A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation
|
|
|
- Malcolm Richardson
- 6 years ago
- Views:
Transcription
1 special article A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation Kevin G. Volpp, M.D., Ph.D., Andrea B. Troxel, Sc.D., Mark V. Pauly, Ph.D., Henry A. Glick, Ph.D., Andrea Puig, B.A., David A. Asch, M.D., M.B.A., Robert Galvin, M.D., M.B.A., Jingsan Zhu, M.B.A., Fei Wan, M.S., Jill DeGuzman, B.S., Elizabeth Corbett, M.L.S., Janet Weiner, M.P.H., and Janet Audrain-McGovern, Ph.D. abstract Background Smoking is the leading preventable cause of premature death in the United States. Previous studies of financial incentives for smoking cessation in work settings have not shown that such incentives have significant effects on cessation rates, but these studies have had limited power, and the incentives used may have been insufficient. Methods We randomly assigned 878 employees of a multinational company based in the United States to receive information about smoking-cessation programs (442 employees) or to receive information about programs plus financial incentives (436 employees). The financial incentives were $100 for completion of a smoking-cessation program, $250 for cessation of smoking within 6 months after study enrollment, as confirmed by a biochemical test, and $400 for abstinence for an additional 6 months after the initial cessation, as confirmed by a biochemical test. Individual participants were stratified according to work site, heavy or nonheavy smoking, and income. The primary end point was smoking cessation 9 or 12 months after enrollment, depending on whether initial cessation was reported at 3 or 6 months. Secondary end points were smoking cessation within the first 6 months after enrollment and rates of participation in and completion of smoking-cessation programs. Results The incentive group had significantly higher rates of smoking cessation than did the information-only group 9 or 12 months after enrollment (14.7% vs. 5.0%, P<0.001) and 15 or 18 months after enrollment (9.4% vs. 3.6%, P<0.001). Incentive-group participants also had significantly higher rates of enrollment in a smoking-cessation program (15.4% vs. 5.4%, P<0.001), completion of a smoking-cessation program (10.8% vs. 2.5%, P<0.001), and smoking cessation within the first 6 months after enrollment (20.9% vs. 11.8%, P<0.001). From the Center for Health Equity Research and Promotion, Philadelphia Veterans Affairs Medical Center (K.G.V., D.A.A.); the Center for Health Incentives, Leonard Davis Institute of Health Economics (K.G.V., A.B.T., M.V.P., H.A.G., D.A.A., J.Z., J.D., J.W.), the Department of Health Care Management, the Wharton School (K.G.V., M.V.P., H.A.G., A.P., D.A.A.), and the Center for Clinical Epidemiology and Biostatistics and Department of Biostatistics and Epidemiology (A.B.T., F.W.), University of Pennsylvania; the Department of Medicine (K.G.V., H.A.G., D.A.A., J.Z., J.D., E.C.) and the Transdisciplinary Tobacco Use Research Center, Department of Psychiatry (J.A.-M.), University of Pennsylvania School of Medicine all in Philadelphia; General Electric, Fairfield, CT (R.G.); and Yale University School of Medicine, New Haven, CT (R.G.). Address reprint requests to Dr. Volpp at the Division of General Internal Medicine and Department of Health Care Management, University of Pennsylvania School of Medicine and the Wharton School, 1232 Blockley Hall, 423 Guardian Dr., Philadelphia, PA , or at volpp70@wharton. upenn.edu. N Engl J Med 2009;360: Copyright 2009 Massachusetts Medical Society. Conclusions In this study of employees of one large company, financial incentives for smoking cessation significantly increased the rates of smoking cessation. (ClinicalTrials.gov number, NCT ) n engl j med 360;7 nejm.org february 12,
2 Smoking remains the leading preventable cause of premature death in the United States, accounting for approximately 438,000 deaths each year. 1 Seventy percent of smokers report that they want to quit, 2 but annually only 2 to 3% of smokers succeed. 3,4 Although smoking-cessation programs and pharmacologic therapies have been associated with higher rates of cessation, rates of participation in such programs and use of such therapies are low. 5,6 Work sites offer a promising venue for encouraging smoking cessation because employers are likely to bear many of the excess health care costs and productivity losses that are due to missed work among smokers. In addition, existing channels of communication can be used to reach smokers and reinforce healthful behavior choices. Previous studies have shown that providing smokers with financial incentives to stop smoking increases enrollment in smoking-cessation programs and short-term cessation rates, 7-10 but the studies have not shown significant increases in long-term cessation rates. Similarly, studies of financial-incentive programs in work settings have not shown significant differences in long-term cessation rates, 11 though the studies generally were limited by small sample sizes and weak financial incentives. In this randomized, controlled trial involving employees at a large, multinational company based in the United States, we tested the effectiveness of a financial incentive of up to $750 in improving long-term rates of smoking cessation. Methods Study Population We recruited study participants from February 2005 through November 2006 among employees at company work sites throughout the United States. Potential participants were identified with the use of a survey that asked employees about their smoking habits, their use of other tobacco products, and their willingness to be contacted by researchers from the University of Pennsylvania about participation in a smoking-cessation study. These surveys were distributed through the firm s intranet and, at sites with high proportions of employees who did not have intranet access, through on-site recruiting. Employees were eligible to participate if they were at least 18 years of age and if they reported that they were currently smoking five or more cigarettes per day. Employees were not included in the study if they were currently using tobacco products other than cigarettes or if they planned to leave the firm within 18 months. All participants were followed for at least 12 months; only those with a confirmed negative result within the first 12 months on a cotinine test, a test that is used for biochemical verification of self-reported abstinence, were followed for an additional 6 months. Study Protocol The protocol was approved by the institutional review board at the University of Pennsylvania, and all participants provided written informed consent before randomization. All study participants received information about community-based smoking-cessation resources within 20 miles of their work site, as well as the standard health benefits provided by the firm, such as coverage of physician visits and bupropion or other drugs prescribed to promote cessation of tobacco use. Participants in the incentive group were also informed that they would receive financial incentives for completion of a community-based smoking-cessation program ($100); for smoking cessation, confirmed with the use of a cotinine test, within 6 months after study enrollment ($250); and for continued abstinence for an additional 6 months after the initial cessation ($400). All participants were contacted 3 months after enrollment and asked whether they had stopped smoking. Participants who reported complete abstinence (not even a puff of a cigarette) for at least 7 days before being contacted were interviewed so that we could obtain a more extensive assessment of smoking behavior. Participants who did not report complete abstinence were recontacted 3 months later (i.e., 6 months after enrollment) for the full follow-up assessment. Six months after completing their first full interview (i.e., at 9 months for those who reported quitting at 3 months; at 12 months for those who were interviewed at 6 months), everyone was interviewed again. Participants who reported during any follow-up interview that they had stopped smoking were asked to provide a saliva or urine sample for confirmation of smoking cessation with the use of a cotinine test. Participants with negative results of cotinine tests at both the interview conducted at 3 or 6 months and the interview conducted at 700 n engl j med 360;7 nejm.org february 12, 2009
3 A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation 9 or 12 months were also interviewed 12 months after completion of their initial full interview (i.e., at 15 or 18 months). Participants received $20 per interview for participating in telephone interviews at baseline, at 3 or 6 months, at 9 or 12 months, and at 15 or 18 months. After each interview, participants who reported that they had stopped smoking received $25 for submitting a sample for biochemical verification of the cotinine level. Randomization Procedures Participants were randomly assigned to a study group after they provided informed consent. Randomization was performed in permuted blocks of four and was stratified according to work site, income (<200%, 200 to 500%, or >500% of the federal poverty level, which was $9,800 for a single person and $20,000 for a family of four in 2006), and heavy or nonheavy smoking (with heavy smoking defined as two or more packs of cigarettes per day). The randomized assignments were concealed until all eligibility criteria had been entered in an electronic tracking system; however, blinding could not be maintained after this point because of the nature of the intervention. Assessments of End Points The primary end point was the participant s selfreport of abstinence at both 3 and 9 months or at both 6 and 12 months after study enrollment. Abstinence was biochemically confirmed by a negative result of a cotinine test performed on a saliva or urine sample. Self-reported continuous abstinence from all tobacco products was defined as abstinence for a minimum of 7 days before the 3- or 6-month interview (point prevalence) and abstinence for the duration of the period from the 3- or 6-month interview to the 9- or 12-month interview (prevalence of prolonged abstinence). Secondary end points included enrollment in a smoking-cessation program; completion of a smoking-cessation program; rates of smoking cessation within 6 months after study enrollment; and rates of smoking cessation at 3, 9, and 15 months or 6, 12, and 18 months after study enrollment. Participants were classified as having completed the smoking-cessation program if they provided a certificate of completion from the sponsoring organization. All follow-up assessments were conducted with Table 1. Characteristics of the Study Participants.* Characteristic Control Group (N = 442) Incentive Group (N = 436) Mean age (yr) Male sex (%) Race or ethnic group (%) White Black Hispanic Other Level of education (%) High school or lower Some college or completion of college Beyond college Income (%) <200% of the poverty level % of the poverty level >500% of the poverty level Smoking habits Mean cigarettes per day (no.) Smoking >2 packs per day (%) Mean previous attempts to quit (no.) Stage of change (%) Precontemplation Contemplation Preparation Self-assessed health (%) Poor Fair Good Very good Excellent Fagerström score for nicotine dependence (%) < * There were no significant differences between the control and incentive groups for any of the variables listed. Race or ethnic group was self-reported. One participant in the control group did not report race or ethnic group and therefore was not included in the denominator for the percentages. Stage of change refers to the smoker s readiness, at baseline, to quit, as defined by DiClemente et al. 16 and Prochaska et al. 17 The Fagerström Test for Nicotine Dependence 14 has a range of 0 to 10, with higher scores indicating more nicotine dependence; a score of 6 or greater was used to classify participants as highly dependent on nicotine. 15 n engl j med 360;7 nejm.org february 12,
4 1903 Patients were assessed for eligibility 1025 Were excluded 771 Were ineligible 254 Did not give consent 878 Underwent randomization 442 Were assigned to control group 436 Were assigned to incentive group 47 Were lost to follow-up by 6 mo 12 Withdrew by 6 mo 50 Were lost to follow-up by 6 mo 16 Withdrew by 6 mo 35 Were lost to follow-up between 6 and 12 mo 12 Withdrew between 6 and 12 mo 43 Were lost to follow-up between 6 and 12 mo 13 Withdrew between 6 and 12 mo 2 Were lost to follow-up between 12 and 18 mo 0 Withdrew between 12 and 18 mo 4 Were lost to follow-up between 12 and 18 mo 1 Withdrew between 12 and 18 mo Figure 1. Assessment for Eligibility, Randomization, and Follow-up. the use of a telephone interview. Self-reported cessation was verified by saliva cotinine tests (with a cotinine level of <15 ng per milliliter considered to be an indication of smoking cessation) 12 or by urine cotinine tests (with a level of <2 ng per milliliter considered to be an indication of smoking cessation). 13 Urine cotinine tests were used only in the case of participants who were using nicotine-replacement therapy, in whom testing of anabasine and anatabine levels in the urine was performed. Participants who reported abstinence but who had saliva or urine cotinine levels above the cutoff points were classified as smokers. Participants who were lost to follow-up were classified as relapsed smokers. The collection of samples was coordinated by the study staff in conjunction with the healthpromotion staff at the firm and, at small work sites that did not have onsite health facilities, through contract with Examination Management Services (Scottsdale, AZ). Participants identities were confirmed and samples were analyzed at the Clinical Pharmacology Laboratory at the University of California, San Francisco. Baseline Variables The pretreatment level of nicotine dependence was assessed according to the number of cigarettes smoked per day, the number of years of smoking, and the score on the Fagerström Test for Nicotine Dependence 14 (which has a range of 0 to 10, with higher scores indicating more nicotine dependence); participants with a score of 6 or greater were classified as highly dependent on nicotine n engl j med 360;7 nejm.org february 12, 2009
5 A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation Table 2. Smoking-Cessation End Points According to Group Assignment.* End Point Control Group (N = 442) Incentive Group (N = 436) P Value Enrollment in smoking-cessation program no. (%) Participation in program 24 (5.4) 67 (15.4) <0.001 Completion of program 11 (2.5) 47 (10.8) <0.001 Smoking cessation at 3 or 6 mo Self-reported 62 (14.0) 102 (23.4) <0.001 Confirmed 52 (11.8) 91 (20.9) <0.001 No sample submitted 9 (2.0) 9 (2.1) 0.79 Positive sample submitted 1 (0.2) 2 (0.5) 0.56 Smoking cessation at 3 or 6 mo with continued abstinence through 9 or 12 mo Self-reported 27 (6.1) 66 (15.1) Confirmed 22 (5.0) 64 (14.7) <0.001 No sample submitted 5 (1.1) 2 (0.5) 0.06 Positive sample submitted 0 0 Self-reported relapse 21 (4.8) 21 (4.8) 0.96 Continued abstinence at 15 or 18 mo among participants who quit at 3 or 6 mo and remained abstinent through 9 or 12 mo Self-reported 17 (3.8) 47 (10.8) <0.001 Confirmed 16 (3.6) 41 (9.4) <0.001 No sample submitted 1 (0.2) 6 (1.4) 0.03 Positive sample submitted 0 0 Self-reported relapse 3 (0.7) 12 (2.8) 0.02 * Smoking cessation was confirmed by means of a negative result on a cotinine test. We also collected information on income, work site, baseline health status, and interest in quitting, as measured by baseline readiness to stop smoking (i.e., the stage of change, as defined by DiClemente et al. 16 and Prochaska et al. 17 ). Statistical Analysis The primary analysis was an unadjusted intentionto-treat analysis of the difference in biochemically confirmed cessation rates between the incentive and control groups at both 3 and 9 months or at both 6 and 12 months. The analysis was performed with the use of Pearson s chi-square test, or with the use of Fisher s exact test, if there were five or fewer participants per cell. We used a similar approach to estimate differences in rates of cessation within 6 months after enrollment; rates of enrollment in and completion of smoking-cessation programs; and cessation rates at 3, 9, and 15 months or 6, 12, and 18 months after study enrollment. Unadjusted odds ratios for cessation were estimated and were compared with odds ratios that were adjusted for the stratification variables (work site, degree of nicotine dependence, and income) as well as the set of baseline covariates shown in Table 1; variable-selection methods were not applied. The similarity of the study groups with respect to covariates at baseline was analyzed by the chi-square test for categorical variables and the Student s t-test or Wilcoxon rank-sum test for continuous variables, as appropriate. Although subgroup analyses were not prespeci- n engl j med 360;7 nejm.org february 12,
6 Table 3. Odds Ratios for Long-Term (9- or 12-Month) Smoking Cessation. Variable Odds Ratio (95% CI) P Value Unadjusted model Incentive group 3.28 ( ) <0.001 Control group 1.00 Model adjusted for stratification variables only Study group <0.001 Incentive 3.19 ( ) Control 1.00 Work site* 0.12 Smoking status 0.15 Heavy 0.34 ( ) Nonheavy 1.00 Poverty level 0.02 >500% of the poverty level 0.96 ( ) % of the poverty level 0.43 ( ) <200% of the poverty level 1.00 Model adjusted for all variables Study group <0.001 Incentive 3.16 ( ) Control 1.00 Work site* 0.20 Age yr 1.05 ( ) <40 yr 1.00 Sex 0.39 Male 0.80 ( ) Female 1.00 Race 0.69 Black 1.02 ( ) White 1.00 Other 0.50 ( ) Ethnic group 0.80 Hispanic 1.20 ( ) Non-Hispanic 1.00 Level of education 0.69 Beyond college 0.92 ( ) College 0.78 ( ) High school or less 1.00 Poverty level 0.04 >500% of the poverty level 1.03 ( ) % of the poverty level 0.48 ( ) <200% of the poverty level n engl j med 360;7 nejm.org february 12, 2009
7 A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation Table 3. (Continued.) Variable Odds Ratio (95% CI) P Value Smoking status 0.20 Heavy 0.38 ( ) Nonheavy 1.00 Previous attempts to stop smoking (top third) 2.00 ( ) 3 or 4 (middle third) 1.58 ( ) 1 or 2 (bottom third) 1.00 Stage of change 0.10 Preparation 2.61 ( ) Contemplation 1.64 ( ) Precontemplation 1.00 Self-reported health 0.37 Excellent 1.85 ( ) Very good 1.14 ( ) Good 1.09 ( ) Fair 0.58 ( ) Poor 1.00 * Odds ratios for work-site groups are not reported, owing to the large number of sites included. Heavy smoking was defined as 40 cigarettes or more per day. One study participant was not included in this analysis, owing to missing data on race or ethnic group. Stage of change refers to the smoker s readiness to quit, as defined by DiClemente et al. 16 and Prochaska et al. 17 fied, we looked at cessation rates in subgroups that were defined by seven baseline variables: sex, income (<200%, 200 to 500%, and >500% of the federal poverty level), heavy smoking ( 2 packs per day) or nonheavy smoking, number of previous attempts to quit (0 to 2, 3 or 4, or 5), stage of quitting as defined by Prochaska et al. 17 (precontemplation, contemplation, or preparation), selfassessed health (poor, fair, good, very good, or excellent), and nicotine dependence as assessed by the score on the Fagerström Test for Nicotine Dependence (<6 or 6). The homogeneity of the association (i.e., interaction) between the group assignment and cessation rates across subgroups was assessed with the use of the Cochran Mantel Haenszel test. Within each subgroup, rates of cessation in the incentive and control groups were compared with the use of chi-square tests. Our power calculations were based on expected 12-month cessation rates of 3.0% in the control group and 9.4% in the incentive group. To have 80% power to show a difference between groups, with a 1% two-sided type I error, we needed to have 360 participants in each group; we increased that number by 15%, to 425 in each group, to account for loss to follow-up resulting from expected employee turnover. No interim analyses were planned or conducted. All reported P values are two-sided and were not adjusted for multiple comparisons. Results Figure 1 shows the numbers of subjects who were screened and who participated in the study intervention and follow-up. Of the 1903 people who initially expressed interest in participating, 878 (46%) were enrolled; 436 participants were randomly assigned to the incentive group, and 442 to the control group. Demographic characteristics, smoking behavior, degree of nicotine dependence, readiness to quit, and health status were similar in the incentive and control groups (Table 1). Participants in both groups smoked approximately one pack of cigarettes per day, and approximately 5 to 6% of the participants were heavy smokers. The majority of the sample had incomes that were greater than 500% of the poverty level, and ap- n engl j med 360;7 nejm.org february 12,
8 Characteristic Sex Male Female Poverty level <200% of poverty level % of poverty level >500% of poverty level Smoking status Nonheavy Heavy (>2 packs/day) Average no. of previous attempts to quit 0 2 (bottom third) 3 4 (middle third) (top third) Stage of change Precontemplation Contemplation Preparation Self-assessed health Poor Fair Good Very good Excellent Fagerström score for nicotine dependence <6 6 Control Incentive Group Group no. who quit/total no. 15/287 7/155 0/7 7/153 15/282 22/415 0/27 5/170 7/118 10/154 2/69 13/282 7/91 1/9 1/41 5/142 11/203 4/47 17/301 5/141 40/286 24/150 2/8 10/140 52/288 62/414 2/22 14/152 17/123 33/161 5/66 40/292 19/78 0/5 3/37 23/147 27/186 11/61 49/290 15/ Odds Ratio for Cessation (log scale) Odds Ratio (95% CI) 2.95 ( ) 4.03 ( ) 5.77 ( ) 1.60 ( ) 3.92 ( ) 3.15 ( ) 6.71 ( ) 3.35 ( ) 2.54 ( ) 3.71 ( ) 2.75 ( ) 3.28 ( ) 3.86 ( ) 0.51 ( ) 3.53 ( ) 5.08 ( ) 2.96 ( ) 2.37 ( ) 3.40 ( ) 3.11 ( ) Figure 2. Rates of Smoking Cessation in Various Subgroups at 9 or 12 Months. Stage of change refers to the smoker s readiness to quit, as defined by DiClemente et al. 16 and Prochaska et al. 17 The Fagerström Test for Nicotine Dependence 14 has a range of 0 to 10, with higher scores indicating greater nicotine dependence; a score of 6 or more was used to classify participants as highly dependent on nicotine. 15 proximately 90% of the participants were white. A similar number of participants in the incentive and control groups were lost to follow-up or withdrew in the first 6 months (66 and 59 participants, respectively; P = 0.45) and through month 12 (56 and 47, respectively; P = 0.25). The 9-month or 12-month rate of cessation, as confirmed by cotinine testing, was 14.7% in the incentive group, as compared with 5.0% in the control group (P<0.001) (Table 2). In both the incentive group and the control group, very few of the participants who reported that they had quit smoking did not submit samples for testing (0.5% and 1.1%, respectively), and no participants who submitted samples had positive test results. The rate of participation in a smoking-cessation program was significantly higher in the incentive group than in the control group (15.4% vs. 5.4%, P<0.001), and a significantly higher percentage of incentive-group participants completed a smoking-cessation program (10.8% vs. 2.5%, P<0.001). Members of the incentive group who participated in a smoking-cessation program had significantly higher rates of cessation than did members of the control group who participated in such a program (46.3% vs. 20.8%, P = 0.03). The cotinine-confirmed cessation rate within 6 months after study enrollment was 20.9% in the incentive group, as compared with 11.8% in the control group (P<0.001). The cessation rate at 15 or 18 months was 9.4% in the incentive group, as compared with 3.6% in the control group (P = 0.001). Among participants who had not stopped smoking in the first 6 months and 706 n engl j med 360;7 nejm.org february 12, 2009
9 A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation thus were ineligible for financial incentives the cotinine-confirmed cessation rates were similar in the incentive and control groups (4.1% and 5.9%, respectively; P = 0.29). The odds ratios for quitting by 9 or 12 months (Table 3) were significantly higher in the incentive group than in the control group in the unadjusted model (odds ratio, 3.28; 95% confidence interval [CI], 1.98 to 5.44), in a logistic-regression model that adjusted for stratification variables only (odds ratio, 3.19; 95% CI, 1.91 to 5.30), and in a model that adjusted for all the variables included in Table 1 (odds ratio, 3.16; 95% CI, 1.88 to 5.32). Exploratory Subgroup Analyses Figure 2 shows 9-month or 12-month cessation rates stratified according to subgroup. For all subgroups, members of the incentive group had higher cessation rates than members of the control group. None of the tests for interaction showed significant differences in this population; instead, the observed patterns showed a consistent effect of the intervention across numerous characteristics. Discussion In this study of employees at a large corporation, rates of prolonged abstinence from smoking after 9 or 12 months of follow-up were 14.7% in the group that was eligible for financial incentives and 5.0% in the control group. Rates of prolonged abstinence at 15 or 18 months in the incentive group remained significantly higher than those in the control group. A 2005 Cochrane Collaboration review of financial incentives for smoking cessation in workplace settings concluded that there was insufficient evidence that these incentives are effective. 11 One reason for this finding may be that many previous studies were not designed with samples that were large enough to detect the differences we observed. A second reason may be that the incentives used in previous studies have generally been small (as little as $10 in some of them). Studies have shown that financial-incentive programs are associated with an increased rate of smoking cessation in the short term, but the programs reported in these studies were not designed to focus on sustaining high rates over time. 7,9 The relationships of the size and structure of incentive payments to rates of smoking cessation remain important empirical questions that need to be addressed in future research. It is possible that larger or smaller payments by employers could be more cost-effective in improving smoking-cessation rates. The optimal design of incentive programs for smoking cessation is also an open question, since extension of the incentives beyond 12 months may result in higher cessation rates over a longer period. The cotinine level, which we used for biochemical verification of 7-day abstinence, is considered to be the best biomarker of smoking cessation. 12 Consistent with the recommendations of the Society for Research on Nicotine and Tobacco, our primary measure of self-reported cessation at both 9 or 12 months and 15 or 18 months was prolonged abstinence. 12 Although prolonged abstinence can be biochemically verified only indirectly because of the limitations of the cotinine test, it is likely that the point prevalence is highly correlated with prolonged abstinence 6 months after short-term quitting was assessed. 12 To date, financial incentives within health care settings have been directed primarily toward providers, through Pay for Performance (P4P) programs. However, given that up to 40% of premature deaths in the United States are due to unhealthful behaviors such as smoking, poor dietary habits, and sedentary lifestyles, 18 incentives directed toward patients rather than providers may have greater potential for changing health behaviors One approach to encouraging smoking cessation would be to adjust health-insurance premiums on the basis of smoking status; however, targeted payments for smoking cessation have the advantage of being unbundled from health-insurance premiums and thus may be more salient to people, thereby having a greater influence on behavior. 22 The financial benefit to employers of having their employees stop smoking is estimated to be about $3,400 per year 23 as a result of increased productivity, decreased absenteeism, and a reduced incidence of illness. There is also strong evidence that employees prefer to work for firms that offer effective and attractive benefit programs. 24 However, few employers offer full coverage of smoking-cessation services or cessation programs in the workplace, 8,25,26 partly because a compelling business case for such coverage has not been made. Notably, neither the financial-incentive intervention nor the control intervention in our study n engl j med 360;7 nejm.org february 12,
10 included the establishment of a smoking-cessation program; both interventions merely encouraged participants to use existing programs. The literature on smoking cessation suggests that most relapses occur within the first month of cessation, that approximately 90% of relapses occur within the first 6 months, 27 and that the likelihood of continuous abstinence over a 20-month period is about 95% for smokers who quit for 1 year or longer. 12 Our finding of relapse rates between the 9- or 12-month visit and the 15- or 18-month visit (27.3% in the control group and 35.9% in the incentive group) that appear to be higher than those reported in the literature may suggest that relapse rates differ according to whether smoking cessation occurs in the presence or absence of incentives. However, since the relapse rates in both the incentive and control groups differ from those in the literature, and in view of the relatively small number of participants in our study who quit smoking, we cannot be confident that the relapse rates do in fact differ from those previously reported. Because approximately 90% of the enrolled population was white and the participants had relatively high education and income levels, our study may have limited generalizability to employees with lower socioeconomic status than that of the participants in our study or to members of a minority racial or ethnic group. In addition, programs that are designed to change behavior may have unintended consequences that we could not observe in our study. However, we think it is unlikely that substantial numbers of people would start smoking in order to be eligible for such incentive programs. Studies such as this one are subject to selection bias, in that smokers who voluntarily enroll in these programs may be more likely (whether they are assigned to the incentive group or the control group) to quit smoking; it is difficult to project how effective these programs would be in a population that included all employees within a given company. In summary, this study shows that smokingcessation rates among company employees who were given both information about cessation programs and financial incentives to quit smoking were significantly higher than the rates among employees who were given program information but no financial incentives. Supported by grants from the Centers for Disease Control and Prevention (RO1 DP and RO1 DP ) and from the Pennsylvania Department of Health, which specifically disclaims responsibility for any analyses, interpretations, or conclusions. Dr. Volpp reports receiving lecture fees from Aetna and grant support from Aetna and Pfizer and owning equity in General Electric; Dr. Pauly, being a board member of Independent Health, owning equity in General Electric, receiving lecture fees from America s Health Insurance Plans, and receiving grant support from Merck and MedStar; Dr. Glick, owning equity in General Electric; and Dr. Galvin, owning equity in General Electric. No other potential conflict of interest relevant to this article was reported. We thank Quincy Greene for his contributions to the analyses for this article and Donna Tomlinson, M.D., for assistance in implementing this study. References 1. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, JAMA 2004;291: [Errata, JAMA 2005;293:293-4, 298.] 2. Cigarette smoking among adults United States, MMWR Morb Mortal Wkly Rep 2002;51: Annual smoking-attributable mortality, years of potential life lost, and productivity losses United States, MMWR Morb Mortal Wkly Rep 2005;54: Tobacco use among adults United States, MMWR Morb Mortal Wkly Rep 2006;55: Zhu S, Melcer T, Sun J, Rosbrook B, Pierce JP. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18: Fiore MC. US Public Health Service Clinical Practice Guideline: treating tobacco use and dependence. Respir Care 2000;45: Volpp KG, Gurmankin Levy A, Asch DA, et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev 2006; 15: Hennrikus DJ, Jeffery RW, Lando HA, et al. The SUCCESS project: the effect of program format and incentives on participation and cessation in worksite smoking cessation programs. Am J Public Health 2002;92: Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Supporter (SOS) program. Tob Control 2000;9:Suppl 3:III67-III Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine Tob Res 2004;6:Suppl 2:S163-S Hey K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database Syst Rev 2005;2:CD SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4: Jacob P III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz N. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev 2002;11: Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86: Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Arch Intern Med 1997;157: DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol 1991;59: n engl j med 360;7 nejm.org february 12, 2009
11 A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation 17. Prochaska JO, DiClemente CC, Velicer WF, Rossi JS. Standardized, individualized, interactive, and personalized selfhelp programs for smoking cessation. Health Psychol 1993;12: Schroeder SA. Shattuck Lecture: We can do better improving the health of the American people. N Engl J Med 2007; 357: Sindelar JL. Paying for performance: the power of incentives over habits. Health Econ 2008;17: Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA 2007;298: Volpp KG, Pauly MV, Loewenstein G, Bangsberg D. P4P4P: an agenda for research on pay-for-performance for patients. Health Aff (Millwood) 2009;28: Thaler RH. Mental accounting and consumer choice. Marketing Sci 1985;4: Annual smoking-attributable mortality, years of potential life lost, and economic costs United States, MMWR Morb Mortal Wkly Rep 2002;51: Pauly MV. Health benefits at work: an economic and political analysis of employment-based health insurance. Ann Arbor: University of Michigan Press, Barbeau EM, Li Y, Sorenson G, Con- lan KM, Youngstrom R, Emmons K. Coverage of smoking cessation treatment by union health and welfare funds. Am J Public Health 2001;91: Aakko E, Piasecki TM, Remington P, Fiore MC. Smoking cessation services offered by health insurance plans for Wisconsin state employees. WMJ 1999;98: Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99: Copyright 2009 Massachusetts Medical Society. posting presentations at medical meetings on the internet Posting an audio recording of an oral presentation at a medical meeting on the Internet, with selected slides from the presentation, will not be considered prior publication. This will allow students and physicians who are unable to attend the meeting to hear the presentation and view the slides. If there are any questions about this policy, authors should feel free to call the Journal s Editorial Offices. n engl j med 360;7 nejm.org february 12,
The impact of asking about interest in free nicotine patches on smoker s stated. intent to change: Real effect or artefact of question ordering?
 Impact of asking about interest in free nicotine patches 1 The impact of asking about interest in free nicotine patches on smoker s stated intent to change: Real effect or artefact of question ordering?
Impact of asking about interest in free nicotine patches 1 The impact of asking about interest in free nicotine patches on smoker s stated intent to change: Real effect or artefact of question ordering?
Reducing Tobacco Use and Secondhand Smoke Exposure: Incentives and Competitions to Increase Smoking Cessation Among Workers
 Reducing Tobacco Use and Secondhand Smoke Exposure: Incentives and Competitions to Increase Smoking Cessation Among Workers Summary Evidence Table Studies of Incentives and Competitions When Implemented
Reducing Tobacco Use and Secondhand Smoke Exposure: Incentives and Competitions to Increase Smoking Cessation Among Workers Summary Evidence Table Studies of Incentives and Competitions When Implemented
A Pragmatic Trial of E-Cigarettes, Incentives, and Drugs for Smoking Cessation
 The new england journal of medicine Special Article A Pragmatic Trial of E-Cigarettes, Incentives, and Drugs for Smoking Cessation Scott D. Halpern, M.D., Ph.D., Michael O. Harhay, Ph.D., Kathryn Saulsgiver,
The new england journal of medicine Special Article A Pragmatic Trial of E-Cigarettes, Incentives, and Drugs for Smoking Cessation Scott D. Halpern, M.D., Ph.D., Michael O. Harhay, Ph.D., Kathryn Saulsgiver,
CHRONIC DISEASE PREVALENCE AMONG ADULTS IN OHIO
 OHIO MEDICAID ASSESSMENT SURVEY 2012 Taking the pulse of health in Ohio CHRONIC DISEASE PREVALENCE AMONG ADULTS IN OHIO Amy Ferketich, PhD Ling Wang, MPH The Ohio State University College of Public Health
OHIO MEDICAID ASSESSMENT SURVEY 2012 Taking the pulse of health in Ohio CHRONIC DISEASE PREVALENCE AMONG ADULTS IN OHIO Amy Ferketich, PhD Ling Wang, MPH The Ohio State University College of Public Health
Michele Clements-Thompson and Robert C. Klesges University of Memphis Prevention Center. Harry Lando University of Minnesota
 Journal of Consulting and Clinical Psychology 1998, Vol. 66, No 6, 1005-1011 Copyright 1998 by the Am n Psychological Association, Inc. 0022-006X/98/S3.0C Relationships Between Stages of Change in Cigarette
Journal of Consulting and Clinical Psychology 1998, Vol. 66, No 6, 1005-1011 Copyright 1998 by the Am n Psychological Association, Inc. 0022-006X/98/S3.0C Relationships Between Stages of Change in Cigarette
The SUCCESS Project: The Effect of Program Format and Incentives on Participation and Cessation in Worksite Smoking Cessation Programs
 The SUCCESS Project: The Effect of Program Format and Incentives on Participation and Cessation in Worksite Smoking Cessation Programs Deborah J. Hennrikus, PhD, Robert W. Jeffery, PhD, Harry A. Lando,
The SUCCESS Project: The Effect of Program Format and Incentives on Participation and Cessation in Worksite Smoking Cessation Programs Deborah J. Hennrikus, PhD, Robert W. Jeffery, PhD, Harry A. Lando,
QUITLINE FOR SMOKERS. Special Article EVIDENCE OF REAL-WORLD EFFECTIVENESS OF A TELEPHONE QUITLINE FOR SMOKERS
 Special Article EVIDENCE OF REAL-WORLD EFFECTIVENESS OF A TELEPHONE QUITLINE FOR SMOKERS SHU-HONG ZHU, PH.D., CHRISTOPHER M. ANDERSON, B.A., GARY J. TEDESCHI, PH.D., BRADLEY ROSBROOK, M.S., CYNTHIA E.
Special Article EVIDENCE OF REAL-WORLD EFFECTIVENESS OF A TELEPHONE QUITLINE FOR SMOKERS SHU-HONG ZHU, PH.D., CHRISTOPHER M. ANDERSON, B.A., GARY J. TEDESCHI, PH.D., BRADLEY ROSBROOK, M.S., CYNTHIA E.
Predictors of smoking cessation among Chinese parents of young children followed up for 6 months
 Title Predictors of smoking cessation among Chinese parents of young children followed up for 6 months Author(s) Abdullah, ASM; Lam, TH; Loke, AY; Mak, YW Citation Hong Kong Medical Journal, 2006, v. 12
Title Predictors of smoking cessation among Chinese parents of young children followed up for 6 months Author(s) Abdullah, ASM; Lam, TH; Loke, AY; Mak, YW Citation Hong Kong Medical Journal, 2006, v. 12
Snowball sampling by mail: application to a survey of smokers in the general population
 International Epidemiological Association 2000 Printed in Great Britain International Journal of Epidemiology 2000;29:43 48 Snowball sampling by mail: application to a survey of smokers in the general
International Epidemiological Association 2000 Printed in Great Britain International Journal of Epidemiology 2000;29:43 48 Snowball sampling by mail: application to a survey of smokers in the general
Fax to Quit: A Model for Delivery of Tobacco Cessation Services to Wisconsin Residents
 Fax to Quit: A Model for Delivery of Tobacco Cessation Services to Wisconsin Residents Robin J. Perry, BS, CHES; Paula A. Keller, MPH; Dave Fraser, MS; Michael C. Fiore, MD, MPH ABSTRACT Research has shown
Fax to Quit: A Model for Delivery of Tobacco Cessation Services to Wisconsin Residents Robin J. Perry, BS, CHES; Paula A. Keller, MPH; Dave Fraser, MS; Michael C. Fiore, MD, MPH ABSTRACT Research has shown
Stages of change analysis of smokers attending clinics for the medically underserved
 December 2002 Vol. 51, No. 12 JFP ONLINE Stages of change analysis of smokers attending clinics for the medically underserved Karen M. Gil, PhD; Susan Labuda Schrop, MS; Sarah C. Kline, BS; Emily A. Kimble,
December 2002 Vol. 51, No. 12 JFP ONLINE Stages of change analysis of smokers attending clinics for the medically underserved Karen M. Gil, PhD; Susan Labuda Schrop, MS; Sarah C. Kline, BS; Emily A. Kimble,
The Transtheoretical Model to Help Clients Thrive
 The Transtheoretical Model to Help Clients Thrive James O. Prochaska, Ph.D. Director and Professor Cancer Prevention Research Center University of Rhode Island Founder Pro-Change Behavior Systems, Inc.
The Transtheoretical Model to Help Clients Thrive James O. Prochaska, Ph.D. Director and Professor Cancer Prevention Research Center University of Rhode Island Founder Pro-Change Behavior Systems, Inc.
Efficacy of Team-Based Financial Incentives for Smoking Cessation in the Workplace
 Brief Communication http://dx.doi.org/10.3349/ymj.2015.56.1.295 pissn: 0513-5796, eissn: 1976-2437 Yonsei Med J 56(1):295-299, 2015 Efficacy of Team-Based Financial Incentives for Smoking Cessation in
Brief Communication http://dx.doi.org/10.3349/ymj.2015.56.1.295 pissn: 0513-5796, eissn: 1976-2437 Yonsei Med J 56(1):295-299, 2015 Efficacy of Team-Based Financial Incentives for Smoking Cessation in
Quit rates among smokers who received pharmacist-provided pharmacotherapy and quitline services versus those who received only quitline services.
 Quit rates among smokers who received pharmacist-provided pharmacotherapy and quitline services versus those who received only quitline services. Jill Augustine, PharmD, MPH 1 ; Ryan Seltzer, PhD 2 ; Martin
Quit rates among smokers who received pharmacist-provided pharmacotherapy and quitline services versus those who received only quitline services. Jill Augustine, PharmD, MPH 1 ; Ryan Seltzer, PhD 2 ; Martin
Long-term Nicotine Replacement Therapy A Randomized Clinical Trial
 Research Original Investigation Long-term Nicotine Replacement Therapy A Randomized Clinical Trial Robert A. Schnoll, PhD; Patricia M. Goelz, MPH; Anna Veluz-Wilkins, MA; Sonja Blazekovic, BA; Lindsay
Research Original Investigation Long-term Nicotine Replacement Therapy A Randomized Clinical Trial Robert A. Schnoll, PhD; Patricia M. Goelz, MPH; Anna Veluz-Wilkins, MA; Sonja Blazekovic, BA; Lindsay
SMOKING CESSATION WORKSHOP. Dr Mark Palayew December
 SMOKING CESSATION WORKSHOP Dr Mark Palayew December 5 2016 Conflicts of Interest None Case 1 Mr. T is a 55 year old smoker 2 packs/day He has been smoking continuously since age 16 When he wakes up at
SMOKING CESSATION WORKSHOP Dr Mark Palayew December 5 2016 Conflicts of Interest None Case 1 Mr. T is a 55 year old smoker 2 packs/day He has been smoking continuously since age 16 When he wakes up at
Patterns of adolescent smoking initiation rates by ethnicity and sex
 ii Tobacco Control Policies Project, UCSD School of Medicine, San Diego, California, USA C Anderson D M Burns Correspondence to: Dr DM Burns, Tobacco Control Policies Project, UCSD School of Medicine,
ii Tobacco Control Policies Project, UCSD School of Medicine, San Diego, California, USA C Anderson D M Burns Correspondence to: Dr DM Burns, Tobacco Control Policies Project, UCSD School of Medicine,
Nicotine Dependence Phenotype, Time to First Cigarette, and Risk of Head and Neck Cancer
 Nicotine Dependence Phenotype, Time to First Cigarette, and Risk of Head and Neck Cancer Joshua E. Muscat, PhD 1 ; Kwangmi Ahn, PhD 2 ; John P. Richie Jr, PhD 3 ; and Steven D. Stellman, PhD 4 BACKGROUND:
Nicotine Dependence Phenotype, Time to First Cigarette, and Risk of Head and Neck Cancer Joshua E. Muscat, PhD 1 ; Kwangmi Ahn, PhD 2 ; John P. Richie Jr, PhD 3 ; and Steven D. Stellman, PhD 4 BACKGROUND:
Extensive research has documented the determinants of
 EUROPEAN JOURNAL OF PUBLIC HEALTH 2002; 12: 302 307 Cigarette smoking and stages of change among men and women in Kiev, Ukraine JENNA M. MCALLISTER, GORDON B. LINDSAY, RAY M. MERRILL, UGO A. PEREGO * 302
EUROPEAN JOURNAL OF PUBLIC HEALTH 2002; 12: 302 307 Cigarette smoking and stages of change among men and women in Kiev, Ukraine JENNA M. MCALLISTER, GORDON B. LINDSAY, RAY M. MERRILL, UGO A. PEREGO * 302
September 14, 2018 James O. Prochaska, Ph.D.
 More Effective and Inclusive Care by Combining Practices for Individual Patients and Entire Populations September 14, 2018 James O. Prochaska, Ph.D. Director and Professor Cancer Prevention Research Center
More Effective and Inclusive Care by Combining Practices for Individual Patients and Entire Populations September 14, 2018 James O. Prochaska, Ph.D. Director and Professor Cancer Prevention Research Center
EliScholar A Digital Platform for Scholarly Publishing at Yale
 Yale University EliScholar A Digital Platform for Scholarly Publishing at Yale Public Health Theses School of Public Health January 2015 Evaluating The Effectiveness Of Smoking Cessation Intervention Program
Yale University EliScholar A Digital Platform for Scholarly Publishing at Yale Public Health Theses School of Public Health January 2015 Evaluating The Effectiveness Of Smoking Cessation Intervention Program
Randomized controlled trial of physical activity counseling as an aid to smoking cessation: 12 month follow-up
 Addictive Behaviors 32 (2007) 3060 3064 Short communication Randomized controlled trial of physical activity counseling as an aid to smoking cessation: 12 month follow-up Michael Ussher a,, Robert West
Addictive Behaviors 32 (2007) 3060 3064 Short communication Randomized controlled trial of physical activity counseling as an aid to smoking cessation: 12 month follow-up Michael Ussher a,, Robert West
Background and Analysis Objectives Methods and Approach
 Cigarettes, Tobacco Dependence, and Smoking Cessation: Project MOM Final Report Lorraine R. Reitzel, Ph.D., The University of Texas, MD Anderson Cancer Center Background and Analysis Objectives Background.
Cigarettes, Tobacco Dependence, and Smoking Cessation: Project MOM Final Report Lorraine R. Reitzel, Ph.D., The University of Texas, MD Anderson Cancer Center Background and Analysis Objectives Background.
Your Guide to Workforce. May 26, Milwaukee, WI. Presented by Brian J. Thomas and James O. Prochaska, Ph.D.
 Wellness That Works: Your Guide to Workforce Health Promotion Presented by Brian J. Thomas and James O. Prochaska, Ph.D. May 26, 2010 8:00 800 10:30 030a.m. Milwaukee, WI Today s Presenters Brian J. Thomas
Wellness That Works: Your Guide to Workforce Health Promotion Presented by Brian J. Thomas and James O. Prochaska, Ph.D. May 26, 2010 8:00 800 10:30 030a.m. Milwaukee, WI Today s Presenters Brian J. Thomas
Selected Agent Characteristics. Product type Nicotine dose levels Constituents (e.g., tar, CO) and ingredients (e.g., additives) Market share
 Tobacco Measurement Stephen Marcus, Ph.D. Tobacco Control Research Branch, Behavioral Research Program, Division of Cancer Control and Population Sciences Public Health Model Agent -- tobacco products
Tobacco Measurement Stephen Marcus, Ph.D. Tobacco Control Research Branch, Behavioral Research Program, Division of Cancer Control and Population Sciences Public Health Model Agent -- tobacco products
The Effect of Smoking Prevalence at Worksites on Individual Cessation Behavior
 J Occup Health 2009; 51: 48 56 Journal of Occupational Health The Effect of Smoking Prevalence at Worksites on Individual Cessation Behavior Chihiro NISHIURA 1, Rie NARAI 2, Takayuki OHGURI 2, Atsushi
J Occup Health 2009; 51: 48 56 Journal of Occupational Health The Effect of Smoking Prevalence at Worksites on Individual Cessation Behavior Chihiro NISHIURA 1, Rie NARAI 2, Takayuki OHGURI 2, Atsushi
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Halpern SD, French B, Small DS, et al. Randomized trial of four financial-incentive
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Halpern SD, French B, Small DS, et al. Randomized trial of four financial-incentive
Breaking the Bank: Cost of Cigarettes in Vermont
 University of Vermont ScholarWorks @ UVM Family Medicine Clerkship Student Projects College of Medicine 2018 Breaking the Bank: Cost of Cigarettes in Vermont Ryan Erik Landvater Larner College of Medicine
University of Vermont ScholarWorks @ UVM Family Medicine Clerkship Student Projects College of Medicine 2018 Breaking the Bank: Cost of Cigarettes in Vermont Ryan Erik Landvater Larner College of Medicine
Hypnosis for smoking cessation: A randomized trial
 Nicotine & Tobacco Research Volume 10, Number 5 (May 2008) 811 818 Hypnosis for smoking cessation: A randomized trial Timothy P. Carmody, Carol Duncan, Joel A. Simon, Sharon Solkowitz, Joy Huggins, Sharon
Nicotine & Tobacco Research Volume 10, Number 5 (May 2008) 811 818 Hypnosis for smoking cessation: A randomized trial Timothy P. Carmody, Carol Duncan, Joel A. Simon, Sharon Solkowitz, Joy Huggins, Sharon
Impact of UNC Health Care s Tobacco-Free Hospital Campus Policy on Hospital Employees
 Impact of UNC Health Care s Tobacco-Free Hospital Campus Policy on Hospital Employees February 5, 2008 Prepared for: UNC Health Care Prepared by: UNC School of Medicine Nicotine Dependence Program For
Impact of UNC Health Care s Tobacco-Free Hospital Campus Policy on Hospital Employees February 5, 2008 Prepared for: UNC Health Care Prepared by: UNC School of Medicine Nicotine Dependence Program For
Acknowledgments. TTM-Tailored Tailored Smoking
 Successes and Failures in Changing Multiple Behaviors in Populations of Primary Care Patients, Employees, and Parents Acknowledgments This project was funded by: National Cancer Institute - NCI PO1 # CA
Successes and Failures in Changing Multiple Behaviors in Populations of Primary Care Patients, Employees, and Parents Acknowledgments This project was funded by: National Cancer Institute - NCI PO1 # CA
Current Cigarette Smoking Among Workers in Accommodation and Food Services United States,
 Current Cigarette Among Workers in Accommodation and Food Services United States, 2011 2013 Girija Syamlal, MPH 1 ; Ahmed Jamal, MBBS 2 ; Jacek M. Mazurek, MD 1 (Author affiliations at end of text) Tobacco
Current Cigarette Among Workers in Accommodation and Food Services United States, 2011 2013 Girija Syamlal, MPH 1 ; Ahmed Jamal, MBBS 2 ; Jacek M. Mazurek, MD 1 (Author affiliations at end of text) Tobacco
Behavioral risk factors such as smoking and obesity
 ORIGINAL ARTICLE Knowledge and Perceptions Among Overweight and Obese Employees About Lifestyle-Related Health Benefit Changes Jiang Li, Laura Linnan, Eric A. Finkelstein, Deborah F. Tate, Carolyn Naseer,
ORIGINAL ARTICLE Knowledge and Perceptions Among Overweight and Obese Employees About Lifestyle-Related Health Benefit Changes Jiang Li, Laura Linnan, Eric A. Finkelstein, Deborah F. Tate, Carolyn Naseer,
Background. Abstinence rates associated with varenicline
 What are the range of abstinence rates for varenicline for smoking cessation? Do they differ based on treatment duration? Are there any studies utilizing 3-4 months of varenicline treatment? Background
What are the range of abstinence rates for varenicline for smoking cessation? Do they differ based on treatment duration? Are there any studies utilizing 3-4 months of varenicline treatment? Background
Self-Efficacy, Decisional Balance and Stages of Change on Dietary Practices among Metabolic Syndrome Persons, Uthai Thani Province
 Self-Efficacy, Decisional Balance and Stages of Change on Dietary Practices among Metabolic Syndrome Persons, Uthai Thani Province Manirat Therawiwat PhD*, Nirat Imamee PhD*, Thaweesak Khamklueng MSc**
Self-Efficacy, Decisional Balance and Stages of Change on Dietary Practices among Metabolic Syndrome Persons, Uthai Thani Province Manirat Therawiwat PhD*, Nirat Imamee PhD*, Thaweesak Khamklueng MSc**
The Pennsylvania State University. The Graduate School. College of Medicine THE USE OF A BRIEF SOLUTION FOCUSED WRITTEN INTERVENTION AND ITS
 The Pennsylvania State University The Graduate School College of Medicine THE USE OF A BRIEF SOLUTION FOCUSED WRITTEN INTERVENTION AND ITS EFFECT ON CURRENT SMOKERS WILLINGNESS TO CONSIDER MAKING A QUIT
The Pennsylvania State University The Graduate School College of Medicine THE USE OF A BRIEF SOLUTION FOCUSED WRITTEN INTERVENTION AND ITS EFFECT ON CURRENT SMOKERS WILLINGNESS TO CONSIDER MAKING A QUIT
INTRODUCTION. The effectiveness of proactive telephone counseling, in particular,
 J Korean Med Sci 2008; 23: 888-94 ISSN 1011-8934 DOI: 10.3346/jkms.2008.23.5.888 Copyright The Korean Academy of Medical Sciences Effectiveness of Proactive Quitline Service and Predictors of Successful
J Korean Med Sci 2008; 23: 888-94 ISSN 1011-8934 DOI: 10.3346/jkms.2008.23.5.888 Copyright The Korean Academy of Medical Sciences Effectiveness of Proactive Quitline Service and Predictors of Successful
Gender Differences in Use of Smoking Cessation Services and Resources: A Real-World Study of Ontario Smokers
 PROJECT NEWS July 2018 Gender Differences in Use of Smoking Cessation Services and Resources: A Real-World Study of Ontario Smokers Smoking cessation greatly reduces the health burden of tobacco use. 1,2
PROJECT NEWS July 2018 Gender Differences in Use of Smoking Cessation Services and Resources: A Real-World Study of Ontario Smokers Smoking cessation greatly reduces the health burden of tobacco use. 1,2
Healthcare financing systems for increasing the use of tobacco dependence treatment (Review)
 Healthcare financing systems for increasing the use of tobacco dependence treatment (Review) Kaper J, Wagena EJ, Severens JL, Van Schayck CP This is a reprint of a Cochrane review, prepared and maintained
Healthcare financing systems for increasing the use of tobacco dependence treatment (Review) Kaper J, Wagena EJ, Severens JL, Van Schayck CP This is a reprint of a Cochrane review, prepared and maintained
Smokers & Their Workplaces
 Smokers & Their Workplaces Opportunities for Research & Intervention Peggy Hannon, PhD, MPH Collaborators Acknowledgements Jeff Harris, MD, MPH, MBA Courtney Hughes, PhD Emily Yette, MPHc This research
Smokers & Their Workplaces Opportunities for Research & Intervention Peggy Hannon, PhD, MPH Collaborators Acknowledgements Jeff Harris, MD, MPH, MBA Courtney Hughes, PhD Emily Yette, MPHc This research
ROLL-YOUR-OWN CIGARETTES AS A RISK FACTOR FOR TOBACCO DEPENDENCE IN NEW ZEALAND
 ROLL-YOUR-OWN CIGARETTES AS A RISK FACTOR FOR TOBACCO DEPENDENCE IN NEW ZEALAND A report prepared as part of a Ministry of Health contract for scientific services by Dr R A Lea Dr P Truman 22 December
ROLL-YOUR-OWN CIGARETTES AS A RISK FACTOR FOR TOBACCO DEPENDENCE IN NEW ZEALAND A report prepared as part of a Ministry of Health contract for scientific services by Dr R A Lea Dr P Truman 22 December
Factors Influencing Smoking Behavior Among Adolescents
 RESEARCH COMMUNICATION Factors Influencing Smoking Behavior Among Adolescents Urmi Sen 1, Arindam Basu 2 Abstract Objective To study the impact of tobacco advertisements and other social factors on the
RESEARCH COMMUNICATION Factors Influencing Smoking Behavior Among Adolescents Urmi Sen 1, Arindam Basu 2 Abstract Objective To study the impact of tobacco advertisements and other social factors on the
Impacts of Early Exposure to Work on Smoking Initiation Among Adolescents and Older Adults: the ADD Health Survey. David J.
 Impacts of Early Exposure to Work on Smoking Initiation Among Adolescents and Older Adults: the ADD Health Survey David J. Lee, PhD University of Miami Miller School of Medicine Department of Public Health
Impacts of Early Exposure to Work on Smoking Initiation Among Adolescents and Older Adults: the ADD Health Survey David J. Lee, PhD University of Miami Miller School of Medicine Department of Public Health
ORIGINAL INVESTIGATION. Nicotine Gum Treatment Before Smoking Cessation
 ORIGINAL INVESTIGATION Nicotine Gum Treatment Before Smoking Cessation A Randomized Trial Jean-François Etter, PhD, MPH; Philippe Huguelet, MD; Thomas V. Perneger, MD, PhD; Jacques Cornuz, MD Background:
ORIGINAL INVESTIGATION Nicotine Gum Treatment Before Smoking Cessation A Randomized Trial Jean-François Etter, PhD, MPH; Philippe Huguelet, MD; Thomas V. Perneger, MD, PhD; Jacques Cornuz, MD Background:
What Do We Know About Best Practice Prenatal Counseling Interventions In Clinical Settings?
 1 What Do We Know About Best Practice Prenatal Counseling Interventions In Clinical Settings? Cathy L. Melvin, PhD, MPH Presented by Catherine L. Rohweder, DrPH The National Conference on Tobacco or Health
1 What Do We Know About Best Practice Prenatal Counseling Interventions In Clinical Settings? Cathy L. Melvin, PhD, MPH Presented by Catherine L. Rohweder, DrPH The National Conference on Tobacco or Health
Systematic review of the effectiveness of stage based interventions to promote smoking cessation
 Systematic review of the effectiveness of stage based interventions to promote smoking cessation Robert Paul Riemsma, Jill Pattenden, Christopher Bridle, Amanda J Sowden, Lisa Mather, Ian S Watt, Anne
Systematic review of the effectiveness of stage based interventions to promote smoking cessation Robert Paul Riemsma, Jill Pattenden, Christopher Bridle, Amanda J Sowden, Lisa Mather, Ian S Watt, Anne
WISCONSIN MEDICAL JOURNAL
 Wisconsin Physicians Advising Smokers to Quit: Results from the Current Population Survey, 1998-1999 and Behavioral Risk Factor Surveillance System, 2000 Anne M. Marbella, MS; Amanda Riemer; Patrick Remington,
Wisconsin Physicians Advising Smokers to Quit: Results from the Current Population Survey, 1998-1999 and Behavioral Risk Factor Surveillance System, 2000 Anne M. Marbella, MS; Amanda Riemer; Patrick Remington,
Effect of a workplace-based group training programme combined with financial incentives on smoking cessation: a cluster-randomised controlled trial
 Effect of a workplace-based group training programme combined with financial incentives on smoking cessation: a cluster-randomised controlled trial Floor A van den Brand, Gera E Nagelhout, Bjorn Winkens,
Effect of a workplace-based group training programme combined with financial incentives on smoking cessation: a cluster-randomised controlled trial Floor A van den Brand, Gera E Nagelhout, Bjorn Winkens,
Impact of Over-the-Counter Sales on Effectiveness of Pharmaceutical Aids for Smoking Cessation JAMA. 2002;288:
 ORIGINAL CONTRIBUTION Impact of Over-the-Counter Sales on Effectiveness of Pharmaceutical Aids for Smoking Cessation John P. Pierce, PhD Elizabeth A. Gilpin, MS Context Successful smoking cessation is
ORIGINAL CONTRIBUTION Impact of Over-the-Counter Sales on Effectiveness of Pharmaceutical Aids for Smoking Cessation John P. Pierce, PhD Elizabeth A. Gilpin, MS Context Successful smoking cessation is
Clean Indoor Air Policies in Wisconsin Workplaces
 Clean Indoor Air Policies in Wisconsin Workplaces Clare E. Guse, MS; Anne M. Marbella, MS; Peter M. Layde, MD, MSc; Ann Christiansen, MPH; Patrick Remington, MD, MPH ABSTRACT Objective: To describe the
Clean Indoor Air Policies in Wisconsin Workplaces Clare E. Guse, MS; Anne M. Marbella, MS; Peter M. Layde, MD, MSc; Ann Christiansen, MPH; Patrick Remington, MD, MPH ABSTRACT Objective: To describe the
Epidemiology of Hardcore Smoking: The Need to Advance the Field
 Epidemiology of Hardcore Smoking: The Need to Advance the Field Gary A. Giovino, Ph.D Transdisciplinary Classification of Hardcore Smokers: How Shall We Define Hardcore? Symposium 10 th Annual Meeting
Epidemiology of Hardcore Smoking: The Need to Advance the Field Gary A. Giovino, Ph.D Transdisciplinary Classification of Hardcore Smokers: How Shall We Define Hardcore? Symposium 10 th Annual Meeting
Smoking Status and Body Mass Index in the United States:
 Smoking Status and Body Mass Index in the United States: 1996-2000 Jun Yang, MD, PhD and Gary Giovino, PhD Roswell Park Cancer Institute Elm and Carlton Streets Buffalo, NY 14263, USA Society for Research
Smoking Status and Body Mass Index in the United States: 1996-2000 Jun Yang, MD, PhD and Gary Giovino, PhD Roswell Park Cancer Institute Elm and Carlton Streets Buffalo, NY 14263, USA Society for Research
ORIGINAL INVESTIGATION
 ORIGINAL INVESTIGATION Chronic Disease Management for Tobacco Dependence A Randomized, Controlled Trial Anne M. Joseph, MD, MPH; Steven S. Fu, MD; Bruce Lindgren, MS; Alexander J. Rothman, PhD; Molly Kodl,
ORIGINAL INVESTIGATION Chronic Disease Management for Tobacco Dependence A Randomized, Controlled Trial Anne M. Joseph, MD, MPH; Steven S. Fu, MD; Bruce Lindgren, MS; Alexander J. Rothman, PhD; Molly Kodl,
Supplementary Online Content
 Supplementary Online Content Baker TB, Piper ME, Stein JH, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical
Supplementary Online Content Baker TB, Piper ME, Stein JH, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical
Diabetes Care Publish Ahead of Print, published online February 25, 2010
 Diabetes Care Publish Ahead of Print, published online February 25, 2010 Undertreatment Of Mental Health Problems In Diabetes Undertreatment Of Mental Health Problems In Adults With Diagnosed Diabetes
Diabetes Care Publish Ahead of Print, published online February 25, 2010 Undertreatment Of Mental Health Problems In Diabetes Undertreatment Of Mental Health Problems In Adults With Diagnosed Diabetes
US College Students Exposure to Tobacco Promotions: Prevalence and Association With Tobacco Use
 US College Students Exposure to Tobacco Promotions: Prevalence and Association With Tobacco Use Nancy A. Rigotti, MD, Susan E. Moran, MD, MSCE, and Henry Wechsler, PhD Tobacco use among young adults in
US College Students Exposure to Tobacco Promotions: Prevalence and Association With Tobacco Use Nancy A. Rigotti, MD, Susan E. Moran, MD, MSCE, and Henry Wechsler, PhD Tobacco use among young adults in
Does cigarette reduction while using nicotine replacement predict quitting? Observational evidence from the Rapid Reduction Trial
 10 th UKSBM ASM; Nottingham 2014 Does cigarette reduction while using nicotine replacement predict quitting? Observational evidence from the Rapid Reduction Trial Lindson-Hawley N; West R; Michie S; Aveyard
10 th UKSBM ASM; Nottingham 2014 Does cigarette reduction while using nicotine replacement predict quitting? Observational evidence from the Rapid Reduction Trial Lindson-Hawley N; West R; Michie S; Aveyard
Wellness on the Run Webinar. Kick the habit. Reducing Tobacco in the Workplace
 Wellness on the Run Webinar Kick the habit. Reducing Tobacco in the Workplace 1 Welcome Today s presentation will begin shortly. In order to hear the audio for this presentation, please turn up your speakers.
Wellness on the Run Webinar Kick the habit. Reducing Tobacco in the Workplace 1 Welcome Today s presentation will begin shortly. In order to hear the audio for this presentation, please turn up your speakers.
Tobacco Dependence Assessment and Treatment
 Tobacco Dependence Assessment and Treatment Jennifer Bluem Moran, M.A. Mayo Clinic Tobacco Treatment Specialist Certification 2013 MFMER slide-1 Outline Motivation Key treatment components Assessment issues
Tobacco Dependence Assessment and Treatment Jennifer Bluem Moran, M.A. Mayo Clinic Tobacco Treatment Specialist Certification 2013 MFMER slide-1 Outline Motivation Key treatment components Assessment issues
MYTH #1. Nicotine is a harmful, medically dangerous drug. Nicotine: Myths, Realities & Treatment OBJECTIVES FACT. CSAM Board Review Course, 10/23/08
 Nicotine: Myths, Realities & Treatment CSAM Board Review Course, Newport Beach, CA Presented by David P.L. Sachs, MD Director, Palo Alto Center for Pulmonary Disease Prevention & Clinical Associate Professor,
Nicotine: Myths, Realities & Treatment CSAM Board Review Course, Newport Beach, CA Presented by David P.L. Sachs, MD Director, Palo Alto Center for Pulmonary Disease Prevention & Clinical Associate Professor,
Cessation and Cessation Measures
 Cessation and Cessation Measures among Adult Daily Smokers: National and State-Specific Data David M. Burns, Christy M. Anderson, Michael Johnson, Jacqueline M. Major, Lois Biener, Jerry Vaughn, Thomas
Cessation and Cessation Measures among Adult Daily Smokers: National and State-Specific Data David M. Burns, Christy M. Anderson, Michael Johnson, Jacqueline M. Major, Lois Biener, Jerry Vaughn, Thomas
Tobacco Use Among Adolescents Enrolled in Wisconsin Medicaid Program
 Tobacco Use Among Adolescents Enrolled in Wisconsin Medicaid Program Tammy Harris Sims, MD, MS; Mario Sims, PhD Abstract Problem: There has been very little study done on tobacco use and cessation among
Tobacco Use Among Adolescents Enrolled in Wisconsin Medicaid Program Tammy Harris Sims, MD, MS; Mario Sims, PhD Abstract Problem: There has been very little study done on tobacco use and cessation among
Stage Based Interventions for Tobacco Cessation
 Precontemplation Stage Based Interventions for Tobacco Cessation Relapse Contemplation Preparation Action Maintenance Theoretical and practical considerations related to Movement through the Stages of
Precontemplation Stage Based Interventions for Tobacco Cessation Relapse Contemplation Preparation Action Maintenance Theoretical and practical considerations related to Movement through the Stages of
COMMUNITY HEALTH NEEDS ASSESSMENT AND IMPLEMENATION PLAN JUNE 2016
 COMMUNITY HEALTH NEEDS ASSESSMENT AND IMPLEMENATION PLAN JUNE 2016 is, and has been an active member of the Healthy Blair County Coalition. Representatives of have been members of the Steering Committee,
COMMUNITY HEALTH NEEDS ASSESSMENT AND IMPLEMENATION PLAN JUNE 2016 is, and has been an active member of the Healthy Blair County Coalition. Representatives of have been members of the Steering Committee,
ORIGINAL INVESTIGATION. Effectiveness of a Computer-Tailored Smoking Cessation Program
 Effectiveness of a Computer-Tailored Smoking Cessation Program A Randomized Trial ORIGINAL INVESTIGATION Jean-François Etter, PhD, MPH; Thomas V. Perneger, MD, PhD Background: From a public health perspective,
Effectiveness of a Computer-Tailored Smoking Cessation Program A Randomized Trial ORIGINAL INVESTIGATION Jean-François Etter, PhD, MPH; Thomas V. Perneger, MD, PhD Background: From a public health perspective,
Wanting to Get Pregnant
 Continuing Medical Education COPD Case Presentation LEARNING OBJECTIVES Those completing this activity will receive information that should allow them to Assist a patient in developing a quit plan; Advise
Continuing Medical Education COPD Case Presentation LEARNING OBJECTIVES Those completing this activity will receive information that should allow them to Assist a patient in developing a quit plan; Advise
Despite substantial declines over the past decade,
 19 The journey to quitting smoking Margot Shields Abstract Objectives This article outlines smoking trends over the past 10 years among the population aged 18 or older. Factors associated with smoking
19 The journey to quitting smoking Margot Shields Abstract Objectives This article outlines smoking trends over the past 10 years among the population aged 18 or older. Factors associated with smoking
Changing for Life: Using the Stages of Change to Support the Recovery Process
 Changing for Life: Using the Stages of Change to Support the Recovery Process James O. Prochaska, Ph.D. Director and Professor Cancer Prevention Research Center University of Rhode Island Founder Pro-Change
Changing for Life: Using the Stages of Change to Support the Recovery Process James O. Prochaska, Ph.D. Director and Professor Cancer Prevention Research Center University of Rhode Island Founder Pro-Change
Baptist Health Beaches Community Health Needs Assessment Priorities Implementation Plans
 Baptist Health Beaches Community Health Needs Assessment Priorities Implementation Plans Health Disparities Heart Disease Stroke Hypertension Diabetes Adult Type II Preventive Health Care Smoking and Smokeless
Baptist Health Beaches Community Health Needs Assessment Priorities Implementation Plans Health Disparities Heart Disease Stroke Hypertension Diabetes Adult Type II Preventive Health Care Smoking and Smokeless
Potential Costs and Benefits of Smoking Cessation for New Jersey
 Potential Costs and Benefits of Smoking Cessation for New Jersey Jill S. Rumberger, PhD Assistant Professor Pennsylvania State University, Capital College, School of Public Affairs, Harrisburg, PA Christopher
Potential Costs and Benefits of Smoking Cessation for New Jersey Jill S. Rumberger, PhD Assistant Professor Pennsylvania State University, Capital College, School of Public Affairs, Harrisburg, PA Christopher
Financial Incentives to help women stop smoking during pregnancy : a new trial Linda Bauld
 Financial Incentives to help women stop smoking during pregnancy : a new trial Linda Bauld on behalf of the CPIT Research Team Feb 2015 Outline Background & Literature Examples from UK practice Methods
Financial Incentives to help women stop smoking during pregnancy : a new trial Linda Bauld on behalf of the CPIT Research Team Feb 2015 Outline Background & Literature Examples from UK practice Methods
MOKP COST CONTAINMENT GRANT FINAL REPORT
 MOKP COST CONTAINMENT GRANT FINAL REPORT Project Title: Helping Recipients Ask: The Effectiveness of Improved Health Education on Increasing Living Donation. Applicant Name: Amy D. Waterman, PhD Address:
MOKP COST CONTAINMENT GRANT FINAL REPORT Project Title: Helping Recipients Ask: The Effectiveness of Improved Health Education on Increasing Living Donation. Applicant Name: Amy D. Waterman, PhD Address:
Compensatory smoking from gradual and immediate reduction in cigarette nicotine content
 Compensatory smoking from gradual and immediate reduction in cigarette nicotine content Dorothy K. Hatsukami, 1 Eric C. Donny, 2 Joseph S. Koopmeiners, 3 Neal L. Benowitz 4 1 University of Minnesota, Tobacco
Compensatory smoking from gradual and immediate reduction in cigarette nicotine content Dorothy K. Hatsukami, 1 Eric C. Donny, 2 Joseph S. Koopmeiners, 3 Neal L. Benowitz 4 1 University of Minnesota, Tobacco
Rates and Predictors of Renewed Quitting After Relapse During a One-Year Follow-Up Among Primary Care Patients
 ann. behav. med. (2015) 49:128 140 DOI 10.1007/s12160-014-9627-6 ORIGINAL ARTICLE Rates and Predictors of Renewed Quitting After Relapse During a One-Year Follow-Up Among Primary Care Patients Krysten
ann. behav. med. (2015) 49:128 140 DOI 10.1007/s12160-014-9627-6 ORIGINAL ARTICLE Rates and Predictors of Renewed Quitting After Relapse During a One-Year Follow-Up Among Primary Care Patients Krysten
Ensuring Medicaid Coverage of Tobacco Cessation
 Ensuring Medicaid Coverage of Tobacco Cessation Why is Medicaid Coverage of Tobacco Cessation Important? In 2014, 32 percent of Medicaid enrollees were smokers, compared with 17 percent of the general
Ensuring Medicaid Coverage of Tobacco Cessation Why is Medicaid Coverage of Tobacco Cessation Important? In 2014, 32 percent of Medicaid enrollees were smokers, compared with 17 percent of the general
10/6/2015. Quotes from the Literature PATIENT, PROGRAM AND POLICY STRATEGIES TO REDUCE TOBACCO USE IN ADDICTION TREATMENT.
 PATIENT, PROGRAM AND POLICY STRATEGIES TO REDUCE TOBACCO USE IN ADDICTION TREATMENT Joseph Guydish, PhD University of California, San Francisco National Conference on Tackling Tobacco Use in Vulnerable
PATIENT, PROGRAM AND POLICY STRATEGIES TO REDUCE TOBACCO USE IN ADDICTION TREATMENT Joseph Guydish, PhD University of California, San Francisco National Conference on Tackling Tobacco Use in Vulnerable
FACTORS ASSOCIATED WITH THE INTENTIONS TO QUIT AND WILLINGNESS TO QUIT AMONG WATERPIPE ADULT SMOKERS IN LEBANON
 FACTORS ASSOCIATED WITH THE INTENTIONS TO QUIT AND WILLINGNESS TO QUIT AMONG WATERPIPE ADULT SMOKERS IN LEBANON Souheil Hallit, Pharm.D., MSc, MPH, PhD candidate Director of Research- Psychiatric Hospital
FACTORS ASSOCIATED WITH THE INTENTIONS TO QUIT AND WILLINGNESS TO QUIT AMONG WATERPIPE ADULT SMOKERS IN LEBANON Souheil Hallit, Pharm.D., MSc, MPH, PhD candidate Director of Research- Psychiatric Hospital
Tanaffos (2002) 1(4), NRITLD, National Research Institute of Tuberculosis and Lung Disease, Iran
 ORIGINAL RESEARCH ARTICLE Tanaffos (2002) 1(4), 61-67 2002 NRITLD, National Research Institute of Tuberculosis and Lung Disease, Iran Effective Factors on Smoking Cessation among the Smokers in the First
ORIGINAL RESEARCH ARTICLE Tanaffos (2002) 1(4), 61-67 2002 NRITLD, National Research Institute of Tuberculosis and Lung Disease, Iran Effective Factors on Smoking Cessation among the Smokers in the First
Tobacco use is Wisconsin s
 Focus on... Smoking Increasing tobacco taxes: An evidencebased measure to reduce tobacco use Marion Ceraso, MHS; David Ahrens, MS; Patrick Remington, MD Tobacco use is Wisconsin s single most important
Focus on... Smoking Increasing tobacco taxes: An evidencebased measure to reduce tobacco use Marion Ceraso, MHS; David Ahrens, MS; Patrick Remington, MD Tobacco use is Wisconsin s single most important
Evaluation of Workplace-based Quit Smoking Programs. Check-in Survey for Employers
 Evaluation of Workplace-based Quit Smoking Programs Check-in Survey for Employers 1 Questions about your organization: Name of Organization: 1. What is your role in this organization? 2. Under which sector
Evaluation of Workplace-based Quit Smoking Programs Check-in Survey for Employers 1 Questions about your organization: Name of Organization: 1. What is your role in this organization? 2. Under which sector
Employers, in their role as health care purchasers, are
 Article Health and Disability Costs of Depressive Illness in a Major U.S. Corporation Benjamin G. Druss, M.D., M.P.H. Robert A. Rosenheck, M.D. William H. Sledge, M.D. Objective: Employers are playing
Article Health and Disability Costs of Depressive Illness in a Major U.S. Corporation Benjamin G. Druss, M.D., M.P.H. Robert A. Rosenheck, M.D. William H. Sledge, M.D. Objective: Employers are playing
Pharmacological interventions for smoking cessation: an overview and network meta-analysis (Review)
 Pharmacological interventions for smoking cessation: an overview and network meta-analysis (Review) Cahill K, Stevens S, Perera R, Lancaster T This is a reprint of a Cochrane review, prepared and maintained
Pharmacological interventions for smoking cessation: an overview and network meta-analysis (Review) Cahill K, Stevens S, Perera R, Lancaster T This is a reprint of a Cochrane review, prepared and maintained
Smoking and the Ø pattern; predictors of transitions through the stages of change
 HEALTH EDUCATION RESEARCH Vol.21 no.3 2006 Theory & Practice Pages 305 314 Advance Access publication 8 June 2006 Smoking and the Ø pattern; predictors of transitions through the stages of change E. F.
HEALTH EDUCATION RESEARCH Vol.21 no.3 2006 Theory & Practice Pages 305 314 Advance Access publication 8 June 2006 Smoking and the Ø pattern; predictors of transitions through the stages of change E. F.
A pilot study of a brief smoking cessation intervention at the student health center.
 University of Texas at El Paso From the SelectedWorks of Theodore V. Cooper 2009 A pilot study of a brief smoking cessation intervention at the student health center. Theodore V. Cooper, University of
University of Texas at El Paso From the SelectedWorks of Theodore V. Cooper 2009 A pilot study of a brief smoking cessation intervention at the student health center. Theodore V. Cooper, University of
The Cochrane Database of Systematic Reviews The Cochrane Library, Copyright 2005, The Cochrane Collaboration Volume (1) 2005 [no page #]
![The Cochrane Database of Systematic Reviews The Cochrane Library, Copyright 2005, The Cochrane Collaboration Volume (1) 2005 [no page #] The Cochrane Database of Systematic Reviews The Cochrane Library, Copyright 2005, The Cochrane Collaboration Volume (1) 2005 [no page #]](/thumbs/75/71744628.jpg) Ovid: Kaper: The Cochrane Library, Volume (1).2005. Pagina 1 di 39 The Cochrane Database of Systematic Reviews The Cochrane Library, Copyright 2005, The Cochrane Collaboration Volume (1) 2005 [no page
Ovid: Kaper: The Cochrane Library, Volume (1).2005. Pagina 1 di 39 The Cochrane Database of Systematic Reviews The Cochrane Library, Copyright 2005, The Cochrane Collaboration Volume (1) 2005 [no page
Harnessing the Power of the DNP: Leading the Development and Implementation of an Evidence Based Clinical Program JOELLE FATHI, DNP, RN, ARNP, CTTS
 Harnessing the Power of the DNP: Leading the Development and Implementation of an Evidence Based Clinical Program JOELLE FATHI, DNP, RN, ARNP, CTTS Objectives Identify innovative ways in which the DNP
Harnessing the Power of the DNP: Leading the Development and Implementation of an Evidence Based Clinical Program JOELLE FATHI, DNP, RN, ARNP, CTTS Objectives Identify innovative ways in which the DNP
Indepen dent Study On Program Effective ness
 Indepen dent Study On Program Effective ness This report was prepared by the Davis-Valente Group in cooperation with the Johns Hopkins University. (NOTE: Graphs have been removed to increase Internet load
Indepen dent Study On Program Effective ness This report was prepared by the Davis-Valente Group in cooperation with the Johns Hopkins University. (NOTE: Graphs have been removed to increase Internet load
Does Motivation Matter? Analysis of a Randomized Trial of Proactive Outreach to VA Smokers
 Does Motivation Matter? Analysis of a Randomized Trial of Proactive Outreach to VA Smokers Elisheva R. Danan, MD, MPH 1,2, Anne M. Joseph, MD, MPH 2, Scott E. Sherman, MD, MPH 3,4, Diana J. Burgess, PhD
Does Motivation Matter? Analysis of a Randomized Trial of Proactive Outreach to VA Smokers Elisheva R. Danan, MD, MPH 1,2, Anne M. Joseph, MD, MPH 2, Scott E. Sherman, MD, MPH 3,4, Diana J. Burgess, PhD
Anna McCullough, MSW, MSPH, Michael Fisher, MD, MPH, Adam O. Goldstein, MD, MPH, Kathryn D. Kramer, PhD, Carol Ripley-Moffitt, MDiv, CCPHC
 Smoking as a Vital Sign: Prompts to Ask and Assess Increase Cessation Counseling Anna McCullough, MSW, MSPH, Michael Fisher, MD, MPH, Adam O. Goldstein, MD, MPH, Kathryn D. Kramer, PhD, Carol Ripley-Moffitt,
Smoking as a Vital Sign: Prompts to Ask and Assess Increase Cessation Counseling Anna McCullough, MSW, MSPH, Michael Fisher, MD, MPH, Adam O. Goldstein, MD, MPH, Kathryn D. Kramer, PhD, Carol Ripley-Moffitt,
THE NEW ZEALAND MEDICAL JOURNAL
 THE NEW ZEALAND MEDICAL JOURNAL Vol 117 No 1190 ISSN 1175 8716 Smoking in a New Zealand university student sample Kypros Kypri and Joanne Baxter Abstract Aims The aims of this study were to estimate the
THE NEW ZEALAND MEDICAL JOURNAL Vol 117 No 1190 ISSN 1175 8716 Smoking in a New Zealand university student sample Kypros Kypri and Joanne Baxter Abstract Aims The aims of this study were to estimate the
Extended interactive voice response telephony (IVR) for relapse prevention after smoking cessation using varenicline and IVR: a pilot study
 McNaughton et al. BMC Public Health 2013, 13:824 RESEARCH ARTICLE Open Access Extended interactive voice response telephony (IVR) for relapse prevention after smoking cessation using varenicline and IVR:
McNaughton et al. BMC Public Health 2013, 13:824 RESEARCH ARTICLE Open Access Extended interactive voice response telephony (IVR) for relapse prevention after smoking cessation using varenicline and IVR:
SMOKING RELAPSE ONE YEAR AFTER DELIVERY AMONG WOMEN WHO QUIT SMOKING DURING PREGNANCY
 International Journal of Occupational Medicine and Environmental Health, 2005;8(2):59 65 SMOKING RELAPSE ONE YEAR AFTER DELIVERY AMONG WOMEN WHO QUIT SMOKING DURING PREGNANCY KINGA POLAŃSKA, WOJCIECH HANKE,
International Journal of Occupational Medicine and Environmental Health, 2005;8(2):59 65 SMOKING RELAPSE ONE YEAR AFTER DELIVERY AMONG WOMEN WHO QUIT SMOKING DURING PREGNANCY KINGA POLAŃSKA, WOJCIECH HANKE,
Beyond Being Lost In Transition: Reviewing the History and Progress in Cancer Survivorship Care
 Beyond Being Lost In Transition: Reviewing the History and Progress in Cancer Survivorship Care Larissa Nekhlyudov, MD, MPH Associate Professor, Harvard Medical School Medical Director, BWH Primary Care
Beyond Being Lost In Transition: Reviewing the History and Progress in Cancer Survivorship Care Larissa Nekhlyudov, MD, MPH Associate Professor, Harvard Medical School Medical Director, BWH Primary Care
Baptist Health Nassau Community Health Needs Assessment Priorities Implementation Plans
 Baptist Health Nassau Community Health Needs Assessment Priorities Implementation Plans Health Disparities Heart Disease Stroke Hypertension Diabetes Adult Type II Preventive Health Care Smoking and Smokeless
Baptist Health Nassau Community Health Needs Assessment Priorities Implementation Plans Health Disparities Heart Disease Stroke Hypertension Diabetes Adult Type II Preventive Health Care Smoking and Smokeless
Minnesota Postsecondary Institutions Tobacco-use Policies and Changes in Student Tobacco-use Rates ( )
 Minnesota Postsecondary Institutions Tobacco-use Policies and Changes in Student Tobacco-use Rates (2007 2013) Boynton Health Service Minnesota Postsecondary Institutions Tobacco-use Policies and Changes
Minnesota Postsecondary Institutions Tobacco-use Policies and Changes in Student Tobacco-use Rates (2007 2013) Boynton Health Service Minnesota Postsecondary Institutions Tobacco-use Policies and Changes
BASELINE PREDICTORS OF SINGULAR ACTION AMONG PARTICIPANTS WITH MULTIPLE HEALTH BEHAVIOR RISKS
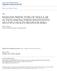 University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2013 BASELINE PREDICTORS OF SINGULAR ACTION AMONG PARTICIPANTS WITH MULTIPLE HEALTH BEHAVIOR RISKS Miryam Yusufov University of
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2013 BASELINE PREDICTORS OF SINGULAR ACTION AMONG PARTICIPANTS WITH MULTIPLE HEALTH BEHAVIOR RISKS Miryam Yusufov University of
Tobacco smoking is the leading preventable. Varenicline Versus Bupropion SR or Placebo for Smoking Cessation: A Pooled Analysis
 Varenicline Versus Bupropion SR or Placebo for Smoking Cessation: A Pooled Analysis Mitchell Nides, PhD; Elbert D. Glover, PhD, FAAHB; Victor I. Reus, MD; Arden G. Christen, DDS, MSA, MA; Barry J. Make,
Varenicline Versus Bupropion SR or Placebo for Smoking Cessation: A Pooled Analysis Mitchell Nides, PhD; Elbert D. Glover, PhD, FAAHB; Victor I. Reus, MD; Arden G. Christen, DDS, MSA, MA; Barry J. Make,
Despite public knowledge of the harmful effects of
 Failure to Obtain Varenicline in Members With Pharmacy Benefits At a Glance Practical Implications e36 Author Information e40 Web Exclusive Original Research Catherine E. Cooke, PharmD, BCPS, PAHM; Elbert
Failure to Obtain Varenicline in Members With Pharmacy Benefits At a Glance Practical Implications e36 Author Information e40 Web Exclusive Original Research Catherine E. Cooke, PharmD, BCPS, PAHM; Elbert
An Expert System Intervention for Smoking Cessation
 Expert System for Smoking Cessation 1 An Expert System Intervention for Smoking Cessation Wayne F. Velicer James O. Prochaska Cancer Prevention Research Center University of Rhode Island Reference: Velicer,
Expert System for Smoking Cessation 1 An Expert System Intervention for Smoking Cessation Wayne F. Velicer James O. Prochaska Cancer Prevention Research Center University of Rhode Island Reference: Velicer,
Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults
 Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults Astha Singhal BDS, MPH, PhD Assistant Professor, Health Policy & Health Services Research Boston University Henry
Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults Astha Singhal BDS, MPH, PhD Assistant Professor, Health Policy & Health Services Research Boston University Henry
