Harmonization of Clinical Laboratory Results is Essential for Quality Patient Care
|
|
|
- Winifred Pope
- 5 years ago
- Views:
Transcription
1 Harmonization of Clinical Laboratory Results is Essential for Quality Patient Care Greg Miller, PhD Virginia Commonwealth University Richmond, VA Presented at the National Association of Chronic Disease Coordinators, Biomarker Standardization Symposium, May 2013
2 Financial disclosures: none
3 What is the problem Ø Many laboratory measurement procedures give different results for the same sample
4 Why does it matter Ø Patients get the wrong treatment Ø Most clinical decisions are informed by laboratory results Ø Many clinical guidelines use a fixed laboratory test value for treatment decisions
5 Diabetes and urine albumin Diabetics are tested annually to identify early kidney damage KDIGO (2012) and other guidelines recommend a decision value of 30 mg/g creatinine Results for urine albumin could differ as much as 78% in this concentration range Ø Based on assessment of 16 routine measurement procedures with 332 patient samples (manuscript submitted)
6 Parathyroid hormone in kidney disease Biological activity resides in N- terminal 34 amino acids. Intact and N-terminal PTH have a short half life in plasma. C-terminal PTH fragments have a long half life and create assay interference issues, especially in renal patients PTH is the key hormone in calcium homeostasis acting on bone, the kidney and the gut 84 AA peptide MW = ~9500 PTH is a key biomarker in renal osteodystrophy PTH slides courtesy of Graham Beastall
7 PTH: Between Method Variability PTH concentra+on (pmol/l) in a single pa+ent. Treatment varia+on caused by comparing highest and lowest PTH concentra+ons in 18 pa+ents. Almond A, Ellis AR, Walker SW Current parathyroid hormone immunoassays do not adequately meet the needs of patients with chronic kidney disease. Ann Clin Biochem 2012; 49: 63 67
8 How to achieve comparable results Ø Calibration of all measurement procedures is traceable to a common reference system Ø All measurement procedures measure the same quantity
9 Case study: cardiovascular disease
10 ISO 17511:2003 In vitro diagnostic medical devices - Measurement of quantities in biological samples - Metrological traceability of values assigned to calibrators and control materials (under revision) Ø CLSI: implementation guideline - X5R (2006) and C29 (in preparation)
11 Traceability (based on ISO 17511) Primary Reference Material (NIST SRM 917b crystalline glucose) Primary Calibrator (glucose in water, 1, 3, 6, 11 mmol/l) Secondary Reference Material (NIST SRM 965b glucose in frozen human serum) A reference system SI unit (glucose, mmol/l) Primary Reference Measurement Procedure (gravimetry, calibrated with NIST mass standards) Secondary Reference Measurement Procedure (IDMS)
12 Traceability (based on ISO 17511) Primary Reference Material (pure substance) Secondary Reference Material (matrix) Mfr Working Calibrator Mfr Product Calibrator Patient sample result (calibrator) SI unit Reference Procedure (e.g. IDMS) Mfr Selected Procedure Mfr Standing Procedure Routine Procedure TRACEABILITY Patient sample results are equivalent to the reference procedure results
13 Traceability (based on ISO 17511) Primary Reference Material (pure substance) Panel of patient samples Mfr Working Calibrator Mfr Product Calibrator Patient sample result (calibrator) SI unit Reference Procedure (e.g. IDMS) Mfr Selected Procedure Mfr Standing Procedure Routine Procedure TRACEABILITY Patient sample results are equivalent to the reference procedure results
14 Measurands for which reference procedures exist or can be developed Measurands for which no reference procedures exist nor are likely to be developed
15 Measurands for which reference procedures exist or can be developed Measurands for which no reference procedures exist nor are likely to be developed
16 What happens when there is no reference measurement procedure
17 Traceability (based on ISO 17511) Secondary Reference Material (matrix) Mfr Working Calibrator Mfr Product Calibrator Patient sample result Value assignment Commutability (calibrator) Mfr Selected Procedure Mfr Standing Procedure Routine Procedure TRACEABILITY Patient sample results are traceable to a reference material
18 Examples: traceable to a reference material (no reference measurement procedure) Ø Human chorionic gonadotropin Ø Prostate-specific antigen Ø Thyroid stimulating hormone Ø Human immunodeficiency virus
19 Value assignment when there is no reference measurement procedure Ø The actual concentration may not be known Ø An arbitrary value can be assigned (e.g. U/L) Ø Since the goal of harmonization is comparable results irrespective of the measurement procedure used, Ø Clinical guidelines can still be implemented
20 Traceability to a Reference Material Secondary Reference Material (calibrator) Procedure 1 Procedure 2 Procedure 3 Procedure n Patient Samples Must be commutable with patient samples for all measurement procedures with which it will be used Results 1 Results 2 Results 3 Results n
21 Commutable means that values measured for a calibration material and for representative clinical samples have the same relationship between measurement procedures for the same measurand.
22 Commutable: same relationship for clinical samples and reference materials 10 Measurement Procedure Clinical Samples Reference Materials Measurement Procedure 1
23 Non-commutable: different relationship for clinical samples and reference materials 10 Measurement Procedure Clinical Samples Reference Materials Measurement Procedure 1
24 Use of a non-commutable material for calibration traceability will cause: Ø Incorrect value assignment for a routine (field) measurement procedure calibrator Ø Incorrect results for patient samples Miller, Myers, Rej. Why commutability matters. Clin Chem 2006; 52:
25 Calibration with non-commutable materials 10 Measurement Procedure Clinical Samples RM as Calibrator Measurement Procedure 1
26 The Problem Many secondary reference materials are not commutable with native clinical samples for routine clinical laboratory procedures
27 The Problem Many secondary reference materials are not commutable with native clinical samples for routine clinical laboratory procedures Ø Historically, commutability of reference materials was not validated for use with routine clinical laboratory measurement procedures
28 The Problem Many secondary reference materials are not commutable with native clinical samples for routine clinical laboratory procedures Ø A manufacturer s standing procedure is frequently the same as the clinical laboratory procedure but may be calibrated with a master lot of calibrator that is traceable to a non-commutable reference material
29 The Problem Breaks the traceability chain Many secondary reference materials are Secondary Reference Material (calibrator) (matrix) not commutable with native clinical Mfr Selected Mfr Working Procedure samples for routine Calibrator clinical laboratory Mfr Standing Mfr Product Procedure procedures Calibrator Patient result Routine Procedure Ø A manufacturer s standing procedure is frequently the same as the clinical laboratory procedure but may be calibrated with a master lot of calibrator that is traceable to a non-commutable reference material
30 The Problem Many secondary reference materials are not commutable with native clinical samples for routine clinical laboratory procedures Ø Even though manufacturers show traceability, the process fails to provide equivalent results for patient samples when different measurement procedures are used
31 Thyroid Stimulating Hormone From the Clinical Practice Guidelines for Hypothyroidism in Adults (American Association of Clinical Endocrinologists and American Thyroid Association): Ø According to NHANES III, the upper normal of serum TSH levels is 4.5 miu/l. Ø According to NACB, the upper normal of serum TSH levels is 2.5 miu/l.
32 Thyroid Stimulating Hormone Neither study mentioned the From the Clinical Practice Guidelines for measurement procedure used to Hypothyroidism in Adults (American Association of make the measurements Clinical Endocrinologists and American Thyroid Association): Ø According to NHANES III, the upper normal of serum TSH levels is 4.5 miu/l. Ø According to NACB, the upper normal of serum TSH levels is 2.5 miu/l.
33 TSH methods All traceable to IS 94/674 (WHO) Mean ±95% CI for 40 patient samples Δ = 0.7 Δ = % Thienpont et al. Clin Chem 2010; 56:
34 Calibration traceability does not ensure accuracy for an individual patient sample Ø Measurement procedure may not be specific for the measurand ð Interfering substances may influence the result Ø Measurand may not be well defined ð Molecular form(s) of clinical interest may not be understood
35 Other examples: Follicle stimulating hormone (Clin Chim Acta 1998;273:103-17) Prostate-specific antigen ( Clin Chem 2006;52: Clin Chem 2011;57:1776-7) C-peptide (Clin Chem 2008;54:1023-6) Insulin (Clin Chem 2009;55: , 2009) Human chorionic gonadotropin (Clin Chem 2009;55: ) Cytomegalovirus (Clin Chem 2009;55: ) Troponin I (Pathology 2010;42:402-8)
36 What do we do?
37 Must change practice to require commutability validation for reference materials intended for use with: Manufacturer s standing procedures Routine clinical laboratory procedures A guideline is available: CLSI C53-A Characterization and Qualification of Commutable Reference Materials for Laboratory Medicine (2010)
38 New biomarkers Sustainable calibration traceability needs to be part of the development process: Ø Definition of the measurand Ø Requirements for measurement specificity Ø Reference measurement procedure Ø Commutable reference materials
39 Ø International Forum organized by AACC in October, 2010 Ø 90 participants from 12 countries Ø Representing 62 organizations & manufacturers
40 Barriers to harmonization Ø Materials are labeled as reference materials that have not been validated to be commutable for the intended measurement procedures Ø Inadequate understanding of the measurand the quantity intended to be measured Ø Inadequate analytical specificity for the measurand
41 Barriers to harmonization Ø Lack of a systematic process to identify and prioritize measurands in need of harmonization Ø Lack of systematic procedures to implement harmonization, in particular: ð when there is no reference measurement procedure ð when there is no reference material
42 Barriers to harmonization Ø Despite many organizations in many countries working to improve harmonization: ð The work is not coordinated to prevent Duplication of effort Different approaches by different groups ð People do not know what others are doing
43 Barriers to harmonization Ø Regulatory requirements ð Changing calibration requires regulatory approval ð Is the clinical benefit worth the cost to meet regulatory requirements
44 The Roadmap Develop an infrastructure to coordinate harmonization activities world wide to include: 1. Prioritization of measurands 2. Gap analysis for what needs to be done 3. Technical processes to achieve harmonization 4. Surveillance of success of harmonization
45 Path Forward Ø Steering Committee Ø 3 Task Forces 1. Administrative operations 2. Checklists for submission and evaluation 3. Tool box of approaches to harmonization
46 AN INFRASTRUCTURE FOR HARMONIZATION Interna2onal Consor2um for Harmoniza2on of Clinical Laboratory Results Approval Strategic Partners Group Council Governance, Administration Harmoniza2on Oversight Group Operations Management Work Groups Harmoniza2on Implementa2on Groups Special Working Groups Secretariat/Host - AACC
47 Stakeholders (Strategic Partners Group): Clinical practice groups Metrology Institutes Laboratory practice groups Standards organizations IVD manufacturers Regulatory organizations Public health organizations PT/EQA organizations Communication Coordination / Cooperation If work is underway, refer to that group If RMP is possible, refer to another group Harmonization Oversight Group When no RMP Evaluate measurand proposals Operation Solicit champion and funding Clinically affected entity Economically affected entity Special Working Group Review priority and technical feasibility Recommendation to Harmonization Oversight Group Harmonization Implementation Group Technical plan Surveillance plan Implement the plans Achieve JCTLM listing
48 An information portal for global standardization / harmonization activities v Communication with Strategic Partners Group v On-line submission of measurands to be harmonized v Information on harmonization status of measurands v Information on global harmonization activities v Useful technical information for harmonization
49 Questions / Comments
IFCC Scientific Division Report to Council, Istanbul, June Ian S.Young Queen s University Belfast Chair, IFCC-SD
 IFCC Scientific Division Report to Council, Istanbul, June 2014 Ian S.Young Queen s University Belfast Chair, IFCC-SD Name Position Country Term Time in Office I. Young Chair UK 2 nd 201401-201612 P.
IFCC Scientific Division Report to Council, Istanbul, June 2014 Ian S.Young Queen s University Belfast Chair, IFCC-SD Name Position Country Term Time in Office I. Young Chair UK 2 nd 201401-201612 P.
IFCC Committee for Standardization of Thyroid Function Tests C-STFT- Update on the project
 IFCC Committee for Standardization of Thyroid Function Tests C-STFT- Update on the project Maria-Magdalena Patru, MD, PhD Director, Medical and Scientific Affairs, Ortho Clinical Diagnostics, Member C-STFT
IFCC Committee for Standardization of Thyroid Function Tests C-STFT- Update on the project Maria-Magdalena Patru, MD, PhD Director, Medical and Scientific Affairs, Ortho Clinical Diagnostics, Member C-STFT
Agenda. 15 Dec
 Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
Implementing Traceability for Heterogeneous Analytes An Industry View JCTLM Symposium on Reference Measurement Systems for Biologicals, December 15, 2004 R. Miller 15 Dec. 2004 1 Agenda Recent trends in
Laboratory and Measurement Issues. Greg Miller, PhD Virginia Commonwealth University Richmond, VA
 Laboratory and Measurement Issues Greg Miller, PhD Virginia Commonwealth University Richmond, VA Outline Serum/plasma creatinine Serum/plasma cystatin C Urine albumin Urine protein Creatinine standardization
Laboratory and Measurement Issues Greg Miller, PhD Virginia Commonwealth University Richmond, VA Outline Serum/plasma creatinine Serum/plasma cystatin C Urine albumin Urine protein Creatinine standardization
Standardization of Thyroid Function Tests
 Standardization of Thyroid Function Tests Saturday, Dec 3 rd 211 39th meeting Location: Nycomed Belgium 18 Brussels Linda Thienpont Linda.thienpont@ugent.be Thyroid dysfunction: clinical importance Relatively
Standardization of Thyroid Function Tests Saturday, Dec 3 rd 211 39th meeting Location: Nycomed Belgium 18 Brussels Linda Thienpont Linda.thienpont@ugent.be Thyroid dysfunction: clinical importance Relatively
GUIDE TO THE EVALUATION OF COMMUTABILITY OF CONTROL MATERIALS
 GUIDE TO THE EVALUATION OF COMMUTABILITY OF CONTROL MATERIALS Ferruccio Ceriotti Servizio di Medicina di Laboratorio, Ospedale San Raffaele, Milano F. Ceriotti, Milano, 27-11-2015 2 Preamble Most of the
GUIDE TO THE EVALUATION OF COMMUTABILITY OF CONTROL MATERIALS Ferruccio Ceriotti Servizio di Medicina di Laboratorio, Ospedale San Raffaele, Milano F. Ceriotti, Milano, 27-11-2015 2 Preamble Most of the
Standardization of. S.M.Boutorabi DCLS,PhD
 Standardization of Creatinine Assay S.M.Boutorabi DCLS,PhD Chronic kidney disease (CKD) is a major public health problem in developed countries Why measure serum creatinine? Jaffe Reaction Creatinine +picric
Standardization of Creatinine Assay S.M.Boutorabi DCLS,PhD Chronic kidney disease (CKD) is a major public health problem in developed countries Why measure serum creatinine? Jaffe Reaction Creatinine +picric
IFCC Working Group on Standardization of Thyroid Function Tests (WG-STFT) Meeting at AACC 2008, Washington DC, Monday July 28 (2:00-5:00 pm)
 IFCC Working Group on Standardization of Thyroid Function Tests (WG-STFT) Meeting at AACC 8, Washington DC, Monday July 8 (: - : pm) PARTICIPANTS The meeting attendance list is attached in annex. REPORT
IFCC Working Group on Standardization of Thyroid Function Tests (WG-STFT) Meeting at AACC 8, Washington DC, Monday July 8 (: - : pm) PARTICIPANTS The meeting attendance list is attached in annex. REPORT
Analytical performance specifications two years after Milan conference. Prof Mauro Panteghini CIRME Scientific Coordinator
 Analytical performance specifications two years after Milan conference Prof Mauro Panteghini CIRME Scientific Coordinator Definition Analytical performance specifications: Criteria that specify (in numerical
Analytical performance specifications two years after Milan conference Prof Mauro Panteghini CIRME Scientific Coordinator Definition Analytical performance specifications: Criteria that specify (in numerical
Quality Initiative In Pathology. Harmonisation of Laboratory Testing
 Quality Initiative In Pathology Harmonisation of Laboratory Testing Harmonisation: what do we mean? Agreement of test results irrespective of the method used or the testing laboratory Requires: Common
Quality Initiative In Pathology Harmonisation of Laboratory Testing Harmonisation: what do we mean? Agreement of test results irrespective of the method used or the testing laboratory Requires: Common
CDC s Clinical Standardization Programs
 CDC s Clinical Standardization Programs Hubert W. Vesper, Ph.D. Director, Clinical Standardization Programs Chief, Protein Biomarker and Lipid Reference Laboratory December 1, 2015, JCTLM Members and Stakeholders
CDC s Clinical Standardization Programs Hubert W. Vesper, Ph.D. Director, Clinical Standardization Programs Chief, Protein Biomarker and Lipid Reference Laboratory December 1, 2015, JCTLM Members and Stakeholders
Moving toward standardization of urine albumin measurements
 dar TF-CKD iz ti special n of ur ne issue: albu The in measurements laboratory diagnostics of chronic kidney diseases Moving toward standardization of urine albumin measurements Jesse C. Seegmiller 1,
dar TF-CKD iz ti special n of ur ne issue: albu The in measurements laboratory diagnostics of chronic kidney diseases Moving toward standardization of urine albumin measurements Jesse C. Seegmiller 1,
Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing. Vincent Delatour, PhD
 Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing Vincent Delatour, PhD 1 Traceability in laboratory medicine : regulatory drivers Reform of medical biology in France
Commutability studies undertaken by the LNE : the case of lipid and lipoprotein testing Vincent Delatour, PhD 1 Traceability in laboratory medicine : regulatory drivers Reform of medical biology in France
Bias in clinical chemistry. Elvar Theodorsson EFLM and Linköping University
 Bias in clinical chemistry Elvar Theodorsson EFLM and Linköping University Bias a controversial subject Different perspectives Reseachers Users Regulatory bodies Standardisation organisations Metrologists
Bias in clinical chemistry Elvar Theodorsson EFLM and Linköping University Bias a controversial subject Different perspectives Reseachers Users Regulatory bodies Standardisation organisations Metrologists
Parathyroid hormone (serum, plasma)
 Parathyroid hormone (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Parathyroid hormone (PTH) 1.2 Alternative names Parathormone 1.3 NMLC code 1.4 Description of analyte PTH is an
Parathyroid hormone (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Parathyroid hormone (PTH) 1.2 Alternative names Parathormone 1.3 NMLC code 1.4 Description of analyte PTH is an
Laboratory Medicine Standardization Activity in Japan
 JCTLM 15/11/2005 Sevres, Paris oratory Medicine Standardization Activity in Japan National Institute Metrology of Japan (NMIJ) Koichi Chiba Fujirebio Inc. Katsuhiko Yamamoto University of Tsukuba Katsuhiko
JCTLM 15/11/2005 Sevres, Paris oratory Medicine Standardization Activity in Japan National Institute Metrology of Japan (NMIJ) Koichi Chiba Fujirebio Inc. Katsuhiko Yamamoto University of Tsukuba Katsuhiko
Reference Material Institute for Clinical Chemistry Standards (ReCCS)
 Certified Reference Material for Measurement of Glucose, Creatinine, Uric Acid and Urea-N in Human Serum JCCRM Certificate of Analysis Intended use This Certified Reference Material CRM is intended primarily
Certified Reference Material for Measurement of Glucose, Creatinine, Uric Acid and Urea-N in Human Serum JCCRM Certificate of Analysis Intended use This Certified Reference Material CRM is intended primarily
Questionnaire. Traceability in EQA. Traceability
 Questionnaire in EQA QUESTIONNAIRE ON TRACEABILITY QUESTIONNAIRE ON TRACEABILITY GENERAL INFORMATION Name EQA organisation Country Specify the total number of measurands in the schemes of your EQA organisation
Questionnaire in EQA QUESTIONNAIRE ON TRACEABILITY QUESTIONNAIRE ON TRACEABILITY GENERAL INFORMATION Name EQA organisation Country Specify the total number of measurands in the schemes of your EQA organisation
Biochemistry Adult Reference Ranges
 Certified correct on 28/06/2016 Biochemistry Adult Reference Ranges Test Reference range Units Reference range from Traceable to standard reference material Albumin 35 50 g/l Pathology IRMM ERM-DA470k/IFCC
Certified correct on 28/06/2016 Biochemistry Adult Reference Ranges Test Reference range Units Reference range from Traceable to standard reference material Albumin 35 50 g/l Pathology IRMM ERM-DA470k/IFCC
Toward Standardization of Insulin Immunoassays
 Clinical Chemistry 55:5 1011 1018 (2009) General Clinical Chemistry Toward Standardization of Insulin Immunoassays W. Greg Miller, 1* Linda M. Thienpont, 2 Katleen Van Uytfanghe, 2 Penelope M. Clark, 3
Clinical Chemistry 55:5 1011 1018 (2009) General Clinical Chemistry Toward Standardization of Insulin Immunoassays W. Greg Miller, 1* Linda M. Thienpont, 2 Katleen Van Uytfanghe, 2 Penelope M. Clark, 3
Use of Target Values in EQA
 Use of Target Values in EQA Dr. Anja Kessler Bonn, Germany 1 External Quality Assessment Example: Progesterone 580 participants Measurement principles: Luminescence detection Radioactivity detection Fluorescence
Use of Target Values in EQA Dr. Anja Kessler Bonn, Germany 1 External Quality Assessment Example: Progesterone 580 participants Measurement principles: Luminescence detection Radioactivity detection Fluorescence
Why Traceability Matters to Patients?
 Why Traceability Matters to Patients? (and you are all patients) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney JCTLM Members and Stakeholders meeting, Paris 2017 Acknowledgements
Why Traceability Matters to Patients? (and you are all patients) Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney JCTLM Members and Stakeholders meeting, Paris 2017 Acknowledgements
Standardisation in Laboratory Medicine: Current Activities of the IFCC
 Standardisation in Laboratory Medicine: Current Activities of the IFCC JEAN-CLAUDE FOREST, MD, PhD Chair, Scientific Division Dept. of Lab. Med., CHUQ, Québec, Canada Sèvres November 2005 Reference Measurement
Standardisation in Laboratory Medicine: Current Activities of the IFCC JEAN-CLAUDE FOREST, MD, PhD Chair, Scientific Division Dept. of Lab. Med., CHUQ, Québec, Canada Sèvres November 2005 Reference Measurement
Recommendations from the Working Group of Senior Scottish Clinical Biochemists on Parathyroid Hormone (PTH) Targets in the Management of Renal Failure
 Recommendations from the Working Group of Senior Scottish Clinical Biochemists on Parathyroid Hormone (PTH) Targets in the Management of Renal Failure Chairman: Simon Walker (SW) Group membership: Jim
Recommendations from the Working Group of Senior Scottish Clinical Biochemists on Parathyroid Hormone (PTH) Targets in the Management of Renal Failure Chairman: Simon Walker (SW) Group membership: Jim
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
Define performance goals in standardization: Is fitness for medical purpose the key?
 Define performance goals in standardization: Is fitness for medical purpose the key? 7th CIRME International Scientific Meeting Metrological traceability and assay standardization Stresa, May 24th, 2013
Define performance goals in standardization: Is fitness for medical purpose the key? 7th CIRME International Scientific Meeting Metrological traceability and assay standardization Stresa, May 24th, 2013
Classification & Clinical Evidence under the IVDR
 Requirements of the IVDR Improving performance, reducing risk Proposed classification rules Annex II to the current IVD Directive addresses the level of risk posed by IVD medical devices by means of a
Requirements of the IVDR Improving performance, reducing risk Proposed classification rules Annex II to the current IVD Directive addresses the level of risk posed by IVD medical devices by means of a
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
Cortisol Assays. The Good, The Bad and The Indifferent. David Ducroq. Cardiff and Vale ulhb WEQAS. Unit 6, Parc Tŷ Glas. Llanishen.
 Cortisol Assays The Good, The Bad and The Indifferent David Ducroq Cardiff and Vale ulhb WEQAS Unit 6, Parc Tŷ Glas Llanishen Cardiff www.weqas.com Summary Brief overview of current methods and challenges
Cortisol Assays The Good, The Bad and The Indifferent David Ducroq Cardiff and Vale ulhb WEQAS Unit 6, Parc Tŷ Glas Llanishen Cardiff www.weqas.com Summary Brief overview of current methods and challenges
Endocrine System. Chemical Control
 Endocrine System Chemical Control Endocrine System - the system that secretes hormones in the body - hormones can last for minutes or for hours - a major gland, once called the master gland, is the pituitary
Endocrine System Chemical Control Endocrine System - the system that secretes hormones in the body - hormones can last for minutes or for hours - a major gland, once called the master gland, is the pituitary
hc Step Serum/Urine, hc Step Detector and HumaPreg Serum/Urine
 Design Verification hc Step Serum/Urine, hc Step Detector and HumaPreg Serum/Urine 1 Function... 2 2 Sensitivity and Dynamic Range... 2 2.1 Analytical Sensitivity... 2 3 Validation of Efficacy... 3 3.1
Design Verification hc Step Serum/Urine, hc Step Detector and HumaPreg Serum/Urine 1 Function... 2 2 Sensitivity and Dynamic Range... 2 2.1 Analytical Sensitivity... 2 3 Validation of Efficacy... 3 3.1
Analyte Specimen Demographic Reference Range Units
 Acetone Negative titer Alanine aminotransferase (ALT/SGPT) 10-49 U/L Albumin 3.2-4.8 g/dl Alcohol < 10 Alpha-fetoprotein (AFP) < 1.3-8.1 ng/ml Alkaline phosphatase 0 7 days 7 30 days 1 3 3 6 6 12 1 3 3
Acetone Negative titer Alanine aminotransferase (ALT/SGPT) 10-49 U/L Albumin 3.2-4.8 g/dl Alcohol < 10 Alpha-fetoprotein (AFP) < 1.3-8.1 ng/ml Alkaline phosphatase 0 7 days 7 30 days 1 3 3 6 6 12 1 3 3
BIOLOGICAL VARIABILITY: THE CIRME CONTRIBUTION. F. Braga Centre for Metrological Traceability in Laboratory Medicine (CIRME)
 BIOLOGICAL VARIABILITY: THE CIRME CONTRIBUTION F. Braga Centre for Metrological Traceability in Laboratory Medicine (CIRME) TOTAL VARIATION (CV (CV T ) T ) PREANALYTICAL VARIATION ANALYTICAL VARIATION
BIOLOGICAL VARIABILITY: THE CIRME CONTRIBUTION F. Braga Centre for Metrological Traceability in Laboratory Medicine (CIRME) TOTAL VARIATION (CV (CV T ) T ) PREANALYTICAL VARIATION ANALYTICAL VARIATION
External Quality Assessment for Calibration Laboratories in Laboratory Medicine - RELA -
 External Quality Assessment for Calibration Laboratories in Laboratory Medicine - RELA - Anja Kessler Bonn, Germany 1 RELA Surveys www.dgkl-rfb.de 2 RELA Annual Process Each participant receives two different
External Quality Assessment for Calibration Laboratories in Laboratory Medicine - RELA - Anja Kessler Bonn, Germany 1 RELA Surveys www.dgkl-rfb.de 2 RELA Annual Process Each participant receives two different
CKD: Bone Mineral Metabolism. Peter Birks, Nephrology Fellow
 CKD: Bone Mineral Metabolism Peter Birks, Nephrology Fellow CKD - KDIGO Definition and Classification of CKD CKD: abnormalities of kidney structure/function for > 3 months with health implications 1 marker
CKD: Bone Mineral Metabolism Peter Birks, Nephrology Fellow CKD - KDIGO Definition and Classification of CKD CKD: abnormalities of kidney structure/function for > 3 months with health implications 1 marker
CCQM-K11: Key Comparison on the Determination of Total Glucose in Human Serum. Final Report May 2003
 CCQM-K11: Key Comparison on the Determination of Total Glucose in Human Serum Final Report May 2003 Michael Welch, Lorna Sniegoski, Reenie Parris, and Willie May Analytical Chemistry Division Chemical
CCQM-K11: Key Comparison on the Determination of Total Glucose in Human Serum Final Report May 2003 Michael Welch, Lorna Sniegoski, Reenie Parris, and Willie May Analytical Chemistry Division Chemical
Measurand characteristics & reference materials for hcg
 Measurand characteristics & reference materials for hcg Dr Cathie Sturgeon on behalf of the IFCC Working Group for hcg C/o Department of Clinical Biochemistry Royal Infirmary of Edinburgh C.Sturgeon@ed.ac.uk
Measurand characteristics & reference materials for hcg Dr Cathie Sturgeon on behalf of the IFCC Working Group for hcg C/o Department of Clinical Biochemistry Royal Infirmary of Edinburgh C.Sturgeon@ed.ac.uk
Bone and Mineral. Comprehensive Menu for the Management of Bone and Mineral Related Diseases
 Bone and Mineral Comprehensive Menu for the Management of Bone and Mineral Related Diseases Innovation to Assist in Clinical Diagnosis and Treatment DiaSorin offers a specialty line of Bone and Mineral
Bone and Mineral Comprehensive Menu for the Management of Bone and Mineral Related Diseases Innovation to Assist in Clinical Diagnosis and Treatment DiaSorin offers a specialty line of Bone and Mineral
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Traceable lipoprotein counting for Cardiovascular disease risk assessment Vincent DELATOUR, PhD
 Traceable lipoprotein counting for Cardiovascular disease risk assessment Vincent DELATOUR, PhD 1 The need for primary methods VIM 2008 : Primary reference measurement procedure : reference measurement
Traceable lipoprotein counting for Cardiovascular disease risk assessment Vincent DELATOUR, PhD 1 The need for primary methods VIM 2008 : Primary reference measurement procedure : reference measurement
Addressing error in laboratory biomarker studies
 Addressing error in laboratory biomarker studies Elizabeth Selvin, PhD, MPH Associate Professor of Epidemiology and Medicine Co-Director, Biomarkers and Diagnostic Testing Translational Research Community
Addressing error in laboratory biomarker studies Elizabeth Selvin, PhD, MPH Associate Professor of Epidemiology and Medicine Co-Director, Biomarkers and Diagnostic Testing Translational Research Community
CALCIUM AND GOOD HEALTH: Where nutritionally essential minerals are concerned, calcium is truly in a class by itself. The daily requirements for
 CALCIUM AND GOOD HEALTH: Where nutritionally essential minerals are concerned, calcium is truly in a class by itself. The daily requirements for calcium are over 1000 milligrams per day for most adults.
CALCIUM AND GOOD HEALTH: Where nutritionally essential minerals are concerned, calcium is truly in a class by itself. The daily requirements for calcium are over 1000 milligrams per day for most adults.
CCQM-K6: Key Comparison on the Determination of Cholesterol In Serum* Final Report December 2001
 CCQM-K6: Key Comparison on the Determination of Cholesterol In Serum* INTRODUCTION Final Report December 2001 Michael J. Welch, Reenie M. Parris, Lorna T. Sniegoski, and Willie E. May Analytical Chemistry
CCQM-K6: Key Comparison on the Determination of Cholesterol In Serum* INTRODUCTION Final Report December 2001 Michael J. Welch, Reenie M. Parris, Lorna T. Sniegoski, and Willie E. May Analytical Chemistry
Cystatin C (serum, plasma, urine)
 Cystatin C (serum, plasma, urine) 1 Name and description of analyte 1.1 Name of analyte Cystatin C (serum, plasma and urine) 1.2 Alternative names Cystatin 3, post-gamma-globulin, neuroendocrine basic
Cystatin C (serum, plasma, urine) 1 Name and description of analyte 1.1 Name of analyte Cystatin C (serum, plasma and urine) 1.2 Alternative names Cystatin 3, post-gamma-globulin, neuroendocrine basic
External quality assessment schemes in Latin America
 In this issue: Laboratory Medicine Perspectives from Latin America External quality assessment schemes in Latin America Gabriel Alejandro Migliarino Gmigliarino Consultants, Buenos Aires, Argentina ARTICLE
In this issue: Laboratory Medicine Perspectives from Latin America External quality assessment schemes in Latin America Gabriel Alejandro Migliarino Gmigliarino Consultants, Buenos Aires, Argentina ARTICLE
Implementing a Reference Measurement System for C-peptide: Successes and Lessons Learned
 Papers in Press. Published June 23, 2017 as doi:10.1373/clinchem.2016.269274 The latest version is at http://hwmaint.clinchem.aaccjnls.org/cgi/doi/10.1373/clinchem.2016.269274 Clinical Chemistry 63:9 000
Papers in Press. Published June 23, 2017 as doi:10.1373/clinchem.2016.269274 The latest version is at http://hwmaint.clinchem.aaccjnls.org/cgi/doi/10.1373/clinchem.2016.269274 Clinical Chemistry 63:9 000
Academic Insights for Biomarker Priorities and Candidate Pilot Project(s)
 Academic Panel Session Academic Insights for Biomarker Priorities and Candidate Pilot Project(s) Moderators: Dr. Chirag Parikh (Yale) Dr. Kumar Sharma (UCSD) Panelists: Dr. Ronald Perrone (Tufts Medical
Academic Panel Session Academic Insights for Biomarker Priorities and Candidate Pilot Project(s) Moderators: Dr. Chirag Parikh (Yale) Dr. Kumar Sharma (UCSD) Panelists: Dr. Ronald Perrone (Tufts Medical
Reference Material Institute for Clinical Chemistry Standards (ReCCS)
 Reference Material Institute for Clinical Chemistry Standards (ReCCS) Certified Reference Material for Measurement of Total Cholesterol and Glycerides in Human Serum Certificate of Analysis Intended use
Reference Material Institute for Clinical Chemistry Standards (ReCCS) Certified Reference Material for Measurement of Total Cholesterol and Glycerides in Human Serum Certificate of Analysis Intended use
In Vitro Diagnostic Testing for Direct Oral Anticoagulants- Premarket Review
 In Vitro Diagnostic Testing for Direct Oral Anticoagulants- Premarket Review Cardiac Safety Research Consortium December 3, 2015 Lea Carrington Division of Immunology and Hematology Devices Office of In
In Vitro Diagnostic Testing for Direct Oral Anticoagulants- Premarket Review Cardiac Safety Research Consortium December 3, 2015 Lea Carrington Division of Immunology and Hematology Devices Office of In
The analytical phase
 The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
Chemical Metrology for Human Health Assessment
 Chemical Metrology for Human Health Assessment Metrology and Physical Constants International School of Physics Enrico Fermi Stephen A. Wise Analytical Chemistry Division Material Measurement Laboratory
Chemical Metrology for Human Health Assessment Metrology and Physical Constants International School of Physics Enrico Fermi Stephen A. Wise Analytical Chemistry Division Material Measurement Laboratory
DBC s panel of thyroid hormone immunoassays is now more comprehensive with the new Reverse T3 ELISA kit. Test Your Hormones
 DBC s panel of thyroid hormone immunoassays is now more comprehensive with the new Reverse T3 ELISA kit Test Your Hormones INTRODUCTION For years thyroid hormone testing has been concentrated on TSH and
DBC s panel of thyroid hormone immunoassays is now more comprehensive with the new Reverse T3 ELISA kit Test Your Hormones INTRODUCTION For years thyroid hormone testing has been concentrated on TSH and
Keywords: albuminuria; albumin/creatinine ratio (ACR); measurements. Introduction
 Clin Chem Lab Med 2015; 53(11): 1737 1743 Beryl E. Jacobson, David W. Seccombe*, Alex Katayev and Adeera Levin A study examining the bias of albumin and albumin/creatinine ratio measurements in urine DOI
Clin Chem Lab Med 2015; 53(11): 1737 1743 Beryl E. Jacobson, David W. Seccombe*, Alex Katayev and Adeera Levin A study examining the bias of albumin and albumin/creatinine ratio measurements in urine DOI
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
Certificate of Analysis JCCRM 811-1
 Reference Material Institute for Clinical Chemistry Standards (ReCCS) Certificate of Analysis Certified Reference Material for Measurement of Uric Acid in Human Serum JCCRM 811-1 Intended use This certified
Reference Material Institute for Clinical Chemistry Standards (ReCCS) Certificate of Analysis Certified Reference Material for Measurement of Uric Acid in Human Serum JCCRM 811-1 Intended use This certified
PREANALYTICAL AND ANALYTICAL ASPECTS AFFECTING CLINICAL RELIABILITY OF PLASMA GLUCOSE RESULTS. Sara Pasqualetti
 PREANALYTICAL AND ANALYTICAL ASPECTS AFFECTING CLINICAL RELIABILITY OF PLASMA GLUCOSE RESULTS Sara Pasqualetti TOTAL VARIABILITY OF LABORATORY TEST RESULTS V TOT = (V P 2 + V A2 + V I2 ) 1/2 PREANALYTICAL
PREANALYTICAL AND ANALYTICAL ASPECTS AFFECTING CLINICAL RELIABILITY OF PLASMA GLUCOSE RESULTS Sara Pasqualetti TOTAL VARIABILITY OF LABORATORY TEST RESULTS V TOT = (V P 2 + V A2 + V I2 ) 1/2 PREANALYTICAL
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2) (b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard
 White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard 7500-10032 Rev F Table of Contents Executive Summary 3 Changes introduced
White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard 7500-10032 Rev F Table of Contents Executive Summary 3 Changes introduced
Cardiac Troponin Testing and Chest Pain Patients: Exploring the Shades of Gray
 Cardiac Troponin Testing and Chest Pain Patients: Exploring the Shades of Gray Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Describe the acute
Cardiac Troponin Testing and Chest Pain Patients: Exploring the Shades of Gray Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Describe the acute
Guide to Fulfillment of Measurement Uncertainty
 DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
Lipid Metabolism and Cardiac Test Markers: Importance of Standardization
 Lipid Metabolism and Cardiac Test Markers: Importance of Standardization Barbara M. Goldsmith, Ph.D., FACB Vice President, Marketing, Membership and Education Clinical and Laboratory Standards Institute
Lipid Metabolism and Cardiac Test Markers: Importance of Standardization Barbara M. Goldsmith, Ph.D., FACB Vice President, Marketing, Membership and Education Clinical and Laboratory Standards Institute
NOTES 11.5: ENDOCRINE SYSTEM. Pages
 NOTES 11.5: ENDOCRINE SYSTEM Pages 1031-1042 ENDOCRINE SYSTEM Communication system that controls metabolism, growth, and development with hormones Maintains homeostasis Hormones: chemical messengers released
NOTES 11.5: ENDOCRINE SYSTEM Pages 1031-1042 ENDOCRINE SYSTEM Communication system that controls metabolism, growth, and development with hormones Maintains homeostasis Hormones: chemical messengers released
Record of the Consultation on Pharmacogenomics/Biomarkers
 This English version of the record of the consultation has been published by PMDA. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. (Attachment
This English version of the record of the consultation has been published by PMDA. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. (Attachment
Using Blood Glucose Meters in the Hospital: Defining Critically Ill and Addressing Accreditation Issues
 Using Blood Glucose Meters in the Hospital: Defining Critically Ill and Addressing Accreditation Issues Stephen E. Kahn, PhD, DABCC, FACB Professor and Vice Chair, Clinical Services, Pathology Loyola University
Using Blood Glucose Meters in the Hospital: Defining Critically Ill and Addressing Accreditation Issues Stephen E. Kahn, PhD, DABCC, FACB Professor and Vice Chair, Clinical Services, Pathology Loyola University
National Voluntary Laboratory Accreditation Program
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 New Mexico Department of Agriculture Metrology Laboratory 3190 South Espina MSC 3170, P.O. Box 30005 Las Cruces, NM 88003-8005 Mr. Steve Sumner Phone: 575-646-1616
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 New Mexico Department of Agriculture Metrology Laboratory 3190 South Espina MSC 3170, P.O. Box 30005 Las Cruces, NM 88003-8005 Mr. Steve Sumner Phone: 575-646-1616
Certified reference materials for ensuring traceability: From experience with steroids and peptide
 1 Certified reference materials for ensuring traceability: From experience with steroids and peptide Akiko Takatsu National Metrology Institute of Japan (NMIJ), National Institute of Advanced Industrial
1 Certified reference materials for ensuring traceability: From experience with steroids and peptide Akiko Takatsu National Metrology Institute of Japan (NMIJ), National Institute of Advanced Industrial
Endocrine part one. Presented by Dr. Mohammad Saadeh The requirements for the Clinical Chemistry Philadelphia University Faculty of pharmacy
 Endocrine part one Presented by Dr. Mohammad Saadeh The requirements for the Clinical Chemistry Philadelphia University Faculty of pharmacy HORMONES Hormones are chemicals released by a cell or a gland
Endocrine part one Presented by Dr. Mohammad Saadeh The requirements for the Clinical Chemistry Philadelphia University Faculty of pharmacy HORMONES Hormones are chemicals released by a cell or a gland
The VDSP is a collaborative venture that was organized in
 PERSPECTIVE JBMR Standardizing Vitamin D Assays: The Way Forward Neil Binkley 1 and Christopher T Sempos 2 for the Vitamin D Standardization Program (VDSP) 1 Osteoporosis Clinical Research Program and
PERSPECTIVE JBMR Standardizing Vitamin D Assays: The Way Forward Neil Binkley 1 and Christopher T Sempos 2 for the Vitamin D Standardization Program (VDSP) 1 Osteoporosis Clinical Research Program and
PREVENTIS. Your partner for telemedicine & point-of-care diagnostic
 PREVENTIS Your partner for telemedicine & point-of-care diagnostic PREVENTIS Diagnostics tests providing rapid results Health Check self-testing at home QuantOn Technology everywhere and always fast and
PREVENTIS Your partner for telemedicine & point-of-care diagnostic PREVENTIS Diagnostics tests providing rapid results Health Check self-testing at home QuantOn Technology everywhere and always fast and
Endocrine secretion cells secrete substances into the extracellular fluid
 Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE. Quantitative (Oxide Semiconductor Alcohol Sensor)
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k060343 B. Purpose for Submission: New Device for the US market. C. Measurand: Breath Alcohol D. Type
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k060343 B. Purpose for Submission: New Device for the US market. C. Measurand: Breath Alcohol D. Type
S150 KEEP Analytical Methods. American Journal of Kidney Diseases, Vol 55, No 3, Suppl 2, 2010:pp S150-S153
 S150 KEEP 2009 Analytical Methods American Journal of Kidney Diseases, Vol 55, No 3, Suppl 2, 2010:pp S150-S153 S151 The Kidney Early Evaluation program (KEEP) is a free, communitybased health screening
S150 KEEP 2009 Analytical Methods American Journal of Kidney Diseases, Vol 55, No 3, Suppl 2, 2010:pp S150-S153 S151 The Kidney Early Evaluation program (KEEP) is a free, communitybased health screening
SydPath Reference Intervals for Clinical Trials (Contract Pathology Unit) Unauthorised Copy
 HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
HAEMATOLOGY APTT 1 150 M 25 35 sec APTT 1 150 F 25 35 sec Basophils Cord 2 weeks M 0.0 0.4 10^9/L Basophils Cord 2 weeks F 0.0 0.4 10^9/L Basophils 2 wks 3 mths M 0.0 0.2 10^9/L Basophils 2 wks 3 mths
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 15197 First edition 2003-05-01 In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus Systèmes d'essais
INTERNATIONAL STANDARD ISO 15197 First edition 2003-05-01 In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus Systèmes d'essais
The Whats and Hows of Reference Intervals. Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney
 The Whats and Hows of Reference Intervals Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements Reference Intervals - Summary What are they
The Whats and Hows of Reference Intervals Graham Jones Department of Chemical Pathology St Vincent s Hospital, Sydney Surabaya Indonesia 2016 Acknowledgements Reference Intervals - Summary What are they
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE A. 510(k) Number: k063276 B. Purpose for Submission: New device C. Measurand: Urinary Glucose, Blood,
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE A. 510(k) Number: k063276 B. Purpose for Submission: New device C. Measurand: Urinary Glucose, Blood,
Product List Autobio Diagnostics Autobio. autobio diagnostics an autobio group company
 Product List Autobio Diagnostics 2009-2010 Autobio.. autobio diagnostics an autobio group company www.autobio-diagnostics.com Autobio Diagnostics Co., Ltd. was established in 1998 and has become one of
Product List Autobio Diagnostics 2009-2010 Autobio.. autobio diagnostics an autobio group company www.autobio-diagnostics.com Autobio Diagnostics Co., Ltd. was established in 1998 and has become one of
The Endocrine System. The Endocrine System
 The Endocrine System Like nervous system, endocrine system provides communication and control. Messages are relayed from one cell to another via chemical messengers (hormones). Unlike nervous system which
The Endocrine System Like nervous system, endocrine system provides communication and control. Messages are relayed from one cell to another via chemical messengers (hormones). Unlike nervous system which
Ca, Mg metabolism, bone diseases. Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary
 Ca, Mg metabolism, bone diseases Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary Calcium homeostasis Ca 1000g in adults 99% in bones (extracellular with Mg, P) Plasma/intracellular
Ca, Mg metabolism, bone diseases Tamás Kőszegi Pécs University, Department of Laboratory Medicine Pécs, Hungary Calcium homeostasis Ca 1000g in adults 99% in bones (extracellular with Mg, P) Plasma/intracellular
work of Prof. Don Catlin
 Hormones work of Prof. Don Catlin "Next generation cheating "losing on artificial hormones Testosterone, stimulants, growth hormone, diuretics, masking compounds, erythropoietin (EPO), more New steroids
Hormones work of Prof. Don Catlin "Next generation cheating "losing on artificial hormones Testosterone, stimulants, growth hormone, diuretics, masking compounds, erythropoietin (EPO), more New steroids
This supplementary material has been provided by the authors to give readers
 Supplementary Online Content LI D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. doi:1.11/jama.217.1375 efigure
Supplementary Online Content LI D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. doi:1.11/jama.217.1375 efigure
BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS
 BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS International Standard ISO 15197:2013 (Second Edition 15/05/2013) In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing
BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS International Standard ISO 15197:2013 (Second Edition 15/05/2013) In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing
Tuberculosis. Ruth McNerney
 Tuberculosis African and interregional harmonization activities to improve access to affordable IVD Ruth McNerney London School of Hygiene & Tropical Medicine Department of Pathogen Molecular Biology,
Tuberculosis African and interregional harmonization activities to improve access to affordable IVD Ruth McNerney London School of Hygiene & Tropical Medicine Department of Pathogen Molecular Biology,
Received: June 06, 2016 Accepted: September 10, https://doi.org/ /bm Biochem Med (Zagreb) 2017;27(3):
 Review Minimum requirements for the estimation of measurement uncertainty: Recommendations of the joint Working group for uncertainty of measurement of the CSMBLM and CCMB Ivana Ćelap* 1,2, Ines Vukasović
Review Minimum requirements for the estimation of measurement uncertainty: Recommendations of the joint Working group for uncertainty of measurement of the CSMBLM and CCMB Ivana Ćelap* 1,2, Ines Vukasović
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
Hormones. BIT 230 Walsh Chapter 8
 Hormones BIT 230 Walsh Chapter 8 Hormones Regulatory molecules Affect all areas of metabolism Endocrine- hormones travel via the bloodstream to its target cell: true hormone Modern definition- any regulatory
Hormones BIT 230 Walsh Chapter 8 Hormones Regulatory molecules Affect all areas of metabolism Endocrine- hormones travel via the bloodstream to its target cell: true hormone Modern definition- any regulatory
Nutrition: obtaining reliable biomarker data to study the health status of populations
 Nutrition: obtaining reliable biomarker data to study the health status of populations Christine M. Pfeiffer, Ph.D. Chief, Nutritional Biomarkers Branch, DLS AAAS 2011 Annual Meeting Science Without Borders
Nutrition: obtaining reliable biomarker data to study the health status of populations Christine M. Pfeiffer, Ph.D. Chief, Nutritional Biomarkers Branch, DLS AAAS 2011 Annual Meeting Science Without Borders
Additional Case Study: Glands and Hormones
 Student Worksheet Additional Case Study: Glands and Hormones LSM 8.5-2 This activity can be done individually or in pairs. Prepare the pieces ahead of time. Materials For each student (or pair): one copy
Student Worksheet Additional Case Study: Glands and Hormones LSM 8.5-2 This activity can be done individually or in pairs. Prepare the pieces ahead of time. Materials For each student (or pair): one copy
PREPARATION STANDARD MATERIAL HELD AT CODE WHO/BS DOCUMENT. Native hormone purified from urine
 WHO International Biological Reference Preparations Held and Distributed by the WHO International Laboratories for Biological Standards ENDOCRINOLOGICAL SUBSTANCES Activin A, human, recombinant, Lyophilized,
WHO International Biological Reference Preparations Held and Distributed by the WHO International Laboratories for Biological Standards ENDOCRINOLOGICAL SUBSTANCES Activin A, human, recombinant, Lyophilized,
numares at a glance company overview 2014 Discover, understand and use biological systems high level NMR analytics
 numares at a glance company overview 2014 Discover, understand and use biological systems high level NMR analytics Three benefits of numares 1 Supporting patients to receive better cure based on effective
numares at a glance company overview 2014 Discover, understand and use biological systems high level NMR analytics Three benefits of numares 1 Supporting patients to receive better cure based on effective
Design Verification IMTEC-TSH R -A C Intended Use... 2 Diagnostic Sensitivity and Diagnostic Specificity... 2 Interferences... 4 Imprecision...
 Design Verification IMTEC-TSH RECEPTOR-ANTIBODIES CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Diagnostic Specificity... 2 2.1 Determination of Diagnostic Sensitivity and Diagnostic Specificity...
Design Verification IMTEC-TSH RECEPTOR-ANTIBODIES CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Diagnostic Specificity... 2 2.1 Determination of Diagnostic Sensitivity and Diagnostic Specificity...
Certified Reference Materials - A Path to Traceable Chemical Measurements
 Certified Reference Materials - A Path to Traceable Chemical Measurements Paul Armishaw 5 May 2016 What I plan to talk about Why traceability so measurements can be compared Traceable to what? SI units
Certified Reference Materials - A Path to Traceable Chemical Measurements Paul Armishaw 5 May 2016 What I plan to talk about Why traceability so measurements can be compared Traceable to what? SI units
SCIEX Vitamin D 200M Assay for the Topaz System
 The First FDA-Cleared LC-MS/MS Assay for Vitamin D SCIEX Vitamin D 200M Assay for the Topaz System The first FDA-cleared LC-MS/MS assay for Vitamin D Vitamin D is an important building block for human
The First FDA-Cleared LC-MS/MS Assay for Vitamin D SCIEX Vitamin D 200M Assay for the Topaz System The first FDA-cleared LC-MS/MS assay for Vitamin D Vitamin D is an important building block for human
See external label 2 C-8 C Σ=96 tests Cat # 3122Z MICROWELL ELISA THYROID STIMULATING HORMONE (TSH) ENZYME IMMUNOASSAY TEST KIT TSH.
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Endocrine System. Chapter 18. Introduction. How Hormones Work. How Hormones Work. The Hypothalamus & Endocrine Regulation
 Introduction Endocrine System Chapter 18 The endocrine system consists of cells, tissues, & organs that secrete into the blood Hormone an organic substance secreted by a cell that has an effect on the
Introduction Endocrine System Chapter 18 The endocrine system consists of cells, tissues, & organs that secrete into the blood Hormone an organic substance secreted by a cell that has an effect on the
Standardization of DiaSorin and Roche automated third generation PTH assays with an International Standard: impact on clinical populations
 DOI 1.1515/cclm-213-127 Clin Chem Lab Med 214; aop Etienne Cavalier *, Pierre Delanaye, Pierre Lukas, Agnes Carlisi, Romy Gadisseur and Jean-Claude Souberbielle Standardization of DiaSorin and Roche automated
DOI 1.1515/cclm-213-127 Clin Chem Lab Med 214; aop Etienne Cavalier *, Pierre Delanaye, Pierre Lukas, Agnes Carlisi, Romy Gadisseur and Jean-Claude Souberbielle Standardization of DiaSorin and Roche automated
CATEGORY Endocrine System Review. Provide labels for the following diagram CHAPTER 13 BLM
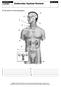 CHAPTER 13 BLM 13.1.1 CATEGORY Endocrine System Review Provide labels for the following diagram. 1. 6. 2. 7. 3. 8. 4. 9. 5. 10. CHAPTER 13 BLM 13.1.2 OVERHEAD Glands and Their Secretions Endocrine gland
CHAPTER 13 BLM 13.1.1 CATEGORY Endocrine System Review Provide labels for the following diagram. 1. 6. 2. 7. 3. 8. 4. 9. 5. 10. CHAPTER 13 BLM 13.1.2 OVERHEAD Glands and Their Secretions Endocrine gland
Hormones. Introduction to Endocrine Disorders. Hormone actions. Modulation of hormone levels. Modulation of hormone levels
 Introduction to Endocrine Disorders Hormones Self-regulating system (homeostasis) Affect: Growth Metabolism Reproduction Fluid and electrolyte balance Hormone actions Endocrine gland Hormone synthesis
Introduction to Endocrine Disorders Hormones Self-regulating system (homeostasis) Affect: Growth Metabolism Reproduction Fluid and electrolyte balance Hormone actions Endocrine gland Hormone synthesis
Ministry of Health and Long-Term Care. Palliative Care. Follow-Up on VFM Section 3.08, 2014 Annual Report RECOMMENDATION STATUS OVERVIEW
 Chapter 1 Section 1.08 Ministry of Health and Long-Term Care Palliative Care Follow-Up on VFM Section 3.08, 2014 Annual Report RECOMMENDATION STATUS OVERVIEW # of Status of Actions Recommended Actions
Chapter 1 Section 1.08 Ministry of Health and Long-Term Care Palliative Care Follow-Up on VFM Section 3.08, 2014 Annual Report RECOMMENDATION STATUS OVERVIEW # of Status of Actions Recommended Actions
