Drug-induced liver injury (DILI)
|
|
|
- Jacob Cox
- 5 years ago
- Views:
Transcription
1 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich Year: 2012 Drug-induced liver injury (DILI) Russmann, Stefan; Kullak-Ublick, Gerd Achim Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: Scientific Publication in Electronic Form Accepted Version Originally published at: Russmann, Stefan; Kullak-Ublick, Gerd Achim (2012). Drug-induced liver injury (DILI). Apple App store for ios (iphone / ipad): European Association for the Study of the Liver (EASL).
2 Author's credits Title: PD Dr. First name: Stefan Surname: Russmann (list Prof. Kullak as senior author here as well) Tel: Institution/Office/Surgery: University Hospital Zurich, Department of Clinical Pharmacology Address: Rämistrasse 100 Zip Code: 8091 City: Zurich Country: Switzerland 1. Definition A very concise definition of the disease (maximum 300 characters, Do not provide a classification of different sub-entities or a differential diagnosis here. Drag your JPEG image(s) here. Minimum size: 480 x 320 pixels Drug-induced liver injury (DILI) refers to liver diseases that are caused by drugs, phytotherapeutics and other potentially toxic substances via a large range of mechanisms. As a general rule, DILI can mimic all forms of acute and chronic liver disease with regard to symptoms, signs and histology. 2. Clinical Presentation 2.1. Symptoms A list of all potential symptoms that may lead patients to seek medical attention (maximum 500 characters, including spaces) Drag your JPEG image(s) here. Minimum size: 480 x 320 pixels Typical symptoms of acute DILI are abdominal discomfort, fatigue, nausea and vomiting. Acute severe and cholestatic DILI may also present with jaundice and dark urine. Asymptomatic presentation is possible for mild forms and early stages. Chronic DILI may present with any symptoms related to liver disease Physical Findings and Signs A list of all physical findings likely to be encountered at first presentation (maximum 500 characters, Drag your JPEG image(s) here. Minimum size: 480 x 320 pixels. Up to 10 representative images can be shown. Jaundice and dark urine are signs of hyperbilirubinemia that may be present in acute severe and cholestatic DILI. Immune-mediated DILI can present with fever, rash and eosinophilia as signs of hypersensitivity. Chronic DILI may present with any signs of hepatic disease Chronology If applicable, provide a chronology of symptoms and physical findings at the time of presentation Drag your JPEG image(s) here. Minimum size: 480 x 320 pixels. Tables and/or graphs will later be (maximum 1,000 characters, Tables and/or graphs will be converted into pop-up converted into pop-up images. images. 3. Diagnosis Generalities - Description A concise description on how you establish a diagnosis of this disease, i.e. the minimum diagnostic criteria (maximum 1,000 characters, Specific classifications of sub-entities can be provided here (e.g. phases of HBV infection, definitions of metabolic syndrome etc.). Tables will be converted into pop-up images. Diagnosis of DILI is primarily clinical and based on three principle criteria: 1. Temporal relationship with drug use; 2. Exclusion of other possible causes (1. and 2. referred to as "intrinsic evidence"); 3. Knowledge of a drug's hepatotoxic potential and signature pattern from previous reports ("extrinsic evidence"). Detailed and if necessary repeated history taking is the key to diagnosis of DILI. Drug history must include prescription and non-prescription drugs, phytotherapeutics, illicit drug use and environmental hepatotoxins.the primary challenge is to establish a precise chronological relationship of all recently used drugs with symptoms, signs and laboratory abnormalities associated with DILI. Specific drugs tend to have characteristic patterns regarding latency, symptoms, signs and histology. These "signature patterns" can make a valuable contribution to expert evaluation of suspected cases, which remains the reference for diagnosis of DILI. In addition, standardized causality assessment scales support an objective and quantitative evaluation of the probability that liver disease is caused by suspected drugs (create link to section 5 scales below). Nevertheless, even standardized causality assessment methods are subject to interrater variability and may not eliminate diagnostic uncertainty. Genetic risk factors for DILI have also been identified, but genetic testing is currently not part of routine diagnostics due to limited positive as well as negative predictive value (Daly AK et al, HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet 2009;41:816-9; Russmann S et al, Pharmacogenetics of drug-induced liver injury. Hepatology 2010;52:748-61). Here is an algorithm for the diagnostic approach to DILI (create link to pop-up image with algorithm A1) Laboratory findings A list of laboratory findings that may help establish a diagnosis (maximum 1,000 characters, including Up to 10 representative images can be shown(format JPG, 480 x 320 pixels) ALT, alkaline phosphatase and bilirubin are the most important diagnostic markers of acute DILI. R-value, criteria for acute DILI and Hy s case calculator They are also the basis for calculation of the R-value that distinguishes hepatocellular, cholestatic R-value and mixed laboratory patterns at initial presentation (create link to R-value calculator), and for the diagnostic threshold criteria of acute DILI (create link to table with acute DILI criteria and Hy's law Note: The R value is calculated with the initial values at first diagnosis. for acute DILI). GGT and AST are also often increased in DILI, but compared to AP and ALT, Required input fields: respectively, their diagnostic value is limited by lower specificity. The calculation of the damage 1. ALT value pattern has also a prognostic value, as hepatocellular DILI is associated with a higher risk of acute 2. ALT ULN liver failure compared to cholestatic DILI. Eosinophilia >6% can be a sign of DILI associated with 3. AP value hypersensitivity. Other laboratory findings are important for the exclusion of other causes (create link 4. AP ULN. to section 4 differential diagnosis). Lymphocyte stimulations and specific antibodies to cytochrome The R-value is then calculated as follows: P450-adducts are specific diagnostic tests but they can lack sensitivity and are not part of routine diagnostics. ALT value / ALT ULN R = AP value / AP ULN Interpretation: R 5 = Hepatocellular pattern of DILI; R >2 and <5 = Mixed pattern of DILI; R 2 = Cholestatic pattern of DILI. Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 1990;11: Criteria of acute DILI 1. ALT elevation 5x ULN or 2. ALP elevation 2x ULN without another cause or 3. "Hy's case" criterium: ALT elevated 3x ULN AND total bilirubin elevation >2x ULN AND initial AP<2xULN. and no other cause can be found to explain th eobserved liver injury Aithal et al, Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89: Hy s law for acute DILI ALT elevated 3x ULN AND total bilirubin elevation >2x ULN AND initial AP<2xULN. and no other cause can be found to explain the observed liver injury References: 1) Guidance for Industry. Drug-Induced Liver Injury: Premarketing Clinical Evaluation. U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). July 2009; 2) Lewis JH. 'Hy's law,' the 'Rezulin Rule,' and other predictors of severe drug-induced hepatotoxicity: putting risk-benefit into perspective. Pharmacoepidemiol Drug Saf 2006;15:221-9; 3) Reuben A. Hy s Law. Hepatology 2004;39:574-8.
3 3.2. Endoscopy findings A list of endoscopy findings that may help establish a diagnosis (maximum 1,000 characters, ERCP for exclusion of obstructive hepatobiliary disease (rare indication) 3.3. Radiology findings A list of radiology findings that may help establish a diagnosis (maximum 1,000 characters, including Abdominal ultrasound, CT or MRI for exclusion of obstructive hepatobiliary disease Histology findings A list of histopathology findings that may help establish a diagnosis (maximum 1,000 characters, Histology is usually not specific for DILI, and liver biopsy is therefore not part of routine diagnostic 3 examples of histology pictures (also provided separately as jpg files): DILI induced by indometacin workup for DILI. However, liver biopsy can be indicated and make an important contribution to patient (presenting predominantly with zone 3 necrosis), oxiplatin (presenting as sinusoidal obstruction management when there is a complex clinical situation with several possible drug and non-drug syndrome with obliteratin of central vein, hepatocellular necrosis, proliferatin of bile ducts and causes. Histology is also useful for grading severity and can identify or establish typical histological inflammatory infiltrates), and phenprocoumon (presenting with hepatocellular necrosis and portal signature patterns of DILI caused by specific drugs and sometimes be the first suggestion of DILI. inflammatory infiltrates with eosinophils). Histology pictures were kindly provided by Prof. Achim The pathologist should use a systematic approach for the evaluation of possible DILI: 1. Identify the Weber, Zurich (which should be acknowledged) pathological pattern of injury and its relative severity. 2. Exclude non-dili etiologies by clinical history and ancillary testing. 3. Compare known patterns of injury of the candidate agents to the biopsy findings, taking into account the temporal sequence of events. 4. Assess the likelihood of DILI as an etiology for the biopsy findings. (Ref.: Kleiner DE. The pathology of drug-induced liver injury. Semin Liver Dis 2009;29:364-72). Indometacin zone 3 necrosis DILI (presenting predominantly oxiplatin DILI (presenting as sinusoidal obstruction syndrome with zone 3 necrosis) with obliteratin of central vein, hepatocellular necrosis, proliferatin of bile ducts and inflammatory infiltrates) phenprocoumon DILI (presenting with hepatocellular necrosis and portal inflammatory infiltrates with eosinophils) 3.5. Other Procedures A list of other findings that may help establish a diagnosis (maximum 1,000 characters, including More than 1000 drugs have been associated with DILI. Information on a drug's hepatotoxic potential and its signature pattern of DILI ("extrinsic evidence") should first be searched in the prescribing information. Searches in the literature, specific DILI databases and standard references (e.g. Kaplowitz N, DeLeve LD [eds]. Drug-induced liver disease. New York, informa healthcare, 2007) are further valuable information sources. Consider also that DILI can present with a large range of clinical phenotypes including immunoallergic hepatitis, autoimmune hepatitis-like, acute hepatic necrosis, acute viral hepatitis-like, acute liver failure, cholestatic hepatitis, bland cholestasis, acute fatty liver with lactic acidosis, nonalcoholic fatty liver, sinusoidal obstruction syndrome (formerly called venoocclusive disease), chronic hepatitis, nodular regeneration, vanishing bile duct syndrome, and cirrhosis. 4. Differential Diagnosis Links - Links to Internet Websites A list of conditions that should be taken into consideration when establishing a differential diagnosis Differential diagnosis may be assisted by selected, representative images (up to 10, format JPG, check-list (maximum 500 characters, 480 x 320 pixels) or shown in the form of a table that will be converted into a pop-up image Viral hepatitis due to HAV, HBV, HCV, HEV, EBV, CMV, HSV. Benign obstructive hepatobiliary disease. Autoimmune hepatitis. Wilson's disease. Hemochromatosis. Alcoholic liver disease. Bacterial or fungal sepsis. Congestive heart failure, ischemic liver. Primary or metastatic malignancy of the liver or biliary tract. Primary biliary cirrhosis. Primary sclerosing cholangitis. Non-alcoholic fatty liver disease and non alcoholic steatohepatitis. Alpha 1- antitrypsin deficiency. You may also provide links to Internet websites. 5. Scores For each score provide an Introduction/Description, Equation and/or Table, an Interpretation and a Reference. Provide scores commonly used and internationally accepted for diagnosis. Each score will open a new screen level (screen level four) Score N 1: RUCAM-CIOMS scale for hepatocellular DILI Score N 2: RUCAM-CIOMS scale for cholestatic or mixed DILI Score N 3: Maria and Victorino (M&V) scale Score N 3: DILIN severity index for DILI Introduction / Description Introduction / Description - In the absence of a gold standard expert evaluation is still considered as the most reliable method for In the absence of a gold standard expert evaluation is still considered as the most reliable method diagnosis of DILI. However, standardized scales improve objectivity and allow quantitative for diagnosis of DILI. However, standardized scales improve objectivity and allow quantitative assessment. Scales are frequently used in the regulatory and industry setting, and the RUCAM- assessment. Scales are frequently used in the regulatory and industry setting, and the RUCAM- CIOMS scale has been found to provide the best overall performance compared to expert evaluation. CIOMS scale has been found to provide the best overall performance compared to expert The M&V scale is somewhat simpler, but it favors hypersensitivity and generally tends to evaluation. The M&V scale is somewhat simpler, but it favors hypersensitivity and generally tends to Introduction / Description - In the absence of a gold standard expert evaluation is still considered as the most reliable method for diagnosis of DILI. However, standardized scales improve objectivity and allow quantitative assessment. Scales are frequently used in the regulatory and industry setting, and the RUCAM-CIOMS scale has been found to provide the best overall performance compared to expert evaluation. The M&V scale is somewhat simpler, but it favors hypersensitivity and generally tends to Introduction / Description - Severity index for DILI based on the DILI Network prospective study. Severity grading for specific symptoms and signs of Criteria for Adverse Events (CTCAE) can be found at: Equation and/or Table -, table, image Equation and/or Table -, table, image Equation and/or Table -, table, image Equation and/or Table -, table, image Provide the formula or calculator or table used for this score here. see A2 see A3 see A4 see A5 RUCAM- CIOMS scale for hepatocellular DILI RUCAM- CIOMS scale for cholesta=c or mixed DILI Maria and Victorino scale for DILI DILI Network (DILIN) severity index for DILI 1. Temporal rela=onship of start of drug to ALT>2x ULN Score Ini%al treatment 5 90 days; subsequent treatment course: 1 15 days 2 Ini%al treatment <5 or >90 days; subsequent treatment course: >15 days 1 From cessa%on of drug: <15 days, or <15 days aber subsequent treatment 1 Otherwise 0 2. AFer drug cessa=on- difference between peak ALT and upper limits normal Decreases >50% within 8 days 3 Decreases >50% within 30 days 2 No informa%on or decrease >50% aber >30 days, or inconclusive 0 Decrease <50% aber 30 days or recurrent increase Risk factors No alcohol use (above recommended limit) 0 Alcohol use (above recommended limit) 1 Age <55 years 0 Age >55 years 1 4. Concomitant drug No concomitant drug administered 0 Concomitant drug with sugges%ve or compa%ble %me of onset - 1 Concomitant known hepatotoxin with sugges%ve or compa%ble %me of onset - 2 Concomitant drug with posi%ve rechallenge or validated diagnos%c test Temporal rela=onship of start of drug to ALP>2x ULN Score Ini%al treatment 5 90 days; subsequent treatment course: 1 90 days 2 Ini%al treatment <5 or >90 days; subsequent treatment course: >90 days 1 From cessa%on of drug: <30 days, or <30 days aber subsequent treatment 1 Otherwise 0 2. AFer drug cessa=on - difference between peak ALP or total bilirubin and ULN Decreases >50% within 180 days 2 Decreases <50% within 180 days 1 Persistence or increase or no informa%on 0 If drug is con%nued inconclusive 0 3. Risk factors No alcohol use (above recommended limit) 0 Alcohol use (above recommended limit) 1 Age <55 years 0 Age >55 years 1 4. Concomitant drug No concomitant drug administered 0 Concomitant drug with sugges%ve or compa%ble %me of onset - 1 Concomitant known hepatotoxin with sugges%ve or compa%ble %me of onset - 2 Concomitant drug with posi%ve rechallenge or validated diagnos%c test Temporal rela=onship between drug intake and the onset of clinical picture Score A. Time from drug intake un%l the onset of clinical or laboratory manifesta%ons 4 days to 8 weeks (or less than 4 days in cases of reexposure) 3 Less than 4 days or more than 4 weeks 1 B. Time from the withdrawal of the drug un%l the onset of manifesta%ons 0 to 7 days 3 8 to 15 days 0 More than 15 days* - 3 C. Time from withdrawal of the drug un%l normaliza%on of laboratory values Less than 6 months (cholesta%c or mixed pabern) or 2 months (hepatocellular) 3 More than 6 months (cholesta%c or mixed) or 2 months (hepatocellular) 0 2. Exclusion of alternate causes Viral hepa%%s (HAV, HBV, HCV, CMV, EBV) Alcoholic liver disease Biliary tree obstruc%on Preexis%ng liver disease Other (pregnancy, acute hypotension) Complete exclusion 3 Par%al exclusion 0 Possible alterna%ve detected - 1 cause Probable alterna%ve cause detected - 3 Categories: 1 - Elevated ALT/ALP concentra%on reaching criteria for DILI, but bilirubin concentra%on < 2 ULN ; 2 - Elevated ALT/ALP concentra%on reaching criteria for DILI, and bilirubin concentra%on 2 ULN or symptoma%c hepa%%s; 3 - Elevated ALT/ALP concentra%on reaching criteria for DILI, bilirubin concentra%on 2 ULN, and one of the following: - Hepa%c failure: - INR Ascites and/or encephalopathy, disease dura%on < 26 weeks, absence of underlying cirrhosis - Other organ failure believed to be due to DILI (e.g. renal, pulmonary); 4 - Death or tranplanta%on due to DILI. 5. Nondrug causes: Six are primary: recent hepa==s A, B, or C, biliary obstruc=on, acute alcoholic hepa==s (AST > 2x ALT), recent hypotension Secondary group: Underlying other disease; possible CMV, EBV or HSV infec=on All primary and secondary causes reasonably ruled out: 2 All 6 primary causes ruled out 1 4 or 5 primary causes ruled out 0 < 4 primary causes ruled out (max. nega%ve score for items 4 and 5: 4) - 2 Nondrug cause highly probable Previous informa=on on hepatotoxicity of the drug in ques=on Package insert or labeling men%on 2 Published case reports but not in label 1 Reac%on unknown 0 7. Rechallenge Posi%ve (ALT doubles with drug in ques%on alone) 3 Compa%ble (ALT doubles with same drugs as given before ini%al reac%on) 1 Nega%ve (Increase in ALT but <2x ULN, same condi%ons as when reac%on occurred) - 2 Not done, or indeterminate result 0 5. Nondrug causes: Six are primary: recent hepa==s A, B, or C, biliary obstruc=on, acute alcoholic hepa==s (AST > 2x ALT), recent hypotension Secondary group: Underlying other disease; possible CMV, EBV or HSV infec=on All primary and secondary causes reasonably ruled out: 2 All 6 primary causes ruled out 1 4 or 5 primary causes ruled out 0 < 4 primary causes ruled out (max. nega%ve score for items 4 and 5: 4) - 2 Nondrug cause highly probable Previous informa=on on hepatotoxicity of the drug in ques=on Package insert or labeling men%on 2 Published case reports but not in label 1 Reac%on unknown 0 7. Rechallenge Posi%ve (ALT doubles with drug in ques%on alone) 3 Compa%ble (ALT doubles with same drugs as given before ini%al reac%on) 1 Nega%ve (Increase in ALT but <2x ULN, same condi%ons as when reac%on occurred) - 2 Not done, or indeterminate result 0 3. Extrahepa=c manifesta=ons Rash, fever, arthralgia, eosinophilia (>6%), cytopenia 4 or more 3 2 or None 0 4. Inten=onal or accidental re- exposure to the drug Posi%ve rechallenge test 3 Nega%ve or absent rechallenge test 0 5. Previous report in the literature of cases of DILI associated with the drug Yes 2 No (drugs marketed for up to 5 years) 0 No (drugs marketed for more than 5 years) - 3 * Except cases of prolonged persistence of the drug in the body aber drug withdrawal (e.g., amiodarone) Normaliza%on decrease to values below 2 the upper limit of normal values. Use the exclusion criteria considered appropriate in each case Interpretation Interpretation Interpretation Interpretation - Provide the interpretation of the score here. Highly probable >8, probable 6-8, possible 3-5, unlikely 1-2, excluded <0. If more than one drug is Highly probable >8, probable 6-8, possible 3-5, unlikely 1-2, excluded <0. If more than one drug suspected the scale should be applied to each drug separately. is suspected the scale should be applied to each drug separately. Definite >17; Probable 14 17; Possible 10 13; Unlikely 6 9; Excluded <6. If more than one drug is suspected the scale should be applied to each drug separately. 1=Mild; 2=Moderate; 3=Severe; 4=Fatal or transplantation
4 Reference Reference Reference Reference - Provide the references of the score here. 1) Danan G, Benichou C. Causality assessment of adverse reactions to drugs--i. A novel method 1) Danan G, Benichou C. Causality assessment of adverse reactions to drugs--i. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46: ) Lucena et al, Comparison of two clinical scales for injuries. J Clin Epidemiol 1993;46: ) Lucena et al, Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology 2001;33: ) Kaplowitz N. Causality causality assessment in hepatotoxicity. Hepatology 2001;33: ) Kaplowitz N. Causality assessment versus guilt-by-association in drug hepatotoxicity. Hepatology 2001;33: ) García- assessment versus guilt-by-association in drug hepatotoxicity. Hepatology 2001;33: ) Cortés et al, Causality assessment methods in drug induced liver injury: Strengths and weaknesses. García-Cortés et al. Causality assessment methods in drug induced liver injury: Strengths and J Hepatol 2011;55: weaknesses. J Hepatol 2011;55: ) Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology 1997;26: ) Lucena et al, Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology 2001;33: ) Kaplowitz N. Causality assessment versus guilt-by-association in drug hepatotoxicity. Hepatology 2001;33: ) García-Cortés et al, Causality assessment methods in drug induced liver injury: Strengths and weaknesses. J Hepatol 2011;55: ) Aithal et al, Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89:80 Network (DILIN) prospective study: rationale, design and conduct. Drug Saf 2009;32: Clinical course - Complications Clinical course - A schematic description of the evolution of the disease over time. This section can be divided into paragraphs with headings (up to 10, each should contain up to 500 characters, Add representative images (up to 10, format JPG, 480 x 320 pixels). Given the extraordinary regenerative capacity of the liver most cases of acute DILI recover without sequelae. However, the risk of acute liver failure increases if the causative drug is not immediately discontinued. Hy's law cases of acute DILI (ALT elevated 3x ULN AND total bilirubin elevation >2x ULN AND initial AP<2xULN) have an ~10% risk of resulting in fatal acute liver failure or liver transplantation. Accordingly, these cases are of high concern and should prompt immediate discontinuation of suspicious drugs with detailed workups and monitoring. Cases with a hepatocellular enzyme pattern have a higher risk of progression to liver failure than those with a cholestatic pattern. Recovery from cholestatic DILI is usually prolonged over several months, but recovery is eventually complete in most patients, whereas permanent vanishing bile duct syndrome is a rare complication. Chronic DILI and sinusoidal obstruction syndrome may lead to cirrhosis. 7. Management and Therapy 7.1. General measures - A concise description of general measures accepted for the management of the disease (e.g. lifestyle changes, type of surveillance) (maximum 1,000 characters, The most important measure is the immediate discontinuation of suspicious drugs, particularly in hepatocellular DILI and Hy's law cases (create link to section 3.1 laboratory findings). On the other hand isolated stable elevations of transaminases without clinical symptoms of liver disease and ALT up to 3 or even 5x ULN may be tolerated and can even be reversible under continued drug therapy. Although, maintenance of the suspicious drug in this setting is only currently justifiable with antituberculosis therapy. Strict monitoring of ALT is required in such patients Specific measures - A list of all specific measures that cannot be included in the list of drugs (e.g. TIPS, paracentesis, surgical procedures, chemioembolization etc.). Each measure comprised in the list will open a new description on screen level four (see below). N-acetylcysteine (NAC) for acetaminophen poisoning is the only approved specific treatment for DILI. Patients that present within 4 hours after ingestion of an acetaminophen dose 7.5 g should also be treated with activated charcoal for decontamination. In addition, NAC has also proved useful in patients with idiosyncratic drug-induced acute liver failure at early stages (Lee et al, Intravenous N- acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009;137:856-64). Assuming a generally protective effect of NAC-mediated antioxidant effects, glutathione replenishment and its good safety profile NAC has therefore also been considered for the treatment of less severe forms of idiosyncratic DILI. Budesonide is sometimes given for presumably immune-mediated DILI. However, those two approaches are not evidence-based Indications - A concise description of indications accepted for the use of each specific measure (maximum 500 characters, If needed, you can provide one or more tables that will be converted into pop-up images. Provide a concise description of the measure here (maximum 1,000 characters, Precautions, limitations and complications Drugs Provide a list of drugs (generic names only, no trade names are allowed) officially admitted for disease management. Avoid traditional or parallel medicine remedies that have not been licensed by official authorities. Each drug comprised in the list will open a new description on screen level four (see below). Drug 1: N-acetylcysteine (NAC) for acetaminophen-poisoning is the only approved specific treatment of DILI Drug N Drug N Drug N 3 Name of the drug - Name of the drug - Name of the drug - N-acetylcysteine (NAC) Drug N 4 Name of the drug Indications Indications Indications Indications - A concise description of indications accepted for the use of each specific drug (maximum 500 characters, Tables will be converted into pop-up images. A concise description of indications accepted for the use of each specific drug (maximum 500 characters, Tables will be converted into pop-up images. A concise description of indications accepted for the use of each specific drug (maximum 500 characters, Tables will be converted into pop-up images. A concise description of indications accepted for the use of each specific drug (maximum 500 characters, including space Indicated for the treatment of paracetamol (acetaminophen) overdose. NAC is most effective if started within 8 hours after ingestion. The Prescott nomogram (attached, create link) should be used to guide treatment with NAC only in an unstaggered (acute ingestion) overdose, and only if the results for paracetamol levels are available within 8 hours of ingestion. The dose required for toxicity may be lower in patients with risk factors such as alcohol abuse or low body weight (diminished cytochrome P450 activity/reduced glutathione levels), or when paracetamol is co-ingested with other drugs, such as codeine which increases gut transit time and enzyme-inducing drugs such as phenytoin and carbemazepine. In such cases, the patient should be considered 'high-risk'. In all other cases of unstaggered (acute ingestion) overdose NAC should be started immediately. In a staggered overdose (multiple overdoses taken over a Posology Posology Posology Posology - A concise description of posologies accepted for the use of each specific drug (maximum 500 A concise description of posologies accepted for the use of each specific drug (maximum 500 characters, Tables will be converted into pop-up images (e.g. in case of characters, Tables will be converted into pop-up images (e.g. in case of adaptation according to body weight, renal insufficiency, age etc.). Also state what specific adaptation according to body weight, renal insufficiency, age etc.). Also state what specific precautions should apply (i.e. on an empty stomach, after meals, at bedtime, after fat-rich meals, with precautions should apply (i.e. on an empty stomach, after meals, at bedtime, after fat-rich meals, water, slow perfusion etc.) with water, slow perfusion etc.) A concise description of posologies accepted for the use of each specific drug (maximum 500 characters, Tables will be converted into pop-up images (e.g. in case of adaptation according to body weight, renal insufficiency, age etc.). Also state what specific precautions should apply (i.e. on an empty stomach, after meals, at bedtime, after fat-rich meals, with water, slow perfusion etc.) A concise description of posologies accepted for the use of each specific drug (maximum 500 characters, including space (e.g. in case of adaptation according to body weight, renal insufficiency, age etc.). Also state what specific precautions sh at bedtime, after fat-rich meals, with water, slow perfusion etc.) There are protocols for intravenous and for oral administration with comparable efficacy. Intravenous application is preferable in patients with vomiting, non-compliance, pancreatitis, bowel complications or acute liver failure. Here is the 20 hour IV protocol according to Prescott et al.: Administer an initial loading dose of 150 mg/kg IV over 15 to 60 minutes (60 min is recommended) Next, administer a 4-hour IV infusion at 50 mg/kg per hour Finally, administer a 16-hour IV infusion at 100 mg/kg per hour The required dose of NAC should be diluted in 5% dextrose as follows: Adults and Children>12 years: Initially 200ml over 15 mins, then 500ml over 4 hours, then 1L over 16 hours Children<12 years, body weight >20kg: Initially 100ml over 15 mins, then 250ml over 4 hours, then 500ml over 16 hours Children body weight <20kg: Initially 3ml/kg over 15 mins, then 7ml/kg over 4 hours, then 14 ml/kg over 16 hours The treatment period may be extended if patients have large ingestions, or elevated serum transaminase activity or increased INR. Prescott et al, Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 1977;2:432-4.
5 Interactions Interactions Interactions Interactions - A list of drugs (generic names only, no trade names are allowed) known for interacting with the drug A list of drugs (generic names only, no trade names are allowed) known for interacting with the drug under description. Add suggested measures in a very concise text (100 characters per each under description. Add suggested measures in a very concise text (100 characters per each interaction, including text). interaction, including text). A list of drugs (generic names only, no trade names are allowed) known for interacting with the drug under description. Add suggested measures in a very concise text (100 characters per each interaction, including text). A list of drugs (generic names only, no trade names are allowed) known for interacting with the drug under description. Ad characters per each interaction, including text). Physicochemical interactions with the thiol group of NAC are possible. As a precaution do not mix with other substances than dextrose or glucose in the infusion. NAC may enhance the vasodilatory effects of nitrates Side Effects Side Effects Side Effects Side Effects - A list of side effects known to be associated with the use of the drug under description. Add suggested measures in a very concise text (100 characters per each side effect). A list of side effects known to be associated with the use of the drug under description. Add suggested measures in a very concise text (100 characters per each side effect). A list of side effects known to be associated with the use of the drug under description. Add suggested measures in a very concise text (100 characters per each side effect). A list of side effects known to be associated with the use of the drug under description. Add suggested measures in a ver The taste and odour of oral NAC may not be tolerated, and nausea and vomiting may be problematic. In Europe NAC is therefore usuallly administered iv. Three to 6% of patients given iv NAC may develop an anaphylactoid reaction, which can be managed by reducing the infusion rate/suspending the infusion until the reaction has settled. Antihistamine and beta2 agonist therapy may be effective Precautions Precautions Precautions Precautions - The decision to stop treatment with NAC should be individualized for each patient based on the course of ALT and INR elevation, and measurements of acetaminophen plasma levels according to the Prescott nomogram (create link to nomogram as in section ) Algorithms - Describe officially accepted algorithms for disease management. As an introduction, you can provide a text of up to 500 characters, including spaces. Algorithms can be provided in the form of tables, that will be converted into pop-up images. Algorithms - Tables Attached is an algorithm for a clinical diagnostic approach to acute DILI. In addition, referral to specialist centre in a timely fashion is paramount in order to achieve good outcomes. The two tables below contain what have have been suggested as referral criteria. We would, however, encourage early referral, with longitudinal assessment by telephone, where appropriate. Referral should not delay start of NAC administration in cases of acetaminophen poisoning if indicated. 8. Links of interest Links of interest - List of links to Internet websites 300 characters max. 1. AASLD-FDA-NIH-PhRMA - Hepatotoxicity Special Interest Group; 2. Drug-Induced Liver Injury Network (DILIN); 3. Spanish DILI Registry
Drug- induced Liver Injury. Guruprasad P. Aithal
 Drug- induced Liver Injury Guruprasad P. Aithal Characterising DILI Deriving case defini=ons Phenotypes and outcomes Manifesta=ons PaBerns Liver histology Cholestasis Terminology Drug- induced Liver disease:
Drug- induced Liver Injury Guruprasad P. Aithal Characterising DILI Deriving case defini=ons Phenotypes and outcomes Manifesta=ons PaBerns Liver histology Cholestasis Terminology Drug- induced Liver disease:
Diagnostic evaluation of suspected Drug-Induced Liver Injury
 Diagnostic evaluation of suspected Drug-Induced Liver Injury Ynto de Boer, MD Department of Gastroenterology and Hepatology Disclosure No disclosures Drug-induced liver injury? Outline What is DILI? What,
Diagnostic evaluation of suspected Drug-Induced Liver Injury Ynto de Boer, MD Department of Gastroenterology and Hepatology Disclosure No disclosures Drug-induced liver injury? Outline What is DILI? What,
Drug Induced Liver Disease Role of the Pathologist. Disclosure. DILI can never be excluded. Romil Saxena, MD. Dr. Saxena has nothing to Disclose
 Drug Induced Liver Disease Role of the Pathologist Romil Saxena, MD Disclosure Dr. Saxena has nothing to Disclose DILI can never be excluded #1 DILI has no specific pattern of injury it can mimic any and
Drug Induced Liver Disease Role of the Pathologist Romil Saxena, MD Disclosure Dr. Saxena has nothing to Disclose DILI can never be excluded #1 DILI has no specific pattern of injury it can mimic any and
EVALUATION OF ABNORMAL LIVER TESTS
 EVALUATION OF ABNORMAL LIVER TESTS MIA MANABAT DO PGY6 MOA 119 TH ANNUAL SPRING SCIENTIFIC CONVENTION MAY 19, 2018 EVALUATION OF ABNORMAL LIVER TESTS Review of liver enzymes vs liver function tests Clinical
EVALUATION OF ABNORMAL LIVER TESTS MIA MANABAT DO PGY6 MOA 119 TH ANNUAL SPRING SCIENTIFIC CONVENTION MAY 19, 2018 EVALUATION OF ABNORMAL LIVER TESTS Review of liver enzymes vs liver function tests Clinical
Drug Induced Liver Injury (DILI)
 Drug Induced Liver Injury (DILI) Aisling Considine- Consultant Hepatology Pharmacist. King s College Hospital NHS Foundation Trust aislingconsidine@nhs.net Drug Induced Liver Injury /Disease Acute Liver
Drug Induced Liver Injury (DILI) Aisling Considine- Consultant Hepatology Pharmacist. King s College Hospital NHS Foundation Trust aislingconsidine@nhs.net Drug Induced Liver Injury /Disease Acute Liver
Drug-induced liver injury
 Drug-induced liver injury Vincent Wong MBChB(Hons), MD, FRCP, FHKCP, FHKAM Professor, Department of Medicine and Therapeutics Director, Cheng Suen Man Shook Foundation Centre for Hepatitis Studies Deputy
Drug-induced liver injury Vincent Wong MBChB(Hons), MD, FRCP, FHKCP, FHKAM Professor, Department of Medicine and Therapeutics Director, Cheng Suen Man Shook Foundation Centre for Hepatitis Studies Deputy
Should There Be a Standard of Care (SOC) for Drug-Induced Liver Injury (DILI)? Leonard B Seeff, MD
 Should There Be a Standard of Care (SOC) for Drug-Induced Liver Injury (DILI)? Leonard B Seeff, MD Drug-Induced Liver Injury: Are We Ready to Look? March 23-24,2011 AASLD, FDA/CDER, PhRMA A Common Statement
Should There Be a Standard of Care (SOC) for Drug-Induced Liver Injury (DILI)? Leonard B Seeff, MD Drug-Induced Liver Injury: Are We Ready to Look? March 23-24,2011 AASLD, FDA/CDER, PhRMA A Common Statement
Approach to the Patient with Liver Disease
 Approach to the Patient with Liver Disease Diagnosis of liver disease Careful history taking Physical examination Laboratory tests Radiologic examination and imaging studies Liver biopsy Liver diseases
Approach to the Patient with Liver Disease Diagnosis of liver disease Careful history taking Physical examination Laboratory tests Radiologic examination and imaging studies Liver biopsy Liver diseases
A Review of Liver Function Tests. James Gray Gastroenterology Vancouver
 A Review of Liver Function Tests James Gray Gastroenterology Vancouver Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted
A Review of Liver Function Tests James Gray Gastroenterology Vancouver Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted
PITFALLS IN THE DIAGNOSIS OF MEDICAL LIVER DISEASE WITH TWO CONCURRENT ETIOLOGIES I HAVE NOTHING TO DISCLOSE CURRENT ISSUES IN ANATOMIC PATHOLOGY 2017
 CURRENT ISSUES IN ANATOMIC PATHOLOGY 2017 I HAVE NOTHING TO DISCLOSE Linda Ferrell PITFALLS IN THE DIAGNOSIS OF MEDICAL LIVER DISEASE WITH TWO CONCURRENT ETIOLOGIES Linda Ferrell, MD, UCSF THE PROBLEM
CURRENT ISSUES IN ANATOMIC PATHOLOGY 2017 I HAVE NOTHING TO DISCLOSE Linda Ferrell PITFALLS IN THE DIAGNOSIS OF MEDICAL LIVER DISEASE WITH TWO CONCURRENT ETIOLOGIES Linda Ferrell, MD, UCSF THE PROBLEM
VIRAL HEPATITIS. Definitions. Acute Liver Disease (Hepatitis A &E, Alcoholic hepatitis, DILI and ALF) Acute Viral Hepatitis Symptoms
 Acute Liver Disease (Hepatitis A &E, Alcoholic hepatitis, DILI and ALF) Definitions AST and ALT Markers of hepatocellular injury Ryan M. Ford, MD Assistant Professor of Medicine Director of Viral Hepatitis
Acute Liver Disease (Hepatitis A &E, Alcoholic hepatitis, DILI and ALF) Definitions AST and ALT Markers of hepatocellular injury Ryan M. Ford, MD Assistant Professor of Medicine Director of Viral Hepatitis
HBV Forum 2 April 18 th 2017 Hilton Amsterdam.
 HBV Forum 2 April 18 th 2017 Hilton Amsterdam www.forumresearch.org Detection, Assessment and Management of DILI During Drug Development for HBV: The IQ DILI Initiative. Arie Regev, MD Global Patient Safety
HBV Forum 2 April 18 th 2017 Hilton Amsterdam www.forumresearch.org Detection, Assessment and Management of DILI During Drug Development for HBV: The IQ DILI Initiative. Arie Regev, MD Global Patient Safety
Abnormal Liver Chemistries. Lauren Myers, MMsc. PA-C Oregon Health and Science University
 Abnormal Liver Chemistries Lauren Myers, MMsc. PA-C Oregon Health and Science University Disclosure 1. The speaker/planner Lauren Myers, MMSc, PA-C have no relevant financial relationships to disclose
Abnormal Liver Chemistries Lauren Myers, MMsc. PA-C Oregon Health and Science University Disclosure 1. The speaker/planner Lauren Myers, MMSc, PA-C have no relevant financial relationships to disclose
What to do with abnormal LFTs? Andrew M Smith Hepatobiliary Surgeon
 What to do with abnormal LFTs? Andrew M Smith Hepatobiliary Surgeon "it looks like there's something wrong.with your television set. Matt Groenig, creator of The Simpsons Probability of an abnormal screening
What to do with abnormal LFTs? Andrew M Smith Hepatobiliary Surgeon "it looks like there's something wrong.with your television set. Matt Groenig, creator of The Simpsons Probability of an abnormal screening
Overview of Causality Assessment in Drug-Induced Liver Injury
 REVIEW Overview of Causality Assessment in Drug-Induced Liver Injury Paul H. Hayashi, M.D., M.P.H. The diagnosis of drug-induced (or herbal/dietary supplement induced) liver injury (DILI) remains largely
REVIEW Overview of Causality Assessment in Drug-Induced Liver Injury Paul H. Hayashi, M.D., M.P.H. The diagnosis of drug-induced (or herbal/dietary supplement induced) liver injury (DILI) remains largely
Observational Medical Outcomes Partnership
 Implications of Health Outcomes of Interest Definitions: Acute Liver Injury Case Study Judy Racoosin, Patrick Ryan on behalf of OMOP Research Team Observational Medical Outcomes Partnership Established
Implications of Health Outcomes of Interest Definitions: Acute Liver Injury Case Study Judy Racoosin, Patrick Ryan on behalf of OMOP Research Team Observational Medical Outcomes Partnership Established
Leonard B. Seeff, M.D. Consultant Einstein Healthcare Network. Click to view Biosketch and Presentation Abstract or page down to review presentation
 Leonard B. Seeff, M.D. Consultant Einstein Healthcare Network Click to view Biosketch and Presentation Abstract or page down to review presentation ALT as a Biomarker for Drug-Induced Liver Injury Leonard
Leonard B. Seeff, M.D. Consultant Einstein Healthcare Network Click to view Biosketch and Presentation Abstract or page down to review presentation ALT as a Biomarker for Drug-Induced Liver Injury Leonard
06 June 2017 DILI Conference XVII FDA/AASLD/Critical Path Institute
 1 DRUG-INDUCED LIVER INJURY How the clinical signature impacts benefits & risks June 6, 2017 Mark Avigan, MD CM Associate Director for Critical Path Initiatives Office of Pharmacovigilance & Epidemiology
1 DRUG-INDUCED LIVER INJURY How the clinical signature impacts benefits & risks June 6, 2017 Mark Avigan, MD CM Associate Director for Critical Path Initiatives Office of Pharmacovigilance & Epidemiology
Acute Liver Failure. Neil Shah, MD UNC School of Medicine High-Impact Hepatology Saturday, Dec 8 th, 2018
 Acute Liver Failure Neil Shah, MD UNC School of Medicine High-Impact Hepatology Saturday, Dec 8 th, 2018 Disclosures None Outline Overview of ALF Management of ALF Diagnosis of ALF Treatments and Support
Acute Liver Failure Neil Shah, MD UNC School of Medicine High-Impact Hepatology Saturday, Dec 8 th, 2018 Disclosures None Outline Overview of ALF Management of ALF Diagnosis of ALF Treatments and Support
Topic review - A patient with impaired liver function. Dr. Karen Hin Kwan Luk 18/1/2018
 Topic review - A patient with impaired liver function Dr. Karen Hin Kwan Luk 18/1/2018 Case M/69 PMH: 1. Diabetes 2. Hypertension 3. Hyperlipidaemia 4. Paroxysmal supraventricular tachycardia 5. Cerebral
Topic review - A patient with impaired liver function Dr. Karen Hin Kwan Luk 18/1/2018 Case M/69 PMH: 1. Diabetes 2. Hypertension 3. Hyperlipidaemia 4. Paroxysmal supraventricular tachycardia 5. Cerebral
2. Liver blood tests and what they mean p2 Acute and chronic liver screen
 Hepatology referral pathways for GP 1 Scope For use within hepatology Contents 2. Liver blood tests and what they mean p2 Acute and chronic liver screen p2 Common reasons for hepatology referral 3. Raised
Hepatology referral pathways for GP 1 Scope For use within hepatology Contents 2. Liver blood tests and what they mean p2 Acute and chronic liver screen p2 Common reasons for hepatology referral 3. Raised
Autoimmune Hepatobiliary Diseases PROF. DR. SABEHA ALBAYATI CABM,FRCP
 Autoimmune Hepatobiliary Diseases PROF. DR. SABEHA ALBAYATI CABM,FRCP Autoimmune hepatobiliary diseases The liver is an important target for immunemediated injury. Three disease phenotypes are recognized:
Autoimmune Hepatobiliary Diseases PROF. DR. SABEHA ALBAYATI CABM,FRCP Autoimmune hepatobiliary diseases The liver is an important target for immunemediated injury. Three disease phenotypes are recognized:
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries
 ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD 1, Stanley M. Cohen, MD, FACG, FAASLD 2, and Joseph K. Lim, MD, FACG, FAASLD 3 1 Division of Gastroenterology/Hepatology,
ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries Paul Y. Kwo, MD, FACG, FAASLD 1, Stanley M. Cohen, MD, FACG, FAASLD 2, and Joseph K. Lim, MD, FACG, FAASLD 3 1 Division of Gastroenterology/Hepatology,
LIVER SPECIALTY CONFERENCE USCAP Maha Guindi, M.D. Clinical Professor of Pathology Cedars-Sinai Medical Center Los Angeles, CA
 LIVER SPECIALTY CONFERENCE USCAP 2016 Maha Guindi, M.D. Clinical Professor of Pathology Cedars-Sinai Medical Center Los Angeles, CA Nothing to disclose Case History 47-year-old male, long standing ileal
LIVER SPECIALTY CONFERENCE USCAP 2016 Maha Guindi, M.D. Clinical Professor of Pathology Cedars-Sinai Medical Center Los Angeles, CA Nothing to disclose Case History 47-year-old male, long standing ileal
Farmadol. Paracetamol 10 mg/ml INFUSION SOLUTION
 Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
Farmadol Paracetamol 10 mg/ml INFUSION SOLUTION Composition Each ml contains: Paracetamol 10 mg Pharmacology Pharmacodynamic properties The precise mechanism of the analgesic and antipyretic properties
HHS Public Access Author manuscript Hepatology. Author manuscript; available in PMC 2017 March 01.
 Febuxostat-Induced Acute Liver Injury Matt Bohm, DO, Raj Vuppalanchi, MD, Naga Chalasani, MD, and Drug Induced Liver Injury Network (DILIN) Department of Medicine, Indiana University School of Medicine,
Febuxostat-Induced Acute Liver Injury Matt Bohm, DO, Raj Vuppalanchi, MD, Naga Chalasani, MD, and Drug Induced Liver Injury Network (DILIN) Department of Medicine, Indiana University School of Medicine,
Noncalculous Biliary Disease Dean Abramson, M.D. Gastroenterologists, P.C. Cedar Rapids. Cholestasis
 Noncalculous Biliary Disease Dean Abramson, M.D. Gastroenterologists, P.C. Cedar Rapids Cholestasis Biochemical hallmark Impaired bile flow from liver to small intestine Alkaline phosphatase is primary
Noncalculous Biliary Disease Dean Abramson, M.D. Gastroenterologists, P.C. Cedar Rapids Cholestasis Biochemical hallmark Impaired bile flow from liver to small intestine Alkaline phosphatase is primary
CrackCast Episode 28 Jaundice
 CrackCast Episode 28 Jaundice Episode overview: 1) Describe heme metabolism 2) List common pre-hepatic/hepatic/post-hepatic causes of jaundice Wisecracks: 1) What are clinical signs of liver disease? 2)
CrackCast Episode 28 Jaundice Episode overview: 1) Describe heme metabolism 2) List common pre-hepatic/hepatic/post-hepatic causes of jaundice Wisecracks: 1) What are clinical signs of liver disease? 2)
Management of autoimmune hepatitis. Pierre-Emmanuel RAUTOU Inserm U970, Paris Service d hépatologie, Hôpital Beaujon, Clichy, France
 Management of autoimmune hepatitis Pierre-Emmanuel RAUTOU Inserm U970, PARCC@HEGP, Paris Service d hépatologie, Hôpital Beaujon, Clichy, France 41 year-old woman, coming to emergency department for fatigue
Management of autoimmune hepatitis Pierre-Emmanuel RAUTOU Inserm U970, PARCC@HEGP, Paris Service d hépatologie, Hôpital Beaujon, Clichy, France 41 year-old woman, coming to emergency department for fatigue
DILI PATHOLOGY. PHILIP KAYE November 2017 BSG Pathology Winter Meeting
 DILI PATHOLOGY PHILIP KAYE November 2017 BSG Pathology Winter Meeting General Mechanisms Role of Liver Biopsy Outline Kleiner Categories Pathology! Differentials Severity Finally Drugs/Toxins may cause
DILI PATHOLOGY PHILIP KAYE November 2017 BSG Pathology Winter Meeting General Mechanisms Role of Liver Biopsy Outline Kleiner Categories Pathology! Differentials Severity Finally Drugs/Toxins may cause
ABNORMAL LIVER FUNCTION TESTS. Dr Uthayanan Chelvaratnam Hepatology Consultant North Bristol NHS Trust
 ABNORMAL LIVER FUNCTION TESTS Dr Uthayanan Chelvaratnam Hepatology Consultant North Bristol NHS Trust INTRODUCTION Liver function tests Cases Non invasive fibrosis measurement Questions UK MORTALITY RATE
ABNORMAL LIVER FUNCTION TESTS Dr Uthayanan Chelvaratnam Hepatology Consultant North Bristol NHS Trust INTRODUCTION Liver function tests Cases Non invasive fibrosis measurement Questions UK MORTALITY RATE
Drug-induced liver injury due to varenicline: a case report
 Sprague and Bambha BMC Gastroenterology 2012, 12:65 CASE REPORT Drug-induced liver injury due to varenicline: a case report David Sprague 1 and Kiran Bambha 2* Open Access Abstract Background: Liver injury
Sprague and Bambha BMC Gastroenterology 2012, 12:65 CASE REPORT Drug-induced liver injury due to varenicline: a case report David Sprague 1 and Kiran Bambha 2* Open Access Abstract Background: Liver injury
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less. For pack sizes of more than 24 tablets
 SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
CITY AND HACKNEY CCG ABNORMAL LIVER FUNCTION TESTS (LFTs) in ADULTS
 CITY AND HACKNEY CCG ABNORMAL LIVER FUNCTION TESTS (LFTs) in ADULTS Interpreting abnormal liver function tests (LFTs) and trying to diagnose any underlying liver disease is a common scenario in Primary
CITY AND HACKNEY CCG ABNORMAL LIVER FUNCTION TESTS (LFTs) in ADULTS Interpreting abnormal liver function tests (LFTs) and trying to diagnose any underlying liver disease is a common scenario in Primary
2. Liver blood tests and what they mean p2 Acute and chronic liver screen
 1 Scope For use within hepatology Contents 2. Liver blood tests and what they mean p2 Acute and chronic liver screen p2 Common reasons for referral 3. Raised ALT +/- GGT p3 4. Non alcoholic fatty liver
1 Scope For use within hepatology Contents 2. Liver blood tests and what they mean p2 Acute and chronic liver screen p2 Common reasons for referral 3. Raised ALT +/- GGT p3 4. Non alcoholic fatty liver
Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need?
 Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need? Rob Goldin r.goldin@imperial.ac.uk Fatty liver disease Is there fatty
Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need? Rob Goldin r.goldin@imperial.ac.uk Fatty liver disease Is there fatty
Diseases of liver. Dr. Mohamed. A. Mahdi 4/2/2019. Mob:
 Diseases of liver Dr. Mohamed. A. Mahdi Mob: 0123002800 4/2/2019 Cirrhosis Cirrhosis is a complication of many liver disease. Permanent scarring of the liver. A late-stage liver disease. The inflammation
Diseases of liver Dr. Mohamed. A. Mahdi Mob: 0123002800 4/2/2019 Cirrhosis Cirrhosis is a complication of many liver disease. Permanent scarring of the liver. A late-stage liver disease. The inflammation
Acute Liver Failure in the USA 2011 Evaluation of diagnostic criteria to approach DILI causality
 Acute Liver Failure in the USA 2011 Evaluation of diagnostic criteria to approach DILI causality William M. Lee, MD Professor of Internal Medicine Meredith Mosle Chair in Liver Diseases UT Southwestern
Acute Liver Failure in the USA 2011 Evaluation of diagnostic criteria to approach DILI causality William M. Lee, MD Professor of Internal Medicine Meredith Mosle Chair in Liver Diseases UT Southwestern
DR J HARTY / DR CM RITCHIE / DR M GIBBONS
 CLINICAL GUIDELINES ID TAG Title: Author: Speciality / Division: Directorate: Paracetamol Poisoning DR J HARTY / DR CM RITCHIE / DR M GIBBONS Medicine Acute Date Uploaded: 16 th September 2014 Review Date
CLINICAL GUIDELINES ID TAG Title: Author: Speciality / Division: Directorate: Paracetamol Poisoning DR J HARTY / DR CM RITCHIE / DR M GIBBONS Medicine Acute Date Uploaded: 16 th September 2014 Review Date
Gastroenterology. Certification Examination Blueprint. Purpose of the exam
 Gastroenterology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the certified gastroenterologist
Gastroenterology Certification Examination Blueprint Purpose of the exam The exam is designed to evaluate the knowledge, diagnostic reasoning, and clinical judgment skills expected of the certified gastroenterologist
1. Based on A.S. s labs and presentation, what type of liver injury would you classify her as experiencing?
 Drug Induced Liver Injury Cases Case #1 A.S., a16 year-old female, was found by her pediatrician to be slightly jaundiced during a routine school physical. She denied any history of liver disease, abdominal
Drug Induced Liver Injury Cases Case #1 A.S., a16 year-old female, was found by her pediatrician to be slightly jaundiced during a routine school physical. She denied any history of liver disease, abdominal
Key Points: Autoimmune Liver Disease: Update for Pathologists from the Hepatologist s Perspective. Jenny Heathcote, MD. University of Toronto
 Autoimmune Liver Disease: Update for Pathologists from the Hepatologist s Perspective Jenny Heathcote, MD University of Toronto Key Points: AILD comprise autoimmune hepatitis, primary biliary cirrhosis
Autoimmune Liver Disease: Update for Pathologists from the Hepatologist s Perspective Jenny Heathcote, MD University of Toronto Key Points: AILD comprise autoimmune hepatitis, primary biliary cirrhosis
Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need?
 Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need? Rob Goldin r.goldin@imperial.ac.uk @robdgol FATTY LIVER DISEASE Brunt
Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need? Rob Goldin r.goldin@imperial.ac.uk @robdgol FATTY LIVER DISEASE Brunt
Paracetamol orodispersible tablets: a risk for severe poisoning in children?
 Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2011 Paracetamol orodispersible tablets: a risk for severe poisoning in children?
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2011 Paracetamol orodispersible tablets: a risk for severe poisoning in children?
Hepatocytes produce. Proteins Clotting factors Hormones. Bile Flow
 R.J.Bailey MD Hepatocytes produce Proteins Clotting factors Hormones Bile Flow Trouble.. for the liver! Trouble for the Liver Liver Gall Bladder Common Alcohol Hep C Fatty Liver Cancer Drugs Viruses Uncommon
R.J.Bailey MD Hepatocytes produce Proteins Clotting factors Hormones Bile Flow Trouble.. for the liver! Trouble for the Liver Liver Gall Bladder Common Alcohol Hep C Fatty Liver Cancer Drugs Viruses Uncommon
Jaundice. Agnieszka Dobrowolska- Zachwieja, MD, PhD
 Jaundice Agnieszka Dobrowolska- Zachwieja, MD, PhD Jaundice definition Jaundice, as in the French jaune, refers to the yellow discoloration of the skin. It arises from the abnormal accumulation of bilirubin
Jaundice Agnieszka Dobrowolska- Zachwieja, MD, PhD Jaundice definition Jaundice, as in the French jaune, refers to the yellow discoloration of the skin. It arises from the abnormal accumulation of bilirubin
William M. Lee, MD. Aim: Discuss Clinical Trends in DILI and Acetaminophen Liver Injury
 Drug-Induced Liver Injury (DILI) including Acetaminophen (APAP) 2014: Practical Tips William M. Lee, MD Clinical Professor Department of Internal Medicine Division of Gastroenterology, Hepatology & Nutrition
Drug-Induced Liver Injury (DILI) including Acetaminophen (APAP) 2014: Practical Tips William M. Lee, MD Clinical Professor Department of Internal Medicine Division of Gastroenterology, Hepatology & Nutrition
Aim: Discuss Clinical Trends in DILI and Acetaminophen Liver Injury
 Drug-Induced Liver Injury (DILI) including Acetaminophen (APAP) 2014: Practical Tips DILI 2014: Aims/Topics Aim: Discuss Clinical Trends in DILI and Acetaminophen Liver Injury William M. Lee, MD Clinical
Drug-Induced Liver Injury (DILI) including Acetaminophen (APAP) 2014: Practical Tips DILI 2014: Aims/Topics Aim: Discuss Clinical Trends in DILI and Acetaminophen Liver Injury William M. Lee, MD Clinical
Yes, the proposed stopping rules are too conservative. John R. Senior, M.D. 25 March 2010 Rethink Stopping Rules 1
 Yes, the proposed stopping rules are too conservative John R. Senior, M.D. 25 March 2010 Rethink Stopping Rules 1 Guidelines for study stop rules: ALT/AST > 8X ULN ALT/AST remains > 5X ULN over 2 wks ALT/AST
Yes, the proposed stopping rules are too conservative John R. Senior, M.D. 25 March 2010 Rethink Stopping Rules 1 Guidelines for study stop rules: ALT/AST > 8X ULN ALT/AST remains > 5X ULN over 2 wks ALT/AST
HIV/AIDS and the Liver : interlinking challenges
 HIV/AIDS and the Liver : interlinking challenges Mark W. Sonderup Division of Hepatology Department of Medicine University of Cape Town & Groote Schuur Hospital Global HIV/AIDS prevalence 37 million people
HIV/AIDS and the Liver : interlinking challenges Mark W. Sonderup Division of Hepatology Department of Medicine University of Cape Town & Groote Schuur Hospital Global HIV/AIDS prevalence 37 million people
What Is Cirrhosis? CIRRHOSIS. Cirrhosis occurs when the liver is. by chronic conditions and diseases. permanently scarred or injured
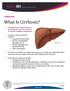 What Is Cirrhosis? Cirrhosis occurs when the liver is permanently scarred or injured by chronic conditions and diseases. Common causes of cirrhosis include: Long-term alcohol abuse. Chronic viral hepatitis
What Is Cirrhosis? Cirrhosis occurs when the liver is permanently scarred or injured by chronic conditions and diseases. Common causes of cirrhosis include: Long-term alcohol abuse. Chronic viral hepatitis
Management of Acute HCV Infection
 Management of Acute HCV Infection This section provides guidance on the diagnosis and medical management of acute HCV infection, which is defined as presenting within 6 months of the exposure. During this
Management of Acute HCV Infection This section provides guidance on the diagnosis and medical management of acute HCV infection, which is defined as presenting within 6 months of the exposure. During this
Autoimmune Hepatitis. Dr. Stefania Casu Hepatology, UVCM, Inselspital Bern. November 14th, 2018
 Autoimmune Hepatitis Dr. Stefania Casu Hepatology, UVCM, Inselspital Bern November 14th, 2018 AIH - Definition Manns MP J Hepatol 2015, vol 62, P 100-111 AIH - Definition Autoimmune hepatitis (AIH) is
Autoimmune Hepatitis Dr. Stefania Casu Hepatology, UVCM, Inselspital Bern November 14th, 2018 AIH - Definition Manns MP J Hepatol 2015, vol 62, P 100-111 AIH - Definition Autoimmune hepatitis (AIH) is
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Ocaliva (obeticholic acid) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ocaliva (obeticholic acid) Prime Therapeutics will review Prior Authorization
Ocaliva (obeticholic acid) Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ocaliva (obeticholic acid) Prime Therapeutics will review Prior Authorization
Leonard B. Seeff, MD Hepatology Consultant, Retired
 Leonard B. Seeff, MD Hepatology Consultant, Retired Subjects with Active Liver Disease Need Special Observation Leonard B. Seeff, MD Hepatology Consultant, Retired DILI Conference XIV 19-20 March 2014
Leonard B. Seeff, MD Hepatology Consultant, Retired Subjects with Active Liver Disease Need Special Observation Leonard B. Seeff, MD Hepatology Consultant, Retired DILI Conference XIV 19-20 March 2014
Liver Transplantation
 1 Liver Transplantation Department of Surgery Yonsei University Wonju College of Medicine Kim Myoung Soo M.D. ysms91@wonju.yonsei.ac.kr http://gs.yonsei.ac.kr History Development of Liver transplantation
1 Liver Transplantation Department of Surgery Yonsei University Wonju College of Medicine Kim Myoung Soo M.D. ysms91@wonju.yonsei.ac.kr http://gs.yonsei.ac.kr History Development of Liver transplantation
Autoimmune Liver Diseases
 2nd Pannonia Congress of pathology Hepato-biliary pathology Autoimmune Liver Diseases Vera Ferlan Marolt Institute of pathology, Medical faculty, University of Ljubljana Slovenia Siofok, Hungary, May 2012
2nd Pannonia Congress of pathology Hepato-biliary pathology Autoimmune Liver Diseases Vera Ferlan Marolt Institute of pathology, Medical faculty, University of Ljubljana Slovenia Siofok, Hungary, May 2012
Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy
 Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy Goals Share an interesting case Important because it highlights a common problem that we re likely to
Fat, ballooning, plasma cells and a +ANA. Yikes! USCAP 2016 Evening Specialty Conference Cynthia Guy Goals Share an interesting case Important because it highlights a common problem that we re likely to
AAIM: GI Workshop Follow Up to Case Studies. Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease
 AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
Improving the Lives of Patients with Liver Diseases
 Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
MANAGEMENT OF DILI in TB/HIV coinfected patients. Chimoio 31 August 2017 by Dr Ndiviwe Mphothulo
 MANAGEMENT OF DILI in TB/HIV coinfected patients Chimoio 31 August 2017 by Dr Ndiviwe Mphothulo ANTI-TB drugs Groups Drugs Group 1: First-line oral drugs Ethambutol (Emb) Pyrazinamide(PZA) Isoniazid (INH)
MANAGEMENT OF DILI in TB/HIV coinfected patients Chimoio 31 August 2017 by Dr Ndiviwe Mphothulo ANTI-TB drugs Groups Drugs Group 1: First-line oral drugs Ethambutol (Emb) Pyrazinamide(PZA) Isoniazid (INH)
Valdoxan (agomelatine) in the treatment of Major Depressive Episodes in Adults. Prescriber Guide Information for Healthcare Professionals
 Important information Do not discard! Valdoxan (agomelatine) in the treatment of Major Depressive Episodes in Adults Prescriber Guide Information for Healthcare Professionals Recommendations regarding:
Important information Do not discard! Valdoxan (agomelatine) in the treatment of Major Depressive Episodes in Adults Prescriber Guide Information for Healthcare Professionals Recommendations regarding:
PBC/AIH variant/ overlap syndrome vs PBC with hepatitic features?
 22 November 2018 BD-IAP UK-LPG Liver Update PBC/AIH variant/ overlap syndrome vs PBC with hepatitic features? in a UDCA non-responder Dina G. Tiniakos Institute of Cellular Medicine, Faculty of Medical
22 November 2018 BD-IAP UK-LPG Liver Update PBC/AIH variant/ overlap syndrome vs PBC with hepatitic features? in a UDCA non-responder Dina G. Tiniakos Institute of Cellular Medicine, Faculty of Medical
Initial Evaluation for HCV Therapy. Hope McGratty PA-C, MPH
 Initial Evaluation for HCV Therapy Hope McGratty PA-C, MPH Conflict of Interest Disclosure Statement None Who are we talking about today? Treatment naïve Chronic infection This patient seems complicated
Initial Evaluation for HCV Therapy Hope McGratty PA-C, MPH Conflict of Interest Disclosure Statement None Who are we talking about today? Treatment naïve Chronic infection This patient seems complicated
LIVER CIRRHOSIS. The liver extracts nutrients from the blood and processes them for later use.
 LIVER CIRRHOSIS William Sanchez, M.D. & Jayant A. Talwalkar, M.D., M.P.H. Advanced Liver Disease Study Group Miles and Shirley Fiterman Center for Digestive Diseases Mayo College of Medicine Rochester,
LIVER CIRRHOSIS William Sanchez, M.D. & Jayant A. Talwalkar, M.D., M.P.H. Advanced Liver Disease Study Group Miles and Shirley Fiterman Center for Digestive Diseases Mayo College of Medicine Rochester,
Ask the Experts Patient Education Program. Understanding the Progression of Liver Disease: Fibrosis
 J. Clint Stanfill, MD April 24, 2018 Ask the Experts Patient Education Program Understanding the Progression of Liver Disease: Fibrosis Fibrosis -replacement of normal liver -reactive phenomenon - stronger
J. Clint Stanfill, MD April 24, 2018 Ask the Experts Patient Education Program Understanding the Progression of Liver Disease: Fibrosis Fibrosis -replacement of normal liver -reactive phenomenon - stronger
DILI: Clinical Pharmacology Considerations for Risk Assessment
 DILI: Clinical Pharmacology Considerations for Risk Assessment Raj Madabushi, PhD Office of Clinical Pharmacology Drug-Induced Liver Injury (DILI) Conference XVII June 06, 2017 Disclaimer: The views expressed
DILI: Clinical Pharmacology Considerations for Risk Assessment Raj Madabushi, PhD Office of Clinical Pharmacology Drug-Induced Liver Injury (DILI) Conference XVII June 06, 2017 Disclaimer: The views expressed
Overview of PSC Making the Diagnosis
 Overview of PSC Making the Diagnosis Tamar Taddei, MD Assistant Professor of Medicine Yale University School of Medicine Overview Definition Epidemiology Diagnosis Modes of presentation Associated diseases
Overview of PSC Making the Diagnosis Tamar Taddei, MD Assistant Professor of Medicine Yale University School of Medicine Overview Definition Epidemiology Diagnosis Modes of presentation Associated diseases
Chronic Liver Disease after Acute Hepatocellular DILI
 Chronic Liver Disease after Acute Hepatocellular DILI Robert J. Fontana, MD University of Michigan Medical Center DILI Natural history of acute DILI Transplant/ death Chronic DILI Chronic DILI Incidence
Chronic Liver Disease after Acute Hepatocellular DILI Robert J. Fontana, MD University of Michigan Medical Center DILI Natural history of acute DILI Transplant/ death Chronic DILI Chronic DILI Incidence
Disclosure. Evaluation of Abnormal Hepatic Enzymes
 Evaluation of Abnormal Hepatic Enzymes Bruce D. Askey, MS, ANP-BC Associate Lecturer North Andover, MA Adult Nurse Practitioner Dept. of Hepatology/Gastroenterology Guthrie Clinic Sayre, Pa Disclosure
Evaluation of Abnormal Hepatic Enzymes Bruce D. Askey, MS, ANP-BC Associate Lecturer North Andover, MA Adult Nurse Practitioner Dept. of Hepatology/Gastroenterology Guthrie Clinic Sayre, Pa Disclosure
NEW ZEALAND DATA SHEET
 1 PRODUCT NAME Acetylcysteine 200 mg/ml Injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml (as N-acetylcysteine) Each 10 ml ampoule contains 2 g N-acetylcysteine Excipients
1 PRODUCT NAME Acetylcysteine 200 mg/ml Injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Acetylcysteine 200 mg per ml (as N-acetylcysteine) Each 10 ml ampoule contains 2 g N-acetylcysteine Excipients
Hepatology for the Nonhepatologist
 Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
ACCME/Disclosures. The Overlap Syndromes: Do They Exist? Key Points and Questions 4/6/2016. Hans Popper Hepatopathology Society
 ACCME/Disclosures The USCAP requires that anyone in a position to influence or control the content of CME disclose any relevant financial relationship WITH COMMERCIAL INTERESTS which they or their spouse/partner
ACCME/Disclosures The USCAP requires that anyone in a position to influence or control the content of CME disclose any relevant financial relationship WITH COMMERCIAL INTERESTS which they or their spouse/partner
So, this is a group of patients that were treated with an anti-inflammatory drug in development within Eli Lilly. The name of the compound was an
 Thank you, Mark and thank you all for being here and sticking with it. This is going to be a little bit of a detour to the left side of the first slide that Mark showed, which is a hypersensitivity allergic-type
Thank you, Mark and thank you all for being here and sticking with it. This is going to be a little bit of a detour to the left side of the first slide that Mark showed, which is a hypersensitivity allergic-type
Current Concepts in Diagnosis and Management of Acute Liver Failure
 Current Concepts in Diagnosis and Management of Acute Liver Failure Oren Fix, MD, MSc, FACP, AGAF, FAASLD Medical Director, Liver Transplant Program Swedish Medical Center Seattle, WA Learning Objectives
Current Concepts in Diagnosis and Management of Acute Liver Failure Oren Fix, MD, MSc, FACP, AGAF, FAASLD Medical Director, Liver Transplant Program Swedish Medical Center Seattle, WA Learning Objectives
Patterns of abnormal LFTs and their differential diagnosis
 Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Outline liver function tests / tests of
Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Outline liver function tests / tests of
CASE 1 Plasma Cell Infiltrates: Significance in post liver transplantation and in chronic liver disease
 CASE 1 Plasma Cell Infiltrates: Significance in post liver transplantation and in chronic liver disease Maria Isabel Fiel, M.D. The Mount Sinai Medical Center New York, New York Case A 57 yo man, 7 months
CASE 1 Plasma Cell Infiltrates: Significance in post liver transplantation and in chronic liver disease Maria Isabel Fiel, M.D. The Mount Sinai Medical Center New York, New York Case A 57 yo man, 7 months
Recent Status of Drug-induced Liver Injury and Its Problems in Japan
 Research and Reviews Recent Status of Drug-induced Liver Injury and Its Problems in Japan JMAJ 53(4): 243 247, 2010 Hajime TAKIKAWA* 1 Abstract Adverse drug reactions are becoming a social issue in recent
Research and Reviews Recent Status of Drug-induced Liver Injury and Its Problems in Japan JMAJ 53(4): 243 247, 2010 Hajime TAKIKAWA* 1 Abstract Adverse drug reactions are becoming a social issue in recent
Liver Disease in the ICU: Acute Liver Failure
 Liver Disease in the ICU: Acute Liver Failure Steven C Pugliese, MD Assistant Professor Division of Pulmonary Sciences and Cri@cal Care Medicine University of Colorado Denver 48 y/o male w/out prior liver
Liver Disease in the ICU: Acute Liver Failure Steven C Pugliese, MD Assistant Professor Division of Pulmonary Sciences and Cri@cal Care Medicine University of Colorado Denver 48 y/o male w/out prior liver
ACETYLCYSTEINE INJECTION
 ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
Primary Sclerosing Cholangitis and Cholestatic liver diseases. Ahsan M Bhatti MD, FACP Bhatti Gastroenterology Consultants
 Primary Sclerosing Cholangitis and Cholestatic liver diseases Ahsan M Bhatti MD, FACP Bhatti Gastroenterology Consultants I have nothing to disclose Educational Objectives What is PSC? Understand the cholestatic
Primary Sclerosing Cholangitis and Cholestatic liver diseases Ahsan M Bhatti MD, FACP Bhatti Gastroenterology Consultants I have nothing to disclose Educational Objectives What is PSC? Understand the cholestatic
Gastrointes*nal and Liver Pathology. Kris*ne Kra5s, M.D.
 Gastrointes*nal and Liver Pathology Kris*ne Kra5s, M.D. GI Pathology Outline Esophagus Stomach Intes*ne Liver Gallbladder Pancreas GI Pathology Outline Esophagus Stomach Intes*ne Liver Hepa**s Alcoholic
Gastrointes*nal and Liver Pathology Kris*ne Kra5s, M.D. GI Pathology Outline Esophagus Stomach Intes*ne Liver Gallbladder Pancreas GI Pathology Outline Esophagus Stomach Intes*ne Liver Hepa**s Alcoholic
Module 1 Introduction of hepatitis
 Module 1 Introduction of hepatitis 1 Training Objectives At the end of the module, trainees will be able to ; Demonstrate improved knowledge of the global epidemiology of the viral hepatitis Understand
Module 1 Introduction of hepatitis 1 Training Objectives At the end of the module, trainees will be able to ; Demonstrate improved knowledge of the global epidemiology of the viral hepatitis Understand
I have no disclosures relevant to this presentation LIVER TESTS: WHAT IS INCLUDED? LIVER TESTS: HOW TO UTILIZE THEM OBJECTIVES
 LIVER TESTS: HOW TO UTILIZE THEM I have no disclosures relevant to this presentation José Franco, MD Professor of Medicine, Surgery and Pediatrics Medical College of Wisconsin OBJECTIVES Differentiate
LIVER TESTS: HOW TO UTILIZE THEM I have no disclosures relevant to this presentation José Franco, MD Professor of Medicine, Surgery and Pediatrics Medical College of Wisconsin OBJECTIVES Differentiate
Chapter 143 Acetaminophen
 Chapter 143 Acetaminophen Episode overview 1) Describe the metabolism of Acetaminophen 2) Describe the 4 stages of Acetaminophen toxicity 3) List 4 mechanism of action of N-acetylcysteine 4) When do you
Chapter 143 Acetaminophen Episode overview 1) Describe the metabolism of Acetaminophen 2) Describe the 4 stages of Acetaminophen toxicity 3) List 4 mechanism of action of N-acetylcysteine 4) When do you
IN THE NAME OF GOD. D r. MANIJE DEZFULI AZAD UNIVERCITY OF TEHRAN BOOALI HOSPITAL INFECTIOUS DISEASES SPECIALIST
 IN THE NAME OF GOD AZAD UNIVERCITY OF TEHRAN BOOALI HOSPITAL D r. MANIJE DEZFULI INFECTIOUS DISEASES SPECIALIST Acute Viral Hepatitis The Anatomy of the Liver Hepatic Physiology Liver: Largest solid organ
IN THE NAME OF GOD AZAD UNIVERCITY OF TEHRAN BOOALI HOSPITAL D r. MANIJE DEZFULI INFECTIOUS DISEASES SPECIALIST Acute Viral Hepatitis The Anatomy of the Liver Hepatic Physiology Liver: Largest solid organ
GASTROENTEROLOGY Maintenance of Certification (MOC) Examination Blueprint
 GASTROENTEROLOGY Maintenance of Certification (MOC) Examination Blueprint ABIM invites diplomates to help develop the Gastroenterology MOC exam blueprint Based on feedback from physicians that MOC assessments
GASTROENTEROLOGY Maintenance of Certification (MOC) Examination Blueprint ABIM invites diplomates to help develop the Gastroenterology MOC exam blueprint Based on feedback from physicians that MOC assessments
Valdoxan (agomelatine) in the treatment of Major Depressive Episodes in Adults. Prescriber Guide Information for Healthcare Professionals
 Important information Do not discard! Valdoxan (agomelatine) in the treatment of Major Depressive Episodes in Adults Prescriber Guide Information for Healthcare Professionals Recommendations regarding:
Important information Do not discard! Valdoxan (agomelatine) in the treatment of Major Depressive Episodes in Adults Prescriber Guide Information for Healthcare Professionals Recommendations regarding:
Patterns of abnormal LFTs and their differential diagnosis
 Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Outline liver function / liver function
Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Outline liver function / liver function
Hepatocellular Carcinoma: Diagnosis and Management
 Hepatocellular Carcinoma: Diagnosis and Management Nizar A. Mukhtar, MD Co-director, SMC Liver Tumor Board April 30, 2016 1 Objectives Review screening/surveillance guidelines Discuss diagnostic algorithm
Hepatocellular Carcinoma: Diagnosis and Management Nizar A. Mukhtar, MD Co-director, SMC Liver Tumor Board April 30, 2016 1 Objectives Review screening/surveillance guidelines Discuss diagnostic algorithm
World Health Organization. Western Pacific Region
 Basic modules for hepatitis 1 Basic Module 1 Liver anatomy and physiology 2 Position of liver Midline Located in right upper abdomen Protected by the right rib cage Right upper Measures: 12 15 cm in vertical
Basic modules for hepatitis 1 Basic Module 1 Liver anatomy and physiology 2 Position of liver Midline Located in right upper abdomen Protected by the right rib cage Right upper Measures: 12 15 cm in vertical
Alpha-1 Antitrypsin Deficiency: Liver Disease
 Alpha-1 Antitrypsin Deficiency: Liver Disease Who is at risk to develop Alpha-1 liver disease? Alpha-1 liver disease may affect children and adults who have abnormal Alpha-1 antitrypsin genes. Keys to
Alpha-1 Antitrypsin Deficiency: Liver Disease Who is at risk to develop Alpha-1 liver disease? Alpha-1 liver disease may affect children and adults who have abnormal Alpha-1 antitrypsin genes. Keys to
Resident, PGY1 David Geffen School of Medicine at UCLA. Los Angeles Society of Pathology Resident and Fellow Symposium 2013
 Resident, PGY1 David Geffen School of Medicine at UCLA Los Angeles Society of Pathology Resident and Fellow Symposium 2013 85 year old female with past medical history including paroxysmal atrial fibrillation,
Resident, PGY1 David Geffen School of Medicine at UCLA Los Angeles Society of Pathology Resident and Fellow Symposium 2013 85 year old female with past medical history including paroxysmal atrial fibrillation,
Drug Induced Liver Injury: A Clinical Perspective. Robert J. Fontana, MD
 Drug Induced Liver Injury: A Clinical Perspective Robert J. Fontana, MD Drug induced liver injury (DILI) is an uncommon cause of acute and chronic liver injury of increasing importance to patients, clinicians,
Drug Induced Liver Injury: A Clinical Perspective Robert J. Fontana, MD Drug induced liver injury (DILI) is an uncommon cause of acute and chronic liver injury of increasing importance to patients, clinicians,
Interpreting Liver Function Tests
 PSH Clinical Guidelines Statement 2017 Interpreting Liver Function Tests Dr. Asad A Chaudhry Consultant Hepatologist, Chaudhry Hospital, Gujranwala, Pakistan. Liver function tests (LFTs) generally refer
PSH Clinical Guidelines Statement 2017 Interpreting Liver Function Tests Dr. Asad A Chaudhry Consultant Hepatologist, Chaudhry Hospital, Gujranwala, Pakistan. Liver function tests (LFTs) generally refer
Current Concepts in the Management and Treatment of PBC & PSC
 Current Concepts in the Management and Treatment of PBC & PSC Michael A Heneghan, MD, MMedSc, FRCPI. Institute of Liver Studies, King s College Hospital, London A family affair? Central vein Hepatocytes
Current Concepts in the Management and Treatment of PBC & PSC Michael A Heneghan, MD, MMedSc, FRCPI. Institute of Liver Studies, King s College Hospital, London A family affair? Central vein Hepatocytes
Evaluation of prognostic markers in severe drug-induced liver disease
 PO Box 2345, Beijing 100023, China World J Gastroenterol 2007 January 28; 13(4): 628-632 World Journal of Gastroenterology ISSN 1007-9327 wjg@wjgnet.com 2007 The WJG Press. All rights reserved. RAPID COMMUNICATION
PO Box 2345, Beijing 100023, China World J Gastroenterol 2007 January 28; 13(4): 628-632 World Journal of Gastroenterology ISSN 1007-9327 wjg@wjgnet.com 2007 The WJG Press. All rights reserved. RAPID COMMUNICATION
Positive Rechallenge: What Clinical Trial Data Tell Us Julie Papay, Pharm.D. UCB BioSciences Global Patient Safety
 Positive Rechallenge: What Clinical Trial Data Tell Us Julie Papay, Pharm.D. UCB BioSciences Global Patient Safety Drug-Induced Liver Injury (DILI) Conference XVI Wednesday 23 March 2016 The comments provided
Positive Rechallenge: What Clinical Trial Data Tell Us Julie Papay, Pharm.D. UCB BioSciences Global Patient Safety Drug-Induced Liver Injury (DILI) Conference XVI Wednesday 23 March 2016 The comments provided
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
How to use statins in patients with chronic liver disease
 REVIEW CME CREDIT MARK W. RUSSO, MD Center for the Study of Hepatitis C, Division of Gastroenterology and Hepatology, Weill Cornell Medical College, New York, NY IRA M. JACOBSON, MD Center for the Study
REVIEW CME CREDIT MARK W. RUSSO, MD Center for the Study of Hepatitis C, Division of Gastroenterology and Hepatology, Weill Cornell Medical College, New York, NY IRA M. JACOBSON, MD Center for the Study
