An Overview of Thyroid Eye Disease (TED), Teprotumumab and OPTIC Phase 3 Trial Topline Results
|
|
|
- Myron Carr
- 5 years ago
- Views:
Transcription
1 An Overview of Thyroid Eye Disease (TED), Teprotumumab and OPTIC Phase 3 Trial Topline Results February 28, 2019 Teprotumumab: fully human monoclonal antibody inhibitor of IGF-1R
2 Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements related to Horizon's expected timing of clinical, regulatory and commercial events, including potential regulatory submissions related to, and potential commercialization of, teprotumumab, the potential benefits and market potential of teprotumumab, Horizon s business strategy and plans and other statements that are not historical facts. These forward-looking statements are based on Horizon's current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks that Horizon s actual future financial and operating results may differ from its expectations or goals; the risk that regulatory submissions and decisions may be delayed and that teprotumumab may not obtain approval by the U.S. Food and Drug Administration; the availability of coverage and adequate reimbursement and pricing from government and third-party payers; risks relating to Horizon s ability to successfully implement its business strategies; risks inherent in developing novel medicine candidates, such as teprotumumab, and existing medicines for new indications; risks associated with regulatory approvals; risks in the ability to recruit, train and retain qualified personnel; competition, including potential generic competition; the ability to protect intellectual property and defend patents; regulatory obligations and oversight, including any changes in the legal and regulatory environment in which Horizon operates and those risks detailed from time-to-time under the caption "Risk Factors" and elsewhere in Horizon's filings and reports with the SEC. Horizon undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information. 2
3 Agenda Horizon Pharma: Building an Innovation-Driven Biopharma Company Thyroid Eye Disease (TED): Disease Overview and Current Treatment Landscape Teprotumumab: Review of Phase 2 Data at 24 and 72 Weeks Teprotumumab: Phase 3 Trial Design and Topline Results Conclusion Q&A Timothy P. Walbert Chairman, President and Chief Executive Officer Horizon Pharma Raymond S. Douglas, M.D., Ph.D. Professor of Surgery Director of Orbital and Thyroid Eye Disease Program Cedars-Sinai Medical Center Shao-Lee Lin, M.D., Ph.D. Executive Vice President, Head of R&D and Chief Scientific Officer Horizon Pharma Shao-Lee Lin, M.D., Ph.D. Executive Vice President, Head of R&D and Chief Scientific Officer Horizon Pharma Timothy P. Walbert Chairman, President and Chief Executive Officer Horizon Pharma 3
4 Horizon Pharma: Building an Innovation-Driven Biopharma Company Timothy P. Walbert Chairman, President and Chief Executive Officer Horizon Pharma 4
5 Key Takeaways for Today s Call Teprotumumab Phase 3 OPTIC trial achieved its primary endpoint, confirming Phase 2 and demonstrating that teprotumumab has the potential to be a disease-modifying therapy (1) No FDA-approved therapies exist for TED; currently used therapies are not disease-modifying Active thyroid eye disease (TED) is a rare and debilitating inflammatory autoimmune disease of the eye; active TED impacts 15,000 to 20,000 U.S. patients annually (2) Expect mid-2019 BLA submission and potential 2020 approval; fast-track and breakthrough designations Estimated U.S. peak net sales potential of >$750M (3) Pre-launch planning underway (1) Smith TJ, et al. N Engl J Med 2017;376: (2) Company analysis of claims data and market research. (3) Horizon Pharma estimate. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 5
6 Our Aspiration Be a leading rare disease biopharma company Delivering innovative therapies to patients Generating high returns for shareholders 66
7 Building a Robust Rare Disease Pipeline MEDICINE / PROGRAM KRYSTEXXA KRYSTEXXA HZN-001 (teprotumumab) (2) HZN-003 PASylated Uricase (3) HemoShear Gout Discovery Collaboration DESCRIPTION MIRROR immunomodulation study: KRYSTEXXA + methotrexate Study in kidney transplant patients with uncontrolled gout (1) OPTIC trial: Phase 3 complete OPTIC-X trial: Phase 3 extension Optimized uricase and optimized PEGylation for uncontrolled gout Optimized uricase and PASylation for uncontrolled gout Exploration of novel approaches to treating gout PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 PHASE 3b / 4 (1) Planned study, expected to begin in the second half of (2) Teprotumumab is a fully human monoclonal antibody (mab) IGF-1R inhibitor in development for active thyroid eye disease (TED). (3) Being developed under a collaboration agreement. MIRROR: Methotrexate to Increase Response Rates in Patients with Uncontrolled GOut Receiving KRYSTEXXA. OPTIC: Treatment of Graves Orbitopathy (Thyroid Eye Disease) to Reduce Proptosis with Teprotumumab Infusions in a Randomized, Placebo-Controlled, Clinical Study. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 7
8 Teprotumumab Exemplifies Our Pipeline Strategy Building a Pipeline for Sustainable Long-Term Growth IDEAL PIPELINE CANDIDATE CRITERIA High unmet need with preference for rare diseases Compelling clinical trial data or proof of concept Key regulatory designations Durable Intellectual Property TEPROTUMUMAB No FDA-approved therapies exist for thyroid eye disease Standard of care proven ineffective; safety concerns Surgery is invasive, complex and often ineffective Impressive Phase 2 results published in The New England Journal of Medicine Dramatic Phase 3 results; achieved primary and all secondary endpoints U.S. Orphan, Fast-Track and Breakthrough Therapy 12-year biologic exclusivity from approval Teprotumumab meets ALL pipeline candidate criteria and has potential to be first FDA-approved therapy to treat active thyroid eye disease Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 8
9 TED: Disease Overview and Current Treatment Landscape Raymond S. Douglas, M.D., Ph.D. Professor of Surgery Director of Orbital and Thyroid Eye Disease Program Cedars-Sinai Medical Center 9
10 Thyroid Eye Disease (TED) Rare and debilitating autoimmune disease of the eye Associated with Graves Disease, but TED is a separate and distinct disease Impacts more women than men; typically happens mid-life; smoking worsens severity Orbital Inflammation & Swelling Begins as treatable active TED and progresses to inactive TED Inflammation behind the eye causes proptosis (bulging of the eyes) Over time turns fibrotic, causing permanent structural damage Proptosis Proptosis causes multiple conditions such as diplopia (double vision), strabismus (misalignment), compressed optic nerve (can be sight threatening), ulcerations, pain; can be disfiguring and emotionally debilitating Corneal Ulceration Corneal Ulceration 10
11 Pathology of TED TED Pathology (1) The body attacks its own orbital cells which overexpress IGF-1R The IGF-1R and TSHR are linked and form a signaling complex This leads to severe inflammation and expansion of tissue, muscle and fat cells behind the eye Causes proptosis (bulging of the eyes) and optic nerve compression (1) Smith TJ and Hedegus L. N Engl J Med 2016;375: IGF-1R: Insulin-like growth factor-1 receptor. TSHR: Thyroid stimulating hormone receptor. 11
12 Epidemiology and Risk Factors of TED TED is Not a Complication of Graves Disease (GD) TED is a separate and distinct disease although it is commonly associated with GD (hyperthyroidism) TED can occur before, during or after diagnosis of GD; can occur without thyroid disease TED can present with hyperthyroid, hypothyroid or euthyroid Phase 2 and Phase 3 trial patients had well-controlled thyroid disease THYROID DYSFUNCTION Hypothyroidism Hyperthyroidism ENVIRONMENT Smoking Radioactive iodine therapy AGE Advancing age Risk Factors for Development or Progression of TED Caucasians > Asians GENDER ANCESTRY Women More frequent Men More severe Source: Internal research. Tanda et al. J Clin Endocrinol Metab Apr;98(4): Wiersinga et al. EJE (2018). 12
13 Disease Progression TED: Disease Progression Active TED Inactive TED Inflammation Fibrosis Up to 3 Years Source: Hiromatsu Y., et al. Intern Med 53: , Beyond 3 Years 13
14 How Do TED Patients Present? Clinical Activity and Severity of TED Active TED Normal Lid swelling / redness Chemosis (swelling of conjunctiva) Swelling of plica and caruncle Conjunctivitis Definable and Identifiable Criteria for Active TED Orbital prolapse of fat tissue Proptosis Lid retraction Source: Hearst Digital Media. Conjunctiva: The mucous membrane that covers the front of the eye and lines the inside of the eyelids. Plica: A small fold of bulbar conjunctiva on the medial canthus of the eye. Caruncle: Small, pink, globular nodule at the inner corner (the medial canthus) of the eye. 14
15 Proptosis (Bulging of the Eye) Swelling or bulging of the eye that can result in: Diplopia (double vision) Difficulty closing eye (sleep, ulcerations) Strabismus (eye misalignment) Optic nerve compression and potentially blindness Periocular muscles Optic nerve Orbital apex Orbital tissue and fat Compression of the optic nerve at orbital apex Healthy Eye and Orbital Tissue in Normal Condition Inflamed and enlarged muscles due to fluid accumulation Increase in orbital tissue and fat In Presence of Active TED Eye is well protected by lid Thin periocular muscles Optic nerve can easily pass through apex Orbit contains a small amount of tissue and fat Lid retraction Eye protrusion Lid and conjunctival redness Source: INDIGO Project (indigo-iapp.eu). 15
16 Diplopia (Double Vision) Two images of a single object are seen resulting from unequal action of the eye muscles Misalignment of one or both eyes Headache Nausea Source: AllAboutVision.com and WebMD. 16
17 Patients Living with TED Have Significant Challenges Living a Normal Life Proptosis You would think, Oh, not being able to close your eye, that's not a big deal, but it was a big deal. It was so easy to irritate. I had to wear sunglasses to sleep; it felt like I was in a stupor. TED has robbed me of a normal life and my looks. I don t know how much more I can take. Corneal Ulceration Diplopia (Double Vision) My social life has basically been non-existent since the bulging started. My colleagues know about the condition but it s difficult talking to an acquaintance about it, or someone I just met it can be kind of embarrassing. Requires Night Protection Source: Horizon Pharma market research and patient interviews. 17
18 Who Diagnoses TED and How is TED Treated? Physicians Involved in Treatment and Diagnosis Primary Care Endocrinologist Ophthalmologist Oculoplastic Surgeon How Is TED Treated Today? Active TED No Approved Treatments Inactive TED Treat with Surgery 18
19 Active TED: Current Approaches are Suboptimal Pathogenic Mechanisms of Disease Not Targeted Current Treatment Approach: Steroids Do not reverse underlying alterations of orbital tissue Do not reverse proptosis or diplopia Significant side effects Significant Unmet Need Exists No disease-modifying medication available Many U.S. physicians watch and wait for active phase to end Significant need for a treatment that can prevent the progression of the disease and the need for multiple corrective surgeries 19
20 Inactive TED: Surgery Only Current Option Exposing Orbit Once TED becomes inactive, long-term damage is done Surgery currently the only option (1) Complex with mixed results Often requires multiple surgeries per eye because accumulation of fibrotic tissue complicates operations Tens of thousands of dollars per surgery While corrective for some, can result in permanent eye misalignment, double vision, lazy eye or blindness Removing Bone Removing Tissue and Fat (1) Surgical treatment can include decompression surgery, eyelid surgery and corrective vision surgery. 20
21 Disease Progression Potential Impact of Disease-Modifying Therapy Active TED Inactive TED Untreated Treatment during active TED Ideal Therapy Up to 3 Years Source: Hiromatsu Y., et al. Intern Med 53: , Beyond 3 Years 21
22 Teprotumumab: Review of Phase 2 Data at 24 and 72 Weeks Shao-Lee Lin, M.D., Ph.D. Executive Vice President, Head of R&D and Chief Scientific Officer Horizon Pharma 22
23 Mechanism of Action of Teprotumumab for TED TED Pathology Teprotumumab Mechanism of Action (1) The body attacks its own orbital cells which overexpress IGF-1R The IGF-1R and TSHR are linked and form a signaling complex This leads to severe inflammation and expansion of tissue, muscle and fat cells behind the eye Causes proptosis (bulging of the eyes) and optic nerve compression Fully human monoclonal antibody inhibitor of IGF-1R Blocks IGF-1R and turns off signaling complex at the source of the disease Intended to reduce inflammation and prevent excessive cell growth behind the eye (1) Smith TJ and Hedegus L. N Engl J Med 2016;375: IGF-1R: Insulin-like growth factor-1 receptor. TSHR: Thyroid stimulating hormone receptor. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 23
24 Screening Randomization Phase 2 Trial Design Week 24 Week 72 Patient Criteria Active TED 18 to 75 years <9 months since active TED onset with no prior treatment CAS 4 FT4 and FT3 <50 percent above or below normal limits Teprotumumab (N=42) 8 infusions: 1 every three weeks Placebo (N=45) 8 infusions: 1 every three weeks 48-Week Off-Treatment Follow-Up Period Primary endpoint at Week 24 Percentage of participants with >2 mm reduction in proptosis and >2 point reduction in Clinical Activity Score (CAS) CAS: Clinical Activity Score, a 7-point scale that measures change in orbital inflammation and pain; a score of >3 indicates active TED. FT4: Free thyroxine. FT3: Free triiodothyronine. Teprotumumab is an investigational candidate, and safety and efficacy have not been established. 24
25 Phase 2 Trial Measures Used Clinical Activity Score (CAS) 1. Spontaneous orbital pain 2. Gaze evoked orbital pain 3. Eyelid swelling that is considered to be due to active GO 4. Eyelid erythema 5. Conjunctival redness that is considered to be due to active GO 6. Chemosis 7. Inflammation of caruncle OR plica Proptosis Measured By Exophthalmometer 0. No diplopia Diplopia Score 1. Intermittent, i.e., diplopia in primary position of gaze, when tired or when first awakening 2. Inconstant, i.e., diplopia at extremes of gaze 3. Constant, i.e., continuous diplopia in primary or reading position Source: Bartalena L, et al. Eur Thyroid J 2016;5:9 26. GO: Graves Orbitopathy, also known as thyroid eye disease, or TED. Chemosis: Swelling of conjunctiva, which is the mucous membrane that covers the front of the eye and lines the inside of the eyelids. Caruncle: Small, pink, globular nodule at the inner corner (the medial canthus) of the eye. Plica: A small fold of bulbar conjunctiva on the medial canthus of the eye. Diplopia: Double vision. 25
26 Phase 2 Trial Results Shows Potential to Be Disease-Modifying and Durable Impressive Response At Week 24, percentage of patients with reduction of >2 mm of proptosis and >2 points in CAS with p<0.001: Teprotumumab patients: 69 percent Placebo patients: 20 percent At Week 24, percentage of proptosis responders with p<0.001: Teprotumumab patients: 71 percent Placebo patients: 20 percent Proptosis and Diplopia: Durable Response Proptosis: Week 24: 71 percent of patients were responders Week 72: 53 percent of responders maintained response approximately 1 year off treatment Diplopia: Week 24: 62 percent of patients were responders Week 72: 69 percent of responders maintained response approximately 1 year off treatment Clinical Activity Score (CAS): a 7-point scale that measures change in orbital inflammation and pain; a score of >3 indicates active TED. Diplopia (double vision) response: improvement of at least one grade of diplopia. Teprotumumab is an investigational candidate, and safety and efficacy have not been established. 26
27 Phase 2 Safety Overview Most adverse events were mild, transient and did not require treatment The most frequent adverse events reported ( 5 percent of teprotumumab and greater than placebo) were nausea, muscle spasms, diarrhea, alopecia, hyperglycemia, dry skin, dysgeusia, headache, paresthesia, hearing impairment and weight loss Hyperglycemia (grade 2 or 3) occurred in some diabetics receiving teprotumumab, and it was well controlled after adjustment of diabetes medication Placebo (N=45) Teprotumumab (N=42) TEAEs 32 (72.7%) 32 (74.4%) SAEs 1 1 (2.3%) 5 (11.6%) Source: Smith TJ, et al. N Engl J Med 2017;376: SAE: Serious adverse event. TEAE: Treatment emergent adverse event. 1 SAE (placebo): Optic neuropathy. 1 SAEs (teprotumumab): severe diarrhea in a patient with history of UC; IBD; E coli infection treated with IV antibiotics; Hashimoto s encephalopathy (Provisional diagnosis after episodic mental confusion with no other neurologic symptoms); urinary retention (after patient had inguinal herniorrhaphy). Teprotumumab is an investigational candidate, and safety and efficacy have not been established. 27
28 Teprotumumab: Phase 3 Trial Design and Topline Results Shao-Lee Lin, M.D., Ph.D. Executive Vice President, Head of R&D and Chief Scientific Officer Horizon Pharma 28
29 Top-line Results OPTIC study met its primary endpoint (82.9% vs 9.5%, p<0.001), demonstrating a dramatic impact on proptosis All secondary endpoints also met statistical significance (p 0.001) Overall responder rate at Week 24 (primary endpoint of Phase 2) (1) CAS responder rate at Week 24 Change in proptosis through Week 24 Diplopia improvement at Week 24 Change in GO-QOL through Week 24 Safety profile consistent with Phase 2 BLA submission targeted for mid-2019 (1) Percent of participants with 2 point reduction in Clinical Activity Score (CAS) and 2 mm reduction in proptosis from baseline, provided there is no corresponding deterioration ( 2-point/mm increase) in CAS or proptosis in the fellow eye. CAS: Clinical Activity Score, a 7-point scale that measures change in orbital inflammation and pain; a score of >3 indicates active TED. Go-QOL: Graves Ophthalmopathy Quality of Life. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 29
30 Phase 3 Trial Design: 24-Week Randomized, Double- Masked, Placebo-Controlled Trial of Teprotumumab Screening Randomization Patient Criteria Active TED 18 to 75 years <9 months since active TED onset with no prior treatment CAS 4 FT4 and FT3 <50 percent above or below normal limits Week 24 Teprotumumab 8 infusions: 1 every three weeks Placebo 8 infusions: 1 every three weeks Off-Treatment Follow-Up Period Primary endpoint at Week 24 Percentage of participants with >2 mm reduction in proptosis CAS: Clinical Activity Score, a 7-point scale that measures change in orbital inflammation and pain; a score of >3 indicates active TED. FT4: Free thyroxine. FT3: Free triiodothyronine. Teprotumumab is an investigational candidate, and safety and efficacy have not been established. 30
31 Subject Disposition 83 underwent randomization 83 received study drug (ITT population) 42 randomized to receive placebo 41 randomized to receive teprotumumab 2 subjects discontinued early Adverse event (1) Withdrew consent (1) 2 subjects discontinued early Adverse event (1) Withdrew consent (1) 40 completed double-masked treatment period 39 completed double-masked treatment period ITT: Intent to treat. 31
32 Key Demographics Placebo (N=42) Teprotumumab (N=41) Age (years) Mean (SD) 48.9 (12.96) 51.6 (12.63) Gender, % Male Female Race, % White Black Asian Other 26.2% 73.8% 88.1% 4.8% 2.4% 4.8% 29.3% 70.7% 85.4% 9.8% 4.90% 0% Years Since Diagnosis of Graves Disease Median (range) ( ) ( ) Months Since Diagnosis of Thyroid Eye Disease Median (range) ( ) ( ) Smoking Status Non-smoker Smoker 81.0% 19.0% 78.0% 22.0% Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 32
33 Proptosis Responders (%) Proptosis Response (Reduction of 2 mm) Over Time 100 Teprotumumab (N=41) Placebo (N=42) Primary Endpoint p<0.001 p< p<0.001 p<0.001 Difference: (95%CI 58.89, 88.01) Baseline Week 6 Week 12 Week 18 Week 24 Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 33
34 Secondary Endpoints Overall responder rate at Week 24 (primary endpoint of Phase 2) (1) Percent of patients with a CAS value of 0 or 1 at Week 24 in the study eye Mean change in proptosis measurement from baseline to Week 24 in the study eye Percent of patients with a change from baseline of at least one grade in diplopia (double vision) Mean change in Graves Ophthalmopathy Quality of Life from baseline to Week 24 All secondary endpoints met statistical significance (p 0.001) (1) Percent of participants with 2 point reduction in Clinical Activity Score (CAS) and 2 mm reduction in proptosis from baseline, provided there is no corresponding deterioration ( 2-point/mm increase) in CAS or proptosis in the fellow eye. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 34
35 Phase 3 Safety Overview Safety profile similar to Phase 2 with no new safety observations Drop-out rate was low (<5%) and balanced across arms No deaths Placebo (N=42) Teprotumumab (N=41) TEAEs 29 (69.0%) 35 (85.4%) SAEs 1 (2.4%) (1) 2 (4.9%) (2) (1) Placebo: visual field defect requiring orbital decompression surgery (patient discontinued study) (2) Teprotumumab: pneumothorax (considered not related to study drug; patient had history of throat cancer with radiation treatment), infusion reaction (patient discontinued study) Vast majority of treatment-emergent adverse events were mild to moderate in intensity and no non-serious events led to discontinuation Note: Table represents number of subjects with TEAEs and SAEs. TEAE: Treatment emergent adverse event. SAE: Serious adverse event. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 35
36 Top-line Results OPTIC study met its primary endpoint (82.9% vs 9.5%, p<0.001), demonstrating a dramatic impact on proptosis All secondary endpoints also met statistical significance (p 0.001) Overall responder rate at Week 24 (primary endpoint of Phase 2) (1) CAS responder rate at Week 24 Change in proptosis through Week 24 Diplopia improvement at Week 24 Change in GO-QOL through Week 24 Safety profile consistent with Phase 2 BLA submission targeted for mid-2019 (1) Percent of participants with 2 point reduction in Clinical Activity Score (CAS) and 2 mm reduction in proptosis from baseline, provided there is no corresponding deterioration ( 2-point/mm increase) in CAS or proptosis in the fellow eye. CAS: Clinical Activity Score, a 7-point scale that measures change in orbital inflammation and pain; a score of >3 indicates active TED. Go-QOL: Graves Ophthalmopathy Quality of Life. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 36
37 Conclusion Timothy P. Walbert Chairman, President and Chief Executive Officer Horizon Pharma 37
38 Key Takeaways for Today s Call Teprotumumab Phase 3 OPTIC trial achieved its primary endpoint, confirming Phase 2 and demonstrating that teprotumumab has the potential to be a disease-modifying therapy (1) No FDA-approved therapies exist for TED; currently used therapies are not disease-modifying Active thyroid eye disease (TED) is a rare and debilitating inflammatory autoimmune disease of the eye; active TED impacts 15,000 to 20,000 U.S. patients annually (2) Expect mid-2019 BLA submission and potential 2020 approval; fast-track and breakthrough designations Estimated U.S. peak net sales potential of >$750M (3) Pre-launch planning underway (1) Smith TJ, et al. N Engl J Med 2017;376: (2) Company analysis of claims data and market research. (3) Horizon Pharma estimate. Teprotumumab is an investigational candidate and its safety and efficacy have not been established. 38
39 Q&A 39
40 An Overview of Thyroid Eye Disease (TED), Teprotumumab and OPTIC Phase 3 Trial Topline Results February 28, 2019 Teprotumumab: fully human monoclonal antibody inhibitor of IGF-1R
THYROID EYE DISEASE. A General Overview
 JAMES R. PATRINELY, MD, FACS CHARLES N.S. SOPARKAR, MD, PHD, FACS 3730 KIRBY DRIVE, SUITE 900, HOUSTON, TX 77098 TELEPHONE (713) 795-0705 FAX (713) 807-0630 THYROID EYE DISEASE A General Overview INTRODUCTION,
JAMES R. PATRINELY, MD, FACS CHARLES N.S. SOPARKAR, MD, PHD, FACS 3730 KIRBY DRIVE, SUITE 900, HOUSTON, TX 77098 TELEPHONE (713) 795-0705 FAX (713) 807-0630 THYROID EYE DISEASE A General Overview INTRODUCTION,
Anatomy: There are 6 muscles that move your eye.
 Thyroid Eye Disease Your doctor thinks you have thyroid orbitopathy. This is an autoimmune condition where your body's immune system is producing factors that stimulate enlargement of the muscles that
Thyroid Eye Disease Your doctor thinks you have thyroid orbitopathy. This is an autoimmune condition where your body's immune system is producing factors that stimulate enlargement of the muscles that
! Women greater than men (4:1)» Typical of other autoimmune diseases
 1 2 3 4 : Overview and Diagnosis Suzanne K. Freitag, M.D. Director, Ophthalmic Plastic Surgery Massachusetts Eye and Ear Infirmary Harvard Medical School! I have no financial disclosures. Learning Objectives!
1 2 3 4 : Overview and Diagnosis Suzanne K. Freitag, M.D. Director, Ophthalmic Plastic Surgery Massachusetts Eye and Ear Infirmary Harvard Medical School! I have no financial disclosures. Learning Objectives!
Horizon Pharma plc. November Isabel M., RAVICTI Patient
 Horizon Pharma plc November 2018 Isabel M., RAVICTI Patient Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements related to Horizon
Horizon Pharma plc November 2018 Isabel M., RAVICTI Patient Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements related to Horizon
What s New in Thyroid Eye Disease? James A. Garrity MD Mayo Clinic Rochester, MN
 What s New in Thyroid Eye Disease? James A. Garrity MD Mayo Clinic Rochester, MN What s New in Thyroid Eye Disease? Diagnosis - TRAb/TSI: diagnosis/prognosis - IgG4: diagnostic confusion Therapy - Surgery:
What s New in Thyroid Eye Disease? James A. Garrity MD Mayo Clinic Rochester, MN What s New in Thyroid Eye Disease? Diagnosis - TRAb/TSI: diagnosis/prognosis - IgG4: diagnostic confusion Therapy - Surgery:
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated
PROMISE 1 Top-Line Data Results. June 27, 2017
 PROMISE 1 Top-Line Data Results dd June 27, 2017 Forward Looking Statements This presentation and the accompanying commentary contains certain forward-looking statements within the meaning of Section 27A
PROMISE 1 Top-Line Data Results dd June 27, 2017 Forward Looking Statements This presentation and the accompanying commentary contains certain forward-looking statements within the meaning of Section 27A
35 th Annual J.P. Morgan Healthcare Conference
 35 th Annual J.P. Morgan Healthcare Conference Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts, regarding management's
35 th Annual J.P. Morgan Healthcare Conference Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts, regarding management's
Thyroid Eye Disease. Patient & Family Guide
 Patient & Family Guide Thyroid Eye Disease 2018 Register using a self check-in kiosk in the main entrance of the Centennial or Dickson building, Victoria General site. Next, go to the Eye Care Centre in
Patient & Family Guide Thyroid Eye Disease 2018 Register using a self check-in kiosk in the main entrance of the Centennial or Dickson building, Victoria General site. Next, go to the Eye Care Centre in
CLINICAL ASSESSMENT OF PATIENTS WITH GRAVES ORBITOPATHY
 44 MEDICINSKI GLASNIK / str. 44-48 Biljana Nedeljković-Beleslin 1 CLINICAL ASSESSMENT OF PATIENTS WITH GRAVES ORBITOPATHY Abstract: Clinical examination is the basis of a good assessment of a patient with
44 MEDICINSKI GLASNIK / str. 44-48 Biljana Nedeljković-Beleslin 1 CLINICAL ASSESSMENT OF PATIENTS WITH GRAVES ORBITOPATHY Abstract: Clinical examination is the basis of a good assessment of a patient with
THYROID EYE DISEASE ORBITAL DECOMPRESSION SURGERY
 THYROID EYE DISEASE ORBITAL DECOMPRESSION SURGERY What is thyroid eye disease (TED)? TED is an autoimmune condition where the body s own immune system attacks the tissues of the thyroid gland and the eye
THYROID EYE DISEASE ORBITAL DECOMPRESSION SURGERY What is thyroid eye disease (TED)? TED is an autoimmune condition where the body s own immune system attacks the tissues of the thyroid gland and the eye
Innovation In Ophthalmology
 Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Patient information leaflet Thyroid Eye Disease
 Patient information leaflet Thyroid Eye Disease What is Thyroid eye Disease? Thyroid Eye Disease (TED) is also known as Graves orbitopathy (GO), thyroid ophthalmopathy and thyroid-associated ophthalmopathy.
Patient information leaflet Thyroid Eye Disease What is Thyroid eye Disease? Thyroid Eye Disease (TED) is also known as Graves orbitopathy (GO), thyroid ophthalmopathy and thyroid-associated ophthalmopathy.
James A. Garrity MD Department of Ophthalmology. Marius N. Stan MD Division of Endocrinology. Mayo Clinic Rochester, MN
 James A. Garrity MD Department of Ophthalmology Marius N. Stan MD Division of Endocrinology Mayo Clinic Rochester, MN Epidemiologic and diagnostic considerations for Graves orbitopathy (GO) 1. How common?
James A. Garrity MD Department of Ophthalmology Marius N. Stan MD Division of Endocrinology Mayo Clinic Rochester, MN Epidemiologic and diagnostic considerations for Graves orbitopathy (GO) 1. How common?
DUPIXENT FDA Approval Call March 28, 2017
 DUPIXENT FDA Approval Call March 28, 2017 1 Sanofi Forward Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as
DUPIXENT FDA Approval Call March 28, 2017 1 Sanofi Forward Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as
Six Things That Changed How I Manage Graves Disease
 Six Things That Changed How I Manage Graves Disease Anthony DeWilde, OD FAAO Kansas City VAMC 6 Things 1. Thyroid status 2. Pathogenesis 3. Ocular signs/symptoms 4. Labs 5. Smoking 6. Mental Health Graves
Six Things That Changed How I Manage Graves Disease Anthony DeWilde, OD FAAO Kansas City VAMC 6 Things 1. Thyroid status 2. Pathogenesis 3. Ocular signs/symptoms 4. Labs 5. Smoking 6. Mental Health Graves
February 23, Q4 and Year-End 2016 Financial Results
 February 23, 2017 Q4 and Year-End 2016 Financial Results 2 RETHINKING CNS Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Jim Doherty, Ph.D.,
February 23, 2017 Q4 and Year-End 2016 Financial Results 2 RETHINKING CNS Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Jim Doherty, Ph.D.,
Soliris in NMOSD Phase 3 PREVENT Study Topline Results September 24, 2018
 Soliris in NMOSD Phase 3 PREVENT Study Topline Results September 24, 2018 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation
Soliris in NMOSD Phase 3 PREVENT Study Topline Results September 24, 2018 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation
Conference Call for Investment Community. Nov 19, 2018
 Conference Call for Investment Community Nov 19, 2018 Agenda for Today s Call Topic Safe Harbor Statement Opening Remarks AR101 for Peanut Allergy Speaker Laura Hansen, PhD, VP, Investor Relations Jayson
Conference Call for Investment Community Nov 19, 2018 Agenda for Today s Call Topic Safe Harbor Statement Opening Remarks AR101 for Peanut Allergy Speaker Laura Hansen, PhD, VP, Investor Relations Jayson
Conference Call to Discuss FDA Approval of ONPATTRO (patisiran)
 Conference Call to Discuss FDA Approval of ONPATTRO (patisiran) August 10, 2018 1 Agenda Welcome Christine Lindenboom Vice President, Investor Relations & Corporate Communications Introduction John Maraganore,
Conference Call to Discuss FDA Approval of ONPATTRO (patisiran) August 10, 2018 1 Agenda Welcome Christine Lindenboom Vice President, Investor Relations & Corporate Communications Introduction John Maraganore,
Committed to Transforming the Treatment Paradigm for Migraine Prevention
 June 14, 2018 Committed to Transforming the Treatment Paradigm for Migraine Prevention September 6, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking
June 14, 2018 Committed to Transforming the Treatment Paradigm for Migraine Prevention September 6, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking
Grave s orbitopathy an approach to clinical evaluation and management
 Grave s orbitopathy an approach to clinical evaluation and management T W N Karunasena 1, M W S Niranjala 2 Sri Lanka Journal of Diabetes, Endocrinology and Metabolism 2012; 2: 106-110 Abstract Grave s
Grave s orbitopathy an approach to clinical evaluation and management T W N Karunasena 1, M W S Niranjala 2 Sri Lanka Journal of Diabetes, Endocrinology and Metabolism 2012; 2: 106-110 Abstract Grave s
Jefferies Healthcare Conference
 Jefferies Healthcare Conference June 7, 2016 NASDAQ: CHMA Forward-Looking Statements These slides contain forward-looking statements and information. The use of words such as may, might, will, should,
Jefferies Healthcare Conference June 7, 2016 NASDAQ: CHMA Forward-Looking Statements These slides contain forward-looking statements and information. The use of words such as may, might, will, should,
Thyroid Gland. Patient Information
 Thyroid Gland Patient Information Contact details for Endocrine and Thyroid Clinics Hawke s Bay Fallen Soldiers Memorial Hospital Villa 16 Phone: 06 8788109 ext 5891 Text: 0274 102 559 Email: endoclinic@hbdhb.govt.nz
Thyroid Gland Patient Information Contact details for Endocrine and Thyroid Clinics Hawke s Bay Fallen Soldiers Memorial Hospital Villa 16 Phone: 06 8788109 ext 5891 Text: 0274 102 559 Email: endoclinic@hbdhb.govt.nz
Lids and Orbits A Patient s Perspective. Dr. Paul Cauchi Consultant Ophthalmologist Southern General and Gartnavel General Hospitals, Glasgow
 Lids and Orbits A Patient s Perspective Dr. Paul Cauchi Consultant Ophthalmologist Southern General and Gartnavel General Hospitals, Glasgow Overview Pathophsiology. Symptoms. Signs. Management. Pathophysiology
Lids and Orbits A Patient s Perspective Dr. Paul Cauchi Consultant Ophthalmologist Southern General and Gartnavel General Hospitals, Glasgow Overview Pathophsiology. Symptoms. Signs. Management. Pathophysiology
Committed to Transforming the Treatment Paradigm for Migraine Prevention
 Committed to Transforming the Treatment Paradigm for Migraine Prevention 36th Annual J.P. Morgan Healthcare Conference January 8, 2018 Forward-Looking Statements This presentation and the accompanying
Committed to Transforming the Treatment Paradigm for Migraine Prevention 36th Annual J.P. Morgan Healthcare Conference January 8, 2018 Forward-Looking Statements This presentation and the accompanying
Innovation In Ophthalmics
 Innovation In Ophthalmics Ophthalmic Innovation Summit @ AAO 2018 October 25, 2018 Mark Iwicki Chairman & CEO, Kala Pharmaceuticals Disclaimers and Notices This presentation contains forward-looking statements
Innovation In Ophthalmics Ophthalmic Innovation Summit @ AAO 2018 October 25, 2018 Mark Iwicki Chairman & CEO, Kala Pharmaceuticals Disclaimers and Notices This presentation contains forward-looking statements
August 7, Q Financial Results
 August 7, 2018 Q2 2018 Financial Results 1 Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
August 7, 2018 Q2 2018 Financial Results 1 Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
NASDAQ: ZGNX. Company Presentation. October 2017
 NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
NASDAQ: ZGNX Company Presentation October 2017 2 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
Phase 3c Topline Results. Page 1
 Phase 3c Topline Results Page 1 Important Information Any statements in this presentation about future expectations, plans and prospects for the Company including the development and regulatory status
Phase 3c Topline Results Page 1 Important Information Any statements in this presentation about future expectations, plans and prospects for the Company including the development and regulatory status
PROMISE 2 Top-Line Data Results January 8, 2018
 PROMISE 2 Top-Line Data Results January 8, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking statements within the meaning of Section 27A
PROMISE 2 Top-Line Data Results January 8, 2018 Forward-Looking Statements This presentation and the accompanying commentary contains certain forward-looking statements within the meaning of Section 27A
November 2, Q Financial Results
 November 2, 2017 Q3 2017 Financial Results Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
November 2, 2017 Q3 2017 Financial Results Agenda Today s Speakers Paul Cox, Senior Director, Investor Relations Jeff Jonas, M.D., Chief Executive Officer Steve Kanes, M.D., Ph.D., Chief Medical Officer
UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam
 UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam as adjunctive treatment of partial-onset seizures in epilepsy Primary efficacy and safety data from the
UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam as adjunctive treatment of partial-onset seizures in epilepsy Primary efficacy and safety data from the
Graves Disease. What is Graves disease?
 Graves Disease What is Graves disease? The thyroid gland s production of thyroid hormones (T 3 and T 4 ) is triggered by thyroidstimulating hormone (TSH), which is made by the pituitary gland. Graves disease,
Graves Disease What is Graves disease? The thyroid gland s production of thyroid hormones (T 3 and T 4 ) is triggered by thyroidstimulating hormone (TSH), which is made by the pituitary gland. Graves disease,
SAKURA 3 Open-Label Phase 3 Safety Study with DaxibotulinumtoxinA for Injection (RT002) for the Treatment of Moderate to Severe Glabellar Lines
 SAKURA 3 Open-Label Phase 3 Safety Study with DaxibotulinumtoxinA for Injection (RT002) for the Treatment of Moderate to Severe Glabellar Lines Presented by Dan Browne, Co-Founder, President & CEO, and
SAKURA 3 Open-Label Phase 3 Safety Study with DaxibotulinumtoxinA for Injection (RT002) for the Treatment of Moderate to Severe Glabellar Lines Presented by Dan Browne, Co-Founder, President & CEO, and
ASCEND Phase 2 Trial of AXS-05 in MDD Topline Results Conference Call
 NASDAQ: AXSM ASCEND Phase 2 Trial of in MDD Topline Results Conference Call January 7, 2019 Overview in MDD ASCEND Phase 2 Trial Topline Results Introduction Mark Jacobson, Senior Vice President, Operations
NASDAQ: AXSM ASCEND Phase 2 Trial of in MDD Topline Results Conference Call January 7, 2019 Overview in MDD ASCEND Phase 2 Trial Topline Results Introduction Mark Jacobson, Senior Vice President, Operations
Thyroid Eye Disease. A Patient s Guide
 Sashank Prasad, MD www.brighamandwomens.org/neuro-ophthalmology A Patient s Guide Symptoms Diagnosis Treatment www.ishaol.com Prognosis What are the symptoms of Thyroid Eye Disease? Patients with Thyroid
Sashank Prasad, MD www.brighamandwomens.org/neuro-ophthalmology A Patient s Guide Symptoms Diagnosis Treatment www.ishaol.com Prognosis What are the symptoms of Thyroid Eye Disease? Patients with Thyroid
XP23829 PHASE 2 PSORIASIS TRIAL PRELIMINARY TOPLINE DATA PRESENTATION SEPTEMBER 15, 2015 COPYRIGHT 2015 XENOPORT, INC. ALL RIGHTS RESERVED.
 XP23829 PHASE 2 PSORIASIS TRIAL PRELIMINARY TOPLINE DATA PRESENTATION SEPTEMBER 15, 2015 COPYRIGHT 2015 XENOPORT, INC. ALL RIGHTS RESERVED. SAFE HARBOR DISCLAIMER These slides and the accompanying oral
XP23829 PHASE 2 PSORIASIS TRIAL PRELIMINARY TOPLINE DATA PRESENTATION SEPTEMBER 15, 2015 COPYRIGHT 2015 XENOPORT, INC. ALL RIGHTS RESERVED. SAFE HARBOR DISCLAIMER These slides and the accompanying oral
February 20, 2019 MANAGEMENT CALL TO DISCUSS PHASE 2 PHOENIX RESULTS AND CKD PROGRAM UPDATES
 February 20, 209 MANAGEMENT CALL TO DISCUSS PHASE 2 PHOENIX RESULTS AND CKD PROGRAM UPDATES Forward-Looking Statements This presentation contains certain forward-looking statements that are made pursuant
February 20, 209 MANAGEMENT CALL TO DISCUSS PHASE 2 PHOENIX RESULTS AND CKD PROGRAM UPDATES Forward-Looking Statements This presentation contains certain forward-looking statements that are made pursuant
EUGOGO INITIAL ASSESSMENT
 EUGOGO INITIAL ASSESSMENT Please complete non-italicised boxes except where indicated, plus relevant italicised ones. 1. Date of inclusion Year of birth Sex male female dd mm yyyy Race Caucasian Black
EUGOGO INITIAL ASSESSMENT Please complete non-italicised boxes except where indicated, plus relevant italicised ones. 1. Date of inclusion Year of birth Sex male female dd mm yyyy Race Caucasian Black
Orbital and Ocular Adnexal Disorders with Red Eyes
 Orbital and Ocular Adnexal Disorders with Red Eyes Jason Lee Associate Consultant Department of Ophthalmology and Visual Sciences Practical Ophthalmology for the Family Physician 21 Jan 2017 No financial
Orbital and Ocular Adnexal Disorders with Red Eyes Jason Lee Associate Consultant Department of Ophthalmology and Visual Sciences Practical Ophthalmology for the Family Physician 21 Jan 2017 No financial
Orbital facia. Periororbital facia Orbital septum Bulbar facia Muscular facia
 Anatomy Orbital facia Periororbital facia Orbital septum Bulbar facia Muscular facia Physiology of symptoms 1) Proptosis ( exophthalmos) Pseudoproptosis Axial Non axial Pulsating Positional Intermittent
Anatomy Orbital facia Periororbital facia Orbital septum Bulbar facia Muscular facia Physiology of symptoms 1) Proptosis ( exophthalmos) Pseudoproptosis Axial Non axial Pulsating Positional Intermittent
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017
 SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
SER-287 Phase 1b topline study results in patients with mild-to-moderate Ulcerative Colitis October 2, 2017 Leading the Microbiome Revolution Forward Looking Statements Some of the statements in this presentation
Tonix Pharmaceuticals Reports Top Line Results From Phase 2b BESTFIT Trial of TNX-102 SL in Patients With Fibromyalgia
 September 29, 2014 Tonix Pharmaceuticals Reports Top Line Results From Phase 2b BESTFIT Trial of TNX-102 SL in Patients With Fibromyalgia Conference Call Today at 8:30 a.m. ET NEW YORK, Sept. 29, 2014
September 29, 2014 Tonix Pharmaceuticals Reports Top Line Results From Phase 2b BESTFIT Trial of TNX-102 SL in Patients With Fibromyalgia Conference Call Today at 8:30 a.m. ET NEW YORK, Sept. 29, 2014
PRO 140. First self-administered antibody therapy for HIV in late-stage clinical development. March
 PRO 140 First self-administered antibody therapy for HIV in late-stage clinical development March 2018 Forward-Looking Statements This presentation includes forward-looking statements and forward-looking
PRO 140 First self-administered antibody therapy for HIV in late-stage clinical development March 2018 Forward-Looking Statements This presentation includes forward-looking statements and forward-looking
IRONWOOD AND FOREST ANNOUNCE POSITIVE LINACLOTIDE RESULTS FROM PHASE 3 TRIAL IN PATIENTS WITH IRRITABLE BOWEL SYNDROME WITH CONSTIPATION
 FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
FOR IMMEDIATE RELEASE Ironwood Contact: Forest Contact: Susan Brady Frank J. Murdolo Corporate Communications Vice President, Investor Relations 617.621.8304 212.224.6714 sbrady@ironwoodpharma.com frank.murdolo@frx.com
This slide kit covers more complex thyroid eye disease.
 An imbalance in the normal level of thyroid hormone in the body can cause thyroid eye disease. If you wish to explore information on the basics of thyroid eye diseases, please first see: https://www.excemed.org/manage-thyroid-online/resources/thyroid-eyedisease
An imbalance in the normal level of thyroid hormone in the body can cause thyroid eye disease. If you wish to explore information on the basics of thyroid eye diseases, please first see: https://www.excemed.org/manage-thyroid-online/resources/thyroid-eyedisease
Liquid Biopsies. Next Generation Cancer Molecular Diagnostics
 Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
GLPG1690 FLORA topline results
 GLPG1690 FLORA topline results Webcast presentation 10 August 2017 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the potential activity
GLPG1690 FLORA topline results Webcast presentation 10 August 2017 Disclaimer This presentation contains forward-looking statements, including (without limitation) statements concerning the potential activity
AVEO and Astellas Report Final Overall Survival Results from TIVO-1
 AVEO and Astellas Report Final Overall Survival Results from TIVO-1 - Median Overall Survival of 28.8 Months Reported for Tivozanib in Patients with Advanced Kidney Cancer - CAMBRIDGE, Mass. and TOKYO,
AVEO and Astellas Report Final Overall Survival Results from TIVO-1 - Median Overall Survival of 28.8 Months Reported for Tivozanib in Patients with Advanced Kidney Cancer - CAMBRIDGE, Mass. and TOKYO,
About X-Linked Hypophosphatemia (XLH)
 Ultragenyx and Kyowa Kirin Announce Publication of Phase 2 Study Results Demonstrating that Crysvita (burosumab) Improved Outcomes in Children with X-linked Hypophosphatemia in the New England Journal
Ultragenyx and Kyowa Kirin Announce Publication of Phase 2 Study Results Demonstrating that Crysvita (burosumab) Improved Outcomes in Children with X-linked Hypophosphatemia in the New England Journal
The New England Journal of Medicine. A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer
 The New England Journal of Medicine A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer May 18, 2015 Forward-Looking Statements Various remarks that we make on this call that are
The New England Journal of Medicine A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer May 18, 2015 Forward-Looking Statements Various remarks that we make on this call that are
Savient's Pegloticase Data in Treatment-Failure Gout Patients Presented at 72nd Annual Meeting of the American College of Rheumatology Conference
 Savient's Pegloticase Data in Treatment-Failure Gout Patients Presented at 72nd Annual Meeting of the American College of Rheumatology Conference EAST BRUNSWICK, N.J., Oct 27, 2008 /PRNewswire-FirstCall
Savient's Pegloticase Data in Treatment-Failure Gout Patients Presented at 72nd Annual Meeting of the American College of Rheumatology Conference EAST BRUNSWICK, N.J., Oct 27, 2008 /PRNewswire-FirstCall
STUDY 1 PHASE 3 TOP-LINE RESULTS. September 2017
 STUDY 1 PHASE 3 TOP-LINE RESULTS September 2017 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
STUDY 1 PHASE 3 TOP-LINE RESULTS September 2017 Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking
What was going on. Thyroid Related Orbitopathy. Pathophysiology. Definition GALLO EYE AND FACIAL PLASTIC SURGERY 3/16/2017
 GALLO EYE AND FACIAL PLASTIC SURGERY What was going on A. She found out the increase to her Premium and Deductible for employee health care next year B. She is getting an over agressive Physical Exam by
GALLO EYE AND FACIAL PLASTIC SURGERY What was going on A. She found out the increase to her Premium and Deductible for employee health care next year B. She is getting an over agressive Physical Exam by
37 th Annual J.P. Morgan Healthcare Conference. January 9, 2019
 37 th Annual J.P. Morgan Healthcare Conference January 9, 2019 Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts,
37 th Annual J.P. Morgan Healthcare Conference January 9, 2019 Forward Looking Statement This presentation includes forward-looking statements. All statements, other than statements of historical facts,
10 Congresso Nazionale Associazione Italiana della Tiroide Cagliari, dicembre 2016 Orbitopa)a basedowiana acuta
 10 Congresso Nazionale Associazione Italiana della Tiroide Cagliari, 15-17 dicembre 2016 Orbitopa)a basedowiana acuta Luigi Bartalena Università dell Insubria a Varese Prevalence of Graves orbitopathy
10 Congresso Nazionale Associazione Italiana della Tiroide Cagliari, 15-17 dicembre 2016 Orbitopa)a basedowiana acuta Luigi Bartalena Università dell Insubria a Varese Prevalence of Graves orbitopathy
Corporate Presentation. March 2019
 Corporate Presentation March 2019 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other
Corporate Presentation March 2019 Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other
New SEL-212 Phase 2 Data Presented at EULAR. June 15, 2018
 New SEL-1 Phase Data Presented at EULAR June 15, 1 Safe Harbor / Disclaimer Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. ( the company
New SEL-1 Phase Data Presented at EULAR June 15, 1 Safe Harbor / Disclaimer Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. ( the company
INVESTOR PRESENTATION
 INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
INVESTOR PRESENTATION May 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities regulation.
Graves Ophthalmopathy Advances in Diagnosis and Treatment CONSTANCE L. FRY, MD ASSOCIATE PROFESSOR OF OPHTHALMOLOGY UT HEALTH SAN ANTONIO
 Graves Ophthalmopathy Advances in Diagnosis and Treatment CONSTANCE L. FRY, MD ASSOCIATE PROFESSOR OF OPHTHALMOLOGY UT HEALTH SAN ANTONIO Ophthalmic Manifestations GO is the #1 cause of unilateral and
Graves Ophthalmopathy Advances in Diagnosis and Treatment CONSTANCE L. FRY, MD ASSOCIATE PROFESSOR OF OPHTHALMOLOGY UT HEALTH SAN ANTONIO Ophthalmic Manifestations GO is the #1 cause of unilateral and
Phase 3 Top-Line Results Show IBRANCE in Combination with Fulvestrant Meets Progression-Free Survival (PFS) Primary Endpoint
 For immediate release: April 15, 2015 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Announces PALOMA-3 Trial for IBRANCE (Palbociclib) Stopped Early Due
For immediate release: April 15, 2015 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Announces PALOMA-3 Trial for IBRANCE (Palbociclib) Stopped Early Due
SUMMARY OF RANDOMIZED CONTROLLED TRIALS COMPARING DIFFERENT ORBITAL RADIOTHERAPY REGIMENTS AND ORBITAL RADIOTHERAPY TO PLACEBO OR CORTICOSTEROIDS
 TABLE e4. SUMMARY OF RANDOMIZED CONTROLLED TRIALS COMPARING DIFFERENT ORBITAL RADIOTHERAPY REGIMENTS AND ORBITAL RADIOTHERAPY TO PLACEBO OR CORTICOSTEROIDS Study Treatment Groups Primary Outcome Measure
TABLE e4. SUMMARY OF RANDOMIZED CONTROLLED TRIALS COMPARING DIFFERENT ORBITAL RADIOTHERAPY REGIMENTS AND ORBITAL RADIOTHERAPY TO PLACEBO OR CORTICOSTEROIDS Study Treatment Groups Primary Outcome Measure
Orbital decompression surgery for proptosis
 Orbital decompression surgery for proptosis Procedure information Information for patients Ophthalmology (Ocular Plastics) Large Print This leaflet explains about the problem you have and how surgery can
Orbital decompression surgery for proptosis Procedure information Information for patients Ophthalmology (Ocular Plastics) Large Print This leaflet explains about the problem you have and how surgery can
Building a Premier Oncology Biotech
 Corporate Deck Building a Premier Oncology Biotech Dr. Helen Torley, President and CEO November 2018 Forward-Looking Statements All of the statements in this presentation that are not statements of historical
Corporate Deck Building a Premier Oncology Biotech Dr. Helen Torley, President and CEO November 2018 Forward-Looking Statements All of the statements in this presentation that are not statements of historical
Revefenacin (TD-4208) Phase 3 Efficacy Results
 Revefenacin (TD-4208) Phase 3 Efficacy Results Once-daily, Nebulized Long-Acting Muscarinic Antagonist (LAMA) October 20, 2016 THERAVANCE, the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE are registered
Revefenacin (TD-4208) Phase 3 Efficacy Results Once-daily, Nebulized Long-Acting Muscarinic Antagonist (LAMA) October 20, 2016 THERAVANCE, the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE are registered
Graves Ophthalmopathy Overview. Graves Disease Hyperthyroidism TSIgs (anti-tsh-receptor-abs) Graves Disease 10/22/2010
 Graves Disease Robert Graves Graves Ophthalmopathy Overview Robert C. Kersten Dept. of Ophthalmology UCSF Triad Hyperthyroidism Eye findings Pretibial Myxedema (phalangeal acropachy-1%) Most common auto-immune
Graves Disease Robert Graves Graves Ophthalmopathy Overview Robert C. Kersten Dept. of Ophthalmology UCSF Triad Hyperthyroidism Eye findings Pretibial Myxedema (phalangeal acropachy-1%) Most common auto-immune
Building a Premier Oncology Biotech
 Wells Fargo Securities Healthcare Conference Building a Premier Oncology Biotech Dr. Helen Torley, President and CEO September 2018 Forward-Looking Statements All of the statements in this presentation
Wells Fargo Securities Healthcare Conference Building a Premier Oncology Biotech Dr. Helen Torley, President and CEO September 2018 Forward-Looking Statements All of the statements in this presentation
ADDITIONAL DOSAGE STRENGTHS OF OTREXUP (METHOTREXATE) INJECTION APPROVED BY FDA
 ADDITIONAL DOSAGE STRENGTHS OF OTREXUP (METHOTREXATE) INJECTION APPROVED BY FDA Now Available 12.5 mg/0.4 ml, 17.5 mg/0.4 ml and 22.5 mg/0.4 ml EWING, N.J., March 29, 2016 Antares Pharma, Inc. (NASDAQ:
ADDITIONAL DOSAGE STRENGTHS OF OTREXUP (METHOTREXATE) INJECTION APPROVED BY FDA Now Available 12.5 mg/0.4 ml, 17.5 mg/0.4 ml and 22.5 mg/0.4 ml EWING, N.J., March 29, 2016 Antares Pharma, Inc. (NASDAQ:
Thyroid Disorders. January 2019
 Thyroid Disorders January 2019 What is the Thyroid? The thyroid is a small butterfly-shaped gland inside the neck, located in front of the trachea (windpipe) and below the larynx (voicebox). It produces
Thyroid Disorders January 2019 What is the Thyroid? The thyroid is a small butterfly-shaped gland inside the neck, located in front of the trachea (windpipe) and below the larynx (voicebox). It produces
Investor Presentation
 Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
Investor Presentation February 2018 2 FORWARD-LOOKING INFORMATION The following presentation contains statements that are considered forward-looking information ( FLI ) within the meaning of securities
- Amendment accelerates anticipated PROSPER top-line results by two years -
 Pfizer Contacts: For Media Dean Mastrojohn (212) 733-6944 dean.mastrojohn@pfizer.com For Investors Ryan Crowe (212) 733-8160 ryan.crowe@pfizer.com Astellas Contact: For Media Tyler Marciniak (847) 736-7145
Pfizer Contacts: For Media Dean Mastrojohn (212) 733-6944 dean.mastrojohn@pfizer.com For Investors Ryan Crowe (212) 733-8160 ryan.crowe@pfizer.com Astellas Contact: For Media Tyler Marciniak (847) 736-7145
EUGOGO FOLLOW-UP assessment
 EUGOGO FOLLOW-UP assessment Please complete non-italicized boxes except where indicated, plus relevant italicized ones. F1. Date follow-up (dd mm yyyy) Visit # Year of birth ( yyyy ) Randomization code
EUGOGO FOLLOW-UP assessment Please complete non-italicized boxes except where indicated, plus relevant italicized ones. F1. Date follow-up (dd mm yyyy) Visit # Year of birth ( yyyy ) Randomization code
Validation of VISA classification for Thyroid Associated Orbitopathy
 25 Original Article Validation of VISA classification for Thyroid Associated Orbitopathy Dr M. Subrahmanyam 1, Dr P. Modini 2, Dr K.J. Sivacharan 3, Dr B. Kavya 4 Abstract Aim : To Validate the VISA classification
25 Original Article Validation of VISA classification for Thyroid Associated Orbitopathy Dr M. Subrahmanyam 1, Dr P. Modini 2, Dr K.J. Sivacharan 3, Dr B. Kavya 4 Abstract Aim : To Validate the VISA classification
Two-stage study designed to evaluate tolerability and efficacy of pracinostat combined with azacitidine in patients with high and very high risk MDS
 Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
Helsinn Group and MEI Pharma Announce First Patient Dosed in Phase 2 Dose-Optimization Study of Pracinostat and Azacitidine in Myelodysplastic Syndrome Two-stage study designed to evaluate tolerability
DARA Reports Year-End 2012 Financial Results
 April 1, 2013 DARA Reports Year-End 2012 Financial Results Company Provides a Commercial Portfolio and Development Pipeline Business Update RALEIGH, NC -- (MARKETWIRE) -- 04/01/13 -- DARA BioSciences,
April 1, 2013 DARA Reports Year-End 2012 Financial Results Company Provides a Commercial Portfolio and Development Pipeline Business Update RALEIGH, NC -- (MARKETWIRE) -- 04/01/13 -- DARA BioSciences,
Investor Presentation March 2015
 Investor Presentation March 2015 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking statements within the
Investor Presentation March 2015 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking statements within the
New Ideas. Better Medicines. Third Quarter Financial Results Conference Call
 New Ideas. Better Medicines. Third Quarter 2018 Financial Results Conference Call Forward-Looking Statements 2 This presentation contains forward-looking statements that involve substantial risks and uncertainties.
New Ideas. Better Medicines. Third Quarter 2018 Financial Results Conference Call Forward-Looking Statements 2 This presentation contains forward-looking statements that involve substantial risks and uncertainties.
UBS Global Healthcare Conference May 19, 2014
 UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
UBS Global Healthcare Conference May 19, 2014 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section
Translating Science. Transforming Lives. ACT DMD Clinical Trial Results
 Translating Science. Transforming Lives ACT DMD Clinical Trial Results FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
Translating Science. Transforming Lives ACT DMD Clinical Trial Results FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
Broad and clinically important benefits beyond the initial registrational endpoints are now reported.
 Biohaven Announces Robust Clinical Data with Single Dose Rimegepant That Defines Acute and Durable Benefits to Patients: The First Oral CGRP Receptor Antagonist to Deliver Positive Data on Pain Freedom
Biohaven Announces Robust Clinical Data with Single Dose Rimegepant That Defines Acute and Durable Benefits to Patients: The First Oral CGRP Receptor Antagonist to Deliver Positive Data on Pain Freedom
Cx601 ADMIRE-CD Top-Line Results Webcast. 24 August 2015
 Cx601 ADMIRE-CD Top-Line Results Webcast 24 August 2015 1 Cx601 ADMIRE-CD Top-Line Results Webcast Speakers Mr Eduardo Bravo, Chief Executive Officer Dr Julián Panés, Head of Gastroenterology Department,
Cx601 ADMIRE-CD Top-Line Results Webcast 24 August 2015 1 Cx601 ADMIRE-CD Top-Line Results Webcast Speakers Mr Eduardo Bravo, Chief Executive Officer Dr Julián Panés, Head of Gastroenterology Department,
LJPC-401 Phase 1 Results and Development Update. September 7, 2016
 LJPC-401 Phase 1 Results and Development Update September 7, 2016 Forward-Looking Statements These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation Reform
LJPC-401 Phase 1 Results and Development Update September 7, 2016 Forward-Looking Statements These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation Reform
Supernus Pharmaceuticals
 Supernus Pharmaceuticals Jefferies 2016 Healthcare Conference May 2016 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include
Supernus Pharmaceuticals Jefferies 2016 Healthcare Conference May 2016 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include
Phase 3 APPROACH Study Top-line Results
 Phase 3 APPROACH Study Top-line Results March 2017 Ionis and Akcea Participants Dr. Stanley Crooke CEO and Chairman Ionis Pharmaceuticals Dr. Louis St. L. O'Dea CMO Akcea Therapeutics Paula Soteropoulos
Phase 3 APPROACH Study Top-line Results March 2017 Ionis and Akcea Participants Dr. Stanley Crooke CEO and Chairman Ionis Pharmaceuticals Dr. Louis St. L. O'Dea CMO Akcea Therapeutics Paula Soteropoulos
ARDELYX REPORTS POSITIVE T3MPO-2 PHASE 3 TRIAL RESULTS IN IBS-C
 ARDELYX REPORTS POSITIVE T3MPO-2 PHASE 3 TRIAL RESULTS IN IBS-C OCTOBER 11, 2017 NASDAQ: ARDX FORWARD-LOOKING STATEMENTS To the extent that statements contained in this presentation are not descriptions
ARDELYX REPORTS POSITIVE T3MPO-2 PHASE 3 TRIAL RESULTS IN IBS-C OCTOBER 11, 2017 NASDAQ: ARDX FORWARD-LOOKING STATEMENTS To the extent that statements contained in this presentation are not descriptions
Pierre Legault CEO June 2, 2014
 April 2012 Pierre Legault CEO June 2, 2014 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
April 2012 Pierre Legault CEO June 2, 2014 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Novartis announces Phase III STRIVE data published in NEJM demonstrating significant and sustained efficacy of erenumab in migraine prevention
 Novartis International AG Novartis Global Communications CH-4002 Basel Switzerland http://www.novartis.com MEDIA RELEASE COMMUNIQUE AUX MEDIAS MEDIENMITTEILUNG Novartis announces Phase III STRIVE data
Novartis International AG Novartis Global Communications CH-4002 Basel Switzerland http://www.novartis.com MEDIA RELEASE COMMUNIQUE AUX MEDIAS MEDIENMITTEILUNG Novartis announces Phase III STRIVE data
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC. August 6, 2015
 Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
Aquinox Q2 /2015 Conference Call: LEADERSHIP Secondary Endpoint Update - AQX-1125 in BPS/IC August 6, 2015 Forward Looking Statements / Safe Harbor This presentation and the accompanying oral commentary
Jefferies Healthcare Conference. June 6, 2018
 Jefferies Healthcare Conference June 6, 2018 Forward-Looking Statements and Non-GAAP Financial Information Some statements in this presentation may be forward-looking statements for purposes of the Private
Jefferies Healthcare Conference June 6, 2018 Forward-Looking Statements and Non-GAAP Financial Information Some statements in this presentation may be forward-looking statements for purposes of the Private
Goiter. This reference summary explains goiters. It covers symptoms and causes of the condition, as well as treatment options.
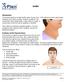 Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
Results Confirm and Extend 40-Week Findings that Treatment with Crysvita is Superior to Conventional Therapy
 Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
Supernus Pharmaceuticals
 Supernus Pharmaceuticals Investor Presentation March 2017 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking
Supernus Pharmaceuticals Investor Presentation March 2017 1 Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking
Rhopressa TM Rocket 2 Phase 3 Topline Results
 Rhopressa TM Rocket 2 Phase 3 Topline Results 1 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved
Rhopressa TM Rocket 2 Phase 3 Topline Results 1 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved
Building a Fully Integrated Biopharmaceutical Company. June 2014
 Building a Fully Integrated Biopharmaceutical Company June 2014 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
Building a Fully Integrated Biopharmaceutical Company June 2014 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE
 37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
37 th ANNUAL JP MORGAN HEALTHCARE CONFERENCE January 2019 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as
CAELUM BIOSCIENCES. Corporate Overview May, 2017
 CAELUM BIOSCIENCES Corporate Overview May, 2017 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning
CAELUM BIOSCIENCES Corporate Overview May, 2017 Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward looking statements within the meaning
Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation
 January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
January 19, 2017 Synergy Pharmaceuticals TRULANCE (Plecanatide) Receives U.S. FDA Approval for the Treatment of Adults with Chronic Idiopathic Constipation NEW YORK--(BUSINESS WIRE)-- Synergy Pharmaceuticals
Acorda Acquisition of Civitas Therapeutics. September 24, 2014
 Acorda Acquisition of Civitas Therapeutics September 24, 2014 Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform
Acorda Acquisition of Civitas Therapeutics September 24, 2014 Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform
