An overview of the disease epidemiology of the four indications of levothyroxine is provided in this section.
|
|
|
- Alban Parrish
- 5 years ago
- Views:
Transcription
1 Page 85 VI.2 VI.2.1 Elements for a Public Summary Overview of Disease Epidemiology An overview of the disease epidemiology of the four indications of levothyroxine is provided in this section. Levothyroxine is indicated to treat benign goitre- a swelling of the neck or larynx resulting from a benign or non-cancerous enlargement of the thyroid gland- in patients with normal thyroid function, to prevent recurrence of goitre after surgery, to replace natural thyroid hormones, when the thyroid gland does not produce enough hormones and to suppress tumor growth in patients with thyroid cancer. Levothyroxine -as contained in levothyroxine sodium tablets- is chemically identical to the hormone naturally produced and secreted by the thyroid gland. Benign goitre (non-cancerous enlargement of the thyroid gland) Worldwide, over 90% cases of goitre are caused by iodine deficiency (a dietary intake of iodine below the required need) (Hoermann, 2005). Goitre is more common among women. If the thyroid gland is producing too much thyroid hormone, radioactive iodine is given to the patient to shrink the gland. If goitre is caused by iodine deficiency, small doses of iodide are given. If the goitre is associated with an underactive thyroid, thyroid hormone, such as levothyroxine is given as treatment. If goitre is interfering with breathing or swallowing, and it has not responded to other forms of treatment, the patient may need surgery to remove part or all of the thyroid gland. This procedure is known as a thyroidectomy (removal of thyroid gland), and is followed by life-long intake of levothyroxine. Prevention of recurrence of goitre after surgery After removal of the thyroid, the intake of levothyroxine can help to avoid the recurrence of goitre. Recurrence of goitre under replacement therapy is reported to happen in about 2-39 % of cases. Recurrence of goitre without replacement therapy with levothyroxine is reported in around 70% (Capellani, 2008). Hypothyroidism (underactive thyroid): to replace natural thyroid hormones, when the thyroid gland does not produce enough hormones. Hypothyroidism, also called underactive thyroid gland, is a disorder in which the thyroid gland does not produce enough thyroid hormone as it should. It can cause a number of symptoms, such as tiredness, poor ability to tolerate cold temperatures, and weight gain. In children, hypothyroidism leads to delays in growth and intellectual development, which, in severe cases was previously known as cretinism. The diagnosis of hypothyroidism, when suspected, can be confirmed with blood tests. Worldwide, too little iodine in the diet is the most common cause of hypothyroidism. In countries with enough dietary iodine, it occurs in 1-2% of the population and it is more common in older women and ten times more common in women than in men (Vanderpump, 2008). In Western countries, hypothyroidism occurs in % of people while 85/108
2 Page 86 subclinical hypothyroidism (relating to a stage in the development of the disease before symptoms become apparent) occurs in % of people. Suppression of tumor growth in patients with thyroid cancer Globally, thyroid cancer accounts for 2.1% of all cancers (Ferlay, 2012). Thyroid cancer accounts for 0.9% of all cancers in men and for 3.5% of all cancers in women. Thyroid cancer is treated by surgically removing all or part of the thyroid gland. This is followed by radioactive iodine ablation of thyroid cells that may remain after this operation. Even after radioactive iodine therapy and surgery, it is possible that thyroid cancer may recur, sometimes years - or even decades - after the initial treatment for the disease. Thyroid hormone therapy uses hormones to help halt the growth of cancer cells by lowering the level of thyroid stimulating hormone, a hormone that directly promotes thyroid gland activity and is associated with thyroid cancer growth. In other thyroid cancers, thyroid hormone may be used to help maintain normal levels of thyroid hormone in the body. VI.2.2 Summary of Treatment Benefits Hypothyroidism, often called underactive thyroid or low thyroid, is a disorder in which the thyroid gland does not produce enough thyroid hormone. It can cause a number of symptoms, such as tiredness, poor ability to tolerate cold temperatures, and weight gain. In children, hypothyroidism leads to delays in growth and intellectual development. Levothyroxine is a synthetic thyroid hormone that is chemically identical to the thyroid hormone which is naturally produced by the cells of the thyroid gland. Levothyroxine sodium tablets are indicated as replacement or supplement of thyroid hormone to prevent the symptoms of hypothyroidism. It is also indicated to treat goitre via its ability to lower the hormone that stimulates goitre growth. Levothyroxine is further indicated as therapy in patients with thyroid cancer to help halt the growth of cancer cells by lowering the level of thyroid stimulating hormone (TSH), a hormone that addresses the thyroid directly and is associated with thyroid cancer growth. Levothyroxine can also be used in the testing of thyroid function. Due to the long-term experience with levothyroxine, the efficacy, safety and tolerability profile of levothyroxine is well established for the therapy of goitre in patients with normal thyroid function, prevention of recurrence of goitre after surgery therapy of hypothyroidism, replacement of natural thyroid hormones when the thyroid gland is under-active, suppression of tumour growth in patients with thyroid cancer as well as the testing of thyroid function. 86/108
3 Page 87 VI.2.3 Unknowns Relating to Treatment Benefits Due to long-term experience with levothyroxine there are no specific unknowns relating to treatment benefits of levothyroxine in therapy of goitre with normal thyroid function, prevention of recurrence of goitre after surgery, replacement of natural thyroid hormones of under-active thyroid gland and suppression of tumor growth and testing of thyroid function. Based on the long-term experience with levothyroxine, there is no indication that levothyroxine is less efficient or has an unfavorable benefit/ risk balance in any specific group of the patient population for whom it is authorized to be used. VI.2.4 Summary of Safety Concerns Important identified risks Risk What is known Preventability Too much thyroid activity, causing symptoms such as headache, muscle weakness or cramps, warmth and redness of the face (flushing), fever, vomiting, problems with menstrual period (disorders of menstruation), increased pressure in the head with eye swelling (Pseudotumor cerebri), trembling, restlessness, sleep disturbances, sweating, weight loss and diarrhea. (Medication-induced hyperthyroidism; drug-induced hyperthyroidism). The term hyperthyroidism refers to any condition in which there is too much thyroid hormone in the body. This could either occur due to an overactive thyroid gland producing a too high amount of thyroid hormone or it could be caused due to intake of too much medication containing thyroid hormone such as levothyroxine sodium tablets. Levothyroxine should be taken strictly as prescribed and not more than the prescribed dosage should be taken. If any of these symptoms of too much thyroid activity are observed, it is advised that the patient contacts his/ her doctor. The treating doctor may decide to interrupt the therapy for several days or reduce the daily dose. The symptoms of medicationinduced hyperthyroidism are described in the patient information leaflet (PIL) of levothyroxine (PIL Levothyroxine, 2015). 87/108
4 Page 88 Risk What is known Preventability Excessive overproduction of thyroid hormones/ excessive levels of thyroid hormones in the blood leading to medical emergency with failure of one or more organ systems (thyrotoxic crisis). Thyrotoxic crisis is an acute, lifethreatening condition. Individuals with existing high thyroid levels in the blood may develop thyrotoxic crisis after experiencing trauma, surgery, severe emotional distress, stroke, heart problems, or blood clots in the lungs. Thyrotoxic crisis may also occur in association with enlargement of the thyroid gland, containing areas that have increased in size and formed nodules. One or more of these nodules produce too much thyroid hormone. Another cause of thyrotoxic crisis may be a complication of a disease of the thyroid gland secreting an overabundance of thyroid hormones. Symptoms of thyrotoxic crisis may include: racing heart rate (tachycardia) that exceeds 140 beats per minute and atrial fibrillation high fever persistent sweating shaking agitation restlessness confusion diarrhea unconsciousness The following precautions are described in the patient information leaflet (PIL) of levothyroxine (PIL Levothyroxine, 2015): Patients with excessive levels of thyroid hormone in the blood (thyrotoxicosis) should not take levothyroxine (described under contraindications in the patient information leaflet of levothyroxine (PIL Levothyroxine, 2015). The doctor will investigate if the patient has a dysfunction of the thyroid gland with uncontrolled overproduction of thyroid hormones (thyroid autonomy), because this condition must be medically controlled before a patient can start taking levothyroxine or before a thyroid suppression test is performed. 88/108
5 Page 89 Risk What is known Preventability Dysfunction of the adrenal gland up to crisis in patients with a medical history of adrenal gland disease (Adrenal crisis in predisposed patients). The adrenal gland is a gland found over each kidney that helps regulate blood pressure and stress. When there is a dysfunctioning of this gland, the body does not produce enough of the hormones cortisol and aldosterone. Cortisol helps responding effectively to stress. It also plays a role in bone health, immune response, and the metabolism of food. People who have a disease of the adrenal glands called Addison s disease do not produce enough cortisol or aldosterone. Low levels of cortisol may cause weakness, fatigue and low blood pressure. Aldosterone regulates sodium and potassium levels. When levels of cortisol fall rapidly, people develop Addisonian crisis. Addisonian crisis, also called acute adrenal insufficiency, is a serious emergency condition and could also be deadly. Those people most at risk for Addisonian crisis are: individuals suffering from Addison s disease people who have damage to the pituitary gland (this is the gland that sits under the brain and makes and secretes many hormones, including some that control other glands), where adrenal insufficiency may be a result patients being treated for any kind of adrenal disease and who do not take their medication people who are experiencing some kinds of physical trauma and stress surgical patients individuals who are experiencing dehydration Levothyroxine should not be taken in patients with untreated dysfunction of the adrenal gland. Before treatment with levothyroxine, the doctor will investigate if the patient has a dysfunction of the adrenal gland, because this condition must be medically controlled before a patient can start taking levothyroxine tablets or before a thyroid suppression test is performed. The precautions stated above are described in the patient information leaflet (PIL Levothyroxine, 2015). 89/108
6 Page 90 Risk What is known Preventability Heart problems such as changes from the normal heart beat, irregular heart beat (cardiac arrhythmias) or rapid heartbeat (tachycardia) or chest pain due to decreased oxygen being supplied to the heart, insufficient blood flow in the blood vessels of the heart (angina pectoris), cardiovascular disorders, e.g. cardiac arrhythmias, tachycardia and angina pectoris) Excess of thyroid hormone increases the heart rate and may also cause irregular heartbeat. The work of the heart is greatly increased with excess thyroid hormone. Excess thyroid hormone increases the force of contraction of the heart muscle, and increases the amount of oxygen demanded by the heart. Levothyroxine should be taken strictly as prescribed and not more than the prescribed dosage should be taken. If any of these heart symptoms are observed, it is advised that the patient contacts his/ her doctor. The treating doctor may decide to interrupt the therapy for several days or reduce the daily dose, to help reduce heart problems The adverse reactions are described in the patient information leaflet of levothyroxine (PIL Levothyroxine, 2015). Over sensitivity, causing allergic reactions (hypersensitivity) The active hormone contained in levothyroxine sodium tablets is chemically identical to the natural hormone, but allergic reactions may occur to any of the ingredients of levothyroxine tablets (see PIL section 6. What levothyroxine sodium tablets contain ). All medicines can cause allergic reactions although serious allergic reactions are rare. Allergic reactions may include swelling of the face or throat (angioedema). Any sudden wheeziness, difficulty in breathing, swelling of the eyelids, face or lips, rash or itching especially affecting the whole body should be reported to a doctor immediately. If a patient had been allergic to any of the ingredients contained in levothyroxine tablets in the past, it is possible that the allergic reaction occurs with levothyroxine sodium tablets intake and this should be discussed with a doctor before intake. The adverse reactions are described in the patient information leaflet of levothyroxine (PIL Levothyroxine, 2015). 90/108
7 Page 91 Important potential risks Risk Use of the drug levothyroxine in order to lose weight (off label use/abuse and misuse for weight reduction) Loss of calcium from bone tissue resulting in bones that break easily; prevalent in postmenopausal women (osteoporosis) Sudden, uncontrolled muscle spasms and loss of consciousness resulting from abnormal brain function in patients with a known history of such disease (Seizures in patients with known history of epilepsy) What is known (Including reason why it is considered a potential risk) Levothyroxine should not be used for weight loss, as this may lead to dangerous side effects which may harm the patient s health. Women in and after the menopause who take levothyroxine may be at an increased risk of developing bones that break easily. Since levothyroxine has been on the market, a few patients have been reported to have experienced osteoporosis while they were taking levothyroxine. However, it is not possible to say whether this is definitely caused by the levothyroxine. The Patient Leaflet advises patients to speak to their doctor, if they are in the menopause or post-menopausal; regular check of thyroid function may be required because of the risk of osteoporosis (MRP PIL Levothyroxine, dated May 2015). The Company is continuously and carefully monitoring all reports on this issue due to its potential seriousness. If more than the prescribed dosage of levothyroxine is taken, sudden, uncontrolled muscle spasms and loss of consciousness resulting from abnormal brain function may be a consequence, especially in patients with a known history of such disease (Kahaly, 1989). The Patient Leaflet advises patients to speak to their doctor, if they experience a seizure (MRP PIL Levothyroxine, dated May 2015). The adverse reactions seizures in isolated cases are described in the section overdose of the patient information leaflet of levothyroxine (PIL Levothyroxine, 2015). Missing information Levothyroxine has been used since its first launch in There is no significant missing information relating to its use. VI.2.5 Summary of Additional Risk Minimization Measures by Safety Concern All medicines have a Summary of Product Characteristics (SmPC) which provides physicians, pharmacists and other health care professionals with details on how to use the medicine, the risks and recommendations for minimizing them. An abbreviated version of this in lay language is provided in the form of the patient information leaflet (PIL). There are no additional risk minimization measures for levothyroxine. 91/108
8 Page 92 VI.2.6 Planned Post Authorization Development Plan The company is currently not planning to conduct further studies which are a condition of the marketing authorization or required for post-authorization development. VI.2.7 Table 8 Summary of Changes to the Risk Management Plan over Time Major changes to the Risk Management Plan over time Version Date Changes performed 2.0 Jan 2015 Signature page Signature of Head of GDS not required any longer as per Company internal SOPs Part I: Product Overview Proposed new formulation added to the Pharmaceutical Form and Strengths for current Further invented trade names outside the EEA added Part II SVI.2 Potential for Transmission of Infectious Agents Information on excipients of new formulation added Part II: SV.2Non-study Post-authorization Exposure Exposure data updated Part III.3 Studies and other activities completed since last update if Pharmacovigilance Plan Study results of the bioequivalence phase I trials with the new formulation of levothyroxine added. Part II: Module SVI - Additional EU Requirements for the Safety Specification Frequencies of cumulative data updated until DLP 31 Dec 2014 SVII.3 Details of Important Identified and Potential Risks from Clinical Development and Post-authorization Experience (including newly identified) Frequency of risks updated cumulatively until DLP 31 Dec 2014 Whole RMP document Where applicable- data updated to DLP 31 Dec /108
9 Page 93 Version Date Changes performed 3.0 Feb 2015 Part II: SV.2 Non-study Post-authorization Exposure Exposure data updated until 15 Feb 2015 Part SVII.3 Important Identified and Potential Risks Important identified risks modified as follows: Important identified risks: clinical signs of hyperthyroidism/ thyrotoxic crisis adrenal insufficiency up to adrenal crisis in predisposed patients cardiovascular disorders (e.g. Cardiac arrhythmias, Tachycardia and Angina pectoris) hypersensitivity Important potential risks: osteoporosis seizures in patients with known history of epilepsy Part SVII.4.2 Important Identified and Potential Interactions Table on interactions moved to part SVII.4.1 Part III.3 Studies and other activities completed since last update of Pharmacovigilance Plan First sentence on the PV system deleted Part II: Module SVI - Additional EU Requirements for the Safety Specification Frequencies of cumulative data updated until DLP 15 Feb 2015 SVII.3 Details of Important Identified and Potential Risks from Clinical Development and Post-authorization Experience (including newly identified) Frequency of risks updated cumulatively until DLP 15 Feb 2015 VI.2.4 Summary of Safety Concerns The following important identified and potential risks were added in lay language: Cardiovascular disorders (e.g. Cardiac arrhythmias, Tachycardia and Angina pectoris Adrenal insufficiency up to adrenal crisis in predisposed patients Seizures in patients with known history of epilepsy Whole RMP document Where applicable- data updated to DLP 15 Feb /108
10 Page 94 Version Date Changes performed 4.0 Aug 2016 Part II: Module SVI - Additional EU Requirements for the Safety Specification Frequencies of cumulative data updated until DLP 05 Oct 2016 SVI.3 Potential for Misuse for Illegal Purposes Section updated regarding the new risk SVI.5 Potential for Off-label Use Section updated Part SVII.3 Important Identified and Potential Risks Addition of the important potential risk off label use/abuse and misuse for weight reduction SVII.3 Details of Important Identified and Potential Risks from Clinical Development and Post-authorization Experience (including newly identified) Addition of the new potential risk off-label use/abuse and misuse for weight reduction Frequency of risks updated cumulatively until DLP 05 Oct 2016 VI.2.4 Summary of Safety Concerns The following important potential risk was added in lay language: Off label use/abuse and misuse for weight reduction Whole RMP document Where applicable- data was updated to DLP 05 Oct 2016 Where applicable- reference to RSI was changed to the MRP SmPC and MRP PIL, dated May 2015), in previous RMP version 3.0, it was usually referenced to the CSDS. 94/108
11 Page 95 Version Date Changes performed 4.1 Aug 2017 Title page of the RMP Deletion of the invented names outside the EEA on the title page of the RMP (request of HA in PVAR DE/H/xxxx/WS/382) Part I: Product(s) Overview Recent RMP Versions under Evaluation: All information in the table of the RMP was deleted/replaced by not applicable (request of HA in PVAR DE/H/xxxx/WS/382) SVII.3 Details of Important Identified and Potential Risks from Clinical Development and Post-authorization Experience (including newly identified) Important Identified Risk: adrenal insufficiency up to adrenal crisis in predisposed patients: - Background incidence/prevalence-: Reconstitution of the accidently deleted information concerning the background incidence and prevalence (request of HA in PVAR DE/H/xxxx/WS/382) Part V: Risk Minimization Measures V.1 Risk Minimization Measures by Safety Concern The following statement has been included (request of HA in PVAR DE/H/xxxx/WS/382): Continuously (at least once monthly), in the context of signal management. A cumulative analysis will be performed at the time of each PSUR (PBRER), or whenever significant new information becomes available. Data lock point DLP in agreement with HA remains 05 Oct 2016 as there were no changes regarding scientific/medical content. 5.0 Oct 2017 Version number of the RMP updated to 5.0 during closing sequence in agreement with HA in FVAR DE/H/xxxx/WS/382. Data lock point DLP in agreement with HA remains 05 Oct 2016 as there were no changes regarding scientific/medical content. 95/108
Summary of Treatment Benefits Page 72 of 111. Page 72
 1.8.2 Page 72 of 111 Page 72 need surgery to remove part or all of the thyroid gland. This procedure is known as a thyroidectomy (removal of thyroid gland), and is followed by life-long intake of levothyroxine.
1.8.2 Page 72 of 111 Page 72 need surgery to remove part or all of the thyroid gland. This procedure is known as a thyroidectomy (removal of thyroid gland), and is followed by life-long intake of levothyroxine.
Elements for a Public Summary
 VI.2 Elements for a Public Summary 25 microgram tablets 50 microgram tablets 75 microgram tablets 100 microgram tablets 125 microgram
VI.2 Elements for a Public Summary 25 microgram tablets 50 microgram tablets 75 microgram tablets 100 microgram tablets 125 microgram
Summary of safety concerns Important identified risks
 Valley VI.2 Elements for a public summary NL/H/3661/001-002/DC - Ivabradin Medical VI.2.1 Overview of disease epidemiology Ivabradine is a medicine used for two long-term (chronic) heart conditions: to
Valley VI.2 Elements for a public summary NL/H/3661/001-002/DC - Ivabradin Medical VI.2.1 Overview of disease epidemiology Ivabradine is a medicine used for two long-term (chronic) heart conditions: to
PACKAGE LEAFLET: INFORMATION FOR THE USER
 PACKAGE LEAFLET PACKAGE LEAFLET: INFORMATION FOR THE USER Euthyrox 25 microgram tablets Euthyrox 50 microgram tablets Euthyrox 75 microgram tablets Euthyrox 88 microgram tablets Euthyrox 100 microgram
PACKAGE LEAFLET PACKAGE LEAFLET: INFORMATION FOR THE USER Euthyrox 25 microgram tablets Euthyrox 50 microgram tablets Euthyrox 75 microgram tablets Euthyrox 88 microgram tablets Euthyrox 100 microgram
ELTROXIN thyroxine (as anhydrous levothyroxine sodium) 50 micrograms, 100 micrograms tablets
 ELTROXIN thyroxine (as anhydrous levothyroxine sodium) 50 micrograms, 100 micrograms tablets Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about
ELTROXIN thyroxine (as anhydrous levothyroxine sodium) 50 micrograms, 100 micrograms tablets Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about
Nocturnal polyuria has been linked to abnormalities of the daily rhythm of (circadian rhythmic) release of naturally occurring antidiuretic hormone.
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Bedwetting Bedwetting (also called primary nocturnal enuresis) is probably the most common developmental problem in children,
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Bedwetting Bedwetting (also called primary nocturnal enuresis) is probably the most common developmental problem in children,
Package leaflet: Information for the user. Optiray 320 mg I/ml, solution for injection or infusion. Active substance: Ioversol
 Package leaflet: Information for the user Optiray 320 mg I/ml, solution for injection or infusion Active substance: Ioversol Read all of this leaflet carefully before you start using this medicine because
Package leaflet: Information for the user Optiray 320 mg I/ml, solution for injection or infusion Active substance: Ioversol Read all of this leaflet carefully before you start using this medicine because
Thyroid Disorders. January 2019
 Thyroid Disorders January 2019 What is the Thyroid? The thyroid is a small butterfly-shaped gland inside the neck, located in front of the trachea (windpipe) and below the larynx (voicebox). It produces
Thyroid Disorders January 2019 What is the Thyroid? The thyroid is a small butterfly-shaped gland inside the neck, located in front of the trachea (windpipe) and below the larynx (voicebox). It produces
PATIENT INFORMATION LEAFLET ZOXADON TABLETS RANGE
 SCHEDULING STATUS: S5 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM: ZOXADON 0,5 mg: Each tablet contains 0,5 mg risperidone. ZOXADON 1 mg: Each tablet contains 1 mg risperidone. ZOXADON 2 mg: Each
SCHEDULING STATUS: S5 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM: ZOXADON 0,5 mg: Each tablet contains 0,5 mg risperidone. ZOXADON 1 mg: Each tablet contains 1 mg risperidone. ZOXADON 2 mg: Each
Desmopressin Version 2.0. Public summary of the Risk Management Plan
 Desmopressin 16.12.2015 Version 2.0 Public summary of the Risk Management Plan VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Bedwetting Bedwetting (also called primary nocturnal
Desmopressin 16.12.2015 Version 2.0 Public summary of the Risk Management Plan VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Bedwetting Bedwetting (also called primary nocturnal
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT OGEN (AN ESTROGEN HORMONE)?
 PATIENT INFORMATION (Updated July 2006) OGEN estropipate tablets, USP Read this PATIENT INFORMATION before you start taking OGEN and read what you get each time you refill OGEN. There may be new information.
PATIENT INFORMATION (Updated July 2006) OGEN estropipate tablets, USP Read this PATIENT INFORMATION before you start taking OGEN and read what you get each time you refill OGEN. There may be new information.
Thyroid Gland. Patient Information
 Thyroid Gland Patient Information Contact details for Endocrine and Thyroid Clinics Hawke s Bay Fallen Soldiers Memorial Hospital Villa 16 Phone: 06 8788109 ext 5891 Text: 0274 102 559 Email: endoclinic@hbdhb.govt.nz
Thyroid Gland Patient Information Contact details for Endocrine and Thyroid Clinics Hawke s Bay Fallen Soldiers Memorial Hospital Villa 16 Phone: 06 8788109 ext 5891 Text: 0274 102 559 Email: endoclinic@hbdhb.govt.nz
Elements for a public summary
 VI.2 Elements for a public summary Part VI.2 Elements for a public summary is applicable for all products that are covered by this RMP, except from the important potential risk of Medication error with
VI.2 Elements for a public summary Part VI.2 Elements for a public summary is applicable for all products that are covered by this RMP, except from the important potential risk of Medication error with
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology VI.2.2 Summary of treatment benefits
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Nausea and vomiting are common ailments had by many individuals. Nausea is defined as the unpleasant, painless sensation that
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Nausea and vomiting are common ailments had by many individuals. Nausea is defined as the unpleasant, painless sensation that
PATIENT INFORMATION LEAFLET DYNARB RANGE
 SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM: DYNARB 75 mg tablets DYNARB 150 mg tablets DYNARB 300 mg tablets Read all of this leaflet carefully before you start taking DYNARB.
SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM: DYNARB 75 mg tablets DYNARB 150 mg tablets DYNARB 300 mg tablets Read all of this leaflet carefully before you start taking DYNARB.
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: , VERSION 1.
 RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
Irbenida H 150mg/12,5mg film-coated tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Irbenida H 150mg/12,5mg film-coated tablets IRBESARTAN/HYDROCHLOROTHIAZIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information
PACKAGE LEAFLET: INFORMATION FOR THE USER Irbenida H 150mg/12,5mg film-coated tablets IRBESARTAN/HYDROCHLOROTHIAZIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information
PACKAGE LEAFLET: INFORMATION FOR THE USER Alfuzosin Sandoz 5 mg, prolonged-release tablets. Alfuzosin hydrochloride
 PACKAGE LEAFLET: INFORMATION FOR THE USER Alfuzosin Sandoz 5 mg, prolonged-release tablets Alfuzosin hydrochloride Read all of this leaflet carefully before you start taking this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE USER Alfuzosin Sandoz 5 mg, prolonged-release tablets Alfuzosin hydrochloride Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the user
 Package leaflet: Information for the user Emerade 150 micrograms solution for injection in pre-filled pen Emerade 300 micrograms solution for injection in pre-filled pen Emerade 500 micrograms solution
Package leaflet: Information for the user Emerade 150 micrograms solution for injection in pre-filled pen Emerade 300 micrograms solution for injection in pre-filled pen Emerade 500 micrograms solution
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr BACLOFEN Baclofen Tablets 10 mg and 20 mg This leaflet is part III of a three-part "Product Monograph" published when BACLOFEN was approved for sale in Canada and is designed
PART III: CONSUMER INFORMATION Pr BACLOFEN Baclofen Tablets 10 mg and 20 mg This leaflet is part III of a three-part "Product Monograph" published when BACLOFEN was approved for sale in Canada and is designed
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Elements for a Public Summary
 VI.2 Elements for a Public Summary Fluticasone propionate / formoterol fumarate are available in pressarised metered dose inhalers (pmdi) under the brand names Flutiform, and in breath actuated inhalers
VI.2 Elements for a Public Summary Fluticasone propionate / formoterol fumarate are available in pressarised metered dose inhalers (pmdi) under the brand names Flutiform, and in breath actuated inhalers
Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine)
 EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
EMA/518024/2015 Summary of the risk management plan (RMP) for Ivabradine Anpharm (ivabradine) This is a summary of the risk management plan (RMP) for Ivabradine Anpharm, which details the measures to be
PATIENT INFORMATION LEAFLET BILOCOR RANGE
 SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM BILOCOR 5 tablets BILOCOR 10 tablets Please read this leaflet carefully before using BILOCOR 5 / 10 mg tablets Keep this leaflet.
SCHEDULING STATUS: S3 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM BILOCOR 5 tablets BILOCOR 10 tablets Please read this leaflet carefully before using BILOCOR 5 / 10 mg tablets Keep this leaflet.
Summary of the risk management plan (RMP) for Pregabalin Mylan (pregabalin)
 EMA/285074/2015 Summary of the risk management plan (RMP) for Pregabalin Mylan (pregabalin) This is a summary of the risk management plan (RMP) for Pregabalin Mylan, which details the measures to be taken
EMA/285074/2015 Summary of the risk management plan (RMP) for Pregabalin Mylan (pregabalin) This is a summary of the risk management plan (RMP) for Pregabalin Mylan, which details the measures to be taken
Goiter. This reference summary explains goiters. It covers symptoms and causes of the condition, as well as treatment options.
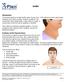 Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
Swiss Summary of the Risk Management Plan (RMP) for Parsabiv (Etelcalcetide)
 Swiss Summary of the Risk Management Plan (RMP) for Parsabiv (Etelcalcetide) RMP Summary: Version 1, November 2017 EU RMP: Version 1.0, November 2016 Page 1 of 6 The Risk Management Plan (RMP) is a comprehensive
Swiss Summary of the Risk Management Plan (RMP) for Parsabiv (Etelcalcetide) RMP Summary: Version 1, November 2017 EU RMP: Version 1.0, November 2016 Page 1 of 6 The Risk Management Plan (RMP) is a comprehensive
PACKAGE LEAFLET: INFORMATION FOR THE USER
 PACKAGE LEAFLET: INFORMATION FOR THE USER Alfuzosin HCl Ranbaxy 10 mg tablets, prolonged-release tablets Alfuzosin hydrochloride Read all of this leaflet carefully before using this medicine. - Keep this
PACKAGE LEAFLET: INFORMATION FOR THE USER Alfuzosin HCl Ranbaxy 10 mg tablets, prolonged-release tablets Alfuzosin hydrochloride Read all of this leaflet carefully before using this medicine. - Keep this
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Alzheimer's disease is the most common form of dementia, a general term for memory loss and other intellectual abilities serious
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Alzheimer's disease is the most common form of dementia, a general term for memory loss and other intellectual abilities serious
Olmesartan is used for treating high blood pressure (hypertension) in adult patients.
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Olmesartan is used for treating high blood pressure (hypertension) in adult patients. Essential hypertension 1 Worldwide, it is
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Olmesartan is used for treating high blood pressure (hypertension) in adult patients. Essential hypertension 1 Worldwide, it is
PATIENT INFORMATION LEAFLET. Testosterone Enantate 250mg/ ml Solution for Injection Testosterone Enantate
 PATIENT INFORMATION LEAFLET Testosterone Enantate 250mg/ ml Solution for Injection Testosterone Enantate Read all of this leaflet carefully before you start using this medicine because it contains important
PATIENT INFORMATION LEAFLET Testosterone Enantate 250mg/ ml Solution for Injection Testosterone Enantate Read all of this leaflet carefully before you start using this medicine because it contains important
I. ALL CLAIMS: HEALTH CARE PROFESSIONALS
 HCP Prescribing Information Date/Version January 2015 Version 2 Page: 1 of 5 I. ALL CLAIMS: HEALTH CARE PROFESSIONALS Indications and Usage Saxenda (liraglutide [rdna origin] injection) is indicated as
HCP Prescribing Information Date/Version January 2015 Version 2 Page: 1 of 5 I. ALL CLAIMS: HEALTH CARE PROFESSIONALS Indications and Usage Saxenda (liraglutide [rdna origin] injection) is indicated as
PATIENT INFORMATION LEAFLET AMLOC RANGE
 SCHEDULING STATUS: S3 PROPRIETARY NAME AND DOSAGE FORM: AMLOC 5 mg tablets AMLOC 10 mg tablet Read all of this leaflet carefully before you start taking AMLOC. Keep this leaflet. You may need to read it
SCHEDULING STATUS: S3 PROPRIETARY NAME AND DOSAGE FORM: AMLOC 5 mg tablets AMLOC 10 mg tablet Read all of this leaflet carefully before you start taking AMLOC. Keep this leaflet. You may need to read it
Summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma (aripiprazole)
 EMA/370707/2016 Summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma (aripiprazole) This is a summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma, which details the
EMA/370707/2016 Summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma (aripiprazole) This is a summary of the risk management plan (RMP) for Aripiprazole Mylan Pharma, which details the
THE THYROID BOOK. Medical and Surgical Treatment of Thyroid Problems
 THE THYROID BOOK Medical and Surgical Treatment of Thyroid Problems Trouble with Your Thyroid Gland The thyroid is a small gland in your neck that plays a big role in how your body functions. It impacts
THE THYROID BOOK Medical and Surgical Treatment of Thyroid Problems Trouble with Your Thyroid Gland The thyroid is a small gland in your neck that plays a big role in how your body functions. It impacts
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
Elements for a Public Summary Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Indication: Treatment of blood clots Blood clots in the large veins of the legs, known as deep vein thrombosis (DVT), are a common
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Indication: Treatment of blood clots Blood clots in the large veins of the legs, known as deep vein thrombosis (DVT), are a common
THYROID AWARENESS. By: Karen Carbone. January is thyroid awareness month. At least 30 million Americans
 THYROID AWARENESS By: Karen Carbone January is thyroid awareness month. At least 30 million Americans have a thyroid disorder and half-15 million-are silent sufferers who are undiagnosed, according to
THYROID AWARENESS By: Karen Carbone January is thyroid awareness month. At least 30 million Americans have a thyroid disorder and half-15 million-are silent sufferers who are undiagnosed, according to
Patient Information VERSACLOZ (VER sa kloz) (clozapine) Oral Suspension
 Patient Information VERSACLOZ (VER sa kloz) (clozapine) Oral Suspension Read this Patient Information before you start taking VERSACLOZ and each time you get a refill. There may be new information. This
Patient Information VERSACLOZ (VER sa kloz) (clozapine) Oral Suspension Read this Patient Information before you start taking VERSACLOZ and each time you get a refill. There may be new information. This
Summary of the risk management plan (RMP) for Kyprolis (carfilzomib)
 EMA/639793/2015 Summary of the risk management plan (RMP) for Kyprolis (carfilzomib) This is a summary of the risk management plan (RMP) for Kyprolis, which details the measures to be taken in order to
EMA/639793/2015 Summary of the risk management plan (RMP) for Kyprolis (carfilzomib) This is a summary of the risk management plan (RMP) for Kyprolis, which details the measures to be taken in order to
PLENADREN EU-RMP VERSION 3.2. Elements for a Public Summary. Overview of Disease Epidemiology
 PLENADREN EU-RMP VERSION 3.2 VI.2 VI.2.1 Elements for a Public Summary Overview of Disease Epidemiology PLENADREN contains hydrocortisone and is used to treat adrenal insufficiency (AI) in adults. AI occurs
PLENADREN EU-RMP VERSION 3.2 VI.2 VI.2.1 Elements for a Public Summary Overview of Disease Epidemiology PLENADREN contains hydrocortisone and is used to treat adrenal insufficiency (AI) in adults. AI occurs
(sunitinib malate) for Kidney Cancer
 Sutent (sunitinib malate) for Kidney Cancer Sutent is a medication used to treat adult patients with kidney cancer that has been surgically removed and at high risk of recurrence, or advanced kidney cancer
Sutent (sunitinib malate) for Kidney Cancer Sutent is a medication used to treat adult patients with kidney cancer that has been surgically removed and at high risk of recurrence, or advanced kidney cancer
PATIENT INFORMATION LEAFLET
 PATIENT INFORMATION LEAFLET PROSTATIN (OCTREOTIDE ACETATE INJECTION 10mcg, 50mcg) Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again.
PATIENT INFORMATION LEAFLET PROSTATIN (OCTREOTIDE ACETATE INJECTION 10mcg, 50mcg) Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again.
Pregnyl Human chorionic gonadotrophin (hcg)
 Pregnyl Human chorionic gonadotrophin (hcg) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Pregnyl. It does not contain all the available information.
Pregnyl Human chorionic gonadotrophin (hcg) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Pregnyl. It does not contain all the available information.
What You Need to Know About LEMTRADA (alemtuzumab) Treatment: A Patient Guide
 For Patients What You Need to Know About LEMTRADA (alemtuzumab) Treatment: A Patient Guide Patients: Your doctor or nurse will go over this patient guide with you. It is important to ask any questions
For Patients What You Need to Know About LEMTRADA (alemtuzumab) Treatment: A Patient Guide Patients: Your doctor or nurse will go over this patient guide with you. It is important to ask any questions
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Sudden pain is an unpleasant sensation resulting from physical hurt caused by injuries of the body, illness or emotional illness.
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Sudden pain is an unpleasant sensation resulting from physical hurt caused by injuries of the body, illness or emotional illness.
Questions to ask your Doctor
 Questions to ask your Doctor What is my current blood pressure? What are my target blood pressure numbers? What blood pressure medication(s) am I currently taking? How is this drug different from what
Questions to ask your Doctor What is my current blood pressure? What are my target blood pressure numbers? What blood pressure medication(s) am I currently taking? How is this drug different from what
Summary of the risk management plan (RMP) for Ketoconazole HRA (ketoconazole)
 EMA/609213/2014 Summary of the risk management plan (RMP) for Ketoconazole HRA (ketoconazole) This is a summary of the risk management plan (RMP) for Ketoconazole HRA, which details the measures to be
EMA/609213/2014 Summary of the risk management plan (RMP) for Ketoconazole HRA (ketoconazole) This is a summary of the risk management plan (RMP) for Ketoconazole HRA, which details the measures to be
NORVASC 5 mg and 10 mg tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER NORVASC 5 mg and 10 mg tablets AMLODIPINE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which
PACKAGE LEAFLET: INFORMATION FOR THE USER NORVASC 5 mg and 10 mg tablets AMLODIPINE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which
Package leaflet: Information for the user. Optiray 300 mg I/ml, solution for injection or infusion, multidose container. Active substance: Ioversol
 Package leaflet: Information for the user Optiray 300 mg I/ml, solution for injection or infusion, multidose container Active substance: Ioversol Read all of this leaflet carefully before you start using
Package leaflet: Information for the user Optiray 300 mg I/ml, solution for injection or infusion, multidose container Active substance: Ioversol Read all of this leaflet carefully before you start using
Summary of the risk management plan (RMP) for Opdivo (nivolumab)
 EMA/285771/2015 Summary of the risk management plan (RMP) for Opdivo (nivolumab) This is a summary of the risk management plan (RMP) for Opdivo, which details the measures to be taken in order to ensure
EMA/285771/2015 Summary of the risk management plan (RMP) for Opdivo (nivolumab) This is a summary of the risk management plan (RMP) for Opdivo, which details the measures to be taken in order to ensure
1. What Miacalcic is and what it is used for
 Package leaflet: Information for the user Miacalcic 100 IU/ml solution for injection and infusion Calcitonin (salmon, synthetic) Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user Miacalcic 100 IU/ml solution for injection and infusion Calcitonin (salmon, synthetic) Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the patient. Acetylsalicylic acid Bluefish 75 mg tablets Acetylsalicylic acid Bluefish 160 mg tablets
 Package leaflet: Information for the patient Acetylsalicylic acid Bluefish 75 mg tablets Acetylsalicylic acid Bluefish 160 mg tablets Acetylsalicylic acid Read all of this leaflet carefully before you
Package leaflet: Information for the patient Acetylsalicylic acid Bluefish 75 mg tablets Acetylsalicylic acid Bluefish 160 mg tablets Acetylsalicylic acid Read all of this leaflet carefully before you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. (Pentazocine Lactate Injection, USP)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION N Talwin (Pentazocine Lactate Injection, USP) Read this carefully before you start taking Talwin. This leaflet is a
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION N Talwin (Pentazocine Lactate Injection, USP) Read this carefully before you start taking Talwin. This leaflet is a
Hyperthyroidism in Cats (icatcare) What is hyperthyroidism?
 Kingsbrook Animal Hospital 5322 New Design Road, Frederick, MD, 21703 Phone: (301) 631-6900 Website: KingsbrookVet.com Hyperthyroidism in Cats (icatcare) Hyperthyroidism [1] What is hyperthyroidism? Hyperthyroidism
Kingsbrook Animal Hospital 5322 New Design Road, Frederick, MD, 21703 Phone: (301) 631-6900 Website: KingsbrookVet.com Hyperthyroidism in Cats (icatcare) Hyperthyroidism [1] What is hyperthyroidism? Hyperthyroidism
Risk What is known Preventability Increased symptoms of congestion when the effects of
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Nasal congestion Nasal congestion and rhinorrhoea (runny nose) are symptoms that commonly occur together. The most common causes
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Nasal congestion Nasal congestion and rhinorrhoea (runny nose) are symptoms that commonly occur together. The most common causes
Summary of the risk management plan (RMP) for Lenvima (lenvatinib)
 EMA/227224/2015 Summary of the risk management plan (RMP) for Lenvima (lenvatinib) This is a summary of the risk management plan (RMP) for Lenvima, which details the measures to be taken in order to ensure
EMA/227224/2015 Summary of the risk management plan (RMP) for Lenvima (lenvatinib) This is a summary of the risk management plan (RMP) for Lenvima, which details the measures to be taken in order to ensure
RABEPRAZOL 10mg and 20mg Gastro-resistant Tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER RABEPRAZOL 10mg and 20mg Gastro-resistant Tablets RABEPRAZOLE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet
PACKAGE LEAFLET: INFORMATION FOR THE USER RABEPRAZOL 10mg and 20mg Gastro-resistant Tablets RABEPRAZOLE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet
SYNACTHEN DEPOT i.m. tetracosactide hexaacetate
 SYNACTHEN DEPOT i.m. tetracosactide hexaacetate 1 mg/ml suspension for injection New Zealand Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given
SYNACTHEN DEPOT i.m. tetracosactide hexaacetate 1 mg/ml suspension for injection New Zealand Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given
FOLLOW DIRECTIONS. How to Use Methadone Safely. U.S. Department of Health & Human Services
 FOLLOW DIRECTIONS How to Use Methadone Safely U.S. Department of Health & Human Services Substance Abuse and Mental Health Services Administration Food and Drug Administration Methadone Methadone provides
FOLLOW DIRECTIONS How to Use Methadone Safely U.S. Department of Health & Human Services Substance Abuse and Mental Health Services Administration Food and Drug Administration Methadone Methadone provides
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Schizophrenia Schizophrenia is a mental illness with a number of symptoms, including confused or unclear thinking and speech,
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Schizophrenia Schizophrenia is a mental illness with a number of symptoms, including confused or unclear thinking and speech,
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN
 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN VI.1 Summary of activities in the risk management plan The summary below was prepared based on the information included in Part II, IV and V of the present
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN VI.1 Summary of activities in the risk management plan The summary below was prepared based on the information included in Part II, IV and V of the present
Summary of the risk management plan (RMP) for Bortezomib Accord (bortezomib)
 EMA/449387/2015 Summary of the risk management plan (RMP) for Bortezomib Accord (bortezomib) This is a summary of the risk management plan (RMP) for Bortezomib Accord, which details the measures to be
EMA/449387/2015 Summary of the risk management plan (RMP) for Bortezomib Accord (bortezomib) This is a summary of the risk management plan (RMP) for Bortezomib Accord, which details the measures to be
PACKAGE LEAFLET: INFORMATION FOR THE USER ANQUIL 0.25mg TABLETS Benperidol
 PACKAGE LEAFLET: INFORMATION FOR THE USER ANQUIL 0.25mg TABLETS Benperidol Please read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again.
PACKAGE LEAFLET: INFORMATION FOR THE USER ANQUIL 0.25mg TABLETS Benperidol Please read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again.
Consumer Medicine Information. This leaflet answers some common questions about UROGRAFIN.
 Urografin Consumer Medicine Information Contrast Imaging Agent Solution for Infusion - amidotrizoate 30% and 76% What is in this leaflet This leaflet answers some common questions about UROGRAFIN. This
Urografin Consumer Medicine Information Contrast Imaging Agent Solution for Infusion - amidotrizoate 30% and 76% What is in this leaflet This leaflet answers some common questions about UROGRAFIN. This
Eloxatin Oxaliplatin concentrated solution for injection
 Eloxatin Oxaliplatin concentrated solution for injection Consumer Medicine Information Please read this leaflet before you are given this medicine. What is in this leaflet This leaflet answers some common
Eloxatin Oxaliplatin concentrated solution for injection Consumer Medicine Information Please read this leaflet before you are given this medicine. What is in this leaflet This leaflet answers some common
THE THYROID GLAND AND YOUR HEALTH
 THE THYROID GLAND AND YOUR HEALTH Your Thyroid is a gland located at the base of your neck, just below your Adam s apple. It is shaped like a butterfly each wing or lobe, of your thyroid lies on either
THE THYROID GLAND AND YOUR HEALTH Your Thyroid is a gland located at the base of your neck, just below your Adam s apple. It is shaped like a butterfly each wing or lobe, of your thyroid lies on either
TREANA 5mg and 10mg Film-coated Tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER TREANA 5mg and 10mg Film-coated Tablets OLANZAPINE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
PACKAGE LEAFLET: INFORMATION FOR THE USER TREANA 5mg and 10mg Film-coated Tablets OLANZAPINE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine,
Package leaflet: Information for the patient. Torarese 2.5 mg tablets Torarese 5 mg tablets Torarese 10 mg tablets Torarese 20 mg tablets.
 Package leaflet: Information for the patient Torarese 2.5 mg tablets Torarese 5 mg tablets Torarese 10 mg tablets Torarese 20 mg tablets Torasemide Read all of this leaflet carefully before you start taking
Package leaflet: Information for the patient Torarese 2.5 mg tablets Torarese 5 mg tablets Torarese 10 mg tablets Torarese 20 mg tablets Torasemide Read all of this leaflet carefully before you start taking
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. ERLEADA apalutamide tablets
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION ERLEADA apalutamide tablets Read this carefully before you start taking ERLEADA and each time you get a refill. This
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION ERLEADA apalutamide tablets Read this carefully before you start taking ERLEADA and each time you get a refill. This
Package leaflet: Information for the user. Zoladex LA 10.8 mg Implant goserelin
 Package leaflet: Information for the user Zoladex LA 10.8 mg Implant goserelin Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Package leaflet: Information for the user Zoladex LA 10.8 mg Implant goserelin Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Package leaflet: Information for the patient. xylometazoline hydrochloride
 Package leaflet: Information for the patient /.../ 0.5 mg/ml nasal spray, solution /.../ 1 mg/ml nasal spray, solution xylometazoline hydrochloride Read all of this leaflet carefully before you start using
Package leaflet: Information for the patient /.../ 0.5 mg/ml nasal spray, solution /.../ 1 mg/ml nasal spray, solution xylometazoline hydrochloride Read all of this leaflet carefully before you start using
MEDICATION GUIDE SUBOXONE (Sub OX own) (buprenorphine and naloxone) Sublingual Tablets (CIII)
 MEDICATION GUIDE SUBOXONE (Sub OX own) (buprenorphine and naloxone) Sublingual Tablets (CIII) IMPORTANT: Keep SUBOXONE in a secure place away from children. Accidental use by a child is a medical emergency
MEDICATION GUIDE SUBOXONE (Sub OX own) (buprenorphine and naloxone) Sublingual Tablets (CIII) IMPORTANT: Keep SUBOXONE in a secure place away from children. Accidental use by a child is a medical emergency
Hyperthyroidism in Cats
 Hyperthyroidism in Cats The thyroid gland is located in the neck and plays a very important role in regulating the body's rate of metabolism. Hyperthyroidism is a disorder characterized by the overproduction
Hyperthyroidism in Cats The thyroid gland is located in the neck and plays a very important role in regulating the body's rate of metabolism. Hyperthyroidism is a disorder characterized by the overproduction
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Intratect 50 g/l and Intratect 100 g/l are solutions for intravenous infusion (infusion into a vein) for restoration and support
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Intratect 50 g/l and Intratect 100 g/l are solutions for intravenous infusion (infusion into a vein) for restoration and support
Patient/Caregiver Information Brochure For Bipolar I Disorder in Adolescents
 Patient/Caregiver Information Brochure For Bipolar I Disorder in Adolescents Date of approval: March 2013 Mercury number: 570HQ13NP00107-01-IE 786203127-001_Patient_Caregiver_IRELAND_v4.indd 1 20/03/2013
Patient/Caregiver Information Brochure For Bipolar I Disorder in Adolescents Date of approval: March 2013 Mercury number: 570HQ13NP00107-01-IE 786203127-001_Patient_Caregiver_IRELAND_v4.indd 1 20/03/2013
Dexamethasone is used to treat cancer. This drug can be given in the vein (IV), by mouth, or as an eye drop.
 Dexamethasone Other Names: Decadron About This Drug Dexamethasone is used to treat cancer. This drug can be given in the vein (IV), by mouth, or as an eye drop. Possible Side Effects (More Common) Increased
Dexamethasone Other Names: Decadron About This Drug Dexamethasone is used to treat cancer. This drug can be given in the vein (IV), by mouth, or as an eye drop. Possible Side Effects (More Common) Increased
Nivolumab. Other Names: Opdivo. About this Drug. Possible Side Effects (More Common) Warnings and Precautions
 Nivolumab Other Names: Opdivo About this Drug Nivolumab is used to treat cancer. It is given in the vein (IV). Possible Side Effects (More Common) Bone marrow depression. This is a decrease in the number
Nivolumab Other Names: Opdivo About this Drug Nivolumab is used to treat cancer. It is given in the vein (IV). Possible Side Effects (More Common) Bone marrow depression. This is a decrease in the number
Package leaflet: Information for the user. Zoledronic Acid Accord 4 mg/5 ml concentrate for solution for infusion Zoledronic acid
 Package leaflet: Information for the user Zoledronic Acid Accord 4 mg/5 ml concentrate for solution for infusion Zoledronic acid Read all of this leaflet carefully before you start taking this medicine
Package leaflet: Information for the user Zoledronic Acid Accord 4 mg/5 ml concentrate for solution for infusion Zoledronic acid Read all of this leaflet carefully before you start taking this medicine
Package leaflet: Information for the user. Pravastatin Actavis 20 mg and 40 mg tablets (pravastatin sodium)
 PACKAGE LEAFLET Package leaflet: Information for the user Pravastatin Actavis 20 mg and 40 mg tablets (pravastatin sodium) Read all of this leaflet carefully before you start taking this medicine because
PACKAGE LEAFLET Package leaflet: Information for the user Pravastatin Actavis 20 mg and 40 mg tablets (pravastatin sodium) Read all of this leaflet carefully before you start taking this medicine because
HERNOVIR 200 mg tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER HERNOVIR 200 mg tablets ACICLOVIR This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER HERNOVIR 200 mg tablets ACICLOVIR This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Sibelium 5 mg Tablets flunarizine
 J-C 2017 Sibelium 5 mg Tablets flunarizine PACKAGE LEAFLET: INFORMATION FOR THE USER Read all of this leaflet carefully before you start taking this medicine because it contains important information for
J-C 2017 Sibelium 5 mg Tablets flunarizine PACKAGE LEAFLET: INFORMATION FOR THE USER Read all of this leaflet carefully before you start taking this medicine because it contains important information for
Pregabalin Aristo Version: RMP-Pregabalin0
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Epilepsy Epilepsy is a long-term condition affecting the brain and is characterised by recurring seizures (or fits). It is one
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Epilepsy Epilepsy is a long-term condition affecting the brain and is characterised by recurring seizures (or fits). It is one
PACKAGE LEAFLET: INFORMATION FOR THE USER. GRAZAX 75,000 SQ-T oral lyophilisate
 PACKAGE LEAFLET: INFORMATION FOR THE USER GRAZAX 75,000 SQ-T oral lyophilisate Standardised allergen extract of grass pollen from Timothy (Phleum pratense) Read all of this leaflet carefully before you
PACKAGE LEAFLET: INFORMATION FOR THE USER GRAZAX 75,000 SQ-T oral lyophilisate Standardised allergen extract of grass pollen from Timothy (Phleum pratense) Read all of this leaflet carefully before you
6.2 Elements for a Public Summary
 6.2 Elements for a Public Summary 6.2.1 Overview of disease epidemiology Invicorp is to be used for erectile dysfunction, also known as impotence, the inability to get and maintain an erection that is
6.2 Elements for a Public Summary 6.2.1 Overview of disease epidemiology Invicorp is to be used for erectile dysfunction, also known as impotence, the inability to get and maintain an erection that is
03-Dec-17. Thyroid Disorders GOITRE. Grossly enlarged thyroid - in hypothyroidism in hyperthyroidism - production of anatomical symptoms
 Thyroid Disorders GOITRE Grossly enlarged thyroid - in hypothyroidism in hyperthyroidism - production of anatomical symptoms 1 Physiological Goiter load on thyroid supply of I - limited stress due to:
Thyroid Disorders GOITRE Grossly enlarged thyroid - in hypothyroidism in hyperthyroidism - production of anatomical symptoms 1 Physiological Goiter load on thyroid supply of I - limited stress due to:
PACKAGE LEAFLET: INFORMATION FOR THE USER. Dantrium 25 mg Capsules / Dantrium 100 mg Capsules Dantrolene sodium
 PACKAGE LEAFLET: INFORMATION FOR THE USER Dantrium 25 mg Capsules / Dantrium 100 mg Capsules Dantrolene sodium Read all of this leaflet carefully before you start taking this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE USER Dantrium 25 mg Capsules / Dantrium 100 mg Capsules Dantrolene sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate)
 EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
Consumer Medicine Information. This leaflet answers some common questions about Calvasc tablets
 Consumer Medicine Information Calvasc Tablets Amlodipine 5 mg tablets and 10 mg tablets What is in this leaflet This leaflet answers some common questions about Calvasc tablets It does not contain all
Consumer Medicine Information Calvasc Tablets Amlodipine 5 mg tablets and 10 mg tablets What is in this leaflet This leaflet answers some common questions about Calvasc tablets It does not contain all
EGLONYL FORTE 200mg tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER EGLONYL FORTE 200mg tablets SULPIRIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER EGLONYL FORTE 200mg tablets SULPIRIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
OXEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation This leaflet is part III of a three-part Product Monograph published when
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation This leaflet is part III of a three-part Product Monograph published when
PACKAGE LEAFLET: INFORMATION FOR THE USER Insomniger 10mg and 20mg Tablets (temazepam)
 PACKAGE LEAFLET: INFORMATION FOR THE USER Insomniger 10mg and 20mg Tablets (temazepam) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
PACKAGE LEAFLET: INFORMATION FOR THE USER Insomniger 10mg and 20mg Tablets (temazepam) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
PACKAGE LEAFLET: INFORMATION FOR THE USER RENNIE DUAL ACTION TABLETS. Calcium carbonate, Magnesium carbonate, Alginic acid Chewable tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER RENNIE DUAL ACTION TABLETS Calcium carbonate, Magnesium carbonate, Alginic acid Chewable tablets Read all of this leaflet carefully because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER RENNIE DUAL ACTION TABLETS Calcium carbonate, Magnesium carbonate, Alginic acid Chewable tablets Read all of this leaflet carefully because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER. Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid
 PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given Zoledronic Acid
PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given Zoledronic Acid
