Fundamentals of Pharmacology for Veterinary Technicians Chapter 1
|
|
|
- Osborne Sharp
- 5 years ago
- Views:
Transcription
1 The original Pure Food and Drug Act is passed by Congress on June 30 and signed by President Theodore Roosevelt. It prohibits interstate commerce in misbranded and adulterated foods and drugs. The Meat Inspection Act is passed the same day (this Act was passed due to concern over insanitary conditions in meatpacking plants and the use of poisonous preservatives and dyes in foods). The Harrison Narcotic Act imposes upper limits on the amount of opium, opium-derived products, and cocaine allowed in products available to the public; requires prescriptions for products exceeding the allowable limit of narcotics; and mandates increased record-keeping for physicians and pharmacists that dispense narcotics. FDA recommends a complete revision of the obsolete 1906 Pure Food and Drug Act. The first bill is introduced into the Senate, launching a five-year legislative battle. Elixir of sulfanilamide, containing the poisonous solvent diethylene glycol, kills 107 persons, many of whom are children, dramatizing the need to establish drug safety before marketing and to enact the pending food and drug law. The federal Food, Drug, and Cosmetic Act of 1938 is passed by Congress, containing new provisions such as authorizing factory inspections and requiring new drugs to be shown safe before marketing. This act started a new system of drug regulation. FDA drastically revises manufacturing and quality control standards due to nearly 300 deaths and injuries as a result of distribution of sulfathiazole tablets tainted with the sedative phenobarbital. The incident prompts the beginning of good manufacturing practices (GMPs). Durham-Humphrey Amendment defines the kinds of drugs that cannot be used safely without medical supervision and restricts their sale to prescription by a licensed practitioner. Drug Abuse Control Amendments are enacted to deal with problems caused by abuse of depressants, stimulants, and hallucinogens. Animal Drug Amendments place all regulation of new animal drugs under one section of the Food, Drug, and Cosmetic Act (Section 512) making approval of animal drugs and medicated feeds more efficient. The Comprehensive Drug Abuse Prevention and Control Act replaces previous laws and categorizes drugs based on abuse and addiction potential. Environmental Protection Agency established; takes over FDA program for setting pesticide tolerances. Over-the-Counter Drug Review is initiated to enhance the safety, effectiveness and appropriate labeling of drugs sold without prescription. Regulation of biologics (including serums, vaccines, and blood products) is transferred from National Institutes of Health to FDA. Orphan Drug Act passed, enabling FDA to promote research and marketing of drugs needed for treating rare diseases. Drug Price Competition and Patent Term Restoration Act expedites the availability of less costly generic drugs by permitting FDA to approve applications to market generic versions of brand-name drugs without repeating the research done to prove them safe and effective. Generic Animal Drug and Patent Term Restoration Act extends to veterinary products benefits given to human drugs under the 1984 Drug Price Competition and Patent Term Restoration Act. Companies can produce and sell generic versions of animal drugs approved after October 1962 without duplicating research done to prove them safe and effective. The act also authorizes extension of animal drug patents. Dietary Supplement Health and Education Act establishes specific labeling requirements, provides a regulatory framework, and authorizes FDA to mandate good manufacturing practice regulations for dietary supplements. This act defines "dietary supplements" and "dietary ingredients" and classifies them as food. Animal Medicinal Drug Use Clarification Act (AMDUCA) allows veterinarians to prescribe extra-label use of veterinary drugs for animals under specific circumstances. In addition, the legislation allows licensed veterinarians to prescribe human drugs for use in animals under certain conditions. Animal Drug Availability Act adds flexibility to animal drug approval process, providing for flexible labeling and more direct communication between drug sponsors and FDA. Food and Drug Administration Modernization Act reauthorizes the Prescription Drug User Fee Act of 1992 and mandates the most wide-ranging reforms in agency practices since Provisions include measures to accelerate review of devices, advertising unapproved uses of approved drugs and devices, health claims for foods in agreement with published data by a reputable public health source, and development of good guidance practices for agency decision-making. Minor Use and Minor Species Health Act is similar to the human Orphan Drug Act of It is intended to provide FDA-authorized drugs for those less common species and indications (provides labeled drugs for needy minor species and provides major species (cats, dogs, horses, cattle, swine, turkey, chickens) with needed therapeutics for uncommon indications called minor uses). The Minor Use and Minor Species Animal Health Act encourages the development of treatments for species that would otherwise attract little interest in the development of veterinary therapies. The Animal Drug User Fee Act (ADUFA) permits FDA to collect subsidies for the review of certain animal drug applications from sponsors, analogous to laws passed for the evaluation of other products FDA regulates, ensuring the safety and effectiveness of drugs for animals and the safety of animals used as foodstuffs. The Animal Generic Drug User Fee Act (AGDUFA) permits FDA to study ways to improve the timeliness and predictability of the animal generic drug review process. The goals are to shorten the time to review and act on submissions by increasing staff and resources. Figure 1-1
2 Figure 1-2
3 Figure 1-3
4 Figure 1-4
5 Table 1-1 Drug Sources Drug Source Example Minerals Botanical (from plants) Animal Synthetic (manmade or engineered) Biological (from molds or bacteria) sulfur, iron, electrolytes digitalis, opioids insulin, thyroid hormone, lanolin aspirin, steroids, procaine antibiotics, ergot Table 1-1
6 Table 1-2 Labeling and Recording Requirements for Use of Extra- Label Drugs Record Requirements When Using Extra-Label Drugs Identify the animals, either as individuals or a group. Animal species treated. Numbers of animals treated. Condition being treated. The established name of the drug and active ingredient. Dosage prescribed or used. Duration of treatment. Specified withdrawal, withholding, or discard time(s), if applicable, for meat, milk, eggs, or animal-derived food. Keep records for two years. FDA may have access to these records to estimate risk to public health. Label Requirements When Using Extra-Label Drugs Name and address of the prescribing veterinarian. Established name of the drug. Any specified directions for use including the class/species or identification of the animal or herd, flock, pen, lot, or other group; the dosage frequency and route of administration; and the duration of therapy. Any cautionary statements. The specified withdrawal, withholding, or discard time for meat, milk, eggs, or any other food. Table 1-2
7 Table 1-3 Controlled Substances Categories and Examples Drug Schedule Schedule I (C-I) Schedule II (C-II) Schedule III (C-III) Schedule IV (C-IV) Schedule V (C-V) Definition of Schedule Substance has high potential for abuse and has no currently accepted medical use; there is a lack of accepted safety for use (considered most dangerous, with virtually no medical benefit) Substance has high potential for abuse but has currently accepted medical use, with severe restrictions Substance has potential for abuse less than schedule I and II drugs and has accepted medical uses Substance has low potential for abuse relative to drugs in schedule III and has accepted medical uses Substance has low potential for abuse relative to drugs in schedule IV and has accepted medical uses Examples heroin, LSD, marijuana cocaine, morphine, amphetamines, pentobarbital, etorphine, fentanyl, codeine acetaminophen/codeine combinations, ketamine, thiamylal, thiopental, hydrocodone diazepam, phenobarbital, butorphanol buprenophrine, diphenoxylate, codeine cough syrups Table 1-3
Which was the greatest problem with patent medicines in early America that lead to drug legislation?
 Pharmacology Connections to Nursing Practice 3rd Edition Adams Test Bank Full Download: http://testbanklive.com/download/pharmacology-connections-to-nursing-practice-3rd-edition-adams-test-bank/ Adams
Pharmacology Connections to Nursing Practice 3rd Edition Adams Test Bank Full Download: http://testbanklive.com/download/pharmacology-connections-to-nursing-practice-3rd-edition-adams-test-bank/ Adams
Chapter 1: Fundamentals of Pharmacology Test Bank
 Chapter 1: Fundamentals of Pharmacology Test Bank MULTIPLE CHOICE 1. By which of the following routes are drugs administered directly into the bloodstream? a. Enteral b. Transdermal c. Transmucosal d.
Chapter 1: Fundamentals of Pharmacology Test Bank MULTIPLE CHOICE 1. By which of the following routes are drugs administered directly into the bloodstream? a. Enteral b. Transdermal c. Transmucosal d.
Fundamentals of Pharmacology
 West Los Angeles College Worksheet #1 Fundamentals of Pharmacology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. By which of the following routes are
West Los Angeles College Worksheet #1 Fundamentals of Pharmacology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. By which of the following routes are
Chloroform Codeine Ether
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Chapter 6, Part 1 GENERAL PRINCIPLES OF PHARMACOLOGY Part 1 Basic Pharmacology Drugs are chemicals used to diagnose, treat, and disease. Pharmacology is the study of drugs
1 2 3 4 5 6 7 8 9 10 11 12 13 14 Chapter 6, Part 1 GENERAL PRINCIPLES OF PHARMACOLOGY Part 1 Basic Pharmacology Drugs are chemicals used to diagnose, treat, and disease. Pharmacology is the study of drugs
Cengage Learning. All rights reserved. No distribution allowed without express authorization. PART 1. Introduction to Pharmacologic Principles
 PART 1 Introduction to Pharmacologic Principles 80333_01_ch01_p001-011 pp3.indd 1 5/5/10 9:51:20 PM Chapter 1 Consumer Safety and Drug Regulations Objectives Upon completion of this chapter, the learner
PART 1 Introduction to Pharmacologic Principles 80333_01_ch01_p001-011 pp3.indd 1 5/5/10 9:51:20 PM Chapter 1 Consumer Safety and Drug Regulations Objectives Upon completion of this chapter, the learner
Sub. S.B. 119 As Passed by the Senate
 AM3404 Sub. S.B. 119 As Passed by the Senate Topic: Opioid Data and Communication Expansion Act moved to amend as follows: In line 1 of the title, after "4723.52," insert "4729.01, 4729.44, 4729.75, 4729.79,
AM3404 Sub. S.B. 119 As Passed by the Senate Topic: Opioid Data and Communication Expansion Act moved to amend as follows: In line 1 of the title, after "4723.52," insert "4729.01, 4729.44, 4729.75, 4729.79,
Do not open the test booklet prior to being told to do so.
 Last Name: Pharmacy 4054 Pharmacy Law Exam II Do not open the test booklet prior to being told to do so. I, the undersigned student, agree to do my best on the exam and that I have only used resources
Last Name: Pharmacy 4054 Pharmacy Law Exam II Do not open the test booklet prior to being told to do so. I, the undersigned student, agree to do my best on the exam and that I have only used resources
Neurobiology of Drug Abuse and Addiction PSYC 450
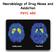 Neurobiology of Drug Abuse and Addiction PSYC 450 Healthy Control Drug Abuser Illicit drug use, lifetime and past year, in Canada 50 Canabis only Any drug Any of 5 drugs (without canabis) (CADUMS 2012)
Neurobiology of Drug Abuse and Addiction PSYC 450 Healthy Control Drug Abuser Illicit drug use, lifetime and past year, in Canada 50 Canabis only Any drug Any of 5 drugs (without canabis) (CADUMS 2012)
From Wikipedia, the free encyclopedia
 1 of 6 11/24/2010 11:43 PM From Wikipedia, the free encyclopedia The regulation of therapeutic goods, that is drugs and therapeutic devices, varies by jurisdiction. In some countries, such as the United
1 of 6 11/24/2010 11:43 PM From Wikipedia, the free encyclopedia The regulation of therapeutic goods, that is drugs and therapeutic devices, varies by jurisdiction. In some countries, such as the United
Non-Prescription Medicinal Products Containing Codeine: Guidance for Pharmacists on Safe Supply to Patients
 Non-Prescription Medicinal Products Containing Codeine: Guidance for Pharmacists on Safe Supply to Patients Pharmaceutical Society of Ireland Version 3 October 2017 Updates made following the enactment
Non-Prescription Medicinal Products Containing Codeine: Guidance for Pharmacists on Safe Supply to Patients Pharmaceutical Society of Ireland Version 3 October 2017 Updates made following the enactment
Test Bank for Basic Pharmacology for Nurses 14th edition by Clayton, Stock and Harroun
 Test Bank for Basic Pharmacology for Nurses 14th edition by Clayton, Stock and Harroun Link download full: https://digitalcontentmarket.org/download/testbank-for-basic-pharmacology-for-nurses-14thedition-by-clayton-stock-harroun
Test Bank for Basic Pharmacology for Nurses 14th edition by Clayton, Stock and Harroun Link download full: https://digitalcontentmarket.org/download/testbank-for-basic-pharmacology-for-nurses-14thedition-by-clayton-stock-harroun
Review of Controlled Drugs and Substances Act
 Review of Controlled Drugs and Substances Act Canadian Medical Association: Submission to Health Canada in response to the consultation on the Controlled Drugs and Substances Act and its regulations A
Review of Controlled Drugs and Substances Act Canadian Medical Association: Submission to Health Canada in response to the consultation on the Controlled Drugs and Substances Act and its regulations A
Nutrition Labeling Laws for Food and Supplements Timeline
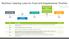 Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
syllabus (.pdf) for Prof. McCarthy s class is also available.
 Rennebohm Hall n University of Wisconsin-Madison School of Pharmacy 777 Highland Avenue n Madison, WI 53705-2222 n 608.262.5378 n aihp@aihp.org This slide presentation was compiled and produced by Robert
Rennebohm Hall n University of Wisconsin-Madison School of Pharmacy 777 Highland Avenue n Madison, WI 53705-2222 n 608.262.5378 n aihp@aihp.org This slide presentation was compiled and produced by Robert
Changes to the Eighth Edition
 Pharmacy Practice and the Law, Eighth Edition Includes Navigate 2 Advantage Access By Richard R. Abood, BS Pharm, JD-Professor Emeritus Pharmacy Practice, Thomas J. Long School of Pharmacy and Health Sciences,
Pharmacy Practice and the Law, Eighth Edition Includes Navigate 2 Advantage Access By Richard R. Abood, BS Pharm, JD-Professor Emeritus Pharmacy Practice, Thomas J. Long School of Pharmacy and Health Sciences,
Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program
 Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program Andrea Leonard-Segal, M.D., M.S. Director, Division of Nonprescription Clinical Evaluation 1 Contents
Naloxone Expanded Access: OTC status Considerations for a Nonprescription Drug Development Program Andrea Leonard-Segal, M.D., M.S. Director, Division of Nonprescription Clinical Evaluation 1 Contents
Drugs. January 30, , 2008 Pearson Education, Inc. Upper Saddle River, NJ 07458
 Drugs January 30, 2018 5-1 Breaking the Ice 1. Introduce Yourself to your neighbor. 2. Discuss/Define the following two words: A. Drugs B. Dependence 3. Find a writing utensil and your notebook! 5-2 Video:
Drugs January 30, 2018 5-1 Breaking the Ice 1. Introduce Yourself to your neighbor. 2. Discuss/Define the following two words: A. Drugs B. Dependence 3. Find a writing utensil and your notebook! 5-2 Video:
9/5/2011. Outline. 1. Past and Current Trends re: RX Abuse 2. Diversion Methods 3. Regulatory Reporting Requirements 4. Q/A
 Prescription Drug Abuse Crises Outline 1. Past and Current Trends re: RX Abuse 2. Diversion Methods 3. Regulatory Reporting Requirements 4. Q/A 1 1970s 1980s 2 The 1990s OXYCODONE Oxycodone/APAP OxyContin
Prescription Drug Abuse Crises Outline 1. Past and Current Trends re: RX Abuse 2. Diversion Methods 3. Regulatory Reporting Requirements 4. Q/A 1 1970s 1980s 2 The 1990s OXYCODONE Oxycodone/APAP OxyContin
Bill C-51 and Natural Health Products - The Facts
 Bill C-51 and Natural Health Products - The Facts 1. How will Bill C-51 change the way natural health products are regulated? Bill C-51 will not affect the way that natural health products are regulated
Bill C-51 and Natural Health Products - The Facts 1. How will Bill C-51 change the way natural health products are regulated? Bill C-51 will not affect the way that natural health products are regulated
SENATE BILL No Introduced by Senator Wieckowski. February 16, 2017
 SENATE BILL No. 504 Introduced by Senator Wieckowski February 16, 2017 An act to add Article 8.5 (commencing with Section 110961) to Chapter 5 of Part 5 of Division 104 of the Health and Safety Code, relating
SENATE BILL No. 504 Introduced by Senator Wieckowski February 16, 2017 An act to add Article 8.5 (commencing with Section 110961) to Chapter 5 of Part 5 of Division 104 of the Health and Safety Code, relating
FDA Regulation of Claims on Dietary Supplement and Food Products
 FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
FDA Regulation of Claims on Dietary Supplement and Food Products Patricia Kaeding August 2004 Topics FDA Overview What are claims Why claims matter Categories of claims Regulatory requirements for types
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS
 WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
MARYLAND BOARD OF PHARMACY
 MARYLAND BOARD OF PHARMACY CONTRACEPTION REGULATIONSAnd Other Legislative Initiatives THE ROAD FROM CONCEPTUALIZATION TO IMPLEMENTATION Deena Speights-Napata Executive Director Maryland Board of Pharmacy
MARYLAND BOARD OF PHARMACY CONTRACEPTION REGULATIONSAnd Other Legislative Initiatives THE ROAD FROM CONCEPTUALIZATION TO IMPLEMENTATION Deena Speights-Napata Executive Director Maryland Board of Pharmacy
M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents
 M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents Communiqué for Health Industry Clients on the Legal Retainer Program Canada s New Natural Health Products Regulations On
M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents Communiqué for Health Industry Clients on the Legal Retainer Program Canada s New Natural Health Products Regulations On
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
DEPARTMENT OF HEALTH & HUMAN SERVICES Food and Drug Apini$r$iy -tq 5-f
 DEPARTMENT OF HEALTH & HUMAN SERVICES Food and Drug Apini$r$iy -tq 5-f J Dallas District 4040 North Central Expressway Dallas, Texas 75204-3145 Ref: 2004-DAL-WL-03 WARNING LETTER CERTIFIED MAIL RETURN
DEPARTMENT OF HEALTH & HUMAN SERVICES Food and Drug Apini$r$iy -tq 5-f J Dallas District 4040 North Central Expressway Dallas, Texas 75204-3145 Ref: 2004-DAL-WL-03 WARNING LETTER CERTIFIED MAIL RETURN
4/26/2013. Libia Lugo Drug Specialist San Juan District Office Investigations Branch
 Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
2017 Legislative Policy Update
 2017 Legislative Policy Update Aliyah N. Horton, CAE Executive Director Maryland Pharmacists Association Presentation Overview Maryland Pharmacists Association General highlights of the session Pharmacy-related
2017 Legislative Policy Update Aliyah N. Horton, CAE Executive Director Maryland Pharmacists Association Presentation Overview Maryland Pharmacists Association General highlights of the session Pharmacy-related
Problems with the 1906 Act
 Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
CO SPONSORED BY. November 14, 2018 DoubleTree by Hilton Hotel Rochester Rochester, MN
 CO SPONSORED BY November 14, 2018 DoubleTree by Hilton Hotel Rochester Rochester, MN Good Manufacturing Practices (GMP) A Regulatory Overview and The Role of EHS in Pharmaceutical Manufacturing Compliance
CO SPONSORED BY November 14, 2018 DoubleTree by Hilton Hotel Rochester Rochester, MN Good Manufacturing Practices (GMP) A Regulatory Overview and The Role of EHS in Pharmaceutical Manufacturing Compliance
Mark W. Caverly, Chief Liaison and Policy Section
 DEA Issues: An Update Harold Rogers National Prescription Drug Monitoring Meeting Meeting September 24 25, 2009 Mark W. Caverly, Chief Liaison and Policy Section Prescription Drug Abuse Prescription Drug
DEA Issues: An Update Harold Rogers National Prescription Drug Monitoring Meeting Meeting September 24 25, 2009 Mark W. Caverly, Chief Liaison and Policy Section Prescription Drug Abuse Prescription Drug
IC ARTICLE 48. CONTROLLED SUBSTANCES
 IC 35-48 ARTICLE 48. CONTROLLED SUBSTANCES IC 35-48-1 Chapter 1. Definitions IC 35-48-1-0.1 Application of certain amendments to chapter Sec. 0.1. The addition of section 9.3 of this chapter by P.L.225-2003
IC 35-48 ARTICLE 48. CONTROLLED SUBSTANCES IC 35-48-1 Chapter 1. Definitions IC 35-48-1-0.1 Application of certain amendments to chapter Sec. 0.1. The addition of section 9.3 of this chapter by P.L.225-2003
PRESCRIPTION DRUG MONITORING PROGRAM ST. CHARLES COUNTY Q1 2018
 PRESCRIPTION DRUG MONITORING PROGRAM ST. CHARLES COUNTY Q1 2018 Contents Executive Summary... 2 User Registration & System Utilization... 4 Dispensation Rates... 6 Dispensation Rates by Geography... 6
PRESCRIPTION DRUG MONITORING PROGRAM ST. CHARLES COUNTY Q1 2018 Contents Executive Summary... 2 User Registration & System Utilization... 4 Dispensation Rates... 6 Dispensation Rates by Geography... 6
Food and Drug Law. Fall Syllabus. Professor Jesson. Hamline University School of Law
 Food and Drug Law Fall 2009 Syllabus Professor Jesson Hamline University School of Law General course Information: Course: Food and Drug Law Credits: 2 Classroom: Law 103 Time: Thursdays, 4-5:50 p.m. Professor:
Food and Drug Law Fall 2009 Syllabus Professor Jesson Hamline University School of Law General course Information: Course: Food and Drug Law Credits: 2 Classroom: Law 103 Time: Thursdays, 4-5:50 p.m. Professor:
PRESCRIPTION DRUG MONITORING PROGRAM
 PRESCRIPTION DRUG MONITORING PROGRAM ST. LOUIS COUNTY Q2 2017 Contents Executive Summary... 2 User Registration & System Utilization... 4 Dispensation Rates... 6 Dispensation Rates by Geography... 6 Dispensation
PRESCRIPTION DRUG MONITORING PROGRAM ST. LOUIS COUNTY Q2 2017 Contents Executive Summary... 2 User Registration & System Utilization... 4 Dispensation Rates... 6 Dispensation Rates by Geography... 6 Dispensation
INTERIM NOTIFICATION PROGRAM LOW RISK VETERINARY HEALTH PRODUCTS OVERVIEW
 INTERIM NOTIFICATION PROGRAM LOW RISK VETERINARY HEALTH PRODUCTS OVERVIEW Table of Contents 1.0 Purpose 2.0 Background 3.0 Scope 4.0 Definitions 5.0 Identification of a Notified Product 6.0 Requirements
INTERIM NOTIFICATION PROGRAM LOW RISK VETERINARY HEALTH PRODUCTS OVERVIEW Table of Contents 1.0 Purpose 2.0 Background 3.0 Scope 4.0 Definitions 5.0 Identification of a Notified Product 6.0 Requirements
Role of PMPs in Preventing Substance Abuse National Conference of State Legislatures December 6, 2006 San Antonio, Tx
 Role of PMPs in Preventing Substance Abuse National Conference of State Legislatures December 6, 2006 San Antonio, Tx Nick Reuter Division of Pharmacologic Therapy Substance Abuse and Mental Health Services
Role of PMPs in Preventing Substance Abuse National Conference of State Legislatures December 6, 2006 San Antonio, Tx Nick Reuter Division of Pharmacologic Therapy Substance Abuse and Mental Health Services
A Bill Regular Session, 2015 SENATE BILL 717
 Stricken language would be deleted from and underlined language would be added to present law. 0 State of Arkansas 0th General Assembly As Engrossed: S// H// A Bill Regular Session, SENATE BILL By: Senator
Stricken language would be deleted from and underlined language would be added to present law. 0 State of Arkansas 0th General Assembly As Engrossed: S// H// A Bill Regular Session, SENATE BILL By: Senator
As Introduced. 132nd General Assembly Regular Session H. B. No
 132nd General Assembly Regular Session H. B. No. 560 2017-2018 Representative Lanese A B I L L To amend sections 923.41, 923.48, and 923.51 of the Revised Code to prohibit pet food from containing remains
132nd General Assembly Regular Session H. B. No. 560 2017-2018 Representative Lanese A B I L L To amend sections 923.41, 923.48, and 923.51 of the Revised Code to prohibit pet food from containing remains
Annual Reports Questionnaire (ARQ) Part III: Extent, patterns and trends in drug use
 Annual Reports Questionnaire (ARQ) Part III: Extent, patterns and trends in drug use Report of the Government of: Reporting Year: Completed on (date): Please return completed questionnaire to: arq@unodc.org
Annual Reports Questionnaire (ARQ) Part III: Extent, patterns and trends in drug use Report of the Government of: Reporting Year: Completed on (date): Please return completed questionnaire to: arq@unodc.org
Aligning Market Objectives and Policy for National Public Health
 Abuse-Deterrent Formulations Summit March 7, 2017 Alexandria, Virginia Aligning Market Objectives and Policy for National Public Health Shruti Kulkarni, Esq. Outside Counsel Twitter: @claad_coalition CLAAD
Abuse-Deterrent Formulations Summit March 7, 2017 Alexandria, Virginia Aligning Market Objectives and Policy for National Public Health Shruti Kulkarni, Esq. Outside Counsel Twitter: @claad_coalition CLAAD
As Introduced. 132nd General Assembly Regular Session H. B. No Representative Ramos Cosponsors: Representatives Seitz, Fedor, Ashford
 132nd General Assembly Regular Session H. B. No. 773 2017-2018 Representative Ramos Cosponsors: Representatives Seitz, Fedor, Ashford A B I L L To amend sections 3719.01 and 3796.20 and to enact section
132nd General Assembly Regular Session H. B. No. 773 2017-2018 Representative Ramos Cosponsors: Representatives Seitz, Fedor, Ashford A B I L L To amend sections 3719.01 and 3796.20 and to enact section
New regulatory requirements DPCS Regulations 2017
 New regulatory requirements DPCS Regulations 2017 Prescribers and pharmacists May 2017 Table of Contents Explanatory notes... 1 Clarifying the meaning of key terms... 1 Chart instructions and prescriptions...
New regulatory requirements DPCS Regulations 2017 Prescribers and pharmacists May 2017 Table of Contents Explanatory notes... 1 Clarifying the meaning of key terms... 1 Chart instructions and prescriptions...
Chapter 23. Medicines and Drugs
 Chapter 23 Medicines and Drugs Lesson 1 The Role of Medicines 1. What are medicines? Drugs that are used to prevent disease or other conditions. 2. What are drugs? Substances other than food that change
Chapter 23 Medicines and Drugs Lesson 1 The Role of Medicines 1. What are medicines? Drugs that are used to prevent disease or other conditions. 2. What are drugs? Substances other than food that change
The Shifting Federal Regulation of Cannabis Products
 The Durham Bar 2019 CLE Program The Shifting Federal Regulation of Cannabis Products Erica M. Jackson, FDA Partner K&L Gates February 6, 2019 Copyright 2018 by K&L Gates LLP. All rights reserved. OVERVIEW
The Durham Bar 2019 CLE Program The Shifting Federal Regulation of Cannabis Products Erica M. Jackson, FDA Partner K&L Gates February 6, 2019 Copyright 2018 by K&L Gates LLP. All rights reserved. OVERVIEW
Drug Trends &Trafficking I/S Brian Dempsey
 Drug Trends &Trafficking I/S Brian Dempsey 412-777-6945 brian.w.dempsey@usdoj.gov Sources Sources 360 Degree Strategy Strategic collaboration and guidance via three interconnected avenues. Community
Drug Trends &Trafficking I/S Brian Dempsey 412-777-6945 brian.w.dempsey@usdoj.gov Sources Sources 360 Degree Strategy Strategic collaboration and guidance via three interconnected avenues. Community
Political Economy of the Medical Products Industry
 Political Economy of the Medical Products Industry Tom Hazlet Survey of Biomedical Regulatory Affairs Spring 2008 Menu Definitions medical products political economy Commodities drugs devices biologics
Political Economy of the Medical Products Industry Tom Hazlet Survey of Biomedical Regulatory Affairs Spring 2008 Menu Definitions medical products political economy Commodities drugs devices biologics
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years
 The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
2004-L SEPTEMBER
 BULLETIN INTELLIGENCE Buprenorphine: Potential for Abuse Product No. 2004-L0424-013 SEPTEMBER 2004 U. S. D E P A R T M E N T O F J U S T I C E NDIC Within the past 2 years buprenorphine a Schedule III
BULLETIN INTELLIGENCE Buprenorphine: Potential for Abuse Product No. 2004-L0424-013 SEPTEMBER 2004 U. S. D E P A R T M E N T O F J U S T I C E NDIC Within the past 2 years buprenorphine a Schedule III
Top 10 narcotic pain pills
 Top 10 narcotic pain pills Click to go to the item or just scroll down the page. Doctors Respond to FDA Panel Recommendations FDA Considers Banning Popular Prescription Pain Medications and. Top 10 Natural
Top 10 narcotic pain pills Click to go to the item or just scroll down the page. Doctors Respond to FDA Panel Recommendations FDA Considers Banning Popular Prescription Pain Medications and. Top 10 Natural
How Are Drugs Discovered?
 DRUG EVALUATION & REGULATION M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 656A, 828-1676, mdamaj@hsc.vcu.edu The Changed Context of Drug Discovery and Development The 1800s:
DRUG EVALUATION & REGULATION M. Imad Damaj, Ph.D. Associate Professor Pharmacology and Toxicology Smith 656A, 828-1676, mdamaj@hsc.vcu.edu The Changed Context of Drug Discovery and Development The 1800s:
Questions & answers on the use of cannabis sativa L. and cannabinoids (such as cannabidiol) as foodstuffs or within foodstuffs
 Questions & answers on the use of cannabis sativa L. and cannabinoids (such as cannabidiol) as foodstuffs or within foodstuffs Table of contents 1. Is there a difference between Cannabis and hemp?... 2
Questions & answers on the use of cannabis sativa L. and cannabinoids (such as cannabidiol) as foodstuffs or within foodstuffs Table of contents 1. Is there a difference between Cannabis and hemp?... 2
Student Number. PHARMACY 543 PHARMACY LAWS & ETHICS MIDTERM EXAMINATION 2 November 10, 1999
 PHARMACY 543 PHARMACY LAWS & ETHICS MIDTERM EXAMINATION 2 November 10, 1999 Questions 1-25 are multiple choice; please record answers on Side 2 of a Standard Answer Sheet, Form 1158. Follow the instructions
PHARMACY 543 PHARMACY LAWS & ETHICS MIDTERM EXAMINATION 2 November 10, 1999 Questions 1-25 are multiple choice; please record answers on Side 2 of a Standard Answer Sheet, Form 1158. Follow the instructions
Minister of Social Affairs. Passed No. 73 RTL 2005, 57, 807 Entry into force
 Issuer: Minister of Social Affairs Type: regulation In force from: 12.02.2011 In force until: Did not enter into force Translation published: 08.10.2014 Conditions and Procedure for Handling of Narcotic
Issuer: Minister of Social Affairs Type: regulation In force from: 12.02.2011 In force until: Did not enter into force Translation published: 08.10.2014 Conditions and Procedure for Handling of Narcotic
Dietary Supplement Health and Education Act of 1994 Public Law rd Congress
 Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Heroin. Brain Research Institute, UCLA Alison Taylor
 Heroin Brain Research Institute, UCLA Alison Taylor Heroin H, Smack, Dope, Junk Can be purchased as a white powder White China or a dark resin Black Tar Opiates Derived from the opium poppy Morphine:
Heroin Brain Research Institute, UCLA Alison Taylor Heroin H, Smack, Dope, Junk Can be purchased as a white powder White China or a dark resin Black Tar Opiates Derived from the opium poppy Morphine:
Ornsurang Teerawat
 ASEAN TRADITIONAL MEDICINES AND HEALTH SUPPLEMENTS REGULATORY AND HARMONIZATION UPDATE Ornsurang Teerawat 21.10.2014 \ http://trainstudenytp.wordpress.com ACCSQ (ASEAN Consultative Committee for Standard
ASEAN TRADITIONAL MEDICINES AND HEALTH SUPPLEMENTS REGULATORY AND HARMONIZATION UPDATE Ornsurang Teerawat 21.10.2014 \ http://trainstudenytp.wordpress.com ACCSQ (ASEAN Consultative Committee for Standard
COPYRIGHTED MATERIAL. Introduction
 1 Introduction Pharmacology is the science of drugs and their effects on biological systems. A drug can be defined as a chemical that can cause a change in a biological system; the important biological
1 Introduction Pharmacology is the science of drugs and their effects on biological systems. A drug can be defined as a chemical that can cause a change in a biological system; the important biological
Schedules of Controlled Substances: Placement of Furanyl fentanyl, 4- Fluoroisobutyryl fentanyl, Acryl fentanyl, Tetrahydrofuranyl fentanyl, and
 This document is scheduled to be published in the Federal Register on 11/29/2018 and available online at https://federalregister.gov/d/2018-26045, and on govinfo.gov Billing Code 4410-09-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 11/29/2018 and available online at https://federalregister.gov/d/2018-26045, and on govinfo.gov Billing Code 4410-09-P DEPARTMENT OF
SECTION 1 LEARNING OBJECTIVES:
 CHAPTER 5 DRUGS SECTION 1 LEARNING OBJECTIVES: 1. Define psychological and physical dependence. 2. Name and classify the commonly abused drugs. 3. Describe the tendency to develop psychological and physical
CHAPTER 5 DRUGS SECTION 1 LEARNING OBJECTIVES: 1. Define psychological and physical dependence. 2. Name and classify the commonly abused drugs. 3. Describe the tendency to develop psychological and physical
Updated: 08/2017 DMMA Approved: 11/2017
 Request for Prior Authorization for Therapy to Treat Binge Eating Disorder Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for medications to treat Binge
Request for Prior Authorization for Therapy to Treat Binge Eating Disorder Website Form www.highmarkhealthoptions.com Submit request via: Fax - 1-855-476-4158 All requests for medications to treat Binge
ANNUAL REPORT
 ANNUAL REPORT - 2015 New Hampshire Prescription Drug Monitoring Program Report for July 1, 2014 June 30, 2015 Published October 2015 Table of Contents Executive Summary... 3-4 NH PDMP Background Information
ANNUAL REPORT - 2015 New Hampshire Prescription Drug Monitoring Program Report for July 1, 2014 June 30, 2015 Published October 2015 Table of Contents Executive Summary... 3-4 NH PDMP Background Information
Proposition 65 and Supplements
 Proposition 65 and Supplements Nutrition Industry Association John Venardos Senior Vice President Worldwide Regulatory & Government Affairs Herbalife International of America, Inc. 5/23/2011 SYNOPSIS Now
Proposition 65 and Supplements Nutrition Industry Association John Venardos Senior Vice President Worldwide Regulatory & Government Affairs Herbalife International of America, Inc. 5/23/2011 SYNOPSIS Now
SUNRISE, FLORIDA ORDINANCE NO.
 SUNRISE, FLORIDA ORDINANCE NO. AN ORDINANCE OF THE CITY OF SUNRISE, FLORIDA, AMENDING CHAPTER 7 LOCAL BUSINESS TAX; LOCAL BUSINESS TAX RECEIPTS, AND BUSINESS REGULATIONS*; BY ADDING ARTICLE XIV MISCELLANEOUS
SUNRISE, FLORIDA ORDINANCE NO. AN ORDINANCE OF THE CITY OF SUNRISE, FLORIDA, AMENDING CHAPTER 7 LOCAL BUSINESS TAX; LOCAL BUSINESS TAX RECEIPTS, AND BUSINESS REGULATIONS*; BY ADDING ARTICLE XIV MISCELLANEOUS
Subtitle E--National Bioengineered Food Disclosure Standard
 GMO Labeling Bill S.764 as of July 14, 2016* [Congressional Bills 114th Congress] [From the U.S. Government Publishing Office]* [S. 764 Enrolled Bill (ENR)] S.764 One Hundred Fourteenth Congress of the
GMO Labeling Bill S.764 as of July 14, 2016* [Congressional Bills 114th Congress] [From the U.S. Government Publishing Office]* [S. 764 Enrolled Bill (ENR)] S.764 One Hundred Fourteenth Congress of the
Issue Overview: Heroin Addiction
 Issue Overview: Heroin Addiction By Lauren Etter, Bloomberg, adapted by Newsela staff on 12.16.16 Word Count 789 Level 1160L TOP: A heroin user prepares to inject himself on March 23, 2016, in New London,
Issue Overview: Heroin Addiction By Lauren Etter, Bloomberg, adapted by Newsela staff on 12.16.16 Word Count 789 Level 1160L TOP: A heroin user prepares to inject himself on March 23, 2016, in New London,
EDUCATIONAL COMMENTARY METHADONE
 EDUCATIONAL COMMENTARY METHADONE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits see the Continuing Education
EDUCATIONAL COMMENTARY METHADONE Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits see the Continuing Education
Iowa District Court Polk County, Iowa. CARL OLSEN, ) ) Petitioner, ) ) vs. ) ) Docket No. CV IOWA BOARD OF PHARMACY ) ) Respondent.
 Iowa District Court Polk County, Iowa CARL OLSEN, ) ) Petitioner, ) ) vs. ) ) Docket No. CV 51068 IOWA BOARD OF PHARMACY ) ) Respondent. ) MEMORANDUM IN SUPPORT OF PETITION FOR JUDICIAL REVIEW Federal
Iowa District Court Polk County, Iowa CARL OLSEN, ) ) Petitioner, ) ) vs. ) ) Docket No. CV 51068 IOWA BOARD OF PHARMACY ) ) Respondent. ) MEMORANDUM IN SUPPORT OF PETITION FOR JUDICIAL REVIEW Federal
Opioid Pain Medications Frequently Asked Questions
 Opioid Pain Medications IT S YOUR HEALTH Opioid Pain Medications What is an opioid? Opioids are a broad range of natural and manufactured (synthetic) compounds that are commonly used for pain relief. There
Opioid Pain Medications IT S YOUR HEALTH Opioid Pain Medications What is an opioid? Opioids are a broad range of natural and manufactured (synthetic) compounds that are commonly used for pain relief. There
Public Health Impacts of 2016 West Virginia Legislation
 Public Health Impacts of 2016 West Virginia Legislation Sherri A. Young, DO, FAAFP WVSOM Summer Seminar North Myrtle Beach, South Carolina June 15, 2016 1 Objectives Passage and implementation of Expedited
Public Health Impacts of 2016 West Virginia Legislation Sherri A. Young, DO, FAAFP WVSOM Summer Seminar North Myrtle Beach, South Carolina June 15, 2016 1 Objectives Passage and implementation of Expedited
on the advertising of medicinal products for human use
 30. 4. 92 Official Journal of the European Communities No L 113 / 13 COUNCIL DIRECTIVE 92/28/EEC of 31 March 1992 on the advertising of medicinal products for human use THE COUNCIL OF THE EUROPEAN COMMUNITIES,
30. 4. 92 Official Journal of the European Communities No L 113 / 13 COUNCIL DIRECTIVE 92/28/EEC of 31 March 1992 on the advertising of medicinal products for human use THE COUNCIL OF THE EUROPEAN COMMUNITIES,
QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances
 QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances Published by authority of the Minister of Health Date Adopted 2017/06/09 Effective Date 2017/06/28 Health Products and Food Branch Our
QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances Published by authority of the Minister of Health Date Adopted 2017/06/09 Effective Date 2017/06/28 Health Products and Food Branch Our
Chapter 7. Appendix A SCHEDULES OF CONTROLLED SUBSTANCES. Forensic Science for High School Kendall/Hunt Publishing Company
 Appendix A SCHEDULES F CTRLLED SUBSTACES Schedule I Substances that have no accepted medical use in the U.S. and have a high abuse potential. May not be prescribed. Schedule II Substances that have a high
Appendix A SCHEDULES F CTRLLED SUBSTACES Schedule I Substances that have no accepted medical use in the U.S. and have a high abuse potential. May not be prescribed. Schedule II Substances that have a high
Risk Evaluation And Mitigation Strategies:
 Risk Evaluation And Mitigation Strategies: The Experts Answer Questions from Health-System Pharmacists A midday symposium about the risk evaluation and mitigation strategies (REMS) required by the Food
Risk Evaluation And Mitigation Strategies: The Experts Answer Questions from Health-System Pharmacists A midday symposium about the risk evaluation and mitigation strategies (REMS) required by the Food
Much Ado About Nothing: Cannabis and the Current Administration
 Much Ado About Nothing: Cannabis and the Current Administration Cannabis Health & Safety Organization 501(c)(3) not-for-profit Independent: No financial stake in or funding from the cannabis industry.
Much Ado About Nothing: Cannabis and the Current Administration Cannabis Health & Safety Organization 501(c)(3) not-for-profit Independent: No financial stake in or funding from the cannabis industry.
FDA Laws & Pharmacy Practice
 Objectives FDA Laws & Pharmacy Practice Tom Hazlet Pharmacy 543 October 6, 2004 Be able to discuss the evolution of food and drug law in the United States Be able to discuss the ways in which pharmacists
Objectives FDA Laws & Pharmacy Practice Tom Hazlet Pharmacy 543 October 6, 2004 Be able to discuss the evolution of food and drug law in the United States Be able to discuss the ways in which pharmacists
U.S. FDA Perspective on Food Supplements/TM
 U.S. FDA Perspective on Food Supplements/TM IKHLAS A. KHAN National Center for Natural Products Research, Department of Pharmacognosy and Research Institute of Pharmaceutical Sciences, School of Pharmacy,
U.S. FDA Perspective on Food Supplements/TM IKHLAS A. KHAN National Center for Natural Products Research, Department of Pharmacognosy and Research Institute of Pharmaceutical Sciences, School of Pharmacy,
Office of University Counsel and Secretary of the Board of Regents
 To: From: University of Colorado Research Faculty President Bruce D. Benson University Counsel Patrick T. O Rourke Date: March 11, 2014 Re: Legality of Marijuana Research Colorado is one of twenty states
To: From: University of Colorado Research Faculty President Bruce D. Benson University Counsel Patrick T. O Rourke Date: March 11, 2014 Re: Legality of Marijuana Research Colorado is one of twenty states
Are You Considering Using CAM?
 Are You Considering Using CAM? Decisions about your health care are important including decisions about whether to use complementary and alternative medicine (CAM). The National Center for Complementary
Are You Considering Using CAM? Decisions about your health care are important including decisions about whether to use complementary and alternative medicine (CAM). The National Center for Complementary
TRAFFIC IN OPIUM AND OTHER DANGEROUS DRUGS
 [Communicated to the Council and the Members of the League.] C. 490, HE. 333. 1938. XI. [O.C./A.R.1937/104.] (Issued in English only.) Geneva, December 2nd, 1938. LEAGUE OF NATIONS TRAFFIC IN OPIUM AND
[Communicated to the Council and the Members of the League.] C. 490, HE. 333. 1938. XI. [O.C./A.R.1937/104.] (Issued in English only.) Geneva, December 2nd, 1938. LEAGUE OF NATIONS TRAFFIC IN OPIUM AND
Confirm Limit--Level of detectable drugs in urine to confirm a positive test.
 Alcohol and Substance Abuse Policy Purpose To establish and maintain a safe, healthy working environment for all PVFD members; reduce the incidence of accidental injury to members and property; reduce
Alcohol and Substance Abuse Policy Purpose To establish and maintain a safe, healthy working environment for all PVFD members; reduce the incidence of accidental injury to members and property; reduce
perpetuate -- and perhaps even intensify -- that controversy. 1 On July 18th, the Fifth Circuit affirmed FDA s longstanding position that
 Food & Drug July 29, 2008 Fifth Circuit Rules that FDA May Regulate Compounded Drugs as New Drugs Update on Medical Center Pharmacy v. Mukasey For decades, the pharmacy compounding industry has disputed
Food & Drug July 29, 2008 Fifth Circuit Rules that FDA May Regulate Compounded Drugs as New Drugs Update on Medical Center Pharmacy v. Mukasey For decades, the pharmacy compounding industry has disputed
Michigan Legislative and Regulatory Pharmacy Initiatives Update
 Michigan Legislative and Regulatory Pharmacy Initiatives Update Michigan Association of Health Plans Summer Conference Carrie Germain, R.Ph. Health Alliance Plan Senior Director, Pharmacy, Quality and
Michigan Legislative and Regulatory Pharmacy Initiatives Update Michigan Association of Health Plans Summer Conference Carrie Germain, R.Ph. Health Alliance Plan Senior Director, Pharmacy, Quality and
H 8313 S T A T E O F R H O D E I S L A N D
 ======== LC00 ======== 01 -- H 1 S T A T E O F R H O D E I S L A N D IN GENERAL ASSEMBLY JANUARY SESSION, A.D. 01 A N A C T RELATING TO FOOD AND DRUGS -- NALOXONE ACCESS Introduced By: Representatives
======== LC00 ======== 01 -- H 1 S T A T E O F R H O D E I S L A N D IN GENERAL ASSEMBLY JANUARY SESSION, A.D. 01 A N A C T RELATING TO FOOD AND DRUGS -- NALOXONE ACCESS Introduced By: Representatives
Medicines in Schedule 1 to the Medicine Regulations 1984 that reference the manufacturer s original pack
 Medicines in Schedule 1 to the Medicine Regulations 1984 that reference the manufacturer s original pack Information paper for the Medicines Classification Committee Medsafe January 2018 1. Purpose The
Medicines in Schedule 1 to the Medicine Regulations 1984 that reference the manufacturer s original pack Information paper for the Medicines Classification Committee Medsafe January 2018 1. Purpose The
Brief History of Methadone Maintenance Treatment
 METHADONE Brief History of Methadone Maintenance Treatment Methadone maintenance treatment was on the cusp of the social revolution in the sixties. Doctors and public health workers had concluded what
METHADONE Brief History of Methadone Maintenance Treatment Methadone maintenance treatment was on the cusp of the social revolution in the sixties. Doctors and public health workers had concluded what
CARD/MAIL/PRE-APPROVAL/PREFERRED RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date:
![CARD/MAIL/PRE-APPROVAL/PREFERRED RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date: CARD/MAIL/PRE-APPROVAL/PREFERRED RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date:](/thumbs/79/80269988.jpg) RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date: CARD/MAIL/PRE-APPROVAL/PREFERRED The Prescription Drug Coverage under this Rider [replaces] [supplements] the Prescription
RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date: CARD/MAIL/PRE-APPROVAL/PREFERRED The Prescription Drug Coverage under this Rider [replaces] [supplements] the Prescription
Effective Date: Approved by: Laboratory Executive Director, Ed Hughes (electronic signature)
 1 Policy #: 803 (PLH-803-02) Effective Date: NA Reviewed Date: 4/11/2008 Subject: URINE DRUG SCREENS Approved by: Laboratory Executive Director, Ed Hughes (electronic signature) Approved by: Laboratory
1 Policy #: 803 (PLH-803-02) Effective Date: NA Reviewed Date: 4/11/2008 Subject: URINE DRUG SCREENS Approved by: Laboratory Executive Director, Ed Hughes (electronic signature) Approved by: Laboratory
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Products
 Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Many drugs of abuse are illegal drugs. Possessing, using, buying, or selling these drugs is illegal for people of any age.
 1 Chapter 12 Section 1 Objectives List six ways illegal drug use can be dangerous. State five reasons a person might try illegal drugs. Identify the reason drug abuse is especially dangerous to teens.
1 Chapter 12 Section 1 Objectives List six ways illegal drug use can be dangerous. State five reasons a person might try illegal drugs. Identify the reason drug abuse is especially dangerous to teens.
Mark M. Yacura. Partner
 Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative
 Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative Presented to: Food Supply Chain Stakeholder Meeting Date: January 27, 2014 Presented by: Daniel Miller, Food
Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative Presented to: Food Supply Chain Stakeholder Meeting Date: January 27, 2014 Presented by: Daniel Miller, Food
I am pleased to highlight for this Honourable House and the public. that today the Government will be laying amendments to the
 MINISTERIAL STATEMENT TO THE HOUSE OF ASSEMBLY BY THE HONOURABLE KIM N. WILSON, JP, MP MINISTER OF HEALTH UPDATES AND MODERNIZATION OF DRUG SCHEDULES 24 November 2017 Mr Speaker and Honourable Members,
MINISTERIAL STATEMENT TO THE HOUSE OF ASSEMBLY BY THE HONOURABLE KIM N. WILSON, JP, MP MINISTER OF HEALTH UPDATES AND MODERNIZATION OF DRUG SCHEDULES 24 November 2017 Mr Speaker and Honourable Members,
What is the difference between Vicoden, OxyContin, Percocet and Percodan
 CHAPTER 5 DRUGS NARCOTICS Narcotics are drugs that induce sleep and relieve pain. The term narcotic is used incorrectly today for example the cocaine is labeled as a narcotic but is actually a stimulant
CHAPTER 5 DRUGS NARCOTICS Narcotics are drugs that induce sleep and relieve pain. The term narcotic is used incorrectly today for example the cocaine is labeled as a narcotic but is actually a stimulant
are being added to products that have the same or similar effects exercised emergency scheduling authority to control five (5)
 ORDINANCE NO.: 12-18 AN ORDINANCE OF THE CITY OF SOUTH DAYTONA, FLORIDA, AMENDING CHAPTER 10 (OFFENSES AND MISCELLANEOUS PROVISIONS), CREATING ARTICLE V. (HERBAL INCENSE AND BATH SALTS), SECTION 10-67
ORDINANCE NO.: 12-18 AN ORDINANCE OF THE CITY OF SOUTH DAYTONA, FLORIDA, AMENDING CHAPTER 10 (OFFENSES AND MISCELLANEOUS PROVISIONS), CREATING ARTICLE V. (HERBAL INCENSE AND BATH SALTS), SECTION 10-67
NARCOTIC NOTES FLIPBOOK BY: PER:
 NARCOTIC NOTES FLIPBOOK BY: PER: 1 https://www.youtube. com/watch?v=lolb21bii-a 2 Laws, Collection, & Preservation Narcotic Drugs = natural or synthetic substance that produces bodily (physiological) or
NARCOTIC NOTES FLIPBOOK BY: PER: 1 https://www.youtube. com/watch?v=lolb21bii-a 2 Laws, Collection, & Preservation Narcotic Drugs = natural or synthetic substance that produces bodily (physiological) or
FlexRx 6-Tier. SM Pharmacy Benefit Guide
 FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
FlexRx 6-Tier SM Pharmacy Benefit Guide Welcome to FlexRx The AllWays Health Partners FlexRx SM program is built for choice, savings, and convenience with benefits including: Low-cost drug tier for many
ELEVEN REASONS WHY S IS BAD PUBLIC POLICY. Clinton Admin. FY00 budget request for FDA tobacco regulation was $34 million.
 ELEVEN REASONS WHY S. 2461 IS BAD PUBLIC POLICY 1. Creates New Bureaucracy. Clinton Admin. FY00 budget request for FDA tobacco regulation was $34 million. S.2461 includes a tax increase of $300 million
ELEVEN REASONS WHY S. 2461 IS BAD PUBLIC POLICY 1. Creates New Bureaucracy. Clinton Admin. FY00 budget request for FDA tobacco regulation was $34 million. S.2461 includes a tax increase of $300 million
