FORMULARY UPDATES ABUHB s Drug Formulary is at:
|
|
|
- Caitlin Shelton
- 5 years ago
- Views:
Transcription
1 Aneurin Bevan University Health Board Medicines & Therapeutics Committee July 2014 PRESCRIBING enewsletter archive at: Dear Gwent Prescriber At its last two meetings (10 th April 2014 and 22 nd May 2014) ABUHB s Medicines & Therapeutics Committee made the following decisions in relation to the ABUHB Formulary: FORMULARY UPDATES ABUHB s Drug Formulary is at: and access to the BNF and the BNF for Children is easy via NHS evidence at: BNF section INDACATEROL/GLYCOPYRRONIUM ( Ultibro Breezhaler ) for COPD ADDED to section as a Green drug (appropriate for initiation and repeat prescribing by all prescribers) in accordance with AWMSG advice No AWMSG s recommendation on Ultibro Breezhaler in COPD (Advice No 0814) can be found at: Full prescribing information on Ultibro is at: _Product_Information/human/002679/WC pdf BNF section EXENATIDE (Byetta ) twice daily REMOVED in accordance with the consensus view of ABUHB s Diabetes Directorate the standardrelease injection (Byetta) has been removed from the Formulary. The MTC noted that NICE s advice on this TWICE DAILY formulation of exenatide was from a non statutory guideline (CG87). Byetta s non Formulary status does NOT require patients established on Byetta to be changed to an alternative GLP 1 agonist. New patients should not be started on Byetta. The ONCE WEEKLY formulation of exenatide (Bydureon ) injection remains in the ABUHB Formulary. Bydureon may be a suitable option in patients with Type 2 diabetes and more severe hyperglycaemia, particularly if third party administration is required (by a district nurse for example). The GLP 1 agonists 1 st & 2 nd line choices 1. Lixisenatide (once daily, less expensive that Liraglutide and Bydureon) will be used as 1 st line GLP 1 injection in suitable patients with Type 2 diabetes and milder degrees of hyperglycaemia (e.g. HbA 1C < 70 mmol/mol) 2. Liraglutide 1.2 mg once daily may be used as 1 st line GLP 1 injection in suitable patients with Type 2 diabetes and more severe hyperglycaemia (e.g. HbA 1C 70 mmol/mol). All GLP 1 agonists are designated Amber in the local Traffic Light system (specialist initiation only and without the need for any formal shared care arrangement). Specialist initiation covers initiation by GPs based in practices that have a relevant accreditation to provide an advanced diabetic service. Comparative acquisition costs (from BNF March 2014) of GLP 1 agonists: Drug Dose Regimen Cost / yr Lixisenatide 20microgram once daily 706 Liraglutide 1.2 once daily Note NICE do NOT recommend 1.8mg once daily 955 Exenatide m/r (Bydureon ) 2mg once weekly 954 Exenatide (Byetta ) 5 to 10microgram twice daily 830 Doses are for general comparison and do not imply therapeutic equivalence. All GLP 1s are administered via subcutaneous injection. Page 1 of 6
2 BNF section COLESEVELAM (Cholestigel ) 625mg tablets ADDED for the OFF LABEL treatment of diarrhoea and other gastrointestinal symptoms in patients with bile acid malabsorption. Prescribers receiving requests to repeat prescribe should note the following restrictions: Colesevelam should only be initiated by specialists in gastroenterology (i.e. it is designated Amber in ABUHB s Traffic Light classification NO shared care arrangements are required). Colesevelam should only be used in individuals with a clinical diagnosis of bile acid malabsorption who have shown an improvement in diarrhoea and gastrointestinal symptoms on colestyramine (following an unsuccessful trial of antimotility drugs) but have not been able to tolerate continued treatment. Current evidence did not support the use of colesevelam for the symptomatic treatment of diarrhoeapredominant IBS. Colesevelam remains non formulary for its licensed indications of primary hypercholesterolaemia (either alone or with a statin) and primary/familial hypercholesterolaemia with ezetimibe (+/ a statin). Patients should be stabilised on colesevelam before GPs are requested to repeat prescribe. Full prescribing information on colesevelam is at: BNF section NALMEFENE ( Selincro ) 18mg tablets ADDED in accordance with AWMSG s advice for it to be used as an option for the reduction of alcohol consumption in adult patients with alcohol dependence who have a high drinking risk level (DRL) without physical withdrawal symptoms and who do not require immediate detoxification. Nalmefene should only be prescribed in conjunction with continuous psychosocial support focused on treatment adherence and reducing alcohol consumption. Nalmefene should be initiated only in patients who continue to have a high DRL two weeks after initial assessment. The MTC has requested the Gwent Substance Misuse Area Planning Board to consider delivering a psychological support programme. The Board will be responding to the MTC once they had scoped existing provision in primary mental health care teams. Until a continuous psychological support programme is widely available in Gwent, nalmefene s Traffic Light is designation is Red (suitable for prescribing only by specialists). This will enable services that are currently able to provide regular ongoing psychosocial support (such as GSSMS) to have access to nalmefene. AWMSG s recommendation on nalmefene (Advice No 0414) can be found at: Full prescribing information on nalmefene at: BNF section VITAMIN B COMPOUND STRONG REMOVED because NICE CG100 Alcohol use disorders: Diagnosis and clinical management of alcoholrelated physical complications (June does not include Vitamin B Compound Strong (or Vitamin B Compound) in its recommendations for preventing Wernicke's encephalopathy: Offer thiamine to people at high risk of developing, or with suspected, Wernicke's encephalopathy in doses toward the upper end of the BNF range Offer prophylactic oral thiamine to harmful or dependent drinkers: if they are malnourished or at risk of malnourishment or if they have decompensated liver disease or if they are in acute withdrawal or before and during a planned medically assisted alcohol withdrawal And Vitamin B Compound Strong and Vitamin B Compound are also both preparations that are considered by the BNF s Joint Formulary Committee to be less suitable for prescribing and both bear this symbol: in the BNF and BNFc. Page 2 of 6
3 If your practice has not yet reviewed and stopped the prescribing of Vitamin B Compound preparations, please contact a member of the medicines management team: Blaenau Gwent Newport Caerphilly Torfaen Monmouthshire BNF section FULTIUM D unit Capsules ADDED these higher strength capsules are now included as an option in the local guidance on Vitamin D insufficiency and deficiency This guidance is available at: ABHBprescribingGuidanceFINAL[June2014].pdf Fultium D 3 capsules (both 400unit and 3200unit) are contra indicated in those with peanut or soya allergy. Full prescribing information on Fultium D unit caps is at: OTHER PRESCRIBING NEWS SHARED CARE PROTOCOLS Prescribers should note the following changes to Gwent Protocols: NALTREXONE NEW Protocol covering use of naltrexone in the maintenance of abstinence from alcohol (now a licensed indication). NICE CG115 (February 2011) Alcohol use disorders: diagnosis, assessment and management of harmful drinking and alcohol dependence advises that naltrexone should be considered (in combination with an individual psychological intervention) for: 1. harmful drinkers and people with mild alcohol dependence who have not responded to psychological interventions alone, or who have specifically requested a pharmacological intervention. 2. people with moderate and severe alcohol dependence after a successful withdrawal. The monitoring (at month 2 and monthly up to month 6 and then 3 monthly thereafter) is an evaluation of treatment adherence and does not involve blood tests. Although there is no standard duration of treatment in maintaining abstinence from alcohol with naltrexone, an initial period of 3 months should be considered, although prolonged administration for up to 12 months may be necessary. All the Gwent Shared Care Protocols can be found at: STRONTIUM now an Amber drug Following the revised MHRA advice (of March 2014) that strontium should only be used by people (postmenopausal women with severe osteoporosis and adult men at high risk of fracture) for whom there are no other treatments for osteoporosis (i.e. for reasons such as contraindications or intolerance), the local Traffic Light classification of strontium ranelate (Proteolos ) has been changed from Green (appropriate for initiation by all prescribers) to Amber without Shared Care (suitable for specialist initiation only). Further practical advice from Gwent Rheumatology specialists on options for managing patients inappropriately on strontium has again gone out to GP practices. This local advice is available (in a 2 page letter) at: ABHBletterUpdateJune2014.pdf The MHRA s advice on strontium is available at: Page 3 of 6
4 FUNGAL NAIL INFECTIONS new local guidance on treatment This guidance is a local adaptation of the wider HPA s 2011 Quick Reference Guide (on fungal nail and skin infections). It covers fungal nail infections only and prescribers should note the key prescribing message in the box below (agreed by the MTC in consultation with ABUHB Dermatology, Podiatry and Microbiology): Topical amorolfine (Loceryl or Curanail ) and topical tioconazole (Trosyl ) are NOT RECOMMENDED as there are no clinically important results from RCTs about their effects in fungal toenail infection. This advice NOT to use topical antifungals also includes: Any treatment of superficial white infection of the top surface of the nail plate or localised distal nail disease Situations where there are contraindications or significant adverse reactions to oral terbinafine or oral itraconazole. Further advice Patients currently mid way through a course of topical treatment should have their treatment reviewed at their next routine appointment. Consideration should be given to switching to more effective oral treatment if required (in line with the detail in the guidance on oral treatments in the table below) and stopping ineffective topical antifungals. Considering the evidence, advising patients to purchase over the counter topical antifungals (e.g. 3mls of amorolfine 5% lacquer), as an alternative to prescribing, is not recommended. CONDITION COMMENTS DRUG DOSE LENGTH OF TREATMENT Dermatophyte and candidal infection of the fingernail or toenail Treat only if infection confirmed by lab. For infections with dermatophytes use oral terbinafine or itraconazole. For infections with candida or non dermatophyte moulds use oral itraconazole. Idiosyncratic liver and other severe reactions occur very rarely with terbinafine and itraconazole. For children seek expert advice Terbinafine Itraconazole 250mg OD 200mg BD fingers toes fingers toes 6 12 weeks 3 6 months 2 courses of 7 days/month 3 courses of 7 days/month The full local guidance (3 pages) is available at: ABUHBquickreferenceGuideFINAL[April2014].pdf NITROFURANTOIN Pulmonary toxicity As UTI causing coliforms are declining in sensitivity to trimethoprim and amoxicillin, local guidance on the treatment of UTIs currently advises In the elderly, or patients who have received antibiotics within the last 3 months consider nitrofurantoin OR co amoxiclav Respiratory physicians in Gwent wish to alert prescribers in Primary Care to nitrofurantoin s potential to cause serious pulmonary reactions. Key prescribing points for all adults on long term nitrofurantoin: To include checks at each issue of a repeat prescription for nitrofurantoin (i.e. when used long term) to ensure there are no new respiratory symptoms (especially breathlessness and/or cough), and/ or three monthly practice spirometry for these patients On finding any evidence of new respiratory symptoms an urgent chest x ray should be performed with consideration of discontinuation of nitrofurantoin and referral to the respiratory physicians. The nitrofurantoin SmPC (at: states: Acute, subacute and chronic pulmonary reactions have been observed in patients treated with nitrofurantoin. If these reactions occur, nitrofurantoin should be discontinued immediately. Chronic pulmonary reactions (including pulmonary fibrosis and diffuse interstitial pneumonitis) can develop insidiously, and may occur commonly in elderly patients. Close monitoring of the pulmonary conditions of patients receiving long term therapy is warranted (especially in the elderly). Page 4 of 6
5 The SmPC also states that if any of the following respiratory reactions occur the drug should be discontinued: Acute pulmonary reactions usually occur within the first week of treatment and are reversible on cessation of therapy. Acute pulmonary reactions are commonly manifested by fever, chills, cough, chest pain, dyspnoea, pulmonary infiltration with consolidation or pleural effusion on chest x ray, and eosinophilia. In subacute pulmonary reactions, fever and eosinophilia occur less often than in the acute form Chronic pulmonary reactions occur rarely in patients who have received continuous therapy for six months or longer and are more common in elderly patients. Changes in ECG have occurred, associated with pulmonary reactions. Minor symptoms such as fever, chills, cough and dyspnoea may be significant. Collapse and cyanosis have been reported rarely. The severity of chronic pulmonary reactions and their degree of resolution appear to be related to the duration of therapy after the first clinical signs appear. It is important to recognise symptoms as early as possible. Pulmonary function may be impaired permanently, even after cessation of therapy. NITROFURANTOIN High cost of 25mg/5ml oral suspension The cost of nitrofurantoin 25mg/5ml oral suspension sugar free is disproportionately high compared to capsules or tablets (300ml is ). Although nitrofurantoin is still a recommended treatment option for UTIs in children in ABUHB s antibiotic guidance for Primary Care, prescribers wishing to use a less costly oral liquid for a UTI should note that the risk of beta lactams causing C. diff overgrowth rises exponentially with age. Therefore cefalexin may be a more appropriate alternative to trimethoprim in children (than adults, in terms of a reduced risk of C. diff developing). DOMPERIDONE risk of cardiac side effects and review of long term use The MHRA has recently recommended changes to the use of domperidone, including restricting the dose, duration of use and its licensed indication (it is now only indicated for the relief of nausea and vomiting), to minimise the known risks of potentially serious cardiac adverse effects. The MTC considered the MHRA s advice and offers the following further practical implementation advice: For gastrostomy tube fed patients on long term domperidone (where domperidone is used to reduce the risk of aspiration pneumonia, to which delayed gastric emptying of feed can contribute) prescribers should seek specialist advice from ABUHB gastroenterology. For those on domperidone for the symptomatic relief of GORD, dyspepsia or gastroparesis refer to the suggested action plan at: Link to MHRA advice (May 2014): ANTIBIOTICS FOR ACUTE EXACERBATIONS OF COPD Revised local guidelines The recommendation to use amoxicillin 500mg for acute exacerbations of COPD has been revised in view of the increasing level of local resistance to amoxicillin. DOXYCYCLINE is now the first choice for treating acute exacerbations of COPD. This change will be reflected in what is supplied in the COPD Rescue Packs. All local guidelines on antibiotics/anti infectives can be found at: Page 5 of 6
6 HAY FEVER local guidance Updated local guidance on the pharmacological management of hay fever can be found on the Prescribing Guidelines / Resources page on the MTC website (in row 3.4 of the table): page.cfm?orgid=814&pid=48819 Prescribing Guidelines / Resources webpage The layout of the Prescribing Guidelines / Resources page on the MTC s website had been changed so that items are now presented in BNF Chapter order so gastro drug related guidance will come in the first section and cardiology drug related guidance in the second. All the pain management guidelines/resources have now been placed on a separate webpage (with a link from section 4): It is hoped that these changes will make local prescribing guidance easier to find. DECLINE FORMS a reminder These Forms provide an easy to use template for GPs to communicate to a hospital specialist their wish to decline an inappropriate request to repeat prescribe. The Forms have been highlighted in previous editions of this newsletter (November 2010 and May 2013). Please note that these Forms are scrutinised by the MTC and can enable ABUHB s Medicines Management team to act to improve prescribing practice in Secondary/Tertiary Care. Recent examples of this are: GP requested to prescribe dronedarone by a consultant cardiologist in Cwm Taf. The cardiology team were alerted to the fact that all Wales advice is that dronedarone should be prescribed and monitored by specialist teams only GP requested to prescribe ulipristal 5mg (Esmya) for uterine fibroids. The consultant gynaecologist was not aware of Esmya s Red status and was happy to prescribe it for the patient. GP requested to prescribe rasagiline. The non Formulary status of rasagiline was clarified with the specialist and a Formulary application is currently being considered. The Decline Forms are available at: The Cost of SPECIALS is there a list of prices somewhere? Part VIIIB of the Drug Tariff covers specials and imported unlicensed medicines and the prices shown will be used for reimbursement regardless of the cost charged to the dispenser by the supplier. The June 2014 list includes over 200 preparations mostly oral solutions and suspensions. Feedback on any item in this enewsletter is welcome. Suggested agenda items for the MTC are also welcome. Trevor Batt Pharmacist & Professional Secretary to: Aneurin Bevan HB Medicines & Therapeutics Committee Aneurin Bevan Health Board Based at Victoria House, Corporation Rd, Newport NP19 0BH trevor.batt@wales.nhs.uk Tel: (DIRECT LINE) Web: Page 6 of 6
FORMULARY UPDATES ABUHB s Drug Formulary is at:
 Aneurin Bevan University Health Board Medicines & Therapeutics Committee PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber At its last
Aneurin Bevan University Health Board Medicines & Therapeutics Committee PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber At its last
Aneurin Bevan Health Board Medicines & Therapeutics Committee. enewsletter
 Aneurin Bevan Health Board Medicines & Therapeutics Committee August 2013 enewsletter Dear Gwent Prescriber At its last two meetings (23 rd May and 11 th July) ABHB s Medicines & Therapeutics Committee
Aneurin Bevan Health Board Medicines & Therapeutics Committee August 2013 enewsletter Dear Gwent Prescriber At its last two meetings (23 rd May and 11 th July) ABHB s Medicines & Therapeutics Committee
GWENT FORMULARY DECISIONS
 Dear Gwent Prescriber At its last two meetings (9 th September and 21 st October 2010) the Gwent Partnership Medicines & Therapeutics Committee (GPMTC) made the following decisions in relation to the Aneurin
Dear Gwent Prescriber At its last two meetings (9 th September and 21 st October 2010) the Gwent Partnership Medicines & Therapeutics Committee (GPMTC) made the following decisions in relation to the Aneurin
FORMULARY UPDATES ABUHB s Drug Formulary is at:
 ANEURIN BEVAN UNIVERSITY HEALTH BOARD MEDICINES & THERAPEUTICS COMMITTEE PRESCRIBING enewsletter 28 September 2016 ARCHIVE at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber
ANEURIN BEVAN UNIVERSITY HEALTH BOARD MEDICINES & THERAPEUTICS COMMITTEE PRESCRIBING enewsletter 28 September 2016 ARCHIVE at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 Dear Gwent Prescriber
EFFECTIVE SHARE CARE AGREEMENT. FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY
 Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
Specialist details Patient identifier Name Tel: EFFECTIVE SHARE CARE AGREEMENT FOR THE off license use of GLP1 mimetics in combination with insulin IN DUDLEY The aim of Effective Shared Care Guidelines
. AREAS OF RESPONSIBILITY FOR SHARED CARE
 SHARED CARE GUIDELINE FOR RILUZOLE IN THE MANAGEMENT OF MOTOR NEURONE DISEASE (MND) SPECIFICALLY AMYOTROPHIC LATERAL SCLEROSIS (ALS) INDICATION This shared care guideline has been prepared to support the
SHARED CARE GUIDELINE FOR RILUZOLE IN THE MANAGEMENT OF MOTOR NEURONE DISEASE (MND) SPECIFICALLY AMYOTROPHIC LATERAL SCLEROSIS (ALS) INDICATION This shared care guideline has been prepared to support the
ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women.
 ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women. Specialist details Patient identifier Name Tel: This effective shared care agreement (ESCA) sets out details for the sharing of
ESCA: Denosumab for the treatment of osteoporosis in postmenopausal women. Specialist details Patient identifier Name Tel: This effective shared care agreement (ESCA) sets out details for the sharing of
Prescribing Framework for Methotrexate for Immunosuppression in ADULTS
 Hull & East Riding Prescribing Committee Prescribing Framework for Methotrexate for Immunosuppression in ADULTS Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
Hull & East Riding Prescribing Committee Prescribing Framework for Methotrexate for Immunosuppression in ADULTS Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker) GP s Name:...
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens
 EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
NHS Kent and Medway Medicines Management. Dronedarone (Multaq ) Shared Care Guideline For Prescribing
 NHS Kent and Medway Medicines Management Dronedarone (Multaq ) Shared Care Guideline For Prescribing Issue No: 2 Review Date (If Applicable): Accountable Officer: Heather Lucas Contact Details: 01233 618158
NHS Kent and Medway Medicines Management Dronedarone (Multaq ) Shared Care Guideline For Prescribing Issue No: 2 Review Date (If Applicable): Accountable Officer: Heather Lucas Contact Details: 01233 618158
SHARED CARE GUIDELINE FOR THE MANAGEMENT OF PATIENTS ON NALTREXONE FOR ALCOHOL DEPENDENCE INDICATION
 SHARED CARE GUIDELINE FOR THE MANAGEMENT OF PATIENTS ON NALTREXONE FOR ALCOHOL DEPENDENCE INDICATION Naltrexone is used as part of a comprehensive programme of treatment against alcoholism to reduce the
SHARED CARE GUIDELINE FOR THE MANAGEMENT OF PATIENTS ON NALTREXONE FOR ALCOHOL DEPENDENCE INDICATION Naltrexone is used as part of a comprehensive programme of treatment against alcoholism to reduce the
SHARED CARE GUIDELINE FOR THE MANAGEMENT OF PATIENTS ON NALTREXONE FOR OPIOID DEPENDENCE
 SHARED CARE GUIDELINE FOR THE MANAGEMENT OF PATIENTS ON NALTREXONE FOR OPIOID DEPENDENCE INDICATION Naltrexone is a pure opiate antagonist licensed as an adjunctive prophylactic therapy in the maintenance
SHARED CARE GUIDELINE FOR THE MANAGEMENT OF PATIENTS ON NALTREXONE FOR OPIOID DEPENDENCE INDICATION Naltrexone is a pure opiate antagonist licensed as an adjunctive prophylactic therapy in the maintenance
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Nalmefene for reducing alcohol consumption in people with alcohol dependence Draft scope (pre-referral) Draft remit/appraisal
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Nalmefene for reducing alcohol consumption in people with alcohol dependence Draft scope (pre-referral) Draft remit/appraisal
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults
 Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
Guidance on Safe Prescribing of Melatonin for Sleep Disorders in Children, Young People and Adults Ref: PHARM-0025-v3 Status: FINAL Document type: Guidelines Guidance on Safe Prescribing of Melatonin Page
Conduct Disorder in Children and Young People (CYP 5-18 years of age) RISPERIDONE Effective Shared Care Agreement (ESCA)
 E102 Conduct Disorder in Children and Young People (CYP 5-18 years of age) RISPERIDONE Effective Shared Care Agreement (ESCA) Patient details Name: Date of birth: NHS number: Contact details Specialist:
E102 Conduct Disorder in Children and Young People (CYP 5-18 years of age) RISPERIDONE Effective Shared Care Agreement (ESCA) Patient details Name: Date of birth: NHS number: Contact details Specialist:
PRESCRIPTION PAD. The Newsletter of the Cumbria Area Prescribing Committee. February 2012 No. 18. Click here to find more. News from the MHRA
 PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
Essential Shared Care Agreement Naltrexone
 In partnership with as part of Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1. The patient s GP 2. Put one copy
In partnership with as part of Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1. The patient s GP 2. Put one copy
Cardiff and Vale (C&V) UHB Corporate Medicines Management Group Shared Care Committee SHARED CARE
 Cardiff and Vale (C&V) UHB Corporate Medicines Management Group Shared Care Committee SHARED CARE Drug: RILUZOLE Protocol number: CV 39 Indication: AMYOTROPHIC LATERAL SCLEROSIS (ALS) General Guidance
Cardiff and Vale (C&V) UHB Corporate Medicines Management Group Shared Care Committee SHARED CARE Drug: RILUZOLE Protocol number: CV 39 Indication: AMYOTROPHIC LATERAL SCLEROSIS (ALS) General Guidance
SHARED CARE AGREEMENT: MELATONIN (CHILDREN)
 NB: This document should be read in conjunction with the current Summary of Product Characteristics (SPC) where appropriate. DRUG AND INDICATION: Generic drug name: Formulations: MELATONIN 3mg immediate
NB: This document should be read in conjunction with the current Summary of Product Characteristics (SPC) where appropriate. DRUG AND INDICATION: Generic drug name: Formulations: MELATONIN 3mg immediate
National Prescribing Indicators Analysis of Prescribing Data to September 2015
 National Prescribing Indicators 2016 Analysis of Prescribing Data to tember National Prescribing Indicators 2016. Analysis of Prescribing Data to tember EXECUTIVE SUMMARY The All Wales Medicines Strategy
National Prescribing Indicators 2016 Analysis of Prescribing Data to tember National Prescribing Indicators 2016. Analysis of Prescribing Data to tember EXECUTIVE SUMMARY The All Wales Medicines Strategy
the person is intolerant of either metformin or a sulphonylurea, or treatment with metformin or a sulphonylurea is contraindicated, and
 Exenatide (Byetta) and Liraglutide (Victoza) prescribing guidance: Notes for initiation in primary care These incretin mimetics are given by subcutaneous injection once or twice daily. They have similar
Exenatide (Byetta) and Liraglutide (Victoza) prescribing guidance: Notes for initiation in primary care These incretin mimetics are given by subcutaneous injection once or twice daily. They have similar
Peterborough Nalmefene Pathway Responsibilities for Specialist Services & GP
 Peterborough Nalmefene Pathway Responsibilities for Specialist Services & GP INTRODUCTION/BACKGROUND In November 2014 NICE (National Institute of Clinical Excellence) published the Technology Appraisal
Peterborough Nalmefene Pathway Responsibilities for Specialist Services & GP INTRODUCTION/BACKGROUND In November 2014 NICE (National Institute of Clinical Excellence) published the Technology Appraisal
BNSSG Shared Care Guidance Please complete all sections
 NHS Bristol CCG NHS North Somerset CCG NHS South Gloucestershire CCG North Bristol NHS Trust University Hospitals Bristol NHS Foundation Trust Weston Area Health NHS Trust BNSSG Shared Care Guidance Please
NHS Bristol CCG NHS North Somerset CCG NHS South Gloucestershire CCG North Bristol NHS Trust University Hospitals Bristol NHS Foundation Trust Weston Area Health NHS Trust BNSSG Shared Care Guidance Please
Prescribing Framework for Naltrexone in Relapse Prevention (Opioid Dependence)
 Hull & East Riding Prescribing Committee Prescribing Framework for Naltrexone in Relapse Prevention (Opioid Dependence) Patients Name: Unit Number: Patients Address:.. G.P s Name:.. Communication We agree
Hull & East Riding Prescribing Committee Prescribing Framework for Naltrexone in Relapse Prevention (Opioid Dependence) Patients Name: Unit Number: Patients Address:.. G.P s Name:.. Communication We agree
Buccal Midazolam For the treatment of prolonged epileptic seizures, clusters of epileptic seizures and status epilepticus.
 Oxfordshire Clinical Commissioning Group, Oxford University Hospitals NHS Trust and Oxfordshire Health NHS Foundation Trust Shared Care Protocol and Information for GPs Buccal Midazolam For the treatment
Oxfordshire Clinical Commissioning Group, Oxford University Hospitals NHS Trust and Oxfordshire Health NHS Foundation Trust Shared Care Protocol and Information for GPs Buccal Midazolam For the treatment
GREATER MANCHESTER INTERFACE PRESCRIBING GROUP
 GREATER MANCHESTER INTERFACE PRESCRIBING GROUP On behalf of the GREATER MANCHESTER MEDICINES MANAGEMENT GROUP SHARED CARE GUIDELINE FOR THE PRESCRIBING OF SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs)
GREATER MANCHESTER INTERFACE PRESCRIBING GROUP On behalf of the GREATER MANCHESTER MEDICINES MANAGEMENT GROUP SHARED CARE GUIDELINE FOR THE PRESCRIBING OF SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs)
Patient Group Direction for Doxycycline (Tetracycline) Version: 01 Start Date: October 2015 Expiry Date: October 2018
 THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
THIS PATIENT GROUP DIRECTION HAS BEEN AGREED BY THE FOLLOWING ORGANISATIONS: CLINICAL COMMISSIONING GROUP: Doncaster CCG Lancashire North CCG Fylde & Wyre CCG East Lancashire CCG Change history Version
SHARED CARE PRESCRIBING GUIDELINE
 SHARED CARE PRESCRIBING GUIDELINE RILUZOLE for the Treatment of MOTOR NEURONE DISEASE NHS Surrey s Medicines Management Committee classification: Amber N.B. The eligibility criteria included here apply
SHARED CARE PRESCRIBING GUIDELINE RILUZOLE for the Treatment of MOTOR NEURONE DISEASE NHS Surrey s Medicines Management Committee classification: Amber N.B. The eligibility criteria included here apply
SULFASALAZINE (Adults)
 Shared Care Guideline DRUG: Introduction: SULFASALAZINE (Adults) Indication: Disease modifying drug for rheumatoid arthritis, psoriatic arthritis, undifferentiated arthritis, spondyloarthropathies, Crohn
Shared Care Guideline DRUG: Introduction: SULFASALAZINE (Adults) Indication: Disease modifying drug for rheumatoid arthritis, psoriatic arthritis, undifferentiated arthritis, spondyloarthropathies, Crohn
CLINICAL PROTOCOL THE PREVENTION OF FATALITIES FROM MEDICATION LOADING DOSES
 National Patient Safety Alert RRR018 Preventing Fatalities From Medication Loading Doses (November 2010) MMCP05 CLINICAL PROTOCOL THE PREVENTION OF FATALITIES FROM MEDICATION LOADING DOSES INTRODUCTION
National Patient Safety Alert RRR018 Preventing Fatalities From Medication Loading Doses (November 2010) MMCP05 CLINICAL PROTOCOL THE PREVENTION OF FATALITIES FROM MEDICATION LOADING DOSES INTRODUCTION
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
Patient Profile. Patient s details Initials: IF Age: 40 Gender: Male. Weight: 139.7kg Height: 510 metres BMI: >47
 Patient Profile Patient background and medication list Reason for selecting profile Interesting depression case whereby there were several opportunities for intervention as a pharmacist to ensure drug-related
Patient Profile Patient background and medication list Reason for selecting profile Interesting depression case whereby there were several opportunities for intervention as a pharmacist to ensure drug-related
Guidance for naltrexone prescribing
 Document level: Drug Alcohol (Trustwide) Code: DA7 Issue number: 2 Guidance for naltrexone prescribing Lead executive Authors details Type of document Target audience Document purpose Lead Clinical Director
Document level: Drug Alcohol (Trustwide) Code: DA7 Issue number: 2 Guidance for naltrexone prescribing Lead executive Authors details Type of document Target audience Document purpose Lead Clinical Director
NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST
 NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: CINACALCET in the management of secondary hyperparathyroidism in adult patients with end-stage renal disease
NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: CINACALCET in the management of secondary hyperparathyroidism in adult patients with end-stage renal disease
JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES
 JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES Authors Dr Ian Benton Respiratory Consultant COCH Penny Rideal Respiratory Nurse COCH Kirti Burgul Respiratory Pharmacist COCH Pam
JOINT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) MANAGEMENT GUIDELINES Authors Dr Ian Benton Respiratory Consultant COCH Penny Rideal Respiratory Nurse COCH Kirti Burgul Respiratory Pharmacist COCH Pam
Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008
 Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008 Shared care agreement has been developed appropriately
Name of Shared Care Agreement: AZATHIOPRINE/6-MERCAPTOPURINE: Oral immunomodulating drugs for inflammatory bowel disease. Reference number: 01/2008 Shared care agreement has been developed appropriately
Lipid Lowering in patients with High Risk of Cardiovascular Disease (Primary Prevention)
 Lipid Lowering in patients with High Risk of Cardiovascular Disease (Primary Prevention) Policy Statement: October 2010 This policy defines the decision made by NHS Wirral following an evidence review
Lipid Lowering in patients with High Risk of Cardiovascular Disease (Primary Prevention) Policy Statement: October 2010 This policy defines the decision made by NHS Wirral following an evidence review
Shared Care Guideline Riluzole Treatment of Motor Neurone Disease
 North Central London Joint Formulary Committee Shared Care Guideline Riluzole Treatment of Motor Neurone Disease Dear GP This shared care agreement outlines suggested ways in which the responsibilities
North Central London Joint Formulary Committee Shared Care Guideline Riluzole Treatment of Motor Neurone Disease Dear GP This shared care agreement outlines suggested ways in which the responsibilities
EFFECTIVE SHARED CARE AGREEMENT (ESCA)
 WORKING IN PARTNERSHIP WITH EFFECTIVE SHARED CARE AGREEMENT (ESCA) DRUG NAME: ACETYLCHOLINESTERASE-INHIBITORS AND MEMANTINE INDICATION/S COVERED: Dementia in Alzheimers Disease Coastal West Sussex Traffic
WORKING IN PARTNERSHIP WITH EFFECTIVE SHARED CARE AGREEMENT (ESCA) DRUG NAME: ACETYLCHOLINESTERASE-INHIBITORS AND MEMANTINE INDICATION/S COVERED: Dementia in Alzheimers Disease Coastal West Sussex Traffic
BRISTOL-MYERS SQUIBB and ASTRAZENECA v SANOFI
 CASE AUTH/2638/9/13 and AUTH/2639/9/13 BRISTOL-MYERS SQUIBB and ASTRAZENECA v SANOFI Promotion of Lyxumia Bristol-Myers Squibb and AstraZeneca jointly complained about cost comparison claims in a Lyxumia
CASE AUTH/2638/9/13 and AUTH/2639/9/13 BRISTOL-MYERS SQUIBB and ASTRAZENECA v SANOFI Promotion of Lyxumia Bristol-Myers Squibb and AstraZeneca jointly complained about cost comparison claims in a Lyxumia
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Overarching Shared Care Guideline for Glucagon-like peptide 1 (GLP 1) agonists, February 2017. PATIENT S NAME: PATIENT S ADDRESS: HOSPITAL NAME
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Overarching Shared Care Guideline for Glucagon-like peptide 1 (GLP 1) agonists, February 2017. PATIENT S NAME: PATIENT S ADDRESS: HOSPITAL NAME
Methylphenidate Shared Care Agreement For attention deficit hyperactivity disorder (ADHD) in adults Effective Shared Care Agreement
 Methylphenidate Shared Care Agreement For attention deficit hyperactivity disorder (ADHD) in adults Effective Shared Care Agreement Section 1: Shared Care arrangements and responsibilities Section 1.1
Methylphenidate Shared Care Agreement For attention deficit hyperactivity disorder (ADHD) in adults Effective Shared Care Agreement Section 1: Shared Care arrangements and responsibilities Section 1.1
Appendix 4I - Melatonin Guidance for the treatment of sleep-wake cycle disorders in children
 Appendix 4I - Melatonin Guidance for the treatment of sleep-wake cycle disorders in children Background Sleep disturbance in children with neurological or behavioural disorders is common and can be a major
Appendix 4I - Melatonin Guidance for the treatment of sleep-wake cycle disorders in children Background Sleep disturbance in children with neurological or behavioural disorders is common and can be a major
ESCA: Cinacalcet (Mimpara )
 ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
ESCA: Cinacalcet (Mimpara ) Effective Shared Care Agreement for the Treatment of Primary hyperparathyroidism when parathyroidectomy is contraindicated or not clinically appropriate. Specialist details
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE SHARED CARE GUIDELINE FOR THE USE OF RILUZOLE IN THE TREATMENT OF MOTOR NEURONE DISEASE
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE SHARED CARE GUIDELINE FOR THE USE OF RILUZOLE IN THE TREATMENT OF MOTOR NEURONE DISEASE PATIENT S NAME: PATIENT S ADDRESS: HOSPITAL NAME AND NUMBER /
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE SHARED CARE GUIDELINE FOR THE USE OF RILUZOLE IN THE TREATMENT OF MOTOR NEURONE DISEASE PATIENT S NAME: PATIENT S ADDRESS: HOSPITAL NAME AND NUMBER /
Prescribing Guidelines Prescribing arrangement for the management of patients transferring from Secondary Care to Primary Care
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Guideline for GP s in primary care for safe prescribing of acamprosate in Sheffield
 Guideline for GP s in primary care for safe prescribing of acamprosate in Sheffield Authors Dr O Lagundoye-Consultant Psychiatrist/Clinical Director MBBS MRCPsych Substance Misuse Service, Sheffield Health
Guideline for GP s in primary care for safe prescribing of acamprosate in Sheffield Authors Dr O Lagundoye-Consultant Psychiatrist/Clinical Director MBBS MRCPsych Substance Misuse Service, Sheffield Health
Public Health Wales Vaccine Preventable Disease Programme
 Public Health Wales Vaccine Preventable Disease Programme Uptake of Seasonal Influenza Vaccination HB specific reports Aneurin Bevan Health Board / July Page of . Background The aim of the Welsh Government
Public Health Wales Vaccine Preventable Disease Programme Uptake of Seasonal Influenza Vaccination HB specific reports Aneurin Bevan Health Board / July Page of . Background The aim of the Welsh Government
Products available Methotrexate tablets 2.5mg ONLY (Methotrexate tablets 10mg are NOT recommended as per NPSA guidance 5 ).
 Methotrexate Traffic light classification- Amber 1 Information sheet for Primary Care Prescribers Part of the Shared Care Protocol: Management of Rheumatological Conditions with Disease-Modifying Anti
Methotrexate Traffic light classification- Amber 1 Information sheet for Primary Care Prescribers Part of the Shared Care Protocol: Management of Rheumatological Conditions with Disease-Modifying Anti
SHARED CARE PROTOCOL CHOLINESTERASE INHIBITORS IN ALZHEIMER S DEMENTIA
 SHARED CARE PROTOCOL CHOLINESTERASE INHIBITORS IN ALZHEIMER S DEMENTIA Introduction Alzheimer s disease is the most common cause of dementia. It is characterised by an insidious onset of global mental
SHARED CARE PROTOCOL CHOLINESTERASE INHIBITORS IN ALZHEIMER S DEMENTIA Introduction Alzheimer s disease is the most common cause of dementia. It is characterised by an insidious onset of global mental
FORMULARY UPDATES ABUHB s Drug Formulary is at:
 Aneurin Bevan University Health Board Medicines & Therapeutics Committee PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 September 2015 Dear Gwent Prescriber
Aneurin Bevan University Health Board Medicines & Therapeutics Committee PRESCRIBING enewsletter archive at: http://www.wales.nhs.uk/sites3/page.cfm?orgid=814&pid=48407 September 2015 Dear Gwent Prescriber
SHARED CARE GUIDELINE
 SHARED CARE GUIDELINE Title: Lithium Treatment in Adults aged 18-65 years Scope: Pennine Care NHS Foundation Trust NHS Bury NHS Oldham NHS Heywood, Middleton and Rochdale NHS Stockport NHS Tameside & Glossop
SHARED CARE GUIDELINE Title: Lithium Treatment in Adults aged 18-65 years Scope: Pennine Care NHS Foundation Trust NHS Bury NHS Oldham NHS Heywood, Middleton and Rochdale NHS Stockport NHS Tameside & Glossop
Bournemouth, Dorset and Poole Prescribing Forum
 SHARED CARE GUIDELINE FOR THE USE OF ATOMOXETINE IN ADULTS WITH ATTENTION DEFICIT HYPERACTIVITY DISORDER INDICATION Atomoxetine is a non-stimulant non-amphetamine inhibitor of noradrenaline reuptake. It
SHARED CARE GUIDELINE FOR THE USE OF ATOMOXETINE IN ADULTS WITH ATTENTION DEFICIT HYPERACTIVITY DISORDER INDICATION Atomoxetine is a non-stimulant non-amphetamine inhibitor of noradrenaline reuptake. It
Prescribing Framework for Naltrexone in Alcohol Relapse Prevention
 Prescribing Framework for Naltrexone in Alcohol Relapse Prevention Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker) GP s Name:... Communication We agree to treat this patient
Prescribing Framework for Naltrexone in Alcohol Relapse Prevention Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker) GP s Name:... Communication We agree to treat this patient
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis. Date: Date:
 Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
SHARED CARE GUIDELINE
 SHARED CARE GUIDELINE Methylphenidate in the treatment of Attention Deficit Hyperactivity Disorder in Children, Young People and Adults Implementation Date: June 2015 Review Date: June 2017 This guidance
SHARED CARE GUIDELINE Methylphenidate in the treatment of Attention Deficit Hyperactivity Disorder in Children, Young People and Adults Implementation Date: June 2015 Review Date: June 2017 This guidance
SHARED CARE PROTOCOL FOR THE PRESCRIBING AND MONITORING OF MEDICINES FOR ATTENTION-DEFICIT HYPERACTIVITY DISORDER (ADHD)
 SHARED CARE PROTOCOL FOR THE PRESCRIBING AND MONITORING OF MEDICINES FOR ATTENTION-DEFICIT HYPERACTIVITY DISORDER (ADHD) 1. Introduction This protocol describes how patients prescribed medicines for ADHD
SHARED CARE PROTOCOL FOR THE PRESCRIBING AND MONITORING OF MEDICINES FOR ATTENTION-DEFICIT HYPERACTIVITY DISORDER (ADHD) 1. Introduction This protocol describes how patients prescribed medicines for ADHD
Chronic Obstructive Pulmonary Disease (COPD) Treatment Guidelines
 Chronic Obstructive Pulmonary Disease (COPD) Treatment Guidelines Where appropriate the following should be offered before commencing inhaled treatment: Offer treatment and support to stop smoking. Smoking
Chronic Obstructive Pulmonary Disease (COPD) Treatment Guidelines Where appropriate the following should be offered before commencing inhaled treatment: Offer treatment and support to stop smoking. Smoking
INTRODUCTION Indication and Licensing
 City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
City and Hackney Clinical Commissioning Group Homerton University Hospital Foundation Trust DRUG NAME: Apixaban (Eliquis ) Transfer of Care document Indication: Treatment of acute venous thromboembolism
for adults engaged with the Family Wellbeing Service Isle of Wight In Community Pharmacy for Isle of Wight Public Health Commissioned Services
 The supply of Champix (Varenicline) Tablets 500mcg and 1mg by registered community pharmacists for smoking cessation / management of nicotine withdrawal for adults engaged with the Family Wellbeing Service
The supply of Champix (Varenicline) Tablets 500mcg and 1mg by registered community pharmacists for smoking cessation / management of nicotine withdrawal for adults engaged with the Family Wellbeing Service
Technology appraisal guidance Published: 26 November 2014 nice.org.uk/guidance/ta325
 Nalmefene for reducing alcohol consumption in people with alcohol dependence Technology appraisal guidance Published: 26 November 2014 nice.org.uk/guidance/ta325 NICE 2018. All rights reserved. Subject
Nalmefene for reducing alcohol consumption in people with alcohol dependence Technology appraisal guidance Published: 26 November 2014 nice.org.uk/guidance/ta325 NICE 2018. All rights reserved. Subject
MELATONIN Information for Primary Care
 North of Tyne Area Prescribing Committee Shared Care Group MELATONIN Information for Primary Care Background Melatonin is a hormone secreted by the pineal gland. It is normally secreted at night and its
North of Tyne Area Prescribing Committee Shared Care Group MELATONIN Information for Primary Care Background Melatonin is a hormone secreted by the pineal gland. It is normally secreted at night and its
NATIONAL PRESCRIBING INDICATORS ANALYSIS OF PRESCRIBING DATA TO DECEMBER 2014
 NATIONAL PRESCRIBING INDICATORS 2014 2015 ANALYSIS OF PRESCRIBING DATA TO DECEMBER 2014 May 2015 This report has been prepared by the Welsh Analytical Prescribing Support Unit (WAPSU), part of the All
NATIONAL PRESCRIBING INDICATORS 2014 2015 ANALYSIS OF PRESCRIBING DATA TO DECEMBER 2014 May 2015 This report has been prepared by the Welsh Analytical Prescribing Support Unit (WAPSU), part of the All
NATIONAL PRESCRIBING INDICATORS ANALYSIS OF PRESCRIBING DATA: DECEMBER April 2013
 NATIONAL PRESCRIBING INDICATORS 2012 2013 ANALYSIS OF PRESCRIBING DATA: DECEMBER 2012 April 2013 This report has been prepared by the Welsh Analytical Prescribing Support Unit (WAPSU), part of the All
NATIONAL PRESCRIBING INDICATORS 2012 2013 ANALYSIS OF PRESCRIBING DATA: DECEMBER 2012 April 2013 This report has been prepared by the Welsh Analytical Prescribing Support Unit (WAPSU), part of the All
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures
 Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Pregabalin Prescribing in Primary Care Audit Results 2012/13
 Executive summary Pregabalin Prescribing in Primary Care Audit Results 2012/13 Pregabalin is extensively used across Aneurin Bevan Health Board (ABHB). It is the second highest medicine in terms of primary
Executive summary Pregabalin Prescribing in Primary Care Audit Results 2012/13 Pregabalin is extensively used across Aneurin Bevan Health Board (ABHB). It is the second highest medicine in terms of primary
Job Title Name Signature Date. Director of Nursing Angela Wallace Signed Angela Wallace 30/6/2014
 PATIENT GROUP DIRECTIONS FOR SUPPLY OF VARENICLINE (CHAMPIX ) BY AUTHORISED COMMUNITY PHARMACISTS WORKING IN FORTH VALLEY Protocol Number 445 Version 1 Date protocol prepared: June 2014 Date protocol due
PATIENT GROUP DIRECTIONS FOR SUPPLY OF VARENICLINE (CHAMPIX ) BY AUTHORISED COMMUNITY PHARMACISTS WORKING IN FORTH VALLEY Protocol Number 445 Version 1 Date protocol prepared: June 2014 Date protocol due
APOMORPHINE (Adults) Shared Care Guidelines DRUG:
 Shared Care Guidelines DRUG: APOMORPHINE (Adults) Indication: Treatment of motor fluctuations in patients with Parkinson's disease which is not sufficiently controlled by oral anti-parkinson medication.
Shared Care Guidelines DRUG: APOMORPHINE (Adults) Indication: Treatment of motor fluctuations in patients with Parkinson's disease which is not sufficiently controlled by oral anti-parkinson medication.
MELATONIN Insomnia and Sleep Disorders in Children
 DOCUMENT TO BE SCANNED INTO ELECTRONIC RECORDS AS AND FILED IN NOTES Patient Name : Date of Birth: NHS No: Name of Referring Consultant: Contact number: INTRODUCTION Melatonin is a pineal hormone which
DOCUMENT TO BE SCANNED INTO ELECTRONIC RECORDS AS AND FILED IN NOTES Patient Name : Date of Birth: NHS No: Name of Referring Consultant: Contact number: INTRODUCTION Melatonin is a pineal hormone which
Prescribing Guidelines Prescribing arrangement for the management of patients transferring from Secondary Care to Primary Care
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
SHARED CARE GUIDELINE: Mycophenolate mofetil or mycophenolic acid for Maintenance of Immunosuppression after Kidney Transplantation in Adults
 NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: Mycophenolate mofetil or mycophenolic acid for Maintenance of Immunosuppression after Kidney Transplantation
NHS LINCOLNSHIRE in association with UNITED LINCOLNSHIRE HOSPITALS TRUST SHARED CARE GUIDELINE: Mycophenolate mofetil or mycophenolic acid for Maintenance of Immunosuppression after Kidney Transplantation
Guidance for Pharmacists on Safe Supply of Oral Methotrexate
 Guidance for Pharmacists on Safe Supply of Oral Methotrexate Pharmaceutical Society of Ireland Version 2 January 2015 Contents 1. Introduction 2 2. Methotrexate 2 3. Guidance 2 3.1 Patient Review 2 3.2
Guidance for Pharmacists on Safe Supply of Oral Methotrexate Pharmaceutical Society of Ireland Version 2 January 2015 Contents 1. Introduction 2 2. Methotrexate 2 3. Guidance 2 3.1 Patient Review 2 3.2
Prescribing Framework for Rivastigmine in the Treatment and Management of Dementia
 Hull & East Riding Prescribing Committee Prescribing Framework for Rivastigmine in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker)
Hull & East Riding Prescribing Committee Prescribing Framework for Rivastigmine in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker)
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE SHARED CARE AGREEMENT
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE SHARED CARE AGREEMENT NALTREXONE 50mg TABLETS For Opioid Relapse Prevention (in formerly opioid dependent patients) This shared care agreement is aimed at all
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE SHARED CARE AGREEMENT NALTREXONE 50mg TABLETS For Opioid Relapse Prevention (in formerly opioid dependent patients) This shared care agreement is aimed at all
Drug Misuse and Dependence Guidelines on Clinical Management
 Department of Health Scottish Office Department of Health Welsh Office Department of Health and Social Services, Northern Ireland Drug Misuse and Dependence Guidelines on Clinical Management An Executive
Department of Health Scottish Office Department of Health Welsh Office Department of Health and Social Services, Northern Ireland Drug Misuse and Dependence Guidelines on Clinical Management An Executive
Treatment monitoring protocol for Dimethyl fumarate therapy in active Relapsing Remitting Multiple Sclerosis
 Treatment monitoring protocol for Dimethyl fumarate therapy in active Relapsing Remitting Multiple Sclerosis This protocol provides monitoring guidance for adult patients requiring Dimethyl fumarate therapy
Treatment monitoring protocol for Dimethyl fumarate therapy in active Relapsing Remitting Multiple Sclerosis This protocol provides monitoring guidance for adult patients requiring Dimethyl fumarate therapy
Specialist Palliative Care Audit and Guidelines Group (SPAGG)
 Specialist Palliative Care Audit and Guidelines Group (SPAGG) Clinical Guideline for the Prescribing and Administration of Furosemide via continuous subcutaneous infusion (CSCI) for Heart Failure Patients
Specialist Palliative Care Audit and Guidelines Group (SPAGG) Clinical Guideline for the Prescribing and Administration of Furosemide via continuous subcutaneous infusion (CSCI) for Heart Failure Patients
NCEPOD - Measuring the Units; A review of patients who died with alcohol-related liver disease
 NCEPOD - Measuring the Units; A review of patients who died with alcohol-related liver disease Hospital Number Admission to hospital 5. Trusts should ensure that medical patients are reviewed by a consultant
NCEPOD - Measuring the Units; A review of patients who died with alcohol-related liver disease Hospital Number Admission to hospital 5. Trusts should ensure that medical patients are reviewed by a consultant
Ciclosporin 25mg, 50mg, 100mg capsules Ciclosporin oral solution 100mg/ml
 Shared Care Protocol Ciclosporin for the treatment of rheumatoid arthritis Name of drug, form and strength Background Ciclosporin 25mg, 50mg, 100mg capsules Ciclosporin oral solution 100mg/ml Ciclosporin
Shared Care Protocol Ciclosporin for the treatment of rheumatoid arthritis Name of drug, form and strength Background Ciclosporin 25mg, 50mg, 100mg capsules Ciclosporin oral solution 100mg/ml Ciclosporin
Document ref. no: Trust Policy and Procedure. PP(16)234 Prescribing, Dispensing and Administration of Methotrexate Policy
 Document ref. no: Trust Policy and Procedure PP(16)234 Prescribing, Dispensing and Administration of Methotrexate Policy For use in: For use by: For use for: Document owner: Status: All Clinical Areas
Document ref. no: Trust Policy and Procedure PP(16)234 Prescribing, Dispensing and Administration of Methotrexate Policy For use in: For use by: For use for: Document owner: Status: All Clinical Areas
SHARED CARE AGREEMENT: METHOTREXATE S/C
 NB: This document should be read in conjunction with the current Summary of Product Characteristics (SPC) and BSR Guideline for disease-modifying anti-rheumatic drug (DMARD) therapy (available at www.rheumatology.org.uk/resources/guidelines/bsr_guidelines.aspx
NB: This document should be read in conjunction with the current Summary of Product Characteristics (SPC) and BSR Guideline for disease-modifying anti-rheumatic drug (DMARD) therapy (available at www.rheumatology.org.uk/resources/guidelines/bsr_guidelines.aspx
Prescribing Framework for Mycophenolate Mofetil for Immunosuppression in ADULTs
 Hull & East Riding Prescribing Committee Prescribing Framework for Mycophenolate Mofetil for Immunosuppression in ADULTs Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker)
Hull & East Riding Prescribing Committee Prescribing Framework for Mycophenolate Mofetil for Immunosuppression in ADULTs Patient s Name:.. NHS Number: Patient s Address:... (Use addressograph sticker)
Medicines Management of Chronic Obstructive Pulmonary Disease (COPD)
 Medicines Management of Chronic Obstructive Pulmonary Disease (COPD) (Chronic & Acute) Guidelines for Primary Care Guideline Authors: Shaneez Dhanji (Wandsworth CCG) Samantha Prigmore (St George s Hospital)
Medicines Management of Chronic Obstructive Pulmonary Disease (COPD) (Chronic & Acute) Guidelines for Primary Care Guideline Authors: Shaneez Dhanji (Wandsworth CCG) Samantha Prigmore (St George s Hospital)
PATIENT GROUP DIRECTIONS FOR SUPPLY OF VARENICLINE (CHAMPIX ) BY AUTHORISED COMMUNITY PHARMACISTS WORKING IN TAYSIDE
 PATIENT GROUP DIRECTIONS FOR SUPPLY OF VARENICLINE (CHAMPIX ) BY AUTHORISED COMMUNITY PHARMACISTS WORKING IN TAYSIDE GENERAL POLICY 2 PATIENT GROUP DIRECTIONS FOR SUPPLY OF VARENICLINE 4 STANDING ORDER
PATIENT GROUP DIRECTIONS FOR SUPPLY OF VARENICLINE (CHAMPIX ) BY AUTHORISED COMMUNITY PHARMACISTS WORKING IN TAYSIDE GENERAL POLICY 2 PATIENT GROUP DIRECTIONS FOR SUPPLY OF VARENICLINE 4 STANDING ORDER
Bournemouth, Dorset and Poole Prescribing Forum
 SHARED CARE GUIDELINES FOR PRESCRIBING OF METHYLPHENIDATE IN ATTENTION DEFICIT HYPERACTIVITY DISORDER IN CHILDREN INDICATION Methylphenidate is generally regarded as a first line choice of treatment for
SHARED CARE GUIDELINES FOR PRESCRIBING OF METHYLPHENIDATE IN ATTENTION DEFICIT HYPERACTIVITY DISORDER IN CHILDREN INDICATION Methylphenidate is generally regarded as a first line choice of treatment for
Neuropathic pain MID ESSEX LOCALITY
 Neuropathic pain Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory nervous system. A. Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) i Read questions to
Neuropathic pain Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory nervous system. A. Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) i Read questions to
Type 2 Diabetes. Stopping Smoking. Consider referral to smoking cessation. Consider referring for weight management advice.
 Type 2 Diabetes Stopping Smoking Consider referral to smoking cessation BMI > 25 kg m² Set a weight loss target of a 5-10% reduction Consider referring for weight management advice Control BP to
Type 2 Diabetes Stopping Smoking Consider referral to smoking cessation BMI > 25 kg m² Set a weight loss target of a 5-10% reduction Consider referring for weight management advice Control BP to
Heart Failure (HF) - Primary Care Flow Charts. Pre diagnosis Symptoms or signs suggestive of HF
 Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts. Symptoms or signs suggestive of HF. Pre diagnosis. Refer to the Heart Failure Clinic at VHK for
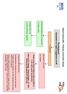 Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Heart Failure (HF) - Primary Care Flow Charts Pre diagnosis Symptoms or signs suggestive of HF 12 lead ECG Normal examination and 12 lead ECG HF highly unlikely Abnormal 12 lead ECG HF Possible Arrange
Prescribing Framework for Galantamine in the Treatment and Management of Dementia
 Hull & East Riding Prescribing Committee Prescribing Framework for Galantamine in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker)
Hull & East Riding Prescribing Committee Prescribing Framework for Galantamine in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker)
Chronic obstructive pulmonary disease
 0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
Aripiprazole Long-Acting Injection (Abilify Maintena ) Guidelines for Prescribing and Administration (Version 3 August 2014)
 1. Key Points. Aripiprazole Long-Acting Injection (Abilify Maintena ) Guidelines for Prescribing and Administration (Version 3 August 2014) 1.1 Aripiprazole long acting injection (LAI) is licensed / indicated
1. Key Points. Aripiprazole Long-Acting Injection (Abilify Maintena ) Guidelines for Prescribing and Administration (Version 3 August 2014) 1.1 Aripiprazole long acting injection (LAI) is licensed / indicated
Degarelix Subcutaneous Injection (Firmagon ) Treatment Guideline
 Mid Essex Locality Degarelix Subcutaneous Injection (Firmagon ) Treatment Guideline Contents FlowChart 2 Summary... 3 Key points... 3 Introduction... 3 Pharmacology... 3 Product information... 4 Place
Mid Essex Locality Degarelix Subcutaneous Injection (Firmagon ) Treatment Guideline Contents FlowChart 2 Summary... 3 Key points... 3 Introduction... 3 Pharmacology... 3 Product information... 4 Place
New Medicines Committee Briefing. July Fosfomycin trometamol for the treatment of multidrug resistant urinary tract infection
 New Medicines Committee Briefing July 2014 Fosfomycin trometamol for the treatment of multidrug resistant urinary tract infection (unlicensed indication) Fosfomycin trometamol to be reviewed for use within:
New Medicines Committee Briefing July 2014 Fosfomycin trometamol for the treatment of multidrug resistant urinary tract infection (unlicensed indication) Fosfomycin trometamol to be reviewed for use within:
Shared Care Agreement for Donepezil
 ESCA: for the treatment of Alzheimer s disease SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR Patient s Name: Date of Birth: NHS Number: ESCA Date: One copy of information leaflet given to
ESCA: for the treatment of Alzheimer s disease SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR Patient s Name: Date of Birth: NHS Number: ESCA Date: One copy of information leaflet given to
Essential Shared Care Agreement Drugs for Dementia
 E098 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1. the patient
E098 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1. the patient
Adult Neurodevelopmental Services. ADHD Shared Protocol
 Adult Neurodevelopmental Services ADHD Shared Protocol Issue 1: April 2016 1 2 Adult Neurodevelopmental Service Shared Care Protocol for Adult Attention Deficit Hyperactivity Disorder (ADHD) 1. BACKGROUND
Adult Neurodevelopmental Services ADHD Shared Protocol Issue 1: April 2016 1 2 Adult Neurodevelopmental Service Shared Care Protocol for Adult Attention Deficit Hyperactivity Disorder (ADHD) 1. BACKGROUND
MANAGEMENT OF TYPE 2 DIABETES
 MANAGEMENT OF TYPE 2 DIABETES 3 Month trial of lifestyle changes. Refer to DESMOND structured education programme. Set glycaemic target HbA1c < 7.0% (53mmol/mol) or individualised If HbA1c > 53mmol/mol
MANAGEMENT OF TYPE 2 DIABETES 3 Month trial of lifestyle changes. Refer to DESMOND structured education programme. Set glycaemic target HbA1c < 7.0% (53mmol/mol) or individualised If HbA1c > 53mmol/mol
