FDA Introductory Remarks Stephanie O. Omokaro, MD
|
|
|
- Rudolph Arnold
- 5 years ago
- Views:
Transcription
1 FDA Introductory Remarks Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research Office of New Drugs Office of Drug Evaluation III Liver Forum 6 - April 18, 2017
2 No conflicts of interest Nothing to disclose Disclosure Statement This talk reflects the views of the author and should not be construed to represent FDA s views or policies 2
3 3
4 2016 in FDA Review NASH Development Programs Pre-submission Meetings INDs Expedited Program Requests initial Pediatric Study Plans Inter-Center Consultations Biomarker Qualification Programs EMA Collaborations 4
5 Regulatory Considerations for Endpoints Early Phase Trials (e.g. proof of concept, dose-ranging): Consider the mechanism and anticipated time course for changes when selecting endpoints and design Liver transaminases have been used; however, changes in transaminases have not been found to be predictive of histological changes in short duration trials Other non-invasive biomarkers (e.g., elastography as measured by MRI, and/or serum biomarkers of disease activity based on drug mechanism) also have been used May reflect the activity of the drug and its effect on the underlying disease process; however, unclear if predictive or correlative with histology Phase 2 Trials: Approach that has been used and found acceptable is histological evaluation Histologically-based NAFLD Activity Score system (NAS) with a decrease of 2 points with at least a 1-point reduction in either lobular inflammation or hepatocellular ballooning and with no concurrent worsening of fibrosis stage 5
6 Phase 3 Trials: Regulatory Considerations for Endpoints Use of biopsy-based surrogate endpoints : A complete resolution of NASH on overall histopathologic interpretation by an experienced pathologist (with a NAS of ballooning of 0 and an inflammation score of 0-1) AND no worsening of fibrosis (NASH/CRN Brunt-Kleiner scale) At least one point improvement in fibrosis score (Brunt-Kleiner scale) AND no worsening of NASH (defined as no worsening in ballooning or inflammation by NAS) Choice of either as a primary endpoint and the other as a key secondary endpoint or assessment of both changes as a co-primary endpoint Subpart H or E generally requires that a trial (phase 4) to verify and describe clinical benefit be ongoing at the time of marketing approval 6
7 Regulatory Considerations for Endpoints Phase 4 Trials: For confirmatory trials, clinical benefit endpoints of: All-cause mortality Liver transplant Hepatic decompensation events Histological progression to cirrhosis Increase of MELD score from below 12 to 15 Acceptable and reflect a meaningful change in clinical status associated with morbidity and mortality. 7
8 Issues with HCC as a Component of a Clinical Benefit Composite Endpoint Multifactorial etiology and complex pathophysiology Majority of events in the endpoint analyses will be primarily cirrhosis events Few events relative to the other components (e.g. death, liver transplant, and MELD score increase) of the composite Not expected to have a significant impact on endpoint analysis Issues of implying that drug reduces HCC when not assessed and shown independently from other components to do so Need for appropriate screening at enrollment (liver ultrasound and alpha fetoprotein) and adequate assessments during the trial 8
9 Unsolved Clinical Development Issues in NASH Investigational Agents: Pathophysiologic concepts of purely antifibrotic or purely antinflammatory drugs. Is it possible in NASH to affect one with out impacting the other? Impact of early hepatotoxic signals on drug development in a population with underlying liver disease Population: Appropriately defining high risk F1 subjects for trial inclusion Placebo/SOC: Precisely defining the placebo effect and exploring it s mechanism and impact Placebo arm sharing across multiple programs and Sponsors Incorporating standardized diet/exercise programs modeled from obesity clinical trials 9
10 Clinical Development Issues in NASH Histology Based Endpoints: Necessity of additional liver-trained pathologists Standardization of the overall histologic interpretation for use across the spectrum of pathologists Understanding of intra- and inter-rater validity to better design clinical trials Role of Non-Invasive Biomarkers: Standardizing methods and protocols for diagnostic imaging Establishing clinically meaningful thresholds Increasing measurement frequency of endpoints (i.e. more data to assure validity) Validation via concurrent biopsies 10
11 Pediatric Development Considerations Enrollment of minors under 21 CFR 50 subpart D requires the investigator demonstrate prospect of direct benefit to the subject as a result of the drug intervention Treatment of fatty liver alone is inadequate to support direct benefit in minors Identification of sub-populations that may benefit from potential treatment such as in adult patients with F2 and F3 fibrosis who are at higher risk for liver-related adverse events Need for pediatric natural history data and incorporation of natural history studies in the initial pediatric study plans (ipsps) 11
12
13 Future Workshops Planned Trial Design, Baseline Parameters and Endpoints for Clinical Trials: Alcoholic Liver Disease (AH) Pediatric Chronic Idiopathic Constipation (CIC) and Irritable Bowel Syndrome (IBS) 13
14
15
16
FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD
 FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) U.S. Food and Drug Administration Center for Drug
FDA Regulatory Considerations for NASH Clinical Trial Endpoints Stephanie O. Omokaro, MD Division of Gastroenterology & Inborn Errors Products (DGIEP) U.S. Food and Drug Administration Center for Drug
DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES
 DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES Kathleen M Donohue MD, MSc Clinical Team Lead Liver & Inborn Errors Division of Gastroenterology & Inborn Errors
DEFINING THE REFERENCE STANDARD FOR BIOMARKERS IN NASH: HISTOLOGY VERSUS CLINICAL OUTCOMES Kathleen M Donohue MD, MSc Clinical Team Lead Liver & Inborn Errors Division of Gastroenterology & Inborn Errors
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms
 SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
SHORTENING TIMELINES AND IMPROVING EFFICIENCY IN DRUG DEVELOPMENT -Seamless drug development paradigms Claudia Filozof, MD PhD Executive Medical Director Covance Challenges in Clinical Development in NASH
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
The EMA reflection paper on chronic liver disease and its implications for drug development in NASH
 The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
The on chronic liver disease and its implications for drug development in NASH Content of the reflection paper and report from stakeholder meeting Elmer Schabel MD No conflict of interest. Content Overview:
Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials
 Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials Yeh-Fong Chen FDA/CDER/OB/DB3 Forum for Collaborative Research (Berkeley) 5/10/2017 Disclaimer This
Utilizing Seamless Adaptive Designs and Considering Multiplicity Adjustment for NASH Clinical Trials Yeh-Fong Chen FDA/CDER/OB/DB3 Forum for Collaborative Research (Berkeley) 5/10/2017 Disclaimer This
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH
 Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
Study Design to Validate Biomarkers of Therapeutic Response in Pre-cirrhotic NASH Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD. Pierre Bedossa. Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE
 PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
PHC, Paris, 30th Jan 2017 PATHOLOGY OF NAFLD Pierre Bedossa Departement of Pathology Hôpital Beaujon University Paris-Diderot Paris - FRANCE 1 PATHOLOGY OF NAFLD NAFLD: a chronic liver disease with a wide
Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Outline. Outline
 Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Preeti Venkataraman, M.D. Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research
Approach to Clinical Trials in Drug Development : Eosinophilic Esophagitis (EoE) Preeti Venkataraman, M.D. Division of Gastroenterology & Inborn Errors Products (DGIEP) Center for Drug Evaluation and Research
Update on Non-Alcoholic Fatty Liver Disease. Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI
 Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
Update on Non-Alcoholic Fatty Liver Disease Timothy R. Morgan, MD Chief, Hepatology, VA Long Beach Professor of Medicine, UCI February 3, 2018 Disclosure Clinical trials: Genfit Speaker s Bureau: none
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE
 PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
PEDIATRIC FOIE GRAS: NON-ALCOHOLIC FATTY LIVER DISEASE Updates on New insights into NAFLD and NASH pathophysiology New AASLD/AGA/ACG guidelines for NAFLD and NASH, as pertains to pediatrics Evidence-based
Forward-looking Statements
 NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
NASDAQ:CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis
 Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis Alina M. Allen, Meng Yin, Sudhakar K. Venkatesh, Taofic Mounajjed, Todd A. Kellogg,
Liver biopsy as the gold standard for diagnosis. Pierre BEDOSSA
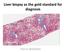 Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
Liver biopsy as the gold standard for diagnosis Pierre BEDOSSA 1 PATHOLOGY OF NAFLD CONFLICTS OF INTEREST Grants and funding from Genfit, Intercept, Allergan, Inventiva, OWL, Echosens CEO and funding of
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
ENCORE-PH Top-line Results
 ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
ENCORE-PH Top-line Results Striving to improve human health December 5, 2018 NASDAQ CNAT Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements
Clinical Trials & Endpoints in NASH Cirrhosis
 Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
Clinical Trials & Endpoints in NASH Cirrhosis April 25, 2018 Peter G. Traber, MD CEO & CMO, Galectin Therapeutics 2018 Galectin Therapeutics NASDAQ: GALT For more information, see galectintherapeutics.com
Improving the Lives of Patients with Liver Diseases
 Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
Improving the Lives of Patients with Liver Diseases Corporate Presentation March 2019 Safe Harbor Statement This presentation contains "forward-looking" statements that involve risks, uncertainties and
NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) NON-ALCOHOLIC STEATOHEPATITIS (NASH) ADDRESSING A GROWING SILENT EPIDEMIC
 NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
NON-ALCOHOLIC FATTY LIVER DISEASE () & NON-ALCOHOLIC STEATOHEPATITIS () ADDRESSING A GROWING SILENT EPIDEMIC PREVALENCE OF / USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology
Hepatology for the Nonhepatologist
 Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
Hepatology for the Nonhepatologist Kenneth E. Sherman, MD, PhD Gould Professor of Medicine Director, Division of Digestive Diseases University of Cincinnati College of Medicine Cincinnati, Ohio Learning
NON-ALCOHOLIC FATTY LIVER DISEASE:
 NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
NON-ALCOHOLIC FATTY LIVER DISEASE: ROLE OF THE PRIMARY PROVIDER Archita P. Desai, MD Assistant Professor of Medicine University of Arizona 25 th Annual Southwestern Conference on Medicine Outline Pathophysiology
INVESTOR PRESENTATION. November 16 th, 2015
 INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
INVESTOR PRESENTATION November 16 th, 2015 1 Disclaimer Important information and forward looking statements GENFIT IS A PUBLIC COMPANY LISTED ON EURONEXT PARIS (COMPARTMENT B) STOCK EXCHANGE SINCE APRIL
Challenges in the Diagnosis of Steatohepatitis
 The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
The Bugaboos of Fatty Liver Disease: Ballooning and Fibrosis Hans Popper Hepatopathology Society Companion Meeting San Antonio, Tx March, 2017 David Kleiner, M.D., Ph.D. NCI/Laboratory of Pathology Challenges
Evercore ISI Presentation- Madrigal
 Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Evercore ISI Presentation- Madrigal Forward-Looking Statements Any statements, other than statements of historical facts, made in this presentation regarding our clinical studies and our research and development
Obesity, Inflammation and Liver Cancer
 Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
Obesity, Inflammation and Liver Cancer Richard Moreau, M.D., 1 INSERM U773, Centre de Recherche Biomédicale Bichat-Beaujon CRB3, 2 Université Denis Diderot Paris 7, 3 Service d Hépatologie, Hôpital Beaujon,
Regulatory Updates Veronica Miller, PhD
 Regulatory Updates Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH PARIS_NASH_2018 2 PARIS NASH 2018 Disclosures Dr. Veronica Miller is an employee of the Forum for Collaborative
Regulatory Updates Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH PARIS_NASH_2018 2 PARIS NASH 2018 Disclosures Dr. Veronica Miller is an employee of the Forum for Collaborative
Update on Vaccine Regulation: Expediting vaccine development. Phil Krause FDA/CBER/OVRR
 Update on Vaccine Regulation: Expediting vaccine development Phil Krause FDA/CBER/OVRR Challenges in vaccine development High cost of development relative to typical profits US markets are often dependent
Update on Vaccine Regulation: Expediting vaccine development Phil Krause FDA/CBER/OVRR Challenges in vaccine development High cost of development relative to typical profits US markets are often dependent
PREVALENCE OF NAFLD & NASH
 - - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
- - PREVALENCE OF & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams et al., Gastroenterology 2011; 140:124-31) Dallas Heart Study Prevalence Numbers (Browning et al., Hepatology 2004;40:1387-95)
Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial
 Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial -- Statistically significantly more patients treated with
Madrigal s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial -- Statistically significantly more patients treated with
Consensus AASLD-EASL HBV Treatment Endpoint and HBV Cure Definition
 Consensus AASLD-EASL HBV Treatment Endpoint and HBV Cure Definition Anna S. Lok, MD, DSc Alice Lohrman Andrews Professor in Hepatology Director of Clinical Hepatology Assistant Dean for Clinical Research
Consensus AASLD-EASL HBV Treatment Endpoint and HBV Cure Definition Anna S. Lok, MD, DSc Alice Lohrman Andrews Professor in Hepatology Director of Clinical Hepatology Assistant Dean for Clinical Research
Liver Pathology and the Clinician in 2015: At the Crossroads. Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York
 Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Liver Pathology and the Clinician in 2015: At the Crossroads Thomas D. Schiano, M.D. Mount Sinai Medical Center New York, New York DISCLOSURES Consultant for: Salix Merck Gilead BMS Synageva Research funding:
Liver Pathology in the 0bese
 Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
Liver Pathology in the 0bese Rob Goldin Centre for Pathology, Imperial College r.goldin@imperial.ac.uk Ludwig et al. Non-alcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease.
DIAGNOSIS OF NASH LIVER BIOPSY. PHC
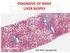 DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
DIAGNOSIS OF NASH LIVER BIOPSY PHC 2018 - www.aphc.info 1 THE NATURAL HISTORY OF NAFLD STEATOSIS (NAFL) STEATOHEPATITIS (NASH) FIBROSIS CIRRHOSIS HCC UNDER THE LENS : THE 3 HISTOLOGICAL COMPONENTS OF NAFLD
A pathologist, a radiologist and a hepatologist walked into a bar
 A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
A pathologist, a radiologist and a hepatologist walked into a bar Brent A. Neuschwander-Tetri, MD, FAASLD Professor of Internal Medicine Director, Division of Gastroenterology and Hepatology Saint Louis
GR-MD-02 for Indication of NASH Cirrhosis: NASH-CX Clinical Trial Results
 GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
GR-MD-02 for Indication of NASH Cirrhosis: NASH-C Clinical Trial Results Supplemental Information to Corporate Presentation February 6, 2018 NASDAQ: GALT www.galectintherapeutics.com 1 2018 2017 Galectin
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abetalipoproteinemia NASH and, 537 Acquired errors of metabolism NASH and, 536 537 Amiodarone steatohepatitis due to, 527 Anticonvulsant mood
Note: Page numbers of article titles are in boldface type. A Abetalipoproteinemia NASH and, 537 Acquired errors of metabolism NASH and, 536 537 Amiodarone steatohepatitis due to, 527 Anticonvulsant mood
Defining the gold standard in biomarker validation for NASH
 Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Defining the gold standard in biomarker validation for NASH Arun J Sanyal M.D. Professor of Medicine, Physiology and Molecular Pathology Virginia Commonwealth University School of Medicine Conflicts of
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease
 Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
Advances in Understanding Hepatic Fibrosis and Chronic Liver Disease Scott Friedman, M.D. Fishberg Professor of Medicine Dean for Therapeutic Discovery Chief, Division of Liver Diseases Icahn School of
Hepatocellular carcinoma
 Hepatocellular carcinoma Mary Ann Y. Huang, M.D., M.S., FAASLD Transplant hepatologist Peak Gastroenterology Associates Porter Adventist Hospital Denver, Colorado Background - Worldwide Hepatocellular
Hepatocellular carcinoma Mary Ann Y. Huang, M.D., M.S., FAASLD Transplant hepatologist Peak Gastroenterology Associates Porter Adventist Hospital Denver, Colorado Background - Worldwide Hepatocellular
M30 Apoptosense ELISA. A biomarker assay for detection and screening of NASH
 M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
M30 Apoptosense ELISA A biomarker assay for detection and screening of NASH NASH A Global Disease In the Western countries, Non-Alcoholic Fatty Liver Disease (NAFLD) is the most common liver disease, strongly
NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES
 NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES Preface Zobair M. Younossi xiii Epidemiology and Natural History of NAFLD and NASH 1 Janus P. Ong and Zobair M. Younossi Understanding
NON-ALCOHOLIC STEATOHEPATITIS AND NON-ALCOHOLIC FATTY LIVER DISEASES Preface Zobair M. Younossi xiii Epidemiology and Natural History of NAFLD and NASH 1 Janus P. Ong and Zobair M. Younossi Understanding
Non-Alcoholic Fatty Liver Disease
 Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
Non-Alcoholic Fatty Liver Disease None Disclosures Arslan Kahloon M.D Chief, Division of Gastroenterology and Hepatology University of Tennessee College of Medicine Chattanooga Objectives Understand the
MEETING PROSPECTUS. International Workshop on NASH Biomarkers
 International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
International Workshop on NASH Biomarkers 2018 18-19 May 2018 Washington, D.C., USA MEETING PROSPECTUS www.expertmedicaleducation.com www.expertmedicalevents.com ADVANCING HEALTH GLOBALLY THROUGH INNOVATIVE
WHEN HCV TREATMENT IS DEFERRED WV HEPC ECHO PROJECT
 WHEN HCV TREATMENT IS DEFERRED WV HEPC ECHO PROJECT October 13, 2016 Reminder - treatment is recommended for all patients with chronic HCV infection Except short life expectancies that cannot be remediated
WHEN HCV TREATMENT IS DEFERRED WV HEPC ECHO PROJECT October 13, 2016 Reminder - treatment is recommended for all patients with chronic HCV infection Except short life expectancies that cannot be remediated
FATTY LIVER DISEASE (NAFLD) (NASH) A GROWING
 NON ALCOHOLIC FATTY LIVER DISEASE () & NON ALCOHOLIC S T E ATO H E PAT I T I S () ADDRESSING A GROWING SILENT EPIDEMIC Prevalence of & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams
NON ALCOHOLIC FATTY LIVER DISEASE () & NON ALCOHOLIC S T E ATO H E PAT I T I S () ADDRESSING A GROWING SILENT EPIDEMIC Prevalence of & USA Prevalence in Middle Age Patients San Antonio, Texas (Williams
Improving Access to Quality Medical Care Webinar Series
 Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
Improving Access to Quality Medical Care Webinar Series Presented by The Arizona Telemedicine Program and the Southwest Telehealth Resource Center 2015 UA Board of Regents Welcome AZ, UT, CO, NM & NV FLEX
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019
 GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
GENFIT: New data to be presented at 2018 AASLD meeting, ahead of key results expected in 2018 and 2019 KOL event to be held ahead of expected data release with elafibranor in PBC by end of 2018 (Phase
EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease.
 Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Commentary. EASL EASD EASO Clinical practice guidelines for the management of nonalcoholic fatty liver disease. Christopher D. Byrne 1,2, Giovanni Targher 3 1 Nutrition and Metabolism, Faculty of Medicine,
Non-alcoholic fatty liver disease: time to take note and manage. Philip Newsome Professor of Hepatology & Director of Centre for Liver Research
 Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
Non-alcoholic fatty liver disease: time to take note and manage Philip Newsome Professor of Hepatology & Director of Centre for Liver Research Disclosures Consultancy, Co-ordinating Investigator roles
DENOMINATOR: All patients aged 18 years and older with a diagnosis of chronic hepatitis C cirrhosis
 Quality ID #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Quality ID #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Understanding your FibroScan Results
 PATIENT & CAREGIVER EDUCATION Understanding your FibroScan Results This information will help you understand your FibroScan results. About FibroScan FibroScan is a specialized ultrasound machine for your
PATIENT & CAREGIVER EDUCATION Understanding your FibroScan Results This information will help you understand your FibroScan results. About FibroScan FibroScan is a specialized ultrasound machine for your
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical
 Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Nonalcoholic Fatty Liver Disease in Children: Typical and Atypical Disclosure Naim Alkhouri, MD discloses the following relationships with commercial companies: Membership in the Speakers Bureau for Alexion
Research in Hepatology What is hot and what is not!
 Research in Hepatology What is hot and what is not! Anna SF Lok, MD University of Michigan Ann Arbor, MI, USA Please consider the environment before printing this PowerPoint Outline How to define hot?
Research in Hepatology What is hot and what is not! Anna SF Lok, MD University of Michigan Ann Arbor, MI, USA Please consider the environment before printing this PowerPoint Outline How to define hot?
Automatic Pathology Software for Diagnosis of Non-Alcoholic Fatty Liver Disease
 Automatic Pathology Software for Diagnosis of Non-Alcoholic Fatty Liver Disease (OTT ID 1236) Inventors: Joseph Bockhorst and Scott Vanderbeck, Department of Computer Science and Electrical Engineering,
Automatic Pathology Software for Diagnosis of Non-Alcoholic Fatty Liver Disease (OTT ID 1236) Inventors: Joseph Bockhorst and Scott Vanderbeck, Department of Computer Science and Electrical Engineering,
Regulatory update from Europe:
 Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
Regulatory update from Europe: Interim endpoints in phase 3 NASH trials Elmer Schabel MD The previously proposed/presented co-primary evaluation of two composite endpoints for the interim has been accepted:
Assessment of Liver Stiffness by Transient Elastography in Diabetics with Fatty Liver A Single Center Cross Sectional observational Study
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 6 Ver. IV (June. 2017), PP 49-53 www.iosrjournals.org Assessment of Liver Stiffness by Transient
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 6 Ver. IV (June. 2017), PP 49-53 www.iosrjournals.org Assessment of Liver Stiffness by Transient
Primary Sclerosing Cholangitis and Cholestatic liver diseases. Ahsan M Bhatti MD, FACP Bhatti Gastroenterology Consultants
 Primary Sclerosing Cholangitis and Cholestatic liver diseases Ahsan M Bhatti MD, FACP Bhatti Gastroenterology Consultants I have nothing to disclose Educational Objectives What is PSC? Understand the cholestatic
Primary Sclerosing Cholangitis and Cholestatic liver diseases Ahsan M Bhatti MD, FACP Bhatti Gastroenterology Consultants I have nothing to disclose Educational Objectives What is PSC? Understand the cholestatic
DENOMINATOR: All patients aged 18 years and older with a diagnosis of chronic hepatitis C cirrhosis
 Quality ID #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care
Quality ID #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care
Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH
 www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
www.alacrita.com Therapeutic Approaches to Cirrhotic versus Pre-Cirrhotic NASH 2nd Annual NASH Summit Europe October 23-24, 2018 Frankfort, Germany Peter G. Traber, MD Partner, Alacrita Consulting Alacrita
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016.
 Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
Early life determinants of Non-Alcoholic Fatty Liver Disease and NASH DR JULIANA MUIVA-GITOBU KENYA PAEDIATRIC ASSOCIATION CONFERENCE APRIL 2016. Outline Definition NAFLD and NASH Magnitude of the problem
Update on Nonalcoholic Fatty Liver Disease. Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic
 Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Update on Nonalcoholic Fatty Liver Disease Kathleen E Corey, MD, MPH, MMSc Director, Mass General Fatty Liver Clinic Outline Defining the phenotypes of nonalcoholic fatty liver disease NAFLD Diagnostics
Non-Invasive Assessment of Liver Fibrosis. Patricia Slev, PhD University of Utah Department of Pathology
 Non-Invasive Assessment of Liver Fibrosis Patricia Slev, PhD University of Utah Department of Pathology Disclosure Patricia Slev has no relevant financial relationships to disclose. 2 Chronic Liver Disease
Non-Invasive Assessment of Liver Fibrosis Patricia Slev, PhD University of Utah Department of Pathology Disclosure Patricia Slev has no relevant financial relationships to disclose. 2 Chronic Liver Disease
End Stage Liver Disease & Disease Specific Indications for Liver Transplant. Susan Kang, RN, MSN, ANP-BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP-BC Introduction (https://www.srtr.org) What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC
 End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
End Stage Liver Disease & Disease Specific Indications for Liver Transplant Susan Kang, RN, MSN, ANP BC Introduction (https://www.srtr.org) 1 What does the liver do? STORAGE METABOLIC DETOXIFICATION SYNTHETIC
Forward-looking Statements
 Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Forward-looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding
Preliminary clinical experience with Shear Wave Dispersion Imaging for liver viscosity
 Preliminary clinical experience with Shear Wave Dispersion Imaging for liver viscosity Dr. Katsutoshi Sugimoto Department of Gastroenterology and Hepatology, Tokyo Medical University, Japan Introduction
Preliminary clinical experience with Shear Wave Dispersion Imaging for liver viscosity Dr. Katsutoshi Sugimoto Department of Gastroenterology and Hepatology, Tokyo Medical University, Japan Introduction
Steatotic liver disease
 Steatotic liver disease Fatty liver disease Prof. Dr. ANNE HOORENS Non-Neoplastic Liver Pathology December 8th 2018 Working Group of Digestive Pathology Belgian Society of Pathology OUTLINE NAFLD = Non-Alcoholic
Steatotic liver disease Fatty liver disease Prof. Dr. ANNE HOORENS Non-Neoplastic Liver Pathology December 8th 2018 Working Group of Digestive Pathology Belgian Society of Pathology OUTLINE NAFLD = Non-Alcoholic
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic
 Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
Non-Alcoholic Fatty Liver Diseasean underestimated epidemic Amir Shlomai MD,PhD Head, Department of Medicine D The Liver Institute Rabin Medical Center, Beilinson Hospital The IASLD semi-annual meeting-
In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease
 REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
REVIEW In Search of New Biomarkers for Nonalcoholic Fatty Liver Disease Ting-Ting Chan, M.R.C.P., and Vincent Wai-Sun Wong, M.D. Nonalcoholic fatty liver disease (NAFLD) affects 15% to 40% of the general
Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need?
 Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need? Rob Goldin r.goldin@imperial.ac.uk @robdgol FATTY LIVER DISEASE Brunt
Basic patterns of liver damage what information can a liver biopsy provide and what clinical information does the pathologist need? Rob Goldin r.goldin@imperial.ac.uk @robdgol FATTY LIVER DISEASE Brunt
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014
 Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Prognosis of NASH VII Workshop Intenracional de Actualizaçao em Hepatologia, Aug 29th 2014 Vlad Ratziu, Université Pierre et Marie Curie, Hôpital Pitié Salpêtrière, Paris, France NASH : a severe hepatic
Corporate Update. NASDAQ: GALT April 9, 2018
 Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
Corporate Update April 9, 2018 NASDAQ: GALT www.galectintherapeutics.com Forward-Looking Statements This presentation contains, in addition to historical information, forward-looking statements within
AAIM: GI Workshop Follow Up to Case Studies. Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease
 AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
AAIM: GI Workshop Follow Up to Case Studies Non-alcoholic Fatty Liver Disease Ulcerative Colitis Crohn s Disease Daniel Zimmerman, MD VP and Medical Director, RGA Global October 2015 Non-alcoholic Fatty
Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic
 NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
NASH and NAFLD Preface: Nonalcoholic Fatty Liver Disease: An Expanding Health Care Epidemic David E. Bernstein xiii Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
Quantitative Assessment of the Liver: Breath Tests. M. Shadab Siddiqui, M.D. Virginia Commonwealth University
 Quantitative Assessment of the Liver: Breath Tests M. Shadab Siddiqui, M.D. Virginia Commonwealth University Objectives Principles of breath tests Breath tests in NAFLD Potential applications of breath
Quantitative Assessment of the Liver: Breath Tests M. Shadab Siddiqui, M.D. Virginia Commonwealth University Objectives Principles of breath tests Breath tests in NAFLD Potential applications of breath
Concepts and Case Study Template for Surrogate Endpoints Workshop. Lisa M. McShane, Ph.D. Biometric Research Program National Cancer Institute
 Concepts and Case Study Template for Surrogate Endpoints Workshop Lisa M. McShane, Ph.D. Biometric Research Program National Cancer Institute Medical Product Development GOAL is to improve how an individual
Concepts and Case Study Template for Surrogate Endpoints Workshop Lisa M. McShane, Ph.D. Biometric Research Program National Cancer Institute Medical Product Development GOAL is to improve how an individual
Transient elastography in chronic viral liver diseases
 4 th AISF POST-MEETING COURSE Roma, 26 Febbraio 2011 Transient elastography in chronic viral liver diseases CRISTINA RIGAMONTI, M.D., Ph.D. Transient elastography (TE): a rapid, non-invasive technique
4 th AISF POST-MEETING COURSE Roma, 26 Febbraio 2011 Transient elastography in chronic viral liver diseases CRISTINA RIGAMONTI, M.D., Ph.D. Transient elastography (TE): a rapid, non-invasive technique
Case #1. Digital Slides 11/6/ year old woman presented with abnormal liver function tests. Liver Biopsy to r/o autoimmune hepatitis
 45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
45 year old woman presented with abnormal liver function tests Liver Biopsy to r/o autoimmune hepatitis Further down. ANA 1: 160; ASMA 1:80 ANA 1: 160; ASMA 1:80 IgG = 14.5 g/l (upper normal range: 16)
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health
 CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
CDHNF & NASPGHAN A Partnership for Research and Education for Children s Digestive and Nutritional Health Obesity and NAFLD Definitions: Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver
Hepatology For The Nonhepatologist
 Hepatology For The Nonhepatologist Andrew Aronsohn, MD Associate Professor of Medicine University of Chicago Chicago, Illinois Learning Objectives After attending this presentation, learners will be able
Hepatology For The Nonhepatologist Andrew Aronsohn, MD Associate Professor of Medicine University of Chicago Chicago, Illinois Learning Objectives After attending this presentation, learners will be able
Pathogenesis and Management of Non-Alcoholic Fatty Liver Disease
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/lipid-luminations/pathogenesis-management-non-alcoholic-fatty-liverdisease/8155/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/lipid-luminations/pathogenesis-management-non-alcoholic-fatty-liverdisease/8155/
Hepatocellular Carcinoma: Epidemiology and Screening
 Hepatocellular Carcinoma: Epidemiology and Screening W. Ray Kim, MD Professor and Chief Gastroenterology and Hepatology Stanford University School of Medicine Case A 67 year old Filipino-American woman
Hepatocellular Carcinoma: Epidemiology and Screening W. Ray Kim, MD Professor and Chief Gastroenterology and Hepatology Stanford University School of Medicine Case A 67 year old Filipino-American woman
Fatty Liver Disease A growing epidemic
 Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Fatty Liver Disease A growing epidemic Updates in GIM for Primary Care Don C. Rockey March 9 th, 2018 Disclosures 2018 Research Funding (all to MUSC) NIH/NIDDK Actelion Pharmaceuticals Gilead Sciences
Non-invasive diagnostic biomarkers
 Non-invasive diagnostic biomarkers Liver Forum, November 12 th, 2015 Rohit Loomba, MD, MHSc Professor of Medicine (with tenure) Director, NAFLD Research Center, Division of Gastroenterology, Department
Non-invasive diagnostic biomarkers Liver Forum, November 12 th, 2015 Rohit Loomba, MD, MHSc Professor of Medicine (with tenure) Director, NAFLD Research Center, Division of Gastroenterology, Department
September 30, Eric BASTINGS, MD. Acting Director Division of Neurology Products (DNP) Center for Drug Evaluation and Research (CDER)
 ISCTM Autumn Conference Options and methods to improve cognitive assessment in clinical trials of AD and its precursors September 30, 2013 Eric BASTINGS, MD Acting Director Division of Neurology Products
ISCTM Autumn Conference Options and methods to improve cognitive assessment in clinical trials of AD and its precursors September 30, 2013 Eric BASTINGS, MD Acting Director Division of Neurology Products
An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications
 REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
REVIEW An Update on the Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Beyond Lifestyle Modifications Naim Alkhouri, M.D.,*, and Andrea Scott, B.S.* Nonalcoholic fatty liver disease (NAFLD)
The FDA and Unmet Needs:
 The FDA and Unmet Needs: The Path to New Therapy for Transplant Recipients Mark D. Stegall, MD James C. Masson Professor of Surgery Research Departments of Surgery and Immunology Disclosures Ad Board Novartis,
The FDA and Unmet Needs: The Path to New Therapy for Transplant Recipients Mark D. Stegall, MD James C. Masson Professor of Surgery Research Departments of Surgery and Immunology Disclosures Ad Board Novartis,
Safety Assessment in Clinical Trials and Beyond
 Safety Assessment in Clinical Trials and Beyond Yuliya Yasinskaya, MD Medical Team Leader Division of Anti-Infective Products Center for Drug Evaluation and Research FDA Clinical Investigator Training
Safety Assessment in Clinical Trials and Beyond Yuliya Yasinskaya, MD Medical Team Leader Division of Anti-Infective Products Center for Drug Evaluation and Research FDA Clinical Investigator Training
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH?
 WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
WHAT CAN YOU USE IN YOUR CLINIC TODAY FOR THE TREATMENT OF NASH? Helena Cortez-Pinto Laboratório de Nutrição, FML, Serviço de Gastrenterologia, Hospital St Maria, Lisboa, Portugal EASL Governing Board:
Personalised medicine with state-of-the-art MR image analysis
 Personalised medicine with state-of-the-art MR image analysis Biomarkers as tests Diagnostic pathways in chronic disease Heart failure EKG BNP Echo/cardiac MRI COPD CXR Pulmonary function tests Chest CT
Personalised medicine with state-of-the-art MR image analysis Biomarkers as tests Diagnostic pathways in chronic disease Heart failure EKG BNP Echo/cardiac MRI COPD CXR Pulmonary function tests Chest CT
Jan vp24.i. GENFIT Overview. January 2014
 Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Jan 2014. vp24.i GENFIT Overview January 2014 1 Disclaimer Forward Looking Statement GENFIT IS A PUBLIC COMPANY LISTED ON NYSE EURONEXT (ALTERNEXT PARIS) STOCK EXCHANGE SINCE 2006. THIS DOCUMENT DOES NOT
Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup
 REVIEW REVIEW Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup Puneet Puri, M.B.B.S., M.D. and Arun J. Sanyal, M.B.B.S., M.D. Nonalcoholic fatty liver disease (NAFLD) is defined
REVIEW REVIEW Nonalcoholic Fatty Liver Disease: Definitions, Risk Factors, and Workup Puneet Puri, M.B.B.S., M.D. and Arun J. Sanyal, M.B.B.S., M.D. Nonalcoholic fatty liver disease (NAFLD) is defined
Phase 2b/3 Topline Trial Results
 Phase 2b/3 Topline Trial Results RP-G28 For the Treatment of Lactose Intolerance March 2017 Forward - Looking Statement To the extent that statements contained in this presentation are not descriptions
Phase 2b/3 Topline Trial Results RP-G28 For the Treatment of Lactose Intolerance March 2017 Forward - Looking Statement To the extent that statements contained in this presentation are not descriptions
Normal ALT for men 30 IU/L 36% US males abnormal. Abnl ALT. Assess alcohol use/meds. Recheck in 6-8 weeks. still pos
 Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
Fatty liver disease Its not just for big boys anymore Ken Flora, MD, FAASLD, FACG, AGAF No disclosures Common situation Normal ALT for men 30 IU/L 36% US males abnormal Normal ALT for women 20 IU/L 28%
NONINVASIVE IMAGING METHODS FOR ASSESSMENT OF LIVER DAMAGE IN NASH
 NONINVASIVE IMAGING METHODS FOR ASSESSMENT OF LIVER DAMAGE IN NASH Bachir Taouli, MD Director of Body MRI and Cancer Imaging Program Department of Radiology / Translational and Molecular Imaging Institute
NONINVASIVE IMAGING METHODS FOR ASSESSMENT OF LIVER DAMAGE IN NASH Bachir Taouli, MD Director of Body MRI and Cancer Imaging Program Department of Radiology / Translational and Molecular Imaging Institute
Nash with cirrhosis icd 10
 Free, official coding info for 2018 ICD-10-CM K75.81 - includes detailed crosswalks, DRG grouping and more. Free, official coding info for 2018 ICD-10-CM K74.69 - includes detailed crosswalks, DRG grouping
Free, official coding info for 2018 ICD-10-CM K75.81 - includes detailed crosswalks, DRG grouping and more. Free, official coding info for 2018 ICD-10-CM K74.69 - includes detailed crosswalks, DRG grouping
Study Endpoint Considerations: Final PRO Guidance and Beyond
 Study Endpoint Considerations: Final PRO Guidance and Beyond Laurie Burke Associate Director for Study Endpoints and Labeling OND/CDER/FDA Presented at: FIRST ANNUAL PATIENT REPORTED OUTCOMES (PRO) CONSORTIUM
Study Endpoint Considerations: Final PRO Guidance and Beyond Laurie Burke Associate Director for Study Endpoints and Labeling OND/CDER/FDA Presented at: FIRST ANNUAL PATIENT REPORTED OUTCOMES (PRO) CONSORTIUM
The Liver for the Nonhepatologist
 The Liver for the Nonhepatologist Michael R. Charlton, MBBS, FRCP Hepatology Director and Medical Director of Liver Transplantation Intermountain Medical Center Salt Lake City, Utah FORMATTED: 05-14-15
The Liver for the Nonhepatologist Michael R. Charlton, MBBS, FRCP Hepatology Director and Medical Director of Liver Transplantation Intermountain Medical Center Salt Lake City, Utah FORMATTED: 05-14-15
Screening for HCCwho,
 Screening for HCCwho, how and how often? Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital HCC Global Epidemiology
Screening for HCCwho, how and how often? Catherine Stedman Associate Professor of Medicine, University of Otago, Christchurch Gastroenterology Department, Christchurch Hospital HCC Global Epidemiology
