PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.
|
|
|
- Susanna Wilcox
- 6 years ago
- Views:
Transcription
1 Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol 500mg, Codeine Phosphate 10mg and Doxylamine Succinate 5.1mg Paracetamol: Codeine Phosphate: Doxylamine Succinate:
2 Product Information Page 2 DESCRIPTION Paracetamol is a white or almost white crystalline powder. It is sparingly soluble in water, freely soluble in alcohol, very slightly soluble in methylene chloride. Paracetamol is an analgesic and antipyretic. Codeine phosphate is a white or almost white, crystalline powder or small, colourless crsytals. It is freely soluble in water, slightly soluble or very slightly soluble in ethanol (96%). Codeine phosphate is a cough suppressant and an analgesic. Doxylamine succinate is a white or creamy white powder with a characterisitc odour. Actives: Each tablet contains Paracetamol 500mg Codeine Phosphate 10mg and Doxylamine Succinate 5.1mg. Excipients: Lactose, Starch-Maize, Povidone, Ethanol, Crospovidone, Cellulose-microcrystalline, Stearic acid, Magnesium Stearate. PHARMACOLOGY Paracetamol is an effective and fast acting analgesic that relieves mild to moderate pain. It is rapidly absorbed from the gastrointestinal tract with peak plasma levels usually reached 30 to 60 minutes after oral administration. It also reduces fever by a direct effect on the heat regulating centres to increase dissipation of body heat. Codeine phosphate is an effective oral analgesic that provides relief from mild to moderate pain. It is also well absorbed from the gastrointestinal tract after oral administration. Doxylamine succinate belongs to the ethanolamine class of antihistamines with sedative properties. Pharmacokinetics Paracetamol: Paracetamol is readily absorbed from the gastrointestinal tract with peak plasma concentrations occurring about 10 to 60 minutes after oral administration. Paracetamol is distributed into most body tissues. Plasma protein binding is negligible at usual therapeutic doses but increases with increasing doses. The elimination half-life varies from about 1 to 3 hours. Paracetamol is metabolised extensively in the liver and excreted in the urine mainly as inactive glucuronide and sulfate conjugates. Less than 5% is excreted unchanged. The metabolites of paracetamol include a minor hydroxylated intermediate which has hepatotoxic activity. This intermediate metabolite is detoxified by conjunction with glutathione, however, it can accumulate following paracetamol overdosage (more than 150mg/kg or 10g total paracetamol ingested) and if left untreated can cause irreversible liver damage. Paracetamol is metabolized differently by premature infants, newborns, infants and young children compared to adults, the sulfate conjugate being predominant.
3 Product Information Page 3 Codeine: Codeine and its salts are well absorbed from the gastrointestinal tract: peak plasmacodeine concentrations occur at about one hour after ingestion of codeine phosphate. Codeine is metabolized by O- and N-demethylation and in the liver (via the cytochrome P450 system) to morphine (about 10 per cent of a codeine dose is demethylated to morphine), norcodeine and other metabolites including normorphine and hydrocodone. Codeine and its metabolites are excreted almost entirely by the kidney, mainly as conjugates with glucuronic acid. Approximately 3% to 16% of a dose is eliminated unchanged in the urine. About 8% of people metabolise drugs poorly via CYP2D6, and are likely to obtain reduced benefit from codeine due to reduced formation of the active metabolite, morphine. The plasma half-life of codeine has been reported to be between 3 and 4 hours after oral administration. Doxylamine succinate: Doxylamine succinate is readily absorbed from the gastrointestinal tract. Following oral administration, the mean peak plasma concentration occurs after 2-3 hours. It has an elimination half-life of about 10 hours in healthy adults. It is excreted in the urine as unchanged doxylamine (60%) and metabolites (nordoxylamine and dinordoxylamine). The major metabolic site is the liver and major metabolic pathways are N-demethylation, N- oxidation, hydroxylation, N-acetylation, N-desalkylation and ether cleavage. INDICATIONS For the effective temporary relief of pain and discomfort associated with neck and back pain, headache, tension headache, migraine headache, toothache, muscle pain, neuralgia, arthritis, rheumatics, osteoarthritis, period pain, symptoms of cold and flu, sore throat, trauma, surgery, dental procedures, other pain where a combined calmative and analgesic action is required. Reduces fever. CONTRAINDICTIONS are contraindicated for use in patients with known hypersensitivity or idiosyncratic reaction to paracetamol, codeine, doxylamine succinate or substances of similar chemical structure or other opiates or other antihistamines of the ethanolamine class (or any of the other ingredients in the product). are also contraindicated for use in patients: with acute respiratory depression
4 Product Information Page 4 with chronic constipation during labour when delivery of a premature infant is anticipated as it may produce codeine withdrawal symptoms in the neonate with active alcoholism with diarrhoea caused by pseudomembranous colitis or poisoning (until the causative organism or toxin has been eliminated from the gastrointestinal tract, since codeine may slow down the elimination, thereby prolonging the diarrhoea). narrow-angle glaucoma stenosing peptic ulcer symptomatic prostatic hypertrophy bladder neck obstruction pyloroduodenal obstruction are contraindicated for use in: newborns or premature infants lactating women patients taking monoamine oxidase inhibitors (MAOIs) PRECAUTIONS Products containing codeine should not be given for prolonged periods as codeine may be habit forming. This medication may be dangerous when used in large amounts or for long periods. Hepatotoxicity may develop following a dose of paracetamol 10g and hepatic failure is known to occur occasionally with the long term use of paracetamol. Patients should be cautioned against the concomitant ingestion of alcohol and antihistamines. should be used with caution in patients with: impaired hepatic function impaired renal function epilepsy decreased respiratory reserve e.g. asthma or chronic obstructive pulmonary disease (COPD) pre-existing respiratory depression raised intracranial pressure or head injury prostatic hypertrophy
5 Product Information Page 5 hypotension hypothyroidism This medicine should also be used with caution in patients who: have a history of drug abuse are taking other respiratory depressants or sedatives, including alcohol have had recent gastrointestinal tract surgery Codeine present in this medicine may obscure the diagnosis or the course of gastrointestinal diseases. Codeine present in this medicine may produce physical and psychological dependence, if used for a prolonged period. may cause drowsiness and may increase the effects of alcohol. Drowsiness may continue the following day. Those affected should not drive or operate machinery; alcohol should be avoided. Impaired renal function: Paracetamol should be given with care to patients with impaired kidney function. Impaired hepatic function: Paracetamol should be given with care to patients with impaired liver function. Use in pregnancy: (Category A). Paracetamol, Codeine Phosphate and Doxylamine Succinate have been taken by a large number of pregnant women and women of child bearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the foetus having been observed. Use in lactation: APOHEALTH PARACETAMOL PLUS CODEINE & Calmative Tablet is not recommended for use by breastfeeding mothers as doxylamine, codeine and paracetamol are excreted in breast milk in small amounts. Effect on ability to drive or operate machinery: Both doxylamine succinate and codeine phosphate may cause drowsiness in some patients; therefore patients should be cautioned about operating vehicle or machinery, or engaging in activities that require them to be fully alert. INTERACTION WITH OTHER MEDICINES The following interactions have been noted:
6 Product Information Page 6 Interactions with Paracetamol: Coumarins: Repeated high doses of paracetamol increase the risk of bleeding in patients taking warfarin and other coumarin derivatives. Dosage reduction of anticoagulants, monitoring of coagulation and bleeding complications is required. Chloramphenicol: Paracetamol may slow down the excretion of chloramphenicol, causing the risk of increased toxicity. Cholestyramine: Cholestyramine reduces the absorption of paracetamol if given within one hour of paracetamol administration. Barbiturates and antiepileptic medications: The likelihood of paracetamol toxicity may be increased by the concomitant use of enzyme inducing agents such as alcohol, barbiturates or anti epileptic drugs. Probenecid: Paracetamol excretion may be affected and plasma concentrations altered when given with probenecid. Concomitant use of paracetamol and isoniazid, phenytoin, zidovudine or sulfinpyrazone has been reported to result in hepatotoxicity. Therefore, patients receiving isoniazid, phenytoin, zidovudine or sulfinpyrazone therapy should avoid large and/or chronic doses of paracetamol. Narcotic analgesics and drugs, which decrease gastric emptying, may decrease the absorption of paracetamol and vice versa. Drugs, which increase gastric emptying, may increase the absorption of paracetamol. Interactions with Codeine: Anticholinergics: Concomitant use of codeine and anticholinergic agents may increase the risk of severe constipation and/or urinary retention. Neuromuscular blocking agents: Codeine may enhance the effects of neuromuscular blocking agents resulting in increased respiratory depression. Antihypertensives: hypotensive effects may be potentiated when used concurrently with codeine and lead to orthostatic hypotension Antiperistaltic antidiarrhoeals (e.g. kaolin, pectin and loperamide): their concurrent use with codeine may increase the risk of severe constipation). Alcohol: Codeine may potentiate the effects of alcohol. Metoclopramide: codeine may antagonise the effects of metoclopramide on gastrointestinal motility. Monoamine oxidase inhibitors (MAOIs): concurrent administration or use within 14 days of ceasing MAOIs may enhance the potential respiratory depressant effects of codeine.
7 Product Information Page 7 Opioid analgesics: concurrent use of codeine and other opioid receptor antagonists is usually inappropriate as additives CNS depression, respiratory depression and hypotensive effects may occur. Substances that inhibit CYP2D6 such as quinidine, phenothiazines and antipsychotic agents can interfere with the metabolism of codeine to morphine, reducing the analgesic effect of codeine. Codeine may potentiate the effects of tranquillisers, sedatives, hypnotics, general anaesthetics and CNS depressants. Interactions with Doxylamine Succinate: Monoamine oxidase inhibitors (MAOIs): concurrent administration or use within 14 days of ceasing MAOIs may enhance or prolong the anticholinergic and CNS depressive effects of doxylamine succinate. ADVERSE EFFECTS Side effects with are infrequent. However, among those reported are anorexia, drowsiness, depression, dizziness, gastrointestinal discomfort (e.g. nausea and diarrhoea), dry mouth and, on rare occasions, rash. Paracetamol may occasionally cause skin reactions, and isolated cases of agranulocytosis and thrombocytopenic purpura have been reported. Doxylamine succinate may cause drowsiness in some individuals. Constipation may occur in association with codeine. Side effects of paracetamol are rare and usually mild, although haematological reactions have been reported. Skin rashes and hypersensitivity reactions occur occasionally. Overdosage with paracetamol, if left untreated, can result in severe, sometimes fatal liver damage and rarely, acute renal tubular necrosis. The most common adverse effects associated with codeine are nausea, vomiting, drowsiness, dizziness and constipation. Other side effects include: cough suppression, respiratory depression, euphoria, dysphoria, skin rashes, histamine release (hypotension, flushing of the face, tachycardia, breathlessness) and other allergic reactions. Central Nervous Systems (CNS) effects: CNS depressive effects of doxylamine succinate include sedation and impaired performance (impaired driving performance, poor work performance,
8 Product Information Page 8 incoordination, reduced motor skills, and impaired information processing). Performance may be impaired in the absence of sedation and may persist the morning after a night-time dose. CNS stimulatory effects of doxylamine succinate may include anxiety, hallucinations, appetite stimulation, muscle dyskinesias and activation of epileptogenic foci. High doses of doxylamine succinate may cause nervousness, tremor, insomnia, agitation, and irritability. Anticholinergic effects: Side effects of doxylamine succinate associated with cholinergic blockage include dryness of the eyes, mouth and nose, blurred vision, urinary hesitancy and retention, constipation and tachycardia. DOSAGE AND ADMINISTRATION Adults and children over 12 years: One or two tablets every four to six hours as needed for relief. Do not exceed eight tablets in a 24 hour period. For short term use only. This product is not recommended for use over long periods. Children under 12 years: Not recommended for use in children under 12 years. OVERDOSAGE In case of overdose, immediately contact the Poisons Information Centre (in Australia, call ; in New Zealand call ), or go to a hospital straight away even if you feel well because of the risk of delayed, serious liver damage with paracetamol. Paracetamol: It has been reported that paracetamol may produce symptoms of acute toxicity in adults following the ingestion of more than 15g. Hepatotoxicity may develop after the ingestion of a single dose of 10 to 15g (200 to 250 mg/kg) and a dose of more than 25g is potentially fatal. Patients may be asymptomatic for several days following ingestion of large doses of paracetamol and laboratory evidence of hepatotoxicity may be delayed for up to one week. Non-fatal hepatic damage is usually reversible. The antidote, N-acetylcysteine, should be administered as early as possible. Codeine: In an evaluation of codeine intoxication in children, symptoms ranked by decreasing order of frequency included sedation, rash, miosis, vomiting, itching, ataxia and swelling of the skin. Respiratory failure may occur. Blood concentrations of codeine ranged from 1.4 to 5.6 microgram/ml in eight adults whose deaths were attributed primarily to codeine overdosage. Doxylamine: Reactions associated with doxylamine overdosage may vary from CNS depression to stimulation. Stimulation is particularly likely in children. Insomnia, nervousness, euphoria, irritability, tremors, nightmares, hallucinations and convulsions can occur. Atropine-like signs and
9 Product Information Page 9 symptoms (e.g. dry mouth, fixed and dilated pupils, flushing and gastrointestinal symptoms) may also occur. PRESENTATION AND STORAGE CONDITIONS are, white, capsule shaped, biconvex, bevel edged uncoated tablets with break line on one side and plain on other side. This product is presented in blister pack sizes of 20 and 40 tablets. Store below 30 o C. NAME AND ADDRESS OF THE SPONSOR Apotex Pty Ltd 16 Giffnock Avenue Macquarie Park, NSW, 2113 POISON SCHEDULE OF THE MEDICINE Schedule 3 - Pharmacist Only Medicine Date of first inclusion in the Australian Register of Therapeutic Goods (the ARTG): 03/11/2014
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML)
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
PRODUCT INFORMATION Panadeine EXTRA
 PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION. Codral* Original Cold & Flu Tablets
 PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
Molecular formula: Molecular weight: C 8 H 9 NO 2 CAS Registry no.:
 Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
PANADOL SINUS DAY & NIGHT TABLETS PRODUCT INFORMATION
 Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION
 Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
PRODUCT INFORMATION. Ammonium chloride is an expectorant that has an irritant effect on mucous membranes.
 PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other.
 Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
Paracetamol, codeine phosphate hemihydrate and promethazine hydrochloride.
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION. Sudafed* Sinus + Allergy & Pain Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus + Allergy & Pain Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION Sudafed* Sinus + Allergy & Pain Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
Active ingredients: Paracetamol Codeine phosphate. Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
 Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION
 RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
PRODUCT INFORMATION PANADEINE FORTE
 PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS
 PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE. Chemical structure: CAS 2 Registry Number: DESCRIPTION
 PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
PANADOL EXTRA PRODUCT INFORMATION
 PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
NEW ZEALAND DATASHEET
 NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
NEW ZEALAND DATA SHEET. Each Paracetamol + Codeine contains 500 mg of paracetamol and 8 mg of codeine phosphate.
 NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
APO-PARACETAMOL/CODEINE 500/30
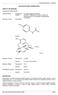 APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
PRODUCT INFORMATION PRODEINE 15 H 3 CO. Paracetamol MW Codeine phosphate MW
 PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
PRODUCT INFORMATION. Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets
 PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION. Benadryl* for the Family Nightime Oral Liquid
 Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
PRODUCT INFORMATION. Codral* Original Cold & Flu + Cough Day & Night Capsules
 PRODUCT INFORMATION Codral* Original Cold & Flu + Cough Day & Night Capsules Description Codral* Original Cold & Flu + Cough Day & Night capsules contain two separate formulations: day capsules and night
PRODUCT INFORMATION Codral* Original Cold & Flu + Cough Day & Night Capsules Description Codral* Original Cold & Flu + Cough Day & Night capsules contain two separate formulations: day capsules and night
DATA SHEET. PANADOL Mini Caps Capsule shaped tablet with a gelatin coating which is one half green and the other half white.
 PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
Your Pharmacy Paracetamol Plus, capsule shaped tablets (caplets)
 New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
PRODUCT INFORMATION. Parke Davis* Day & Night Original Cough Cold & Flu Capsules
 PRODUCT INFORMATION Parke Davis* Day & Night Original Cough Cold & Flu Capsules Product description Parke Davis* Day & Night Original Cough Cold & Flu capsules contain two separate formulations: day capsules
PRODUCT INFORMATION Parke Davis* Day & Night Original Cough Cold & Flu Capsules Product description Parke Davis* Day & Night Original Cough Cold & Flu capsules contain two separate formulations: day capsules
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution DATA SHEET
 PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
APOHEALTH Cold & Flu Relief Day/Night Tablets
 Day/Night Tablets Contains the active ingredients Paracetamol, Pseudoephedrine hydrochloride, Codeine phosphate and Chlorpheniramine maleate Consumer Medicine Information For a copy of a large print leaflet,
Day/Night Tablets Contains the active ingredients Paracetamol, Pseudoephedrine hydrochloride, Codeine phosphate and Chlorpheniramine maleate Consumer Medicine Information For a copy of a large print leaflet,
Migraleve, Migraleve Pink and Migraleve Yellow Product Information
 Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
Each 5ml of Sinarest LP New Syrup contains: Phenylephrine
 Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES
 PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
NEW ZEALAND DATA SHEET ACUPAN TM. 3. PHARMACEUTICAL FORM White, round, biconvex, film-coated tablets (7 mm diameter) engraved APN on one face.
 1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
PRODUCT INFORMATION ACTACODE
 PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
Chemists Own Strong Pain Extra Paracetamol and Codeine Phosphate
 Chemists Own Strong Pain Extra Paracetamol and Codeine Phosphate Consumer Medicine Information(CMI) Please read this information before you start taking this medicine. What is in this CMI? This CMI answers
Chemists Own Strong Pain Extra Paracetamol and Codeine Phosphate Consumer Medicine Information(CMI) Please read this information before you start taking this medicine. What is in this CMI? This CMI answers
2. QUALITATIVE AND QUANTITATIVE COMPOSITION. Each capsule contains PARACETAMOL 500mg For a full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
PANADOL OSTEO PRODUCT INFORMATION
 PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules.
 Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30
 PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
APO-Paracetamol/Codeine 500/30 Contains the active ingredients, paracetamol and codeine phosphate
 APO-Paracetamol/Codeine 500/30 Contains the active ingredients, paracetamol and codeine phosphate Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
APO-Paracetamol/Codeine 500/30 Contains the active ingredients, paracetamol and codeine phosphate Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
Metabolism Paracetamol is metabolised in the liver and excreted in the urine mainly as glucuronide and sulphate conjugates.
 FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
PHARMACY ACTION COLD & FLU DAY & NIGHT paracetamol, pseudoephedrine hydrochloride, chlorphenamine maleate
 CONSUMER MEDICINE INFORMATION PHARMACY ACTION COLD & FLU DAY & NIGHT paracetamol, pseudoephedrine hydrochloride, chlorphenamine maleate What is in this leaflet This leaflet answers some common questions
CONSUMER MEDICINE INFORMATION PHARMACY ACTION COLD & FLU DAY & NIGHT paracetamol, pseudoephedrine hydrochloride, chlorphenamine maleate What is in this leaflet This leaflet answers some common questions
DATA SHEET. Paracetamol (BP) 500 mg and Phenylephrine Hydrochloride (BP) 5 mg
 COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
PRODUCT INFORMATION. AKINETON (biperiden hydrochloride 2 mg tablets)
 PRODUCT INFORMATION AKINETON (biperiden hydrochloride 2 mg tablets) NAME OF THE MEDICINE Non-proprietary name: biperiden hydrochloride Structural formula (biperiden): Chemical name (biperiden): α-5-norbornen-2-yl-α-phenyl-1-piperidine
PRODUCT INFORMATION AKINETON (biperiden hydrochloride 2 mg tablets) NAME OF THE MEDICINE Non-proprietary name: biperiden hydrochloride Structural formula (biperiden): Chemical name (biperiden): α-5-norbornen-2-yl-α-phenyl-1-piperidine
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS
 AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Codral Original Day & Night Cold & Flu Tablets Paracetamol, Pseudoephedrine hydrochloride, Codeine phosphate, Triprolidine hydrochloride
 Codral Original Day & Night Cold & Flu Tablets Paracetamol, Pseudoephedrine hydrochloride, Codeine phosphate, Triprolidine hydrochloride Consumer Medicine Information What is in this leaflet This leaflet
Codral Original Day & Night Cold & Flu Tablets Paracetamol, Pseudoephedrine hydrochloride, Codeine phosphate, Triprolidine hydrochloride Consumer Medicine Information What is in this leaflet This leaflet
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
Codral Original Day & Night Cold & Flu Tablets
 & Flu Tablets Paracetamol, Pseudoephedrine hydrochloride, Codeine phosphate, Triprolidine hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions
& Flu Tablets Paracetamol, Pseudoephedrine hydrochloride, Codeine phosphate, Triprolidine hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions
PRODUCT INFORMATION LOFENOXAL
 PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
Before you use DEMAZIN Day & Night Cold & Flu. What is in this leaflet. What DEMAZIN Day & Night Cold & Flu is used for. Consumer Medicine Information
 Tablets Day tablets: Pseudoephedrine hydrochloride, paracetamol and codeine phosphate; Night tablets: Pseudoephedrine hydrochloride, paracetamol and chlorpheniramine maleate Consumer Medicine Information
Tablets Day tablets: Pseudoephedrine hydrochloride, paracetamol and codeine phosphate; Night tablets: Pseudoephedrine hydrochloride, paracetamol and chlorpheniramine maleate Consumer Medicine Information
Mersyndol Forte Paracetamol, codeine phosphate hemihydrate, doxylamine succinate
 Mersyndol Forte Paracetamol, codeine phosphate hemihydrate, doxylamine succinate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Mersyndol Forte.
Mersyndol Forte Paracetamol, codeine phosphate hemihydrate, doxylamine succinate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Mersyndol Forte.
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
Dexchlorpheniramine maleate (CAS no ) is described chemically as (+)-2-[pchloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine
![Dexchlorpheniramine maleate (CAS no ) is described chemically as (+)-2-[pchloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine Dexchlorpheniramine maleate (CAS no ) is described chemically as (+)-2-[pchloro-α-[2-(dimethylamino)ethyl]benzyl]pyridine](/thumbs/76/74308383.jpg) PRODUCT INFORMATION NAME OF THE MEDICINE POLARAMINE (dexchlorpheniramine maleate) DESCRIPTION Polaramine (dexchlorpheniramine maleate) is the dextro-isomer of chlorpheniramine maleate. It is an antihistamine
PRODUCT INFORMATION NAME OF THE MEDICINE POLARAMINE (dexchlorpheniramine maleate) DESCRIPTION Polaramine (dexchlorpheniramine maleate) is the dextro-isomer of chlorpheniramine maleate. It is an antihistamine
Data Sheet. Phenergan
 Data Sheet Phenergan Name of Medicine Phenergan Tablets Phenergan Elixir Non-proprietary Name Promethazine hydrochloride Chemical Structure Promethazine hydrochloride has the following structural formula:
Data Sheet Phenergan Name of Medicine Phenergan Tablets Phenergan Elixir Non-proprietary Name Promethazine hydrochloride Chemical Structure Promethazine hydrochloride has the following structural formula:
CONSUMER MEDICINE INFORMATION Please read this information before you start using this medicine.
 Panadol Sinus Pain & Congestion Relief Day & Night Caplets Day caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride; Night caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride / 2 mg
Panadol Sinus Pain & Congestion Relief Day & Night Caplets Day caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride; Night caplet: 500 mg Paracetamol / 5 mg Phenylephrine hydrochloride / 2 mg
TRIPROLIDINE. Please read this leaflet and the packaging of the medicine you purchased, carefully before you start using triprolidine.
 TRIPROLIDINE New Zealand Consumer Medicine Information What is in this leaflet The medicine you have purchased contains triprolidine. This leaflet is intended to provide information on the active ingredient
TRIPROLIDINE New Zealand Consumer Medicine Information What is in this leaflet The medicine you have purchased contains triprolidine. This leaflet is intended to provide information on the active ingredient
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES
 PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF DAY & NIGHT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF DAY & NIGHT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
PRODEINE paracetamol and codeine phosphate hemihydrate
 PRODEINE paracetamol and codeine phosphate hemihydrate Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about Prodeine. It does not, however, contain
PRODEINE paracetamol and codeine phosphate hemihydrate Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about Prodeine. It does not, however, contain
PRODUCT INFORMATION LEAFLET
 PRODUCT INFORMATION LEAFLET 1. Product Name Brand Name: Crocin Cold & Flu Max Generic Name: Paracetamol, Caffeine and Phenylephrine Hydrochloride Tablets 2. Qualitative & Quantitative Composition Each
PRODUCT INFORMATION LEAFLET 1. Product Name Brand Name: Crocin Cold & Flu Max Generic Name: Paracetamol, Caffeine and Phenylephrine Hydrochloride Tablets 2. Qualitative & Quantitative Composition Each
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
PRODUCT INFORMATION PRODEINE FORTE H 3 CO. Paracetamol MW Codeine phosphate hemihydrate MW
 PRODUCT INFORMATION PRODEINE FORTE NAME OF THE MEDICINE Australian Approved Name Paracetamol and codeine phosphate hemihydrate Chemical Structure H N CH 3 HO O H.H 3 PO 4. 1/2 H 2 0 NH CH 3 H 3 CO Paracetamol
PRODUCT INFORMATION PRODEINE FORTE NAME OF THE MEDICINE Australian Approved Name Paracetamol and codeine phosphate hemihydrate Chemical Structure H N CH 3 HO O H.H 3 PO 4. 1/2 H 2 0 NH CH 3 H 3 CO Paracetamol
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL COLD & FLU MAX + DECONGESTANT HOT LEMON POWDER NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
PRODUCT INFORMATION PANADOL COLD & FLU MAX + DECONGESTANT HOT LEMON POWDER NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 Phenylephrine Hydrochloride
APOHEALTH Diarrhoea Relief PLUS
 APOHEALTH Diarrhoea Relief PLUS Chewable Tablets Contains the active ingredient Loperamide hydrochloride and Simethicone Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195
APOHEALTH Diarrhoea Relief PLUS Chewable Tablets Contains the active ingredient Loperamide hydrochloride and Simethicone Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195
CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET. Proprietary (Trade) Name: PANADOL
 CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 120 mg/5ml (Children
CHILDREN S PANADOL COLOURFREE SUSPENSION PANADOL SUPPOSITORIES 125 MG PANADOL SUPPOSITORIES 250 MG DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 120 mg/5ml (Children
Maxilief Effervescent Tablets
 Package Leaflet: Information for the user Maxilief Effervescent Tablets Paracetamol Codeine Phosphate Hemihydrate Caffeine 500mg 8mg 30mg Read all of this leaflet carefully before you start taking this
Package Leaflet: Information for the user Maxilief Effervescent Tablets Paracetamol Codeine Phosphate Hemihydrate Caffeine 500mg 8mg 30mg Read all of this leaflet carefully before you start taking this
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
1 NAME OF THE MEDICINAL PRODUCT Tipol 75mg Suppositories Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 75mg of paracetamol For a full list of excipients,
NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%.
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NAPHCON-A Eye Drops naphazoline hydrochloride 0.025% and pheniramine maleate 0.3%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Naphcon-A contains naphazoline hydrochloride
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Codeine Phosphate Tablets (PSM) 15 mg, 30 mg & 60 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Codeine Phosphate Hemihydrate BP 15 mg
1. PRODUCT NAME Codeine Phosphate Tablets (PSM) 15 mg, 30 mg & 60 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Codeine Phosphate Hemihydrate BP 15 mg
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Paracetamol 80mg Suppositories 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each suppository contains 80mg Paracetamol For a full list of
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
Package leaflet: Information for the patient
 Package leaflet: Information for the patient Triflu Paracetamol, Ascorbic Acid, Pheniramine Maleate 500 mg/200 mg/ 25 mg granules for oral solution in sachet Read all of this leaflet carefully before you
Package leaflet: Information for the patient Triflu Paracetamol, Ascorbic Acid, Pheniramine Maleate 500 mg/200 mg/ 25 mg granules for oral solution in sachet Read all of this leaflet carefully before you
MIDAZOLAM APOTEX Solution for Injection Contains the active ingredient midazolam
 MIDAZOLAM APOTEX Solution for Injection Contains the active ingredient midazolam Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read this leaflet
MIDAZOLAM APOTEX Solution for Injection Contains the active ingredient midazolam Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read this leaflet
New Zealand Data Sheet
 New Zealand Data Sheet POLARAMINE Tablets, film-coated dexchlorpheniramine maleate 2 mg Syrup - dexchlorpheniramine maleate 2 mg/5 ml (0.4 mg/ml) 1. Name of the Medicinal Product Polaramine (dexchlorpheniramine
New Zealand Data Sheet POLARAMINE Tablets, film-coated dexchlorpheniramine maleate 2 mg Syrup - dexchlorpheniramine maleate 2 mg/5 ml (0.4 mg/ml) 1. Name of the Medicinal Product Polaramine (dexchlorpheniramine
SUMMARY OF PRODUCT CHARACTERISTICS FOR BENZODIAZEPINES AS ANXIOLYTICS OR HYPNOTICS
 SUMMARY OF PRODUCT CHARACTERISTICS FOR BENZODIAZEPINES AS ANXIOLYTICS OR HYPNOTICS Guideline Title Summary of Product Characteristics for Benzodiazepines as Anxiolytics or Hypnotics Legislative basis Directive
SUMMARY OF PRODUCT CHARACTERISTICS FOR BENZODIAZEPINES AS ANXIOLYTICS OR HYPNOTICS Guideline Title Summary of Product Characteristics for Benzodiazepines as Anxiolytics or Hypnotics Legislative basis Directive
PANADOL Night Caplets 500mg Paracetamol & 25 mg Diphenhydramine Hydrochloride
 PANADOL Night Caplets 500mg Paracetamol & 25 mg Diphenhydramine Hydrochloride Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about PANADOL Night. It does
PANADOL Night Caplets 500mg Paracetamol & 25 mg Diphenhydramine Hydrochloride Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about PANADOL Night. It does
AVIOMARIN 50 mg tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER AVIOMARIN 50 mg tablets DIMENHYDRINATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER AVIOMARIN 50 mg tablets DIMENHYDRINATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PRODUCT INFORMATION H 3 CO. paracetamol MW codeine phosphate hemihydrate MW
 RODUCT INFORMATION RODEINE NAME OF THE MEDICINE Non-proprietary Name aracetamol, codeine phosphate hemihydrate Chemical Structure H N CH 3 HO O H.H 3 O 4. 1/2 H 2 0 NH CH 3 H 3 CO paracetamol MW 151.17
RODUCT INFORMATION RODEINE NAME OF THE MEDICINE Non-proprietary Name aracetamol, codeine phosphate hemihydrate Chemical Structure H N CH 3 HO O H.H 3 O 4. 1/2 H 2 0 NH CH 3 H 3 CO paracetamol MW 151.17
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less. For pack sizes of more than 24 tablets
 SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
Excipients: water d / and. 1 ml ampoule (5) packings Valium planimetric (1) packs cardboard.
 SUPRASTIN Active material: Xloropiramin When ATH: R06AC03 CCF: Gistaminovыh blocker H 1 -receptors. Allergy medication ICD-10 codes (testimony): H10.1, J06.9, J30.1, J30.3, L20.8, L23, L24, L29, L30.0,
SUPRASTIN Active material: Xloropiramin When ATH: R06AC03 CCF: Gistaminovыh blocker H 1 -receptors. Allergy medication ICD-10 codes (testimony): H10.1, J06.9, J30.1, J30.3, L20.8, L23, L24, L29, L30.0,
LEAFLET: INFORMATION FOR THE USER. Freshalgin 500th mg tablets Metamizole sodium (M etamizol sodium)
 LEAFLET: INFORMATION FOR THE USER Freshalgin 500th mg tablets Metamizole sodium (M etamizol sodium) Read this leaflet carefully before you start taking this medicine. This medicine is available without
LEAFLET: INFORMATION FOR THE USER Freshalgin 500th mg tablets Metamizole sodium (M etamizol sodium) Read this leaflet carefully before you start taking this medicine. This medicine is available without
Package leaflet: Information for the user CODEINE PHOSPHATE TABLETS BP 15mg and 30mg (Codeine Phosphate)
 Package leaflet: Information for the user CODEINE PHOSPHATE TABLETS BP 15mg and 30mg (Codeine Phosphate) Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the user CODEINE PHOSPHATE TABLETS BP 15mg and 30mg (Codeine Phosphate) Read all of this leaflet carefully before you start taking this medicine because it contains important
Core Safety Profile. Pharmaceutical form(s)/strength: Tablets 5 mg and 10 mg BE/H/PSUR/0002/002 Date of FAR:
 Core Safety Profile Active substance: Clotiazepam Pharmaceutical form(s)/strength: Tablets 5 mg and 10 mg P-RMS: BE/H/PSUR/0002/002 Date of FAR: 16.06.2011 4.3 Contraindications is contraindicated
Core Safety Profile Active substance: Clotiazepam Pharmaceutical form(s)/strength: Tablets 5 mg and 10 mg P-RMS: BE/H/PSUR/0002/002 Date of FAR: 16.06.2011 4.3 Contraindications is contraindicated
PRESCRIBING INFORMATION. N MERSYNDOL with codeine (acetaminophen 325 mg, codeine phosphate 8 mg and doxylamine succinate 5 mg)
 PRESCRIBING INFORMATION N MERSYNDOL with codeine (acetaminophen 325 mg, codeine phosphate 8 mg and doxylamine succinate 5 mg) Analgesic, Antihistamine sanofi-aventis Canada Inc. 2905 Place Louis R.-Renaud
PRESCRIBING INFORMATION N MERSYNDOL with codeine (acetaminophen 325 mg, codeine phosphate 8 mg and doxylamine succinate 5 mg) Analgesic, Antihistamine sanofi-aventis Canada Inc. 2905 Place Louis R.-Renaud
European PSUR Work Sharing Project CORE SAFETY PROFILE. Lendormin, 0.25mg, tablets Brotizolam
 European PSUR Work Sharing Project CORE SAFETY PROFILE Lendormin, 0.25mg, tablets Brotizolam 4.2 Posology and method of administration Unless otherwise prescribed by the physician, the following dosages
European PSUR Work Sharing Project CORE SAFETY PROFILE Lendormin, 0.25mg, tablets Brotizolam 4.2 Posology and method of administration Unless otherwise prescribed by the physician, the following dosages
PATIENT INFORMATION LEAFLET
 PPM Code: E1981 PATIENT INFORMATION LEAFLET SCHEDULING STATUS: S2 PROPRIETARY NAME DOSAGE FORM: film coated tablets Read all of this leaflet carefully because it contains important information for you.
PPM Code: E1981 PATIENT INFORMATION LEAFLET SCHEDULING STATUS: S2 PROPRIETARY NAME DOSAGE FORM: film coated tablets Read all of this leaflet carefully because it contains important information for you.
Ausfam PRODUCT INFORMATION NAME OF THE MEDICINE DESCRIPTION PHARMACOLOGY
 Ausfam PRODUCT INFORMATION NAME OF THE MEDICINE Famotidine. The chemical name for famotidine is N 2 -(aminosulphonyl)-3-[[[2-[(diamino methylene)amino]thiazol-4yl]methyl]thio]propanamidine. Its structural
Ausfam PRODUCT INFORMATION NAME OF THE MEDICINE Famotidine. The chemical name for famotidine is N 2 -(aminosulphonyl)-3-[[[2-[(diamino methylene)amino]thiazol-4yl]methyl]thio]propanamidine. Its structural
PRODUCT INFORMATION ANAGRAINE. Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol.
 PRODUCT INFORMATION ANAGRAINE COMPOSITION Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol. ACTIONS Metoclopramide stimulates gastrointestinal tract motility and accelerates gastric
PRODUCT INFORMATION ANAGRAINE COMPOSITION Each ANAGRAINE tablet contains 5 mg metoclopramide and 500 mg paracetamol. ACTIONS Metoclopramide stimulates gastrointestinal tract motility and accelerates gastric
Panafen Plus PRODUCT INFORMATION
 Panafen Plus PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Ibuprofen Codeine phosphate Ibuprofen Chemical structure: Chemical name: 2-(4-isobutylphenyl) propionic acid Molecular formula:
Panafen Plus PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Ibuprofen Codeine phosphate Ibuprofen Chemical structure: Chemical name: 2-(4-isobutylphenyl) propionic acid Molecular formula:
NOTOPAIN CAPLETS. Diclofenac Sodium + Paracetamol. Composition. Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg.
 NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
