ORANGE BOOK ORANGE BOOK
|
|
|
- Kelley Howard
- 6 years ago
- Views:
Transcription
1 1
2 INTRODUCTION: DEFINITION: The official title of the book is Approved Drug Products with Therapeutic Equivalence Evaluations. Orange book is a publication by the Food and Drug Administration which contains a list of approved drug products with therapeutic equivalence evaluations. It is prepared by the Orange Book Staff, Center for Drug Evaluation and Research (CDER). It contains identified drug products on the basis of safety and effectiveness by the Food and Drug Administration under the Federal Food, Drug, and Cosmetics Act. 2
3 HISTORY: YEAR May 31, 1978 January, 1979 January 12, 1979 ACTION The Commissioner of the Food and Drug Administration sent a letter to officials of each state stating FDA's intent to provide a list of all prescription drug products that are approved by FDA for safety and effectiveness, along with therapeutic equivalence determinations for multi-source prescription products. The list was distributed (included only currently marketed prescription drug products approved by FDA through NDAs and ANDAs under the provision of section 505 of the Act) A complete discussion of the background and basis of FDA s therapeutic equivalence evaluation policy was published in the Federal Register (44 FR 2932) October 31, The final rule (includes FDA s response to the public comments on the proposal) was published in the Federal Register (45 FR 72582). The list incorporated appropriate corrections and additions.
4 OBJECTIVE: 1. To allow review of patterns of access and usage. 2. To allow discovery of use of unusual privileges. 3. To allow discovery of repeated attempts to bypass protections. 4. To serve as a deterrent by its existence. 5. To supply an additional form of user assurance. 4
5 5 NEED OF : Health Care Costs Continue to Increase So health cost of patient increases since branded drugs are costly Clinicians are increasingly encouraged to prescribe GENERIC drugs for effective treatment Before substituting a generic product, physicians and other decisions makers should consider the potential clinical and pharmacoeconomic consequences like over and Undertreatment, Adverse effects, Additional expenses and Cost savings To help avoid complications arising from product substitution, the FDA established a list of generic drugs that can be safely and appropriately substituted for brand products The FDA has prepared a list of drugs that are bioequivalent they can be substituted for each other These drugs are listed in a federal publication called Approved Drug Products With Therapeutic Equivalence Evaluations known as the Orange Book
6 CONTENT OF : 6 PREFACE TO 32 nd EDITION 1. INTRODUCTION 1.1 Content and Exclusion 1.2 Therapeutic Equivalence-Related Terms 1.3 Statistical Criteria for Bioequivalence 1.4 Reference Listed Drug 1.5 General Policies and Legal Status 1.6 Practitioner/User Responsibilities 1.7 Therapeutic Equivalence Evaluations Codes 1.8 Description of Special Situations 1.9 Therapeutic Equivalence Code Change for a Drug Entity 1.10 Change of the Therapeutic Equivalence Evaluation for a Single Product 1.11 ORANGE Discontinued BOOK Section
7 2. HOW TO USE THE DRUG PRODUCTS LISTS 2.1 Key Sections for Using the Drug Product Lists 2.2 Drug Product Illustration 2.3 Therapeutic Equivalence Evaluations Illustration 7 3. DRUG PRODUCT LISTS: Prescription Drug Product List OTC Drug Product List Drug Products with Approval under Section 505 of the Act Administered by the Center for Biologics Evaluation and Research List Discontinued Drug Product List Orphan Products Designations and Approvals List Drug Products Which Must Demonstrate in vivo Bioavailability
8 4. APPENDICES A. Product Name Index A-1 B. Product Name Index Listed by C. Uniform Terms 5. PATENT AND EXCLUSIVITY INFORMATION ADDENDUM A. Patent and Exclusivity Lists B. Patent and Exclusivity Terms 8
9 1. INTRODUCTION 1.1 Content And Exclusion: The List is composed of four parts: 1. Approved prescription drug with therapeutic equivalence evaluations; 2. Approved over-the-counter (OTC) drug products for those drugs that may not be marketed without NDAs or ANDAs because they are not covered under existing OTC monographs; 3. Drug products with approval under Section 505 of the Act administered by the Center for Biologics Evaluation and Research; and 4. A cumulative list of approved products that have been discontinued from marketing. 9
10 The List also includes: Indices of prescription and OTC drug by trade or established name (if no trade name exists) and by applicant name (holder of the approved application). The latter list includes applicants names as abbreviated in this publication An Addendum contains drug patent and exclusivity information for the Prescription and OTC Drug Product Lists, and for the Drug Products with Approval under Section 505 of the Act Administered by the Center for Biologics Evaluation and Research. It includes only full approval products and not tentative approval products. The List excludes: Prior to the 6th Edition, the publication had excluded OTC drug products with approval under Section 505 of the Act administered by the Center for Biologics Evaluation and Research. Distributors or re-packagers of products. 10
11 1.2 Therapeutic-Equivalence Related Terms 1. Pharmaceutical Equivalents: Drug products are considered pharmaceutical equivalents if they contain the same active ingredient(s), are of the same dosage form, route of administration and are identical in strength or concentration (e.g., chlordiazepoxide hydrochloride, 5mg capsules). 2. Pharmaceutical Alternatives: Drug products are considered pharmaceutical alternatives if they contain the same therapeutic moiety, but are different salts, esters, or complexes of that moiety, or are different dosage forms or strengths (e.g., tetracycline hydrochloride, 250mg capsules vs. tetracycline phosphate complex, 250mg capsules; quinidine sulfate, 200mg tablets vs. quinidine sulfate, 200mg capsules). 11
12 3.Therapeutic Equivalents: Drug products are considered to be therapeutic equivalents only if they are pharmaceutical equivalents and if they can be expected to have the same clinical effect and safety profile when administered to patients under the conditions specified in the labeling. 4.Bioavailability: This term means the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action. 5.Bioequivalent Drug Products: This term describes pharmaceutical equivalent or pharmaceutical alternative products that display comparable bioavailability when studied under similar experimental conditions. 12
13 1.3 Statistical criteria for Bioequiavalence Under the Drug Price Competition and Patent Term Restoration Act of 1984, (Hatch-Waxman Act) manufacturers seeking approval to market a generic drug product must submit data demonstrating that the drug product is bioequivalent to the pioneer (innovator) drug product. A major premise underlying the 1984 law is that bioequivalent drug products are therapeutically equivalent, and therefore, interchangeable. Methods used to define bioequivalence as per 21 CFR includes Pharmacokinetic (PK) studies, Pharmacodynamic (PD) studies, Comparative clinical trials, and In-vitro studies The ORANGE choice BOOK of study used is based on the site of action of the drug and the ability of the study design to compare drug delivered to that site by the two 13
14 Two situations are tested with this statistical methodology: The first of the two one-sided tests determines whether a generic product (test), when substituted for a brand-name product (reference) is significantly less bioavailable. The second of the two one-sided tests determines whether a brand-name product when substituted for a generic product is significantly less bioavailable. Based on the opinions of FDA medical experts, a difference of greater than 20% for each of the above tests was determined to be significant, and therefore, undesirable for all drug products. 14 Numerically, this is expressed as a limit of test-product average/referenceproduct average of 80% for the first statistical test and a limit of referenceproduct average/test-product average of 80% for the second statistical test. By convention, all data is expressed as a ratio of the average response (AUC and Cmax) for test/reference, so the limit expressed in the second statistical test ORANGE is 125% BOOK(reciprocal of 80%).
15 For statistical reasons, all data is log-transformed prior to conducting statistical testing. In practice, these statistical tests are carried out using an analysis of variance procedure (ANOVA) and calculating a 90% confidence interval for each pharmacokinetic parameter (Cmax and AUC). This system of assessing bioequivalence of generic products assures that these substitutable products do not deviate substantially in in-vivo performance from the reference product. The primary concern from the regulatory point of view is the protection of patient against approval of products that are not bioequivalent. 15
16 1.4 REFERENCE LISTED DRUG (RLD) A reference listed drug (21 CFR (a)(3)) means the listed drug identified by FDA as the drug product upon which, an applicant rely on seeking approval for its ANDA. FDA has identified in the Prescription Drug Product and OTC Drug Product Lists those Reference Listed Drugs, to which the in vivo bioequivalence (reference standard) and, in some instances, the in vitro bioequivalence of the applicant's product is compared. 16
17 The reference listed drug is identified by the symbol "+" in the Prescription and Over-the-Counter (OTC) Drug Product Lists. By designating a single reference listed drug as the standard to which all generic versions must be shown to be bioequivalent, FDA hopes to avoid possible significant variations among generic drugs and their brand name counterpart. 17
18 1.5 General Policies and Legal Status The list contains public information and advice for product selection. These evaluations do not constitute determinations that any product is in violation of the Act or that any product is preferable to any other. 18
19 Responsibilities Professional care and judgement should be exercised in using the list. Practitioner should be aware of the multi-source and single-source drug products. Multisource Drug Product means those pharmaceutical equivalents available from more than one manufacturer. For such products, a therapeutic equivalence (TE) code is included and, in addition, product information is highlighted in bold face and underlined. Single-Source Drug Product is also included on the list but no therapeutic equivalence code is included with such products. If only one approved product is available for particular active ingredient, dosage form, route of administration, and strength then it is known as single-source drug product. Products on the List are identified by the names of the holders of approved applications (applicants) who may not necessarily be the manufacturer of the product: Applicants: (1) Manufacturer 19 (2) Contract Manufacturer
20 1.7 Therapeutic Equivalence Evaluation Codes: The coding system for therapeutic equivalence evaluations is constructed users to determine quickly whether the Agency has evaluated a particular approved product as therapeutically equivalent to other pharmaceutically equivalent products (first letter) and to provide additional information on the basis of FDA's evaluations (second letter). The two basic categories into which multisource drugs have been placed are indicated by the first letter as follows: CODE: A CODE: B 20
21 CODE- A Drug product that FDA considers to be therapeutically equivalent to other pharmaceutically equivalent products, i.e., drug products for which: There are no known or suspected bio-equivalence problems. These are designated as AA, AN, AO, AP or AT, depending on the dosage form; or Actual or potential bio-equivalence problems have been resolved with adequate in-vivo and/or in-vitro evidence supporting bio-equivalence. These are designated as AB. 21
22 Drug product designated with code A fall under the two main policies: 1.A therapeutically equivalent rating is assigned to pharmaceutically equivalent products so long as they are manufactured in accordance with Current Good Manufacturing Practice regulations and meet the other requirements of their approved applications (these are designated AA, AN, AO, AP, or AT, depending on the dosage form, as described below); or 2.For those DESI (Drug Efficacy Study Implementation) drug products and for post-1962 drug products in a dosage form presenting a potential bioequivalence problem, an evaluation of therapeutic equivalence is assigned to pharmaceutical equivalents only if the approved application contains adequate scientific evidence establishing through in vivo and/or in vitro studies the bioequivalence of the product to a selected reference product (these products are designated as AB). 22
23 CODE INDEX 23 AA: Products in conventional dosage forms not presenting bioequivalence problems. AB, AB1, AB2, AB3: Products meeting necessary bio-equivalence requirements. (In certain instances, a number is added to end of the AB code to make a three character code, which is assigned only in situations when more than one reference listed drug of the same strength has been designated under the same heading. Two or more reference listed drugs are generally selected only when there are at-least two potential reference drug products which are not bio-equivalent to each other). AN: AO: Solutions and powder for aerosolization Injectable oil solutions AP: Injectable aqueous solutions and intra-venous non-aqueous solutions AT: Topical products
24 CODE-B Drug product that FDA at this time, considers not to be therapeutically equivalent to other pharmaceutically equivalent products, i.e. Drug products for which actual or potential bioequivalence problems have not been resolved by adequate evidence of bio-equivalence. Often the problem is with specific dosage forms rather than with active ingredients. These are designated as BC, BD, BE, BN, BP, BR, BS, BT, BX or B*. Drug product designated with code B fall under the three main policies: The drug products contain active ingredients or are manufactured in dosage forms that have been identified by the Agency as having documented bio-equivalence problems or a significant potential for such problems and for which no adequate studies demonstrating bioequivalence have been submitted to FDA; or 2. The quality standards are inadequate or FDA has an insufficient basis to determine therapeutic equivalence; or 3. The drug products are under regulatory review.
25 CODE INDEX B*: Drug products requiring further FDA investigation and review to determine therapeutic equivalence BC: Extended release dosage forms (capsules, injectables and tablets) BD: Active ingredients and dosage forms with documented bio-equivalence problems. BE: Delayed release oral dosage forms BN: Products in aerosol-nebulizer drug delivery systems BP: Active ingredients and dosage forms with potential bio-equivalence problems BR: Suppositories or enemas that delivers drug for systemic absorption BS: Products having drug standard deficiencies BX: Drug products for which the data are in-sufficient to determine therapeutic equivalence 25
26 1.8 Description of special situations: Certain drugs listed in the orange book present special situations that merit further discussion. Following is a description of those special situations: Amino-acid and Protein Hydrolysate injections: These products differ in the amount and kinds of amino-acids they contain, and therefore, are not considered pharmaceutical equivalents. For this reasons, these products are not considered therapeutically equivalent. At the same time, the Agency believes that it is appropriate to point out that where nitrogen balance is the sole therapeutic objective and individual amino acid content is not a consideration, pharmaceutical alternatives with the same total amount of nitrogen content may be considered therapeutically equivalent. Follitropin alpha and beta: Based on available data derived from physico-chemical tests and bio-assay, follitropin alpha and follitropin beta are indistinguishable. 26
27 Gaviscon Gaviscon is an OTC product which has been marketed since September The active ingredients in this product, aluminum hydroxide and magnesium trisilicate, were reviewed by the Agency's OTC Antacid Panel and were considered to be safe and effective ingredients (Category I) by that Panel. However, the tablet failed to pass the antacid test which is required of all antacid products. The Agency, therefore, placed the tablet in Category III for lack of effectiveness. 27
28 Later a full NDA with clinical studies was submitted by Marion Laboratories, Inc., and approved by FDA on December 9, Gaviscon s activity in treating reflux acidity is made possible by the physical-chemical properties of the inactive ingredients, sodium bicarbonate and alginic acid. Therefore, all ANDAs which cite Gaviscon tablets as the listed drug must contain the inactive ingredients sodium bicarbonate and alginic acid. A full NDA will be required to support the effectiveness of the drug product if different inactive ingredients are to be substituted for sodium bicarbonate or alginic acid or if different proportions of these ingredients are to be used. 28
29 Patent certification (s) Reference listed drug based upon a suitability petition: An abbreviated new drug application that refers to a Reference Listed Drug (RLD) approved pursuant to a suitability petition must demonstrate that the proposed product is bioequivalent to the RLD, and it must include appropriate patent certification(s) and an exclusivity statement with respect to the listed drug which served as the basis for the approved suitability petition. 29
30 Waived exclusivity If a new drug application (NDA) submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act (Act) qualifies for exclusivity under sections 505(c)(3)(D) and 505(j)(5)(D), the exclusivity is listed in the Patent and Exclusivity Section of the Orange Book. If a drug product has received this exclusivity, the FDA will delay the approval of a 505(b)(2) application or an abbreviated new drug application (ANDA) under section 505(j) of the Act until the expiration of the exclusivity. If the listed drug is also protected by one or more patents, the approval date for the 505(b)(2) application or ANDA will be determined by the latest expiring patent or exclusivity listed in the Orange Book. 30
31 Therapeutic Equivalence Code Change for a Drug Entity The Agency will use the following procedures when, in response to a petition or on its own initiative, it is considering a change in the therapeutic equivalence code for approved multi-source drug products. Such changes will generally occur when the Agency becomes aware of new scientific information affecting the therapeutic equivalence of an entire category of drug products in the List (e.g., information concerning the active ingredient or the dosage form), rather than information concerning a single drug product within the category. These procedures will be used when a change in therapeutic equivalence code is under consideration for all drug products found in the Prescription Drug Product List under a specific drug entity and dosage form.
32 32 The change may be from the code signifying that the drug does not present a bioequivalence problem (e.g., AA) to a code signifying a bioequivalence problem (e.g., BP), or vice versa. This procedure does not apply to a change of a particular product code (e.g., a change from BP to AB or from AB to BX). Before making a change in a therapeutic equivalence code for an entire category of drugs, the Agency will announce in the Introduction to the Cumulative Supplement that it is considering the ORANGE change BOOK and will invite comment.
33 1.10 Change of the Therapeutic Equivalence Evaluation for a Single Product The aforementioned procedure does not apply to a change in a single drug product code. For example, a change in a single drug product's code from BP to AB as a result of the submission of an acceptable bioequivalence study ordinarily will not be the subject of notice and comment. Likewise, a change in a single drug product's code from AB to BX (e.g., as a result of new information raising a significant question as to bioequivalence) does not require notice and comment. 33
34 1.11 Discontinued section Approved products are added to the Discontinued Section of the Orange Book when the applicant holder notifies the Orange Book staff of the products not marketed status. Products may also be added if annual reports indicate the product is no longer marketed or other Agency administrative action (e.g., Withdrawal of an Application). 34
35 Active Ingredient DISCONTINUED DRUG PRODUCT LIST Dosage Form; Route Strength Proprietary Name Applicant ASCORBIC ACID; BIOTIN; CYANOCOBALAMIN; DEXPANTHENOL; ERGOCALCIFEROL; FOLIC ACID; NIACINAMIDE; PYRIDOXINE HYDROCHLORIDE; RIBOFLAVIN 5'- PHOSPHATE SODIUM; THIAMINE HYDROCHLORIDE; VITAMIN A PALMITATE; VITAMIN E 35 INJECTABLE; INJECTION 50MG/ML;0.03MG/ML; MG/ML;7.5MG/ML; 100 IU/ML;0.2MG/ML; 20MG/ML;2MG/ML; 1.8MG/ML;1.5MG/ML; 1,650 IU/ML;5 IU/ML BEROCCA PN ROCHE
36 2. HOW TO USE THE DRUG PRODUCT LISTS: 2.1 Key Sections for Using the Drug Product Lists This part of the publication contains illustrations, along with Drug Product Lists, indices, and lists of abbreviations and terms which facilitate their use Illustration: Depicts the format found in the Prescription Drug Product List Drug Product Lists: The Prescription and OTC drug product lists, arranged alphabetically by active ingredient (s), contains product identification information (active ingredients, dosage forms, routes of administration, product names, application holders, strengths) for single and multiple ingredient drug products. Also shown are the application number and drug product number. 36
37 Product Name Index: This is an index of Prescription and OTC Drug Products by established or trade names. These terms are listed in Appendix A Product Name Index Listed by Applicant: This is an index of Prescription and OTC Drug Products that cross references applicants to drug products. These terms are listed in Appendix B. Uniform terms: To improve readability, uniform terms are used to designate dosage forms, routes of administration and abbreviations used to express strengths. These terms are listed in the Appendix C. 37
38 3. DRUG PRODUCT LISTS: Prescription Drug Product List OTC Drug Product List Drug Products with Approval under Section 505 of the Act Administered by the Center for Biologics Evaluation and Research List Discontinued Drug Product List Orphan Products Designations and Approvals List Drug Products Which Must Demonstrate in vivo Bioavailability Only if Product Fails to Achieve Adequate Dissolution 4. APPENDICES A. Product Name Index A-1 B. Product Name Index Listed by C. Uniform Terms 5. PATENT AND EXCLUSIVITY INFORMATION ADDENDUM A. Patent and Exclusivity Lists 38 B. Patent ORANGE and BOOK Exclusivity Terms
39 GREEN BOOK The Generic Animal Drug and Patent Restoration act requires that each sponsor of an approved animal drug must submit to the FDA certain information regarding patents held for the animal drug or its method of use. The Act requires that this information, as well as a list of all animal drug products approved for safety and effectiveness, be made available to the public. This list must be updated monthly under the provisions of the Act. The list, known as the Green Book was therefore first published in January Updates have been added monthly since then. 39
APPROVED DRUG PRODUCTS with THERAPEUTIC EQUIVALENCE EVALUATIONS
 APPROVED DRUG PRODUCTS WITH THERAPEUTIC EQUIVALENCE EVALUATIONS 34 th EDITION THE PRODUCTS IN THIS LIST HAVE BEEN APPROVED UNDER SECTION 505 OF THE FEDERAL FOOD, DRUG, AND COSMETIC ACT. U.S. DEPARTMENT
APPROVED DRUG PRODUCTS WITH THERAPEUTIC EQUIVALENCE EVALUATIONS 34 th EDITION THE PRODUCTS IN THIS LIST HAVE BEEN APPROVED UNDER SECTION 505 OF THE FEDERAL FOOD, DRUG, AND COSMETIC ACT. U.S. DEPARTMENT
Clinical Endpoint Bioequivalence Study Review in ANDA Submissions. Ying Fan, Ph.D.
 Clinical Endpoint Bioequivalence Study Review in ANDA Submissions Ying Fan, Ph.D. 1 Disclaimer This presentation constitutes an informal communication that represents the best judgment of the speaker at
Clinical Endpoint Bioequivalence Study Review in ANDA Submissions Ying Fan, Ph.D. 1 Disclaimer This presentation constitutes an informal communication that represents the best judgment of the speaker at
January 2005 Legal Aspects of Pharmacy: Using the Orange Book for Bioequivalence Evaluations H03
 W-F Professional Associates, Inc. 400 Lake Cook Rd., Suite 207 Deerfield, IL 60015 847-945-8050 January 2005 Legal Aspects of Pharmacy: Using the Orange Book for Bioequivalence Evaluations 707-000-05-001-H03
W-F Professional Associates, Inc. 400 Lake Cook Rd., Suite 207 Deerfield, IL 60015 847-945-8050 January 2005 Legal Aspects of Pharmacy: Using the Orange Book for Bioequivalence Evaluations 707-000-05-001-H03
Interchangeable Drug Products - Additional Criteria
 Interchangeable Drug Products - Additional Criteria Principle: Decisions respecting interchangeability and drug lists remain in the domain of the institution responsible for the costs of the product which
Interchangeable Drug Products - Additional Criteria Principle: Decisions respecting interchangeability and drug lists remain in the domain of the institution responsible for the costs of the product which
DRUG PRODUCT PERFORMANCE: CONSIDERATIONS FOR INTERCHANGEABILITY OF MULTISOURCE DRUG SUBSTANCES AND DRUG PRODUCTS
 DRUG PRODUCT PERFORMANCE: CONSIDERATIONS FOR INTERCHANGEABILITY OF MULTISOURCE DRUG SUBSTANCES AND DRUG PRODUCTS Leon Shargel, Ph.D. Applied Biopharmaceutics, LLC Raleigh, NC 27603 ABSTRACT Drug product
DRUG PRODUCT PERFORMANCE: CONSIDERATIONS FOR INTERCHANGEABILITY OF MULTISOURCE DRUG SUBSTANCES AND DRUG PRODUCTS Leon Shargel, Ph.D. Applied Biopharmaceutics, LLC Raleigh, NC 27603 ABSTRACT Drug product
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
ANDA Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY December 3, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
I. BACKGROUND. Docket No. FDA-2009-P Dear Dr. Aikman:
 DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 Mark S. Aikman, Pharm.D. Vice President, Regulatory Affairs and Quality Assurance Osmotica Pharmaceutical Corp. 1205
DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 Mark S. Aikman, Pharm.D. Vice President, Regulatory Affairs and Quality Assurance Osmotica Pharmaceutical Corp. 1205
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
PEDIATRIC INFUVITE MULTIPLE VITAMINS FOR INFUSION National Drug Code Directory
 54643-5646-0 PEDIATRIC INFUVITE MULTIPLE VITAMINS FOR INFUSION National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration
54643-5646-0 PEDIATRIC INFUVITE MULTIPLE VITAMINS FOR INFUSION National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration
FDB FOOD AND DRUGS BOARD G H A N A GUIDELINES FOR CONDUCTING BIOEQUIVALENCE STUDIES
 FDB FOOD AND DRUGS BOARD G H A N A GUIDELINES FOR CONDUCTING BIOEQUIVALENCE STUDIES 1 SCOPE In pursuance of section 47 of the Food and Drugs Law 1992, P.N.D.C.L 305B, as amended by Act 523, 1996, these
FDB FOOD AND DRUGS BOARD G H A N A GUIDELINES FOR CONDUCTING BIOEQUIVALENCE STUDIES 1 SCOPE In pursuance of section 47 of the Food and Drugs Law 1992, P.N.D.C.L 305B, as amended by Act 523, 1996, these
Brand and Generic Drugs. Educational Objectives. Absorption
 Peter J. Rice, PharmD, PhD Associate Professor of Pharmacology East Tennessee State University Educational Objectives Pharmacokinetic Processes Distribution Metabolism Excretion Similarities Active ingredient(s)
Peter J. Rice, PharmD, PhD Associate Professor of Pharmacology East Tennessee State University Educational Objectives Pharmacokinetic Processes Distribution Metabolism Excretion Similarities Active ingredient(s)
Generic Drug Approval Process
 1 Generic Drug Approval Process Sharon Ricciardo Katherine P. Weld. M.S., Ph.D. AAVPT Veterinary Drug Regulatory Life Cycle Course March 2, 2011 Overview Background Approval Process Bioequivalence Special
1 Generic Drug Approval Process Sharon Ricciardo Katherine P. Weld. M.S., Ph.D. AAVPT Veterinary Drug Regulatory Life Cycle Course March 2, 2011 Overview Background Approval Process Bioequivalence Special
Clinical Studies in BE Evaluation of Generic Products. Brenda S. Gierhart, M.D. Medical Officer, Division of Clinical Review, Office of Generic Drugs
 Clinical Studies in BE Evaluation of Generic Products Brenda S. Gierhart, M.D. Medical Officer, Division of Clinical Review, Office of Generic Drugs 1 Disclaimer The opinions and information in this presentation
Clinical Studies in BE Evaluation of Generic Products Brenda S. Gierhart, M.D. Medical Officer, Division of Clinical Review, Office of Generic Drugs 1 Disclaimer The opinions and information in this presentation
PREFACE. Saskatchewan is participating in the Common Drug Review (CDR).
 PREFACE OBJECTIVES The Drug Plan has been established to: provide coverage to Saskatchewan residents for quality pharmaceutical products of proven therapeutic effectiveness; reduce the direct cost of prescription
PREFACE OBJECTIVES The Drug Plan has been established to: provide coverage to Saskatchewan residents for quality pharmaceutical products of proven therapeutic effectiveness; reduce the direct cost of prescription
4/26/2013. Libia Lugo Drug Specialist San Juan District Office Investigations Branch
 Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
ANDA Labeling Question Based Review September 11, 2013 GPhA/FDA ANDA Labeling Workshop/USP User Forum
 ANDA Labeling Question Based Review September 11, 2013 GPhA/FDA ANDA Labeling Workshop/USP User Forum Sarah Park, PharmD Jeanne Skanchy, RPh. Labeling Reviewers Office of Generic Drugs/Division of Labeling
ANDA Labeling Question Based Review September 11, 2013 GPhA/FDA ANDA Labeling Workshop/USP User Forum Sarah Park, PharmD Jeanne Skanchy, RPh. Labeling Reviewers Office of Generic Drugs/Division of Labeling
LACHMAN CONSULTANT SERVICES, INC STEWART AVENUE, WESTBURY, NY (516) " FAX (516) $,7,1 OVERNIGHT COURIER 11/16/09
 LACHMAN CONSULTANT SERVICES, INC. CONSULTANTS TO THE PHARMACEUTICAL AND ALLIED INDUSTRIES 1600 STEWART AVENUE, WESTBURY, NY 11590 (516) 222-6222 " FAX (516) 683-18$,7,1 November 16, 2009 OVERNIGHT COURIER
LACHMAN CONSULTANT SERVICES, INC. CONSULTANTS TO THE PHARMACEUTICAL AND ALLIED INDUSTRIES 1600 STEWART AVENUE, WESTBURY, NY 11590 (516) 222-6222 " FAX (516) 683-18$,7,1 November 16, 2009 OVERNIGHT COURIER
FEB 28 A 9 :09
 InvaGen Pharmaceutic als. Inc. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4 2 3 7 11 FEB 28 A 9 :09 Division of Dockets Management Food and Drug Administration Department of Health
InvaGen Pharmaceutic als. Inc. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4 2 3 7 11 FEB 28 A 9 :09 Division of Dockets Management Food and Drug Administration Department of Health
Drug/Device Combination Products: Bioequivalence
 Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
Drug/Device Combination Products: Bioequivalence Three stories:. The story of Nasal and Inhalation Product BE 2. The story of the Generic Auto-Injector 3. The story of User Interface Considerations Bioequivalence
In-Vitro Bioequivalence Studies for Oral Solid Dose products using the Morphologi G3-ID
 In-Vitro Bioequivalence Studies for Oral Solid Dose products using the Morphologi G3-ID PARTICLE SHAPE MOLECULAR SIZE CHEMICAL IDENTIFICATION Introduction A generic drug is defined as being "identical
In-Vitro Bioequivalence Studies for Oral Solid Dose products using the Morphologi G3-ID PARTICLE SHAPE MOLECULAR SIZE CHEMICAL IDENTIFICATION Introduction A generic drug is defined as being "identical
Office of Generic Drugs. April 14, 2010
 Considerations for PET ANDAs Office of Generic Drugs April 14, 2010 1 Agenda Office of Generic Drugs: background information RLDs and Suitability Petitions Submission requirements for an ANDA Approval
Considerations for PET ANDAs Office of Generic Drugs April 14, 2010 1 Agenda Office of Generic Drugs: background information RLDs and Suitability Petitions Submission requirements for an ANDA Approval
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Center for Drug Evaluation and Research
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Center for Drug Evaluation and Research DATE: February 16, 2017 TO: FROM: Ganciclovir injection 500mg/250ml (NDA
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Center for Drug Evaluation and Research DATE: February 16, 2017 TO: FROM: Ganciclovir injection 500mg/250ml (NDA
jjc Troxyca (Oxycodone Hydrochloride and Naltrexone Hydrochloride) ER capsules (new drug application (NDA) ) TO:
 TO: FROM: THROUGH: Troxyca (Oxycodone Hydrochloride and Naltrexone Hydrochloride) ER capsules (new drug application (NDA) 207621) Emily Helms Williams Wi Regulatory Counsel / Office of Regulatory Policy
TO: FROM: THROUGH: Troxyca (Oxycodone Hydrochloride and Naltrexone Hydrochloride) ER capsules (new drug application (NDA) 207621) Emily Helms Williams Wi Regulatory Counsel / Office of Regulatory Policy
BIOEQUIVALENCE AND THERAPEUTIC EQUIVALENCE. Soula Kyriacos, B.Pharm, PhD Head R&D, Pharmaline November 2016
 BIOEQUIVALENCE AND THERAPEUTIC EQUIVALENCE Soula Kyriacos, B.Pharm, PhD Head R&D, Pharmaline November 2016 Introduction Early 1970 s FDA regulations for submission of BA data 1984 US Congress passed the
BIOEQUIVALENCE AND THERAPEUTIC EQUIVALENCE Soula Kyriacos, B.Pharm, PhD Head R&D, Pharmaline November 2016 Introduction Early 1970 s FDA regulations for submission of BA data 1984 US Congress passed the
Understanding Regulatory Global Requirements for Nasal Drug Products. Julie D. Suman, Ph.D. April 8, 2016
 Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
Understanding Regulatory Global Requirements for Nasal Drug Products Julie D. Suman, Ph.D. April 8, 2016 AGENDA NDA vs ANDA Regulatory Approaches for Bioequivalence (BE) FDA Drug Specific Guidances FDA,
Hogan Lovells. December 11, 2013 BY HAND DELIVERY
 Hogan Lovells 13 777 BY HAND DELIVERY Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, Maryland 20852 Re: CITIZEN PETITION SUPPLEMENT On behalf of AbbVie Inc. ("AbbVie") 1, the undersigned
Hogan Lovells 13 777 BY HAND DELIVERY Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, Maryland 20852 Re: CITIZEN PETITION SUPPLEMENT On behalf of AbbVie Inc. ("AbbVie") 1, the undersigned
the May 2010 Draft Guidance on Azelaic Acid, and you request that the FDA take the following actions relating to those comments:
 (.t ~stltvic's. ~"~Iy"~ DEPARTMENT OF HEALTH &. HUMAN SERVICES JUN 22 2012 Food and Drug Administration Rockvile MD 20857. David L. Rosen Foley & Lardner LLP 3000 K Street, N.W., Suite 500 Washington,
(.t ~stltvic's. ~"~Iy"~ DEPARTMENT OF HEALTH &. HUMAN SERVICES JUN 22 2012 Food and Drug Administration Rockvile MD 20857. David L. Rosen Foley & Lardner LLP 3000 K Street, N.W., Suite 500 Washington,
Guide to Interchangeable Medicines
 Guide to Interchangeable Medicines AUT-G0115-6 01 JUNE 2018 This guide does not purport to be an interpretation of law and/or regulations and is for guidance purposes only. CONTENTS 1 BACKGROUND 3 1.1
Guide to Interchangeable Medicines AUT-G0115-6 01 JUNE 2018 This guide does not purport to be an interpretation of law and/or regulations and is for guidance purposes only. CONTENTS 1 BACKGROUND 3 1.1
Case 8:14-cv DKC Document 2-4 Filed 11/17/14 Page 1 of 17. Exhibit 3
 Case 8:14-cv-03607-DKC Document 2-4 Filed 11/17/14 Page 1 of 17 Exhibit 3 Case 8:14-cv-03607-DKC Document 2-4 Filed 11/17/14 Page 2 of 17 Mallinckrodt: Chart Documenting Generic Substitution Laws for 50
Case 8:14-cv-03607-DKC Document 2-4 Filed 11/17/14 Page 1 of 17 Exhibit 3 Case 8:14-cv-03607-DKC Document 2-4 Filed 11/17/14 Page 2 of 17 Mallinckrodt: Chart Documenting Generic Substitution Laws for 50
Withdrawal of Proposed Rule on Supplemental Applications Proposing Labeling Changes for
 This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Reference ID: NDA was approved on December 12, The product was not formulated with properties to deter abuse,
 tablets (NDA 020553). 1 According to Purdue, the reformulated OxyContin (OCR) had controlled-release features that would be less easily compromised by tampering than the original OxyContin (OC), and thereby
tablets (NDA 020553). 1 According to Purdue, the reformulated OxyContin (OCR) had controlled-release features that would be less easily compromised by tampering than the original OxyContin (OC), and thereby
Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic Drug Products for. Over-the-Counter Human Use; Amendment of Monograph for OTC Nasal
 1 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 341 [Docket No. 1976N 0052N] (formerly 76N 052N) RIN 0910 AF34 Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic
1 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 341 [Docket No. 1976N 0052N] (formerly 76N 052N) RIN 0910 AF34 Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic
InvaGen. FDP..90 fit P(Do47. Citizen petitian
 InvaGen Pharmaceuticals, I nt. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4:91. 6 11. Division of Dockets Management Food and Drug Administration Department of Health and Human Services
InvaGen Pharmaceuticals, I nt. InvaGen Pharmaceuticals, Inc. Te1:631-231-3233 Fax:631-231-4248 4:91. 6 11. Division of Dockets Management Food and Drug Administration Department of Health and Human Services
***II POSITION OF THE EUROPEAN PARLIAMENT
 EUROPEAN PARLIAMENT 1999 2004 Consolidated legislative document 13 March 2002 2000/0080(COD) PE2 ***II POSITION OF THE EUROPEAN PARLIAMENT adopted at second reading on 13 March 2002 with a view to the
EUROPEAN PARLIAMENT 1999 2004 Consolidated legislative document 13 March 2002 2000/0080(COD) PE2 ***II POSITION OF THE EUROPEAN PARLIAMENT adopted at second reading on 13 March 2002 with a view to the
Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program
 Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program Andrea Leonard-Segal, M.D., M.S. Director, Division of Nonprescription Clinical Evaluation 1 Contents
Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program Andrea Leonard-Segal, M.D., M.S. Director, Division of Nonprescription Clinical Evaluation 1 Contents
IMPACT OF PHARMACY REGULATION AND PAYMENT ON GENERIC DRUG USE IN THE MEDICAID PROGRAMS: 1991 TO 2008
 IMPACT OF PHARMACY REGULATION AND PAYMENT ON GENERIC DRUG USE IN THE MEDICAID PROGRAMS: 1991 TO 2008 A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL UNIVERSITY OF MINNESOTA BY DANIEL A.
IMPACT OF PHARMACY REGULATION AND PAYMENT ON GENERIC DRUG USE IN THE MEDICAID PROGRAMS: 1991 TO 2008 A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL UNIVERSITY OF MINNESOTA BY DANIEL A.
DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT
 c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION
 rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION HAR 1 0 2017 Anthony Maffia Vice President, Regulatory Affairs Sandoz Inc. 1 00 College Road West Princeton, NJ 08540 Dear Mr. Maffia: Re: Docket No. FDA-2016-P-3356
rj.;!i U.S. FOOD & DRUG - ADMIN ISTRATION HAR 1 0 2017 Anthony Maffia Vice President, Regulatory Affairs Sandoz Inc. 1 00 College Road West Princeton, NJ 08540 Dear Mr. Maffia: Re: Docket No. FDA-2016-P-3356
JAN MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY. Docket No.
 DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
Martin Shimer Deputy Director, Division of Legal and Regulatory Support Office of Generic Drug Policy
 M E M O R A N D U M DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR DRUG EVALUATION AND RESEARCH DATE: November 9, 2017 FROM: Martin Shimer Deputy
M E M O R A N D U M DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE FOOD AND DRUG ADMINISTRATION CENTER FOR DRUG EVALUATION AND RESEARCH DATE: November 9, 2017 FROM: Martin Shimer Deputy
LIDOCAINE HYDROCHLORIDE AND EPINEPHRINE National Drug Code Directory
 0409-3178-03 LIDOCAINE HYDROCHLORIDE AND EPINEPHRINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA)
0409-3178-03 LIDOCAINE HYDROCHLORIDE AND EPINEPHRINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA)
Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen
 Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
DIVALPROEX SODIUM National Drug Code Directory
 57237-046-01 DIVALPROEX SODIUM National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of
57237-046-01 DIVALPROEX SODIUM National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of
DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL. of 31 March 2004
 30.4.2004 Official Journal of the European Union L 136/85 DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 amending, as regards traditional herbal medicinal products,
30.4.2004 Official Journal of the European Union L 136/85 DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 amending, as regards traditional herbal medicinal products,
Compare Results. 153 Replacements 26 Insertions 344 Deletions. Total Changes. Styling and. Content. 0 Annotations. Old File: New File:
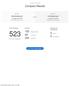 3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
Topics covered by the talk
 04/02/2016 Finished product monographs containing chemically defined active substances Dr Dirk Leutner Scientific Officer, European Pharmacopoeia Department European Directorate for the Quality of Medicines
04/02/2016 Finished product monographs containing chemically defined active substances Dr Dirk Leutner Scientific Officer, European Pharmacopoeia Department European Directorate for the Quality of Medicines
Current Challenges and Opportunities in Demonstrating Bioequivalence
 Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
Current Challenges and Opportunities in Demonstrating Bioequivalence Gur Jai Pal Singh, Ph.D. Watson Laboratories, Inc. Corona, California, USA Demonstrating Bioequivalence of Locally Acting Orally Inhaled
AbbVie Inc., et al.; Proposal to Withdraw Approval of Abbreviated New Drug Applications for
 This document is scheduled to be published in the Federal Register on 03/27/204 and available online at http://federalregister.gov/a/204-06802, and on FDsys.gov 460-0-P DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 03/27/204 and available online at http://federalregister.gov/a/204-06802, and on FDsys.gov 460-0-P DEPARTMENT OF HEALTH AND HUMAN SERVICES
DEXAMETHASONE SODIUM PHOSPHATE National Drug Code Directory
 0641-6146-25 DEXAMETHASONE SODIUM PHOSPHATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current
0641-6146-25 DEXAMETHASONE SODIUM PHOSPHATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current
PHENYTOIN SODIUM National Drug Code Directory
 57664-808-88 PHENYTOIN SODIUM National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of
57664-808-88 PHENYTOIN SODIUM National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of
O-CAL FA MULTIVITAMIN National Drug Code Directory
 0813-9616-01 O-CAL FA MULTIVITAMIN National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list
0813-9616-01 O-CAL FA MULTIVITAMIN National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list
LOREAL USA. February 6, Theresa Michelle, M.D.
 Theresa Michelle, M.D. LOREAL ~OREAL USA PRODUCTS, Inc. - Clark, NJ 07066 2) Chemical structure: 1) Product name: Drometrizole trisiloxane The following information is being provided for the purposes of
Theresa Michelle, M.D. LOREAL ~OREAL USA PRODUCTS, Inc. - Clark, NJ 07066 2) Chemical structure: 1) Product name: Drometrizole trisiloxane The following information is being provided for the purposes of
Bruce Wilkinson. Formulary & Benefit (F&B) Copay Summary Recommendation
 Bruce Wilkinson Formulary & Benefit (F&B) Copay Summary Recommendation F&B Summary Copay Usage Goal: Use the Summary Copay files in a consistent manner with other F&B file types, specifically formulary
Bruce Wilkinson Formulary & Benefit (F&B) Copay Summary Recommendation F&B Summary Copay Usage Goal: Use the Summary Copay files in a consistent manner with other F&B file types, specifically formulary
FOLET DHA National Drug Code Directory
 67555-153-30 FOLET DHA National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
67555-153-30 FOLET DHA National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
Coventry Health Care of Georgia, Inc.
 Coventry Health Care of Georgia, Inc. PRESCRIPTION DRUG RIDER (for High Deductible Health Plans) This Prescription Drug Rider is an attachment to the Coventry Health Care of Georgia, Inc. ( Health Plan
Coventry Health Care of Georgia, Inc. PRESCRIPTION DRUG RIDER (for High Deductible Health Plans) This Prescription Drug Rider is an attachment to the Coventry Health Care of Georgia, Inc. ( Health Plan
ANTACID LABELLING STANDARD
 ANTACID LABELLING STANDARD CATEGORY: DESCRIPTION: Antacid Single and multiple ingredient drugs, containing medicinal ingredients suitable for self-medication, which are intended to relieve symptoms such
ANTACID LABELLING STANDARD CATEGORY: DESCRIPTION: Antacid Single and multiple ingredient drugs, containing medicinal ingredients suitable for self-medication, which are intended to relieve symptoms such
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies
 ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies ISSUING BODY: The Ministry of Health published in: the Official
ORDER of the Minister of Health No. 269/2017 of 14 March 2017 on mandatory provision of medicinal product adequate and continuous supplies ISSUING BODY: The Ministry of Health published in: the Official
The proposed rule is significant, and the requirements and exceptions are complex. Key provisions of the proposal are described below.
 ADVISORY Food & Drug FDA ISSUES PROPOSED RULE TO ESTABLISH A UNIQUE DEVICE IDENTIFICATION SYSTEM FOR MEDICAL DEVICES July 16, 2012 On July 11, 2012, the Food and Drug Administration (FDA) published in
ADVISORY Food & Drug FDA ISSUES PROPOSED RULE TO ESTABLISH A UNIQUE DEVICE IDENTIFICATION SYSTEM FOR MEDICAL DEVICES July 16, 2012 On July 11, 2012, the Food and Drug Administration (FDA) published in
CITRANATAL DHA National Drug Code Directory
 0178-0894-30 CITRANATAL DHA National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all
0178-0894-30 CITRANATAL DHA National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all
MYCAMINE National Drug Code Directory
 0469-3250-10 MYCAMINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
0469-3250-10 MYCAMINE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
To Substitute or Not to Substitute: That Is the Question
 To Substitute or Not to Substitute: That Is the Question Published Online: Monday, March 14th, 2011 Tina Zerilli, PharmD; Andy He, PharmD Candidate 2011; Joseph P. Nathan, MS, PharmD; and Sara Grossman,
To Substitute or Not to Substitute: That Is the Question Published Online: Monday, March 14th, 2011 Tina Zerilli, PharmD; Andy He, PharmD Candidate 2011; Joseph P. Nathan, MS, PharmD; and Sara Grossman,
ANTICOAGULANT CITRATE DEXTROSE A ACD-A National Drug Code Directory
 43203-852-47 ANTICOAGULANT CITRATE DEXTROSE A ACD-A National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with
43203-852-47 ANTICOAGULANT CITRATE DEXTROSE A ACD-A National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with
SCIENTIFIC DISCUSSION. Antimycobacterial (J04AC01).
 SCIENTIFIC DISCUSSION Name of the Finished Pharmaceutical Product: Manufacturer of Prequalified Product: Active Pharmaceutical Ingredients (APIs): International Nonproprietary Name: Pharmaco-therapeutic
SCIENTIFIC DISCUSSION Name of the Finished Pharmaceutical Product: Manufacturer of Prequalified Product: Active Pharmaceutical Ingredients (APIs): International Nonproprietary Name: Pharmaco-therapeutic
REGULATORY PERSPECTIVE. Dr. Raghunandan H V Associate Professor JSSCP, JSSU, Mysore
 1 REGULATORY PERSPECTIVE Dr. Raghunandan H V Associate Professor JSSCP, JSSU, Mysore Contents 2 1. Role of Dissolution Testing in Generic Drug Approval 2. Dissolution Testing Recommendation for Solid Oral
1 REGULATORY PERSPECTIVE Dr. Raghunandan H V Associate Professor JSSCP, JSSU, Mysore Contents 2 1. Role of Dissolution Testing in Generic Drug Approval 2. Dissolution Testing Recommendation for Solid Oral
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE National Drug Code Directory
 64720-132-10 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments
64720-132-10 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE, DEXTROAMPHETAMINE SULFATE AND AMPHETAMINE SULFATE National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments
NOV
 DEPARTMENT OF HEALTH &, HUMAN SERVICES ------------------------------------------ NOV 17 2010 Food and Drug Administration Rockville M D 20857 Mark S. Aikman, Pharm.D. Vice President, Regulatory Affairs
DEPARTMENT OF HEALTH &, HUMAN SERVICES ------------------------------------------ NOV 17 2010 Food and Drug Administration Rockville M D 20857 Mark S. Aikman, Pharm.D. Vice President, Regulatory Affairs
Mark M. Yacura. Partner
 Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Received: ; Revised; Accepted: A REVIEW ON BIOAVAILABILITY AND BIOEQUIVALENCE STUDY Shashi Kant*, Bharat Parashar
 International Journal of Institutional Pharmacy and Life Sciences 2(5): September-October 2012 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES Pharmaceutical Sciences Review Article!!!
International Journal of Institutional Pharmacy and Life Sciences 2(5): September-October 2012 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES Pharmaceutical Sciences Review Article!!!
Requirements to the Registration of Medicinal products in the Republic of Armenia
 Requirements to the Registration of Medicinal products in the Republic of Armenia Yerevan 2010 Requirements to the Registration of Medicinal products in the Republic of Armenia Current requirements to
Requirements to the Registration of Medicinal products in the Republic of Armenia Yerevan 2010 Requirements to the Registration of Medicinal products in the Republic of Armenia Current requirements to
Chloroform Codeine Ether
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Chapter 6, Part 1 GENERAL PRINCIPLES OF PHARMACOLOGY Part 1 Basic Pharmacology Drugs are chemicals used to diagnose, treat, and disease. Pharmacology is the study of drugs
1 2 3 4 5 6 7 8 9 10 11 12 13 14 Chapter 6, Part 1 GENERAL PRINCIPLES OF PHARMACOLOGY Part 1 Basic Pharmacology Drugs are chemicals used to diagnose, treat, and disease. Pharmacology is the study of drugs
KETAMINE HYDROCHLORIDE National Drug Code Directory
 42023-138-10 KETAMINE HYDROCHLORIDE The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured,
42023-138-10 KETAMINE HYDROCHLORIDE The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured,
National Drug Code Directory
 The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
FM-IN-FC-01 Rev.1. PDF processed with CutePDF evaluation edition
 ก 2553 ก ก ก (DIRECTIVE 2002/46/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof 10 June 2002 on the approximation of the laws of the Member States relating to food supplements) : ก ก ก ก : ก ก : 10
ก 2553 ก ก ก (DIRECTIVE 2002/46/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof 10 June 2002 on the approximation of the laws of the Member States relating to food supplements) : ก ก ก ก : ก ก : 10
Decentralised Procedure. Public Assessment Report. Nurofen Immedia 200mg Weichkapseln Ibuprofen DE/H/1482/001/DC
 Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Nurofen Immedia 200mg Weichkapseln Ibuprofen DE/H/1482/001/DC Applicant: Reckitt Benckiser Reference
Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Nurofen Immedia 200mg Weichkapseln Ibuprofen DE/H/1482/001/DC Applicant: Reckitt Benckiser Reference
Dietary Supplement Health and Education Act of 1994 Public Law rd Congress
 Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
DIANEAL LOW CALCIUM WITH DEXTROSE National Drug Code Directory
 0941-0459-08 DIANEAL LOW CALCIUM WITH DEXTROSE The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured,
0941-0459-08 DIANEAL LOW CALCIUM WITH DEXTROSE The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured,
M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents
 M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents Communiqué for Health Industry Clients on the Legal Retainer Program Canada s New Natural Health Products Regulations On
M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents Communiqué for Health Industry Clients on the Legal Retainer Program Canada s New Natural Health Products Regulations On
Can a system like the U.S. OTC monographs work for ENDS?
 Can a system like the U.S. OTC monographs work for ENDS? Jack Henningfield, PhD Vice President, Research and Health Policy PinneyAssociates and Professor, Behavioral Biology, Adjunct Department of Psychiatry
Can a system like the U.S. OTC monographs work for ENDS? Jack Henningfield, PhD Vice President, Research and Health Policy PinneyAssociates and Professor, Behavioral Biology, Adjunct Department of Psychiatry
The solid choice for Omega-3
 The solid choice for Omega-3 The highest-load powder in its class. Superior bioavailability, stability and compressibility. Enabling smaller, simpler and more satisfying supplements. Unmatched properties
The solid choice for Omega-3 The highest-load powder in its class. Superior bioavailability, stability and compressibility. Enabling smaller, simpler and more satisfying supplements. Unmatched properties
National Drug Code Directory
 The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
December 4, 2017 VIA ELECTRONIC SUBMISSION
 VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
Bioequivalence of Oral Generic Product with An Alternate Administration
 Bioequivalence of Oral Generic Product with An Alternate Administration Minglei Cui, Ph.D. CDR, U.S. Public Health Service Division of Bioequivalence 2 Office of Generic Drugs CDER/FDA 1 Disclaimer & Disclosure
Bioequivalence of Oral Generic Product with An Alternate Administration Minglei Cui, Ph.D. CDR, U.S. Public Health Service Division of Bioequivalence 2 Office of Generic Drugs CDER/FDA 1 Disclaimer & Disclosure
Food Additives Program
 U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
Understanding the Value of Generic Drugs
 Understanding the Value of Generic Drugs Generic drugs can help you get the most out of your health care budget. 05.02.306.1 (8/05) Many people believe that when something costs more, it must be of higher
Understanding the Value of Generic Drugs Generic drugs can help you get the most out of your health care budget. 05.02.306.1 (8/05) Many people believe that when something costs more, it must be of higher
FlexRx 6-Tier. SM Pharmacy Benefit Guide
 FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
National Drug Code Directory
 The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or
Botanicals and botanical preparations in the EU: evolution through time towards full harmonisation. Opportunities and challenges.
 Botanicals and botanical preparations in the EU: evolution through time towards full harmonisation. Opportunities and challenges.part III- Marketing botanical food supplements in Italy Vittorio Silano
Botanicals and botanical preparations in the EU: evolution through time towards full harmonisation. Opportunities and challenges.part III- Marketing botanical food supplements in Italy Vittorio Silano
H 7816 S T A T E O F R H O D E I S L A N D
 LC00 01 -- H 1 S T A T E O F R H O D E I S L A N D IN GENERAL ASSEMBLY JANUARY SESSION, A.D. 01 A N A C T RELATING TO BUSINESSES AND PROFESSIONS - PHARMACIES Introduced By: Representatives Serpa, Canario,
LC00 01 -- H 1 S T A T E O F R H O D E I S L A N D IN GENERAL ASSEMBLY JANUARY SESSION, A.D. 01 A N A C T RELATING TO BUSINESSES AND PROFESSIONS - PHARMACIES Introduced By: Representatives Serpa, Canario,
Generic Medicines in Australia. Andrew McLachlan
 Generic Medicines in Australia Andrew McLachlan andrewm@pharm.usyd.edu.au The aim of this presentation Overview of medicines policy and regulation in Australia Generic medicines in the Australia market
Generic Medicines in Australia Andrew McLachlan andrewm@pharm.usyd.edu.au The aim of this presentation Overview of medicines policy and regulation in Australia Generic medicines in the Australia market
Decentralised Procedure. Public Assessment Report. Zolmitriptan Renantos 2.5 mg Orodispersible film 5 mg Orodispersible film DE/H/2295/ /DC
 Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Zolmitriptan Renantos 2.5 mg Orodispersible film 5 mg Orodispersible film DE/H/2295/001-002/DC Applicant:
Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Zolmitriptan Renantos 2.5 mg Orodispersible film 5 mg Orodispersible film DE/H/2295/001-002/DC Applicant:
FREEDOM OF INFORMATION SUMMARY
 Date of Approval: June 18, 2004 FREEDOM OF INFORMATION SUMMARY Original Abbreviated New Animal Drug Application Ivermectin Chewable Tablets (ivermectin) Antilarval For use in dogs to prevent canine heartworm
Date of Approval: June 18, 2004 FREEDOM OF INFORMATION SUMMARY Original Abbreviated New Animal Drug Application Ivermectin Chewable Tablets (ivermectin) Antilarval For use in dogs to prevent canine heartworm
HAVRIX National Drug Code Directory
 58160-826-52 HAVRIX National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
58160-826-52 HAVRIX National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs
PLEASE NOTE. For more information concerning the history of this Act, please see the Table of Public Acts.
 PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to July 1, 2012. It is intended for information and reference purposes only. This
PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to July 1, 2012. It is intended for information and reference purposes only. This
Optimum Bioenergy International Corp. 12/21/17
 Optimum Bioenergy International Corp. 12/21/17 Office of Human and Animal Food Division 5 West 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Sent Via UPS Signature Required WARNING LETTER December 21,
Optimum Bioenergy International Corp. 12/21/17 Office of Human and Animal Food Division 5 West 1431 Harbor Bay Parkway Alameda, CA 94502-7070 Sent Via UPS Signature Required WARNING LETTER December 21,
