Stimulants such as cocaine and opioid agonists such as heroin are among the most widely abused illicit drugs
|
|
|
- Annabelle Maxwell
- 6 years ago
- Views:
Transcription
1 Effects of Mu Opioid Agonists Alone and in Combination with Cocaine and D-Amphetamine in Rhesus Monkeys Trained to Discriminate Cocaine S. Stevens Negus, Ph.D., Michael B. Gatch, Ph.D., and Nancy K. Mello, Ph.D. Psychomotor stimulants and mu opioid agonists are often used together by polydrug abusers, and it has been suggested that this form of polydrug abuse may result from the ability of stimulants and mu agonists to enhance each other s abuse-related effects. To investigate this possibility, the present study examined stimulant-opioid interactions in rhesus monkeys trained to discriminate cocaine. Specifically, the effects of the mu opioid agonists heroin, alfentanil, fentanyl, and morphine administered alone or in combination with cocaine or d-amphetamine were examined in five monkeys trained to discriminate 0.4 mg/kg cocaine (IM) from saline in a two-lever, food-reinforced drug discrimination procedure. When administered alone, the rapid onset mu agonists heroin ( mg/kg) and alfentanil ( mg/kg) substituted completely for cocaine in three of five monkeys but produced primarily saline-appropriate responding in the other two monkeys. The slower onset mu agonists fentanyl ( mg/kg) and morphine ( mg/kg) substituted for cocaine in only one of five monkeys. When administered as pretreatments to cocaine, morphine and fentanyl increased levels of cocaine-appropriate responding produced by low doses of cocaine in some monkeys. Morphine pretreatment also increased levels of cocaine-appropriate responding produced by low doses of amphetamine in some monkeys. However, in other monkeys, morphine and fentanyl pretreatment did not alter the discriminative stimulus effects of cocaine or amphetamine. These results indicate that there are substantial individual difference in the effects of mu agonists in cocaine-discriminating rhesus monkeys. In some monkeys, mu agonists mimic or enhance the discriminative stimulus of cocaine, whereas in other monkeys, mu agonists neither mimic nor enhance the effects of stimulants. [Neuropsychopharmacology 18: , 1998] 1998 American College of Neuropsychopharmacology. Published by Elsevier Science Inc. KEY WORDS: Cocaine; D-Amphetamine; Heroin; Alfentanil; Fentanyl; Morphine; Drug discrimination; Rhesus monkeys Stimulants such as cocaine and opioid agonists such as heroin are among the most widely abused illicit drugs From the Alcohol and Drug Abuse Research Center, McLean Hospital, Harvard Medical School, Belmont, Massachusetts. Address correspondence to: S. Stevens Negus, Ph.D., Alcohol and Drug Abuse Research Center, McLean Hospital, Harvard Medical School, 115 Mill Street, Belmont, MA Received April 18, 1997; revised August 8, 1997; accepted August 18, in the United States, and these drugs are increasingly used together in a drug combination known as speedball (Substance Abuse and Mental Health Services Administration 1996a,b). Although the use and abuse of stimulant-opioid combinations could result simply from the coincident availability of both drugs, stimulantopioid combinations may also produce interacting effects that contribute to enhanced abuse potential. Some drug users have reported that stimulant-opioid combinations produce greater euphoric effects than either drug alone or that use of stimulants and opioids in combination ameliorates the undesirable effects of each NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO American College of Neuropsychopharmacology Published by Elsevier Science Inc X/98/$ Avenue of the Americas, New York, NY PII S X(97)
2 326 S.S. Negus et al. NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 drug (Brecher 1972; Kosten et al. 1986, 1987). In addition to these anecdotal reports, controlled laboratory studies in humans have found that stimulant-opioid combinations produce subjective effects that may differ quantitatively or qualitatively from the effects produced by either drug alone (Foltin and Fischman 1992; Walsh et al. 1996; Foltin et al. 1995; Preston et al. 1996). The relatively high incidence of speedball abuse has also stimulated preclinical research designed to investigate possible behavioral interactions between stimulants and opioids. For example, we recently examined the effects of heroin administered alone or in combination with cocaine in rhesus monkeys trained to discriminate cocaine from saline (Mello et al. 1995). When heroin was administered alone, it produced a dose-dependent and complete substitution for cocaine in three of the five monkeys tested. In the other two monkeys, heroin produced primarily saline-appropriate responding up to doses that eliminated responding. Thus, there was considerable individual variability in the effects of heroin in these monkeys. When heroin was administered as a pretreatment to cocaine, doses of heroin that did not produce cocaine-like effects usually did not alter the discriminative stimulus effects of cocaine. Higher pretreatment doses of heroin usually produced levels of cocaine-appropriate responding similar to those produced by heroin alone. Taken together, these results suggested that heroin and cocaine produced similar discriminative stimulus effects in at least some monkeys, but that pretreatments with heroin did not consistently enhance the discriminative stimulus effects of cocaine. The effects of heroin administered alone or in combination with cocaine were antagonized by the mu opioid receptor-selective antagonist quadazocine, suggesting that these effects of heroin were mediated by mu opioid receptors (Mello et al. 1995). Similarly, other studies have also suggested that the effects of heroin in rhesus monkeys are mediated primarily by mu opioid receptors (Bertalmio et al. 1992). However, heroin differs from most other mu opioid agonists in at least two respects. First, heroin is a highly lipophilic drug that rapidly crosses the blood-brain barrier and that consequently has a rapid rate of onset following systemic administration (Oldendorf et al. 1972; Hartvig et al. 1984). Second, heroin has a relatively low affinity for opioid receptors that does not correlate with its relatively high potency in vivo (Inturrisi et al. 1983; Bertalmio et al. 1992). For example, heroin was reported to be 10-fold less potent than morphine in competing for tritiated [D-Ala 2, NMePhe 4, Gly 5-01]-enkephalin (DAMGO) binding in rhesus monkey brain, but 10-fold more potent than morphine in a drug discrimination assay in monkeys (Bertalmio et al. 1992). Findings such as these have led to the hypothesis that heroin produces its opioid agonist effects indirectly by acting as a prodrug that is deacetylated to form the active metabolites monoacetyl morphine and morphine (Way et al. 1960; Inturrisi et al. 1983, 1984; Bertalmio et al. 1992). As a result of heroin s distinctive attributes, it is possible that the effects of heroin in cocaine discriminating monkeys could differ from the effects of other mu agonists. To investigate this possibility, one goal of the present study was to compare the effects of heroin with the effects of the other opioid agonists morphine, fentanyl and alfentanil. As with heroin, the behavioral effects of morphine, fentanyl, and alfentanil are thought to be mediated primarily by mu opioid receptors in rhesus monkeys (Dykstra et al. 1987; Walker et al. 1993; Emmerson et al. 1994; Gerak et al. 1994). In addition, these mu agonists have different rates of onset (e.g., Bertalmio and Woods 1987) and may provide insight into the role of rate of onset in determining the effects of mu agonists in cocaine-discriminating monkeys. To verify differences in rate of onset of these mu agonists for the purposes of the present study, the time course of each agonist was examined in an assay of thermal antinociception in which mu agonists produce robust and reliable antinociceptive effects (Dykstra et al. 1987). A second goal of this study was to examine the ability of morphine to enhance the cocaine-like stimulus effects of another psychomotor stimulant, d-amphetamine. Cocaine and amphetamine share discriminative stimulus effects in animals (e.g., Colpaert et al. 1979; de la Garza and Johanson 1983, 1985) and subjective effects in humans (Fischman et al. 1976), and amphetamine may be used instead of cocaine as the stimulant component in speedball abuse (Kramer et al. 1967: Langrod 1970; Brecher 1972). However, the effects of mu opioid agonists on the discriminative stimulus effects of amphetamine in rhesus monkeys are unknown. Subjects METHODS Five male rhesus monkeys (Macaca mulatta) were studied in the drug discrimination experiments, and these were the same monkeys that were used in our previous study of the effects of heroin in cocaine-discriminating monkeys (Mello et al. 1995). Two female and two male rhesus monkeys were studied in the warm water tailwithdrawal experiments. All monkeys had an experimental history involving the evaluation of monoaminergic and/or opioid compounds. Monkeys weighed kg and were maintained on a diet of fresh fruit, vegetables, and Lab Diet Jumbo Monkey biscuits (PMI Feeds, Inc., St. Louis, MO). In addition, monkeys in the cocaine discrimination experiments could receive 1-g banana pellets (P.J. Noyes Co., Lancaster, NH) during daily operant sessions. Water was continuously available, and a 12-h light-dark cycle was in effect (lights on from 7 A.M. to 7 P.M.).
3 NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 Mu Opioids and Cocaine Discrimination 327 Animal maintenance and research were conducted in accordance with the guidelines provided by the National Institutes of Health (NIH) Committee on Laboratory Animal Resources. The facility was licensed by the U.S. Department of Agriculture, and protocols were approved by the Institutional Animal Care and Use Committee. The health of the monkeys was periodically monitored by consulting veterinarians. Monkeys had visual, auditory, and olfactory contact with other monkeys throughout the study. Monkeys also had access to puzzle feeders, mirrors, and chew toys to provide environmental enrichment. Operant procedures provided an opportunity for environmental manipulation and enrichment in the cocaine discrimination monkeys (Line et al. 1989). Drug Discrimination Procedures Apparatus. Drug discrimination procedures were identical to those used in our previous studies (e.g., Mello et al. 1995). Each monkey was housed individually in a well-ventilated, stainless steel chamber ( cm). The home cages of all monkeys were modified to include an operant panel (28 28 cm) mounted on the front wall. Three square translucent response keys ( cm) were arranged 2.54 cm apart in a horizontal row 3.2 cm from the top of the operant panel. Each key could be transilluminated by red or green stimulus lights (Superbright LEDs). The operant panel also supported an externally mounted pellet dispenser (Gerbrands, Model G5210, Arlington, MA) that delivered 1 g fruit-flavored food pellets to a food receptacle mounted on the cage beneath the operant response panel. Operation of the operant panels and data collection were accomplished with Apple IIGS computers located in a separate room. Discrimination Training. Discrimination sessions consisted of multiple cycles and were conducted 5 days per week. Each cycle consisted of a 15-min time-out period followed by a 5-min response period. During the timeout period, all stimulus lights were off, and responding had no scheduled consequences. During the response period, the right and left response keys were transilluminated red or green, and monkeys could receive up to 10 food pellets by responding under a fixed ratio (FR) 30 schedule of food presentation. For two of the five monkeys, the left key was illuminated green and the right key was illuminated red. For the other three monkeys, the colors of the response keys were reversed. The center key was not illuminated at any time, and responding on the center key had no scheduled consequences. If all available food pellets were delivered before the end of the 5-min response period, the stimulus lights transilluminating the response keys were turned off, and responding had no scheduled consequences for the remainder of that response period. On training days, monkeys were given an IM injection of either saline or 0.40 mg/kg cocaine 5 min after the beginning of each time-out period (i.e., 10 min before the response period). After the administration of saline, responding on only the green key (the salineappropriate key) produced food, whereas following administration of 0.40 mg/kg cocaine, only responding on the red key (the drug-appropriate key) produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. Sessions consisted of one to five cycles each day, and if the training dose of cocaine was administered, it was administered only during the last cycle. During the response period of each cycle, three dependent variables were determined: (1) percent injection-appropriate responding prior to delivery of the first reinforcer [(injection-appropriate responses emitted prior to 1st reinforcer total responses emitted prior to delivery of 1st reinforcer) 100]; (2) percent injection-appropriate responding for the entire response period [(injection-appropriate responses emitted during response period total responses emitted during response period) 100]; and (3) response rate (total responses emitted during response period total time stimulus lights were illuminated). Monkeys were considered to have acquired cocaine discrimination when the following three criteria were met for seven of eight consecutive training session: (1) the percent injection-appropriate responding prior to delivery of the first reinforcer was greater than or equal to 80% for all cycles; (2) the percent injection-appropriate responding for the entire cycle was greater than or equal to 90% for all cycles; (3) response rates during saline training cycles were greater than 1.0 response per second. Discrimination Testing. Once monkeys met criterion levels of cocaine discrimination, testing began. Test sessions were identical to training sessions except that responding on either key produced food, and test compounds were administered as described below. Three series of experiments were conducted to characterize the effects of mu opioid agonists in these monkeys. The first series of experiments examined the effects of cocaine, the other central nervous system (CNS) stimulant d-amphetamine, and the mu opioid agonists heroin, alfentanil, fentanyl, and morphine administered alone. Monkeys received an injection 5 min after the beginning of each cycle of a multiple cycle session, and each injection increased the total, cumulative dose of the test drug by 1/4 or 1/2 log units. Each drug was studied in each monkey up to doses that either substituted completely for the cocaine training stimulus (i.e., produced 90% cocaine-appropriate responding for the entire cycle) or that decreased responding to 0.1 responses/s. Initially, all substitution studies were conducted using standard cycles consisting of a 15-min time-out period followed by
4 328 S.S. Negus et al. NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 a 5-min response period, and test compounds were administered 10 min before each response period (i.e., a 10-min pretreatment interval). However, parallel studies using a warm-water tail-withdrawal assay of antinociception (see below) indicated that IM administered fentanyl and morphine had relatively slow rates of onset, suggesting that longer pretreatment intervals may have been required to allow these mu agonists to reach peak effect. Consequently, both fentanyl and morphine were also studied using longer pretreatment intervals designed to assure that response periods began at the time of peak drug effect. Specifically, fentanyl was studied using 16 min pretreatment intervals, and morphine was studied using 32-min pretreatment intervals. Only four monkeys were studied using these longer pretreatment intervals, because one monkey died of unrelated causes before these experiments could be conducted. Since morphine and fentanyl did not consistently substitute for cocaine, a second series of experiments examined the effects of morphine and fentanyl on the cumulative cocaine dose-effect curve. Both morphine and fentanyl were administered 20 min prior to the administration of the first dose of cocaine (i.e., 30 min prior to the first response period). In addition, the effects of pretreatment with both the opioid antagonist naltrexone and morphine on the cocaine dose-effect curve were determined. In these experiments, naltrexone was administered 15 min before morphine, and morphine was administered 20 min before the first dose of cocaine. A third series of experiments examined the effects of morphine pretreatment on the cumulative amphetamine dose-effect curve. Morphine doses were administered 20 min before the first dose of amphetamine. Training sessions were usually conducted on Mondays, Wednesdays, and Thursdays, and test sessions were conducted on Tuesdays and Fridays. Test sessions were conducted only if the three criteria listed above under criteria for discrimination were met during the training day immediately preceding the test day. If responding did not meet criterion levels of discrimination performance, then training was continued until criterion levels of performance were obtained for at least 2 consecutive days. Data Analysis. Individual subject graphs of the percent cocaine-appropriate responding (for the entire response period) and response rates were plotted as a function of the cumulative dose of cocaine (log scale). The percent cocaine-appropriate responding for a given cycle was calculated and reported only if the monkey emitted enough responses to earn at least one food pellet (i.e., 30 responses, equivalent to a response rate of 0.1 responses/s). For experiments examining the effects of cocaine, d-amphetamine and mu opioid agonists administered alone, a drug was considered to have substituted for cocaine in a monkey if at least one dose of the drug elicited 90% cocaine-appropriate responding. For experiments examining the effects of mu agonist pretreatments on the cocaine and amphetamine doseeffect curves, the cocaine and amphetamine dose-effect curves following mu opioid pretreatment were visually compared to a range of control dose-effect curves. Warm-Water Tail-Withdrawal Procedure Apparatus and Procedure. The results of the initial drug discrimination studies suggested that the ability of mu agonists to mimic the discriminative stimulus effects of cocaine may have been related to their rate of onset (see results below). To provide a direct comparison of the rates of onset of heroin, alfentanil, fentanyl, and morphine following IM administration in rhesus monkeys, the effects of these mu agonists were examined in a warm-water tail-withdrawal assay of antinociception using water heated to 50 C as the noxious stimulus. This assay was used to evaluate time course because mu agonists produce robust and reliable antinociceptive effects in this procedure and because the antinociceptive effects of heroin, alfentanil, fentanyl, and morphine in this procedure are mediated primarily by mu opioid receptors (Dykstra et al. 1987; Walker et al. 1993; Emmerson et al. 1994; Gerak et al. 1994; Negus et al., unpublished observations). The monkeys were seated in acrylic restraint chairs so that their tails moved freely. The bottom 10 cm of each monkey s shaved tail was immersed in a thermal container of warm water. If the subject did not withdraw its tail within 20 s, the tail was removed from the water by the experimenter, and a latency of 20 s was assigned to that measurement. Tail-withdrawal latencies were measured using two different water temperatures: 42 and 50 C. Water heated to 42 C was used as an innocuous control stimulus. Water heated to 50 C was used as the experimental, noxious stimulus. Experiments were conducted no more than twice a week. An Apple IIe microcomputer (Apple Computers, Inc., Cupertino, CA) was used to measure and record time intervals. Each test session consisted of multiple cycles. Before the first cycle, baseline latencies to tail-withdrawal from 42 and 50 C water were determined. Following preliminary experiments to identify the potency and time course of each agonist, subsequent experiments used two types of testing sequences. During cumulative dosing experiments, a single drug dose was administered at the start of each of four to five sequential cycles, and tail-withdrawal latencies from 42 and 50 C water were measured at the end of each cycle. The two temperatures were presented in a pseudo random order during each cycle. The duration of each cycle was 15 min for alfentanil and heroin and 30 min for fentanyl and morphine. During time course experiments, equiantinociceptive doses of each mu agonist were administered at the beginning of the session, and tail with-
5 NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 Mu Opioids and Cocaine Discrimination 329 drawal latencies from 50 C water were measured at 2, 4, 8, 16, 32, 64, and 128 min after injection. All drugs were administered IM to mimic the route of administration used in the drug discrimination studies. Data Analysis. Tail-withdrawal latency values from 50 C water were graphed as a function of both drug dose (for dose-effect studies) and time after drug injection (for time course studies). Equi-effective antinociceptive doses of each drug were identified from the initial dose-effect studies as the lowest dose to produce a mean tail-withdrawal latency of 15 s. These equieffective doses were then examined in the time course experiments. Time course data were statistically analyzed using a one-factor ANOVA, with time after injection as the single factor. A significant ANOVA was followed by individual means comparisons using the Newman-Keuls post hoc test (Winer 1971). The criterion for significance was set at p.01. The time to maximal effect was defined as the interval between the time of drug injection and the earliest time when the following two criteria were met: (1) the mean tail-withdrawal latency was significantly greater than baseline, and (2) tail-withdrawal latencies did not increase significantly at later times. Drugs Cocaine hydrochloride, heroin hydrochloride, alfentanil hydrochloride, morphine sulfate, naltrexone hydrochloride (all provided by the National Institute on Drug Abuse, Bethesda, MD), d-amphetamine sulfate (Sigma, St. Louis, MO) and fentanyl citrate (Research Biochemicals International, Natick, MA) were dissolved in sterile water. Doses are expressed as mg/kg of the salt form of the compound. All drugs were administered IM in the thigh. RESULTS Warm-Water Tail-Withdrawal Procedure The relative rates of onset of IM heroin, alfentanil, fentanyl, and morphine were characterized using a warmwater tail-withdrawal assay of thermal antinociception. The monkeys always left their tails in 42 C water for the full 20 s, indicating that tail immersion alone did not elicit tail withdrawal. Baseline tail-withdrawal latencies from 50 C water averaged 1.09 ( 0.33) s. Heroin, alfentanil, fentanyl, and morphine all produced dose-dependent increases in tail-withdrawal latencies from 50 C water (Figure 1, top panel). The lowest doses to elicit tailwithdrawal latencies 15 s for each drug were 0.32 mg/ kg heroin, 0.1 mg/kg alfentanil, mg/kg fentanyl, and 10 mg/kg morphine. The bottom panel of Figure 1 shows the time courses of these equi-effective doses of each mu agonist. Alfentanil and heroin displayed the most rapid onsets of action. Both drugs produced significant (p.01) and maximal increases in tail-withdrawal latency after 8 min. The effects of alfentanil then decreased rapidly, whereas the Figure 1. Antinociceptive effects of mu opioid agonists in a warm-water tail-withdrawal assay. Abscissa (top panel): Cumulative dose of drug in mg/kg (log scale). Abscissa (bottom panel): Time in min after injection of drug. Ordinates: Latency in sec to tail withdrawal from water heated to 50 C. Each point shows mean data from three or four monkeys. Points above BL in the bottom panel show baseline tailwithdrawal latencies prior to administration of each mu agonist.
6 330 S.S. Negus et al. NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 maximal effect of heroin was sustained at 16 and 32 min before declining at 64 min. Fentanyl produced a significant but submaximal increase in tail-withdrawal latency after 8 min (p.01); however, fentanyl did not produce its maximal effect until 16 min. Morphine produced a significant but submaximal increase in tail-withdrawal latency after 16 min, and morphine produced its maximal effect after 32 min. Thus, the order of these drugs was, from fastest to slowest rate of onset, alfentanil heroin fentanyl morphine. Cocaine Discrimination Control Performance. During training sessions immediately preceding test sessions, monkeys responded almost exclusively on the saline-appropriate key during saline cycles ( % saline-appropriate responding) and almost exclusively on the cocaine-appropriate key during cocaine training cycles ( % cocaineappropriate responding). Mean response rates during saline and cocaine training cycles were and responses/s, respectively. Effects of Mu Agonists Alone. Figure 2 shows the effects of heroin and alfentanil administered alone in individual monkeys. Heroin and alfentanil substituted completely for cocaine in monkeys 89B036, 186F, and 153F. Doses of heroin and alfentanil that produced high levels of cocaine-appropriate responding in these monkeys also usually decreased response rates. Heroin and alfentanil up to doses that eliminated responding produced primarily saline-appropriate responding in monkeys L990 and 150F. Doses of alfentanil ( mg/kg) and heroin ( mg/kg) that were behaviorally active in these cocaine discriminating monkeys were identical to doses of these compounds that produced antinociceptive effects in the warm-water tail-withdrawal procedure. Figure 3 shows the effects of fentanyl and morphine administered alone in the same monkeys. When fentanyl and morphine were administered using the standard 10-min pretreatment interval, neither mu agonist substituted completely for cocaine in any monkey (see open symbols in Figure 3). However, intermediate levels of cocaine-appropriate responding (between 10 and 90%) were occasionally observed, especially in monkey 89B036, and both mu agonists produced dose-dependent decreases in response rates. Since time course studies using the warm-water tail-withdrawal procedure indicated that equieffective IM doses of fentanyl and morphine reached peak effect at 16 and 32 min Figure 2. Effects of heroin and alfentanil alone in individual monkeys. Abscissae: Dose drug in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in response/s. In this and all other figures, each column of two panels shows data from an individual monkey, and the monkey s identification number is shown above the top panels.
7 NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 Mu Opioids and Cocaine Discrimination 331 after administration, respectively, the effects of fentanyl and morphine were subsequently examined using longer pretreatment intervals (16 min for fentanyl and 32 min for morphine; see closed symbols in Figure 3). Under these conditions, both fentanyl and morphine substituted completely for cocaine in monkey 186F, a monkey in which both heroin and alfentanil also substituted for cocaine. In the other monkeys, however, fentanyl and morphine still failed to substitute for cocaine. In fact, fentanyl and morphine produced similar or lower levels of cocaine-appropriate responding in these monkeys when the longer pretreatment interval was used than when the shorter pretreatment interval was used. As with alfentanil and heroin, doses of fentanyl ( mg/kg) and morphine ( mg/kg) that were behaviorally active in the cocaine discriminating monkeys also produced thermal antinociception in the warmwater tail-withdrawal procedure. Effects of Mu Agonist Pretreatment on Cocaine Discrimination. Figure 4 shows the effects of cocaine administered alone or following pretreatment with morphine in each of the five monkeys. Cocaine produced a dose-dependent and complete substitution for the training dose of cocaine in all five monkeys, while having little effect on response rates. Pretreatment with morphine ( mg/kg) increased levels of cocaine-appropriate responding produced by low doses of cocaine in monkeys 186F and 89B036. Doses of morphine that altered cocaine discrimination in these monkeys also produced substantial decreases in response rates. In monkeys 153F, L990, and 150F, morphine pretreatment ( mg/kg) had little or no effect on the cocaine discrimination dose-effect curve, although morphine decreased response rates in these monkeys (for clarity, only the highest two doses of morphine tested are shown for each monkey). The effects of 3.2 mg/kg morphine on cocaine discrimination and response rates in monkeys 89B036 and 186F could be blocked by pretreatment with the opioid antagonist naltrexone (0.1 mg/kg) (Figure 5). Naltrexone (0.1 mg/kg) also blocked the rate decreasing effects of 3.2 mg/kg morphine in monkeys 153F, L990, and 150F (data not shown). The effects of pretreatment with fentanyl on the cocaine dose-effect curve were examined in two monkeys, monkeys 186F and 150F (Figure 6). These two monkeys Figure 3. Effects of fentanyl and morphine alone in individual monkeys. Abscissae: Dose of drug in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in responses/s. Fentanyl and morphine were evaluated using two different pretreatment intervals (i.e., the interval between administration of each dose of drug and the beginning of the subsequent response period during which cocaine-appropriate responding and response rates were evaluated). Fentanyl dose-effect curves were determined using pretreatment intervals of 10 min and 16 min, whereas morphine dose-effect curves were determined using pretreatment intervals of 10 min and 32 min.
8 332 S.S. Negus et al. NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 Figure 4. Effects of cocaine administered alone or following morphine pretreatment in individual monkeys. Abscissae: Dose of cocaine in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in responses/s. The shaded area shows the range of values obtained during four determinations of the doseeffect curve for cocaine alone. Symbols show the effects of cocaine following pretreatment with different doses of morphine. The dotted line in the upper panel for monkey 186F indicates that the monkey did not respond and that a value for percent cocaine responding could not be determined at a dose (0.013 mg/kg) intermediate between the two connected points. were selected for studies with fentanyl, because they represented the different types of responses obtained with morphine pretreatment (i.e., monkey 186F was representative of animals in which morphine enhanced the discriminative stimulus effects of low doses of cocaine, whereas monkey 150F was representative of monkeys in which morphine did not alter the discriminative stimulus effects of cocaine). Like morphine, fentanyl increased levels of cocaine-appropriate responding produced by low doses of cocaine in monkey 186F, but had no effect on cocaine discrimination in monkey 150F. A higher dose of fentanyl (0.1 mg/kg) eliminated responding in monkey 150F (data not shown). Effects of Morphine on the Cocaine-Like Discriminative Stimulus Effects of Amphetamine. Figure 7 shows the effects of amphetamine administered alone or after pretreatment with morphine. Like cocaine, amphetamine produced a dose-dependent and complete substitution for the training dose of cocaine in all five monkeys. Morphine ( mg/kg) increased levels of cocaineappropriate responding produced by low doses of amphetamine in monkeys 89B036, 186F, L990, and 153F. However, morphine pretreatment had little effect on the cocaine-like discriminative stimulus effects of amphetamine in monkey 150F. Morphine produced dosedependent decreases in response rates relative to amphetamine alone in all monkeys. DISCUSSION Cocaine-Like Stimulus Effects of Mu Agonists In the present study, we replicated our earlier finding that heroin substitutes completely for cocaine in some monkeys (Mello et al. 1995). Importantly, heroin produced complete substitution in the same three monkeys (186F, 89B036, and 153F) as in the original study, whereas heroin failed to substitute for cocaine in the other two monkeys (L990 and 150F). Thus, although there are individual differences in the ability of heroin to produce cocaine-like discriminative stimulus effects, the effects of heroin in any one monkey appear to be consistent through time. The reasons for these individual differences are not clear; however, these individual differences were probably not the result of different behavioral histories, because the behavioral histories of
9 NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 Mu Opioids and Cocaine Discrimination 333 Figure 5. Effects of cocaine administered alone or following pretreatment with morphine or morphine naltrexone in monkeys 186F and 89B036. Details as in Figure 3. Figure 6. Effects of cocaine administered alone or following fentanyl pretreatment in monkeys 186F and 150F. Details as in Figure 3. the five monkeys used in the discrimination studies were nearly identical. We extended these findings by examining the effects of the other mu opioid agonists alfentanil, fentanyl, and morphine. Alfentanil produced a dose-dependent and complete substitution for cocaine in the same three monkeys that discriminated heroin as cocaine (monkeys 186F, 89B036, and 153F). Fentanyl and morphine produced lower levels of cocaine-appropriate responding than heroin and alfentanil, and complete substitution of fentanyl and morphine for cocaine was observed in only one monkey (186F). These studies provide additional evidence to suggest that there are individual differences in the effects of mu agonists in cocainediscriminating rhesus monkeys. The differential ability of heroin, alfentanil, fentanyl, and morphine to mimic the discriminative stimulus effects of cocaine may be related to their different rates of onset. We evaluated the rates of onset of these mu agonists in a warm-water tail-withdrawal assay of thermal nociception. As noted above in the Methods section, this assay was used to evaluate time course because mu agonists produce robust and reliable antinociceptive effects in this procedure and because the antinociceptive effects of heroin, alfentanil, fentanyl, and morphine in this procedure are mediated primarily by mu opioid receptors. (Dykstra et al. 1987; Walker et al. 1993; Emmerson et al. 1994; Gerak et al. 1994; Negus et al., unpublished observations). When equi-antinociceptive IM doses of each mu agonist were compared, heroin and alfentanil displayed the most rapid rates of onset, whereas fentanyl and morphine had slower rates of onset. The rates of onset of these mu agonists after IM administration have not been directly compared before; however, these results concur with previous reports of the time courses of these mu agonists in rhesus monkeys (Bertalmio and Woods 1987; Gatch et al. 1995) and humans (Reichle et al. 1962; Kaiko et al. 1981; Jaffe and Martin 1990). The ability of the rapid onset mu agonists heroin and alfentanil to produce higher levels of cocaineappropriate responding than the slower onset mu agonists fentanyl and morphine suggests that rate of onset may be one determinant of the ability of mu agonists to substitute for the discriminative stimulus effects of cocaine. Many previous studies have found that mu agonists usually do not substitute for cocaine in subjects trained to discriminate cocaine from saline (Colpaert 1978;
10 334 S.S. Negus et al. NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 Figure 7. Effects of d-amphetamine administered alone or following morphine pretreatment in individual monkeys. Abscissae: Dose amphetamine in mg/kg (log scale). Ordinates (top panels): Percent cocaine-appropriate responding. Ordinates (bottom panels): Response rate in responses/s. The shaded area shows the range of values obtained during two determinations of the dose-effect curve for amphetamine alone. Open symbols show the effects of amphetamine following morphine pretreatment. Dykstra et al. 1992; Spealman and Bergman 1992, 1994; Broadbent et al. 1995). However, as in the present study, there have been exceptions to this general finding, and these exceptions may also be related to the rate of onset of mu agonist effects. For example, morphine administered IM failed to substitute for cocaine in several studies, including the present one. However, morphine administered via the IV route, which produces immediate absorption and rapid distribution, produced high levels of cocaine-appropriate responding (80 100%) in two monkeys trained to discriminate IV cocaine from saline (Ando and Yanagita 1978). Similarly, morphine failed to substitute for cocaine in rats, but fentanyl, which has a more rapid rate of onset, substituted completely for cocaine in six of 10 rats tested (Colpaert 1978). Taken together, these findings suggest that mu agonists may mimic the discriminative stimulus effects of cocaine in some subjects, especially when the onset of mu agonist effects is rapid. In our previous study, we found that the cocainelike stimulus effects and rate-decreasing effects of heroin were antagonized by the mu-selective antagonist quadazocine, suggesting that these effects of heroin were mediated by mu opioid receptors (Mello et al. 1995). In the present study, the mu-selective agonist alfentanil was approximately 3- to 10-fold more potent than heroin in producing cocaine-like stimulus effects. Moreover, all four mu agonists produced dose-dependent decreases in response rates, and the potency order of these drugs in decreasing response rates was identical to their potency order in producing antinociception in the warm-water tail-withdrawal procedure (fentanyl alfentanil heroin morphine). The similar relative potencies of these drugs in producing antinociception, decreases in response rates, and cocaine-like stimulus effects (for heroin and alfentanil) is consistent with the conclusion that all these effects were mediated by a common pharmacological mechanism of action, probably activation of mu opioid receptors. Effects of Mu Agonists in Combination with Cocaine Although morphine and fentanyl did not consistently substitute for cocaine when administered alone, both mu agonists increased levels of cocaine-appropriate responding produced by low doses of cocaine in some monkeys. However, as with the discriminative stimulus
11 NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 Mu Opioids and Cocaine Discrimination 335 effects of mu agonists alone, there were striking individual differences in the effects of mu agonists administered as pretreatments to cocaine. At one extreme, morphine and fentanyl were most effective in shifting the cocaine dose-effect curve upward and to the left in a monkey (186F) in which mu agonists administered alone substituted completely for cocaine. The effects of morphine on both cocaine-appropriate responding and response rates were antagonized by a relatively low dose of the opioid antagonist naltrexone in monkey 186F as well as in monkey 89B036, further suggesting that these effects were mediated by mu opioid receptors. At the other extreme, morphine and fentanyl were ineffective in altering the cocaine dose-effect curve in a monkey (150F) in which even rapid onset mu agonists failed to substitute for cocaine. The individual differences in the effects of morphine and fentanyl on cocaine discrimination were probably not the result of different behavioral histories across monkeys, because as noted above, the behavioral histories of all five monkeys were similar. Previous studies of the effects of mu agonists on cocaine discrimination have also reported highly variable results. In squirrel monkeys, morphine and several other mu agonists produced leftward shifts in the cocaine discrimination dose-effect curve in all monkeys tested (Spealman and Bergman 1992, 1994). On the other hand, fentanyl did not alter the cocaine discrimination dose-effect curve in rats (Broadbent et al. 1995). In another study in rats, buprenorphine increased levels of cocaine-appropriate responding occasioned by a low dose of cocaine, but decreased levels of cocaineappropriate responding occasioned by higher doses of cocaine (Dykstra et al. 1992). The effects of mu agonists on cocaine discrimination have not been evaluated in humans; however, several studies have examined the effects of mu agonists on cocaine-induced subjective effects, and the results of these studies are also variable. For example, the subjective effects of acute administration of morphine/cocaine combinations were usually similar to the effects of either morphine or cocaine alone, suggesting that morphine did not consistently enhance the subjective effects of cocaine (Foltin and Fischman 1992). However, methadone maintenance has been reported to enhance the subjective effects of cocaine (Foltin et al. 1995; Preston et al. 1996). Taken together, these results suggest that interactions between mu opioid agonists and cocaine are complex, and that mu agonists do not uniformly enhance the effects of cocaine in all subjects under all conditions. In contrast to the inconsistent effects that have been obtained with mu opioid agonists, drugs acting as direct or indirect dopamine agonists usually produce high levels of cocaine-appropriate responding when administered alone (Kleven et al. 1990; Spealman et al. 1991) and leftward shifts in the cocaine-dose effect curve when administered in combination with cocaine (e.g., Cunningham and Callahan 1991; Spealman 1996). Indeed, these findings have provided important evidence for the hypothesis that the discriminative stimulus effects of cocaine are mediated primarily by the effects of cocaine on dopaminergic systems. Effects of Mu Agonists in Combination with Amphetamine Amphetamine produced a dose-dependent and complete substitution for the cocaine training stimulus in all five monkeys tested in this study. This finding agrees with many previous reports that cocaine and amphetamine produce similar discriminative stimulus effects in animals (e.g., Colpaert et al. 1979; de la Garza and Johanson 1983, 1985). In addition, morphine shifted the dose-effect curve for amphetamine-induced cocaineappropriate responding upward and to the left in some monkeys. Previous studies have not examined the effects of mu agonists on the discriminative stimulus effects of amphetamine in primates. However, clinical evidence indicates that amphetamines have been abused as the psychostimulant component of speedball combinations (Kramer et al. 1967; Langrod 1970; Brecher 1972), which suggests that combinations of mu opioids and amphetamine may produce interacting effects. In addition, the present findings agree with previous reports that mu agonists and amphetamine produced additive or greater than additive effects on antinociception (Ahmed et al. 1970; Richards 1975) and on rates of behavior maintained under some schedules of food reinforcement (Lucot et al. 1979). However, it should be noted that mu agonists and amphetamine do not always enhance each other s effects (e.g., Fog 1970), and in one interesting exception, amphetamine decreased the discriminative stimulus effects of morphine in rats trained to discriminate a low dose (3.2 mg/kg), but not a high dose (5.6 mg/kg), of morphine from saline (Young et al. 1992). In the present study, morphine appeared to be more effective in enhancing the cocaine-like stimulus effects of amphetamine than in enhancing the effects of cocaine itself. In monkeys 186F and 89B036, morphine increased the stimulus effects of both cocaine and amphetamine, but morphine/amphetamine combinations produced less severe rate-decreasing effects than morphine/cocaine combinations. As a result, the effects of morphine could be observed across a broader range of amphetamine doses. In monkeys L990 and 153F, morphine at doses up to 10 mg/kg had little or no effect on the cocaine dose-effect curve, but morphine at doses of mg/kg produced clear leftward and/or upward shifts in the amphetamine dose-effect curves. Morphine failed to alter the amphetamine dose-effect curve only in monkey 150F, a monkey in which mu agonists also failed to alter the cocaine dose-effect curve. It is not
12 336 S.S. Negus et al. NEUROPSYCHOPHARMACOLOGY 1998 VOL. 18, NO. 5 clear why morphine enhanced the cocaine-like discriminative stimulus effects of amphetamine more than those of cocaine itself. Although cocaine and amphetamine share many common physiological and behavioral effects, they have different mechanisms of action. Specifically, cocaine acts primarily by blocking the reuptake of the monoamine neurotransmitters dopamine, norepinephrine, and serotonin (Koe 1976; Taylor and Ho 1978), whereas amphetamine blocks the reuptake and promotes the release of monoamine neurotransmitters (Coyle and Snyder 1969; Taylor and Ho 1978; Langer and Arbilla 1984). It is possible the morphine interacts to a greater degree with monoamine releasers than with monoamine uptake blockers. Furthermore, previous studies have suggested that endogenous opioid systems may be differentially involved in modulating the effects of cocaine and amphetamine. For example, the opioid antagonist naloxone blocks many neurochemical and behavioral effects of amphetamine in rats, but is less effective in blocking the effects of cocaine (Jones and Holtzman 1994; Schad et al. 1995). Indeed, one study found that naloxone attenuated the locomotor activating effects of amphetamine but enhanced the locomotor activating effects of cocaine. These results were interpreted to suggest that endogenous opioid systems differentially regulate the behavioral effects of amphetamine and cocaine. The findings of the present study suggest that exogenous opioid agonists may also differentially regulate the behavioral effects of these two psychomotor stimulants. Conclusions The results of the present study demonstrate that there is considerable individual variability in the effects of mu agonists in rhesus monkeys trained to discriminate cocaine from saline. At one extreme, mu agonists (and especially rapid onset mu agonists) produced high levels of cocaine-appropriate responding, and slower onset mu agonists enhanced the effects of cocaine and amphetamine. At the other extreme, mu agonists neither substituted for cocaine nor altered the effects of cocaine or amphetamine. The observed degree of variability suggests that the behavioral interactions between CNS stimulants and mu opioid agonists in rhesus monkeys are complex. Moreover, one implication of these findings is that, in humans, different individuals may be differentially sensitive to the abuse-related effects of stimulant/opioid speedball combinations. ACKNOWLEDGMENTS The authors thank Peter Fivel, Nicholas Diaz-Migoyo, and Josephine Avery for technical contributions and Elizabeth Hall for providing veterinary oversight. The authors also thank Dr. Bill Morse for his comments on an earlier version of the article. This work was supported by Grants DA 04059, DA 07252, DA and K from the National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland. REFERENCES Ahmed SS, Abraham GJS, Assari M (1970): Dose-dependent modification of codeine analgesia by d-amphetamine in albino rats. Arch Int Pharmacodyn Ther 184: Ando K, Yanagita T (1978): The discriminative stimulus properties of intravenously administered cocaine in rhesus monkeys. In Colpaert FC, Rosecrans JS (eds), Stimulus Properties of Drugs: Ten Years of Progress. Amsterdam, Elsevier, pp Bertalmio AJ, Medzihradsky F, Winger G, Woods JH (1992): Differential influence of N-dealkylation on the stimulus properties of some opioid agonists. J Pharmacol Exp Ther 261: Bertalmio AJ, Woods JH (1987): Differentiation between mu and kappa receptor-mediated effects in opioid drug discrimination: Apparent pa 2 analysis. J Pharmacol Exp Ther 243: Brecher EM (1972): Licit and Illicit Drugs. Boston, Little, Brown and Co. Broadbent J, Gaspard TM, Dworkin SI (1995): Assessment of the discriminative stimulus effects of cocaine in the rat: Lack of interaction with opioids. Pharmacol Biochem Behav 51: Colpaert FC (1978): Discriminative stimulus properties of narcotic analgesic drugs. Pharmacol Biochem Behav 9: Colpaert FC, Niemegeers CJE, Janssen PAJ (1979): Discriminative stimulus properties of cocaine: Neuropharmacological characteristics as derived from stimulus generalization experiments. Pharmacol Biochem Behav 10: Coyle JT, Snyder SH (1969): Catecholamine uptake by synaptosomes in homogenates of rat brain: Stereospecificity in different areas. J Pharmacol Exp Ther 170: Cunningham KA, Callahan PM (1991): Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology 104: de la Garza R, Johanson CE (1983): The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav 19: de la Garza R, Johanson CE (1985): Discriminative stimulus properties of cocaine in pigeons. Psychopharmacology 85:23 30 Dykstra LA, Doty P, Johnson AB, Picker MJ (1992): Discriminative stimulus properties of cocaine, alone and in combination with buprenorphine, morphine and naltrexone. Drug Alcohol Depend 30: Dykstra LA, Gmerek DE, Winger G, Woods JH (1987): Kappa opioids in rhesus monkeys. II. Analysis of antagonistic actions of quadazocine and -funaltrexamine. J Pharmacol Exp Ther 242: Emmerson PJ, Liu M-R, Woods JH, Medzihradsky F (1994): Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 271:
Discriminative Stimulus Effects of a Cocaine/Heroin Speedball Combination in Rhesus Monkeys 1
 0022-3565/98/2853-1123$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 3 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2853-1123$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 3 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
The Effects of Buprenorphine on Self-administration of Cocaine and Heroin Speedball Combinations and Heroin Alone by Rhesus Monkeys 1
 0022-3565/98/2852-0444$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2852-0444$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Effects of a Novel Fentanyl Derivative on Drug Discrimination and Learning in Rhesus Monkeys
 PII S0091-3057(99)00058-1 Pharmacology Biochemistry and Behavior, Vol. 64, No. 2, pp. 367 371, 1999 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/99/$ see front matter Effects
PII S0091-3057(99)00058-1 Pharmacology Biochemistry and Behavior, Vol. 64, No. 2, pp. 367 371, 1999 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0091-3057/99/$ see front matter Effects
Choice Between Heroin and Food in Non-Dependent and Heroin-Dependent Rhesus Monkeys: Effects of Naloxone, Buprenorphine and Methadone
 JPET Fast This article Forward. has not Published been copyedited on and February formatted. The 2, final 2006 version as DOI:10.1124/jpet.105.095380 may differ from this version. Choice Between Heroin
JPET Fast This article Forward. has not Published been copyedited on and February formatted. The 2, final 2006 version as DOI:10.1124/jpet.105.095380 may differ from this version. Choice Between Heroin
Cocaine-Like Discriminative Stimulus Effects of Heroin: Modulation by Selective
 JPET Fast Forward. Published on February 25, 2004 as DOI:10.1124/jpet.104.065631 JPET #65631 Cocaine-Like Discriminative Stimulus Effects of Heroin: Modulation by Selective Monoamine Transport Inhibitors*
JPET Fast Forward. Published on February 25, 2004 as DOI:10.1124/jpet.104.065631 JPET #65631 Cocaine-Like Discriminative Stimulus Effects of Heroin: Modulation by Selective Monoamine Transport Inhibitors*
Cocaine-Like Discriminative Stimulus Effects of Heroin: Modulation by Selective Monoamine Transport Inhibitors
 0022-3565/04/3101-342 348$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 310, No. 1 Copyright 2004 by The American Society for Pharmacology and Experimental Therapeutics 65631/1150088
0022-3565/04/3101-342 348$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 310, No. 1 Copyright 2004 by The American Society for Pharmacology and Experimental Therapeutics 65631/1150088
Antagonism of the discriminative stimulus properties of cocaine with the combination of a dopamine Dl and D2 antagonist
 Antagonism of the discriminative stimulus properties of cocaine with the combination of a dopamine Dl and D2 antagonist Theo F. Meert, Patrick De Haes, Nancy Aerts and Gilbert Clincke Department of Neuropsychopharmacology,
Antagonism of the discriminative stimulus properties of cocaine with the combination of a dopamine Dl and D2 antagonist Theo F. Meert, Patrick De Haes, Nancy Aerts and Gilbert Clincke Department of Neuropsychopharmacology,
Asymmetric Generalization and Interaction Profiles in Rhesus Monkeys Discriminating Intravenous Cocaine or Intravenous Heroin from Vehicle
 22-3565/1/3323-985 995$2. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 332, No. 3 Copyright 21 by The American Society for Pharmacology and Experimental Therapeutics 162941/3559918 JPET
22-3565/1/3323-985 995$2. THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 332, No. 3 Copyright 21 by The American Society for Pharmacology and Experimental Therapeutics 162941/3559918 JPET
Behavioral Effects of Cocaine: Interactions with D1 Dopaminergic Antagonists and Agonists in Mice and Squirrel Monkeys
 0022-3565/99/2911-0265$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 291, No. 1 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/99/2911-0265$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 291, No. 1 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Effects of d-amphetamine and Buprenorphine Combinations on Speedball (Cocaine + Heroin) Self-Administration by Rhesus Monkeys
 (2007) 32, 1985 1994 & 2007 Nature Publishing Group All rights reserved 0893-133X/07 $30.00 www.neuropsychopharmacology.org Effects of d-amphetamine and Buprenorphine Combinations on Speedball (Cocaine
(2007) 32, 1985 1994 & 2007 Nature Publishing Group All rights reserved 0893-133X/07 $30.00 www.neuropsychopharmacology.org Effects of d-amphetamine and Buprenorphine Combinations on Speedball (Cocaine
Psychopharmacology 9 Springer-Verlag 1982
 Psychopharmacology (1982) 76:172-176 Psychopharmacology 9 Springer-Verlag 1982 Discriminative Stimulus Effects of Pentobarbital in Rhesus Monkeys: Tests of Stimulus Generalization and Duration of Action
Psychopharmacology (1982) 76:172-176 Psychopharmacology 9 Springer-Verlag 1982 Discriminative Stimulus Effects of Pentobarbital in Rhesus Monkeys: Tests of Stimulus Generalization and Duration of Action
Cocaine and other indirect-acting monoamine agonists differentially attenuate a. naltrexone discriminative stimulus in morphine-treated rhesus monkeys
 JPET Fast This article Forward. has not Published been copyedited on and October formatted. 20, The final 2003 version as DOI:10.1124/jpet.103.058917 may differ from this version. Cocaine and other indirect-acting
JPET Fast This article Forward. has not Published been copyedited on and October formatted. 20, The final 2003 version as DOI:10.1124/jpet.103.058917 may differ from this version. Cocaine and other indirect-acting
Effects of Chronic Buspirone Treatment on Cocaine Self-Administration
 (2013) 38, 455 467 & 2013 American College of. All rights reserved 0893-133X/13 www.neuropsychopharmacology.org Effects of Chronic Buspirone Treatment on Cocaine Self-Administration Nancy K Mello*,1, Peter
(2013) 38, 455 467 & 2013 American College of. All rights reserved 0893-133X/13 www.neuropsychopharmacology.org Effects of Chronic Buspirone Treatment on Cocaine Self-Administration Nancy K Mello*,1, Peter
Cocaine and Other Indirect-Acting Monoamine Agonists Differentially Attenuate a Naltrexone Discriminative Stimulus in Morphine-Treated Rhesus Monkeys
 0022-3565/04/3081-111 119$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 308, No. 1 Copyright 2004 by The American Society for Pharmacology and Experimental Therapeutics 58917/1116389
0022-3565/04/3081-111 119$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 308, No. 1 Copyright 2004 by The American Society for Pharmacology and Experimental Therapeutics 58917/1116389
Ellen A. Walker, Mitchell J. Picker, Arthur Granger, and Linda A. Dykstra
 JPET Fast This article Forward. has not been Published copyedited on and March formatted. 25, The 4 final version as DOI:1.1124/jpet.13.5853 may differ from this version. Effects of opioids in morphine-treated
JPET Fast This article Forward. has not been Published copyedited on and March formatted. 25, The 4 final version as DOI:1.1124/jpet.13.5853 may differ from this version. Effects of opioids in morphine-treated
Introduction ORIGINAL INVESTIGATION. selor&:g. Zernig J.W. Lewis J.H. Woods
 Psychopharmacology (1997) 129:233 242 Springer-Verlag 1997 ORIGINAL INVESTIGATION selor&:g. Zernig J.W. Lewis J.H. Woods Clocinnamox inhibits the intravenous self-administration of opioid agonists in rhesus
Psychopharmacology (1997) 129:233 242 Springer-Verlag 1997 ORIGINAL INVESTIGATION selor&:g. Zernig J.W. Lewis J.H. Woods Clocinnamox inhibits the intravenous self-administration of opioid agonists in rhesus
Discriminative Stimulus Effects of Intravenous Heroin and Its Metabolites in Rhesus Monkeys: Opioid and Dopaminergic Mechanisms
 0022-3565/01/2992-760 767$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 299, No. 2 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 4042/938691
0022-3565/01/2992-760 767$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 299, No. 2 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 4042/938691
Effects of Phentermine on Responding Maintained under Multiple Fixed-Ratio Schedules of Food and Cocaine Presentation in the Rhesus Monkey 1
 0022-3565/99/2882-0550$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 288, No. 2 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/99/2882-0550$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 288, No. 2 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Discriminative stimulus effects of a centrally administered, delta-opioid peptide (o-pen2-o-pens-enkephalin) in pigeons
 Psychopharmacology (1996) 127:225-230 Springer-Verlag 1996 David C. Jewett Henry I. Mosberg James H. Woods Discriminative stimulus effects of a centrally administered, delta-opioid peptide (o-pen2-o-pens-enkephalin)
Psychopharmacology (1996) 127:225-230 Springer-Verlag 1996 David C. Jewett Henry I. Mosberg James H. Woods Discriminative stimulus effects of a centrally administered, delta-opioid peptide (o-pen2-o-pens-enkephalin)
Effects of methcathinone and 3-Cl-methcathinone (PAL-434) in cocaine discrimination or self-administration in rhesus monkeys
 International Journal of Neuropsychopharmacology (213), 16, 1985 1998. CINP 213 This is a work of the U.S. Government and is not subject to copyright protection in the United States. doi:1.117/s146114571359x
International Journal of Neuropsychopharmacology (213), 16, 1985 1998. CINP 213 This is a work of the U.S. Government and is not subject to copyright protection in the United States. doi:1.117/s146114571359x
Drug Discrimination in Methamphetamine-Trained Monkeys: Effects of Monoamine Transporter Inhibitors
 0022-3565/04/3112-720 727$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 311, No. 2 Copyright 2004 by The American Society for Pharmacology and Experimental Therapeutics 71035/1174932
0022-3565/04/3112-720 727$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 311, No. 2 Copyright 2004 by The American Society for Pharmacology and Experimental Therapeutics 71035/1174932
Discrete versus cumulative dosing in dose response discrimination studies
 Ž. European Journal of Pharmacology 326 1997 113 118 Discrete versus cumulative dosing in dose response discrimination studies Martin D. Schechter Department of Pharmacology, Northeastern Ohio UniÕersities
Ž. European Journal of Pharmacology 326 1997 113 118 Discrete versus cumulative dosing in dose response discrimination studies Martin D. Schechter Department of Pharmacology, Northeastern Ohio UniÕersities
Comparative Behavioral Pharmacology of Cocaine and the Selective Dopamine Uptake Inhibitor RTI-113 in the Squirrel Monkey 1
 0022-3565/00/2922-0521$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 292, No. 2 Copyright 2000 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/00/2922-0521$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 292, No. 2 Copyright 2000 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Negative Reinforcing Properties of Morphine-Antagonists in Naive Rhesus Monkeys* **
 Psychopharmacologia (Berl.) 33, 247--258 (1973) 9 by Springer-Verlag 1973 Negative Reinforcing Properties of Morphine-Antagonists in Naive Rhesus Monkeys* ** F. Hoffmeister and W. Wuttke Institute of Pharmacology,
Psychopharmacologia (Berl.) 33, 247--258 (1973) 9 by Springer-Verlag 1973 Negative Reinforcing Properties of Morphine-Antagonists in Naive Rhesus Monkeys* ** F. Hoffmeister and W. Wuttke Institute of Pharmacology,
UNIVERSITY OF NORTH CAROLINA CHAPEL HILL AND UNIVERSITY OF NORTH CAROLINA WILMINGTON
 JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 2005, 83, 281 296 NUMBER 3(MAY) MORPHINE TOLERANCE AS A FUNCTION OF RATIO SCHEDULE: RESPONSE REQUIREMENT OR UNIT PRICE? CHRISTINE E. HUGHES, STACEY C. SIGMON,
JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 2005, 83, 281 296 NUMBER 3(MAY) MORPHINE TOLERANCE AS A FUNCTION OF RATIO SCHEDULE: RESPONSE REQUIREMENT OR UNIT PRICE? CHRISTINE E. HUGHES, STACEY C. SIGMON,
Delayed Matching-To-Sample Test in Macaques
 C O N F I D E N T I A L Delayed Matching-To-Sample Test in Macaques DATE This study was conducted under the terms of a Materials Transfer and Services Agreement between NeuroDetective International and
C O N F I D E N T I A L Delayed Matching-To-Sample Test in Macaques DATE This study was conducted under the terms of a Materials Transfer and Services Agreement between NeuroDetective International and
The Role of Peripheral Mu Opioid Receptors in the Modulation of Capsaicin-Induced Thermal Nociception in Rhesus Monkeys 1,2
 0022-3565/98/2861-0150$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 286, No. 1 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2861-0150$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 286, No. 1 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
 No! No! No! No! With the possible exception of humans Public Health Question Does the compound have the potential to be abused? Public Health Question Does the compound have the potential to be abused?
No! No! No! No! With the possible exception of humans Public Health Question Does the compound have the potential to be abused? Public Health Question Does the compound have the potential to be abused?
Effects of Dopamine D 1-like and D 2-like Agonists in Rats that Self-Administer Cocaine 1
 0022-3565/99/2911-0353$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 291, No. 1 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/99/2911-0353$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 291, No. 1 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Psychomotor stimulant effects of β-phenylethylamine in monkeys treated with MAO-B inhibitors
 Psychopharmacology (2001) 159:21 30 DOI 10.1007/s002130100890 ORIGINAL INVESTIGATION J. Bergman S. Yasar G. Winger Psychomotor stimulant effects of β-phenylethylamine in monkeys treated with MAO-B inhibitors
Psychopharmacology (2001) 159:21 30 DOI 10.1007/s002130100890 ORIGINAL INVESTIGATION J. Bergman S. Yasar G. Winger Psychomotor stimulant effects of β-phenylethylamine in monkeys treated with MAO-B inhibitors
Dissociable Effects of the Cannabinoid Receptor Agonists
 1521-0103/12/3432-389 400$25.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 343, No. 2 Copyright 2012 by The American Society for Pharmacology and Experimental Therapeutics 197780/3801449
1521-0103/12/3432-389 400$25.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 343, No. 2 Copyright 2012 by The American Society for Pharmacology and Experimental Therapeutics 197780/3801449
OP01 [Mar96] With regards to pethidine s physical properties: A. It has an octanol coefficient of 10 B. It has a pka of 8.4
![OP01 [Mar96] With regards to pethidine s physical properties: A. It has an octanol coefficient of 10 B. It has a pka of 8.4 OP01 [Mar96] With regards to pethidine s physical properties: A. It has an octanol coefficient of 10 B. It has a pka of 8.4](/thumbs/90/104143670.jpg) Opioid MCQ OP01 [Mar96] With regards to pethidine s physical properties: A. It has an octanol coefficient of 10 B. It has a pka of 8.4 OP02 [Mar96] Which factor does NOT predispose to bradycardia with
Opioid MCQ OP01 [Mar96] With regards to pethidine s physical properties: A. It has an octanol coefficient of 10 B. It has a pka of 8.4 OP02 [Mar96] Which factor does NOT predispose to bradycardia with
Efficacy and the discriminative stimulus effects of negative GABA A modulators, or inverse
 JPET This Fast article Forward. has not been Published copyedited and on formatted. May 16, The 26 final as version DOI:1.1124/jpet.16.13168 may differ from this version. Efficacy and the discriminative
JPET This Fast article Forward. has not been Published copyedited and on formatted. May 16, The 26 final as version DOI:1.1124/jpet.16.13168 may differ from this version. Efficacy and the discriminative
MACIEJ GASIOR, 1 MARIA JASZYNA, 2 JAMIE PETERS, 3 and STEVEN R. GOLDBERG
 0022-3565/00/2953-1101 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 295, No. 3 U.S. Government work not protected by U.S. copyright 2783/864754 JPET 295:1101 1111, 2000 Printed in U.S.A.
0022-3565/00/2953-1101 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 295, No. 3 U.S. Government work not protected by U.S. copyright 2783/864754 JPET 295:1101 1111, 2000 Printed in U.S.A.
Sensitization and tolerance to the discriminative stimulus effects of mu-opioid agonists
 Psychopharmacology (1994) 114: 601-610 Psychopharmacology Springer-Verlag 1994 Sensitization and tolerance to the discriminative stimulus effects of mu-opioid agonists Carol A. Paronis*, Stephen G. Holtzman
Psychopharmacology (1994) 114: 601-610 Psychopharmacology Springer-Verlag 1994 Sensitization and tolerance to the discriminative stimulus effects of mu-opioid agonists Carol A. Paronis*, Stephen G. Holtzman
Instrumental Conditioning I
 Instrumental Conditioning I Basic Procedures and Processes Instrumental or Operant Conditioning? These terms both refer to learned changes in behavior that occur as a result of the consequences of the
Instrumental Conditioning I Basic Procedures and Processes Instrumental or Operant Conditioning? These terms both refer to learned changes in behavior that occur as a result of the consequences of the
CAROL 0. ECKERMAN UNIVERSITY OF NORTH CAROLINA. in which stimulus control developed was studied; of subjects differing in the probability value
 JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 1969, 12, 551-559 NUMBER 4 (JULY) PROBABILITY OF REINFORCEMENT AND THE DEVELOPMENT OF STIMULUS CONTROL' CAROL 0. ECKERMAN UNIVERSITY OF NORTH CAROLINA Pigeons
JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 1969, 12, 551-559 NUMBER 4 (JULY) PROBABILITY OF REINFORCEMENT AND THE DEVELOPMENT OF STIMULUS CONTROL' CAROL 0. ECKERMAN UNIVERSITY OF NORTH CAROLINA Pigeons
Behavioral Pharmacology
 Psych 181: Dr. Anagnostaras Lecture 4 Behavioral Pharmacology Behavioral Pharmacology Behavioral Pharmacology The study of the relationship between the physiological actions of drugs and their effects
Psych 181: Dr. Anagnostaras Lecture 4 Behavioral Pharmacology Behavioral Pharmacology Behavioral Pharmacology The study of the relationship between the physiological actions of drugs and their effects
Overview of Pharmacodynamics. Psyc 472 Pharmacology of Psychoactive Drugs. Pharmacodynamics. Effects on Target Binding Site.
 Pharmacodynamics Overview of Pharmacodynamics Psychology 472: Pharmacology of Psychoactive Drugs Generally is defined as effects of drugs on a systems Can be associated with any system Neural, Heart, Liver,
Pharmacodynamics Overview of Pharmacodynamics Psychology 472: Pharmacology of Psychoactive Drugs Generally is defined as effects of drugs on a systems Can be associated with any system Neural, Heart, Liver,
Dr Alistair Dunn. General Practitioner Whangarei
 Dr Alistair Dunn General Practitioner Whangarei 1982 Temgesic = Buprenorphine Sublingual opiate analgesic 0.2 mg Popular drug of abuse 1991 Temgesic NX = Bup & Nalxone Nalxone added to deter abuse 1999
Dr Alistair Dunn General Practitioner Whangarei 1982 Temgesic = Buprenorphine Sublingual opiate analgesic 0.2 mg Popular drug of abuse 1991 Temgesic NX = Bup & Nalxone Nalxone added to deter abuse 1999
ASSOCIATED WITH NALORPHINE IN
 JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR CONDITIONED SUPPRESSION BY A STIMULUS ASSOCIATED WITH NALORPHINE IN MORPHINE-DEPENDENT MONKEYS1 STEVEN R. GOLDBERG AND CHARLES R. SCHUSTER UNIVERSITY OF
JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR CONDITIONED SUPPRESSION BY A STIMULUS ASSOCIATED WITH NALORPHINE IN MORPHINE-DEPENDENT MONKEYS1 STEVEN R. GOLDBERG AND CHARLES R. SCHUSTER UNIVERSITY OF
Methadone Maintenance
 Methadone Maintenance A Practical Guide to Pharmacotherapy Methadone/Buprenorphine 101 Workshop, April 1, 2017 Ron Joe, MD, DABAM Objectives I. Pharmacology Of Methadone II. Practical Application of Pharmacology
Methadone Maintenance A Practical Guide to Pharmacotherapy Methadone/Buprenorphine 101 Workshop, April 1, 2017 Ron Joe, MD, DABAM Objectives I. Pharmacology Of Methadone II. Practical Application of Pharmacology
Local administration of 9 -tetrahydrocannabinol attenuates capsaicin-induced thermal nociception in rhesus monkeys: a peripheral cannabinoid action
 Psychopharmacology (1999) 143:322 326 Springer-Verlag 1999 RAPID COMMUNICATION Mei-Chuan Ko James H. Woods Local administration of 9 -tetrahydrocannabinol attenuates capsaicin-induced thermal nociception
Psychopharmacology (1999) 143:322 326 Springer-Verlag 1999 RAPID COMMUNICATION Mei-Chuan Ko James H. Woods Local administration of 9 -tetrahydrocannabinol attenuates capsaicin-induced thermal nociception
Evaluation of morphine-like effects of the mixed mu/delta agonist morphine-6-o-sulfate in rats: Drug discrimination and physical dependence
 Received: 8 February 2018 Accepted: 10 April 2018 DOI: 10.1002/prp2.403 ORIGINAL ARTICLE Evaluation of morphine-like effects of the mixed mu/delta agonist morphine-6-o-sulfate in rats: Drug discrimination
Received: 8 February 2018 Accepted: 10 April 2018 DOI: 10.1002/prp2.403 ORIGINAL ARTICLE Evaluation of morphine-like effects of the mixed mu/delta agonist morphine-6-o-sulfate in rats: Drug discrimination
ACTIVITY USING RATS A METHOD FOR THE EVALUATION OF ANALGESIC. subject and a variety of stimuli employed. In the examination of new compounds
 Brit. J. Pharmacol. (1946), 1, 255. A METHOD FOR THE EVALUATION OF ANALGESIC ACTIVITY USING RATS BY 0. L. DAVIES, J. RAVENT6S, AND A. L. WALPOLE From Imperial Chemical Industries, Ltd., Biological Laboratories,
Brit. J. Pharmacol. (1946), 1, 255. A METHOD FOR THE EVALUATION OF ANALGESIC ACTIVITY USING RATS BY 0. L. DAVIES, J. RAVENT6S, AND A. L. WALPOLE From Imperial Chemical Industries, Ltd., Biological Laboratories,
The Dopamine Transporter and Cocaine Medication Development: Drug Self-Administration in Nonhuman Primates
 0022-3565/01/2981-1 6$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 298, No. 1 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 900018/911057
0022-3565/01/2981-1 6$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 298, No. 1 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 900018/911057
CS DURATION' UNIVERSITY OF CHICAGO. in response suppression (Meltzer and Brahlek, with bananas. MH to S. P. Grossman. The authors wish to
 JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 1971, 15, 243-247 NUMBER 2 (MARCH) POSITIVE CONDITIONED SUPPRESSION: EFFECTS OF CS DURATION' KLAUS A. MICZEK AND SEBASTIAN P. GROSSMAN UNIVERSITY OF CHICAGO
JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 1971, 15, 243-247 NUMBER 2 (MARCH) POSITIVE CONDITIONED SUPPRESSION: EFFECTS OF CS DURATION' KLAUS A. MICZEK AND SEBASTIAN P. GROSSMAN UNIVERSITY OF CHICAGO
QUANTIFICATION OF DRUG CHOICE WITH THE GENERALIZED MATCHING LAW IN RHESUS MONKEYS MIKHAIL N. KOFFARNUS AND JAMES H. WOODS
 JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 2008, 89, 209 224 NUMBER 2(MARCH) QUANTIFICATION OF DRUG CHOICE WITH THE GENERALIZED MATCHING LAW IN RHESUS MONKEYS MIKHAIL N. KOFFARNUS AND JAMES H. WOODS
JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 2008, 89, 209 224 NUMBER 2(MARCH) QUANTIFICATION OF DRUG CHOICE WITH THE GENERALIZED MATCHING LAW IN RHESUS MONKEYS MIKHAIL N. KOFFARNUS AND JAMES H. WOODS
OPIOID ANTINOCICEPTION AND ANTIHYPERALGESIA IN COMBINATION WITH METABOTROPIC GLUTAMATE RECEPTOR ANTAGONISM. Dana Elaine Daugherty
 OPIOID ANTINOCICEPTION AND ANTIHYPERALGESIA IN COMBINATION WITH METABOTROPIC GLUTAMATE RECEPTOR ANTAGONISM Dana Elaine Daugherty A thesis submitted to the faculty of the University of North Carolina at
OPIOID ANTINOCICEPTION AND ANTIHYPERALGESIA IN COMBINATION WITH METABOTROPIC GLUTAMATE RECEPTOR ANTAGONISM Dana Elaine Daugherty A thesis submitted to the faculty of the University of North Carolina at
The stimulus effect of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline is similar to that of cocaine but different from that of amphetamine
![The stimulus effect of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline is similar to that of cocaine but different from that of amphetamine The stimulus effect of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline is similar to that of cocaine but different from that of amphetamine](/thumbs/90/102639907.jpg) Pharmacology, Biochemistry and Behavior 71 (2002) 205 213 www.elsevier.com/locate/pharmbiochembeh The stimulus effect of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline is similar to that of cocaine
Pharmacology, Biochemistry and Behavior 71 (2002) 205 213 www.elsevier.com/locate/pharmbiochembeh The stimulus effect of 5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline is similar to that of cocaine
A comparison of MDMA and d-amphetamine in the drug discrimination paradigm.
 A comparison of MDMA and d-amphetamine in the drug discrimination paradigm. D.N. Harper, A. Crowther, S. Schenk School of Psychology Victoria University of Wellington, New Zealand AIM To compare the subjective
A comparison of MDMA and d-amphetamine in the drug discrimination paradigm. D.N. Harper, A. Crowther, S. Schenk School of Psychology Victoria University of Wellington, New Zealand AIM To compare the subjective
Pharmacology, Biochemistry and Behavior
 Pharmacology, Biochemistry and Behavior 90 (2008) 453 462 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Relationship
Pharmacology, Biochemistry and Behavior 90 (2008) 453 462 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Relationship
The Effects of Chronic Cocaine Self-Administration on the Menstrual Cycle in Rhesus Monkeys
 0022-3565/97/2811-0070$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 281, No. 1 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/97/2811-0070$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 281, No. 1 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Assessment of the Relative Reinforcing Strength of Cocaine in. Socially Housed Monkeys Using a Choice Procedure
 JPET Fast This article Forward. has not Published been copyedited on and August formatted. 30, The 2004 final version as DOI:10.1124/jpet.104.073411 may differ from this version. 1 Assessment of the Relative
JPET Fast This article Forward. has not Published been copyedited on and August formatted. 30, The 2004 final version as DOI:10.1124/jpet.104.073411 may differ from this version. 1 Assessment of the Relative
Adolescent Prozac Exposure Enhances Sensitivity to Cocaine in Adulthood INTRODUCTION
 INTRODUCTION Epidemiologic reports indicate that mood disorders in children and adolescents are quite common, with up to 70% of depressed children and adolescents experiencing a recurrence within 5 years
INTRODUCTION Epidemiologic reports indicate that mood disorders in children and adolescents are quite common, with up to 70% of depressed children and adolescents experiencing a recurrence within 5 years
GOALS AND OBJECTIVES
 SUBOXONE AND VIVITROL: ARE THERE DISPARITIES SURFACING IN MEDICATION ASSISTED TREATMENTS? P R E S E N T E D B Y D R. K I AM E M AH A N I A H & D R. M Y E C H I A M I N T E R - J O R D AN GOALS AND OBJECTIVES
SUBOXONE AND VIVITROL: ARE THERE DISPARITIES SURFACING IN MEDICATION ASSISTED TREATMENTS? P R E S E N T E D B Y D R. K I AM E M AH A N I A H & D R. M Y E C H I A M I N T E R - J O R D AN GOALS AND OBJECTIVES
Naltrexone reduces ethanol- and sucrose-reinforced responding in rhesus monkeys
 Psychopharmacology (1998) 139:53 61 Springer-Verlag 1998 ORIGINAL INVESTIGATION Keith L. Williams Gail Winger Eric D. Pakarinen James H. Woods Naltrexone reduces ethanol- and sucrose-reinforced responding
Psychopharmacology (1998) 139:53 61 Springer-Verlag 1998 ORIGINAL INVESTIGATION Keith L. Williams Gail Winger Eric D. Pakarinen James H. Woods Naltrexone reduces ethanol- and sucrose-reinforced responding
TRAMADOL, A CENTRALLY ACTING OPIOID : ANTICONVULSANT EFFECT AGAINST MAXIMAL ELECTROSHOCK SEIZURE IN MICE
 Indian J Physiol Pharmacol 1998; 42 (3) : 407-411 TRAMADOL, A CENTRALLY ACTING OPIOID : ANTICONVULSANT EFFECT AGAINST MAXIMAL ELECTROSHOCK SEIZURE IN MICE ANSHU MANOCHA, KRISHNA K. SHARMA* AND PRAMOD K.
Indian J Physiol Pharmacol 1998; 42 (3) : 407-411 TRAMADOL, A CENTRALLY ACTING OPIOID : ANTICONVULSANT EFFECT AGAINST MAXIMAL ELECTROSHOCK SEIZURE IN MICE ANSHU MANOCHA, KRISHNA K. SHARMA* AND PRAMOD K.
Some Parameters of the Second-Order Conditioning of Fear in Rats
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Behavior and Biological Sciences Papers in the Biological Sciences 1969 Some Parameters of the Second-Order Conditioning
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Behavior and Biological Sciences Papers in the Biological Sciences 1969 Some Parameters of the Second-Order Conditioning
Prevention of Development of Tolerance and Dependence to Opiate in Mice by BR-16A (Mentat) A Herbal Psychotropic Preparation
 [Indian Journal of Experimental Biology (1992): (30), 885] Prevention of Development of Tolerance and Dependence to Opiate in Mice by BR-16A (Mentat) A Herbal Psychotropic Preparation Kulkarni, S.K. and
[Indian Journal of Experimental Biology (1992): (30), 885] Prevention of Development of Tolerance and Dependence to Opiate in Mice by BR-16A (Mentat) A Herbal Psychotropic Preparation Kulkarni, S.K. and
Buprenorphine pharmacology
 Buprenorphine pharmacology Victorian Opioid Management ECHO Department of Addiction Medicine St Vincent s Hospital Melbourne 2018 Page 1 Opioids full, partial, antagonist Full Agonists - bind completely
Buprenorphine pharmacology Victorian Opioid Management ECHO Department of Addiction Medicine St Vincent s Hospital Melbourne 2018 Page 1 Opioids full, partial, antagonist Full Agonists - bind completely
Actigraphy-based sleep parameters during the reinstatement of methamphetamine self-administration in rhesus monkeys.
 Actigraphy-based sleep parameters during the reinstatement of methamphetamine self-administration in rhesus monkeys. Laís F. Berro, Emory University Monica L. Andersen, Universidade Federal de São Paulo
Actigraphy-based sleep parameters during the reinstatement of methamphetamine self-administration in rhesus monkeys. Laís F. Berro, Emory University Monica L. Andersen, Universidade Federal de São Paulo
Psycho pharmacology. Similarity of the Discriminative Stimulus Effects of Ketamine, Cyclazocine, and Dextrorphan in the Pigeon
 Psychopharmacology (1981) 73:286-291 Psycho pharmacology 9 Springer-Verlag 1981 Similarity of the Discriminative Stimulus Effects of Ketamine, Cyclazocine, and Dextrorphan in the Pigeon Seymore Hefting,
Psychopharmacology (1981) 73:286-291 Psycho pharmacology 9 Springer-Verlag 1981 Similarity of the Discriminative Stimulus Effects of Ketamine, Cyclazocine, and Dextrorphan in the Pigeon Seymore Hefting,
EDUCATIONAL COMMENTARY METHADONE
 EDUCATIONAL COMMENTARY METHADONE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits see the Continuing Education
EDUCATIONAL COMMENTARY METHADONE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits see the Continuing Education
Supporting Information
 Supporting Information Schlosburg et al. 10.1073/pnas.1219159110 SI Materials and Methods: Quantification of Heroin Metabolites Sample Collection. Trunk blood was collected in a 1:1 ratio with acetate
Supporting Information Schlosburg et al. 10.1073/pnas.1219159110 SI Materials and Methods: Quantification of Heroin Metabolites Sample Collection. Trunk blood was collected in a 1:1 ratio with acetate
Effects of the dopamine D2 agonist, quinpirole, on time and number processing in rats
 Pharmacology, Biochemistry and Behavior 68 (2001) 147±155 www.elsevier.com/locate/pharmbiochembeh Effects of the dopamine D2 agonist, quinpirole, on time and number processing in rats Angelo Santi*, Romina
Pharmacology, Biochemistry and Behavior 68 (2001) 147±155 www.elsevier.com/locate/pharmbiochembeh Effects of the dopamine D2 agonist, quinpirole, on time and number processing in rats Angelo Santi*, Romina
Slide 1. Slide 2. Slide 3. Opioid (Narcotic) Analgesics and Antagonists. Lesson 6.1. Lesson 6.1. Opioid (Narcotic) Analgesics and Antagonists
 Slide 1 Opioid (Narcotic) Analgesics and Antagonists Chapter 6 1 Slide 2 Lesson 6.1 Opioid (Narcotic) Analgesics and Antagonists 1. Explain the classification, mechanism of action, and pharmacokinetics
Slide 1 Opioid (Narcotic) Analgesics and Antagonists Chapter 6 1 Slide 2 Lesson 6.1 Opioid (Narcotic) Analgesics and Antagonists 1. Explain the classification, mechanism of action, and pharmacokinetics
Stimulus control of foodcup approach following fixed ratio reinforcement*
 Animal Learning & Behavior 1974, Vol. 2,No. 2, 148-152 Stimulus control of foodcup approach following fixed ratio reinforcement* RICHARD B. DAY and JOHN R. PLATT McMaster University, Hamilton, Ontario,
Animal Learning & Behavior 1974, Vol. 2,No. 2, 148-152 Stimulus control of foodcup approach following fixed ratio reinforcement* RICHARD B. DAY and JOHN R. PLATT McMaster University, Hamilton, Ontario,
Kappa Opioids in Rhesus Monkeys. II. Analysis of the Antagonistic Actions of Quadazocine and fl-funaltrexam ine
 22-3565/87/2422-O421$2.O/ THE Jouiw*.. or Piunco.ocv AND EXPERMENTAL TnswsuTtcs CopyrightO 1987 by The American Society for Pharmacology and Experimental Therapeutics Vol. 242, No. 2 Printed in U.S.A.
22-3565/87/2422-O421$2.O/ THE Jouiw*.. or Piunco.ocv AND EXPERMENTAL TnswsuTtcs CopyrightO 1987 by The American Society for Pharmacology and Experimental Therapeutics Vol. 242, No. 2 Printed in U.S.A.
Introduction SHORT COMMUNICATION. Irma V. Belozertseva Anton Yu. Bespalov
 Naunyn-Schmiedeberg s Arch Pharmacol (1998) 358:270 274 Springer-Verlag 1998 SHORT COMMUNICATION Irma V. Belozertseva Anton Yu. Bespalov Effects of NMDA receptor channel blockers, dizocilpine and memantine,
Naunyn-Schmiedeberg s Arch Pharmacol (1998) 358:270 274 Springer-Verlag 1998 SHORT COMMUNICATION Irma V. Belozertseva Anton Yu. Bespalov Effects of NMDA receptor channel blockers, dizocilpine and memantine,
WR Fentanyl Symposium. Opioids, Overdose, and Fentanyls
 Opioids, Overdose, and Fentanyls Outline: What are opioids? Why are we experiencing and opioid crisis? Potency, purity, and product How do opioids cause overdose and overdose deaths? What is naloxone and
Opioids, Overdose, and Fentanyls Outline: What are opioids? Why are we experiencing and opioid crisis? Potency, purity, and product How do opioids cause overdose and overdose deaths? What is naloxone and
INTRODUCING NEW STIMULI IN FADING
 JOURNL OF THE EXPERMENTL NLYSS OF BEHVOR 1979, 32, 121-127 NUMBER (JULY) CQUSTON OF STMULUS CONTROL WHLE NTRODUCNG NEW STMUL N FDNG LNNY FELDS THE COLLEGE OF STTEN SLND fter establishing a discrimination
JOURNL OF THE EXPERMENTL NLYSS OF BEHVOR 1979, 32, 121-127 NUMBER (JULY) CQUSTON OF STMULUS CONTROL WHLE NTRODUCNG NEW STMUL N FDNG LNNY FELDS THE COLLEGE OF STTEN SLND fter establishing a discrimination
Interaction between Mu and Delta Opioid Receptor Agonists in an Assay of Capsaicin- Induced Thermal Allodynia in Rhesus Monkeys
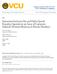 Virginia Commonwealth University VCU Scholars Compass Pharmacology and Toxicology Publications Dept. of Pharmacology and Toxicology 2012 Interaction between Mu and Delta Opioid Receptor Agonists in an
Virginia Commonwealth University VCU Scholars Compass Pharmacology and Toxicology Publications Dept. of Pharmacology and Toxicology 2012 Interaction between Mu and Delta Opioid Receptor Agonists in an
Reports from the Research Laboratories. of the Department of Psychiatry. Self-Administration of Amphetamine and Cocaine by Rats by ROY PICKENS.
 Reports from the Research Laboratories of the Department of Psychiatry SelfAdministration of Amphetamine and Cocaine by Rats by ROY PCKENS and TRAVS THOMPSON MSDf\1\ P5 R311r Report Number PR664 September
Reports from the Research Laboratories of the Department of Psychiatry SelfAdministration of Amphetamine and Cocaine by Rats by ROY PCKENS and TRAVS THOMPSON MSDf\1\ P5 R311r Report Number PR664 September
6/6/2018. Nalbuphine: Analgesic with a Niche. Mellar P Davis MD FCCP FAAHPM. Summary of Advantages. Summary of Advantages
 Nalbuphine: Analgesic with a Niche Mellar P Davis MD FCCP FAAHPM 1 Summary of Advantages Safe in renal failure- fecal excretion Analgesia equal to morphine with fewer side effects Reduced constipation
Nalbuphine: Analgesic with a Niche Mellar P Davis MD FCCP FAAHPM 1 Summary of Advantages Safe in renal failure- fecal excretion Analgesia equal to morphine with fewer side effects Reduced constipation
Role of Dopamine D2-like Receptors in Cocaine Self-Administration: Studies with D2 Receptor Mutant Mice and Novel D2 Receptor Antagonists
 The Journal of Neuroscience, April 1, 2002, 22(7):2977 2988 Role of Dopamine D2-like Receptors in Cocaine Self-Administration: Studies with D2 Receptor Mutant Mice and Novel D2 Receptor Antagonists S.
The Journal of Neuroscience, April 1, 2002, 22(7):2977 2988 Role of Dopamine D2-like Receptors in Cocaine Self-Administration: Studies with D2 Receptor Mutant Mice and Novel D2 Receptor Antagonists S.
Opioids- Indica-ons, Equivalence, Dependence and Withdrawal Methadone Maintenance (OST) Paul Glue
 Opioids- Indica-ons, Equivalence, Dependence and Withdrawal Methadone Maintenance (OST) Paul Glue Scope Pharmacology of Opioids Equivalence Dependence and Withdrawal Methadone Maintenance (OST) 3 Opioid
Opioids- Indica-ons, Equivalence, Dependence and Withdrawal Methadone Maintenance (OST) Paul Glue Scope Pharmacology of Opioids Equivalence Dependence and Withdrawal Methadone Maintenance (OST) 3 Opioid
STUDIES OF WHEEL-RUNNING REINFORCEMENT: PARAMETERS OF HERRNSTEIN S (1970) RESPONSE-STRENGTH EQUATION VARY WITH SCHEDULE ORDER TERRY W.
 JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 2000, 73, 319 331 NUMBER 3(MAY) STUDIES OF WHEEL-RUNNING REINFORCEMENT: PARAMETERS OF HERRNSTEIN S (1970) RESPONSE-STRENGTH EQUATION VARY WITH SCHEDULE
JOURNAL OF THE EXPERIMENTAL ANALYSIS OF BEHAVIOR 2000, 73, 319 331 NUMBER 3(MAY) STUDIES OF WHEEL-RUNNING REINFORCEMENT: PARAMETERS OF HERRNSTEIN S (1970) RESPONSE-STRENGTH EQUATION VARY WITH SCHEDULE
Medication-Assisted Treatment and HIV/AIDS: Aspects in Treating HIV- Infected Drug Users.
 Slide #1 Medication-Assisted Treatment and HIV/AIDS: Aspects in Treating HIV- Infected Drug Users. R. Douglas Bruce, MD, MA, MSc Assistant Professor Yale AIDS Program Medical Director South Central Rehabilitation
Slide #1 Medication-Assisted Treatment and HIV/AIDS: Aspects in Treating HIV- Infected Drug Users. R. Douglas Bruce, MD, MA, MSc Assistant Professor Yale AIDS Program Medical Director South Central Rehabilitation
Abuse Potential of Morphine/ Dextromethorphan Combinations
 S26 Journal of Pain and Symptom Management Vol. 19 No. 1(Suppl.) January 2000 Proceedings Supplement NMDA-Receptor Antagonists: Evolving Role in Analgesia Abuse Potential of Morphine/ Dextromethorphan
S26 Journal of Pain and Symptom Management Vol. 19 No. 1(Suppl.) January 2000 Proceedings Supplement NMDA-Receptor Antagonists: Evolving Role in Analgesia Abuse Potential of Morphine/ Dextromethorphan
The discriminative effects of the κ-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects
 DOI 1.17/s213-9-1771-5 ORIGINAL INVESTIGATION The discriminative effects of the κ-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects Eduardo R. Butelman
DOI 1.17/s213-9-1771-5 ORIGINAL INVESTIGATION The discriminative effects of the κ-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects Eduardo R. Butelman
Enadoline and Butorphanol: Evaluation of -Agonists on Cocaine Pharmacodynamics and Cocaine Self-Administration in Humans
 0022-3565/01/2991-147 158$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 299, No. 1 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 3969/928004
0022-3565/01/2991-147 158$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 299, No. 1 Copyright 2001 by The American Society for Pharmacology and Experimental Therapeutics 3969/928004
Reports from the Research Laboratories
 Reports from the Research Laboratories of the Department of Psychiatry University of Minnesota Effects of Morphine on Behavior Maintained by Four Simple Food Reinforcement Schedules by T. Thompson, J.
Reports from the Research Laboratories of the Department of Psychiatry University of Minnesota Effects of Morphine on Behavior Maintained by Four Simple Food Reinforcement Schedules by T. Thompson, J.
PAIN & ANALGESIA. often accompanied by clinical depression. fibromyalgia, chronic fatigue, etc. COX 1, COX 2, and COX 3 (a variant of COX 1)
 Pain - subjective experience associated with detection of tissue damage ( nociception ) acute - serves as a warning chronic - nociception gone bad often accompanied by clinical depression fibromyalgia,
Pain - subjective experience associated with detection of tissue damage ( nociception ) acute - serves as a warning chronic - nociception gone bad often accompanied by clinical depression fibromyalgia,
STIMULUS FUNCTIONS IN TOKEN-REINFORCEMENT SCHEDULES CHRISTOPHER E. BULLOCK
 STIMULUS FUNCTIONS IN TOKEN-REINFORCEMENT SCHEDULES By CHRISTOPHER E. BULLOCK A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR
STIMULUS FUNCTIONS IN TOKEN-REINFORCEMENT SCHEDULES By CHRISTOPHER E. BULLOCK A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR
The generality of within-session patterns of responding: Rate of reinforcement and session length
 Animal Learning & Behavior 1994, 22 (3), 252-266 The generality of within-session patterns of responding: Rate of reinforcement and session length FRANCES K. MCSWEENEY, JOHN M. ROLL, and CARI B. CANNON
Animal Learning & Behavior 1994, 22 (3), 252-266 The generality of within-session patterns of responding: Rate of reinforcement and session length FRANCES K. MCSWEENEY, JOHN M. ROLL, and CARI B. CANNON
Does nicotine alter what is learned about non-drug incentives?
 East Tennessee State University Digital Commons @ East Tennessee State University Undergraduate Honors Theses 5-2014 Does nicotine alter what is learned about non-drug incentives? Tarra L. Baker Follow
East Tennessee State University Digital Commons @ East Tennessee State University Undergraduate Honors Theses 5-2014 Does nicotine alter what is learned about non-drug incentives? Tarra L. Baker Follow
Increasing the persistence of a heterogeneous behavior chain: Studies of extinction in a rat model of search behavior of working dogs
 Increasing the persistence of a heterogeneous behavior chain: Studies of extinction in a rat model of search behavior of working dogs Eric A. Thrailkill 1, Alex Kacelnik 2, Fay Porritt 3 & Mark E. Bouton
Increasing the persistence of a heterogeneous behavior chain: Studies of extinction in a rat model of search behavior of working dogs Eric A. Thrailkill 1, Alex Kacelnik 2, Fay Porritt 3 & Mark E. Bouton
A Behavioral Economic Analysis of Concurrent Ethanol- and Water-Reinforced Responding in Different Preference Conditions
 0145-6008/00/2407-0980$03.00/0 ALCOHOLSM: CLNCAL AND EXPERMENTAL RESEARCH Vol. 24, No. 7 July 2000 A Behavioral Economic Analysis of Concurrent Ethanol- and Water-Reinforced Responding in Different Preference
0145-6008/00/2407-0980$03.00/0 ALCOHOLSM: CLNCAL AND EXPERMENTAL RESEARCH Vol. 24, No. 7 July 2000 A Behavioral Economic Analysis of Concurrent Ethanol- and Water-Reinforced Responding in Different Preference
THE OPIUM POPPY OPIOID PHARMACOLOGY 2/18/16. PCTH 300/305 Andrew Horne, PhD MEDC 309. Papaver somniferum. Poppy Seeds Opiates
 OPIOID PHARMACOLOGY PCTH 300/305 Andrew Horne, PhD andrew.horne@ubc.ca MEDC 309 THE OPIUM POPPY Papaver somniferum Sleep-bringing poppy Poppy Seeds Opiates Opium Poppy Straw 1 OPIATES VS. OPIOIDS Opiates:
OPIOID PHARMACOLOGY PCTH 300/305 Andrew Horne, PhD andrew.horne@ubc.ca MEDC 309 THE OPIUM POPPY Papaver somniferum Sleep-bringing poppy Poppy Seeds Opiates Opium Poppy Straw 1 OPIATES VS. OPIOIDS Opiates:
PHRM20001: Pharmacology - How Drugs Work!
 PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
PHRM20001: Pharmacology - How Drugs Work Drug: a chemical that affects physiological function in a specific way. Endogenous substances: hormones, neurotransmitters, antibodies, genes. Exogenous substances:
Assessment of Opioid Partial Agonist Activity with a Three- Choice Hydromorphone Dose-Discrimination Procedure 1
 0022-3565/99/2893-1350$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 289, No. 3 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/99/2893-1350$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 289, No. 3 Copyright 1999 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Buprenorphine as a Treatment Option for Opioid Use Disorder
 Buprenorphine as a Treatment Option for Opioid Use Disorder Joji Suzuki, MD Assistant Professor of Psychiatry Harvard Medical School Director, Division of Addiction Psychiatry Brigham and Women s Hospital
Buprenorphine as a Treatment Option for Opioid Use Disorder Joji Suzuki, MD Assistant Professor of Psychiatry Harvard Medical School Director, Division of Addiction Psychiatry Brigham and Women s Hospital
Fentanyls and Naloxone. Opioids, Overdose, and Naloxone
 Opioids, Overdose, and Naloxone Presenter Disclosure Presenter s Name: Michael Beazely I have no current or past relationships with commercial entities Speaking Fees for current program: I have received
Opioids, Overdose, and Naloxone Presenter Disclosure Presenter s Name: Michael Beazely I have no current or past relationships with commercial entities Speaking Fees for current program: I have received
8 Respiratory depression by tramadol in the cat: involvement of opioid receptors?
 8 Respiratory depression by tramadol in the cat: involvement of opioid receptors? A MAJOR ADVERSE effect of opioid analgesics is respiratory depression which is probably mediated by an effect on µ-opioid
8 Respiratory depression by tramadol in the cat: involvement of opioid receptors? A MAJOR ADVERSE effect of opioid analgesics is respiratory depression which is probably mediated by an effect on µ-opioid
Conditioned Effects Produced by Naltrexone Doses That Reduce Ethanol-Reinforced Responding in Rhesus Monkeys. Keith L. Williams and James H.
 145-X/99/234-78$3./ ALCOHOLISM: CLINICAL AND EXPERIMENTAL RCSEARC.II Vol. 23, No. 4 April 1999 Conditioned Effects Produced by Naltrexone Doses That Reduce Ethanol-Reinforced Responding in Rhesus Monkeys
145-X/99/234-78$3./ ALCOHOLISM: CLINICAL AND EXPERIMENTAL RCSEARC.II Vol. 23, No. 4 April 1999 Conditioned Effects Produced by Naltrexone Doses That Reduce Ethanol-Reinforced Responding in Rhesus Monkeys
An overview of Medication Assisted Treatment (MAT) and acute pain management on MAT
 An overview of Medication Assisted Treatment (MAT) and acute pain management on MAT Goals of Discussion Recognize opioid use disorder (OUD) Discuss the pharmacology of medication assisted treatments (MAT)
An overview of Medication Assisted Treatment (MAT) and acute pain management on MAT Goals of Discussion Recognize opioid use disorder (OUD) Discuss the pharmacology of medication assisted treatments (MAT)
Opioid Abuse: A Dark Tunnel
 Opioid Abuse: A Dark Tunnel Sandra D. Comer, Ph.D. Professor of Neurobiology Division on Substance Abuse Department of Psychiatry Columbia University New York State Psychiatric Institute New York, NY Charles
Opioid Abuse: A Dark Tunnel Sandra D. Comer, Ph.D. Professor of Neurobiology Division on Substance Abuse Department of Psychiatry Columbia University New York State Psychiatric Institute New York, NY Charles
Antinociceptive Interactions between the Imidazoline I 2 Receptor Agonist 2-BFI and Opioids in Rats: Role of Efficacy at the m-opioid Receptor
 1521-0103/357/3/509 519$25.00 http://dx.doi.org/10.1124/jpet.116.232421 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 357:509 519, June 2016 Copyright ª 2016 by The American
1521-0103/357/3/509 519$25.00 http://dx.doi.org/10.1124/jpet.116.232421 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 357:509 519, June 2016 Copyright ª 2016 by The American
Problems of Drug Dependence, 1993: Proceedings of the 55th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc.
 Problems of Drug Dependence, 1993: Proceedings of the 55th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. Volume I: Plenary Session Symposia and Annual Reports Editor: Louis
Problems of Drug Dependence, 1993: Proceedings of the 55th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. Volume I: Plenary Session Symposia and Annual Reports Editor: Louis
