nerve Block & Home Pump For Shoulder Surgery Department of Anesthesiology and Perioperative Medicine www.happypatient.org Updated 10/09 Why? To Help Control Postoperative Pain at Home A pump connected
> Cue vaping device. > One e-liquid cartridge. > USB charging cord. > One Buddy Tip for sharing CUE. > This Quick Start Guide
 What s in the box? > Cue vaping device > One e-liquid cartridge > USB charging cord > One Buddy Tip for sharing CUE > This Quick Start Guide Getting started y Charge the CUE device When the light turns
What s in the box? > Cue vaping device > One e-liquid cartridge > USB charging cord > One Buddy Tip for sharing CUE > This Quick Start Guide Getting started y Charge the CUE device When the light turns
Comfortmax Thermax Hot/Cold Water Circulation System. Instructions for Use
 Comfortmax Thermax Hot/Cold Water Circulation System Instructions for Use THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS
Comfortmax Thermax Hot/Cold Water Circulation System Instructions for Use THIS DEVICE CAN BE COLD ENOUGH TO CAUSE SERIOUS INJURY. SERIOUS ADVERSE REACTIONS AND SAFETY HAZARDS MAY OCCUR WHEN USING THIS
KNEE ARTHROSCOPY SURGERY
 KNEE ARTHROSCOPY SURGERY SUMMARY OF PROCEDURE Arthroscopy involves looking at the inside of the knee joint with a small telescope and camera (arthroscope). The image is projected onto a television monitor
KNEE ARTHROSCOPY SURGERY SUMMARY OF PROCEDURE Arthroscopy involves looking at the inside of the knee joint with a small telescope and camera (arthroscope). The image is projected onto a television monitor
Breathe deep...and live. The SCI patient s guide to increasing independence with the NeuRx Diaphragm Pacing System
 Breathe deep...and live. The SCI patient s guide to increasing independence with the NeuRx Diaphragm Pacing System PACING THE DIAPHRAGM: The NeuRx DPS Program for People with Spinal Cord Injury (SCI) and
Breathe deep...and live. The SCI patient s guide to increasing independence with the NeuRx Diaphragm Pacing System PACING THE DIAPHRAGM: The NeuRx DPS Program for People with Spinal Cord Injury (SCI) and
Remote control 2 guide
 Remote control 2 guide Thank you Thank you for choosing remote control 2 for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare
Remote control 2 guide Thank you Thank you for choosing remote control 2 for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare
TWISTING STAIR STEPPER WITH BANDS NO. 068 USER MANUAL
 TWISTING STAIR STEPPER WITH BANDS NO. 068 USER MANUAL IMPORTANT: Read all instructions carefully before using this product. Retain owner s manual for future reference. For customer service, please contact:
TWISTING STAIR STEPPER WITH BANDS NO. 068 USER MANUAL IMPORTANT: Read all instructions carefully before using this product. Retain owner s manual for future reference. For customer service, please contact:
UMC HEALTH SYSTEM Lubbock, Texas :
 Consent for Commonly Performed Procedures in the Adult Critical Care Units I, the undersigned, understand that the adult intensive and intermediate care units ( critical care units ) are places where seriously
Consent for Commonly Performed Procedures in the Adult Critical Care Units I, the undersigned, understand that the adult intensive and intermediate care units ( critical care units ) are places where seriously
Medescan Nebuliser Med - S600A Instructions Manual
 Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
Device and Activator. Table of Contents INFORMATION FOR PATIENTS AND INSTRUCTIONS FOR USE
 Device and Activator INFORMATION FOR PATIENTS AND INSTRUCTIONS FOR USE Table of Contents 1. Purpose of the inflow Device...2 2. Description of the inflow Device...2 3. When Should This Device Not Be Used?...3
Device and Activator INFORMATION FOR PATIENTS AND INSTRUCTIONS FOR USE Table of Contents 1. Purpose of the inflow Device...2 2. Description of the inflow Device...2 3. When Should This Device Not Be Used?...3
CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear)
 CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear) Table of Contents Part 1 Learning about the Catheter...2 Part 2 Caring for Your Child s Catheter...3 A. Preventing
CARE OF A TUNNELED CATHETER (HICKMAN & BROVIAC ) with a Needleless Connector (MicroClave Clear) Table of Contents Part 1 Learning about the Catheter...2 Part 2 Caring for Your Child s Catheter...3 A. Preventing
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide FreeStyle LibreLink app A FreeStyle Libre product IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide FreeStyle LibreLink app A FreeStyle Libre product IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the
Cardiac Resynchronisation Therapy Patient Information
 Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
Melbourne Heart Rhythm Cardiac Resynchronisation Therapy Patient Information Normal Heart Function The heart is a pump responsible for maintaining blood supply to the body. It has four chambers. The two
Cardiac Catheterization Lab Procedures
 Patient Education Cardiac Catheterization Lab Procedures This booklet describes cardiac catheterization. It also details how to prepare for the procedure and the care needed after it is done. Before You
Patient Education Cardiac Catheterization Lab Procedures This booklet describes cardiac catheterization. It also details how to prepare for the procedure and the care needed after it is done. Before You
USER S MANUAL. Visit for more information on e-pulse products and offers.
 USER S MANUAL Visit www.epulsemassage.com for more information on e-pulse products and offers. Manufactured for and distributed by Enovative Technologies, LLC 2016 Enovative Technologies, LLC INTRODUCTION
USER S MANUAL Visit www.epulsemassage.com for more information on e-pulse products and offers. Manufactured for and distributed by Enovative Technologies, LLC 2016 Enovative Technologies, LLC INTRODUCTION
You have been referred to the Hamilton General Hospital to assess if having your mitral heart valve repaired with a mitral clip is right for you.
 Mitral Clip Procedure You have been referred to the Hamilton General Hospital to assess if having your mitral heart valve repaired with a mitral clip is right for you. 2 Mitral Clip Procedure What is mitral
Mitral Clip Procedure You have been referred to the Hamilton General Hospital to assess if having your mitral heart valve repaired with a mitral clip is right for you. 2 Mitral Clip Procedure What is mitral
COPYRIGHT Ramsey Medical, Inc.
 Now your petmap+ii can monitor ECG 1 This manual applies to the operation of the ECG Module (REF# 9026), available as an option with the petmap+ii. The ECG Module requires a petmap+ii to be useful. Read
Now your petmap+ii can monitor ECG 1 This manual applies to the operation of the ECG Module (REF# 9026), available as an option with the petmap+ii. The ECG Module requires a petmap+ii to be useful. Read
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
Compressor Nebulizer. Model UN-014
 Compressor Nebulizer Model UN-014 Instruction Manual Original Manuel d instructions Traduction Manual de Instrucciones Traducción Manuale di Istruzioni Traduzione ITALIANO ESPAÑOL FRANÇAIS ENGLISH 1WMPD4003398
Compressor Nebulizer Model UN-014 Instruction Manual Original Manuel d instructions Traduction Manual de Instrucciones Traducción Manuale di Istruzioni Traduzione ITALIANO ESPAÑOL FRANÇAIS ENGLISH 1WMPD4003398
About Your Ventricular Assist Device (VAD) Surgery
 About Your Ventricular Assist Device (VAD) Surgery Why do I need surgery for a Heart Pump or Ventricular Assist Device (VAD)? VAD s may help you live longer. These electric powered heart pumps are put
About Your Ventricular Assist Device (VAD) Surgery Why do I need surgery for a Heart Pump or Ventricular Assist Device (VAD)? VAD s may help you live longer. These electric powered heart pumps are put
Botulinum Toxin Injections
 KAISER PERMANENTE SAN FRANCISCO DEPARTMENT OF NEUROLOGY Office Procedures included: - Botulinum Toxin Injections - Electroencephalogram (EEG) - Electromyography (EMG) and Nerve Condition Studies (NCS)
KAISER PERMANENTE SAN FRANCISCO DEPARTMENT OF NEUROLOGY Office Procedures included: - Botulinum Toxin Injections - Electroencephalogram (EEG) - Electromyography (EMG) and Nerve Condition Studies (NCS)
SaeboStim Micro. Saebo INSTRUCTION MANUAL
 SaeboStim Micro Saebo INSTRUCTION MANUAL Introduction Saebo is pleased to provide you with the latest in evidence-based upper limb rehabilitation. The SaeboStim Micro provides sensory electrical stimulation
SaeboStim Micro Saebo INSTRUCTION MANUAL Introduction Saebo is pleased to provide you with the latest in evidence-based upper limb rehabilitation. The SaeboStim Micro provides sensory electrical stimulation
Medtronic MiniMed Insulin Infusion Pumps
 Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Medtronic MiniMed Insulin Infusion Pumps Patients should always discuss potential risks and benefits with a physician. Please review the product manual prior to use for detailed instructions and disclosure.
Home Sleep Test. User Manual. One Night Test. For help at any time, call Also, please visit to watch our video.
 Home Sleep Test User Manual For help at any time, call 1-877-753-3776. Also, please visit www.novasom.com to watch our video. One Night Test Before You Begin Before You Begin The AccuSom unit: cannot be
Home Sleep Test User Manual For help at any time, call 1-877-753-3776. Also, please visit www.novasom.com to watch our video. One Night Test Before You Begin Before You Begin The AccuSom unit: cannot be
Quick Reference Guide
 FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
FLASH GLUCOSE MONITORING SYSTEM Quick Reference Guide IMPORTANT USER INFORMATION Before you use your System, review all the product instructions and the Interactive Tutorial. The Quick Reference Guide
Your surgeon will order pre-operative testing before you have surgery.
 Tests You May Need Prior to Surgery Your surgeon will order pre-operative testing before you have surgery. These tests give your surgeon valuable information regarding your current health condition. Below
Tests You May Need Prior to Surgery Your surgeon will order pre-operative testing before you have surgery. These tests give your surgeon valuable information regarding your current health condition. Below
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide)
 Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Allergy Reliever. User Manual. AR 1 Series
 Allergy Reliever User Manual AR 1 Series Content Introduction Parts Warnings Using the Allergy Reliever Battery Information Specification Maintenance and Cautions Explanation of Symbols on Unit 2 3 4 5
Allergy Reliever User Manual AR 1 Series Content Introduction Parts Warnings Using the Allergy Reliever Battery Information Specification Maintenance and Cautions Explanation of Symbols on Unit 2 3 4 5
Take control of your pain therapy. Getting started with Algovita SCS.
 Take control of your pain therapy. Getting started with Algovita SCS. The two-step approach to spinal cord stimulation therapy. You ve talked with your doctor. You ve discussed it with your family. You
Take control of your pain therapy. Getting started with Algovita SCS. The two-step approach to spinal cord stimulation therapy. You ve talked with your doctor. You ve discussed it with your family. You
Out of pain. Into relief.
 Spinal Cord Stimulation for Chronic Pain USING YOUR TRIAL STIMULATION SYSTEM Out of pain. Into relief. SCREENING TRIAL GUIDE AND PATIENT DIARY You are taking an important step in having a trial for spinal
Spinal Cord Stimulation for Chronic Pain USING YOUR TRIAL STIMULATION SYSTEM Out of pain. Into relief. SCREENING TRIAL GUIDE AND PATIENT DIARY You are taking an important step in having a trial for spinal
Function of Cell Membrane
 Function of Cell Membrane Electrical Potential Concept Electrical Circuit Human Cell electrical circuit Is Human Cell rechargeable? Why Negative Potential Is Important. Why Positive Potential Can Be Harmful
Function of Cell Membrane Electrical Potential Concept Electrical Circuit Human Cell electrical circuit Is Human Cell rechargeable? Why Negative Potential Is Important. Why Positive Potential Can Be Harmful
Compressor Nebulizer. Guidebook
 NSTRU Compressor Nebulizer Guidebook MODEL: CN02WS (MQ6003) READ ALL INSTRUCTION BEFORE USE Airial TM Compressor Nebulizer INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
NSTRU Compressor Nebulizer Guidebook MODEL: CN02WS (MQ6003) READ ALL INSTRUCTION BEFORE USE Airial TM Compressor Nebulizer INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
PEMF Users Manual. IMPORTANT: Read Me Before Powering On Your Machine!
 PEMF 4000 Users Manual IMPORTANT: Read Me Before Powering On Your Machine! 2013 Lemuria Technologies LLC At the time of publishing the PEMF 4000 is not approved by the FDA. It is for experimental use or
PEMF 4000 Users Manual IMPORTANT: Read Me Before Powering On Your Machine! 2013 Lemuria Technologies LLC At the time of publishing the PEMF 4000 is not approved by the FDA. It is for experimental use or
GASTRECTOMY. Date of Surgery. Please bring this booklet the day of your surgery. QHC#34
 GASTRECTOMY Date of Surgery Please bring this booklet the day of your surgery. QHC#34 What is a Gastrectomy? A Gastrectomy is the surgical removal of all or part of the stomach. The stomach is the digestion
GASTRECTOMY Date of Surgery Please bring this booklet the day of your surgery. QHC#34 What is a Gastrectomy? A Gastrectomy is the surgical removal of all or part of the stomach. The stomach is the digestion
Colonic (Large Intestine) Manometry
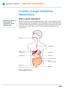 Patient and Family Education Colonic (Large Intestine) Manometry Manometry measures the pressure of contractions of the digestive tract. What is colonic manometry? The colon (also known as the large intestine)
Patient and Family Education Colonic (Large Intestine) Manometry Manometry measures the pressure of contractions of the digestive tract. What is colonic manometry? The colon (also known as the large intestine)
